Abstract
Background
Component‐resolved diagnostics (CRD) are promising tools for diagnosing food allergy, offering the potential to determine specific phenotypes and to develop patient‐tailored risk profiles. Nevertheless, the diagnostic accuracy of these tests varies across studies; thus, their clinical utility remains unclear. Therefore, we synthesized the evidence from studies investigating the diagnostic accuracy, risk assessment ability, and cost‐effectiveness of CRD for food allergy.
Methods
We systematically searched 10 electronic databases and four clinical trial registries for studies published from January 2000 to February 2017. The quality of included studies was assessed using QUADAS‐2. Due to heterogeneity, we narratively synthesized the evidence.
Results
Eleven studies met inclusion criteria, altogether recruiting 1098 participants. The food allergies investigated were cow's milk, hen's egg, peanut, hazelnut, and shrimp. The components with the highest diagnostic accuracy for each allergen, along with their sensitivity‐specificity pairs, were as follows: Bos d 4 for cow's milk (62.0% and 87.5%), Gal d 1 for hen's egg (84.2% and 89.8% for heated egg, and 60.6% and 97.1% for raw egg), Ara h 6 for peanut (94.9% and 95.1%), Cor a 14 for hazelnut (100% and 93.8%), and Lit v 1 for shrimp (82.8% and 56.3%) allergy.
Conclusion
Selected components of cow's milk, hen's egg, peanut, hazelnut, and shrimp allergen showed high specificity, but lower sensitivity. However, few studies exist for each component, and studies vary widely regarding the cutoff values used, making it challenging to synthesize findings across studies. Further research is needed to determine clinically appropriate cutoff values, risk assessment abilities, and cost‐effectiveness of CRD approaches.
Keywords: component‐resolved diagnostics, cost‐effectiveness, diagnostic test accuracy systematic review, food allergy, risk assessment
Abbreviations
- aa
amino acid
- APT
atopy patch test
- CRD
component‐resolved diagnostics
- DBPCFC
double‐blind, placebo‐controlled food challenge
- DTA
diagnostic test accuracy
- HSROC
hierarchical summary receiver operating characteristic
- IgE
immunoglobulin E
- NPV
negative predictive value
- PPV
positive predictive value
- QUADAS‐2
quality assessment of diagnostic accuracy studies‐2
- ROB
risk of bias
- ROC
receiver operating characteristic
- sIgE
specific immunoglobulin E
- SPT
skin prick test
1. INTRODUCTION
The high prevalence of food allergy is now an emerging global public health concern.1 Estimates of the prevalence of food allergy vary, but overall lifetime prevalence has been estimated to be between 4% and 7% for children and between 3% and 6% for adults in economically developed countries.2, 3 The quality of life of patients with food allergy is often severely affected, resulting in considerable morbidity and healthcare utilization, including risk of accidental exposure leading to life‐threatening anaphylactic reactions.4
An accurate diagnosis of food allergy is essential to provide appropriate, potentially life‐saving advice on how to prevent and manage allergic reactions and prevent unnecessary dietary restrictions.1, 4 The diagnosis of food allergy is dependent on a thorough clinical history as well as an objective marker of allergic sensitization and, in some cases, oral food challenge tests.5 Current first‐line tests to assess allergen sensitization are skin prick tests (SPT) and/or immunoassays of serum food‐specific IgE (sIgE) levels. However, these approaches have a high rate of false‐positive results and are poor predictors of the severity of allergic reactions.4 Thus, diagnostic confirmation with (ideally) a double‐blinded placebo‐controlled food challenge (DBPCFC) is often required.5 While DBPCFCs are considered the gold standard diagnostic tests, they are costly, technically challenging, time‐consuming, labor‐intensive, and are associated with important safety risks, as they can trigger anaphylactic reactions.4
Given the limitations of conventional methods for diagnosing food allergy, new molecular‐based diagnostic techniques—collectively referred to as component‐resolved diagnostics (CRD)—have emerged as promising diagnostic tools.6 While current approaches evaluate patients’ reactivity to whole food extracts, CRD involves detecting sIgE levels to individual allergenic molecules or the epitopes of those allergens.7 This approach may enhance determination of specific food allergy phenotypes, assist in the development of patient‐tailored risk profiles for specific food allergens, and improve detection of possible cases of cross‐reactivity between different allergenic molecules.8
Over the last decade, researchers have compared CRD to conventional diagnostic approaches for food allergy.9 Through this work, the major allergen components in different food allergies have been identified. However, the diagnostic accuracy of identified components varies across studies, and thus, the diagnostic value and clinical utility of CRD remains unclear.9, 10 CRD approaches are also expensive, which raises questions about their cost‐effectiveness.11
While the diagnostic accuracy of various tests for food allergy was evaluated in our previous systematic review, CRD was not included.12 A health technology assessment was carried out to evaluate multiplex CRD assays, but clinical effectiveness (rather than diagnostic accuracy) was investigated.13 To the best of our knowledge, only one CRD‐specific diagnostic test accuracy (DTA) review has been conducted, but it focused solely on peanut allergy diagnosis.14 This review concluded that Ara h 2 showed superior diagnostic accuracy than SPT and sIgE tests, and therefore has the potential to replace first‐line tests for the diagnosis of peanut allergy. Given the increasing body of work, there is a need to undertake a more comprehensive evidence synthesis on the diagnostic accuracy of CRD. We therefore conducted a systematic review to: (i) determine the accuracy of CRD for the diagnosis of food allergy, focusing on the “big eight” food allergies (ie, cow's milk, wheat, hen's egg, peanut, soy, tree nuts, fish, and shellfish allergy); (ii) estimate the effectiveness and cost‐effectiveness of CRD in comparison with conventional techniques for the diagnosis of these food allergies; and (iii) summarize the evidence on the ability of CRD to predict the severity of allergic reactions. We focused on these eight food allergies to align with the foods considered in our previous systematic reviews for the European Academy of Allergy and Clinical Immunology.2, 12
2. METHODS
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist guided the reporting of this systematic review (see Table S1).15 Our protocol was published16 and preregistered (PROSPERO:CRD42016053512).
2.1. Eligibility criteria
We included prospective, retrospective, cross‐sectional, and case‐control studies that examined the accuracy of CRD in diagnosing cow's milk, hen's egg, wheat, soybean, peanut, tree nuts, fish, or shellfish allergy in children or adults. Studies were required to have sufficient data to calculate the following four relevant diagnostic measures: sensitivity, specificity, positive predicted value (PPV), and negative predictive value (NPV). Additionally, all studies were required to have a defined study population with either consecutive or random sampling of participants. Studies in which the recruitment technique used to select participants was not indicated were included, and the lack of information regarding their sampling methodology was noted during the quality assessment process. The reference standard was DBPCFC used in at least 50% of the participants.
2.2. Search strategy
Although CRD methods were originally described in the 1990s,17 their application to food allergy diagnosis was not clinically implemented until the 2000s.1 Hence, we chose the beginning of 2000 as the starting time for the literature search. We searched the following databases from January 2000 to February 2017: AMED (Ovid), CAB Abstracts (Ovid), the Cochrane Library, CINAHL (EBSCO), EMBASE (Ovid), Global Health (Ovid), PsycINFO (Ovid), Web of Science Core Collection (Thomson Reuters), WHO's Global Health Library and the Health Economic Evaluations Database. Our full search strategy is included in the online supplement (Table S2). We also contacted international experts who have published in the field, screened the references cited in identified studies, and used the citation‐tracking feature of Google Scholar to find any additional studies. The list of contacted experts can be found in the online supplement (Table S3). Additionally, the International Standard Randomized Controlled Trial Number (ISRCTN) Registry, ClinicalTrials.gov, the Australian and New Zealand Clinical Trials Registry, and WHO's International Clinical Trials Registry Platform (ICTRP) were searched to identify relevant ongoing studies. No language restrictions were applied.
2.3. Study selection and data collection
Two reviewers (JFK and NM) independently screened titles and abstracts and then reviewed full‐texts to identify eligible studies. Authors of studies for which further details were required to determine inclusion or exclusion were contacted to obtain further information to enable a decision. For papers in languages other than English, speakers of the language in question were contacted to determine eligibility. Both reviewers independently extracted data from included studies using a form developed specifically for this systematic review. Study characteristics, DTA measures (ie, sensitivity, specificity, PPV and NPV), and 2 × 2 contingency tables (reflecting the number of true positives, true negatives, false positives, and false negatives) were extracted. DTA measures and 95% confidence intervals were calculated from 2 × 2 contingency tables when not provided by authors.18, 19, 20 The two reviewers assessed the quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool.21 Discrepancies were resolved by discussion and consensus, or when necessary, arbitrated by a third reviewer (BN).
2.4. Data synthesis, analysis, and reporting
Diagnostic accuracy measures (sensitivity, specificity, PPV, and NPV) of individual studies were summarized in tables and presented by allergy type and individual allergen component. We had planned to conduct meta‐analyses of the evidence with respect to each allergen component by fitting a bivariate model (when included studies used a common threshold) or a hierarchical summary receiver operating characteristic (HSROC) model (when included studies used multiple thresholds). However, we were unable to do this, as the number of studies for each component was too small to permit quantitative syntheses. In a simulated analysis based on the Bayesian approach, it was recommended that a minimum of four studies were required to reasonably fit these models.22 In a very few cases, we had a maximum of three studies per allergen component; the most common was two studies. The statistical programs we tried to use to fit the models (R and Stata) indicated that the models lacked convergence, as a result of containing too few studies. For these reasons, we narratively synthesized the evidence.
3. RESULTS
3.1. Study selection
A total of 10 380 articles were identified through the literature search carried out on June 15, 2016. After excluding duplicate articles, 6853 titles and abstracts were screened against the inclusion and exclusion criteria; of these, 195 full‐text papers were assessed. Thirteen articles reporting 11 studies met our criteria and were thus included.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 Additionally, one potentially relevant ongoing clinical trial was found in ClinicalTrials.gov (details can be found in Table S4). The literature search was updated on February 9, 2017, to incorporate newly published papers. No additional relevant studies were identified in the updated search. The study screening and selection processes are summarized in Figure 1. A list of potentially relevant studies can be found in the Online supplement (Tables S5 and S6). The authors of these studies did not reply to a request for further information.
Figure 1.
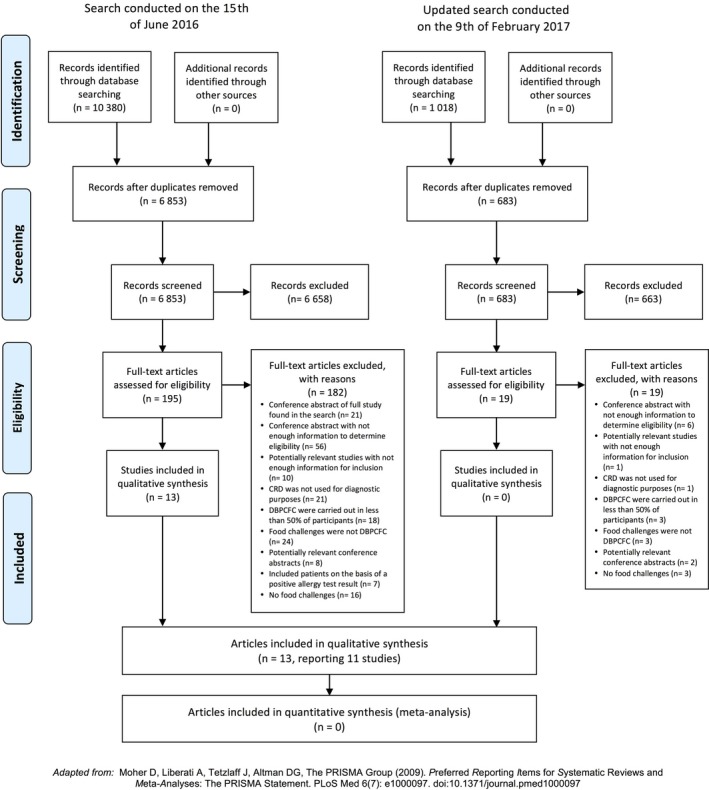
PRISMA Diagram for the literature search
3.2. Characteristics of included studies
Table 1 summarizes the main characteristics of the 11 studies included. The studies altogether recruited a total of 1098 participants. Nine studies were carried out in Western Europe,23, 24, 25, 26, 27, 28, 30, 32, 33 and two multicenter studies analyzed data from multiple countries.31, 34 Two studies used a case‐control design,32, 34 while nine were cross‐sectional studies.23, 24, 25, 26, 27, 28, 30, 31, 33 Two of the cross‐sectional studies used consecutive sampling to recruit participants,26, 28 while the sampling strategy used in the remaining seven cross‐sectional studies was unclear.23, 24, 25, 27, 30, 31, 33 Taking all studies together, 87% of participants underwent DBPCFC to verify their food allergy status.
Table 1.
Main characteristics of included studies
| First author, year | Country of origin | Reference no. | Study design | Food allergy studied | Population | Sample size | Components tested | Index test | Number of patients that underwent DBPCFC | QUADAS‐2 risk of bias domains 1, 2, 3, 4 applicability domains 1, 2, 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Alessandri 2012a | Italy | 23 | Cross‐sectional | Cow Milk | Children with suspected cow's milk allergy | 66 | Bos d 4, 5, and 8 | ImmunoCAP | 66 (100%) |

|
| Alessandri 2012b | Italy | 24 | Cross‐sectional | Hen's Egg | Children with suspected hen's egg allergy | 68 | Gal d 1,2, 3, and 5 | ISAC 103 | 68 (100%) |

|
| Ando 2008 | Japan | 25 | Cross‐sectional | Hen's Egg | Children with suspected hen's egg allergy | 108 | Gal d 1, and 2 | ImmunoCAP | 108 (100%) |

|
| Ayuso 2012 | Spain | 26 | Cross‐sectional | Shrimp | Patients with reported immediate shrimp‐allergic reactions | 37 | Pen a 1; and Lit v 1, 2, 3, and 4 | ImmunoCAP and Immunoblot | 31 (83.8%) |

|
| Brandstrom 2015 | Sweden | 27 | Cross‐sectional | Hazelnut | Children referred for oral challenge for hazelnut allergy suspicion | 40 | Cor a 1, 8, 9, and 14; and Bet v 1 | ImmunoCAP | 40 (100%) |

|
| Klemans 2014 (& Klemans 2013) | The Netherlands | 28 , 29 | Cross‐sectional | Peanut | Adults who underwent a DBPCFC for peanut allergy suspicion | 107 | Ara h 2 and 6 | ISAC 112 | 107 (100%) |

|
| Kukkonen 2015 | Finland | 30 | Cross‐sectional | Peanut | Children with suspected peanut allergy | 102 | Ara h 1, 2, 3, 6, 8, and 9 | ImmunoCap and ISAC112 | 102 (100%) |

|
| Lieberman 2013 | USA and Sweden | 31 | Cross‐sectional | Peanut | Children referred to an allergy center for evaluation of peanut allergy | 167 | Ara h 1, 2, 3, and 8 | ImmunoCAP | 119 (71.3%) |

|
| Masthoff 2013 (& Masthoff 2015) | The Netherlands | 32,35 | Case‐Control | Hazelnut | Patients sensitized to hazelnut extract | 161 | Cor a 1, 8, 9, and 14; and Bet v 1 | ImmunoCAP | 137 (85.1%) |

|
| Ott 2008 | Germany | 33 | Cross‐sectional | Hen's Egg and Cow's Milk | Children with suspected hen's egg or cow's milk allergy | 130 | Gal d 1, 2 and 4; Bos d 4, and 5, and Caseins | ISAC version CRD 51 | 103 (71%) |

|
| Pascal 2015 | Brazil, Spain, and USA | 34 | Case‐control | Shrimp | Patients sensitized to shrimp | 103 | Lit v 1, Lit v 2, Lit v 3, and Lit v 4 | Immunoblot | 78 (76%) |

|
The included studies analyzed the diagnostic accuracy of CRD for the following types of food allergy: cow's milk (n = 2),23, 33 hen's egg (n = 3),24, 25, 33 peanut (n = 3),28, 30, 31 hazelnut (n = 2),27, 32 and shrimp (n = 2).26, 34 No studies investigated the other allergies of interest (i.e, wheat, soybean, and fish). All but one study analyzed a single type of allergy; this study analyzed the diagnostic accuracy of CRD for both cow's milk and hen's egg.33 Five studies used the ImmunoCAP test to measure sIgE levels,23, 25, 26, 27, 31, 32 three studies used microarray techniques (ISAC CRD 51, ISAC 103, and ISAC 112),24, 28, 33 one study used a combination of ImmunoCAP and a microarray technique (ISAC 112),30 and one study used an immunoblotting technique for sIgE detection.34 Two studies additionally analyzed the DTA of individual component epitopes using immunoblotting techniques.26, 34
3.3. Quality assessment of included studies
Table 1 includes a summary of the QUADAS‐2 quality assessment for each study. Table S7 in the supplementary information provides the detailed QUADAS‐2 assessments.
3.3.1. Patient selection
Two studies were rated as high risk of bias (ROB) in this domain because of the use of a case‐control design.32, 34 Eight other studies were found to have an unclear ROB, mainly because they did not explicitly indicate their sampling methodology and/or did not avoid inappropriate exclusions.23, 24, 25, 27, 28, 30, 31, 33 The remaining study had a low ROB.26
3.3.2. Index test
Two studies had high ROB in this domain because they did not use prespecified thresholds for determining positive results.27, 33 Eight studies had unclear ROB because they did not report whether index test results were interpreted without knowledge of DBPCFC results.23, 24, 25, 26, 28, 31, 32, 34 Only one study had low ROB in this domain.30 Two studies used immunoblotting assays to analyze sIgE reactivity against allergens; because these assays do not provide quantitative sIgE levels, these two studies were scored as unsure in terms of their applicability to this review's research question.26, 34
3.3.3. Reference standard
One study did not specify the criteria used to classify DBPCFC results and was thus scored as having an unclear ROB in this domain.26 Two studies were scored as unsure in terms of their applicability to this review's research question.30, 32 The reason for this appraisal was that the purpose of this review was to assess the accuracy of CRD and its ability to predict allergy severity (both of which are assessed through objective symptoms in a DBPCFC), and the two aforementioned studies included patients with mild or subjective DBPCFC symptoms in the same group as patients with negative DBPCFC.
3.3.4. Patient flow and timing
Two studies had a high ROB in this domain because less than 100% of patients underwent DBPCFCs, and not all patient data were included in their data analysis.26, 34 Three studies specified the time interval between index and reference tests, compared all patients against the same reference standard, included all patients in data analysis, and were thus ranked as low ROB in this domain.24, 25, 27 The remaining six studies failed to meet at least one of those criteria and were thus scored as having an unclear ROB.23, 28, 30, 31, 32, 33
3.4. Diagnostic accuracy of CRD
DTA measures for all the 11 studies are presented in Table 2. The information in this table includes data points for all sIgE cutoff values that the included studies used to define test positivity, as some studies used multiple values. In the following narrative synthesis, we present the results of the diagnostic accuracy of all components per food allergy type for only cutoff values with the highest diagnostic potential as defined in each study.
Table 2.
Summary DTA measures of CRD components tested by allergy type
| Food allergy | Component | Study | Year | Reference no. | Country | Index test | Cutoff value | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cow's milk | Bos d 4 | Alessandri | 2012a | 23 | Italy | ISAC 103 | >0 (kUa/L) | 56.0 (41.3‐70.0) | 87.5 (61.7‐98.5) | 93.3 (78.9‐98.1) | 38.9 (38.9‐47.8) |
| Cow's milk | Bos d 4 | Alessandri | 2012a | 23 | Italy | ISAC 103 | >0.01 (kUa/L) | 62.0 (47.2‐75.4) | 87.5 (61.7‐98.5) | 93.9 (80.6‐98.3) | 42.4 (33.1‐52.4) |
| Cow's milk | Bos d 4 | Ott | 2008 | 33 | Germany | ISAC CRD51 | >0.1 (FI) | 50.0 (34.2‐65.8) | 93.0 (80.9‐98.5) | 87.5 (69.3‐95.6) | 65.6 (58.2‐72.3) |
| Cow's milk | Bos d 4 | Alessandri | 2012a | 23 | Italy | ImmunoCAP | >0.35 (kUa/L) | 80.0 (66.3‐90.0) | 50.0 (24.7‐75.4) | 83.3 (75.0‐89.3) | 44.4 (27.6‐62.6) |
| Cow's milk | Bos d 4 | Alessandri | 2012a | 23 | Italy | ImmunoCAP | >1.02 (kUa/L) | 58.0 (43.2‐71.8) | 81.3 (54.4‐96.0) | 90.6 (77.2‐96.5) | 38.2 (29.3‐48.1) |
| Cow's milk | Bos d 5 | Alessandri | 2012a | 23 | Italy | ImmunoCAP | >0 (kUa/L) | 90.0 (78.2‐96.7) | 50.0 (24.7‐75.4) | 84.9 (77.4‐90.3) | 61.5 (37.9‐80.8) |
| Cow's milk | Bos d 5 | Alessandri | 2012a | 23 | Italy | ISAC 103 | >0 (kUa/L) | 40.0 (26.4‐54.8) | 93.8 (69.8‐99.8) | 95.2 (74.4‐99.3) | 33.3 (27.8‐39.3) |
| Cow's milk | Bos d 5 | Alessandri | 2012a | 23 | Italy | ISAC 103 | >0.01 (kUa/L) | 44.0 (30.0‐58.8) | 93.8 (69.8‐99.8) | 95.7 (76.3‐99.3) | 34.9 (28.9‐41.4) |
| Cow's milk | Bos d 5 | Ott | 2008 | 33 | Germany | ISAC CRD 51 | >0.1 (FI) | 23.8 (12.1‐39.5) | 95.3 (84.2‐99.4) | 83.3 (53.8‐95.6) | 56.2 (51.7‐60.6) |
| Cow's milk | Bos d 5 | Alessandri | 2012a | 23 | Italy | ImmunoCAP | >0.35 (kUa/L) | 82.0 (68.6‐91.4) | 62.5 (35.4‐84.8) | 87.2 (78.2‐92.9) | 52.6 (35.5‐69.2) |
| Cow's milk | Bos d 8 | Alessandri | 2012a | 23 | Italy | ISAC 103 | >0 (kUa/L) | 54.0 (39.3‐68.2) | 81.3 (54.4‐96.0) | 90.0 (75.9‐96.3) | 36.1 (27.9‐45.3) |
| Cow's milk | Bos d 8 | Alessandri | 2012a | 23 | Italy | ISAC 103 | >0.01 (kUa/L) | 56.0 (41.3‐70.0) | 81.3 (54.4‐96.0) | 90.3 (76.6‐96.4) | 37.1 (28.6‐46.6) |
| Cow's milk | Bos d 8 | Alessandri | 2012a | 23 | Italy | ImmunoCAP | >0.35 (kUa/L) | 88.0 (75.7‐95.5) | 56.3 (29.9‐80.3) | 86.3 (78.1‐91.7) | 60.0 (38.7‐78.1) |
| Cow's milk | Bos d 8 | Alessandri | 2012a | 23 | Italy | ImmunoCAP | >0.44 (kUa/L) | 82.0 (68.6‐91.4) | 62.5 (35.4‐84.8) | 87.2 (78.2‐92.9) | 52.6 (35.5‐69.2) |
| Cow's milk | α‐casein | Ott | 2008 | 33 | Germany | ISAC CRD 51 | >0.1 (FI) | 26.2 (13.9‐42.0) | 97.7 (87.7‐99.9) | 91.7 (59.8‐98.8) | 57.5 (52.9‐62.0) |
| Cow's milk | β‐casein | Ott | 2008 | 33 | Germany | ISAC CRD 51 | >0.1 (FI) | 26.2 (13.9‐42.0) | 93.0 (80.9‐98.5) | 78.6 (52.4‐92.4) | 56.3 (51.4‐61.1) |
| Cow's milk | κ‐casein | Ott | 2008 | 33 | Germany | ISAC CRD 51 | >0.1 (FI) | 38.1 (23.6‐54.4) | 88.4 (74.9‐96.1) | 76.2 (56.3‐88.8) | 59.4 (53.0‐65.5) |
| Heated Hen's Egg | Gal d 1 | Alessandri | 2012b | 24 | Italy | ISAC 103 | >0.01 (kUa/L) | 84.2 (60.4‐96.6) | 89.8 (77.8‐96.6) | 76.2 (57.7‐88.3) | 93.6 (83.8‐97.7) |
| Heated Hen's Egg | Gal d 1 | Ando | 2008 | 25 | Japan | ImmunoCAP | >0.37 (kUa/L) | 97.4 (86.2‐99.9) | 35.7 (24.6‐48.1) | 45.1 (40.7‐49.7) | 96.2 (77.9‐99.4) |
| Heated Hen's Egg | Gal d 1 | Ando | 2008 | 25 | Japan | ImmunoCAP | >4.4 (kUa/L) | 76.3 (59.8‐88.6) | 81.4 (70.3‐89.8) | 69.0 (57.0‐79.0) | 86.4 (78.0‐91.9) |
| Heated Hen's Egg | Gal d 2 | Alessandri | 2012b | 24 | Italy | ISAC 103 | >0.01 (kUa/L) | 52.6 (28.9‐75.6) | 83.7 (70.3‐92.7) | 55.6 (36.8‐72.9) | 82.0 (73.6‐88.1) |
| Heated Hen's Egg | Gal d 2 | Ando | 2008 | 25 | Japan | ImmunoCAP | >0.37 (kUa/L) | 100.0 (90.8‐100) | 21.4 (12.5‐32.9) | 40.9 (37.9‐43.9) | 100.0 (NA) |
| Heated Hen's Egg | Gal d 2 | Ando | 2008 | 25 | Japan | ImmunoCAP | >6.33 (kUa/L) | 73.7 (56.9‐86.6) | 72.9 (60.9‐82.8) | 59.6 (49.0‐69.3) | 83.6 (76.6‐89.9) |
| Heated Hen's Egg | Gal d 3 | Alessandri | 2012b | 24 | Italy | ISAC 103 | >0.01 (kUa/L) | 21.1 (6.1‐45.6) | 93.9 (83.1‐98.7) | 57.1 (24.7‐84.4) | 75.4 (70.6‐79.6) |
| Raw Hen's Egg | Gal d 1 | Ott | 2008 | 33 | Germany | ISAC CRD 51 | >0 (FI) | 57.8 (42.2‐72.3) | 86.7 (59.5‐98.3) | 92.9 (77.7‐98.0) | 40.6 (31.6‐50.4) |
| Raw Hen's Egg | Gal d 1 | Alessandri | 2012b | 24 | Italy | ISAC 103 | >0.01 (kUa/L) | 60.6 (42.1‐77.1) | 97.1 (85.1‐99.9) | 95.2 (74.0‐99.3) | 72.3 (63.1‐80.0) |
| Raw Hen's Egg | Gal d 1 | Ando | 2008 | 25 | Japan | ImmunoCAP | >0.37 (kUa/L) | 86.6 (76.0‐93.7) | 41.5 (26.3‐57.9) | 70.7 (64.8‐76.1) | 65.4 (48.2‐79.3) |
| Raw Hen's Egg | Gal d 1 | Ando | 2008 | 25 | Japan | ImmunoCAP | >2.26 (kUa/L) | 73.1 (60.9‐83.2) | 82.9 (67.9‐92.9) | 87.5 (77.8‐93.3) | 65.4 (55.4‐74.2) |
| Raw Hen's Egg | Gal d 2 | Ott | 2008 | 33 | Germany | ISAC CRD 51 | >0 (FI) | 57.8 (42.2‐72.3) | 80.0 (51.9‐95.7) | 89.7 (75.3‐96.1) | 38.7 (29.2‐49.1) |
| Raw Hen's Egg | Gal d 2 | Alessandri | 2012b | 24 | Italy | ISAC 103 | >0.01 (kUa/L) | 42.4 (25.5‐60.8) | 88.6 (73.3‐96.8) | 77.8 (56.2‐90.5) | 62.0 (54.3‐69.1) |
| Raw Hen's Egg | Gal d 2 | Ando | 2008 | 25 | Japan | ImmunoCAP | >0.37 (kUa/L) | 97.0 (89.6‐99.6) | 31.7 (18.1‐48.1) | 69.9 (65.2‐74.2) | 86.7 (60.7‐96.5) |
| Raw Hen's Egg | Gal d 2 | Ando | 2008 | 25 | Japan | ImmunoCAP | >3.88 (kUa/L) | 76.1 (64.1‐85.7) | 82.9 (67.9‐92.9) | 87.9 (78.6‐93.6) | 68.0 (57.6‐76.9) |
| Raw Hen's Egg | Gal d 3 | Alessandri | 2012b | 24 | Italy | ISAC 103 | >0.01 (kUa/L) | 18.2 (7.0‐35.5) | 97.1 (85.1‐99.9) | 85.7 (43.3‐97.9) | 55.7 (51.5‐59.9) |
| Raw Hen's Egg | Gal d 4 | Ott | 2008 | 33 | Germany | ISAC CRD 51 | >0 (FI) | 17.8 (8.0‐32.1) | 100.0 (78.2‐100) | 100.0 (NA) | 28.8 (26.1‐31.7) |
| Peanut | Ara h 1 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >0.35 kUa/L | 63.9 (50.6‐75.8) | 87.8 (73.8‐95.9) | 88.6 (77.1‐94.8) | 62.1 (53.5‐70.0) |
| Peanut | Ara h 1 | Lieberman | 2013 | 31 | USA and Sweden | ImmunoCAP | >0.35 kUa/L | 56.6 (46.6‐66.2) | 86.9 (75.8‐94.2) | 88.2 (79.4‐93.6) | 53.5 (47.6‐59.4) |
| Peanut | Ara h 1 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >0.8 kUa/L | 60.7 (47.3‐72.9) | 95.1 (83.5‐99.4) | 94.9 (82.5‐98.6) | 61.9 (54.2‐69.1) |
| Peanut | Ara h 2 | Klemans | 2014 | 28 | The Netherlands | ISAC 112 | >0.3 ISU/L | 69.2 (56.6‐80.1) | 90.5 (77.4‐97.3) | 91.8 (81.4‐96.7) | 65.5 (56.6‐73.5) |
| Peanut | Ara h 2 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >0.35 kUa/L | 95.1 (86.3‐99.0) | 73.2 (57.1‐85.8) | 84.1 (76.0‐89.8) | 90.9 (76.6‐96.8) |
| Peanut | Ara h 2 | Lieberman | 2013 | 31 | USA and Sweden | ImmunoCAP | >0.35 kUa/L | 80.2 (71.3‐87.3) | 91.8 (81.9‐97.3) | 94.4 (88.0‐97.5) | 72.7 (64.4‐79.8) |
| Peanut | Ara h 2 | Klemans | 2014 | 28 | The Netherlands | ISAC 112 | >1.0 ISU/L | 58.5 (45.6‐70.6) | 95.2 (83.8‐99.4) | 95.0 (82.9‐98.7) | 59.7 (52.4‐66.6) |
| Peanut | Ara h 2 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >1.8 kUa/L | 80.3 (68.2‐89.4) | 95.1 (83.5‐99.4) | 96.1 (86.3‐99.0) | 76.5 (66.1‐84.4) |
| Peanut | Ara h 3 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >0.35 kUa/L | 57.4 (46.1‐70.0) | 90.2 (76.9‐97.3) | 89.7 (77.1‐95.8) | 58.7 (51.1‐66.0) |
| Peanut | Ara h 3 | Lieberman | 2013 | 31 | USA and Sweden | ImmunoCAP | >0.35 kUa/L | 48.1 (38.3‐58.1) | 90.2 (79.8‐96.3) | 89.5 (79.5‐94.9) | 50.0 (45.0‐55.0) |
| Peanut | Ara h 3 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >0.8 kUa/L | 55.7 (42.5‐68.5) | 95.1 (83.5‐99.4) | 94.4 (81.2‐98.5) | 59.1 (51.9‐65.9) |
| Peanut | Ara h 6 | Kukkonen | 2015 | 30 | Finland | ISAC 112 | >0.3 ISU | 98.3 (90.9‐100) | 90.2 (76.9‐97.3) | 93.5 (85.1‐97.4) | 97.4 (84.1‐99.6) |
| Peanut | Ara h 6 | Klemans | 2014 | 28 | The Netherlands | ISAC 112 | >0.3 ISU/L | 70.8 (58.2‐81.4) | 85.7 (71.5‐94.6) | 88.5 (78.2‐94.2) | 65.5 (56.0‐73.8) |
| Peanut | Ara h 6 | Kukkonen | 2015 | 30 | Finland | ISAC 112 | >0.8 ISU/L | 94.9 (85.9‐98.9) | 95.1 (83.5‐99.4) | 96.6 (87.9‐99.1) | 92.9 (81.2‐97.5) |
| Peanut | Ara h 6 | Klemans | 2014 | 28 | The Netherlands | ISAC 112 | >1.0 ISU/L | 61.5 (48.6‐73.4) | 95.2 (83.8‐99.4) | 95.2 (83.6‐98.7) | 61.5 (53.9‐68.7) |
| Peanut | Ara h 8 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >0.35 kUa/L | 78.7 (66.3‐88.1) | 14.6 (5.6‐29.2) | 57.8 (53.3‐62.2) | 31.6 (16.0‐52.7) |
| Peanut | Ara h 8 | Lieberman | 2013 | 31 | USA and Sweden | ImmunoCAP | >0.35 kUa/L | 34.9 (25.9‐44.8) | 42.6 (30.0‐55.9) | 51.4 (43.0‐59.7) | 27.4 (21.4‐34.2) |
| Peanut | Ara h 9 | Kukkonen | 2015 | 30 | Finland | ImmunoCAP | >0.35 kUa/L | 14.8 (7.0‐26.2) | 85.4 (70.8‐94.4) | 60.0 (36.6‐79.6) | 40.2 (36.4‐44.2) |
| Hazelnut | Cor a 1 | Masthoff | 2012 | 32 | The Netherlands | ImmunoCAP | >0.35 kUa/L | 79.7 (69.2‐88.0) | 7.3 (2.7‐15.3) | 45.3 (42.2‐48.5) | 27.3 (13.4‐47.6) |
| Hazelnut | Cor a 8 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >0.35 kUa/L | 6.3 (2.1‐14.2) | 96.3 (89.7‐99.2) | 62.5 (29.2‐87.1) | 51.6 (49.9‐53.4) |
| Hazelnut | Cor a 9 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >0.35 kUa/L | 59.5 (47.9‐70.4) | 87.8 (78.7‐94.0) | 82.5 (71.9‐89.6) | 69.2 (63.0‐74.8) |
| Hazelnut | Cor a 9 | Bransdstrom | 2015 | 27 | Sweden | ImmunoCAP | >0.65 kUa/L | 100.0 (63.1‐100) | 71.9 (53.3‐86.3) | 47.1 (33.8‐60.7) | 100.0 (NA) |
| Hazelnut | Cor a 9 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >1 kUa/L | 54.4 (42.8‐65.7) | 97.6 (91.7‐99.7) | 95.6 (84.4‐98.9) | 69.0 (63.5‐73.9) |
| Hazelnut | Cor a 9 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >5 kUa/L | 30.4 (20.5‐41.8) | 98.8 (93.4‐100) | 96.0 (76.9‐99.4) | 59.6 (56.0‐63.1) |
| Hazelnut | Cor a 14 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >0.35 kUa/L | 54.4 (42.8‐65.7) | 85.4 (75.8‐92.2) | 78.2 (67.2‐86.3) | 66.0 (60.1‐71.6) |
| Hazelnut | Cor a 14 | Bransdstrom | 2015 | 27 | Sweden | ImmunoCAP | >0.64 kUa/L | 100.0 (63.1‐100) | 93.8 (79.2‐99.2) | 80.0 (51.1‐93.9) | 100.0 (NA) |
| Hazelnut | Cor a 14 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >1 kUa/L | 50.6 (39.1‐62.1) | 86.6 (77.3‐93.1) | 78.4 (66.8‐86.8) | 64.5 (58.9‐69.8) |
| Hazelnut | Cor a 14 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >5 kUa/L | 39.2 (28.4‐50.9) | 98.8 (93.4‐100) | 96.9 (81.3‐100) | 62.8 (58.5‐66.9) |
| Hazelnut | Bet v 1 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >0.35 kUa/L | 81.0 (70.6‐89.0) | 7.3 (2.7‐15.3) | 45.7 (42.7‐48.8) | 28.6 (14.1‐49.5) |
| Hazelnut | Bet v 2 | Masthoff | 2013 | 32 | The Netherlands | ImmunoCAP | >0.35 kUa/L | 10.1 (4.5‐19.0) | 81.7 (71.6‐89.4) | 34.8 (19.3‐54.3) | 48.6 (45.4‐51.7) |
| Shrimp | Lit v 1 | Pascal | 2015 | 34 | Brazil, Spain and USA | Immunoblot | NA | 82.8 (70.6‐91.4) | 56.3 (29.9‐80.3) | 87.3 (79.5‐92.4) | 47.4 (30.7‐64.7) |
| Shrimp | Lit v 4 | Pascal | 2015 | 34 | Brazil, Spain and USA | Immunoblot | NA | 34.5 (22.5‐48.1) | 93.8 (69.8‐99.8) | 95.2 (74.4‐99.3) | 28.3 (24.0‐33.1) |
| Shrimp | rPen a 1 | Ayuso | 2012 | 26 | Spain | ImmunoCAP | >0.35 kUa/L | 88.2 (63.6‐98.5) | 23.8 (8.2‐47.2) | 48.4 (41.1‐55.8) | 71.4 (35.6‐91.9) |
3.4.1. Cow's milk allergy
Two studies evaluated CRD for cow's milk allergy,23, 33 and the following components were assessed: Bos d 4 (α‐lactalbumin), Bos d 5 (β‐lactoglobulin), Bos d 8 (caseins), and the caseins (α‐, β‐, and κ‐) separately. The reported sensitivity‐specificity for these components were as follows: for Bos d 4, 62.0% and 87.5% (with a cutoff value defining a positive test of >0.01 kUa/L),23 and 50.0% and 93.0% (at >0.1 FI)33; for Bos d 5, 82.0% and 62.5% (at >0.35 kUa/L),23 and 23.8% and 95.3% (at >0.1 FI)33; for Bos d 8, 88.0% and 56.3% (at >0.35 kUa/L)23; and the casein with the highest DTA was κ‐casein with a sensitivity‐specificity pair of 38.1% and 88.4% (at >0.1 FI).33
3.4.2. Hen's egg allergy
Three studies evaluated CRD for hen's egg allergy,24, 25, 33 and the following components were assessed: Gal d 1 (ovomucoid), Gal d 2 (ovalbumin), Gal d 3 (ovotransferrin), and Gal d 4 (lysozyme). Two studies investigated heated egg and raw egg allergy separately,24, 25 while the third study analyzed only raw egg allergy.33 For heated egg allergy, the reported sensitivity‐specificity for these components were as follows: for Gal d 1, 84.2% and 89.8% (at >0.01 kUa/L),24 and 76.3% and 81.4% (at >4.4 kUa/L)25; for Gal d 2, 52.6% and 83.7% (at >0.01 kUa/L),24 and 73.7% and 72.9% (at >6.33 kUa/L);25 and for Gal d 3, 21.1% and 93.9% (at >0.01 kUa/L).24 For raw egg allergy, the reported sensitivity‐specificity for these components were as follow: for Gal d 1, 60.6% and 97.1% (at >0.01 kUa/L),24 73.1% and 82.9% (at >2.26 kUa/L),25 and 57.8% and 86.7% (at >0 FI)33; for Gal d 2, 42.4% and 88.6% (at >0.01 kUa/L),24 and 76.1% and 82.9% (at >3.88 kUa/L),25 and 57.8% and 80.0% (at >0 FI)33; for Gal d 3, 18.2% and 97.1% (at >0.01 kUa/L)24; and for Gal d 4, 17.8% and 100% (at >0 FI).33
3.4.3. Peanut allergy
Three studies evaluated the diagnostic accuracy of CRD for peanut allergy,28, 30, 31 and the following components were assessed: Ara h 1 (cupin, a 7S globulin), Ara h 2 (conglutin, a 2S albumin), Ara 3 (cupin, a 11S globulin), Ara h 6 (conglutin, a 2S albumin), Ara h 8 (Bet v 1 homologue), and Ara h 9 (LTP). The reported sensitivity‐specificity for these components were as follows: for Ara h 1, 56.6% and 86.9% (at >0.35 kUa/L),31 and 60.7% and 95.1% (at >0.8 kUa/L)30; for Ara h 2, 69.2% and 90.5% (at >0.3 ISU/L),28 80.2% and 91.8% (at >0.35 kUa/L),31 and 80.3% and 95.1% (at >1.8 kUa/L)30; for Ara h 3, 48.1% and 90.2% (at >0.35 kUa/L),31 and 55.7% and 95.1% (at >0.8 kUa/L)30; for Ara h 6, 61.5% and 95.2% (at >1.0 ISU/L)28, and 94.9% and 95.1% (at >0.8 ISU)30; for Ara h 8, 34.9% and 42.6% (at >0.35 kUa/L),31 and 78.7% and 14.6% (at >0.35 kUa/L)30; and for Ara h 9, 14.8% and 85.4% (at >0.35 kUa/L).30
3.4.4. Hazelnut allergy
Two studies evaluated the diagnostic accuracy of CRD for hazelnut allergy,27, 32 and the following components were assessed: Cor a 1 (PR‐10 protein), Cor a 8 (LTP), Cor a 9 (11S seed storage globulin), and Cor a 14 (2S albumin). Additionally, one of the studies investigated whether sensitization to the allergens Bet v 1 (PR‐10 protein) and Bet v 2 (profilin) (from the European White Birch) could also predict hazelnut allergy.32 The reported sensitivity‐specificity for these components were as follows: for Cor a 1, 79.7% and 7.3% (at >0.35 kUa/L32; for Cor a 8, 6.3% and 96.3% (at >0.35 kUa/L)32; for Cor a 9, 100% and 71.9% (at >0.65 kUa/L),27 and 54.4% and 97.6% (at >1 kUa/L)32; for Cor a 14, 100% and 93.8% (at >0.64 kUa/L),27 and 54.4% and 85.4% (at >0.35 kUa/L)32; for Bet v 1, 81.0% and 7.3% (at 0.35 kUa/L)32; and for Bet v 2, 10.1% and 81.7% (at 0.35 kUa/L).32
3.4.5. Shrimp allergy
Two studies reported data on CRD for shrimp allergy.26, 34 One study tested the component Pen a 1 (tropomyosin) using the ImmunoCAP test,26 and the other study investigated the components Lit v 1 (tropomyosin) and Lit v 4 (sarcoplasmic calcium‐binding protein) through an immunoblotting technique.34 Additionally, both studies investigated the diagnostic value of several individual epitopes in shrimp through an immunoblotting technique. The components (and their epitopes) tested in this manner were as follows: Lit v 1, Lit v 2 (arginine kinase), Lit v 3 (myosin light chain), and Lit v 4. The reported sensitivity‐specificity pair of Lit v 1 was 82.8% and 56.3%, and of Lit v 4 was 34.5% and 93.8%,34 and the reported sensitivity‐specificity pair of Pen a 1 was 88.2% and 23.8% (at >0.35 kUa/L).26 With regards to epitope data, the epitopes with highest DTA were found on Lit v 1 and Lit v 2.26, 34 Table S8 in the online supplement presents the full DTA data by epitope.
3.5. Cost‐effectiveness of CRD
None of the studies meeting our inclusion criteria evaluated the cost‐effectiveness of CRD or made mention of any economic considerations. The only relevant evidence identified was two manufacturer‐authored abstracts featuring Markov simulation‐based cost‐utility models for CRD vs DBPCFC for peanut allergy and one with unspecified methodology featuring multiple food allergies; contacting the authors confirmed there were no accompanying peer‐reviewed papers.36, 37, 38
3.6. Risk assessment ability of CRD
Two studies assessing CRD for hen's egg allergy found that sIgE levels for all components tested were higher in patients with more severe allergies.24, 25 Another study found similar results for cow's milk‐allergic patients.23 For peanut allergy, one study found that patients with more severe food challenge reactions had higher sIgE levels to Ara h 1, 2, 3, and 6 than did patients with no reaction or mild symptoms.30 Furthermore, this study found that all severe allergic patients were sensitized to Ara h 2 or Ara h 6, and none of them were sensitized to Ara h 1, 3, or 9 without Ara h 2.30 For hazelnut allergy, one study found that higher sIgE levels to Cor a 9 and Cor a 14 were associated with more severe reactions in food challenges, but found no correlation between sIgE levels to Cor a 1 or Cor a 8 and reaction severity.32
4. DISCUSSION
4.1. Summary of key findings
This systematic review included 11 studies that assessed the accuracy of CRD in diagnosing cow's milk, hen's egg, peanut, hazelnut, and shrimp allergies. Overall, the components tested by the studies included in this review were as follows: Bos d 4, Bos d 5, Bos d 8, and the caseins for cow's milk allergy; Gal d 1, Gal d 2, Gal d 3, and Gal d 4 for hen's egg allergy; Ara h 1, Ara h 2, Ara 3, Ara h 6, Ara h 8, and Ara h 9 for peanut allergy; Cor a 1, Cor a 8, Cor a 9, Cor a 14, Bet v 1, and Bet v 2 for hazelnut allergy; and Pen a 1, Lit v 1, and Lit v 4 for shrimp allergy. No studies meeting our inclusion criteria investigated CRD for diagnosing wheat, soy, and fish allergies. The components with the highest diagnostic accuracy reported, along with their sensitivity‐specificity pairs, were as follows: Bos d 4 for cow's milk allergy (62.0% and 87.5%), 23 Gal d 1 for hen's egg allergy (84.2% and 89.8% for heated egg, and 60.6% and 97.1% for raw egg),24 Ara h 6 for peanut allergy (94.9% and 95.1%),30 Cor a 14 for hazelnut allergy (100% and 93.8%),27 and Lit v 1 for shrimp (82.8% and 56.3%).34 Additionally, two studies found that individual epitopes in shrimp's Lit v 1 and Lit v 2 could potentially have high diagnostic accuracy measures.26, 34
Of the included studies, one study had a high ROB score in two of the four QUADAS‐2 domains,34 and four studies had one such score.26, 27, 32, 33 The remaining six studies were scored low or unclear ROB in all four domains.23, 24, 25, 28, 30, 31
4.2. Strengths and limitations
To the best of our knowledge, this is the first systematic review analyzing the evidence on the diagnostic accuracy of CRD for a range of food allergies. The strengths of this study include the use of a highly sensitive search strategy with no language restrictions, which allowed a comprehensive literature search, conducted across several databases and clinical trial registries. The inclusion criteria for this review were carefully selected to provide clinically relevant information.16 Similar work in the field, including a RAND report and a systematic review by the European Academy of Allergy and Clinical Immunology (EAACI) Food Allergy and Anaphylaxis Guidelines Group,12, 39 was used as bases for the inclusion criteria of this review. Furthermore, the internal validity of the studies included in this review was strong, as they all used DBPCFC as the reference standard in at least 50% of participants. A limitation of this review is that due to the large degree of heterogeneity between studies that met the inclusion criteria (in terms of the components tested, the particular CRD assay employed, and the cutoff values used), a quantitative synthesis of data could not be undertaken. Additionally, we acknowledge that food allergies other than the ones we focused on are becoming increasingly important. As our search strategy was broadly formulated, we were, in response to expert peer‐review feedback, able to check for any other potentially relevant studies for other foods. We were however unable to find any such studies. In the future, in the context of planned updates to this review, we plan to formally include terms for other foods (eg, apple, cherry, and peach) as this may impact on the sensitivity of our searches.
4.3. Comparison of findings with the wider literature
The results of this review corroborate the findings of a previous DTA systematic review on CRD for peanut allergy,14 suggesting that sIgE levels to the component Ara h 2 can provide diagnostic measures with very high accuracy. Our review, however, used more rigorous inclusion criteria than the previous one, strengthening the DTA evidence in relation to this component. The studies included in our review that analyzed peanut components also found that sIgE levels to the components Ara h 1, 3, 8, and 9 showed varying results, most with underperforming diagnostic values.14 Nevertheless, this is the first review to present evidence on the diagnostic value of Ara h 6 as a CRD component, which was found to have higher sensitivity and specificity values than Ara h 2.
When comparing the diagnostic accuracy of CRD found in our review with that of first‐line diagnostic tests for food allergy (atopy patch tests (APT), SPT, and sIgE) reported in a previous systematic review,12 results vary by allergy. For cow's milk allergy, Bos d 4 and Bos d 8 have similar DTA results to APT and sIgE (these tests showed sensitivity‐specificity pairs of 52.8% and 88.1%, and 87.3% and 47.7%, respectively); additionally, these components displayed lower sensitivity and higher specificity than SPT (which showed a sensitivity‐specificity pair of 87.9% and 67.5%).12 For hen's egg allergy, Gal d 1 had lower sensitivity and higher specificity than SPT and sIgE (these tests showed sensitivity‐specificity pairs of 92.4% and 58.1%, and 93.4% and 49.2%, respectively) for both raw and heated egg.12 For peanut allergy, Ara h 6 showed higher DTA measures than SPT and sIgE (these tests showed sensitivity‐specificity pairs of 94.7% and 61.0%, and 96.3% and 59.3%, respectively).12 Although the previous systematic review did not carry out meta‐analysis for hazelnut and shrimp allergies, results from individual studies suggest that Cor a 9 and Cor a 14 may have higher DTA measures than SPT and sIgE for hazelnut allergy and that Lit v 1 shows marginally lower sensitivity and higher sensitivity than SPT and sIgE.12
4.4. Implications for research
We have identified important research gaps in this field. First, there is a limited body of methodologically robust evidence to assess the accuracy of CRD in diagnosing food allergies. From potentially relevant studies identified through database searches, 21 were excluded because they did not carry out DBPCFC in at least 50% of participants, and 27 were excluded because their food challenges were not double‐blind placebo‐controlled. Furthermore, we did not identify any methodologically strong studies assessing the diagnostic accuracy of CRD for wheat, soy, or fish allergies. Therefore, there is a need for more DTA studies using DBPCFC as the reference standard in >50% of participants to better assess the diagnostic accuracy of CRD for food allergies. Alternatively, because of the challenges that DBPCFC pose for researchers and patients, there is a need to systematically assess whether other types of diagnoses, such as a combination of open food challenges and other markers of sensitization as recently proposed,40 could be used as a reference standard for DTA studies.
Second, this review found that at present, a quantitative synthesis of CRD diagnostic accuracy data is not possible because of the paucity of studies for each of the components that have been studied. Given that CRD is still in development, there is a need for more studies and a consensus reached on the optimal cutoff values that will facilitate quantitative evidence synthesis and data pooling across studies. Alternatively, the heterogeneity of cutoff values could be alleviated if all DTA studies reported appendices with sIgE concentration values for each participant. This would allow reviewers to obtain DTA summary measures from all studies in a transparent and homogenous manner.
Third, there is a need to standardize all CRD assays to ensure that results are comparable between different tests. This includes the individual allergen components used, the results obtained from assays from different manufacturers, and the results obtained from microarray and single‐component tests.
Finally, there is a dearth of evidence on the cost‐effectiveness and the risk assessment ability of CRD relative to current care models. In principle, it may be possible to utilize some of the data obtained here for use in economic modeling to facilitate comparisons of the relative value of any trade‐off between sensitivity and specificity in a more formal manner. Such analyses were beyond the scope of this review.
4.5. Implications for patient care
Selected CRD components have the potential to diagnose food allergies with a higher specificity, but lower sensitivity than current first‐line tests. Furthermore, risk assessments carried out by five of the included studies suggest that quantitative measurements of sIgE levels to key components have the potential to identify patients with more severe allergic phenotypes. Such is the case for Ara h 2 and Ara h 6 for peanut allergy, and Cor a 9 and Cor a 14 for hazelnut allergy. Nevertheless, further research is necessary to draw stronger conclusions and to determine the components with the highest diagnostic value, as well as the clinically appropriate cutoff values.
Importantly, all studies included in this review recruited patients with suspected allergies. Therefore, it is likely that the prevalence of allergies in the study populations is considerably higher than in more population‐based settings, rendering the tests’ PPVs higher and NPVs lower than they would be in populations with lower allergy prevalence.
5. CONCLUSIONS
The findings of this review suggest that some CRD components have the potential to diagnose cow's milk, hen's egg, peanut, hazelnut, and shrimp allergies with high specificity, but low sensitivity. Nevertheless, at present, there is not enough methodologically robust evidence to draw definite conclusions. Further studies employing DBPCFC as the reference standard are urgently needed to effectively evaluate the DTA and cost‐effectiveness of CRD, as well as standardization of the components assessed and CRD assays used, and consensus on study reporting.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
AS conceived this study, and together with BN, NM, and ASt, secured the funding for the work. The systematic review was led by JFK and NM with ASt advising on the health economics. JFK drafted the manuscript, which was commented on by other authors. All authors approved the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
We are thankful to Marshall Dozier, Senior Liaison Librarian, and Liaison Director for the College of Medicine and Veterinary Medicine at the University of Edinburgh for assistance in developing the search strategy. We are grateful to Dr. Adrianna Machinena‐Spera and Dr. Komei Ito for providing additional details about their studies, and we thank Dr. Adriano Mari and Dr. Claudia Alessandri for providing their original data. We would also like to thank the panel of experts we contacted for further references. We also appreciate the help of Kei Yamaya, Christine Blandhol, Lennart Hoedemakers, and Son Tran Tuan, who helped us make eligibility decisions for studies published in languages other than English. We are also grateful to the Asthma UK Centre for Applied Research Patient Advisory Group for providing perspectives of patients and this public throughout this work. BN acknowledges the support of Knut and Alice Wallenberg Foundation and the Wallenberg Centre for Molecular and Translational Medicine, Institute of Medicine, University of Gothenburg. We thank Abby King for her helpful comments on the paper. Finally, we thank the expert peer reviewer for their thoughtful constructive feedback on an earlier version of this manuscript.
Flores Kim J, McCleary N, Nwaru BI, Stoddart A, Sheikh A. Diagnostic accuracy, risk assessment, and cost‐effectiveness of component‐resolved diagnostics for food allergy: A systematic review. Allergy. 2018;73:1609–1621. 10.1111/all.13399
REFERENCES
- 1. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291‐307. [DOI] [PubMed] [Google Scholar]
- 2. Nwaru BI, Hickstein L, Panesar SS, et al. The epidemiology of food allergy in Europe: a systematic review and meta‐analysis. Allergy. 2014;69:62‐75. [DOI] [PubMed] [Google Scholar]
- 3. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594‐602. [DOI] [PubMed] [Google Scholar]
- 4. Muraro A, Werfel T, Hoffmann‐Sommergruber K, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008‐1025. [DOI] [PubMed] [Google Scholar]
- 5. Bischoff SC. Food allergies. Curr Gastroenterol Rep. 2006;8:374‐382. [DOI] [PubMed] [Google Scholar]
- 6. Schussler E, Kattan J. Allergen component testing in the diagnosis of food allergy. Curr Allergy Asthma Rep. 2015;15:55. [DOI] [PubMed] [Google Scholar]
- 7. Kalayci O. In‐vitro diagnosis of allergic diseases. Clin Biochem. 2014;47:728‐729. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann‐Sommergruber K, Pfeifer S, Bublin M. Applications of molecular diagnostic testing in food allergy. Curr Allergy Asthma Rep. 2015;15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luengo O, Cardona V. Component resolved diagnosis: when should it be used? Clin Transl Allergy. 2014;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010;40:1442‐1460. [DOI] [PubMed] [Google Scholar]
- 11. Wolthers OD. Component‐resolved diagnosis in pediatrics. ISRN Pediatr. 2012;2012:806920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soares‐Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta‐analysis. Allergy. 2014;69:76‐86. [DOI] [PubMed] [Google Scholar]
- 13. Westwood M, Ramaekers B, Lang S, et al. ImmunoCAP® ISAC and Microtest for multiplex allergen testing in people with difficult to manage allergic disease: a systematic review and cost analysis. Health Technol Assess. 2016;20:1‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klemans RJB, van Os‐Medendorp H, Blankestijn M, Bruijnzeel‐Koomen CAFM, Knol EF, Knulst AC. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. Clin Exp Allergy. 2015;45:720‐730. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flores Kim J, Nwaru BI, Mccleary N, Stoddart A, Sheikh A. Investigating the accuracy, risk impact, and cost‐effectiveness of component‐resolved diagnostic test for food allergy: a systematic review protocol. Primary Care Respir Med. 2017;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Gronlund H. The recombinant allergen‐based concept of component‐resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exp Allergy. 1999;29:896‐904. [DOI] [PubMed] [Google Scholar]
- 18. Altman DG, Bland JM. Diagnostic tests. 1: sensitivity and specificity. BMJ. 1994;308:1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Altman DG, Bland JM. Statistics notes: diagnostic tests 2: predictive values. BMJ. 1994;309:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857‐872. [DOI] [PubMed] [Google Scholar]
- 21. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529‐536. [DOI] [PubMed] [Google Scholar]
- 22. Dendukuri N, Schiller I, Joseph L, Pai M. Bayesian meta‐analysis of the accuracy of a test for tuberculous pleuritis in the absence of a gold standard reference. Biometrics. 2012;68:1285‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alessandri C, Sforza S, Palazzo P, et al. Tolerability of a fully maturated cheese in cow's milk allergic children: biochemical, immunochemical, and clinical aspects. PLoS One. 2012;7:e40945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alessandri C, Zennaro D, Scala E, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen's egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2012;42:441‐450. [DOI] [PubMed] [Google Scholar]
- 25. Ando H, Moverare R, Kondo Y, et al. Utility of ovomucoid‐specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008;122:583‐588. [DOI] [PubMed] [Google Scholar]
- 26. Ayuso R, Sanchez‐Garcia S, Pascal M, et al. Is epitope recognition of shrimp allergens useful to predict clinical reactivity? Clin Exp Allergy. 2012;42:293‐304. [DOI] [PubMed] [Google Scholar]
- 27. Brandstrom J, Nopp A, Johansson SGO, et al. Basophil allergen threshold sensitivity and component‐resolved diagnostics improve hazelnut allergy diagnosis. Clin Exp Allergy. 2015;45:1412‐1418. [DOI] [PubMed] [Google Scholar]
- 28. Klemans RJ, Knol EF, Bruijnzeel‐Koomen CA, Knulst AC. The diagnostic accuracy of specific IgE to Ara h 6 in adults is as good as Ara h 2. Allergy. 2014;69:1112‐1114. [DOI] [PubMed] [Google Scholar]
- 29. Klemans RJB, Liu X, Knulst AC, et al. IgE binding to peanut components by four different techniques: Ara h 2 is the most relevant in peanut allergic children and adults. Clin Exp Allergy. 2013;43:967‐974. [DOI] [PubMed] [Google Scholar]
- 30. Kukkonen AK, Pelkonen AS, Makinen‐Kiljunen S, Voutilainen H, Makela MJ. Ara h 2 and Ara 6 are the best predictors of severe peanut allergy: a double‐blind placebo‐controlled study. Allergy. 2015;70:1239‐1245. [DOI] [PubMed] [Google Scholar]
- 31. Lieberman JA, Glaumann S, Batelson S, Borres MP, Sampson HA, Nilsson C. The utility of peanut components in the diagnosis of IgE‐mediated peanut allergy among distinct populations. J Allergy Clin Immunol Pract. 2013;1:75‐82. [DOI] [PubMed] [Google Scholar]
- 32. Masthoff LJ, Mattsson L, Zuidmeer‐Jongejan L, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013;132:393‐399. [DOI] [PubMed] [Google Scholar]
- 33. Ott H, Baron JM, Heise R, et al. Clinical usefulness of microarray‐based IgE detection in children with suspected food allergy. Allergy. 2008;63:1521‐1528. [DOI] [PubMed] [Google Scholar]
- 34. Pascal M, Grishina G, Yang AC, et al. Molecular diagnosis of shrimp allergy: efficiency of several allergens to predict clinical reactivity. J Allergy Clin Immunol Pract. 2015;3:521‐529. [DOI] [PubMed] [Google Scholar]
- 35. Masthoff LJ, van Hoffen E, Mattsson L, et al. Peanut allergy is common among hazelnut‐sensitized subjects but is not primarily the result of IgE cross‐reactivity. Allergy. 2015;70:265‐274. [DOI] [PubMed] [Google Scholar]
- 36. Hermansson LL, Glaumann S, Mascialino B, Wang LL, Nilsson C. Cost‐utility of molecular IgE in vitro diagnostics (IVD) in children suspected with peanut allergy compared to most used diagnostics in selected Asian markets. Value Health. 2012;15:A640. [Google Scholar]
- 37. Hermansson LL, Mascialino B, Glaumann S, Borres M, Hubben G, Nilsson C. Is molecular allergology cost‐effective and cost saving in children with suspected peanut allergy compared to double blind placebo controlled food challenge (DBPCFC) and skin prick test in US, Europe and Asia? J Allergy Clin Immunol. 2013;1:AB58. [Google Scholar]
- 38. Hermansson LL, Pensamo E, Korhonen K, Rantanen S, Isoaho R, Savolainen J. Health economic benefit of including component resolved diagnostics (CRD) (immunocap ISAC) in in vitro diagnostic (IVD) algorithm in prospective trial with suspected food allergic school children in Finland. Value Health. 2014;17:A176. [DOI] [PubMed] [Google Scholar]
- 39. Chafen JJS, Newberry S, Riedl M, et al. Prevalence, Natural History, Diagnosis, and Treatment of Food Allergy: A Systematic Review of the Evidence. Santa Monica, CA: RAND Corporation; 2010. [Google Scholar]
- 40. Datema MR, van Ree R, Asero R, et al. CRD and beyond: multivariable regression models to predict severity of hazelnut allergy. Allergy. 2017;73:549‐559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


