Abstract
Objective
To assess the reliability and usefulness of an EEG-based brain-computer interface (BCI) for patients with advanced amyotrophic lateral sclerosis (ALS) who used it independently at home for up to 18 months.
Methods
Of 42 patients consented, 39 (93%) met the study criteria, and 37 (88%) were assessed for use of the Wadsworth BCI. Nine (21%) could not use the BCI. Of the other 28, 27 (men, age 28–79 years) (64%) had the BCI placed in their homes, and they and their caregivers were trained to use it. Use data were collected by Internet. Periodic visits evaluated BCI benefit and burden and quality of life.
Results
Over subsequent months, 12 (29% of the original 42) left the study because of death or rapid disease progression and 6 (14%) left because of decreased interest. Fourteen (33%) completed training and used the BCI independently, mainly for communication. Technical problems were rare. Patient and caregiver ratings indicated that BCI benefit exceeded burden. Quality of life remained stable. Of those not lost to the disease, half completed the study; all but 1 patient kept the BCI for further use.
Conclusion
The Wadsworth BCI home system can function reliably and usefully when operated by patients in their homes. BCIs that support communication are at present most suitable for people who are severely disabled but are otherwise in stable health. Improvements in BCI convenience and performance, including some now underway, should increase the number of people who find them useful and the extent to which they are used.
Brain-computer interfaces (BCIs) enable people to interact with the world through brain signals (e.g., EEG activity) rather than muscles.1 The principal rationale for their development has been that BCIs could restore communication and control to people with severe neuromuscular disabilities. However, despite >4,000 BCI research studies,2 only 3 case reports3–5 have described independent home use of a BCI for communication (i.e., use that is not supervised by an investigator) by people who were severely disabled. Thus, beyond this anecdotal information, the translational potential of BCIs—whether they can function reliably and usefully when operated by patients in home environments—remains unexplored.
People with advanced amyotrophic lateral sclerosis (ALS) are a logical population in which to address this critical question. They often lose the capacity to communicate as their disease progresses. Actual or anticipated loss of communication contributes to the fact that >90% of people in the United States with ALS decline long-term mechanical ventilation when it becomes necessary.6–10 Thus, BCIs that can preserve communication might affect clinical recommendations and patient decisions.1,11,12
Encouraged by the several case reports,3–5 this study investigated independent home use of an EEG-based BCI by a group of people who were severely disabled. It set out to determine whether the BCI system could function reliably when used by patients in their homes, the extent of use, and whether clinical personnel could provide effective technical support.13 It also assessed BCI effect on the patient and the primary caregiver.
Methods
These issues were addressed in the context of the US Department of Veterans Affairs (VA) nationally integrated health care system.14 The study was motivated largely by the high incidence of ALS in veterans.15 We enrolled patients with advanced ALS from 5 VA medical centers. VA personnel recruited the patients, placed the BCIs in their homes, trained the patients and their caregivers (i.e., the BCI system assistants [SAs]), and provided technical support as needed. Wadsworth Center personnel provided the BCI systems, trained VA personnel in their use, and supported the VA personnel as needed throughout the study.
Standard protocol approvals, registrations, and patient consents
The VA Central Institutional Review Board reviewed and approved the protocol. All patients and their SAs gave informed consent.
Patients
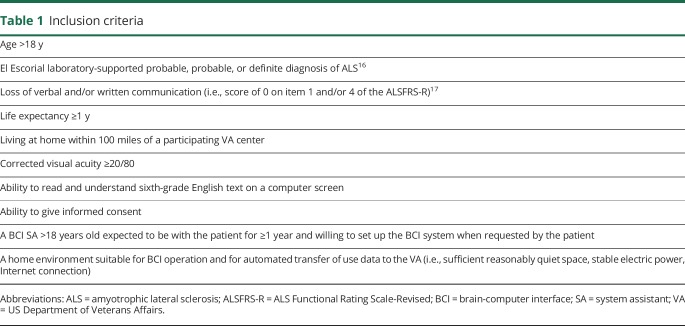
Table 1 lists the inclusion criteria. Patients who appeared to meet these criteria were identified by investigators at the participating VA centers from their practices or the National Registry of Veterans With ALS18 or were referred from nearby VA facilities. After the patients and their SAs provided informed consent, they were evaluated at home by the site investigator (i.e., a neurologist) to confirm that they met the inclusion criteria. If they did, their ability to use the BCI was assessed by their accuracy on a copy-spelling task. This task had a 6 × 6 matrix of possible selections; thus, chance accuracy was 1/36, or 3%. The inclusion criterion was ≥70% accuracy on the task for at least 2 of 2 or 3 sessions administered within 3 weeks. This criterion was based on the highly nonlinear relationship between accuracy and effective communication rate.19 Patients satisfying this criterion were asked to become BCI home users.
Table 1.
Inclusion criteria
Study design
After patients agreed to become home users, the BCI system was placed in the home, and the patient and SA were trained to use it and its applications over two to six 1- to 2-hour home visits. Once they demonstrated mastery of the system by using it successfully without study personnel present (success was documented from data obtained over the Internet), they entered the 12- to 18-month home-use phase of the study.
Throughout this period, complete data on BCI use, including EEG activity, were automatically collected daily through a secure Internet connection. Every 3 months, study personnel visited the home to interview the user and SA, to address their questions and concerns, and to check the BCI. The patients could use the BCI to answer interview questions.
Occasional ad hoc visits (actual or virtual) were made as needed to introduce new or updated BCI applications, to retrain the patient and SA after a hiatus in BCI use (e.g., due to hospitalization), to train a new SA, to resolve a technical problem, or to otherwise improve BCI performance and convenience.
The BCI home system
The Wadsworth BCI home system comprises a laptop computer with BCI2000 software,20,21 an 8-channel EEG amplifier, a 50-cm monitor and stand, a power conditioner, connecting cables and power cords, an external speaker, a web camera, and an electrode cap with tin electrodes at locations Fz, Cz, Pz, P3, P4, PO7, PO8, and Oz.22 A commercial version of this system would cost $5,000.
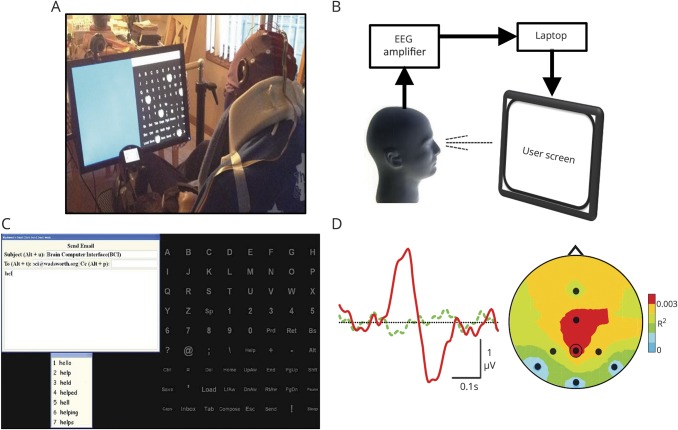
Figure 1A shows a patient using the BCI and the major components of the system (figure 1B).
Figure 1. BCI system and its operation.
(A) A home user operating the brain-computer interface (BCI). (B) The EEG amplifier amplifies and digitizes the 8 EEG channels. The laptop computer analyzes the EEG, controls the user screen, and stores all data. (C) BCI operation illustrated with the e-mail application. The 72-item matrix of possible selections (i.e., letters, numbers, functions) is equivalent to a full keyboard. To make a selection, the user pays attention to the desired item as groups of items flash in rapid succession. The e-mail message appears at the top left. A predictive speller option (Word Q3; Mayer-Johnson) is at the bottom left (e.g., after spelling “hel,” the user can complete the word “hello” simply by selecting the numeral 1). (D) BCI detection of the user's selection. (Left) The user's average EEG response at location Pz to the flash of a group of items that includes the desired item (solid red) differs from the average response to the flash of a group that does not include it (dashed green). The BCI detects this difference and selects the desired item. (Right) Topographic distribution (238 milliseconds after flash; nose at top) of the difference between the 2 responses (measured as R2). The EEG recording sites are indicated (Pz is circled). The difference between the responses has a typical posterior-central focus. See elsewhere23–26 for details.
The 72-item matrix of possible selections (i.e., letters, numbers, functions) (figure 1C) provides the functionality of a full keyboard. To select an item, the user pays attention to the desired item as groups of 5 to 6 items flash in rapid succession (typically every 125 milliseconds). As successive selections are made, the e-mail message appears (top left). A predictive speller (Word Q3; Mayer-Johnson, Pittsburgh, PA) located at the bottom left enables the user to complete words more quickly (e.g., after spelling “hel,” the user can finish the word “hello” by selecting the numeral 1). To determine which item the user wants to select, the BCI calculates for each EEG location the user's average responses to each of the 72 items. It then compares the 72 average responses at specific locations in terms of their voltages for specific latency periods after the flash. (The specific locations and latency periods are those previously defined for the individual by the BCI calibration procedure.) This comparison detects a user-specific difference between the response to the item the user wants to select and the responses to the other 71 items (e.g., figure 1D). Full details on the system, its calibration, and its operation have been published,13,23–26 and an illustrative video is available at neurotechcenter.org/videos/cbs-60-minutes.
The BCI applications include a simple setup program for the SA; a menu that enables the patient to select applications; a copy-spelling/calibration program to measure accuracy and to update system parameters; a communication program (WordPad) with word prediction and print and speech outputs; an e-mail program with word prediction; an Internet news reader; and audio and video content (e.g., audio books, YouTube videos, picture albums).26 Figure 1C illustrates the e-mail application. Figure 1D shows how the BCI enables the patient to select an item from the 72-item matrix.
Outcome measures
The outcomes were (1) BCI use (total time, time per application, days per week, selection rate, and accuracy [which was assessed by the copy-spelling program]), (2) effect on the user (McGill Quality of Life [MQoL]27) and perception of BCI benefit and burden, (3) effect on the SA (perception of BCI benefit and burden), (4) technical support (number and nature of issues, resolution time), and (5) patient attrition (study stage and reason). BCI benefit and burden were assessed by the user's responses (on a 7-point Likert scales from 1 [strongly disagree] to 7 [strongly agree]) to the 2 statements: “The benefit I derive from using the BCI is much greater than any difficulty associated with using the BCI” and “The burden associated with using the BCI is so great I would rather not use the BCI,” as well as by the SA's responses to equivalent questions.
Data at study entry
Data collected at study entry included demographics (e.g., age, sex, education level), medical history, and medications; ALS Functional Rating Scale–Revised (ALSFRS-R)17; and MQoL.27 The MQoL questionnaire assessed the patient's overall QoL (physical and psychological symptoms, physical and existential well-being, and support systems) on a scale of 0 to 10 (10 highest). These data were updated during follow-up visits (typically every 3 months).
Data analysis
Data analysis was based on the intention-to-treat principle.28 Thus, the study population comprised the 42 individuals who appeared to meet the study criteria, were interested in using the BCI, and were consented. Some left the study before or after completing training and beginning BCI use; the reasons are described. For those who completed training and became BCI home users, the extent, nature, effect, and support requirements of their BCI use are described in detail. Thus, the presentation provides a comprehensive picture of the outcome when a group of veterans severely disabled by ALS indicated interest in undertaking BCI home use.
Data availability
Deidentified data sets will be shared for specified uses after approval by the VA.
Results
BCI users
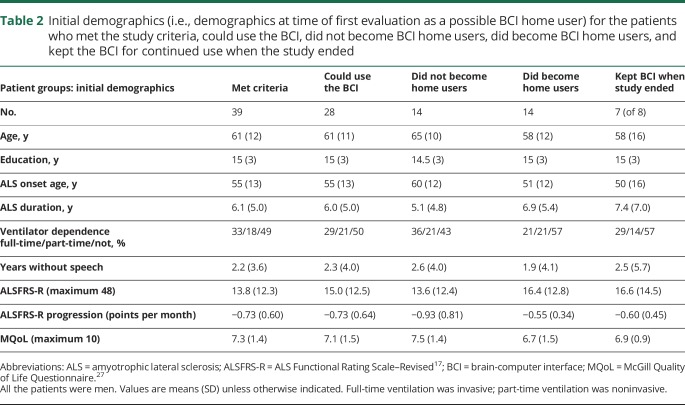
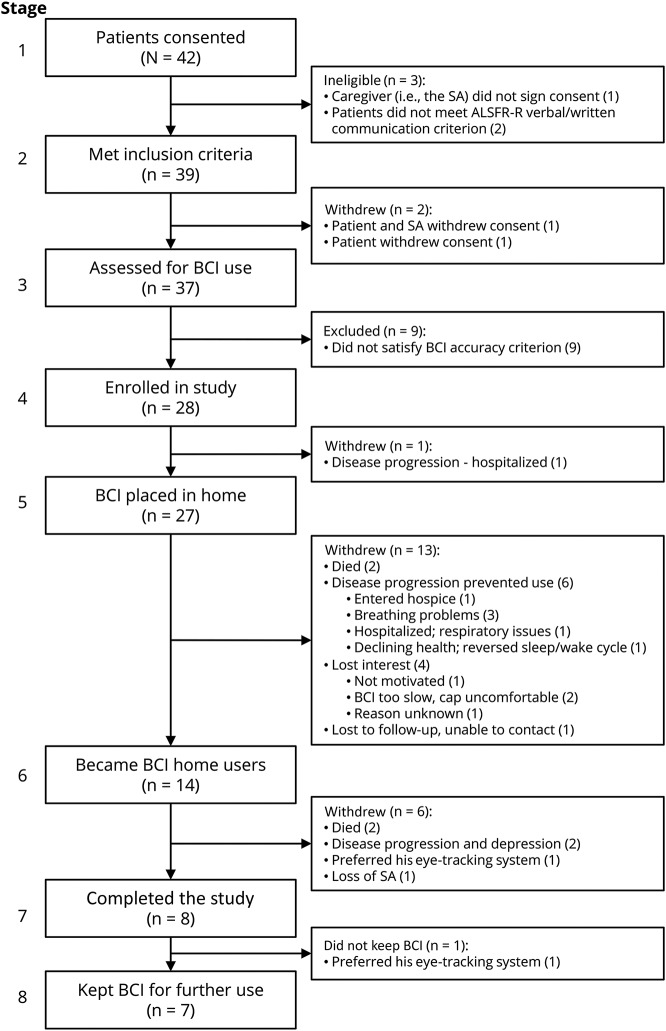
Forty-two patients provided informed consent. All were men (94.1% of veterans ≥50 years old are male29). While the absence of women is a limitation of the study, we know of no published data suggesting that men and women differ in BCI performance (nor have we noted such a difference in our own studies over the past 30 years). Table 2 provides demographics for the 39 who satisfied the inclusion criteria and for study-related subsets. Figure 2 describes their progression through the 8 stages of the study, indicating the attrition at each stage and the reasons for it. The predominant reason for attrition before stage 4 was insufficient BCI accuracy; consistent with previous work,23 impaired vision (e.g., due to ptosis, cataracts, diplopia) was the major cause.
Table 2.
Initial demographics (i.e., demographics at time of first evaluation as a possible BCI home user) for the patients who met the study criteria, could use the BCI, did not become BCI home users, did become BCI home users, and kept the BCI for continued use when the study ended
Figure 2. Patient progression through the 8 stages of the study.
Reasons for attrition at each stage are indicated. ALSFRS-R = ALS Functional Rating Scale–Revised; BCI = brain-computer interface; SA = system assistant.
As figure 2 shows, 28 patients were able to use the BCI (stage 4). Fourteen left the study before stage 6, 9 because of death or rapid disease progression (often resulting in death shortly afterward), 4 because of limitations of the BCI, and 1 who was lost to follow-up. The other 14 reached stage 6: they and their SAs completed 4 (±2 SD) training sessions over 3.8 (±2.1 SD) weeks and demonstrated their ability to use the BCI without study personnel present. They thereby became BCI home users.
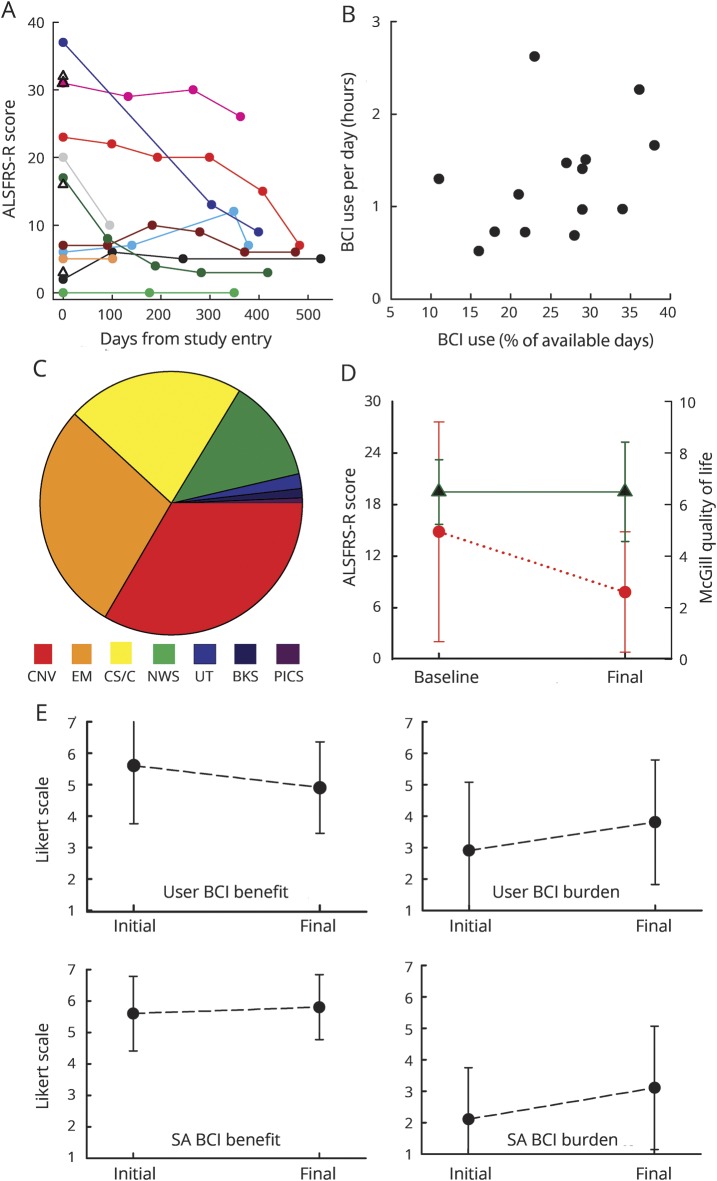
These 14 patients used the BCI independently in their homes for 2 to 17 (average 9 ± 5 [SD], median 12) months. Two died during the study. Four withdrew because of increased illness (2), loss of the SA (1), or preference for another device (1). Eight were still using the BCI when the study closed, and 7 chose to keep it for continued use. Of these 7, 6 could neither speak nor write at the end of the study, and 5 were totally (3) or partially (2) ventilator dependent. Figure 3A shows the home users' ALSFRS-R scores at study entry and at later times. The scores of those with higher initial scores (i.e., >10) declined, while the scores of those with low initial scores showed little change.
Figure 3. ALSFRS-R scores and BCI use data.
(A) ALS Functional Rating Scale–Revised (ALSFRS-R) scores for each brain-computer interface (BCI) home user (10 with ≥2 scores, 4 with 1 score at study entry). (B) Average duration of BCI use per day vs days used (in percent of days on which the BCI was available for use) for each of the BCI home users. (C) Average breakdown of BCI use time across home users. (Many used the CS/C program far more than needed for the study or for BCI calibration; they appeared to view it as a worthwhile exercise.) (D) Baseline and final average (±SD) ALSFRS-R (circle) and McGill Quality of Life (triangle) scores for the 10 BCI home users with values at ≥2 times. (E) Initial and final average (±SD) BCI benefit (left) and BCI burden (right) scores for the 8 home users (top) and their system assistants (SAs) (bottom) with values at ≥2 times. BKS = audio books; CNV = conversation via WordPad; CS/C = copy-spelling/calibration; EM = e-mail; NWS = Internet news reader; PICS = Pictures; UT = YouTube.
BCI use
The BCI home use periods of the 14 BCI home users totaled 3,904 days. BCI use was not possible for 1,664 of these days (i.e., hiatus days) because of hospitalization, acute illness, home construction, work, travel, or SA absence. Thus, on the average, each user had 160 (±117 SD) days when BCI use was possible and 119 (±112 SD) hiatus days when it was not. Over the BCI days, copy-spelling accuracy averaged 73% (±11% SD) and did not change significantly over the months of independent use (p = 0.44 for paired t test of users' average copy-spelling accuracies in first 4 independent BCI sessions vs last 4 independent sessions).
The user and SA determined when the BCI was used. Figure 3B plots for each user the average duration of daily use vs percent of days of possible BCI use on which he used the BCI. Daily use averaged 1.3 (±0.6 SD) hours, and the percent of days the BCI was used averaged 26% (±8% SD). Selections per minute for the BCI applications averaged 2.9 (±1.3 SD) across users and typically remained stable over the study.
Figure 3C shows the average percent of BCI time devoted to each application and the wide range across individuals. Communication (i.e., conversation or e-mail) averaged 62% (±20% SD) of BCI time; the users used the BCI to communicate with others, to interact via e-mail, and to participate in interviews at follow-up visits.
Each time the BCI was used, the SA spent 13 (±6 SD) minutes placing the electrode cap and initiating system operation, 15 (±9) minutes removing the cap and cleaning up, and 4 (±6 SD) minutes on related tasks.
User QoL and perception of BCI value
Figure 3D shows average baseline and final ALSFRS-R and MQoL scores. Notably, the average MQoL score did not decline despite the decline in neuromuscular function indicated by the drop in average ALSFRS-R score.
At study entry and each subsequent visit, the users and SAs completed questionnaires assessing BCI benefit and BCI burden on Likert scales (see Methods). Figure 3E summarizes the results. As indicated there, most users and SAs felt that BCI use was more beneficial than burdensome. Over the study, the user BCI benefit score fell slightly from 5.6 (±1.8 SD) to 4.9 (±1.5) (p = 0.02, initial vs final value by paired t test); the SA BCI benefit score and the user and SA BCI burden scores did not change significantly. Neither the benefit score, the burden score, nor their difference correlated with the ALSFRS-R score. Notably, 7 of the 8 users (88%) in the study when it ended elected to keep their BCIs for continued use.
BCI technical support
Users, SAs, other caregivers, significant others, or study personnel initiated 134 technical support requests. These concerned training (38%), personal preferences (29%), software (20%), or hardware (13%). For only 21 requests (16%) did the issue prevent BCI use (median downtime 3, mean 62, range 0.1–288 hours). Overall BCI availability (probability that it was in good working order when the user and the BCI were both available) was 0.98.
Discussion
In previous BCI-based communication studies of people with severe disabilities (aside from several case reports3–5), the investigators directly managed BCI operation: they studied patients in the laboratory or they took their systems to the patients' homes and studied them there. These studies generated substantial expectations for the clinical value of BCIs.
This study tested these expectations. It asked, “Will a BCI function reliably and usefully in independent home use by people with advanced ALS?” The answer it provides is complicated, reflecting both the BCI and the population in which it was tested. Some patients did use the BCI throughout the study, and 7 of the 8 remaining in the study when it ended kept the BCI for future use. However, these patients were a minority of the 37 initially assessed for BCI use (i.e., figure 2, stage 3). As figure 2 shows, several factors accounted for the high attrition. The major factor was ALS: 13 patients died during the study or withdrew because of rapid, often preterminal, disease progression. The other factors reflected the limitations of the BCI: 9 patients could not use it; 4 lost interest; and 2 preferred another assistive communication device. These results and their implications are considered first in terms of the BCI itself and second in terms of the population selected for BCI use.
The BCI performed reliably in independent use by the patients and their SAs. That is, the BCI and its applications functioned properly; technical problems were rare and were usually handled quickly and effectively by the VA and/or Wadsworth personnel. As noted, the BCI was in good working order 98% of the time. BCI training required an initial time commitment by the patient and the SA; and BCI setup and cleanup occupied ≈30 minutes. While these requirements are modest, they contributed to attrition and to the fact that BCI use was sometimes not possible (e.g., during hospitalization). Advances now underway such as dry-electrode technology, telemetry, and improved portability would reduce these requirements and could thereby increase the ease and convenience of BCI use and decrease the interruptions caused by acute illnesses or other life events.30,31 The need for an SA willing and able to support BCI use was a limitation for some users. For 4, loss of the SA contributed to their withdrawal from the study, and for others, limited availability of the SA reduced BCI use. For people who are severely disabled, this dependence on another is currently unavoidable. However, further improvements in the simplicity of BCI support should ease this limitation by reducing demands on the SA.30,31 Furthermore, if BCIs that depend on auditory rather than visual stimuli32–34 can be improved beyond their present extremely low communication rates, they could extend BCI use to those people (24% in this study) who cannot use current vision-based BCIs.23
Copy-spelling accuracy over the period of home use averaged 73% (±11% SD). This average accuracy, while adequate for useful communication, was significantly and substantially less than the average accuracy of 86% (±10% SD) for these same individuals during the initial evaluation of their ability to use the BCI (p = 0.003 by paired t test). Previous data from people with ALS23 and the stability of home-use accuracy over the course of the present study indicate that this difference is not attributable to disease progression. It probably reflects the difference between BCI operation (i.e., initial setup, electrode placement) managed by experts in controlled conditions and BCI operation managed by users and their SAs in more variable conditions. This difference is likely to degrade the performance of most BCI systems when they are deployed for independent home use. Thus, BCIs that are robust and easy to use can be expected to fare best in clinical translation.
As figure 3C indicates, communication (i.e., WordPad, e-mail) dominated BCI use. At the same time, the users differed widely in their preferred applications. This variation, together with the variation across users in number of training visits needed, indicates the importance of user-specific training and a broad range of appealing applications. These are essential if a BCI, or any assistive technology, is to be both useful and actually used.35 Unlike other therapies (e.g., medications), an assistive technology typically requires an initial and continuing commitment of time and effort by the user and often the caregiver as well. This is essential for mastering the technology, defining how it can best serve the user, and integrating it into daily life.
As figure 3B indicates, those who used the BCI most did so 2 to 3 times a week. While some still retained functional speech or keyboarding ability and several used another assistive device, others relied primarily on partner-assisted scanning (e.g., observation of small eye movements) for most interactions with caregivers and others. They had created their own no- or low-technology communication methods that required coordination with another person and thus did not provide truly autonomous communication. Nevertheless, all the home users used the BCI for autonomous communication (i.e., through WordPad and/or e-mail), and most also took advantage of other applications such as the Internet news reader.
As figure 3E summarizes, most users and SAs felt that the BCI benefit exceeded the burden. BCI benefit could be enhanced by increasing the speed and accuracy of selection36 and by providing new applications (e.g., full Internet access, artistic expression37). The burden is likely to be reduced in the future by dry-electrode technology, which will simplify placement and removal of the recording cap and enable development of more comfortable and cosmetic caps.30,31 Such improvements should facilitate the initial and continuing commitment of the users and their SAs, reduce attrition, and increase BCI use and benefit.
Given the impressive capabilities of conventional assistive communication devices (e.g., eye-tracking systems38), BCIs that support communication are at present most suitable for people whose disabilities prevent or greatly impair their ability to use conventional devices. Thus, the study set out to recruit patients who were severely disabled by ALS.17 At the same time, it sought patients who were in otherwise stable health and were expected to remain so for the duration of the study. As the results show, the study recruited people who were severely disabled, but it was less successful in recruiting patients whose health remained stable. Of the 28 who were able to use the BCI, nearly half died or withdrew as a result of increased illness (often preterminal) before or after becoming BCI home users. Retention might have been improved by limiting recruitment to those who had already made the difficult decision to accept full-time ventilation when it became necessary. Table 2 suggests that people who were younger, had more slowly progressive ALS, and/or had had ALS for a longer time were more likely to persist in BCI use and to retain the system when the study ended (although, with the limited sample size, significant statistical differences are not present).
The high disease-related attrition suggests that BCI home use is likely to be greater in populations who have similarly severe but stable disabilities (e.g., people with severe cerebral palsy, brainstem stroke, or high-level spinal cord injury). This inference is supported by the present data: among the 15 patients who could use the BCI and were not subsequently lost to disease-related attrition, 8 (53%) used the BCI throughout the study, and 7 elected to keep it after the study ended.
The MQoL assessments (i.e., figure 3D) indicated that the users' QoL was similar to that of normal volunteers39–41 and was stable over the study period, despite further decline in motor function (i.e., figure 3A). These findings are consistent with other studies of people with ALS.42 Thus, the results do not provide clear evidence that the BCI affected QoL.
These results support 3 statements about the Wadsworth BCI home system. First, it can function reliably and usefully when used by patients in their homes. Second, patient selection is important; the BCI is most suitable for people who are severely disabled but are otherwise in stable health. Third, improvements in its convenience and performance, some of which are now underway, should increase the numbers of people who find it useful and increase the extent to which they use it.
While this discussion has addressed issues raised by independent home use of the Wadsworth BCI, similar issues are likely to arise for other BCIs, noninvasive or implanted, as they attempt the transition to the real world. Many different BCIs are under development; each has its own problems and uncertainties.43–45 Noninvasive BCIs face issues of capacity, convenience, and artifact avoidance; implanted BCIs face unresolved questions about biological and functional longevity and uncertainties about the extent to which they offer greater capacities that justify the risks and expense of surgery. Given appropriate expertise and care in their development, most BCIs will probably provide high hardware/software reliability, as does the Wadsworth home system. At the same time, most BCIs are likely to encounter some performance loss in the transition from short-term use by experts in protected environments to long-term use by nonexperts in daily life; the simplest systems are likely to suffer the least loss. The user-related issues raised for the Wadsworth BCI are issues that apply to assistive communication technologies in general, whether conventional (i.e., muscle based) or BCI based. These devices are most successful for those able to commit time and effort to their mastery and use; thus, reasonably stable health is important. Furthermore, to be adopted and used, a device must adapt to each user's individual capabilities, needs, and desires, and it must do so better or more conveniently than other devices (e.g., eye-gaze systems) or no device (e.g., partner-assisted scanning). These realities should influence BCI research and development.46 Their unique conceptual appeal notwithstanding, BCIs that restore communication simply extend the spectrum of assistive communication devices. Thus, they will stand or fall on their ability to serve users better than conventional alternatives.
Acknowledgment
The authors thank the veterans, their caregivers, and their families for their participation and their unwavering support, and Drs. Peter Brunner, Amir Eftekhar, and Jonathan Carp for their helpful suggestions on the manuscript.
Glossary
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale–Revised
- BCI
brain-computer interface
- MQoL
McGill Quality of Life
- QoL
quality of life
- SA
system assistant
- VA
US Department of Veterans Affairs
Footnotes
Editorial, page 109
Author contributions
Jonathan R. Wolpaw and Richard S. Bedlack, joint principal investigators, designed the trial, participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Domenic J. Reda designed the trial, participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Robert J. Ringer designed the trial, participated in data analysis, data interpretation, and revision of the manuscript. Patricia G. Banks participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Theresa M. Vaughan designed the trial, participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Susan M. Heckman, Lynn M. McCane, Charles S. Carmack, and Stefan Winden participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Dennis J. McFarland designed the trial, participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Eric W. Sellers designed the trial, participated in data analysis, data interpretation, and revision of the manuscript. Hairong Shi participated in data analysis, data interpretation, statistical analysis, and revision of the manuscript. Tamara Paine participated in data analysis, data interpretation, and revision of the manuscript. Donald S. Higgins, Albert C. Lo, and Huned S. Patwa participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Katherine J. Hill designed the trial, participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript. Grant D. Huang designed the trial, participated in data analysis, data interpretation, and revision of the manuscript. Robert L. Ruff, joint principal investigator, designed the trial, participated in data collection, participated in data analysis, data interpretation, and revision of the manuscript.
Study funding
This study (Cooperative Studies Program No. 567) was supported by the Cooperative Studies Program, Office of Research and Development, Department of Veterans Affairs. The opinions expressed here are those of the authors and do not necessarily reflect the position or policy of the VA. The National Center for Adaptive Neurotechnologies at the Wadsworth Center is supported by the National Institute of Biomedical Imaging and Bioengineering of the NIH (grant 1P41EB018783).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Wolpaw JR, Wolpaw EW. Brain-computer interfaces: something new under the sun. In: Wolpaw JR, Wolpaw EW, editors. Brain-Computer Interfaces: Principles and Practice. 1st ed. Oxford: Oxford University Press; 2012:3–12. [Google Scholar]
- 2.All articles with “brain-computer interface,” “brain-machine interface,” or “direct brain interface” in the title or abstract. Accessed November 21, 2017. Scopus (scopus.com/).
- 3.Birbaumer N, Ghanayim N, Hinterberger T, et al. A spelling device for the paralyzed. Nature 1999;398:297–298. [DOI] [PubMed] [Google Scholar]
- 4.Sellers W, Vaughan TM, Wolpaw JR. A brain-computer interface for long-term independent home use. Amyotroph Lateral Scler 2010;11:449–455. [DOI] [PubMed] [Google Scholar]
- 5.Vansteensel MJ, Pels EGM, Bleichner MG, et al. Fully implanted brain-computer interface in a locked-in patient with ALS. New Engl J Med 2016;375:2060–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss H, Casey P, Stocking CB, Roos RP, Brooks BR, Siegler M. Home ventilation for amyotrophic lateral sclerosis patients: outcomes, costs, and patient, family, and physician attitudes. Neurology 1993;43:438–443. [DOI] [PubMed] [Google Scholar]
- 7.Moss H, Oppenheimer EA, Casey P, et al. Patients with amyotrophic lateral sclerosis receiving long-term mechanical ventilation: advance care planning and outcomes. Chest 1996;110:249–255. [DOI] [PubMed] [Google Scholar]
- 8.Heiman-Patterson T, Aboussouan LS. Respiratory care in amyotrophic lateral sclerosis. In: Mitsumoto H, Przedborski S, Gordon PH, editors. Amyotrophic Lateral Sclerosis. New York: Taylor & Francis Group; 2006:737–755. [Google Scholar]
- 9.Maessen M, Veldink JH, van den Berg LH, Schouten HJ, van der Wal G, Onwuteaka-Philipsen BD. Requests for euthanasia: origin of suffering in ALS, heart failure, and cancer patients. J Neurol 2010;257:1192–1198. [DOI] [PubMed] [Google Scholar]
- 10.Körner S, Sieniawski M, Kollewe K, et al. Speech therapy and communication device: impact on quality of life and mood in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:20–25. [DOI] [PubMed] [Google Scholar]
- 11.Farwell A, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephal Clin Neurophysiol 1988;70:510–523. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg LR, Cudkowicz ME. Locked in but not out? Neurology 2014;82:1852–1853. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan TM, Sellers EW, Wolpaw JR. Clinical evaluations of BCIs. In: Wolpaw JR, Wolpaw EW, editors. Brain-Computer Interfaces: Principles and Practice. 1st ed. Oxford: Oxford University Press; 2012:325–336. [Google Scholar]
- 14.Nelson K, Sun H, Dolan E, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage 2014;37:331–338. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Amyotrophic Lateral Sclerosis in Veterans: Review of the Scientific Literature. Washington: National Academies Press; 2006. [Google Scholar]
- 16.Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases: El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 17.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function: BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 18.Allen KD, Kasarskis EJ, Bedlack RS, et al. The National Registry of Veterans With Amyotrophic Lateral Sclerosis. Neuroepidemiol 2008;30:180–190. [DOI] [PubMed] [Google Scholar]
- 19.Sellers EW, Krusienski DJ, McFarland DJ, Vaughan TM, Wolpaw JR. A P300 event-related potential brain-computer interface (BCI): the effects of matrix size and inter stimulus interval on performance. Biol Psychol 2006;73:242–252. [DOI] [PubMed] [Google Scholar]
- 20.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng 2004;51:1034–1043. [DOI] [PubMed] [Google Scholar]
- 21.Schalk G, Mellinger J, editors. A Practical Guide to Brain-Computer Interfacing With BCI2000. London: Springer; 2010. [Google Scholar]
- 22.Sharbrough F, Chatrian GE, Lesser RP, Luders H, Nuwer M, Picton TW. AEEGS guidelines for standard electrode position nomenclature. J Clin Neurophysiol 1991;8:200–202. [PubMed] [Google Scholar]
- 23.McCane LM, Sellers EW, McFarland DJ, et al. Brain-computer interface (BCI) evaluation in people with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krusienski DJ, Sellers EW, McFarland DJ, Vaughan TM, Wolpaw JR. Toward enhanced P300 speller performance. J Neurosci Methods 2008;167:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellers EW, Arbel Y, Donchin E. BCIs that use P300 event-related potentials. In: Wolpaw JR, Wolpaw EW, editors. Brain-Computer Interfaces: Principles and Practice. 1st ed. Oxford: Oxford University Press; 2012:215–226. [Google Scholar]
- 26.Winden S, Carmack CS, Corda DE, et al. BCI-360: full-service support for independent home-based BCI and for translational studies. Presented at the 2012 Annual Meeting of the Society for Neuroscience; October 13-17; New Orleans, LA.
- 27.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med 1997;11:3–20. [DOI] [PubMed] [Google Scholar]
- 28.Detry MA, Lewis RJ. The intention-to-treat principle: how to assess the true effect of choosing a medical treatment. JAMA 2014;312:85–86. [DOI] [PubMed] [Google Scholar]
- 29.US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Available at: va.gov/vetdata/veteranpopulation.asp (table 6L). Accessed June 2018. [Google Scholar]
- 30.Gargiulo G, Calvo RA, Bifulco P, et al. A new EEG recording system for passive dry electrodes. Clin Neurophysiol 2010;121:686–693. [DOI] [PubMed] [Google Scholar]
- 31.Guger C, Krausz G, Allison BZ, Edlinger G. Comparison of dry and gel based electrodes for p300 brain-computer interfaces. Front Neurosci 2012;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreuder M, Rost T, Tangermann M. Listen, you are writing! Speeding up online spelling with a dynamic auditory BCI. Front Neurosci 2011;5:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill NJ, Ricci E, Haider S, et al. A practical, intuitive brain-computer interface for communicating “yes” or “no” by listening. J Neural Eng 2014;11:035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhary U, Xia B, Silvoni S, Cohen LG, Birbaumer N. Brain–computer interface–based communication in the completely locked-in state. PLoS Biol 2017;15:e1002593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Scherer MJ. The change in emphasis from people to person: introduction to the special issue on assistive technology. Disabil Rehabil 2002;24:1–4. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann T, Schulz SM, Grunzinger C, Kubler A. Flashing characters with famous faces improves ERP-based brain-computer interface performance. J Neural Eng 2011;8:056016. [DOI] [PubMed] [Google Scholar]
- 37.Holz EM, Botrel L, Kaufmann T, Kübler A. Long-term independent brain-computer interface home use improves quality of life of a patient in the locked-in state: a case study. Arch Phys Med Rehab 2015;96(suppl 3):S16–S26. [DOI] [PubMed] [Google Scholar]
- 38.Ball LJ, Nordness AS, Fager SK, et al. Eye-gaze access to AAC technology for people with amyotrophic lateral sclerosis. J Med Speech Lang Pathol 2010;18:11–23. [Google Scholar]
- 39.Rousseau MC, Pietra S, Nadji M, Billette de Villemeur T. Evaluation of quality of life in complete locked-in syndrome patients. J Palliat Med 2013;16:1455–1458. [DOI] [PubMed] [Google Scholar]
- 40.Lule D, Hacker S, Ludolph A, Birbaumer N, Kübler A. Depression and quality of life in patients with amyotrophic lateral sclerosis. Dtsch Arztebl Int 2008;105:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linse K, Rüger W, Joos M, Schmitz-Peiffer H, Storch A, Hermann A. Eye-tracking-based assessment suggests preserved well-being in locked-in patients. Ann Neurol 2017;81:310–315. [DOI] [PubMed] [Google Scholar]
- 42.Robbins RA, Simmons Z, Bremer BA, Walsh SM, Fischer S. Quality of life in ALS is maintained as physical function declines. Neurology 2001;56:442–444. [DOI] [PubMed] [Google Scholar]
- 43.Wolpaw JR, Wolpaw EW. The future of BCIs: meeting the expectations. In: Wolpaw JR, Wolpaw EW, editors. Brain-Computer Interfaces: Principles and Practice. 1st ed. Oxford: Oxford University Press; 2012:387–392. [Google Scholar]
- 44.Chaudhary U, Birbaumer N, Ramos-Murguialday A. Brain-computer interfaces for communication and rehabilitation. Nat Rev Neurol 2016;12:513–525. [DOI] [PubMed] [Google Scholar]
- 45.Riccio A, Pichiorri F, Schettini F, et al. Interfacing brain with computer to improve communication and rehabilitation after brain damage. Prog Brain Res 2016;228:357–387. [DOI] [PubMed] [Google Scholar]
- 46.Blabe H, Gilja1 V, Chestek CA, Shenoy KV, Anderson KD, Henderson JM. Assessment of brain-machine interfaces from the perspective of people with paralysis. J Neural Eng 2015;12:043002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data sets will be shared for specified uses after approval by the VA.