The intestinal immune system is crucial for protection from pathogenic infection and maintenance of mucosal homeostasis. We studied the intestinal immune microenvironment in a Salmonella enterica serovar Typhimurium intestinal infection mouse model.
KEYWORDS: CD4+ T cells, galectin-9, intestinal mucosal immunity, macrophages, Salmonella Typhimurium infection, Tim-3
ABSTRACT
The intestinal immune system is crucial for protection from pathogenic infection and maintenance of mucosal homeostasis. We studied the intestinal immune microenvironment in a Salmonella enterica serovar Typhimurium intestinal infection mouse model. Intestinal lamina propria macrophages are the main effector cells in innate resistance to intracellular microbial pathogens. We found that S. Typhimurium infection augmented Tim-3 expression on intestinal lamina propria CD4+ T cells and enhanced galectin-9 expression on F4/80+ CD11b+ macrophages. Moreover, CD4+ T cells promoted the activation and bactericidal activity of intestinal F4/80+ CD11b+ macrophages via the Tim-3/galectin-9 interaction during S. Typhimurium infection. Blocking the Tim-3/galectin-9 interaction with α-lactose significantly attenuated the bactericidal activity of intracellular S. Typhimurium by macrophages. Furthermore, the Tim-3/galectin-9 interaction promoted the formation and activation of inflammasomes, which led to caspase-1 cleavage and interleukin 1β (IL-1β) secretion. The secretion of active IL-1β further improved bactericidal activity of macrophages and galectin-9 expression on macrophages. These results demonstrated the critical role of the cross talk between CD4+ T cells and macrophages, particularly the Tim-3/galectin-9 interaction, in antimicrobial immunity and the control of intestinal pathogenic infections.
INTRODUCTION
The intestinal mucosal immune system is an intricate collection of organs, tissues, and cells spread along or associated with the intestine, including the mesenteric lymph nodes (MLN), lamina propria (LP), Peyer's patches, intestinal epithelial cells (IEC), and the intestinal intraepithelial lymphocytes (IELs). The intestinal LP is the main effector of the intestinal immune response and consists of a large numbers of leukocytes such as T cells, B cells, macrophages, dendritic cells (DCs), neutrophils, and mast cells. The gastrointestinal mucosa is the largest reservoir of macrophages in the body. Strategically positioned in the subepithelial LP, intestinal macrophages are the first phagocytic cells of the innate immune system to interact with microorganisms and microbial products that have breached the epithelium, reflecting their active and essential roles in the defense against invading pathogens and maintaining epithelial integrity and mucosal homeostasis (1, 2). CD4+ T cells account for 60 to 70% of T cells in the intestinal LP. Unlike T cells in the peripheral blood, due to continuous contact with food antigens or bacterial pathogens, intestinal LP CD4+ T cells are characterized by high activation and expression of the CD25 and HLA-DR antigens and relatively reduced proliferation ability (3).
Studies have shown that infected macrophages can interact with CD4+ T cells in a variety of ways in the process of contact with a bacterial pathogen. Macrophages interact with T cells through costimulatory molecules such as CD40, CD80, CD86, and major histocompatibility complex class II (MHC-II), which activate antigen-specific CD4+ T cells (4–6). Macrophages can also promote T cell proliferation by secreting tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), IL-6, and IL-12 (7). Meanwhile, activated CD4+ T cells can secrete gamma interferon (IFN-γ), which promotes the activation of macrophages and the production of reactive oxygen species (ROS) and cytokines. The interaction between CD4+ T cells and macrophages promotes resistance to intracellular pathogens (6, 8). However, the cross talk between macrophages and CD4+ T cells in response to bacterial infection in the intestinal mucosal immune microenvironment and the underlying mechanisms remain to be fully understood.
In the present study, we explored the intestinal immune microenvironment of an intestinal infection mouse model created by orally administering mice with Salmonella enterica serotype Typhimurium. Our results revealed that F4/80+ CD11b+ macrophages accumulated in the small intestinal LP of the infected mice. Depletion of CD4+ T cells significantly weakened the activation and function of intestinal macrophages. Further research demonstrated that F4/80+ CD11b+ macrophages and CD4+ T cells interacted through the interaction of galectin-9 and Tim-3, which triggered the activation of inflammasomes, promoted the production of IL-1β, and enhanced the antibacterial function of macrophages. These results indicate the critical role of the cross talk between CD4+ T cells and macrophages, particularly the interaction between Tim-3 and galectin-9, in antimicrobial immunity and in the control of intestinal pathogenic infections.
RESULTS
Intestinal macrophages and CD4+ T cells in the LP increase and activate during S. Typhimurium infection.
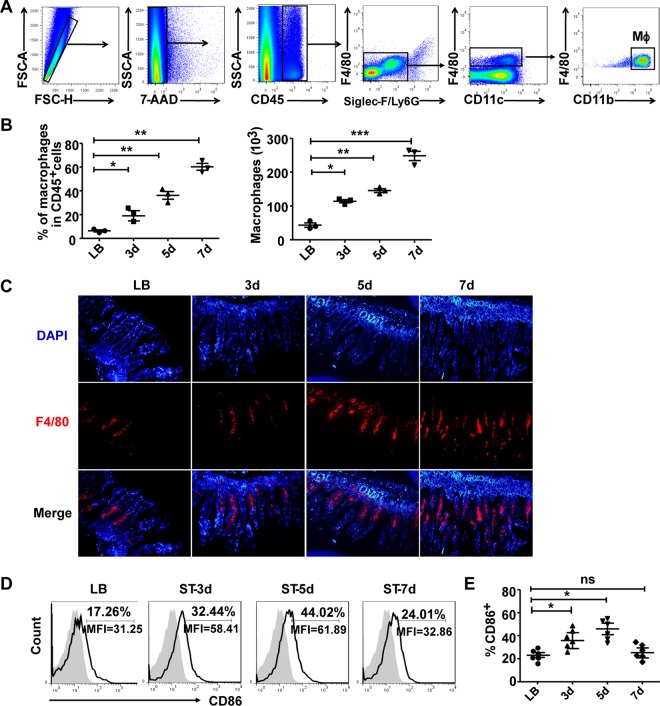
To determine the role of intestinal lymphocytes in the defense and clearance of invading bacteria, we first established an S. Typhimurium-infected mouse enteritis model as described previously (9, 10). The mice in this model displayed weight loss, intestinal inflammation, and high bacterial loads in the MLN, liver, and spleen (see Fig. S1 in the supplemental material). As the macrophages in the intestine LP are among the main effector cells in innate resistance to intracellular microbial pathogens, we isolated intestine LP leukocytes to observe the changes of LP macrophages during S. Typhimurium infection by fluorescence-activated cell sorting (FACS). As shown in Fig. 1A, the isolated LP leukocytes were first gated (FSC-H versus FSC-A) to exclude doublets or cell aggregates and further stained with 7-aminoactinomycin D (7-AAD) to exclude dead cells. The survival rate of LP leukocytes was higher than 90%. Leukocytes were then selected by the expression of CD45, before granulocytes were excluded by gating out SiglecF+ eosinophils and Ly6G+ neutrophils. Mucosal DCs were excluded on the basis of their high levels of CD11c expression and lack of F4/80. Thus, CD45+ SiglecF− Ly6G− CD11cint F4/80+ CD11b+ cells were regarded as intestinal mucosal macrophages (11).
FIG 1.
Intestinal lamina propria F4/80+ CD11b+ cells recruit and express activating markers after infection. Total small intestinal lamina propria (LP) cells were prepared from uninfected or S. Typhimurium-infected C57BL/6 mice. (A) Gating strategy used to identify F4/80+CD11b+ intestinal macrophages. The isolated LP leukocytes were first gated (FSC-H versus FSC-A) to exclude doublets or cell aggregates and further stained with 7-AAD to exclude dead cells; leukocytes were then selected by the expression of CD45 before granulocytes were excluded by gating out SiglecF+ eosinophils and Ly6G+ neutrophils. Mucosal DCs were excluded on the basis of their high levels of CD11c expression and lack of F4/80. (B) The percentages and numbers of F4/80+ CD11b+ intestinal macrophages were determined by flow cytometry. (C) The changes of F4/80+ macrophages in LP at the indicated days (d) after S. Typhimurium infection were determined by immunofluorescence staining. (D and E) The expression of the costimulatory molecules CD86 on F4/80+ CD11b+ macrophages was detected and statistically analyzed. Data are representative of those from three independent experiments (means ± SDs). ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the LB group). ns, not significant.
We then examined the percentages and numbers of LP macrophages at different time points after S. Typhimurium infection. Both the percentages and absolute numbers of the F4/80+ CD11b+ macrophage population increased significantly from 3 days after infection compared to those in mice in the mock infection group (Fig. 1B and C). We further analyzed the activation and functional status of these macrophages by detecting the expression of costimulatory molecules and intracellular cytokines. The results showed that CD86+ macrophages were significantly elevated on days 3 and 5 after infection (Fig. 1D and E). The costimulatory molecules CD80 and CD40, MHC-II, inflammatory cytokines TNF-α and IL-1β, and ROS were all markedly augmented (Fig. S2).
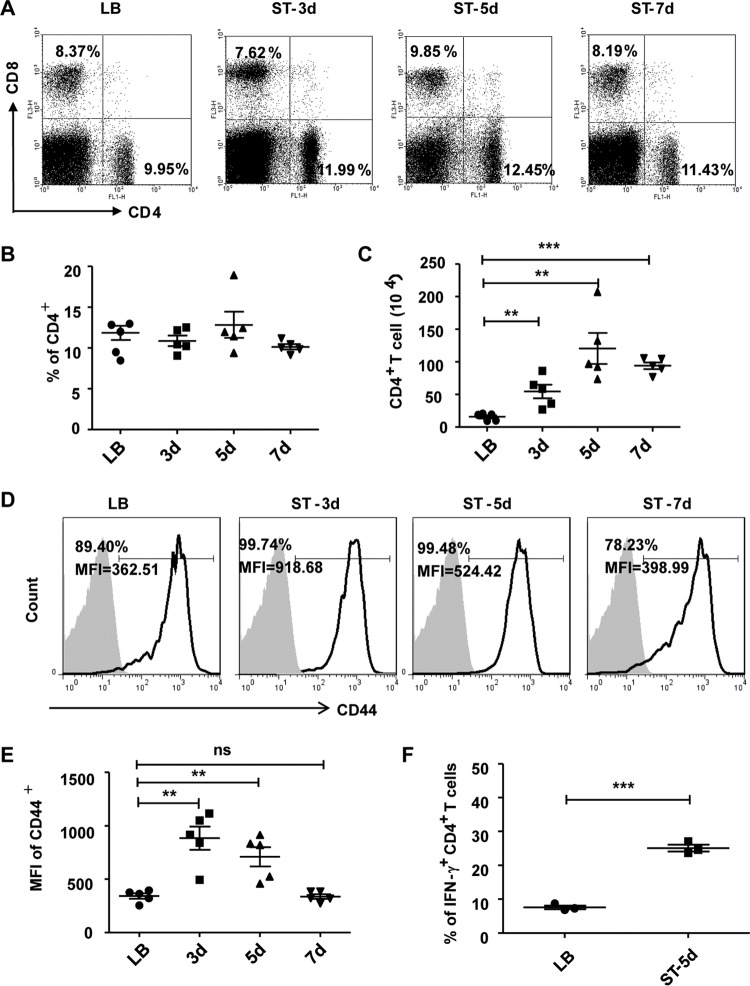
Furthermore, we measured the changes in the levels of CD4+ T and CD8+ T cells in the LP. As shown in Fig. 2, while the percentage of CD4+ T cells did not change, the numbers of CD4+ T cells were significantly higher from days 3 to 7 in the infection model than in the uninfected control (Fig. 2A to C). CD44 expression, which is indicative of activation status, was significantly enhanced, particularly in the mean fluorescence intensity (MFI), at days 3 and 5 after infection (Fig. 2D and E). The production of the TH1 cytokine IFN-γ was also markedly augmented at day 5 after infection (Fig. 2F). There were no significant differences in the numbers and activation of CD8+ T cells between the infection and control groups (data not shown). These data suggested that macrophages and CD4+ T cells might play more important roles in the defense against S. Typhimurium infection.
FIG 2.
Intestinal lamina propria CD4+ T cells increase and activate after infection with S. Typhimurium. (A) Small intestinal LP cells were isolated at various time points after infection. The cells were treated with fluorescence-labeled monoclonal antibodies (MAbs) against CD3, CD4, and CD8, and the percentages of CD4+ T and CD8+ T cells were determined by flow cytometry. (B and C) Statistical analysis of the percentages (B) and absolute numbers (C) of CD4+ T cells at days 3, 5, and 7 after S. Typhimurium infection. (D and E) The expression of CD44 on CD4+ T cells was detected (D) and the mean fluorescence intensity (MFI) was statistically analyzed (E). (F) The intracellular expression of IFN-γ by LP CD4+ T cells was detected and analyzed. Data are expressed as the means ± SDs from at least three independent experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the LB group).
CD4+ T cells promote the bactericidal activity of intestinal LP macrophages.
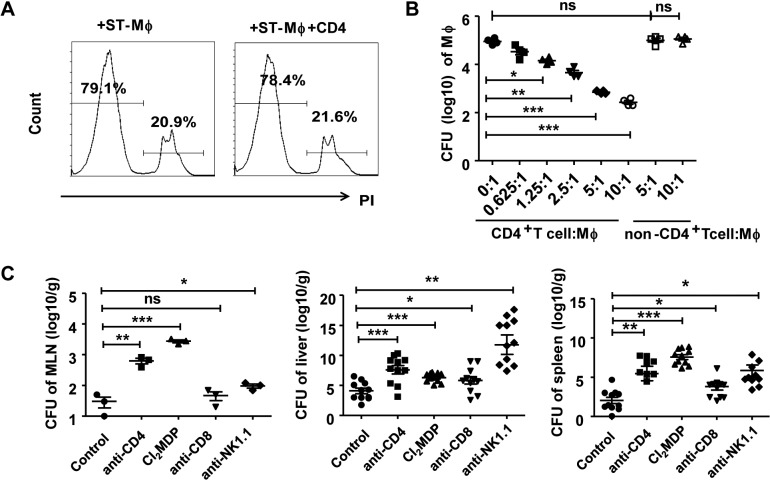
Because CD4+ T cells usually play a supporting role in macrophage activation, we aimed to determine whether CD4+ T cells could affect the function of intestinal macrophages. S. Typhimurium-infected intestinal macrophages were sorted by FACS (the viability of macrophages is shown in Fig. 3A) and cocultured with increasing numbers of CD4+ T cells or non-CD4+ T cells (that is, LP mononuclear cells with CD4+ T cell depletion) for 12 h, and the bacterial loads in macrophages were determined. The results revealed a progressive decrease in the bacterial load in macrophages cocultured with CD4+ T cells compared with macrophages alone or macrophages that were cocultured with non-CD4+ T cells (Fig. 3B). To further determine the role of CD4+ T cells on bactericidal activity of macrophages in vivo, the CD4+ T cell population was depleted 3 days before establishing the S. Typhimurium infection (depletion efficiency is shown in Fig. S3A). S. Typhimurium bacterial loads were detected in the MLN (3 days after infection) and liver and spleen (5 days after infection), and the bacterial load was higher in the CD4+ T cell-depleted mice than in the S. Typhimurium-infected mice containing the CD4+ T cell population (Fig. 3C). Due to the fact that previous reports had shown that depletion of T cells might impair the levels of neutrophils and thus affect the bacterial numbers in infection sites (12), we observed the changes of neutrophils after CD4+ T cell depletion. The results showed that S. Typhimurium infection augmented the recruitment of neutrophils into intestinal lamina propria, but depletion of CD4+ T cells had no impact on the levels of neutrophils (Fig. S4). Similarly, macrophages were depleted 3 days prior to infection (Fig. S3B), and the bacterial load also markedly increased (Fig. 3C). These results demonstrated that both macrophages and CD4+ T cells are indispensable for defense against S. Typhimurium infection. Depletion of CD8+ T cells and NK cells (Fig. S3C and D) also augmented the bacterial loads compared to those in control groups, suggesting that these cells also contribute to clearance of S. Typhimurium (Fig. 3C).
FIG 3.
CD4+ T cells promote the antibacterial activity of intestinal macrophages. S. Typhimurium-infected intestinal macrophages were cultured alone or in the presence of increasing amounts of CD4+ T cells or non-CD4+ T cells (control) for 12 h. (A) The viability of sorted macrophages and macrophages cocultured with CD4+ T cells was detected by FACS. (B) The bacterial loads in macrophages were detected. Mice intraperitoneally injected with antibodies for depleting cell populations (anti-CD4, anti-CD8α, and anti-NK1.1) or Cl2MDP-liposomes separately were infected with S. Typhimurium after 3 days. (C) The CFU of the MLN (3 days after infection) and liver and spleen (5 days after infection) were detected. Data are representative of those from at least three independent experiments. Error bars indicate SDs. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the control group).
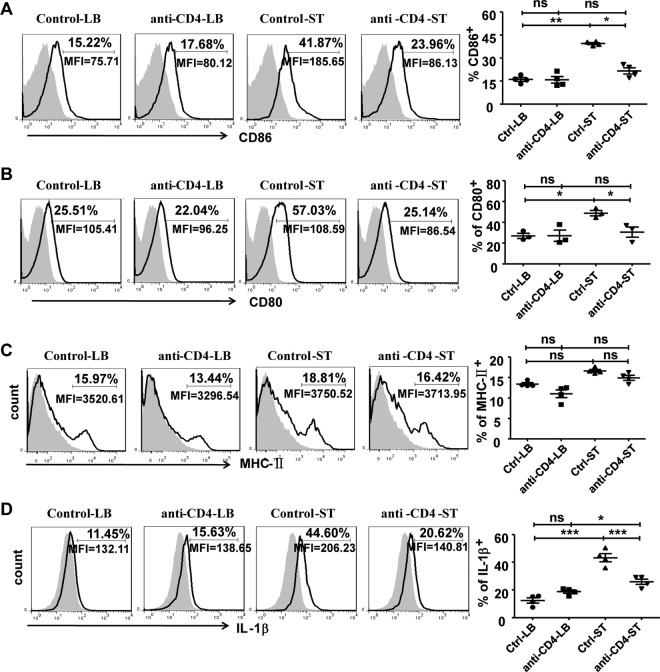
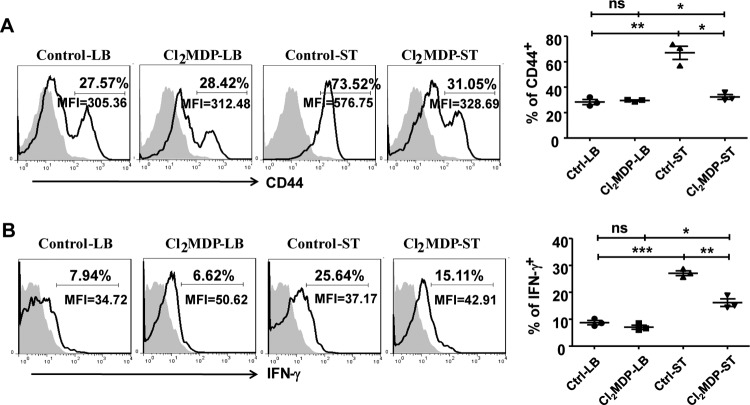
To further explore how CD4+ T cells affect intestinal macrophages, we analyzed macrophage activation from CD4+ T cell-depleted mice and control group 5 days after infection. There was a significant increase in both the percentage and number of macrophages in both CD4+ T cell-depleted and nondepleted infected mice. There was no significant difference in macrophage numbers between these two groups, which indicates that CD4+ T cell depletion did not impair the numbers of LP macrophages during the infection (data not shown). However, the expression of CD86 and CD80 on macrophages was significantly reduced in the CD4+ T cell-depleted mice during infection compared with that in the control group (Fig. 4A and B), but MHC class II expression was not impaired (Fig. 4C). These results demonstrate that the absence of CD4+ T cells attenuated the activation of intestinal macrophages during infection, indicating that CD4+ T cells can promote the activation of macrophages in the LP of the small intestine during S. Typhimurium infection.
FIG 4.
Depletion of CD4+ T cells attenuated the activation of and IL-1β production by intestinal macrophages during S. Typhimurium infection. Mice were intraperitoneally injected with anti-CD4 antibodies. Three days later, treated mice were orally infected with S. Typhimurium. Five days after infection, the small intestine LP lymphocytes were isolated and stained with fluorescence-labeled MAbs for flow cytometry. Expression of CD86 (A), CD80 (B), MHC-II (C), and IL-1β (D) by F4/80+ CD11b+ macrophages is shown. Data are expressed as the means ± SDs from at least three independent experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the control group).
Further, CD4+ T cell depletion significantly attenuated the production of IL-1β by intestinal LP macrophages in the infection model (Fig. 4D). However, depletion of this cell population did not affect the production of TNF-α and ROS (Fig. S5).
Intestinal LP macrophages promote CD4+ T cell activation.
To investigate whether the intestinal LP macrophages influenced the activation and function of CD4+ T cells, we observed the activation of CD4+ T cells and the secretion of IFN-γ in the small intestine LP in macrophage-depleted mice infected with S. Typhimurium. The results revealed that the CD44 expression and IFN-γ secretion by CD4+ T cells significantly increased in the infection model, while depletion of macrophages reduced both CD44 expression and IFN-γ secretion (Fig. 5). These results suggested that macrophages could enhance the activation and function of CD4+ T cells. Taken together, these data indicated a reciprocal activation between macrophages and CD4+ T cells in the small intestine LP during S. Typhimurium infection.
FIG 5.
Intestinal macrophages promoted the activation of CD4+ T cells during S. Typhimurium infection. Mice were intraperitoneally injected with Cl2MDP-liposomes. Three days later, the treated mice were orally infected with S. Typhimurium. Five days after infection, the small intestinal LP lymphocytes were isolated and stained with fluorescence-labeled MAbs for analysis by flow cytometry. CD44 (A) and intracellular IFN-γ (B) expression by CD4+ T cells is shown. Data are expressed as the means ± SDs from at least three independent experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the control group).
Tim-3/galectin-9 interaction enhances the activation and bactericidal activity of intestinal LP macrophages.
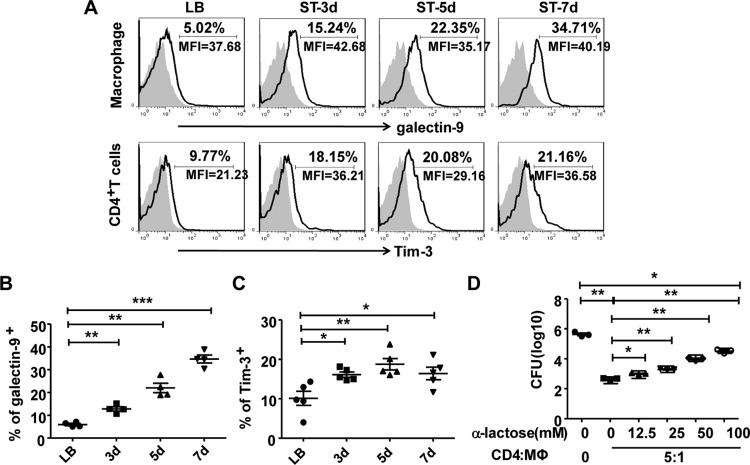
We explored the mechanism of interaction between intestinal macrophages and CD4+ T cells. Previous studies have shown that Tim-3, which is expressed on Th1 cells, interacts with its ligand galectin-9, which is expressed by Mycobacterium tuberculosis-infected alveolar macrophages, to restrict intracellular bacterial growth (13, 14). We investigated whether this Tim-3/galectin-9 interaction occurs in the interaction between CD4+ T cells and macrophages during S. Typhimurium infection. To this end, we analyzed the levels of expression of Tim-3 on CD4+ T cells and galectin-9 on macrophages in tissue samples obtained from uninfected control and S. Typhimurium-infected model mice. As shown in Fig. 6A and B, galectin-9 expression on intestinal F4/80+ CD11b+ LP macrophages increased gradually after S. Typhimurium infection. Similarly, immunofluorescence staining also showed higher levels of galectin-9 in LP macrophages after infection with S. Typhimurium than in the uninfected control (Fig. S6A). Meanwhile, the galectin-9 receptor Tim-3 on CD4+ T cells of the small intestine LP also showed enhanced expression in infected mice compared with that in the uninfected control group (Fig. 6A and C). To further confirm the role of Tim-3/galectin-9 in the cross talk between CD4+ T cells and macrophages, infected intestinal LP macrophages were cocultured with isolated CD4+ T cells in the presence of increasing concentrations of α-lactose, which is known to competitively block Tim-3/galectin-9 interaction in vitro (13). Blocking the Tim-3/galectin-9 interaction with α-lactose significantly inhibited the antimicrobial activity of macrophages in a dose-dependent manner (Fig. 6D). These results indicate that the Tim-3/galectin-9 interaction is required to promote the effect of CD4+ T cells on the antimicrobial activity of macrophages. Further, we also investigated whether Tim-3/galectin-9 interaction might induce the apoptosis of CD4+ T cells in the coculture system containing CD4+ T cells and infected intestinal LP macrophages. Increasing concentrations of α-lactose did not influence the expression of CD44 and the production of IFN-γ (Fig. S6B); however, α-lactose treatment markedly reduced the apoptosis of CD4+ T cells in a dose-dependent manner (Fig. S6C). These results demonstrated that Tim-3/galectin-9 interaction may induce the apoptosis of Tim-3+ CD4+ T cells as expected, but it does not influence CD4+ T cell activation and IFN-γ production.
FIG 6.
The Tim-3/galectin pathway is involved in the cross talk between intestinal CD4+ T cells and macrophages. The expression levels of galectin-9 on intestinal macrophages (A and B) and Tim-3 on CD4+ T cells (A and C) were determined after S. Typhimurium infection. Then S. Typhimurium-infected intestinal macrophages were cultured alone or with CD4+ T cells in the presence of increasing amounts of α-lactose. The numbers of CFU in the macrophages were determined (D). Data are expressed as the means ± SDs from at least three separate experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the control group).
Tim-3/galectin-9 interaction triggers inflammasome activation and IL-1β secretion.
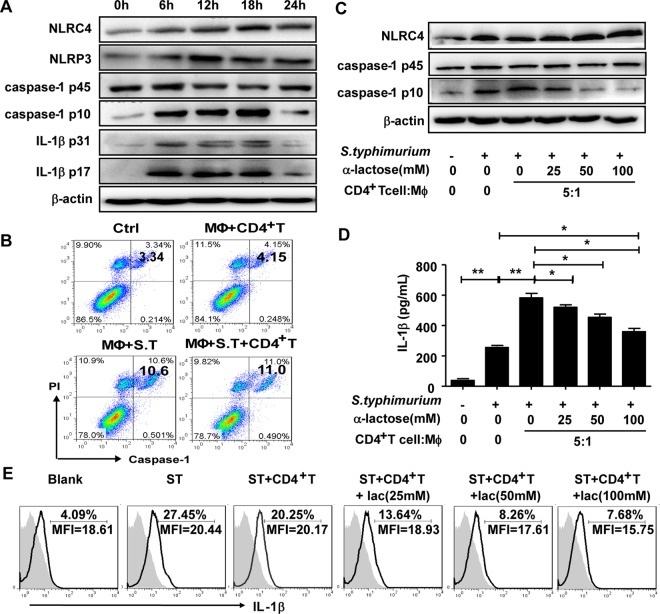
Recent studies have shown that microbial infection triggers the assembly of inflammasome complexes that promote caspase-1-dependent secretion of IL-1β, which plays an important role in the defense against bacterial infection (15–19). As illustrated in Fig. 4D, IL-1β production by intestinal LP macrophages was significantly attenuated in the infection model mice if the CD4+ T cells were depleted. Therefore, we hypothesized that during the contact of CD4+ T cells and macrophages, Tim-3/galectin-9 interaction might promote inflammasome activation, which would then trigger caspase-1 cleavage and the release of mature IL-1β from infected macrophages. To verify this hypothesis, we first detected the expression and activation of inflammasomes, caspase-1, and IL-1β in the S. Typhimurium-infected intestinal LP macrophages. Our results revealed that S. Typhimurium infection significantly promoted the expression of the NLRC4 and NLRP3 inflammasomes. The mature forms of caspase-1 (caspase-1 p10), pro-IL-1β (IL-1β p31), and IL-1β (IL-1β p17) showed a marked increase at 6 h after infection and peaked at 18 h postinfection (Fig. 7A). It is known that inflammasome activation and caspase-1 cleavage also lead to a particular form of cell death called pyroptosis (20). We investigated whether S. Typhimurium infection resulted in the pyroptosis of LP macrophages and whether CD4+ T cells were involved. As shown in Fig. 7B, infection indeed promoted pyroptosis of macrophages; however, coculture with CD4+ T cells did not affect the percentages of LP macrophages that underwent pyroptosis. These results suggested that CD4+ T cells did not play a significant role in the induction of pyroptosis of LP macrophages. After coculture of S. Typhimurium-infected intestinal LP macrophages and CD4+ T cells with various concentrations of α-lactose, the levels of expression of NLRC4 inflammasome and caspase-1 were detected by Western blotting (Fig. 7C), the IL-1β level in the supernatant was measured by enzyme-linked immunosorbent assay (ELISA), and the intracellular IL-1β levels were determined by FACS (Fig. 7D and E). The results showed that at high concentrations, α-lactose inhibited the activation of NLRC4 and active caspase-1, as well as the production of IL-1β. These results indicated that interaction of Tim-3 with galectin-9 was required to promote inflammasome activation and active IL-1β secretion from infected intestinal LP macrophages.
FIG 7.
Tim-3/galectin-9 interaction promotes inflammasome activation, which causes caspase-1 cleavage and IL-1β secretion. The expression levels of NLRs, procaspase-1, caspase-1, pro-IL-1β, and mature IL-1β in macrophages were assessed at the indicated infection time points by Western blotting (A). S. Typhimurium-infected macrophages were cultured alone or with CD4+ T cells. One hour later, the double positivity for capase-1 fluorescent inhibitor probe (FAM-YVAD-FMK) and PI in macrophages was detected by FACS (B). S. Typhimurium-infected intestinal macrophages was cultured alone or with CD4+ T cells in the presence of increasing amounts of α-lactose. Macrophages were then isolated from the culture system by discarding CD4+ T cells using the EasySep Mouse CD4 Positive Selection kit. The expression levels of NLRC4, procaspase-1, and caspase-1 were detected by Western blotting (C). IL-1β expression levels in the cocultures were determined by ELISA (D) and intracellular IL-1β expression was detected by FACS (E). Data are representative of those from three independent experiments. Error bars indicate SDs from three replicate cultures. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the control group).
IL-1β improves the bactericidal activity of intestinal LP macrophages.
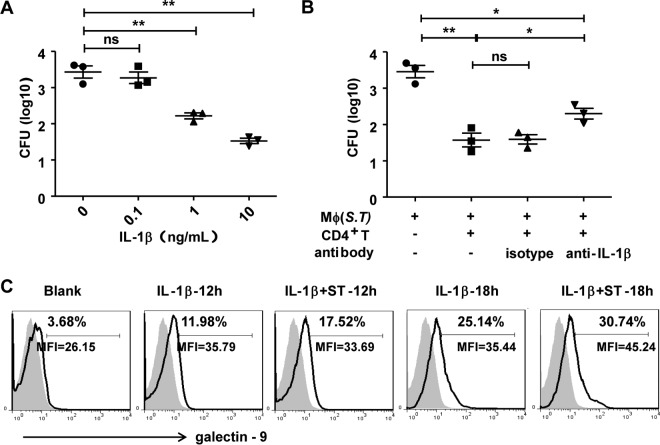
To further confirm the role of IL-1β in the defense against S. Typhimurium infection, S. Typhimurium-infected intestinal LP macrophages were stimulated with different concentrations of recombinant mouse IL-1β. The results showed that IL-1β reduced the bacterial load of infected intestinal LP macrophages in a dose-dependent manner (Fig. 8A). Addition of anti-IL-1β antibodies to the coculture system resulted in significant attenuation of the CD4+ T cell-mediated promotion of bactericidal activity by macrophages (Fig. 8B). These results indicate that IL-1β plays an important role in activating the bactericidal activity of macrophages. In addition, IL-1β treatment enhanced galectin-9 expression on macrophages (Fig. 8C), which might further promote cross talk between macrophages and CD4+ T cells.
FIG 8.
IL-1β improves the bactericidal activity of intestinal macrophages and the expression of galectin-9. S. Typhimurium-infected intestinal macrophages were cultured alone or in the presence of increasing amounts of IL-1β for 12 h (A). S. Typhimurium-infected intestinal macrophages were cultured alone or with CD4+ T cells in the presence of anti-IL-1β antibodies or isotype (B). The numbers of CFU in the macrophages were determined (A and B). Intestinal macrophages were cultured alone or infected with S. Typhimurium and then treated with 10 ng/ml of IL-1β. Galectin-9 expression on intestinal macrophages was detected by FACS (C). Data are representative of those from three independent experiments. Error bars indicate SDs from three replicate cultures. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (compared with the control group).
DISCUSSION
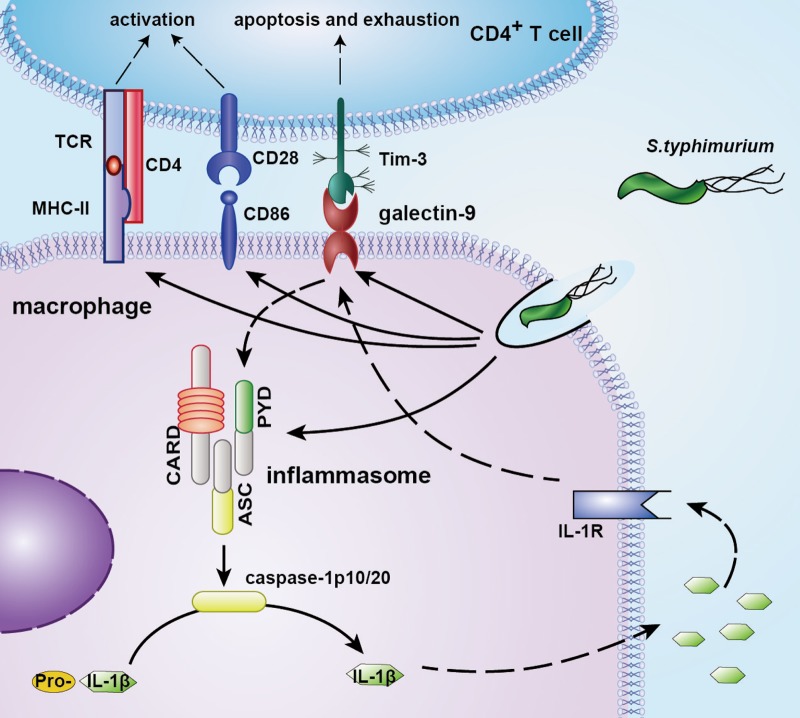
The mucosal immune system forms the largest part of the body's immune tissues, containing approximately three-quarters of all lymphocytes, and plays a crucial role in defense against invading pathogens (1, 2). Macrophages are important components of protective immunity. They play a crucial role in defense against intracellular and extracellular bacteria and function via multiple antimicrobial mechanisms even at initial contact with pathogens (4, 21–23). CD4+ T cells usually help and promote the activation and bactericidal activity of macrophages, thus contributing to the body's resistance to intracellular microbial pathogens. However, in the intestinal mucosal immune microenvironment, whether cross talk occurs between macrophages and CD4+ T cells in response to bacterial infection and its underlying mechanisms are still not completely clarified. In this study, we found that F4/80+ CD11b+ macrophages and CD4+ T cells accumulated in the small intestine LP after Salmonella infection. S. Typhimurium infection increased galectin-9 expression on F4/80+ CD11b+ macrophages in the LP and Tim-3 expression on CD4+ T cells. CD4+ T cells promoted intestinal macrophage activation and bactericidal activity via Tim-3 and galectin-9 interaction, which triggered the formation and activation of inflammasomes, leading to caspase-1 cleavage and active IL-1β secretion accompanied by low levels of pyroptosis of LP macrophages. Furthermore, the secretion of active IL-1β further improved the bactericidal activity of intestinal macrophages and galectin-9 expression on intestinal macrophages (Fig. 9). To our knowledge, this is the first study to show the critical role of the cross talk between intestinal CD4+ T cells and macrophages, particularly the Tim-3/galectin-9 interaction, in antibacterial immunity and in the control of intestinal pathogen infections.
FIG 9.
Proposed model for mechanisms of cross talk between intestinal macrophages and CD4+ T cells involved in the Tim-3/galectin-9 pathway. S. Typhimurium infection promotes the accumulation of macrophages and CD4+ T cells in the small intestinal LP. Meanwhile, the expressions of galectin-9 on intestinal macrophages and Tim-3 on CD4+ T cells are enhanced following infection. CD4+ T cells promote the activation and bactericidal activity of intestinal macrophages via Tim-3/galectin-9 interaction, which triggers the formation and activation of inflammasomes, leading to the cleavage of caspase-1 and IL-1β. The secretion of active IL-1β further improves the bactericidal activity of intestinal macrophages and expression of galectin-9 on macrophages.
The intestinal LP is the main effector site for clearing invading pathogens. Intestinal macrophages are one of the most abundant types of leukocytes, especially in the intestinal LP (2). These cells exert highly phagocytic and bactericidal activity and play an indispensable role in protective immunity by clearance of bacteria during invasion of pathogenic bacteria. The intestinal LP also contains large numbers of CD4+ T cells, which contributes to the maintenance of intestinal homeostasis and protective immunity against pathogens. CD4+ T cells are known to interact with macrophages via CD40− CD40L and cytokine secretion, promoting their activation and function (4, 7). Bidirectional regulation between macrophages and CD4+ T cells in the intestinal mucosal immune system in response to foreign pathogens has gained a great deal of research interest in recent years (24, 25). Using an S. Typhimurium infection model, the present study demonstrated the cross talk between intestinal LP CD4+ T cells and macrophages. By using in vitro coculture system and in vivo cell depletion, we found that CD4+ T cells promote the activation and bactericidal activity of intestinal macrophages; the activation of intestinal macrophage also promoted the activation of and IFN-γ production by CD4+ T cells. The synergistic effect of CD4+ T cells and macrophages accelerates the clearance of S. Typhimurium.
Tim-3 was initially regarded as an inhibitory receptor expressed on activated CD4+ T and CD8+ T cells, and it negatively regulates T cell responses by inducing T cell death or exhaustion after binding to its ligand galectin-9 (26). Tim-3 signaling serves as a checkpoint for T cell activation, and blockade with anti-Tim-3 monoclonal antibodies (MAbs) or in combination with blockade of other checkpoints (e.g., anti-PD-1 MAbs) to improve immunity and restore exhausted or impaired CD8+ T cells is becoming a potential therapeutic target for cancer and chronic viral infections (27–29). We observed reciprocal activation between CD4+ T cells and macrophages, and blocking the interaction of Tim-3 and galectin-9 with α-lactose did not impair CD4+ T cell activation. These results indicate that other molecules, such as CD40− CD40L or cytokines, may contribute to the macrophage-mediated promotion of CD4+ T cell activation.
Although most studies have focused on the suppressive effect of Tim-3 signaling on T cells, some studies have investigated the influence of Tim-3/galectin-9 interaction on galectin-9+ cells. Some researchers reported that galectin-9 induced dendritic cell maturation and differentiation, suggesting that it might be a key immune regulator connecting innate and adaptive immunity (30, 31). A previous study demonstrated the critical role of the Tim-3/galectin-9 interaction in promoting antimicrobial immunity by activating galectin-9+ macrophages in the lungs (13, 14). It was found that Tim-3/galectin-9 binding activates Mycobacterium tuberculosis-infected macrophages, stimulating bactericidal activity by inducing caspase-1-dependent IL-1β secretion in both humans and mice. In the present study, Tim-3/galectin-9 interaction was found to promote macrophage activation and bactericidal activity in the intestinal LP in the S. Typhimurium infection model. We further confirmed that Tim-3/galectin-9 ligation triggered the formation and activation of inflammasomes, leading to caspase-1 cleavage and IL-1β secretion. The active IL-1β further augmented the bactericidal activity of macrophages and galectin-9 expression on macrophages. Thus, by providing a feedback-signaling loop involving IL-1β to upregulate galectin-9, Tim-3/galectin-9 interaction plays a key role in a bidirectional regulatory circuit that activates intestinal macrophages to inhibit intracellular pathogens. Tim-3 and galectin-9 can serve as potential therapeutic targets to modulate antimicrobial immunity, particularly against intracellular bacterial infections. Although Tim-3/galectin-9 signaling induces effector T cell apoptosis, its effect on antimicrobial immunity in vivo is likely to be a balance between its potential negative effects on T cells and its ability to stimulate bactericidal pathways in macrophages to clear intracellular pathogens. Further studies using different models are required to explore how these functions mediated by Tim-3 and galectin-9 are balanced during bacterial infection in vivo.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice (6 to 8 weeks of age) were purchased from Beijing HuaFuKang Bioscience Co., Ltd. (Beijing, China) and maintained under specific-pathogen-free conditions. All animal experiments and protocols were conducted in accordance with the guidelines for experimental animals from Shandong University. The study protocol was approved by the Committee on the Ethics of Animal Experiments of Shandong University.
Bacterial strains and growth conditions.
Virulent S. enterica serotype Typhimurium (ATCC 14028) was cultured in Luria-Bertani (LB; 10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl/liter) broth or agar plates. The bacteria were cultured overnight at 37°C, and on the following day, bacterial cultures at saturation density were diluted (1/100) and cultured to the mid-logarithmic growth phase (optical density at 600 nm [OD600], 0.5 to 0.6). The cell suspensions were then centrifuged and washed twice in LB broth before use. The cell count was calculated from a standard curve of CFU versus OD600.
In vivo infection with S. Typhimurium.
Mice were orally administered 0.2-ml suspensions of wild-type S. Typhimurium (5 × 105 bacterial cells), using a standard gastric intubation needle (9). Mice in the mock-infected control group received 0.2 ml of sterile LB broth.
Cell depletion.
To deplete CD4+ T, CD8+ T, and NK cells, monoclonal antibodies (MAbs) were purified from GK1.5 (anti-CD4), 2.43 (anti-CD8α), and PK136 (anti-NK1.1) hybridoma cell lines (32). Briefly, mice were intraperitoneally injected with 1 mg of MAb for 3 days. For depletion of macrophages, mice were intraperitoneally administered 200 μl of Cl2MDP-liposomes (obtained from Shanghai qcbio Science & Technologies Co., Ltd., China) 3 days prior to induction of infection.
Isolation of lamina propria leukocytes.
For the isolation of LP leukocytes, the small intestines were removed, cleaned of mesentery and fat, and flushed with sterile phosphate-buffered saline (PBS). After Peyer's patches were excised, LP leukocytes were isolated as described previously (33). Briefly, the small intestines were cut into 3- to 4-in. segments, turned inside out, and incubated in prewarmed 0.155-mg/ml dithiothreitol (Sigma, San Diego, CA), 0.001 M EDTA, and 1% fetal bovine serum (FBS) in RPMI medium for 15 min in a shaking incubator at 37°C. After removal of intraepithelial lymphocytes, the tissue pieces were agitated in RPMI medium to wash away residual extraction medium, chopped finely, and incubated in prewarmed 0.5-mg/ml dispase (Invitrogen, San Diego, CA), 1.5-mg/ml collagenase II (Gibco, Grand Island, NY), and 1% FBS in RPMI medium for 30 min in a shaking incubator at 37°C. The digested tissues were filtered through a 100-μm cell strainer and washed with RPMI medium containing 1% FBS three times, and cells were suspended in RPMI medium with 1% FBS.
Flow cytometric analysis and sorting of cells.
For cell surface molecular staining, LP leukocytes were isolated, blocked, and incubated with fluorescent MAbs. IFN-γ and IL-1β expression was evaluated using an intracellular staining set (R&D Systems, MN). The antibodies utilized are listed in Table S1 in the supplemental material. The stained cells were analyzed using a flow cytometer (FACSCalibur or FACSAria III; BD Biosciences, San Jose, CA), and the data were analyzed with WinMDI 2.9 software (Scripps Research Institute, La Jolla, CA) or FlowJo software (Treestar Inc., Ashland, OR). LP macrophages were sorted as live gated CD45+ SiglecF− Ly6G− CD11cint CD11b+ F4/80+. Intestinal LP CD4+ T cells were isolated using the CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany); the non-CD4+ T cells were LP mononuclear cells without CD4+ T cells.
Quantification of bacterial burden.
The liver, spleen, small intestine, and MLN were obtained at different points after infection and homogenized with a tissue homogenizer (Tiangen, Beijing, China). The cells were then lysed with 0.1% Triton X-100 in 1× PBS to release bacteria. Serial dilutions of the cell lysate prepared with 1× PBS were plated onto LB agar plates, and bacterial colonies were incubated overnight at 37°C and then counted in triplicate.
To detect infected macrophages and to measure the CFU, peritoneal macrophages were isolated and resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 15% FBS at a density of 1.2 × 106 cells/ml, after which the cells were incubated in a 12-well plate. After adhering to the bottom, macrophages were infected with S. Typhimurium at a multiplicity of infection (MOI) of 10:1. At 1 h after culture, fresh culture medium containing 100 μg/ml of gentamicin was added and the cells were cultured for an additional 1 h and then washed with PBS. The gentamicin concentration was subsequently reduced to 10 μg/ml for the remainder of the infection. After 6 h, the cells were washed with PBS and lysed with 0.1% Triton X-100 to release bacteria. Serial dilutions were plated to determine the CFU (34–36).
Western blotting.
Western blot analysis was carried out as described previously (32). The following antibodies were used: anti-NLRP3 (Adipogen, San Diego, CA), anti-NLRC4, anti-IL-1β p31/17 (Cell Signaling Technology, Danvers, MA), anti-caspase-1 p45/10 (Abcam, Cambridge, UK), and anti-β-actin (Santa Cruz Biotechnology, CA) antibodies. Immunoreactive protein bands were visualized using the Molecular Imager ChemiDoc XRS system (Bio-Rad).
Histopathology.
Tissues from the small intestine were excised on days 1, 3, and 5, fixed in 10% neutral buffered formalin, and embedded in paraffin. Then, the tissues were cut into 4-μm sections, fixed to slides, deparaffinized, and stained with hematoxylin and eosin.
Immunofluorescence staining.
To detect macrophages in the small intestine, cryostat sections (5 μm) were cut, air dried, fixed in acetone, and blocked with 1% bovine serum albumin (BSA)–10% normal goat serum for 1 h at room temperature. The primary antibody solution (anti-F4/80 MAb, clone C-7 [Santa Cruz Biotechnology, CA]; rabbit anti-galectin-9 polyclonal antibody, bs-0604R [Bioss, China]; and fluorescein isothiocyanate [FITC]-labeled anti-Salmonella antibody, ab69253) was dropped to cover the tissue sections and incubated overnight at 4°C. After being washed in PBS, the slides were incubated for 1 h at room temperature with the corresponding secondary fluorescence-conjugated Ab, DyLight 594-conjugated goat anti-mouse IgG (Abbkine, CA), for 1 h, washed three times, and incubated with Hoechst 33342 (Beyotime, Jiangsu, China). Fluorescent images were visualized using an OLYMPUS inverted microscope.
Measurement of apoptosis.
CD4+ T cells were stained with annexin V-FITC and propidium iodide (PI; BestBio, Shanghai, China) to detect apoptosis and analyzed by flow cytometry. The percentage of annexin V-positive cells represented the proportion of apoptotic cells.
Measurement of pyroptosis.
The intestinal LP macrophages were sorted by flow cytometry and then added to a cell culture plate. The next day macrophages were infected with S. Typhimurium at an MOI of 10:1 or cocultured with CD4+ T cells isolated from LP lymphocytes by magnetically activated cell sorting (MACS). At 1 h after culture, fresh culture medium containing 100 μg/ml of gentamicin was added and cultured for an additional 1 h, the cell culture was collected and spun to obtain a pellet, the supernatant was discarded, and then the intestinal LP macrophages were stained with FAM-YVAD-FMK FLICA and propidium iodide (FAM-FLICA caspase assays, catalog number 98; Immunochemistry Technologies, LLC) to detect pyroptosis and analyzed by flow cytometry. The percentage of caspase-1+ PI+ cells represented the proportion of cells undergoing pyroptosis.
Measurement of ROS production.
Cells were incubated with 10 μM dichlorofluorescein diacetate (DCFH-DA; Sigma, San Diego, CA) for 20 min at 37°C and subsequently washed (37). ROS production was measured from dichlorofluorescein (DCF) fluorescence intensity by using a FACSCalibur flow cytometer (BD Biosciences).
ELISA.
The IL-1β levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Cusabio, Wuhan, China) according to the manufacturer's instructions.
Statistical analysis.
All analyses were carried out using GraphPad Prism 5 (GraphPad, La Jolla, CA). Data were statistically analyzed using one-way analysis of variance (ANOVA). Values were compared between the two groups using the Student t test. A P value of less than 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (no. 81472646, no. 81771686, and no. 91442114 to C.Z.).
We declare no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00769-17.
REFERENCES
- 1.Mowat AM, Bain CC. 2011. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun 3:550–564. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platt AM, Mowat AM. 2008. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett 119:22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Shale M, Schiering C, Powrie F. 2013. CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev 252:164–182. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dibra D, Li S. 2013. The cell-to-cell coordination between activated T cells and CpG-stimulated macrophages synergistically induce elevated levels of IL-10 via NF-kappaB1, STAT3, and CD40/CD154. Cell Commun Signal 11:95. doi: 10.1186/1478-811X-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju C, Tacke F. 2016. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol 13:316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan AT, Goldrath AW, Glass CK. 2017. Metabolic and epigenetic coordination of T cell and macrophage immunity. Immunity 46:714–729. doi: 10.1016/j.immuni.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts CA, Dickinson AK, Taams LS. 2015. The interplay between monocytes/macrophages and CD4(+) T cell subsets in rheumatoid arthritis. Front Immunol 6:571. doi: 10.3389/fimmu.2015.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CH, Liu H, Ge B. 2017. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol 14:963–975. doi: 10.1038/cmi.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Zhang C, Zhou Z, Zhang J, Zhang J, Tian Z. 2012. Small intestinal intraepithelial lymphocytes expressing CD8 and T cell receptor gammadelta are involved in bacterial clearance during Salmonella enterica serovar Typhimurium infection. Infect Immun 80:565–574. doi: 10.1128/IAI.05078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Liu M, Zuo Z, Liu J, Yu X, Guan Y, Zhan R, Han Q, Zhang J, Zhou R, Sun R, Tian Z, Zhang C. 2017. TLR9 Regulates the NF-kappaB-NLRP3-IL-1beta pathway negatively in Salmonella-induced NKG2D-mediated intestinal inflammation. J Immunol 199:761–773. doi: 10.4049/jimmunol.1601416. [DOI] [PubMed] [Google Scholar]
- 11.Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. 2014. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, Baumler AJ. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun 76:2008–2017. doi: 10.1128/IAI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. 2010. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med 207:2343–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sada-Ovalle I, Chavez-Galan L, Torre-Bouscoulet L, Nava-Gamino L, Barrera L, Jayaraman P, Torres-Rojas M, Salazar-Lezama MA, Behar SM. 2012. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J Immunol 189:5896–5902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynosky-Dolfi MA, Snyder AG, Philip NH, Doonan PJ, Poffenberger MC, Avizonis D, Zwack EE, Riblett AM, Hu B, Strowig T, Flavell RA, Jones RG, Freedman BD, Brodsky IE. 2014. Oxidative metabolism enables Salmonella evasion of the NLRP3 inflammasome. J Exp Med 211:653–668. doi: 10.1084/jem.20130627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. 2012. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol 188:5623–5635. doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, Behar SM. 2013. IL-1beta promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol 190:4196–4204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Cao X. 2016. Cellular and molecular regulation of innate inflammatory responses. Cell Mol Immunol 13:711–721. doi: 10.1038/cmi.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao T. 2017. Innate immunity and inflammation. Cell Mol Immunol 14:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Ning X, Jiang Z. 2017. Caspases control antiviral innate immunity. Cell Mol Immunol 14:736–747. doi: 10.1038/cmi.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan C. 2016. Macrophages' choice: take it in or keep it out. Immunity 45:710–711. doi: 10.1016/j.immuni.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Varol C, Mildner A, Jung S. 2015. Macrophages: development and tissue specialization. Annu Rev Immunol 33:643–675. [DOI] [PubMed] [Google Scholar]
- 23.De Santis M, Locati M, Selmi C. 2018. The elegance of a macrophage. Cell Mol Immunol 15:196–198. doi: 10.1038/cmi.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossini V, Zhurina D, Radulovic K, Manta C, Walther P, Riedel CU, Niess JH. 2014. CX3CR1(+) cells facilitate the activation of CD4 T cells in the colonic lamina propria during antigen-driven colitis. Mucosal Immunol 7:533–548. doi: 10.1038/mi.2013.70. [DOI] [PubMed] [Google Scholar]
- 25.Panea C, Farkas AM, Goto Y, Abdollahi-Roodsaz S, Lee C, Koscso B, Gowda K, Hohl TM, Bogunovic M, Ivanov II. 2015. Intestinal monocyte-derived macrophages control commensal-specific Th17 responses. Cell Rep 12:1314–1324. doi: 10.1016/j.celrep.2015.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. 2011. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol 32:345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. 2016. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol 34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Zhang S, Hu Y, Yang Z, Li J, Liu X, Deng L, Wang Y, Zhang X, Jiang T, Lu X. 2016. Targeting PD-1 and Tim-3 pathways to reverse CD8 T-cell exhaustion and enhance ex vivo T-cell responses to autologous dendritic/tumor vaccines. J Immunother 39:171–180. doi: 10.1097/CJI.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z. 2012. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol 42:1180–1191. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- 30.Ma CJ, Li GY, Cheng YQ, Wang JM, Ying RS, Shi L, Wu XY, Niki T, Hirashima M, Li CF, Moorman JP, Yao ZQ. 2013. Cis association of galectin-9 with Tim-3 differentially regulates IL-12/IL-23 expressions in monocytes via TLR signaling. PLoS One 8:e72488. doi: 10.1371/journal.pone.0072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Kivit S, Kostadinova AI, Kerperien J, Ayechu Muruzabal V, Morgan ME, Knippels LMJ, Kraneveld AD, Garssen J, Willemsen LEM. 2017. Galectin-9 produced by intestinal epithelial cells enhances aldehyde dehydrogenase activity in dendritic cells in a PI3K- and p38-dependent manner. J Innate Immun 9:609–620. doi: 10.1159/000479817. [DOI] [PubMed] [Google Scholar]
- 32.Guo Q, Lan P, Yu X, Han Q, Zhang J, Tian Z, Zhang C. 2014. Immunotherapy for hepatoma using a dual-function vector with both immunostimulatory and pim-3-silencing effects. Mol Cancer Ther 13:1503–1513. [DOI] [PubMed] [Google Scholar]
- 33.Couter CJ, Surana NK. 2016. Isolation and flow cytometric characterization of murine small intestinal lymphocytes. J Vis Exp 2016(111):e54114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan R, Han Q, Zhang C, Tian Z, Zhang J. 2015. Toll-like receptor 2 (TLR2) and TLR9 play opposing roles in host innate immunity against Salmonella enterica serovar Typhimurium infection. Infect Immun 83:1641–1649. doi: 10.1128/IAI.02870-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos AJM, Durkin CH, Helaine S, Boucrot E, Holden DW. 2016. Clustered intracellular Salmonella enterica serovar Typhimurium blocks host cell cytokinesis. Infect Immun 84:2149–2158. doi: 10.1128/IAI.00062-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva-Herzog E, Detweiler CS. 2010. Salmonella enterica replication in hemophagocytic macrophages requires two type three secretion systems. Infect Immun 78:3369–3377. doi: 10.1128/IAI.00292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu X, Lan P, Hou X, Han Q, Lu N, Li T, Jiao C, Zhang J, Zhang C, Tian Z. 2017. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1beta production via suppressing the NF-kappaB pathway and ROS production. J Hepatol 66:693–702. doi: 10.1016/j.jhep.2016.12.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.