Abstract
Brown adipose tissue (BAT) possesses a unique uncoupling protein (UCP1) which, when activated, enables the rapid generation of heat and the oxidation of lipids or glucose or both. It is present in small amounts (~15–350 mL) in adult humans. UCP1 is rapidly activated at birth and is essential in preventing hypothermia in newborns, who rapidly generate large amounts of heat through non-shivering thermogenesis. Since the “re-discovery” of BAT in adult humans about 10 years ago, there has been an exceptional amount of research interest. This has been accompanied by the establishment of beige fat, characterised as discrete areas of UCP1-containing cells dispersed within white adipocytes. Typically, the amount of UCP1 in these depots is around 10% of the amount found in classic BAT. The abundance of brown/beige fat is reduced with obesity, and the challenge is to prevent its loss with ageing or to reactivate existing depots or both. This is difficult, as the current gold standard for assessing BAT function in humans measures radio-labelled glucose uptake in the fasted state and is usually dependent on cold exposure and the same subject can be found to exhibit both positive and negative scans with repeated scanning. Rodent studies have identified multiple pathways that may modulate brown/beige fat function, but their direct relevance to humans is constrained, as these studies typically are undertaken in cool-adapted animals. BAT remains a challenging organ to study in humans and is able to swiftly adapt to changes in the thermal environment and thus enable rapid changes in heat production and glucose oxidation.
Keywords: Brown adipose tissue
Introduction
The subject of brown adipose tissue (BAT) has become increasingly topical and controversial since its re-discovery in adult humans in 2007 1. The simultaneous publication of three studies in the New England Journal of Medicine demonstrating the unequivocal detection of brown fat in adult humans 2– 4 paved the way for an exponential rise in publications on this subject 5. Brown fat is important because, though present in relatively small amounts in the body, it has the potential to rapidly produce large amounts of heat and thus impact on both energy balance and glucose and lipid homeostasis 6, 7. This is exemplified in the rapid activation of brown fat around the time of birth and the critical role it plays in the prevention of hypothermia 8. The “re-discovery” of brown fat has been accompanied by the identification of beige adipocytes (that is, small clusters of brown-like white adipocytes within white fat depots) 9, 10. Furthermore, lineage-tracing experiments in mice indicated that classic brown adipocytes, characterised as possessing the unique mitochondrial uncoupling protein 1 (UCP1), have a common lineage with skeletal muscle and are very different from the cellular origins of beige and white adipocytes 8, 11.
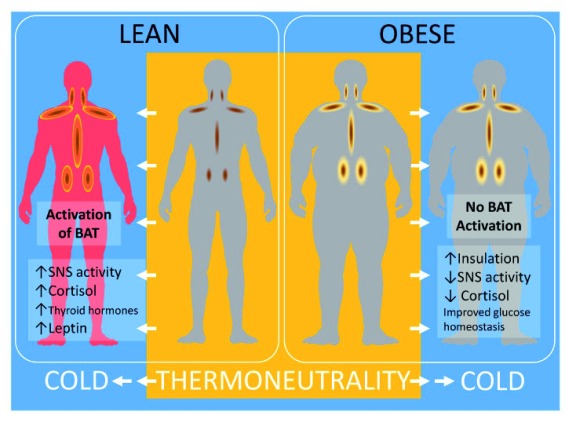
When UCP1 is stimulated, usually by the sympathetic nervous system, this results in the free flow of protons across the inner mitochondrial membrane 12, thereby bypassing the need to convert ADP to ATP, as occurs in the mitochondria of all other tissues. The primary stimulus for uncoupling remains contentious but is considered to be the release of fatty acids from lipid either within or surrounding brown adipocytes 13. Brown fat has the potential to produce far more heat per unit mass than any other organ in the body 8. Furthermore, the amount of UCP1 in classic brown fat is 10 times greater than that in the beige fat of rodents 14, meaning that the latter has a much smaller capacity to impact on whole-body energy balance. Beige fat, however, has the largest potential as a therapeutic target in the prevention of obesity or diabetes (or both) because it can be present in many white depots as clusters of pre-adipocytes that then can be recruited 14. However, the capacity of beige fat to modulate metabolism (especially glucose oxidation) may be mediated in part by mechanisms that do not involve UCP1 and have been proposed to be non-canonical 15. As summarised in Figure 1, the amount of activity of brown fat is reduced with raised white fat mass in obesity and its accompanying metabolically compromised endocrine environment.
Figure 1. Summary of the impact of obesity on brown adipose tissue function and regulation in adult humans and the potential benefits of chronic cold exposure.
ATGL, adipose triglyceride lipase; BAT, brown adipose tissue; FFA, free fatty acid; HSL, hormone-sensitive lipase; MGL, monoglyceride lipase; NEFA, non-esterified fatty acid; SNS, sympathetic nervous system; T, temperature; TG, triglyceride; WAT, white adipose tissue.
Advances in our understanding of the amount and activity of brown fat in adult humans
The gold standard by which the activity of brown fat is assessed in adult humans is still positron emission tomography-computed tomography (PET-CT) 16. This was the original method used to identify brown fat, and the same technique is used clinically around the world. It is a method that identifies brown fat from the uptake of radio-labelled glucose (fludeoxyglucose, or 18FDG) and is measured relative to the amount of glucose uptake in other tissues 16. However, to gain a significant signal within brown fat, the subject needs to be both fasted and cold-exposed 17. A better tracer than glucose for assessing brown fat thermogenesis is acetate 18, which is converted into acetyl-CoA within the cell and then incorporated into components of the citric acid cycle (a reaction that does not occur for 18FDG). Consequently, as brown fat rapidly turns over, the radio-labelled carbons in acetate are released as carbon dioxide and the amount of positron label lost is directly proportional to the metabolic activity of the tissue 19, which in the case of brown fat is substantial 18. When brown fat is activated by cold exposure, lipids stored within the depot are mobilised and oxidised to release heat 19. Acetate, however, is rarely used as a tracer, as it needs to be freshly prepared and, as it is not routinely used for cancer detection, such a facility is seldom available. This technical challenge means that our understanding of brown fat metabolism in humans remains constrained.
Quantification of brown fat in humans
It is now recognised that the amount of brown fat is under-assessed in most studies which use radio-labelled glucose in PET-CT and varies considerably between individuals, with current estimates now ranging from about 30 to 350 mL in healthy subjects 20, 21. PET-CT studies examining the impact of an intervention on brown fat function in humans have to subdivide participants into brown fat “positive/+ve” and “negative/−ve” sub-groups 22 or only study BAT+ve individuals 23. This could be considered a rather arbitrary classification, as all adults have the potential to exhibit a brown fat+ve response when repeatedly assessed with PET-CT 21. However, one small study has shown that a BAT+ve scan is associated with greater UCP1 within supraclavicular brown fat 24. The same study demonstrated a large increase in UCP1 gene expression with cold exposure, although there was appreciable variation in response between individuals. Taken together, these findings indicate the rapid response of brown fat to cold thermal stimulation for which increased gene expression 24 could be a longer-term response that parallels the pronounced change in substrate uptake, compared with warm conditions, as recently indicated in human supraclavicular brown fat 23. It has also been suggested, from PET-CT studies, that more brown fat is present in females than in males, although these results are more likely to reflect the greater sensitivity to cold by females 6.
The practical and health limitations of using PET-CT, which involves significant exposure to radiation, prevent its widespread use on healthy populations, meaning other methods of assessing brown fat function in vivo are required. These include thermal imaging for which a close correlation between brown fat function, as measured with PET-CT, has been established 25. Furthermore, there are currently no reports of any individuals undergoing thermal imaging who do not have a hot spot that co-locates with the supraclavicular depot (that then increases in temperature with mild hand cooling) 17. Thermal imaging has also demonstrated a marked responsiveness of supraclavicular brown fat to diet 26 and its contribution to dietary-induced thermogenesis. It is therefore able to provide novel insights into the impact of diet on brown fat that cannot be readily obtained from PET-CT studies.
Primary activators of brown fat
Reduced ambient temperature in humans remains the most potent stimulator of brown fat 27 and is not unexpected given that cold exposure to the extra-uterine environment is the primary catalyst for the onset of non-shivering thermogenesis at birth 8. This adaptation is accompanied by a rapid rise in a range of metabolic hormones, including catecholamines, thyroid hormones, cortisol, and leptin 7, with leptin co-locating onto the nucleus during cold induction of beige adipocytes 28. The extent to which repeated cold challenges can be used to enhance brown fat function in adults remains an important milestone for current research.
Importantly, glucose uptake in supraclavicular brown fat increases substantially with cold exposure and is positively correlated with the magnitude of cold-induced thermogenesis 23. Even in subjects who are classified as BAT−ve, an increase in mean glucose uptake is seen with cooling, although it is about 50% less than in those participants who are BAT+ve 24. A comparable adaptation has been observed in individuals who are diabetic, in whom the only study conducted to date indicates an improvement in glucose homeostasis 29. This was not fully explained, however, by the increase in glucose uptake within brown fat as measured by PET-CT 29. These findings are important given the potentially large amounts of brownable fat (up to 1,500 mL) as indicated in young healthy obese adults 30.
The exact amount of glucose oxidised by brown fat remains to be fully established, and it has been conservatively calculated that 300 mL of brown fat could dispose of at least 9 g of glucose per day 20. This value could be much greater on the basis of the threefold to fivefold increase in glucose uptake recently measured in supraclavicular fat with moderate cold exposure 23. With the innovative developments in studying brown fat mitochondria, further refinements in these calculations are likely. This is because two types of mitochondria have now been identified within rodent interscapular brown fat—the peridroplet and cytoplasmic domains—the latter of which regulates lipid supply, whereas the cytoplasmic domain could be more important in regulating glucose oxidation 31. Further adaptations as shown in beige fat of mice following cold exposure include the appearance of dense intra-adipose sympathetic arborisations 32, but have yet to be confirmed in humans. The capacity to predict which experimental models of browning are most relevant to humans could be further enhanced by the systematic integration of transcriptional profiles using online resources that are now being developed 33. The full extent to which changes in brown fat function can contribute to inadequate glucose homeostasis remains to be fully explored. It is noteworthy, however, that two recent studies have indicated that raised temperature is associated with increased risk of diabetes, either during pregnancy in Canada 34 or in all adults across the United States 35. Taken together, these findings emphasise the impact of climate change on human health and the extent to which adverse effects may be dependent on the body’s natural heat generator (that is, brown fat) 5.
Other targeted approaches to stimulate brown fat in humans have included the use of the β3-adrenoreceptor antagonist mirabegron (at a relatively high dose of 200 mg). Mirabegron promotes glucose uptake in a wide range of brown fat depots 36, as determined with PET-CT, as well as stimulating metabolic rate, although these two measures were not well correlated. Thus, it is possible that the effects of mirabegron are related more to effects on glucose metabolism in brown fat 37 rather than to heat production per se, but this needs further studying. Surprisingly, despite being published nearly 4 years ago, this study remains the only one of its kind. Both acute and chronic stimulation of the stress-sympathetic nervous system would appear to offer a route by which brown fat activity can be enhanced. For example, 24-hour infusion of hydrocortisone in adult males increases the temperature of the supraclavicular depot that co-locates with brown fat 26. In children, the stress associated with severe burn injury promotes the appearance of UCP1 in subcutaneous fat after 4 weeks 38.
Limitation on our understanding of regulation of brown fat metabolism from rodent studies
The past 5 years have seen a prolific amount of research into brown fat, which suggests that it may have the capacity to improve metabolic homeostasis in adults, but such a goal will not be a straightforward outcome to achieve. This is, in part, because of the very different metabolic roles for brown fat in rodents and humans together with the divergence in experimental protocols used in animal studies 39 that do not readily compare with the human situation 5. However, the potential for UCP1 to generate heat appears to be similar between species 40, although the exact range of mechanisms involved remains to be fully established 13. It should be noted that laboratory rodents are typically maintained within a highly artificial environment, are usually fed a highly processed diet that is the same each day, experience no change in photoperiod (that is, fixed 12-hour day and 12-hour night) or ambient temperature, and have limited (if any) exposure to pathogens 41. Furthermore, the main brown (or beige) fat depot in adult humans is located within the supraclavicular region 42, and although this is also present in rodents 43, it has seldom been examined, as the interscapular and inguinal depots are primarily investigated.
Thus, it is important that the results from the plethora of rodent-based investigations are considered in light of the depot examined and the relevance to human adipose tissue of the identified pathways.
Although the focus of most rodent studies has been on identifying novel pathways that could be targeted to promote brown fat function, these have had relatively little impact on enabling sustainable interventions in adult humans. This could be for a number of reasons that now are starting to gain more consideration across the scientific community. The main concern is the thermal environment in which rodents are maintained when brown fat is examined, as it is clear that 20–21 °C represents a substantial thermal stress and that thermoneutrality is approximately 28–30 °C 39. Moreover, many rodent studies go on to examine the effect of further exposure to what, for laboratory mice, would be extreme cold (that is, about 6 °C), which results in maximal brown fat activation. This is rather an abrupt challenge and without a gradual decline in temperature, as would occur in the wild, is an unphysiological adaptation. In terms of susceptibility to metabolic-related disease, this is best illustrated in a mouse model of non-alcoholic fatty liver disease (NAFLD). Housing at thermoneutrality exacerbates the magnitude of NAFLD as well as removing any difference between sexes 44. Not surprisingly, those mice housed at 30 °C are characterised as possessing less brown fat and have lower plasma corticosterone concentrations which, in humans, are known to positively impact on brown fat function in some 26, 45 but not all 46 studies. Furthermore, thermoneutral housing accelerates atherosclerosis through increased metabolic inflammation, which surprisingly is uncoupled from insulin resistance 47. The link among inflammation, obesity-induced insulin resistance, and atherosclerosis has been clear for decades 48. It is likely, therefore, that these results in rodents have been confounded by chronic mild-cold stress and that better modelling of human physiology, especially with regard to the role of brown fat in metabolic disease, will be needed in future. The critical role of temperature has been highlighted in vitro 49, under which conditions the appearance of UCP1 also appears to be dependent on reduced ambient temperature 28.
Conclusions
The re-discovery of BAT in adults has led to the recognition that promoting its activity could be an effective new strategy to improve metabolic homeostasis in a sedentary world where obesity and diabetes are prevalent. Although to date most studies in humans have focused on promoting heat production in brown fat, given the recent findings on increased glucose metabolism, this could be a more promising area in future research.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Shingo Kajimura, Diabetes Center, University of California, San Francisco, California, USA
Francesc Villarroya, Biochemistry and Molecular Biology, University of Barcelona, Barcelona, 08028, Spain
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Nedergaard J, Bengtsson T, Cannon B: Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52. 10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- 2. Cypess AM, Lehman S, Williams G, et al. : Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. : Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8. 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Virtanen KA, Lidell ME, Orava J, et al. : Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Symonds ME, Aldiss P, Dellschaft N, et al. : Brown adipose tissue development and function and its impact on reproduction. J Endocrinol. 2018;238(1):R53–R62. 10.1530/JOE-18-0084 [DOI] [PubMed] [Google Scholar]
- 6. Cannon B, Nedergaard J: Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214(Pt 2):242–53. 10.1242/jeb.050989 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Symonds ME: Brown adipose tissue growth and development. Scientifica (Cairo). 2013;2013: 305763. 10.1155/2013/305763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Symonds ME, Pope M, Budge H: The Ontogeny of Brown Adipose Tissue. Annu Rev Nutr. 2015;35:295–320. 10.1146/annurev-nutr-071813-105330 [DOI] [PubMed] [Google Scholar]
- 9. Timmons JA, Wennmalm K, Larsson O, et al. : Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104(11):4401–6. 10.1073/pnas.0610615104 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Cannon B, Nedergaard J: Cell biology: Neither brown nor white. Nature. 2012;488(7411):286–7. 10.1038/488286a [DOI] [PubMed] [Google Scholar]
- 11. Seale P, Bjork B, Yang W, et al. : PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Cannon B, Nedergaard J: Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 13. Cannon B, Nedergaard J: What Ignites UCP1? Cell Metab. 2017;26(5):697–8. 10.1016/j.cmet.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 14. Nedergaard J, Cannon B: UCP1 mRNA does not produce heat. Biochim Biophys Acta. 2013;1831(5):943–9. 10.1016/j.bbalip.2013.01.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Ikeda K, Kang Q, Yoneshiro T, et al. : UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23(12):1454–65. 10.1038/nm.4429 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Cypess AM, Haft CR, Laughlin MR, et al. : Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 2014;20(3):408–15. 10.1016/j.cmet.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Law J, Chalmers J, Morris DE, et al. : The use of infrared thermography in the measurement and characterization of brown adipose tissue activation. Temperature. 2017;5(2):147–61. 10.1080/23328940.2017.1397085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ouellet V, Labbé SM, Blondin DP, et al. : Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–52. 10.1172/JCI60433 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Cannon B, Nedergaard J: Yes, even human brown fat is on fire! J Clin Invest. 2012;122(2):486–9. 10.1172/JCI60941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerngroß C, Schretter J, Klingenspor M, et al. : Active Brown Fat During 18F-FDG PET/CT Imaging Defines a Patient Group with Characteristic Traits and an Increased Probability of Brown Fat Redetection. J Nucl Med. 2017;58(7):1104–1110. 10.2967/jnumed.116.183988 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Leitner BP, Huang S, Brychta RJ, et al. : Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A. 2017;114(32):8649–54. 10.1073/pnas.1705287114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Yoneshiro T, Matsushita M, Hibi M, et al. : Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am J Clin Nutr. 2017;105(4):873–81. 10.3945/ajcn.116.144972 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Weir G, Ramage LE, Akyol M, et al. : Substantial Metabolic Activity of Human Brown Adipose Tissue during Warm Conditions and Cold-Induced Lipolysis of Local Triglycerides. Cell Metab. 2018;27(6):1348–1355.e4. 10.1016/j.cmet.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Chondronikola M, Volpi E, Børsheim E, et al. : Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–99. 10.2337/db14-0746 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Law J, Morris DE, Izzi-Engbeaya C, et al. : Thermal Imaging Is a Noninvasive Alternative to PET/CT for Measurement of Brown Adipose Tissue Activity in Humans. J Nucl Med. 2018;59(3):516–22. 10.2967/jnumed.117.190546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scotney H, Symonds ME, Law J, et al. : Glucocorticoids modulate human brown adipose tissue thermogenesis in vivo. Metabolism. 2017;70:125–32. 10.1016/j.metabol.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cypess AM, Chen YC, Sze C, et al. : Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109(25):10001–5. 10.1073/pnas.1207911109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Velickovic K, Lugo Leija HA, Bloor I, et al. : Low temperature exposure induces browning of bone marrow stem cell derived adipocytes in vitro. Sci Rep. 2018;8(1):4974. 10.1038/s41598-018-23267-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanssen MJ, Hoeks MJ, Brans B, et al. : Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21(8):863–5. 10.1038/nm.3891 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Hanssen MJ, van der Lans AA, Brans B, et al. : Short-term Cold Acclimation Recruits Brown Adipose Tissue in Obese Humans. Diabetes. 2016;65(5):1179–89. 10.2337/db15-1372 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Benador IY, Veliova M, Mahdaviani K, et al. : Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018;27(4):869–885.e6. 10.1016/j.cmet.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Jiang H, Ding X, Cao Y, et al. : Dense Intra-adipose Sympathetic Arborizations Are Essential for Cold-Induced Beiging of Mouse White Adipose Tissue. Cell Metab. 2017;26(4):686–692.e3. 10.1016/j.cmet.2017.08.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Cheng Y, Jiang L, Keipert S, et al. : Prediction of Adipose Browning Capacity by Systematic Integration of Transcriptional Profiles. Cell Rep. 2018;23(10):3112–25. 10.1016/j.celrep.2018.05.021 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Booth GL, Luo J, Park AL, et al. : Influence of environmental temperature on risk of gestational diabetes. CMAJ. 2017;189(19):E682–E689. 10.1503/cmaj.160839 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Blauw LL, Aziz NA, Tannemaat MR, et al. : Diabetes incidence and glucose intolerance prevalence increase with higher outdoor temperature. BMJ Open Diabetes Res Care. 2017;5(1):e000317. 10.1136/bmjdrc-2016-000317 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Cypess AM, Weiner LS, Roberts-Toler C, et al. : Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–8. 10.1016/j.cmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Hainer V: Beta3-adrenoreceptor agonist mirabegron - a potential antiobesity drug? Expert Opin Pharmacother. 2016;17(16):2125–7. 10.1080/14656566.2016.1233177 [DOI] [PubMed] [Google Scholar]
- 38. Sidossis LS, Porter C, Saraf MK, et al. : Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22(2):219–27. 10.1016/j.cmet.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Maloney SK, Fuller A, Mitchell D, et al. : Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda). 2014;29(6):413–20. 10.1152/physiol.00029.2014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Porter C, Herndon DN, Chondronikola M, et al. : Human and Mouse Brown Adipose Tissue Mitochondria Have Comparable UCP1 Function. Cell Metab. 2016;24(2):246–55. 10.1016/j.cmet.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Symonds ME, Sebert S, Budge H: The obesity epidemic: from the environment to epigenetics - not simply a response to dietary manipulation in a thermoneutral environment. Front Genet. 2011;2:24. 10.3389/fgene.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cypess AM, White AP, Vernochet C, et al. : Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19(5):635–9. 10.1038/nm.3112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Mo Q, Salley J, Roshan T, et al. : Identification and characterization of a supraclavicular brown adipose tissue in mice. JCI Insight. 2017;2(11): pii: 93166. 10.1172/jci.insight.93166 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Giles DA, Moreno-Fernandez ME, Stankiewicz TE, et al. : Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med. 2017;23(7):829–38. 10.1038/nm.4346 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Robinson LJ, Law JM, Symonds ME, et al. : Brown adipose tissue activation as measured by infrared thermography by mild anticipatory psychological stress in lean healthy females. Exp Physiol. 2016;101(4):549–57. 10.1113/EP085642 [DOI] [PubMed] [Google Scholar]
- 46. Ramage LE, Akyol M, Fletcher AM, et al. : Glucocorticoids Acutely Increase Brown Adipose Tissue Activity in Humans, Revealing Species-Specific Differences in UCP-1 Regulation. Cell Metab. 2016;24(1):130–41. 10.1016/j.cmet.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tian XY, Ganeshan K, Hong C, et al. : Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metab. 2016;23(1):165–78. 10.1016/j.cmet.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Osborn O, Olefsky JM: The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–74. 10.1038/nm.2627 [DOI] [PubMed] [Google Scholar]
- 49. Ye L, Wu J, Cohen P, et al. : Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A. 2013;110(30):12480–5. 10.1073/pnas.1310261110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation