ABSTRACT
BACKGROUND AND PURPOSE
Tai Chi is a mind‐body exercise that has been shown to improve both mental and physical health. As a result, recent literature suggests the use of Tai Chi to treat both physical and psychological disorders. However, the underlying physiological changes have not been characterized. The aim of this pilot study is to assess the changes in brain metabolites and muscle energetics after Tai Chi training in an aging population using a combined brain‐muscle magnetic resonance spectroscopy (MRS) examination.
METHODS
Six healthy older adults were prospectively recruited and enrolled into a 12‐week Tai Chi program. A brain 1H MRS and a muscle 31P MRS were scanned before and after the training, and postprocessed to measure N‐acetylaspartate to creatine (NAA/Cr) ratios and phosphocreatine (PCr) recovery time. Wilcoxon‐signed rank tests were utilized to assess the differences between pre‐ and post‐Tai Chi training.
RESULTS
A significant within‐subject increase in both the NAA/Cr ratios (P = .046) and the PCr recovery time (P = .046) was observed between the baseline and the posttraining scans. The median percentage changes were 5.38% and 16.51% for NAA/Cr and PCr recovery time, respectively.
CONCLUSIONS
Our pilot study demonstrates significant increase of NAA/Cr ratios in posterior cingulate gyrus and significantly improved PCr recovery time in leg muscles in older adults following short‐term Tai Chi training, and thus provides insight into the beneficial mechanisms.
Keywords: Tai Chi, magnetic resonance spectroscopy, N‐acetylaspartate, phosphocreatine recovery time
Introduction
Tai Chi is a mind‐body exercise that utilizes continuous, curved, and spiral body movements.1 It is a moderate‐intensity aerobic exercise that utilizes 50–58% of heart rate reserve during Tai Chi practice, and requires strong concentration with breathing control when performed properly.1, 2, 3 This combination results in physical benefits, such as improvements in aerobic capacity, muscle strength, balance, and motor control, as well as psychological benefits of improved attentiveness, reduced stress and anxiety, and cardiovascular improvements.1, 4 Numerous studies have shown that Tai Chi results in the strengthening of muscles in the lower limb particularly in knee flexor and extensor muscle groups such as the quadriceps.5, 6, 7 Multiple studies have shown the benefits of Tai Chi with improved cognition.8 Furthermore, a growing body of evidence consisting of morphological magnetic resonance imaging (MRI) and functional MRI data suggests that Tai Chi can induce beneficial neuroplasticity.9, 10, 11 As a result, recent literature suggests the use of Tai Chi to treat both physical and psychological disorders, including stroke, Parkinson's disease, traumatic brain injury, and depression.1, 12, 13, 14 However, the beneficial mechanisms of Tai Chi are not well understood. Noninvasive and objective measures are required to better understand the physiological changes that the brain and body undergo during Tai Chi training.
Magnetic resonance spectroscopy (MRS) can noninvasively measure the endogenous chemicals of body tissues. In the brain, 1H MRS has long been used to measure concentrations of different metabolites to study the biochemical processes. Previous studies in aerobic exercise have shown that N‐acetylaspartate (NAA) is a neuronal marker that can assess neuronal health.15, 16, 17 In the leg muscles, 31P MRS can quantify mitochondrial function by measuring the rate of recovery of phosphocreatine (PCr) following exercise.18 Together, these measures can be used to track changes in the brain and muscle due to mind‐body interventions such as Tai Chi and yoga.
The goal of this pilot study is to assess the changes that occur in brain metabolites and muscle energetics after Tai Chi training in the aging population using a combined brain‐muscle MRS examination. To the best of our knowledge, this is the first study to investigate the responses of brain and leg muscles to Tai Chi exercise by noninvasive MRS.
Methods
Participants
This pilot study was approved by the Brigham and Women's Hospital Institutional Review Board, and all subjects provided written informed consent. Healthy older adults from the local community center were prospectively recruited based on the following inclusion and exclusion criteria.
The inclusion criteria were: (1) age 55 or older, (2) no Tai Chi training in the past 12 months, and (3) willing to participate in a 12‐week Tai Chi class.
The exclusion criteria included: (1) signs or symptoms of upper motor neuron syndrome; (2) any major systemic illness or unstable condition, which could interfere with protocol compliance, including the diagnosis of major depression; (3) active psychiatric disease that would interfere with participation; (4) psychiatric diagnoses; (5) agitation, or behavioral problems within the last 3 months; (6) history of alcohol/substance abuse or dependence within the past 2 years; (7) any neurological diseases; (8) participation in other clinical studies involving neuropsychological measures collected more than one time per year; and (9) unable to undergo MRI for any reason such as claustrophobia or presence of magnetic resonance (MR) incompatible implants.
Tai Chi Training
After enrollment, all participants received a 12‐week Tai Chi training under the instruction of a certified teacher at Greater Boston Golden Age Center (Boston, MA, USA). All subjects underwent at least two practices per week, each practice of 60 minutes.
The 1H brain and 31P muscle MRS scans were conducted in a single session before and after Tai Chi training.
1H Brain MRS Acquisition
1H brain MRS was performed on a clinical 3.0 Tesla MR scanner (Siemens TIM Skyra, VD13) with a 32‐channel head coil. Axial 3‐dimensional magnetization prepared rapid‐acquisition gradient echo (3D‐MPRAGE, Repetition time (TR)/Echo time (TE) = 1,760 ms/3.43 ms, Field of View (FOV) = 268 × 209 mm2, matrix = 268 × 209) images were acquired prior to spectroscopy, and reconstructed in the sagittal and coronal planes with 2 mm slice resolution for accurate localization of the voxel.
The 1H brain MRS utilized a single voxel point‐resolved spectroscopy (PRESS) sequence. The acquisition parameters included: TR/TE = 2,000 ms/30 ms, voxel size = 30 × 30 × 30 mm3, 64 averages, 833 ms dwell time, 1,024 points, and total scan time = 3 minutes. The voxel was positioned in the posterior cingulate gyrus (PCG, Fig 1) to include as much of the gray matter as possible while avoiding the scalp tissue. The PCG was chosen due to its sensitivity to a broad range of diseases and because it is one of the most homogenous parts of the brain, thus providing excellent quality spectra.19, 20 Each voxel underwent automated optimization including 3‐dimensional shimming, transmit gain, frequency adjustment, and water suppression. When the full width at half maximum (FWHM) of the water signal was ≥14 Hz, manual shimming was performed to optimize the magnetic field homogeneity of the selected spectroscopy volume of interest to a line width of <14 Hz FWHM of the water signal.
Figure 1.
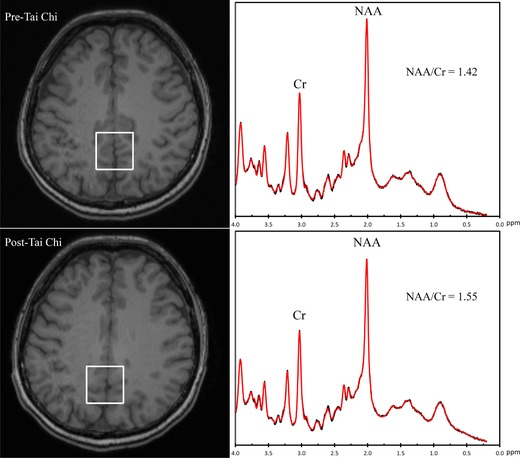
Representative 1H MRI images (left) from subject 5 and the voxel placed in the posterior cingulate gyrus area from which the localized 1H MRS spectra (right) were obtained. N‐acetylaspartate to creatine(NAA/Cr) ratios increased from 1.42 before to 1.55 after 12 weeks of Tai Chi training.
A screenshot of the pretraining 1H MRS voxel position was saved, and care was taken to place the excitation volume to anatomically match the initial MRS scan in the posttraining follow‐up.
1H Brain MRS Data Analysis
LCModel software (Version 6.2, LCMODEL Inc., Oakville, Canada) was used for the 1H brain single voxel spectroscopy spectral fitting, using simulated spectra of 20 metabolites as a customized basis set. All spectra were quality controlled by examining the FWHM of the unsuppressed water spectrum and signal‐to‐noise ratio (SNR). Total NAA to total creatine (Cr) ratios were calculated in the LCModel output, and included in the further analysis.
31P MRS Acquisition
31P data acquisition was performed in the same scanner as the 1H brain MRS. A single channel 31P tuned transmit/receive surface coil was strapped to the right thigh at the vastus medialis muscle of the quadriceps. First, the transmitter gain of a 500‐μs hard excitation pulse was adjusted to determine the value giving the highest PCr peak. A simple pulse‐acquire sequence (TR = 2,000 ms, bandwidth = 3,000 Hz) was then performed continuously during 10‐minute scan consisting of: 2 minutes of rest, 3 minutes of leg‐lifting exercise, and 5 minutes of recovery rest. During the protocol, 300 free induction decays (FIDs), with 2,048 complex data points per FID, were acquired continuously.
Exercise During 31P MRS Acquisition
The 31P MRS scan leg‐lifting exercise was done inside the MR scanner. Subjects performed this exercise in a supine position with a custom‐built dynamic knee extension apparatus. This apparatus has a bar that rests across the subjects’ shins and attaches to weights in the back of the MR scanner by means of a pulley. The subjects extended their knees, pushing up the bar to lift the weights. Subjects were familiarized with the leg‐lifting exercise and asked to perform constant‐load leg lifting at a steady rate (every 2 seconds) until exhaustion, limited by symptoms, or 3 minutes duration. The constant‐load was set to 30% of the maximum work capacity at the time of familiarization, which was determined by a 3‐second knee extension test using different weights.
31P MRS Data Analysis
The 31P MRS data was postprocessed offline, with the spectral improvement by Fourier thresholding (SIFT) method described elsewhere.21 Briefly, the SIFT was applied to improve SNR and smooth temporal trajectories of the 300 spectra. The spectra were then fit using a simulated basis set to obtain the 31P metabolite areas.21 The postexercise PCr amplitudes were fit to a monoexponential recovery function to determine the recovery time constant τ.
Statistical Analysis
Related‐samples Wilcoxon‐signed rank tests were used to assess the differences in NAA/Cr ratios and PCr recovery time between pre‐ and post‐Tai Chi training. A P‐value of <.05 was considered significant. Statistical analyses were performed using SPSS (Version 21, IBM Corporation, NY, USA).
Results
Participants
Seven subjects were enrolled in this study. One subject withdrew due to conflicts with the Tai Chi class schedule, so six subjects completed the 12‐week Tai Chi program. All six subjects were Tai Chi‐naive at their first scan. The changes of the NAA/Cr ratios and the PCr recovery time between pre‐ and post‐Tai Chi training, as well as the subject demographic characteristics, are summarized in Table 1. Representative 1H brain MRS and 31P muscle MRS are shown in Figures 1 and 2.
Table 1.
Subject Demographics and Changes of N‐Acetylaspartate/Creatine Ratios and Phosphocreatine Recovery Time
| Subjects | Gender* | Age (Year) | BMI† (kg/m2) | ∆ NAA/Cr Ratios (Percentage Change)‡ | ∆ PCr Recovery Time, Seconds (Percentage Change)§ |
|---|---|---|---|---|---|
| 1 | F | 69 | 20.34 | .17 (12.85%) | –3.97 (–16.82%) |
| 2 | F | 69 | 26.22 | –.04 (–2.38%) | –26.04 (–53.66%) |
| 3 | M | 70 | 26.56 | .05 (3.78%) | –15.65 (–42.61%) |
| 4 | F | 72 | 23.24 | .08 (5.48%) | –3.54 (–11.33%) |
| 5 | F | 51 | 22.72 | .13 (9.08%) | –4.35 (–16.20%) |
| 6 | F | 51 | 23.62 | .08 (5.27%) | 1.66 (9.56%) |
*F = female; M = male.
†BMI = body mass index.
‡NAA = N‐acetylaspartate; Cr = creatine; ∆ NAA/Cr Ratios = NAA/Crpost‐Tai Chi – NAA/Crpre‐Tai Chi; Percentage Change = (∆ NAA/Cr Ratios)/(NAA/Crpre‐Tai Chi).
§PCr = phosphocreatine; ∆ PCr Recovery Time = PCr Recovery Timepost‐Tai Chi – PCr Recovery Timepre‐Tai Chi; Percentage Change = (∆ PCr Recovery Time)/(PCr Recovery Timepre‐Tai Chi).
Figure 2.
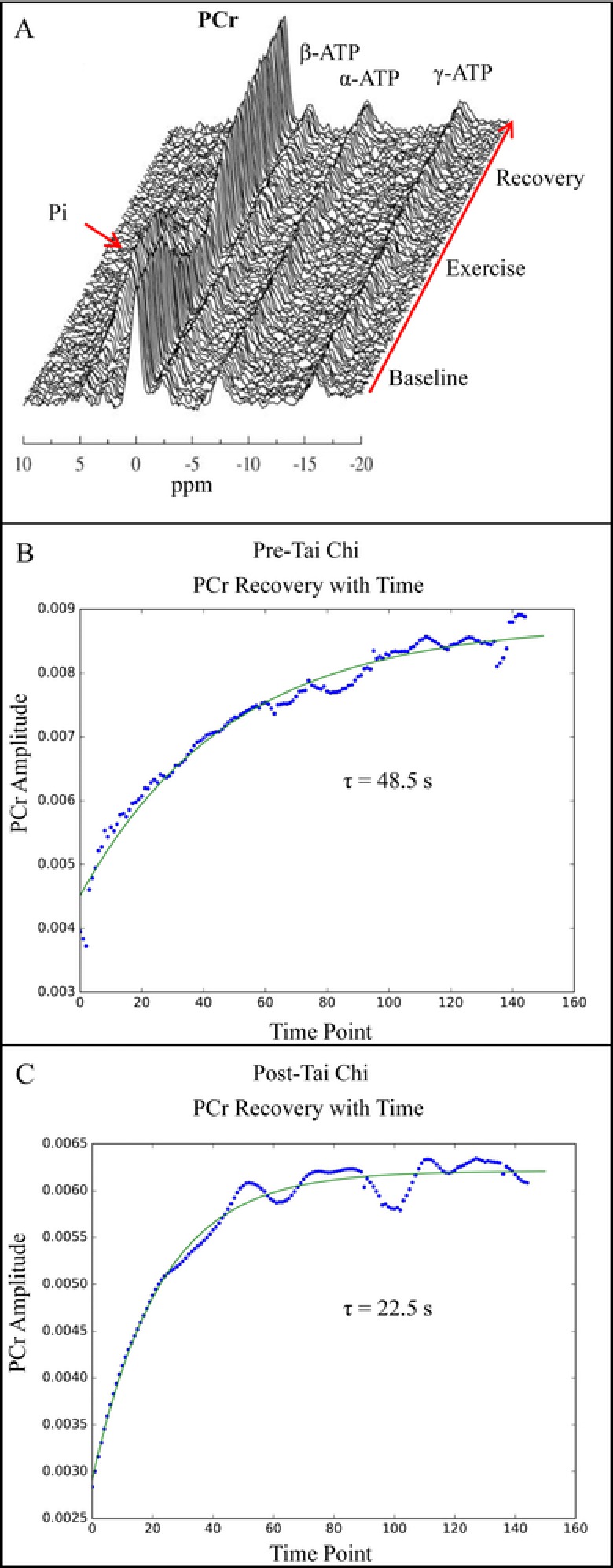
Panel A: A stacked plot of 300 31P spectra after spectral improvement by Fourier thresholding processing. Panels B and C: Temporal plots of phosphocreatine (PCr) areas during the recovery of subject 2 from lifting exercise and the result of a monoexponential fit (green line), which yields the PCr recovery rate. PCr recovery time decreased from 48.5 to 22.5 seconds after 12 weeks of Tai Chi training. α, β, γ‐ATP = adenosine triphosphate resonances (alpha, beta, and gamma); Pi = inorganic phosphate; ppm = parts per million; τ = recovery time constant.
Changes of NAA/Cr Ratios
A significant within‐subject increase in NAA/Cr ratios was observed between the baseline and the posttraining scans (P = .046). Five of six subjects showed an increase in NAA/Cr ratios after 12 weeks of Tai Chi training (Fig 3). The median percentage change was 5.38%, and the mean NAA/Cr ratio difference was .08 ± .07 (mean ± standard deviation) between baseline and posttraining scans.
Figure 3.
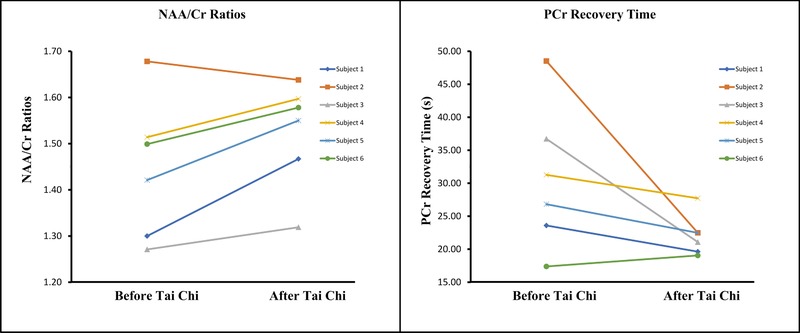
The changes of N‐acetylaspartate to creatine(NAA/Cr) ratios (left panel) and phosphocreatine (PCr) recovery rates (right panel). There were significant within‐subject increase of NAA/Cr ratios (P = .046) and decrease of PCr recovery time (P = .046) after 12 weeks of Tai Chi training.
PCr Recovery
There was a significant within‐subject decrease in PCr recovery time between the pre‐ and the post‐Tai Chi scans (P = .046). Improved PCr recovery rates were observed in five of six participants following 12 weeks of Tai Chi training as shown in Figure 3. The PCr recovery time decreased 16.51% (median), or 8.65 ± 10.24 (mean ± standard deviation) from baseline scan, after Tai Chi training.
Discussion
In our study, we found significantly increased NAA/Cr ratios in posterior cingulate gyrus and significantly improved PCr recovery time in leg muscles in older adults with 12 weeks of Tai Chi training. Our results suggest that Tai Chi, as a mind‐body exercise, may effectively promote neuroplasticity and increase lower extremity muscle oxidative capacity in older adults.
NAA is a metabolite found almost exclusively within cell bodies of neurons, and directly reflecting neuron cell number.22 A decrease of NAA level is an indication of neuronal loss, as is typically observed in healthy aging, Alzheimer's disease, stroke, multiple sclerosis, schizophrenia, epilepsy, and bipolar disorder.17, 23, 24, 25 A growing body of research indicates that aerobic fitness promotes neuronal integrity and viability. A study of 137 healthy older adults demonstrated that higher aerobic fitness level correlated with increased NAA levels in the frontal cortex.15 Another study compared neurochemical concentrations between sedentary and endurance‐trained middle‐aged adults, and found that endurance‐trained adults had significantly higher NAA/Cr in the frontal gray matter.16 Our results demonstrated significantly increased NAA/Cr in healthy older adults after 12 weeks of Tai Chi training, suggesting either increased neuroplasticity, or potentially, a protective effect of Tai Chi on neurons. Of note, different from the prior studies, we acquired the MRS data in posterior cingulate gyrus, which has been shown to be more sensitive to global changes in the brain.19 Furthermore, the PCG plays an important role in cognitive processes such as attention,26 which has been shown to be enhanced by Tai Chi.8 Finally, the PCG was also selected because it is highly reproducible with a covariance of 2‐2.5% for NAA.27
Our results also showed significant increase in the PCr recovery of the thigh muscles in our subjects after 12 weeks of Tai Chi training. PCr recovery time, measured by 31P MRS, is a reliable indicator of skeletal muscle mitochondrial metabolism.18, 28 It is very reproducible with a covariance of 8%.29 It reflects the mitochondrial ATP synthesis during recovery from exercise, which is driven by oxidative metabolism.30, 31 Our findings of decreased PCr recovery time indicate that Tai Chi training accelerates ATP synthesis and improves muscle mitochondrial function of older adults. Choi et al accessed the muscle bioenergetics by postexercise PCr recovery rate in a study with 126 participants and showed that muscle bioenergetics were highly correlated with walking speed.32 Furthermore, a study by Zane et al with 326 participants demonstrated that impaired muscle mitochondrial energetics accessed by PCr resynthesis rate affected muscle strength, and through this mechanism, had a negative effect on walking performance.33 Thus, our results provide an insight into the physiological mechanisms of the increased lower extremity muscular strength and improved motor control following Tai Chi exercise. Our pilot study is, however, limited by a small sample size, and these encouraging findings need to be confirmed in a larger study.
Higher NAA level in prefrontal cortex has also been observed in subjects with higher aerobic fitness level in prior studies.15, 16 Thus, multivoxel MRS sequence could be added in the protocol to explore the global effect that Tai Chi training has on the brain. Moreover, multiple covariates especially aerobic fitness level before enrollment could be analyzed to help explain the variation of changes of NAA and PCr recovery time discovered in our pilot study.15 Finally, different control groups including regular aerobic exercise or yoga could be designed to better understand the effect of Tai Chi training. In conclusion, our pilot study demonstrates significant increase of NAA/Cr ratios in posterior cingulate gyrus and significantly improved PCr recovery time in the leg muscles of older adults following short‐term Tai Chi training. Our results support the recent findings of the physical and psychological benefits of Tai Chi exercise, and provide an insight into the beneficial mechanisms. Further well‐designed and larger studies are needed to validate our findings.
Acknowledgments and Disclosures: This study was funded by the Pilot Research Grant from Osher Center for Integrative Medicine at Brigham and Women's Hospital and Harvard Medical School. The authors would like to thank the China Scholarship Council for financial support for Min Zhou. The authors have no competing interests related to this study.
References
- 1. Lan C, Chen SY, Lai JS, et al. Tai Chi Chuan in medicine and health promotion. Evid Based Complement Alternat Med 2013;2013:502131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lan C, Chen SY, Lai JS, et al. Heart rate responses and oxygen consumption during Tai Chi Chuan practice. Am J Chin Med 2001;29:403‐10. [DOI] [PubMed] [Google Scholar]
- 3. Lan C, Chen SY, Lai JS. Relative exercise intensity of Tai Chi Chuan is similar in different ages and gender. Am J Chin Med 2004;32:151‐60. [DOI] [PubMed] [Google Scholar]
- 4. Field T. Tai Chi research review. Complement Ther Clin Pract 2011;17:141‐6. [DOI] [PubMed] [Google Scholar]
- 5. Chen YS, Crowley Z, Zhou S, et al. Effects of 12‐week Tai Chi training on soleus H‐reflex and muscle strength in older adults: a pilot study. Eur J Appl Physiol 2012;112:2363‐8. [DOI] [PubMed] [Google Scholar]
- 6. Li JX, Xu DQ, Hong Y. Changes in muscle strength, endurance, and reaction of the lower extremities with Tai Chi intervention. J Biomech 2009;42:967‐71. [DOI] [PubMed] [Google Scholar]
- 7. Lu X, Hui‐Chan CW, Tsang WW. Tai Chi, arterial compliance, and muscle strength in older adults. Eur J Prev Cardiol 2013;20:613‐9. [DOI] [PubMed] [Google Scholar]
- 8. Wayne PM, Walsh JN, Taylor‐Piliae RE, et al. Effect of Tai Chi on cognitive performance in older adults: systematic review and meta‐analysis. J Am Geriatr Soc 2014;62:25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei GX, Xu T, Fan FM, et al. Can Taichi reshape the brain? A brain morphometry study. PLoS One 2013;8:e61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li R, Zhu X, Yin S, et al. Multimodal intervention in older adults improves resting‐state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Front Aging Neurosci 2014;6:39 10.3389/fnagi.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei GX, Dong HM, Yang Z, et al. Tai Chi Chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front Aging Neurosci 2014;6:74 10.3389/fnagi.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Black DS, Irwin MR, Olmstead R, et al. Tai Chi meditation effects on nuclear factor‐kappaB signaling in lonely older adults: a randomized controlled trial. Psychother Psychosom 2014;83:315‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait Posture 2008;28:456‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song QH, Shen GQ, Xu RM, et al. Effect of Tai Chi exercise on the physical and mental health of the elder patients suffered from anxiety disorder. Int J Physiol Pathophysiol Pharmacol 2014;6:55‐60. [PMC free article] [PubMed] [Google Scholar]
- 15. Erickson KI, Weinstein AM, Sutton BP, et al. Beyond vascularization: aerobic fitness is associated with N‐acetylaspartate and working memory. Brain Behav 2012;2:32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzales MM, Tarumi T, Kaur S, et al. Aerobic fitness and the brain: increased N‐acetyl‐aspartate and choline concentrations in endurance‐trained middle‐aged adults. Brain Topogr 2013;26:126‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moffett JR, Ross B, Arun P, et al. N‐acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 2007;81:89‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kemp GJ, Ahmad RE, Nicolay K, et al. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiol (Oxf) 2015;213:107‐44. [DOI] [PubMed] [Google Scholar]
- 19. Lin A, Tran T, Bluml S, et al. Guidelines for acquiring and reporting clinical neurospectroscopy. Semin Neurol 2012;32:432‐53. [DOI] [PubMed] [Google Scholar]
- 20. Shao R, Keuper K, Geng X, et al. Pons to posterior cingulate functional projections predict affective processing changes in the elderly following eight weeks of meditation training. EBioMedicine 2016;10:236‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rowland B, Merugumala SK, Liao H, et al. Spectral improvement by Fourier thresholding of in vivo dynamic spectroscopy data. Magn Reson Med 2016;76:978‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng LL, Newell K, Mallory AE, et al. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging 2002;20:527‐33. [DOI] [PubMed] [Google Scholar]
- 23. Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Res Brain Res Rev 2004;44:83‐102. [DOI] [PubMed] [Google Scholar]
- 24. Kantarci K, Jack CR, Jr. , Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: a 1H MRS study. Neurology 2000;55:210‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Angelie E, Bonmartin A, Boudraa A, et al. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. AJNR Am J Neuroradiol 2001;22:119‐27. [PMC free article] [PubMed] [Google Scholar]
- 26. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014;137:12‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terpstra M, Cheong I, Lyu T, et al. Test‐retest reproducibility of neurochemical profiles with short‐echo, single‐voxel MR spectroscopy at 3T and 7T. Magn Reson Med 2016;76:1083‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson CH, Kemp GJ, Sanderson AL, et al. Skeletal muscle mitochondrial function studied by kinetic analysis of postexercise phosphocreatine resynthesis. J Appl Physiol (1985) 1995;78:2131‐9. [DOI] [PubMed] [Google Scholar]
- 29. McCully KK, Turner TN, Langley J, et al. The reproducibility of measurements of intramuscular magnesium concentrations and muscle oxidative capacity using 31P MRS. Dyn Med 2009;8:5 10.1186/1476-5918-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J 1993;291:681‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forbes SC, Paganini AT, Slade JM, et al. Phosphocreatine recovery kinetics following low‐ and high‐intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol 2009;296:R161‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi S, Reiter DA, Shardell M, et al. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci 2016;71:1638‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zane AC, Reiter DA, Shardell M, et al. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell 2017;16:461‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]


