Abstract
Objectives
To identify risk factors for antepartum stillbirth, including fetal growth restriction, among women with well‐dated pregnancies and access to antenatal care.
Design
Population‐based, prospective, observational study.
Setting
Eight international urban populations.
Population
Pregnant women and their babies enrolled in the Newborn Cross‐Sectional Study of the INTERGROWTH‐21st Project.
Methods
Cox proportional hazard models were used to compare risks among antepartum stillborn and liveborn babies.
Main outcome measures
Antepartum stillbirth was defined as any fetal death after 16 weeks’ gestation before the onset of labour.
Results
Of 60 121 babies, 553 were stillborn (9.2 per 1000 births), of which 445 were antepartum deaths (7.4 per 1000 births). After adjustment for site, risk factors were low socio‐economic status, hazard ratio (HR): 1.6 (95% CI, 1.2–2.1); single marital status, HR 2.0 (95% CI, 1.4–2.8); age ≥40 years, HR 2.2 (95% CI, 1.4–3.7); essential hypertension, HR 4.0 (95% CI, 2.7–5.9); HIV/AIDS, HR 4.3 (95% CI, 2.0–9.1); pre‐eclampsia, HR 1.6 (95% CI, 1.1–3.8); multiple pregnancy, HR 3.3 (95% CI, 2.0–5.6); and antepartum haemorrhage, HR 3.3 (95% CI, 2.5–4.5). Birth weight <3rd centile was associated with antepartum stillbirth [HR, 4.6 (95% CI, 3.4–6.2)]. The greatest risk was seen in babies not suspected to have been growth restricted antenatally, with an HR of 5.0 (95% CI, 3.6–7.0). The population‐attributable risk of antepartum death associated with small‐for‐gestational‐age neonates diagnosed at birth was 11%.
Conclusions
Antepartum stillbirth is a complex syndrome associated with several risk factors. Although small babies are at higher risk, current growth restriction detection strategies only modestly reduced the rate of stillbirth.
Tweetable abstract
International stillbirth study finds individual risks poor predictors of death but combinations promising.
Keywords: Antepartum stillbirth, birth weight, fetal growth restriction, INTERGROWTH‐21st
Tweetable abstract
International stillbirth study finds individual risks poor predictors of death but combinations promising.
Introduction
The prevention of stillbirth remains a major global challenge. In 2015, an estimated 2.6 million babies were stillborn.1 By 28 weeks’ gestation approximately 1 in every 250 pregnancies will result in a stillbirth in high‐income countries, with rates in low‐ and middle‐income countries as high as 1 in 22. Stillbirth is the final common endpoint of several pathologies,2, 3 yet most are unexpected and many unexplained.4
Stillbirth is classified, according to its timing, aetiology and possible preventive strategies, as death occurring before the initiation of labour (antepartum) or during labour and delivery (intrapartum). Most intrapartum stillbirths occur because of poor‐quality care during childbirth; improvements in intrapartum care, closer fetal monitoring and access to operative delivery have dramatically reduced the number of such deaths.5, 6 However, the prevention of antepartum stillbirths has proved to be more challenging.
First, the pathways linking clinical and pregnancy conditions to antepartum stillbirth are poorly understood, making it difficult to screen women at increased risk in whom interventions could potentially be implemented. Second, while antepartum stillbirth has been associated with socio‐demographic factors and medical conditions,7, 8, 9, 10, 11, 12 most studies report associations that are not sufficiently strong and that are of uncertain relevance outside their specific population. Finally, fetal growth restriction (FGR) is frequently cited as preceding antepartum stillbirth;5, 6, 7, 8, 9 however, the magnitude of the causal association is difficult to ascertain, as FGR is almost always diagnosed retrospectively based on weight at birth.
Our primary aim in this study was to investigate risk factors specific for antepartum stillbirth, including poor fetal growth, in a large, population‐based study of fetal growth and pregnancy outcome among women with ultrasound‐dated pregnancies who were receiving good antenatal care in eight urban areas around the world.13
Methods
Study design and participants
The Newborn Cross‐Sectional Study (NCSS) component of the INTERGROWTH‐21st Project was a large, multi‐country, multi‐ethnic, population‐based study conducted between May 14 2009 and August 2 2013. The primary aim was to study fetal growth and development from early pregnancy until 2 years of age.13 Populations were selected at the cluster level. Study protocols with detailed criteria for population selection have been previously published.14, 15, 16, 17, 18, 19, 20, 21 Clusters were defined as urban areas, representing either a complete city or a demarcated geographical or political area within a city, where most women accessed antenatal care and delivered in medical facilities; altitude was ≤1600 m; perinatal mortality rate was <20 per 1000 live births; mean birthweight was >3100 g; and low‐birthweight rates (<2500 g) were <10%. In addition, >75% of mothers had an educational level greater than a locally defined minimum threshold and there was an absence of known, major, environmental contaminants assessed using a specifically designed data collection form.22 Sites selected were: the cities of Pelotas, Brazil; Turin, Italy; Muscat, Oman; Oxford, UK; Seattle, WA, USA; Shunyi County in Beijing, China; the central area of Nagpur, Maharashtra, India; and the Parklands suburb of Nairobi, Kenya. Within these sites, hospitals and clinics were selected to ensure coverage of at least 80% of births. All participating hospitals agreed to a policy of routinely estimating gestational age using ultrasound before 24 weeks’ gestation with a strict, standardised protocol to measure either fetal crown–rump length (if ≤14 weeks’ gestation) or head circumference (if >14 and ≤24 weeks’ gestation).13 Information was obtained on all births from 16 weeks’ gestation. Stillbirth was defined as fetal death between 16 weeks and birth, in keeping with our previous publications on preterm birth.23
Independent variables
Data were prospectively collected using a standardised collection form and a secure online data entry and management system (MedSciNet).24 Low socio‐economic status was country specific. Smoking was defined as any tobacco use in the current pregnancy; alcohol intake was recorded if >5 units per week. Full descriptions are available from the study website, http://www.intergrowth21.tghn.org.
Outcomes from previous pregnancies were self‐reported. Pregnancy‐induced hypertension was defined as blood pressure ≥140/90 mmHg, after 20 weeks’ gestation in a previously normotensive woman without proteinuria. Pre‐eclampsia was defined by consensus as blood pressure ≥140/90 mmHg, or an increase of 30 mmHg systolic or 15 mmHg diastolic over baseline, detected on at least two occasions more than 6 hours apart, associated with proteinuria after 20 weeks’ gestation. Severe pre‐eclampsia/ eclampsia/HELLP (Haemolysis, Elevated Liver enzymes and Low Platelet count) syndrome was defined as blood pressure ≥160 mmHg systolic and/or ≥110 mmHg diastolic on two occasions at least 4 hours but no more than 168 hours apart, or if the first measurement was immediately followed by treatment with an antihypertensive, either of these scenarios being associated with proteinuria. Eclampsia was defined as the occurrence of convulsions unrelated to other cerebral conditions with symptoms or signs of pre‐eclampsia. Antepartum fetal distress was defined on the basis of clinical suspicion with or without non‐invasive fetal monitoring.
We explored the relationship between FGR, small‐for‐gestational‐age (SGA) at birth, and antepartum stillbirth using three approaches. First, the relationship between the detection of FGR during pregnancy and antepartum stillbirth was examined. Detected FGR was defined as documented poor fetal growth prior to death, based either on ultrasound evidence of poor serial growth, or size/abdominal circumference <10th centile with abnormal Doppler studies. Second, the association between stillbirth and SGA at birth, defined as birth weight <3rd centile for gestational age, and gender for babies born after 24 weeks using the INTERGROWTH‐21st Newborn Size at Birth Standards, was assessed.25 Third, we evaluated the ultrasound growth trajectories for the stillbirths that occurred within the Fetal Growth Longitudinal Study (FGLS) of the INTERGROWTH‐21st Project.13 A subset was selected of 4607 women who were at low risk, at the individual level, of growth problems at the start of pregnancy, and who were monitored with serial ultrasound scans every 5 weeks until delivery.26
Statistical analysis
The sample size was based on the primary objective.27 In a post‐hoc estimation for 440 antepartum stillbirths with a 5% prevalence of risk factors, a relative risk of 2.1 could be demonstrated with 80% power, and statistical significance was set at a two‐tailed P of <0.05.
We estimated total stillbirth (antepartum and intrapartum) rates per 1000 live‐ and stillborn babies delivered after 16 weeks’ gestation. We performed time‐to‐event analysis using Cox Proportional Hazard Models. Antepartum stillbirth was defined as the event and gestational age in weeks was the time‐dependent variable. Preterm births were censored. This is the recommended method for stillbirth analysis, as it accounts for the cumulative risk of death with ongoing duration of pregnancy.28, 29 In order to account for clustering within sites, all hazard ratios (HR) were adjusted for country using the UK as the reference. An exploratory analysis was performed to identify associations between potential risk factors and antepartum stillbirth, compared with live births. Variables with fewer than five cases are not presented.30 Variables missing more than 5% of responses were excluded from further models (i.e. maternal education). Exclusion of education avoided multi‐colinearity with socio‐economic status. The proportional hazard assumption was tested using Shoenfeld residuals, which were plotted against each covariate and the graphs inspected for any trend in the residuals.
We used a hierarchical approach to model building for confounder selection.31 This approach recognises that distal disease determinants such as socio‐economic factors can potentially affect all other variables as well as the outcome (antepartum stillbirth) directly or indirectly. In addition, the relationship between variables is often temporal, with most standard regression methods failing to take this into account. Adopting a hierarchical approach helps to ensure that distal determinant effects on the outcome are not underestimated.32 A directed acyclic graph was developed based on known clinical associations (Figure S1). Sequential models incorporated variables from the preceding model only if P was <0.2.33 Biologically plausible interaction terms were identified a priori, and only retained if P < 0.05, with effect consistent with action.
Positive (LR+) and negative (LR−) likelihood ratios were computed if an association with antepartum stillbirth was found in the multivariate models, and for plausible risk‐factor combinations. LRs remain constant despite differing risk‐factor prevalence and are, therefore, clinically useful in interpreting how a variable changes the post‐test probability of disease. LRs between 2 and 5 (or LRs 0.5 and 0.2 for protective factors) are accepted indicators of a small change in the post‐test probability of a condition, between 5 and 10 (0.2 and 0.1) a moderate change in risk, and above 10 (below 0.1) a large change.34
Attributable risk (%) in the total population was estimated as the proportion of antepartum stillbirths that could potentially be reduced by eliminating a risk factor. Statistical calculations were performed using spss Statistics version 21 (IBM, Armonk, NY, USA).
Results
Study population
Across the eight study sites, 59 137 women delivered 60 268 babies. We excluded 105 babies with unknown gestational age at delivery, 15 with gestational age <16 weeks, and 27 born after 43 weeks. Thus, 60 121 babies born to 59 052 mothers were included, ranging from 6431 babies in Seattle, USA, to 8206 in Oxford, UK. Descriptive characteristics of the mothers and babies are presented in Table S1.
A total of 59 568 (singleton 57 508, multiple 2060) were liveborn and 553 (511 singleton, 42 multiple) were stillborn (overall stillbirth rate 9.2 per 1000 live and stillborn babies, range 5.1–15.1 across sites). Of all the stillbirths, 445 (singleton 413, multiple 32) were antepartum and 108 (singleton 98, multiple 10) were intrapartum. The overall late fetal death rate according to the WHO definition (>28 weeks’ gestation) was 5.4 (range, 3.1–9.1 across sites).
Antepartum stillbirth accounted for a high proportion of fetuses born before 24 weeks’ gestation (98 deaths; 73.1% of all births before 24 weeks); 168 (37.8%) of the antepartum stillborn babies delivered after 33 weeks.
Among the antepartum stillbirths, 14 (3.1%) had congenital malformations diagnosed either at birth or by ultrasound during pregnancy (Table S2).
Epidemiological associations and prognostic factors for antepartum stillbirth
No significant association with antepartum stillbirth was demonstrated with smoking, reported illicit drug use, high‐risk occupation or body mass index (Table S3). Women with a history of either a previous perinatal loss, preterm delivery, a baby born with high (>4500 g) or low (<2500 g) birthweight, or miscarriages in the preceding two pregnancies, had a higher risk of antepartum stillbirth (Table S4). When pregnancy‐related conditions were examined (Table S5), there was an increasing risk of stillbirth with severity of hypertensive disease, i.e. pregnancy‐induced hypertension increased the HR of antepartum stillbirth by 1.75, pre‐eclampsia by 3.23 and severe pre‐eclampsia/HELLP/eclampsia by 5.53.
After adjustment for potential confounders including study site, low socio‐economic standing and single marital status showed independent associations with antepartum stillbirth; single, widowed or divorced women were at twice the risk of antepartum stillbirth than were those married or cohabiting (adjusted HR, 2.00) (Table 1). Maternal age of at least 40 also doubled the risk compared with that of mothers aged between 18 and 25 years (adjusted HR, 2.23). Prior essential hypertension or HIV/AIDS both increased HR of antepartum stillbirth approximately fourfold (adjusted HR, 3.98 and 4.26, respectively). Women who had previously had two miscarriages had a modest increase in risk (adjusted HR, 1.82). Bleeding in pregnancy was both an independent risk (adjusted HR, 3.33), and potential effect modifier of the effect of severe pre‐eclampsia, increasing the adjusted HR from 2.80 to 4.20. The highest population‐attributable percentages for individual risk factors were for low socio‐economic standing (9.7%), antepartum haemorrhage (9.0%) and multiple pregnancy (7.4%).
Table 1.
Association of maternal factors with antepartum stillbirth in a population of 60 121 babies in the INTERGROWTH‐21st Project
| Variable | Adjusted hazard ratio (95% CI) | Population‐attributable percentage (%) |
|---|---|---|
| Low socio‐economic standing | 1.6 (1.2–2.1)* | 9.7 |
| Single marital status | 2.0 (1.4–2.8)* | 4.8 |
| Maternal age (years) | ||
| <18 | 0.8 (0.4–1.6)** | |
| 18–25 | Ref | |
| 26–34 | 1.1 (0.9–1.4)** | |
| 35–39 | 1.2 (0.8–1.7)** | |
| ≥40 | 2.2 (1.4–3.7)** | 3.0 |
| Prior history of hypertension | 4.0 (2.7–5.9)*** | 5.5 |
| HIV/AIDS diagnosed before pregnancy | 4.3 (2.0–9.1)*** | 0.3 |
| Last two pregnancies ended in miscarriage | 1.8 (1.1–3.0)*** | 4.3 |
| Pre‐eclampsia | 1.6 (1.1–3.8)**** | 1.4 |
| Severe pre‐eclampsia/eclampsia/HELLP without antepartum haemorrhage | 2.8 (1.5–5.1)**** | 1.6 |
| Severe pre‐eclampsia/eclampsia/HELLP with antepartum haemorrhage | 4.2 (1.3–13.6)**** | 2.2 |
| Antepartum haemorrhage without severe pre‐eclampsia | 3.3 (2.5–4.5)**** | 9.0 |
| Multiple pregnancy | 3.3 (2.0–5.6)**** | 7.4 |
| Fetal distress suspected in pregnancy | 2.1 (1.3–2.7)***** | 3.4 |
All models adjusted for country of birth and fetal gender. In addition, the following stepwise adjustments were made: *Model 1, socio‐economic deprivation and marital status; **Model 2, I < 0.2 in Model 1 + maternal age, body mass index, height, weight, parity + smoking, illicit drug use, >5 units of alcohol per week + high‐risk occupation; ***Model 3, P < 0.2 in Model 2+ pre‐existing maternal medical conditions and past obstetric outcomes; ****Model 4, P < 0.2 in Model 3 + maternal illnesses and conditions that develop during in pregnancy; *****Model 5, P < 0.2 in Model 4+ fetal‐related conditions.
The overall cumulative probability of antepartum stillbirth in this population was 0.007 (Table 2). The highest probabilities of antepartum stillbirth were for women with the combinations of severe pre‐eclampsia and antepartum haemorrhage (APH) (probability 0.18, LR+ 31); in multiple pregnancies where fetal distress was suspected (probability 0.10, LR+ 16.0), and in women aged >40 with a history of essential hypertension [probability 0.09, LR+ 14.0 (95% CI, 7.76–25.0)]. All other risk factors explored demonstrated relatively weak associations, with poor negative predictive ability (LR− all close to 1.0).
Table 2.
Liklihood ratios for risk factors associated with antepartum stillbirth in a population of 60 121 babies in the INTERGROWTH‐21st Project
| Risk factor | Antepartum stillbirth (%) | Live birth (%) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|
| Low socio‐economic standing | 34.6 | 22.7 | 1.5 (1.3–1.7) | 0.9 (0.8–0.9) |
| Single marital status | 11.2 | 5.0 | 2.3 (1.7–2.9) | 0.9 (0.9–1.0) |
| Maternal age ≥40 years | 5.0 | 2.4 | 2.1 (1.4–3.1) | 1.0 (1.0–1.0) |
| Pre‐existing hypertension | 7.7 | 1.8 | 4.5 (3.2–6.3) | 1.0 (1.0–1.0) |
| HIV/AIDS diagnosed prior to pregnancy | 1.6 | 0.3 | 5.5 (2.7–11.5) | 1.0 (1.0–1.0) |
| Last two pregnancies ended in miscarriage | 6.1 | 3.4 | 1.8 (1.2–2.6) | 1.0 (1.0–1.0) |
| Bleeding after 15 weeks’ gestation | 13.7 | 4.4 | 3.1 (2.5–4.0) | 0.9 (0.9–0.9) |
| Pre‐eclampsia | 6.1 | 2.4 | 2.5 (1.7–3.7) | 1.0 (0.9–1.0) |
| Severe pre‐eclampsia/ HELLP/ eclampsia | 3.9 | 0.8 | 5.0 (3.1–8.1) | 1.0 (1.0–1.0) |
| Multiple pregnancy | 7.2 | 3.4 | 2.1 (1.5–3.0) | 1.0 (0.9–1.0) |
| Fetal distress suspected in pregnancy | 4.0 | 1.7 | 2.4 (1.5–3.7) | 1.0 (1.0–1.0) |
| FGR suspected in pregnancy a | 10.1 | 4.4 | 2.3 (1.7–3.2) | 0.9 (0.9–1.0) |
| SGA at birth a | 16.7 | 3.5 | 4.8 (3.8–6.1) | 0.9 (0.8–0.9) |
| Selected risk factor combinations: | ||||
| Single marital + antepartum haemorrhage | 2.5 | 0.5 | 5.2 (2.9–9.4) | 1.0 (1.0–1.0) |
| Maternal age >40+ pre‐existing hypertension | 2.7 | 0.2 | 14.0 (7.8–25.0) | 1.0 (1.0–1.0) |
| Pre‐eclampsia + suspected FGR | 2.0 | 0.3 | 6.8 (3.5–13.0) | 1.0 (1.0–1.0) |
| Severe pre‐eclampsia/eclampsia/HELLP + antepartum haemorrhage | 0.9 | 0.0 | 31.0 (13.0–73.0) | 0.8 (0.6–1.0) |
| Multiple pregnancy + suspected fetal distress | 1.6 | 0.1 | 16.0 (7.2–34.0) | 1.0 (1.0–1.0) |
FGR, fetal growth restriction; LR, likelihood ratio; SGA, small‐for‐gestational‐age.
For babies born after 24 weeks only.
Association between fetal growth restriction and antepartum stillbirth
In the entire INTERGROWTH‐21st population, evidence of altered growth or small fetal size on ultrasound doubled the risk of antepartum stillbirth [adjusted HR, 2.1 (95% CI, 1.4 –3.1)]. In addition, we studied the association between antepartum stillbirth and small size at birth. Stillborn babies were more likely to be SGA at birth [16.8% compared with 3.5% for babies born after 24 week’ gestation, with an adjusted HR of 4.6 (95% CI, 3.4–6.2)] (Table 3). Interestingly, among the SGA babies at birth, if FGR was identified during pregnancy, the risk of stillbirth was lower than for those babies in whom growth restriction was not suspected [adjusted HR 3.5 (95% CI, 1.9–6.4), compared with HR 5.0 (95% CI, 3.6–7.0)], suggesting, perhaps, that effective action had been implemented in these women. The population‐attributable risk for stillbirth associated with SGA at birth in the entire INTRGROWTH‐21st population was 11%. If we were able to ‘transform’ all FGR not suspected before birth to ‘suspected’ FGR then the attributable risk for them would have been 9%.
Table 3.
Association between fetal growth restriction and antepartum stillbirth in a population of 59 792 babies in the INTERGROWTH‐21st Project
| Variable | Prevalence (%) | Adjusted HR | Population‐attributable risk (%) | |
|---|---|---|---|---|
| Liveborn (n = 59 445) | Stillborn (n = 347) | |||
| FGR suspected during pregnancy | 4.4 | 10.1 | 2.1 (1.4–3.1) | |
| SGA at birth | 3.5 | 16.7 | 4.6 (3.4–6.2) | 11.1 |
| SGA at birth: FGR suspected during pregnancy | 0.9 | 3.5 | 3.5 (1.9–6.4) | 2.2 |
| SGA at birth: FGR not suspected during pregnancy | 2.6 | 13.3 | 5.0 (3.6–7.0) | 9.4 |
HR, hazard ratio.
FGR defined as an antenatal diagnosis of growth restriction based upon ultrasound findings of poor interval growth or estimated weight or AC <10th centile for gestational age with abnormal functional studies. SGA defined as birth weight <3rd centile for gestational age and gender using the INTERGROWTH‐21st Newborn Size at Birth Standards, adjusted for study country and other conditions listed in Table 2.
We conducted a detailed subgroup analysis among the 4607 women in the low‐risk FGLS cohort for whom serial ultrasound data were available. Among the 18 antenatal stillbirths that were tracked, their ultrasound parameters were almost all within the 3rd and 97th centiles of the INTERGROWTH‐21st fetal growth standards. No evidence of decreased velocity in either skeletal growth (head circumference) or fat‐dependent measures (abdominal circumference) could be demonstrated among these 18 fetuses that died before labour (Figure 1).
Figure 1.
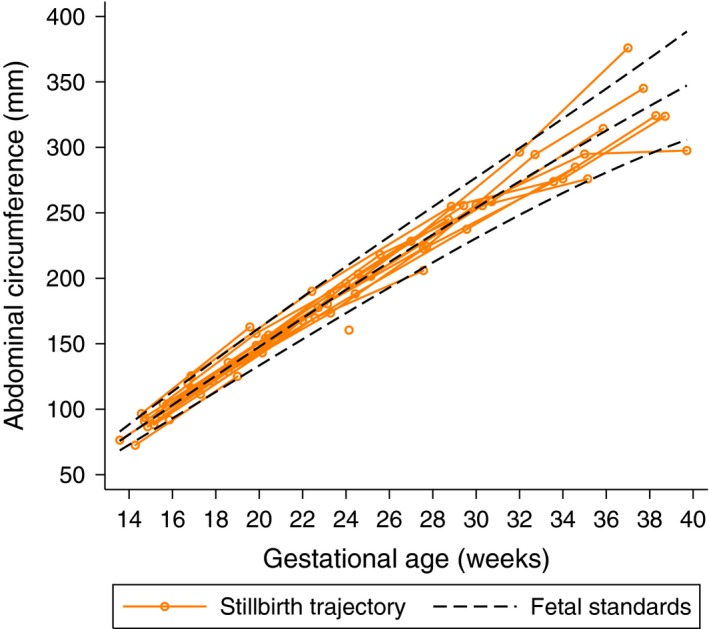
Individual growth trajectories of 18 babies who were stillborn in the Fetal Growth Longitudinal Study. Dashed black lines represent 10th, 50th and 90th centiles of the INTERGROWTH‐21st Fetal Growth Standards (stillbirths were excluded from the calculation of these values).
Discussion
Main findings
The principal results from our multi‐country study of antepartum stillbirth are that: (1) the rate can be as low as 10 per 1000 births when adequate health care, education, nutrition and maternity care are provided to a population; (2) the several known clinical and demographic factors identified are individually relatively poor predictors of antepartum death; (3) the attributable fraction of antepartum death that could be prevented by modifying these individual risk factors is <10%; (4) in the general population, FGR is a risk factor for antepartum stillbirth; however among women at low risk who were followed with repeated ultrasound measurements, abnormal growth may play a lesser role in leading to stillbirth.
Of clinical relevance are the predictive probabilities of combinations of risk factors, e.g. women aged 40 or older with essential hypertension, those with multiple pregnancies who develop fetal distress and those with pre‐eclampsia who experience APH.
Strengths and limitations of the study
Our study has several strengths: there was high (>80%) coverage of all births within the target populations as defined by geographic and social criteria,35 and we differentiated between antepartum and intrapartum stillbirth, which are clearly separate entities. Any definition of stillbirth that fails to differentiate these entities, although simpler for routine data collection, has limited value for evaluating and implementing preventive strategies. We also describe antepartum stillbirth from 16 weeks’ gestation in eight diverse locations around the world where women had access to early dating by ultrasound, providing a more comprehensive view of this entity than those limited to traditional definitions, i.e. those including only late antepartum death. We applied a high degree of methodological standardisation to ensure the collection of robust, unbiased, clinical and demographic data, including gestational age dating by early ultrasound, and used a hierarchical approach to analysis that demonstrated the importance of distal determinants on antepartum stillbirth. We also present risk models based on common risk factors that could be introduced into clinical practice immediately.
Pathological and microbiological examinations of the fetus/placenta were not performed in NCSS. Placental histopathology, although retrospective, can be useful for confirming the likely cause(s) of death in some cases of stillbirth.36 Therefore, we acknowledge that a limitation of this study is that we cannot claim the conditions we examined were causal. Instead, what we present are prognostic factors that, either singularly or in combination, increase the risk of stillbirth. In future studies, it is hoped that use of the new International Classification of Disease (ICD‐11) will provide a universally applicable, standard classification system for stillbirths that can be applied in settings with and without access to pathology.
Interpretation
Our data demonstrate that poor fetal growth and SGA at birth, particularly when not suspected during pregnancy, are asociated with antepartum stillbirth.9 However, this association was less evident in the subset of women with longitudinal ultrasound data, selected as they were, for low risk of growth problems. It is possible that, by using SGA based on weight at birth (as a proxy for FGR), the contribution of FGR to stillbirth may be overestimated, as fetuses lose weight after death.37 While some studies have attempted to correct for this potential bias,38 in clinical practice the exact time of death is rarely known. While we acknowledge that the number of cases of stillbirth was small in the low‐risk group, we cannot exclude the possibility that babies exhibiting poor growth were delivered earlier based on this finding. It should be borne in mind, however, that not all the pathways leading to antepartum stillbirth are chronic enough to cause an alteration in growth velocity, highlighting the heterogenous nature of this complex syndrome. This is important when evaluating the role of routine third‐trimester ultrasound scans to prevent antepartum stillbirth in low‐risk populations.
In our study population, the overall attributable risk of SGA for antepartum stillbirth was 11%, i.e. only 11% of all antepartum deaths could be prevented by eliminating all cases of FGR, which is in itself impossible. However, given that correct antenatal detection of SGA in the whole population was 27% – a rate consistent with that in the literature – the impact of screening low‐risk women for FGR to reduce stillbirth may be very limited.
The Every Newborn Action Plan 2014 has set a target for all countries to achieve stillbirth rates of <10 per 1000 births (after 28 weeks’ gestation) by 2035.39 We believe that this target is achievable across the world provided that the social, nutritional and health‐care needs of women are met. The disparities currently seen in stillbirth rates among countries mostly reflect differences in population health, nutritional status, reporting systems and access to care, and are less likely to be related to any underlying ethnic or genetic differences.6
Our findings reinforce recommendations to improve the detection and management of hypertensive disorders of pregnancy and other pregnancy conditions including the association with FGR. It is noteworthy, however, that several of the ‘lifestyle’ risk factors that have previously been associated with stillbirth, such as obesity and smoking, were not independently associated in this population. This is probably owing to the relatively low prevalence of these conditions in some of the study sites, emphasising the importance of context.
Although we focused on antepartum stillbirth, intrapartum stillbirth still accounted for 20% of deaths in our study, compared to the global figure of around 45%.5 Providing all women with access to high‐quality intrapartum care remains crucial; however, our study emphasises that improving intrapartum care alone will not be enough to reduce all preventable deaths. The evidence we present on clinical risk‐factor associations adds to the developing knowledge of other types of prediction models to identify pregnancies at risk of stillbirth, from biomarkers and uterine blood flow studies. For example, there is a suggestion that low serum levels of pregnancy‐associated plasma protein‐A during the first trimester and an increased pulsatility index from Doppler ultrasonography during the second trimester could be good predictors of stillbirth, with a moderate LR+ ranging from 10.0 to 15.0.40 The presence of these risk factors, in addition to the patterns of clinical variables we have reported, could guide decision‐making about the timing of delivery to prevent antepartum stillbirth.
Conclusion
We have confirmed that antepartum stillbirth is a complex syndrome that requires comprehensive interventions to meet the social, nutritional and health‐care requirements of all pregnant women worldwide. The promotion of interventions that are targeted at only one risk factor is unlikely to make a large impact on rates of antepartum stillbirth. Combinations of risk prediction models, biomarkers and growth trajectories could help to identify women at risk of specific forms of stillbirth.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Contribution to authorship
All authors are members of the INTERGROWTH‐21st Consortium and developed the conceptual ideas for the planned secondary analyses of the INTERGROWTH‐21st Project. JEH and DF wrote the first versions of the manuscript with subsequent contributions from JV, SHK, CGV, FCB, AL and ZAB. JV and SHK are the co‐directors of the INTERGROWTH‐21st Project; LCI was the Project Leader; ATP was the ultrasound lead and JAN the engineering lead for ultrasound standardisation across sites; MGG, FG, MP, FCB, IOF, RP, WS and YAJ implemented the study in their respective countries and were responsible for site coordination, data collection and integrity. DGA and EOO were the medical statisticians for the INTERGROWTH‐21st Project. All co‐authors read the report and approved the final manuscript.
Ethical approval
The INTERGROWTH‐21st Project was approved by the Oxfordshire Research Ethics Committee ‘C’ (reference:08/H0606/139) in December 2008, the research ethics committees of the individual participating institutions, and corresponding health authorities where the project was implemented.
Funding
The INTERGROWTH‐21st Project was supported by a grant from the Bill and Melinda Gates Foundation. The sponsor had no role in the study design, data collection, analysis or interpretation or in the writing of the report.
Supporting information
Figure S1. The hierarchical approach to analysis for stillbirth using a directed acyclic approach.
Table S1. Descriptive characteristics of the mothers and babies.
Table S2. Details of congenital abnormalities detected in antepartum death.
Table S3. Maternal characteristics comparing antepartum stillborn with live born babies.
Table S4. Associations between Maternal past medical and obstetric history and antepartum stillbirth.
Table S5. Conditions first detected in pregnancy and pregnancy‐specific conditions and antepartum stillbirth.
Hirst JE, Villar J, Victora CG, Papageorghiou AT, Finkton D, Barros FC, Gravett MG, Giuliani F, Purwar M, Frederick IO, Pang R, Cheikh Ismail L, Lambert A, Stones W, Jaffer YA, Altman DG, Noble JA, Ohuma EO, Kennedy SH, Bhutta ZA, for the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH‐21st) . The antepartum stillbirth syndrome: risk factors and pregnancy conditions identified from the INTERGROWTH‐21st Project. BJOG 2018; 125:1145–1153.
References
- 1. Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2016;4:e98–108. [DOI] [PubMed] [Google Scholar]
- 2. Froen JF, Pinar H, Flenady V, Bahrin S, Charles A, Chauke L, et al. Causes of death and associated conditions (Codac): a utilitarian approach to the classification of perinatal deaths. BMC Pregnancy Childbirth 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Headley E, Gordon A, Jeffery H. Reclassification of unexplained stillbirths using clinical practice guidelines. Aust NZ J Obstet Gynaecol 2009;49:285–9. [DOI] [PubMed] [Google Scholar]
- 4. de Bernis L, Kinney MV, Stones W, Ten Hoope‐Bender P, Vivio D, Leisher SH, et al. Stillbirths: ending preventable deaths by 2030. Lancet 2016;387:703–16. [DOI] [PubMed] [Google Scholar]
- 5. Lawn JE, Lee AC, Kinney M, Sibley L, Carlo WA, Paul VK, et al. Two million intrapartum‐related stillbirths and neonatal deaths: where, why, and what can be done? Int J Gynaecol Obstet 2009;107(Suppl 1):S5–18, S19. [DOI] [PubMed] [Google Scholar]
- 6. Bhutta ZA, Yakoob MY, Lawn JE, Rizvi A, Friberg IK, Weissman E, et al. Stillbirths: what difference can we make and at what cost? Lancet 2011;377:1523–38. [DOI] [PubMed] [Google Scholar]
- 7. Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet 2011;377:1331–40. [DOI] [PubMed] [Google Scholar]
- 8. Fretts R. Etiology and prevention of stillbirth. Am J Obstet Gynecol 2005;193:1923–35. [DOI] [PubMed] [Google Scholar]
- 9. Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ 2013;346:f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCowan L, George‐Haddad M, Stacey T, Thompson J. Fetal growth restriction and other risk factors for stillbirth in a New Zealand setting. Aus NZ J Obstet Gynaecol 2007;47:450–6. [DOI] [PubMed] [Google Scholar]
- 11. Gordon A, Raynes‐Greenow C, McGeechan K, Morris J, Jeffery H. Risk factors for antepartum stillbirth and the influence of maternal age in New South Wales Australia: a population based study. BMC Pregnancy Childbirth 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stillbirth Collaborative Research Network Writing Group . Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 2011;306:2469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villar J, Altman DG, Purwar M, Noble JA, Knight HE, Ruyan P, et al. The objectives, design and implementation of the INTERGROWTH‐21st Project. BJOG 2013;120(Suppl 2):9–26. [DOI] [PubMed] [Google Scholar]
- 14. Giuliani F, Bertino E, Oberto M, Di Nicola P, Gilli G, Knight H, et al. Implementation of the INTERGROWTH‐21st Project in Italy. BJOG 2013;120(Suppl 2):100–4. [DOI] [PubMed] [Google Scholar]
- 15. Roseman F, Knight HE, Giuliani F, Lloyd S, Di Nicola P, Laister A, et al. Implementation of the INTERGROWTH‐21st Project in the UK. BJOG 2013;120(Suppl 2):117–22. [DOI] [PubMed] [Google Scholar]
- 16. Carvalho M, Vinayak S, Ochieng R, Choksey V, Musee N, Stones W, et al. Implementation of the INTERGROWTH‐21st Project in Kenya. BJOG 2013;120(Suppl 2):105–10. [DOI] [PubMed] [Google Scholar]
- 17. Jaffer YA, Al Abri J, Abdawani J, Knight HE, Cheikh Ismail L, International F, et al. Implementation of the INTERGROWTH‐21st Project in Oman. BJOG 2013;120(Suppl 2):111–16. [DOI] [PubMed] [Google Scholar]
- 18. Pan Y, Wu MH, Wang JH, Pang RY, Knight HE, Cheikh Ismail L, et al. Implementation of the INTERGROWTH‐21st Project in China. BJOG 2013;120(Suppl 2):87–93. [DOI] [PubMed] [Google Scholar]
- 19. Purwar M, Kunnawar N, Deshmukh S, Singh A, Mulik I, Taori V, et al. Implementation of the INTERGROWTH‐21st project in India. BJOG 2013;120(Suppl 2):94–9. [DOI] [PubMed] [Google Scholar]
- 20. Silveira MF, Barros FC, Sclowitz IK, Domingues MR, Mota DM, Fonseca SS, et al. Implementation of the INTERGROWTH‐21st Project in Brazil. BJOG 2013;120(Suppl 2):81–6. [DOI] [PubMed] [Google Scholar]
- 21. Dighe MK, Frederick IO, Andersen HF, Gravett MG, Abbott SE, Carter AA, et al. Implementation of the INTERGROWTH‐21st Project in the United States. BJOG 2013;120(Suppl 2):123–8. [DOI] [PubMed] [Google Scholar]
- 22. Eskenazi B, Bradman A, Finkton D, Purwar M, Noble JA, Pang R, et al. A rapid questionnaire assessment of environmental exposures to pregnant women in the INTERGROWTH‐21st Project. BJOG 2013;120(Suppl 2):129–38. [DOI] [PubMed] [Google Scholar]
- 23. Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol 2012;206:119–23. [DOI] [PubMed] [Google Scholar]
- 24. Ohuma EO, Hoch L, Cosgrove C, Knight HE, Cheikh Ismail L, Juodvirsiene L, et al. Managing data for the international, multicentre INTERGROWTH‐21st Project. BJOG 2013;120(Suppl 2):64–70. [DOI] [PubMed] [Google Scholar]
- 25. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross‐Sectional Study of the INTERGROWTH‐21st Project. Lancet 2014;384:857–68. [DOI] [PubMed] [Google Scholar]
- 26. Papageorghiou AT, Kennedy SH, Salomon LJ, Ohuma EO, Cheikh Ismail L, Barros FC, et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown–rump length in the first trimester. Ultrasound Obstet Gynecol 2014;44:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altman DG. Ohuma EO; International Fetal and Newborn Growth Consortium for the 21st Century. Statistical considerations for the development of prescriptive fetal and newborn growth standards in the INTERGROWTH‐21st Project. BJOG 2013;120(Suppl 2):71–6. [DOI] [PubMed] [Google Scholar]
- 28. Platt RW, Joseph KS, Ananth CV, Grondines J, Abrahamowicz M, Kramer MS. A proportional hazards model with time‐dependent covariates and time‐varying effects for analysis of fetal and infant death. Am J Epidemiol 2004;160:199–206. [DOI] [PubMed] [Google Scholar]
- 29. Smith GC. Estimating risks of perinatal death. Am J Obstet Gynecol 2005;192:17–22. [DOI] [PubMed] [Google Scholar]
- 30. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710–8. [DOI] [PubMed] [Google Scholar]
- 31. Victora CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol 1997;26:224–7. [DOI] [PubMed] [Google Scholar]
- 32. Victora CG, Fuchs SC, Flores JA, Fonseca W, Kirkwood B. Risk factors for pneumonia among children in a Brazilian metropolitan area. Pediatrics 1994;93:977–85. [PubMed] [Google Scholar]
- 33. Maldonado G, Greenland S. Simulation study of confounder‐selection strategies. Am J Epidemiol 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- 34. Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet 2005;365:1500–5. [DOI] [PubMed] [Google Scholar]
- 35. Szklo M. Population‐based cohort studies. Epidemiol Rev 1998;20:81–90. [DOI] [PubMed] [Google Scholar]
- 36. Tellefsen CH, Vogt C. How important is placental examination in cases of perinatal deaths? Pediatr Dev Pathol 2011;14:99–104. [DOI] [PubMed] [Google Scholar]
- 37. Sebire NJ. Detection of fetal growth restriction at autopsy in non‐anomalous stillborn infants. Ultrasound Obstet Gynecol 2014;43:241–4. [DOI] [PubMed] [Google Scholar]
- 38. Conway DL, Hansen NI, Dudley DJ, Parker CB, Reddy UM, Silver RM, et al. An algorithm for the estimation of gestational age at the time of fetal death. Paediatr Perinat Epidemiol 2013;27:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet 2014;384:189–205. [DOI] [PubMed] [Google Scholar]
- 40. Conde‐Agudelo A, Bird S, Kennedy SH, Villar J, Papageorghiou AT. First‐ and second‐trimester tests to predict stillbirth in unselected pregnant women: a systematic review and meta‐analysis. BJOG 2015;122:41–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The hierarchical approach to analysis for stillbirth using a directed acyclic approach.
Table S1. Descriptive characteristics of the mothers and babies.
Table S2. Details of congenital abnormalities detected in antepartum death.
Table S3. Maternal characteristics comparing antepartum stillborn with live born babies.
Table S4. Associations between Maternal past medical and obstetric history and antepartum stillbirth.
Table S5. Conditions first detected in pregnancy and pregnancy‐specific conditions and antepartum stillbirth.


