Abstract
Schizophrenia, bipolar disorder and major depressive disorder (MDD) have all been associated with aberrant blood cytokine levels; however, neither the pattern of cytokine alterations nor the impact of clinical status have been compared across disorders. We performed a meta-analysis of blood cytokines in acutely and chronically ill patients with these major psychiatric disorders. Articles were identified by searching the PubMed, PsycInfo and Web of Science, and the reference lists of these studies. Sixty-eight studies met the inclusion criteria (40 schizophrenia, 10 bipolar disorder and 18 MDD) for acutely ill patients. Forty-six studies met the inclusion criteria (18 schizophrenia, 16 bipolar disorder and 12 MDD) for chronically ill patients. Levels of two cytokines (interleukin- 6 (IL-6), tumor necrosis factor-α (TNF-α)), one soluble cytokine receptor (sIL-2R), and one cytokine receptor antagonist (IL-1RA) were significantly increased in acutely ill patients with schizophrenia, bipolar mania and MDD compared with controls (P < 0.01). Following treatment of the acute illness, IL-6 levels significantly decreased in both schizophrenia and MDD (P < 0.01); sIL-2R levels increased in schizophrenia; and IL-1RA levels in bipolar mania decreased. In chronically ill patients, the levels of IL-6 were significantly increased in schizophrenia, euthymic (but not depressed) bipolar disorder and MDD compared with controls (P < 0.01). The levels of IL-1β and sIL-2R were significantly increased in both chronic schizophrenia and euthymic bipolar disorder. Overall, there were similarities in the pattern of cytokine alterations in schizophrenia, bipolar disorder and MDD during acute and chronic phases of illness, raising the possibility of common underlying pathways for immune dysfunction. Effects of treatment on cytokines were more robust for schizophrenia and MDD, but were more frequently studied than for acute mania. These findings have important implications for our understanding of the pathophysiology and treatment of major psychiatric disorders.
INTRODUCTION
The investigation of immune system abnormalities in psychiatric disorders has become a popular area of research over the past decade. This interest has been at least partially stimulated by our increased understanding of the interactions that occur between the immune system and the brain in other chronic medical disorders. Advances in molecular biology and genetics have led to the identification of associations between genes involved in the regulation of the immune system and increased risk of schizophrenia, bipolar disorder and major depressive disorder (MDD).1–3 These disorders are also associated with abnormalities in immune cell numbers, inflammatory markers and antibody titers.4–9 There is mixed evidence in different psychiatric disorders, suggesting that adjunctive treatment with immunomodulatory agents may be associated with improvement in psychopathology.10–13 Taken together, these findings suggest that we need to more extensively evaluate the hypothesis that immune dysfunction manifested by an increase in proinflammatory and decrease in anti-inflammatory biomarkers may be involved in the pathogenesis of major psychiatric disorders in some individuals.
Aberrant blood levels of components of the cytokine network have been reported in schizophrenia, bipolar disorder and MDD.14–20 Cytokines are key signaling molecules of the immune system that exert effects in the periphery and brain. They are produced by immune and non-immune cells, and exert their effects by binding specific receptors on a variety of target cells. Cytokine receptors also exist in soluble forms, which can inhibit (e.g., soluble interleukin-2 receptor (sIL-2R)) or enhance (e.g., sIL-6R)) the biological activity of cytokines. And, there are endogenous cytokine receptor antagonists (e.g., interleukin-6 (IL-6) receptor antagonist (IL-1RA) that compete with cytokines for membrane receptors. Cytokines are key regulators of acute and chronic inflammation, a complex but vital biological response that impacts all organ systems. Cytokines help coordinate the function of both the innate and adaptive components of the immune system as well as a host of other physiological processes throughout the body.21,22
Previous meta-analyses in schizophrenia and bipolar disorder have found that blood levels of some components of the cytokine network may vary with clinical status,16–18 but this has not been investigated in MDD. At this time, we do not know if or when changes in blood cytokine network proteins represent state or trait markers of these disorders.
Despite the evidence for cytokine network alterations in major psychiatric disorders, there is tremendous between-study heterogeneity with respect to: cytokine components studied; effects of clinical course and illness duration; effects of potential confounding factors (e.g., medications, smoking, body mass index (BMI), assay methodology, fasting status); and associations between cytokine component measurement and psychopathology. Meta-analysis is one approach that can bring increased clarity to an area of research with significant heterogeneity,23 and thus is well suited to the study of the cytokine network in psychiatric disorders. At this time, no one has used meta-analysis to compare and contrast the patterns of cytokine alterations across schizophrenia, bipolar disorder and MDD. This paper presents meta-analyses comparing and contrasting blood cytokine levels in acutely and chronically ill patients with these psychiatric disorders, and the effects of treatment of the acute episode on cytokine levels. In doing so, we investigate potential common underlying pathways for immune dysfunction across these disorders, identify important gaps in the literature and discuss implications for the research agenda in this field.
MATERIALS AND METHODS
Study selection
Studies of blood cytokine (and cytokine receptor or antagonist) levels in schizophrenia, bipolar disorder and MDD were identified from two sources. First, we identified studies from recent meta-analyses of cytokines in these disorders.14–20 Second, studies were systematically searched using Medline (PubMed; National Center for Biotechnology Information, US National Library of Medicine, Bethesda, MD, USA), PsycInfo (via Ovid; American Psychological Association, Washington, DC, USA) and Thomson Reuters (formerly ISI) Web of Science (Science Citation Index and Social Sciences Citation Index; Thomson Reuters, Charlottesville, VA, USA) in July 2014 and again in March 2015. The primary search strategies were: (1) ‘(inflammation OR cytokine OR interleukin OR interferon OR ‘tumor necrosis factor’) AND (schizophrenia OR psychosis)’, (2) ‘(inflammation OR cytokine OR interleukin OR interferon OR ‘tumor necrosis factor’) AND (bipolar OR mania)’ and (3) ‘(inflammation OR cytokine OR interleukin OR interferon OR ‘tumor necrosis factor’) AND (depression OR ‘major depressive disorder’), limiting results to human studies in English. From all sources, we identified 164 potential studies for schizophrenia, 68 for bipolar disorder and 122 for MDD. The majority of initial matches were excluded because they were review articles, did not present cytokine data, measured only in vitro cytokine production or were genetic studies related to cytokines. We excluded studies of in vitro cytokine production because cytokine production in stimulated, separated mononuclear cells do not necessarily reflect endogenous immune system functioning.
The inclusion criteria were: (1a) studies assessing blood (plasma/serum) cytokine network component levels in acutely ill patients with schizophrenia, bipolar mania or MDD and healthy controls, (1b) studies assessing blood cytokine network component levels in acutely ill patients at baseline (i.e., first clinical contact for illness episode) and again following a period of treatment for the episode or (1c) studies assessing blood cytokine network component levels in chronically ill patients with schizophrenia, euthymic bipolar disorder, bipolar depression or MDD compared with healthy controls, (2) blood samples collected within 1 week of hospitalization and (3) studies in English. For patients with schizophrenia or bipolar disorder, we defined acutely ill as hospitalization for acute psychosis or acute mania. For patients with MDD, acutely ill was defined as either hospitalization for depression or outpatients with clinical data indicative of an acute depressive episode (i.e., mean Hamilton Rating Scale for Depression score >20 or mean Montgomery Asberg Depression Rating Scale score >25). For patients with schizophrenia, we defined chronically ill as being treated as an outpatient. Patients with euthymic bipolar disorder and bipolar depression were chosen for the meta-analysis of chronically ill patients because subjects with mania usually require in-patient care. Finally, for patients with MDD, chronically ill was defined as outpatients with clinical data that did not indicate an acute depressive episode (i.e., mean Hamilton Rating Scale for Depression score <20 or mean Montgomery Asberg Depression Rating Scale score <25). If the acutely/chronically ill status of subjects was unclear, we attempted to contact study authors for clarification. Although we included studies with blood samples collected within 1 week of hospitalization, this was carried out at the time of admission/before initiation of psychotropic medications in a majority of studies, with the exception of acute mania (35/40 studies for acute schizophrenia; 2/10 for mania (most were hospitalized for acute mania despite already being on medication—all samples were drawn at the time of hospitalization); 16/17 studies for MDD). For studies of cytokine levels in schizophrenia, we further stratified subjects with first-episode psychosis and acute exacerbation of chronic schizophrenia, to investigate potential effects of antipsychotic medications and disease course. Insufficient data were available for stratified analyses of first-episode mania or MDD. There were only a few studies evaluating components of the cytokine network in treatment-resistant subjects and so these studies were excluded.
The exclusion criteria were: (1) studies without a control group (except for studies of treatment of the acute illness episode), (2) studies that did not present either mean and standard deviations or median and interquartile range for cytokine levels (after attempting to contact the study authors), (3) studies of clinically stable or treatment-resistant patients, (4) studies with significant overlap in study population and (5) genetic and gene expression studies related to cytokine network components. Owing to the potential for low concentrations of some cytokine network components, the methods of the potential studies were reviewed to evaluate assay sensitivity. An assay result was excluded if: (1) the mean concentration was less than the lower limit of assay detection, (2) concentrations were not detectable in >50% of subjects or (3) either the intra-assay coefficient of variation was >10% or the interassay coefficient of variation was >15%.
After independent searches, review of study methods by two authors (BJM and DRG) and attempts to contact other authors, 68 studies met the inclusion criteria (40 schizophrenia, 10 bipolar disorder and 18 MDD) for acutely ill patients, and 46 studies met the inclusion criteria (18 studies of schizophrenia, 16 studies of bipolar disorder and 12 studies of MDD) for chronically ill patients.24–134 There was universal agreement on the included studies. There was no overlap in samples of acutely and chronically ill patients. All studies of outpatients with acute MDD measured cytokines before and after treatment of depression. A flow chart summarizing the study selection process is presented in Supplementary Material.
Data extraction and meta-analysis
Data were extracted (sample size, mean ± s.d. or median/interquartile range for patients and controls) for every cytokine network component assessed in each study for each disorder. If necessary, we estimated the mean ± s.d. from the median/interquartile range using the following formulas: (1) mean = (2m+a+b)/4, where m is the median and a and b are the 25th and 75th percentiles, respectively,135 and (2) interquartile range = 1.35 × s.d.136 One author (DRG) extracted all data, which was independently verified by another author (BJM). We then calculated effect size (ES) estimates (Hedges’ g) for every cytokine in each study for each disorder, and these data are included in Supplementary Material. Fixed-effects pooled ES estimates and 95% confidence intervals (95% CIs) were calculated using the Mantel and Haenszel method. Separate meta-analyses were performed for each individual cytokine network component for each disorder (versus controls), as well as for changes in cytokine levels following treatment of acute illness. Separate meta-analyses were performed for acutely and chronically ill patients. P-values were considered statistically significant at the α = 0.05 level. The statistical analyses were performed in Stata 10.0 (StataCorp, College Station, TX, USA).
The meta-analysis procedure also calculates a χ2 value for the heterogeneity in ES estimates, using Cochran’s Q-statistic137 and I2, the proportion of the variation in ES attributable to between-study heterogeneity. χ2 was considered significant for P < 0.10.138 For many cytokine network components for each disorder, χ2 was significant, so we performed a sensitivity analysis. This was carried out by removing one study at a time and repeating the meta-analysis procedure, to examine its impact on the odds ratio and between-study heterogeneity.139
Given the significant heterogeneity for many cytokine network components, we performed a series of meta-regressions for IL-6 and tumor necrosis factor-α (TNF-α) in acutely ill patients and IL-6 in chronically ill patients—the most frequently studied cytokines in each disorder—to explore possible moderating variables of age, sex, illness duration, smoking, body mass index and, for bipolar disorder, the proportion of subjects taking lithium and antipsychotic medications. We were not able to perform metaregression analyses for other cytokine network components because of the small number of available studies. The potential for publication bias was examined with Sterne’s funnel plot analysis140 and Egger’s regression intercept141 and can be found in Supplementary Materials.
RESULTS
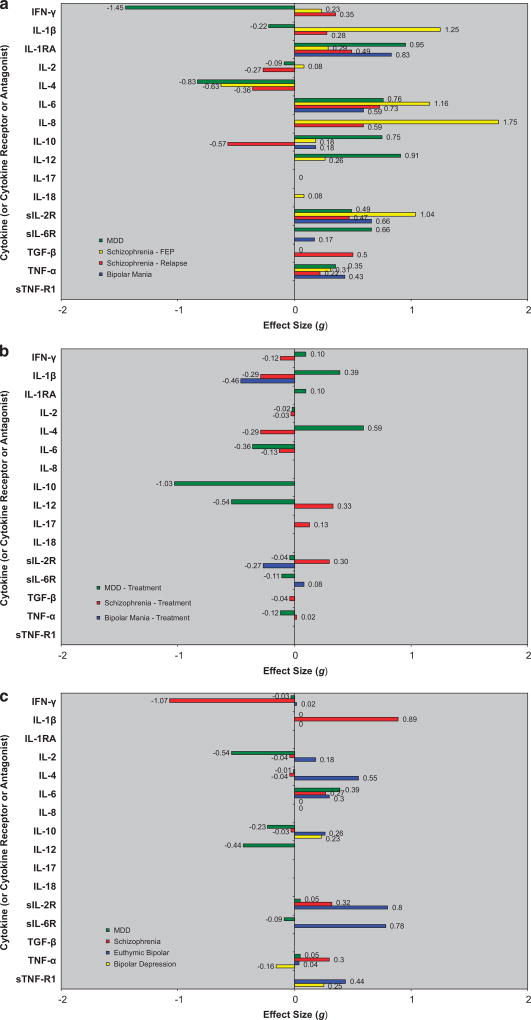
To summarize our findings: the levels of IL-6 (medium-large ES, range 0.59–1.16), TNF-α (small ES, range 0.22–0.43), sIL-2R (medium-large ES, range 0.47–1.04) and IL-1RA (small-large ES, range 0.29–0.95) were significantly increased in acutely ill patients with schizophrenia, bipolar mania and MDD versus controls (P < 0.01 for each). Following treatment of acute illness, IL-6 (small ES, range − 0.13 to − 0.36) levels significantly decreased in both schizophrenia and MDD (P < 0.01 for each); there were no significant changes in TNF-α; sIL-2R (small ES = 0.30) levels increased in schizophrenia; and IL-1RA (medium ES = − 0.46) levels in bipolar mania decreased. In chronically ill patients, levels of IL-6 (small-to-medium ES, range 0.27–0.39) were significantly increased in patients with schizophrenia, euthymic bipolar disorder and MDD compared with controls (P < 0.01 for each). Levels of IL-1β and sIL-2R were significantly increased in chronically ill patients with schizophrenia and euthymic bipolar disorder. Findings for each disorder are presented in greater detail below.
Schizophrenia
Table 1 and Figures 1a–c present ES estimates with 95% CIs for cytokine alterations in schizophrenia.
Table 1.
Blood cytokine network alterations in schizophrenia
| 1a. Blood cytokine network levels in first-episode psychosis versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 7 | 452 | 747 | 0.23 | 0.09–0.37 | <0.01 | 37.28 | <0.01 | 83.9 | 25,29–31,39,46,47 | |
| IL-1β | 6 | 333 | 298 | 1.25 | 1.07–1.42 | <0.01 | 78.36 | <0.01 | 93.6 | 31,43,57–60 | |
| IL-1RA | 2 | 194 | 376 | 0.29 | 0.10–0.48 | <0.01 | 1.25 | 0.26 | 20.1 | 24,29 | |
| IL-2 | 5 | 140 | 300 | 0.08 | −0.14 to 0.29 | 0.48 | 29.5 | <0.01 | 86.4 | 31,39,43,47,60 | |
| IL-4 | 4 | 193 | 322 | −0.63 | −0.84 to −0.42 | <0.01 | 74.95 | <0.01 | 96.0 | 25,31,46,47 | |
| IL-6 | 11 | 506 | 577 | 1.16 | 1.03–1.30 | <0.01 | 135.67 | <0.01 | 92.6 | 24,25,30,31,35,41,43,47,48,58,59 | |
| IL-8 | 2 | 49 | 49 | 1.75 | 1.27–2.24 | <0.01 | 12.43 | <0.01 | 92.0 | 31,51 | |
| IL-10 | 4 | 357 | 461 | 0.18 | 0.04–0.31 | 0.01 | 38.52 | <0.01 | 92.2 | 29,31,48,61 | |
| IL-12 | 3 | 258 | 463 | 0.26 | 0.05–0.47 | 0.02 | 69.17 | <0.01 | 97.1 | 28,29,45 | |
| IL-17 | 2 | 157 | 96 | 0.00 | −0.26 to 0.26 | 0.99 | 7.91 | <0.01 | 87.4 | 25,30 | |
| IL-18 | 3 | 335 | 403 | 0.08 | −0.07 to 0.23 | 0.28 | 0.87 | 0.65 | 0.0 | 29,62,63 | |
| sIL-2 R | 3 | 30 | 97 | 1.04 | 0.55–1.52 | <0.01 | 10.18 | <0.01 | 80.3 | 24,37,54 | |
| sIL-6 R | |||||||||||
| TGF-β | 3 | 169 | 298 | 0.58 | 0.36–0.80 | <0.01 | 11.86 | <0.01 | 83.1 | 25,46,47 | |
| TNF-α | 9 | 587 | 842 | 0.31 | 0.22–0.39 | <0.01 | 238.22 | <0.01 | 96.6 | 29,31,35,47,48,57–60 | |
| sTNF-R1 | |||||||||||
| 1b. Blood cytokine network levels in acutely ill patients with schizophrenia versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 4 | 162 | 266 | 0.35 | 0.13–0.57 | <0.01 | 5.34 | 0.15 | 43.9 | 25,42,47 | |
| IL-1β | 3 | 131 | 151 | 0.28 | 0.04–0.52 | 0.02 | 7.41 | 0.02 | 73.0 | 27,32,43 | |
| IL-1RA | 2 | 32 | 94 | 0.49 | 0.07–0.90 | 0.02 | 8.19 | <0.01 | 87.8 | 40,50 | |
| IL-2 | 2 | 43 | 199 | −0.27 | −0.61 to 0.07 | 0.12 | 14.08 | <0.01 | 92.9 | 43,47 | |
| IL-4 | 5 | 169 | 350 | −0.36 | −0.56 to −0.16 | <0.01 | 50.46 | <0.01 | 92.1 | 25,42,46,47,52 | |
| IL-6 | 9 | 278 | 468 | 0.73 | 0.56–0.90 | <0.01 | 134.19 | <0.01 | 94.0 | 25,33,36,38,42,43,47,49,52 | |
| IL-8 | 2 | 46 | 52 | 0.59 | 0.19–1.00 | <0.01 | 2.06 | 0.15 | 51.4 | 42,52 | |
| IL-10 | 2 | 46 | 52 | −0.57 | 0.98 −0.17 | <0.01 | 3.42 | 0.07 | 70.7 | 42,52 | |
| IL-12 | |||||||||||
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 3 | 58 | 120 | 0.47 | 0.14–0.80 | 0.01 | 4.46 | 0.11 | 55.1 | 32,30,50 | |
| sIL-6 R | |||||||||||
| TGF-β | 6 | 243 | 382 | 0.50 | 0.32–0.68 | <0.01 | 14.21 | 0.01 | 64.8 | 25,32,46,47,49 | |
| TNF-α | 7 | 269 | 449 | 0.22 | 0.05–0.39 | 0.01 | 141.05 | <0.01 | 95.7 | 27,33,40,42,47,49,52 | |
| sTNF-R1 | |||||||||||
| 1c. Blood changes in cytokine network levels following treatment of acutely ill patients with schizophrenia | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean duration | Mean ES Change | 95% CI | P-value | Heterogeneity | I2 | References | |||
|
|
|
||||||||||
| Studies | Pre | Post | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 4 | 265 | 265 | 36 | −0.12 | −0.29 to 0.05 | 0.16 | 1.57 | 0.81 | 0.0 | 26,30,44,47 |
| IL-1β | 4 | 189 | 189 | 75 | −0.29 | −0.49 to −0.09 | <0.01 | 4.90 | 0.18 | 38.7 | 43,44,57,59 |
| IL-1RA | |||||||||||
| IL-2 | 4 | 132 | 132 | 49 | −0.03 | −0.27 to 0.22 | 0.84 | 8.27 | 0.04 | 63.7 | 43,44,47,53 |
| IL-4 | 2 | 186 | 186 | 43 | −0.29 | −0.50 to −0.09 | 0.01 | 0.36 | 0.83 | 0.0 | 26,47 |
| IL-6 | 11 | 521 | 500 | 56 | −0.13 | −0.25 to −0.01 | 0.04 | 20.55 | 0.04 | 46.5 | 26,30,33,36,43,42,47,48,53,59 |
| IL-8 | |||||||||||
| IL-10 | |||||||||||
| IL-12 | 3 | 104 | 104 | 42 | 0.33 | 0.06–0.60 | 0.02 | 3.77 | 0.15 | 46.9 | 28,44,45 |
| IL-17 | 2 | 193 | 193 | 29 | 0.13 | −0.07 to 0.33 | 0.22 | 0.49 | 0.78 | 0.0 | 26,30 |
| IL-18 | |||||||||||
| sIL-2 R | 3 | 90 | 90 | 69 | 0.30 | 0.01 to 0.60 | 0.04 | 2.19 | 0.34 | 8.6 | 51,55,56 |
| sIL-6 R | |||||||||||
| TGF-β | 4 | 286 | 286 | 43 | −0.04 | −0.21 to 0.12 | 0.61 | 22.63 | <0.01 | 82.3 | 26,46,47,49 |
| TNF-α | 6 | 310 | 310 | 73 | 0.02 | −0.14 to 0.18 | 0.84 | 15.53 | 0.01 | 67.8 | 33,47,53,57,59 |
| sTNF-R1 | |||||||||||
| 1d. Blood cytokine network alterations in chronically ill patients with schizophrenia versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 4 | 198 | 132 | −1.07 | −1.35 to −0.80 | <0.01 | 164.70 | <0.01 | 98.2 | 75,77,80,91 | |
| IL-1β | 4 | 330 | 303 | 0.89 | 0.73–1.05 | <0.01 | 101.94 | <0.01 | 96.1 | 75,78,81,91 | |
| IL-1RA | |||||||||||
| IL-2 | 6 | 193 | 171 | −0.04 | −0.24 to 0.16 | 0.68 | 55.25 | <0.01 | 91.0 | 76,80,84,88,90,91 | |
| IL-4 | 2 | 73 | 46 | −0.04 | −0.42 to 0.34 | 0.83 | 0.87 | 0.35 | 0.0 | 80,91 | |
| IL-6 | 12 | 711 | 674 | 0.27 | 0.17 to 0.38 | <0.01 | 110.59 | <0.01 | 88.2 | 75,78,80,81,83,85–91 | |
| IL-8 | |||||||||||
| IL-10 | 4 | 118 | 126 | −0.03 | −0.24 to 0.18 | 0.81 | 4.06 | 0.40 | 1.4 | 75,80,85,91 | |
| IL-12 | |||||||||||
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 3 | 116 | 135 | 0.32 | 0.40–0.86 | <0.01 | 1.42 | 0.70 | 0.0 | 78–80 | |
| sIL-6 R | |||||||||||
| TGF-β | |||||||||||
| TNF-α | 9 | 508 | 509 | 0.30 | 0.18–0.43 | <0.01 | 46.77 | <0.01 | 80.8 | 75,78,80–83,85,90,91 | |
| sTNF-R1 | |||||||||||
Abbreviations: ES, effect size; CI, confidence interval; IFN, interferon; IL, interleukin; IL-1RA, interleukin-2 receptor antagonist; Pt, patients; sIL, soluble interelukin; TGF, tumor growth factor; TNF, tumor necrosis factorr.
Bold and italicized values are statistically significant.
Figure 1.
Blood cytokine network alterations in studies of patients with schizophrenia, bipolar disorder and major depressive disorder (MDD) versus controls. (a) Acutely ill patients with schizophrenia, bipolar mania and MDD versus controls. (b) Changes in blood cytokine network levels following treatment of acute illness in schizophrenia, bipolar mania and MDD. (c) Chronically ill patients with schizophrenia, bipolar disorder and MDD versus control.
First-episode psychosis versus controls
The ESs indicated that levels of interferon-γ (IFN-γ), IL-1RA, IL-1β, IL-6, IL-8, IL-10, IL-12, sIL-2R, TGF-β and TNF-α were all significantly increased (P < 0.01 for each), and levels of IL-4 were significantly decreased (P < 0.01), but IL-2, IL-17 and IL-18 levels did not differ in first-episode psychosis versus controls. Between-study heterogeneity was significant for all cytokines except for IL-1RA.
In sensitivity analyses, heterogeneity was no longer significant, but the ES remained significant, after removing one study for IFN-γ,31 sIL-2R37 and TGF-β.47 For IL-12, the ES was no longer significant after removing one study.28 Between-study heterogeneity remained significant in sensitivity analyses for IL-1β, IL-4, IL-6 and TNF-α. Sensitivity analysis was not possible for IL-8 because of the small number of studies.
A funnel plot and results of Egger’s test showed no evidence for publication bias for IL-6 (P = 0.624). Egger’s test showed significant evidence for publication bias for TNF-α (P = 0.049), but the funnel plot suggests that this may reflect true between-study heterogeneity (see Supplementary Material). In meta-regression analyses, age, sex, illness duration, smoking and BMI were all unrelated to IL-6 and TNF-α in first-episode psychosis (P > 0.05 for each).
Acute exacerbation of chronic schizophrenia versus controls
The ESs indicated that the levels of IFN-γ, IL-1RA, IL-1β, IL-6, IL-8, IL-12, sIL-2R, TGF-β and TNF-α were all significantly increased (P < 0.04 for each) and IL-4 and IL-10 levels were significantly decreased (P < 0.01 for each) in acutely ill patients with chronic schizophrenia versus controls. ES differences for IL-2 levels were not significantly different between patients and controls. ESs were similar in direction, although generally of smaller magnitude, than studies of first-episode psychosis versus controls. Between-study heterogeneity was significant for all cytokines (or receptors or antagonists) except for IFN-γ, IL-8, IL-12 and sIL-2R. In sensitivity analyses, the heterogeneity was no longer significant, but the ES estimate remained significant, after removing one study for IL-1β32 and IL-6.47 The heterogeneity remained significant in a sensitivity analysis for IL-4, TGF-β and TNF-α. Sensitivity analyses were not possible for IL-1RA, IL-8 and IL-10 because of the small number of studies.
A funnel plot and results of Egger’s test showed no evidence for publication bias for IL-6 (P = 0.752) or TNF-α (P = 0.993). In metaregression analyses, age, sex, illness duration and BMI were all unrelated to IL-6; age, illness duration and smoking were all unrelated to TNF-α in schizophrenia (P > 0.05 for each). Sex (P = 0.068) and BMI (P = 0.092) showed a trend-level association, with a higher proportion of females and higher BMI associated with larger ESs for TNF-α.
Treatment of acute psychosis
Following treatment for acute psychosis (range 36–75 days), there were significant small ES decreases in levels of IL-1β (P < 0.01), IL-4 (P = 0.01) and IL-6 levels (P = 0.04). There were significant small ES increases in IL-12 (P = 0.02) and sIL-2R (P = 0.04) levels. Between-study heterogeneity was significant for IL-6, and remained significant in a sensitivity analysis. There were no significant ES changes in levels of IFN-γ, IL-2, TGF-β or TNF-α.
Chronically ill patients with schizophrenia versus controls
There were small-to-medium ESs for elevations of IL-6 (ES = 0.27), TNF-α (ES = 0.30) and sIL-2R (ES = 0.32), a large ES for elevation of IL-1β (ES = 0.89), and a large ES for decrease in IFN-γ (ES = − 1.07) for patients with schizophrenia versus controls. By contrast, there was not a significant ES difference in the levels of IL-2, IL-4 or IL-10 compared with controls. Between-study heterogeneity was significant for IL-1β, IL-6 and TNF-α, but not for sIL-2R. Between-study heterogeneity remained significant in sensitivity analyses for IFN-γ, IL-1β, IL-6 and TNF-α.
A funnel plot and results of Egger’s test (P = 0.069) found no significant evidence for publication bias. The metaregression analyses for age, sex, illness duration, smoking and BMI were all unrelated to the association between IL-6 and schizophrenia (P > 0.05 for each).
Summary of findings for schizophrenia
After sensitivity analyses (where possible), IFN-γ, IL-1RA, IL-1β, IL-6, IL-8, IL-10, sIL-2R, TGF-β and TNF-α levels were significantly increased and IL-4 levels significantly decreased in first-episode psychosis versus controls (P < 0.01 for each). Levels of IFN-γ, IL-1RA, IL-1β, IL-6, IL-8, IL-12, sIL-2R, TGF-β and TNF-α were all significantly increased and the levels of IL-4 and IL-10 were significantly decreased in acutely ill patients with chronic schizophrenia versus controls (P < 0.01 for each). Following treatment of acute psychosis, there were significant decreases in IL-1β, IL-4 and IL-6, and increases in IL-12 and sIL-2R. In chronically ill patients, after sensitivity analyses (where possible) the levels of IL-1β, IL-6, sIL-2R and TNF-α were significantly increased and IFN-γ levels significantly decreased in chronically ill patients with schizophrenia compared with controls (P < 0.01 for each).
Bipolar disorder
Table 2 and Figures 1a–c present ES estimates with 95% CIs for cytokine alterations in bipolar disorder.
Table 2.
Blood cytokine network alterations in bipolar disorder
| 2a. Blood cytokine network levels in acute ill patients with bipolar mania versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | |||||||||||
| IL-1β | |||||||||||
| IL-1RA | 2 | 46 | 53 | 0.83 | 0.42–1.25 | <0.01 | 0.05 | 0.82 | 0.0 | 67,73 | |
| IL-2 | |||||||||||
| IL-4 | |||||||||||
| IL-6 | 2 | 44 | 160 | 0.59 | 0.25–0.93 | <0.01 | 3.49 | 0.06 | 71.4 | 64,68 | |
| IL-8 | |||||||||||
| IL-10 | 2 | 61 | 116 | 0.18 | −0.11 to 0.47 | 0.22 | 1.21 | 0.55 | 0.0 | 64,66 | |
| IL-12 | |||||||||||
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 3 | 82 | 90 | 0.66 | 0.35–0.97 | <0.01 | 1.81 | 0.41 | 0.0 | 70–72 | |
| sIL-6 R | 3 | 82 | 90 | 0.17 | −0.13 to 0.48 | 0.26 | 0.04 | 0.98 | 0.0 | 70–72 | |
| TGF-β | |||||||||||
| TNF-α | 3 | 73 | 137 | 0.43 | 0.12–0.74 | <0.01 | 12.68 | <0.01 | 84.2 | 64,66,68 | |
| sTNF-R1 | |||||||||||
| 2b. Changes in blood cytokine network levels following treatment of acutely ill bipolar mania | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean duration | Mean ES change | 95% CI | P-value | Heterogeneity | I2 | References | |||
|
|
|
||||||||||
| Studies | Pre | Post | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | |||||||||||
| IL-1β | 2 | 46 | 62 | N/A | −0.46 | −0.86 to −0.07 | 0.02 | 0.22 | 0.64 | 0.0 | 67,73 |
| IL-1RA | |||||||||||
| IL-2 | |||||||||||
| IL-4 | |||||||||||
| IL-6 | |||||||||||
| IL-8 | |||||||||||
| IL-10 | |||||||||||
| IL-12 | |||||||||||
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 3 | 82 | 82 | N/A | −0.27 | −0.58 to 0.03 | 0.08 | 0.12 | 0.94 | 0.0 | 70–72 |
| sIL-6 R | 3 | 82 | 82 | N/A | 0.08 | −0.23 to 0.39 | 0.60 | 0.15 | 0.93 | 0.0 | 70–72 |
| TGF-β | |||||||||||
| TNF-α | |||||||||||
| sTNF-R1 | |||||||||||
| 2c. Blood cytokine network alterations in chronically ill patients with euthymic bipolar disorder versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 3 | 91 | 122 | 0.02 | −0.26 to 0.30 | 0.89 | 3.20 | 0.36 | 6.2 | 95,101,108 | |
| IL-1β | 2 | 125 | 158 | 0.31 | 0.07–0.55 | 0.01 | 0.01 | 0.92 | 0.0 | 105,108 | |
| IL-1RA | |||||||||||
| IL-2 | 2 | 46 | 44 | 0.18 | −0.24 to 0.60 | 0.40 | 2.87 | 0.24 | 30.3 | 95,100 | |
| IL-4 | 3 | 0.55 | 0.15–0.95 | 0.01 | 58.31 | <0.01 | 94.9 | 95,96,100,95,96,101,103,104 | |||
| IL-6 | 7 | 213 | 540 | 0.30 | 0.13–0.47 | <0.01 | 27.57 | <0.01 | 78.2 | 106,108 | |
| IL-8 | |||||||||||
| IL-10 | 7 | 166 | 273 | 0.26 | 0.06–0.46 | 0.01 | 27.74 | <0.01 | 74.8 | 95,96,100,102–104,108 | |
| IL-12 | |||||||||||
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 3 | 199 | 230 | 0.80 | 0.59–1.00 | <0.01 | 24.63 | <0.01 | 91.9 | 93,96,107 | |
| sIL-6 R | 2 | 98 | 153 | 0.78 | 0.52–1.05 | <0.01 | 2.14 | 0.14 | 53.4 | 93,98 | |
| TGF-β | |||||||||||
| TNF-α | 7 | 236 | 296 | 0.04 | −0.14 to 0.22 | 0.66 | 19.08 | 0.01 | 58.1 | 94,95,99,100 | |
| sTNF-R1 | 4 | 133 | 310 | 0.44 | 0.19–0.69 | <0.01 | 6.80 | 0.08 | 55.9 | 102–104,108 | |
| 2d. Blood cytokine network alterations in chronically ill patients with bipolar depression versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | |||||||||||
| IL-1β | |||||||||||
| IL-1RA | |||||||||||
| IL-2 | |||||||||||
| IL-4 | |||||||||||
| IL-6 | 3 | 102 | 344 | 0.00 | −0.23 to 0.23 | 0.98 | 6.94 | 0.03 | 71.2 | 95,101,103 | |
| IL-8 | |||||||||||
| IL-10 | 2 | 44 | 105 | 0.23 | −0.14 to 0.60 | 0.22 | 0.80 | 0.37 | 0.0 | 95,103 | |
| IL-12 | |||||||||||
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | |||||||||||
| sIL-6 R | |||||||||||
| TGF-β | |||||||||||
| TNF-α | 2 | 44 | 105 | −0.16 | −0.53 to 0.21 | 0.40 | 1.93 | 0.16 | 48.3 | 95,103 | |
| sTNF-R1 | 2 | 72 | 369 | 0.25 | 0.00–0.51 | 0.05 | 3.99 | 0.05 | 74.9 | 91,101 | |
Abbreviations: ES, effect size; CI, confidence interval; IFN, interferon; IL, interleukin; IL-1RA, interleukin-2 receptor antagonist; N/A, not available; Pt, patients; sIL, soluble interelukin; TGF, tumor growth factor; TNF, tumor necrosis factor.
Bold and italicized values are statistically significant.
Bipolar mania versus controls
The ESs indicated that the levels of IL-1RA, IL-6, sIL-2R and TNF-α were all significantly increased in acute mania versus controls (P < 0.01 for each). Between-study heterogeneity was significant for IL-6 and TNF-α, but not for IL-1RA and sIL-2R. In a sensitivity analysis for TNF-α, the heterogeneity was no longer significant, but the ES estimate was also not significant after removal of one study.69 Sensitivity analysis was not possible for IL-6 because of the small number of studies. By contrast, there was not a significant ES difference in levels of IL-10 or sIL-6R.
A funnel plot, Egger’s test for potential publication bias and metaregression analyses were not performed because of a small number of studies for IL-6 and TNF-α.
Treatment of acute mania
Following treatment for acute mania, there was a significant ES decrease in IL-1RA levels (P = 0.02) and a trend toward a decrease in sIL-2R levels (P = 0.08). By contrast, there was not a significant change in ES for sIL-6R levels. Between-study heterogeneity was not significant for any cytokines.
Chronically ill patients with euthymic bipolar disorder versus controls
Blood levels in patients with euthymic bipolar disorder compared with controls indicated a small-to-medium ES for elevations of IL-10 (ES = 0.26), IL-6 (ES = 0.30), IL-1β (ES = 0.31) and sTNF-R1 (ES = 0.44), and medium-to-large ES for elevation of IL-4 (ES = 0.55), sIL-6R (ES = 0.78) and sIL-2R (ES = 0.80). Between-study heterogeneity was significant for IL-4, IL-6, IL-10, sIL-2R and sTNF-R1, but not sIL-6R or IL-1β. In sensitivity analyses, the heterogeneity was no longer significant, but the effect size estimate remained significant, after removing one study for sTNF-R1.114 Between-study heterogeneity remained significant in sensitivity analyses for IL-4, IL-6, IL-10 and sIL-2R. Sensitivity analyses were not possible for either IL-1β or sIL-6R because of the small number of studies. By contrast, there was not a significant ES difference in levels of IFN-γ, IL-2 or TNF-α compared with controls.
A funnel plot and results of Egger’s test (P = 0.398) showed no significant evidence for publication bias. The metaregression analyses for age, sex, illness duration, body mass index and the proportion of subjects taking lithium and antipsychotics were all unrelated to the association between IL-6 and bipolar disorder (P > 0.05 for each).
Chronically ill patients with bipolar depression versus controls
ES differences for IL-6, IL-10 and TNF-α levels were not significantly different between patients and controls. There was a trend for increased sTNF-R levels (P = 0.052).
Summary of findings for bipolar disorder
After sensitivity analyses, IL-1RA, IL-6 and sIL-2R levels were significantly increased in subjects with acute mania versus controls (P < 0.01 for each). Following treatment of acute mania, there was a significant decrease in IL-1RA levels (P = 0.02) and a trend for a decrease in sIL-2R. In chronically ill patients, after sensitivity analyses (where possible) levels of IL-1β, IL-4, IL-6, IL-10, sIL-2R, sIL-6R and sTNF-R1 were significantly increased in chronically ill patients with euthymic bipolar disorder compared with controls (P ≤ 0.01 for each) but there were no differences between patients with bipolar depression and controls.
Major depressive disorder
Table 3 and Figures 1a–c present ES estimates with 95% CIs for cytokine alterations in MDD.
Table 3.
Blood cytokine network alterations in MDD
| 3a. Blood cytokine network levels in acutely ill patients with MDD versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 2 | 61 | 54 | −1.45 | −1.87 to −1.04 | <0.01 | 1.06 | 0.30 | 5.7 | 78,79 | |
| IL-1β | 4 | 116 | 112 | −0.22 | −0.49 to 0.06 | 0.13 | 80.87 | <0.01 | 94.0 | 78,79,81,84 | |
| IL-1RA | 2 | 44 | 25 | 0.95 | 0.42–1.48 | <0.01 | 0.97 | 0.33 | 0.0 | 82,86 | |
| IL-2 | 3 | 84 | 79 | −0.09 | −0.47 to 0.29 | 0.64 | 108.92 | <0.01 | 98.2 | 78,79,90 | |
| IL-4 | 2 | 53 | 57 | −0.83 | −1.26 to −0.40 | <0.01 | 40.86 | <0.01 | 97.6 | 78,90 | |
| IL-6 | 10 | 306 | 216 | 0.76 | 0.56–0.95 | <0.01 | 96.09 | <0.01 | 90.6 | 74,75,81,82,84–89 | |
| IL-8 | |||||||||||
| IL-10 | 4 | 112 | 103 | 0.75 | 0.46–1.04 | <0.01 | 37.32 | <0.01 | 92.0 | 78–80,82 | |
| IL-12 | 4 | 117 | 172 | 0.91 | 0.64–1.17 | <0.01 | 44.69 | <0.01 | 93.3 | 45,78,83,90 | |
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 5 | 247 | 170 | 0.49 | 0.29–0.70 | <0.01 | 11.04 | 0.05 | 54.7 | 77,81,85,87,88 | |
| sIL-6 R | 3 | 145 | 68 | 0.66 | 0.36–0.96 | <0.01 | 2.68 | 0.26 | 25.3 | 85,86,88 | |
| TGF-β | |||||||||||
| TNF-α | 8 | 296 | 281 | 0.35 | 0.17–0.53 | <0.01 | 136.00 | <0.01 | 94.1 | 77,78,80,81,84,86,88,90 | |
| sTNF-R1 | |||||||||||
| 3b. Changes in blood cytokine network levels following treatment of acutely ill patients with MDD | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean duration | Mean ES change | 95% CI | P-value | Heterogeneity | I2 | References | |||
|
|
|
||||||||||
| Studies | Pre | Post | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 2 | 54 | 61 | 112 | 0.10 | −0.27 to 0.47 | 0.59 | 0.58 | 0.45 | 0.0 | 78,79 |
| IL-1β | 4 | 89 | 82 | 74 | 0.39 | 0.06–0.72 | 0.02 | 54.55 | <0.01 | 92.7 | 78,79,81,84 |
| IL-1RA | 2 | 34 | 34 | 39 | 0.10 | −0.38 to 0.58 | 0.70 | 2.30 | 0.13 | 56.5 | 82,86 |
| IL-2 | 3 | 84 | 77 | 93 | −0.02 | −0.38 to 0.34 | 0.92 | 82.08 | <0.01 | 97.6 | 78,79,90 |
| IL-4 | 3 | 84 | 77 | 93 | 0.59 | 0.25–0.92 | <0.01 | 38.97 | <0.01 | 94.9 | 78,79,90 |
| IL-6 | 7 | 116 | 116 | 45 | −0.36 | −0.62 to −0.09 | 0.01 | 17.95 | 0.01 | 61.0 | 74,81,82,84–87 |
| IL-8 | |||||||||||
| IL-10 | 2 | 54 | 61 | 112 | −1.03 | −1.45 to −0.61 | <0.01 | 30.41 | <0.01 | 96.7 | 78,79 |
| IL-12 | 4 | 86 | 86 | 60 | −0.54 | −0.86 to −0.23 | <0.01 | 20.67 | <0.01 | 85.5 | 45,78,83,90 |
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 4 | 139 | 139 | 60 | −0.04 | −0.28 to 0.19 | 0.72 | 3.33 | 0.50 | 0.0 | 77,81,85,87 |
| sIL-6 R | 2 | 42 | 42 | 63 | −0.11 | 0.53–0.32 | 0.93 | 0.85 | 0.36 | 0.0 | 85,87 |
| TGF-β | |||||||||||
| TNF-α | 6 | 205 | 205 | 54 | −0.12 | −0.32 to 0.08 | 0.22 | 45.66 | <0.01 | 84.7 | 76–78,81,84,90 |
| sTNF-R1 | |||||||||||
| 3c. Blood cytokine network alterations in chronically ill patients with MDD versus controls | |||||||||||
|
| |||||||||||
| Cytokine component | N | Mean ES | 95% CI | P-value | Heterogeneity | I2 | References | ||||
|
|
|
||||||||||
| Studies | Pt | Control | χ2 | P-value | |||||||
|
| |||||||||||
| IFN-γ | 4 | 195 | 226 | −0.03 | −0.24 to 0.16 | 0.82 | 158.68 | <0.01 | 98.1 | 64,109,112,117 | |
| IL-1β | 3 | 105 | 157 | 0.21 | −0.04 to 0.47 | 0.10 | 17.32 | <0.01 | 88.5 | 64,111,112 | |
| IL-1RA | |||||||||||
| IL-2 | 2 | 82 | 82 | −0.54 | −0.90 to −0.17 | <0.01 | 84.19 | <0.01 | 98.8 | 64,117 | |
| IL-4 | 2 | 82 | 113 | −0.01 | −0.30 to 0.28 | 0.94 | 4.82 | 0.03 | 79.3 | 64,112 | |
| IL-6 | 7 | 205 | 211 | 0.39 | 0.20–0.59 | <0.01 | 31.44 | <0.01 | 77.7 | 64,110,112,115,116,118,119 | |
| IL-8 | 3 | 126 | 137 | 0.08 | −0.16 to 0.32 | 0.49 | 15.03 | <0.01 | 80.0 | 64,112,116 | |
| IL-10 | 4 | 138 | 148 | −0.23 | −0.546 to 0.01 | 0.06 | 34.64 | <0.01 | 88.5 | 64,110,112,116 | |
| IL-12 | 2 | 82 | 113 | −0.44 | −0.75 to −0.14 | <0.01 | 30.32 | <0.01 | 96.7 | 64,112 | |
| IL-17 | |||||||||||
| IL-18 | |||||||||||
| sIL-2 R | 2 | 117 | 80 | 0.05 | −0.20 to 0.30 | 0.68 | 10.84 | 0.01 | 72.3 | 113,118 | |
| sIL-6 R | 2 | 66 | 42 | −0.09 | −0.43 to 0.26 | 0.63 | 0.94 | 0.63 | 0.0 | 115,116 | |
| TGF-β | |||||||||||
| TNF-α | 7 | 346 | 351 | 0.05 | −0.10 to 0.19 | 0.52 | 75.26 | <0.01 | 86.7 | 64,109,111–114,116,109 | |
| sTNF-R1 | |||||||||||
Abbreviations: ES, effect size; CI, confidence interval; IFN, interferon; IL, interleukin; IL-1RA, interleukin-2 receptor antagonist; N/A, not available; Pt, patients; sIL, soluble interelukin; TGF, tumor growth factor; TNF, tumor necrosis factor.
Bold and italicized values are statistically significant.
Acutely ill MDD versus controls
The ESs indicated that the levels of IL-1RA, IL-6, IL-10, IL-12, sIL-2R, sIL-6R and TNF-α were all significantly increased (P < 0.01) and levels of IFN-γ and IL-4 were significantly decreased (P < 0.01) in acutely ill patients with MDD versus controls. There were not significant differences in ESs for levels of IL-1β and IL-2. Between-study heterogeneity was significant for IL-4, IL-6, IL-10, IL-12 sIL-2R and TNF-α, but not IL-1RA, IFN-γ or sIL-6R. In sensitivity analyses, heterogeneity was no longer significant and the ES remained significant, after removing one study for sIL-2R.88 Between-study heterogeneity remained significant in sensitivity analyses for IL-6, IL-10, IL-12 and TNF-α. Sensitivity analysis was not possible for IL-4 because of the small number of studies.
A funnel plot and results of Egger’s test showed no evidence for publication bias for IL-6 (P = 0.118) or TNF-α (P = 0.166). In metaregression analyses, age and sex were unrelated to IL-6; age, sex and BMI were unrelated to TNF-α in depression (P > 0.05).
Treatment of acute depression
Following treatment for acute depression (range 39–112 days), there were significant ES changes indicating a decrease in IL-6, IL-10 and IL-12 levels (P < 0.02 for each), and an increase in IL-4 (P < 0.01) and IL-1β (P = 0.02) levels. Between-study heterogeneity was significant for all of these cytokines. The heterogeneity remained significant in sensitivity analyses for IL-1β, IL-6 and IL-12. For IL-4, the heterogeneity was no longer significant, but neither was the ES estimate after removal of one study.90 The small number of studies precluded a sensitivity analysis for IL-10.
Chronically ill patients with MDD versus controls
Blood levels in patients with MDD compared with controls indicated a small-to-medium ES for increase for IL-6 levels (ES = 0.39) and decrease for IL-12 levels (ES = − 0.44), and medium ES for decrease in IL-2 (ES = − 0.54). Between-study heterogeneity was significant for all of these cytokines. In sensitivity analysis, the heterogeneity was no longer significant and the ES estimate remained significant, after removing one study for IL-6.112 The small number of studies precluded sensitivity analyses for IL-2 or IL-12. The ES differences were not significant for, IL-1β, IL-4, IL-8, IL-10, sIL-2R, sIL-6R or TNF-α.
Egger’s test (P = 0.037) of the IL-6 data revealed significant evidence for publication bias, but the funnel plot suggests this may also reflect true between-study heterogeneity (see Supplementary Material). The metaregression analyses found that IL-6 levels were not effected by age, sex or smoking (P > 0.05).
Summary of findings for MDD
After sensitivity analyses, IL-1RA, IL-6, IL-10, IL-12, sIL-2R, sIL-6R and TNF-α levels were all significantly increased and IFN-γ and IL-4 levels were significantly decreased in subjects with MDD versus controls (P < 0.01). Following treatment of acute depression, there was a significant decrease in IL-6, IL-10 and IL-12 levels, and an increase in IL-4 and IL-1β levels (P < 0.02). In chronically ill patients, after sensitivity analyses (where possible) levels of IL-6 were significantly increased and levels of IL-2 and IL-12 significantly decreased in chronically ill patients with MDD compared with controls (P < 0.01) (Tables 1–3 and Figures 1a–c).
DISCUSSION
Overall, there are similarities in the ES direction and, to a lesser extent, magnitude of blood cytokine network component levels in schizophrenia, bipolar disorder and MDD during acute and chronic phases of illness, raising the possibility of common underlying pathways for immune dysfunction in these disorders (Figures 1a and c). The levels of two cytokines (IL-6 and TNF-α), one cytokine receptor antagonist (IL-1RA), thought to reflect increased IL-1 activity, and one soluble cytokine receptor (sIL-2R), thought to function in a counter-regulatory manner, were significantly elevated in all three syndromes during acute illness episodes. Following treatment of acute illness, there were similarities in the pattern of change of levels of these cytokine network components: IL-6 levels significantly decreased in both schizophrenia and MDD; there were no significant changes in TNF-α levels in either schizophrenia or MDD; sIL-2R levels increased in schizophrenia, but were not significantly changed in either bipolar mania or MDD; and IL-1RA levels in bipolar mania decreased, but were not significantly changed in MDD. In chronically ill patients, the levels of IL-6 were statistically significantly elevated in all three disorders, and the levels of sIL-2R and the cytokine IL-1β were significantly elevated in schizophrenia and bipolar disorder.
It is intriguing that all of the cytokines that were elevated in acutely ill patients with all three syndromes are modulated through the nuclear factor-κB, signaling pathway that is commonly activated in inflammatory and autoimmune disease.142–144 This suggests that acutely symptomatic patients may share a profile of immune system activation that commonly is observed during an acute inflammatory state. Particularly in light of work demonstrating that acute stress increases IL-6 and other inflammatory markers, one may speculate that there may be a common stress-related phenomenon occurring in acutely ill patients across these disorders.99,100 This hypothesis-generating observation requires more extensive investigation. In addition to the common cytokine network alterations across all three disorders, the pattern of other cytokine network alterations suggests that treatment of acute illness may lead to resolution of inflammation and an increase in anti-inflammatory biomarkers; this observation requires more extensive exploration (Figure 1b). Although speculative, the common findings in chronically ill patients also might reflect the influence of chronic stress on the immune system.145,146 Chronic stress may lead to a dysregulation of the immune response with decreased glucocorticoid receptor sensitivity, impaired feedback of the HPA axis and subsequent increases in proinflammatory cytokines as well as suppression of anti-inflammatory cytokines and/or counter-regulatory mechanisms.147–149 Elevations in the cytokines IL-1β and IL-6 suggest the possibility of monocyte/macrophage activation,150,151 whereas elevations in sIL-2 R, which is constitutively produced by activated T cells, suggests a counter-regulatory response to T-cell activation.152 Elevations in both IL-6 and sIL-2 R in chronically ill patients suggests a role for persistent immune activation for some patients with these disorders. Our findings suggest that it would be useful to compare and contrast inflammatory markers in the same subjects during both acute and chronic phases of illness.
The strengths of our study include comparisons of cytokine network alterations across three major psychiatric disorders, which has not been previously performed. We also investigated cytokine network component levels in both acute and chronic phases of illness, as well as the effects of treatment of acute illness episodes on these biomarkers. There are always limitations to studies. Our findings should be interpreted with caution in light of relatively small number of studies meeting the inclusion criteria for this meta-analysis and small sample size available for many of the cytokine network comparisons. This limits our ability to make inferences regarding molecular mechanisms of similarities and differences across disorders. A second limitation is that one cannot account for either the type or the length of pharmacologic intervention used in the studies included in our meta-analysis. Furthermore, our classification of acute and chronic illness was limited by not having symptom measures for each patient in each study to make a determination of illness phase. Instead, we classified acute and chronic illness in a way that we felt most appropriately captured illness phase given the limitation of not having individual level data. A limitation of the field is the lack of agreement on a uniform panel of cytokine network components that are consistently studied. Consequently, the field may continue to focus on assays of individual cytokines, which, as observed in this meta-analysis, limits our ability to compare across studies. Furthermore, it may be less important to measure individual cytokines, but instead measure overall patterns of immune activation, for example, with a panel of relevant cytokines or with cytokine ratios (e.g. IFN/IL-4; TNF/IL-10). One also needs to acknowledge the problem of important potential confounding, and some potentially moderating, factors such as smoking, body mass index, medical comorbidities, level and type of psychopathology, genetic heterogeneity, sample collection and processing, time of day, whether plasma or serum were assayed, type of assay used and how and how long samples are stored before being analyzed.153–155 Although we did not find evidence for moderating effects of age, sex, illness duration, BMI and medications on IL-6 levels in either acutely ill or chronically ill subjects, this does not preclude moderating effects between such clinical and demographic factors and other cytokine network components.156–160 These factors should be carefully considered when one weighs the significance of meta-analytic findings in psychoneuroimmunology.
It is important to emphasize nuances in the interpretation of the ES estimates in the analyses of acutely ill patients. ESs for acutely ill subjects versus controls represent the magnitude of the difference in cytokine levels between two different subject groups. By contrast, ESs for treatment of acute illness reflect the change in cytokine levels in the same subjects before and after treatment, and thus tend to be smaller (see Figure 1). Another caveat is that analysis of impact of treatment on cytokine levels represents a subset of the analysis that contrasts patients with the three disorders with matched control samples. A final issue that must be taken into consideration when interpreting our findings is the understanding that many of the ES differences for statistically significant findings fall into the small ES category. This suggests that either the observed change is either relatively minor and may not be clinically significant, or, alternatively, immune disequilibrium occurs in only a minority of subjects with these syndromes.
Our findings raise many questions. What are the most useful set of cytokines to measure? Should we measure the same panel of cytokines across disorders? Should future studies preferentially measure cytokine network components where there is more robust evidence (to replicate findings), or those that have not been thoroughly investigated, or some combination of both? The creation of an agreed upon standard for assay methodology is critical to the advancement of the field: should we be using a multiplex system or an ELISA-based system? What are the normal ranges for these measures? What are the most reasonable statistical approaches to employ with data that are frequently highly skewed? Another important question is whether measurement of peripheral levels of cytokine network components is sufficient? Evaluation of specific leukocyte populations via flow cytometry or coupling peripheral findings with brain imaging may be more informative. We need to determine whether traditional measures like demographic and clinical (e.g., psychopathology, cognition, medical comorbidities) features are associated with cytokine network alterations. We still need to more closely evaluate the effects of all aspects of treatment of the acute illness on cytokine levels through the serial measurement of intraindividual changes in cytokines across the course of illness. More studies in first-episode drug-naïve subjects would clarify which changes in the cytokine network are intrinsically associated with these disorders and which might be better attributed to medication effects. What are the effects of course of illness and treatment-resistant illness on cytokine levels? We excluded treatment-resistant schizophrenia because of preliminary evidence that these patients may represent a subset with unique cytokine alterations,161–163 and there were insufficient number of studies in MDD and bipolar disorder to make meaningful comparisons. Future studies of the effects of treatment-resistant illness on immune function are needed.
In conclusion, acutely ill and chronically ill subjects with schizophrenia, bipolar disorder and MDD manifested alterations in blood cytokine levels most consistent with an inflammatory profile and T-cell activation. In general, treatment of these disorders led to a decrease in cytokines with proinflammatory functions and an increase in cytokines with anti-inflammatory functions. Despite between-study heterogeneity, there were remarkable similarities in these patterns that suggest that there may be common mechanisms at least partially responsible for these findings. The small to moderate effect sizes observed most likely reflects that immune system involvement occurs in only a subset of patients with each of these syndromes. This may explain why treatment studies that have targeted inflammation in these syndromes have yielded mixed results. Future studies of anti-inflammatory therapies may want to include only patients who clearly manifest signs of inflammation. The results from this meta-analysis reflect a need to more systematically evaluate how and what we measure in the immune system. Longitudinal data with a uniform sample of patients could clarify what role the cytokine network has in mediating the course of illness for these disorders. More extensive and systematic investigation is clearly warranted.
Supplementary Material
Acknowledgments
In the past 12 months, Dr Goldsmith received grant funding from the Janssen Academic Research Mentoring program. Dr Goldsmith has also recieved training support from the National Institute of Mental Health (R25MH101079). In the past 12 months, Dr Rapaport is a member of the scientific advisory board for Pax (unpaid) and the Depression and Bipolar Alternative Therapies Foundation, and a consultant for the American Psychiatric Association. In the past 12 months, Dr Miller has received grant support from the National Institute of Mental Health (1K23MH098014-01) and the American Psychiatric Association; Research support from the National Institutes of Health Clinical Loan Repayment Program and Georgia Regents University; Honoraria from Psychiatric Times; and Speaker fees for lectures from the University of Nevada, Reno and Emory University.
Footnotes
Previous Presentation: This manuscript has been presented at the American College of Neuropsychopharmacology annual meeting, 7–11 December 2014, Phoenix, Arizona, the biennial International Congress on Schizophrenia Research, 28 March–1 April 2015, Colorado Springs, Colorado, the Society of Biological Psychiatry annual meeting, 14–16 May 2015, Toronto, Canada, and the American Society of Clinical Psychopharmacology annual meeting, 22–25 June 2015, Miami, FL, USA.
CONFLICT OF INTEREST
Dr Goldsmith has nothing to disclose relevant to the present work. Dr Rapaport has nothing to disclose relevant to the present work. Dr Miller has nothing to disclose relevant to present work.
AUTHOR CONTRIBUTIONS
Drs Goldsmith and Miller designed the study, managed the literature searches and the analyses. Drs Goldsmith, Rapaport and Miller wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia that overlaps with bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbosa IG, Machado-Vieira R, Soares JC, Teixeira AL. The immunology of bipolar disorder. Neuroimmunomodulation. 2014;21:117–122. doi: 10.1159/000356539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezeoke A, Mellor A, Buckley P, Miller BJ. A systematic quantitative review of blood autoantibody elevations in schizophrenia. Schizophr Res. 2013;150:245–251. doi: 10.1016/j.schres.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. 2013;8:900–920. doi: 10.1007/s11481-013-9462-8. [DOI] [PubMed] [Google Scholar]
- 7.Miller B, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73:993–999. doi: 10.1016/j.biopsych.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller N. Immunology of major depression. Neuroimmunomodulation. 2014;21:123–130. doi: 10.1159/000356540. [DOI] [PubMed] [Google Scholar]
- 9.Pearlman DM, Najjar S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res. 2014;157:249–258. doi: 10.1016/j.schres.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Ayorech Z, Tracy DK, Baumeister D, Giaroli G. Taking the fuel out of the fire: evidence for the use of anti-inflammatory agents in the treatment of bipolar disorders. J Affect Disord. 2015;174:467–478. doi: 10.1016/j.jad.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 12.Nitta M, Kishimoto T, Müller N, Weiser M, Davidson M, Kane JM, et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. 2013;39:1230–1241. doi: 10.1093/schbul/sbt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–191. doi: 10.1093/schbul/sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Ho RCM, Mak A. Interleukin (IL)-6, tumor necrosis factor alpha (TNF-α) and solubleinterleukin-2 receptors (sIL-2 R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Munkholm K, Vinberg M, Kessing LV. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2013;144:16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naïve first episode psychosis: a systematic review and meta-analysis. Schizophr Resh. 2014;155:101–108. doi: 10.1016/j.schres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingman WV, Robertson SA. The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev. 2009;20:233–239. doi: 10.1016/j.cytogfr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama K. Serum levels of soluble IL-2 receptor alpha, IL-6 and IL-1 receptor antagonist in schizophrenia before and during neuroleptic administration. Schizophr Res. 1999;37:97–106. doi: 10.1016/s0920-9964(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 25.Borovcanin M, Jovanovic I, Radosavljevic G, Dejanovic SD, Bankovic D, Arsenijevic N, et al. Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J Psychiatric Res. 2012;46:1421–1426. doi: 10.1016/j.jpsychires.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Borovcanin M, Jovanovic I, Radosavljevic G, Dejanovic SD, Stefanovic V, Arsenijevic N, et al. Antipsychotics can modulate the cytokine profile in schizophrenia: attenuation of the type-2 inflammatory response. Schizophr Res. 2013;147:103–109. doi: 10.1016/j.schres.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Chen S-L, Lee S-Y, Chang Y-H, Chen S-H, Chu C-H, Tzeng N-S, et al. Inflammation in patients with schizophrenia: the therapeutic benefits of risperidone plus add-on dextromethorphan. J Neuroimmun Pharmacol. 2012;7:656–664. doi: 10.1007/s11481-012-9382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crespo-Facorro B, Carrasco-Marín E, Pérez-Iglesias R, Pelayo-Terán JM, Fernandez-Prieto L, Leyva-Cobián F, et al. Interleukin-12 plasma levels in drug-naïve patients with a first episode of psychosis: effects of antipsychotic drugs. Psychiatry Res. 2008;158:206–216. doi: 10.1016/j.psychres.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 29.de Witte L, Tomasik J, Schwarz E, Guest PC, Rahmoune H, Kahn RS, et al. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res. 2014;154:23–29. doi: 10.1016/j.schres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Ding M, Song X, Zhao J, Gao J, Li X, Yang G, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Progr Neuro-Psychopharmacol Biol Psychiatry. 2014;51:78–82. doi: 10.1016/j.pnpbp.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, et al. Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun. 2013;31:90–95. doi: 10.1016/j.bbi.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJM, et al. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;14:746–755. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
- 33.Dunjic-Kostic B, Jasovic-Gasic M, Ivkovic M, Radonjic NV, Pantovic M, Damjanovic A, et al. Serum levels of interleukin-6 and tumor necrosis factor-alpha in exacerbation and remission phase of schizophrenia. Psychiatr Danub. 2013;25:55–61. [PubMed] [Google Scholar]
- 34.El Kissi Y, Samoud S, Mtiraoui A, Letaief, Hannachi N, Ayachi M, et al. Increased interleukin-17 and decreased BAFF serum levels in drug-free acute schizophrenia. Psychiatry Res. 2015;225:58–63. doi: 10.1016/j.psychres.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Egea E, Bernardo M, Donner T, Congent I, Parellada E, Justicia A, et al. Metabolic profile of antipsychotic-naïve individuals with nonaffective psychosis. Br J Psychiatry. 2009;194:434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Interleukin-6 (IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- 37.Ganguli R, Rabin BS. Increased serum interleukin 2 receptor concentration in schizophrenic and brain-damaged subjects. Arch Gen Psychiatry. 1989;46:292. doi: 10.1001/archpsyc.1989.01810030098018. [DOI] [PubMed] [Google Scholar]
- 38.Ganguli R, Yang Z, Shurin G, Chengappa KN, Brar JS, Gubbi AV, et al. Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry Res. 1994;51:1–10. doi: 10.1016/0165-1781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 39.Gattaz WF, Dalgalarrondo P, Schröder HC. Abnormalities in serum concentrations of interleukin-2, interferon-α and interferon-γ in schizophrenia not detected. Schizophr Res. 1992;6:237–241. doi: 10.1016/0920-9964(92)90006-q. [DOI] [PubMed] [Google Scholar]
- 40.Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kühn M, Schuld A, et al. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33:407–418. doi: 10.1016/s0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 41.Kalmady SV, Venkatasubramania G, Shivakumar V, Gautham S, Subramaniam A, Jose DA, et al. Relationship between interleukin-6 gene polymorphism and hippocampal volume in antipsychotic-naïve schizophrenia: evidence for differential susceptibility? PLoS One. 2014;9:e96021. doi: 10.1371/journal.pone.0096021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaminska T, Wysocka A, Marmurowska-Michalowska H, Dubas-Slemp H, Kandefer-Szerszen M. Investigation of serum cytokine levels and cytokine production in whole blood cultures of paranoid schizophrenic patients. Arch Immunol Ther Exp (Warsz) 2001;49:439–445. [PubMed] [Google Scholar]
- 43.Kim YK, Kim L, Lee MS. Relationships between interleukins, neurotransmitters and psychopathology in drug-free male schizophrenics. Schizophr Res. 2000;44:165–175. doi: 10.1016/s0920-9964(99)00171-1. [DOI] [PubMed] [Google Scholar]
- 44.Kim DJ, Kim W, Yoon SJ, Go HJ, Choi BM, Jun TY, et al. Effect of risperidone on serum cytokines. Int J Neurosci. 2001;111:11–19. doi: 10.3109/00207450108986549. [DOI] [PubMed] [Google Scholar]
- 45.Kim YK, Suh IB, Kim H, Han CS, Lim CS, Choi SH, et al. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: Effects of psychotropic drugs. Mol Psychiatry. 2002;7:1107–1114. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- 46.Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Progr Neuropsychopharmacol Biol Psychiatry. 2004;28:1129–1134. doi: 10.1016/j.pnpbp.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 47.Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication naïve and medication-free schizophrenic patients. Neuropsychobiology. 2009;59:123–129. doi: 10.1159/000213565. [DOI] [PubMed] [Google Scholar]
- 48.Kubistova A, Joracek J, Novak T. Increased interleukin-6 and tumor necrosis factor alpha in first episode schizophrenia patients versus healthy controls. Psychiatr Danub. 2012;24:153–156. [PubMed] [Google Scholar]
- 49.Lin CC, Chang MC, Chang PY, Huang TL. Increased interleukin-6 level in Taiwanese schizophrenic patients. Chang Gunng Med J. 2011;34:375–381. [PubMed] [Google Scholar]
- 50.Maes M, Bosmans E, Ranjan R, Vandoolaeghe E, Meltzer HY, De Ley M, et al. Lower plasma CC16, a natural anti-inflammatory protein, and increased plasma interleukin-1 receptor antagonist in schizophrenia: effects of antipsychotic drugs. Schizophr Res. 1996;21:39–50. doi: 10.1016/0920-9964(96)00029-1. [DOI] [PubMed] [Google Scholar]
- 51.Müller N, Empl M, Riedel M, Schwarz M, Ackenheil M. Neuroleptic treatment increases soluble IL-2 receptors and decreases soluble IL-6 receptors in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1997;247:308–313. doi: 10.1007/BF02922260. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien SM, Scully P, Dinan TG. Increased tumor necrosis factor alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160:256–262. doi: 10.1016/j.psychres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Pae CU, Yoon CH, Kim TS, Kim JJ, Park SH, Lee CU, et al. Antipsychotic treatment may alter T-helper (TH) 2 arm cytokines. Int Immunopharmacol. 2006;6:666–671. doi: 10.1016/j.intimp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Rapaport MH, Lohr JB. Serum-soluble interleukin-2 receptors in neuroleptic-naïve schizophrenic subjects and in medicated schizophrenic subjects with and without tardive dyskinesia. Acta Psychiatr Scand. 1994;90:311–315. doi: 10.1111/j.1600-0447.1994.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz MJ, Riedel M, Gruber R, Muller N, Ackenheil M. Autoantibodies against 60-kDa heat shock protein in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1998;248:282–288. doi: 10.1007/s004060050051. [DOI] [PubMed] [Google Scholar]
- 56.Sirota P, Meiman M, Herschko R, Bessler H. Effect of neuroleptic administration on serum levels of soluble IL-2 receptor-alpha and IL-1 receptor antagonist in schizophrenic patients. Psychiatry Res. 2005;134:151–159. doi: 10.1016/j.psychres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65:481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 58.Song X, Fan X, Song X, Zhang J, Zhang W, Li X, et al. Elevated levels of adiponectin and other cytokines in drug naïve first episode schizophrenia patients with normal weight. Schizophr Res. 2013;150:269–273. doi: 10.1016/j.schres.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 59.Song X, Fan X, Li X, Zhang W, Gao J, Zhao J, et al. Changes in pro-inflammatory cytokines and body weight during 6-montth risperidone treatment in drug naïve first-episode schizophrenia. Psychopharmacology. 2014;231:319–325. doi: 10.1007/s00213-013-3382-4. [DOI] [PubMed] [Google Scholar]
- 60.Theodoropoulou S, Spanakos G, Baxevanis CN, Economou M, Gritzapis AD, Papamichail MP, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47:13–25. doi: 10.1016/s0920-9964(00)00007-4. [DOI] [PubMed] [Google Scholar]
- 61.Xiu MH, Yang GG, Tan YL, Chen DC, Tan SP, Wag ZR, et al. Decreased interleukin-10 serum levels in first-episode drug-naïve schizophrenia: relationship to psychopathology. Schizophr Res. 2014;156:9–14. doi: 10.1016/j.schres.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 62.Xiu MH, Chen DC, Wang D, Zhang K, Dong A, Tang W, et al. Elevated interleukin-18 serum levels in chronic schizophrenia: association with psychopathology. J Psychiatr Res. 2012;46:1093–1098. doi: 10.1016/j.jpsychires.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 63.Zhang XY, Tang W, Xiu MH, Chen DC, Yang FD, Tan YL, et al. Interleukin-18 and cognitive impairment in first episode and drug naïve schizophrenia versus healthy controls. Brain Behav Immun. 2013;32:105–111. doi: 10.1016/j.bbi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PVS, Kauer-Sant’Anna M, Flamt F, et al. Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res. 2011;45:156–161. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 65.Kim YK, Myint AM, Lee BH, Han CS, Lee SW, Leonard BE, et al. T-helper types 1, 2, and 3 cytokine interactions in symptomatic manic patients. Psychiatry Res. 2004;129:267–272. doi: 10.1016/j.psychres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Li H, Hong W, Zhang C, Wu Z, Wang Z, Yuan C, et al. IL-23 and TGF-β1 levels as potential predictive biomarkers in treatment of bipolar I disorder with acute manic episode. J Affect Disord. 2015;174:361–366. doi: 10.1016/j.jad.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 67.Liu HC, Yang YY, Chou YM, Chen KP, Shen WW, Leu SJ. Immunological variables in acute mania of bipolar disorder. J Neuroimmunol. 2004;150:116–122. doi: 10.1016/j.jneuroim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Munkholm K, Weikop P, Kessing LV, Vinberg M. Elevated levels of IL-6 and IL-18 in manic hypomanic states in rapid cycling bipolar disorder patients. Brain Behav Immun. 2014;43:205–213. doi: 10.1016/j.bbi.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 69.O’Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J Affect Disord. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Tsai SY, Chen KP, Yang YY, Chen CC, Lee JC, Singh VK, et al. Activation of indices of cell-mediated immunity in bipolar mania. Biol Psychiatry. 1999;45:989–994. doi: 10.1016/s0006-3223(98)00159-0. [DOI] [PubMed] [Google Scholar]
- 71.Tsai SY, Yang YY, Kuo CJ, Chen CC, Leu SJC. Effects of symptomatic severity on elevation of plasma soluble interleukin-2 receptor in bipolar mania. J Affect Disord. 2001;64:185–193. doi: 10.1016/s0165-0327(00)00252-4. [DOI] [PubMed] [Google Scholar]
- 72.Tsai SY, Lee HC, Chen CC, Lee CH. Plasma levels of soluble transferrin receptors and clara cell protein (CC16) during bipolar mania and subsequent remission. J Psychiatr Res. 2003;37:229–235. doi: 10.1016/s0022-3956(02)00103-6. [DOI] [PubMed] [Google Scholar]
- 73.Tsai SY, Chung KH, Wu JY, Kuo CJ, Lee HC, Huang SH. Inflammatory markers and their relationships with leptin and insulin from acute mania to full remission in bipolar disorder. J Affect Disord. 2012;136:110–116. doi: 10.1016/j.jad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Basterzi AD, Avdemir C, Kisa C, Aksaray S, Tuzer V, Yazici K, et al. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum Psychopharmacol. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- 75.Berk M, Wadee AA, Kuschke RH, O’Neill-Kerr A. Acute phase proteins in major depression. J Psychosomat Res. 1997;43:529–534. doi: 10.1016/s0022-3999(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 76.Dome P, Halmai Z, Dobos J, Lazary J, Gonda X, Kenessey I, et al. Investigation of circulating endothelial progenitor cells and angiogenic and inflammatory cytokines during recovery from an episode of major depression. J Affect Disord. 2012;136:1159–1163. doi: 10.1016/j.jad.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 77.Eller T, Vaasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 78.Fornaro M, Rocchi G, Escelsior A, Contini P, Martino M. Might different cytokine trends in depressed patients receiving duloxetine indicate differential biological backgrounds. J Affect Disord. 2013;145:300–307. doi: 10.1016/j.jad.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Hernandez ME, Mendieta D, Martinez-Fong D, Loria F, Moreno J, Estrada I, et al. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. Eur Neuropsychopharmacol. 2008;18:917–924. doi: 10.1016/j.euroneuro.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Huang TL, Lee CT. T-helper 1/T-helper 2 cytokine imbalance and clinical phenotypes of acute-phase major depression. Psychiatry Clin Neurosci. 2007;61:415–420. doi: 10.1111/j.1440-1819.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- 81.Kagaya A, Kugaya A, Takebayashi M, Fukue-Saeki M, Saeki T, Yamawaki S, et al. Plasma concentrations on interleukin-1beta, interleukin-6, soluble interleukin-2 receptor and tumor necrosis factor alpha of depressed patients in Japan. Neuropsychobiology. 2001;43:59–62. doi: 10.1159/000054867. [DOI] [PubMed] [Google Scholar]
- 82.Kubera M, Kenis G, Bosans E, Zieba A, Dudek D, Mowak G, et al. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Polish J Pharmacol. 2000;52:237–241. [PubMed] [Google Scholar]
- 83.Lee K-M, Kim Y-K. The role of IL-12 and TGF-β1 in the pathophysiology of major depressive disorder. Int Immunopharmacol. 2006;6:1298–1304. doi: 10.1016/j.intimp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Leo R, DiLorenzo G, Tesauro M, Razzini C, Forleo GB, Chiricolo G, et al. Association between enchanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J Clin Psychiatry. 2006;67:1760–1766. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- 85.Maes M, Meltzer HY, Bosmans E, Bergmans R, Vadoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 86.Maes M, Bosmans E, De Jongh R, Kenis G, Vadoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 87.Mikova O, Yakimova R, Bosmans, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001;11:203–208. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 88.Sluzewska A, Rybakowski J, Bostmans E, Sobieska M, Berghmans R, Maes M, et al. Indictors of immune activation in major depression. Psychiatry Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 89.Su SC, Sun MT, Wen MJ, Lin CH, Chen YC, Hung YJ. Brain-derived neurotrophic factor, adiponectin, and proinflammatory markers in various subtypes of depression in young men. Int J Psychiatry Med. 2011;42:211–226. doi: 10.2190/PM.42.3.a. [DOI] [PubMed] [Google Scholar]
- 90.Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Asmari AK, Khan MW. Inflammation and schizophrenia: Alterations in cytokine levels and perturbation in antioxidative defense systems. Hum Exp Toxicol. 2014;33:115–122. doi: 10.1177/0960327113493305. [DOI] [PubMed] [Google Scholar]
- 92.Asevedo E, Rizzo LB, Gadelha A, Mansur R, Ota VK, Berberian AA, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194–198. doi: 10.1016/j.physbeh.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 93.Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–294. doi: 10.1177/0269881108089583. [DOI] [PubMed] [Google Scholar]
- 94.Beumer W, Drexhage RC, De Wit H, Versnel MA, Drexhage HA, Cohen D. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychneuroendocrinology. 2012;37:1901–1911. doi: 10.1016/j.psyneuen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Bresee C, Rapaport MH. Persistently increased serum soluble interleukin-2 receptors in continuously ill patients with schizophrenia. Int J Neuropsychopharmacol. 2009;12:861–865. doi: 10.1017/S1461145709000315. [DOI] [PubMed] [Google Scholar]
- 96.Chiang SSW, Ridel M, Schwarz M, Muller N. Is T-helper type 2 shift schizophrenia-specific? Primary results from a comparison of related psychiatric disorders and healthy controls. Psychiatry Clin Neurosci. 2013;67:228–236. doi: 10.1111/pcn.12040. [DOI] [PubMed] [Google Scholar]
- 97.Drexhage RC, Padmos RC, de Wit H, Versnel MA, Hooijkaas H, van der Lely AJ, et al. Patients with schizophrenia show raised serum levels of the proinflammatory chemokine CCL2: association with the metabolic syndrome in patients? Schizophr Res. 2008;102:352–355. doi: 10.1016/j.schres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 98.Francesconi LP, Cereser KM, Mascarenhas, Stertz L, Gama CS, Belmonte-de-Abreu P. Increased annexin-V and decreased TNF-alpha serum levels in chronic-medicated patients with schizophrenia. Neurosci Lett. 2011;502:143–146. doi: 10.1016/j.neulet.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 99.Kuo F-C, Lee C-H, Hsieh C-H, Kuo P, Chen Y-C, Hung Y-J. Lifestyle modification and behavior therapy effectively reduce body weight and increase serum level of brain-derived neurotrophic factor in obsess non-diabetic patients with schizophrenia. Psychiatry Res. 2013;209:150–154. doi: 10.1016/j.psychres.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 100.Liu H, Kang Y, Liang J, Li C, Xiu M, Chen D, et al. Lower serum interleukin-2 levels in schizophrenic patients with tardive dyskinesia. Psychiatry Res. 2012;198:329–331. doi: 10.1016/j.psychres.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 101.Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JCP, et al. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res. 2012;46:819–824. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 102.Sasayama D, Wakabayash C, Hori H, Teraishi T, Hattori K, Ota M, et al. Association of plasma IL-6 and soluble IL-6 receptor levels with the Asp358Ala polymorphism of the IL-6 receptor gene in schizophrenic patients. J Psychiatr Res. 2011;45:1439–1444. doi: 10.1016/j.jpsychires.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 103.Sasayama D, Hattori K, Wakabayash C, Teraishi T, Hori H, Ota M, et al. Increased cerebrospinal fluid inteleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47:401–406. doi: 10.1016/j.jpsychires.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Singh B, Bera NK, Nayak CR, Chaudhuri TK. Decreased serum levels of interleukin-2 and interleukin-6 in Indian Bengalee schizophrenic patients. Cytokine. 2009;47:1–5. doi: 10.1016/j.cyto.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Stojanovic A, Martorell, Montalvo I, Ortega L, Monseny R, Vilella E, et al. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychneuroendocrinology. 2014;41:23–32. doi: 10.1016/j.psyneuen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Suvisaari J, Loo BM, Saarni SE, Haukka J, Perala J, Saarni SI, et al. Inflammation in psychotic disorders: a population-based study. Psychiatry Res. 2011;189:305–311. doi: 10.1016/j.psychres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 107.Dimitrov DH, Lee S, Yantis J, Valdez C, Paredes RM, Braida N, et al. Differential correlations between inflammatory cytokine sand psychopathology in veterans with schizophrenia: potential role for Il-17 pathway. Schizohpr Res. 2013;151:29–35. doi: 10.1016/j.schres.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 108.Noto C, Gadelha A, Belangero SI, Spindola LM, Rocha NP, de Miranda AS, et al. Circulating levels of sTNFR1 as a marker of severe clinical course in schizophrenia. J Psychiatr Res. 2013;47:467–471. doi: 10.1016/j.jpsychires.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 109.Bai YM, Su TP, Tsai SJ, Wen-Fei C, Li CT, Pei-Chi T, et al. Comparison of inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. J Affect Disord. 2014;166:187–192. doi: 10.1016/j.jad.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 110.Barbosa IG, Rocha NP, de Miranda AS, Magalhaes PV, Huguet RB, de Souza LP, et al. Increased levels of adipokines in bipolar. J Psychiatr Res. 2012;46:389–393. doi: 10.1016/j.jpsychires.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Barbosa IG, Nogueira CR, Rocha NP, Queiroz AL, Vago JP, Tavares LP, et al. Altered intracellular signaling cascades in peripheral blood mononuclear celles from BD patients. J Psychiatr Res. 2013;47:1949–1954. doi: 10.1016/j.jpsychires.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 112.Brietzke E, Kauer-Sant-Anna M, Teixeira AL, Kapczinski F. Abnormalities in serum chemokine levels in euthymic patients with bipolar disorder. Brain Behav Immun. 2009;23:1079–1082. doi: 10.1016/j.bbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 113.Brietzke E, Teixeira AL. Similar immune profile in bipolar disorder and schizophrenia, selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. 2010;12:453–454. doi: 10.1111/j.1399-5618.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 114.Cetin T, Guloksuz S, Cetin EA, Gazioglu SB, Deniz G, Oral ET, et al. Plasma concentrations of soluble cytokine receptors in euthymic bipolar paitents with and without subsyndromal symptoms. BMC Psychiatry. 2012;12:158. doi: 10.1186/1471-244X-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doganavsargil-Baysal O, Cinemre B, Aksoy UM, Akbas H, Metin O, Fettahoglu C, et al. Levels of TNF-α, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Hum Psychopharmacol. 2013;28:160–167. doi: 10.1002/hup.2301. [DOI] [PubMed] [Google Scholar]
- 116.Guloksuz S, Cetin EA, Cetin T, Geniz G, Oral ET, Nutt DJ. Cytokine levels in euthymic bipolar patients. J Affect Disord. 2010;126:458–462. doi: 10.1016/j.jad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 117.Hope S, Dieset I, Agartz I, Steen NE, Ueland T, Melle I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45:1608–1616. doi: 10.1016/j.jpsychires.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 118.Hsu JW, Lirng JF, Wang SJ, Lin CL, Yang KC, Liao MH, et al. Association of thalamic serotonin transporter and interleukin-10 in bipolar I disorder: a SPECT study. Bipolar Disord. 2014;16:241–248. doi: 10.1111/bdi.12164. [DOI] [PubMed] [Google Scholar]
- 119.Lotrich FE, Butters MA, Aizenstein H, Marron MM, Reynolds CF, Gildengers AG. The relationship between interleukin-1 receptor antagonist and cognitive function in older adults with bipolar disorder. Int J Geriatr Psychiatry. 2014;29:635–644. doi: 10.1002/gps.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mota R, Gazal M, Acosta BA, de Leon PB, Jansen K, Pinheiro RT, et al. Interleukin-1β is associated with depressive episode in major depression but not in bipolar disorder. J Psychaitr Res. 2013;47:2011–2014. doi: 10.1016/j.jpsychires.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 121.Rapaport MH. Immune parameters in euthymic bipolar patients and normal volunteers. J Affect Disord. 1994;32:149–156. doi: 10.1016/0165-0327(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 122.Remlinger-Molenda A, Wojciak P, Michalak M, Karczewski J, Rybakowski JK. Selected cytokine profiles during remission in bipolar patients. Neuropsychobiology. 2012;66:193–198. doi: 10.1159/000339949. [DOI] [PubMed] [Google Scholar]
- 123.Baek D, Park Y. Association between erythrocyte n-3 polyunsaturated fatty acids and biomarkers of inflammation and oxidative stress in patients with and without depression. Prostaglandins Leukot Essent Fatty Acids. 2013;89:291–296. doi: 10.1016/j.plefa.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 124.Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, et al. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatric Res. 2009;43:962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 125.Diniz BS, Teixeira AL, Talib L, Gattaz WF, Forlenza OV. Interleukin-1β serum levels is increased in antidepressant-free elderly depressed patients. Am J Geriatr Psychiatry. 2010;18:172–176. doi: 10.1097/JGP.0b013e3181c2947f. [DOI] [PubMed] [Google Scholar]
- 126.Einvik G, Vistnes M, Hrubos-Strom H, Randby A, Namtvedt SK, Nordhus IH, et al. Circulating cytokine concentrations are no associated with major depressive disorder in a community-based cohort. Gen Hosp Psychiatry. 2012;34:262–267. doi: 10.1016/j.genhosppsych.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 127.Eller T, Aluoja A, Maron E, Vasar V. Soluble interleukin-2 receptor and tumor necrosis factor levels in depressed patients in Estonia. Medicina (Kaunas) 2009;45:971–977. [PubMed] [Google Scholar]
- 128.Maes M, Ringel K, Kubera M, Berk M, Rybakowski J. Increased autoimmune activity against 5-HT: a key component of depression that is associated with inflammation and activation of cell-mediated immunity, and with severity and staging of depression. J Affect Disord. 2012;136:386–392. doi: 10.1016/j.jad.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 129.Motovala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 130.O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatric Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 131.Pavon L, Sandoval-Lopez G, Hernandez ME, Loria F, Estrada I, Perez M, et al. Th2 cytokine response in major depressive disorder patients before treatment. J Neuroimmunol. 2006;172:156–165. doi: 10.1016/j.jneuroim.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 132.Pike JL, Irwin MR. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain Behav Immun. 2006;20:169–174. doi: 10.1016/j.bbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 133.Simon NM, McNamara K, Chow CW, Maser RS, Papkostas GI, Pollack MH, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoshimura R, Umene-Nakano W, Hoshuyama T, Ikenouchi-Sugita A, Hori K, Katsuki A, et al. Plasma levels of brain-derived neurotrophic factor and interleukin-6 in patients with dysthymic disorder: comparison with age- and sexmatched major depressed patients and healthy controls. Hum Psychopharmacol. 2010;25:566–569. doi: 10.1002/hup.1155. [DOI] [PubMed] [Google Scholar]
- 135.Hernandez AV, Guarnizo M, Miranda Y, et al. Association between insulin resistance and breast carcinoma: a systematic review and meta-analysis. PLoS One. 2014;9:e99317. doi: 10.1371/journal.pone.0099317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Higgins J. Cochrane Handbook for Systematic Reviews of Interventions Version 510. The Cochrane Collaboration; [updated March 2011]. 2011. Deeks J Selecting studies and collecting data. Available at: www.cochrane-handbook.org. [Google Scholar]
- 137.Cochran WB. The comparison of percentages in matched samples. Biometrika. 1950;37:256–266. [PubMed] [Google Scholar]
- 138.Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24:126–151. doi: 10.1177/016327870102400203. [DOI] [PubMed] [Google Scholar]
- 139.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; [updated March 2011]. 2011. 9.7. Sensitivity analyses. Available at: www.cochrane-handbook.org. [Google Scholar]
- 140.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 141.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akira S, Kishimoto T. NF-IL6 and NF-kappa B in cytokine gene regulation. Adv Immunol. 1997;65:1–46. [PubMed] [Google Scholar]
- 143.Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann NY Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thomas R. The TRAF6-NF kappa B signaling pathway in autoimmunity: not just inflammation. Arthritis Res Ther. 2005;7:170–173. doi: 10.1186/ar1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 2013;38:2676–2685. doi: 10.1016/j.psyneuen.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2012;31:264–268. doi: 10.1037/a0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 148.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 149.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroendocrine substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Bran Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 150.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 151.Piccioli P, Rubartelli A. The secretion of IL-1Β and options for release. Semin Immunol. 2013;25:425–429. doi: 10.1016/j.smim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 152.Caruso C, Candore G, Cigna D, Colucci AT, Modica MA. Biological significance of soluble IL-2 receptor. Mediat Inflamm. 1993;2:3–21. doi: 10.1155/S0962935193000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.De Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keustermans GC, Hoeks SM, Meerding JM, Prakken BJ, de Jager W. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods. 2013;61:10–17. doi: 10.1016/j.ymeth.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 155.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kechel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A. 2008;63:879–888. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Berardis D, Conti CM, Serroni N, Moschetta FS, Olivieri L, Carano A, et al. The effect of newer serotonin–noradrenalin antidepressants on cytokine production: a review of the current literature. Int J Immunopathol Pharmacol. 2010;23:417–422. doi: 10.1177/039463201002300204. [DOI] [PubMed] [Google Scholar]
- 158.Młodzikowska-Albrecht J, Steinborn B, Zarowski M. Cytokines, epilepsy and epileptic drugs—is there a mutual influence? Pharmacol Rep. 2007;59:129–138. [PubMed] [Google Scholar]
- 159.Raghavendra PB, Lee E, Parameswaran N. Regulation of macrophage biology by lithium: a new look at an old drug. J Neuroimmune Pharmacol. 2014;9:277–284. doi: 10.1007/s11481-013-9516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tourjman V, Kouassi É, Koué MÈ, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, et al. Antipsychotics' effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151:43–47. doi: 10.1016/j.schres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 161.Klemettilia JP, Kampman O, Seppala N, Viikki M, Hamalainen M, Moilanen E, et al. Cytokine and adipokine alterations in patients with schizophrenia treated with clozapine. Psychiatry Res. 2014;218:277–283. doi: 10.1016/j.psychres.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 162.Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura GJ, Piolo R, Boin F, et al. Increased serum interleukin-8 and interleukin-10 in schizophrenic patients resistant to treatment with neuroleptics and the stimulatory effects of clozapine on serum leukemia inhibitory factor receptor. Schizophr Res. 2002;54:281–291. doi: 10.1016/s0920-9964(00)00094-3. [DOI] [PubMed] [Google Scholar]
- 163.Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32:9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.