SUMMARY
Pneumocystis, a unique atypical fungus with an elusive lifestyle, has had an important medical history. It came to prominence as an opportunistic pathogen that not only can cause life-threatening pneumonia in patients with HIV infection and other immunodeficiencies but also can colonize the lungs of healthy individuals from a very early age. The genus Pneumocystis includes a group of closely related but heterogeneous organisms that have a worldwide distribution, have been detected in multiple mammalian species, are highly host species specific, inhabit the lungs almost exclusively, and have never convincingly been cultured in vitro, making Pneumocystis a fascinating but difficult-to-study organism. Improved molecular biologic methodologies have opened a new window into the biology and epidemiology of Pneumocystis. Advances include an improved taxonomic classification, identification of an extremely reduced genome and concomitant inability to metabolize and grow independent of the host lungs, insights into its transmission mode, recognition of its widespread colonization in both immunocompetent and immunodeficient hosts, and utilization of strain variation to study drug resistance, epidemiology, and outbreaks of infection among transplant patients. This review summarizes these advances and also identifies some major questions and challenges that need to be addressed to better understand Pneumocystis biology and its relevance to clinical care.
KEYWORDS: Pneumocystis, molecular biology, epidemiology, genome features, strain variation, transmission
INTRODUCTION
Pneumocystis is a ubiquitous unicellular fungus that is distributed worldwide. Initially believed to be a single protozoan species infecting a broad range of mammalian hosts, it has now been recognized as a genus of fungi comprising a group of highly diversified species with an apparently strict host species specificity. The species that infects humans is Pneumocystis jirovecii. In immunosuppressed individuals, especially those with untreated human immunodeficiency virus (HIV) infection, Pneumocystis can cause a severe, potentially fatal pneumonia, commonly known as Pneumocystis pneumonia (PCP, the preferred acronym in the Pneumocystis research community) and also occasionally referred to as P. jirovecii pneumonia (PJP) and pneumocystosis. Immunocompetent individuals can be infected by P. jirovecii, but this infection is usually asymptomatic or manifested only as a mild respiratory infection. In fact, based on serologic and PCR assays, most infants appear to have been infected by 1 year of age (1, 2).
Pneumocystis was first recognized in the mid-20th century as an important human pathogen causing epidemics of interstitial pneumonia in premature infants and malnourished children in Europe (3, 4). During the 1960s to 1970s, PCP was reported with increasing frequency for immunocompromised patients, especially cancer patients, as intensive chemotherapeutic or anti-inflammatory regimens were initially being developed. However, it was in the early 1980s that Pneumocystis rose to national prominence, when the occurrence of PCP (as well as Kaposi's sarcoma) in previously healthy young men led to the initial recognition of the AIDS epidemic (5). Prior to the widespread utilization of combination antiretroviral therapy (cART), there were >20,000 new cases of PCP per year in the United States alone (6). Although there have been dramatic declines in its incidence following the introduction of cART, PCP remains one of the most common and serious opportunistic infections in the HIV/AIDS population (7). PCP cases now occur primarily in persons who are unaware of their HIV infection or are not receiving cART therapy or PCP prophylaxis (8–10) and who have advanced immunosuppression, with CD4 counts of <100 cells/μl (11). Recently, the incidence of PCP has been growing in patients without HIV infection as a result of more widespread use of potent immunosuppressive agents, including various immunodepleting monoclonal antibodies (12, 13), and greater utilization of organ transplantation; multiple outbreaks have been reported for transplant patients, especially renal transplant patients, in the past 2 decades (14, 15). Increasingly, Pneumocystis colonization is also being identified in patients with a variety of pulmonary conditions, and it may be contributing to more rapid declines in pulmonary function (16).
Symptoms of PCP are nonspecific and include fever, nonproductive cough, shortness of breath, chest pain, and fatigue. Physical examination also gives nonspecific findings, and pulmonary auscultation is usually normal. Chest radiographs usually display diffuse interstitial or alveolar infiltrates but are normal in some cases. Note that PCP can present with a broad range of radiographic patterns, including asymmetrical or lobar infiltrates, nodules, cavities, pleural effusions, and pneumothorax (17). Arterial blood gases may show hypoxemia that can be brought on or exacerbated by exercise. While most HIV-infected patients have CD4 cell counts of <200 cells/μl (18, 19) prior to the development of PCP, the CD4 count is a less reliable predictor of risk in the non-HIV-infected population (20, 21).
Although the lungs are the primary site of infection or colonization, rare cases of extrapulmonary involvement of Pneumocystis have been reported for humans, as extensively reviewed by Ng et al. (22). Commonly involved extrapulmonary sites include the eyes, ears, lymph nodes, liver, spleen, and bone marrow, while systemic dissemination has also been reported. More than half of the patients with these cases had known concurrent PCP.
As Pneumocystis cannot reliably be cultured in vitro, diagnosis of PCP has relied primarily on the microscopic detection of organisms in respiratory specimens, such as bronchoalveolar lavage (BAL) fluid, induced sputum, and lung biopsy specimens, after chemical staining (e.g., using methenamine silver, toluidine blue O, or Diff-Quik methods) (23–26) or immunofluorescence staining, which can be performed using commercially available anti-Pneumocystis monoclonal antibodies. The latter is more sensitive and specific than colorimetric staining (27–29). Serologic tests to detect anti-P. jirovecii antibodies have not proven useful clinically for establishing a diagnosis of PCP or for assessing prognosis (30–35). Detection of β-1,3-glucan, a component of the cell wall, in serum or BAL fluid appears to be sensitive but not specific (23, 24, 36–38). In recent years, multiple PCR assays have been developed for detection of P. jirovecii, most commonly utilizing primers for the mitochondrial large-subunit rRNA gene (mtLSU) or the multicopy major surface glycoprotein (msg) gene family (39–46), and these assays appear to be 10 to 100 times more sensitive than microscopic detection of stained organisms. Several commercially available diagnostic PCR kits are approved for clinical use in a number of countries, primarily in Europe (47–50); none are currently FDA approved for use in the United States.
The first-line drug for treatment of PCP is the combination of trimethoprim and sulfamethoxazole (TMP-SMX), which can be administered orally or intravenously (51). This combination is highly effective and generally well tolerated. For patients who cannot tolerate this regimen or for whom it fails, alternative therapies include clindamycin-primaquine, dapsone-trimethoprim, intravenous pentamidine, and atovaquone. Prophylaxis with TMP-SMX, dapsone, atovaquone, and aerosol pentamidine is effective at preventing PCP in at-risk populations. The widespread use of TMP-SMX has raised concerns about the development of drug resistance by P. jirovecii (52–58).
This review summarizes recent advances in the biology and epidemiology of Pneumocystis, with a focus on its taxonomic classification, atypical fungal nature, genome features, life cycle, strain variation, and transmission.
BIOLOGY OF PNEUMOCYSTIS
Atypical Fungal Nature
Although Pneumocystis was originally classified as a protozoan, it is now unequivocally recognized as a fungus based on overwhelming genomic evidence and phylogenic analyses, as described in detail below (see “Species and Taxonomy”). Such a classification is also supported by studies of cell wall composition and metabolic pathways. However, despite many common characteristics, Pneumocystis is often regarded as an atypical fungus, with substantial differences from other fungi. The knowledge of the atypical nature of Pneumocystis is evolving gradually with advances in Pneumocystis research. Earlier reviews on this topic can be found elsewhere (59, 60).
Following its reclassification as a fungus in the late 1980s (61, 62), considerable efforts were made to test the anti-Pneumocystis activities of classical antifungal drugs, including azoles, such as fluconazole, which targets ergosterol synthesis, and amphotericin B, which binds to ergosterol in the cell membrane (63–65). Unexpectedly, Pneumocystis was found to be resistant to these drugs, presumably due to its absence of ergosterol, the major sterol in the cell membranes of most fungi (64, 66). Instead of ergosterol, cholesterol was found to be the major sterol in Pneumocystis (64, 67, 68), though the metabolic pathways for both sterols remained poorly understood until the recent sequencing of Pneumocystis genomes. Based on genome analysis, Pneumocystis lacks several key enzyme genes involved in ergosterol biosynthesis (69). In addition, a homolog of Dhcr24 (24-dehydrocholesterol reductase; EC 1.3.1.72), which is necessary for cholesterol biosynthesis in mammals, is missing in both human and rodent Pneumocystis spp. It has been hypothesized that Pneumocystis may sequester cholesterol from its host (67, 69, 70).
Another major atypical feature of Pneumocystis is the pleomorphic shape and fragile cell wall of the trophic form, in contrast to the rigid cell wall of typical fungi. It has long been known that fungal cell walls are rich in glycoproteins, mannans, glucans, chitin, and chitosan. Early studies found that the Pneumocystis cell wall contains abundant glycoproteins and, in cysts only, β-glucans (71–73); however, other components were not well characterized until recently. Genome analysis in combination with experimental validation (described in greater detail below) has revealed that constituents characteristic of cell walls in other fungi, including outer chain N-mannans (the high-mannose residues of glycoproteins) and chitin, are absent in Pneumocystis (69). Thus, at present, Pneumocystis is the only fungus lacking chitin in its cell wall. Consistent with earlier studies, Pneumocystis has all the enzymes required for biosynthesis and degradation of β-1,3-glucan and β-1,6-glucan (69) but has lost genes found in many other fungi that are required for biosynthesis and degradation of α-glucan.
The third atypical feature of Pneumocystis is the inability of researchers to propagate the organism in vitro, despite extensive efforts that have utilized fungal culture media and other culture systems, including coculture with mammalian cells. Whole-genome analysis has provided some possible insight into this. Compared to those of other fungi, Pneumocystis has a highly compact genome, with a consequent loss of many biological pathways (69), which potentially renders Pneumocystis highly dependent on the host to complement these losses, as discussed in detail below. This suggests that successful culture will depend on a better understanding of the specific nutrients that need to be supplemented and the mechanism through which Pneumocystis acquires these nutrients. It is plausible that Pneumocystis cannot be cultured axenically but will require a feeding cell layer to complement certain as-yet-undefined biologic processes.
Finally, another unique feature of Pneumocystis, which is related to its host dependence, is its adaptation to and possible coevolution with its host species; as a consequence, each Pneumocystis species appears to exclusively infect one host species and is unable to infect a different host species (74). This is distinctly different from the case for many other pathogenic fungi that can inhabit diverse environmental niches and can also infect different host species. No apparent clues to this specificity have been identified in genome analyses or other studies.
Species and Taxonomy
Although Pneumocystis has been found in nearly every mammalian species examined, to date, mainly using PCR-based methods, only a limited number of Pneumocystis species have formally been classified and named at the species level according to the International Code of Botanical Nomenclature rules; these are P. jirovecii (infecting humans and named in honor of Otto Jirovec) (75), P. carinii (infecting rats and named in honor of Antonio Carini) (76), P. murina (infecting mice) (77), P. wakefieldiae (infecting rats and named in honor of Ann Wakefield) (78), and P. oryctolagi (infecting rabbits) (79). Pneumocystis organisms identified from other mammals have usually been named using a trinomial system of special form (formae speciales) names associated with host genera following the nomenclature system recommended in 1994 by the Pneumocystis workshop (80). For example, the Pneumocystis sp. infecting rhesus monkeys (Macaca mulatta) is referred to as Pneumocystis carinii f. sp. macacae (81).
Whether the organisms detected in macaques, bats, horses, dogs, ferrets, and other host species represent unique Pneumocystis species remains to be clarified, but these observations support the notion that these organisms are highly ubiquitous and that each mammalian species is infected by at least one Pneumocystis species. Interestingly, based on single-locus PCR studies, wild rats (82), macaques (83, 84), bats (85), and dogs (86) are often infected by more than one Pneumocystis population, with sequence divergence approaching or exceeding interspecies levels, raising the question of whether these animals may be infected with more than one distinct species.
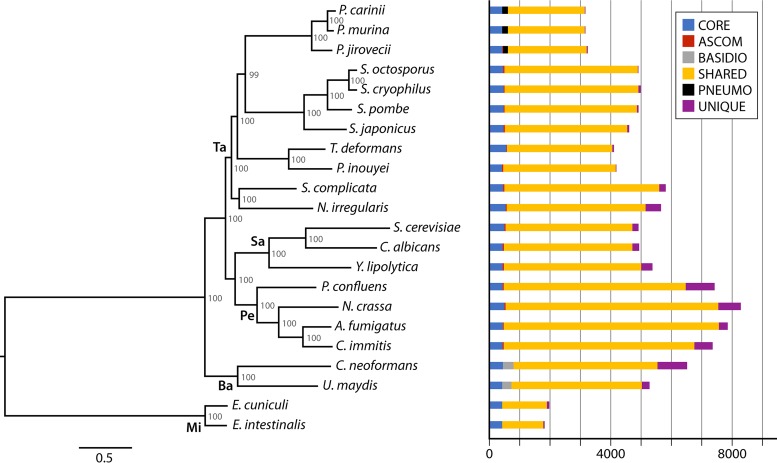
To complicate our understanding of this enigmatic organism, the taxonomic classification of Pneumocystis has undergone dramatic changes since its discovery. Initially, Pneumocystis was widely believed to be a protozoan based on some morphological features and drug sensitivity. It was not until 1988 that 16S rRNA gene analysis strongly suggested Pneumocystis to be a fungus (61). This finding has been confirmed unequivocally by all subsequent molecular phylogenetic analyses. Nevertheless, its taxonomic position within the fungal kingdom has varied in different studies depending on the genetic loci and fungal species involved (reviewed in reference 75). Most studies based on single-gene data sets and common fungal species have placed Pneumocystis into the Ascomycota phylum, with Schizosaccharomyces pombe or Saccharomyces cerevisiae often being the closest relative (61, 62, 87, 88), while there are rare reports of placement in the Basidiomycota (89) or another phylum (90). An improved taxonomic classification gradually emerged synchronously with the increasing recognition of an early-diverging Ascomycota lineage, initially (in 1994) named Archiascomycetes (91) and subsequently renamed Taphrinomycotina (92), as a monophyletic subphylum in parallel with Saccharomycotina and Pezizomycotina (93). Currently, Taphrinomycotina includes seven highly heterogeneous genera: Pneumocystis, Schizosaccharomyces, Taphrina, Saitoella, Neolecta, Protomyces, and Archaeorhizomyces (93–96). Over the last decade, growing phylogenetic analyses based on multigene and genome-wide (phylogenomic) data sets have strongly supported the grouping of Pneumocystis within Taphrinomycotina as a monophyletic subphylum (69, 93, 95–103). However, its position within this subphylum remains unclear. A few phylogenomic studies have shown that Pneumocystis is closer to Taphrina than to Schizosaccharomyces, with strong bootstrap support (69, 97), but this relationship was not confirmed in other phylogenomic studies including different gene sets and/or different Taphrinomycotina members (95, 100, 102, 103). Figure 1 shows a phylogenomic tree inferred from 248 single-copy core orthologs among sequenced species representing all known genera of Taphrinomycotina. In this tree, the Pneumocystis genus and the Schizosaccharomyces genus cluster as sister groups basal to the group formed by Taphrina and Protomyces; our understanding of their interrelationships remains dynamic and can change as additional species are included. In order to define the precise relationship of Pneumocystis with other Taphrinomycotina members, it may be necessary to use additional integrated phylogenomic analyses (104) and broader taxon sampling of Taphrinomycotina.
FIG 1.
Phylogenetic relationships and ortholog conservation for Pneumocystis and related fungi. The maximum likelihood tree was inferred from 248 single-copy core orthologs. Ortholog conservation patterns highlighted include core orthologs found in all genomes (CORE), ascomycete-specific orthologs found in all ascomycetes but not in Microsporidia (ASCOM), basidiomycete-specific orthologs found in both Cryptococcus neoformans and Ustilago maydis but not in all other fungi (BASIDIO), Pneumocystis-specific orthologs (PNEUMO), orthologs shared in any two or more genomes (SHARED), and orthologs unique to only one genome (UNIQUE). Numbers on the branches of the tree indicate bootstrap support values. The phyla and subphyla are indicated on the main branches as follows: Ta, Taphrinomycotina; Sa, Saccharomycotina; Pe, Pezizomycotina; Ba, Basidiomycota; and Mi, Microsporidia.
Phylogenetic relationships within the Pneumocystis genus have also been studied extensively, mainly utilizing single-gene data sets. All studies have consistently shown that all known Pneumocystis species form a monophyletic group, while the relationships between different Pneumocystis species vary between studies, depending on the genes and species used. For the three most extensively studied species, it is very clear that the two species infecting rodents, P. carinii and P. murina, are phylogenetically closer to each other than to P. jirovecii, as determined by single- and multiple-gene analyses (77, 105–107) as well as by phylogenomic analyses using full mitochondrial and nuclear genome data sets (69, 99).
Phylogenetic analysis has extended our understanding of the host species specificity of Pneumocystis. In a study of ∼20 different primate species or subspecies (including humans) based on sequence analysis of multiple Pneumocystis genes, a unique Pneumocystis sequence for all genes was obtained from each primate species or subspecies (108, 109), despite high genetic similarities among these host species. For example, Pneumocystis organisms from the Chinese rhesus macaque (M. mulatta) and the crab-eating macaque (Macaca fascicularis) differ by 3.5% in mtLSU sequence (108), though the genomes of these two macaque host species differ by only 0.34% (110), which further highlights the exceptionally high-level host species specificity. Parallel analysis of these Pneumocystis organisms and their primate host species found that the phylogenetic relationships between Pneumocystis organisms were well correlated with the phylogenetic relationships between their respective host species (108, 109). These findings suggest that the host species specificity of Pneumocystis might result from a long history of coevolution with or adaptation to its hosts. The hypothesis of coevolution is further supported by phylogenetic analysis of Pneumocystis species from broader mammalian taxa, including primates, rodents, carnivores, bats, lagomorphs, marsupials, and ungulates (111). Given the strict host species specificity and assuming coevolution with its host, each Pneumocystis species can serve as a signature of its host species, and studies of Pneumocystis phylogeny may complement studies of the phylogeny of the host.
The species divergence times for three Pneumocystis species were estimated based on a small number of genes (112). According to these estimations, P. murina and P. carinii diverged from each other between 51 and 71 million years ago, which appears to be earlier than the divergence time between rats and mice (∼12 to 24 million years ago) (113); P. carinii and P. jirovecii diverged from each other between 90 and 100 million years ago, similar to the divergence time between humans and rodents (∼80 million years ago) (114). These estimates should be interpreted with caution due to their reliance on nucleotide variations calculated from only a few genes, which may not contain enough information for estimating speciation timing. The availability of whole-genome sequences for multiple Pneumocystis species and strains, together with new, advanced bioinformatics tools, will potentially improve these estimates.
Morphology and Hypothetical Life Cycle
Although Pneumocystis was identified more than 100 years ago, its life cycle remains poorly understood, largely due to the inability of researchers to culture the organism continuously in vitro. As extracellular parasites, Pneumocystis organisms have been found almost exclusively in the alveolar space in the lungs of mammals. At either the light or electron microscopic level, the morphologies of Pneumocystis spp. from different mammalian species are generally not distinguishable, although some subtle electron microscope-based differences have been reported (115). It has been hypothesized that the Pneumocystis life cycle consists of asexual and sexual phases, with two primary morphological forms: the trophic form (or trophozoite) and the cyst (ascus) form (116–118). While there are intermediate stages between these two forms, they are less defined. In fact, neither “cyst” nor “trophozoite” has been used to describe any stage of other fungi; both have been used for Pneumocystis because it was originally classified as a protozoan, for which trophozoite (Greek for “animal that feeds”) is the active, replicating stage in the host, usually associated with pathogenesis, while cyst often refers to the dormant stage, with a thick protective cell wall enabling the parasite to survive in the outside environment (119). With the recognition that Pneumocystis is a fungus, the trophic form is thought to be equivalent to vegetative yeast and the cyst form to the asci of ascomycete fungi.
The trophic form is highly pleomorphic, varying in size from ∼2 to 10 μm (for the long dimension), with a thin, flexible cell wall (∼20 to 30 nm). In infected lungs, trophic forms are often clustered together or tightly attached to type I pneumocytes, and they usually predominate over cyst forms by a ratio of ∼10 to 20:1. The majority of the trophic forms are haploid, but a minor population appears to be diploid (120, 121). Each trophic form contains a single nucleus, which is surrounded by cytoplasmic organelles, including mitochondria, rough and smooth endoplasmic reticula (ER), Golgi vesicles, and cytoplasmic vacuoles (122, 123). On the surface of the trophic form, there are many protrusions, termed tubular extensions or filopodia (122–126), which often protrude toward the host cell or penetrate into invaginations of the host cell (127, 128). The function of these structures remains unknown, but they have been hypothesized to play a role in nutrient uptake by interdigitating with the host membrane (125, 126, 129). The trophic forms are believed to replicate asexually by binary fission (130–132). In the sexual phase, two trophic forms can potentially mate and develop into cysts.
The cyst form has a spherical shape (∼5 to 8 μm in diameter) with a thick, smooth cell wall (∼100 to 160-nm thick) that is rich in β-glucans. Each mature cyst typically contains eight intracystic bodies (spores), which may represent precursors to trophic forms. Each intracystic body contains a nucleus, mitochondria, and abundant endoplasmic reticula (128). Compared to those of the trophic form, the cyst form has rare tubular extensions, which are typically attached just to the surface of the cell wall but do not extend into host cell invaginations. Studies of Pneumocystis-infected mice treated with β-glucan synthetase inhibitors have demonstrated that the cyst is the infective form responsible for transmission to new hosts (132). After inhalation, cysts are presumably deposited to the alveoli and release eight spores that subsequently develop into trophic forms and begin the life cycle again.
The occurrence of a sexual phase is supported by the observation of synaptonemal structures within Pneumocystis cells (118, 133, 134), the identification of a conserved meiotic pathway (135), and the presence and transcription of many sex-related genes in Pneumocystis genomes (69, 97, 136–138). Recent studies of the genomic structure at the mating-type (mat) loci suggested that sexual reproduction in Pneumocystis is achieved by a self-fertilizing mechanism known as primary homothallism (139), in which both mating-type idiomorphs are present within a single genome (140). Nevertheless, the identity and organization of the mat genes in Pneumocystis remain uncertain due to their significant divergence from those of a closely related sibling species, S. pombe, one of the best-studied fungal models for sexual reproduction (141). In addition, some key components involved in the mating process, including the mating factors (map2 and mfm1/2/3), have not been identified in any Pneumocystis species. Mating and sexual reproduction presumably play a crucial role in the survival of Pneumocystis; elucidation of the related genetic pathways should improve the understanding of PCP pathogenesis and may identify new strategies to prevent or better manage disease in immunocompromised patients.
Genome Features
The mysterious lifestyle of Pneumocystis raised important questions about its genome. This led to an international proposal for a Pneumocystis genome sequencing project, which was announced in 1997 (142), only 1 year after the release of the first eukaryotic genome sequence, that of S. cerevisiae (143). However, this project progressed very slowly, lagging substantially behind those for many other pathogens, principally as a result of difficulties in obtaining high-quality DNA samples due to the lack of an efficient in vitro culture system. It was not until 2006 that the first Pneumocystis genome assembly was reported, though it was only a partial, highly fragmentary assembly for P. carinii (144). In 2012, the first P. jirovecii assembly, which utilized powerful next-generation sequencing (NGS) technologies, was published (97). Further application of multiple NGS technologies resulted in very-high-quality (at or near the chromosomal level) genome assemblies for P. murina, P. carinii, and P. jirovecii (69), allowing for a more reliable and thorough comparative genomic analysis (Table 1). Analyses of these genomes have yielded important insights into the biology of Pneumocystis (69, 97, 136, 145–147). This section briefly outlines various genome features, including genome structure, gene content, metabolic capacity, coding strategies for cell wall components, introns, and alternative splicing.
TABLE 1.
Genome statistics for Pneumocystis spp. and other fungi
| Species | Size (Mb) | No. of genes | No. of exons per gene | No. of tRNAs | No. of rRNAs | GC (%) |
|---|---|---|---|---|---|---|
| P. murina | 7.5 | 3,623 | 6.08 | 47 | 5 | 26.9 |
| P. carinii | 7.7 | 3,646 | 5.97 | 45 | 5 | 33.2 |
| P. jirovecii | 8.4 | 3,761 | 5.78 | 46 | 5 | 28.4 |
| T. deformans | 13.4 | 4,651 | 1.64 | 81 | 4 | 49.5 |
| S. pombe | 12.6 | 5,155 | 1.99 | 171 | 43 | 36.1 |
| S. cerevisiae | 12.1 | 5,863 | 1.06 | 275 | 25 | 38.3 |
| C. albicans | 14.3 | 6,189 | 1.06 | 126 | 4 | 33.4 |
| A. fumigatus | 29.4 | 9,782 | 2.92 | 179 | 34 | 49.8 |
| C. immitis | 28.9 | 9,757 | 3.31 | 121 | 41 | 46.0 |
| E. cuniculi | 2.5 | 1,996 | 1.00 | 46 | 9 | 47.3 |
| E. intestinalis | 2.2 | 1,833 | 1.01 | 46 | 13 | 41.5 |
Genome structure.
The genomes of the two species infecting rodents, P. carinii and P. murina, have very similar sizes and chromosomal organizations, with few chromosomal rearrangements, involving ∼60- to 260-kb segments in five chromosomes of each species (69). In contrast, compared to that of rodent Pneumocystis, the P. jirovecii genome is highly rearranged both inter- and intrachromosomally, with each of the 17 largest scaffolds (potentially representing chromosomes) mapped to two to five different chromosomes of P. murina. A similar organization was seen in mitochondrial genome (mitogenome) studies. While all three species have nearly the same set of genes, the mitogenomes of P. murina and P. carinii are linear, with the same gene order; in contrast, P. jirovecii bears a circular mitogenome with a different gene order (99). The substantial variation in both the nuclear and mitochondrial genomes between human and rodent Pneumocystis spp. highlights the possibility that there may be clinically relevant differences between animal models of PCP and human disease.
Genome contraction.
Reductive evolution, which results in a loss of genes and a reduction in genome size, is a pervasive process that has long been considered a hallmark of parasitism and presumably occurs in part because the host can complement necessary biological functions; in fact, there is a growing body of evidence recognizing its impact as a major evolutionary force affecting a broad range of organisms (reviewed in reference 148). Pneumocystis species follow this paradigm, since they are the only lineage within the Taphrinomycotina to have evolved for animal parasitism, and they also harbor the smallest genome size. The Pneumocystis genomes sequenced, to date, have all demonstrated a contracted genome compared to those of other closely related fungi (Table 1).
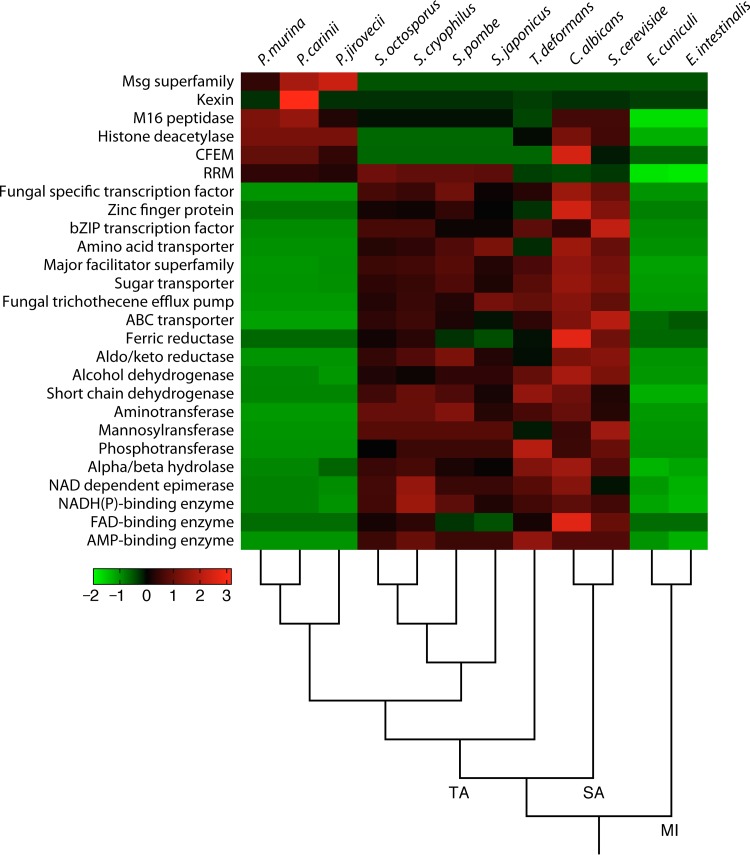
Genome reduction takes place via elimination of genes, reduction of gene length, reduction of intergenic spaces, contraction of gene families, and simplification of gene structures (e.g., loss of introns). Evidence of nearly all these processes can be found in the Pneumocystis genomes. Massive gene losses have been identified through comparative genomics (see “Lost metabolic functions,” below). Intergenic spaces, which cover ∼33% of the Pneumocystis genome, have reduced lengths compared to those in other ascomycetes (69). Gene families other than the subtelomeric msg superfamily display a net contraction (Fig. 2), and only a few instances of expansions have been reported (69, 145). The only discordant feature compared to those of other parasites is the high intron density (see “Introns and alternative splicing,” below).
FIG 2.
Top enriched and depleted protein families in Pneumocystis. Significantly enriched and depleted Pfam domains (Fisher's exact test; q < 0.05) were included in the heat map if the domains appeared at least twice in the following comparisons: Pneumocystis versus Schizosaccharomyces, Pneumocystis versus Schizosaccharomyces and Taphrina deformans, Pneumocystis versus S. cerevisiae and C. albicans, Pneumocystis versus Encephalitozoon cuniculi and E. intestinalis, and Pneumocystis versus all others shown. CFEM, common in fungal extracellular membrane domain; RRM, RNA recognition motif. The heat map is color coded based on Z scores from −2 to 3, as indicated by the key. Fungal species are ordered based on their phylogenetic relationships, as indicated at the bottom. The subphyla are indicated on the main branches, as follows: TA, Taphrinomycotina; SA, Saccharomycotina; and MI, Microsporidia. (Modified from Fig. 2 in reference 69.)
Understanding gene loss may give insights into the basis for Pneumocystis host specificity. However, the patterns of gene loss in multiple Pneumocystis species are roughly similar (69), which suggests that gene deletions occurred in a common ancestor, became fixed (i.e., not lethal), and were transmitted vertically, although formal validation of this scenario awaits genome sequencing for additional species. If this holds true, analysis of gene loss in currently sequenced species alone cannot fully explain the host specificity.
Lost metabolic functions.
Many opportunistic pathogens, including bacteria (e.g., Pseudomonas aeruginosa [149]) and fungi (e.g., Aspergillus fumigatus and Coccidioides immitis [Table 1]), have large genomes encoding complex and often redundant metabolic pathways to allow the pathogens to live within diverse environments, including both inside and outside the host. While initial analysis of an early partial genome of P. carinii suggested the presence of most standard metabolic pathways (136), recent studies of the nearly complete genome of P. carinii as well as those of P. murina and P. jirovecii demonstrated a loss of many metabolic pathways (Table 2), with retention presumably of those critical to survival in the host environment (69, 97, 145).
TABLE 2.
Loss of biological pathways in Pneumocystis
| Category | Type of loss | Biological pathway or component affected |
|---|---|---|
| Stress responses | Loss of many transcription factors | pH sensing |
| Osmotic stress response | ||
| Oxidative stress response | ||
| Cell wall stress response | ||
| Protein synthesis | Loss of de novo synthesis | All 20 amino acids |
| Carbohydrate metabolism | Loss of pathway | Gluconeogenesis |
| Glyoxylate cycle | ||
| Fermentation | ||
| Lipid metabolism | Loss of synthesis | Ergosterol |
| Cholesterol | ||
| myo-Inositol | ||
| Choline | ||
| Ether lipids | ||
| Complex sphingolipids | ||
| Phosphatidylinositol | ||
| Phosphatidylcholine | ||
| Glycerol | ||
| Loss of function | Fatty acid β-oxidation | |
| Cofactor metabolism | Loss of synthesis | Coenzyme A |
| Thiamine | ||
| Biotin | ||
| Siderophores | ||
| Reductive iron uptake | ||
| Loss of enzyme | Carbonic anhydrase |
(i) Amino acid metabolism.
Amino acid metabolism is shifted to wholly scavenge from the host instead of performing de novo biosynthesis. All three Pneumocystis species lack ∼80% of the genes for amino acid biosynthesis that are present in yeast (97, 150), and they are also defective in inorganic nitrogen and sulfur assimilation (145). As a result, Pneumocystis cannot synthesize any of the 20 standard amino acids. Moreover, there is only one potential plasma membrane-localized amino acid transporter (Ptr2) in each Pneumocystis species, in sharp contrast to the case in yeast species, which have more than 20 such transporters. Nevertheless, nearly 50% of the 26 amino acid transporters associated with mitochondria and vacuoles in yeast are conserved in Pneumocystis. These findings suggest that Pneumocystis scavenges amino acids from its host. This hypothesis is supported by the retention or relative expansion of genes encoding proteases and proteasome proteins in Pneumocystis and the presence of abundant amino acids and peptides in alveolar fluid of the hosts (151, 152). Similarly, polyamine biosynthesis is completely lost in Pneumocystis, while one polyamine transporter is retained, supporting a mechanism for direct uptake of polyamines from the host.
(ii) Nucleotide metabolism.
Nucleotide metabolism is focused solely on de novo biosynthesis of fundamental cellular components. The Pneumocystis genomes have retained the complete de novo biosynthesis pathways for purine and pyrimidine nucleotides but have lost nucleotide salvage and degradation pathways (69, 145), consistent with the reported absence of a thymidine salvage pathway (153). This is unusual for a highly compact genome, since the de novo biosynthetic pathways involve substantially more chemical reactions and thus require more energy than those for salvage pathways. In fact, although de novo nucleotide synthesis is a fundamental biological process that is highly conserved in fungi and other organisms, most organisms, including all known human fungal pathogens, also maintain functional salvage and degradation pathways (154). The exception to this are the intracellular fungal pathogen Microsporidia (155, 156) and parasitic protozoa (157, 158), which lack de novo nucleotide synthesis pathways but preserve the nucleotide salvage and degradation pathways. The retention of the energy-expensive de novo synthesis pathways in Pneumocystis suggests an absolute necessity to keep these pathways, presumably as a result of a lack of nucleosides and nucleobases in the host alveolar environment. These findings suggest that inhibitors of de novo nucleotide synthesis may be effective drugs for treating PCP.
(iii) Carbohydrate metabolism.
Carbohydrate metabolism is streamlined to produce energy for cell wall synthesis, with limited output of metabolites. Pneumocystis genomes carry all genes required for glucose uptake and catabolism through glycolysis and the tricarboxylic acid cycle, as well as all key genes required for oxidative phosphorylation. In addition, all the enzymes required to catalyze mannose and fructose to glucose and for the metabolism of glycogen and trehalose are preserved, though critical enzymes required for converting sucrose or galactose to glucose are not present. The synthesis of glycogen is supported by the detection of abundant glycogen granules in the Pneumocystis cytoplasm in many electron microscopic studies (115, 159, 160). These findings indicate that Pneumocystis relies largely on glucose utilization via oxidative pathways for energy production.
The most striking losses in carbohydrate metabolism include the central regulatory enzyme (Fbp1) for gluconeogenesis, two key enzymes (Mls1 and Icl1) for glyoxylation, and all enzymes for pyruvate fermentation. Simultaneous loss of these pathways not only suggests an inability of Pneumocystis to use nonsugar carbon sources (including fatty acids and simple carbon compounds, such as ethanol and acetate) for energy production, further supporting a high reliance on glucose, but also indicates a limited capacity for biosynthesis of complex structural polysaccharides in the cell wall, as discussed below. In addition, the loss of the glyoxylate pathway may explain in part the apparent lack of virulence of Pneumocystis given that this pathway is believed to be required for virulence of some pathogenic fungi (161–163) as well as bacteria (164).
(iv) Lipid metabolism.
Lipid metabolism is optimized to exploit host resources, resulting in distinctive lipid profiles compared to those of other fungi. Pneumocystis has unique sterol biosynthesis pathways contributing to distinct sterol compositions in the plasma membrane (69, 165, 166). Based on genome analysis, both human and rodent Pneumocystis spp. are able to synthesize lanosterol, zymosterol, episterol, and fecosterol but cannot further metabolize them to ergosterol due to the lack of one or two key late-stage enzymes (Erg3 and Erg5) (69), consistent with the results of membrane chemical composition analysis (64, 67, 167, 168) and with resistance to antifungal agents targeting ergosterol (64). In addition, all potential pathways for cholesterol biosynthesis found in mammals and a few fungal species appear to be disabled due to the absence of the key enzyme Dhcr24 in both human and rodent Pneumocystis spp. and of another two enzymes (Erg3 and Dhcr7) in rodent Pneumocystis only. Nevertheless, cholesterol has been identified as the most abundant sterol in both human and rodent Pneumocystis spp. (64, 165, 169–171). There has been evidence suggesting that Pneumocystis can scavenge cholesterol from its host (67, 69, 169), but the genes involved in cholesterol acquisition are not identified in the genome data. Alternative possibilities include the possibility that the Dhcr24 homolog in Pneumocystis could not be identified based on sequence homology due to high sequence divergence or that the enzyme activities of Dhcr24 could be replaced by those of other enzymes. The utilization of cholesterol in the Pneumocystis membrane is very unusual within the fungal kingdom, especially since organisms within the most closely related subdivision, Taphrinomycotina (166), utilize ergosterol (fission yeasts) (172) and brassicasterol (Taphrina and Protomyces) (173, 174). The reason for utilization of cholesterol rather than ergosterol is not clear but may reflect optimized cellular and physiological functions as a result of adaptation to the host environment. First, it is less energetically expensive to synthesize cholesterol (175) or to scavenge it from the host than to synthesize ergosterol de novo. Second, since membranes containing cholesterol are less rigid than membranes containing ergosterol, utilization of cholesterol may create a more flexible cell wall and thus may have promoted development of trophic forms. Third, as the major sterol in mammalian cell membranes, cholesterol is an efficient mechanical stabilizer (176). Utilization of cholesterol may allow Pneumocystis to better interact (interdigitate) with the cholesterol-containing host cell membrane and to stabilize its cell structure. Lastly, studies of plant fungal pathogens have suggested that ergosterol is a pathogen-associated molecular pattern (PAMP) (177, 178), and loss of ergosterol in Pneumocystis may represent a mechanism of immune evasion.
In addition to the loss of de novo biosynthesis of ergosterol and/or cholesterol, each Pneumocystis species lacks enzymes for de novo biosynthesis of many other lipids, including glycerol, ether lipids, phosphatidylcholine, phosphatidylinositol, and complex sphingolipids, as well as other components (such as myo-inositol and choline) related to synthesis of complex lipids (69). Except for glycerol, for which two potential transporters are encoded in the genome (responsible for direct uptake and export), there are no direct transporters predicted for any of these lipids. Nevertheless, alternative mechanisms may supply these lipids. For example, each Pneumocystis species encodes the Dnf1-Lem3 flippase complex, involved in uptake of external lysophosphatidylcholine that can be converted to phosphatidylcholine and, subsequently, to choline. There is also a potential transporter (Git1) involved in uptake of external glycerophosphoinositol that may be hydrolyzed into inositol (179), though the enzyme responsible for this hydrolysis has not been identified definitively in Pneumocystis or other fungi. However, other studies have reported the identification of direct inositol transporters (147, 180). This discrepancy awaits further investigation using yeast complementation assays and other approaches.
Fatty acid metabolism in Pneumocystis is also unique among fungi. The absence of fas1 and fas2 genes in Pneumocystis suggests a loss of the cytosolic fatty acid synthesis (FAS) pathway, which is highly conserved in other fungi and eukaryotes. In addition, Pneumocystis lacks the majority of genes required for fatty acid β-oxidation that are conserved in other fungi, implying that fatty acids cannot be used by Pneumocystis for energy generation, further highlighting its dependence on glucose for energy production. Despite the loss of the cytosolic FAS pathway, rodent Pneumocystis, but not P. jirovecii, conserved the complete mitochondrial FAS pathway, which is composed of eight monofunctional enzymes resembling the bacterial FAS system, in contrast to the cytosolic eukaryotic multifunctional FAS complex. This selective conservation in rodent Pneumocystis spp. suggests its vital role in these organisms' survival, given that mitochondrial FAS is believed to be essential for cellular respiration, RNA processing, and mitochondrial biogenesis in eukaryotes (181–183). P. jirovecii lacks one of the eight genes (Mct1) involved in mitochondrial FAS, raising the possibility that this pathway is disabled in this organism. However, given the retention of the seven other genes of this pathway in this organism, as well as all the genes required for the downstream processes in the ER, including fatty acid chain elongation, desaturation, and hydroxylation, in all sequenced Pneumocystis species, it seems likely that P. jirovecii has also retained mitochondrial FAS activities. It is possible that the mct1 gene in P. jirovecii could not be identified due to sequence divergence or incomplete assembly of the genome, or the Mct1 activity in P. jirovecii may be replaced by activities of other enzymes.
(v) Cofactor metabolism.
Cofactor metabolism is greatly consolidated. Pneumocystis genomes lack almost all genes involved in pantothenate biosynthesis and transport. However, they encode the enzymes required for synthesis of coenzyme A (CoA) from pantothenate and also encode a carrier protein (Leu5) to facilitate transport of CoA into mitochondria, suggesting a possible scavenging mechanism for pantothenate or its metabolites by another process, such as endocytosis, as discussed below. While Pneumocystis genomes are also missing key genes needed for de novo synthesis of thiamine (vitamin B1), biotin (vitamin H), siderophores, and ubiquinone, they do encode transporters for each of them. P. carinii and P. murina have retained complete pathways for both biosynthesis and salvage of the coenzyme NAD. However, P. jirovecii is missing most of the enzymes required for NAD synthesis de novo but has preserved a salvage pathway to produce NAD by using nicotinic acid mononucleotide transported from an exogenous source via Tna1. All three Pneumocystis species lack almost all genes required for assimilation of reductive iron and biosynthesis of siderophores (184), but they do encode five proteins containing fungus-specific CFEM (common in fungal extracellular membrane) domains (185), raising the possibility that, like Candida albicans (186, 187), Pneumocystis can use a subset of these to scavenge iron from host hemoglobin and heme.
As discussed above, there is a lack of both de novo biosynthesis and direct transporters for some nutrients, implying other mechanisms for nutrient uptake. Genome analysis suggests that endocytosis may serve this purpose, as evidenced by the preservation of nearly all proteins associated with clathrin-dependent endocytosis and the presence of vesicles and other structures (including tubule-, filopodium- and basket-like structures) in numerous electron microscopic studies of different Pneumocystis species (115, 126, 188, 189).
In summary, the streamlining of Pneumocystis genomes has resulted in a significant reduction and consolidation of metabolic pathways compared to those of other closely related fungal species, presumably reflecting adaptation to the mammalian hosts, on which these organisms are highly dependent for nutrients and a stable environment. These reduced pathways may explain the slow growth of Pneumocystis organisms (190, 191) and their failure to grow continuously in vitro.
Cell wall reduction.
Two main types of macromolecules are typically found in the cell walls of fungi: polysaccharides of different types (mainly chitin, chitosan, glucans, and mannans) and proteins with various modifications. These components are cross-linked to maintain the cell shape and structural integrity, protecting the cell from its surroundings and allowing the cell to interact with other cells and the environment (192–194). The importance of the cell wall in fungi is reflected by the fact that, in yeast, one-fifth of the genome is devoted to cell wall biosynthesis (193, 195). Given that the pathways needed for cell wall biosynthesis are largely limited to fungi, enzymes required for cell wall metabolism are potential targets for antifungal chemotherapies and fungicides. Due largely to an inability to culture Pneumocystis, its cell wall composition and structure have not been completely defined.
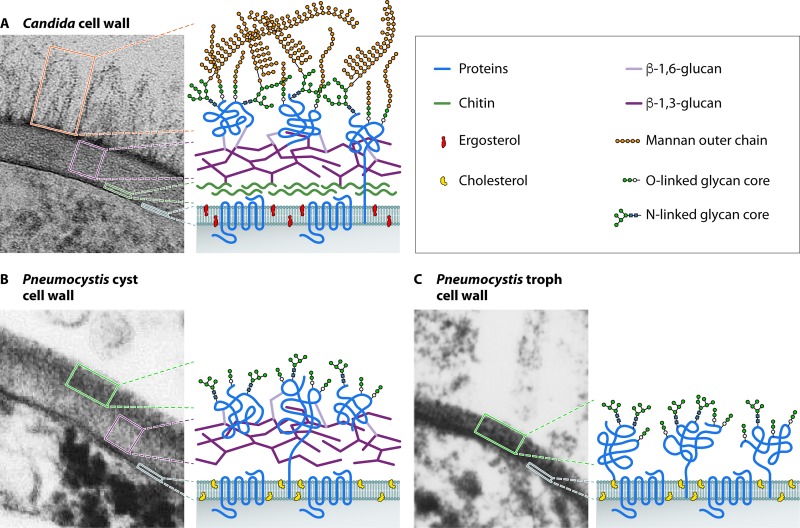
Early biochemical studies demonstrated an abundance of glycoproteins in the cell wall for all forms of the organism (196–202), while β-glucans were found only in cysts (71–73, 203, 204). Based on ultrastructural studies, the cell wall of the trophic form measures 20 to 30 nm and consists of two layers: an electron-dense outer layer and an inner layer containing the plasma membrane (205, 206). The cyst cell wall is ∼100 nm thick and consists of a distinct, tightly packed, electron-dense outer layer and an inner plasma membrane separated by an electron-lucent middle layer (Fig. 3). Thiery's reagent stained only the electron-lucent middle layer (207), suggesting a limited abundance and distribution of polysaccharides. This is in contrast to the cell wall of C. albicans, which is ∼200 to 300 nm thick, with five to nine layers (representing different dominant polysaccharides) distinguishable by Thiery's staining (208). The recent sequencing of Pneumocystis genomes has allowed an improved understanding of the molecular landscape of the Pneumocystis cell wall (69, 97, 145), as illustrated in Fig. 3 and summarized below.
FIG 3.
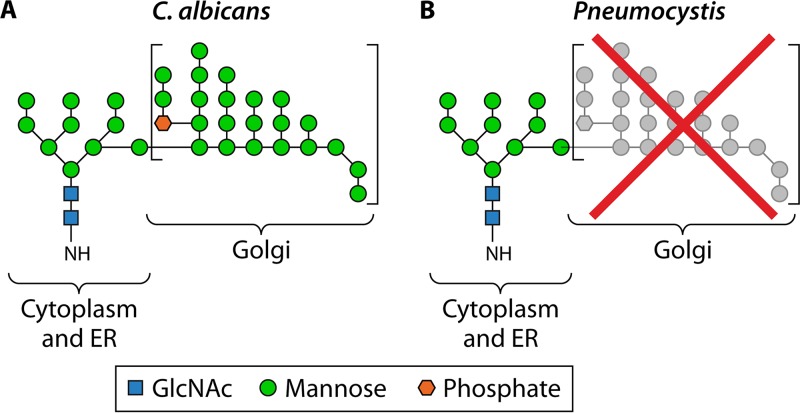
Cell wall structure of Pneumocystis compared to that of C. albicans. (A) C. albicans cell wall. The inner layer contains chitin and β-glucans, whereas the outer layer contains hypermannosylated N- and O-linked glycans (mannans) that are covalently linked with proteins to form glycoproteins. The plasma membrane contains ergosterol. (Electron micrograph courtesy of Louise Walker and Neil Gow, University of Aberdeen, United Kingdom; reprinted with permission.) (B) Cell wall of Pneumocystis cysts (asci). The inner layer contains β-glucans and no chitin, whereas the outer layer is highly enriched in proteins that are glycosylated via N- and O-linked glycans, but without the mannan outer chains. The plasma membrane contains cholesterol instead of ergosterol. (C) Cell wall of Pneumocystis trophic forms. The cell wall is the same as that of Pneumocystis cysts, except for the absence of β-glucans.
(i) Complete loss of chitin biosynthesis and degradation pathways.
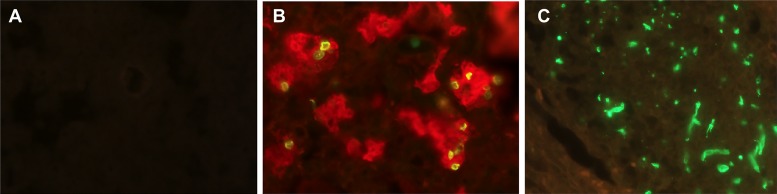
None of the Pneumocystis genomes sequenced (69) encodes chitin synthase, an enzyme critical for the synthesis of chitin, in contrast to all other sequenced fungal species, which contain up to 10 chitin synthase genes (209). Pneumocystis also lacks chitinases, which are required for chitin degradation during cell wall remodeling, encoded by up to 30 or more genes in other fungi (210–212). The absence of both chitin synthase and chitinase strongly suggests that the Pneumocystis cell wall lacks chitin, which was confirmed experimentally by mass spectrometric analysis of the cell wall content and by the absence of staining with a recombinant chitin binding domain specific for chitin (Fig. 4). While each Pneumocystis species encodes homologs of a few accessory proteins, such as Chs5 (213), not directly associated with chitin synthesis or degradation, these proteins do not have domains associated with either chitin synthase or chitinase activity. Note that the Chs5 protein of S. cerevisiae is part of a complex associated with exporting membrane proteins, including chitin synthase (214). A lack of chitin has not been reported previously for any other fungi.
FIG 4.
Loss of chitin in Pneumocystis cell wall. P. murina-infected lung tissue (A) and C. albicans-infected kidney tissue (positive control) (C) were stained with a recombinant chitin binding domain (green). Chitin staining is absent in P. murina (A) but readily detected in C. albicans (C). (B) Pneumocystis organisms are demonstrated by dual staining with anti-Msg (red), which labels both trophic forms and cysts, and dectin-Fc (green), which labels β-1,3-glucan in cysts. Original magnification, ×400.
(ii) Presence of β-glucans but not α-glucans.
Both human and rodent Pneumocystis spp. have complete pathways for the biosynthesis and degradation of β-1,3-glucan and β-1,6-glucan (69), consistent with the detection of both types of glucans in the cell wall of cysts in previous studies (71, 73, 215–217) (Fig. 4). As in other fungi, β-1,3-glucan is found in the inner layer of the cyst cell wall (73) (Fig. 3). In the trophic form, both types of glucans are absent (71, 73, 215–217). Pneumocystis spp. are missing all genes required for biosynthesis and degradation of α-glucan (69), which is present in many other fungi and is able to prevent innate immune recognition by the β-glucan receptor (218). The retention of β-glucans in the absence of α-glucan, chitin, and mannans (see below) suggests that β-glucans are absolutely necessary for the survival of Pneumocystis cysts, supporting β-glucans as potential targets for treatment of PCP. Indeed, several studies have shown that inhibitors of β-1,3-glucan synthase are highly effective at reducing cyst numbers in animal models of PCP (132, 204, 216, 217, 219–221). In other fungi, β-1,3-glucan is found as a branched polymer with β-1,6 side interchains and is covalently linked to other wall components, such as chitin, mannans, and glycoproteins (193, 195, 222). How β-1,3- and β-1,6-glucans interact with each other and with other cyst cell wall components in Pneumocystis is currently unknown, though a recent study found that β-glucans are masked by surface proteins (216). Since β-glucans are well-known activators of innate immunity during infection by Pneumocystis (223–229) and other pathogens (230–232), their absence in the trophic form and their masking in cysts may represent a mechanism to escape host innate immunity. Given that immunocompromised hosts were likely rarely encountered during the evolution of Pneumocystis, this mechanism presumably evolved during infection of immunocompetent hosts, in whom the organism burden is low. In immunosuppressed hosts with high organism loads, release of β-glucans, presumably as a result of organism death, is a critical factor contributing to deleterious host inflammatory responses (216).
(iii) Partial loss of protein glycosylation.
Pneumocystis genomes encode all enzymes residing in the endoplasmic reticulum that are necessary for biosynthesis of the core structure of N- and O-linked glycans (consisting of up to nine mannose residues), but they do not encode any enzymes to add mannose outer chains, including α-1,6-, α-1,2-, and α-1,3-mannosyltransferases as well as mannan polymerase complex I and complex II, all of which are located in the Golgi apparatus (69). These findings suggest that in contrast to those of other fungi, cell wall proteins of Pneumocystis have low levels of mannosylation (Fig. 5). This hypothesis was confirmed by direct examination of glycosylation in purified P. carinii Msg proteins (69). By liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of peptide-N-glycosidase F (PNGase F)-released N-linked glycans, the predominant N-linked glycan identified was M5N2 (hexose5 HexNAc2). Although trace levels of M6N2 to M9N2 were found, nothing larger than M9N2 was definitively identified. Additionally, glycopeptide mapping of Msg tryptic digests identified 15 Msg isoforms, with 31 N-linked glycans in, all with M5N2 as the major constituent. In only one Msg isoform, M6N2 was also identified as a minor constituent.
FIG 5.
Lack of N-linked hypermannose (mannan) outer chains in Pneumocystis. Like C. albicans, Pneumocystis spp. are able to synthesize the N-linked glycan core structure (containing up to nine mannose residues, as indicated on the left) in the cytoplasm and the endoplasmic reticulum (ER). However, due to the loss of multiple enzymes, Pneumocystis spp. are unable to synthesize the α-1,6-linked mannose backbone as well as the α-1,2- and α-1,3-linked mannose outer chains seen in C. albicans (square brackets), which are synthesized in the Golgi apparatus. (Diagrams of the N-linked mannan structure in C. albicans and Pneumocystis were adapted from reference 69.)
(iv) Expansion of the complex surface protein superfamily.
Fungal wall proteins often form large families with a common multidomain structure (233, 234). They are usually heavily glycosylated and form a dense protein coat to mask the inner polysaccharide layer (193, 234). They can function in mediating developmental states, optimizing mobility and adhesion ability, protecting the organism from harmful environmental stresses, and adapting to various niches. Characterization of Pneumocystis cell wall proteins is very challenging due in large part to the lack of a reproducible culture method, which prevents isolation of Pneumocystis proteins in large quantities and with high purity. Nevertheless, numerous studies have found that the most abundant cell wall protein in both the cyst and trophic forms of all Pneumocystis species studied, to date, is the major surface glycoprotein (Msg), also known as gp95, gp115, gp120, and gpA (196, 197, 201, 235–239).
Early studies of Pneumocystis in humans, rats, and mice showed that the Msg protein is encoded by a gene family with an estimated ∼30 to 100 copies per genome (236–238, 240, 241). msg genes (up to ∼3 kb each) are closely related to but clearly distinct from each other and are clustered in the subtelomeric regions of multiple chromosomes (69, 242). These features make it difficult to accurately determine the total number or complete sequences of msg family members. Thanks to advances in DNA sequencing technology, especially the PacBio long-read sequencing platform (243, 244), nearly complete sets of msg genes from three Pneumocystis species (P. jirovecii, P. carinii, and P. murina) were determined as part of the Pneumocystis genome project (69). This genome study identified multiple additional genes that are related to msg, based on the presence of one or more conserved domains, which are collectively termed the msg superfamily. Each Pneumocystis genome encodes 60 to 180 members of the Msg superfamily, which is the largest family of surface proteins found in fungi, to date (245). In each species, msg superfamily genes account for 3 to 6% of an otherwise highly compact genome, suggesting a vital role in the organism's survival in its host.
The availability of a nearly complete Msg repertoire in three Pneumocystis species has allowed, for the first time, a detailed analysis of the Msg domain structure, phylogeny, and classification. Like the cell wall proteins in other fungi (233, 234), Msg proteins are also composed of multiple conserved domains, named N1, M1 to M6, C1, and C2 (69). These domains are unique to Pneumocystis and are not shared with any other fungal species. The Msg superfamily can be grouped, based on phylogeny and domain organization, into five families, termed Msg-A to Msg-E. The sequences of these proteins show not only conservation among different families across different Pneumocystis species but also species-specific expansions or contractions.
The Msg-A family is the largest family and includes three subfamilies: Msg-A1, -A2, and -A3. The majority of members of this family contain all nine Msg signature domains, while a small number contain only three to eight domains. The Msg-A1 subfamily primarily includes all classical Msgs, which are conserved in all known Pneumocystis species and whose gene expression is controlled by a unique, single-copy subtelomeric expression site known as the upstream conserved sequence (UCS) (246–249). The UCS is expressed in frame with one of the multiple msg gene variants; the region between the UCS and its downstream msg gene is termed the conserved recombination joint element (CRJE), which is highly conserved among all classical msg genes and potentially serves as an anchor for recombination (250). Different msg variants are presumably expressed by recombination downstream of the UCS. The Msg-A2 subfamily primarily represents msg-related (msr) genes present in both P. carinii (251, 252) and P. murina but absent in P. jirovecii (69). Each msr gene contains a highly conserved exon at the 5′ end; its expression is not dependent on the UCS. The Msg-A3 subfamily includes genes with substantial sequence identity to the Msg-A1 and Msg-A2 subfamilies, but without the CRJE of the classical msg genes or the highly conserved exon 1 of the msr genes. This subfamily has 33 members in P. jirovecii but only 1 and 2 members in P. murina and P. carinii, respectively.
The Msg-B family is present only in P. jirovecii; most genes encode only three Msg signature domains. The Msg-C family is encoded by a tandem array of six genes in P. murina, with each copy containing three Msg signature domains. Only one and two short copies are present in P. carinii and P. jirovecii, respectively. The Msg-D family is related to the previously reported A12 antigen gene in P. murina (253, 254); this family is encoded by a single gene in P. murina and P. carinii but is expanded to 20 copies in P. jirovecii, with the majority encoding six Msg signature domains. The Msg-E family is related to two previously reported p55 genes (255–260); there are five to seven Msg-E genes in each Pneumocystis species, and each of them encodes only one Msg signature domain.
Currently, the functions of the vast majority of Msgs remain poorly understood or uncharacterized. The best-studied proteins are the classical Msgs of the Msg-A1 subfamily. It has long been hypothesized that this subfamily plays a critical role in pathogen-host interactions, including adhesion to host cells and extracellular matrix proteins (261–264), evasion of host immune attacks via antigenic variation (237, 265–268), and masking of immune activation by β-glucans (216). Evidence in support of their role in antigenic variation includes the preservation of all required components of the DNA recombination machinery (69), the clustering of msg genes almost exclusively in subtelomeric regions to facilitate recombination (69, 242), the presence of strong serological responses to Msg proteins in patients with PCP (30, 269–271), the selective expression of Msg variants among different organisms in the same infected lung (267), and the occurrence of discordant antibody and cellular responses to Msg variants in animal models (268). The last observation suggests that antigenic variation may target T-cell responses, not antibody responses. In addition, transcriptome sequencing (RNA-Seq) data indicate that all msg genes in P. murina and P. carinii are transcribed in a population of organisms; the UCS gene in both species is the most highly expressed protein-encoding gene, consistent with a very high level of expression of the msg-A1 gene subfamily as a whole (69). Due to the lack of a culture system and an inability to genetically manipulate Pneumocystis, it is not currently feasible to use more direct methods to address the underlying mechanism of msg gene recombination and antigenic variation, such as by studying organisms expressing only one msg gene. Although they are recognized as glycoproteins, classical Msg proteins are not highly mannosylated (69), unlike glycoproteins in other fungi (272, 273).
The functions of all nonclassical msg genes remain unknown, although studies of animal models have suggested that p55-related proteins in the Msg-E family (258, 259, 274, 275) and A12-related proteins in the Msg-D family (253, 254) are antigenic and can potentially generate protective immune responses in hosts. Given that the Pneumocystis genome is highly compact and that the recombination system associated with classical msg genes is presumably sufficient for antigenic variation and immune evasion, the diverse nonclassical Msgs may provide other advantages for Pneumocystis to survive in the host, such as mediation of life-stage development (276), optimization of cell mobility and adhesion ability, and adaptation to specific host niches.
Despite the Msg proteins being classified as cell wall proteins, their subcellular locations and interactions with other cell wall components remain largely uncharacterized. Cell surface proteins, including the classical Msgs, mask β-glucans, but the chemical linkages between them are still unknown. One study suggested that the p55 antigen in P. carinii may be at least partially masked by β-glucans (257). While Pneumocystis genomes encode all key enzymes required for glycosylphosphatidylinositol (GPI) anchor synthesis (69), there is a lack of direct proof of GPI-anchored proteins on the cell wall, though potentially functional GPI signal sequences have been reported from indirect studies of the ferret Pneumocystis glycoprotein (277) and the P. carinii kexin genes (278).
(v) Other cell wall components.
Melanin is present in some pathogenic fungi, with an important role in protecting against harmful environmental exposures that can include ionizing radiation, UV light, or oxidizing agents (279). While there are reports of detection of melanin in the Pneumocystis cell wall (280, 281), none of the key enzymes involved in melanin biosynthesis, including polyketide synthase, laccase, tyrosinase, and phenoloxidase (279, 282), is encoded in the Pneumocystis genome. It is possible that the melanin biosynthesis pathway was lost in Pneumocystis as a result of adaptation to an environment largely devoid of exposure to UV light and ionizing radiation.
In summary, compared to those of other fungi, the Pneumocystis cell wall has a significantly reduced composition, thickness, and rigidity. This reduction presumably reflects its adaptation to the mammalian host environment. Given that chitin, mannan, and β-glucans are all known PAMPs that trigger the host innate immunity through host pattern recognition receptors (HPRRs) that include DC-SIGN, dectin-1, and dectin-2 (272, 283), their simultaneous loss or masking may represent a highly efficient mechanism adapted by Pneumocystis organisms, especially the trophic form, to evade host innate immunity. The absence of these PAMPs is consistent with the absence in the Pneumocystis genome of LysM effector genes, which are widely conserved among fungi and function to suppress the PAMP-triggered host innate immune response (284, 285). The potential for antigenic variation conferred by the Msg family may provide additional protection from host adaptive immunity. Given the losses noted above, retention of β-glucans in cysts is noteworthy, suggesting that they are essential to the organism, possibly by providing an aerodynamically efficient cell wall enabling airborne transmission to other hosts (132) and/or contributing to formation of protective biofilms with Msg proteins (286, 287). The absence of both β-glucan and chitin in the cell wall suggests that trophic forms are fragile (with a “wall-less” state) and presumably unable to withstand harsher environmental conditions outside the host lung environment. This may reflect the adaptation of Pneumocystis to the stable environment in the host lungs. The absence of β-glucan and chitin in trophic forms may also confer some advantages on Pneumocystis, including a cell wall with greater malleability, which may permit a closer connection with host cells to obtain nutrients.
Introns and alternative splicing.
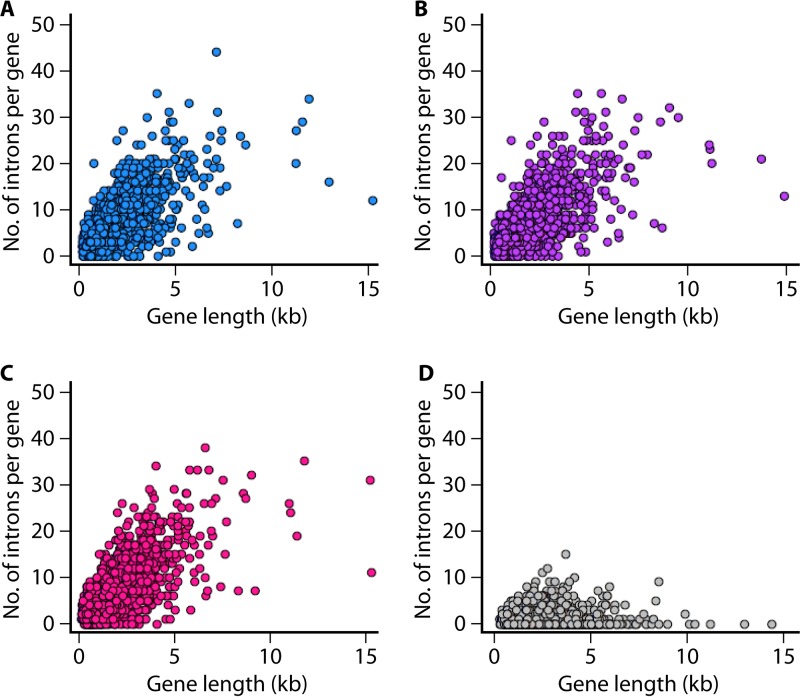
Introns can provide mRNA stability, act as regulators of gene expression (288), and promote proteome diversity via alternative splicing. Introns can also mediate RNA interference and microRNA biogenesis (289, 290). Despite the reduced genome and low gene content, the intron density is exceptionally high in Pneumocystis (averaging about 5 introns per gene) compared to those in many other fungi in which widespread loss of introns is apparent, as in S. pombe (Fig. 6) and Microsporidia (291, 292). Pneumocystis introns are small (48 bp, on average) and have a strong adenine and thymine (A+T) bias. They harbor canonical spliceosomal splicing motifs (293) and are present throughout the genomes. Intron-rich genes (>4 introns) represent 42 to 46% of the gene content.
FIG 6.
High intron densities in Pneumocystis genomes. The graphs show numbers of introns per gene as a function of gene length (measured in kilobases) for three Pneumocystis species as well as for the fission yeast Schizosaccharomyces pombe. Each dot represents a single gene. Intron densities per gene for 1,624 orthologous genes are systematically higher for Pneumocystis spp. than those for S. pombe. Intron positions and sizes were extracted from annotated GenBank files for P. jirovecii (accession no. GCA_001477535.1) in panel A, P. carinii (accession no. GCA_001477545.1) in panel B, P. murina (accession no. GCF_000349005.1) in panel C, and S. pombe (accession no. GCF_000002945.1) in panel D.
Transcription and splicing of intron-rich genes require a considerable expenditure of energy and cellular resources, as evidenced by the relative expansion in the Pneumocystis genome of RNA recognition motif (RRM)-containing genes (Fig. 2) as well as genes related to mRNA surveillance and the spliceosome (69). Such deployment in an otherwise highly compact genome suggests an essential role of introns in the organism's survival. It is possible that alternative splicing allows Pneumocystis to regulate gene expression and to increase transcript diversity from its reduced genome. In consonance with this hypothesis, earlier studies showed that Pneumocystis spp. are apparently able to use alternative splicing to respond to changes in their environment, e.g., different isoforms of the IMP dehydrogenase are produced by P. carinii in response to different short-term culture conditions (294).
The self-splicing group I introns in Pneumocystis have been well studied because of their potential as drug targets (295). They have been identified in numerous genes, such as rRNA genes (296). These introns catalyze self-excision from RNA, a process that can be inhibited by drugs, such as pentamidine and its analogues (297), which correlates with growth inhibition in C. albicans (298). Significant intron variations in rRNA genes within and among multiple Pneumocystis species have been described (299). The intron of the msg expression site of P. jirovecii is also variable, which can help in identifying strain variation (300).
Approximately 30 to 40% of introns are efficiently removed from transcripts (69), indicating a high intron retention rate, which is consistent with the dominant tendency in the fungal kingdom. The nonsense-mediated mRNA decay machinery is conserved in Pneumocystis, which suggests that mRNAs containing nonspliced introns, which encode aberrant proteins, are tagged for destruction and recycled. Although the functions of some genes of Pneumocystis can be evaluated via complementation in other fungi, direct testing of Pneumocystis introns in fungal genetic models, such as S. pombe, has failed because of an inability of the yeast spliceosome to splice them (301).
The origin and mode of acquisition of introns are unknown. The high intron density suggests that there is an advantage to conserving them, or alternatively, these elements may represent a transient stage after a massive intron proliferation. This is intriguing because intron gain is rare in many eukaryotes (302, 303); retracing the intron evolutionary history of many eukaryotic lineages shows little support for intron creation (304).
Introns evolve without the biologic constraints placed on exons and, as a consequence, have higher evolutionary change rates. This accelerated evolution can erase sequence homology clues necessary to determine their origin as well as the mechanisms that created them. Intron densities are roughly similar in all three Pneumocystis species (69), and their locations are often highly conserved, suggesting a single origin.
EPIDEMIOLOGY OF PNEUMOCYSTIS
Methods for Molecular Typing
A variety of molecular typing methods have been utilized to study strain variation and the epidemiology of Pneumocystis infection in humans. Early reviews on this topic are available (305–307). The present review serves as an update on this expanding field, with an emphasis on newer typing methods leading to new insights into the epidemiology of PCP.
Single-locus Sanger DNA sequencing.
Traditional Sanger DNA sequencing remains the most commonly used approach for single-locus typing of P. jirovecii. This method has the advantage of being able to detect all known or potentially new sequence variants in the target regions. While the emergence of newer typing methods has decreased its popularity, Sanger sequencing is still an attractive choice in many circumstances, especially with the advent of fast and low-cost commercial sequencing services. The main disadvantages of Sanger sequencing include its low throughput and the inability to differentiate mixed sequences within an amplicon, which are frequently encountered for some loci, as discussed below. Circumventing such limitations usually requires subcloning before sequencing.
Almost all genetic markers used for P. jirovecii genotyping were initially validated by Sanger sequencing, including the internal transcribed spacer 1 and 2 (ITS1 and ITS2, respectively) regions and the intron of the 26S subunit (26S rRNA) of the nuclear rRNA operon (308, 309), mitochondrial small- and large-subunit rRNA genes (mtSSU and mtLSU, respectively) (310, 311), and genes encoding cytochrome b (cob) (312, 313), thymidylate synthase (ts) (314), beta-tubulin (β-tub) (315), superoxide dismutase (sod) (106), the multifunctional product of arom (310, 316), dihydropteroate synthase (dhps) (56, 317), dihydrofolate reductase (dhfr) (54, 318), kexin (kex1) (319), and thioredoxin reductase 1 (trr1) (320).
Among these loci, ITS1 and ITS2 are the most polymorphic loci and have been used widely, often simultaneously, for typing P. jirovecii (305, 306, 309). So far, at least 60 and 62 unique genotypes at ITS1 and ITS2, respectively, have been reported in GenBank, based on worldwide studies. One drawback with these two loci is the presence of poly(T) and poly(A) tracts, which often have variable lengths within the same strains and which prevent Sanger sequencing from accurately determining the sequences downstream of these tracts (308, 309, 321). It remains unclear whether the variation in these tracts is caused by slipped-strand mispairing during in vivo DNA replication or represents artifacts generated during PCR and sequencing. Since this results in reporting the presence of two or more sequence populations within the same patient samples (309, 322–324), such polymorphisms are typically not used to identify different strains.
While mtLSU and mtSSU have also been used frequently for P. jirovecii strain typing, both appear to be less discriminatory than ITS1 and ITS2 (306, 325). Approximately 5 and 25 unique genotypes at mtLSU and mtSSU, respectively, have been reported in GenBank, based on worldwide studies. Recently, sequencing of the complete mitochondrial genomes of multiple P. jirovecii isolates identified a 1-kb noncoding region rich in polymorphic sites, including both tandem repeats and single nucleotide polymorphisms (SNPs) (99); targeted sequencing of this region identified at least 20 unique P. jirovecii genotypes in 23 clinical samples, suggesting its potential utility for typing of human isolates.
MLST.
Multilocus sequence typing (MLST) involves PCR amplification followed by DNA sequencing of multiple genes (326). The sequence of each gene in an isolate is digitally assigned a distinct allele, and the combination of alleles at all genes in each isolate defines the allelic profile or sequence type (327, 328).
Almost all known genetic markers for P. jirovecii have been evaluated for their potential to develop an MLST system (14, 329–336). While MLST has higher discriminatory power than that of single-locus typing methods, no consensus MLST scheme is currently available. Various schemes involving different genetic loci have been reported (307, 333–338), making it difficult to compare data from different laboratories (339). In fact, the application of MLST to P. jirovecii has lagged behind that for many other pathogens (340), due in part to the previously limited availability of sequenced genetic loci for this pathogen. Recent reports of whole-genome sequences of P. jirovecii (69, 97), together with increasing application of NGS, should facilitate the development of MLST methods for this pathogen.
Since it is costly and labor-intensive to amplify and sequence individual loci from individual patients, an attempt has been made to overcome this drawback by employing either DNA pooling strategies (341, 342) or simultaneous amplification of multiple loci followed by single-base extension analysis (343, 344). These approaches have potential for high-throughput application but have not been evaluated by different laboratories.
RFLP.
Restriction fragment length polymorphism analysis (RFLP), the most popular method for studying genetic variation during the 1980s and early 1990s (345), is still used frequently for typing of many organisms. This method usually involves PCR amplification of the genetic targets followed by restriction enzyme digestion, resulting in restriction fragments which are then separated by size by use of gel electrophoresis; hybridization can usually increase the sensitivity of DNA band detection but is not always needed. Similarities or differences in the band patterns reflect sequence similarities or differences. The advantages of this method include no requirement for expensive instruments, a potentially short processing time (without hybridization), and a higher sensitivity (with hybridization). The major disadvantages are its reliance on the availability of restriction sites within the targeted DNA and the interrogation of fewer polymorphic sites than those used for sequencing. Other potential disadvantages include challenges in interpretation of band patterns with minor differences and in data exchangeability between different laboratories. RFLP has been used most extensively to detect mutations in the dhps gene of P. jirovecii (346–354). Recently, RFLP was adapted to identify polymorphisms of the P. jirovecii msg repertoire (355–357); we refer to this method as msg-RFLP henceforth.
The msg-RFLP system targets an ∼1,300-bp fragment of the msg gene family in P. jirovecii referred to as the classical msg genes or the msg-A1 subfamily, comprising ∼80 genes per genome based on whole-genome sequencing (69). This target is amplified by PCR, using primers targeting highly conserved regions, followed by restriction digestion and then conventional agarose gel electrophoresis (Fig. 7) (355, 356). The main strength of msg-RFLP is that rather than examining a single or very limited number of nucleotide polymorphisms, as is the case with many available typing methods, it interrogates the entire msg repertoire of the P. jirovecii genome, thus permitting an exceptionally powerful discriminability. Indeed, in the initial report, no two isolates from different AIDS patients with PCP showed identical RFLP patterns (356). In contrast, sequential samples from the same patient (obtained within intervals of ≤3 months) displayed identical patterns, indicating a high stability within the same patient. Despite its high discriminability and stability, msg-RFLP has a major disadvantage in that it requires a minimum of ∼1,000 msg gene copies per RFLP PCR, which can be quantified by real-time quantitative PCR (356). Samples with low msg copy numbers may produce weak signals or inconsistent results. This system has been used successfully to investigate outbreaks of PCP, as discussed below.
FIG 7.
msg-RFLP analysis of P. jirovecii. Msg is encoded by a multicopy msg gene family (msg-A1 subfamily) in P. jirovecii, with an estimated 80 to 90 variable copies per genome. DNAs were extracted from respiratory samples from patients with PCP. The downstream region of the msg gene repertoire was amplified by PCR, using primers targeting conserved regions, followed by restriction digestion with the enzyme DraI and then electrophoresis in conventional agarose gels stained with SYBR green. Labels at the top represent the DNA marker (lane M) and individual patient samples (numbered lanes). (A) Samples from different HIV-infected patients with PCP, except for samples 4a and 4b, which were sequential samples from the same patient. (B) Samples from different renal transplant patients with PCP from an outbreak in Germany (345). Note that the RFLP patterns among samples from unrelated HIV patients are different from each other, whereas the RFLP patterns among the renal transplant patients are identical to each other.
SSCP.
Originally developed to detect polymorphisms of human DNA, single-strand conformation polymorphism analysis (SSCP) relies on the ability of individual nucleotide polymorphisms to change the mobility of single-stranded DNA under nondenaturing electrophoretic conditions (358). The method consists of PCR amplification followed by gel electrophoresis under nondenaturing conditions. SSCP is a relatively simple and effective method for identifying nucleotide variations within and between amplicons (∼100 to 500 bp), with the potential for rapid screening of large numbers of samples per day. The main disadvantage of this method is that its detection efficiency varies depending on various parameters, such as the size and base composition of the sequence, the electrophoresis temperature, and/or the gel composition. Thus, it is possible that some nucleotide changes may not be identified. The genetic targets typically utilized in SSCP typing of P. jirovecii include ITS1, the 26S rRNA gene, mtSSU, mtLSU, β-tub, and dhps (55, 321, 335, 338, 359–364). The main strength of SSCP for typing of P. jirovecii is its ability to distinguish multiple sequences in patients with coinfections, with a detection threshold of as little as 10%, which is usually not achievable by direct Sanger sequencing (361). This is important for studying the epidemiology of PCP given the high prevalence of coinfections (up to 92%) based on studies at various genetic loci (300, 307, 309, 361, 365–368).
VNTR analysis.
Unlike typing methods that rely on identifying nucleotide substitutions or indels in nonrepetitive loci, variable-number tandem-repeat (VNTR) analysis relies on quantifying the repeat copy numbers of short tandem repeats, also known as microsatellites. VNTR loci generally mutate at 10 to 100,000 times higher frequencies than those of nonrepetitive sequences in the genome (369), as a result of slipped-strand mispairing during DNA replication (370).
The first VNTR locus used for typing of P. jirovecii is located in the intron of the UCS of the msg gene, which contains a 10-bp VNTR motif (247, 300). The number of repeats can be determined by PCR amplification of the VNTR-containing region followed by high-resolution denaturing gel electrophoresis (300) (Fig. 8) or fluorescence capillary electrophoresis (371). A major benefit of this method is the high sensitivity for detection of a minor population in mixed populations, which can be seen in up to 92% of patients with PCP (see Table S1 in the supplemental material).
FIG 8.
Quantification of the tandem repeat copy number in the msg-UCS of P. jirovecii. The tandem repeat region in the intron of the single-copy msg-UCS gene was amplified by PCR, separated in an acrylamide sizing gel, and stained by silver staining as described by Ma et al. (300). Numbers above each lane represent individual patients. Lanes M contain a DNA size marker, with the number of repeats indicated above each DNA band. Each band within a lane represents a unique Pneumocystis strain identified in that patient. For example, lane 1 was obtained from a patient infected with three strains, lane 2 from a patient infected with two strains, and lane 5 from a patient infected with a single strain.
The discriminatory ability of the VNTR assay can be improved by sequencing the amplified DNA, since the 10-bp repeat units are not identical but have at least three different sequence types (types 1, 2, and 3) (Table S2). Isolates with the same number of repeat units can have different distribution patterns of repeat types. Application of this VNTR method to different PCP patient populations worldwide has found that the number of repeat units varies from 2 to 6, with 2, 3, and 4 repeats being the most common (300, 319, 371–374). The 6-repeat allele contains only one repeat pattern, while all other alleles reported, to date, harbor two or more different repeat patterns. In addition, SNPs are present in two positions upstream and five positions downstream of the repeat region (Table S2). Recently, an additional VNTR locus containing a 5-bp repeat unit was identified 228 bp downstream of the above-mentioned 10-bp VNTR locus, and it also contains three different sequence types (types a, b, and c) (Table S2) (373). Combining these two VNTR markers and their adjacent SNPs can improve the discriminatory power (319, 373). In fact, simultaneous use of VNTRs at different loci, termed multilocus VNTR analysis (MLVA), is a very popular approach in studying many organisms (375). This approach is facilitated by the availability of P. jirovecii genome sequences (69, 97), which has allowed the identification of a large number of tandem repeats. Two MLVA schemes, based on six or eight VNTR markers (with only one locus common to both), have been reported for samples from different patient populations (368, 376). Both schemes achieved a high discriminatory power, though additional studies with this approach are needed, including the selection of the best VNTR markers and standardization of PCR amplification protocols, repeat number quantification methods, and data reporting and transfer systems.
Contributions of NGS to molecular typing.
NGS is invaluable for the discovery, validation, and evaluation of genetic markers for strain typing. The 2012 release of the first P. jirovecii genome assembly, for a Swiss strain (97), allowed the identification of multiple VNTR loci, leading to the development of the first two MLVA schemes, reported in 2014 and 2015 (368, 376). The 2015 release of the second P. jirovecii genome assembly, for an American strain, allowed, for the first time, a genome-wide comparison of these two geographically diverse strains; 24,902 SNPs were identified by such an analysis (∼1 per 337 bases) (69).
While whole-genome sequencing and genome-wide SNP analysis are more informative than traditional typing methods (377, 378), their application to P. jirovecii is still challenging, not only because the cost of NGS is still fairly high, preventing its routine use, but also because it is difficult to obtain sufficient quantities of high-quality P. jirovecii DNA for NGS, due partly to the absence of a reliable culture method. Studies have reported enrichment to about 20% for P. jirovecii DNA by use of immunoaffinity purification followed by random whole-genome amplification (97) or oligonucleotide hybrid selection (69). However, these approaches are expensive, time-consuming, and labor-intensive; additionally, their reproducibility has not yet been established, especially for utilizing the small amounts of Pneumocystis typically available in clinical samples. There is a clear need to develop more cost-effective and innovative strategies for whole-genome sequencing of P. jirovecii.
Despite the difficulty in using NGS for P. jirovecii whole-genome sequencing, NGS was applied successfully in three studies for targeted sequencing of several commonly used genetic markers for P. jirovecii, including mtLSU, ITS2, dhfr, cob, sod, and β-tub (379–381). In these studies, three or four genetic loci from different clinical samples were amplified by PCR, pooled after barcoding, and then subjected to NGS. This approach enabled highly efficient detection of all known and new SNPs in the targets. Together with the availability of the P. jirovecii whole-genome sequence, this approach could be used to evaluate additional loci for the development of novel and more efficient MLST as well as MLVA systems.
Transmission
As ubiquitous fungal pathogens distributed throughout the world, Pneumocystis organisms found in humans and animals are morphologically similar but genetically distinct. While many questions are still unanswered regarding their epidemiology and transmission, advances in the molecular biology of Pneumocystis spp. have opened a window and afforded a better view of the following three basic questions. Where is the reservoir, what is the infectious form, and how are these organisms transmitted? Understanding these questions is essential for the development of effective control and prevention measures.
Where is the reservoir of Pneumocystis?
Initially after the recognition that Pneumocystis causes pneumonia in humans (382), PCP was believed to be a zoonosis, largely because of the morphological similarity and wide distribution of this pathogen across mammals (383). In addition, this belief was supported by a few studies showing cross-infection of nude or scid mice by intrarespiratory inoculation of Pneumocystis organisms from rats and humans (384–386). However, increasing reports of an inability to experimentally transmit Pneumocystis between various mammalian species (74, 387–391), as well as the presence of significant genetic differences among Pneumocystis organisms from different animal species (60, 392–395), led to the recognition that Pneumocystis organisms are host species specific. The organism specific for humans, P. jirovecii, has been found only in humans, not in any other mammalian species, including nonhuman primates (108, 111). Pneumocystis organisms from animals have never reliably been found in humans; rare reports of P. carinii or P. wakefieldiae DNA (identified by PCR) in patient samples (97, 308, 396–398) may represent experimental contamination (60). Taken together, the high host species specificity and the results of genome-wide sequence analyses exclude the possibility that Pneumocystis infection in humans represents zoonotic transmission.
After excluding animals as the reservoir, the next question is whether an environmental reservoir (outside the mammalian host) exists for Pneumocystis. This question has been addressed in ∼20 studies by use of PCR to detect Pneumocystis DNA or RNA (Table S3). The vast majority of these studies were carried out on air samples. Wakefield reported the detection of both P. jirovecii and P. carinii DNAs in outdoor air in rural areas of England (399). In the same study and another six studies (400–405), indoor air was also collected from animal facilities housing Pneumocystis-infected rats or rabbits, and Pneumocystis DNA was identified by PCR in all these studies. Two of these studies compared the genotypes found in air samples and infected-animal lung samples and found identical genotypes (402, 405). One study reported the detection of Pneumocystis DNA in air samples from zoological parks for monkeys, with identical sequences seen between air samples and infected-monkey lung samples (108). Another 12 studies all reported the detection of P. jirovecii DNA in indoor air samples from various locations, including homes and hospital rooms occupied by PCP patients and hospital rooms and ward corridors without PCP patients. In seven of these studies, the genotypes of the air samples were well matched to those of patient samples from the same room (325, 406–411).
The consistent detection of Pneumocystis DNA in the air in many independent studies from diverse locations strongly suggests the presence of viable Pneumocystis organisms in the air, especially in close proximity to infected hosts. Direct proof of this hypothesis would require identification of intact, viable organisms, which is challenging and has never been reported due to the very low organism load in the air (399) and the inability to culture Pneumocystis. Nevertheless, Chin et al. (412) reported the detection of Pneumocystis organisms with a thick wall by immunofluorescence staining of materials retrieved from air blower prefilters and air filters attached to microisolator cages housing P. carinii-infected rats. After storage at −80°C or room temperature for 3 to 21 weeks, these prefilters were placed in cages with immunosuppressed P. carinii-free rats; 76% of these rats developed PCP, in contrast to only 4% of control rats kept under the same conditions except for exposure to autoclaved air prefilters (413), suggesting that the organisms remain viable and infective outside mammals. The viability of organisms is also supported by the detection of P. jirovecii RNA by reverse transcription-PCR for air samples from an HIV clinic (409) and from hospital rooms occupied by patients with PCP (408).
One study from the United States reported the detection of P. jirovecii DNA in pond water samples (414). It remains uncertain if the DNA in water represents deposition of organisms from air or infected humans, or possibly a PCR artifact. Further, a study using immunosuppressed rats suggested that water is not an environmental source of Pneumocystis infection (415). Note that waterborne fungal pathogens are very rare, with only two species reported so far, including the microsporidia Encephalitozoon intestinalis and Encephalitozoon bieneusi, which cause gastrointestinal tract infections through contaminated water (416, 417).
Another potential environmental source of Pneumocystis is the soil. Many human fungal pathogens, such as Cryptococcus, Coccidioides, Blastomyces, Aspergillus, Histoplasma, and Sporothrix, normally live in soil or soil-like environments but are able to infect humans when conditions are appropriate (418, 419). One study from Japan reported the detection of P. jirovecii DNA from a hospital floor swab, which showed a genotype matching the PCP outbreak strain (420) and which may represent deposition from air. There has been no report describing Pneumocystis in soil samples, although certain soil exposures were linked to a higher risk of PCP in humans in one study (421). Nevertheless, Hughes and colleagues found that immunosuppressed rats did not develop PCP after exposure to soil spiked with P. carinii cysts (422), which argues against soil as an environmental source of Pneumocystis infection. This is consistent with the results of NGS deep sequencing of fungal communities in >14,600 soil samples from across the world, in which ∼100,000 fungal operational taxonomic units were identified, none of which matched Pneumocystis sequences (423, 424). While the failure to detect Pneumocystis sequences in these studies does not necessarily indicate its absolute absence in soil, these data suggest that, unlike the case for other fungal pathogens, soil is not a reservoir of Pneumocystis.
Based on recent genome analyses of human and rodent Pneumocystis spp., Pneumocystis has adapted to growth as an obligate pulmonary pathogen which depends on the lung for a stable supply of gas and nutrients as well as a stress-free environment (including stable pH, temperature, and osmatic pressure). This organism, particularly its trophic form, appears to be unable to survive in a harsh environment with low CO2 concentrations and other potentially harmful factors. From this point of view, soil, water, and ambient air all do not favor the growth of Pneumocystis due to the presence of too much stress and a lack of accessible nutrients and sufficient CO2 concentrations (i.e., ∼0.25% in soil, ∼0.001% in surface water, and ∼0.04% in ambient air versus ∼5% in the lung). Moreover, Pneumocystis lacks carbonic anhydrase, an enzyme critical for regulation of pH. S. cerevisiae strains that lack this enzyme are able to grow normally in 5% CO2 but demonstrate severe growth restriction in ambient air; extrapolating to Pneumocystis, vegetative growth would be difficult outside the host. However, given the presence of a relatively rigid cell wall containing β-glucans, along with the consistent detection of DNA in the air, the cyst form is likely able to survive in the air, at least transiently or in a dormant state following exhalation from the infected host. The air serves as the medium for transmission rather than as a reservoir.
Thus, current evidence, including the high level of dependence on the host, the strict host species specificity, and the potential coevolution with its host, points strongly to the conclusion that Pneumocystis can propagate only within its specific mammalian host, which also serves as the natural reservoir of infection. This is strikingly different from the case for other fungal pathogens that normally reside in the environment without a requirement for a specific mammalian host for propagation and development. The multilayered immune systems of the mammalian host play an important role in inhibiting and killing fungal invaders. However, unique among fungal pathogens, Pneumocystis has developed multilayered mechanisms to avoid both host innate and acquired immune defenses, allowing the organism to live and replicate within the host, as discussed above.
What is the infectious form of Pneumocystis?
Based on the discussion above, the cyst form of Pneumocystis may be the only form that can survive transiently outside the mammalian host, suggesting its potential role as the infectious form responsible for transmission. While this possibility has been hypothesized since at least the 1980s (425, 426), it has only recently been supported by studies with immunosuppressed rat and mouse PCP models (132). Treatment of Pneumocystis-infected rats and mice with echinocandins, which inhibit β-1,3-glucan synthesis, resulted in elimination of cysts while leaving trophic forms largely unaffected. Animals receiving this treatment could not transmit Pneumocystis to cohoused immunosuppressed Pneumocystis-free animals, while untreated infected animals were able to transmit infection, supporting the hypothesis of the cyst being the infectious form. This finding was further strengthened by a study using nude rats and cell-sorted P. carinii cyst and trophic forms (427). Rats infected with cysts alone were able to transmit the infection to receiver rats after 12 h of cohousing, while rats infected with trophic forms alone were not.
Theoretically, the cyst can initiate infection efficiently, since each cyst contains eight intracystic bodies/spores (191, 428), which presumably are released and develop into trophic forms that attach to type I pneumocytes (429–431). Further, assuming it is exhaled as a particle from an infected lung, the size of the cyst (∼5 to 8 μm) is in a range that can be deposited directly in the alveolar space following inhalation (432).
How is Pneumocystis transmitted?
The question of how Pneumocystis is transmitted has been investigated extensively, beginning soon after Pneumocystis was recognized as the causative agent of interstitial plasma cell pneumonia that occurred in malnourished infants (382). The lungs have been shown to be the primary infection site in patients and animals, consistent with genome analysis, which suggests that Pneumocystis has adapted efficiently to life in the lung environment of its mammalian host and thus is unlikely to inhabit extrapulmonary sites except in extraordinarily rare circumstances, as noted above. This concept is consistent with the absence of Pneumocystis DNA in NGS deep sequencing of fungal amplicons or metagenomic DNAs from human gut and skin samples (433–436). The absence of Pneumocystis in the skin and gut indicates that infection is not transmitted by skin-to-skin contact or the fecal-oral route.
There is overwhelming evidence that infection with Pneumocystis is acquired by inhalation. Airborne transmission has been proven under controlled conditions with various animal models, including rats, mice, rabbits, and macaques, and has also been suggested in numerous reports of humans, as reviewed by Morris et al. (437) and Nevez et al. (438). The question that remains unanswered is whether the mode of transmission is via host-to-host spread or involves a common environmental source. Given the absence of an identified environmental reservoir, in addition to the evidence supporting mammalian hosts infected with Pneumocystis as the natural reservoir of infection, with cysts as the transmissive agent (as discussed above), it seems likely that this pathogen is transmitted via airborne spread of cysts from infected hosts, rather than from a common environmental source, to susceptible hosts. Infected hosts presumably discharge infectious particles containing cysts through wheezing, coughing, or sneezing, and these are subsequently inhaled by susceptible hosts. The finding that the incidence and organism burdens of P. jirovecii in air samples decreased as the distance from PCP patients increased (439) suggests that close person-to-person contact (e.g., in the same room) can facilitate transmission.
Various animal studies have confirmed that both immunocompetent and immunodeficient hosts can become reservoirs of Pneumocystis and that airborne transmission can occur between immunocompetent hosts (440, 441), between immunodeficient hosts (132, 427, 442, 443), or between immunocompetent and immunodeficient hosts (190, 440, 444, 445), as illustrated in Fig. 9. A 12- to 24-h period of cohousing is sufficient to allow transmission in animal models (427, 440). For humans, airborne transmission has been suggested by the occurrence of clustered cases or outbreaks of PCP in different settings, including solid organ transplant units, hematology wards, pediatric oncology wards, and wards of other medical specialties (discussed below in more detail). Potential human reservoirs include people with active PCP and people with Pneumocystis colonization, which is defined as the presence of Pneumocystis organisms (usually identified by PCR-based techniques) without signs or symptoms of acute pneumonia (446, 447) and which was reviewed extensively by Morris and Norris (16).
FIG 9.
Hypothetical transmission mode of Pneumocystis in animals and humans. In this mode, cysts (indicated by green, as seen with immunofluorescence staining) serve as the infectious form and mammalian hosts (e.g., rodents on the left and humans on the right) as the reservoir for infection; transmission occurs via the airborne route between immunocompetent hosts, between immunodeficient hosts, or between immunocompetent and immunodeficient hosts. The healthy population (indicated by three individuals) is substantially larger than the immunodeficient population (indicated by one individual).
Colonization occurs with a highly variable prevalence in both healthy and diseased individuals. The prevalence of P. jirovecii colonization among healthy adults in most studies varies from 0 to 20% (16). Higher prevalences of 32 to 100% have been reported for immunocompetent infants based on microscopic or PCR studies of autopsy or nasopharyngeal samples (1, 2, 448–450). Primary exposure to Pneumocystis likely occurs commonly at an early age, as evidenced by the increasing serum antibody titers against Pneumocystis found in the first few years of life (2, 451, 452). Healthy pregnant women have been found to have a colonization prevalence of 16% during the third trimester, based on PCR studies of nasopharyngeal swabs (450, 453). Health care workers (HCWs) in close contact with PCP patients with active PCP may have an increased risk of colonization (24%) compared to that for noncontact HCWs (11%) (454). This observation is consistent with the demonstration of higher P. jirovecii antibody titers in contact HCWs than in noncontact HCWs (455, 456). These studies suggest that HCWs may participate in transmission, though no definitive studies have addressed this directly (457).
Compared to immunocompetent individuals, immunocompromised patients generally have a higher colonization prevalence. Particularly for patients with HIV infection, colonization rates of 20 to 69% have been reported (16). Colonization is also commonly seen in patients without HIV infection but with other immunocompromised conditions, including cancers, immunosuppressive therapies, autoimmune disorders, and chronic lung diseases, especially chronic obstructive pulmonary disease (COPD). These colonized patients may be a source of transmission of P. jirovecii to HCWs and other susceptible individuals.
The clinical significance of colonization remains uncertain. One study suggested that P. jirovecii colonization was associated with the pathogenesis of sudden infant death syndrome (SIDS) (458); however, this was not supported by later studies that utilized better controls (1, 459, 460). Given the high prevalence of P. jirovecii colonization in numerous studies of COPD patients (10 to 55%), as reviewed by Khodavaisy et al. (461), it has been hypothesized that colonization may contribute to the development of some lung diseases, particularly COPD (16, 462). In support of this hypothesis, studies with different patient populations have demonstrated that P. jirovecii colonization is associated with a higher risk of airway obstruction (463) and severe COPD (464) and an increased systemic inflammatory response in COPD (465, 466). In addition, studies of nonhuman primates with humanized simian-human immunodeficiency virus (SHIV) infection have suggested that Pneumocystis colonization may contribute to the development of COPD (467). However, whether this represents a causal association or indicates that damaged lungs provide a better environment for Pneumocystis colonization remains to be determined.
Understanding the reservoir of infection, mode of transmission, and susceptibility of the host has important implications for practicing and developing infection control strategies. According to the latest guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents, issued in 2017 by the U.S. Department of Health and Human Services and the Infectious Diseases Society of America (IDSA) (51), PCP infection control measures include treating patients with PCP together with prophylaxis of susceptible individuals (including HIV-infected adults and adolescents with CD4 counts of <200 cells/μl or CD4 percentages of <14%); respiratory isolation is not recommended as standard practice due to a lack of sufficient data. Nevertheless, since 2007, the Centers for Disease Control and Prevention (CDC) has recommended that a patient with PCP should not be placed in the same room with an immunodeficient patient (468). Note that the most effective approach for ending outbreaks of PCP has been widespread institution of prophylaxis among transplant recipients (14, 469, 564).
Strain Variation of P. jirovecii
Strain variation of Pneumocystis has been less well characterized than that for many other pathogens due to an inability to culture the organism. To date, no type strain (or reference strain) is available from any public microbial collection. Strain variation has been defined exclusively by molecular studies; no clear phenotypic variation has been observed within any Pneumocystis species. Strain variation of Pneumocystis was first observed in the early 1990s among Pneumocystis organisms from rats, based on electrophoretic karyotyping (470–472). Different karyotypes were identified among Pneumocystis organisms from different rat colonies. Some of these karyotypic differences were due to infections with P. carinii and P. wakefieldiae, either alone or in mixed infections (299, 473, 474). Electrophoretic karyotypes for P. jirovecii have also been determined (471), but application of this technique has been hampered severely by the limited amounts of organisms available from clinical samples. Therefore, determination of strain variation of P. jirovecii has relied largely on analysis of genetic loci after PCR amplification.
Prevalence of coinfection with multiple P. jirovecii strains in humans.
Coinfections with two or more P. jirovecii genotypes in the same patients have been observed for almost all genetic loci studied. The prevalence of coinfection is highly variable (0 to 92%), depending on the genetic loci and the detection methods used (e.g., Sanger sequencing, gel electrophoresis, or NGS). It appears clear that the detection method has a more significant impact on the prevalence than the genetic locus utilized. The majority of the strain variation studies, especially those before 2000, used Sanger sequencing and revealed coinfection prevalences of up to 30% (see Table S1 in the supplemental material). In contrast, other methods, including NGS and high-resolution gel electrophoresis (for SSCP and VNTR analyses), have consistently shown high prevalences of coinfection (up to 92%).
The lower prevalence revealed by Sanger sequencing is attributed to the low sensitivity of this method for detecting the minority sequence in samples containing mixed populations. Sanger sequencing is unable to detect a minority genotype if it is below 10 to 20% of the population (361, 475). These observations suggest that the coinfection prevalence has been underestimated in studies using Sanger sequencing. Indeed, a very recent study that analyzed 25 P. jirovecii samples by NGS identified a coinfection prevalence of 92% using mtLSU, 80% using ITS2, and 32% using dhfr as the PCR target (379). In this study, NGS was able to detect a minority genotype with a frequency as low as 0.5%; five patients (20%) showed coinfection with six distinct genotypes for mtLSU. Coinfection with six or seven genotypes was also reported in a study of ITS regions by Sanger sequencing (309), which represents the greatest heterogeneity of coinfection with Pneumocystis strains reported so far.
Relatively high prevalences of coinfection (23 to 70%) were also detected by VNTR analyses based on high-resolution gel (300) and capillary (367, 368, 376, 379) electrophoresis (Table S1). It is noteworthy that some VNTR loci, especially mono-, di-, and trinucleotide repeats, are highly unstable as a result of slipped-strand mispairing during in vivo DNA replication (370). Intrastrain instability has been observed in some di- or trinucleotide repeats of P. jirovecii (368). Variation detected at such loci may reflect their instability but not coinfection with different strains.
Despite the high prevalence of coinfection, there are no published studies on how coinfection occurs and what its potential consequences are. It is unknown if coinfection results from a single exposure to multiple strains simultaneously, resulting, e.g., from exposure to a host with preexisting infection with multiple strains, or from multiple exposures to different strains over time; mutation of an existing strain under selection pressures, including host immunity and drug pressure, seems unlikely to account for the substantial differences seen at multiple loci. It is also unknown how coinfections are distributed among immunocompetent and immunodeficient populations, how coinfecting strains interact with each other and with the host, and whether they affect host immune responses. Furthermore, it is unknown whether coinfection affects transmission and virulence (if it exists), alters disease dynamics, or affects treatment outcomes, as demonstrated in studies of more than 50 other human pathogens (476–478).
In addition to coinfection with multiple strains of P. jirovecii, there are considerable case reports of pulmonary coinfection of P. jirovecii with one or more other pathogens, including Aspergillus spp. (479–482), Cryptococcus spp. (483–485), Candida spp. (486), Histoplasma capsulatum (487), Mycobacterium tuberculosis (488–491), Legionella pneumophila (492–495), Salmonella enterica serovar Enteritidis (496), cytomegalovirus (497–506), influenza virus (507–509), herpesvirus 6 (510), Strongyloides stercoralis (511, 512), Toxoplasma gondii (513), and Trichomonas vaginalis (514). The majority of these cases occurred in patients with severe immunodeficiency. Most likely, these coinfections are more difficult to treat, potentially resulting in poorer outcomes. Further studies are need to elucidate how these coinfections behave in the lungs and what impacts they have on each other.
Strain variation provides insights into the pathogenesis of PCP.
A long-debated question has been whether clinical PCP results from reactivation of latent infection or from acquisition of a new infection. This question has been reviewed in detail previously (16, 437, 515). Briefly, epidemiological evidence in favor of the former mechanism includes the high seroprevalence of anti-Pneumocystis antibodies in healthy children early in life (452, 516), the high prevalence of colonization of Pneumocystis detected by PCR in immunocompetent infants (1) and neonatal rats (517), and the high prevalence of PCP in children with immunodeficiency or malnutrition (4, 518, 519). Epidemiological evidence supporting the latter mechanism includes the presence of Pneumocystis DNA in the air (Table S3), animal-to-animal air transmission under controlled conditions, geographic clustering of PCP in HIV-infected patients (520, 521), and outbreaks of PCP in various organ transplant centers (14, 522, 523). However, it is hard to differentiate these two mechanisms based simply on these epidemiological data.
Strain variation studies performed by genotyping have significantly extended our understanding of these two mechanisms. There is increasingly convincing evidence from genotyping studies supporting recent acquisition in at least some cases. This includes the presence of different genotypes between the first and second episodes in patients with recurrent infection (366, 524, 525); outbreaks of PCP caused by a single strain within one or more organ transplant centers, based on highly discriminative msg-RFLP or MLST, as discussed below (355, 357, 526, 527); and the occurrence of sulfa resistance-associated dhps mutations in patients without prior exposure to sulfa drugs (54, 57, 528, 529). Recent acquisition is further supported by the high prevalence of multistrain coinfections as discussed in the preceding section. These coinfections, particular those involving a mixture of three to seven strains, may represent continuous acquisition of new strains from different infected or colonized individuals over time.
Nonetheless, genotyping studies have also provided evidence in favor of reactivation of latent infection, including the presence of the same genotypes between the first and second episodes in patients with recurrent infection (366) and the high diversity of genotypes among different patients in many studies, especially the msg-RFLP studies (356, 530). The msg-RFLP system is highly discriminatory and has found remarkable variation among unrelated P. jirovecii isolates: no two isolates from >50 different patients shared an identical genotype profile. This implies that each patient is infected with a unique strain. This finding, together with the high level of adaptation to the lung environment and strict host specificity, suggests possible colonization and persistence in a latent state. The existence of a latent state for Pneumocystis is supported by its slow growth and ability to simultaneously evade both the host innate and acquired immunities as suggested by genome analysis (69). However, proving reactivation of a latent infection in humans is extremely difficult and would ideally require demonstrating genotypic identity between a strain acquired earlier in life (e.g., during primary infection as an infant) and one that is subsequently causing clinical pneumonia many years later.
Thus, while there is strong evidence to support recent acquisition in some cases, reactivation in other cases cannot currently be excluded. It is possible that both mechanisms can occur. A more detailed understanding of these mechanisms may facilitate the rational design of approaches for disease management and control. If reactivation of latent infection is the primary mechanism, there is no need for respiratory isolation of patients with PCP other than protection of susceptible individuals by prophylaxis. If de novo infection is the primary mechanism, preventing infected patients from transmitting Pneumocystis to others, particularly to immunocompromised patients, would have a more important role.
Strain variation and PCP outbreaks in organ transplant patients.
As an infectious agent, Pneumocystis appears to be generally less pathogenic or virulent than many other human pathogens. However, outbreaks of PCP have been reported under various conditions across the world. The epidemic of interstitial pneumonia in European premature infants and malnourished children in the mid-20th century led to the initial recognition of Pneumocystis as a human pathogen (3, 4). Multiple outbreaks of PCP among apparently healthy individuals in the early 1980s (5, 531–533) not only heralded the onset of the HIV/AIDS epidemic but also led to the recognition of PCP as the leading cause of morbidity and mortality in HIV/AIDS. Although the incidence of PCP in HIV-infected patients dramatically declined with the introduction of cART and prophylaxis, its incidence among non-HIV-infected immunocompromised patients has been increasing. Over the last 2 decades, outbreaks of PCP have been reported frequently, as recently reviewed by de Boer et al. (14) and Yiannakis and Boswell (15). These two reviews summarize 30 nosocomial outbreaks of PCP between 1982 and 2013, involving 486 patients across 12 countries, predominantly in Europe. One of the most striking features of these outbreaks is that the majority (83%) of them occurred in patients undergoing solid organ transplantation, particularly renal transplantation. A minor portion of outbreaks occurred in pediatric oncology (6%), hematological malignancy (6%), and rheumatoid arthritis (3%) patients. In all these outbreaks, the affected patients received no or suboptimal prophylaxis for PCP.
These outbreaks have attracted intense global efforts to understand their causes, underlying mechanisms, and potential intervention strategies. Early epidemiological investigations implicated certain immunosuppressive regimens as a risk factor for PCP development in outbreaks (534, 535), but this observation was not supported in other studies (536). In the majority of epidemiological studies, contact tracing analysis found colocalization of cases within clinic areas, suggesting the possibility of person-to-person or nosocomial spread of infection. Molecular typing studies, primarily for outbreaks in renal transplant patients occurring after 2000, provided strong evidence in support of this (15). All these typing studies, which primarily utilized MLST, identified cases with an identical genotype profile in more than one patient. More strikingly, a predominant strain, often even a single strain, of P. jirovecii was identified in 13 (81%) of the 16 outbreaks. Furthermore, three distant outbreaks, in Switzerland and Germany, were linked to a single P. jirovecii strain (355, 537). These findings strongly support the recent acquisition of infection through person-to-person transmission and also highlight that outbreaks of PCP can be understood better by an improved understanding of organism strain variation as well as patterns of transmission.
However, the origins of these outbreaks have not been defined. It remains unknown why such outbreaks prevail in renal transplant recipients and whether they are due to the introduction of specific P. jirovecii strains (e.g., with enhanced virulence) into the transplant environment or to specific conditions that may increase patients' susceptibility to infection (such as certain immunosuppressive regimens or rejection treatment protocols). The experience from these outbreaks illustrates the practical utility of strain variation in elucidating the epidemiology of PCP, underlines the necessity of utilizing prophylaxis in renal transplant recipients, and supports the recommendation to avoid placement of a patient with PCP in the same room as that of an immunocompromised patient per the current CDC guidelines (468).
Strain variation and drug resistance.
The widespread use of TMP-SMX over the last 3 decades for both the treatment and prevention of PCP has raised concern about the development of drug resistance by P. jirovecii. The lack of a culture system has precluded the use of routine in vitro susceptibility testing to determine drug resistance in Pneumocystis. To overcome this problem, researchers have attempted to address this concern indirectly by determining genetic variations in the P. jirovecii dhfr and dhps genes, the targets of TMP and sulfa (including SMX), respectively, in clinical strains and then correlating strain variations with clinical characteristics of the PCP patients and with homologous mutations known to be associated with drug resistance in other organisms.
Genetic variation in the P. jirovecii dhps gene was first described in 1997 for six patient isolates; nonsynonymous nucleotide mutations involving amino acid changes were identified at six codons, suggesting a positive selective pressure, possibly as a result of sulfa exposure. Subsequently, two of these mutations (at amino acids 55 and 57) were identified in numerous studies of PCP patients throughout the world, with prevalences of 3 to 81% (reviewed in references 53, 538, and 539). The majority of these studies demonstrated that both mutations are associated with prior exposure to and failure of TMP-SMX and dapsone, primarily when administered as prophylaxis. Both mutations are at the active sites of the Dhps enzyme, based on homology to the crystal structure of Dhps in Escherichia coli (540). Mutations at or very near these positions are likely to alter the local structure and thus affect the binding of the substrate and sulfa. Mutations at these positions confer resistance to sulfa drugs in other organisms, including E. coli (541), Streptococcus pneumoniae (542), Neisseria meningitidis (543), and Plasmodium falciparum (544). All these observations strongly suggest that P. jirovecii has developed some level of resistance to sulfa drugs.
The clinical significance of dhps mutations in relation to sulfa resistance remains unclear. Several studies showed associations of dhps mutations with poor outcomes for HIV-infected patients with PCP, including increased mortality (545) and treatment failure (58, 546), but these associations were not supported in other studies (547, 548).
Genetic variations in the P. jirovecii dhfr gene have been less well studied. In about a dozen studies of clinical isolates worldwide (54, 528, 549–551), no dhfr mutations were detected, other than rare synonymous nucleotide substitutions. In contrast, extensive mutations at more than 30 amino acid positions were reported in another half dozen studies, from Japan (318, 552), Portugal (320, 553), South Africa (554), Switzerland, and France (555). Each of these mutations occurred at a low frequency (≤1%); a small portion of isolates (2%) contained mutations at two or more positions simultaneously (555, 556). The greatest number of dhfr mutations was reported by Nahimana et al. (555), who identified 16 mutations in 11 (33%) of 33 clinical isolates. In the same study, patients with failure of prophylaxis, including that with a Dhfr inhibitor, were found to be more likely to harbor at least one dhfr mutation than those without such prophylaxis, though no single mutation or pattern of mutations was associated with failure. Notably, mutations were seen primarily in patients receiving pyrimethamine rather than trimethoprim as part of their prophylactic regimens; this drug is used infrequently as prophylaxis in other centers. By in vitro testing of recombinant P. jirovecii Dhfr enzymes containing known mutations, six Dhfr variants were found to be resistant to TMP, with Ki values that were 4- to 100-fold higher than those for the native enzyme (52), suggesting that these mutations may contribute to clinical resistance.
The consistently lower prevalence of mutations in dhfr than in dhps in the vast majority of reported studies suggests less selective pressure on dhfr than on dhps and further suggests that the dhps mutations are not random occurrences but rather the result of drug pressure. In agreement with the hypothesis of low pressure on dhfr, in vitro inhibition assays using recombinant enzymes (557) and yeast complementation (558) demonstrated that TMP as well as, to a lesser extent, pyrimethamine is a relatively poor inhibitor of wild-type P. jirovecii Dhfr. These findings support the concept that TMP contributes little to the effectiveness of TMP-SMX against P. jirovecii, which may in fact function as sulfa monotherapy (26, 54).
In addition to the emergence of sulfa resistance, there are a few studies suggesting that P. jirovecii may also be developing resistance to atovaquone, an alternative regimen for treating and preventing PCP (559). Atovaquone targets the mitochondrial gene cytochrome b (cob). Mutations in cob associated with atovaquone resistance have been well documented for malaria (560). Sequence analysis of the P. jirovecii cob genes from >70 clinical isolates from the United States and the United Kingdom identified seven different mutations, with only two present in more than one isolate (313, 561). These mutations were significantly more frequent in patients who received atovaquone, implying a positive selection pressure (561). When these seven mutations were introduced into the S. cerevisiae cob gene, five resulted in increases of the atovaquone 50% inhibitory concentration (IC50), to >150 nM, compared to 25 nM for the wild type (562). In addition, structural modeling analysis suggests that these mutations directly interfere with atovaquone binding (562). These data support the hypothesis that cob mutations are involved in the development of atovaquone resistance in P. jirovecii. However, no reports, to date, have demonstrated an association of these mutations with treatment outcomes.
Based on currently available data, genotypic resistance testing remains an investigational tool to be utilized in a research setting, and clinical decisions should not be made based on such testing; prophylaxis should continue to be administered to susceptible individuals. Most patients with retrospectively identified dhps mutations responded appropriately to treatment with standard doses of TMP-SMX. dhps mutations thus appear to represent a low-level resistance that can be overcome by higher doses of TMP-SMX. Given that Pneumocystis has already shown that it can develop mutations in response to drug pressure, a greater concern is that subsequent additional mutations may result in high-level resistance, potentially leading to the loss of the most effective drug for managing Pneumocystis infection.
Note that mutations in all three drug targets described above (dhps, dhfr, and cob) were also detected in PCP patients who had no known prior exposure to the implicated drugs. The presence of mutations in these patients may suggest direct acquisition of mutant P. jirovecii strains via person-to-person transmission. Therefore, these mutations, together with synonymous nucleotide changes in these genes, may be useful as markers in epidemiological studies (320, 336, 339, 563).
CONCLUDING REMARKS
Pneumocystis is truly a unique organism with unusual lifestyle and biological traits, which makes it a fascinating and at the same time difficult organism to study. Thanks to advances in science and technology and the continuing commitment of the Pneumocystis research community, a new window has been opened into the biology and epidemiology of Pneumocystis. Much of the mystery surrounding this somewhat elusive pathogen has begun to unravel, especially its refined taxonomic classification as a member of the Taphrinomycotina, its extremely reduced genome and concomitant inability to metabolize and grow independently of a mammalian host, its reliance on cysts as the infectious form and on mammalian hosts as the infection reservoir for airborne transmission, its widespread colonization in both immunocompetent and immunodeficient hosts, and its strain variation related to drug resistance, pathogenesis, and outbreaks of infection among transplant patients. These advances have greatly improved our understanding of Pneumocystis as a prominent pathogen. However, significant questions remain unanswered about many aspects of Pneumocystis biology and epidemiology. The most challenging issue is the lack of a continuous in vitro culture method, which has increasingly been recognized as the major bottleneck of Pneumocystis research. In order to resolve this issue, it may be necessary to leverage new and innovative strategies, which may include collaboration across different research teams. A reliable culture system is urgently needed to provide a better understanding of the Pneumocystis life cycle, host specificity, strain variation, and mechanisms of antigenic variation and development of drug resistance. This will allow the development of improved methods for identifying potential new therapeutic targets and screening of new drugs and will potentially allow the development of methods to genetically manipulate the organism. Nonetheless, the application of modern molecular biologic techniques has provided a strong foundation upon which to build the next major advances in understanding the biology and epidemiology of this family of atypical fungi.
ACKNOWLEDGMENTS
Work in our laboratory was supported by federal funds from the Intramural Research Program of the U.S. National Institutes of Health Clinical Center.
Initial Pneumocystis genome analysis was carried out in collaboration with Christina A. Cuomo and Zehua Chen and their colleagues at the Broad Institute of Harvard and the Massachusetts Institute of Technology and with Richard A. Lempicki and Da Wei Huang and their colleagues at Leidos BioMedical Research, Inc. We thank Louise Walker and Neil Gow, University of Aberdeen, United Kingdom, for providing the electron microscopic picture of Candida albicans as well as for helpful comments on Fig. 3; Leho Tedersoo, University of Tartu, Estonia, for information about Pneumocystis sequences in global soil samples; Serena Dollive and Frederic Bushman, University of Pennsylvania, for confirming the absence of Pneumocystis sequences in their human stool microbiome data; and Julia Segre and Heidi Kong, National Institutes of Health, for information about Pneumocystis sequences in human skin microbiome studies. We are also grateful to the following investigators for discussions and comments on cholesterol biosynthesis pathways: Melanie Cushion, University of Cincinnati; W. David Nes, Texas Tech University; Andrew Brown, University of New South Wales, Australia; and Masato Ohashi, National Institute for Physiological Sciences, Japan. Special thanks are given to artist Patrick Lane of ScEYEnce Studios for improving the figures.
We declare that we have no competing interests.
Biographies

Liang Ma received his M.D. and Ph.D. from Chongqing Medical University in China. After completing a guest research fellowship at the Gifu University School of Medicine in Japan, he came to the National Institutes of Health (NIH), Bethesda, MD, as a Fogarty Visiting Fellow. Subsequently, he joined the faculty at Loyola University Chicago and then at the Louisiana State University Health Science Center in New Orleans, LA. In 2009, he returned to the NIH as a Staff Scientist in the Critical Care Medicine Department of the NIH Clinical Center. He has extensive experience in utilizing molecular biology to study various human pathogens that include bacteria, protozoa, helminths, and fungi. His current research interests focus on the biology and epidemiology of Pneumocystis. In collaboration with investigators in North American, Asia, and Europe, he is investigating various aspects of Pneumocystis infection, including genomics, evolution, proteomics, metabolism, and host immune responses.

Ousmane H. Cissé is currently a Fogarty Postdoctoral Visiting Fellow in the Critical Care Department at the National Institutes of Health, where he has worked since 2015. Ousmane completed his Pharm.D. studies at the University of Bamako (Mali) in 2005 and worked as a research scientist at the University of Bamako from 2005 to 2008. He received his Ph.D. from the University of Lausanne (Switzerland) in 2013 and completed a Swiss National Science Foundation-funded postdoctoral fellowship at the University of California Riverside (USA) from 2013 to 2015. His research interests lie in the area of evolutionary microbiology, ranging from population genetics to the emergence of complex multicellular phenotypes, with a special interest in fungal pathogens. Ousmane has been active in the field of Pneumocystis for 6 years and firmly believes that the study of Pneumocystis species provides a rare opportunity to better understand fundamental aspects of pathogen evolution.

Joseph A. Kovacs received his medical degree from Cornell University Medical College. Following his residency in internal medicine at The New York Hospital/Cornell, he completed a fellowship in infectious diseases and critical care at the NIH, Bethesda, MD. He remained at the NIH and is currently a Senior Investigator and Section Chief in the Critical Care Medicine Department, NIH Clinical Center, and an attending physician in the NIAID-CCMD HIV/AIDS program. He is also an Associate Clinical Professor of Medicine, The George Washington University School of Medicine, and an attending physician at the Medstar Washington Hospital Center. His research interests include understanding the basic biology of and host immune responses to Pneumocystis infection and developing improved methods for diagnosis, treatment, and prevention of Pneumocystis pneumonia. His clinical research focuses on better understanding the infectious and noninfectious complications of HIV infection and evaluating novel immune-based therapies for HIV infection.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CMR.00009-18.
REFERENCES
- 1.Beard CB, Fox MR, Lawrence GG, Guarner J, Hanzlick RL, Huang L, del Rio C, Rimland D, Duchin JS, Colley DG. 2005. Genetic differences in Pneumocystis isolates recovered from immunocompetent infants and from adults with AIDS: epidemiological implications. J Infect Dis 192:1815–1818. doi: 10.1086/497381. [DOI] [PubMed] [Google Scholar]
- 2.Vargas SL, Hughes WT, Santolaya ME, Ulloa AV, Ponce CA, Cabrera CE, Cumsille F, Gigliotti F. 2001. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis 32:855–861. doi: 10.1086/319340. [DOI] [PubMed] [Google Scholar]
- 3.Gajdusek DC. 1957. Pneumocystis carinii; etiologic agent of interstitial plasma cell pneumonia of premature and young infants. Pediatrics 19:543–565. [PubMed] [Google Scholar]
- 4.Goldman AS, Goldman LR, Goldman DA. 2005. What caused the epidemic of Pneumocystis pneumonia in European premature infants in the mid-20th century? Pediatrics 115:e725–e736. doi: 10.1542/peds.2004-2157. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1982. A cluster of Kaposi's sarcoma and Pneumocystis carinii pneumonia among homosexual male residents of Los Angeles and Orange Counties, California. MMWR Morb Mortal Wkly Rep 31:305–307. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2001. HIV and AIDS—United States, 1981–2000. MMWR Morb Mortal Wkly Rep 50:430–434. [PubMed] [Google Scholar]
- 7.Buchacz K, Lau B, Jing Y, Bosch R, Abraham AG, Gill MJ, Silverberg MJ, Goedert JJ, Sterling TR, Althoff KN, Martin JN, Burkholder G, Gandhi N, Samji H, Patel P, Rachlis A, Thorne JE, Napravnik S, Henry K, Mayor A, Gebo K, Gange SJ, Moore RD, Brooks JT, North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. 2016. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000–2010. J Infect Dis 214:862–872. doi: 10.1093/infdis/jiw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fei MW, Kim EJ, Sant CA, Jarlsberg LG, Davis JL, Swartzman A, Huang L. 2009. Predicting mortality from HIV-associated Pneumocystis pneumonia at illness presentation: an observational cohort study. Thorax 64:1070–1076. doi: 10.1136/thx.2009.117846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fei MW, Sant CA, Kim EJ, Swartzman A, Davis JL, Jarlsberg LG, Huang L. 2009. Severity and outcomes of Pneumocystis pneumonia in patients newly diagnosed with HIV infection: an observational cohort study. Scand J Infect Dis 41:672–678. doi: 10.1080/00365540903051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundberg BE, Davidson AJ, Burman WJ. 2000. Epidemiology of Pneumocystis carinii pneumonia in an era of effective prophylaxis: the relative contribution of non-adherence and drug failure. AIDS 14:2559–2566. doi: 10.1097/00002030-200011100-00019. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AJ, O'Donnell AE. 2001. Pulmonary manifestations of HIV infection in the era of highly active antiretroviral therapy. Chest 120:1888–1893. doi: 10.1378/chest.120.6.1888. [DOI] [PubMed] [Google Scholar]
- 12.Reid AB, Chen SC, Worth LJ. 2011. Pneumocystis jirovecii pneumonia in non-HIV-infected patients: new risks and diagnostic tools. Curr Opin Infect Dis 24:534–544. doi: 10.1097/QCO.0b013e32834cac17. [DOI] [PubMed] [Google Scholar]
- 13.Roux A, Gonzalez F, Roux M, Mehrad M, Menotti J, Zahar JR, Tadros VX, Azoulay E, Brillet PY, Vincent F. 2014. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect 44:185–198. doi: 10.1016/j.medmal.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 14.de Boer MG, de Fijter JW, Kroon FP. 2011. Outbreaks and clustering of Pneumocystis pneumonia in kidney transplant recipients: a systematic review. Med Mycol 49:673–680. [DOI] [PubMed] [Google Scholar]
- 15.Yiannakis EP, Boswell TC. 2016. Systematic review of outbreaks of Pneumocystis jirovecii pneumonia: evidence that P. jirovecii is a transmissible organism and the implications for healthcare infection control. J Hosp Infect 93:1–8. doi: 10.1016/j.jhin.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Morris A, Norris KA. 2012. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 25:297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs JA, Masur H. 2009. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 301:2578–2585. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs A, Frederick T, Church J, Eller A, Oxtoby M, Mascola L. 1991. CD4 T-lymphocyte counts and Pneumocystis carinii pneumonia in pediatric HIV infection. JAMA 265:1698–1703. [PubMed] [Google Scholar]
- 19.Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saah A. 1990. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS Cohort Study Group. N Engl J Med 322:161–165. [DOI] [PubMed] [Google Scholar]
- 20.Baulier G, Issa N, Gabriel F, Accoceberry I, Camou F, Duffau P. 2018. Guidelines for prophylaxis of Pneumocystis pneumonia cannot rely solely on CD4-cell count in autoimmune and inflammatory diseases. Clin Exp Rheumatol 36:490–493. [PubMed] [Google Scholar]
- 21.Guo F, Chen Y, Yang SL, Xia H, Li XW, Tong ZH. 2014. Pneumocystis pneumonia in HIV-infected and immunocompromised non-HIV infected patients: a retrospective study of two centers in China. PLoS One 9:e101943. doi: 10.1371/journal.pone.0101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng VL, Yajko DM, Hadley WK. 1997. Extrapulmonary pneumocystosis. Clin Microbiol Rev 10:401–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White PL, Backx M, Barnes RA. 2017. Diagnosis and management of Pneumocystis jirovecii infection. Expert Rev Anti Infect Ther 15:435–447. doi: 10.1080/14787210.2017.1305887. [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Ren Y, Wang X, Li R. 2016. Recent advances in the diagnosis of Pneumocystis pneumonia. Med Mycol J 57:E111–E116. doi: 10.3314/mmj.16-00019. [DOI] [PubMed] [Google Scholar]
- 25.Tasaka S, Tokuda H. 2013. Recent advances in the diagnosis of Pneumocystis jirovecii pneumonia in HIV-infected adults. Expert Opin Med Diagn 7:85–97. doi: 10.1517/17530059.2012.722080. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs JA, Gill VJ, Meshnick S, Masur H. 2001. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia. JAMA 286:2450–2460. doi: 10.1001/jama.286.19.2450. [DOI] [PubMed] [Google Scholar]
- 27.Blumenfeld W, Kovacs JA. 1988. Use of a monoclonal antibody to detect Pneumocystis carinii in induced sputum and bronchoalveolar lavage fluid by immunoperoxidase staining. Arch Pathol Lab Med 112:1233–1236. [PubMed] [Google Scholar]
- 28.Kovacs JA, Ng VL, Masur H, Leoung G, Hadley WK, Evans G, Lane HC, Ognibene FP, Shelhamer J, Parrillo JE, Vee JG. 1988. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med 318:589–593. doi: 10.1056/NEJM198803103181001. [DOI] [PubMed] [Google Scholar]
- 29.Siegel M, Masur H, Kovacs J. 2016. Pneumocystis jirovecii pneumonia in human immunodeficiency virus infection. Semin Respir Crit Care Med 37:243–256. doi: 10.1055/s-0036-1579556. [DOI] [PubMed] [Google Scholar]
- 30.Djawe K, Huang L, Daly KR, Levin L, Koch J, Schwartzman A, Fong S, Roth B, Subramanian A, Grieco K, Jarlsberg L, Walzer PD. 2010. Serum antibody levels to the Pneumocystis jirovecii major surface glycoprotein in the diagnosis of P. jirovecii pneumonia in HIV+ patients. PLoS One 5:e14259. doi: 10.1371/journal.pone.0014259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop LR, Kovacs JA. 2003. Quantitation of anti-Pneumocystis jiroveci antibodies in healthy persons and immunocompromised patients. J Infect Dis 187:1844–1848. doi: 10.1086/375354. [DOI] [PubMed] [Google Scholar]
- 32.Gingo MR, Lucht L, Daly KR, Djawe K, Palella FJ, Abraham AG, Bream JH, Witt MD, Kingsley LA, Norris KA, Walzer PD, Morris A. 2011. Serologic responses to Pneumocystis proteins in HIV patients with and without Pneumocystis jirovecii pneumonia. J Acquir Immune Defic Syndr 57:190–196. doi: 10.1097/QAI.0b013e3182167516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walzer PD, Djawe K, Levin L, Daly KR, Koch J, Kingsley L, Witt M, Golub ET, Bream JH, Taiwo B, Morris A. 2009. Long-term serologic responses to the Pneumocystis jirovecii major surface glycoprotein in HIV-positive individuals with and without P. jirovecii infection. J Infect Dis 199:1335–1344. doi: 10.1086/597803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daly KR, Huang L, Morris A, Koch J, Crothers K, Levin L, Eiser S, Satwah S, Zucchi P, Walzer PD. 2006. Antibody response to Pneumocystis jirovecii major surface glycoprotein. Emerg Infect Dis 12:1231–1237. doi: 10.3201/eid1708.060230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daly KR, Koch J, Levin L, Walzer PD. 2004. Enzyme-linked immunosorbent assay and serologic responses to Pneumocystis jiroveci. Emerg Infect Dis 10:848–854. doi: 10.3201/eid1005.030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. 2013. Accuracy of beta-d-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect 19:39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 37.Li WJ, Guo YL, Liu TJ, Wang K, Kong JL. 2015. Diagnosis of Pneumocystis pneumonia using serum (1-3)-beta-d-glucan: a bivariate meta-analysis and systematic review. J Thorac Dis 7:2214–2225. doi: 10.3978/j.issn.2072-1439.2015.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White PL, Price JS, Posso RB, Barnes RA. 2017. An evaluation of the performance of the Dynamiker(R) fungus (1-3)-beta-d-glucan assay to assist in the diagnosis of invasive aspergillosis, invasive candidiasis and Pneumocystis pneumonia. Med Mycol 55:843–850. doi: 10.1093/mmy/myx004. [DOI] [PubMed] [Google Scholar]
- 39.Wakefield AE, Guiver L, Miller RF, Hopkin JM. 1991. DNA amplification on induced sputum samples for diagnosis of Pneumocystis carinii pneumonia. Lancet 337:1378–1379. doi: 10.1016/0140-6736(91)93062-E. [DOI] [PubMed] [Google Scholar]
- 40.Huang SN, Fischer SH, O'Shaughnessy E, Gill VJ, Masur H, Kovacs JA. 1999. Development of a PCR assay for diagnosis of Pneumocystis carinii pneumonia based on amplification of the multicopy major surface glycoprotein gene family. Diagn Microbiol Infect Dis 35:27–32. doi: 10.1016/S0732-8893(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 41.Fischer S, Gill VJ, Kovacs J, Miele P, Keary J, Silcott V, Huang S, Borio L, Stock F, Fahle G, Brown D, Hahn B, Townley E, Lucey D, Masur H. 2001. The use of oral washes to diagnose Pneumocystis carinii pneumonia: a blinded prospective study using a polymerase chain reaction-based detection system. J Infect Dis 184:1485–1488. doi: 10.1086/324520. [DOI] [PubMed] [Google Scholar]
- 42.Larsen HH, Huang L, Kovacs JA, Crothers K, Silcott VA, Morris A, Turner JR, Beard CB, Masur H, Fischer SH. 2004. A prospective, blinded study of quantitative touch-down polymerase chain reaction using oral-wash samples for diagnosis of Pneumocystis pneumonia in HIV-infected patients. J Infect Dis 189:1679–1683. doi: 10.1086/383322. [DOI] [PubMed] [Google Scholar]
- 43.Torres J, Goldman M, Wheat LJ, Tang X, Bartlett MS, Smith JW, Allen SD, Lee CH. 2000. Diagnosis of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected patients with polymerase chain reaction: a blinded comparison to standard methods. Clin Infect Dis 30:141–145. doi: 10.1086/313584. [DOI] [PubMed] [Google Scholar]
- 44.Fauchier T, Hasseine L, Gari-Toussaint M, Casanova V, Marty PM, Pomares C. 2016. Detection of Pneumocystis jirovecii by quantitative PCR to differentiate colonization and pneumonia in immunocompromised HIV-positive and HIV-negative patients. J Clin Microbiol 54:1487–1495. doi: 10.1128/JCM.03174-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atzori C, Lu JJ, Jiang B, Bartlett MS, Orlando G, Queener SF, Smith JW, Cargnel A, Lee CH. 1995. Diagnosis of Pneumocystis carinii pneumonia in AIDS patients by using polymerase chain reactions on serum specimens. J Infect Dis 172:1623–1626. doi: 10.1093/infdis/172.6.1623. [DOI] [PubMed] [Google Scholar]
- 46.Lipschik GY, Gill VJ, Lundgren JD, Andrawis VA, Nelson NA, Nielsen JO, Ognibene FP, Kovacs JA. 1992. Improved diagnosis of Pneumocystis carinii infection by polymerase chain reaction on induced sputum and blood. Lancet 340:203–206. doi: 10.1016/0140-6736(92)90469-J. [DOI] [PubMed] [Google Scholar]
- 47.Montesinos I, Delforge ML, Ajjaham F, Brancart F, Hites M, Jacobs F, Denis O. 2017. Evaluation of a new commercial real-time PCR assay for diagnosis of Pneumocystis jirovecii pneumonia and identification of dihydropteroate synthase (DHPS) mutations. Diagn Microbiol Infect Dis 87:32–36. doi: 10.1016/j.diagmicrobio.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Hoarau G, Le Gal S, Zunic P, Poubeau P, Antok E, Jaubert J, Nevez G, Picot S. 2017. Evaluation of quantitative FTD-Pneumocystis jirovecii kit for Pneumocystis infection diagnosis. Diagn Microbiol Infect Dis 89:212–217. doi: 10.1016/j.diagmicrobio.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Guillaud-Saumur T, Nevez G, Bazire A, Virmaux M, Papon N, Le Gal S. 2017. Comparison of a commercial real-time PCR assay, RealCycler(R) PJIR kit, Progenie Molecular, to an in-house real-time PCR assay for the diagnosis of Pneumocystis jirovecii infections. Diagn Microbiol Infect Dis 87:335–337. doi: 10.1016/j.diagmicrobio.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Sasso M, Chastang-Dumas E, Bastide S, Alonso S, Lechiche C, Bourgeois N, Lachaud L. 2016. Performances of four real-time PCR assays for diagnosis of Pneumocystis jirovecii pneumonia. J Clin Microbiol 54:625–630. doi: 10.1128/JCM.02876-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. 2017. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf Accessed 19 October 2017.
- 52.Queener SF, Cody V, Pace J, Torkelson P, Gangjee A. 2013. Trimethoprim resistance of dihydrofolate reductase variants from clinical isolates of Pneumocystis jirovecii. Antimicrob Agents Chemother 57:4990–4998. doi: 10.1128/AAC.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang L, Crothers K, Atzori C, Benfield T, Miller R, Rabodonirina M, Helweg-Larsen J. 2004. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg Infect Dis 10:1721–1728. doi: 10.3201/eid1010.030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma L, Borio L, Masur H, Kovacs JA. 1999. Pneumocystis carinii dihydropteroate synthase but not dihydrofolate reductase gene mutations correlate with prior trimethoprim-sulfamethoxazole or dapsone use. J Infect Dis 180:1969–1978. doi: 10.1086/315148. [DOI] [PubMed] [Google Scholar]
- 55.Ma L, Kovacs JA, Cargnel A, Valerio A, Fantoni G, Atzori C. 2002. Mutations in the dihydropteroate synthase gene of human-derived Pneumocystis carinii isolates from Italy are infrequent but correlate with prior sulfa prophylaxis. J Infect Dis 185:1530–1532. doi: 10.1086/340220. [DOI] [PubMed] [Google Scholar]
- 56.Mei Q, Gurunathan S, Masur H, Kovacs JA. 1998. Failure of co-trimoxazole in Pneumocystis carinii infection and mutations in dihydropteroate synthase gene. Lancet 351:1631–1632. [DOI] [PubMed] [Google Scholar]
- 57.Kazanjian P, Locke AB, Hossler PA, Lane BR, Bartlett MS, Smith JW, Cannon M, Meshnick SR. 1998. Pneumocystis carinii mutations associated with sulfa and sulfone prophylaxis failures in AIDS patients. AIDS 12:873–878. doi: 10.1097/00002030-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Kazanjian P, Armstrong W, Hossler PA, Burman W, Richardson J, Lee CH, Crane L, Katz J, Meshnick SR. 2000. Pneumocystis carinii mutations are associated with duration of sulfa or sulfone prophylaxis exposure in AIDS patients. J Infect Dis 182:551–557. doi: 10.1086/315719. [DOI] [PubMed] [Google Scholar]
- 59.Cailliez JC, Seguy N, Denis CM, Aliouat EM, Mazars E, Polonelli L, Camus D, Dei-Cas E. 1996. Pneumocystis carinii: an atypical fungal micro-organism. J Med Vet Mycol 34:227–239. doi: 10.1080/02681219680000401. [DOI] [PubMed] [Google Scholar]
- 60.Stringer JR. 1996. Pneumocystis carinii: what is it, exactly? Clin Microbiol Rev 9:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edman JC, Kovacs JA, Masur H, Santi DV, Elwood HJ, Sogin ML. 1988. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature 334:519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- 62.Stringer SL, Stringer JR, Blase MA, Walzer PD, Cushion MT. 1989. Pneumocystis carinii: sequence from ribosomal RNA implies a close relationship with fungi. Exp Parasitol 68:450–461. doi: 10.1016/0014-4894(89)90130-6. [DOI] [PubMed] [Google Scholar]
- 63.Bartlett MS, Eichholtz R, Smith JW. 1985. Antimicrobial susceptibility of Pneumocystis carinii in culture. Diagn Microbiol Infect Dis 3:381–387. doi: 10.1016/0732-8893(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 64.Bartlett MS, Queener SF, Shaw MM, Richardson JD, Smith JW. 1994. Pneumocystis carinii is resistant to imidazole antifungal agents. Antimicrob Agents Chemother 38:1859–1861. doi: 10.1128/AAC.38.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cushion MT, Chen F, Kloepfer N. 1997. A cytotoxicity assay for evaluation of candidate anti-Pneumocystis carinii agents. Antimicrob Agents Chemother 41:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaneshiro ES, Cushion MT, Walzer PD, Jayasimhulu K. 1989. Analyses of Pneumocystis fatty acids. J Protozool 36:69S–72S. [PubMed] [Google Scholar]
- 67.Kaneshiro ES, Amit Z, Chandra J, Baughman RP, Contini C, Lundgren B. 1999. Sterols of Pneumocystis carinii hominis organisms isolated from human lungs. Clin Diagn Lab Immunol 6:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaneshiro ES, Ellis JE, Jayasimhulu K, Beach DH. 1994. Evidence for the presence of “metabolic sterols” in Pneumocystis: identification and initial characterization of Pneumocystis carinii sterols. J Eukaryot Microbiol 41:78–85. doi: 10.1111/j.1550-7408.1994.tb05938.x. [DOI] [PubMed] [Google Scholar]
- 69.Ma L, Chen Z, Huang DW, Kutty G, Ishihara M, Wang H, Abouelleil A, Bishop L, Davey E, Deng R, Deng X, Fan L, Fantoni G, Fitzgerald M, Gogineni E, Goldberg JM, Handley G, Hu X, Huber C, Jiao X, Jones K, Levin JZ, Liu Y, Macdonald P, Melnikov A, Raley C, Sassi M, Sherman BT, Song X, Sykes S, Tran B, Walsh L, Xia Y, Yang J, Young S, Zeng Q, Zheng X, Stephens R, Nusbaum C, Birren BW, Azadi P, Lempicki RA, Cuomo CA, Kovacs JA. 2016. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat Commun 7:10740. doi: 10.1038/ncomms10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Furlong ST, Koziel H, Bartlett MS, McLaughlin GL, Shaw MM, Jack RM. 1997. Lipid transfer from human epithelial cells to Pneumocystis carinii in vitro. J Infect Dis 175:661–668. doi: 10.1093/infdis/175.3.661. [DOI] [PubMed] [Google Scholar]
- 71.Kottom TJ, Limper AH. 2000. Cell wall assembly by Pneumocystis carinii. Evidence for a unique gsc-1 subunit mediating beta-1,3-glucan deposition. J Biol Chem 275:40628–40634. [DOI] [PubMed] [Google Scholar]
- 72.Linke MJ, Cushion MT, Walzer PD. 1989. Properties of the major antigens of rat and human Pneumocystis carinii. Infect Immun 57:1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nollstadt KH, Powles MA, Fujioka H, Aikawa M, Schmatz DM. 1994. Use of beta-1,3-glucan-specific antibody to study the cyst wall of Pneumocystis carinii and effects of pneumocandin B0 analog L-733,560. Antimicrob Agents Chemother 38:2258–2265. doi: 10.1128/AAC.38.10.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Durand-Joly I, Aliouat EM, Recourt C, Guyot K, Francois N, Wauquier M, Camus D, Dei-Cas E. 2002. Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J Clin Microbiol 40:1862–1865. doi: 10.1128/JCM.40.5.1862-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Redhead SA, Cushion MT, Frenkel JK, Stringer JR. 2006. Pneumocystis and Trypanosoma cruzi: nomenclature and typifications. J Eukaryot Microbiol 53:2–11. doi: 10.1111/j.1550-7408.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 76.Frenkel JK. 1999. Pneumocystis pneumonia, an immunodeficiency-dependent disease (IDD): a critical historical overview. J Eukaryot Microbiol 46:89S–92S. [PubMed] [Google Scholar]
- 77.Keely SP, Fischer JM, Cushion MT, Stringer JR. 2004. Phylogenetic identification of Pneumocystis murina sp. nov., a new species in laboratory mice. Microbiology 150:1153–1165. doi: 10.1099/mic.0.26921-0. [DOI] [PubMed] [Google Scholar]
- 78.Cushion MT, Keely SP, Stringer JR. 2004. Molecular and phenotypic description of Pneumocystis wakefieldiae sp. nov., a new species in rats. Mycologia 96:429–438. [PubMed] [Google Scholar]
- 79.Dei-Cas E, Chabe M, Moukhlis R, Durand-Joly I, Aliouat EM, Stringer JR, Cushion M, Noel C, de Hoog GS, Guillot J, Viscogliosi E. 2006. Pneumocystis oryctolagi sp. nov., an uncultured fungus causing pneumonia in rabbits at weaning: review of current knowledge, and description of a new taxon on genotypic, phylogenetic and phenotypic bases. FEMS Microbiol Rev 30:853–871. doi: 10.1111/j.1574-6976.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- 80.Bartlett M, Cushion MT, Fishman JA, Kaneshiro ES, Lee CH, Leibowitz MJ, Lu J-J, Lundgren B, Peters SE, Smith JW, Smulian AG, Staben C, Stringer JR, Stringer SL, Wakefield AE, Walzer PD, Weinberg GA. 1994. Revised nomenclature for Pneumocystis carinii. The Pneumocystis Workshop. J Eukaryot Microbiol 41:121S–122S. [PubMed] [Google Scholar]
- 81.Durand-Joly I, Wakefield AE, Palmer RJ, Denis CM, Creusy C, Fleurisse L, Ricard I, Gut JP, Dei-Cas E. 2000. Ultrastructural and molecular characterization of Pneumocystis carinii isolated from a rhesus monkey (Macaca mulatta). Med Mycol 38:61–72. [DOI] [PubMed] [Google Scholar]
- 82.Palmer RJ, Settnes OP, Lodal J, Wakefield AE. 2000. Population structure of rat-derived Pneumocystis carinii in Danish wild rats. Appl Environ Microbiol 66:4954–4961. doi: 10.1128/AEM.66.11.4954-4961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guillot J, Demanche C, Norris K, Wildschutte H, Wanert F, Berthelemy M, Tataine S, Dei-Cas E, Chermette R. 2004. Phylogenetic relationships among Pneumocystis from Asian macaques inferred from mitochondrial rRNA sequences. Mol Phylogenet Evol 31:988–996. doi: 10.1016/j.ympev.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 84.Norris KA, Wildschutte H, Franko J, Board KF. 2003. Genetic variation at the mitochondrial large-subunit rRNA locus of Pneumocystis isolates from simian immunodeficiency virus-infected rhesus macaques. Clin Diagn Lab Immunol 10:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akbar H, Pincon C, Aliouat-Denis CM, Derouiche S, Taylor ML, Pottier M, Carreto-Binaghi LH, Gonzalez-Gonzalez AE, Courpon A, Barriel V, Guillot J, Chabe M, Suarez-Alvarez RO, Aliouat EM, Dei-Cas E, Demanche C. 2012. Characterizing Pneumocystis in the lungs of bats: understanding Pneumocystis evolution and the spread of Pneumocystis organisms in mammal populations. Appl Environ Microbiol 78:8122–8136. doi: 10.1128/AEM.01791-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.English K, Peters SE, Maskell DJ, Collins ME. 2001. DNA analysis of Pneumocystis infecting a Cavalier King Charles spaniel. J Eukaryot Microbiol 2001(Suppl):106S. doi: 10.1111/j.1550-7408.2001.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 87.Baldauf SL, Palmer JD. 1993. Animals and fungi are each other's closest relatives: congruent evidence from multiple proteins. Proc Natl Acad Sci U S A 90:11558–11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fletcher LD, McDowell JM, Tidwell RR, Meagher RB, Dykstra CC. 1994. Structure, expression and phylogenetic analysis of the gene encoding actin I in Pneumocystis carinii. Genetics 137:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wakefield AE, Peters SE, Banerji S, Bridge PD, Hall GS, Hawksworth DL, Guiver LA, Allen AG, Hopkin JM. 1992. Pneumocystis carinii shows DNA homology with the ustomycetous red yeast fungi. Mol Microbiol 6:1903–1911. doi: 10.1111/j.1365-2958.1992.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe J, Hori H, Tanabe K, Nakamura Y. 1989. Phylogenetic association of Pneumocystis carinii with the ‘Rhizopoda/Myxomycota/Zygomycota group’ indicated by comparison of 5S ribosomal RNA sequences. Mol Biochem Parasitol 32:163–167. doi: 10.1016/0166-6851(89)90067-4. [DOI] [PubMed] [Google Scholar]
- 91.Nishida H, Sugiyama J. 1994. Archiascomycetes: detection of a major new lineage within the Ascomycota. Mycoscience 35:361–366. doi: 10.1007/BF02268506. [DOI] [Google Scholar]
- 92.Eriksson OE, Winka K. 1997. Supraordinal taxa of Ascomycota. Myconet 1:1–16. [Google Scholar]
- 93.Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Koljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 94.Menkis A, Urbina H, James TY, Rosling A. 2014. Archaeorhizomyces borealis sp. nov. and a sequence-based classification of related soil fungal species. Fungal Biol 118:943–955. doi: 10.1016/j.funbio.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 95.Rosling A, Cox F, Cruz-Martinez K, Ihrmark K, Grelet GA, Lindahl BD, Menkis A, James TY. 2011. Archaeorhizomycetes: unearthing an ancient class of ubiquitous soil fungi. Science 333:876–879. doi: 10.1126/science.1206958. [DOI] [PubMed] [Google Scholar]
- 96.Sugiyama J, Hosaka K, Suh SO. 2006. Early diverging Ascomycota: phylogenetic divergence and related evolutionary enigmas. Mycologia 98:996–1005. [DOI] [PubMed] [Google Scholar]
- 97.Cisse OH, Pagni M, Hauser PM. 2012. De novo assembly of the Pneumocystis jirovecii genome from a single bronchoalveolar lavage fluid specimen from a patient. mBio 4:e00428-12. doi: 10.1128/mBio.00428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O'Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schussler A, Longcore JE, O'Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, et al. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 99.Ma L, Huang DW, Cuomo CA, Sykes S, Fantoni G, Das B, Sherman BT, Yang J, Huber C, Xia Y, Davey E, Kutty G, Bishop L, Sassi M, Lempicki RA, Kovacs JA. 2013. Sequencing and characterization of the complete mitochondrial genomes of three Pneumocystis species provide new insights into divergence between human and rodent Pneumocystis. FASEB J 27:1962–1972. doi: 10.1096/fj.12-224444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsai IJ, Tanaka E, Masuya H, Tanaka R, Hirooka Y, Endoh R, Sahashi N, Kikuchi T. 2014. Comparative genomics of Taphrina fungi causing varying degrees of tumorous deformity in plants. Genome Biol Evol 6:861–872. doi: 10.1093/gbe/evu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, Steenkamp ET, Brinkmann H, Forget L, Philippe H, Lang BF. 2009. Phylogenomic analyses predict sistergroup relationship of nucleariids and fungi and paraphyly of zygomycetes with significant support. BMC Evol Biol 9:272. doi: 10.1186/1471-2148-9-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Riley R, Haridas S, Wolfe KH, Lopes MR, Hittinger CT, Goker M, Salamov AA, Wisecaver JH, Long TM, Calvey CH, Aerts AL, Barry KW, Choi C, Clum A, Coughlan AY, Deshpande S, Douglass AP, Hanson SJ, Klenk HP, LaButti KM, Lapidus A, Lindquist EA, Lipzen AM, Meier-Kolthoff JP, Ohm RA, Otillar RP, Pangilinan JL, Peng Y, Rokas A, Rosa CA, Scheuner C, Sibirny AA, Slot JC, Stielow JB, Sun H, Kurtzman CP, Blackwell M, Grigoriev IV, Jeffries TW. 2016. Comparative genomics of biotechnologically important yeasts. Proc Natl Acad Sci U S A 113:9882–9887. doi: 10.1073/pnas.1603941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen TA, Cisse OH, Yun Wong J, Zheng P, Hewitt D, Nowrousian M, Stajich JE, Jedd G. 2017. Innovation and constraint leading to complex multicellularity in the Ascomycota. Nat Commun 8:14444. doi: 10.1038/ncomms14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delsuc F, Brinkmann H, Philippe H. 2005. Phylogenomics and the reconstruction of the tree of life. Nat Rev Genet 6:361–375. [DOI] [PubMed] [Google Scholar]
- 105.Aliouat-Denis CM, Chabe M, Demanche C, Aliouat EM, Viscogliosi E, Guillot J, Delhaes L, Dei-Cas E. 2008. Pneumocystis species, co-evolution and pathogenic power. Infect Genet Evol 8:708–726. doi: 10.1016/j.meegid.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 106.Denis CM, Mazars E, Guyot K, Odberg-Ferragut C, Viscogliosi E, Dei-Cas E, Wakefield AE. 2000. Genetic divergence at the SODA locus of six different formae speciales of Pneumocystis carinii. Med Mycol 38:289–300. [DOI] [PubMed] [Google Scholar]
- 107.Ma L, Imamichi H, Sukura A, Kovacs JA. 2001. Genetic divergence of the dihydrofolate reductase and dihydropteroate synthase genes in Pneumocystis carinii from 7 different host species. J Infect Dis 184:1358–1362. doi: 10.1086/324208. [DOI] [PubMed] [Google Scholar]
- 108.Demanche C, Berthelemy M, Petit T, Polack B, Wakefield AE, Dei-Cas E, Guillot J. 2001. Phylogeny of Pneumocystis carinii from 18 primate species confirms host specificity and suggests coevolution. J Clin Microbiol 39:2126–2133. doi: 10.1128/JCM.39.6.2126-2133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hugot JP, Demanche C, Barriel V, Dei-Cas E, Guillot J. 2003. Phylogenetic systematics and evolution of primate-derived Pneumocystis based on mitochondrial or nuclear DNA sequence comparison. Syst Biol 52:735–744. doi: 10.1080/10635150390250893. [DOI] [PubMed] [Google Scholar]
- 110.Yan G, Zhang G, Fang X, Zhang Y, Li C, Ling F, Cooper DN, Li Q, Li Y, van Gool AJ, Du H, Chen J, Chen R, Zhang P, Huang Z, Thompson JR, Meng Y, Bai Y, Wang J, Zhuo M, Wang T, Huang Y, Wei L, Li J, Wang Z, Hu H, Yang P, Le L, Stenson PD, Li B, Liu X, Ball EV, An N, Huang Q, Zhang Y, Fan W, Zhang X, Li Y, Wang W, Katze MG, Su B, Nielsen R, Yang H, Wang J, Wang X, Wang J. 2011. Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat Biotechnol 29:1019–1023. doi: 10.1038/nbt.1992. [DOI] [PubMed] [Google Scholar]
- 111.Guillot J, Demanche C, Hugot JP, Berthelemy M, Wakefield AE, Dei-Cas E, Chermette R. 2001. Parallel phylogenies of Pneumocystis species and their mammalian hosts. J Eukaryot Microbiol 2001(Suppl):113S–115S. doi: 10.1111/j.1550-7408.2001.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 112.Keely SP, Fischer JM, Stringer JR. 2003. Evolution and speciation of Pneumocystis. J Eukaryot Microbiol 50(Suppl):624–626. doi: 10.1111/j.1550-7408.2003.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 113.Kimura Y, Hawkins MT, McDonough MM, Jacobs LL, Flynn LJ. 2015. Corrected placement of Mus-Rattus fossil calibration forces precision in the molecular tree of rodents. Sci Rep 5:14444. doi: 10.1038/srep14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mouse Genome Sequencing Consortium, Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, et al. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 115.Nielsen MH, Settnes OP, Aliouat EM, Cailliez JC, Dei-Cas E. 1998. Different ultrastructural morphology of Pneumocystis carinii derived from mice, rats, and rabbits. APMIS 106:771–779. doi: 10.1111/j.1699-0463.1998.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 116.Cushion MT. 1998. Taxonomy, genetic organization, and life cycle of Pneumocystis carinii. Semin Respir Infect 13:304–312. [PubMed] [Google Scholar]
- 117.Aliouat-Denis CM, Martinez A, Aliouat EM, Pottier M, Gantois N, Dei-Cas E. 2009. The Pneumocystis life cycle. Mem Inst Oswaldo Cruz 104:419–426. doi: 10.1590/S0074-02762009000300004. [DOI] [PubMed] [Google Scholar]
- 118.Matsumoto Y, Yoshida Y. 1984. Sporogony in Pneumocystis carinii: synaptonemal complexes and meiotic nuclear divisions observed in precysts. J Protozool 31:420–428. doi: 10.1111/j.1550-7408.1984.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 119.Baron S. 1996. Medical microbiology. University of Texas Medical Branch at Galveston, Galveston, TX. [PubMed] [Google Scholar]
- 120.Martinez A, Aliouat EM, Standaert-Vitse A, Werkmeister E, Pottier M, Pincon C, Dei-Cas E, Aliouat-Denis CM. 2011. Ploidy of cell-sorted trophic and cystic forms of Pneumocystis carinii. PLoS One 6:e20935. doi: 10.1371/journal.pone.0020935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wyder MA, Rasch EM, Kaneshiro ES. 1998. Quantitation of absolute Pneumocystis carinii nuclear DNA content. Trophic and cystic forms isolated from infected rat lungs are haploid organisms. J Eukaryot Microbiol 45:233–239. [DOI] [PubMed] [Google Scholar]
- 122.Yoshikawa H, Morioka H, Yoshida Y. 1987. Freeze-fracture studies on Pneumocystis carinii. II. Fine structure of the trophozoite. Parasitol Res 73:132–139. [DOI] [PubMed] [Google Scholar]
- 123.Vossen ME, Beckers PJ, Meuwissen JH, Stadhouders AM. 1978. Developmental biology of Pneumocystis carinii, and alternative view on the life cycle of the parasite. Z Parasitenkd 55:101–118. doi: 10.1007/BF00384826. [DOI] [PubMed] [Google Scholar]
- 124.Ham EK, Greenberg D, Reynolds RC, Singer DB. 1971. Ultrastructure of Pneumocystis carinii. Exp Mol Pathol 14:362–372. doi: 10.1016/0014-4800(71)90007-4. [DOI] [PubMed] [Google Scholar]
- 125.Vavra J, Kucera K. 1970. Pneumocystis carinii delanoe, its ultrastructure and ultrastructural affinities. J Protozool 17:463–483. doi: 10.1111/j.1550-7408.1970.tb04715.x. [DOI] [PubMed] [Google Scholar]
- 126.Millard PR, Wakefield AE, Hopkin JM. 1990. A sequential ultrastructural study of rat lungs infected with Pneumocystis carinii to investigate the appearances of the organism, its relationships and its effects on pneumocytes. Int J Exp Pathol 71:895–904. [PMC free article] [PubMed] [Google Scholar]
- 127.Dei-Cas E, Jackson H, Palluault F, Aliouat EM, Hancock V, Soulez B, Camus D. 1991. Ultrastructural observations on the attachment of Pneumocystis carinii in vitro. J Protozool 38:205S–207S. [PubMed] [Google Scholar]
- 128.Yoshida Y. 1989. Ultrastructural studies of Pneumocystis carinii. J Protozool 36:53–60. doi: 10.1111/j.1550-7408.1989.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 129.Henshaw NG, Carson JL, Collier AM. 1985. Ultrastructural observations of Pneumocystis carinii attachment to rat lung. J Infect Dis 151:181–186. doi: 10.1093/infdis/151.1.181. [DOI] [PubMed] [Google Scholar]
- 130.Cushion MT. 2004. Pneumocystis: unraveling the cloak of obscurity. Trends Microbiol 12:243–249. doi: 10.1016/j.tim.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 131.de Souza W, Benchimol M. 2005. Basic biology of Pneumocystis carinii: a mini review. Mem Inst Oswaldo Cruz 100:903–908. doi: 10.1590/S0074-02762005000800013. [DOI] [PubMed] [Google Scholar]
- 132.Cushion MT, Linke MJ, Ashbaugh A, Sesterhenn T, Collins MS, Lynch K, Brubaker R, Walzer PD. 2010. Echinocandin treatment of Pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS One 5:e8524. doi: 10.1371/journal.pone.0008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peters SE, English K, Rana A, Akter S, Malik S, Warburton NC, Duckett JG. 2001. Synaptonemal complexes in the pre-cyst of Pneumocystis carinii. J Eukaryot Microbiol 2001(Suppl):134S. doi: 10.1111/j.1550-7408.2001.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 134.Gong YX, Chang ZS, Zhang ZG, Zeng XZ, Tan JS, Zhao R, Wang YS. 2006. Observation on the ultrastructure of Pneumocystis carinii. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 24:449–452. [PubMed] [Google Scholar]
- 135.Burgess JW, Kottom TJ, Limper AH. 2008. Pneumocystis carinii exhibits a conserved meiotic control pathway. Infect Immun 76:417–425. doi: 10.1128/IAI.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cushion MT, Smulian AG, Slaven BE, Sesterhenn T, Arnold J, Staben C, Porollo A, Adamczak R, Meller J. 2007. Transcriptome of Pneumocystis carinii during fulminate infection: carbohydrate metabolism and the concept of a compatible parasite. PLoS One 2:e423. doi: 10.1371/journal.pone.0000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smulian AG, Sesterhenn T, Tanaka R, Cushion MT. 2001. The ste3 pheromone receptor gene of Pneumocystis carinii is surrounded by a cluster of signal transduction genes. Genetics 157:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vohra PK, Sanyal B, Thomas CF Jr. 2004. Biochemical requirements for PCBCK1 kinase activity, the Pneumocystis carinii MEKK involved in cell wall integrity. FEMS Microbiol Lett 235:153–156. doi: 10.1111/j.1574-6968.2004.tb09580.x. [DOI] [PubMed] [Google Scholar]
- 139.Almeida JM, Cisse OH, Fonseca A, Pagni M, Hauser PM. 2015. Comparative genomics suggests primary homothallism of Pneumocystis species. mBio 6:e02250-14. doi: 10.1128/mBio.02250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ni M, Feretzaki M, Sun S, Wang X, Heitman J. 2011. Sex in fungi. Annu Rev Genet 45:405–430. doi: 10.1146/annurev-genet-110410-132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klar AJ. 2007. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu Rev Genet 41:213–236. doi: 10.1146/annurev.genet.39.073103.094316. [DOI] [PubMed] [Google Scholar]
- 142.Cushion MT, Arnold J. 1997. Proposal for a Pneumocystis genome project. J Eukaryot Microbiol 44:7S. doi: 10.1111/j.1550-7408.1997.tb05737.x. [DOI] [PubMed] [Google Scholar]
- 143.Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG. 1996. Life with 6000 genes. Science 274:546, 563–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 144.Slaven BE, Meller J, Porollo A, Sesterhenn T, Smulian AG, Cushion MT. 2006. Draft assembly and annotation of the Pneumocystis carinii genome. J Eukaryot Microbiol 53(Suppl 1):S89–S91. doi: 10.1111/j.1550-7408.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 145.Cisse OH, Pagni M, Hauser PM. 2014. Comparative genomics suggests that the human pathogenic fungus Pneumocystis jirovecii acquired obligate biotrophy through gene loss. Genome Biol Evol 6:1938–1948. doi: 10.1093/gbe/evu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hauser PM. 2014. Genomic insights into the fungal pathogens of the genus Pneumocystis: obligate biotrophs of humans and other mammals. PLoS Pathog 10:e1004425. doi: 10.1371/journal.ppat.1004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Porollo A, Sesterhenn TM, Collins MS, Welge JA, Cushion MT. 2014. Comparative genomics of Pneumocystis species suggests the absence of genes for myo-inositol synthesis and reliance on inositol transport and metabolism. mBio 5:e01834. doi: 10.1128/mBio.01834-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Albalat R, Canestro C. 2016. Evolution by gene loss. Nat Rev Genet 17:379–391. doi: 10.1038/nrg.2016.39. [DOI] [PubMed] [Google Scholar]
- 149.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 150.Hauser PM, Burdet FX, Cisse OH, Keller L, Taffe P, Sanglard D, Pagni M. 2010. Comparative genomics suggests that the fungal pathogen Pneumocystis is an obligate parasite scavenging amino acids from its host's lungs. PLoS One 5:e15152. doi: 10.1371/journal.pone.0015152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noel-Georis I, Bernard A, Falmagne P, Wattiez R. 2002. Database of bronchoalveolar lavage fluid proteins. J Chromatogr B Analyt Technol Biomed Life Sci 771:221–236. doi: 10.1016/S1570-0232(02)00114-9. [DOI] [PubMed] [Google Scholar]
- 152.Viswan A, Sharma RK, Azim A, Sinha N. 2016. NMR-based metabolic snapshot from minibronchoalveolar lavage fluid: an approach to unfold human respiratory metabolomics. J Proteome Res 15:302–310. doi: 10.1021/acs.jproteome.5b00919. [DOI] [PubMed] [Google Scholar]
- 153.Vestereng VH, Kovacs JA. 2004. Inability of Pneumocystis organisms to incorporate bromodeoxyuridine suggests the absence of a salvage pathway for thymidine. Microbiology 150:1179–1182. doi: 10.1099/mic.0.26890-0. [DOI] [PubMed] [Google Scholar]
- 154.Chitty JL, Fraser JA. 2017. Purine acquisition and synthesis by human fungal pathogens. Microorganisms 5:E33. doi: 10.3390/microorganisms5020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cuomo CA, Desjardins CA, Bakowski MA, Goldberg J, Ma AT, Becnel JJ, Didier ES, Fan L, Heiman DI, Levin JZ, Young S, Zeng Q, Troemel ER. 2012. Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res 22:2478–2488. doi: 10.1101/gr.142802.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dean P, Hirt RP, Embley TM. 2016. Microsporidia: why make nucleotides if you can steal them? PLoS Pathog 12:e1005870. doi: 10.1371/journal.ppat.1005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de Koning HP, Bridges DJ, Burchmore RJ. 2005. Purine and pyrimidine transport in pathogenic protozoa: from biology to therapy. FEMS Microbiol Rev 29:987–1020. doi: 10.1016/j.femsre.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 158.Fairlamb AH. 1989. Novel biochemical pathways in parasitic protozoa. Parasitology 99(Suppl):S93–S112. doi: 10.1017/S003118200008344X. [DOI] [PubMed] [Google Scholar]
- 159.Palluault F, Pietrzyk B, Dei-Cas E, Slomianny C, Soulez B, Camus D. 1991. Three-dimensional reconstruction of rabbit-derived Pneumocystis carinii from serial-thin sections. I. Trophozoite. J Protozool 38:402–407. doi: 10.1111/j.1550-7408.1991.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 160.Yu JR, Pyon JK, Seo M, Jung BS, Cho SR, Lee SH, Hong ST. 2001. Localization of cytoskeletal proteins in Pneumocystis carinii by immuno-electron microscopy. Korean J Parasitol 39:13–21. doi: 10.3347/kjp.2001.39.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Idnurm A, Howlett BJ. 2002. Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryot Cell 1:719–724. doi: 10.1128/EC.1.5.719-724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lorenz MC, Fink GR. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 163.Wang ZY, Thornton CR, Kershaw MJ, Debao L, Talbot NJ. 2003. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol Microbiol 47:1601–1612. doi: 10.1046/j.1365-2958.2003.03412.x. [DOI] [PubMed] [Google Scholar]
- 164.Munoz-Elias EJ, McKinney JD. 2005. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med 11:638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaneshiro ES. 2002. Sterol biosynthesis in Pneumocystis: unique steps that define unique targets. Drug Resist Updat 5:259–268. doi: 10.1016/S1368-7646(02)00122-X. [DOI] [PubMed] [Google Scholar]
- 166.Weete JD, Abril M, Blackwell M. 2010. Phylogenetic distribution of fungal sterols. PLoS One 5:e10899. doi: 10.1371/journal.pone.0010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Giner JL, Zhao H, Beach DH, Parish EJ, Jayasimhulu K, Kaneshiro ES. 2002. Comprehensive and definitive structural identities of Pneumocystis carinii sterols. J Lipid Res 43:1114–1124. doi: 10.1194/jlr.M200113-JLR200. [DOI] [PubMed] [Google Scholar]
- 168.Joffrion TM, Cushion MT. 2010. Sterol biosynthesis and sterol uptake in the fungal pathogen Pneumocystis carinii. FEMS Microbiol Lett 311:1–9. doi: 10.1111/j.1574-6968.2010.02007.x. [DOI] [PubMed] [Google Scholar]
- 169.Kaneshiro ES. 2004. Sterol metabolism in the opportunistic pathogen Pneumocystis: advances and new insights. Lipids 39:753–761. doi: 10.1007/s11745-004-1292-5. [DOI] [PubMed] [Google Scholar]
- 170.Kaneshiro ES, Wyder MA. 2000. C27 to C32 sterols found in Pneumocystis, an opportunistic pathogen of immunocompromised mammals. Lipids 35:317–324. doi: 10.1007/s11745-000-0528-8. [DOI] [PubMed] [Google Scholar]
- 171.Zhou W, Nguyen TT, Collins MS, Cushion MT, Nes WD. 2002. Evidence for multiple sterol methyl transferase pathways in Pneumocystis carinii. Lipids 37:1177–1186. doi: 10.1007/s11745-002-1018-8. [DOI] [PubMed] [Google Scholar]
- 172.Iwaki T, Iefuji H, Hiraga Y, Hosomi A, Morita T, Giga-Hama Y, Takegawa K. 2008. Multiple functions of ergosterol in the fission yeast Schizosaccharomyces pombe. Microbiology 154:830–841. doi: 10.1099/mic.0.2007/011155-0. [DOI] [PubMed] [Google Scholar]
- 173.van Eijk GW, Roeymans HJ. 1982. Distribution of carotenoids and sterols in relation to the taxonomy of Taphrina and Protomyces. Antonie Van Leeuwenhoek 48:257–264. doi: 10.1007/BF00400385. [DOI] [PubMed] [Google Scholar]
- 174.Weete JD, Sancholle MS, Montant C. 1983. Effects of triazoles on fungi. II. Lipid composition of Taphrina deformans. Biochim Biophys Acta 752:19–29. [Google Scholar]
- 175.Parks LW, Casey WM. 1995. Physiological implications of sterol biosynthesis in yeast. Annu Rev Microbiol 49:95–116. doi: 10.1146/annurev.mi.49.100195.000523. [DOI] [PubMed] [Google Scholar]
- 176.Dupont S, Lemetais G, Ferreira T, Cayot P, Gervais P, Beney L. 2012. Ergosterol biosynthesis: a fungal pathway for life on land? Evolution 66:2961–2968. doi: 10.1111/j.1558-5646.2012.01667.x. [DOI] [PubMed] [Google Scholar]
- 177.Klemptner RL, Sherwood JS, Tugizimana F, Dubery IA, Piater LA. 2014. Ergosterol, an orphan fungal microbe-associated molecular pattern (MAMP). Mol Plant Pathol 15:747–761. doi: 10.1111/mpp.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Laquitaine L, Gomes E, Francois J, Marchive C, Pascal S, Hamdi S, Atanassova R, Delrot S, Coutos-Thevenot P. 2006. Molecular basis of ergosterol-induced protection of grape against Botrytis cinerea: induction of type I LTP promoter activity, WRKY, and stilbene synthase gene expression. Mol Plant Microbe Interact 19:1103–1112. doi: 10.1094/MPMI-19-1103. [DOI] [PubMed] [Google Scholar]
- 179.Patton-Vogt J. 2007. Transport and metabolism of glycerophosphodiesters produced through phospholipid deacylation. Biochim Biophys Acta 1771:337–342. doi: 10.1016/j.bbalip.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 180.Cushion MT, Collins MS, Sesterhenn T, Porollo A, Vadukoot AK, Merino EJ. 2016. Functional characterization of Pneumocystis carinii inositol transporter 1. mBio 7:e01851-16. doi: 10.1128/mBio.01851-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hiltunen JK, Okubo F, Kursu VA, Autio KJ, Kastaniotis AJ. 2005. Mitochondrial fatty acid synthesis and maintenance of respiratory competent mitochondria in yeast. Biochem Soc Trans 33:1162–1165. [DOI] [PubMed] [Google Scholar]
- 182.Hiltunen JK, Schonauer MS, Autio KJ, Mittelmeier TM, Kastaniotis AJ, Dieckmann CL. 2009. Mitochondrial fatty acid synthesis type II: more than just fatty acids. J Biol Chem 284:9011–9015. doi: 10.1074/jbc.R800068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kastaniotis AJ, Autio KJ, Keratar JM, Monteuuis G, Makela AM, Nair RR, Pietikainen LP, Shvetsova A, Chen Z, Hiltunen JK. 2017. Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology. Biochim Biophys Acta 1862:39–48. doi: 10.1016/j.bbalip.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 184.Kornitzer D. 2009. Fungal mechanisms for host iron acquisition. Curr Opin Microbiol 12:377–383. doi: 10.1016/j.mib.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 185.Kulkarni RD, Kelkar HS, Dean RA. 2003. An eight-cysteine-containing CFEM domain unique to a group of fungal membrane proteins. Trends Biochem Sci 28:118–121. doi: 10.1016/S0968-0004(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 186.Kuznets G, Vigonsky E, Weissman Z, Lalli D, Gildor T, Kauffman SJ, Turano P, Becker J, Lewinson O, Kornitzer D. 2014. A relay network of extracellular heme-binding proteins drives C. albicans iron acquisition from hemoglobin. PLoS Pathog 10:e1004407. doi: 10.1371/journal.ppat.1004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weissman Z, Kornitzer D. 2004. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol 53:1209–1220. doi: 10.1111/j.1365-2958.2004.04199.x. [DOI] [PubMed] [Google Scholar]
- 188.Bartlett MS, Goheen MP, Lee CH, Shaw MM, Durkin MM, Smith JW. 1994. Close association of Pneumocystis carinii from infected rat lung with culture cells as shown by light and electron microscopy. Parasitol Res 80:208–215. doi: 10.1007/BF00932676. [DOI] [PubMed] [Google Scholar]
- 189.Long GG, White JD, Stookey JL. 1975. Pneumocystis carinii infection in splenectomized owl monkeys. J Am Vet Med Assoc 167:651–654. [PubMed] [Google Scholar]
- 190.Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, Kovacs JA. 2004. Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. J Infect Dis 189:1540–1544. doi: 10.1086/382486. [DOI] [PubMed] [Google Scholar]
- 191.Aliouat EM, Dujardin L, Martinez A, Duriez T, Ricard I, Dei-Cas E. 1999. Pneumocystis carinii growth kinetics in culture systems and in hosts: involvement of each life cycle parasite stage. J Eukaryot Microbiol 46:116S–117S. [PubMed] [Google Scholar]
- 192.Erwig LP, Gow NA. 2016. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 193.Gow NAR, Latge JP, Munro CA. 2017. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 5:FUNK-0035-2016. doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Klis FM, Boorsma A, De Groot PW. 2006. Cell wall construction in Saccharomyces cerevisiae. Yeast 23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- 195.Lesage G, Bussey H. 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 70:317–343. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.De Stefano JA, Myers JD, Du Pont D, Foy JM, Theus SA, Walzer PD. 1998. Cell wall antigens of Pneumocystis carinii trophozoites and cysts: purification and carbohydrate analysis of these glycoproteins. J Eukaryot Microbiol 45:334–343. doi: 10.1111/j.1550-7408.1998.tb04545.x. [DOI] [PubMed] [Google Scholar]
- 197.Gigliotti F, Ballou LR, Hughes WT, Mosley BD. 1988. Purification and initial characterization of a ferret Pneumocystis carinii surface antigen. J Infect Dis 158:848–854. doi: 10.1093/infdis/158.4.848. [DOI] [PubMed] [Google Scholar]
- 198.Linke MJ, Walzer PD. 1989. Analysis of a surface antigen of Pneumocystis carinii. J Protozool 36:60S–61S. [DOI] [PubMed] [Google Scholar]
- 199.Nakamura Y, Tanabe K, Egawa K. 1989. Structure of major surface determinants and DNA diagnosis of Pneumocystis carinii. J Protozool 36:58S–60S. [DOI] [PubMed] [Google Scholar]
- 200.Radding JA, Armstrong MY, Ullu E, Richards FF. 1989. Identification and isolation of a major cell surface glycoprotein of Pneumocystis carinii. Infect Immun 57:2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tanabe K, Takasaki S, Watanabe J, Kobata A, Egawa K, Nakamura Y. 1989. Glycoproteins composed of major surface immunodeterminants of Pneumocystis carinii. Infect Immun 57:1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lundgren B, Lipschik GY, Kovacs JA. 1991. Purification and characterization of a major human Pneumocystis carinii surface antigen. J Clin Invest 87:163–170. doi: 10.1172/JCI114966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Matsumoto Y, Matsuda S, Tegoshi T. 1989. Yeast glucan in the cyst wall of Pneumocystis carinii. J Protozool 36:21S–22S. [DOI] [PubMed] [Google Scholar]
- 204.Schmatz DM, Romancheck MA, Pittarelli LA, Schwartz RE, Fromtling RA, Nollstadt KH, Vanmiddlesworth FL, Wilson KE, Turner MJ. 1990. Treatment of Pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci U S A 87:5950–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Barton EG Jr, Campbell WG Jr. 1967. Further observations on the ultrastructure of Pneumocystis. Arch Pathol 83:527–534. [PubMed] [Google Scholar]
- 206.Yoneda K, Walzer PD, Richey CS, Birk MG. 1982. Pneumocystis carinii: freeze-fracture study of stages of the organism. Exp Parasitol 53:68–76. doi: 10.1016/0014-4894(82)90093-5. [DOI] [PubMed] [Google Scholar]
- 207.Palluault F, Dei-Cas E, Slomianny C, Soulez B, Camus D. 1990. Golgi complex and lysosomes in rabbit derived Pneumocystis carinii. Biol Cell 70:73–82. doi: 10.1016/0248-4900(90)90362-7. [DOI] [PubMed] [Google Scholar]
- 208.Poulain D, Hopwood V, Vernes A. 1985. Antigenic variability of Candida albicans. Crit Rev Microbiol 12:223–270. doi: 10.3109/10408418509104430. [DOI] [PubMed] [Google Scholar]
- 209.Lenardon MD, Munro CA, Gow NA. 2010. Chitin synthesis and fungal pathogenesis. Curr Opin Microbiol 13:416–423. doi: 10.1016/j.mib.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gao Q, Jin K, Ying SH, Zhang Y, Xiao G, Shang Y, Duan Z, Hu X, Xie XQ, Zhou G, Peng G, Luo Z, Huang W, Wang B, Fang W, Wang S, Zhong Y, Ma LJ, St Leger RJ, Zhao GP, Pei Y, Feng MG, Xia Y, Wang C. 2011. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet 7:e1001264. doi: 10.1371/journal.pgen.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hartl L, Zach S, Seidl-Seiboth V. 2012. Fungal chitinases: diversity, mechanistic properties and biotechnological potential. Appl Microbiol Biotechnol 93:533–543. doi: 10.1007/s00253-011-3723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kubicek CP, Herrera-Estrella A, Seidl-Seiboth V, Martinez DA, Druzhinina IS, Thon M, Zeilinger S, Casas-Flores S, Horwitz BA, Mukherjee PK, Mukherjee M, Kredics L, Alcaraz LD, Aerts A, Antal Z, Atanasova L, Cervantes-Badillo MG, Challacombe J, Chertkov O, McCluskey K, Coulpier F, Deshpande N, von Dohren H, Ebbole DJ, Esquivel-Naranjo EU, Fekete E, Flipphi M, Glaser F, Gomez-Rodriguez EY, Gruber S, Han C, Henrissat B, Hermosa R, Hernandez-Onate M, Karaffa L, Kosti I, Le Crom S, Lindquist E, Lucas S, Lubeck M, Lubeck PS, Margeot A, Metz B, Misra M, Nevalainen H, Omann M, Packer N, Perrone G, Uresti-Rivera EE, Salamov A, Schmoll M, Seiboth B, Shapiro H, Sukno S, et al. 2011. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol 12:R40. doi: 10.1186/gb-2011-12-4-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Villegas LR, Kottom TJ, Limper AH. 2012. Chitinases in Pneumocystis carinii pneumonia. Med Microbiol Immunol 201:337–348. doi: 10.1007/s00430-012-0239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sanchatjate S, Schekman R. 2006. Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface. Mol Biol Cell 17:4157–4166. doi: 10.1091/mbc.e06-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kottom TJ, Hebrink DM, Jenson PE, Gudmundsson G, Limper AH. 2015. Evidence for proinflammatory beta-1,6 glucans in the Pneumocystis carinii cell wall. Infect Immun 83:2816–2826. doi: 10.1128/IAI.00196-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kutty G, Davis AS, Ferreyra GA, Qiu J, Huang DW, Sassi M, Bishop L, Handley G, Sherman B, Lempicki R, Kovacs JA. 2016. Beta-glucans are masked but contribute to pulmonary inflammation during Pneumocystis pneumonia. J Infect Dis 214:782–791. doi: 10.1093/infdis/jiw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kutty G, Davis AS, Ma L, Taubenberger JK, Kovacs JA. 2015. Pneumocystis encodes a functional endo-beta-1,3-glucanase that is expressed exclusively in cysts. J Infect Dis 211:719–728. doi: 10.1093/infdis/jiu517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rappleye CA, Eissenberg LG, Goldman WE. 2007. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A 104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Matsumoto Y, Yamada M, Amagai T. 1991. Yeast glucan of Pneumocystis carinii cyst wall: an excellent target for chemotherapy. J Protozool 38:6S–7S. [PubMed] [Google Scholar]
- 220.Schmatz DM, Powles M, McFadden DC, Pittarelli LA, Liberator PA, Anderson JW. 1991. Treatment and prevention of Pneumocystis carinii pneumonia and further elucidation of the P. carinii life cycle with 1,3-beta-glucan synthesis inhibitor L-671,329. J Protozool 38:151S–153S. [PubMed] [Google Scholar]
- 221.Sun P, Tong Z. 2014. Efficacy of caspofungin, a 1,3-beta-d-glucan synthase inhibitor, on Pneumocystis carinii pneumonia in rats. Med Mycol 52:798–803. doi: 10.1093/mmy/myu060. [DOI] [PubMed] [Google Scholar]
- 222.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. 2011. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Carmona EM, Lamont JD, Xue A, Wylam M, Limper AH. 2010. Pneumocystis cell wall beta-glucan stimulates calcium-dependent signaling of IL-8 secretion by human airway epithelial cells. Respir Res 11:95. doi: 10.1186/1465-9921-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. 2005. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol 32:490–497. doi: 10.1165/rcmb.2004-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hoffman OA, Standing JE, Limper AH. 1993. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J Immunol 150:3932–3940. [PubMed] [Google Scholar]
- 226.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 227.Vassallo R, Standing J, Limper AH. 1999. Beta-glucan from Pneumocystis carinii stimulates TNF alpha release from alveolar macrophages. J Eukaryot Microbiol 46:145S. [PubMed] [Google Scholar]
- 228.Vassallo R, Standing JE, Limper AH. 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol 164:3755–3763. doi: 10.4049/jimmunol.164.7.3755. [DOI] [PubMed] [Google Scholar]
- 229.Vassallo R, Thomas CF Jr, Vuk-Pavlovic Z, Limper AH. 2000. Mechanisms of defence in the lung: lessons from Pneumocystis carinii pneumonia. Sarcoidosis Vasc Diffuse Lung Dis 17:130–139. [PubMed] [Google Scholar]
- 230.Brown GD, Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 231.Drummond RA, Brown GD. 2011. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 232.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dranginis AM, Rauceo JM, Coronado JE, Lipke PN. 2007. A biochemical guide to yeast adhesins: glycoproteins for social and antisocial occasions. Microbiol Mol Biol Rev 71:282–294. doi: 10.1128/MMBR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Klis FM, Brul S, De Groot PW. 2010. Covalently linked wall proteins in ascomycetous fungi. Yeast 27:489–493. doi: 10.1002/yea.1747. [DOI] [PubMed] [Google Scholar]
- 235.Gigliotti F. 1992. Host species-specific antigenic variation of a mannosylated surface glycoprotein of Pneumocystis carinii. J Infect Dis 165:329–336. doi: 10.1093/infdis/165.2.329. [DOI] [PubMed] [Google Scholar]
- 236.Haidaris PJ, Wright TW, Gigliotti F, Haidaris CG. 1992. Expression and characterization of a cDNA clone encoding an immunodominant surface glycoprotein of Pneumocystis carinii. J Infect Dis 166:1113–1123. doi: 10.1093/infdis/166.5.1113. [DOI] [PubMed] [Google Scholar]
- 237.Kovacs JA, Powell F, Edman JC, Lundgren B, Martinez A, Drew B, Angus CW. 1993. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem 268:6034–6040. [PubMed] [Google Scholar]
- 238.Wright TW, Bissoondial TY, Haidaris CG, Gigliotti F, Haidaris PJ. 1995. Isoform diversity and tandem duplication of the glycoprotein A gene in ferret Pneumocystis carinii. DNA Res 2:77–88. doi: 10.1093/dnares/2.2.77. [DOI] [PubMed] [Google Scholar]
- 239.Maddison SE, Hayes GV, Ivey MH, Tsang VC, Slemenda SB, Norman LG. 1982. Fractionation of Pneumocystis carinii antigens used in an enzyme-linked immunosorbent assay for antibodies and in the production of antiserum for detecting Pneumocystis carinii antigenemia. J Clin Microbiol 15:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Garbe TR, Stringer JR. 1994. Molecular characterization of clustered variants of genes encoding major surface antigens of human Pneumocystis carinii. Infect Immun 62:3092–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Keely SP, Stringer JR. 2009. Complexity of the MSG gene family of Pneumocystis carinii. BMC Genomics 10:367. doi: 10.1186/1471-2164-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Keely SP, Renauld H, Wakefield AE, Cushion MT, Smulian AG, Fosker N, Fraser A, Harris D, Murphy L, Price C, Quail MA, Seeger K, Sharp S, Tindal CJ, Warren T, Zuiderwijk E, Barrell BG, Stringer JR, Hall N. 2005. Gene arrays at Pneumocystis carinii telomeres. Genetics 170:1589–1600. doi: 10.1534/genetics.105.040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jiao X, Zheng X, Ma L, Kutty G, Gogineni E, Sun Q, Sherman BT, Hu X, Jones K, Raley C, Tran B, Munroe DJ, Stephens R, Liang D, Imamichi T, Kovacs JA, Lempicki RA, Huang DW. 2013. A benchmark study on error assessment and quality control of CCS reads derived from the PacBio RS. J Data Mining Genomics Proteomics 4:16008. doi: 10.4172/2153-0602.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liang M, Raley C, Zheng X, Kutty G, Gogineni E, Sherman BT, Sun Q, Chen X, Skelly T, Jones K, Stephens R, Zhou B, Lau W, Johnson C, Imamichi T, Jiang M, Dewar R, Lempicki RA, Tran B, Kovacs JA, Huang DW. 2016. Distinguishing highly similar gene isoforms with a clustering-based bioinformatics analysis of PacBio single-molecule long reads. BioData Min 9:13. doi: 10.1186/s13040-016-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Deitsch KW, Lukehart SA, Stringer JR. 2009. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol 7:493–503. doi: 10.1038/nrmicro2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Edman JC, Hatton TW, Nam M, Turner R, Mei Q, Angus CW, Kovacs JA. 1996. A single expression site with a conserved leader sequence regulates variation of expression of the Pneumocystis carinii family of major surface glycoprotein genes. DNA Cell Biol 15:989–999. doi: 10.1089/dna.1996.15.989. [DOI] [PubMed] [Google Scholar]
- 247.Kutty G, Ma L, Kovacs JA. 2001. Characterization of the expression site of the major surface glycoprotein of human-derived Pneumocystis carinii. Mol Microbiol 42:183–193. doi: 10.1046/j.1365-2958.2001.02620.x. [DOI] [PubMed] [Google Scholar]
- 248.Sunkin SM, Stringer JR. 1996. Translocation of surface antigen genes to a unique telomeric expression site in Pneumocystis carinii. Mol Microbiol 19:283–295. doi: 10.1046/j.1365-2958.1996.375905.x. [DOI] [PubMed] [Google Scholar]
- 249.Wada M, Sunkin SM, Stringer JR, Nakamura Y. 1995. Antigenic variation by positional control of major surface glycoprotein gene expression in Pneumocystis carinii. J Infect Dis 171:1563–1568. doi: 10.1093/infdis/171.6.1563. [DOI] [PubMed] [Google Scholar]
- 250.Stringer JR. 2007. Antigenic variation in Pneumocystis. J Eukaryot Microbiol 54:8–13. doi: 10.1111/j.1550-7408.2006.00225.x. [DOI] [PubMed] [Google Scholar]
- 251.Huang SN, Angus CW, Turner RE, Sorial V, Kovacs JA. 1999. Identification and characterization of novel variant major surface glycoprotein gene families in rat Pneumocystis carinii. J Infect Dis 179:192–200. doi: 10.1086/314558. [DOI] [PubMed] [Google Scholar]
- 252.Schaffzin JK, Sunkin SM, Stringer JR. 1999. A new family of Pneumocystis carinii genes related to those encoding the major surface glycoprotein. Curr Genet 35:134–143. doi: 10.1007/s002940050442. [DOI] [PubMed] [Google Scholar]
- 253.Wells J, Gigliotti F, Simpson-Haidaris PJ, Haidaris CG. 2004. Epitope mapping of a protective monoclonal antibody against Pneumocystis carinii with shared reactivity to Streptococcus pneumoniae surface antigen PspA. Infect Immun 72:1548–1556. doi: 10.1128/IAI.72.3.1548-1556.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wells J, Haidaris CG, Wright TW, Gigliotti F. 2006. Active immunization against Pneumocystis carinii with a recombinant P. carinii antigen. Infect Immun 74:2446–2448. doi: 10.1128/IAI.74.4.2446-2448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ma L, Kutty G, Jia Q, Kovacs JA. 2003. Characterization of variants of the gene encoding the p55 antigen in Pneumocystis from rats and mice. J Med Microbiol 52:955–960. doi: 10.1099/jmm.0.05131-0. [DOI] [PubMed] [Google Scholar]
- 256.Smulian AG, Stringer JR, Linke MJ, Walzer PD. 1992. Isolation and characterization of a recombinant antigen of Pneumocystis carinii. Infect Immun 60:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Broomall KR, Morris RE, Walzer PD, Smulian AG. 1998. Zymolyase treatment exposes p55 antigen of Pneumocystis carinii. J Eukaryot Microbiol 45:284–289. doi: 10.1111/j.1550-7408.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 258.Smulian AG, Sullivan DW, Theus SA. 2000. Immunization with recombinant Pneumocystis carinii p55 antigen provides partial protection against infection: characterization of epitope recognition associated with immunization. Microbes Infect 2:127–136. doi: 10.1016/S1286-4579(00)00275-6. [DOI] [PubMed] [Google Scholar]
- 259.Theus SA, Sullivan DW, Walzer PD, Smulian AG. 1994. Cellular responses to a 55-kilodalton recombinant Pneumocystis carinii antigen. Infect Immun 62:3479–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Smulian AG, Theus SA, Denko N, Walzer PD, Stringer JR. 1993. A 55 kDa antigen of Pneumocystis carinii: analysis of the cellular immune response and characterization of the gene. Mol Microbiol 7:745–753. doi: 10.1111/j.1365-2958.1993.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 261.McCormack FX, Festa AL, Andrews RP, Linke M, Walzer PD. 1997. The carbohydrate recognition domain of surfactant protein A mediates binding to the major surface glycoprotein of Pneumocystis carinii. Biochemistry 36:8092–8099. doi: 10.1021/bi970313f. [DOI] [PubMed] [Google Scholar]
- 262.Vuk-Pavlovic Z, Standing JE, Crouch EC, Limper AH. 2001. Carbohydrate recognition domain of surfactant protein D mediates interactions with Pneumocystis carinii glycoprotein A. Am J Respir Cell Mol Biol 24:475–484. doi: 10.1165/ajrcmb.24.4.3504. [DOI] [PubMed] [Google Scholar]
- 263.Zimmerman PE, Voelker DR, McCormack FX, Paulsrud JR, Martin WJ II. 1992. 120-kD surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J Clin Invest 89:143–149. doi: 10.1172/JCI115554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Williams MD, Wright JR, March KL, Martin WJ II. 1996. Human surfactant protein A enhances attachment of Pneumocystis carinii to rat alveolar macrophages. Am J Respir Cell Mol Biol 14:232–238. doi: 10.1165/ajrcmb.14.3.8845173. [DOI] [PubMed] [Google Scholar]
- 265.Wada M, Kitada K, Saito M, Egawa K, Nakamura Y. 1993. cDNA sequence diversity and genomic clusters of major surface glycoprotein genes of Pneumocystis carinii. J Infect Dis 168:979–985. doi: 10.1093/infdis/168.4.979. [DOI] [PubMed] [Google Scholar]
- 266.Linke MJ, Smulian AG, Stringer JR, Walzer PD. 1994. Characterization of multiple unique cDNAs encoding the major surface glycoprotein of rat-derived Pneumocystis carinii. Parasitol Res 80:478–486. doi: 10.1007/BF00932694. [DOI] [PubMed] [Google Scholar]
- 267.Angus CW, Tu A, Vogel P, Qin M, Kovacs JA. 1996. Expression of variants of the major surface glycoprotein of Pneumocystis carinii. J Exp Med 183:1229–1234. doi: 10.1084/jem.183.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bishop LR, Helman D, Kovacs JA. 2012. Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice. BMC Immunol 13:39. doi: 10.1186/1471-2172-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Blount RJ, Jarlsberg LG, Daly KR, Worodria W, Davis JL, Cattamanchi A, Djawe K, Andama A, Koch J, Walzer PD, Huang L, International HIV-Associated Opportunistic Pneumonias (IHOP) Study. 2012. Serologic responses to recombinant Pneumocystis jirovecii major surface glycoprotein among Ugandan patients with respiratory symptoms. PLoS One 7:e51545. doi: 10.1371/journal.pone.0051545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Daly KR, Fichtenbaum CJ, Tanaka R, Linke MJ, O'Bert R, Thullen TD, Hui MS, Smulian AG, Walzer PD. 2002. Serologic responses to epitopes of the major surface glycoprotein of Pneumocystis jiroveci differ in human immunodeficiency virus-infected and uninfected persons. J Infect Dis 186:644–651. doi: 10.1086/341565. [DOI] [PubMed] [Google Scholar]
- 271.Daly KR, Koch JV, Shire NJ, Levin L, Walzer PD. 2006. Human immunodeficiency virus-infected patients with prior Pneumocystis pneumonia exhibit increased serologic reactivity to several major surface glycoprotein clones. Clin Vaccine Immunol 13:1071–1078. doi: 10.1128/CVI.00140-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, Kullberg BJ, Torensma R, Williams DL, Figdor CG. 2008. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem 283:20590–20599. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Machova E, Fiacanova L, Cizova A, Korcova J. 2015. Mannoproteins from yeast and hyphal form of Candida albicans considerably differ in mannan and protein content. Carbohydr Res 408:12–17. doi: 10.1016/j.carres.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 274.Fan H, Guo JY, Ma SL, Zhang N, An CL. 2016. Synthetic p55 tandem DNA vaccine against Pneumocystis carinii in rats. Microbiol Immunol 60:397–406. doi: 10.1111/1348-0421.12386. [DOI] [PubMed] [Google Scholar]
- 275.Feng Y, Guo S, Jiang T, Han X, Liu P, Wu T, Luo Y. 2011. Active immunization against Pneumocystis carinii with p55-v3 DNA vaccine in rats. Can J Microbiol 57:375–381. doi: 10.1139/w11-023. [DOI] [PubMed] [Google Scholar]
- 276.Haidaris PJ, Wright TW, Gigliotti F, Fallon MA, Whitbeck AA, Haidaris CG. 1993. In situ hybridization analysis of developmental stages of Pneumocystis carinii that are transcriptionally active for a major surface glycoprotein gene. Mol Microbiol 7:647–656. doi: 10.1111/j.1365-2958.1993.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 277.Guadiz G, Haidaris CG, Maine GN, Simpson-Haidaris PJ. 1998. The carboxyl terminus of Pneumocystis carinii glycoprotein A encodes a functional glycosylphosphatidylinositol signal sequence. J Biol Chem 273:26202–26209. doi: 10.1074/jbc.273.40.26202. [DOI] [PubMed] [Google Scholar]
- 278.Palmer RJ, Wakefield AE. 2001. Functional glycosylphosphatidylinositol anchor signal sequences in the Pneumocystis carinii PRT1 protease family. Am J Respir Cell Mol Biol 25:466–473. doi: 10.1165/ajrcmb.25.4.4514. [DOI] [PubMed] [Google Scholar]
- 279.Eisenman HC, Casadevall A. 2012. Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 93:931–940. doi: 10.1007/s00253-011-3777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Icenhour CR, Kottom TJ, Limper AH. 2003. Evidence for a melanin cell wall component in Pneumocystis carinii. Infect Immun 71:5360–5363. doi: 10.1128/IAI.71.9.5360-5363.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Icenhour CR, Kottom TJ, Limper AH. 2006. Pneumocystis melanins confer enhanced organism viability. Eukaryot Cell 5:916–923. doi: 10.1128/EC.00176-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jacobson ES. 2000. Pathogenic roles for fungal melanins. Clin Microbiol Rev 13:708–717. doi: 10.1128/CMR.13.4.708-717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Amarsaikhan N, Templeton SP.. 2015. Co-recognition of beta-glucan and chitin and programming of adaptive immunity to Aspergillus fumigatus. Front Microbiol 6:344. doi: 10.3389/fmicb.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.de Jonge R, van Esse HP, Kombrink A, Shinya T, Desaki Y, Bours R, van der Krol S, Shibuya N, Joosten MH, Thomma BP. 2010. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science 329:953–955. doi: 10.1126/science.1190859. [DOI] [PubMed] [Google Scholar]
- 285.Kombrink A, Thomma BP. 2013. LysM effectors: secreted proteins supporting fungal life. PLoS Pathog 9:e1003769. doi: 10.1371/journal.ppat.1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cushion MT, Collins MS. 2011. Susceptibility of Pneumocystis to echinocandins in suspension and biofilm cultures. Antimicrob Agents Chemother 55:4513–4518. doi: 10.1128/AAC.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cushion MT, Collins MS, Linke MJ. 2009. Biofilm formation by Pneumocystis spp. Eukaryot Cell 8:197–206. doi: 10.1128/EC.00202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rose AB, Carter A, Korf I, Kojima N. 2016. Intron sequences that stimulate gene expression in Arabidopsis. Plant Mol Biol 92:337–346. doi: 10.1007/s11103-016-0516-1. [DOI] [PubMed] [Google Scholar]
- 289.Ying SY, Lin SL. 2009. Intron-mediated RNA interference and microRNA biogenesis. Methods Mol Biol 487:387–413. doi: 10.1007/978-1-60327-547-7_19. [DOI] [PubMed] [Google Scholar]
- 290.Chung WJ, Agius P, Westholm JO, Chen M, Okamura K, Robine N, Leslie CS, Lai EC. 2011. Computational and experimental identification of mirtrons in Drosophila melanogaster and Caenorhabditis elegans. Genome Res 21:286–300. doi: 10.1101/gr.113050.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Stajich JE, Dietrich FS, Roy SW. 2007. Comparative genomic analysis of fungal genomes reveals intron-rich ancestors. Genome Biol 8:R223. doi: 10.1186/gb-2007-8-10-r223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Irimia M, Rukov JL, Penny D, Roy SW. 2007. Functional and evolutionary analysis of alternatively spliced genes is consistent with an early eukaryotic origin of alternative splicing. BMC Evol Biol 7:188. doi: 10.1186/1471-2148-7-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Slaven BE, Porollo A, Sesterhenn T, Smulian AG, Cushion MT, Meller J. 2006. Large-scale characterization of introns in the Pneumocystis carinii genome. J Eukaryot Microbiol 53(Suppl 1):S151–S153. doi: 10.1111/j.1550-7408.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 294.Ye D, Lee CH, Queener SF. 2001. Differential splicing of Pneumocystis carinii f. sp. carinii inosine 5′-monophosphate dehydrogenase pre-mRNA. Gene 263:151–158. doi: 10.1016/S0378-1119(00)00577-1. [DOI] [PubMed] [Google Scholar]
- 295.Testa SM, Gryaznov SM, Turner DH. 1999. In vitro suicide inhibition of self-splicing of a group I intron from Pneumocystis carinii by an N3′ → P5′ phosphoramidate hexanucleotide. Proc Natl Acad Sci U S A 96:2734–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sogin ML, Edman JC. 1989. A self-splicing intron in the small subunit rRNA gene of Pneumocystis carinii. Nucleic Acids Res 17:5349–5359. doi: 10.1093/nar/17.13.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Liu Y, Tidwell RR, Leibowitz MJ. 1994. Inhibition of in vitro splicing of a group I intron of Pneumocystis carinii. J Eukaryot Microbiol 41:31–38. doi: 10.1111/j.1550-7408.1994.tb05931.x. [DOI] [PubMed] [Google Scholar]
- 298.Miletti KE, Leibowitz MJ. 2000. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob Agents Chemother 44:958–966. doi: 10.1128/AAC.44.4.958-966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu Y, Leibowitz MJ. 1993. Variation and in vitro splicing of group I introns in rRNA genes of Pneumocystis carinii. Nucleic Acids Res 21:2415–2421. doi: 10.1093/nar/21.10.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ma L, Kutty G, Jia Q, Imamichi H, Huang L, Atzori C, Beckers P, Groner G, Beard CB, Kovacs JA. 2002. Analysis of variation in tandem repeats in the intron of the major surface glycoprotein expression site of the human form of Pneumocystis carinii. J Infect Dis 186:1647–1654. doi: 10.1086/345721. [DOI] [PubMed] [Google Scholar]
- 301.Thomas CF Jr, Leof EB, Limper AH. 1999. Analysis of Pneumocystis carinii introns. Infect Immun 67:6157–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Csuros M, Rogozin IB, Koonin EV. 2011. A detailed history of intron-rich eukaryotic ancestors inferred from a global survey of 100 complete genomes. PLoS Comput Biol 7:e1002150. doi: 10.1371/journal.pcbi.1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Roy SW, Irimia M. 2009. Mystery of intron gain: new data and new models. Trends Genet 25:67–73. doi: 10.1016/j.tig.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 304.Roy SW, Irimia M. 2012. Genome evolution: where do new introns come from? Curr Biol 22:R529–R531. doi: 10.1016/j.cub.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 305.Hauser PM. 2004. The development of a typing method for an uncultivable microorganism: the example of Pneumocystis jirovecii. Infect Genet Evol 4:199–203. doi: 10.1016/j.meegid.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 306.Beard CB, Roux P, Nevez G, Hauser PM, Kovacs JA, Unnasch TR, Lundgren B. 2004. Strain typing methods and molecular epidemiology of Pneumocystis pneumonia. Emerg Infect Dis 10:1729–1735. doi: 10.3201/eid1010.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hauser PM, Blanc DS, Bille J, Francioli P. 1998. Typing methods to approach Pneumocystis carinii genetic heterogeneity. FEMS Immunol Med Microbiol 22:27–35. doi: 10.1111/j.1574-695X.1998.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 308.Lu JJ, Bartlett MS, Shaw MM, Queener SF, Smith JW, Ortiz-Rivera M, Leibowitz MJ, Lee CH. 1994. Typing of Pneumocystis carinii strains that infect humans based on nucleotide sequence variations of internal transcribed spacers of rRNA genes. J Clin Microbiol 32:2904–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lee CH, Helweg-Larsen J, Tang X, Jin S, Li B, Bartlett MS, Lu JJ, Lundgren B, Lundgren JD, Olsson M, Lucas SB, Roux P, Cargnel A, Atzori C, Matos O, Smith JW. 1998. Update on Pneumocystis carinii f. sp. hominis typing based on nucleotide sequence variations in internal transcribed spacer regions of rRNA genes. J Clin Microbiol 36:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wakefield AE. 1998. Genetic heterogeneity in human-derived Pneumocystis carinii. FEMS Immunol Med Microbiol 22:59–65. doi: 10.1111/j.1574-695X.1998.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 311.Tsolaki AG, Miller RF, Wakefield AE. 1999. Oropharyngeal samples for genotyping and monitoring response to treatment in AIDS patients with Pneumocystis carinii pneumonia. J Med Microbiol 48:897–905. doi: 10.1099/00222615-48-10-897. [DOI] [PubMed] [Google Scholar]
- 312.Walker DJ, Meshnick SR. 1998. Drug resistance in Pneumocystis carinii: an emerging problem. Drug Resist Updat 1:201–204. doi: 10.1016/S1368-7646(98)80040-X. [DOI] [PubMed] [Google Scholar]
- 313.Walker DJ, Wakefield AE, Dohn MN, Miller RF, Baughman RP, Hossler PA, Bartlett MS, Smith JW, Kazanjian P, Meshnick SR. 1998. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis 178:1767–1775. doi: 10.1086/314509. [DOI] [PubMed] [Google Scholar]
- 314.Mazars E, Odberg-Ferragut C, Dei-Cas E, Fourmaux MN, Aliouat EM, Brun-Pascaud M, Mougeot G, Camus D. 1995. Polymorphism of the thymidylate synthase gene of Pneumocystis carinii from different host species. J Eukaryot Microbiol 42:26–32. doi: 10.1111/j.1550-7408.1995.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 315.Edlind TD, Bartlett MS, Weinberg GA, Prah GN, Smith JW. 1992. The beta-tubulin gene from rat and human isolates of Pneumocystis carinii. Mol Microbiol 6:3365–3373. doi: 10.1111/j.1365-2958.1992.tb02204.x. [DOI] [PubMed] [Google Scholar]
- 316.Banerji S, Lugli EB, Miller RF, Wakefield AE. 1995. Analysis of genetic diversity at the arom locus in isolates of Pneumocystis carinii. J Eukaryot Microbiol 42:675–679. doi: 10.1111/j.1550-7408.1995.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 317.Lane BR, Ast JC, Hossler PA, Mindell DP, Bartlett MS, Smith JW, Meshnick SR. 1997. Dihydropteroate synthase polymorphisms in Pneumocystis carinii. J Infect Dis 175:482–485. doi: 10.1093/infdis/175.2.482. [DOI] [PubMed] [Google Scholar]
- 318.Takahashi T, Endo T, Nakamura T, Sakashitat H, Kimurat K, Ohnishit K, Kitamura Y, Iwamoto A. 2002. Dihydrofolate reductase gene polymorphisms in Pneumocystis carinii f. sp. hominis in Japan. J Med Microbiol 51:510–515. doi: 10.1099/0022-1317-51-6-510. [DOI] [PubMed] [Google Scholar]
- 319.Esteves F, Tavares A, Costa MC, Gaspar J, Antunes F, Matos O. 2009. Genetic characterization of the UCS and Kex1 loci of Pneumocystis jirovecii. Eur J Clin Microbiol Infect Dis 28:175–178. doi: 10.1007/s10096-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 320.Esteves F, Gaspar J, Tavares A, Moser I, Antunes F, Mansinho K, Matos O. 2010. Population structure of Pneumocystis jirovecii isolated from immunodeficiency virus-positive patients. Infect Genet Evol 10:192–199. doi: 10.1016/j.meegid.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 321.Ma L, Kovacs JA. 2001. Genetic analysis of multiple loci suggests that mutations in the Pneumocystis carinii f. sp. hominis dihydropteroate synthase gene arose independently in multiple strains. Antimicrob Agents Chemother 45:3213–3215. doi: 10.1128/AAC.45.11.3213-3215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Li K, He A, Cai WP, Tang XP, Zheng XY, Li ZY, Zhan XM. 2013. Genotyping of Pneumocystis jirovecii isolates from Chinese HIV-infected patients based on nucleotide sequence variations in the internal transcribed spacer regions of rRNA genes. Med Mycol 51:108–112. doi: 10.3109/13693786.2012.695458. [DOI] [PubMed] [Google Scholar]
- 323.Nimri LF, Moura IN, Huang L, del Rio C, Rimland D, Duchin JS, Dotson EM, Beard CB. 2002. Genetic diversity of Pneumocystis carinii f. sp. hominis based on variations in nucleotide sequences of internal transcribed spacers of rRNA genes. J Clin Microbiol 40:1146–1151. doi: 10.1128/JCM.40.4.1146-1151.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Robberts FJ, Liebowitz LD, Chalkley LJ. 2004. Genotyping and coalescent phylogenetic analysis of Pneumocystis jiroveci from South Africa. J Clin Microbiol 42:1505–1510. doi: 10.1128/JCM.42.4.1505-1510.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Le Gal S, Blanchet D, Damiani C, Gueguen P, Virmaux M, Abboud P, Guillot G, Kerangart S, Merle C, Calderon E, Totet A, Carme B, Nevez G. 2015. AIDS-related Pneumocystis jirovecii genotypes in French Guiana. Infect Genet Evol 29:60–67. doi: 10.1016/j.meegid.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 326.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 95:3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Maiden MC, Jansen van Rensburg MJ, Bray JE, Earle SG, Ford SA, Jolley KA, McCarthy ND. 2013. MLST revisited: the gene-by-gene approach to bacterial genomics. Nat Rev Microbiol 11:728–736. doi: 10.1038/nrmicro3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Perez-Losada M, Cabezas P, Castro-Nallar E, Crandall KA. 2013. Pathogen typing in the genomics era: MLST and the future of molecular epidemiology. Infect Genet Evol 16:38–53. doi: 10.1016/j.meegid.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 329.Schmoldt S, Schuhegger R, Wendler T, Huber I, Sollner H, Hogardt M, Arbogast H, Heesemann J, Bader L, Sing A. 2008. Molecular evidence of nosocomial Pneumocystis jirovecii transmission among 16 patients after kidney transplantation. J Clin Microbiol 46:966–971. doi: 10.1128/JCM.02016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gianella S, Haeberli L, Joos B, Ledergerber B, Wuthrich RP, Weber R, Kuster H, Hauser PM, Fehr T, Mueller NJ. 2010. Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl Infect Dis 12:1–10. doi: 10.1111/j.1399-3062.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 331.Curran T, McCaughey C, Coyle PV. 2013. Pneumocystis jirovecii multilocus genotyping profiles in Northern Ireland. J Med Microbiol 62:1170–1174. doi: 10.1099/jmm.0.057794-0. [DOI] [PubMed] [Google Scholar]
- 332.Debourgogne A, Favreau S, Ladriere M, Bourry S, Machouart M. 2014. Characteristics of Pneumocystis pneumonia in Nancy from January 2007 to April 2011 and focus on an outbreak in nephrology. J Mycol Med 24:19–24. doi: 10.1016/j.mycmed.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 333.Depypere M, Saegeman V, Lagrou K. 2016. Typing of Pneumocystis jirovecii by multilocus sequencing: evidence of outbreak? Eur J Clin Microbiol Infect Dis 35:911–916. doi: 10.1007/s10096-016-2615-y. [DOI] [PubMed] [Google Scholar]
- 334.Desoubeaux G, Dominique M, Morio F, Thepault RA, Franck-Martel C, Tellier AC, Ferrandiere M, Hennequin C, Bernard L, Salame E, Bailly E, Chandenier J. 2016. Epidemiological outbreaks of Pneumocystis jirovecii pneumonia are not limited to kidney transplant recipients: genotyping confirms common source of transmission in a liver transplantation unit. J Clin Microbiol 54:1314–1320. doi: 10.1128/JCM.00133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hauser PM, Francioli P, Bille J, Telenti A, Blanc DS. 1997. Typing of Pneumocystis carinii f. sp. hominis by single-strand conformation polymorphism of four genomic regions. J Clin Microbiol 35:3086–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Matos O, Esteves F. 2010. Pneumocystis jirovecii multilocus gene sequencing: findings and implications. Future Microbiol 5:1257–1267. doi: 10.2217/fmb.10.75. [DOI] [PubMed] [Google Scholar]
- 337.Phipps LM, Chen SC, Kable K, Halliday CL, Firacative C, Meyer W, Wong G, Nankivell BJ. 2011. Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients. Transplantation 92:1327–1334. doi: 10.1097/TP.0b013e3182384b57. [DOI] [PubMed] [Google Scholar]
- 338.Hauser PM, Francioli P, Bille J, Telenti A, Blanc DS. 1997. Typing of Pneumocystis carinii sp. f. hominis by PCR-SSCP of four genomic regions. J Eukaryot Microbiol 44:16S. doi: 10.1111/j.1550-7408.1997.tb05744.x. [DOI] [PubMed] [Google Scholar]
- 339.Maitte C, Leterrier M, Le Pape P, Miegeville M, Morio F. 2013. Multilocus sequence typing of Pneumocystis jirovecii from clinical samples: how many and which loci should be used? J Clin Microbiol 51:2843–2849. doi: 10.1128/JCM.01073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Maiden MC. 2006. Multilocus sequence typing of bacteria. Annu Rev Microbiol 60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 341.Sham P, Bader JS, Craig I, O'Donovan M, Owen M. 2002. DNA pooling: a tool for large-scale association studies. Nat Rev Genet 3:862–871. doi: 10.1038/nrg930. [DOI] [PubMed] [Google Scholar]
- 342.Esteves F, Gaspar J, de Sousa B, Antunes F, Mansinho K, Matos O. 2012. Pneumocystis jirovecii multilocus genotyping in pooled DNA samples: a new approach for clinical and epidemiological studies. Clin Microbiol Infect 18:E177–E184. doi: 10.1111/j.1469-0691.2012.03828.x. [DOI] [PubMed] [Google Scholar]
- 343.Esteves F, Gaspar J, De Sousa B, Antunes F, Mansinho K, Matos O. 2011. Clinical relevance of multiple single-nucleotide polymorphisms in Pneumocystis jirovecii pneumonia: development of a multiplex PCR-single-base-extension methodology. J Clin Microbiol 49:1810–1815. doi: 10.1128/JCM.02303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Steemers FJ, Chang W, Lee G, Barker DL, Shen R, Gunderson KL. 2006. Whole-genome genotyping with the single-base extension assay. Nat Methods 3:31–33. doi: 10.1038/nmeth842. [DOI] [PubMed] [Google Scholar]
- 345.Narayanan S. 1991. Applications of restriction fragment length polymorphism. Ann Clin Lab Sci 21:291–296. [PubMed] [Google Scholar]
- 346.Helweg-Larsen J, Eugen-Olsen J, Lundgren B. 2000. Rapid detection of dihydropteroate polymorphism in AIDS-related Pneumocystis carinii pneumonia by restriction fragment length polymorphism. Scand J Infect Dis 32:481–483. doi: 10.1080/003655400458730. [DOI] [PubMed] [Google Scholar]
- 347.Latouche S, Lacube P, Maury E, Bolognini J, Develoux M, Girard PM, Godet C, Lebrette MG, Mayaud C, Guillot J, Roux P. 2003. Pneumocystis jirovecii dihydropteroate synthase genotypes in French patients with pneumocystosis: a 1998-2001 prospective study. Med Mycol 41:533–537. doi: 10.1080/13693780310001615394. [DOI] [PubMed] [Google Scholar]
- 348.Valerio A, Tronconi E, Mazza F, Fantoni G, Atzori C, Tartarone F, Duca P, Cargnel A. 2007. Genotyping of Pneumocystis jiroveci pneumonia in Italian AIDS patients. Clinical outcome is influenced by dihydropteroate synthase and not by internal transcribed spacer genotype. J Acquir Immune Defic Syndr 45:521–528. [DOI] [PubMed] [Google Scholar]
- 349.Monroy-Vaca EX, de Armas Y, Illnait-Zaragozi MT, Torano G, Diaz R, Vega D, Alvarez-Lam I, Calderon EJ, Stensvold CR. 2014. Prevalence and genotype distribution of Pneumocystis jirovecii in Cuban infants and toddlers with whooping cough. J Clin Microbiol 52:45–51. doi: 10.1128/JCM.02381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Montes-Cano MA, de la Horra C, Martin-Juan J, Varela JM, Torronteras R, Respaldiza N, Medrano FJ, Calderon EJ. 2004. Pneumocystis jiroveci genotypes in the Spanish population. Clin Infect Dis 39:123–128. doi: 10.1086/421778. [DOI] [PubMed] [Google Scholar]
- 351.Le Gal S, Damiani C, Perrot M, Rouille A, Virmaux M, Quinio D, Moalic E, Saliou P, Berthou C, Le Meur Y, Totet A, Nevez G. 2012. Circulation of Pneumocystis dihydropteroate synthase mutants in France. Diagn Microbiol Infect Dis 74:119–124. doi: 10.1016/j.diagmicrobio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 352.Jarboui MA, Sellami A, Sellami H, Cheikhrouhou F, Makni F, Ayadi A. 2011. Dihydropteroate synthase gene mutations in Pneumocystis jiroveci strains isolated from immunocompromised patients. Pathol Biol (Paris) 59:222–225. doi: 10.1016/j.patbio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 353.Friaza V, Morilla R, Respaldiza N, de la Horra C, Calderon EJ. 2010. Pneumocystis jiroveci dihydropteroate synthase gene mutations among colonized individuals and Pneumocystis pneumonia patients from Spain. Postgrad Med 122:24–28. doi: 10.3810/pgm.2010.11.2219. [DOI] [PubMed] [Google Scholar]
- 354.Esteves F, Gaspar J, Marques T, Leite R, Antunes F, Mansinho K, Matos O. 2010. Identification of relevant single-nucleotide polymorphisms in Pneumocystis jirovecii: relationship with clinical data. Clin Microbiol Infect 16:878–884. doi: 10.1111/j.1469-0691.2009.03030.x. [DOI] [PubMed] [Google Scholar]
- 355.Sassi M, Ripamonti C, Mueller NJ, Yazaki H, Kutty G, Ma L, Huber C, Gogineni E, Oka S, Goto N, Fehr T, Gianella S, Konrad R, Sing A, Kovacs JA. 2012. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of Pneumocystis: implications for transmission and virulence. Clin Infect Dis 54:1437–1444. doi: 10.1093/cid/cis217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ripamonti C, Orenstein A, Kutty G, Huang L, Schuhegger R, Sing A, Fantoni G, Atzori C, Vinton C, Huber C, Conville PS, Kovacs JA. 2009. Restriction fragment length polymorphism typing demonstrates substantial diversity among Pneumocystis jirovecii isolates. J Infect Dis 200:1616–1622. doi: 10.1086/644643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rostved AA, Sassi M, Kurtzhals JA, Sorensen SS, Rasmussen A, Ross C, Gogineni E, Huber C, Kutty G, Kovacs JA, Helweg-Larsen J. 2013. Outbreak of Pneumocystis pneumonia in renal and liver transplant patients caused by genotypically distinct strains of Pneumocystis jirovecii. Transplantation 96:834–842. doi: 10.1097/TP.0b013e3182a1618c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. 1989. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A 86:2766–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Hauser PM, Blanc DS, Bille J, Telenti A, Francioli P. 1997. Development of a molecular typing method for Pneumocystis carinii sp. f. hominis. APMIS 77(Suppl):7–10. doi: 10.1111/j.1600-0463.1997.tb05373.x. [DOI] [PubMed] [Google Scholar]
- 360.Hauser PM, Blanc DS, Sudre P, Senggen Manoloff E, Nahimana A, Bille J, Weber R, Francioli P. 2001. Genetic diversity of Pneumocystis carinii in HIV-positive and -negative patients as revealed by PCR-SSCP typing. AIDS 15:461–466. doi: 10.1097/00002030-200103090-00004. [DOI] [PubMed] [Google Scholar]
- 361.Ma L, Kovacs JA. 2001. Rapid detection of mutations in the human-derived Pneumocystis carinii dihydropteroate synthase gene associated with sulfa resistance. Antimicrob Agents Chemother 45:776–780. doi: 10.1128/AAC.45.3.776-780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Mathis A, Weber R, Kuster H, Speich R. 1996. Reliable one-tube nested PCR for detection and SSCP-typing of Pneumocystis carinii. J Eukaryot Microbiol 43:7S. doi: 10.1111/j.1550-7408.1996.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 363.Nahimana A, Blanc DS, Francioli P, Bille J, Hauser PM. 2000. Typing of Pneumocystis carinii f. sp. hominis by PCR-SSCP to indicate a high frequency of co-infections. J Med Microbiol 49:753–758. doi: 10.1099/0022-1317-49-8-753. [DOI] [PubMed] [Google Scholar]
- 364.Nahimana A, Rabodonirina M, Helweg-Larsen J, Meneau I, Francioli P, Bille J, Hauser PM. 2003. Sulfa resistance and dihydropteroate synthase mutants in recurrent Pneumocystis carinii pneumonia. Emerg Infect Dis 9:864–867. doi: 10.3201/eid0907.020753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Hauser PM, Blanc DS, Telenti A, Nahimana A, Bille J, Francioli P. 1998. Potential coinfections complicate typing of Pneumocystis carinii f. sp. hominis. J Clin Microbiol 36:3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Tsolaki AG, Miller RF, Underwood AP, Banerji S, Wakefield AE. 1996. Genetic diversity at the internal transcribed spacer regions of the rRNA operon among isolates of Pneumocystis carinii from AIDS patients with recurrent pneumonia. J Infect Dis 174:141–156. doi: 10.1093/infdis/174.1.141. [DOI] [PubMed] [Google Scholar]
- 367.Alanio A, Gits-Muselli M, Guigue N, Desnos-Ollivier M, Calderon EJ, Di Cave D, Dupont D, Hamprecht A, Hauser PM, Helweg-Larsen J, Kicia M, Lagrou K, Lengerova M, Matos O, Melchers WJG, Morio F, Nevez G, Totet A, White LP, Bretagne S. 2017. Diversity of Pneumocystis jirovecii across Europe: a multicentre observational study. EBioMedicine 22:155–163. doi: 10.1016/j.ebiom.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gits-Muselli M, Peraldi MN, de Castro N, Delcey V, Menotti J, Guigue N, Hamane S, Raffoux E, Bergeron A, Valade S, Molina JM, Bretagne S, Alanio A. 2015. New short tandem repeat-based molecular typing method for Pneumocystis jirovecii reveals intrahospital transmission between patients from different wards. PLoS One 10:e0125763. doi: 10.1371/journal.pone.0125763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Gemayel R, Vinces MD, Legendre M, Verstrepen KJ. 2010. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu Rev Genet 44:445–477. doi: 10.1146/annurev-genet-072610-155046. [DOI] [PubMed] [Google Scholar]
- 370.Levinson G, Gutman GA. 1987. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol 4:203–221. [DOI] [PubMed] [Google Scholar]
- 371.Jarboui MA, Mseddi F, Sellami H, Sellami A, Mahfoudh N, Makni F, Makni H, Ayadi A. 2013. A comparison of capillary electrophoresis and direct sequencing in upstream conserved sequence region analysis of Pneumocystis jirovecii strains. J Med Microbiol 62:560–564. doi: 10.1099/jmm.0.045336-0. [DOI] [PubMed] [Google Scholar]
- 372.Sun L, Huang M, Wang J, Xue F, Hong C, Guo Z, Gu J. 2015. Genotyping of Pneumocystis jirovecii isolates from human immunodeficiency virus-negative patients in China. Infect Genet Evol 31:209–215. doi: 10.1016/j.meegid.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 373.Jarboui MA, Mseddi F, Sellami H, Sellami A, Makni F, Ayadi A. 2013. Genetic diversity of Pneumocystis jirovecii strains based on sequence variation of different DNA region. Med Mycol 51:561–567. doi: 10.3109/13693786.2012.744879. [DOI] [PubMed] [Google Scholar]
- 374.Gupta R, Mirdha BR, Guleria R, Kumar L, Luthra K, Agarwal SK, Sreenivas V. 2013. Genetic characterization of UCS region of Pneumocystis jirovecii and construction of allelic profiles of Indian isolates based on sequence typing at three regions. Infect Genet Evol 13:180–186. doi: 10.1016/j.meegid.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 375.Chiou CS. 2010. Multilocus variable-number tandem repeat analysis as a molecular tool for subtyping and phylogenetic analysis of bacterial pathogens. Expert Rev Mol Diagn 10:5–7. doi: 10.1586/erm.09.76. [DOI] [PubMed] [Google Scholar]
- 376.Parobek CM, Jiang LY, Patel JC, Alvarez-Martinez MJ, Miro JM, Worodria W, Andama A, Fong S, Huang L, Meshnick SR, Taylor SM, Juliano JJ. 2014. Multilocus microsatellite genotyping array for investigation of genetic epidemiology of Pneumocystis jirovecii. J Clin Microbiol 52:1391–1399. doi: 10.1128/JCM.02531-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Chan JZ, Pallen MJ, Oppenheim B, Constantinidou C. 2012. Genome sequencing in clinical microbiology. Nat Biotechnol 30:1068–1071. doi: 10.1038/nbt.2410. [DOI] [PubMed] [Google Scholar]
- 378.Didelot X, Bowden R, Wilson DJ, Peto TEA, Crook DW. 2012. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet 13:601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Alanio A, Gits-Muselli M, Mercier-Delarue S, Dromer F, Bretagne S. 2016. Diversity of Pneumocystis jirovecii during infection revealed by ultra-deep pyrosequencing. Front Microbiol 7:733. doi: 10.3389/fmicb.2016.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Charpentier E, Garnaud C, Wintenberger C, Bailly S, Murat JB, Rendu J, Pavese P, Drouet T, Augier C, Malvezzi P, Thiebaut-Bertrand A, Mallaret MR, Epaulard O, Cornet M, Larrat S, Maubon D. 2017. Added value of next-generation sequencing for multilocus sequence typing analysis of a Pneumocystis jirovecii pneumonia outbreak. Emerg Infect Dis 23:1237–1245. doi: 10.3201/eid2308.161295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Urabe N, Ishii Y, Hyodo Y, Aoki K, Yoshizawa S, Saga T, Murayama SY, Sakai K, Homma S, Tateda K. 2016. Molecular epidemiologic analysis of a Pneumocystis pneumonia outbreak among renal transplant patients. Clin Microbiol Infect 22:365–371. doi: 10.1016/j.cmi.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 382.Vanek J, Jirovec O. 1952. Parasitic pneumonia. Interstitial plasma cell pneumonia of premature infants, caused by Pneumocystis carinii. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg 158:120–127. (In German.) [PubMed] [Google Scholar]
- 383.Kucera K. 1967. Pneumocystosis as an anthropozoonosis. Ann Parasitol Hum Comp 42:465–481. doi: 10.1051/parasite/1967425465. [DOI] [PubMed] [Google Scholar]
- 384.Furuta T, Ueda K. 1987. Intra- and inter-species transmission and antigenic difference of Pneumocystis carinii derived from rat and mouse. Jpn J Exp Med 57:11–17. [PubMed] [Google Scholar]
- 385.Sethi KK. 1992. Multiplication of human-derived Pneumocystis carinii in severe combined immunodeficient (SCID) mice. Experientia 48:63–66. doi: 10.1007/BF01923610. [DOI] [PubMed] [Google Scholar]
- 386.Walzer PD, Schnelle V, Armstrong D, Rosen PP. 1977. Nude mouse: a new experimental model for Pneumocystis carinii infection. Science 197:177–179. doi: 10.1126/science.301657. [DOI] [PubMed] [Google Scholar]
- 387.Aliouat EM, Mazars E, Dei-Cas E, Delcourt P, Billaut P, Camus D. 1994. Pneumocystis cross infection experiments using SCID mice and nude rats as recipient host, showed strong host-species specificity. J Eukaryot Microbiol 41:71S. [PubMed] [Google Scholar]
- 388.Furuta T, Fujita M, Mukai R, Sakakibara I, Sata T, Miki K, Hayami M, Kojima S, Yoshikawa Y. 1993. Severe pulmonary pneumocystosis in simian acquired immunodeficiency syndrome induced by simian immunodeficiency virus: its characterization by the polymerase-chain-reaction method and failure of experimental transmission to immunodeficient animals. Parasitol Res 79:624–628. doi: 10.1007/BF00932502. [DOI] [PubMed] [Google Scholar]
- 389.Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ. 1993. Pneumocystis carinii is not universally transmissible between mammalian species. Infect Immun 61:2886–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Aliouat EM, Mazars E, Dei-Cas E, Cesbron JY, Camus D. 1993. Intranasal inoculation of mouse, rat or rabbit-derived Pneumocystis in SCID mice. J Protozool Res 3:94–98. [Google Scholar]
- 391.Atzori C, Agostini F, Angeli A, Mainini A, Micheli V, Cargnel A. 1999. P. carinii host specificity: attempt of cross infections with human derived strains in rats. J Eukaryot Microbiol 46(Suppl):112S. [PubMed] [Google Scholar]
- 392.Shah JS, Pieciak W, Liu J, Buharin A, Lane DJ. 1996. Diversity of host species and strains of Pneumocystis carinii is based on rRNA sequences. Clin Diagn Lab Immunol 3:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Sinclair K, Wakefield AE, Banerji S, Hopkin JM. 1991. Pneumocystis carinii organisms derived from rat and human hosts are genetically distinct. Mol Biochem Parasitol 45:183–184. doi: 10.1016/0166-6851(91)90042-5. [DOI] [PubMed] [Google Scholar]
- 394.Wakefield AE, Fritscher CC, Malin AS, Gwanzura L, Hughes WT, Miller RF. 1994. Genetic diversity in human-derived Pneumocystis carinii isolates from four geographical locations shown by analysis of mitochondrial rRNA gene sequences. J Clin Microbiol 32:2959–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Weinberg GA, Durant PJ. 1994. Genetic diversity of Pneumocystis carinii derived from infected rats, mice, ferrets, and cell cultures. J Eukaryot Microbiol 41:223–228. doi: 10.1111/j.1550-7408.1994.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 396.Lee CH, Lu JJ, Bartlett MS, Durkin MM, Liu TH, Wang J, Jiang B, Smith JW. 1993. Nucleotide sequence variation in Pneumocystis carinii strains that infect humans. J Clin Microbiol 31:754–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Lu JJ, Bartlett MS, Shaw MM, Smith JW, Lee CH. 1994. Typing of Pneumocystis carinii strains that infect humans based on nucleotide sequence variations of internal transcribed spacers of rRNA genes. J Eukaryot Microbiol 41:102S. [PubMed] [Google Scholar]
- 398.Lu JJ, Bartlett MS, Smith JW, Lee CH. 1995. Typing of Pneumocystis carinii strains with type-specific oligonucleotide probes derived from nucleotide sequences of internal transcribed spacers of rRNA genes. J Clin Microbiol 33:2973–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Wakefield AE. 1996. DNA sequences identical to Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. hominis in samples of air spora. J Clin Microbiol 34:1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Bartlett MS, Lee CH, Lu JJ, Bauer NL, Bettz JF, McLaughlin GL, Smith JW. 1994. Pneumocystis carinii detected in air. J Eukaryot Microbiol 41:75S. [PubMed] [Google Scholar]
- 401.Choukri F, Aliouat EM, Menotti J, Totet A, Gantois N, Garin YJ, Bergeron V, Dei-Cas E, Derouin F. 2011. Dynamics of Pneumocystis carinii air shedding during experimental pneumocystosis. J Infect Dis 203:1333–1336. doi: 10.1093/infdis/jir018. [DOI] [PubMed] [Google Scholar]
- 402.Latouche S, Olsson M, Polack B, Brun-Pascaud M, Bernard C, Roux P. 1997. Detection of Pneumocystis carinii f. sp. in air samples collected in animal rooms. J Eukaryot Microbiol 44:46S–47S. doi: 10.1111/j.1550-7408.1997.tb05768.x. [DOI] [PubMed] [Google Scholar]
- 403.Menotti J, Emmanuel A, Bouchekouk C, Chabe M, Choukri F, Pottier M, Sarfati C, Aliouat EM, Derouin F. 2013. Evidence of airborne excretion of Pneumocystis carinii during infection in immunocompetent rats. Lung involvement and antibody response. PLoS One 8:e62155. doi: 10.1371/journal.pone.0062155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Olsson M, Sukura A, Lindberg LA, Linder E. 1996. Detection of Pneumocystis carinii DNA by filtration of air. Scand J Infect Dis 28:279–282. doi: 10.3109/00365549609027173. [DOI] [PubMed] [Google Scholar]
- 405.Philippe L, Rene C, Guillot J, Berthalemy M, Polack B, Laine V, Lacube P, Chermette R, Roux P. 1999. Impaction versus filtration for the detection of Pneumocystis carinii DNA in air. J Eukaryot Microbiol 46:94S. [PubMed] [Google Scholar]
- 406.Bartlett MS, Vermund SH, Jacobs R, Durant PJ, Shaw MM, Smith JW, Tang X, Lu JJ, Li B, Jin S, Lee CH. 1997. Detection of Pneumocystis carinii DNA in air samples: likely environmental risk to susceptible persons. J Clin Microbiol 35:2511–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Frealle E, Valade S, Guigue N, Hamane S, Chabe M, Le Gal S, Damiani C, Totet A, Aliouat EM, Nevez G, Menotti J. 2017. Diffusion of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis colonization: frequency and putative risk factors. Med Mycol 55:568–572. doi: 10.1093/mmy/myw113. [DOI] [PubMed] [Google Scholar]
- 408.Latouch S, Totet A, Lacube P, Bolognini J, Nevez G, Roux P. 2001. Development of an RT-PCR on the heat shock protein 70 gene for viability detection of Pneumocystis carinii f. sp. hominis in patients with pneumocystosis and in air sample. J Eukaryot Microbiol 2001(Suppl):176S–177S. doi: 10.1111/j.1550-7408.2001.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 409.Maher NH, Vermund SH, Welsh DA, Dillon HK, Awooda A, Unnasch TR. 2001. Development and characterization of a molecular viability assay for Pneumocystis carinii f. sp. hominis. J Infect Dis 183:1825–1827. doi: 10.1086/320738. [DOI] [PubMed] [Google Scholar]
- 410.Olsson M, Lidman C, Latouche S, Bjorkman A, Roux P, Linder E, Wahlgren M. 1998. Identification of Pneumocystis carinii f. sp. hominis gene sequences in filtered air in hospital environments. J Clin Microbiol 36:1737–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Damiani C, Choukri F, Le Gal S, Menotti J, Sarfati C, Nevez G, Derouin F, Totet A. 2012. Possible nosocomial transmission of Pneumocystis jirovecii. Emerg Infect Dis 18:877–878. doi: 10.3201/eid1805.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Chin K, Luttrell TD, Roe JD, Shadzi S, Wyder MA, Kaneshiro ES. 1999. Putative Pneumocystis dormant forms outside the mammalian host, and long-term culture derived from them: initial characterizations. J Eukaryot Microbiol 46:95S–99S. [PubMed] [Google Scholar]
- 413.Kaneshiro ES, Maiorano JN. 1996. Survival and infectivity of Pneumocystis carinii outside the mammalian host. J Eukaryot Microbiol 43:35S. doi: 10.1111/j.1550-7408.1996.tb04971.x. [DOI] [PubMed] [Google Scholar]
- 414.Casanova-Cardiel L, Leibowitz MJ. 1997. Presence of Pneumocystis carinii DNA in pond water. J Eukaryot Microbiol 44:28S. doi: 10.1111/j.1550-7408.1997.tb05752.x. [DOI] [PubMed] [Google Scholar]
- 415.Hughes WT. 1982. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J Infect Dis 145:842–848. doi: 10.1093/infdis/145.6.842. [DOI] [PubMed] [Google Scholar]
- 416.Dowd SE, Gerba CP, Pepper IL. 1998. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol 64:3332–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Nwachcuku N, Gerba CP. 2004. Emerging waterborne pathogens: can we kill them all? Curr Opin Biotechnol 15:175–180. doi: 10.1016/j.copbio.2004.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Ajello L. 1956. Soil as natural reservoir for human pathogenic fungi. Science 123:876–879. doi: 10.1126/science.123.3203.876. [DOI] [PubMed] [Google Scholar]
- 419.Baumgardner DJ. 2012. Soil-related bacterial and fungal infections. J Am Board Fam Med 25:734–744. doi: 10.3122/jabfm.2012.05.110226. [DOI] [PubMed] [Google Scholar]
- 420.Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. 2009. Outbreak of Pneumocystis jiroveci pneumonia in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation 88:380–385. doi: 10.1097/TP.0b013e3181aed389. [DOI] [PubMed] [Google Scholar]
- 421.Navin TR, Rimland D, Lennox JL, Jernigan J, Cetron M, Hightower A, Roberts JM, Kaplan JE. 2000. Risk factors for community-acquired pneumonia among persons infected with human immunodeficiency virus. J Infect Dis 181:158–164. doi: 10.1086/315196. [DOI] [PubMed] [Google Scholar]
- 422.Hughes WT, Bartley DL, Smith BM. 1983. A natural source of infection due to Pneumocystis carinii. J Infect Dis 147:595. doi: 10.1093/infdis/147.3.595. [DOI] [PubMed] [Google Scholar]
- 423.Tedersoo L, Bahram M, Polme S, Koljalg U, Yorou NS, Wijesundera R, Villarreal Ruiz L, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, Poldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Partel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson KH, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo LD, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, De Kesel A, Dang T, Chen X, Buegger F, Brearley FQ, Bonito G, Anslan S, Abell S, Abarenkov K. 2014. Fungal biogeography. Global diversity and geography of soil fungi. Science 346:1256688. doi: 10.1126/science.1256688. [DOI] [PubMed] [Google Scholar]
- 424.Tedersoo L, Anslan S, Bahram M, Põlme S, Riit T, Liiv I, Kõljalg U, Kisand V, Nilsson H, Hildebrand F, Bork P, Abarenkov K. 2015. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 10:1–43. doi: 10.3897/mycokeys.10.4852. [DOI] [Google Scholar]
- 425.Anonymous. 1985. Pneumocystis—an orphan organism? Lancet i:676–677. [PubMed] [Google Scholar]
- 426.Gutierrez Y. 1989. The biology of Pneumocystis carinii. Semin Diagn Pathol 6:203–211. [PubMed] [Google Scholar]
- 427.Martinez A, Halliez MC, Aliouat EM, Chabe M, Standaert-Vitse A, Frealle E, Gantois N, Pottier M, Pinon A, Dei-Cas E, Aliouat-Denis CM. 2013. Growth and airborne transmission of cell-sorted life cycle stages of Pneumocystis carinii. PLoS One 8:e79958. doi: 10.1371/journal.pone.0079958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Cushion MT, Stringer JR. 2010. Stealth and opportunism: alternative lifestyles of species in the fungal genus Pneumocystis. Annu Rev Microbiol 64:431–452. doi: 10.1146/annurev.micro.112408.134335. [DOI] [PubMed] [Google Scholar]
- 429.Long EG, Smith JS, Meier JL. 1986. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Invest 54:609–615. [PubMed] [Google Scholar]
- 430.Lanken PN, Minda M, Pietra GG, Fishman AP. 1980. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol 99:561–588. [PMC free article] [PubMed] [Google Scholar]
- 431.Yoneda K, Walzer PD. 1980. Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infect Immun 29:692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. 1986. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Sci 17:811–825. doi: 10.1016/0021-8502(86)90035-2. [DOI] [Google Scholar]
- 433.Dollive S, Peterfreund GL, Sherrill-Mix S, Bittinger K, Sinha R, Hoffmann C, Nabel CS, Hill DA, Artis D, Bachman MA, Custers-Allen R, Grunberg S, Wu GD, Lewis JD, Bushman FD. 2012. A tool kit for quantifying eukaryotic rRNA gene sequences from human microbiome samples. Genome Biol 13:R60. doi: 10.1186/gb-2012-13-7-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. 2013. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One 8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Oh J, Byrd AL, Deming C, Conlan S, NISC Comparative Sequencing Program, Kong HH, Segre JA. 2014. Biogeography and individuality shape function in the human skin metagenome. Nature 514:59–64. doi: 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Oh J, Freeman AF, NISC Comparative Sequencing Program, Park M, Sokolic R, Candotti F, Holland SM, Segre JA, Kong HH. 2013. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res 23:2103–2114. doi: 10.1101/gr.159467.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Morris A, Beard CB, Huang L. 2002. Update on the epidemiology and transmission of Pneumocystis carinii. Microbes Infect 4:95–103. doi: 10.1016/S1286-4579(01)01514-3. [DOI] [PubMed] [Google Scholar]
- 438.Nevez G, Chabe M, Rabodonirina M, Virmaux M, Dei-Cas E, Hauser PM, Totet A. 2008. Nosocomial Pneumocystis jirovecii infections. Parasite 15:359–365. doi: 10.1051/parasite/2008153359. [DOI] [PubMed] [Google Scholar]
- 439.Choukri F, Menotti J, Sarfati C, Lucet JC, Nevez G, Garin YJ, Derouin F, Totet A. 2010. Quantification and spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin Infect Dis 51:259–265. doi: 10.1086/653933. [DOI] [PubMed] [Google Scholar]
- 440.Chabe M, Dei-Cas E, Creusy C, Fleurisse L, Respaldiza N, Camus D, Durand-Joly I. 2004. Immunocompetent hosts as a reservoir of Pneumocystis organisms: histological and RT-PCR data demonstrate active replication. Eur J Clin Microbiol Infect Dis 23:89–97. doi: 10.1007/s10096-003-1092-2. [DOI] [PubMed] [Google Scholar]
- 441.Gigliotti F, Harmsen AG, Wright TW. 2003. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun 71:3852–3856. doi: 10.1128/IAI.71.7.3852-3856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Kling HM, Shipley TW, Patil SP, Kristoff J, Bryan M, Montelaro RC, Morris A, Norris KA. 2010. Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection. Infect Immun 78:4320–4330. doi: 10.1128/IAI.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Khalife S, Chabe M, Gantois N, Audebert C, Pottier M, Hlais S, Pincon C, Chassat T, Pierrot C, Khalife J, Aliouat-Denis CM, Aliouat EM. 2016. Relationship between Pneumocystis carinii burden and the degree of host immunosuppression in an airborne transmission experimental model. J Eukaryot Microbiol 63:309–317. doi: 10.1111/jeu.12280. [DOI] [PubMed] [Google Scholar]
- 444.An CL, Gigliotti F, Harmsen AG. 2003. Exposure of immunocompetent adult mice to Pneumocystis carinii f. sp. muris by cohousing: growth of P. carinii f. sp. muris and host immune response. Infect Immun 71:2065–2070. doi: 10.1128/IAI.71.4.2065-2070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Dumoulin A, Mazars E, Seguy N, Gargallo-Viola D, Vargas S, Cailliez JC, Aliouat EM, Wakefield AE, Dei-Cas E. 2000. Transmission of Pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts. Eur J Clin Microbiol Infect Dis 19:671–678. doi: 10.1007/s100960000354. [DOI] [PubMed] [Google Scholar]
- 446.Huang L, Morris A, Limper AH, Beck JM, ATS Pneumocystis Workshop Participants. 2006. An official ATS Workshop summary: recent advances and future directions in Pneumocystis pneumonia (PCP). Proc Am Thorac Soc 3:655–664. doi: 10.1513/pats.200602-015MS. [DOI] [PubMed] [Google Scholar]
- 447.Pifer LL, Niell HB, Morrison BJ, Counce JD Jr, Freeman JM, Woods DR, Neely CL. 1984. Pneumocystis carinii antigenemia in adults with malignancy, infection, or pulmonary disease. J Clin Microbiol 20:887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Ozkoc S, Koker M, Onder M, Delibas SB. 2016. Prevalence of Pneumocystis jirovecii colonization in autopsy cases in Turkey. J Med Microbiol 65:1152–1157. doi: 10.1099/jmm.0.000337. [DOI] [PubMed] [Google Scholar]
- 449.Vargas SL, Ponce CA, Gallo M, Perez F, Astorga JF, Bustamante R, Chabe M, Durand-Joly I, Iturra P, Miller RF, Aliouat EM, Dei-Cas E. 2013. Near-universal prevalence of Pneumocystis and associated increase in mucus in the lungs of infants with sudden unexpected death. Clin Infect Dis 56:171–179. doi: 10.1093/cid/cis870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Vera C, Aguilar YA, Velez LA, Rueda ZV. 2017. High transient colonization by Pneumocystis jirovecii between mothers and newborn. Eur J Pediatr 176:1619–1627. doi: 10.1007/s00431-017-3011-z. [DOI] [PubMed] [Google Scholar]
- 451.Respaldiza N, Medrano FJ, Medrano AC, Varela JM, de la Horra C, Montes-Cano M, Ferrer S, Wichmann I, Gargallo-Viola D, Calderon EJ. 2004. High seroprevalence of Pneumocystis infection in Spanish children. Clin Microbiol Infect 10:1029–1031. doi: 10.1111/j.1469-0691.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- 452.Wakefield AE, Stewart TJ, Moxon ER, Marsh K, Hopkin JM. 1990. Infection with Pneumocystis carinii is prevalent in healthy Gambian children. Trans R Soc Trop Med Hyg 84:800–802. doi: 10.1016/0035-9203(90)90087-U. [DOI] [PubMed] [Google Scholar]
- 453.Vargas SL, Ponce CA, Sanchez CA, Ulloa AV, Bustamante R, Juarez G. 2003. Pregnancy and asymptomatic carriage of Pneumocystis jiroveci. Emerg Infect Dis 9:605–606. doi: 10.3201/eid0905.020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Miller RF, Ambrose HE, Wakefield AE. 2001. Pneumocystis carinii f. sp. hominis DNA in immunocompetent health care workers in contact with patients with P. carinii pneumonia. J Clin Microbiol 39:3877–3882. doi: 10.1128/JCM.39.11.3877-3882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Leigh TR, Millett MJ, Jameson B, Collins JV. 1993. Serum titres of Pneumocystis carinii antibody in health care workers caring for patients with AIDS. Thorax 48:619–621. doi: 10.1136/thx.48.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Tipirneni R, Daly KR, Jarlsberg LG, Koch JV, Swartzman A, Roth BM, Walzer PD, Huang L. 2009. Healthcare worker occupation and immune response to Pneumocystis jirovecii. Emerg Infect Dis 15:1590–1597. doi: 10.3201/eid1510.090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Valade S, Azoulay E, Damiani C, Derouin F, Totet A, Menotti J. 2015. Pneumocystis jirovecii airborne transmission between critically ill patients and health care workers. Intensive Care Med 41:1716–1718. doi: 10.1007/s00134-015-3835-9. [DOI] [PubMed] [Google Scholar]
- 458.Vargas SL, Ponce CA, Hughes WT, Wakefield AE, Weitz JC, Donoso S, Ulloa AV, Madrid P, Gould S, Latorre JJ, Avila R, Benveniste S, Gallo M, Belletti J, Lopez R. 1999. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin Infect Dis 29:1489–1493. doi: 10.1086/313521. [DOI] [PubMed] [Google Scholar]
- 459.Vargas SL, Ponce CA, Galvez P, Ibarra C, Haas EA, Chadwick AE, Krous HF. 2007. Pneumocystis is not a direct cause of sudden infant death syndrome. Pediatr Infect Dis J 26:81–83. doi: 10.1097/01.inf.0000247071.40739.fd. [DOI] [PubMed] [Google Scholar]
- 460.Vargas SL, Ponce CA, Luchsinger V, Silva C, Gallo M, Lopez R, Belletti J, Velozo L, Avila R, Palomino MA, Benveniste S, Avendano LF. 2005. Detection of Pneumocystis carinii f. sp. hominis and viruses in presumably immunocompetent infants who died in the hospital or in the community. J Infect Dis 191:122–126. doi: 10.1086/426451. [DOI] [PubMed] [Google Scholar]
- 461.Khodavaisy S, Mortaz E, Mohammadi F, Aliyali M, Fakhim H, Badali H. 2015. Pneumocystis jirovecii colonization in chronic obstructive pulmonary disease (COPD). Curr Med Mycol 1:42–48. doi: 10.18869/acadpub.cmm.1.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Christensen PJ, Preston AM, Ling T, Du M, Fields WB, Curtis JL, Beck JM. 2008. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect Immun 76:3481–3490. doi: 10.1128/IAI.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Morris A, Alexander T, Radhi S, Lucht L, Sciurba FC, Kolls JK, Srivastava R, Steele C, Norris KA. 2009. Airway obstruction is increased in Pneumocystis-colonized human immunodeficiency virus-infected outpatients. J Clin Microbiol 47:3773–3776. doi: 10.1128/JCM.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. 2004. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med 170:408–413. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 465.Calderon EJ, Rivero L, Respaldiza N, Morilla R, Montes-Cano MA, Friaza V, Munoz-Lobato F, Varela JM, Medrano FJ, Horra CL. 2007. Systemic inflammation in patients with chronic obstructive pulmonary disease who are colonized with Pneumocystis jiroveci. Clin Infect Dis 45:e17–e19. doi: 10.1086/518989. [DOI] [PubMed] [Google Scholar]
- 466.Varela JM, Respaldiza N, Sanchez B, de la Horra C, Montes-Cano M, Rincon M, Dapena J, Gonzalez-Becerra C, Medrano FJ, Calderon E. 2003. Lymphocyte response in subjects with chronic pulmonary disease colonized by Pneumocystis jirovecii. J Eukaryot Microbiol 50(Suppl):672–673. doi: 10.1111/j.1550-7408.2003.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 467.Shipley TW, Kling HM, Morris A, Patil S, Kristoff J, Guyach SE, Murphy JE, Shao X, Sciurba FC, Rogers RM, Richards T, Thompson P, Montelaro RC, Coxson HO, Hogg JC, Norris KA. 2010. Persistent Pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis 202:302–312. doi: 10.1086/653485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. 2007. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. http://www.cdc.gov/hicpac/2007IP/2007isolationprecautions.html Accessed 21 November 2017. [DOI] [PMC free article] [PubMed]
- 469.de Boer MG, Kroon FP, le Cessie S, de Fijter JW, van Dissel JT. 2011. Risk factors for Pneumocystis jirovecii pneumonia in kidney transplant recipients and appraisal of strategies for selective use of chemoprophylaxis. Transpl Infect Dis 13:559–569. doi: 10.1111/j.1399-3062.2011.00645.x. [DOI] [PubMed] [Google Scholar]
- 470.Cushion MT, Kaselis M, Stringer SL, Stringer JR. 1993. Genetic stability and diversity of Pneumocystis carinii infecting rat colonies. Infect Immun 61:4801–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Hong ST, Steele PE, Cushion MT, Walzer PD, Stringer SL, Stringer JR. 1990. Pneumocystis carinii karyotypes. J Clin Microbiol 28:1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Lundgren B, Cotton R, Lundgren JD, Edman JC, Kovacs JA. 1990. Identification of Pneumocystis carinii chromosomes and mapping of five genes. Infect Immun 58:1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Cushion MT, Zhang J, Kaselis M, Giuntoli D, Stringer SL, Stringer JR. 1993. Evidence for two genetic variants of Pneumocystis carinii coinfecting laboratory rats. J Clin Microbiol 31:1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Liu Y, Rocourt M, Pan S, Liu C, Leibowitz MJ. 1992. Sequence and variability of the 5.8S and 26S rRNA genes of Pneumocystis carinii. Nucleic Acids Res 20:3763–3772. doi: 10.1093/nar/20.14.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Alanio A, Olivi M, Cabaret O, Foulet F, Bellanger AP, Millon L, Berceanu A, Cordonnier C, Costa JM, Bretagne S. 2015. Correlation between Pneumocystis jirovecii mitochondrial genotypes and high and low fungal loads assessed by single nucleotide primer extension assay and quantitative real-time PCR. J Eukaryot Microbiol 62:650–656. doi: 10.1111/jeu.12222. [DOI] [PubMed] [Google Scholar]
- 476.Alizon S, de Roode JC, Michalakis Y. 2013. Multiple infections and the evolution of virulence. Ecol Lett 16:556–567. doi: 10.1111/ele.12076. [DOI] [PubMed] [Google Scholar]
- 477.Balmer O, Tanner M. 2011. Prevalence and implications of multiple-strain infections. Lancet Infect Dis 11:868–878. doi: 10.1016/S1473-3099(11)70241-9. [DOI] [PubMed] [Google Scholar]
- 478.Susi H, Barres B, Vale PF, Laine AL. 2015. Co-infection alters population dynamics of infectious disease. Nat Commun 6:5975. doi: 10.1038/ncomms6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Marinosa M, Soler A, Nogues X, Pedro-Botet J. 2004. Pulmonary coinfection by Pneumocystis carinii and Aspergillus fumigatus in a seronegative arthritis patient treated with low-dose methotrexate. Clin Rheumatol 23:555–556. doi: 10.1007/s10067-004-0900-0. [DOI] [PubMed] [Google Scholar]
- 480.Markantonatou AM, Ioakimidou A, Arvaniti K, Manou E, Papadopoulos V, Kiriklidou P, Samaras K, Kioumi A, Vyzantiadis TA. 2017. Pulmonary co-infections by Pneumocystis jirovecii and Aspergillus fumigatus in non-HIV patients: a report of two cases and literature review. Mycoses 60:626–633. doi: 10.1111/myc.12642. [DOI] [PubMed] [Google Scholar]
- 481.Orsi CF, Bettua C, Pini P, Venturelli C, La Regina A, Morace G, Luppi M, Forghieri F, Bigliardi S, Luppi F, Codeluppi M, Girardis M, Blasi E. 2015. Detection of Pneumocystis jirovecii and Aspergillus spp. DNA in bronchoalveolar lavage fluids by commercial real-time PCR assays: comparison with conventional diagnostic tests. New Microbiol 38:75–84. [PubMed] [Google Scholar]
- 482.Langlois ME, Lorillou M, Ferry T, Chidiac C, Valour F. 2015. Cystic lung lesions revealing a Pneumocystis jirovecii and Aspergillus flavus co-infection in an HIV-infected patient. Int J Infect Dis 37:143–144. doi: 10.1016/j.ijid.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 483.Desai A, Fe A, Desai A, Ilowite J, Cunha BA, Mathew JP. 2016. A case of pneumonia caused by Pneumocystis jirovecii and Cryptococcus neoformans in a patient with HTLV-1 associated adult T-cell leukemia/lymphoma: Occam's razor blunted. Conn Med 80:81–83. [PubMed] [Google Scholar]
- 484.Pena ZG, Byers HR, Lehmer LM, Smith CA, Ragsdale BD. 2013. Mixed Pneumocystis and Cryptococcus cutaneous infection histologically mimicking xanthoma. Am J Dermatopathol 35:e6–e10. doi: 10.1097/DAD.0b013e318266b59a. [DOI] [PubMed] [Google Scholar]
- 485.Javier B, Susana L, Santiago G, Alcides T. 2012. Pulmonary coinfection by Pneumocystis jiroveci and Cryptococcus neoformans. Asian Pac J Trop Biomed 2:80–82. doi: 10.1016/S2221-1691(11)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Prestes-Carneiro LE, Junior NGS, Oliveira MR, Auer LS, Duarte AJS, Almeida A, Vasconcelos DM. 2014. Recurrent pneumocystosis pneumonia/chronic obstructive pulmonary disease and mild immunodeficiency in a human immunodeficiency virus-negative subject. JMM Case Rep 1:1–5. doi: 10.1099/jmmcr.0.001578. [DOI] [Google Scholar]
- 487.Gago S, Esteban C, Valero C, Zaragoza O, Puig de la Bellacasa J, Buitrago MJ. 2014. A multiplex real-time PCR assay for identification of Pneumocystis jirovecii, Histoplasma capsulatum, and Cryptococcus neoformans/Cryptococcus gattii in samples from AIDS patients with opportunistic pneumonia. J Clin Microbiol 52:1168–1176. doi: 10.1128/JCM.02895-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Boondireke S, Mungthin M, Tan-ariya P, Boonyongsunchai P, Naaglor T, Wattanathum A, Treewatchareekorn S, Leelayoova S. 2010. Evaluation of sensitivity of multiplex PCR for detection of Mycobacterium tuberculosis and Pneumocystis jirovecii in clinical samples. J Clin Microbiol 48:3165–3168. doi: 10.1128/JCM.00323-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Jensen L, Jensen AV, Praygod G, Kidola J, Faurholt-Jepsen D, Changalucha J, Range N, Friis H, Helweg-Larsen J, Jensen JS, Andersen AB. 2010. Infrequent detection of Pneumocystis jirovecii by PCR in oral wash specimens from TB patients with or without HIV and healthy contacts in Tanzania. BMC Infect Dis 10:140. doi: 10.1186/1471-2334-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Nowaseb V, Gaeb E, Fraczek MG, Richardson MD, Denning DW. 2014. Frequency of Pneumocystis jirovecii in sputum from HIV and TB patients in Namibia. J Infect Dev Ctries 8:349–357. doi: 10.3855/jidc.3864. [DOI] [PubMed] [Google Scholar]
- 491.To KK, Hung IF, Xu T, Poon RW, Ip WC, Li PT, Li CP, Lau SK, Yam WC, Chan KH, Yuen KY. 2013. Clinical significance of Pneumocystis jiroveci in patients with active tuberculosis. Diagn Microbiol Infect Dis 75:260–265. doi: 10.1016/j.diagmicrobio.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 492.Dworzack DL, Ferry JJ, Clark RB. 1989. Co-infection with Legionella pneumophila and Pneumocystis carinii in a patient with chronic lymphocytic leukemia. Nebr Med J 74:73–75. [PubMed] [Google Scholar]
- 493.Abernathy-Carver KJ, Fan LL, Boguniewicz M, Larsen GL, Leung DY. 1994. Legionella and Pneumocystis pneumonias in asthmatic children on high doses of systemic steroids. Pediatr Pulmonol 18:135–138. doi: 10.1002/ppul.1950180303. [DOI] [PubMed] [Google Scholar]
- 494.Arakaki N, Higa F, Tateyama M, Yamazato Y, Yara S, Ishimine T, Toyama M, Miyara T, Koide M, Saito A. 1999. Concurrent infection with Legionella pneumophila and Pneumocystis carinii in a patient with adult T cell leukemia. Intern Med 38:160–163. doi: 10.2169/internalmedicine.38.160. [DOI] [PubMed] [Google Scholar]
- 495.Musallam N, Bamberger E, Srugo I, Dabbah H, Glikman D, Zonis Z, Kessel A, Genizi J. 2014. Legionella pneumophila and Pneumocystis jirovecii coinfection in an infant treated with adrenocorticotropic hormone for infantile spasm: case report and literature review. J Child Neurol 29:240–242. doi: 10.1177/0883073813511148. [DOI] [PubMed] [Google Scholar]
- 496.Salelles P, Roig P, Orti A, Navarro V, Ortiz de la Tabla V, Galant J, Merino J. 1997. Pulmonary coinfection by Salmonella enteritidis and Pneumocystis carinii in a patient with the acquired immunodeficiency syndrome. Eur J Clin Microbiol Infect Dis 16:773–774. doi: 10.1007/BF01709265. [DOI] [PubMed] [Google Scholar]
- 497.Shah K, Cherabuddi K, Beal SG, Kalyatanda G. 2017. Refractory acute respiratory failure due to Pneumocystis jiroveci (PCP) and cytomegalovirus (CMV) pneumonitis: a case report and review of literature. IDCases 10:42–45. doi: 10.1016/j.idcr.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Chuganji E, Abe T, Kobayashi H, Nakano N, Kanai T, Ohara G, Takayashiki N, Noguchi M, Morishita Y, Aoki M, Tokuda Y. 2014. Fatal pulmonary co-infection with Pneumocystis and cytomegalovirus in a patient with acquired immunodeficiency syndrome. Intern Med 53:1575–1578. doi: 10.2169/internalmedicine.53.2171. [DOI] [PubMed] [Google Scholar]
- 499.Pliquett RU, Asbe-Vollkopf A, Hauser PM, Presti LL, Hunfeld KP, Berger A, Scheuermann EH, Jung O, Geiger H, Hauser IA. 2012. A Pneumocystis jirovecii pneumonia outbreak in a single kidney-transplant center: role of cytomegalovirus co-infection. Eur J Clin Microbiol Infect Dis 31:2429–2437. doi: 10.1007/s10096-012-1586-x. [DOI] [PubMed] [Google Scholar]
- 500.Kim T, Moon SM, Sung H, Kim MN, Kim SH, Choi SH, Jeong JY, Woo JH, Kim YS, Lee SO. 2012. Outcomes of non-HIV-infected patients with Pneumocystis pneumonia and concomitant pulmonary cytomegalovirus infection. Scand J Infect Dis 44:670–677. doi: 10.3109/00365548.2011.652665. [DOI] [PubMed] [Google Scholar]
- 501.Ishiguro T, Takayanagi N, Kawabata Y, Sugita Y. 2011. Intestinal perforation due to concomitant cytomegalovirus infection during treatment for Pneumocystis jirovecii pneumonia in a patient with rheumatoid arthritis. Intern Med 50:1835–1837. doi: 10.2169/internalmedicine.50.5437. [DOI] [PubMed] [Google Scholar]
- 502.Vetter M, Battegay M, Trendelenburg M. 2010. Primary cytomegalovirus infection with accompanying Pneumocystis jiroveci pneumonia in a patient with large-vessel vasculitis. Infection 38:331–334. doi: 10.1007/s15010-010-0024-1. [DOI] [PubMed] [Google Scholar]
- 503.Sritippayawan S, Jitchaiwat S, Chatchatee P, Prapphal N, Deerojanawong J, Samransamruajkit R. 2006. Disseminated cytomegalovirus infection associated with Pneumocystis carinii pneumonia in a previously normal infant. Scand J Infect Dis 38:312–314. doi: 10.1080/00365540500353259. [DOI] [PubMed] [Google Scholar]
- 504.Gupta RK, Naran S, Lallu S, Fauck R. 2004. Cytodiagnosis of simultaneous pulmonary infection due to cytomegalovirus and Pneumocystis carinii in a sample of bronchoalveolar lavage. Diagn Cytopathol 30:341. doi: 10.1002/dc.20029. [DOI] [PubMed] [Google Scholar]
- 505.du Bois RM, Branthwaite MA, Mikhail JR, Batten JC. 1981. Primary Pneumocystis carinii and cytomegalovirus infections. Lancet ii:1339. [DOI] [PubMed] [Google Scholar]
- 506.Wang NS, Huang SN, Thurlbeck WM. 1970. Combined Pneumocystis carinii and cytomegalovirus infection. Arch Pathol 90:529–535. [PubMed] [Google Scholar]
- 507.Pulcini C, Hasseine L, Mondain V, Baudin G, Roger PM. 2012. Possible pandemic HIN1 influenza complicated by Pneumocystis jirovecii pneumonia in an HIV-infected patient. J Mycol Med 22:88–91. doi: 10.1016/j.mycmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 508.van Kampen JJ, Bielefeld-Buss AJ, Ott A, Maaskant J, Faber HJ, Lutisan JG, Boucher CA. 2013. Case report: oseltamivir-induced resistant pandemic influenza A (H1N1) virus infection in a patient with AIDS and Pneumocystis jirovecii pneumonia. J Med Virol 85:941–943. doi: 10.1002/jmv.23560. [DOI] [PubMed] [Google Scholar]
- 509.Burke J, Soubani AO. 2017. Influenza and Pneumocystis jirovecii pneumonia in an allogeneic hematopoietic stem cell transplantation recipient: co-infection or superinfection? Transpl Infect Dis 20:e12802. doi: 10.1111/tid.12802. [DOI] [PubMed] [Google Scholar]
- 510.Vuorinen T, Kotilainen P, Lautenschlager I, Kujari H, Krogerus L, Oksi J. 2004. Interstitial pneumonitis and coinfection of human herpesvirus 6 and Pneumocystis carinii in a patient with hypogammaglobulinemia. J Clin Microbiol 42:5415–5418. doi: 10.1128/JCM.42.11.5415-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Bava AJ, Romero M, Prieto R, Troncoso A. 2011. A case report of pulmonary coinfection of Strongyloides stercoralis and Pneumocystis jiroveci. Asian Pac J Trop Biomed 1:334–336. doi: 10.1016/S2221-1691(11)60056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Udaka M, Maehara N, Tamaki K, Fukuhara H, Kaneshima H, Nakamura H, Irabu Y, Shimoji K, Kitsukawa K, Shigeno Y. 1990. A case of Pneumocystis carinii pneumonia with hyperinfection of Strongyloides stercoralis complicated with smoldering adult T-cell leukemia. Kansenshogaku Zasshi 64:630–635. doi: 10.11150/kansenshogakuzasshi1970.64.630. [DOI] [PubMed] [Google Scholar]
- 513.Levy PY, Brouqui P, Dumon H, Gallais H. 1990. Pneumonia in AIDS: a case of co-infection caused by Toxoplasma gondii and Pneumocystis carinii. Presse Med 19:673. [PubMed] [Google Scholar]
- 514.Duboucher C, Noel C, Durand-Joly I, Gerbod D, Delgado-Viscogliosi P, Jouveshomme S, Leclerc C, Cartolano GL, Dei-Cas E, Capron M, Viscogliosi E. 2003. Pulmonary coinfection by Trichomonas vaginalis and Pneumocystis sp. as a novel manifestation of AIDS. Hum Pathol 34:508–511. doi: 10.1016/S0046-8177(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 515.Alanio A, Bretagne S. 2017. Pneumocystis jirovecii detection in asymptomatic patients: what does its natural history tell us? F1000Res 6:739. doi: 10.12688/f1000research.10619.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Peglow SL, Smulian AG, Linke MJ, Pogue CL, Nurre S, Crisler J, Phair J, Gold JW, Armstrong D, Walzer PD. 1990. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis 161:296–306. doi: 10.1093/infdis/161.2.296. [DOI] [PubMed] [Google Scholar]
- 517.Icenhour CR, Rebholz SL, Collins MS, Cushion MT. 2002. Early acquisition of Pneumocystis carinii in neonatal rats as evidenced by PCR and oral swabs. Eukaryot Cell 1:414–419. doi: 10.1128/EC.1.3.414-419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Simonds RJ, Hughes WT, Feinberg J, Navin TR. 1995. Preventing Pneumocystis carinii pneumonia in persons infected with human immunodeficiency virus. Clin Infect Dis 21(Suppl 1):S44–S48. doi: 10.1093/clinids/21.Supplement_1.S44. [DOI] [PubMed] [Google Scholar]
- 519.Simonds RJ, Oxtoby MJ, Caldwell MB, Gwinn ML, Rogers MF. 1993. Pneumocystis carinii pneumonia among US children with perinatally acquired HIV infection. JAMA 270:470–473. [PubMed] [Google Scholar]
- 520.Dohn MN, White ML, Vigdorth EM, Ralph Buncher C, Hertzberg VS, Baughman RP, George Smulian A, Walzer PD. 2000. Geographic clustering of Pneumocystis carinii pneumonia in patients with HIV infection. Am J Respir Crit Care Med 162:1617–1621. doi: 10.1164/ajrccm.162.5.9707101. [DOI] [PubMed] [Google Scholar]
- 521.Morris AM, Swanson M, Ha H, Huang L. 2000. Geographic distribution of human immunodeficiency virus-associated Pneumocystis carinii pneumonia in San Francisco. Am J Respir Crit Care Med 162:1622–1626. doi: 10.1164/ajrccm.162.5.2002065. [DOI] [PubMed] [Google Scholar]
- 522.Cordonnier C, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Alanio A, Hauser PM, Lagrou K, Melchers WJ, Helweg-Larsen J, Matos O, Bretagne S, Maertens J, Fifth European Conference on Infections in Leukemia (ECIL-5), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS), and the European LeukemiaNet (ELN). 2016. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 71:2379–2385. doi: 10.1093/jac/dkw155. [DOI] [PubMed] [Google Scholar]
- 523.Kostakis ID, Sotiropoulos GC, Kouraklis G. 2014. Pneumocystis jirovecii pneumonia in liver transplant recipients: a systematic review. Transplant Proc 46:3206–3208. doi: 10.1016/j.transproceed.2014.09.156. [DOI] [PubMed] [Google Scholar]
- 524.Keely SP, Stringer JR, Baughman RP, Linke MJ, Walzer PD, Smulian AG. 1995. Genetic variation among Pneumocystis carinii hominis isolates in recurrent pneumocystosis. J Infect Dis 172:595–598. doi: 10.1093/infdis/172.2.595. [DOI] [PubMed] [Google Scholar]
- 525.Keely SP, Baughman RP, Smulian AG, Dohn MN, Stringer JR. 1996. Source of Pneumocystis carinii in recurrent episodes of pneumonia in AIDS patients. AIDS 10:881–888. doi: 10.1097/00002030-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 526.Vindrios W, Argy N, Le Gal S, Lescure FX, Massias L, Le MP, Wolff M, Yazdanpanah Y, Nevez G, Houze S, Dorent R, Lucet JC. 2017. Outbreak of Pneumocystis jirovecii infection among heart transplant recipients: molecular investigation and management of an inter-human transmission. Clin Infect Dis 65:1120–1126. doi: 10.1093/cid/cix495. [DOI] [PubMed] [Google Scholar]
- 527.Mulpuru S, Knoll G, Weir C, Desjardins M, Johnson D, Gorn I, Fairhead T, Bissonnette J, Bruce N, Toye B, Suh K, Roth V. 2016. Pneumocystis pneumonia outbreak among renal transplant recipients at a North American transplant center: risk factors and implications for infection control. Am J Infect Control 44:425–431. doi: 10.1016/j.ajic.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 528.Deng X, Zhuo L, Lan Y, Dai Z, Chen WS, Cai W, Kovacs JA, Ma L, Tang X. 2014. Mutational analysis of Pneumocystis jirovecii dihydropteroate synthase and dihydrofolate reductase genes in HIV-infected patients in China. J Clin Microbiol 52:4017–4019. doi: 10.1128/JCM.01848-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Huang L, Beard CB, Creasman J, Levy D, Duchin JS, Lee S, Pieniazek N, Carter JL, del Rio C, Rimland D, Navin TR. 2000. Sulfa or sulfone prophylaxis and geographic region predict mutations in the Pneumocystis carinii dihydropteroate synthase gene. J Infect Dis 182:1192–1198. doi: 10.1086/315824. [DOI] [PubMed] [Google Scholar]
- 530.Kutty G, Maldarelli F, Achaz G, Kovacs JA. 2008. Variation in the major surface glycoprotein genes in Pneumocystis jirovecii. J Infect Dis 198:741–749. doi: 10.1086/590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. 1981. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 305:1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 532.Masur H, Michelis MA, Greene JB, Onorato I, Stouwe RA, Holzman RS, Wormser G, Brettman L, Lange M, Murray HW, Cunningham-Rundles S. 1981. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med 305:1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- 533.Follansbee SE, Busch DF, Wofsy CB, Coleman DL, Gullet J, Aurigemma GP, Ross T, Hadley WK, Drew WL. 1982. An outbreak of Pneumocystis carinii pneumonia in homosexual men. Ann Intern Med 96:705–713. doi: 10.7326/0003-4819-96-6-705. [DOI] [PubMed] [Google Scholar]
- 534.Hardy AM, Wajszczuk CP, Suffredini AF, Hakala TR, Ho M. 1984. Pneumocystis carinii pneumonia in renal-transplant recipients treated with cyclosporine and steroids. J Infect Dis 149:143–147. doi: 10.1093/infdis/149.2.143. [DOI] [PubMed] [Google Scholar]
- 535.Lufft V, Kliem V, Behrend M, Pichlmayr R, Koch KM, Brunkhorst R. 1996. Incidence of Pneumocystis carinii pneumonia after renal transplantation. Impact of immunosuppression. Transplantation 62:421–423. doi: 10.1097/00007890-199608150-00022. [DOI] [PubMed] [Google Scholar]
- 536.Branten AJ, Beckers PJ, Tiggeler RG, Hoitsma AJ. 1995. Pneumocystis carinii pneumonia in renal transplant recipients. Nephrol Dial Transplant 10:1194–1197. [PubMed] [Google Scholar]
- 537.Hauser P, Rabodonirina M, Nevez G. 2013. Pneumocystis jirovecii genotypes involved in Pneumocystis pneumonia outbreaks among renal transplant recipients. Clin Infect Dis 56:165–166. doi: 10.1093/cid/cis810. [DOI] [PubMed] [Google Scholar]
- 538.Huang L, Cattamanchi A, Davis JL, den Boon S, Kovacs J, Meshnick S, Miller RF, Walzer PD, Worodria W, Masur H, International HIV-Associated Opportunistic Pneumonias (IHOP) Study, Lung HIV Study. 2011. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc 8:294–300. doi: 10.1513/pats.201009-062WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Stein CR, Poole C, Kazanjian P, Meshnick SR. 2004. Sulfa use, dihydropteroate synthase mutations, and Pneumocystis jirovecii pneumonia. Emerg Infect Dis 10:1760–1765. doi: 10.3201/eid1010.040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Achari A, Somers DO, Champness JN, Bryant PK, Rosemond J, Stammers DK. 1997. Crystal structure of the anti-bacterial sulfonamide drug target dihydropteroate synthase. Nat Struct Biol 4:490–497. doi: 10.1038/nsb0697-490. [DOI] [PubMed] [Google Scholar]
- 541.Vedantam G, Nichols BP. 1998. Characterization of a mutationally altered dihydropteroate synthase contributing to sulfathiazole resistance in Escherichia coli. Microb Drug Resist 4:91–97. doi: 10.1089/mdr.1998.4.91. [DOI] [PubMed] [Google Scholar]
- 542.Lopez P, Espinosa M, Greenberg B, Lacks SA. 1987. Sulfonamide resistance in Streptococcus pneumoniae: DNA sequence of the gene encoding dihydropteroate synthase and characterization of the enzyme. J Bacteriol 169:4320–4326. doi: 10.1128/jb.169.9.4320-4326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Fermer C, Swedberg G. 1997. Adaptation to sulfonamide resistance in Neisseria meningitidis may have required compensatory changes to retain enzyme function: kinetic analysis of dihydropteroate synthases from N. meningitidis expressed in a knockout mutant of Escherichia coli. J Bacteriol 179:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Triglia T, Menting JG, Wilson C, Cowman AF. 1997. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A 94:13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Helweg-Larsen J, Benfield TL, Eugen-Olsen J, Lundgren JD, Lundgren B. 1999. Effects of mutations in Pneumocystis carinii dihydropteroate synthase gene on outcome of AIDS-associated P. carinii pneumonia. Lancet 354:1347–1351. doi: 10.1016/S0140-6736(99)03320-6. [DOI] [PubMed] [Google Scholar]
- 546.Takahashi T, Hosoya N, Endo T, Nakamura T, Sakashita H, Kimura K, Ohnishi K, Nakamura Y, Iwamoto A. 2000. Relationship between mutations in dihydropteroate synthase of Pneumocystis carinii f. sp. hominis isolates in Japan and resistance to sulfonamide therapy. J Clin Microbiol 38:3161–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Crothers K, Beard CB, Turner J, Groner G, Fox M, Morris A, Eiser S, Huang L. 2005. Severity and outcome of HIV-associated Pneumocystis pneumonia containing Pneumocystis jirovecii dihydropteroate synthase gene mutations. AIDS 19:801–805. doi: 10.1097/01.aids.0000168974.67090.70. [DOI] [PubMed] [Google Scholar]
- 548.Rabodonirina M, Vaillant L, Taffe P, Nahimana A, Gillibert RP, Vanhems P, Hauser PM. 2013. Pneumocystis jirovecii genotype associated with increased death rate of HIV-infected patients with pneumonia. Emerg Infect Dis 19:21–28. doi: 10.3201/eid1901.120140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Taylor SM, Meshnick SR, Worodria W, Andama A, Cattamanchi A, Davis JL, Yoo SD, Byanyima P, Kaswabuli S, Goodman CD, Huang L, International HIV-Associated Opportunistic Pneumonias Study. 2012. Low prevalence of Pneumocystis pneumonia (PCP) but high prevalence of Pneumocystis dihydropteroate synthase (dhps) gene mutations in HIV-infected persons in Uganda. PLoS One 7:e49991. doi: 10.1371/journal.pone.0049991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Munoz C, Zuluaga A, Restrepo A, Tobon A, Cano LE, Gonzalez A. 2012. Molecular diagnosis and detection of Pneumocystis jirovecii DHPS and DHFR genotypes in respiratory specimens from Colombian patients. Diagn Microbiol Infect Dis 72:204–213. doi: 10.1016/j.diagmicrobio.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 551.Siripattanapipong S, Leelayoova S, Mungthin M, Worapong J, Tan-Ariya P. 2008. Study of DHPS and DHFR genes of Pneumocystis jirovecii in Thai HIV-infected patients. Med Mycol 46:389–392. doi: 10.1080/13693780701883722. [DOI] [PubMed] [Google Scholar]
- 552.Takahashi T, Kanda T, Iwamoto A. 2002. Genetic diversity of drug targets including dihydropteroate synthase, dihydrofolate reductase and cytochrome b, in Pneumocystis carinii f. sp. hominis isolates in Japan. Res Commun Mol Pathol Pharmacol 112:159–176. [PubMed] [Google Scholar]
- 553.Costa MC, Esteves F, Antunes F, Matos O. 2006. Genetic characterization of the dihydrofolate reductase gene of Pneumocystis jirovecii isolates from Portugal. J Antimicrob Chemother 58:1246–1249. doi: 10.1093/jac/dkl411. [DOI] [PubMed] [Google Scholar]
- 554.Robberts FJ, Chalkley LJ, Weyer K, Goussard P, Liebowitz LD. 2005. Dihydropteroate synthase and novel dihydrofolate reductase gene mutations in strains of Pneumocystis jirovecii from South Africa. J Clin Microbiol 43:1443–1444. doi: 10.1128/JCM.43.3.1443-1444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Nahimana A, Rabodonirina M, Bille J, Francioli P, Hauser PM. 2004. Mutations of Pneumocystis jirovecii dihydrofolate reductase associated with failure of prophylaxis. Antimicrob Agents Chemother 48:4301–4305. doi: 10.1128/AAC.48.11.4301-4305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Singh Y, Mirdha BR, Guleria R, Khalil S, Panda A, Chaudhry R, Mohan A, Kabra SK, Kumar L, Agarwal SK. 2015. Molecular detection of DHFR gene polymorphisms in Pneumocystis jirovecii isolates from Indian patients. J Infect Dev Ctries 9:1250–1256. doi: 10.3855/jidc.6810. [DOI] [PubMed] [Google Scholar]
- 557.Ma L, Kovacs JA. 2000. Expression and characterization of recombinant human-derived Pneumocystis carinii dihydrofolate reductase. Antimicrob Agents Chemother 44:3092–3096. doi: 10.1128/AAC.44.11.3092-3096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Ma L, Jia Q, Kovacs JA. 2002. Development of a yeast assay for rapid screening of inhibitors of human-derived Pneumocystis carinii dihydrofolate reductase. Antimicrob Agents Chemother 46:3101–3103. doi: 10.1128/AAC.46.9.3101-3103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.El-Sadr WM, Murphy RL, Yurik TM, Luskin-Hawk R, Cheung TW, Balfour HH Jr, Eng R, Hooton TM, Kerkering TM, Schutz M, van der Horst C, Hafner R. 1998. Atovaquone compared with dapsone for the prevention of Pneumocystis carinii pneumonia in patients with HIV infection who cannot tolerate trimethoprim, sulfonamides, or both. Community Program for Clinical Research on AIDS and the AIDS Clinical Trials Group. N Engl J Med 339:1889–1895. [DOI] [PubMed] [Google Scholar]
- 560.Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. 2000. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother 44:2100–2108. doi: 10.1128/AAC.44.8.2100-2108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Kazanjian P, Armstrong W, Hossler PA, Lee CH, Huang L, Beard CB, Carter J, Crane L, Duchin J, Burman W, Richardson J, Meshnick SR. 2001. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. J Infect Dis 183:819–822. doi: 10.1086/318835. [DOI] [PubMed] [Google Scholar]
- 562.Kessl JJ, Hill P, Lange BB, Meshnick SR, Meunier B, Trumpower BL. 2004. Molecular basis for atovaquone resistance in Pneumocystis jirovecii modeled in the cytochrome bc(1) complex of Saccharomyces cerevisiae. J Biol Chem 279:2817–2824. doi: 10.1074/jbc.M309984200. [DOI] [PubMed] [Google Scholar]
- 563.Esteves F, de Sousa B, Calderon EJ, Huang L, Badura R, Maltez F, Bassat Q, de Armas Y, Antunes F, Matos O. 2016. Multicentre study highlighting clinical relevance of new high-throughput methodologies in molecular epidemiology of Pneumocystis jirovecii pneumonia. Clin Microbiol Infect 22:566.e9–566.e19. doi: 10.1016/j.cmi.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 564.Di Cocco P, Orlando G, Bonanni L, D'Angelo M, Clemente K, Greco S, Gravante G, Madeddu F, Scelzo C, Famulari A, Pisani F. 2009. A systematic review of two different trimetoprim-sulfamethoxazole regimens used to prevent Pneumocystis jirovecii and no prophylaxis at all in transplant recipients: appraising the evidence. Transplant Proc 41:1201–1203. doi: 10.1016/j.transproceed.2009.03.004. [DOI] [PubMed] [Google Scholar]