Abstract
Hematopoietic stem cell transplantation following myeloablative chemotherapy is a curative treatment for many hematopoietic malignancies. However, profound granulocytopenia during the interval between transplantation and marrow recovery exposes recipients to risks of fatal infection, a significant source of transplant-associated morbidity and mortality. We have previously described the discovery of a small molecule, SW033291, that potently inhibits the prostaglandin degrading enzyme 15-PGDH, increases bone marrow prostaglandin E2, and accelerates hematopoietic recovery following murine transplant. Here we describe the efficacy of (+)-SW209415, a second-generation 15-PGDH inhibitor, in an expanded range of models relevant to human transplantation. (+)-SW209415 is 10,000-fold more soluble, providing the potential for intravenous delivery, while maintaining potency in inhibiting 15-PGDH, increasing in vivo prostaglandin E2, and accelerating hematopoietic regeneration following transplantation. In additional models, (+)-SW209415: (i) demonstrated synergy with granulocyte colony-stimulating factor, the current standard of care; (ii) maintained efficacy as transplant cell dose was escalated; (iii) maintained efficacy when transplant donors and recipients were aged; and (iv) potentiated homing in xenotransplants using human hematopoietic stem cells. (+)-SW209415 showed no adverse effects, no potentiation of in vivo growth of human myeloma and leukemia xenografts, and, on chronic high-dose administration, no toxicity as assessed by weight, blood counts and serum chemistry. These studies provide independent chemical confirmation of the activity of 15-PGDH inhibitors in potentiating hematopoietic recovery, extend the range of models in which inhibiting 15-PGDH demonstrates activity, allay concerns regarding potential for adverse effects from increasing prostaglandin E2, and thereby, advance 15-PGDH as a therapeutic target for potentiating hematopoietic stem cell transplantation.
Introduction
Hematopoietic stem cells (HSC) are rare, primitive cells in the blood and bone marrow which give rise to all differentiated blood cells.1–6 HSC are transplanted therapeutically after myeloablative chemotherapy as part of potentially curative treatment regimes for a variety of malignant and non-malignant disorders.7–10 However, profound neutropenia during the period while awaiting hematopoietic recovery after hematopoietic stem cell transplantation (HSCT) results in a high risk of opportunistic infection, which is a major source of morbidity, mortality, and prolonged hospitalization associated with the procedure.11,12 Strategies to accelerate hematopoietic regeneration following HSCT are thus of therapeutic interest. The eicosanoid signaling molecule prostaglandin E2 (PGE2), synthesized by the cyclooxygenase isoenzymes, has been recognized by several investigators as of interest for supporting hematopoietic regeneration. Ex vivo incubation of whole bone marrow with PGE2 has been shown to enhance HSC homing and proliferation in murine and non-human primate transplant models, and non-randomized clinical studies suggested potential for similar benefit in humans.13–17 In addition, in vivo treatment with PGE2 shows protective effects on murine hematopoietic cell populations following sublethal radiation via upregulation of cellular survival pathways.18,19 These observations suggest that in vivo modulation of PGE2 signaling could also potentiate hematopoietic recovery following HSCT.
We have previously identified 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the enzyme that mediates the first and rate-limiting step in PGE2 degradation in vivo, as a therapeutic target that, by acting on the bone marrow HSC niche, can potentiate hematopoietic recovery after HSCT.20 In particular, we described the discovery of SW033291, a potent small molecule 15-PGDH inhibitor (Ki = 0.1 nM).20 In in vivo studies, we have shown that, by inhibiting 15-PGDH, SW033291 doubles bone marrow PGE2 levels, induces bone marrow stromal production of CXCL12 and stem cell factor, potentiates HSC homing to the bone marrow niche, potentiates bone marrow colony-forming capacity, and, in murine HSCT, accelerates recovery of neutrophil counts by 6 days, enhances survival, and shows no long-term effects on serial transplantation capacity.20 In subsequent medicinal chemistry studies we have now developed SW209415, a second-generation 15-PGDH inhibitor, which has a 10,000-fold enhanced aqueous solubility of 4300 mg/mL (as an HCl salt) as compared to SW033921.21 This improved aqueous solubility of SW209415 would enable administration in a human-acceptable intravenous formulation.21 SW209415 modifies SW033291 by substituting a dimethyl-imidazole in place of a previous phenyl group and substituting a thiazole ring in place of a prior thiophene (Online Supplementary Figure S1). Moreover, we identified that all of the 15-PGDH inhibitory activity of both SW033291 and SW209415 lies in their respective (+)-enantiomers.21 We herein now describe the in vivo biological activities of (+)-SW209415 in modulating tissue PGE2, and in enhancing recovery from HSCT in an expanded range of biological models relevant to human HSCT, and furthermore evaluate concerns that 15-PGDH inhibitors, by increasing PGE2, could potentiate the in vivo growth of cancers. These studies provide positive findings that advance 15-PGDH as a therapeutic target for potentiating HSCT.
Methods
Bone marrow homing
Bone marrow homing to the recipient niche was measured by labeling donor marrow with 5 μM CellTrace CFSE (Life Technologies) for 30 min at 37°C and transplanting 10×106 labeled cells into lethally irradiated recipient mice (of the same age, gender, and strain). Recipient mice were give three intraperitoneal injections of vehicle or 5 mg/kg (+)-SW209415.
Colony-forming unit – spleen assay
Eight-week old C57BL/6J mice were lethally irradiated with 11 Gy and transplanted with 200×103 total bone marrow cells. Recipients were treated intraperitoneally with either vehicle or 2.5 mg/kg (+)-SW209415 twice daily for 12 days. On day 12 mice were sacrificed and spleens harvested and assessed for colony counts and SKL determination.
Bone marrow transplantation
Eight-week old female C57BL/6J mice were lethally irradiated with 11 Gy and transplanted with 500×103 total bone marrow cells 16 h after irradiation. Recipients were treated intraperitoneally with either vehicle or 2.5 mg/kg (+)-SW209415 twice daily through the course of the experiment. In studies involving granulocyte colony-stimulating factor (G-CSF), cohorts of mice were additionally treated once daily subcutaneously with 250 mg/kg human G-CSF or the combination of human G-CSF and (+)-SW209415.
Human bone marrow and umbilical cord blood studies
Discarded, de-identified human umbilical cord blood (3 unique samples) and adult bone marrow aspirates [2 unique samples from a 28-year old male (28/M) and a 50-year old male (50/M)] were obtained from the Case Western Reserve University Hematopoietic Biorepository with permission from the Institutional Review Board. Buffy coat from umbilical cord samples and total marrow from aspirates were incubated with carboxyfluorescein succinimidyl ester (CFSE) and the homing assay performed as described above. Engraftment at 12 weeks was studied by transplanting 1×106 mononuclear cells into each recipient NSG mouse. This was performed using two adult bone marrow aspirates (37 M and 41 M). Mice were treated twice daily for 21 days after the transplant with vehicle or 2.5 mg/kg (+)-SW209415 and bled at serial time-points through to day 84. Peripheral blood multilineage differentiation was assessed via flow cytometry (using CD3, B220, and CD11b gated from human CD45 cells).
Xenograft studies
In vivo growth of human acute myeloid leukemia (AML) and human multiple myeloma (MM) was established by transplanting 5×106 total human AML cells (cultured from a human AML patient at University Hospitals) or MM cells (ATCC line RPMI 8226), via the tail vein, into NSG mice receiving 2.5 Gy irradiation. Treatment was initiated 2 weeks after transplantation, with mice then started on twice daily intraperitoneal injections with either vehicle or 2.5 mg/kg (+)-SW209415 until the animals began to demonstrate noticeable signs of hunching and lethargy, at which point they were sacrificed to assess human CD45+ cells in the bone marrow of those that had received AML cells and human CD38+ cells in the bone marrow of those that had received MM cells.
Results
(+)-SW209415 inhibits 15-PGDH and induces prostaglandin E2 in vivo
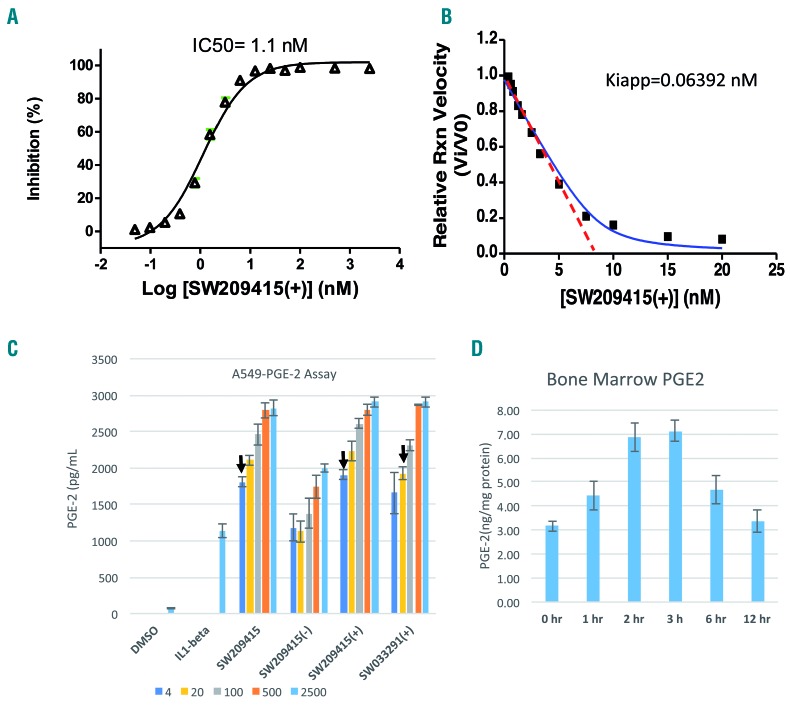
We first examined the potency of (+)-SW209415 in inhibiting enzymatic activity of recombinant 15-PGDH protein in vitro in a titration experiment in which (+)-SW209415 was added to ~5 nM 15-PGDH protein. The half minimal inhibitory concentration (IC50) of (+)-SW209415 was 1.1 nM, consistent with this compound acting as a tight-binding inhibitor of 15-PGDH (and suggesting approximately 50% activity of the recombinant protein) (Figure 1A). A Morrison design experiment estimated that the Kiapp for (+)-SW209415 is 0.06 nM (Figure 1B). (+)-SW209415 thus retains the tight-binding inhibitor characteristics of the parent SW033291 compound. To test the potency in inhibiting 15-PGDH in cells, (+)-SW209415 was added to cultures of A549 cells stimulated with interleukin-1β, and increases in levels of PGE2 secreted into the culture media were determined. In this cell-based assay, (+)-SW209415 showed a half maximal effective concentration (EC50) of approximately 10 nM, demonstrating improved potency compared to SW033291 which had an EC50 of 40 nM (Figure 1C, arrows denote approximate EC50 values). Furthermore, (+)-SW209415 retained high potency in inducing PGE2 in vivo in the bone marrow of treated mice: mice treated in vivo with a single intraperitoneal dose of 2.5 mg/kg showing a 2-fold induction of bone marrow PGE2 at 2 and 3 h following treatment, with levels falling at 6 h and returning to baseline at 12 h after treatment (Figure 1D).21 This PGE2 response matches the persistent 2-fold elevation of PGE2 observed in the 15-PGDH knockout mouse. Last, in vivo administration of (+)-SW209415 recapitulated the induction of two cytokines, stem cell factor and CXCL12, in the CD45− bone marrow stromal cell population, which was previously demonstrated by SW03329120 (Online Supplementary Figure S2).
Figure 1.
(+)-SW209415 inhibits 15-PGDH and induces prostaglandin E2 (PGE2) in vivo. (A) Increasing concentrations of (+)-SW209415 were added to 5 nM of 15-PGDH protein and enzyme activity was measured in duplicate determinations, with percent inhibition at each concentration depicted in the graph. (B) Graph of the relative initial 15-PGDH enzyme reaction velocities Vi/V0 versus concentration of (+)-SW209415. Vi values are the averages of triplicate determinations. (C) PGE2 levels in medium of A549 cells following stimulation with interleukin-1β and treatment with increasing concentrations of (+)-SW209415. The arrows indicate the approximate EC50 values. The graph shows the means of four independent determinations ± Standard Error of Mean. (D) Eight-week old C57/Bl6J female mice were administered a single intraperitoneal dose of 2.5 mg/kg (+)-SW209415. Animals were sacrificed at the time points indicated after the injection and bone marrow flushed for analysis of PGE2 via enzyme-linked immunosorbent assay (3 mice per time-point). hr: hour.
(+)-SW209415 promotes hematopoietic homing, engraftment and recovery after hematopoietic stem cell transplantation
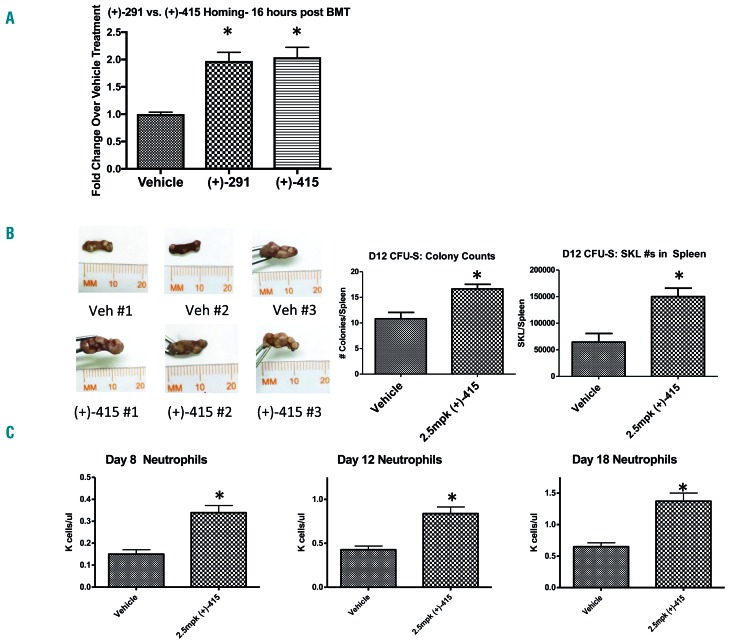
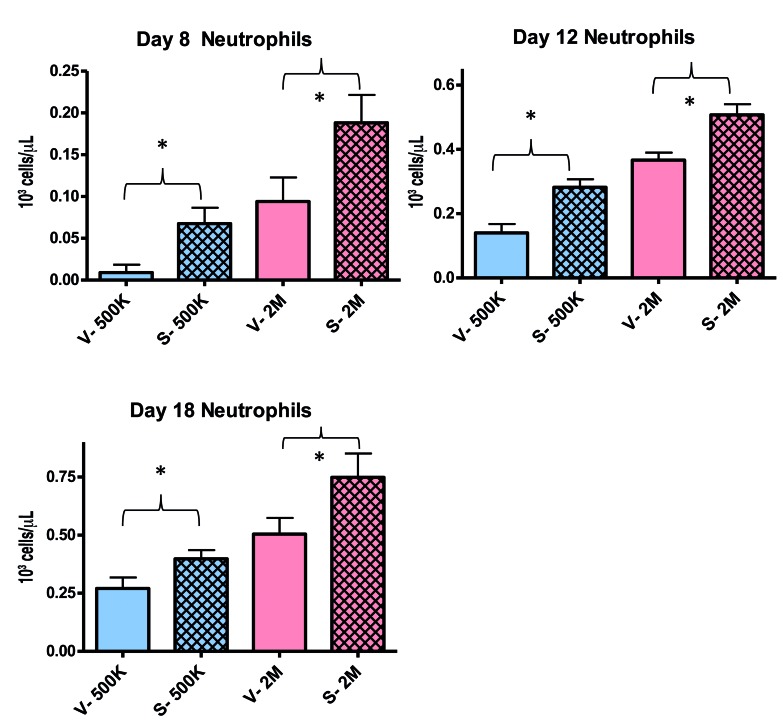
We next investigated the effects of (+)-SW209415 on steps in hematopoietic recovery following murine HSCT. We studied four distinct biological steps in recovery from HSCT: (i) homing of transplanted HSC to recipient marrow; (ii) expansion of transplanted HSC as hematopoietic colonies in the spleen; (iii) expansion of the stem cell-enriched SKL population in the bone marrow; and (iv) production of mature neutrophils in the peripheral blood. We first investigated the effects of (+)-SW209415 on enhancing efficiency of HSC homing to bone marrow following transplantation. CFSE-labeled cells that successfully homed to the recipient’s bone marrow were quantified 16 h following the transplant. Three doses of (+)-SW209415 induced a 2-fold increase in donor marrow cell homing to the marrow cavity of recipient mice tibiae (P=0.0002), with an activity essentially identical to that of three doses of (+)-SW033291 (Figure 2A). Homing of SKL cells to the bone marrow niche was increased by 1.8-fold in (+)-SW209415-treated mice (P=0.0029) (Online Supplementary Figure S3). We next examined the effects of (+)-SW209415 on splenic hematopoiesis following HSCT, where engraftment of individual HSC in the spleen can be assessed by their generation of macroscopic multilineage hematopoietic colonies termed CFU-S. On post-transplant day 12, (+)-SW209415-treated mice demonstrated a significant increase in total spleen colony counts (1.53-fold, P=0.003), an increased total spleen weight (Online Supplementary Figure S4), and a significantly higher number of splenic SKL cells (2.32-fold, P=0.02) (Figure 2B). We observed a similar 2.75-fold expansion of bone marrow SKL cells on day 18 in the same arm of (+)-SW209415-treated mice (P=0.0002). Thus (+)-SW209415 potentiates engraftment and expansion of donor hematopoietic cells in both bone marrow and spleen. Last, we examined the effect of (+)-SW209415 on the recovery of blood counts. (+)-SW209415-treated mice attained double the neutrophil counts of vehicle-treated control animals at each of post-HSCT days 8 (P<0.0001), 12 (P<0.0001), and 18 (P<0.0001) (Figure 2C). In addition, (+)-SW209415-treated mice demonstrated higher platelet counts on each of days 8 (P=0.005), 12 (P=0.06), and 18 (P=0.0005) and, in particular, showed a significantly higher day 8 nadir count (98×109/L in (+)-SW209415 treated mice versus 73×109/L in vehicle-treated controls; P=0.005) (Online Supplementary Figure S5).
Figure 2.
(+)-SW209415 promotes early hematopoietic homing, engraftment and recovery after bone marrow transplantation (BMT). (A) Lethally irradiated (11 Gy) 8-week old female C57/BL6J mice were transplanted with 107 CFSE-labeled total bone marrow cells and treated intraperitoneally with either vehicle or 2.5 mg/kg (+)-SW209415 immediately after irradiation, after BMT, and 8 hours (h) after BMT. Mice were sacrificed 16 h after the BMT and CFSE+ cells were measured in two femora + two tibiae/mouse. The graph shows mean values and Standard Error of Mean (SEM) (6 mice/arm). *Student t-test; P<0.05. (B) Lethally irradiated (11 Gy) 8-week old female C57/BL6J mice were transplanted with 200×103 total bone marrow cells and treated as in (A). The mice were sacrificed on day 12 after BMT. The spleens were harvested, fixed in Bouin solution, and the colonies counted (6 mice/arm). A second cohort of mice were sacrificed on day 12 and a single cell suspension prepared for SKL analysis via FACS (3 mice/arm). Representative photographs of day 12 spleens are shown on the left, with means and SEM of CFU-S and SKL numbers represented graphically on the right. *Student t-test; with a P<0.05. (C) Lethally irradiated (11 Gy) 8-week old female C57/BL6J mice were transplanted with 500×103 total bone marrow cells and treated intraperitoneally with either vehicle or 2.5 mg/kg (+)-SW209415 (twice daily). Peripheral blood counts were measured on days 8, 12, and 18 after BMT. The graph shows the mean values and SEM (13 mice/arm). *Student t-test; P<0.05.
To further distinguish differential effects of (+)-SW209415 on early versus later steps in hematopoietic reconstitution, we compared the effects of giving (+)-SW209415 for only the first 7 days following bone marrow transplantation (BMT) versus maintaining continuous dosing of (+)-SW209415 for 18 days. In this experiment mice that received continuous twice-daily doses of (+)-SW209415 attained neutrophil counts almost 3-fold higher on days 12 and 18 after BMT than those achieved in the vehicle-treated control animals, while mice that received only 7 days of (+)-SW209415 showed no significant difference in neutrophil counts on day 12 and a moderate increase on day 18 (Online Supplementary Figure S6). Moreover, while mice that received (+)-SW209415 continuously had a nearly 4-fold increase in bone marrow SKL cells (from 3707 to 14007, P=0.0026), mice that received only 7 days of (+)-SW209415 had only a 1.54-fold increase that did not reach statistical significance (Online Supplementary Figure S7). Collectively, these observations suggest that continuous in vivo dosing with the 15-PGDH inhibitor (+)-SW209415 optimally promotes improvement in hematopoietic recovery following murine BMT.
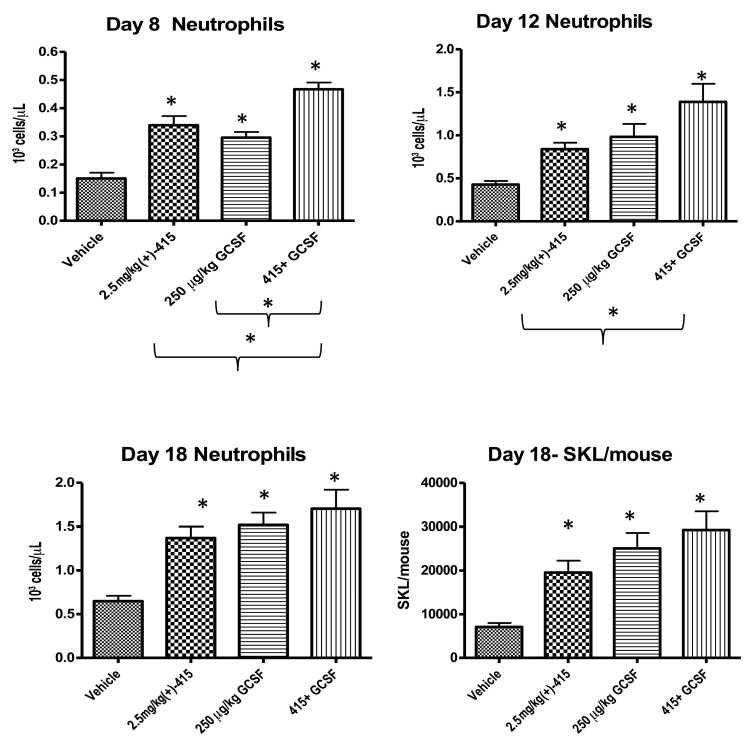
(+)-SW209415 accelerates neutrophil recovery after bone marrow transplantation even in mice treated with granulocyte colony-stimulating factor
G-CSF (clinical names, Lenograstim and Filgrastim) is a Food and Drug Administration-approved glycoprotein that is the standard of care used to accelerate granulocyte recovery after human HSCT. As initial human studies of (+)-SW209415 would almost certainly be done in HSCT patients also receiving G-CSF, we used mice to model the effects of (+)-SW209415 in comparison to and in combination with G-CSF. On post-transplant day 8 neutrophil counts were significantly higher in (+)-SW209415-treated mice (339×103 cells/μL, P<0.0001), and also in G-CSF-treated mice (295×103 cells/μL, P<0.0001), than in mice treated with vehicle control (150×103 cells/μL). However, mice treated with the combination of (+)-SW209415 and G-CSF developed 466×103 neutrophils/μL, which was significantly higher than the levels in mice treated with either (+)-SW209415 (P=0.0041) or G-CSF (P<0.0001) alone (Figure 3). Moreover, although, as compared to vehicle control, either (+)-SW209415 or G-CSF alone significantly increased neutrophil counts on post-HSCT days 8, 12 and 18, mice treated with the combination of (+)-SW209415 and G-CSF showed the highest neutrophil counts on each of these days (P=0.002 for the combination vs. (+)-SW209415, P=0.01 for the combination vs. G-CSF) (Figure 3). Furthermore, on day 12, the increase in the number of neutrophils in mice treated with the combination of (+)-SW209415 and G-CSF (an increase of 963×103 cells/μL over that in vehicle-treated mice) essentially equaled the additive increments of the two agents when given individually (412×103/μL for (+)-SW209415 and 555×103/μL for G-CSF, or the predicted additive effect of 967×103 cells/μL). Similarly, on day 8, the increase in number of neutrophils in mice treated with the combination of (+)-SW209415 and G-CSF (an increase of 316×103 cells/μL over that in the vehicle-treated mice) nearly equaled the additive increments produced by the two agents when given individually (189×103/μL for (+)-SW209415 and 145×103/μL for G-CSF, or the additive effect of 334×103 cells/μL). Furthermore, on post-HSCT day 18, mice treated with the combination of (+)-SW209415 plus G-CSF also showed the highest numbers of bone marrow SKL cells (Figure 3). In overview, treatment with (+)-SW209415 significantly accelerates neutrophil recovery after HSCT even in mice that are also receiving G-CSF, suggesting that this approach can provide an additive benefit over current standard therapy.
Figure 3.
(+)-SW209415 accelerates neutrophil recovery after bone marrow transplantation (BMT) in mice treated with granulocyte colony-stimulating factor. Lethally irradiated (11 Gy) 8-week old female C57/BL6J mice were transplanted with 500×103 total bone marrow cells and treated with either vehicle, 2.5 mg/kg (+)-SW209415 (twice daily, intraperitoneally), 250 mg/kg human G-CSF (once daily, subcutaneously), or the combination. The graphs display the means and Standard Error of Mean of peripheral blood neutrophil counts on days 8, 12, and 18 after BMT and bone marrow SKL cell numbers determined in mice sacrificed on day 18 (13 mice/arm). *Student t-test P<0.05.
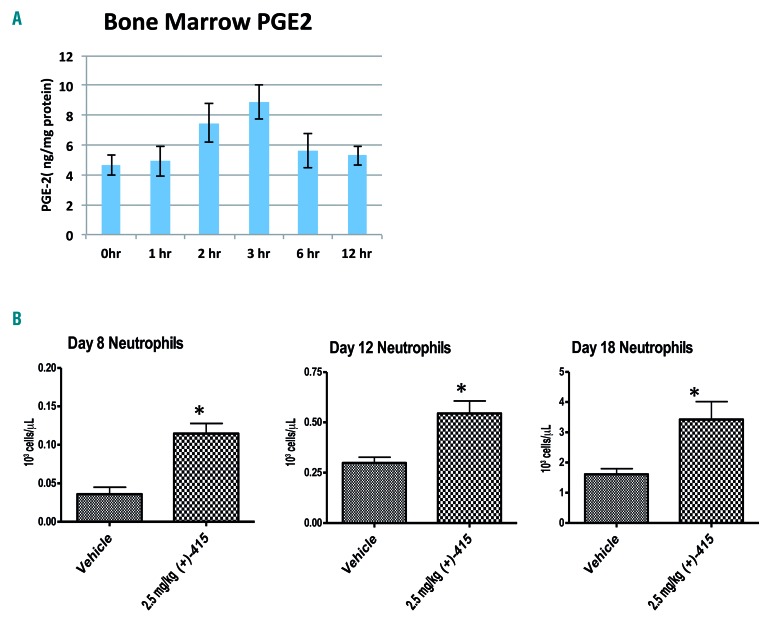
(+)-SW209415 is effective in promoting hematopoietic recovery after bone marrow transplantation in aged mice
Our prior studies demonstrating the effects of the 15-PGDH inhibitor SW033291 on accelerating hematopoietic reconstitution were performed with donor and recipient mice of 8–10 weeks of age, and thus roughly approximating humans of late teenage years.22 Humans undergoing HSCT are, however, primarily middle-aged and older, and the potency of human HSC in hematopoietic reconstitution is recognized to decline with age.23–25 To investigate potential age-related dependencies of the effects of 15-PGDH inhibition, both in inducing tissue PGE2 and in promoting hematopoietic reconstitution, we also studied mice aged 52 weeks old, an age at which females are approaching reproductive senescence and exhibiting other biological changes of late middle-aged humans. Reassuringly, in these older mice a single dose of (+)-SW209415 induced a 2-fold increase in bone marrow PGE2 at 3 h after injection (Figure 4A), essentially recapitulating the same pharmacodynamic effect demonstrated in 8-week old mice (Figure 1D). We next investigated the activity of (+)-SW209415 in accelerating recovery from HSCT in these older mice, performing murine HSCT in which both the bone marrow donor and lethally irradiated bone marrow recipient mice were 52 weeks old. Most notably, compared to control older mice, (+)-SW209415-treated older mice had double the number of neutrophils on each of days 8, 12, and 18 after transplantation (P=0.01) (Figure 4B). Moreover, we also observed an ~2-fold upward trend in the total number of SKL cells in the (+)-SW209415-treated cohort (Online Supplementary Figure S7). In overview, these observations confirm that the approach of inhibiting 15-PGDH to accelerate recovery from HSCT remains efficacious even when both transplant donor and recipient animals are older, which may model older persons.
Figure 4.
(+)-SW209415 is effective in promoting hematopoietic recovery after bone marrow transplantation (BMT) in aged mice. (A) Fifty-two-week old aged C57/Bl6J female mice were administered a single intraperitoneal dose of 2.5 mg/kg (+)-SW209415. Animals were sacrificed at the time points indicated after injection and bone marrow flushed for analysis of PGE2 via enzyme-linked immunosorbent assay (3 mice/arm). (B) Fifty-two-week old female donor and lethally irradiated (11 Gy) recipient C57/BL6J aged mice were used in this study. Mice were transplanted with 500×103 total bone marrow cells and treated with either vehicle or 2.5 mg/kg (+)-SW209415 (twice daily, intraperitoneally). The graphs display the means and Standard Error of Mean of peripheral blood neutrophil counts on day 8, 12, and 18 after BMT (10 mice/arm). *Student t-test P<0.05.
(+)-SW209415 accelerates hematopoietic recovery in hematopoietic stem cell transplant recipient mice that receive a “high-dose” inoculum of donor cells
(+)-SW209415 demonstrates activity in accelerating hematopoietic recovery in multiple different models of HSCT. However, hematopoietic recovery following HSCT can also be accelerated by providing a larger inoculum of transplanted donor cells. To investigate whether the benefits from inhibiting 15-PGDH are independent of the dose of transplanted donor cells, we re-examined our initial studies of murine HSCT using an escalated dose of 2×106 donor bone marrow cells. As expected, compared to mice transplanted with 500,000 donor cells, mice receiving a larger inoculum of 2×106 donor cells showed greater neutrophil recovery on each of post-transplant days 8 (P=0.009), 12 (P<0.0001), and 18 (P=0.01), achieving an effect quite similar to that of accelerating a 500,000 donor cell transplant with (+)-SW209415 (Figure 5). Notably though, treatment with (+)-SW209415 significantly further accelerated hematopoietic recovery even in mice transplanted with the higher cell dose. Indeed, mice receiving 2×106 donor cells plus (+)-SW209415 showed greater neutrophil recovery than corresponding mice receiving vehicle control on each of post-HSCT days 8 (P=0.04), 12 (P=0.0019), and 18 (P=0.04) (Figure 5). Furthermore, in mice transplanted with 2×106 donor bone marrow cells, added treatment with (+)-SW209415 increased post-HSCT day 18 numbers of marrow SKL cells by 55%, which is approximately the same proportion as the corresponding day 18 increase in neutrophil counts (Online Supplementary Figure S8). These data demonstrate that (+)-SW209415 continues to provide acceleration of hematopoietic recovery from HSCT even when the inoculum of transplanted donor cells is markedly increased.
Figure 5.
(+)-SW209415 accelerates hematopoietic recovery in hematopoietic stem cell transplantation (HSCT) recipient mice that receive “high-dose” donor cell inoculum. Lethally irradiated (11 Gy) 8-week old female C57/BL6J mice were transplanted with either 500 × 103 (blue bars) or 2×106 (red bars) total bone marrow cells and treated with either vehicle (V), or 2.5 mg/kg (+)-SW209415 (S). Graphs display means and Standard Error of Mean of peripheral blood neutrophil counts as measured on days 8, 12, and 18 after BMT (12 mice/arm). *Student t-test P<0.05.
(+)-SW209415 promotes bone marrow homing of human umbilical cord and human bone marrow hematopoietic stem cells
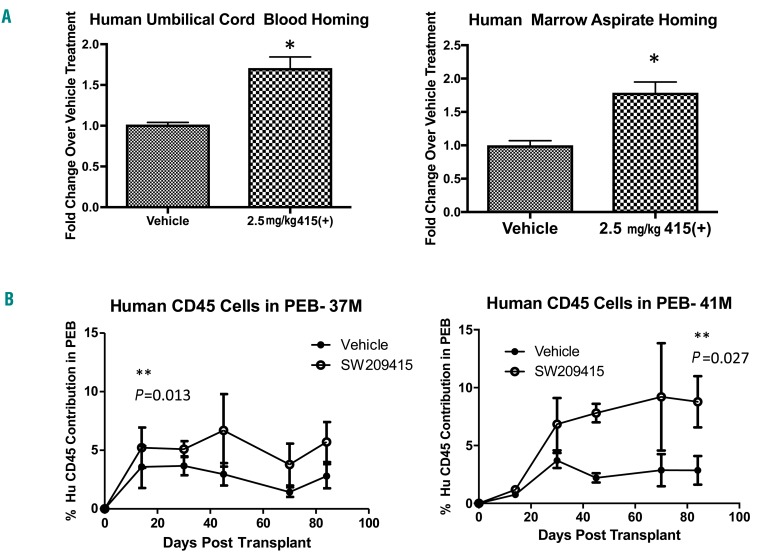
To further model the potential efficacy of (+)-SW209415 in human HSCT, we examined the effects of (+)-SW209415 in accelerating bone marrow homing of xenotransplanted human bone marrow and human umbilical cord blood cells. In these studies, lethally irradiated immunodeficient NSG mice received inocula of CFSE-labeled total human cells, and the frequency of the labeled cells was determined at 16 h after transplantation in recipient mouse femora and tibiae. We found that administering (+)-SW209415 increased homing of human bone marrow aspirate-derived cells by ~1.78-fold (P=0.0008) in a pooled analysis of two donors and increased homing of human umbilical cord blood-derived cells by ~1.71-fold (P=0.0001) in a pooled analysis of three donors (Figure 6A). A similar ~2-fold effect of (+)-SW209415 on increasing homing of purified human CD34+ cells was obtained in mice that were transplanted with CFSE-labeled isolated human CD34+ cells from two bone marrow aspirate donors (Online Supplementary Figure S9). Beneficial effects of (+)-SW209415 were maintained in follow-up studies assessing 12-week engraftment of human cells. In these experiments, NSG mice were transplanted with 1×106 mononuclear cells/mouse from total bone marrow aspirates of 2 donors and effects of (+)SW209415 human engraftment were assessed 84 days later. Treatment with (+)-SW209415 on days 1–21 days after BMT resulted in a 3.16-fold increase in day 84 human CD45+ bone marrow cells (P=0.024) (Online Supplementary Figure S10) and a similar 2- to 3-fold sustained increase in human peripheral blood chimerism from days 30–84 (P<0.03) (Figure 6B). CD45+ human-derived cells were identified in each of the myeloid, T-cell, and B-cell compartments, at frequencies ranging from 3–10% of human cells (data not shown). These data support that effects of (+)-SW209415 in accelerating HSCT recovery will translate to humans as well.
Figure 6.
(+)-SW209415 promotes bone marrow homing of human umbilical cord and human bone marrow hematopoiesis stem cells. (A) Irradiated (2.5Gy) 8-week old female NSG mice were transplanted with 20*106 CFSE-labeled total human umbilical cord blood buffy coat (3 samples transplanted into 4 mice/condition/sample) or total human marrow aspirate cells (2 samples transplanted into 3 mice/condition/sample). Mice were treated with either vehicle or 2.5 mg/kg (+)-SW209415 immediately after irradiation, after bone marrow transplantation (BMT), and 8 hours (h) after BMT. Graphs show mean and Standard Error of Mean for % of CFSE-labeled human donor cells homing to murine marrow. *Student t-test P<0.05. (B) Irradiated (2.5 Gy) 8-week old male NSG mice were transplanted with 1*106 white blood cells from 2 unique bone marrow aspirate donors and treated with either vehicle or 2.5mg/kg (+)-SW209415 twice daily for 21 days. Peripheral blood was collected at multiple intervals after transplant and human CD45 measured via flow cytometry.
(+)-SW209415 does not affect in vivo growth of human hematopoietic cancer cells
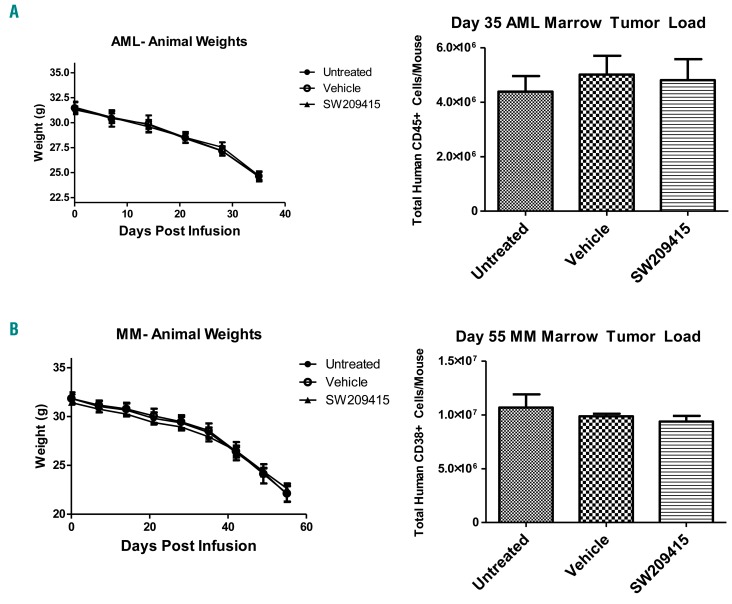
Although hematopoietic growth factors such as G-CSF have been safely administered to human HSCT patients with hematologic malignancies, concerns have been raised regarding potential effects of increasing bone marrow PGE2 on growth of human cancer cells.26 To address this issue, we examined the effects of (+)-SW209415 on in vivo growth of xenografted cancer cells from a human AML and from a human MM, two cancers that are commonly treated by HSCT. We established cohorts of NSG mice xenotransplanted with primitive human AML blasts or with human MM cells, and treated these mice with (+)-SW209415 2.5 mg/kg twice daily for 21 days, or with vehicle control. In both AML and MM xenotransplanted cohorts, administering (+)-SW209415 showed no effect on the rate of decline of animal weights and no effect on the number of human cancer cells that expanded in the mouse bone marrow (Figure 7A,B). While further studies with additional hematopoietic tumor models are warranted, these first experimental results do not support the hypothesis that a doubling of bone marrow PGE2 will potentiate the in vivo growth of human cancer cells.
Figure 7.
(+)-SW209415 does not effect in vivo growth of human hematopoietic cancer cells. Irradiated (2.5Gy) 8-week old male NSG mice were transplanted with 5*106 human acute myeloid leukemia (AML) (A) or multiple myeloma (MM) cells (B). Two weeks after transplant mice began intraperitoneal treatment with either vehicle or 2.5 mg/kg (+)-SW209415 twice daily for 21 days. Upon signs of impending animal death (hunching, limited mobility) mice were sacrificed and the frequency of human CD45+ cells (for AML) or human CD38+ cells (for MM) in mouse marrow was measured via flow cytometry (4 mice/arm). Graphs show means and Standard Error of Mean for animal weights and for human cancer cell numbers in the mouse bone marrow.
Humans with homozygous inactivating mutations of 15-PGDH show chronically elevated PGE2 levels, but except for development of digital clubbing are otherwise phenotypically normal.27–32 However, a subset of individuals with mutations that inactivate SLCO2A1, the transporter that carries PGE2 to 15-PGDH, have been reported to develop myelofibrosis.33 As myelofibrosis has been observed in only some of these individuals, the question has arisen as to whether this finding is related to the increased PGE2 seen with loss of SLCO2A1, or whether it might be due to other genetic defects in these individuals who were all offspring of consanguineous marriages. To investigate this issue, we examined bone marrow from mice following 30 days of twice daily treatment with 2.5 mg/kg (+)-SW209415 or with vehicle control. The potential development of bone marrow fibrosis was assessed by reticulin staining. Essentially no myelofibrosis was detected in either control or (+)-SW209415-treated mice (Online Supplementary Figure S11). While longer term studies remain warranted, these initial results mitigate concerns of bone marrow fibrosis as a potential toxicity from treatment with (+)-SW209415.
(+)-SW209415 does not show off-target toxicity
As an additional evaluation, we examined for potential off-target toxicities that might be induced by (+)-SW209415. Mice were treated twice daily for 21 days with vehicle or with escalating doses of (+)-SW209415 over a range of doses from the therapeutically active dose of 2.5 mg/kg up to 10-fold above this, at 25 mg/kg. Parallel pharmacokinetic experiments confirmed dose-proportional increases in maximum and total plasma exposure (Cmax, area under the curve) over this dosing range. Administering (+)-SW209415 did not produce any adverse effects on mouse weights (Online Supplementary Figure S12), activity, or grooming. Additionally, administering (+)-SW209415 did not have adverse effects on peripheral blood counts (Online Supplementary Figure S12) or on any of a battery of serum chemistry values (Online Supplementary Table S1). These data suggest that (+)-SW209415 is without off-target toxicity at doses of up to 10-fold over the therapeutically active range.
Discussion
In summary, (+)-SW209415, a second-generation 15-PGDH inhibitor, provides independent chemical support that 15-PGDH is a therapeutic target whose inhibition accelerates hematopoietic recovery following HSCT. Moreover, these studies show that (+)-SW209415 advantages hematopoietic recovery across a broad range of models that newly interrogate key features of human HSCT. Firstly, these studies demonstrate that (+)-SW209415 accelerates neutrophil recovery after HSCT in mice that are also treated with G-CSF, which supports that (+)-SW209415 will provide added benefit in human HSCT. Secondly, these studies demonstrate that (+)-SW209415 promotes hematopoietic recovery following HSCT in models in which both HSC donors and HSCT recipients are older. The current findings demonstrate that the pharmacodynamic activity of 15-PGDH and the downstream responses that potentiate bone marrow recovery both remain robust in older animals. Thirdly, these findings show that (+)-SW209415 advantages hematopoietic recovery, even when recovery is independently accelerated by administering a 4-fold increased number of donor HSC. This observation also suggests that (+)-SW209415 would provide benefit at doses of HSC typically employed in human HSCT. Fourthly, and further supporting potential applicability of these findings to human HSCT, (+)-SW209415 potentiates homing of human HSC (that have multilineage differentiation capacity) when assayed by xenotransplantation into lethally irradiated NSG mice. Our further findings that the efficacy of (+)-SW209415 is maximal when the drug is administered twice daily throughout the full period of bone marrow recovery informs the optimal method for administering 15-PGDH inhibitors in HSCT and also supports that the mechanism of action of (+)-SW209415 is distinct from approaches that have examined acute ex vivo exposure of donor cells to PGE2 analogs prior to graft administration.14–17 These findings, across multiple different models, are consistent with and extend our prior observations with SW033291, a first-generation 15-PGDH inhibitor, that demonstrated inhibiting 15-PGDH increased bone marrow stem cells as assessed by both cell surface markers and by marrow colony-forming assays.20
These demonstrations of efficacy are further buttressed by findings supporting the safety of (+)-SW209415, both when tested for evidence of hypothesized or potential on-target toxicities and when tested for off-target effects. We have previously shown that administering a 15-PGDH inhibitor to mice receiving a bone marrow transplant had no adverse effects, as assayed by ability to serially transplant the regenerated bone marrow, and also did not show any evidence of tumor induction during 7 months following whole body irradiation.20 These new studies also produce no evidence that inhibiting tissue 15-PGDH has any effect on in vivo growth of either human MM cells or human AML cells. Although studies in additional human tumor models remain warranted, the absence of any effect of increased PGE2 on the in vivo growth of these cancer cells allays, at least, in part the hypothetical concern of this as a potential on-target toxicity of therapies employing 15-PGDH inhibitors.26 This observed lack of effect of PGE2 modulation on growth of established human cancer cells is consistent with clinical observations of a lack of effects of non-steroidal anti-inflammatory drugs, which lower tissue PGE2, in clinical trials testing these drugs as therapeutic agents for treating established cancers,34–37 and is consistent with prior hypotheses that oncogene activation may render tumor stem cells independent of PGE2 signaling.38 Furthermore, we found no evidence of off-target toxicity associated with (+)-SW209415 when chronically administered at doses 10-fold above the dose that is therapeutically effective in potentiating BMT recovery, thus demonstrating a substantial therapeutic index for this chemical scaffold.
In conclusion, our results identify (+)-SW209415 as a promising second-generation 15-PGDH inhibitor that validates the efficacy and safety of targeting 15-PGDH in multiple murine models and thereby advances 15-PGDH as a therapeutic target for potentiating HSCT.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/6/1054
Funding
This work was funded by NIH grants R35 CA197442, R01 216863, and U54 HL119810, by a Harrington Discovery Institute Scholar Innovator Award, by an award from the Case Western Reserve University Council to Advance Human Health, by the Welch Foundation (I-1612), and by a sponsored research agreement to Case Western Reserve University from Rodeo Therapeutics. This research was also supported by the Radiation Resources Core Facility (P30CA043703), the Hematopoietic Biorepository and Cellular Therapy Core Facility (P30CA043703), and the Cytometry & Imaging Microscopy Core Facility of Case Comprehensive Cancer Center (P30CA043703).
References
- 1.Rossi L, Lin KK, Boles NC, et al. Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell. 2012;11(3):302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126(22):2443–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96(6):3120–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nygren JM, Bryder D. A novel assay to trace proliferation history in vivo reveals that enhanced divisional kinetics accompany loss of hematopoietic stem cell self-renewal. PLoS One. 2008;3(11):e3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA. 2003;100 Suppl 1:11842–11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97(10):2962–2971. [DOI] [PubMed] [Google Scholar]
- 8.Bird JM, Russell NH, Samson D. Minimal residual disease after bone marrow transplantation for multiple myeloma: evidence for cure in long-term survivors. Bone Marrow Transplant. 1993;12(6):651–654. [PubMed] [Google Scholar]
- 9.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16): 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shenoy S. Hematopoietic stem-cell transplantation for sickle cell disease: current evidence and opinions. Ther Adv Hematol. 2013;4(5):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86(7):2856–2862. [PubMed] [Google Scholar]
- 12.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015;50(4):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagedorn EJ, Durand EM, Fast EM, Zon LI. Getting more for your marrow: boosting hematopoietic stem cell numbers with PGE2. Exp Cell Res. 2014;329(2):220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goessling W, Allen RS, Guan X, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8(4):445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122(17):3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter RL, Georger MA, Bromberg O, et al. Prostaglandin E2 increases hematopoietic stem cell survival and accelerates hematopoietic recovery after radiation injury. Stem Cells. 2013;31(2):372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoggatt J, Singh P, Stilger KN, et al. Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells Mol Dis. 2013;50(3):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Desai A, Yang SY, et al. Tissue regeneration. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science. 2015;348(6240):aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antczak MI, Zhang Y, Wang C, et al. Inhibitors of 15-prostaglandin dehydrogenase to ptentiate tissue repair. J Med Chem. 2017;60(9):3979–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. [DOI] [PubMed] [Google Scholar]
- 23.Popplewell LL, Forman SJ. Is there an upper age limit for bone marrow transplantation? Bone Marrow Transplant. 2002;29(4):277–284. [DOI] [PubMed] [Google Scholar]
- 24.Kim MJ, Kim MH, Kim SA, Chang JS. Age-related deterioration of hematopoietic stem cells. Int J Stem Cells. 2008;1(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13(5):376–389. [DOI] [PubMed] [Google Scholar]
- 26.FitzGerald GA. Biomedicine. Bringing PGE(2) in from the cold. Science. 2015;348(6240):1208–1209. [DOI] [PubMed] [Google Scholar]
- 27.Bergmann C, Wobser M, Morbach H, et al. Primary hypertrophic osteoarthropathy with digital clubbing and palmoplantar hyperhidrosis caused by 15-PGHD/HPGD loss-of-function mutations. Exp Dermatol. 2011;20(6):531–533. [DOI] [PubMed] [Google Scholar]
- 28.Diggle CP, Carr IM, Zitt E, et al. Common and recurrent HPGD mutations in Caucasian individuals with primary hypertrophic osteoarthropathy. Rheumatology (Oxford). 2010;49(6):1056–1062. [DOI] [PubMed] [Google Scholar]
- 29.Erken E, Koroglu C, Yildiz F, Ozer HT, Gulek B, Tolun A. A novel recessive 15-hydroxyprostaglandin dehydrogenase mutation in a family with primary hypertrophic osteoarthropathy. Mod Rheumatol. 2015;25(2):315–321. [DOI] [PubMed] [Google Scholar]
- 30.Sinibaldi L, Harifi G, Bottillo I, et al. A novel homozygous splice site mutation in the HPGD gene causes mild primary hypertrophic osteoarthropathy. Clin Exp Rheumatol. 2010;28(2):153–157. [PubMed] [Google Scholar]
- 31.Uppal S, Diggle CP, Carr IM, et al. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet. 2008;40(6):789–793. [DOI] [PubMed] [Google Scholar]
- 32.Yuksel-Konuk B, Sirmaci A, Ayten GE, et al. Homozygous mutations in the 15-hydroxyprostaglandin dehydrogenase gene in patients with primary hypertrophic osteoarthropathy. Rheumatol Int. 2009; 30(1):39–43. [DOI] [PubMed] [Google Scholar]
- 33.Diggle CP, Parry DA, Logan CV, et al. Prostaglandin transporter mutations cause pachydermoperiostosis with myelofibrosis. Hum Mutat. 2012;33(8):1175–1181. [DOI] [PubMed] [Google Scholar]
- 34.Lilenbaum R, Socinski MA, Altorki NK, et al. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second-line treatment of non-small-cell lung cancer. J Clin Oncol. 2006;24(30):4825–4832. [DOI] [PubMed] [Google Scholar]
- 35.El-Rayes BF, Zalupski MM, Manza SG, et al. Phase-II study of dose attenuated schedule of irinotecan, capecitabine, and celecoxib in advanced colorectal cancer. Cancer Chemother Pharmacol. 2008;61(2): 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies JM, Goldberg RM. First-line therapeutic strategies in metastatic colorectal cancer. Oncology (Williston Park). 2008;22(13): 1470–1479. [PubMed] [Google Scholar]
- 37.Maiello E, Giuliani F, Gebbia V, et al. FOLFIRI with or without celecoxib in advanced colorectal cancer: a randomized phase II study of the Gruppo Oncologico dell’Italia Meridionale (GOIM). Ann Oncol. 2006;17(Suppl 7):vii55–59. [DOI] [PubMed] [Google Scholar]
- 38.Markowitz SD. Aspirin and colon cancer–targeting prevention? N Engl J Med. 2007;356(21):2195–2198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.