Abstract
The bone marrow microenvironment harbors and protects leukemic cells from apoptosis-inducing agents via mechanisms that are incompletely understood. We previously showed SDF-1 (CXCL-12), a chemokine readily abundant within the bone marrow microenvironment, induces apoptosis in acute myeloid leukemia (AML) cells that express high levels of the SDF-1 receptor CXCR4. However, differentiating osteoblasts, found within this niche, protect co-cultured acute myeloid leukemia (AML) cells from apoptosis. Additionally, this protection was abrogated upon treatment of the differentiating osteoblasts with histone deacetylase inhibitors (HDACi). Here, we begin to characterize and target the molecular mechanisms that mediate this osteoblast protection. Quantitative RT-PCR revealed that HDACi treatment of differentiating osteoblasts (mouse MC3T3 osteoblast cell line) reduced expression of multiple genes required for osteoblast differentiation, including genes important for producing mineralized bone matrix. Interestingly, pretreating differentiating osteoblasts with cyclosporine A (CSA), a drug known to inhibit osteoblast differentiation, similarly impaired osteoblast-mediated protection of co-cultured AML cells (KG1a and U937 human AML cell lines). Both HDACi and CSA reduced osteoblast expression of the key mineralization enzyme tissue non-specific alkaline phosphatase (TNAP, Alpl). Moreover, specifically reducing TNAP expression or activity in differentiating osteoblasts significantly impaired the ability of the osteoblasts to protect co-cultured AML cells. Together, our results indicate that inhibiting osteoblast matrix mineralization by specifically targeting TNAP is sufficient to significantly impair osteoblast-mediated protection of AML cells. Therefore, designing combination therapies that additionally target the osteoblast-produced mineralized bone matrix may improve treatment of AML by reducing the protection of leukemic cells within the bone marrow microenvironment.
Keywords: AML, osteoblast, HDACi, cyclosporine, TNAP
INTRODUCTION
In recent years, the protective regions of the bone marrow microenvironment, including the endosteal niche, have come to the forefront as targets to enhance the elimination of acute myeloid leukemia (AML) cells. While many patients typically achieve remission with standard chemotherapeutic treatments, the subsequent relapse experienced by most patients is likely due to those AML cells that survive the treatments within the endosteal niche, the area where the ossified bone and bone marrow interact (1–5). The osteoblast lineage cells that reside in this niche promote the survival of various cell types including leukemic cells (6–11); however, the molecular mechanisms that mediate osteoblast protection of AML cells are incompletely characterized. Therefore, further characterization of these osteoblast protective mechanisms is critical to identifying treatments that will target osteoblast protection and thus permit killing of AML cells within the endosteal niche.
We previously demonstrated that differentiating osteoblasts, but not earlier precursors of the osteoblast lineage, have the ability to protect AML cells in vitro from apoptosis-inducing agents (12, 13). Interestingly, SDF-1, a chemokine abundantly expressed by multiple cells within the bone marrow, induces apoptosis of AML cells expressing high levels of its receptor, CXCR4 (14). Others further confirmed the apoptosis-stimulating properties of CXCR4 in AML cells by showing that a fully humanized anti-CXCR4 antibody directly induces apoptosis in AML by interacting with and subsequently inducing signaling via CXCR4 (15). Nonetheless, AML cells survive in the bone marrow, suggesting that the endosteal niche provides protection from SDF-1-induced apoptosis. Utilizing co-culture systems, we showed that differentiating osteoblasts protect AML cells from SDF-1-induced apoptosis (12). In addition, we showed that this protective effect is disrupted by pretreating the osteoblasts with histone deacetylase inhibitors (HDACi) (12, 16). HDACi are a class of drugs currently being evaluated in numerous malignancies, including AML, that exert their effects by inhibiting deacetylation of histones as well as other proteins to keep chromatin in a more open state, thus causing alterations in gene expression in both malignant cells and the osteoblast-lineage cells of the bone marrow microenvironment (17–27). Indeed, HDACi-mediated gene alterations inhibit differentiation of immature osteoblasts (26, 27).
Another drug capable of inhibiting differentiation of osteoblast-lineage cells of the bone marrow microenvironment is Cyclosporine A (CSA). CSA has been utilized to prevent the rejection of organ transplants; however, one of the side effects associated with this medication is osteoporosis (28, 29). CSA has been shown to alter osteoblast differentiation and affect bone matrix synthesis (30–32). Interestingly, CSA has been shown to reduce activity of the key bone matrix mineralization enzyme tissue non-specific alkaline phosphatase (TNAP, Alpl) (30–32). TNAP has been shown to play an essential role in bone mineralization in the skeletal disorder hypophosphatemia (33). TNAP cleaves inorganic pyrophosphate into inorganic phosphate, which combines with calcium and other ions to form hydroxyapatite, the hardened component of bone (34–36). This mineralized matrix then serves as a repository for growth factors, which have been shown to promote the survival and proliferation of metastatic prostate or breast cancer cells that localize to the bone marrow (37).
Here, we demonstrate that TNAP expression is critical for osteoblast-mediated protection of AML cells in vitro. We show that either HDACi or CSA treatment of differentiating osteoblasts reduces the expression of TNAP and inhibits the protective properties of differentiating osteoblasts. Therefore, targeting osteoblast differentiation, particularly matrix mineralization, may be an effective strategy to more completely eliminate AML cells from the bone marrow microenvironment and prevent relapse of disease.
MATERIALS AND METHODS
Materials
Ascorbic acid, β-glycerophosphate, dimethylsulfoxide (DMSO), AMD3100, SDF-1, and the protease inhibitor cocktail, were acquired from Sigma (St. Louis, MO, USA). SDF-1 was obtained from R&D Systems (Minneapolis, MN, USA). APC-conjugated annexin-V was purchased from BD Biosciences (San Jose, CA, USA). Suberoylanilide hydroxamic acid (SAHA, vorinostat) was procured from the Cancer Therapy Evaluation Program, National Cancer Institute (Bethesda, MD, USA). LBH-589 (panobinostat) was obtained from Selleckchem (Houston, TX, USA). CSA and TNAP inhibitor MLS-0038949 were purchased from Millipore (Burlington, MA, USA). Live/dead viability assays were obtained from Invitrogen (Waltham, MA, USA). The ON-TARGET Plus Control siRNA pool was purchased from GE Healthcare (Dharmacon) (Lafayette, CO, USA). The Alpl Silencer Select siRNA (ID: s62206) was purchased from Thermo Fisher Scientific (Ambion) (Waltham, MA, USA). Alpl (TNAP) mouse reactive polyclonal goat IgG antibody (AF2910) was purchased from R&D Systems (Minneapolis, MN, USA), and ERK 2 rabbit polyclonal IgG antibody (sc-154) was purchased from Santa Cruz (Dallas, Texas, USA).
Cells
The KG1a and U937 human AML cell lines (ATCC, Manassas, VA, USA) were maintained as previously described (12). KG1a-CXCR4 and U937-CXCR4 AML cells were generated by transfecting a plasmid encoding CXCR4-YFP fluorescent fusion protein (38) into KG1a or U937 cells as described (14). MC3T3 sc4 murine calvarial osteoblasts (ATCC, Manassas, VA, USA) are a robust and well characterized osteoblast model that were cultured in minimum MC3T3 maintenance medium (α-MEM without ascorbic acid (Invitrogen, Carlsbad, VA, USA), 10% FCS (volume/volume), and 1% penicillin/streptomycin (volume/volume)) (39). For use in assays, MC3T3 cells were plated in 12-well plates. Upon reaching confluence, MC3T3 cells were treated (defined as Day 0) with osteogenic differentiation medium (α-MEM, 10% FCS (volume/volume), 1% penicillin/streptomycin (volume/volume), 50 μg/ml ascorbic acid, and 4 mM β-glycerophosphate).
Co-cultures, HDACi Treatment, CSA Treatment, siRNA Treatment, TNAP Inhibitor Treatment, and Apoptosis Assay
On Day −1, MC3T3 cells were plated in 12 well plates in maintenance medium. Where indicated, prior to plating, MC3T3 cells were transfected with 0.8 nanomoles of either control or Alpl (TNAP) siRNA via electroporation as described (16)). On Day 0, osteogenic differentiation medium was added to MC3T3 cells (+/− either 0.1% DMSO, 0.025 mg/ml CSA, or 10 μM TNAP inhibitor (MLS-0038949) as indicated). The CSA dose was selected to inhibit TNAP activity and mineralization of MC3T3 cells (31). The TNAP inhibitor dose used inhibits TNAP activity (40). On Day 1, either vehicle (0.1% DMSO), 10 μM SAHA, or 1 μM LBH-589 was added where indicated. Because of the short half-life of SAHA (41, 42), the 10 μM SAHA dose was selected to ensure persistent histone H3-acetylation (a marker of SAHA activity) within SAHA-treated MC3T3 cells for the duration of the 30 hour pretreatment course (13, 16, 25). The 1 μM LBH-589 dose showed persistent histone H3-acetylation in LBH-589-treated MC3T3 cells for the duration of the 30 hour pretreatment course as well (13, 16). On Day 2, the cells were rinsed with PBS, fresh medium consisting of RPMI and 10% FCS (volume/volume) was added to the cells, and 1 x 106 KG1a-CXCR4 AML cells per well were added to the differentiating MC3T3 cell cultures. Where indicated, some of these KG1a-CXCR4 AML cells were pretreated with 30 μM AMD3100 to inhibit SDF-1/CXCR4 signaling. After one hour of co-culture, cells in selected wells were challenged with 1.3 × 10−8 M SDF-1. On Day 3, the apoptosis of KG1a-CXCR4 AML cells was assayed via use of APC-conjugated annexin-V staining and flow cytometry to detect surface phosphatidylserine.
Live/dead cell viability assay
The live/dead cell assays were imaged with a LSM780 laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) with ZEN software (Carl Zeiss), an EC PlnN 10x/0.3 DICI objective, a total magnification of 100x, and excitation/emission wavelengths of 561 nm/626 nm (dead; red) and 488 nm/522 nm (live; green). The percentage of dead (red) cells was calculated by dividing the total number of dead (red) cells by an average total number of live (green) cells plus the dead (red) cells.
qRT-PCR for osteoblast differentiation markers
On Day 0, MC3T3 osteoblasts were treated with osteogenic differentiation medium. On Day 1, the MC3T3 cells were treated with HDACi (10 μM SAHA or 1 μM LBH-589) or vehicle control (0.1% DMSO) and harvested on Day 2 for qRT-PCR. The RNeasy Plus Kit (Qiagen, Hilden, Germany) was used to isolate RNA, and Superscript III First-strand Synthesis System was used to reverse transcribe the RNA into cDNA (Invitrogen, Carlsbad, CA). The qRT-PCR reactions to measure gene expression utilized 25 ng of cDNA per 20 μl with QuantiTect SYBR Green PCR Kit (Qiagen, Hilden, Germany) and the CFX384 Real-time System (Bio-Rad, Hercules, CA, USA). The housekeeping gene Gapdh was used to normalize transcript levels. The 2ΔΔCt method was used to quantify gene expression levels. Osteogenic genes assayed were Runx2, Sp7, Satb2, Ogn, Omd, Sparc, Ibsp, Bglap, Alpl (TNAP), and Col1a1. Reference genes used were Hprt, Akt1, Atf1 and Sp1. Ezh1 and Hist2h4 were used as controls to show that not all genes are decreased in expression upon HDACi treatment. The qRT-PCR primer sets used are described in Table I.
Table I.
qRT-PCR Primers.
| Gene ID | Forward Primer | Reverse Primer |
|---|---|---|
| Runx2 | CCTGAACTCTGCACCAAGTCCT | TCATCTGGCTCAGATAGGAGGG |
| Sp7 | GGCTTTTCTGCGGCAAGAGGTT | CGCTGATGTTTGCTCAAGTGGTC |
| Satb2 | CAAGAGTGGCATTCAACCGCAC | TCCACTTCAGGCAGGTTGAGGA |
| Ogn | CTCGTTACATTCGGGAGCGA | GCTGCACTGATGGGGTTAGA |
| Omd | TGCACATTCAGCAACTCAACC | TGCAGTCACAGCCTCAATGT |
| Sparc | CCCCTCAGCAGACTGAAGTT | ACAGGTACCCCTGTCTCCTC |
| Ibsp | GAATGGCCTGTGCTTTCTCG | CCGGTACTTAAAGACCCCGTT |
| Bglap | GCAATAAGGTAGTGAACAGACTCC | CCATAGATGCGTTTGTAGGCGG |
| Alpl | CCAGAAAGACACCTTGACTGTGG | TCTTGTCCGTGTCGCTCACCAT |
| Col1a1 | CCTCAGGGTATTGCTGGACAAC | CAGAAGGACCTTGTTTGCCAGG |
| Atf1 | AACCTCATGGGTTCTCCAGCGA | CTCCAACATCCAATCTGTCCCG |
| Sp1 | CTCCAGACCATTAACCTCAGTGC | CACCACCAGATCCATGAAGACC |
| Hprt1 | CTGGTGAAAAGGACCTCTCGAAG | CCAGTTTCACTAATGACACAAACG |
| Ezh1 | CGAGTCTTCCACGGCACCTATT | GCTCATCTGTTGGCAGCTTTAGG |
| Akt1 | CACACGTCAAGCGACCCATGAA | TCTTCTCGCTCTCGTTCAGCAG |
| Hist2h4 | AAGGTTCTCCGCGACAACATCC | GTCGCGGATCACATTCTCAAGG |
| Gapdh | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
Statistical analysis methods
Two-tailed t-tests were used for statistical analysis (Microsoft Excel) with the means of the two distributions being considered significantly different if p<0.05, unless otherwise specified. For qRT-PCR, transcripts of the indicated genes were normalized to Gapdh (set to 100) and the means + standard deviation for expression of each gene are shown for three independent experiments.
RESULTS
HDACi inhibit expression of osteogenic genes in MC3T3 cells
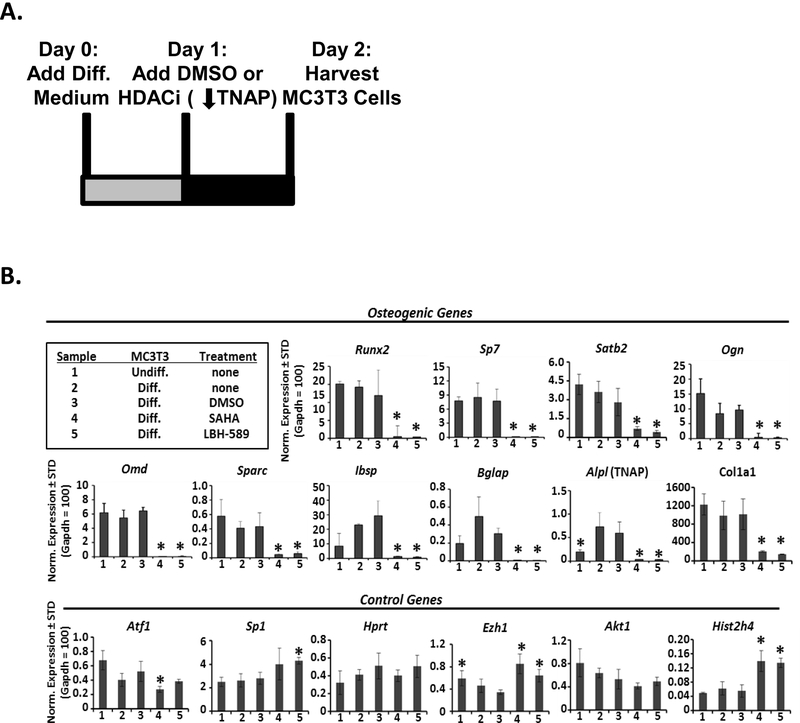
We previously demonstrated that HDACi treatment during the early stages of MC3T3 osteoblast differentiation inhibits the osteoblast-mediated protection of co-cultured AML cells from SDF-1-induced apoptosis (16). Additionally, we showed that HDACi treatment of primary bone marrow-derived mesenchymal stromal/stem cells (BMSC) during the early stages of osteoblast differentiation led to downregulation of Col1a1, osteocalcin, and TNAP, which are key osteogenic genes (26). We therefore hypothesized that downregulation of one or more of these osteoblast-specific genes required for differentiation might also inhibit osteoblast-mediated protection of AML cells. We began by determining which osteogenic genes displayed altered expression at the time when the AML cells were added to the co-cultures with the osteoblasts. The MC3T3 osteoblast cells were treated with osteogenic differentiation medium on Day 0, treated with either HDACi (SAHA or LBH-589) or vehicle on Day 1, and then cells were harvested on Day 2 for qRT-PCR (Fig. 1A). The two day time point was selected because this was the point in differentiation that robust protection of AML cells from SDF-1 began to be observed (12). As expected, compared to undifferentiated MC3T3 cells, Day 2 differentiating MC3T3 cells began to show increases in osteoblast differentiation genes including Alpl, Bglap, and Ibsp (Fig. 1B). In contrast, both SAHA- and LBH-589-treated differentiating MC3T3 cells displayed significantly decreased expression of these and other genes required for osteoblast differentiation: Runx2, Sp7, Satb2, Ogn, Omd, Sparc, Ibsp, Bglap, Alpl (TNAP), and Col1a1 (Fig. 1B). Nonetheless, the expression levels of several reference genes, including Hprt and Akt1 were not significantly altered by HDACi treatment, while less than two-fold differences were seen in the expression levels of reference genes Atf1 and Sp1 (Fig. 1B). In contrast to the osteogenic genes, Ezh1 and Hist2h4 were up-regulated with the addition of SAHA and LBH-589, demonstrating that all genes are not decreased upon HDACi treatment (Fig. 1B). These results identify several key osteoblast differentiation genes that are downregulated by HDACi, including Alpl (which encodes TNAP).
FIGURE 1. HDACi inhibit expression of osteogenic genes in MC3T3 cells.
(A) Depiction of the timeline describing the MC3T3 osteoblast cell line culture for qRT-PCR. On Day 0, MC3T3 osteoblasts were treated with osteogenic differentiation medium. On Day 1, the MC3T3 cells were treated with HDACi (10 μM SAHA or 1 μM LBH-589) or vehicle control (0.1% DMSO) and harvested on Day 2 for qRT-PCR. (B) qRT-PCR results were normalized to Gapdh, which was set to 100. Each bar represents mean+standard deviation for three independent experiments with * representing statistical significance (p<0.05) from DMSO treated cells.
CSA reduces MC3T3 osteoblast-mediated protection of KG1a-CXCR4 and U937-CXCR4 AML cells from SDF-1-induced apoptosis
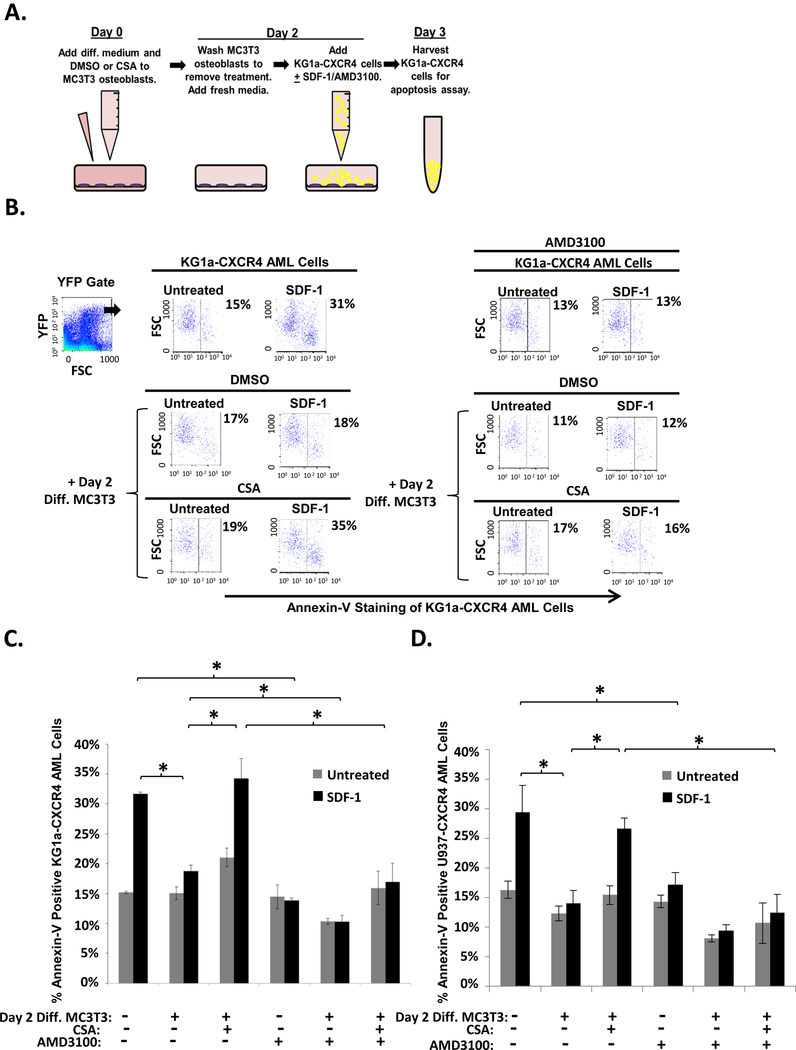
We next asked if other agents that inhibit MC3T3 osteoblast differentiation could also block the protective properties of the differentiating osteoblasts similar to HDACi treatment. It was previously shown that high doses of CSA inhibit both TNAP activity and matrix mineralization in differentiating MC3T3 osteoblasts (30–32). To assess whether CSA could inhibit osteoblast protection, we utilized our previously defined co-culture model that combines MC3T3 osteoblasts with the KG1a AML cell line transiently transfected with CXCR4-YFP (KG1a-CXCR4 cells). KG1a-CXCR4 cells mimic primary AML patient isolates that express cell-surface CXCR4 and respond to SDF-1 by undergoing apoptosis (14). Additionally, the YFP tag permits unambiguous identification of the KG1a-CXCR4 AML cells following co-culture. As shown in Fig. 2A, on Day 0, confluent MC3T3 cells received both differentiation medium and either CSA or vehicle. On Day 2, the MC3T3 cells were washed with PBS to remove the CSA and then KG1a-CXCR4 AML cells were added to the differentiating MC3T3 cell cultures and challenged with SDF-1; where indicated, SDF-1/CXCR4 antagonist AMD3100 was also added to cultures as a control (Fig. 2A). On Day 3, the apoptosis of KG1a-CXCR4 AML cells was assessed via annexin-V staining and flow cytometry. As expected, SDF-1 induced apoptosis of KG1a-CXCR4 cells, but co-culturing KG1a-CXCR4 AML cells together with differentiating MC3T3 cells protected the AML cells from SDF-1-induced apoptosis (Fig. 2B-C). Interestingly, CSA pretreatment of the differentiating MC3T3 osteoblasts significantly inhibited their ability to protect KG1a-CXCR4 AML cells from SDF-1-induced apoptosis (Fig. 2B-C). To confirm that CSA was not directly inducing apoptosis of the AML cells, we utilized AMD3100, a small molecule inhibitor of SDF-1/CXCR4 interactions that prevents SDF-1-induced apoptosis of AML cells (14). As shown in Fig. 2B-C, AMD3100 significantly inhibited SDF-1-induced apoptosis of KG1a-CXCR4 AML cells in both the presence and absence of differentiating osteoblasts as well as in the presence and absence of CSA-pretreated osteoblasts (Fig. 2B-C). Thus, CSA does not directly induce apoptosis of AML cells. These experiments were repeated as in Fig. 2A with a second human AML cell line, U937 (Fig. 2D). CSA pretreatment of the differentiating MC3T3 osteoblasts significantly inhibited their ability to protect U937-CXCR4 AML cells from SDF-1-induced apoptosis. Similar to KG1a-CXCR4 cells, AMD3100 significantly inhibited SDF-1-induced apoptosis of U937-CXCR4 AML cells even in the presence of CSA-pretreated osteoblasts (Fig. 2D). Together, the results in Fig. 2 demonstrate that pretreatment of differentiating osteoblasts with CSA, a known inhibitor of osteoblast differentiation, significantly inhibits the osteoblasts’ ability to protect AML cells from SDF-1-induced apoptosis (Fig. 2B-C).
FIGURE 2. CSA reduces MC3T3 osteoblast-mediated protection of KG1a-CXCR4 and U937-CXCR4 AML cells from SDF-1-induced apoptosis.
(A) Depiction of MC3T3 osteoblast and KG1a-CXCR4 AML cell line co-culture model. On Day 0, MC3T3 osteoblasts were treated with osteogenic differentiation media and either 0.1% DMSO or 0.025 mg/mL CSA. On Day 2, the osteoblasts were washed to remove the DMSO or CSA pretreatment and were then co-cultured with KG1a-CXCR4 AML cells. The indicated wells were treated with 30 μM AMD3100 1 hr prior to 1.3 × 10−8 M SDF-1 challenge. On Day 3, the KG1a-CXCR4 AML cells were harvested and assayed for apoptosis utilizing annexin-V staining and flow cytometry. (B) A representative experiment as performed in (A) shows the percentage of annexin-V positive KG1a-CXCR4 cells from each culture within the YFP gate indicated (gating on cells with high levels of CXCR4 expression as the CXCR4 is YFP-tagged). (C) Summary of multiple experiments performed as in (A). Bars depict mean results + S.E.M., n=3; *, indicates p<0.05. (D) Summary of multiple experiments performed as in (A) except with U937-CXCR4 AML cells instead of KG1a-CXCR4 AML cells. Bars depict mean results + S.E.M., n=3; *, indicates p<0.05.
CSA pretreatment of early differentiating MC3T3 osteoblasts reduces TNAP expression
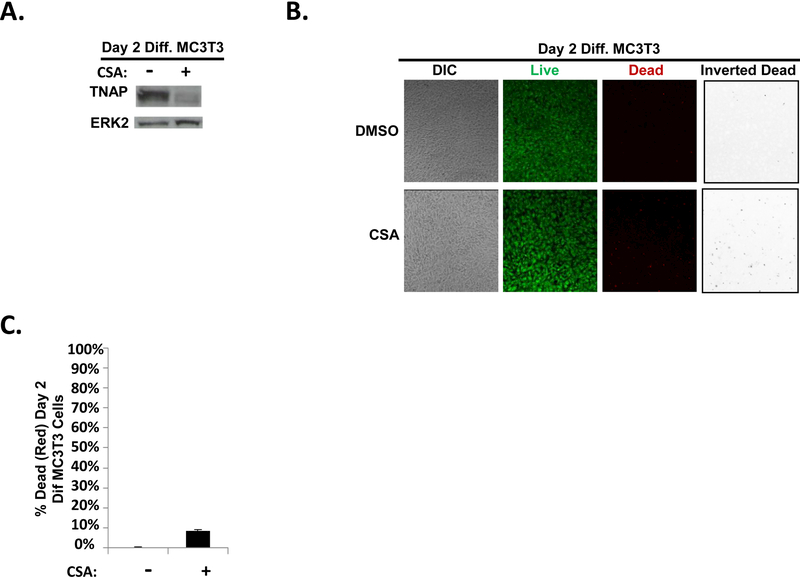
To characterize the molecular mechanism of this response, we explored the role of TNAP. TNAP is an osteoblast-produced alkaline phosphatase enzyme encoded by the Alpl gene and is required for calcification of the bone matrix (34–36). CSA treatment of osteoblasts has been shown to inhibit TNAP activity (31). Furthermore, in Fig. 1b,we showed that Alpl mRNA levels were decreased by HDACi treatment of differentiating MC3T3 osteoblasts coincident, as was previously shown, with the loss of the ability of these osteoblasts to protect AML cells from apoptosis (16). We therefore hypothesized that TNAP expression is also decreased by CSA treatment of differentiating MC3T3 osteoblasts. Indeed, CSA treatment of differentiating MC3T3 cells resulted in decreased expression of TNAP protein as compared to vehicle control (Fig. 3A). To confirm that CSA treatment simply did not kill the majority of the MC3T3 cells, live/dead assays were conducted to assess MC3T3 viability after CSA treatment. These results showed that while there was a slight increase in dead (red) MC3T3 cells with CSA treatment (~8.5% dead cells) as compared to vehicle control treatment (~0.3%), the majority of CSA-treated MC3T3 cells in these experiments still formed a confluent monolayer of predominantly live (green) cells (Fig. 3B-C). Together, the results in Fig. 2&3 indicate that CSA treatment of differentiating MC3T3 osteoblasts significantly reduces both TNAP expression as well as the ability of the differentiating MC3T3 osteoblasts to protect co-cultured AML cells from SDF-1-induced apoptosis.
FIGURE 3. CSA pretreatment of early differentiating MC3T3 osteoblasts reduces TNAP expression.
(A) MC3T3 cells were treated as in Fig. 2A except whole cell lysates were harvested on Day 2 (without the addition of KG1a-CXCR4 AML cells, AMD3100, or SDF-1). Immunoblot depicting the effect of CSA on TNAP expression in MC3T3 cells. The same membrane was stripped and re-probed for total ERK2 as a control, n=3. (B) MC3T3 cells were treated as in Fig. 2a except live/dead staining and imaging occurred on Day 2 (without the addition of KG1a-CXCR4 AML cells, AMD3100, or SDF-1). Live/dead staining and confocal imaging of live (green) and dead (red) cells were used to ensure MC3T3 cell viability in the presence of CSA. Images were acquired on three separate days for a total of 15 images analyzed for each condition. (C) Statistical summary of (B). Bars depict mean results + S.E.M., n=3.
Depletion of TNAP via siRNA reduces MC3T3 osteoblast-mediated protection of KG1a-CXCR4 and U937-CXCR4 AML cells from SDF-1-induced apoptosis
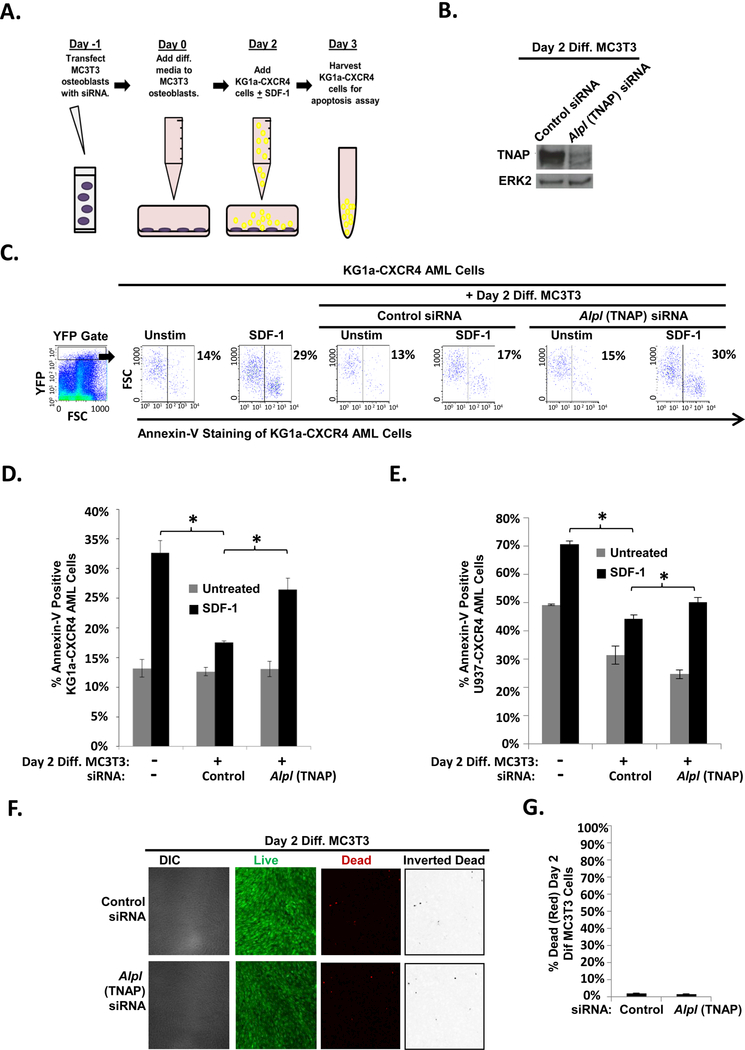
Either HDACi or CSA treatment of differentiating MC3T3 cells decreased expression of TNAP and inhibited osteoblast-mediated protection of AML cells. Therefore, we next determined if TNAP expression is required for osteoblasts to mediate protection of co-cultured AML cells. MC3T3 cells were transfected with either control or Alpl (TNAP) siRNA on Day −1 (Fig. 4A). Subsequently, differentiation was initiated on Day 0. On Day 2, KG1a-CXCR4 cells were added +/− SDF-1 and then on Day 3, the KG1a-CXCR4 cells were harvested and assayed for apoptosis (Fig 4A). Alpl (TNAP) siRNA inhibited upregulation of TNAP in Day 2 differentiating MC3T3 cells (Fig. 4B). Interestingly, transfection of differentiating MC3T3 cells with Alpl (TNAP) siRNA significantly inhibited their ability to protect KG1a-CXCR4 AML cells from SDF-1-induced apoptosis compared to control siRNA-transfected MC3T3 cells (Fig. 4C-D). These experiments were repeated as in Fig. 4A with a second human AML cell line, U937 (Fig. 4E). Alpl (TNAP) siRNA transfection of the differentiating MC3T3 osteoblasts significantly inhibited their ability to protect U937-CXCR4 AML cells from SDF-1-induced apoptosis (Fig. 4E). To confirm that transfection with Alpl (TNAP) siRNA simply did not kill the majority of the MC3T3 cells, live/dead assays were conducted to assess MC3T3 viability. These results showed that dead (red) MC3T3 cell levels were similar between transfection with control siRNA (~1.7%) and Alpl siRNA (~1.5%), and there was a confluent monolayer of predominantly live (green) cells (Fig. 4F-G). Together, these results demonstrate that reduction of TNAP expression reduces MC3T3 osteoblast-mediated protection of AML from SDF-1 induced apoptosis.
FIGURE 4. Depletion of TNAP via siRNA reduces MC3T3 osteoblast-mediated protection of KG1a-CXCR4 and U937-CXCR4 AML cells from SDF-1-induced apoptosis.
(A) Depiction of MC3T3 osteoblast and KG1a-CXCR4 AML cell line co-culture model. On Day −1, MC3T3 osteoblasts were electroporated with 0.8 nanomoles of control or Alpl (TNAP) siRNA. The maintenance medium was changed 6 hours later. On Day 0, MC3T3 osteoblasts were treated with osteogenic differentiation media. On Day 2, the osteoblasts were washed and then co-cultured with KG1a-CXCR4 AML cells. The indicated samples were challenged with 1.3 × 10−8 M SDF-1. On Day 3, the KG1a-CXCR4 AML cells were harvested and assayed for apoptosis utilizing annexin-V staining and flow cytometry. (B) MC3T3 cells were treated as in (A) except whole cell lysates were harvested on Day 2 (without the addition of KG1a-CXCR4 AML cells or SDF-1). Immunoblot of TNAP expression in control siRNA- and TNAP-siRNA transfected MC3T3 cells. The same membrane was stripped and re-probed for total ERK2 as a control, n=3. (C) A representative experiment as performed in (A) shows the percentage of annexin-V positive KG1a-CXCR4 cells from each culture within the YFP gate indicated (gating on cells with high levels of CXCR4 expression as the CXCR4 is YFP tagged). (D) Summary of multiple experiments performed as in (A). Bars depict mean results + S.E.M., n=3; *, indicates p<0.05. (E) Summary of multiple experiments performed as in (A) except with U937-CXCR4 AML cells instead of KG1a-CXCR4 AML cells. Bars depict mean results + S.E.M., n=4; *, indicates p<0.05. (F) MC3T3 cells were treated as in Fig. 4a except live/dead staining and imaging occurred on Day 2 (without the addition of KG1a-CXCR4 AML cells, AMD3100, or SDF-1). Live/dead staining and confocal imaging of live (green) and dead (red) cells were used to ensure MC3T3 cell viability in the presence of Alpl (TNAP) siRNA. Images were acquired on three separate days for a total of 15 images analyzed for each condition. (G) Statistical summary of (F). Bars depict mean results + S.E.M., n=3.
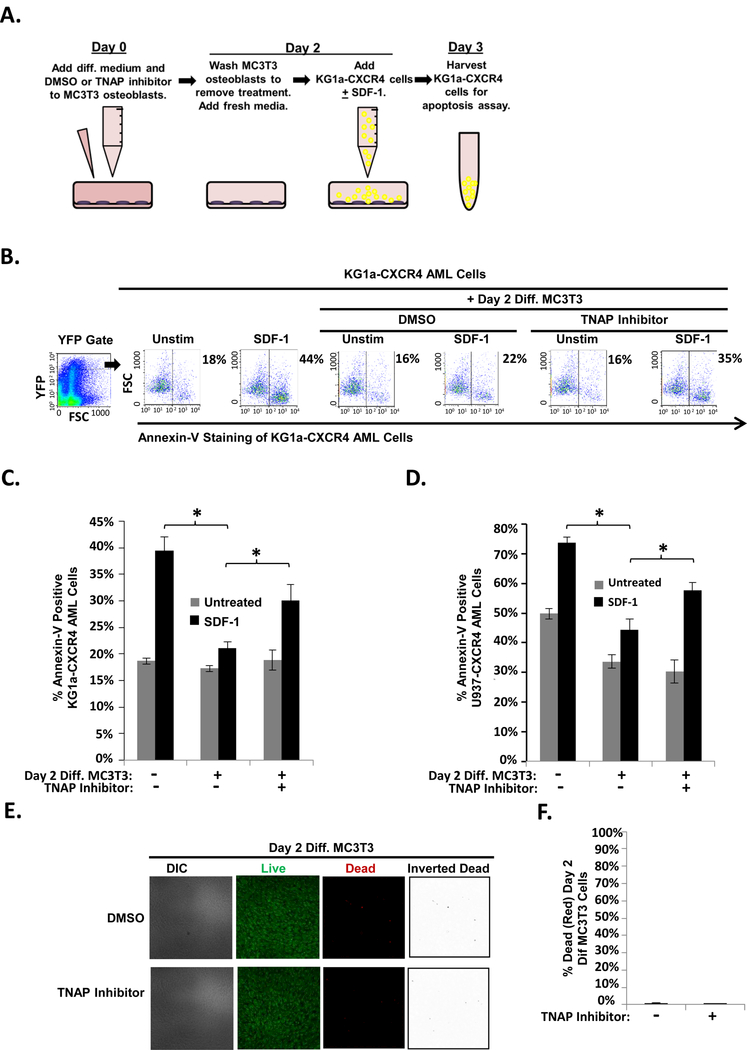
TNAP inhibition reduces MC3T3 osteoblast-mediated protection of KG1a-CXCR4 and U937-CXCR4 AML cells from SDF-1-induced apoptosis
To further confirm that TNAP was necessary for MC3T3 osteoblast-mediated protection of AML cells from SDF-1 induced apoptosis, MC3T3 osteoblasts were pretreated with a TNAP inhibitor. TNAP inhibitor MLS-0038949 specifically inhibits TNAP activity (40). As shown in Fig. 5A, on Day 0 confluent MC3T3 cells received both differentiation medium and either TNAP inhibitor or vehicle. On Day 2, the MC3T3 cells were washed with PBS to remove the TNAP inhibitor and then KG1a-CXCR4 AML cells were added to the differentiating MC3T3 cell cultures and challenged with SDF-1; (Fig. 5A). On Day 3, the apoptosis of KG1a-CXCR4 AML cells was assessed via annexin-V staining and flow cytometry. The results show that pretreating differentiating osteoblasts with the TNAP inhibitor significantly impaired their ability to protect KG1a-CXCR4 AML cells from SDF-1-induced apoptosis (Fig. 5B-C). Similar results were obtained with U937-CXCR4 cells (Fig. 5D). To confirm that pretreatment of differentiating osteoblasts with the TNAP inhibitor simply did not kill the majority of the MC3T3 cells, live/dead assays were conducted to assess MC3T3 viability. These results showed that dead (red) MC3T3 cell levels were similar between vehicle treatment (~0.7%) and TNAP inhibitor treatment (~0.7%), and there was a confluent monolayer of predominantly live (green) cells (Fig. 5E-F). Together, the results in this paper indicate that both HDACi and CSA effectively downregulate TNAP expression and that decreased expression or enzymatic activity of TNAP in differentiating osteoblasts is sufficient to reduce osteoblast-mediated protection of AML cells (Fig. 6).
FIGURE 5. TNAP inhibition reduces MC3T3 osteoblast-mediated protection of KG1a-CXCR4 and U937-CXCR4 AML cells from SDF-1-induced apoptosis.
(A) Depiction of MC3T3 osteoblast and KG1a-CXCR4 AML cell line co-culture model. On Day 0, MC3T3 osteoblasts were treated with osteogenic differentiation media and either 0.1% DMSO or 10μM TNAP inhibitor MLS-0038949. On Day 2, the osteoblasts were washed to remove the DMSO or TNAP inhibitor pretreatment and were then co-cultured with KG1a-CXCR4 AML cells. The indicated wells were challenged with 1.3 × 10−8 M SDF-1. On Day 3, the KG1a-CXCR4 AML cells were harvested and assayed for apoptosis utilizing annexin-V staining and flow cytometry. (B) A representative experiment as performed in (A) shows the percentage of annexin-V positive KG1a-CXCR4 cells from each culture within the YFP gate indicated (gating on cells with high levels of CXCR4 expression as the CXCR4 is YFP tagged). (C) Summary of multiple experiments performed as in (A). Bars depict mean results + S.E.M., n=3; *, indicates p<0.05. (D) Summary of multiple experiments performed as in (A) except with U937-CXCR4 AML cells instead of KG1a-CXCR4 AML cells. Bars depict mean results + S.E.M., n=3; *, indicates p<0.05. (E) MC3T3 cells were treated as in Fig. 4a except live/dead staining and imaging occurred on Day 2 (without the addition of KG1a-CXCR4 AML cells, AMD3100, or SDF-1). Live/dead staining and confocal imaging of live (green) and dead (red) cells were used to ensure MC3T3 cell viability in the presence of TNAP inhibitor. Images were acquired on three separate days for a total of 15 images analyzed for each condition. (F) Statistical summary of (F). Bars depict mean results + S.E.M., n=3.
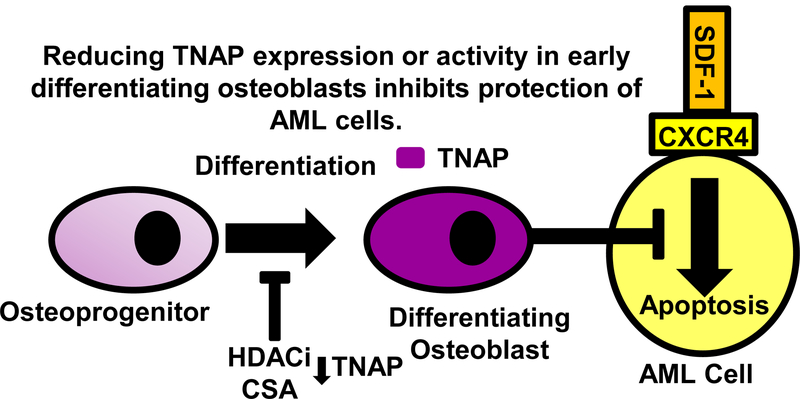
FIGURE 6. Reduction of TNAP expression or activity in early differentiating osteoblasts inhibits protection of AML cells.
Differentiating osteoblasts protect AML cells from SDF-1 induced apoptosis. HDACi and CSA inhibit osteoblast differentiation and TNAP expression as well as osteoblast-mediated protection of AML cells. Reducing TNAP expression or activity reduces osteoblast-mediated protection of AML cells.
DISCUSSION
The endosteal niche within the protective bone marrow microenvironment provides a safe harbor for AML cells during traditional chemotherapy (1, 2, 4, 5); however, the molecular mechanisms provided by this niche are incompletely understood. We previously showed that a chemokine found within the endosteal niche, SDF-1, induces apoptosis of AML cells that express high levels of its receptor, CXCR4 (12). However, differentiating osteoblasts protected AML cells from SDF-1-induced apoptosis, thus likely permitting the AML cells to thrive in the bone marrow (12, 14). Interestingly, HDACi treatment of the differentiating osteoblasts was sufficient to inhibit osteoblast-mediated protection of AML cells from SDF-1 (16). Therefore, characterizing the mechanisms behind HDACi inhibition of osteoblast-mediated protection could lead to the ability to design more targeted combination therapies for AML.
To identify HDACi-mediated mechanisms that regulate osteoblast protection, we first performed mRNA expression analysis via qRT-PCR, which demonstrated that expression of several key differentiation genes was inhibited by HDACi treatment of differentiating osteoblasts, including Alpl, which encodes TNAP. These results combined with our previous findings that osteoblast precursors as well as HDACi-treated osteoblasts fail to protect AML cells suggested that osteoblast differentiation is necessary for protection of AML cells (12, 16). Similarly, pretreatment of differentiating osteoblasts with a different inhibitor of osteoblast differentiation, CSA, also inhibited the ability of osteoblasts to protect AML cells. Since, like HDACi, CSA reduced the expression of TNAP in differentiating MC3T3 osteoblasts, we specifically reduced the expression of TNAP in differentiating osteoblasts via siRNA and demonstrated that downregulation of TNAP was sufficient to inhibit their protection of AML cells from SDF-1. In addition, TNAP inhibitor treatment of differentiating MC3T3 osteoblasts reduced protection of AML cells from SDF-1. Together, our results suggest that manipulating osteoblast differentiation, specifically TNAP-mediated matrix mineralization, could inhibit the protective properties of differentiating osteoblasts within the endosteal niche of the bone marrow.
The ability of HDACi and CSA to inhibit both osteoblast differentiation and osteoblast-mediated protection of AML cells suggests that osteoblast differentiation is necessary to protect AML cells from apoptosis-inducing agents. This result is consistent with the clinical observations indicating that an increase in osteoblast-secreted factors correlates with both worse prognosis and the development of chemoresistance in AML (43, 44). Interestingly, mesenchymal stromal cells from AML patients are primed for osteogenic differentiation and express higher levels of TNAP compared to those of healthy controls (45). Furthermore, leukemic induction of osteogenic differentiation of these mesenchymal stromal cells promoted AML cell growth (45). Our results are also consistent with the previous observation that HDACi-mediated gene alterations inhibit differentiation of immature osteoblasts (26, 27). Supporting the role of HDAC involvement in bone development, HDAC3-deficient endochondral bone exhibits reduced expression of extracellular matrix production, bone development, and ossification genes (46). HDACi also directly alter gene expression in malignancies, which makes them attractive options to target malignancies (17–23). However, while HDACi treatment does not eradicate CXCR4 expression in AML cells, it can decrease CXCR4 expression levels, which theoretically may partially impair SDF-1/CXCR4 induced apoptosis of AML cells (47). Thus, we explored alternative strategies to target TNAP expression/activity with drugs that work via different mechanisms of action, including CSA or a TNAP inhibitor. Interestingly, changes in mineralization are observed in malignancy within the bone (48). Sclerostin is a negative regulator of Wnt signaling and bone formation, is produced by osteocytes and malignancies within the bone, and can inhibit osteoblast mineralization and the expression of osteoblast-specific proteins (48). It is unknown whether sclerostin contributes as an attempted protective counter-measure or actively contributes to disease pathogenesis (48).
To our knowledge, this is the first report demonstrating that the key matrix mineralization enzyme TNAP is required for osteoblast-mediated protection of AML cells. Osteoblasts form the mineralized matrix that serves as a reservoir for growth factors that are secreted by many cell types within the bone marrow and that are released upon bone resorption by osteoclasts (37). Malignant cells can influence the behavior of osteoblasts and osteoclasts, and in turn the growth factors of the mineralized matrix influence the behavior of the malignant cells (37). A “vicious cycle” between osteoblasts, osteoclasts, and malignant cells can aid in tumor cell survival and proliferation (37). Decreasing the expression of TNAP could alter matrix structure and thereby disrupt accumulation of key growth factor(s) required for the survival of AML cells. Numerous growth factors have already been found in the mineralized bone matrix, including Insulin-like growth factor-1 and 2 (IGF-1 or IGF-2) and TGF-β, which have been reported to support AML cell survival (37, 49, 50). Because inhibiting TNAP reduces mineralization, prolonged TNAP inhibition could cause osteopenia. As such, TNAP inhibition would need to be used at strategic time points in treatment such as with induction therapy to maximize the effects on the bone marrow microenvironment during the peak time of malignancy eradication (51). In addition, TNAP inhibition (be it through HDACi, CSA, or potentially new TNAP inhibitor treatment) could be combined with bisphosphonate treatment to prevent osteoclast resorption of bone and further halt the “vicious cycle” (51).
Our results reported here add to the accumulating evidence indicating the complex roles of SDF-1 and its receptor CXCR4 in regulating the leukemic bone marrow microenvironment. The SDF-1/CXCR4 axis has been shown to impact multiple pathways of leukemic and other cells of the bone marrow that regulate cell growth, adhesion, migration, gene expression, and survival (52). Using an in vitro approach, we previously established that SDF-1/CXCR4 signaling in AML cells potently induces an apoptosis signaling pathway via decreased expression of Bcl-XL, upregulation of Noxa, and upregulation of Bak (14). In addition, we found that conditions within the bone marrow microenvironment such as hypoxia and differentiating osteoblasts protect AML cells from SDF-1/CXCR4-induced apoptosis (12, 14). Others subsequently confirmed the apoptosis-stimulating properties of CXCR4 in AML cells, for example, showing that a fully humanized anti-CXCR4 antibody directly induces apoptosis in hematological malignancies, including AML, by interacting with and subsequently inducing signaling via CXCR4 (15, 53). In addition to causing apoptosis in AML cells, CXCR4 signaling has been shown to be capable of inducing apoptosis in T cells, colorectal carcinoma cells, and several other cell types (54–60). In contrast, other studies have revealed a protective effect of SDF-1/CXCR4 interactions on human myeloid tumor cells and have additionally shown that CXCR4 antagonists can impair leukemic cell survival and promote differentiation (47, 61–64). Moreover, CXCR4 antagonists have been shown to mobilize AML cells from the bone marrow (65–69). Nevertheless, CXCR4 antagonists in disease models are not as effective as hoped, possibly due to the leukemic stem cells resisting mobilization (65–69), and/or the inhibition of SDF-1/CXCR4 - stimulated apoptosis signaling in these leukemic cells (14, 15, 53). Our results reported here suggest that therapies that include target the protective osteoblast-derived mechanisms of the bone marrow microenvironment such as TNAP may be an effective strategy during treatment of AML.
In summary, our results shown here demonstrate that inhibiting osteoblast differentiation and specifically matrix mineralization via targeting TNAP could transform the bone marrow microenvironment into a less hospitable environment for AML cells. Additionally, TNAP may also play a role in promoting the survival of metastatic breast or prostate cancer cells that localize to the bone marrow (37). Therefore, combination therapies that involve the manipulation of the osteoblast mineralized matrix could be more efficacious than standard therapies alone in preventing relapse.
Acknowledgments
The Joanne G. and Gary N. Owen Fund in Immunology Research, the Alma B. Stevenson Endowment Fund for Medical Research, the Mayo Clinic Center for Biomedical Discovery, and NIH R21CA194217 (K.E.H.) supported this work. The Mayo Clinic Medical Scientist Training Program Robert L. Howell Physician-Scientist Scholarship supported R.M.S. NIH F32 AR066508 supported A.D. NIH R01AR049069 supported A.J.v.W.
ABBREVIATIONS
- AML
acute myeloid leukemia
- HDACi
histone deacetylase inhibitors
- SAHA
suberoylanilide hydroxamic acid, vorinostat
- LBH589
panobinostat
- TNAP
protein name for tissue nonspecific alkaline phosphatase
- Alpl
gene name for tissue nonspecific alkaline phosphatase
- BMSC
bone marrow-derived mesenchymal stromal/stem cells
Footnotes
Conflict of Interest: We have no conflicts of interest to disclose
REFERENCES
- 1.Estey EH. 2013. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol 88: 318-327. [DOI] [PubMed] [Google Scholar]
- 2.Burnett A, Wetzler M, and Lowenberg B. 2011. Therapeutic advances in acute myeloid leukemia. J Clin Oncol 29: 487-494. [DOI] [PubMed] [Google Scholar]
- 3.Marin JJ, Briz O, Rodriguez-Macias G, Diez-Martin JL, and Macias RI. 2016. Role of drug transport and metabolism in the chemoresistance of acute myeloid leukemia. Blood Rev 30: 55-64. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya M, Abe A, Katsumi A, Xu J, Ito M, Arai F, Suda T, Kiyoi H, Kinoshita T, and Naoe T. 2007. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia 21: 136-142. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, Fukata M, Miyamoto T, Lyons B, Ohshima K, Uchida N, Taniguchi S, Ohara O, Akashi K, Harada M, and Shultz LD. 2007. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25: 1315-1321. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto S, Mihara K, Downing JR, Pui CH, and Campana D. 2007. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 117: 1049-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levesque JP, Helwani FM, and Winkler IG. 2010. The endosteal 'osteoblastic' niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 24: 1979-1992. [DOI] [PubMed] [Google Scholar]
- 8.Ehninger A, and Trumpp A. 2011. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med 208: 421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dazzi F, Ramasamy R, Glennie S, Jones SP, and Roberts I. 2006. The role of mesenchymal stem cells in haemopoiesis. Blood Rev 20: 161-171. [DOI] [PubMed] [Google Scholar]
- 10.Konopleva M, Tabe Y, Zeng Z, and Andreeff M. 2009. Therapeutic targeting of microenvironmental interactions in leukemia: mechanisms and approaches. Drug Resist Updat 12: 103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macanas-Pirard P, Broekhuizen R, Gonzalez A, Oyanadel C, Ernst D, Garcia P, Montecinos VP, Court F, Ocqueteau M, Ramirez P, and Nervi B. 2017. Resistance of leukemia cells to cytarabine chemotherapy is mediated by bone marrow stroma, involves cell-surface equilibrative nucleoside transporter-1 removal and correlates with patient outcome. Oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer KN, Dudakovic A, McGee-Lawrence ME, Philips RL, Hess AD, Smith BD, van Wijnen AJ, Karp JE, Kaufmann SH, Westendorf JJ, and Hedin KE. 2014. Osteoblasts protect AML cells from SDF-1-induced apoptosis. J Cell Biochem 115: 1128-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterner RM KK, Al-Kali A, Patnaik MM, Gangat N, Litzow MR, Kaufmann SH, Westendorf JJ, van Wijnen AJ, Hedin KE. 2017. Histone deacetylase inhibitors reduce differentiating osteoblast-mediated protection of acute myeloid leukemia cells from cytarabine. Oncotarget 8: 94569-94579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer KN, Peterson KL, Schneider PA, Meng XW, Dai H, Hess AD, Smith BD, Rodriguez-Ramirez C, Karp JE, Kaufmann SH, and Hedin KE. 2013. CXCR4 chemokine receptor signaling induces apoptosis in acute myeloid leukemia cells via regulation of the Bcl-2 family members Bcl-XL, Noxa, and Bak. J Biol Chem 288: 22899-22914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, Cohen LJ, Korman AJ, and Cardarelli PM. 2013. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res 19: 357-366. [DOI] [PubMed] [Google Scholar]
- 16.Kremer KN, Dudakovic A, Hess AD, Smith BD, Karp JE, Kaufmann SH, Westendorf JJ, van Wijnen AJ, and Hedin KE. 2015. Histone Deacetylase Inhibitors Target the Leukemic Microenvironment by Enhancing a Nherf1-Protein Phosphatase 1alpha-TAZ Signaling Pathway in Osteoblasts. J Biol Chem 290: 29478-29492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Manero G, Tambaro FP, Bekele NB, Yang H, Ravandi F, Jabbour E, Borthakur G, Kadia TM, Konopleva MY, Faderl S, Cortes JE, Brandt M, Hu Y, McCue D, Newsome WM, Pierce SR, de Lima M, and Kantarjian HM. 2012. Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome. J Clin Oncol 30: 2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, Loboda A, Fantin VR, Randolph SS, Hardwick JS, Reilly JF, Chen C, Ricker JL, Secrist JP, Richon VM, Frankel SR, and Kantarjian HM. 2008. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 111: 1060-1066. [DOI] [PubMed] [Google Scholar]
- 19.Gojo I, Tan M, Fang HB, Sadowska M, Lapidus R, Baer MR, Carrier F, Beumer JH, Anyang BN, Srivastava RK, Espinoza-Delgado I, and Ross DD. 2013. Translational phase I trial of vorinostat (suberoylanilide hydroxamic acid) combined with cytarabine and etoposide in patients with relapsed, refractory, or high-risk acute myeloid leukemia. Clin Cancer Res 19: 1838-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschbaum M, Gojo I, Goldberg SL, Bredeson C, Kujawski LA, Yang A, Marks P, Frankel P, Sun X, Tosolini A, Eid JE, Lubiniecki GM, and Issa JP. 2014. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol 167: 185-193. [DOI] [PubMed] [Google Scholar]
- 21.Prebet T, and Vey N. 2011. Vorinostat in acute myeloid leukemia and myelodysplastic syndromes. Expert Opin Investig Drugs 20: 287-295. [DOI] [PubMed] [Google Scholar]
- 22.Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, Masson E, Rae P, Laird G, Sharma S, Kantarjian H, Dugan M, Albitar M, and Bhalla K. 2006. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 12: 4628-4635. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, Newsome WM, Miller WH, Jr., Rousseau C, Kalita A, Bonfils C, Dubay M, Patterson TA, Li Z, Besterman JM, Reid G, Laille E, Martell RE, and Minden M. 2008. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood 112: 981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudakovic A, Camilleri ET, Lewallen EA, McGee-Lawrence ME, Riester SM, Kakar S, Montecino M, Stein GS, Ryoo HM, Dietz AB, Westendorf JJ, and van Wijnen AJ. 2015. Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. J Cell Physiol 230: 52-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudakovic A, Evans JM, Li Y, Middha S, McGee-Lawrence ME, van Wijnen AJ, and Westendorf JJ. 2013. Histone deacetylase inhibition promotes osteoblast maturation by altering the histone H4 epigenome and reduces Akt phosphorylation. J Biol Chem 288: 28783-28791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGee-Lawrence ME, McCleary-Wheeler AL, Secreto FJ, Razidlo DF, Zhang M, Stensgard BA, Li X, Stein GS, Lian JB, and Westendorf JJ. 2011. Suberoylanilide hydroxamic acid (SAHA; vorinostat) causes bone loss by inhibiting immature osteoblasts. Bone 48: 1117-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder TM, Nair AK, Staggs R, Lamblin AF, and Westendorf JJ. 2007. Gene profile analysis of osteoblast genes differentially regulated by histone deacetylase inhibitors. BMC Genomics 8: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamler R, and Epstein S. 2006. Nonsteroid immune modulators and bone disease. Ann N Y Acad Sci 1068: 284-296. [DOI] [PubMed] [Google Scholar]
- 29.Sass DA, Bowman AR, Yuan Z, Ma Y, Jee WS, and Epstein S. 1997. Alendronate prevents cyclosporin A-induced osteopenia in the rat. Bone 21: 65-70. [DOI] [PubMed] [Google Scholar]
- 30.Fornoni A, Cornacchia F, Howard GA, Roos BA, Striker GE, and Striker LJ. 2001. Cyclosporin A affects extracellular matrix synthesis and degradation by mouse MC3T3-E1 osteoblasts in vitro. Nephrol Dial Transplant 16: 500-505. [DOI] [PubMed] [Google Scholar]
- 31.Yeo H, Beck LH, McDonald JM, and Zayzafoon M. 2007. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone 40: 1502-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira RO, Thiago LS, Oliveira FL, Balduino A, Borojevic R, Duarte ME, and Farias ML. 2009. Cyclosporine A, but not tacrolimus, is associated with impaired proliferation and differentiation of human osteoblast-like cells in vitro. Med Sci Monit 15: BR65-70. [PubMed] [Google Scholar]
- 33.Mori M, DeArmey SL, Weber TJ, and Kishnani PS. 2016. Case series: Odontohypophosphatasia or missed diagnosis of childhood/adult-onset hypophosphatasia? - Call for a long-term follow-up of premature loss of primary teeth. Bone Rep 5: 228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narisawa S, Frohlander N, and Millan JL. 1997. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn 208: 432-446. [DOI] [PubMed] [Google Scholar]
- 35.Terkeltaub RA. 2001. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol 281: C1-C11. [DOI] [PubMed] [Google Scholar]
- 36.Johnson K, Goding J, Van Etten D, Sali A, Hu SI, Farley D, Krug H, Hessle L, Millan JL, and Terkeltaub R. 2003. Linked deficiencies in extracellular PP(i) and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. J Bone Miner Res 18: 994-1004. [DOI] [PubMed] [Google Scholar]
- 37.Kingsley LA, Fournier PG, Chirgwin JM, and Guise TA. 2007. Molecular biology of bone metastasis. Mol Cancer Ther 6: 2609-2617. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, and Hedin KE. 2006. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity 25: 213-224. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, and Franceschi RT. 1999. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res 14: 893-903. [DOI] [PubMed] [Google Scholar]
- 40.Dahl R, Sergienko EA, Su Y, Mostofi YS, Yang L, Simao AM, Narisawa S, Brown B, Mangravita-Novo A, Vicchiarelli M, Smith LH, O'Neill WC, Millan JL, and Cosford ND. 2009. Discovery and validation of a series of aryl sulfonamides as selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). J Med Chem 52: 6919-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, Curly T, Moskowitz C, Portlock C, Horwitz S, Zelenetz AD, Frankel S, Richon V, Marks P, and Kelly WK. 2006. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol 24: 166-173. [DOI] [PubMed] [Google Scholar]
- 42.Konsoula R, and Jung M. 2008. In vitro plasma stability, permeability and solubility of mercaptoacetamide histone deacetylase inhibitors. Int J Pharm 361: 19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liersch R, Gerss J, Schliemann C, Bayer M, Schwoppe C, Biermann C, Appelmann I, Kessler T, Lowenberg B, Buchner T, Hiddemann W, Muller-Tidow C, Berdel WE, and Mesters R. 2012. Osteopontin is a prognostic factor for survival of acute myeloid leukemia patients. Blood 119: 5215-5220. [DOI] [PubMed] [Google Scholar]
- 44.De Toni F, Racaud-Sultan C, Chicanne G, Mas VM, Cariven C, Mesange F, Salles JP, Demur C, Allouche M, Payrastre B, Manenti S, and Ysebaert L. 2006. A crosstalk between the Wnt and the adhesion-dependent signaling pathways governs the chemosensitivity of acute myeloid leukemia. Oncogene 25: 3113-3122. [DOI] [PubMed] [Google Scholar]
- 45.Battula VL, Le PM, Sun JC, Nguyen K, Yuan B, Zhou X, Sonnylal S, McQueen T, Ruvolo V, Michel KA, Ling X, Jacamo R, Shpall E, Wang Z, Rao A, Al-Atrash G, Konopleva M, Davis RE, Harrington MA, Cahill CW, Bueso-Ramos C, and Andreeff M. 2017. AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpio LR, Bradley EW, McGee-Lawrence ME, Weivoda MM, Poston DD, Dudakovic A, Xu M, Tchkonia T, Kirkland JL, van Wijnen AJ, Oursler MJ, and Westendorf JJ. 2016. Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling. Sci Signal 9: ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandawat A, Fiskus W, Buckley KM, Robbins K, Rao R, Balusu R, Navenot JM, Wang ZX, Ustun C, Chong DG, Atadja P, Fujii N, Peiper SC, and Bhalla K. 2010. Pan-histone deacetylase inhibitor panobinostat depletes CXCR4 levels and signaling and exerts synergistic antimyeloid activity in combination with CXCR4 antagonists. Blood 116: 5306-5315. [DOI] [PubMed] [Google Scholar]
- 48.Weivoda MM, Youssef SJ, and Oursler MJ. 2017. Sclerostin expression and functions beyond the osteocyte. Bone 96: 45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doepfner KT, Spertini O, and Arcaro A. 2007. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia 21: 1921-1930. [DOI] [PubMed] [Google Scholar]
- 50.Tabe Y, Shi YX, Zeng Z, Jin L, Shikami M, Hatanaka Y, Miida T, Hsu FJ, Andreeff M, and Konopleva M. 2013. TGF-beta-Neutralizing Antibody 1D11 Enhances Cytarabine-Induced Apoptosis in AML Cells in the Bone Marrow Microenvironment . PLoS One 8: e62785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drake MT, Clarke BL, and Khosla S. 2008. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83: 1032-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, and Ratajczak MZ. 2004. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol 35: 233-245. [DOI] [PubMed] [Google Scholar]
- 53.Kashyap MK, Kumar D, Jones H, Amaya-Chanaga CI, Choi MY, Melo-Cardenas J, Ale-Ali A, Kuhne MR, Sabbatini P, Cohen LJ, Shelat SG, Rassenti LZ, Kipps TJ, Cardarelli PM, and Castro JE. 2016. Ulocuplumab (BMS-936564 / MDX1338): a fully human anti-CXCR4 antibody induces cell death in chronic lymphocytic leukemia mediated through a reactive oxygen species-dependent pathway. Oncotarget 7: 2809-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colamussi ML, Secchiero P, Gonelli A, Marchisio M, Zauli G, and Capitani S. 2001. Stromal derived factor-1 alpha (SDF-1 alpha) induces CD4+ T cell apoptosis via the functional up-regulation of the Fas (CD95)/Fas ligand (CD95L) pathway. J Leukoc Biol 69: 263-270. [PubMed] [Google Scholar]
- 55.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien WA, and Verdin E. 1998. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature 395: 189-194. [DOI] [PubMed] [Google Scholar]
- 56.Drury LJ, Wendt MK, and Dwinell MB. 2010. CXCL12 chemokine expression and secretion regulates colorectal carcinoma cell anoikis through Bim-mediated intrinsic apoptosis. PLoS One 5: e12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castedo M, Perfettini JL, Andreau K, Roumier T, Piacentini M, and Kroemer G. 2003. Mitochondrial apoptosis induced by the HIV-1 envelope. Ann N Y Acad Sci 1010: 19-28. [DOI] [PubMed] [Google Scholar]
- 58.Ullrich CK, Groopman JE, and Ganju RK. 2000. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood 96: 1438-1442. [PubMed] [Google Scholar]
- 59.Endo M, Inatsu A, Hashimoto K, Takamune N, Shoji S, and Misumi S. 2008. Human immunodeficiency virus-induced apoptosis of human breast cancer cells via CXCR4 is mediated by the viral envelope protein but does not require CD4. Curr HIV Res 6: 34-42. [DOI] [PubMed] [Google Scholar]
- 60.Lusso P. 2006. HIV and the chemokine system: 10 years later. EMBO J 25: 447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abe-Suzuki S, Kurata M, Abe S, Onishi I, Kirimura S, Nashimoto M, Murayama T, Hidaka M, and Kitagawa M. 2014. CXCL12+ stromal cells as bone marrow niche for CD34+ hematopoietic cells and their association with disease progression in myelodysplastic syndromes. Lab Invest 94: 1212-1223. [DOI] [PubMed] [Google Scholar]
- 62.Abraham M, Klein S, Bulvik B, Wald H, Weiss ID, Olam D, Weiss L, Beider K, Eizenberg O, Wald O, Galun E, Avigdor A, Benjamini O, Nagler A, Pereg Y, Tavor S, and Peled A. 2017. The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16–1 expression. Leukemia 31: 2336-2346. [DOI] [PubMed] [Google Scholar]
- 63.Tavor S, Eisenbach M, Jacob-Hirsch J, Golan T, Petit I, Benzion K, Kay S, Baron S, Amariglio N, Deutsch V, Naparstek E, and Rechavi G. 2008. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia 22: 2151-5158. [DOI] [PubMed] [Google Scholar]
- 64.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, and Calandra G. 2005. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 106: 1867-1874. [DOI] [PubMed] [Google Scholar]
- 65.Clements D, Markwick LJ, Puri N, and Johnson SR. 2010. Role of the CXCR4/CXCL12 axis in lymphangioleiomyomatosis and angiomyolipoma. J Immunol 185: 1812-1821. [DOI] [PubMed] [Google Scholar]
- 66.Monaco G, Konopleva M, Munsell M, Leysath C, Wang RY, Jackson CE, Korbling M, Estey E, Belmont J, and Andreeff M. 2004. Engraftment of acute myeloid leukemia in NOD/SCID mice is independent of CXCR4 and predicts poor patient survival. Stem Cells 22: 188-201. [DOI] [PubMed] [Google Scholar]
- 67.Stolzel F, Wermke M, Rollig C, Thiede C, Platzbecker U, and Bornhauser M. 2010. Mobilization of PML/RARalpha negative peripheral blood stem cells with a combination of G-CSF and CXCR4 blockade in relapsed acute promyelocytic leukemia pre-treated with arsenic trioxide. Haematologica 95: 171-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, Konoplev S, Andreeff M, and Konopleva M. 2009. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood 113: 6215-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, Shemtov N, Deutsch V, Naparstek E, Nagler A, and Lapidot T. 2004. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res 64: 2817-2824.</References> [DOI] [PubMed] [Google Scholar]