Merkel cell carcinoma is a rare and aggressive neuroendocrine skin cancer, with a rising incidence. This article evaluates the feasibility of using cabozantinib in patients with advanced Merkel cell carcinoma whose disease failed platinum‐based therapy.
Keywords: Merkel cell carcinoma, Cabozantinib, Platinum‐failure, Correlative studies
Abstract
Background.
This study sought to determine the efficacy and safety profile of cabozantinib in patients with advanced Merkel cell carcinoma (MCC).
Experimental Design.
This prospective, phase II, single‐institution trial enrolled patients with platinum‐failure, recurrent/metastatic MCC to receive cabozantinib 60 mg orally daily until disease progression, withdrawal from study, or severe toxicity. The primary endpoint was disease control rate. Secondary endpoints included overall survival (OS), progression‐free survival (PFS), and toxicity. Immunohistochemistry for VEGFR‐2, MET, and HGF expression and next‐generation sequencing of tumor tissue were performed and correlated with outcome.
Results.
Eight patients were accrued from January 24, 2014, to June 8, 2016. The study was closed prematurely because of toxicity and lack of responses. The most frequent adverse events were grades 1 and 2 and included anorexia, fatigue, nausea, hypothyroidism, and dysgeusia. Two patients developed nonhealing, painful ulcers and tumor‐skin fistula. One patient had stable disease for 8 months. One patient withdrew from the study after 2 weeks of therapy because of adverse events. Three patients required dose reduction because of toxicity. Median PFS and OS were 2.1 and 11.2 months, respectively. No expression of MET, HGF, or VEGFR‐2 was identified in tumor cells by immunohistochemistry of patients’ tissue samples.
Conclusion.
Cabozantinib was poorly tolerated and did not demonstrate activity in patients with recurrent/metastatic, platinum‐failure MCC. It is unclear whether preselection of patients with the specific upregulation or genetic alteration in the targets for cabozantinib would have changed the results of this study. (Clinical trial identification number: NCT02036476)
Implications for Practice.
This phase II study demonstrated poor tolerability and lack of activity of cabozantinib in an unselected group of patients with advanced Merkel cell carcinoma. Although it is unclear whether preselection of patients with the specific upregulation and genetic alterations in targets for cabozantinib would have changed the results of this study, this would have likely led to an extremely rare patient population that would take many years to accrue.
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin cancer with a rising incidence [1], [2]. Although fewer than 10% of the patients present with advanced disease at diagnosis, approximately half of those with locoregional disease die of recurrence or metastasis [1]. A single‐institution, retrospective study of 62 patients with metastatic disease reported a response rate of 55% among patients who received first‐line chemotherapy and 23% among those after second‐line chemotherapy, with a median progression‐free survival (PFS) of 94 days and 61 days, respectively, and a median overall survival (OS) of only 9.5 months [3]. Given activity seen in other high‐grade neuroendocrine tumors and the lack of prospective randomized trials comparing different chemotherapy regimens in MCC, a platinum and etoposide combination has been historically used as first‐line therapy and a doxorubicin‐based regimen as second‐line therapy in patients with metastatic disease. Despite the relatively high response rates, the toxic death rate with these regimens has been described as 3.4% for this patient population [4].
Most recently, the anti‐programmed cell death ligand 1 (anti‐PD‐L1) avelumab received U.S. Food and Drug Administration (FDA) approval for patients with advanced MCC after a phase II study demonstrated a response rate of 31.8% in 88 patients with stage IV MCC who had failed one or more lines of systemic therapy [5]. On extended follow‐up, the response rate increased to 33%, including 10 complete and 19 partial responses, with the median duration of response not yet reached [6]. Another phase II trial evaluating the anti‐programmed cell death‐1 (anti‐PD‐1) pembrolizumab in 26 patients with metastatic MCC demonstrated a response rate of 56% and a median PFS of 9 months [7]. Despite these positive results, many patients do not benefit from or are not candidates for immunotherapy, leaving them with less than optimal systemic therapy options.
Sennino et al have demonstrated a synergistic effect with dual vascular endothelial growth factor receptor 2 (VEGFR‐2; KDR) and mesenchymal‐epithelial transition factor (c‐MET) inhibition, preventing tumor invasion and metastasis in pancreatic neuroendocrine tumors [8]. These targets are important mediators of tumor growth and/or angiogenesis, with c‐MET particularly associated with tumor invasiveness and metastasis [9]. c‐MET is upregulated by PAX5, a transcription factor that has been shown to be overexpressed in MCC [10]. Furthermore, our group has detected expression of c‐MET in MCC samples by RNA sequencing [11]. VEGFR‐2 expression has been described in intratumoral vessels of MCC, and its expression has been associated with increased tumor volume and metastatic potential [12].
Cabozantinib (XL184, Exelixis, South San Francisco, CA, http://www.exelixis.com) is an oral small molecule, multiple tyrosine kinase inhibitor, approved by the FDA in 2012 for the treatment of patients with progressive metastatic medullary thyroid cancer and in 2016 for patients with advanced renal cell carcinoma who have received prior antiangiogenic therapy [13], [14], [15]. Cabozantinib inhibits several receptor tyrosine kinases, including, but not limited to, c‐MET and VEGFR‐2 [14].
Given the preclinical data on the effects of c‐MET and VEGFR‐2 inhibition in neuroendocrine tumors, we sought to evaluate the feasibility of using cabozantinib in patients with advanced MCC whose disease failed platinum‐based therapy.
Materials and Methods
Study Population and Treatment
This was a prospective, investigator‐initiated, single‐arm, open‐label, phase II trial conducted at the Dana‐Farber Cancer Institute (ClinicalTrials.gov identification number: NCT02036476). Eligibility criteria included measurable, histologically or cytologically confirmed metastatic or regionally recurrent MCC; age ≥18 years; Eastern Cooperative Oncology Group performance status (PS) ≤1; and normal organ and marrow function. Patients must have progressed through platinum‐based therapy. Any number of prior chemotherapy or radiation therapy regimens was allowed, with the exception of a prior c‐MET inhibitor, and as long as at least 2 weeks had passed since cessation of prior treatment. Patients were excluded if they had evidence of active brain metastasis; tumor invading the gastrointestinal tract, trachea, or bronchus; evidence of cavitary lesions, nonhealing wounds, or active (or a risk of) bleeding; or use of therapeutic doses of anticoagulants. This study was approved by the Dana‐Farber/Harvard Cancer Center Institutional Review Board, and each patient signed informed consent prior to initiation screening procedures and study treatment.
Treatment Regimen and Toxicity Assessment
Cabozantinib was administered as an oral daily dose of 60 mg until progression of disease or significant toxicity. Dose reductions to 40 mg and then 20 mg were allowed for significant toxicities, and treatment was discontinued if patients were unable to tolerate 20 mg dosing. One cycle was defined as 28 days regardless of treatment delays.
History, physical examination, and routine laboratory studies, including complete blood count, chemistry panel, liver and thyroid function tests, and prothrombin time/partial thromboplastin time tests were performed at baseline and every 2 weeks. Electrocardiograms were performed every 4 weeks. Positron emission tomography and/or computed tomography scans were performed at baseline, 6 weeks, and 12 weeks from treatment initiation and then every 9 weeks until disease progression or withdrawal from study.
Toxicity Monitoring.
Toxicities were graded using the National Cancer Institute Common Toxicity Criteria scale, version 4.0, and assessed at the biweekly clinic visits. If a subject developed an unacceptable toxicity determined to be related to the study treatment, and if supportive care measures and/or a dose reduction failed to lessen the toxicity to acceptable levels, study treatment was withheld. Unacceptable toxicities included intolerable grade 2 toxicities (except alopecia); rash of any grade that could not be adequately managed with supportive care and/or a dose reduction; any grade 3 or 4 toxicity that, despite optimal management, posed a significant clinical risk (including nausea, vomiting, diarrhea, hypertension); urine protein‐creatinine ratio >2; grade 4 thrombocytopenia or anemia; grade 4 neutropenia of >5 days duration; grade ≥3 neutropenia of any duration with fever (>38.5°C); or documented infection.
Tumor Analysis
Patient Tissue Samples.
Archival formalin‐fixed paraffin‐embedded specimens were obtained from all patients. Hematoxylin and eosin stained sections confirmed presence of tumor in a total of eight samples obtained from seven patients. Six of these samples were recurrent cutaneous lesions, and two were metastases (one to lymph node and one to liver). For one patient, two samples of cutaneous recurrences were available, including one obtained prior to cabozantinib treatment and one obtained during cabozantinib treatment.
Hybrid capture sequencing of more than 300 cancer genes was performed using the OncoPanel platform on archived tumor tissues after patients’ consent. OncoPanel surveyed exonic DNA sequences of over 300 cancer genes to identify single nucleotide variants and insertion deletion events as well as copy number variations, in addition to 113 introns from 35 genes to detect rearrangements [16], [17], [18].
Immunohistochemistry.
All samples with confirmed tumor (n = 8) were stained for c‐MET (SP44, 1:100, Spring Biosciences, Pleasanton, CA), phosphorylated MET (p‐MET; D26, 1:50, Cell Signaling Technology, Danvers, MA), HGF (D6S7D, 1:50, Cell Signaling Technology, Danvers, MA), and VEGFR‐2 (D5B1, 1:100, Cell Signaling Technology, Danvers, MA) by incubating sections with primary antibody dilutions for 30 (c‐MET and p‐MET) to 60 (HGF and VEGFR‐2) minutes at room temperature. For signal detection, a polymer reagent and DAB chromagen were employed (Bond Polymer Refine Detection System, Leica Biosystems, Wetzlar, Germany). All appropriate positive and negative controls were reviewed.
Statistical Analysis
The primary objective of this trial was to determine the activity of cabozantinib in patients with advanced MCC who had progressed after platinum‐based therapy. Cabozantinib would be considered an active drug if there were evidence that the true disease control rate (DCR) was at least 40%. DCR was defined as achievement of confirmed objective response (complete or partial response) or stable disease that lasted at least 3 months from the baseline disease evaluation. We planned to enroll 12 treated patients in this trial. If three or more patients showed disease control, cabozantinib would be considered an active drug for this patient population. With this design, using the exact binomial, the probability of concluding the proposed treatment as promising was 0.92 if the true but unknown DCR was 40% but 0.11 if the true DCR was 10%. Overall response rate was also of interest in this trial, but the true response rate was likely less than 40%. If there were two or more patients with complete or partial response, the treatment would also be considered promising. There was at least 80% probability of observing 2 or more responses in 12 treated patients if the true but known response rate was at least 23%. PFS and OS were described using the Kaplan‐Meier method.
Results
Patient Demographics
From January 24, 2014, to June 8, 2016, 27 patients were screened, 9 deemed eligible, and 8 (6 male and 6 white, median age of 66.5 years [range 39–75]) ultimately enrolled in the study (Table 1). Reasons for ineligibility included tumor around major vessels or organs, active central nervous system disease, comorbidities, poor PS, no prior chemotherapy, and no evidence of disease progression after chemotherapy. All accrued patients progressed after platinum/etoposide therapy prior to entering the trial; prior lines of therapy are reported in Table 2. None of the eight patients was immunosuppressed.
Table 1. Patient and tumor characteristics.
Immunohistochemical analysis performed with a murine monoclonal IgG2b antibody (clone CM2B4, sc‐136172, Santa Cruz Biotechnology, Dallas, TX).
Abbreviations: MCPyV, Merkel cell polyomavirus; NE, not evaluable; PD, progressive disease; SD, stable disease.
Table 2. Prior lines of systemic therapy.
Abbreviations: mTOR, mammalian target of rapamycin; PI3K, phosphoinositol‐3 kinase.
Efficacy
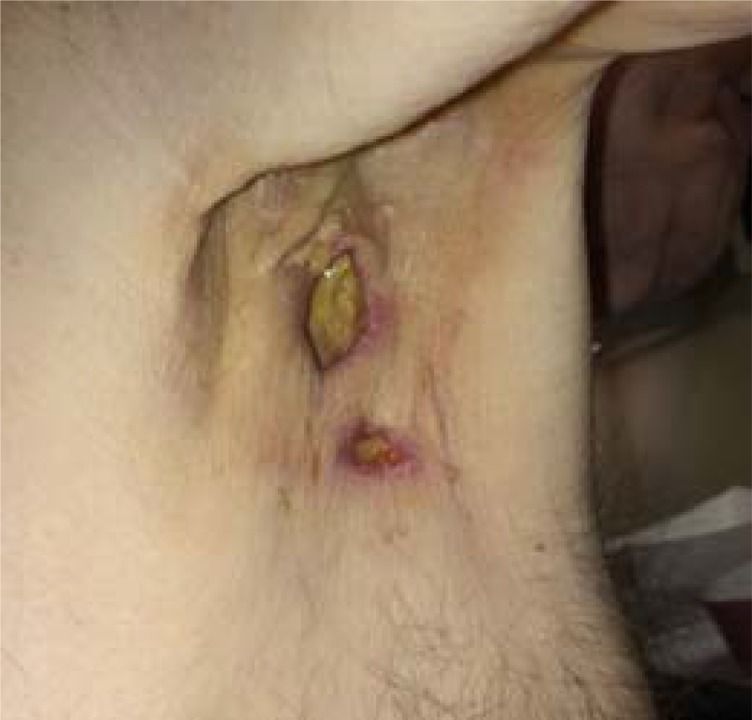
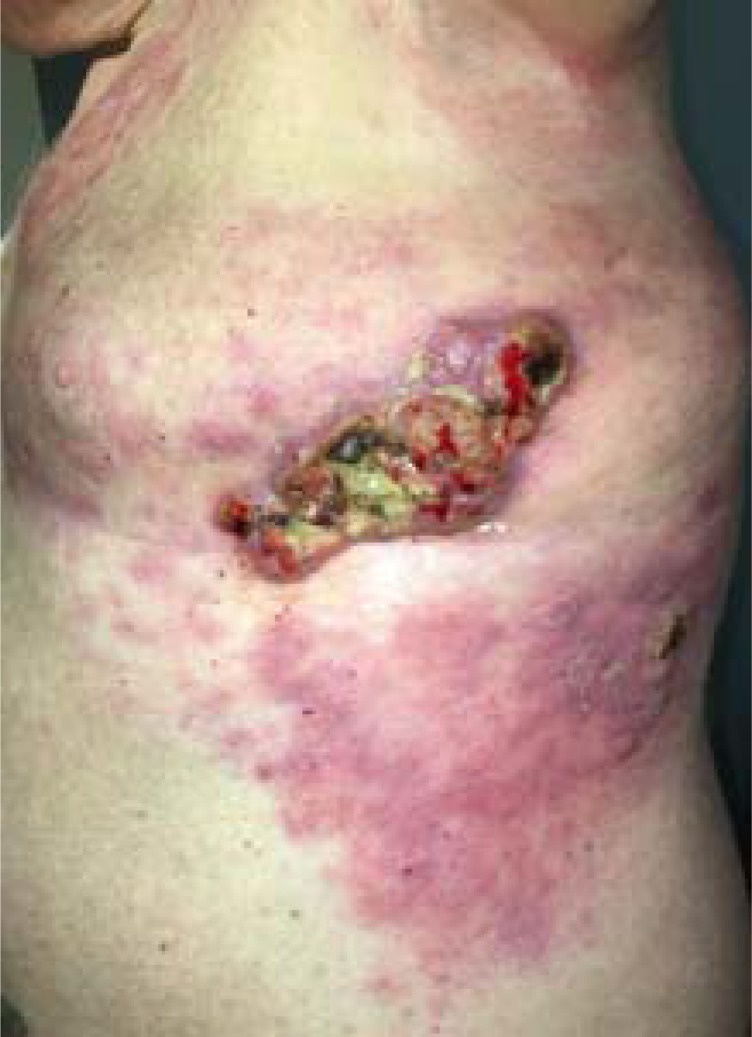
One patient was not evaluable for response because of withdrawal 2 weeks after starting on the study drug. There were no complete or partial responses among the seven evaluable patients. The median number of treatment weeks among the eight patients was 6.5 (range 2–23). One patient achieved stable disease for 8 months but discontinued therapy after five cycles because of axillary fistula formation (Fig. 1) and nonhealing wound ulcers. The median PFS and OS were 2.1 and 11.2 months, respectively.
Figure 1.
Left axillary fistula formation between tumor and skin on patient 5.
Safety and Tolerability
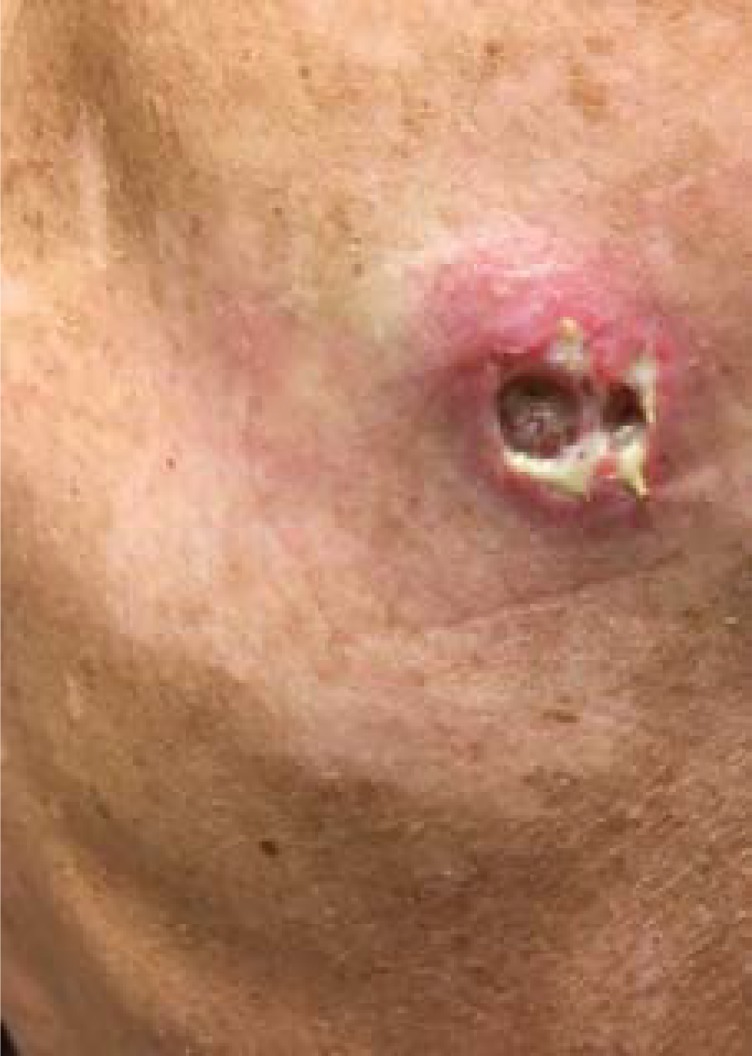
Toxicities are listed in Table 3. The most frequent adverse events (AEs) were grades 1 and 2 and included anorexia, fatigue, nausea, hypothyroidism, and dysgeusia. Grade 3 toxicities included oral pain, hypophosphatemia, hyponatremia, proteinuria, and headache. The adverse events that caused early withdrawal of one patient included oral pain and fatigue. Three (37.5%) patients required dose reduction to 40 mg orally daily because of electrolyte abnormalities, proteinuria, headache, and oral pain. In addition to the patient with prolonged stable disease who developed the fistula and nonhealing ulcers, two other patients also developed poor wound healing at tumor and biopsied sites (Figs. 2, 3).
Table 3. Treatment‐related adverse events.
Figure 2.
Poor wound healing from tumor biopsy site on right upper back of patient 7.
Figure 3.
Necrosis and ulceration of the large tumor on left lateral chest wall, with evidence of disease progression around it, on patient 8.
Table 4. OncoPanel results.
Abbreviations: AKT, akt serine‐threonine kinase; ERBB, ErbB2 receptor tyrosine kinase; mTOR, mammalian target of rapamycin; n/a, not applicable; PI3K, phosphoinositol‐3 kinase.
Correlative Studies
Tumor samples were available for next‐generation sequencing (OncoPanel) from all patients. Potentially significant somatic alterations were detected in five of eight cases, including PI3K/AKT/mTOR, ERBB and Hedgehog pathways (Table 3). No VEGFR‐2 (KDR) or MET mutations were identified.
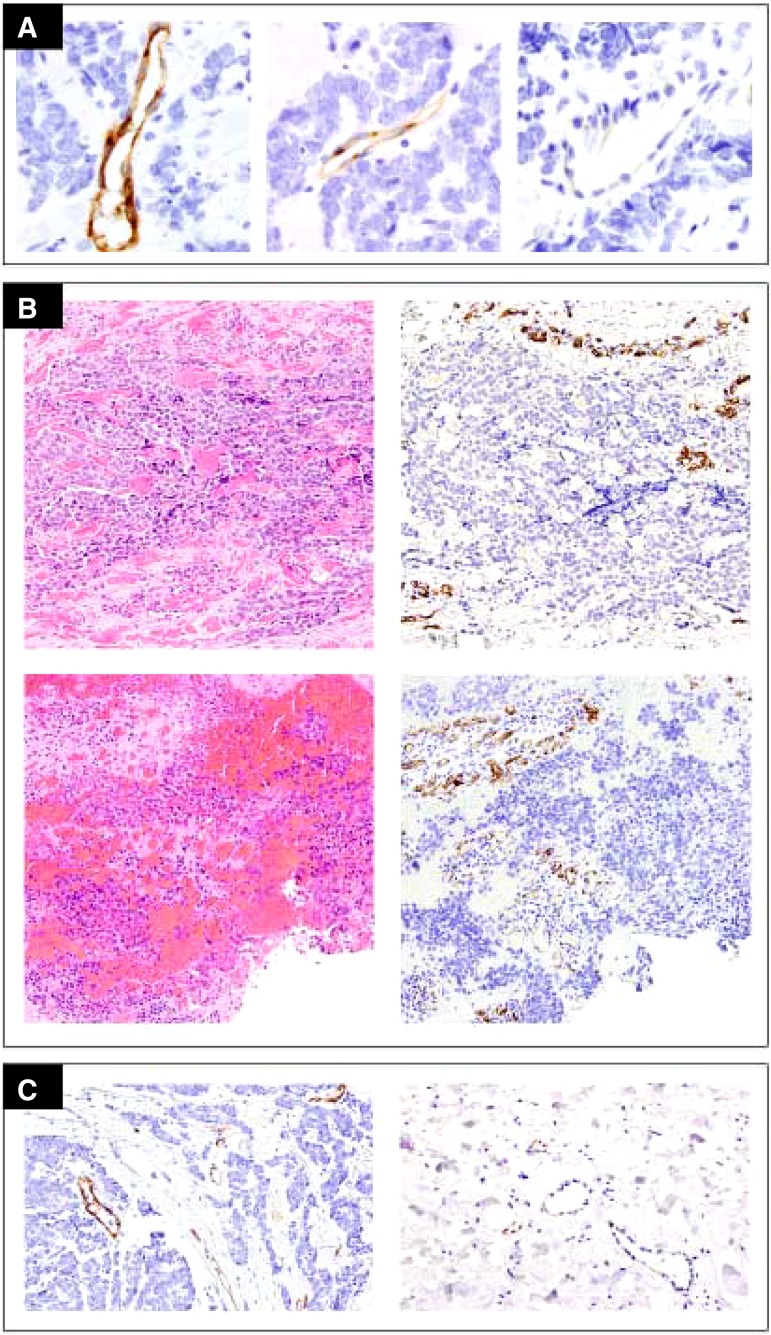
Pretreatment tissue samples were available for seven patients, five of them corresponding to recurrent cutaneous lesions and two to distant metastases (one to lymph nodes and one to the liver). For one of these patients, an on‐treatment sample from a cutaneous recurrence was also available. Immunohistochemical evaluation of all samples (n = 8) with appropriate positive and negative controls showed no expression of c‐MET, p‐MET, HGF, or VEGFR‐2 in MCC tumor cells. Although VEGFR‐2 was detected in endothelial cells, intratumoral variability of staining intensity was seen in all cases (Fig. 4A). The paired samples corresponding to cutaneous recurrences obtained before and during cabozantinib therapy from a single patient showed no difference in the staining intensity or the proportion of VEGFR‐2+ intratumoral vessels (Fig. 4B, upper and lower rows, respectively). Markedly increased staining intensity and proportion of VEGFR‐2+ vessels were consistently seen within tumor stroma compared with the more subtle immunoreactivity seen in adjacent benign tissue (Fig. 4C, left and right, respectively).
Figure 4.
Results of VEGFR‐2 detection by immunohistochemistry. No expression of VEGFR‐2 was detected on Merkel cell carcinoma cells. (A): Variable, strong to minimal, VEGFR‐2 staining intensity was present in intratumoral vessels. (B): Paired pre‐cabozantinib (upper row) and on‐treatment (lower row) samples showed no appreciable changes in intensity or extent of VEGFR‐2 staining in intratumoral vessels. (C): Vessels within the tumor and away from the tumor, nonmalignant surrounding tissue. The proportion of positive vessels and staining intensity was markedly increased in intratumoral vessels (left) compared with vessels in adjacent benign tissue (right).
Discussion
Despite the recent advances in the treatment of advanced MCC with the introduction of immune checkpoint inhibitors, a large proportion of patients do not benefit from or are not candidates for this type of therapy. Although cytotoxic chemotherapy remains an option for these patients, the high response rates seen must be reconciled with the toxicity and relatively short duration of response in this older population. The development of targeted therapies remains an area of active investigation and an important goal for these patients.
Expression of VEGFR‐2, a mediator of angiogenesis, by endothelial cells of tumor stroma vessels has been correlated with poor prognosis in MCC [12]. Davids et al reported a partial response to pazopanib, a second‐generation tyrosine kinase inhibitor against VEGFR‐1, ‐2 and ‐3, platelet‐derived growth factor (PDGF)‐α and ‐β, and c‐kit, in a 69‐year‐old woman with metastatic MCC whose disease had progressed through prior therapies [19]. In this report, tumor immunohistochemistry was negative for c‐kit, VEGFR‐1, ‐2 and ‐3, and PDGF‐A, but positive for VEGF and PDGFR‐β (weakly). A PDGFR‐α mutation in codon 478 was identified and may have contributed to the treatment response. More recently, the results of a phase II study of pazopanib in metastatic MCC demonstrated partial responses in 3 of 18 patients (17%) [20]. Although a primary endpoint was met, the trial recruitment was stopped early because of slow accrual. Six other patients had stable disease, for a DCR of 50%, and the median duration of treatment was 8 weeks; two patients discontinued treatment because of toxicity and 13 others because of progression. Median PFS was 2.8 months, and median OS was 8.9 months [20]. Serious adverse events related to drug were reported in seven (39%) patients, including one death secondary to gastrointestinal bleeding.
In this phase II study, cabozantinib monotherapy did not meet the prespecified efficacy endpoint and was poorly tolerated in this patient population. The best response was stable disease of 8 months in one patient. Next‐generation sequencing identified potentially oncogenic alterations in genes affecting a variety of signaling pathways previously implicated in MCC tumorigenesis [21], [22], [23], but no genetic alterations were detected in either MET or VEGFR‐2. These findings were consistent with the exome sequencing results of 49 MCC cases previously reported by Goh et al. [24]. Merkel cell polyomavirus (MCPyV)‐negative MCCs had a high mutation burden, with frequent mutations in RB1 and TP53. Additional mutations were seen in ASXL1, MLL2, MLL3, MAP3K1, TRAF7, ATM, MSH2, and BRCA1. In contrast, MCPyV‐positive MCCs harbored few mutations, with none in the genes reported above. HRAS, KRAS, PIK3CA, PTEN, TSC1, Notch1, and Notch2 mutations were seen in both MCPyV‐positive and ‐negative subgroups [24].
Overall, the genetic results demonstrate a diversity of potential oncogenic mechanisms at play in MCC and no clear dependence on MET or VEGFR‐2 signaling. Of note, none of the sequenced tumors had evidence of retinoblastoma pathway mutations; the lack of genetic inactivation of this pathway is associated with polyomavirus infection [23], [25].
Recruitment was stopped early, after eight patients were enrolled, given the slow accrual, lack of response, and poor tolerability in this patient population. Safety data were consistent with those observed in other studies with cabozantinib, with no occurrences of new or unexpected AEs. In a phase III study of cabozantinib 60 mg daily dosing in men with previously treated metastatic castration‐resistant prostate cancer, 67% and 33% of patients on the cabozantinib arm (n = 682; median age 69.5 years) had AEs that led to dose reductions or treatment discontinuation, respectively [27]. Another phase II trial with cabozantinib at the same dose in 19 patients (median age 67 years) with advanced cholangiocarcinoma demonstrated a 63% rate of dose reduction, also because of toxicity [28]. A phase III trial in medullary thyroid cancer using a higher dose of cabozantinib (140 mg) in 219 patients (median age of 55 years) reported AEs leading to dose reduction and treatment discontinuation in 79% and 16% of patients, respectively [29]. Although, based on these results, one may consider this agent to be poorly tolerated, particularly in the heavily pretreated, elderly population, dose reduction may not necessarily indicate poor tolerability. Cabozantinib has a broad exposure profile, and the need for dose reduction is highly correlated with individual patient exposure characteristics [30].
Our study is limited by the small sample size and single‐arm design. Although cabozantinib is synergistic with immune checkpoint blockade, resulting in a combinatorial strategy in other cancers [31], [32], the toxicity and lack of activity observed in this phase II trial make it difficult to consider further studies with cabozantinib in patients with MCC.
Conclusion
Single‐agent cabozantinib did not demonstrate activity in this group of patients with advanced platinum‐failure MCC. Although it is unclear whether preselection of patients with the specific upregulation and genetic alterations in targets for cabozantinib would have changed the results of this study, this would have likely led to an extremely rare patient population that would take many years to accrue.
Acknowledgments
The authors thank Exelixis for drug supply and financial support to conduct this study. The authors also thank the patients and their families for participating in this study.
Footnotes
For Further Reading: Allison S. Betof, Ryan D. Nipp, Anita Giobbie‐Hurder et al. Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma. The Oncologist 2017;22:963–971; first published on May 5, 2017.
Implications for Practice: Immunotherapy has revolutionized treatment for patients with metastatic melanoma, yet data are lacking regarding the effectiveness and tolerability of these treatments for older patients. In this study, we demonstrated that patients with melanoma safely tolerate immunotherapy and achieve similar outcomes regardless of their age. Specifically, we utilized data from two academic cancer centers and found no significant difference in overall survival, progression free survival, or immune‐related toxicities, other than arthritis, across age groups. As the population ages, studies such as this will become critical to help us understand how best to treat older adults with cancer.
Author Contributions
Conception/design: Guilherme Rabinowits, Paul J. Catalano, Ayushi Tyagi, Geoffrey I. Shapiro, Robert Haddad, James A. DeCaprio
Provision of study material or patients: Guilherme Rabinowits, Patricia McHugh, Hailey Becker, Megan M. Reilly, Julian Huang, Manisha Thakuria
Data analysis: Guilherme Rabinowits, Cecilia Lezcano, Paul J. Catalano, Ayushi Tyagi, Scott C. Bresler, Lynette M. Sholl, James A. DeCaprio
Manuscript writing: Guilherme Rabinowits
Final approval of manuscript: Guilherme Rabinowits, Cecilia Lezcano, Paul J. Catalano, Patricia McHugh, Hailey Becker, Megan M. Reilly, Julian Huang, Ayushi Tyagi, Manisha Thakuria, Scott C. Bresler, Lynette M. Sholl, Geoffrey I. Shapiro, Robert Haddad, James A. DeCaprio
Disclosures
Guilherme Rabinowits: EMD Serono, Pfizer (C/A); EMD Serono, Millenium, Exelixis (RF‐institution); Geoffrey I. Shapiro: Pfizer, Eli Lilly & Co., G1 Therapeutics, Roche, Vertex Pharmaceuticals (C/A); Pfizer, Eli Lilly & Co. (RF); Robert Haddad: Bristol‐Myers Squibb, Merck, AstraZeneca, Pfizer, Eisai (C/A); Bristol‐Myers Squibb, Merck, AstraZeneca, Pfizer, Celgene (RF‐institution); James A. DeCaprio: Tango Therapeutics (C/A), Constellation Pharmaceuticals (RF). The other authors reported no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Lemos BD, Storer BE, Iyer JG et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 2010;63:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodgson NC. Merkel cell carcinoma: Changing incidence trends. J Surg Oncol 2005;89:1–4. [DOI] [PubMed] [Google Scholar]
- 3. Iyer JG, Blom A, Doumani R et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med 2016;5:2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tai PT, Yu E, Winquist E et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: Case series and review of 204 cases. J Clin Oncol 2000;18:2493–2499. [DOI] [PubMed] [Google Scholar]
- 5. Kaufman HL, Russell J, Hamid O et al. Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: A multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol 2016;17:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufman HL, Russell JS, Hamid O et al. Durable responses to avelumab (anti‐PD‐L1) in patients with Merkel cell carcinoma progressed after chemotherapy: 1‐year efficacy update. Cancer Res 2017;77(suppl 13):CT079A. [Google Scholar]
- 7. Nghiem PT, Bhatia S, Lipson EJ et al. PD‐1 Blockade with pembrolizumab in advanced Merkel‐cell carcinoma. N Engl J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sennino B, Ishiguro‐Oonuma T, Wei Y et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c‐Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2012;2:270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boccaccio C, Comoglio PM. Invasive growth: A MET‐driven genetic programme for cancer and stem cells. Nat Rev Cancer 2006;6:637–645. [DOI] [PubMed] [Google Scholar]
- 10. Dong HY, Liu W, Cohen P, Mahle CE, Zhang W. B‐cell specific activation protein encoded by the PAX‐5 gene is commonly expressed in merkel cell carcinoma and small cell carcinomas. Am J Surg Pathol 2005;29:687–692. [DOI] [PubMed] [Google Scholar]
- 11. Starrett GJ, Marcelus C, Cantalupo PG et al. Merkel cell polyomavirus exhibits dominant control of the tumor genome and transcriptome in virus‐associated Merkel cell carcinoma. MBio 2017;8:pii.e02079–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kukko H, Koljonen V, Lassus P et al. Expression of vascular endothelial growth factor receptor‐2 in Merkel cell carcinoma. Anticancer Res 2007;27:2587–2589. [PubMed] [Google Scholar]
- 13. Viola D, Cappagli V, Elisei R. Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Future Oncol 2013;9:1083–1092. [DOI] [PubMed] [Google Scholar]
- 14. Yakes FM, Chen J, Tan J et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298–2308. [DOI] [PubMed] [Google Scholar]
- 15. Tannir NM, Schwab G, Grünwald V. Cabozantinib: An active novel multikinase inhibitor in renal cell carcinoma. Curr Oncol Rep 2017;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagle N, Berger MF, Davis MJ et al. High‐throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov 2012;2:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia EP, Minkovsky A, Jia Y et al. Validation of OncoPanel: A targeted next‐generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med 2017;141:751–758. [DOI] [PubMed] [Google Scholar]
- 18. Sholl LM, Do K, Shivdasani P et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016;1:e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davids MS, Charlton A, Ng SS et al. Response to a novel multitargeted tyrosine kinase inhibitor pazopanib in metastatic Merkel cell carcinoma. J Clin Oncol 2009;27:e97–e100. [DOI] [PubMed] [Google Scholar]
- 20. Nathan PD, Gaunt P, Wheatley K et al. UKMCC‐01: A phase II study of pazopanib (PAZ) in metastatic Merkel cell carcinoma. J Clin Oncol 2016;34(suppl 15):9542A. [Google Scholar]
- 21. Veija T, Sarhadi VK, Koljonen V et al. Hotspot mutations in polyomavirus positive and negative Merkel cell carcinomas. Cancer Genet 2016;209:30–35. [DOI] [PubMed] [Google Scholar]
- 22. Nardi V, Song Y, Santamaria‐Barria JA et al. Activation of PI3K signaling in Merkel cell carcinoma. Clin Cancer Res 2012;18:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harms PW, Vats P, Verhaegen ME et al. The distinctive mutational spectra of polyomavirus‐negative Merkel cell carcinoma. Cancer Res 2015;75:3720–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goh G, Walradt T, Markarov V et al. Mutational landscape of MCPyV‐positive and MCPyV‐negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 2016;7:3403–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cimino PJ, Robirds DH, Tripp SR et al. Retinoblastoma gene mutations detected by whole exome sequencing of Merkel cell carcinoma. Mod Pathol 2014;27:1073–1087. [DOI] [PubMed] [Google Scholar]
- 26. Smith MR, Sweeney CJ, Corn PG et al. Cabozantinib in chemotherapy‐pretreated metastatic castration‐resistant prostate cancer: Results of a phase II nonrandomized expansion study. J Clin Oncol 2014;32:3391–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith M, De Bono J, Sternberg C et al. Phase III study of cabozantinib in previously treated metastatic castration‐resistant prostate cancer: COMET‐1. J Clin Oncol 2016;34:3005–3013. [DOI] [PubMed] [Google Scholar]
- 28. Goyal L, Zheng H, Yurgelun MB et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017;123:1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elisei R, Schlumberger MJ, Müller SP et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miles D, Jumbe NL, Lacy S et al. Population pharmacokinetic model of cabozantinib in patients with medullary thyroid carcinoma and its application to an exposure‐response analysis. Clin Pharmacokinet 2016;55:93–105. [DOI] [PubMed] [Google Scholar]
- 31. Kwilas AR, Ardiani A, Donahue RN et al. Dual effects of a targeted small‐molecule inhibitor (cabozantinib) on immune‐mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med 2014;12:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Apolo AB, Mortazavi A, Stein M et al. A phase I study of cabozantinib plus nivolumab (CaboNivo) in patients (pts) refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. Ann Oncol 2016;27(suppl 6):774PDA. [Google Scholar]