Summary
Chronic HIV-1 infection is associated with lower frequencies and functional impairment of mucosa-associated invariant T (MAIT) cells. We evaluated IL-7 treatment to restore MAIT cells in peripheral blood of chronically HIV-1 infected individuals on ART. IL-7 led to increased relative and absolute levels of MAIT cells and this expansion occurred primarily in the CD8+ subset. These results suggest that IL-7 may represent a therapeutic intervention for the restoration of MAIT cells in chronic HIV-1 infection.
Mucosa-associated Invariant T (MAIT) cells represent a subset of unconventional, innate-like T cells that are abundant in healthy individuals, constituting 1 to 10% of peripheral blood T cells[1] and are present in mucosa, liver, and mesenteric lymph nodes.[2] MAIT cells are characterized by the expression of a semi-invariant Vα7.2-carrying T cell receptor (TCR), IL-18Rα, the promyelocytic leukemia zinc finger protein (PLZF),[1] and high levels of CD161 and the alpha chain of the IL-7 receptor (IL-7R).[2]
The majority of MAIT cells are either CD8αα or CD8αβ, with smaller CD4/8 double-negative (DN) and CD4+ populations.[1] MAIT cells recognize microbial vitamin B2 biosynthetic intermediates presented by the nonclassical MHC-1–related protein 1 (MR1),[3] and respond to such antigens with production of proinflammatory cytokines[1] and cellular cytotoxicity[2, 3]. During chronic HIV-1 infection, there is numerical and functional loss of MAIT cells that persists despite long-term antiretroviral therapy (ART).[2, 4] Approaches to restore MAIT cells may improve mucosal and anti-bacterial immunity in HIV-infected people.
IL-7 is required for the development and homeostasis of T cells.[5] In HIV-infected patients, IL-7 administration induces T cell expansions in peripheral blood and gut mucosa.[6] Notably, IL-7 dramatically enhances the function of MAIT cells from both healthy individuals and HIV-infected patients in vitro.[2] We hypothesized that in vivo administration of IL-7 in chronically HIV-1 infected patients on ART may overcome the inability of long-term ART alone to restore MAIT cells. To address this hypothesis, we evaluated the numbers, phenotype, and functionality of MAIT cells before and after IL-7 administration.
HIV-1 infected patients with CD4 counts of 101–400 cells/µL, plasma HIV RNA < 50 copies/mL who were on ART for at least 12 months were enrolled in a single arm, open-label, multicenter study (NCT01190111) approved by the study sites Ethics committees. HIV-uninfected individuals (n=7) were recruited at the blood bank of the NIH. All study participants signed informed consent. Patients were treated with three subcutaneous injections of IL-7 (CYT107) at a dose of 10 mg/kg each. PBMCs were collected at baseline, before IL-7 administration, and at week 12 post-IL-7. The details of the clinical trial have been previously published.[7, 8] Seven HIV-infected men with a median age of 55 years (range 43–58 years), median CD4 T-lymphocyte count of 270 cells/µL (range 135–364 cells/µL) and plasma HIV RNA < 50 copies/mL were included in this study based on sample availability. Cryopreserved PBMCs were used for immunophenotyping. The functional assays for activation of MAIT cells in the PBMC mixture with IL-12 and IL-18 or E. coli (ratio E. coli CFU:PBMC of 3) were performed for 24 hours as previously described.[2] The antibodies used were: anti-CD4 APC-Cy7, anti-CD3 AmCyan and Alexa Fluor (AF) 700, anti-CD8 Pacific Blue and Brilliant Violet (BV) 711, anti-CD161 PECy5, anti-TNF BV650 from BD Biosciences; anti-TCRVα7.2 PerCPCy5.5 and PECy7, anti-CD4 BV711, anti-CD8 BV570, anti-CD161 BV605, anti-GzmB FITC, anti-IFNγ BV785, and anti-perforin (Prf) BV421 from Biolegend; anti-CD69 PE-Texas Red, LIVE/DEAD Fixable Blue Dead Cells Stain (Thermo Fisher). Stained cells were fixed in Cytofix/Cytoperm or in Transcription Factor Fixation/Permeabilization buffer (BD Biosciences and eBioscience, respectively).
Samples were acquired on an LSRII flow cytometer (BD Biosciences). Software-based compensation was performed using the compensation platform in FlowJo software versions 9.9 and 10.0.8 (TreeStar).
The Wilcoxon signed-rank test was used to assess significance. Statistical analyses were performed using Prism version 7.0a, and two-sided p-values < 0.05 were considered significant.
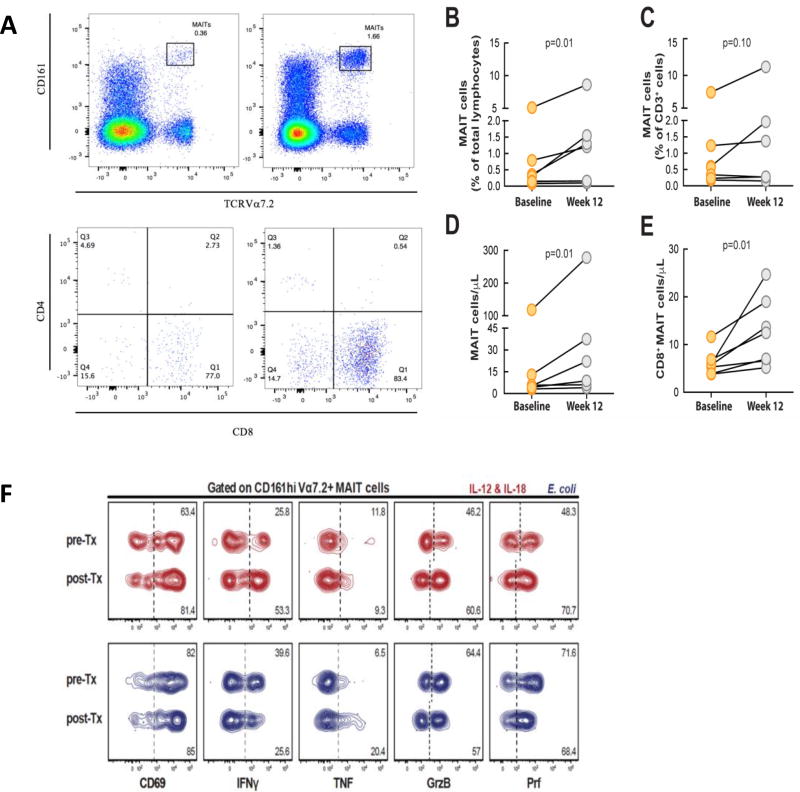
The frequency of MAIT cells was higher in HIV-negative controls compared to the chronically HIV-infected subjects (median (IQR) = 1% (0.87–5.12) vs. 0.5% (0.2–1.2); p = 0.07), consistent with previous observations[4], however the difference did not reach statistical significance. We evaluated the percentage and absolute number of MAIT cells from baseline to week 12 post-IL-7 treatment (Fig. 1A). The percentage of MAIT cells among total lymphocytes increased significantly (median (IQR) = 1.2% (0.1–1.5) vs. 0.3% (0.1–0.7), p = 0.01) (Fig. 1B). Since IL-7 expands overall T cells in HIV-infected persons[9], we examined changes in the percentage of MAIT cells within the T cell compartment. The proportion of MAIT cells within the CD3+ cell population trended towards an increase, although the change did not reach statistical significance (median (IQR) = 1.3% (0.27–2.12) vs. 0.5% (0.2–1.2), p = 0.1) (Fig. 1C). However, the absolute number of MAIT cells increased significantly at 12 weeks post-IL-7 administration compared to baseline (median (IQR) 22 (6–75.9) cells/µL vs. 5.2 (4.2–12.9) cells/µL, p = 0.01) (Fig. 1D). Furthermore, the absolute number of CD8+ MAIT cells increased after IL-7 treatment (median (IQR) 20.5 (4–33.5) cells/µL vs. 4 (2–11), p = 0.01) (Fig. 1E).
Figure 1. MAIT cell characterization and changes in the MAIT cell population after treatment with IL-7.
(A) MAIT cells identified by Vα7.2 and CD161 staining and flow cytometry. Expression of CD8 and CD4 in MAIT cells. (B) Percentage of MAIT cells among total lymphocytes increased (p = 0.01) after treatment with IL-7. (C) There was no significant increase in the percentage of MAIT cells within the total CD3+ cells. (D) The absolute number of MAIT cells increased significantly after IL-7 treatment (p = 0.01). (E) There was an increase in the absolute number of CD8+ MAIT cells after IL-7 treatment. (F) Function of MAIT cells in a HIV-1 infected patient before and after rhIL-7 treatment after stimulation with E. coli.
It was previously reported that MAIT cells have reduced expression of transcription factors T-bet and EOMES in chronically HIV-infected individuals, and this was associated with loss of MAIT cells in the periphery and impaired effector function.[2] In the same study, addition of IL-7 to MAIT cells in vitro enhanced their effector responses to bacterial stimulation.[2] Due to limited sample availability, we were only able to test responses to E. coli stimulation in one patient, and MAIT cells in this patient maintained responsiveness and effector function after IL-7 treatment (Fig. 1F). Although we did not study MAIT cells in mucosal samples, this will be of interest for future studies to evaluate the potential significance of increased MAIT cells in mucosal immunity.
In conclusion, we found a significant increase in the frequency and number of MAIT cells in the periphery of chronically HIV-infected individuals 12 weeks post-IL-7 treatment. This is striking given that no significant restoration of MAIT cells has been seen during long-term ART.[4] IL-7 treatment thus represents a potential therapeutic strategy to reconstitute the number of MAIT cells in chronically HIV-infected individuals. Rescue of these cells may improve immune reconstitution in HIV-infected individuals and could potentially result in a reduced risk of microbial co-infections. Future larger studies could better determine if this immunotherapy can restore both the frequency and functionality of MAIT cells in disease settings.
Acknowledgments
Financial support: This research was supported by grants to JKS from the Swedish Research Council (Dnr 2016-03052), the Swedish Cancer Society (CAN 2014/879), and the US National Institutes of Health (R01DK108350). JD was supported by Fundação para a Ciência e a Tecnologia Doctoral Fellowship SFRH/BD/85290/2012. EL is supported by grants from the Jonas Söderquist Foundation for Virology and Immunology, Swedish Research Council Grant 2015-00174, and Marie Skłodowska Curie Actions, Cofund, Project INCA 600398. It was also suppoted by the intramural research program of NIAID, NIH. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Footnotes
Disclaimer: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Authorship: Contribution: O.S. and E.R. designed and performed phenotyping experiments, analyzed the data and wrote the manuscript. J.D. designed and performed functional experiments and analyzed the data. I.S., J.K.S. and E.L. conceived and designed research. I.S. and J.K.S. supervised research. All authors contributed to manuscript writing.
Potential conflicts of interest: The authors declare no competing financial interests.
References
- 1.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 2.Leeansyah E, Svard J, Dias J, Buggert M, Nystrom J, Quigley MF, et al. Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection. PLoS Pathog. 2015;11(8):e1005072. doi: 10.1371/journal.ppat.1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 4.Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121(7):1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasson SC, Zaunders JJ, Kelleher AD. The IL-7/IL-7 receptor axis: understanding its central role in T-cell homeostasis and the challenges facing its utilization as a novel therapy. Curr Drug Targets. 2006;7(12):1571–1582. doi: 10.2174/138945006779025365. [DOI] [PubMed] [Google Scholar]
- 6.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113(25):6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10(1):e1003890. doi: 10.1371/journal.ppat.1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55(2):291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119(4):997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]