Plasmodium falciparum merozoite surface protein 3 (MSP3) is an abundantly expressed secreted merozoite surface protein and a leading malaria vaccine candidate antigen. However, it is unclear how MSP3 is retained on the surface of merozoites without a glycosylphosphatidylinositol (GPI) anchor or a transmembrane domain.
KEYWORDS: Plasmodium, parasite, merozoite surface proteins, malaria vaccine, Plasmodium falciparum, apicomplexan parasites
ABSTRACT
Plasmodium falciparum merozoite surface protein 3 (MSP3) is an abundantly expressed secreted merozoite surface protein and a leading malaria vaccine candidate antigen. However, it is unclear how MSP3 is retained on the surface of merozoites without a glycosylphosphatidylinositol (GPI) anchor or a transmembrane domain. In the present study, we identified an MSP3-associated network on the Plasmodium merozoite surface by immunoprecipitation of Plasmodium merozoite lysate using antibody to the N terminus of MSP3 (anti-MSP3N) followed by mass spectrometry analysis. The results suggested the association of MSP3 with other merozoite surface proteins: MSP1, MSP6, MSP7, RAP2, and SERA5. Protein-protein interaction studies by enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR) analysis showed that MSP3 complex consists of MSP1, MSP6, and MSP7 proteins. Immunological characterization of MSP3 revealed that MSP3N is strongly recognized by hyperimmune serum from African and Asian populations. Furthermore, we demonstrate that human antibodies, affinity purified against recombinant MSP3N (rMSP3N), promote opsonic phagocytosis of merozoites in cooperation with monocytes. At nonphysiological concentrations, anti-MSP3N antibodies inhibited the growth of P. falciparum in vitro. Together, the data suggest that MSP3 and especially its N-terminal region containing known B/T cell epitopes are targets of naturally acquired immunity against malaria and also comprise an important candidate for a multisubunit malaria vaccine.
INTRODUCTION
Malaria remains one of the world's greatest public health problems (1). The treatment and prevention of malaria are achieved through antimalaria drugs to eliminate the parasite and by insecticides to eliminate the mosquito vector. However, the emergence of insecticide-resistant vectors and drug-resistant parasites calls for investment in new and improved control tools. Malaria vaccines hold the potential to dramatically alleviate the burden of malaria that has proven very difficult to roll back. Plasmodium falciparum merozoites, the parasite form that invades erythrocytes, are exposed to circulating antibodies, which make merozoite surface proteins (MSPs) likely targets of naturally acquired immunity (NAI) (2, 3). Accordingly, several MSPs have been associated with protection against clinical malaria in longitudinal cohort studies (4). The mechanisms through which immunity is conferred remain unknown; however, some data indicate that antibody-dependent cellular inhibition (ADCI) (5, 6), opsonic phagocytosis (OP) (7, 8), and complement-mediated lysis (9) may play a role. Of these immune mechanisms, both ADCI and OP are initiated by antibody-mediated opsonization of free merozoites (10).
A number of MSPs, such as MSP1, MSP4, MSP5, and MSP10, are anchored to the merozoite membrane (11, 12), while others, such as MSP6, MSP7, and MSP9, are attached to the merozoite surface through protein-protein interactions (13–15). Some of these MSPs bind human erythrocytes and play a role in the invasion of red cells (9, 16). P. falciparum MSP3 (PfMSP3; previously identified as a secreted polymorphic antigen associated with merozoites [SPAM]) is an ∼43-kDa soluble protein associated with the merozoite membrane surface. MSP3 was first identified as a target of hyperimmune serum from Papua New Guinea and later as a target of antibodies effective in ADCI (17, 18). The deduced amino acid sequence of MSP3 predicts several domains (19): an N-terminal signal peptide sequence, a central domain of imperfect Ala heptad repeats, a second central domain rich in Glu residues, and a C-terminal region that contains a leucine zipper-like motif (17, 19, 20). While the C-terminal domain of MSP3 (MSP3C; corresponding to amino acids [aa] 196 to 379 in the K1 allele sequence) is relatively conserved, the N-terminal region (corresponding to aa 52 to 195 of K1 MSP3) is polymorphic, with many amino acid substitutions and several large indels (21). These polymorphisms define two major allele types, K1 and 3D7 (22). Biophysical studies suggest that the full-length MSP3 forms elongated oligomeric structures through protein-protein interactions between residues in the C-terminal region (12, 19, 23). Studies performed on long, synthetic peptides from the oligomeric region suggested that the C-terminal residues may be involved in erythrocyte binding activity. The C-terminal region of MSP3 has also been reported to be involved in trafficking of MSP9 (15). However, it is still not clear that how MSP3 is staged onto the merozoite surface, despite the absence of a terminal domain as well as a glycosylphosphatidylinositol (GPI) anchor sequence.
Several lines of evidence have suggested that MSP3 is a strong vaccine candidate antigen. First, seroepidemiological studies have demonstrated that levels of MSP3-specific antibodies are associated with protection against clinical malaria (9, 18, 24–31). Second, functional studies have demonstrated that MSP3-specific antibodies have the capacity to kill the parasite in vitro (8, 18). Third, the clinical trials of MSP3-based vaccines suggest that MSP3 may elicit at least partial protective immunity in humans (32, 33). In the present study, we performed immunoprecipitation (IP) of P. falciparum merozoite lysate with antibodies raised against MSP3 consisting of aa 21 to 238 (MSP321–238, or MSP3N) and identified an MSP3-associated protein complex. In vitro protein-protein interaction techniques validated the association of proteins associated with MSP3 complex. Further, we performed human antibody-mediated protective efficacy studies that suggest the MSP3N region as a target of biologically active antibodies.
RESULTS
MSP3 localizes on merozoite surface in association with MSP165, MSP6, and MSP7.
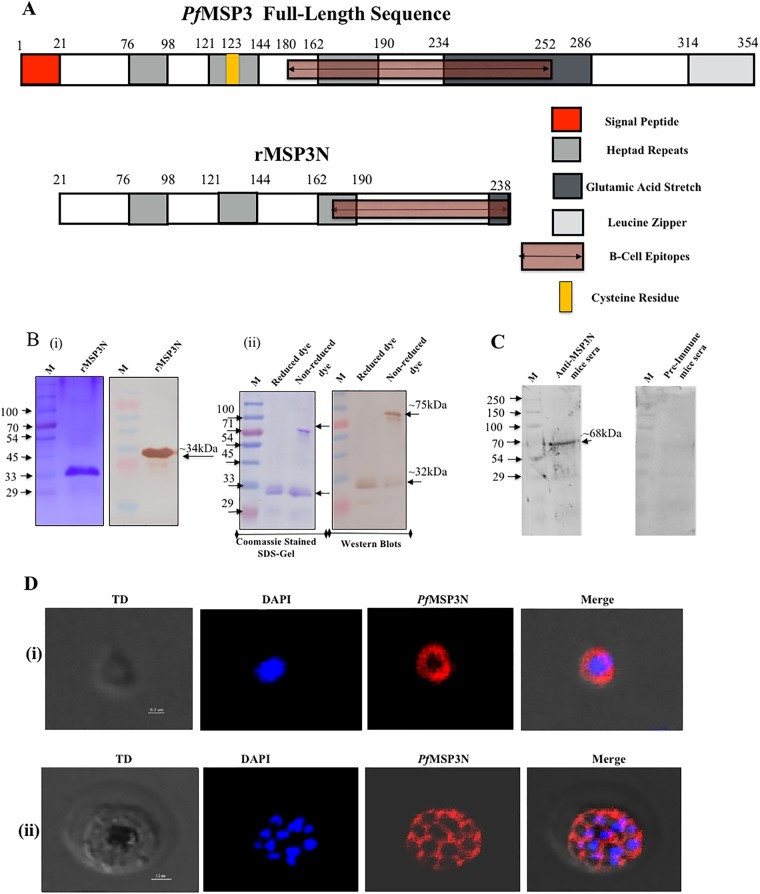
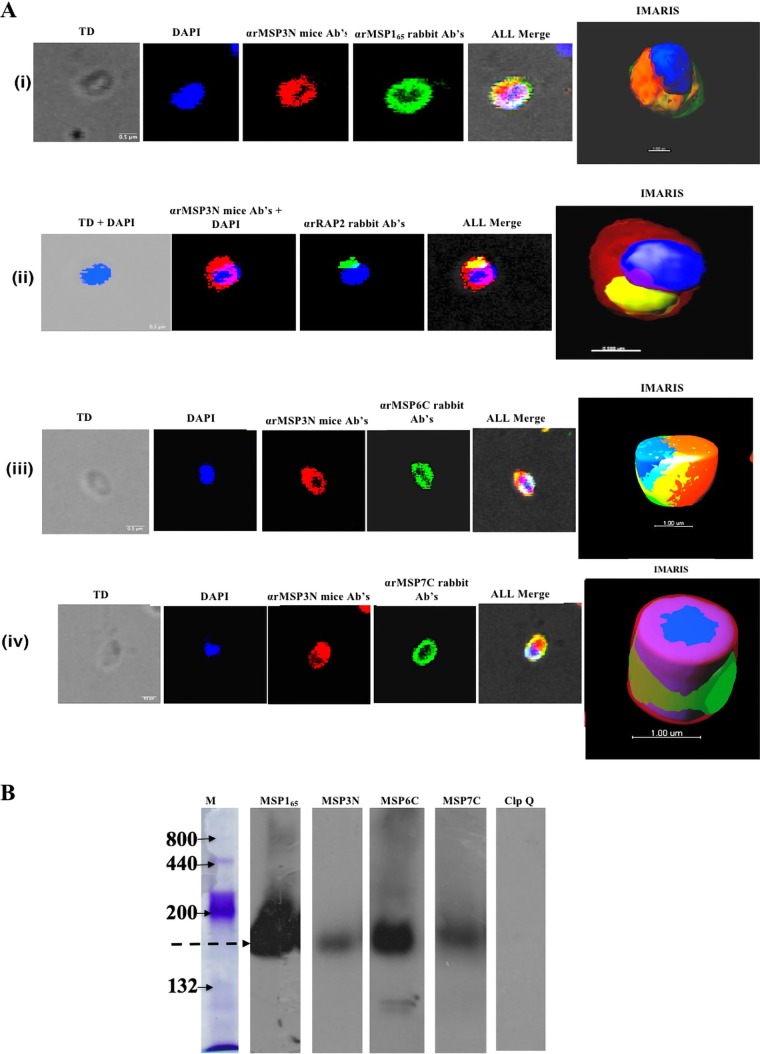
To analyze the localization of MSP3 and its interactions with other proteins on the merozoite surface, we produced a His-tagged recombinant protein, MSP3N, encompassing amino acid residues 21 to 238 (Fig. 1A). Recombinant MSP3N (rMSP3N) was solubilized in 8 M urea, purified on a Ni-nitrilotriacetic acid (Ni-NTA+) column, and refolded by gradual removal of urea by dialysis. Refolded rMSP3N migrated as a single protein band of ∼34 kDa under reducing conditions and as two bands of ∼30 kDa and ∼70 kDa under nonreducing conditions (Fig. 1B). The ∼70-kDa protein species represents a dimer, formed through an intermolecular disulfide bond at Cys123, and both of these MSP3 species of rMSP3N were recognized by anti-His antibodies (Fig. 1B). Specific antibodies were generated against rMSP3N in mice and rabbits. Purified anti-MSP3N antibodies recognized a band of ∼68 kDa in the merozoite extract (Fig. 1C), corresponding to native MSP3. As expected, an immunofluorescence assay (IFA) with mouse anti-rMSP3N antibodies localized MSP3 over the entire surface of P. falciparum merozoites (Fig. 1D). To determine whether MSP3 is associated with the merozoite surface through protein-protein interactions, we isolated MSP3-associated protein complexes through immunoprecipitation using anti-MSP3N sera from both rabbits and mice. The liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of four independent immunoprecipitation experiments showed that MSP3 is associated with other merozoite surface proteins (Tables 1 and 2). The proteins detected in at least three experiments are represented in Table 1. The association of MSP3 with other proteins identified by immunoprecipitation and mass spectrometry analysis was further evaluated by other biochemical methods. For the interaction analysis, the C-terminal region of MSP6 consisting of C-terminal residues 161 to 373 (MSP6C161–372), MSP7C195–352, and residues 22 to 298 of RAP2 (RAP222–398) were produced in Escherichia coli (see Fig. S2 in the supplemental material) and subsequently used to generate specific antisera in mice and rabbits. Fragments of MSP1 protein consisting of aa 1052 to 1664, designated MSP165, and SERA5 (serine repeat antigen 5) were generated as described previously (28, 34). The five antisera were used to study the colocalization of these proteins on merozoites using immunofluorescence assay (IFA). MSP3 was found to colocalize with MSP165, MSP6C, MSP7C, and RAP2 (Fig. 2A). The IFA studies on MSP3N showed the highest colocalization with MSP165, followed by MSP6C, MSP7C, and RAP2 (Pearson's correlation coefficients of 0.73, 0.65, 0.69, and 0.55, respectively). To prove that these proteins form part of a single protein complex, parasite invasion supernatant was run on a blue native PAGE (BN-PAGE) gel and subjected to immunoblot analysis. Anti-MSP3N, anti-MSP6C, and anti-MSP7C antibodies recognized a similarly sized band of ∼150 kDa, suggesting the existence of a protein complex that is shed during the invasion process (Fig. 2B). P. falciparum ClpQ (PfClpQ), an apicoplast parasite protein, served as a negative control. Taken together, these results suggest that MSP3 participates in one or more complexes with MSP165, MSP6, and MSP7 on the merozoite surface.
FIG 1.
Expression and localization of rMSP3N in P. falciparum asexual blood stages. (A) Schematic diagram showing the organization of PfMSP3. Numbers represent amino acid positions. (B) Coomassie-stained SDS-PAGE gel and immunoblot showing the purified rMSP3N are shown at left (i). At right, an SDS-PAGE gel and Western blot show migration of rMSP3N in reduced and nonreduced dye (ii). Lane M, molecular mass marker. (C) Western blot showing recognition of native PfMSP3 antigen in merozoite lysate by mouse anti-rMSP3N antibodies. (D) Confocal images showing immunostaining in asexual blood stage of P. falciparum using anti-rMSP3N mouse serum. Row i, merozoite stage; row ii, schizont stage. TD, transmitted light channel. Imaris software was used to convert confocal images into clear informative schematics.
TABLE 1.
Identification of Plasmodium falciparum malarial proteins
| Locus taga | Protein name | % sequence coverageb | Scoreb | No. of unique peptidesb | Peptide sequencesc |
|---|---|---|---|---|---|
| PF3D7_0930300 | PfMSP-1 | 35.04 | 802.83 | 53 | FNIDSLFTDPLELEYYLR, TLSEVSIQTEDNYANLEK, LEALEDAVLTGYSLFQK, YLIDGYEEINELLYK, EAEIAETENTLENTK, LLEVYNLTPEEENELK, FSSSNNSVYNVQK, LLSTGLVQNFPNTIISK, FLPFLTNIETLYNNLVNK, KLEALEDAVLTGYSLFQK, LNSLNNPHNVLQNFSVFFNK, NNDTYFNDDIK, LNFYFDLLR, VTTVVTPPQPDVTPSPLSVR, SYENFLPEAK, IDDYLINLK, LNDNLHLGK, YYNGESSPLK, FYENILK |
| PF3D7_0207600 | PfSERA5 | 13.96 | 135.37 | 11 | LPSNGTTGEQGSSTGTVR, TNNAISFESNSGSLEK, cDTLASNcFLSGNFNIEK |
| PF3D7_1035400 | PfMSP-3 | 19.56 | 47.29 | 5 | EASSYDYILGWEFGGGVPEHK, GNNQIDSTLKDLVEELSK, GNNQIDSTLK |
| PF3D7_0207700 | PfSERA4 | 15.85 | 89.67 | 11 | NSYVYGQDTTPVEnEAPR, YNHEFEVGDNScSR |
| PF3D7_1035500 | PfMSP6 | 13.0 | 38.38 | 3 | YSSPSDINAQNLISNK, KPDNEITNEVK, NEIDSTINNLVQEMIHLFSNN |
| PF3D7_0501600 | PfRAP2 | 15.7 | 50.83 | 4 | DINPLFINDFILILNDK, ENYYnSDIAGPAR, YTEISVLNYVR |
| PF3D7_1335100 | MSP7 | 21.15 | 90.76 | 7 | NEQEISTQGQEVQKPAQGGESTFQK, INLDEYGK, EYEDFVLNSK |
Proteins from P. falciparum 3D7 were identified by LC-MS/MS analysis of immunoprecipitates from merozoite lysate using anti-MSP3N antibody.
Values are the averages of four experiments or (for PfMSP6) three experiments.
Common peptides in four experiments or (for PfMSP6) three experiments.
TABLE 2.
Representation of the proteins identified by LC-MS/MS analysis after immunoprecipitation
| Locus taga | Protein name | IP using anti-MSP3N antiserum from:b |
|||
|---|---|---|---|---|---|
| Rabbit |
Mouse |
||||
| Expt. 1 | Expt. 2 | Expt. 1 | Expt. 2 | ||
| PF3D7_0930300 | MSP1 | + | + | + | + |
| PF3D7_0207600 | SERA5 | + | + | + | + |
| PF3D7_1035400 | MSP3 | + | + | + | + |
| PF3D7_0207700 | SERA4 | + | + | + | + |
| PF3D7_103550 | MSP6 | + | − | + | + |
| PF3D7_0501600 | RAP2 | + | + | + | + |
| PF3D7_1335100 | MSP7 | + | + | + | + |
Proteins were identified from P. falciparum 3D7.
Specific antibodies were generated against rMSP3N in mice and rabbits. None of these proteins were detected by preimmune rabbit or mouse antiserum.
FIG 2.
Association of rMSP3N with merozoite-expressed proteins. (A) Colocalization studies on Plasmodium falciparum merozoite-stage parasites using immunofluorescence assay. TD, transmitted light channel; Ab, antibody. Imaris software was used to convert confocal images into clear informative schematics. (B) Western blot analysis of invasion supernatant to find the existence of the merozoite surface protein complex shed during parasite invasion. PfClpQ is a negative control.
In vitro protein-protein interaction between MSP3 and other merozoite surface/rhoptry proteins confirms their association with the merozoite surface.
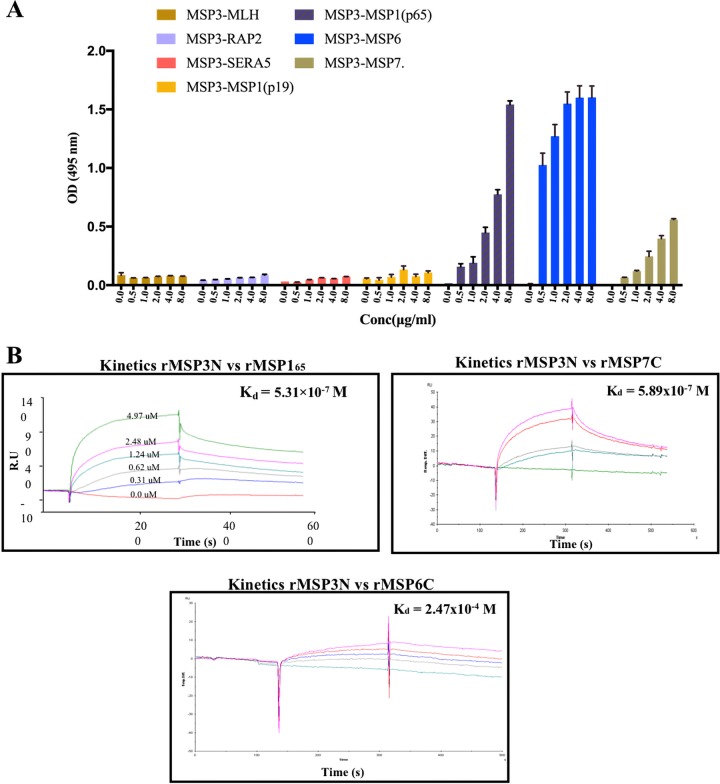
To confirm the MSP3-associated interactome, protein-protein interaction analysis between MSP3 and MSP165, MSP6, MSP7, RAP2, or SERA5 was performed by an enzyme-linked immunosorbent assay (ELISA) protein binding assay and surface plasmon resonance (SPR). For the ELISA-based protein-protein interaction analysis, 96-well ELISA plates were coated with rMSP3N, followed by the addition of increasing concentrations of the prey proteins rMSP165, rMSP6C, rMSP7C, rRAP2, and rSERA5. The respective interactions were analyzed using specific antibodies against prey proteins. A concentration-dependent binding between rMSP3N and the prey proteins rMSP165, rMSP6C, and rMSP7C was observed (Fig. 3A). In contrast, recombinant RAP2, SERA5, and a MutL homolog (MLH, nonspecific protein) did not show interactions with rMSP3N even at the highest concentrations used. To quantitate the interaction between rMSP3N and rMSP165, rMSP3N and rMSP6C, and rMSP3N and rMSP7C, SPR analysis was performed. For the SPR analysis either rMSP165 or rMSP3N was immobilized on CM5 chips. Recombinant MSP3N was injected over immobilized rMSP165, and rMSP6C, rMSP7C, rRAP2, and rSERA5 were injected over immobilized rMSP3N. Recombinant MSP3 bound to rMSP165 and rMSP7C in a dose-dependent manner, with equilibrium dissociation constants (Kd) of 5.31 × 10−7 M and 5.89 × 10−7 M, respectively (Fig. 3B). The interaction between rMSP3N and rMSP6C in SPR was moderate, with a Kd value of 2.47 × 10−4 M (Fig. 3B). Recombinant RAP2 and rSERA5 did not show any binding to rMSP3N in the SPR analysis (Fig. S4). Together, these results demonstrated that MSP3 is part of a merozoite surface protein complex consisting of MSP165, MSP6, and MSP7.
FIG 3.
Existence of a merozoite surface protein complex in P. falciparum asexual blood stages. (A) ELISA binding analysis, confirming concentration-dependent interaction of rMSP3N with rMSP165, rMSP7C, and rMSP6C while rRAP2, rSERA5, and rMLH did not show any binding to rMSP3N even at the highest concentrations (Conc). Bars represent the SEM, calculated from two sets of experiments. (B) SPR analysis showing interaction between rMSP3N and rMSP165 and between rMSP7C and rMSP6C. Kd is the dissociation constant representing the measure of affinities.
Seroprevalence of MSP3 antibodies during natural malaria infection.
The antigenicity of MSP3N was assessed using serum collected from regions in Africa and India where malaria is endemic. Twenty-one out of 28 (75%) Liberian samples and 19 out of 28 (67.9%) Indian samples were seropositive for rMSP3N (Fig. 4), suggesting that the N-terminal region of MSP3 is well recognized during natural P. falciparum infection in geographically distinct locations.
FIG 4.
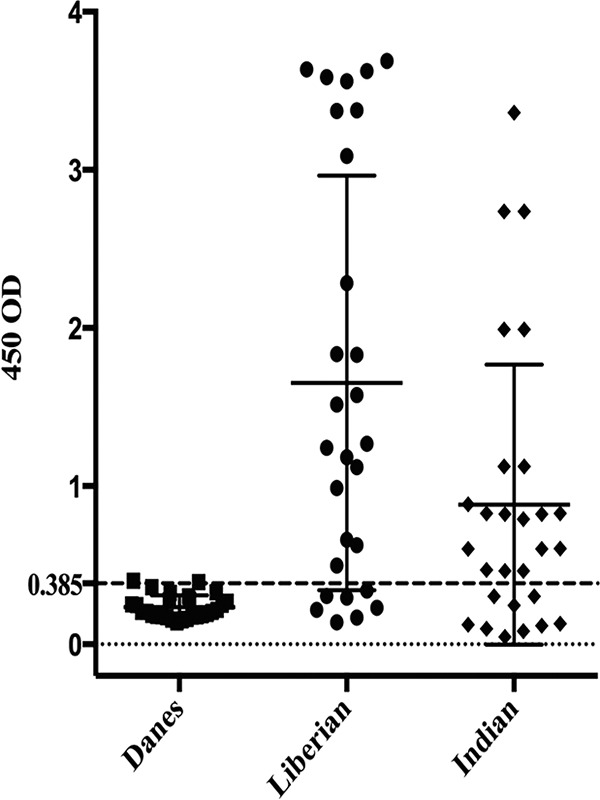
Naturally acquired humoral IgGs against rMSP3N in Indian and Liberian populations. ELISA was performed to check seroprevalence against rMSP3N in 28 naturally infected sera from areas of malaria endemicity in Liberia and India. The dotted line represents positivity threshold limits calculated by mean relativities plus twice the SEM of results from 28 serum samples from Danish nonimmune volunteers.
Functional analysis of rMSP3N-specific antibodies.
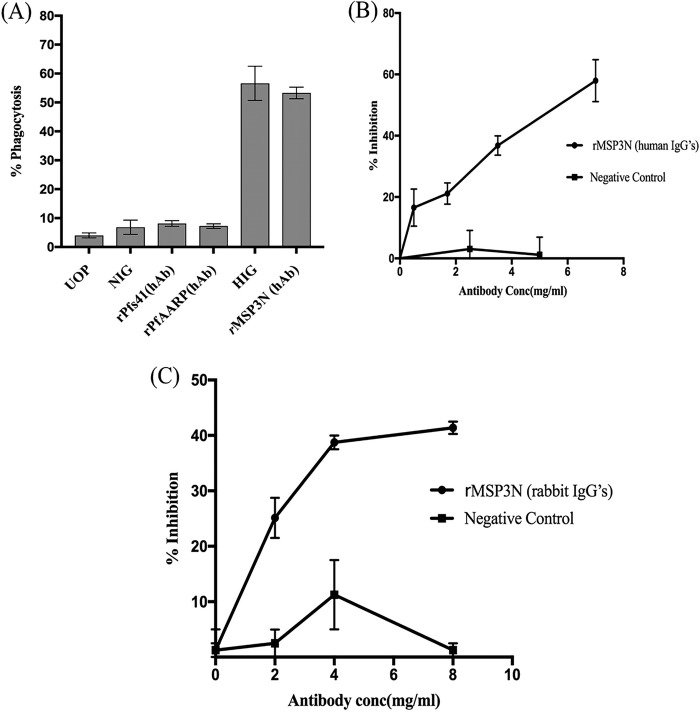
The biological activity of MSP3N-specific antibodies was assessed by opsonic phagocytosis assay (OPA) of merozoites and an in vitro growth inhibition assay (GIA). MSP3N-specific IgG antibodies were affinity purified from a pool of Liberian serum samples. MSP3N-specific antibodies showed strong opsonizing activity for intact merozoites (53%) compared to that for unopsonized control merozoites (7%) at a final concentration of 0.035 mg/ml (Fig. 5A). Single-factor analysis of variance (ANOVA) was performed to statistically evaluate the obtained results (Tables S3A and B). This activity was observed in three important levels of phagocytosis with MSP3N-specific human antibodies matching the levels observed with total IgG (0.035 mg/ml) purified from the same malaria-hyperimmune Liberian adults (Fig. 5A). In comparison, low levels of opsonizing activity were detected with human antibodies affinity purified against recombinant P. falciparum apical asparagine-rich protein (PfAARP) and rPfs41 (Fig. 5A) as these antigens are localized internally in the parasite and are less exposed to the host immune system. To examine whether the MSP3N-specific antibodies also had a direct growth-inhibitory effect, increasing concentrations of purified human antibodies were added to a highly synchronized P. falciparum culture at the late schizont stage. At 36 h postinfection (hpi), MSP3N-specific antibodies significantly inhibited parasite invasion in a concentration-dependent manner, with 36.8% and 57.9% inhibition at 3.5 and 7.0 mg/ml, respectively (Fig. 5B and Tables S4A and B). To confirm the growth-inhibitory effects of the human antibodies, GIA was repeated with rabbit anti-MSP3N antibodies. A maximum of 35% inhibition was observed at a concentration of 8 mg/ml (Fig. 5C and Tables S5A and B), suggesting that MSP3N-specific antibodies promote GIA at nonphysiological concentrations. In contrast, total IgGs from preimmune rabbits and purified human antibodies against rhoptry protein rRhopH3N showed <10% invasion inhibition (Fig. 5B). The results demonstrate that high levels of neutralizing antibodies are generated against MSP3N during natural P. falciparum infection. Together, these results show that MSP3N generates phagocytosis as well as humoral responses during natural infections.
FIG 5.
Biofunctional assays on rMSP3N to evaluate vaccine efficacy. (A) Bar graph showing percentage of opsonic phagocytosis of merozoites by THP-1 cells mediated by either test or control antibodies at a 0.035-mg/ml concentration. Human MSP3N-specific purified antibodies promoted strong opsonic phagocytosis activity, which was similar to that of the positive control (HIG). Opsonic phagocytosis activity is expressed as the average of triplicate measurements with standard errors of the means. HIG, hyperimmune immunoglobulin G; NIG, malaria-naive immunoglobulin G; UOP, unopsonized merozoites; MSP, merozoite surface protein; hAb, human antibody. (B) Line graph showing invasion inhibition assay on schizont-stage parasites using rabbit anti-rMSP3N total purified IgG at increasing concentrations; rabbit preimmune total IgG served as a negative control. (C) Line graph representing invasion inhibition using anti-MSP3N human IgGs and merozoite-PfRhopH3N human antibodies in a concentration-dependent manner. FACS analysis was done to determine parasitemia after 40 h of incubation. Bars indicate SEM of duplicate measurements.
DISCUSSION
Among the several merozoite surface antigens that are being considered for malaria vaccine development, merozoite surface protein 3 is well studied for its role in antibody-dependent cellular inhibition and therefore is considered one of the leading malaria vaccine candidate antigens (9, 31, 35–38). Although PfMSP3 is abundantly expressed on the merozoite surface, it is still uncertain how this protein is associated with the merozoite surface. To establish the PfMSP3-associated network on the Plasmodium merozoite surface, we carried out a detailed protein-protein interaction analysis by performing immuno-pulldown of parasite lysate proteins using antibodies against an rMSP3 N-terminal fragment, a technique previously used to identify protein-protein interactions (28, 34, 39, 40). Five parasite proteins, MSP1, MSP6, MSP7, RAP2, and SERA5, which may be directly or indirectly involved in the invasion process were identified together with MSP3 in four independent LC-MS/MS experiments. Out of these five proteins, MSP1 showed the highest confidence score, thereby indicating that PfMSP1 may be the anchor protein on the merozoite surface to which MSP3 is associated. The interaction between PfMSP3 and PfMSP1 has been reported previously (16). Recently Lin et al. reported two separate complexes of processed MSP1 fragments, p38/p30 (102 kDa) and p38/p42 (89.7 kDa), which serve as the backbone for forming multiprotein complexes of various sizes on the merozoite surface (16), and MSP3 was found to be associated with p83/p30 and p38/p42 MSP1 complexes individually or in combination with other merozoite proteins on the surface of merozoites (16, 41–43). Our results further supported the existence of MSP1-MSP3 complex on the merozoite surface. To further validate the association of the MSP3 complex, we next applied two protein-protein interaction techniques, ELISA binding assays and SPR analysis, using recombinant MSP165, MSP6C, MSP7C, RAP2, and SERA5 proteins. Both of these techniques demonstrated strong interaction of rMSP3N with rMSP165, rMSP6C, and rMSP7C. In contrast, we did not observe interactions between MSP3N and rRAP2 or rSERA5. Together, these results indicated a protein complex consisting of MSP165, MSP3, MSP6, and MSP7 on the merozoite surface. An interesting finding of the study was that the PfMSP165 protein that contains p38 and p33 domains was involved in the interaction between MSP3N and MSP165. Another finding of the present study is that the MSP3 N-terminal region (aa 21 to 238) is mainly involved in the interaction with PfMSP1. These results are in line with previous reports suggesting that MSP3 exists in a complex as an elongated dimer/tetramer on the merozoite surface and that its coiled-coil domain-adherent structure that is represented by its N-terminal domain is mainly involved in formation of various large complexes with other proteins on the merozoite surface (12, 16, 19, 26). The long, extended fibrillary nature of the MSP3 molecule complexed with other GPI-anchored or non-GPI-anchored merozoite surface proteins thus allows flexibility of the merozoite complex of proteins to trap or reach the ligands of the erythrocytes (5, 8, 24, 31, 35–38). A number of previous studies have identified the C-terminal region (aa 184 to 252) of MSP3 as a target of protective antibody responses (9, 21, 30, 38, 44, 45). Since the recombinant MSP3 protein used here covers the N-terminal region, a serological analysis was performed to confirm its antigenicity using serum samples from India and Liberia. We found a high prevalence of IgG antibodies against rMSP3N in both populations. The higher seroprevalence in Liberian (73%) than in Indian (63%) samples is in agreement with Liberia being an area of hyperendemicity and the blood donors having attained solid immunity against clinical malaria (46). Antibodies against multiple merozoite surface antigens, including MSP2, MSP3, and glutamate-rich protein (GLURP), seems, at least in part, to mediate their functional activities in collaboration with blood monocytes (7, 8, 10, 18). Our finding that antibodies against the K1 allelic version of MSP3 promote strong opsonic phagocytosis of freshly purified merozoites extends functional studies of MSP3 IgG from Kenyan individuals (7, 8). We found more than 54% phagocytosis of merozoites opsonized with human MSP3N IgG at 0.035 mg/ml, which is clearly within the physiological range in this population where malaria is endemic. More generally, some studies seem to suggest the benefit of using conserved merozoite antigenic domains, due to their cross-reactive nature, for vaccine development (47); others have highlighted the importance of combining conserved and polymorphic regions for an effective vaccine (48). Our results support the notion that polymorphic domains of MSP3 may also be important as they are targets of opsonizing antibodies. Recently, we have shown that a hybrid protein (GMZ2) composed of MSP3 and GLURP elicits modest (14%) protection against clinical malaria in a multicenter phase 2 efficacy trial (32, 33). Whether this protective immunity was mediated by opsonizing antibodies against MSP3 or GLURP remains to be investigated. Of interest was the finding that the deletion of MSP3 did not affect the opsonizing activity of total IgG (47), suggesting that multiple antibody specificities act in concert.
Since antibodies against MSPs have been found to mediate direct inhibition of merozoite invasion, both human and rabbit antibodies against MSP3N were tested in the GIA (11, 16, 18, 25–27, 40–42, 45, 49, 50). Both of the antibody preparations showed moderate inhibition of parasite growth at very high antibody concentrations, suggesting that MSP3-specific antibodies do not play a major role in invasion inhibition during natural infection. In contrast, our results confirm and extend previous studies suggesting that antibodies to MSP3 (and other MSPs) promote their protective effects through collaboration with monocytes. Whether antibodies against MSP3 also promote neutrophil-mediated effector mechanisms, including phagocytosis and respiratory burst or complement-mediated lysis, is unknown.
In conclusion, we have demonstrated that MSP3 is retained on the merozoite surface by associating with a network of merozoite surface proteins. We further show that the N-terminal region of MSP3 (aa 21 to 238) is an important immunogen that generates protective immune responses against malaria, mainly through cooperation with immune cells. Taken together, our data advocate the inclusion of MSP3 in future malaria subunit vaccine(s).
MATERIALS AND METHODS
In vitro P. falciparum culture.
P. falciparum strain 3D7 was cultured on human erythrocytes (4% hematocrit) in RPMI 1640 medium (Invitrogen) supplemented with 10% O+ human serum using the standard protocol described by Trager and Jensen (51). Parasite cultures were synchronized by two consecutive sorbitol treatments at intervals of 4 h, following a protocol described previously (52). P. falciparum 3D7 merozoite isolation was done as described previously (53). Briefly, highly synchronized P. falciparum 3D7 strains were grown in vitro at high parasitemia in 2% hematocrit and allowed to mature into late schizonts followed by release of merozoites (40, 53). P. falciparum NF54 merozoites used in opsonic phagocytosis assays were isolated as described previously (7).
Cloning of MSP3N21–238, RAP224–398, MSP6C161–372, and MSP7C195–351.
The recombinant gene fragments MSP6C161–372, MSP7C195–351, and RAP224–398 were PCR amplified from P. falciparum genomic DNA using primer pairs given in Table S1 in the supplemental material and cloned into the pGEMT vector (Promega Corp., USA). Subsequently, the RAP2 fragment was subcloned into pET-28b vector between NcoI and XhoI restriction sites. The MSP6C and MSP7C fragments were ligated into pET-28a vector between BamHI and XhoI restriction sites. The expression clone of the MSP3N21–238 fragment in pET-28a was borrowed from Imam et al. (26).
Expression and purification of recombinant proteins.
The large N-terminal fragment of MSP3N in a pET-28a expression construct was transformed into SHuffle T7 competent E. coli cells (NEB) for expression; the cells were induced with 0.5 mM isopropyl-d-thiogalactopyranoside (IPTG) (Sigma Chemical Co.) for 8 h at 30°C. The induced cell pellet was disrupted by sonication in Tris-NaCl lysis buffer (0.05 M Tris, pH 8.0, and 0.15 M NaCl, 0.01 M dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 1% Triton X-100) with 9-s pulses at 9-s intervals 10 times using a miniprobe. The soluble and insoluble fractions were separated by centrifugation at 12,000 × g for 30 min and analyzed by SDS-PAGE. The MSP3N protein was found expressed in the insoluble fractions. The insoluble fractions were washed four times with Tris-NaCl lysis buffer without Triton X-100. The inclusion bodies thus obtained were solubilized in 8 M urea buffer (0.5 M Tris, 0.150 M NaCl). The suspension was incubated for 1 h at room temperature (RT) and then centrifuged at 12,000 × g for 30 min at 4°C. The supernatant containing solubilized protein was kept for binding with Ni-NTA+ resin for 1 to 2 h at RT with constant shaking. After binding, the suspension was packed in a purification column, and flowthrough was collected. The resin was washed four times with 8 M urea buffer containing 10 mM imidazole. Bound protein(s) eluted in 8 M urea buffer containing different concentrations of imidazole. Eluted protein fractions were analyzed by 12% SDS-PAGE. The pure eluted protein fractions were pooled and concentrated to proceed to the refolding process. The refolding method was adopted from a standard universal protocol described by Tsumoto et al. (54). The protein was refolded gradually by decreasing the urea concentration in the refolding buffer (0.05 M Tris, pH 8, 1 mM EDTA, 0.5 M arginine, 0.4 mM Triton X-100, 1 mM reduced glutathione, 0.5 mM oxidized glutathione). Urea concentration was gradually decreased by dialyzing the protein at every 2 h in refolding buffers containing 6 M, 4 M, 2 M, and 1 M urea, and finally the refolded proteins were concentrated and dialyzed against 0.05 M Tris, pH 8, and 0.15 M NaCl and stored at −80°C. The purified protein was analyzed on a 12% SDS polyacrylamide gel under both reducing and nonreducing conditions (26). The expression vector construct of the RAP2 gene in the pET-28b vector was expressed in BL21(DE3) cells using 0.5 mM IPTG for 6 h. The recombinant RAP2 protein was found expressed in the insoluble fraction, so the purification and refolding were carried out as described for the rMSP3N protein. The pET-28a vector constructs containing MSP6C and the MSP7C fragment were transformed into Rosetta (DE3) cells and induced with 0.5 mM IPTG for 8 h at 37 °C. The induced cell pellet was disrupted similarly as described above for the proteins. Both of the proteins were present in the soluble fractions. The supernatant was allowed to bind Ni-NTA+ resin for 2 h to proceed to affinity purification. Imidazole at 10 mM in Tris-NaCl lysis buffer without Triton X-100 at pH 8 was used for washing the protein-bound Ni-NTA+ resin. The pure protein fractions were eluted using various imidazole concentrations (50 mM, 100 mM, 250 mM, 500 mM, and 1 M) in the Tris-NaCl lysis buffer without Triton X-100. The fractions were analyzed on a 12% SDS-PAGE gel; purified fractions were pooled, dialyzed in 1× phosphate-buffered saline (PBS) (137 mM NaCl, 10 mM Na2HPO4, 1.8 mM NaH2PO4 at pH 7.2) buffer using 10-kDa dialysis bags, concentrated by using ultrapure sucrose (Sigma), and finally stored at −80°C in 100-μl aliquots. The expressed proteins were confirmed by Western blotting with monoclonal anti-His antibody (Sigma). The rMSP165 (consisting of aa 1052 to 1664 of MSP1) and rSERA5 proteins along with their specific anti-rabbit sera were based on Paul et al. and Kanodia et al., respectively (28, 34).
Antibody generation and purification.
Polyclonal antibodies against the merozoite surface/rhoptry antigens MSP3N, MSP6C, MSP7C, and RAP2 were generated in mice and rabbits using Freund's adjuvants as per the protocol described in Paul et al. (28). Total IgGs were purified from mouse and rabbit final-bleed sera using protein A/G-agarose beads (Pierce) according to Eliasson et al. (55). Also, specific human anti-rMSP3N, -rRAP2, -rRhopH3, and -rAARP antibodies were isolated from the pool of hyperimmune malaria-infected Liberian patient sera by using N-hydroxysuccinimide (NHS)-activated affinity purification as described in Hermanson et al. (56). The purity and specificity of the purified human IgGs were checked by reduced SDS-PAGE and Western blot analysis, and cross-reactivity of human purified antibodies was determined by ELISA.
Immunoblot analysis of P. falciparum merozoite lysate.
In brief, freshly isolated P. falciparum merozoites were harvested and solubilized in radioimmunoprecipitation assay (RIPA) buffer and incubated for 1 h on ice, and the supernatant was collected by centrifugation at 15,000 × g for 15 min. The merozoite lysate was mixed with SDS sample buffer and boiled at 98°C for 10 min for proper separation by SDS-PAGE. After insoluble material was removed by centrifugation (15,000 × g for 15 min), the boiled parasite lysate was run on an SDS-PAGE gel and transferred to nitrocellulose membrane. The membrane was incubated with mouse anti-rMSP3N (1:100) or antiserum followed by IRDye 800CW (Li-Cor) goat anti-mouse secondary IgGs (1:5,000). The specific protein band was detected through an infrared (IR) blot scanner (57).
Indirect immunofluorescent assay.
Indirect immunofluorescence assays were performed on the merozoite stage of P. falciparum 3D7 parasites as described earlier (12, 26, 28, 40). Parasite smears on the glass slide were fixed using prechilled methanol-acetone for 20 min and allowed to air dry at room temperature (RT). Slides were blocked with 3% bovine serum albumin (BSA) in PBS, pH 7.4, for 2 h at 37°C and washed three times with PBS and PBS with Tween 20 (PBST). The primary antibodies were used at the following dilutions: mouse anti-rMSP3N, rabbit anti-rMSP6C, and rabbit anti-rMSP7C at 1:100; anti-rabbit rRAP2 at 1:250; human anti-rMSP3N purified antibodies at 1:500 (made in 1% BSA in PBS). The primary antibodies were overlaid on the glass slides and incubated in a moist chamber at 37°C for 2 h, and after appropriate washes, the secondary antibody conjugated with Alexa 484 or Alexa 584 dye was added and incubated for 1 h in the dark. The 4′,6′-diamidino-2-phenylindole (DAPI)-antifade solution was added just before the slides were mounted with coverslips and stored at 4°C. Confocal laser scanning IFAs were performed with an A1 confocal microscope (Nikon). Images were analyzed using Nikon NIS Elements, version 4.0, software. Imaris file format images were created using the software Imaris, version 4.0 (Bitplane).
Immunoprecipitation reaction.
Immunoprecipitation was performed using a Pierce cross-link immunoprecipitation kit (ThermoFisher Scientific Inc., USA) as per the manufacturer's protocol. Merozoites were isolated from highly synchronized P. falciparum cultures. Merozoite lysate was prepared in IP lysis buffer (250 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5% glycerol, pH 7.4). Polyclonal rabbit and mouse rMSP3N antibodies were used (20 μg) to cross-link with protein A/G-agarose Plus separately and incubated with parasite lysate (∼1 mg per reaction volume). After 12 h of incubation, protein A/G-agarose beads were washed with wash buffer, and bound proteins were eluted from the beads using the elution buffer (Tris-glycine, pH 2.8). The experiment with each antibody was performed twice, and the respective preimmune antibodies were used as controls.
Proteins in the immunoprecipitated samples were digested by the in-solution trypsin digestion method. Samples were first reduced with 10 mM DTT (final concentration) for 1 h at room temperature (RT). After reduction, samples were alkylated with 40 mM iodoacetamide (Sigma-Aldrich, USA) for 1 h at RT in the dark. Proteins were digested by the addition of trypsin at a ratio of 1:50 (wt/wt) trypsin to protein at 37°C overnight. After digestion, extracted peptides were acidified to 0.1% formic acid and analyzed by an Orbitrap Velos Pro mass spectrometer coupled with a Nano-LC 1000 instrument (ThermoFisher Scientific, Inc., USA). The raw data were analyzed using Proteome Discoverer, version 1.4, using the SEQUEST algorithm. The proteins which were identified in at least three out of total four experiments were chosen to be represented in Tables 1 and 2.
Protein-protein interaction analysis.
In vitro interactions between the members of the merozoite surface proteins were examined by ELISA- and surface plasmon resonance (SPR)-based binding techniques (28, 39, 40). In brief, ELISA plates were coated with 100 ng of MSP3N protein overnight (O/N) at 4°C. The next day, the ELISA plates were washed three times with 1× PBS, followed by three washes with 1× PBS containing 0.01% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate). ELISA plates were blocked with 5% milk at 37°C for 90 min and washed as described above. The recombinant proteins rMSP6C, rMSP7C, rRAP2, and rMSP165 at concentrations ranging from 0.5 to 8 μg/ml were made in ELISA binding buffer (phosphate buffer) and added to the ELISA plates and incubated at 37°C for 3 h. Antigen-specific rabbit polyclonal antibodies detected bound proteins using horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibodies (1:3,000) in the presence of phenylenediamine dihydrochloride (Sigma-Aldrich) by measuring the resulting absorbance at 490 nm in an ELISA microplate reader. All experiments were done in duplicates, and means ± standard error of the means (SEM) were calculated. First, rMSP165 was immobilized on the flow cell CM5 sensor chip; rMSP3N at increasing concentrations was injected over the immobilized MSP165 as well as on the reference flow cell at a flow rate of 20 μl min−1. Next, on the new CM5 chip, rMSP3N was immobilized, and increasing concentrations of recombinant MSP6C, MSP7C, RAP2, and SERA5 proteins were injected over MSP3N as well as on the reference flow cell at a flow rate of 20 μl min−1. The kinetic parameters of the interaction and binding responses in the steady-state region of the sensorgram were analyzed using Biacore evaluation software, version 4.1.1 (GE Healthcare).
Complex identification in postinvasion culture supernatant.
Highly synchronized P. falciparum cultures were maintained at high parasitemia, and after the invasion of the parasites into erythrocytes, the culture supernatant was collected by centrifugation at 2,000 rpm for 5 min. The supernatants were further centrifuged at 15,000 × g for 10 min to remove insoluble debris and then concentrated 20 times by using a 3,000-Da Amicon filter and stored at −80°C in small aliquots. Blue native PAGE (BN-PAGE) analysis was carried out to separate out various complexes present in the invasion supernatant. The following protocol for BN-PAGE analysis with slight modifications was adopted from Wittig et al. (58). Western blot analysis was carried out after BN-PAGE using various antibodies to rMSP3N, rMSP165, rMSP6C, and rMSP7C to find the association of these proteins to various complexes separated by BN-PAGE analysis. The blots were developed using an ECL kit (Thermo Scientific Pierce) (59).
An enzyme-linked immunosorbent assay (ELISA) was performed as described earlier (24). Sera from 28 P. falciparum malaria patients in India and Liberia were used, while sera from 28 Danish volunteers were used as negative controls. The mean optical density plus two standard deviations of samples from 28 nonimmune Danish volunteers was used to determine the positive threshold.
Briefly, 96-well polystyrene flat-bottom plates (Nunc-Maxisorp; Thermo Scientific) were coated with 100 μl of 5 μg/ml rMSP3N protein in carbonate-bicarbonate buffer and incubated overnight at 4°C. After a blocking step, the wells were incubated with Indian, Liberian, or Danish serum samples (1:200 dilution) at RT for 1 h, followed by incubation with HRP-conjugated goat anti-human IgG (1:3,000; Sigma) for 1 h at RT. The bound antibody was detected with tetramethylbenzidine (TMB) solution (Sigma). Plates were extensively washed between each incubation period with PBST (PBS containing 0.1% Tween).
Statistical analysis.
Graphs and statistical analysis were performed in GraphPad Prism, version 7. The single-factor ANOVA was performed using Microsoft Excel.
Invasion inhibition assay.
An invasion inhibition assay was performed as previously described (28, 39). Briefly, ring-stage parasites were synchronized by sorbitol lysis and allowed to mature into schizont stage. The hematocrit and parasitemia of 2% and 1%, respectively, were adjusted. Purified rabbit anti-rMSP3N- and human anti-rMSP3N-specific IgGs were added to the parasite cultures in 96-well plates at increasing concentrations ranging from 1 to 8 mg/ml for rabbit and from 2 to 7 mg/ml for human IgGs. The cultures were incubated for 40 h to allow for schizont rupture and merozoite invasion, and the parasitemia was counted using a fluorescence-activated cell sorter (FACS) through ethidium bromide (EtBr) staining. The percent inhibition was calculated relative to that of preimmune serum for rabbit IgG and RhopH3a purified antibodies from human IgG. Error bars were calculated by taking the standard errors of the mean (SEMs) from duplicate measurements for rabbit IgGs and quadruplicate measurements for human IgGs.
OPA.
An opsonic phagocytosis assay (OPA) was performed as previously described (7). In brief, ethidium bromide (EtBr)-stained Plasmodium falciparum NF54 merozoites were opsonized with human anti-rMSP3N or control IgG at 0.035 mg/ml. Fifty-microliter aliquots of merozoites (4 × 105) were added to fetal calf serum-coated 96-well U-bottom plates containing THP-1 cells (1 × 1−5 cells/150 μl/well) and incubated for 40 min in a 5% carbon dioxide-humidified incubator for phagocytosis to take place. Phagocytosis was stopped by prechilled centrifugation of plates at 500 × g for 5 min. Samples were washed twice with ice-cold FACS buffer (PBS, 0.5% BSA, 2 mM EDTA) and then finally resuspended in 200 μl of FACS fixative (FACS buffer plus 2% paraformaldehyde) before being analyzed by a Beckman Coulter cytometer (Cytomics FC 500 MPL). Several controls were included for each assay: (i) unopsonized merozoites (UOP), (ii) merozoites opsonized with malaria-native immunoglobulin (NIG) from Danes, or (iii) hyperimmune immunoglobulin (HIG) from malaria-exposed Liberian adults. Percent phagocytosis refers to the percentage of THP-1 cells containing EtBr-stained merozoites.
Ethical approval and consent to participate.
All animal experiments conducted were approved by the Institutional Animals Ethics Committee of the International Centre for Genetic Engineering, and Biotechnology (IAEC-ICGEB) under approval number ICGEB/AH/2015/01/MAL-74. Written informed consents were obtained from patients for all Indian serum samples. Liberian and Danish serum samples were from the collection of Michael Theisen of Statens Serum Institut, Copenhagen, Denmark.
Availability of data and materials.
All data generated or analyzed during this study are included in this published article (and its supplemental material).
Supplementary Material
ACKNOWLEDGMENTS
G.P. acknowledges financial support from the Department of Biotechnology, Government of India, in the form of a DBT-SRF project fellowship. The study was also supported by the Department of Biotechnology, Government of India, under grants BT/PR-5267/MED/15/87/2012 and BT/IN/Denmark/13/SS/2013 (supervised by P.M. and A.M.). The funders had no role in the design of the study and collection, analysis, interpretation of data, and writing of the manuscript.
We thank the Rotary Blood Bank (India) for providing human red blood cells and V. S. Chauhan and Puneet Gupta for providing PfMSP119 protein and anti-PfMSP119 antibody. We also thank Renu Tuteja for providing rPfMLH protein.
A.D., B.K.C., S.M., G.P., I.H.K., I.K., S.R., and P.G. performed research experiments. K.M., A.P., S.K.S., P.G., S.K.G., and A.P. contributed expertise and necessary tools. A.D., P.M., A.M., and M.T. designed the study, interpreted data and wrote the manuscript; S.K.G. and M.T. contributed to experimental design, data interpretation, and manuscript preparation.
We declare that we have no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00067-18.
REFERENCES
- 1.WHO. 2017. World malaria report 2017. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world-malaria-report-2017/en/. [Google Scholar]
- 2.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. 2010. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A 107:6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG. 2013. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. 2016. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 40:343–372. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiendrebeogo RW, Adu B, Singh SK, Dziegiel MH, Nebie I, Sirima SB, Christiansen M, Dodoo D, Theisen M. 2015. Antibody-dependent cellular inhibition is associated with reduced risk against febrile malaria in a longitudinal cohort study involving Ghanaian children. Open Forum Infect Dis 2:ofv044. doi: 10.1093/ofid/ofv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kana IH, Adu B, Tiendrebeogo RW, Singh SK, Dodoo D, Theisen M. 2017. Naturally acquired antibodies target the glutamate-rich protein on intact merozoites and predict protection against febrile malaria. J Infect Dis 215:623–630. doi: 10.1093/infdis/jiw617. [DOI] [PubMed] [Google Scholar]
- 8.Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, McCallum FJ, Reiling L, Jaworowski A, Anders RF, Marsh K, Beeson JG. 2014. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 12:108. doi: 10.1186/1741-7015-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, Cheng YS, Stubbs J, Tetteh KK, Conway DJ, McCarthy JS, Muller I, Marsh K, Anders RF, Beeson JG. 2015. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 42:580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill DL, Eriksson EM, Li Wai Suen CSN, Chiu CY, Ryg-Cornejo V, Robinson LJ, Siba PM, Mueller I, Hansen DS, Schofield L. 2013. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS One 8:e74627. doi: 10.1371/journal.pone.0074627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan VS, Yazdani SS, Gaur D. 2010. Malaria vaccine development based on merozoite surface proteins of Plasmodium falciparum. Hum Vaccin 6:757–762. doi: 10.4161/hv.6.9.12468. [DOI] [PubMed] [Google Scholar]
- 12.Imam M, Singh S, Kaushik NK, Chauhan VS. 2014. Plasmodium falciparum merozoite surface protein 3: oligomerization, self-assembly, and heme complex formation. J Biol Chem 289:3856–3868. doi: 10.1074/jbc.M113.520239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaur D, Mayer DC, Miller LH. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol 34:1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Kushwaha A, Rao PP, Duttu VS, Malhotra P, Chauhan VS. 2000. Expression and characterisation of Plasmodium falciparum acidic basic repeat antigen expressed in Escherichia coli. Mol Biochem Parasitol 106:213–224. doi: 10.1016/S0166-6851(99)00212-1. [DOI] [PubMed] [Google Scholar]
- 15.Mills KE, Pearce JA, Crabb BS, Cowman AF. 2002. Truncation of merozoite surface protein 3 disrupts its trafficking and that of acidic-basic repeat protein to the surface of Plasmodium falciparum merozoites. Mol Microbiol 43:1401–1411. doi: 10.1046/j.1365-2958.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin CS, Uboldi AD, Epp C, Bujard H, Tsuboi T, Czabotar PE, Cowman AF. 2016. Multiple Plasmodium falciparum merozoite surface protein 1 complexes mediate merozoite binding to human erythrocytes. J Biol Chem 291:7703–7715. doi: 10.1074/jbc.M115.698282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McColl DJ, Silva A, Foley M, Kun JF, Favaloro JM, Thompson JK, Marshall VM, Coppel RL, Kemp DJ, Anders RF. 1994. Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol Biochem Parasitol 68:53–67. doi: 10.1016/0166-6851(94)00149-9. [DOI] [PubMed] [Google Scholar]
- 18.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, Filgueira MC, Tartar A, Druilhe P. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594–1602. [PubMed] [Google Scholar]
- 19.Burgess BR, Schuck P, Garboczi DN. 2005. Dissection of merozoite surface protein 3, a representative of a family of Plasmodium falciparum surface proteins, reveals an oligomeric and highly elongated molecule. J Biol Chem 280:37236–37245. doi: 10.1074/jbc.M506753200. [DOI] [PubMed] [Google Scholar]
- 20.McColl DJ, Anders RF. 1997. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3). Mol Biochem Parasitol 90:21–31. doi: 10.1016/S0166-6851(97)00130-8. [DOI] [PubMed] [Google Scholar]
- 21.Huber W, Felger I, Matile H, Lipps HJ, Steiger S, Beck HP. 1997. Limited sequence polymorphism in the Plasmodium falciparum merozoite surface protein 3. Mol Biochem Parasitol 87:231–234. doi: 10.1016/S0166-6851(97)00067-4. [DOI] [PubMed] [Google Scholar]
- 22.Okenu DM, Thomas AW, Conway DJ. 2000. Allelic lineages of the merozoite surface protein 3 gene in Plasmodium reichenowi and Plasmodium falciparum. Mol Biochem Parasitol 109:185–188. doi: 10.1016/S0166-6851(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 23.Gondeau C, Corradin G, Heitz F, Le Peuch C, Balbo A, Schuck P, Kajava AV. 2009. The C-terminal domain of Plasmodium falciparum merozoite surface protein 3 self-assembles into alpha-helical coiled coil tetramer. Mol Biochem Parasitol 165:153–161. doi: 10.1016/j.molbiopara.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Courtin D, Oesterholt M, Huismans H, Kusi K, Milet J, Badaut C, Gaye O, Roeffen W, Remarque EJ, Sauerwein R. 2009. The quantity and quality of African children's IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One 4:e7590. doi: 10.1371/journal.pone.0007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta PK, Mukherjee P, Dhawan S, Pandey AK, Mazumdar S, Gaur D, Jain SK, Chauhan VS. 2014. Production and preclinical evaluation of Plasmodium falciparum MSP-119 and MSP-311 chimeric protein, PfMSP-Fu24. Clin Vaccine Immunol 21:886–897. doi: 10.1128/CVI.00179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imam M, Devi YS, Verma AK, Chauhan VS. 2011. Comparative immunogenicities of full-length Plasmodium falciparum merozoite surface protein 3 and a 24-kilodalton N-terminal fragment. Clin Vaccine Immunol 18:1221–1228. doi: 10.1128/CVI.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, Druilhe P. 2000. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun 68:2617–2620. doi: 10.1128/IAI.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul G, Deshmukh A, Kaur I, Rathore S, Dabral S, Panda A, Singh SK, Mohmmed A, Theisen M, Malhotra P. 2017. A novel Pfs38 protein complex on the surface of Plasmodium falciparum blood-stage merozoites. Malar J 16:79. doi: 10.1186/s12936-017-1716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saul A, Lawrence G, Allworth A, Elliott S, Anderson K, Rzepczyk C, Martin LB, Taylor D, Eisen DP, Irving DO, Pye D, Crewther PE, Hodder AN, Murphy VJ, Anders RF. 2005. A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA720 adjuvant. Vaccine 23:3076–3083. doi: 10.1016/j.vaccine.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Soe S, Mejia JP, Roussilhon C, Theisen M, Corradin G, Druilhe P. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J Infect Dis 190:1010–1018. doi: 10.1086/423208. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Soe S, Weisman S, Barnwell JW, Perignon JL, Druilhe P. 2009. A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4:e5410. doi: 10.1371/journal.pone.0005410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirima SB, Mordmuller B, Milligan P, Ngoa UA, Kironde F, Atuguba F, Tiono AB, Issifou S, Kaddumukasa M, Bangre O, Flach C, Christiansen M, Bang P, Chilengi R, Jepsen S, Kremsner PG, Theisen M, GMZ2 Trial Study Group . 2016. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine 34:4536–4542. doi: 10.1016/j.vaccine.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 33.Sirima SB, Cousens S, Druilhe P. 2011. Protection against malaria by MSP3 candidate vaccine. N Engl J Med 365:1062–1064. doi: 10.1056/NEJMc1100670. [DOI] [PubMed] [Google Scholar]
- 34.Kanodia S, Kumar G, Rizzi L, Pedretti A, Hodder AN, Romeo S, Malhotra P. 2014. Synthetic peptides derived from the C-terminal 6kDa region of Plasmodium falciparum SERA5 inhibit the enzyme activity and malaria parasite development. Biochim Biophys Acta 1840:2765–2775. doi: 10.1016/j.bbagen.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Chan JA, Fowkes FJ, Beeson JG. 2014. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell Mol Life Sci 71:3633–3657. doi: 10.1007/s00018-014-1614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros MM, Fotoran WL, dalla Martha RC, Katsuragawa TH, Pereira da Silva LH, Wunderlich G. 2013. Natural antibody response to Plasmodium falciparum merozoite antigens MSP5, MSP9 and EBA175 is associated to clinical protection in the Brazilian Amazon. BMC Infect Dis 13:608. doi: 10.1186/1471-2334-13-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver KL, Higgins SJ, McDonald CR, Kain KC. 2010. Complement driven innate immune response to malaria: fuelling severe malarial diseases. Cell Microbiol 12:1036–1045. doi: 10.1111/j.1462-5822.2010.01492.x. [DOI] [PubMed] [Google Scholar]
- 38.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG Jr, Plowe CV. 2011. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chugh M, Sundararaman V, Kumar S, Reddy VS, Siddiqui WA, Stuart KD, Malhotra P. 2013. Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum. Proc Natl Acad Sci U S A 110:5392–5397. doi: 10.1073/pnas.1218412110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranjan R, Chugh M, Kumar S, Singh S, Kanodia S, Hossain MJ, Korde R, Grover A, Dhawan S, Chauhan VS, Reddy VS, Mohmmed A, Malhotra P. 2011. Proteome analysis reveals a large merozoite surface protein-1 associated complex on the Plasmodium falciparum merozoite surface. J Proteome Res 10:680–691. doi: 10.1021/pr100875y. [DOI] [PubMed] [Google Scholar]
- 41.Lin CS, Uboldi AD, Marapana D, Czabotar PE, Epp C, Bujard H, Taylor NL, Perugini MA, Hodder AN, Cowman AF. 2014. The merozoite surface protein 1 complex is a platform for binding to human erythrocytes by Plasmodium falciparum. J Biol Chem 289:25655–25669. doi: 10.1074/jbc.M114.586495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kauth CW, Woehlbier U, Kern M, Mekonnen Z, Lutz R, Mücke N, Langowski J, Bujard H. 2006. Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J Biol Chem 281:31517–31527. doi: 10.1074/jbc.M604641200. [DOI] [PubMed] [Google Scholar]
- 43.Kadekoppala M, Holder AA. 2010. Merozoite surface proteins of the malaria parasite: the MSP1 complex and the MSP7 family. Int J Parasitol 40:1155–1161. doi: 10.1016/j.ijpara.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Opi DH, Uyoga S, Orori EN, Williams TN, Rowe JA. 2016. Red blood cell complement receptor one level varies with Knops blood group, α(+)thalassaemia and age among Kenyan children. Genes Immun 17:171–178. doi: 10.1038/gene.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez LE, Curtidor H, Ocampo M, Garcia J, Puentes A, Valbuena J, Vera R, López R, Patarroyo ME. 2005. Identifying Plasmodium falciparum merozoite surface antigen 3 (MSP3) protein peptides that bind specifically to erythrocytes and inhibit merozoite invasion. Protein Sci 14:1778–1786. doi: 10.1110/ps.041304505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theisen M, Vuust J, Gottschau A, Jepsen S, Hogh B. 1995. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin Diagn Lab Immunol 2:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill DL, Wilson DW, Sampaio NG, Eriksson EM, Ryg-Cornejo V, Harrison GLA, Uboldi AD, Robinson LJ, Beeson JG, Siba P, Cowman AF, Hansen DS, Mueller I, Schofield L. 2016. Merozoite antigens of Plasmodium falciparum elicit strain-transcending opsonizing immunity. Infect Immun 84:2175–2184. doi: 10.1128/IAI.00145-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polley SD, Tetteh KK, Lloyd JM, Akpogheneta OJ, Greenwood BM, Bojang KA, Conway DJ. 2007. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J Infect Dis 195:279–287. doi: 10.1086/509806. [DOI] [PubMed] [Google Scholar]
- 49.Mazumdar S, Mukherjee P, Yazdani SS, Jain SK, Mohmmed A, Chauhan VS. 2010. Plasmodium falciparum merozoite surface protein 1 (MSP-1)-MSP-3 chimeric protein: immunogenicity determined with human-compatible adjuvants and induction of protective immune response. Infect Immun 78:872–883. doi: 10.1128/IAI.00427-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachdeva S, Mohmmed A, Dasaradhi PV, Crabb BS, Katyal A, Malhotra P, Chauhan VS. 2006. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-1(42) using human compatible adjuvants. Vaccine 24:2007–2016. doi: 10.1016/j.vaccine.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 51.Trager W, Jensen JB. 2005. Human malaria parasites in continuous culture. 1976. J Parasitol 91:484–486. doi: 10.1645/0022-3395(2005)091[0484:HMPICC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 53.Hill DL, Eriksson EM, Schofield L. 2014. High yield purification of Plasmodium falciparum merozoites for use in opsonizing antibody assays. J Vis Exp 89:e51590. doi: 10.3791/51590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsumoto K, Ejima D, Kumagai I, Arakawa T. 2003. Practical considerations in refolding proteins from inclusion bodies. Protein Expr Purif 28:1–8. doi: 10.1016/S1046-5928(02)00641-1. [DOI] [PubMed] [Google Scholar]
- 55.Eliasson M, Andersson R, Olsson A, Wigzell H, Uhlen M. 1989. Differential IgG-binding characteristics of staphylococcal protein A, streptococcal protein G, and a chimeric protein AG. J Immunol 142:575–581. [PubMed] [Google Scholar]
- 56.Hermanson GT, Mallia AK, Smith PK. 1992. Immobilized affinity ligand techniques. Academic Press, San Diego, CA. [Google Scholar]
- 57.Mathews ST, Plaisance EP, Kim T. 2009. Imaging systems for Westerns: chemiluminescence vs. infrared detection. Methods Mol Biol 536:499–513. doi: 10.1007/978-1-59745-542-8_51. [DOI] [PubMed] [Google Scholar]
- 58.Wittig I, Braun HP, Schagger H. 2006. Blue native PAGE. Nat Protoc 1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 59.Mattson DL, Bellehumeur TG. 1996. Comparison of three chemiluminescent horseradish peroxidase substrates for immunoblotting. Anal Biochem 240:306–308. doi: 10.1006/abio.1996.0364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplemental material).