Abstract
Pulmonary hypertension is a multifactorial disease with a high morbidity and mortality. Right ventricular function is the most important predictor of morbidity and mortality in patients suffering from pulmonary hypertension, but currently there are no approved treatments directly supporting the failing right ventricle. Levosimendan is a calcium sensitizing agent with inotropic, pulmonary vasodilatory, and cardioprotective properties. Given its pharmacodynamic profile, levosimendan could be a potential novel agent for the treatment of right ventricular failure caused by pulmonary hypertension. The aim of this review is to provide an overview of the current knowledge on the effects of levosimendan in pulmonary hypertension and right heart failure.
Keywords: right heart failure, levosimendan, calcium sensitizer, pulmonary hypertension, pulmonary arterial hypertension, congenital heart disease
Introduction
Pulmonary hypertension (PH) is defined as an increased mean pulmonary arterial pressure ≥25 mmHg at rest, as assessed by right heart catheterization.1 PH can be caused by various conditions and the treatment options depend upon the type of PH. The World Health Organization has classified PH into five clinical subgroups:2 pulmonary arterial hypertension (PAH) (group 1), PH due to left heart disease (LHD) (group 2), PH due to lung diseases and/or hypoxemia (group 3), PH due to chronic thromboembolism (CTEPH) (group 4), and PH with unclear and/or multifactorial mechanisms (group 5). In PAH, the pathological changes are mainly in the small pulmonary arteries and may occur idiopathic, familial, or associated, for example, with congenital heart disease (CHD). Treatments of PAH primarily target pulmonary vascular dysfunction in order to lower right ventricle (RV) afterload and thereby improve RV function. PH induced by increased pressure in the pulmonary veins is seen in LHD, whereas hypoxia is considered a major pathophysiological driver of PH in chronic pulmonary diseases. In these subgroups of PH, the treatment is directed towards the underlying heart or lung disease. In chronic thromboembolic PH, the disease is predominantly located in the central pulmonary arteries and treatment is primarily to relieve vascular obstruction by pulmonary endarterectomy or alternatively balloon pulmonary angioplasty. In all of the PH subgroups, RV function is the main predictor of morbidity and mortality; however, there are currently no approved treatments supporting the RV directly.
There is currently no consensus definition of RV failure and RV dysfunction. To maintain cardiac output in the setting of an acute pulmonary arterial pressure elevation, for example, a large pulmonary embolism, the RV increases its contractility.3 Failure to adapt acutely leads to RV dilatation and dysfunction clinically evidenced by hypotension and cardiogenic shock. When pulmonary arterial pressure increases more slowly, the RV dilates and preserves cardiac output using Starling’s law. Generally, RV function is preserved until late phases of the disease. Ultimately, the RV fails and gets spherical, tricuspid regurgitation develops and leads to more right heart failure. A spiral process follows resulting in venous system congestion and symptomatic disease. Symptoms include fatigue, exertional dyspnea, ankle swelling, dizziness, right upper abdominal discomfort or pain, and epigastric fullness.
The calcium sensitizer levosimendan that is used in left heart failure has also been suggested in the management of PH and right heart failure, due to its pharmacodynamic profile with inotropic and pulmonary vasodilatory effects. However, evidence for levosimendan treatment in PH is currently lacking, and it is not possible to extrapolate knowledge from studies with levosimendan on the left ventricle (LV) to the RV. In this review, we summarize and discuss the existing data on the use of levosimendan in both experimental models of PH and clinical PH in order to guide potential clinical use and future research.
Levosimendan
Mechanism of action
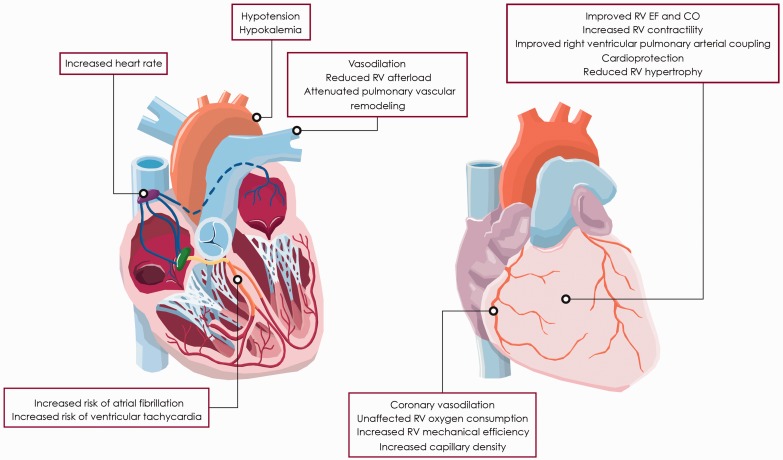
The mechanism of action is complex and at least three key pharmacological effects have been identified, including an inotropic effect, vasodilation, and cardioprotection (Fig. 1). Levosimendan is a calcium sensitizer that displays positive inotropic effects by increasing the affinity of myocardial troponin C to calcium. In contrast to other inotropes, the positive inotropic effect of levosimendan does not occur at the expense of calcium overload or increased myocardial oxygen demand.4,5 Additionally, diastolic function does not deteriorate as the calcium sensitizing effect is related to intracellular calcium levels, which decreases during diastole yielding an improvement in diastolic function.6 Levosimendan displays vasodilatory effects by the opening of adenosine triphosphate (ATP)-dependent K+ channels in vascular smooth muscle cells. Vasodilation occurs in both arterial and venous smooth muscle cells, causing a reduction in both RV preload and afterload.7 The cardioprotective properties are related to opening of mitochondrial ATP-dependent K+ channels in the cardiomyocytes, thereby providing protection against ischemia-reperfusion injury, apoptosis, and oxidative stress.8 Dilatation of coronary arteries and improvement in oxygen supply has also been assumed to protect the myocardium against ischemia.9 Although levosimendan is a potent and selective inhibitor of phosphodiesterase type 3, this action does not seem to contribute to its inotropic and vasodilator effect at therapeutic doses.10
Fig. 1.
Cardiovascular effects of levosimendan in the setting of PH and associated right heart failure. Abbreviations: CO, cardiac output; EF, ejection fraction.
Pharmacokinetics
Levosimendan has a short elimination half-life of approximately 1 h, whereas the active metabolite OR-1896 has an elimination half-life of 70–80 h allowing hemodynamic effects to persist 7–9 days after a 24 h infusion of levosimendan.11 About 5% of levosimendan is converted to the metabolite OR-1855 in the intestine and then acetylated to OR-1896 in the liver. The active metabolite OR-1896 displays hemodynamic properties similar to those of levosimendan. In patients with severe renal dysfunction or moderate hepatic dysfunction, the pharmacokinetic of levosimendan is unaltered, although the elimination of the metabolites may be prolonged. Importantly, no development of tolerance,11 and no rebound effect after withdrawal have been reported.8
Dosage and administration
Today, the recommended dose to obtain favorable hemodynamic effects in left heart failure is a bolus dose of 12 µg/kg during 10 min followed by continuous infusion of 0.1 µg/kg/min for 24 h, which can be reduced to 0.05 µg/kg/min or increased to 0.2 µg/kg/min.12 No dosage adjustments are required in patients with mild to moderate renal failure or mild to moderate hepatic failure. Contraindications are severe hypotension (systolic blood pressure < 85 mmHg), tachycardia, severe renal or hepatic failure, mechanical obstruction to ventricular filling or outflow, and a history of torsades de pointes ventricular tachycardia.
Adverse events
Generally, levosimendan has been well tolerated in patients with acute left heart failure considering the high risk nature of these patients. The most common adverse effects reported are hypotension, headache, nausea, and dizziness secondary to the vasodilating effect.13 Levosimendan infusion is associated with an increased incidence of atrial fibrillation compared both with dobutamine and with placebo.14,15 However, with respect to ventricular arrhythmias, conflicting results have been presented. In the SURVIVE study, no difference between the incidence of ventricular tachycardia in the levosimendan and dobutamine group was found.14 In the REVIVE study, an increased incidence of ventricular tachycardia was reported with levosimendan compared to placebo.15 This discrepancy may be caused by the high-sustained infusion, the administration of other intravenously drugs along with the more high-risk nature of patients enrolled in the REVIVE trial. Finally, hypokalemia has been reported more frequently with levosimendan compared with dobutamine, but the mechanism underlying hypokalemia is unresolved.
Levosimendan in PH
Preclinical studies
Given the small and heterogeneous patient groups with PH, different animal models of PH are essential for evaluation of the safety and efficacy of novel therapeutic agents. There is no gold standard for animal models of PH and right heart failure and such models obviously carry important limitations. However, they might serve as a rational basis for designing relevant clinical investigations. The effect of levosimendan on PH and associated RV failure has been assessed using a wide range of different animal models of acute and chronic PH, and several of these preclinical studies have showed beneficial effects of levosimendan. Supplementary Table 1 online summarizes the main preclinical studies.
In animal models of acute pressure overload induced RV failure caused by constriction of the pulmonary artery, thromboxane mimetics, repeated acute pulmonary embolisms, or hypoxic pulmonary vasoconstriction, acute administration of levosimendan has been reported to improve RV contractility and function, reduce RV afterload by pulmonary vasorelaxant effects, and most importantly, restore right ventricular–pulmonary arterial coupling.16–21 Additionally, it was found that levosimendan increased coronary blood flow.19 In contrast to these beneficial studies, negative results were described in a study with levosimendan in endotoxin-induced PH in rats.22 In this experiment, no improvement in RV or LV function was found and levosimendan was associated with hyperlactatemia, acidosis, and an increase in plasma proinflammatory cytokines.
When investigating the acute effect of levosimendan in open chest pigs with normal pulmonary circulation, levosimendan was found to increase RV contractility, but it did not change pulmonary vascular resistance and right ventricular–pulmonary arterial coupling,23 indicating that the pulmonary vasodilatory effect of levosimendan is primarily displayed in the setting of PH. In this study no changes in right coronary artery flow and RV oxygen consumption were found and RV mechanical efficiency deteriorated slightly at the highest dose of levosimendan.
Recently, the role of levosimendan in chronic PH and associated RV failure has been investigated using the monocrotaline rat model, the pulmonary trunk banding rat model, and the Sugen-hypoxia rat model. First, Revermann et al. demonstrated that three weeks of levosimendan treatment in the monocrotaline rat model attenuated the development of PAH by inhibiting proliferation of pulmonary arterial smooth muscle cells, pulmonary vascular medial wall thickness, and RV hypertrophy.24 Vildbrad et al. showed that acute administration of levosimendan in the monocrotaline and pulmonary trunk banding models improved RV contractility and RV lusitropy in both models and improved RV stroke volume in the monocrotaline model, but not in the pulmonary trunk banding model.25 This difference may be explained by the absence of RV afterload reduction in the pulmonary trunk banding model due to the fixed RV afterload. However, Hillgaard et al. reported that long-term treatment in the pulmonary trunk banding model improved RV function and contractility despite a fixed afterload.26 Translation of these two rat models to human PAH is problematic since the pulmonary trunk banding model does not provide information about the pulmonary vascular effects of levosimendan, and the monocrotaline model has been widely criticized for inducing a systemic proinflammatory and procoagulant response, which causes myocarditis and thickening of the coronary arterioles.27 Based on this concern, Hansen et al. studied the effects of long-term treatment in the Sugen-hypoxia rat model, which is characterized by vascular changes that closely mimics those in PAH patients.28 It was demonstrated that levosimendan in this model improved both RV hemodynamics, pulmonary vasculopathy, and RV remodeling. Hemodynamic improvement was demonstrated by improved RV function, reduced RV afterload, and improved right ventricular–pulmonary arterial coupling. Effects on pulmonary vasculopathy was reported by attenuation of pulmonary arterial occlusive lesions, and reduced RV remodeling was demonstrated by increased capillary density, reduced cardiomyocyte size, and reduced atrial natriuretic peptide and B-type natriuretic peptide. Additionally, Hansen et al. revealed that long-term treatment in the pulmonary trunk banding rat model improved RV function without increasing RV myocardial oxygen consumption leading to improved myocardial external efficiency,29 which is consistent with the calcium sensitizing effect and emphasizes the potential therapeutic value of levosimendan therapy in PH.
Overall the existing preclinical data provides strong evidence of beneficial effects of levosimendan and motivates clinical testing of levosimendan in PH and associated RV failure in well-designed clinical studies.
Levosimendan in PAH
Results similar to what has been shown in preclinical studies, have been reported in patients with PAH, however data are very sparse. Supplementary Table 2 online summarizes the few clinical studies and case-reports on levosimendan treatment in PAH patients. A placebo-controlled pilot study conducted in 28 patients with PH of different etiologies, including idiopathic PAH, PH due to LHD, and PH due to chronic thromboembolic disease,30 investigated the acute (24 h) and long-term (2 months) effects of repetitive intravenous infusions of levosimendan. Patients with right heart failure, NYHA class III-IV, mean right atrial pressure ≥4 mmHg, mean pulmonary arterial pressure ≥30 mmHg, and a positive vasoreactive response were included in the study. Patients were randomized 2:1 to levosimendan or placebo and the study drug was administered five times with 2 weeks of interval. The initial levosimendan infusion caused a decrease in pulmonary vascular resistance and mean pulmonary arterial pressure, which was maintained during the treatment course. Similar results were observed in a prospective, single-arm study performed in 9 patients with idiopathic PAH and associated right heart failure, where a 24 h levosimendan infusion led to a reduction in pulmonary vascular resistance, increased exercise tolerance, and reduced NT-proBNP. At 12 week follow-up, the improved functional status was preserved, and the hemodynamic improvement was maintained.31 In a prospective non-randomized open-label study of 45 PH patients with severe acute right heart failure, levosimendan improved WHO functional class, Borg dyspnea score, 6-min walk test, biochemical markers and echocardiographic parameters of RV function.32 On the contrary, a paradoxical rise in pulmonary arterial pressure were described in two idiopathic PAH patients with RV failure and a negative vasoreactivity test.33 The authors discussed whether this could be due to improved RV function in the setting of a relatively fixed pulmonary arterial resistance, but no cardiac output or pulmonary vascular resistance values were reported.
Further clinical studies are obviously required to confirm the efficacy and safety of levosimendan in PAH. Patients in such trials should be adequately characterized hemodynamically including status on pulmonary vasoreactivity.
Levosimendan in PH due to LHD
PH and RV dysfunction often complicates advanced left heart failure and have negative impact on the prognosis. PH related to LHD represents the most frequent type of PH and constitutes 65–80% of PH cases.34 It may be difficult to distinguish correctly between PAH and PH due to LHD, however it has important therapeutic consequences. Supplementary Table 3 online summarizes the clinical studies on levosimendan treatment in patients with PH due to LHD.
One of the first studies conducted was a placebo-controlled study of 54 patients with advanced heart failure due to LV systolic dysfunction (NYHA III-IV, LVEF <35%), which demonstrated that a 24 h infusion with levosimendan improved echocardiographic parameters of RV systolic and diastolic function, reduced systolic pulmonary arterial pressure, and improved neurohormonal status.35 Similar randomized clinical trials or prospective, single-arm studies in patients with LHD have been performed and showed improved RV contractility, reduced RV afterload, and improved clinical symptoms with levosimendan treatment.36–38
However, these studies were not designed to investigate patients with significant RV dysfunction. Subsequently, Poelzl et al. administered open label levosimendan in 18 patients with predominantly RV failure and LV ejection fraction ≤30%, cardiac index ≤2.5 L/min/m2, right atrial pressure ≥10 mmHg, and pulmonary capillary wedge pressure ≥15 mmHg and found an improvement in RV and LV contractility but no difference in RV afterload.39 Levosimendan was found to offer more favorable effects compared with dobutamine in a study by Yilmaz et al.,40 where 40 patients with acutely decompensated systolic heart failure and moderate-to-severe RV dysfunction were randomized to open label levosimendan or dobutamine. Both treatments improved RV ejection fraction and decreased systolic pulmonary arterial pressure, however, tricuspid annular plane systolic excursion, 24 h urine output, and creatinine was improved in the levosimendan group compared with the dobutamine group. Similar results were reported by Duygu et al. in an echocardiographic study comprising 62 patients with acute left heart failure randomized to levosimendan or dobutamine,41 where levosimendan was found to be superior to dobutamine in improving RV systolic and diastolic function and reducing systolic pulmonary arterial pressure. In a randomized clinical trial in patients with PH undergoing valve replacement, levosimendan was found as effective as milrinone in improving biventricular function and reducing mean pulmonary arterial pressure and pulmonary vascular resistance.42 However, levosimendan resulted in a greater increase in heart rate, decrease in systemic vascular resistance, and a greater need for norepinephrine.
Another key finding is that levosimendan improves cardiac output in patients with left heart failure without increasing biventricular oxygen consumption leading to an improvement in RV mechanical efficiency.5
No studies investigating the effect of levosimendan on PH due to LV failure with preserved ejection fraction has been performed to our knowledge.
Overall, levosimendan seems to be favorable in treating patients with PH due to LHD because of its ability to improve RV function, decrease pulmonary arterial pressure, improve clinical symptoms, and improve RV mechanical efficiency. Additionally, levosimendan was found superior or as effective as dobutamine in improving RV function and reducing pulmonary arterial pressure. Due to the small number of patients included in the studies of levosimendan in PH due to LHD, the clinical importance of levosimendan in PH due to LHD needs further investigation in future studies with inclusion of more patients and longer time of follow up.
Levosimendan in CHD
Given the inotropic effect and pulmonary vasodilating properties, levosimendan may offer therapeutic benefits in pediatric patients with CHD and PH. The pharmacokinetic profile of levosimendan has been characterized in pediatric patients with CHD assessed for cardiac surgery and was found comparable to that in adult patients with heart failure.43 However, the active metabolite OR-1896 has been suggested to exert prolonged hemodynamic effects in neonates as the active metabolite OR-1896 remained detectable in plasma 14 days after a 48 h levosimendan infusion in neonates going through cardiac surgery.44 Pediatric patients were given the same dose of levosimendan as adults, which implies a bolus of 6–24 µg/kg followed by continuous infusion of 0.05–0.2 µg/kg/min or continuous infusion only. No important adverse effects or unexpected adverse drug reactions has been reported in children undergoing cardiac surgery, only transitory tachycardia or hypotension has been described at start of the infusion.43
Levosimendan has mainly been studied in the perioperative setting of CHD because preoperative PH may exacerbate in the postoperative period, and levosimendan has been suggested effective in preventing and treating low cardiac output syndrome and PH after cardiac surgery. Only one single clinical trial of levosimendan treatment in patients with PH due to CHD has been conducted. The randomized double-blinded study by Ebade et al. demonstrated that levosimendan was superior to dobutamine in improving cardiac index and lowering mean pulmonary arterial pressure in children younger than 4 years with PH undergoing cardiac surgery.45 In addition, four cases have been reported on the use of levosimendan in children with CHD and PH. Lechner et al. described a neonate who underwent arterial switch operation and suffered from postoperative low cardiac output syndrome and PH.46 Levosimendan was administered after an insufficient response to conventional inotropic treatment, and led to an improvement in LV function, and a reduction in left atrial pressure and systolic pulmonary arterial pressure. Braun et al. reported a 2 months old child with postoperative acute left heart failure, PH, and right-to-left shunt after cardiac surgery due to CHD and PH.47 Levosimendan infusion caused an improvement in LV function, a halving of pulmonary arterial pressure, and resolution of right-to-left shunting. Likewise, Luther et al. reported improved LV function and reduced pulmonary vascular resistance in a 9 months old child with LV dysfunction and refractory PH due to congenital stenosis of the left coronary artery.48 Contrary to these three favorable cases, a case report described levosimendan-induced increase in pulmonary arterial pressure in a child with LV dysfunction and PH due to CHD and a negative vasoreactive response.49
In adults, only one case report of levosimendan treatment in CHD and PH has been published. Rafiq et al. reported a 30 years old patient with end stage heart failure and PH due to CHD who improved in exercise tolerance, number of hospitals admissions, and expected survival after levosimendan therapy 1–2 times per month for 1 year.50 End stage heart failure due to CHD in adults is a frequent clinical problem particularly when all therapeutic, surgical, and device options have been depleted. Levosimendan might potentially play a therapeutic role in improving prognosis and quality of life in this specific group of patients.
Levosimendan in PH due to lung disease and thromboembolism
Only very few levosimendan studies have been conducted in PH associated with lung disease and in PH related to pulmonary thromboembolism. In lung disease, a randomized, placebo-controlled clinical trial by Morelli et al. studied 35 patients with PH related to acute respiratory distress syndrome and septic shock.51 A 24 h infusion of levosimendan was reported to reduce mean pulmonary arterial pressure and pulmonary vascular resistance, and improve RV function and mixed venous oxygen saturation compared with placebo. In acute and chronic pulmonary thromboembolism, only two case reports have been published. Powell et al. reported a case with a 72 year old man suffering from life-threating acute pulmonary embolism.52 Due to a history of upper gastrointestinal bleeding and a poor pre-morbid status, medical, invasive, and surgical treatments were unfavorable. The patient received a 24 h levosimendan infusion that, after transient hypotension and tachycardia, improved the patient with hemodynamic stabilization, increasing PaO2, and improvement in metabolic parameters. Pitsiou et al. reported a case with a 49 year old man with non-operable CTEPH and decompensated RV failure.53 The patient was treated with furosemide, inhaled iloprost, and 24 h levosimendan infusion. He improved during the next days, and at follow up at one month, he presented with clinical stabilization and was referred for transplant evaluation.
Conclusions
The present literature on levosimendan in PH, despite limited in its extent, suggests that levosimendan is potentially favorable in treating PH and associated RV failure resulting from different etiologies such as PAH, LHD, and CHD. The existing literature does not provide adequate evidence to currently recommend the use of levosimendan in PH and associated RV failure. Larger, well-designed and sufficiently powered clinical trials should be carried out to evaluate the clinical efficacy and safety of levosimendan in this important group of patients with a high morbidity and mortality.
Supplemental Material
Supplemental material for Levosimendan in pulmonary hypertension and right heart failure by Mona Sahlholdt Hansen, Asger Andersen and Jens Erik Nielsen-Kudsk in Pulmonary Circulation
Conflict of interest
The authors declares that there is no conflict of interest
Funding
This work was supported by the Novo Nordisk Foundation (grant number NNF17OC0024868).
References
- 1.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009; 34: 1219–1263. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim BS. Right ventricular failure. E-J Cardiol Pract 2016; 14(32).
- 4.Lancaster MK, Cook SJ. The effects of levosimendan on [Ca2+]i in guinea-pig isolated ventricular myocytes. Eur J Pharmacol 1997; 339: 97–100. [DOI] [PubMed] [Google Scholar]
- 5.Ukkonen H, Saraste M, Akkila J, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther 2000; 68: 522–531. [DOI] [PubMed] [Google Scholar]
- 6.Haikala H, Nissinen E, Etemadzadeh E, et al. Troponin C-mediated calcium sensitization induced by levosimendan does not impair relaxation. J Cardiovasc Pharmacol 1995; 25: 794–801. [DOI] [PubMed] [Google Scholar]
- 7.Yokoshiki H, Katsube Y, Sunagawa M, et al. Levosimendan, a novel Ca2+ sensitizer, activates the glibenclamide-sensitive K+ channel in rat arterial myocytes. Eur J Pharmacol 1997; 333: 249–259. [DOI] [PubMed] [Google Scholar]
- 8.Parissis JT, Rafouli-Stergiou P, Paraskevaidis I, et al. Levosimendan: from basic science to clinical practice. Heart Fail Rev 2009; 14: 265–275. [DOI] [PubMed] [Google Scholar]
- 9.Kaheinen P, Pollesello P, Levijoki J, et al. Levosimendan increases diastolic coronary flow in isolated guinea-pig heart by opening ATP-sensitive potassium channels. J Cardiovasc Pharmacol 2001; 37: 367–374. [DOI] [PubMed] [Google Scholar]
- 10.Papp Z, Csapo K, Pollesello P, et al. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc Drug Rev 2005; 23: 71–98. [DOI] [PubMed] [Google Scholar]
- 11.Kivikko M, Antila S, Eha J, et al. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int J Clin Pharmacol Ther 2002; 40: 465–471. [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016; 69: 1167. [DOI] [PubMed] [Google Scholar]
- 13.Mebazaa A, Erhardt L. Levosimendan: a new dual-action drug in the treatment of acute heart failure. Int J Clin Pract 2003; 57: 410–416. [PubMed] [Google Scholar]
- 14.Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 2007; 297: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 15.Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 2013; 1: 103–111. [DOI] [PubMed] [Google Scholar]
- 16.De Witt BJ, Ibrahim IN, Bayer E, et al. An analysis of responses to levosimendan in the pulmonary vascular bed of the cat. Anesth Analg 2002; 94: 1427–1433. [DOI] [PubMed] [Google Scholar]
- 17.Kerbaul F, Rondelet B, Demester JP, et al. Effects of levosimendan versus dobutamine on pressure load-induced right ventricular failure. Crit Care Med 2006; 34: 2814–2819. [DOI] [PubMed] [Google Scholar]
- 18.Kerbaul F, Gariboldi V, Giorgi R, et al. Effects of levosimendan on acute pulmonary embolism-induced right ventricular failure. Crit Care Med 2007; 35: 1948–1954. [DOI] [PubMed] [Google Scholar]
- 19.Missant C, Rex S, Segers P, et al. Levosimendan improves right ventriculovascular coupling in a porcine model of right ventricular dysfunction. Crit Care Med 2007; 35: 707–715. [DOI] [PubMed] [Google Scholar]
- 20.Schwarte LA, Schwartges I, Thomas K, et al. The effects of levosimendan and glibenclamide on circulatory and metabolic variables in a canine model of acute hypoxia. Intensive Care Med 2011; 37: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiklund A, Kylhammar D, Radegran G. Levosimendan attenuates hypoxia-induced pulmonary hypertension in a porcine model. J Cardiovasc Pharmacol 2012; 59: 441–449. [DOI] [PubMed] [Google Scholar]
- 22.Chew MS, Hawthorne WJ, Bendall J, et al. No beneficial effects of levosimendan in acute porcine endotoxaemia. Acta Anaesthesiol Scand 2011; 55: 851–861. [DOI] [PubMed] [Google Scholar]
- 23.Leather HA, Ver Eycken K, Segers P, et al. Effects of levosimendan on right ventricular function and ventriculovascular coupling in open chest pigs. Crit Care Med 2003; 31: 2339–2343. [DOI] [PubMed] [Google Scholar]
- 24.Revermann M, Schloss M, Mieth A, et al. Levosimendan attenuates pulmonary vascular remodeling. Intensive Care Med 2011; 37: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 25.Vildbrad MD, Andersen A, Holmboe S, et al. Acute effects of levosimendan in experimental models of right ventricular hypertrophy and failure. Pulm Circ 2014; 4: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillgaard TK, Andersen A, Andersen S, et al. Levosimendan prevents pressure-overload-induced right ventricular failure. J Cardiovasc Pharmacol 2016; 67: 275–282. [DOI] [PubMed] [Google Scholar]
- 27.Guihaire J, Bogaard HJ, Flecher E, et al. Experimental models of right heart failure: a window for translational research in pulmonary hypertension. Semin Respir Crit Care Med 2013; 34: 689–699. [DOI] [PubMed] [Google Scholar]
- 28.Hansen MS, Andersen A, Holmboe S, et al. Levosimendan prevents and reverts right ventricular failure in experimental pulmonary arterial hypertension. J Cardiovasc Pharmacol 2017; 70: 232–238. [DOI] [PubMed] [Google Scholar]
- 29.Hansen MS, Andersen A, Tolbod L, et al. Levosimendan improves cardiac function and myocardial efficiency in rats with right ventricular failure. Pulm Circ 2018; 8: 2045893217743122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleber F, Bollmann T, Borst M, et al. Repetitive dosing of intravenous levosimendan improves pulmonary hemodynamics in patients with pulmonary hypertension: results of a pilot study. J Clin Pharmacol 2009; 49: 109–115. [DOI] [PubMed] [Google Scholar]
- 31.Martynyuk TV, Arkhipova OA, Kobal EA, et al. Possibilities of using levosimendan in patients with idiopathic pulmonary hypertension. Terapevticheskii Arkhiv 2012; 84: 83–88. [PubMed] [Google Scholar]
- 32.Jiang R, Zhao QH, Wu WH, et al. Efficacy and safety of a calcium sensitizer, levosimendan, in patients with right heart failure due to pulmonary hypertension. Clin Respir J 2018; 12: 1518–1525. [DOI] [PubMed] [Google Scholar]
- 33.Cavusoglu Y, Beyaztas A, Birdane A, et al. Levosimendan is not effective in reducing pulmonary pressures in patients with idiopathic pulmonary arterial hypertension: report of two cases. J Cardiovasc Med 2009; 10: 503–507. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37: 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parissis J, Paraskevaidis I, Bistola V, et al. Effects of levosimendan on right ventricular function in patients with advanced heart failure. Am J Cardiol 2006; 98: 1489–1492. [DOI] [PubMed] [Google Scholar]
- 36.Russ MA, Prondzinsky R, Carter JM, et al. Right ventricular function in myocardial infarction complicated by cardiogenic shock: Improvement with levosimendan. Crit Care Med 2009; 37: 3017–3023. [DOI] [PubMed] [Google Scholar]
- 37.Slawsky MT, Colucci WS, Gottlieb SS, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 2000; 102: 2222–2227. [DOI] [PubMed] [Google Scholar]
- 38.Alibaz-Oner F, Gurbuz OZ, Oner E, et al. Impact of levosimendan on right ventricular functions by using novel tissue Doppler derived indices in patients with ischaemic left ventricular failure. Kardiol Polska 2013; 71: 1036–1041. [DOI] [PubMed] [Google Scholar]
- 39.Poelzl G, Zwick RH, Grander W, et al. Safety and effectiveness of levosimendan in patients with predominant right heart failure. Herz 2008; 33: 368–373. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz M, Yontar C, Erdem A, et al. Comparative effects of levosimendan and dobutamine on right ventricular function in patients with biventricular heart failure. Heart Vessels 2009; 24: 16–21. [DOI] [PubMed] [Google Scholar]
- 41.Duygu H, Ozerkan F, Zoghi M, et al. Effect of levosimendan on right ventricular systolic and diastolic functions in patients with ischaemic heart failure. Int J Clin Pract 2008; 62: 228–233. [DOI] [PubMed] [Google Scholar]
- 42.Mishra A, Kumar B, Dutta V, et al. Comparative effect of levosimendan and milrinone in cardiac surgery patients with pulmonary hypertension and left ventricular dysfunction. J Cardiothor Vasc Anesth 2016; 30: 639–646. [DOI] [PubMed] [Google Scholar]
- 43.Turanlahti M, Boldt T, Palkama T, et al. Pharmacokinetics of levosimendan in pediatric patients evaluated for cardiac surgery. Pediatr Crit Care Med 2004; 5: 457–462. [DOI] [PubMed] [Google Scholar]
- 44.Pellicer A, Riera J, Lopez-Ortego P, et al. Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatr Res 2013; 73: 95–103. [DOI] [PubMed] [Google Scholar]
- 45.Ebade A, Khalil M, Mohamed A. Levosimendan is superior to dobutamine as an inodilator in the treatment of pulmonary hypertension for children undergoing cardiac surgery. J Anesth 2013; 27: 334–339. [DOI] [PubMed] [Google Scholar]
- 46.Lechner E, Moosbauer W, Pinter M, et al. Use of levosimendan, a new inodilator, for postoperative myocardial stunning in a premature neonate. Pediatr Crit Care Med 2007; 8: 61–63. [DOI] [PubMed] [Google Scholar]
- 47.Braun JP, Schneider M, Kastrup M, et al. Treatment of acute heart failure in an infant after cardiac surgery using levosimendan. Eur J Cardio-Thor Surg 2004; 26: 228–230. [DOI] [PubMed] [Google Scholar]
- 48.Luther YC, Schulze-Neick I, Stiller B, et al. Levosimendan – long-term inodilation in an infant with myocardial infarction. Z Kardiol 2004; 93: 234–239. [DOI] [PubMed] [Google Scholar]
- 49.Schwienbacher M, Schweigmann U, Neu N, et al. Heart transplantation in a 14-year-old boy in the presence of severe out-of-proportion pulmonary hypertension due to restrictive left heart disease: a case report. Case Rep Cardiol 2013; 2013: 418565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rafiq I, Freeman LJ. Pulsed levosimendan therapy in the management of chronic end stage cardiac failure in ‘adult congenital heart disease’. Int J Cardiol 2015; 195: 283–284. [DOI] [PubMed] [Google Scholar]
- 51.Morelli A, Teboul JL, Maggiore SM, et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med 2006; 34: 2287–2293. [DOI] [PubMed] [Google Scholar]
- 52.Powell BP, Simes D. Levosimendan in acute pulmonary embolism. Anaesth Intensive Care 2007; 35: 771–772. [DOI] [PubMed] [Google Scholar]
- 53.Pitsiou G, Paspala A, Bagalas V, et al. Inhaled iloprost plus levosimendan to decompensate right heart failure due to chronic thromboembolic pulmonary hypertension. Anaesth Intensive Care 2013; 41: 554–556. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Levosimendan in pulmonary hypertension and right heart failure by Mona Sahlholdt Hansen, Asger Andersen and Jens Erik Nielsen-Kudsk in Pulmonary Circulation