Abstract
Oxidative stress plays a key role in age-related macular degeneration and hereditary retinal degenerations. Light damage in rodents has been used extensively to model oxidative stress-induced photoreceptor degeneration, and photo-oxidative injury from blue light is particularly damaging to photoreceptors. The endogenous factors protecting photoreceptors from oxidative stress, including photo-oxidative stress, are continuing to be elucidated. In this study, we evaluated the effect of blue light exposure on photoreceptors and its relationship to Nrf2 using cultured murine photoreceptor (661W) cells. 661W cells were exposed to blue light at 2500 lux. Exposure to blue light for 6 to 24 hours resulted in a significant increase in intracellular reactive oxygen species (ROS) and death of 661W cells in a time-dependent fashion. Blue light exposure resulted in activation of Nrf2, as indicated by an increase in nuclear translocation of Nrf2. This was associated with a significant induction of expression of Nrf2 as well as an array of Nrf2 target genes, including antioxidant genes, as indicated by quantitative reverse transcription PCR (qRT-PCR). In order to determine the functional role of Nrf2, siRNA-mediated knockdown studies were performed. Nrf2-knockdown in 661W cells resulted in significant exacerbation of blue light-induced reactive oxygen species levels as well as cell death. Taken together, these findings indicate that Nrf2 is an important endogenous protective factor against oxidative stress in photoreceptor cells. This suggests that drugs targeting Nrf2 could be considered as a neuroprotective strategy for photoreceptors in AMD and other retinal conditions.
Keywords: photoreceptor, oxidative stress, Nrf2, neuroprotection
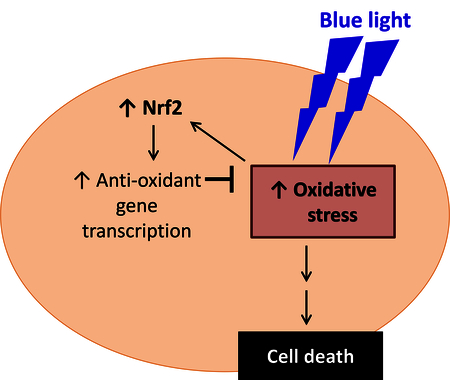
1. Introduction
Oxidative stress is thought to have a major role in the progression of age-related macular degeneration (AMD)(Beatty et al., 2000; Winkler et al., 1999; Zarbin, 2004) and is also implicated in photoreceptor cell death in hereditary retinal degenerations, including cone photoreceptors in mouse models of retinitis pigmentosa (RP) (Komeima et al., 2006; Shen et al., 2005). Light-induced damage, including photo-oxidative stress, has received significant attention as a pathogenic contributor to photoreceptor death (Organisciak and Vaughan, 2010). This effect is augmented by oxygen and its initiation of free-radical chain reactions (Young, 1988), especially as the outer retina has a higher concentration of oxygen from the choriocapillaris. The blue wavelength (higher energy) end of the visible spectrum (400–500 nm)is much more injurious than the green and red portion (500–700 nm)(Young, 1988). Blue light exposure may therefore contribute to the pathogenesis of AMD (Glazer-Hockstein and Dunaief, 2006; Margrain et al., 2004). The contribution of blue light in the years ahead may become intensified with the increasing exposure to video display terminals that emit an abundance of blue light and the increased reliance on light-emitting diodes (LEDs) as light sources. Indeed, phototoxicity of LEDs on the retina has been demonstrated to exert strong damage to photoreceptors (Jaadane et al., 2015). In addition to AMD, light induced injury has been used for several decades to model oxidative stress-induced photoreceptor degeneration (Organisciak and Vaughan, 2010).
Therapies for nonexudative age-related macular degeneration and hereditary retinal degenerations remain limited. In order to develop additional treatment strategies to preserve photoreceptors, greater insights need to be gained relating to the factors involved in photoreceptor health. In particular, it would be of great interest to gain further insights into the endogenous protective mechanisms in photoreceptors.
Nrf2 (NF-E2-related factor 2) is a stress-response transcription factor which plays a role in cellular protection from endogenous and exogenous stresses in multiple tissues. Nrf2 is an important regulator of the antioxidant response in many cell types, serving as a protective mechanism against oxidative stress (Kensler et al., 2007). In the absence of stress, Nrf2 resides in the cytoplasm bound to the actin-anchored protein Keap1, which targets Nrf2 toward proteosomal degradation. Multiple inducers including reactive oxygen species can disrupt the interaction of Keap1 with Nrf2, allowing the nuclear translocation of Nrf2. Nrf2 then binds to the antioxidant response element and activates the expression of an array of cytoprotective and antioxidant genes (Kensler et al., 2007). With respect to neurodegeneration, Nrf2 has received attention for its protective role against oxidative stress and neuroinflammation in the central nervous system (Buendia et al., 2016; Esteras et al., 2016). In the retina, Nrf2 was found to be protective in retinal ganglion cells in a rodent model of optic nerve crush injury (Himori et al., 2013). Our lab has reported that Nrf2 plays a cytoprotective role in retinal ganglion cells in the context of retinal ischemia-reperfusion injury (Cho et al., 2015; Wei et al., 2011; Xu et al., 2015). Strikingly, Xiong et al. recently demonstrated that AAV-mediated gene transfer of Nrf2 improved cone photoreceptor cell survival in murine models of retinitis pigmentosa (Xiong et al., 2015), indicating that exogenous activation of Nrf2 can confer photoreceptor protection in these genetic models.
In the current study, we sought to gain further insights into the role of Nrf2 in photoreceptor health. Important issues of interest include implicating the role of Nrf2 as an endogenous cell-intrinsic protective mechanism in photoreceptors under stress and its specific function in the context of blue-light induced photo-oxidative injury. For this purpose, we used the mouse-derived 661W photoreceptor cell line (Tan et al., 2004), which has yielded multiple insights into light-induced retinal damage (Santos et al., 2012; Yang et al., 2007), including blue light-induced oxidative stress and cell death (Kuse et al., 2014; Ogawa et al., 2014).
2. Methods
2.1. 661W cells
661W cells were obtained from Dr. Muayyad Al-Ubaidi, under a Material Transfer Agreement with the University of Oklahoma Health Sciences Center (Oklahoma City, OK). 661W is a mouse cone photoreceptor cell line immortalized by expression of simian virus (SV)40 T antigen (T-ag) driven by the human IRBP promoter (al-Ubaidi et al., 1992) and exhibit cellular and biochemical features characteristic of cone photoreceptor cells (Tan et al. 2004). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) plus 10%FBS (fetal bovine serum).For DCF and cell viability assays, 661W cells were seeded at a density of 3000 cells per well in 96-well plates. For Nrf2 target gene expression and western blot analyses, 661W cells were seeded at a density of 5 × 105 cells per well in 6-well plates and 2.5 × 106 cells per well in 60mm plates, respectively.
2.2. Blue light irradiation
661W cells were incubated in 5% CO2 at 37°C for 24 hours. Medium was replaced with DMEM containing 1% FBS, and then the plates were placed above a blue light source(HQRP 13.8W 225 LED Blue Spectrum Hydroponic Plant Grow Light Panel/Lamp, US)at a distance to keep the light intensity at 2500 lux (Fig. 1). The wavelength of the blue light source was 465 nm. The energy was measured by Dr.Meter LX1010B 100,000 Light Meter with LCD Display (Hisgadget Inc., US). For DCF and cell viability assays, control groups were maintained in the same plate and covered by aluminum foil. For nuclear translocation and target gene expression studies, control plates were concurrently placed in a separate incubator without blue light source for the duration of the study.
Fig. 1.

The light-emitting diode-based blue light source used in the current study. The rack above the light panel maintains the desired distance between the cell culture plates and the blue light source. The light intensity was measured by Dr. Meter LX1010B 100,000 Light Meter with LCD Display (Hisgadget Inc, US) as 2500 lux.
2.3. DCF assay
ROS production was quantified by dichlorofluorescein (DCF) assay. After blue light irradiation, medium was removed, and 5-(and-6)-chloromethyl-20,70-dichlorodihydrofluoresceindiacetate (CM-H2DCFDA) (10 μmol/L, Invitrogen) was then added to cells at a final concentration of 10 μΜ, followed by incubation for 1 hr. Fluorescence was then measured with a spectrophotometer (BMG LabtechInc., Durham, NC, USA) at 482 nm (excitation) and 535 nm (emission).Fluorescence measured immediately after addition of reagent (without subsequent incubation) served as the background signal.
2.4. Cell viability (ATP content) assay
The ATP content, which reflects the number of viable cells, was measured using the Celltiter-Glo luminescent cell viability assay (Promega, Madison, WI, USA). 100μL Celltiter-Glo reagent was added to each well containing 100μL growth medium. The plates were placed on an orbital shaker for 10 minutes to mix contents and induce cell lysis. After incubation at room temperature for 20 min, luminescence was read with a spectrophotometer (BMG LabtechInc., Durham, NC, USA).
2.5. Cell death study
After 24 hours of exposure to blue light, Hoechst 33342 (2.75μg/mL, Life Technologies) and ethidiumhomodimer-1 (2μM, Life Technologies) were added to the plates. Double staining was performed for cell scanning. Hoechst 33342 stained the nuclei of all cells, while ethidium homodimer-1 stained only the dead cells. The cells were counted using the CellomicsArrayScan VTl HCS reader (Thermo Fisher Scientific, Waltham, MA). The percentage of ethidium homodimer-1-positive cells was calculated.
2.6. Western blot analysis
For analysis of Nrf2 nuclear translocation, nuclear extracts from cultured cells were prepared using NEPER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Waltham, MA, USA). The concentrations of the extracted nuclear samples were measured using the DC™ Protein Assay Kit (BioRad Laboratories, Hercules, CA, USA). 15 micrograms of protein were subjected to western blot analysis for each treatment condition. Anti-Nrf2 (Cell Signaling Technology, Danvers, MA, USA) and anti-Lamin B antibodies (Santa Cruz Biotechnology, Dallas, Texas, USA) were used for Western blot analysis.
2.7. Real-time PCR analysis
RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA,USA), and single-stranded cDNA was synthesized using MMLVReverse Transcriptase (Invitrogen). Real-time PCR was performed withQuantiTect SYBR Green PCR Kit (Qiagen) with StepOnePlus real-timePCR system (Applied Biosystems). The qPCR primers wereHO-1: (5’-ATGACACCAAGGACCAGAGC-3’)and (5’-GTGTAAGGACCCATCGGAGA-3’);NQO1: (5’-CAGCTCACCGAGAGCCTAGT-3’) and (5’-ACCACCTCCCATCCTTTCTT-3’); GCLC: (5’-ACCATCATCAATGGGAAGGA-3’) and (5’-GCGATAAACTCCCTCATCCA-3’); GCLM: (5’ TGGAGCAGCTGTATCAGTGG-3’) and (5’-AGAGCAGTTCTTTCGGGTCA-3’); GSTM1: (5’-AGTGGGTGGGAAAGGGTCATTACA-3’)and (5’-TAGCCAAGGCTTCTGCTGGTACTT-3’); TXNRD1: (5’-AACTTTCAGAAGGGCCAGGT-3’) and (5’-GTAGACAGGGGCGAAGACTG-3’);GAPDH:(5’-CGACTTCAACAGCAACTCCCACTCTTCC-3’) and (5’-TGGGTGGTCCAGGGTTTCTTACTCCTT-3’) was used for normalization.
2.8. Nrf2 knockdown
661W Cells were seeded to be 70% confluent on the day of transfection. Lipofectamine® RNAiMAX reagent (Invitrogen,Thermo Fisher Scientific, Waltham, MA, USA ) and siRNA were diluted in Opti-MEM® Medium (Thermo Fisher Scientific, Waltham, MA, USA ) separately. Diluted siRNA was added to diluted Lipofectamine® RNAiMAX at a ratio of 1:1. After incubation for 5 minutes at room temperature, the complex was added to cells. Transfection was performed with either negative control siRNA and Nrf2 siRNA (s70522)(AppliedBiosystems, Foster city, CA, USA). The efficacy of siRNA-mediated Nrf2 knockdown in transfected cells was evaluated by measures of mRNA level by real-time PCR.
2.9. Statistical analysis
Data were presented as mean ± SD. The Student’s t-test was used for two groups’ comparison. A value of p <0.05 was considered statistically significant.
3. Results
3.1. Blue light induces ROS and cell death in 661W cells
We first investigated production of reactive oxygen species (ROS) by 661W cells after blue LED light irradiation for 6 to 24 hours at 2500 lux. We found that blue LED light exposure resulted in 1.45-fold increase in ROS production after irradiation for 6 hours, 1.72-fold increase for 16 hours, and 1.96-fold (p < 0.001; n=5) increase for 24 hours compared with control group (Fig. 2A). We next evaluated 661W cell viability, as reflected by ATP content, to determine whether this blue light-induced oxidative stress was associated with photoreceptor cell death. ATP content was progressively decreased in a time-dependent fashion, to 87% (p < 0.05; n=5) after irradiation for 6 hours, 68% for 16 hours, and 49% (p < 0.001, n=5) after blue light exposure for 24 hours compared with control group (Fig. 2B).
Fig. 2.
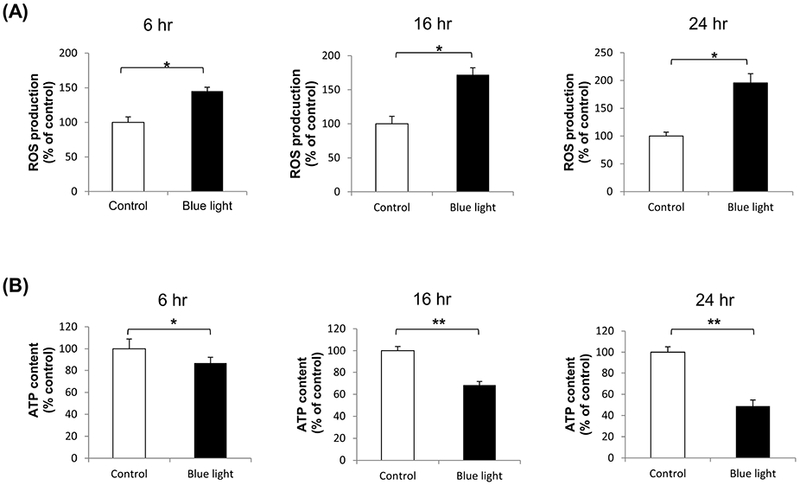
The effect of blue light on ROS production and cell viability. (A) Intracellular ROS levels were increased after exposure to blue light for 6 hours to 24 hours. There was 1.45-fold increase in ROS after irradiation for 6 hours, 1.72-fold increase for 16 hours, and 1.96-fold for 24 hours. ROS levels were determined by dichlorofluorescein (DCF) assay. Data were expressed as mean + SD. n = 5, *p< 0.001. (B) Cell survival, reflected by ATP content, was detected using Celltiter-Glo luminescent cell viability assay. After blue light irradiation for 6 hours, 16 hours, and 24 hours, ATP content was decreased to 87%, 68%, and 49%, respectively. Data were expressed as mean + SD. n = 5, *p < 0.05, **p < 0.001.
3.2. Blue light induces Nrf2 nuclear translocation in 661W cells
Reactive oxygen species are known to activate Nrf2 in multiple cell types. Given the blue light induction of ROS in 661W cells (Fig. 2A), we investigated whether blue light exposure resulted in activation of Nrf2, which is reflected by nuclear translocation (Kensler et al., 2007). We analyzed the nuclear translocation of Nrf2 after blue light irradiation for 6 and 16 hours. Western blot analysis demonstrated a statistically significant increase in nuclear Nrf2 in irradiated cells as compared to control cells at 6 hours of blue light exposure, with a further dramatic increase at 16 hours (Fig. 3A-B).
Fig. 3.
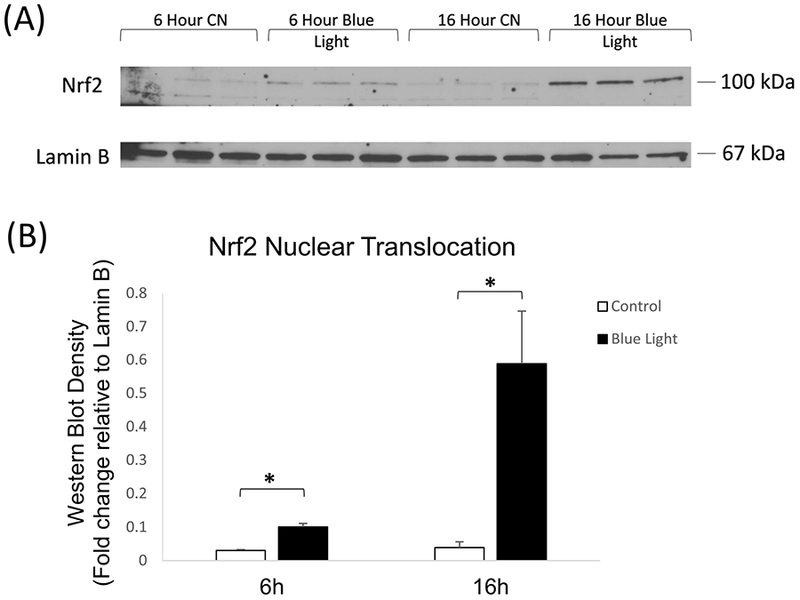
(A) Representative western blot analysis of Nrf2 nuclear translocation showed significant increase in nuclear localization after 6 and 16 hours irradiation with 465 nm blue light at 2500 lux. (B) Quantitation of western blot analysis of Nrf2 nuclear translocation by densitometry indicated significant induction by blue light after 6 and 24 hours of blue light irradiation. n = 3, *p < 0.05.
3.3. Blue light induces Nrf2 target gene expression
In association with the induction of Nrf2 nuclear translocation in 661W cells by blue light, we investigated the associated induction of Nrf2 target genes. We also analyzed induction of Nrf2 itself. For this purpose, mRNA levels were measured by real-time PCR for Nrf2 and an array of target genes which we previously reported to be induced by Nrf2 in the retina (Cho et al., 2015; Wei et al., 2011; Xu et al., 2015). After exposure to blue light at 2500 lux for 24 hours, the expression of Nrf2 was increased (p < 0.05, n=3). In addition, expression of multiple Nrf2 target genes was significantly increased (p < 0.01, n=3), including HO-1, NQO1,GCLC, GCLM, GSTM1, and TXNRD1 (Fig. 4).
Fig. 4.
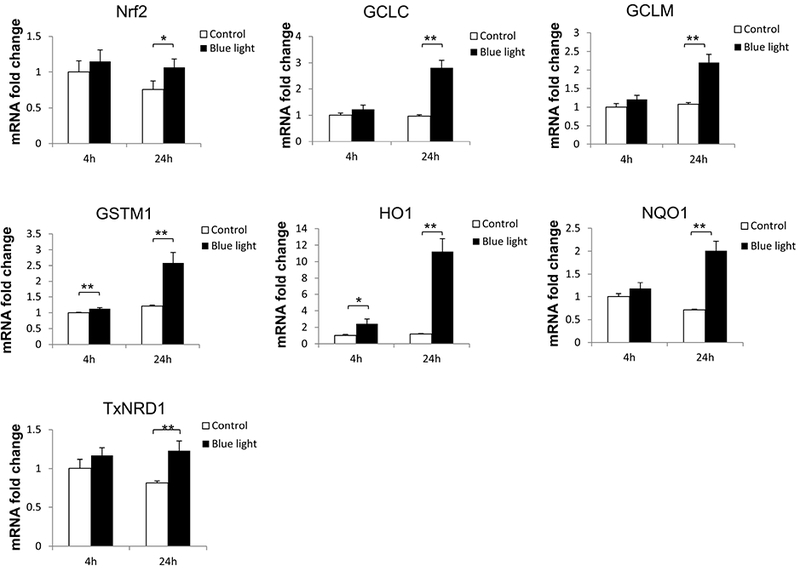
Blue light induced Nrf2 target gene expression. After exposure to blue light for 24 hours, multiple Nrf2-responsive antioxidant gene expression were significantly increased. Data were expressed as mean + SD. n = 3, *p < 0.05. **p < 0.01.
3.4. Blue light-induced ROS and cell death are accentuated by Nrf2 knockdown
Since blue light exposure activated Nrf2 in 661W cells, we were interested in determining the functional role of Nrf2 under these blue light conditions. For this purpose, we performed Nrf2 knockdown studies by transfection of 661W cells with Nrf2 siRNA. Knockdown efficiency of Nrf2 was determined to be 70% (p < 0.001, n=3) (Fig. 5A). Given its known role in regulating oxidative stress, we first evaluated whether Nrf2 knockdown affects levels of reactive oxygen species in 661W cells. We found that after blue light irradiation for 24 hours, there was 1.8-fold increase in ROS in control siRNA-transfected 661W cells. In contrast, there was a 2.6-fold increase of ROS production in cells transfected by Nrf2 siRNA (p < 0.01, n=4) (Fig. 5B), indicating that Nrf2 knockdown exacerbated intracellular ROS induced by exposure to blue light. We also investigated whether Nrf2 knockdown could affect cell viability by measuring ATP content. After exposing 661W cells to blue light for 24 hours, we found ATP content to be decreased significantly in cells transfected by Nrf2 siRNA compared to the control group transfected with control siRNA (p< 0.001, n=4–6)(Fig. 5C), indicating the important cytoprotective role of Nrf2 against blue light-induced cell damage. We also evaluated cell death by double staining with Hoechst 33342 (blue) and ethidium homodimer-1 (red). There was a marked increase in 661W cell death after exposure to blue light for 24 hours, and the severity was much higher in cells transfected with Nrf2 siRNA compared to the control group transfected with control siRNA (Fig. 6A). The percentage of dead cells calculated (numbers of ethidium homodimer-positive cells/Hoechst 33342-positive cells) was significantly higher in cells deficient inNrf2 (p < 0.001, n=6) (Fig. 6B), further supporting the importance of Nrf2 against blue light-induced cell death.
Fig. 5.
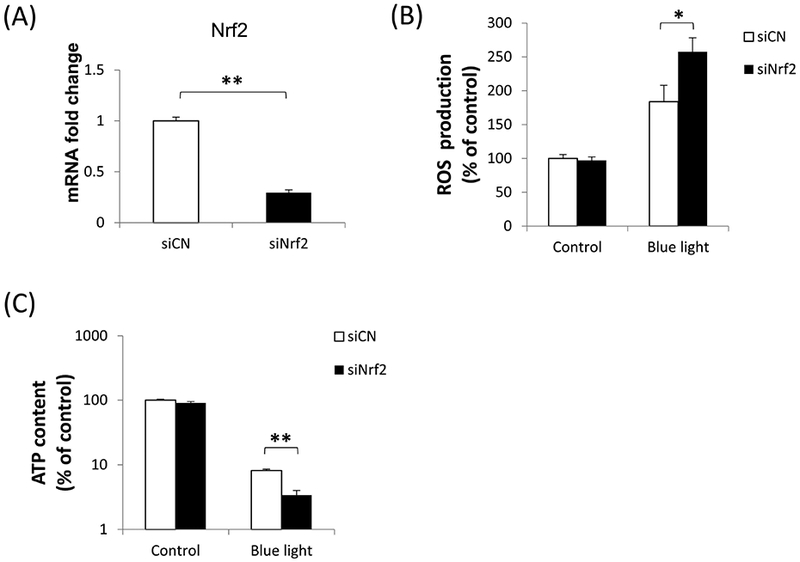
Blue light effects on ROS production and cell viability were accentuated by Nrf2 deficiency. (A) The efficiency of Nrf2 knockdown by siRNA was tested by mRNA level, indicating 70% knockdown. (B) After blue light irradiation for 24 hours, intracellular ROS production was significantly higher in Nrf2 knockdown cells. n=4, *p < 0.01. (C) Nrf2 knockdown exacerbated the decrease in ATP content, a measure of cell viability, induced by blue light irradiation for 24 hours. n=4–6, **p < 0.001. White bars: control siRNA; black bars: Nrf2 siRNA.
Fig. 6.
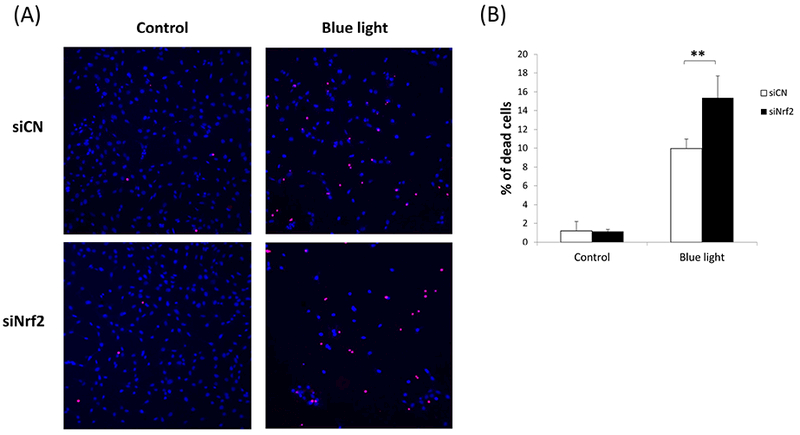
Blue light-induced cell death was accentuated by Nrf2 deficiency. (A) Double staining with Hoechst 33342 (blue) and ethidium homodimer-1 (red) showed marked cell loss after blue light irradiation, and the severity was much higher in cells undergoing Nrf2 knockdown. (B) There was a significantly higher rate of cell death in cells deficient in Nrf2. n=6, **p < 0.001.
4. Discussion
Oxidative stress and light-induced damage have both received extensive attention as pathogenic contributors to photoreceptor injury. Tremendous efforts have been directed toward the identification of protective mechanisms that could reduce photoreceptor death in clinical entities including AMD and inherited retinal degenerations. The stress-response transcription factor Nrf2 is drawing increasing attention as a neuroprotective factor. With respect to the retina, several reports have emerged indicating the protective effect of Nrf2 on retinal ganglion cells, including studies from our lab that have focused on retinal ischemia-reperfusion injury (Cho et al., 2015; Wei et al., 2011; Xu et al., 2015). In view of the important role of Nrf2 in regulating oxidative stress, it is somewhat surprising that Nrf2 has received less attention with regard to photoreceptor health. However, a recent study reported that AAV-mediated gene transfer of Nrf2 was beneficial in reducing cone photoreceptor death in mouse models of RP (Xiong et al., 2015). This indicated that exogenous approaches for activating retinal Nrf2 could delay or arrest cone photoreceptor death in hereditary retinal degenerations.
In the current study, we sought to gain greater insight into the endogenous activity and function of Nrf2 in photoreceptors, specifically with respect to blue light-induced photoreceptor damage. For this purpose, we utilized the 661W photoreceptor cell line. We found that blue light exposure resulted in activation of Nrf2 in 661W cells, with associated induction of an array of Nrf2 target genes, including antioxidant genes. This suggests that Nrf2 is operative in photoreceptors under blue light-induced stress. In order to determine and confirm the functional impact of Nrf2 activation, we performed Nrf2 knockdown studies under blue light exposure. These studies demonstrated significantly greater induction of reactive oxygen species and cell death, indicating that Nrf2 acts as an endogenous protective mechanism in photoreceptor in this setting of blue light exposure. With regard to Nrf2’s effects on cell viability, we utilized a luminescent assay of ATP. Although ATP is a widely accepted marker of viable cells, the linearity between ATP content and cell number can be affected by conditions such as oxidative stress. We therefore also performed a complementary study of cell death (Figure 6C), which confirmed the critical role of Nrf2 on this endpoint under blue light conditions. Together these results suggest that Nrf2 is an important endogenous cell-intrinsic protective mechanism against oxidative stress in photoreceptors and further supports its potential as a therapeutic target for photoreceptor neuroprotection. In our in vitro cell culture system, cells were maintained in low serum medium containing phenol red. The effect of this colored medium on the blue light-induced cell damage is considered to be negligible since the blue light source was situated below the cells. However, we cannot rule out the possibility that the data could be affected by reaction between light and colored compounds.
The blue light (high energy) end of the visible spectrum is considered to have the potential to be particularly injurious to the retina. For this reason, investigators have evaluated potential strategies and mechanisms for protection of photoreceptors against this stimulus. Studies using 661W cells have helped to demonstrate possible therapeutic strategies in this regard. Kuse et al. demonstrated the protective role of N-acetylcysteine (NAC) in blue light-induced photoreceptor cell damage and suggested that NAC could promote cell survival by scavenging ROS produced by blue light exposure (Kuse et al., 2014). Using the same blue light model in 661W cells, they also demonstrated that Bilberry and lingoberry extract containing high amounts of polyphenols could lower ROS and inhibited the activation of p38 MAPK and NF-kB induced by blue light(Ogawa et al., 2014). However, there has been relatively little attention regarding Nrf2 as a possible mechanism and target for attenuating photo-oxidative stress in photoreceptors. Our study suggests that Nrf2 is an endogenous mechanism for protection against blue light, presumably via Nrf2 activation by blue light-induced reactive oxygen species which could modulate the Nrf2 inhibitor Keap1, causing it to release Nrf2 and enabling nuclear translocation of Nrf2.
In the current study, we used the 661W cell line as the model system for studying the relationship between blue light stress and Nrf2 activation in photoreceptors. This mouse photoreceptor-derived cell line (Tan et al., 2004)has been a useful in vitro model for studying photoreceptor biology as it exhibits both cellular and biochemical characteristics of cone photoreceptor cells, including expression of photoreceptor-specific proteins(Tan et al., 2004; Tang et al., 2011). These cells have also been demonstrated to exhibit photoreceptor functions including retinoid processing (Kanan et al., 2008) and importantly, display high susceptibility to light stress only in the presence of the chromophore 9-cis retinal (Kanan et al., 2007). However, it should be noted that they do not form outer segment-like membranes, possibly due to lack of physical contact with RPE cells, although they do project neuronal processes (Tan et al., 2004). Therefore, future follow-up studies of blue light and Nrf2, both in primary photoreceptor cells and in animal models will be invaluable in definitively establishing Nrf2 biology in photoreceptors under blue light stress.
In our study, we demonstrated a cell-intrinsic neuroprotective role of Nrf2 in a photoreceptor cell line against the photo-oxidative damage induced by blue light irradiation. Beyond the endogenous protective effect of Nrf2, it is quite plausible to speculate on the clinical utility of targeting Nrf2, in order to boost and maximize its beneficial effects on photoreceptors. Indeed, a recent study used an AAV-mediated approach for gene transfer of Nrf2 into the retina, which served to reduce cone photoreceptor death in mouse models of RP (Xiong et al., 2015). Beyond this approach, it would certainly be feasible to consider pharmacologic strategies to activate Nrf2, including drugs that can disrupt the inhibitory effects of Keap1 on Nrf2 (Kensler et al., 2007). For instance, triterpenoid derivatives including CDDO-Im, which are potent Nrf2 activators, were shown to protect ARPE-19 retinal pigment epithelial cells from oxidant-induced cell death (Pitha-Rowe et al., 2009). Our previous work has found that CDDO-Im treatment is effective in the activation of Nrf2 target genes and significantly reduces cell death of ganglion cells in mice following retinal ischemia-reperfusion injury (Xu et al., 2015). Similarly, we also found that the Nrf2 activator monomethyl fumarate (MMF) reduces neurodegeneration in the ganglion cell layer and improves retinal function after retinal ischemia-reperfusion injury in an Nrf2-dependent manner (Cho et al., 2015). It will be of great interest to investigate the pharmacologic activation of Nrf2 in photoreceptors, with respect to both cell culture and animal models. The use of Nrf2 activating drugs constitutes a promising strategy for consideration and further investigation in the attenuation of photoreceptor death in various retinal conditions.
4.1. Conclusions
The data presented in the current study indicates that Nrf2 is an endogenous, cell-intrinsic neuroprotective mechanism in the 661W photoreceptor cell line subjected to blue light exposure. This suggests that Nrf2 might serve more broadly to protect photoreceptors against oxidative stress and potentially other insults. In the future, it will be of great interest to conduct in vivo studies to further elucidate the role Nrf2 in protecting retinal photoreceptors from blue light and other stressors. Moreover, Nrf2 targeting drugs may be potential resources in reducing photo-oxidative damage, and possibly damage from other etiologies, in photoreceptors. Further investigations are warranted to explore the importance of Nrf2 and the full potential of Nrf2 activation as a therapeutic strategy for photoreceptors.
Blue light increases ROS levels and cell death in the 661W photoreceptor cell line
Blue light induces nuclear translocation of Nrf2
Blue light increases expression of Nrf2 and Nrf2-target genes
Nrf2 knockdown renders 661W cells more vulnerable to blue light-induced photo-oxidative stress, with accentuation of both ROS and cell death.
Nrf2 knockdown exacerbates blue light-induced ROS and cell death in 661W cells
Acknowledgments
This work was supported by research grants from the National Institutes of Health (EY022383 and EY022683; to E.J.D.) and Core Grant P30EY001765, Imaging and Microscopy Core Module. We thank Cindy Berlinicke for her help with the quantitative analysis of the cell staining with the CellomicsArrayScan VTI HCS reader.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Ubaidi MR, Font RL, Quiambao AB, Keener MJ, Liou GI, Overbeek PA, Baehr W, 1992. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. The Journal of cell biology 119, 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M, 2000. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Survey of ophthalmology 45, 115–134. [DOI] [PubMed] [Google Scholar]
- Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, Leon R, 2016. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacology & therapeutics 157, 84–104. [DOI] [PubMed] [Google Scholar]
- Cho H, Hartsock MJ, Xu Z, He M, Duh EJ, 2015. Monomethyl fumarate promotes Nrf2-dependent neuroprotection in retinal ischemia-reperfusion. Journal of neuroinflammation 12, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteras N, Dinkova-Kostova AT, Abramov AY, 2016. Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biological chemistry 397, 383–400. [DOI] [PubMed] [Google Scholar]
- Glazer-Hockstein C, Dunaief JL, 2006. Could blue light-blocking lenses decrease the risk of age-related macular degeneration? Retina 26, 1–4. [DOI] [PubMed] [Google Scholar]
- Himori N, Yamamoto K, Maruyama K, Ryu M, Taguchi K, Yamamoto M, Nakazawa T, 2013. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. Journal of neurochemistry 127, 669–680. [DOI] [PubMed] [Google Scholar]
- Jaadane I, Boulenguez P, Chahory S, Carre S, Savoldelli M, Jonet L, Behar-Cohen F, Martinsons C, Torriglia A, 2015. Retinal damage induced by commercial light emitting diodes (LEDs). Free radical biology & medicine 84, 373–384. [DOI] [PubMed] [Google Scholar]
- Kanan Y, Kasus-Jacobi A, Moiseyev G, Sawyer K, Ma JX, Al-Ubaidi MR, 2008. Retinoid processing in cone and Muller cell lines. Experimental eye research 86, 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Moiseyev G, Agarwal N, Ma JX, Al-Ubaidi MR, 2007. Light induces programmed cell death by activating multiple independent proteases in a cone photoreceptor cell line. Investigative ophthalmology & visual science 48, 40–51. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S, 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47, 89–116. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA, 2006. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America 103, 11300–11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H, 2014. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Scientific reports 4, 5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrain TH, Boulton M, Marshall J, Sliney DH, 2004. Do blue light filters confer protection against age-related macular degeneration? Progress in retinal and eye research 23, 523–531. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Kuse Y, Tsuruma K, Kobayashi S, Shimazawa M, Hara H, 2014. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC complementary and alternative medicine 14, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak DT, Vaughan DK, 2010. Retinal light damage: mechanisms and protection. Progress in retinal and eye research 29, 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha-Rowe I, Liby K, Royce D, Sporn M, 2009. Synthetic triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Investigative ophthalmology & visual science 50, 5339–5347. [DOI] [PubMed] [Google Scholar]
- Santos AM, Lopez-Sanchez N, Martin-Oliva D, de la Villa P, Cuadros MA, Frade JM, 2012. Sortilin participates in light-dependent photoreceptor degeneration in vivo. PloS one 7, e36243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA, 2005. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. Journal of cellular physiology 203, 457–464. [DOI] [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR, 2004. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investigative ophthalmology & visual science 45, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PH, Buhusi MC, Ma JX, Crouch RK, 2011. RPE65 is present in human green/red cones and promotes photopigment regeneration in an in vitro cone cell model. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 18618–18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, Sporn MB, Handa JT, Duh EJ, 2011. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free radical biology & medicine 51, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P, 1999. Oxidative damage and age-related macular degeneration. Molecular vision 5, 32. [PMC free article] [PubMed] [Google Scholar]
- Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL, 2015. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. The Journal of clinical investigation 125, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Cho H, Hartsock M, Mitchell KL, Gong J, Wu L, Wei Y, Wang S, Thimmulappa RK, Sporn MB, Biswal S, Welsbie DS, Duh EJ, 2015. Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion. Journal of neurochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO, 2007. Role of NF-kappaB and MAPKs in light-induced photoreceptor apoptosis. Investigative ophthalmology & visual science 48, 4766–4776. [DOI] [PubMed] [Google Scholar]
- Young RW, 1988. Solar radiation and age-related macular degeneration. Survey of ophthalmology 32, 252–269. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, 2004. Current concepts in the pathogenesis of age-related macular degeneration. Archives of ophthalmology 122, 598–614. [DOI] [PubMed] [Google Scholar]


