ABSTRACT
Medicinal mushroom Ganoderma sp. is considered to be a key source for the production of therapeutic agents. Our current review indicates that a limited number (<19%; 79 out of >430) of isolated compounds have been tested and known to be active against several microorganisms and parasites. In this review, we aim to summarise all the antimicrobial and anti-parasitic works on Ganoderma sp. displayed on web of science, google scholar and endnote X7 from 1932 to August 2016. We further present and discuss the structure of active compounds against microorganisms and parasites. In addition, we also discuss the possible further research to identify lead compounds from Ganoderma sp. as a novel strategy to combat the potential global emergence of bad bugs and parasites.
KEYWORDS: Ganoderma sp, antimicrobial, anti-parasitic, triterpenoid, quinone structures
1. Introduction
Ganoderma sp. is a medicinal mushroom producing a group of frequently studied bioactive compounds. They belong to the kingdom of Fungi, division of Basidiomycota, class of Agaricomycetes, order Polyporales, family of Ganodermataceae and genus of Ganoderma. A search for “Ganoderma” in the database Index Fungorum displayed 409 species records, including synonyms (http://www.speciesfungorum.org). Ganoderma sp., especially G. lucidum, G. tsugae and G. applanatum, are well studied and have been in use in East Asian countries since the ancient times for the treatment of various diseases (Ofodile et al. 2005; Paterson 2006; Ferreira et al. 2015). Triterpenes and polysaccharides are considered key constituents isolated from fruiting bodies, gills, spores and mycelia for their bioactivities (Xia et al. 2014).
Literature reviews suggest, besides its antimicrobial activities, Ganoderma sp. components exhibit a variety of bioactivities, including anti-tumour, immune-modulatory, antioxidant, antihypertensive and anti-androgenic. Moreover, Ganoderma sp. is widely used for the remedy of various chronic diseases such as cancers, diabetes, hypertension and hepatitis (Ofodile et al. 2005; Zhang et al. 2015). To date, most of the reviews on Ganoderma sp. have been focused on its anticancer and antioxidant activities and immune modulation (Sanodiya et al. 2009). Therefore, our basic aim is to provide a glimpse on the antimicrobial and anti-parasitic activities of Ganoderma sp. In addition, we also provide possible future prospect for research on Ganoderma sp. and its compounds.
In this review, we have performed literature searches in English (ISI Web of Science and Google Scholar) and Endnote X7 (online search, Pub Med) to find publications that described Ganoderma sp. for antimicrobial activities. We have used the keywords “Ganoderma” and “Antimicrobial”. Finally, we filtered individual references to determine the relevancy to our study. The inclusion criterion was the study that provided data or results or discussion on the antimicrobial activities of Ganoderma sp.
2. Antimicrobial and anti-parasitic bioactive compounds
Ganoderma sp. has been reported as important sources of antimicrobial bioactive compounds. Terpenes, terpenoids and polyketides of farnesyl quonines types are the major secondary metabolites (SMs) produced by Ganoderma sp. In Ganoderma sp., more than 316 terpenes have been reported, with the majority of compounds from G. lucidium (Xia et al. 2014).
Chemical analysis of numerous Ganoderma sp. has showed Ganoderma Triterpenes (GTs) are mainly lanostanoid-type triterpene (Zhang et al. 2015). Among them, majority contain 30 or 27 carbon atoms, and some occasionally may contain 24 carbon atoms. These compounds possess the same parent skeleton, namely a trans-configuration of rings A/B, B/C, C/D and 10β, 13β, 14α, 17β substituent. In addition, the substituents are always found at the C-3, 7, 11, 12, 15, 22, 23, 24 and 25 positions of the parent nucleus (Xia et al. 2014). Thirty carbon terpenoids are usually formed by the fusion of two smaller terpenoids precursors, each containing 15 carbons sesquiterpene. Head-to-tail fashions linking of isoprene units to form linear chains and various cyclisations and rearrangements is the core mechanism to give cyclic terpenoids (Mothana et al. 2000; Hill & Connolly 2013). The parent carbon skeleton of antimicrobial and anti-parasitic GTs is shown in Figure 1, from which it can be concluded that GTs are the most common antimicrobial and anti-parasitic compounds reported from Ganoderma sp.
Figure 1.
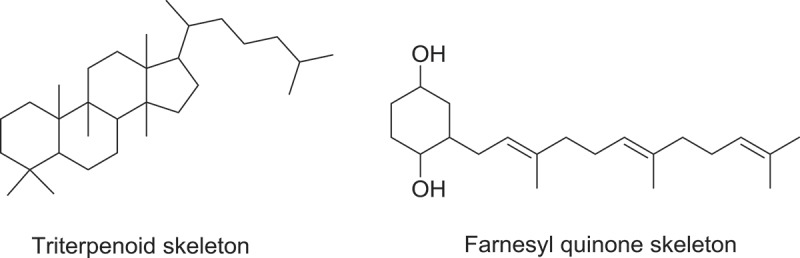
Parent carbon skeletons of triterpenoid and farnesyl quinone type of polyketide from Ganoderma sp. with antimicrobial and anti-parasitic activities.
Farnesyl quinone, a polyketide type, is the second most common antimicrobial and anti-parasitic compound from Ganoderma sp. Quinones are known to be oxidised derivatives of aromatic compounds and are often readily made from reactive aromatic compounds with electron-donating substituent such as catechols and phenols. Besides GTs, polypeptides, small peptides such as ganodermin, polysaccharide such as sacchachitin, and chitosan also possess antimicrobial and anti-parasitic properties (Mothana et al. 2000; Wang & Ng 2006; Sanodiya et al. 2009; Chuang et al. 2013). Structures of antimicrobial and anti-parasitic compounds from Ganoderma sp. are shown in Figure 2.
Figure 2.
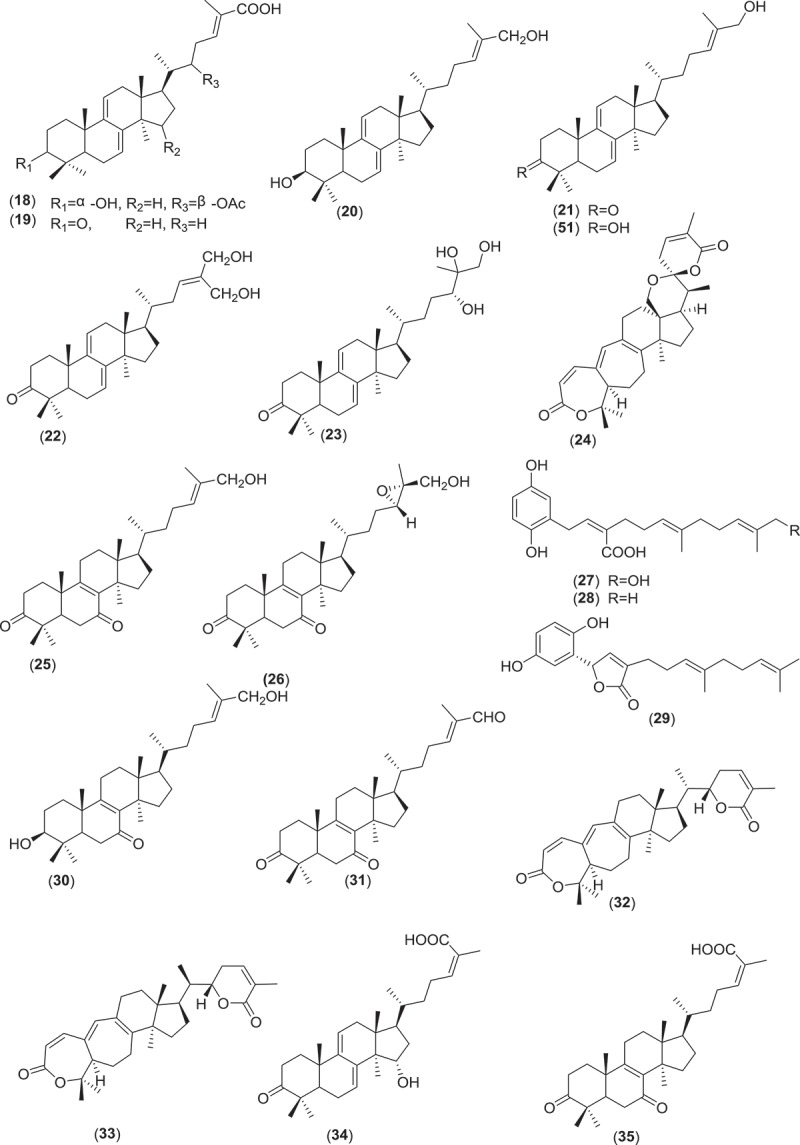
(Continued).
Figure 2.
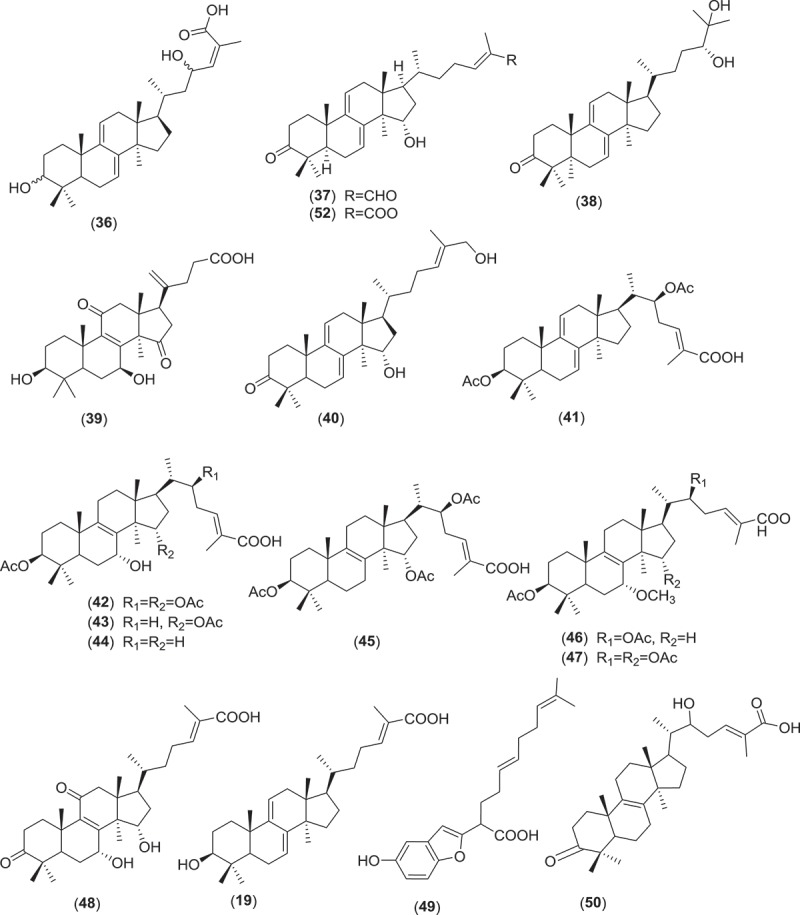
(Continued).
Figure 2.
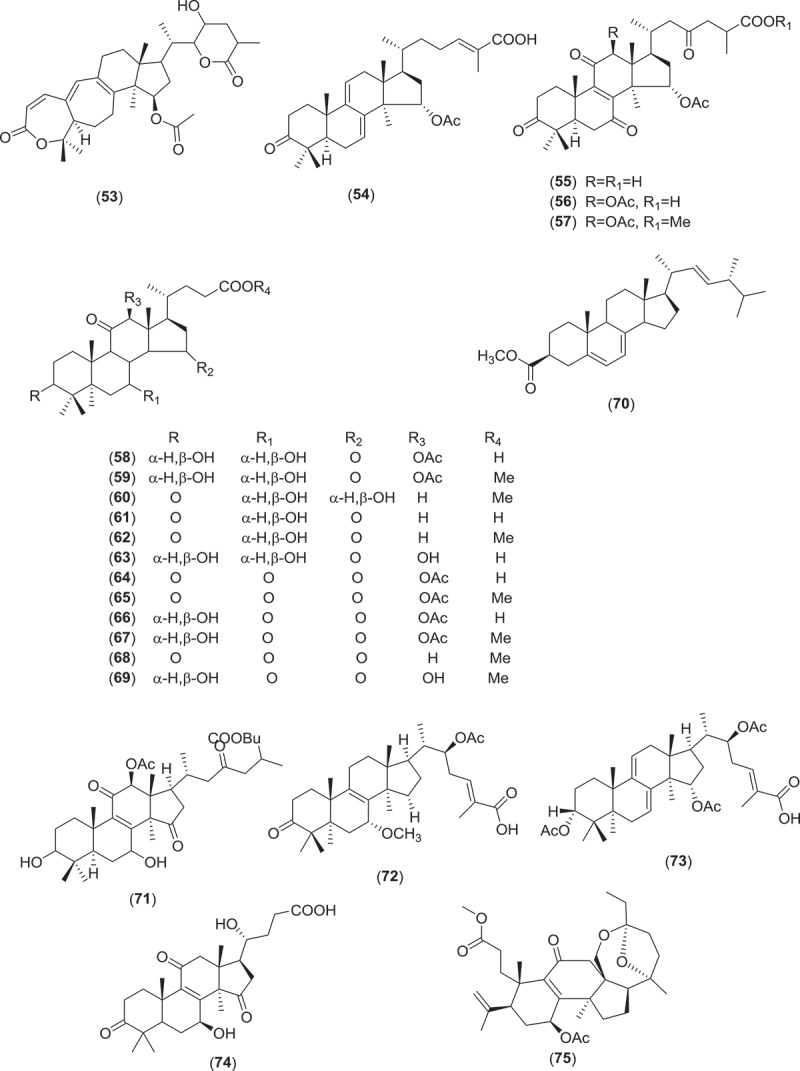
(Continued).
Figure 2.
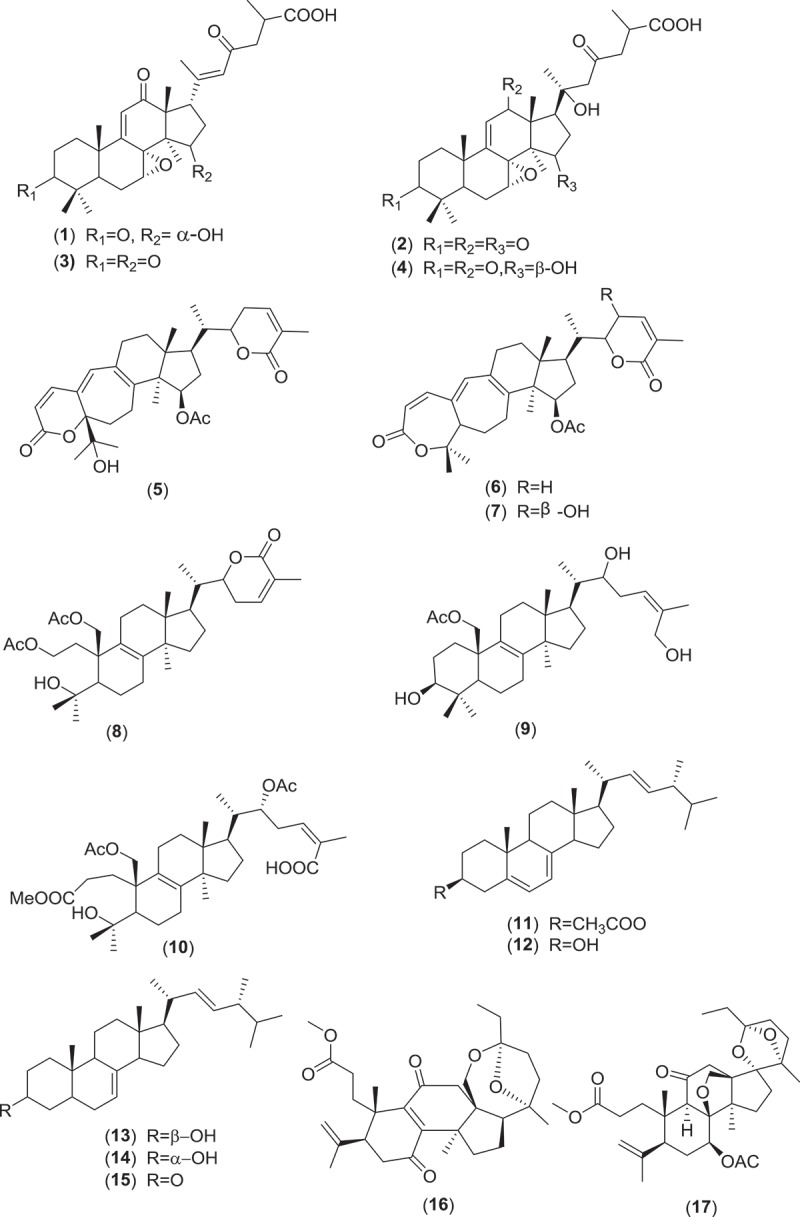
Structure of compounds with antimicrobial and anti-parasitic activities from Ganoderma sp.
3. Isolation of antimicrobial and anti-parasitic bioactive compounds
Extracts from fruiting bodies, both wild and cultivated, and mycelia from fermentation broth (Tables 1–4) are used for the isolation of antimicrobial and anti-parasitic bioactive compounds. Literatures divulge that most commonly ethanol (EtoAc) (Tables 1–4) is used to prepare crude extract; sometimes some researchers preferred other solvents such as chloroform (CHCl3), EtOH, and acetone (Isaka et al. 2016). In addition, our review reveals that hexane and ether are poorly used for the preparation of extract from Ganoderma sp. Moreover, some techniques such as microwave, ultrasound and enzyme treatments can facilitate the breakdown of the cell wall (Ferreira et al. 2015). Solvents like MeOH, EtOH, CH2Cl2, CHCl3 and aqueous – both cold and hot – are used for further purifications and isolation. Techniques such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and column chromatography (CC) are used to facilitate the purification and isolation process (Huie & Di 2004). The general procedures of the isolation of antimicrobial and anti-parasitic compounds are shown in Figure 3. In addition, this outline can be used for other chemical investigations from Ganoderma sp.
Table 1.
Details of antibacterial activities of Ganoderma sp. parts, products and compounds.
| Ganodermasp. | Extraction Solvent | Parts/products/compounds | Tested bacteria strains | Method | MIC/MBC | References |
|---|---|---|---|---|---|---|
| G. atrum | EtOH soluble acidic components | Fruiting bodies | S. aureus sub species Aureus, E. coli, B.subtilis, P. vulgaris | Micro dilution | 1.56-25mg/ml/3.125-25mg/ml | (Li et al. 2012) |
| G. lucidium | 96%EtOH | Fruiting bodies | H. pylori ATCC 43504, S. aureus ATCC 26003 | Micro plate Agar, Disc fusion Assay |
<1.0mg/ml &<10mg/ml resp./ND | (Shang et al. 2013) |
| G. colossum | Hexane: CH2Cl2(2:7) | Colossolactone E(6), 23 hydroxycolossolactone E(53) | B. subtilisIMI 347329, P.syringae var IMI 34748(ACTCC 19310) | TLC Agar Overlay | ND | (Lauretta Nwanneka Ofodile et al. 2011) |
| G. pfeifferiBres. | CH2 Cl2 | Ganomycins A- B(27–28) | S. aureus(ATCC 6538), B. subtilis(SBUG 14), E. coli(SBUG 13), P. mirabilis (SBUG 47), S. marcescens(SBUG 9), M. flavus(SBUG 16) | Micro dilution | 2.5-25µg/mL/ND | (Mothana et al. 2000) |
| G. applanatum | 96%EtOH | Mycelia extract | B. cereus (clinical isolate), M. flavusATCC10240, S. aureusATCC6538, L. monocytogenesNCTC7973, E. coliATCC35218, E. cloacae (human isolate), P. aeruginosaATCC27853 and S. typhimuriumATCC13311 | Colorimetric Microbial Viability Assay | 1.16–1.90mg/ml/2.54–4.00mg/ml | (Cilerdzic et al. 2016) |
| G. carnosum | 96%EtOH | Mycelia extract | B. cereus (clinical isolate), M. flavusATCC10240, S. aureusATCC6538, L. monocytogenesNCTC7973, E. coli ATCC35218, E. cloacae (human isolate), P. aeruginosaATCC27853 and S. typhimuriumATCC13311 | Colorimetric Microbial Viability Assay | 1.16–4.00mg/ml/1.16–4.00mg/ml | (Cilerdzic et al. 2016) |
| G. lucidium | 96%EtOH | Mycelia extract | B. cereus (clinical isolate), M. flavusATCC10240, S. aureusATCC6538, L. monocytogenesNCTC7973, E. coli ATCC35218, E. cloacae (human isolate), P. aeruginosaATCC27853 and S. typhimuriumATCC13311 | Colorimetric Microbial Viability Assay | 1.00–1.67mg/ml/1.16-400mg/ml | (Cilerdzic et al. 2016) |
| G. colossusm | CH2Cl2, MeOH, H2O | Fruiting bodies | S. aureus(ATCC 29213), B. subtilis(ATCC 6059), E. coli (ATCC 25922),P. aeruginosa(ATCC 27853), M. flavus(SBUG 16) | Agar Diffusion | ND | (Al-Fatimi et al. 2005) |
| G. resinaceum | CH2Cl2, MeOH, H2O | Fruiting bodies | S. aureus (ATCC 29213), B. subtilis(ATCC 6059), E. coli (ATCC 25922),P. aeruginosa(ATCC 27853), M. flavus(SBUG 16) | Agar Diffusion | ND | (Al-Fatimi et al. 2005) |
| G. applanatum | MeOH | Fruiting bodies | E.coli(ATCC 25922) | Micro dilution | ND | (Zengin et al. 2015) |
| G.lucidum | EtOH and H2O | Fruiting bodies | S. aureus(MTCC 96), B. cereus (MTCC 430), P. aeruginosa(MTCC 424) | Micro dilution | 80-200mg/ml/ND | (Karwa & Rai 2012) |
| G. lucidum | Hexane and chloroform | Fruiting bodies | S. aureus(ATCC 6538), B. subtilis(ATCC 6633) | Agar Diffusion | 6.25mg/ml/ND | (Vazirian et al. 2014) |
| G. lucidum | Hexane and chloroform | Ergosta-5,7,22-trien-3β-yl acetate(11),ergosta-7,22-dien-3β-yl acetate(70),ergosta-7,22-dien-3-one(15), ergosta-7,22-dien-3β-ol(13), ergosta-5,7,22 trien,-3β-ol(12), ganodermadiol(20) |
S. aureus(ATCC 6538), B. subtilis(ATCC 6633) | 2.5-5mg/ml/ND | (Vazirian et al. 2014) | |
| G.lucidum | Hot H2O | Carpophores | B. anthracisATCC 6603, B. cereus ATCC 27348, B. subtilisATCC 6633, M. luteus ATCC 9341, S. aureus ATCC 25923,E.coilATCC 259 22, K. oxytocaATCC 8724, K. pneumoniaeATCC 10031, P. vulgaris ATCC 27853, S. typhiATCC 6229 | Micro dilution | 1.25–5.0mg/ml/ND | (Yoon et al. 1994) |
| G. lucidium | 96% EtOH | Basidiocarps | B. cereus (clinical isolate), M. flavusATCC10240, S. aureusATCC6538, L. monocytogenesNCTC7973, E. coli ATCC35218, E. cloacae (human isolate), P. aeruginosaATCC27853 & S. typhimuriumATCC13311 | Disc-diffusion & Micro dilution | 1–3.4mg/ml/1.4–4.0mg/ml | (Ćilerdžić et al. 2014) |
| G.lucidium | 95% EtOH | 12b-acetoxy-3β,7 β -dihydroxy-11,15,23-trioxolanost-8-en-26-oic acid butyl ester(71) | S. aureus(ATCC 6538) & B.subtilis(ATCC6633) | Micro dilution | 68.5 µM&123.8 µM | (Liu et al. 2014) |
| G. lucidum | MeOH | NG | S. aureus(ATCC 6538),B.cereus(clinical isolate), L. monocytogenes(NCTC 7973), M. flavus(ATCC 10240), P. aeruginosa(ATCC 27853), E. coli (ATCC 35210), S. typhimurium(ATCC 13311), E. cloacae (human isolate) | Micro dilution | 0.0125–0.75mg/ml/0.035–1.5mg/ml | (Heleno et al. 2013) |
| G. lucidium | H2O | Mycelia(Protein extract) | S. epidermidis, B. subtilis, B .cereus E. coli, P. aeruginosa | Micro dilution | 20–81.5mg/ml/ND | (Sa-Ard et al. 2015) |
| G. lucidium | H2O | Fruiting bodies(Protein extract) | S. epidermidis, S. aureus, B. subtilis, B. cereus, E. coli, P. aeruginosa | 81.5-512mg/ml/ND | (Sa-Ard et al. 2015) | |
| G. orbiforme | MeOH, EtOAc, Acetone | Mycelia# | M. tuberculosis | Green Fluorescent Protein Micro Plate Assay | 0.781-50µg/mL/ND | (Isaka et al. 2016) |
MeOH-Methanol; EtOH-Ethanol; dH2O- Distilled water; NG- Data Not Given; ZOI-Zone Of Inhibition; MIC- Minimum Inhibitory Concentration; MBC- Minimum Bactericidal Concentration; S. aureus- Staphylococcus aureus; B. subtilis- Bacillus subtilis;, #(astraodoric acid B(50), ganorbiformin F(72), ganoderic acid TR(34), ganoderic acid T(73), ganoderic acid S(18), (22S,24E)-3β,15α,22-triacetoxylanosta-8,24-dien-26-oic acid(41), (24E)-3β-acetoxy-7α-hydroxylanosta-8,24-dien-26-oic acid(44), (24E)-3β,15α-diacetoxy-7α-hydroxylanosta-8,24-dien-26-oic acid(43), (22S,24E)-7α-hydroxy-3β,15α,22-triacetoxylanosta-8,24-dien-26-oic acid(42), (22S,24E)-3β,22-diacetoxy-7α-methoxylanosta-8,24-dien-26-oic acid(46), (22S,24E)-7α-Methoxy-3β,15α,22-triacetoxylanosta-8,24-dien-26-oic acid(47), (22S,24E)-3β,22-diacetoxylanosta-7,9(11),24-trien-26-oic acid(45).
Table 4.
Details of anti-parasitic activities of Ganoderma sp. parts and compounds.
| Ganodermasp. | Extraction Solvent | Parts/compounds | Test Parasite | Method | LD50/IC50 value | References |
|---|---|---|---|---|---|---|
| Ganodermasp. | EtOAc&MeOH | Fruiting bodies(schisanlactone B(32), Ganodermalactone F(24), colossolactone E(6)) | P. falciparum | Micro culture Radioisotope Technique | 6.0–10.0 μM | (Lakornwong et al. 2014) |
| G. lucidum | EtOAc&MeOH | Fruiting bodies* | P. falciparum | Micro culture Radioisotope Technique | 6.0-20μM | (Adams et al. 2010) |
| G. boninense | EtOH | Ganoboninketals A(75) Ganoboninketals B-C(16–17) | P. falciparum | DNA Fluorescence Signal Test |
4.0, 7.9, and 1.7 μM | (Ma et al. 2014) |
| G. lucidum | EtOH | Crude extract | P. berghei | In Vivo Malarial activity | (Oluba et al. 2012) | |
| G.lucidum | NG | Lectin | H. glycines | Parasite Mortality Test | (>10 mg/ml/2hrs,4.5 mg/ml/24hrs, 1.7 mg/ml/48hrs | (Zhao et al. 2009) |
| G. lucidum | NG | Lectin | D. dipsaci | Parasite Mortality Test | >10 mg/ml | (Zhao et al. 2009) |
EtoAc: Ethyl Acetate; EtOH: ethanol; MeOH: Methanol; P. falciparum: Plasmodium falciparum; H. glycines: Heteroderaglycines; D. dipsaci: Ditylenchusdipsaci; NG: Data Not Given; μM: Micro Mole; mg/ml: milligram/millilitre; *(Ganodericacid DM(35), Ganoderic Acid TR 1(52),Ganoderic Aldehyde TR(37),23-Hydroxyganoderic Acid S(36), Ganoderic acid S(18), Ganodermanondiol(37), Ganofuran B(49)).
Figure 3.
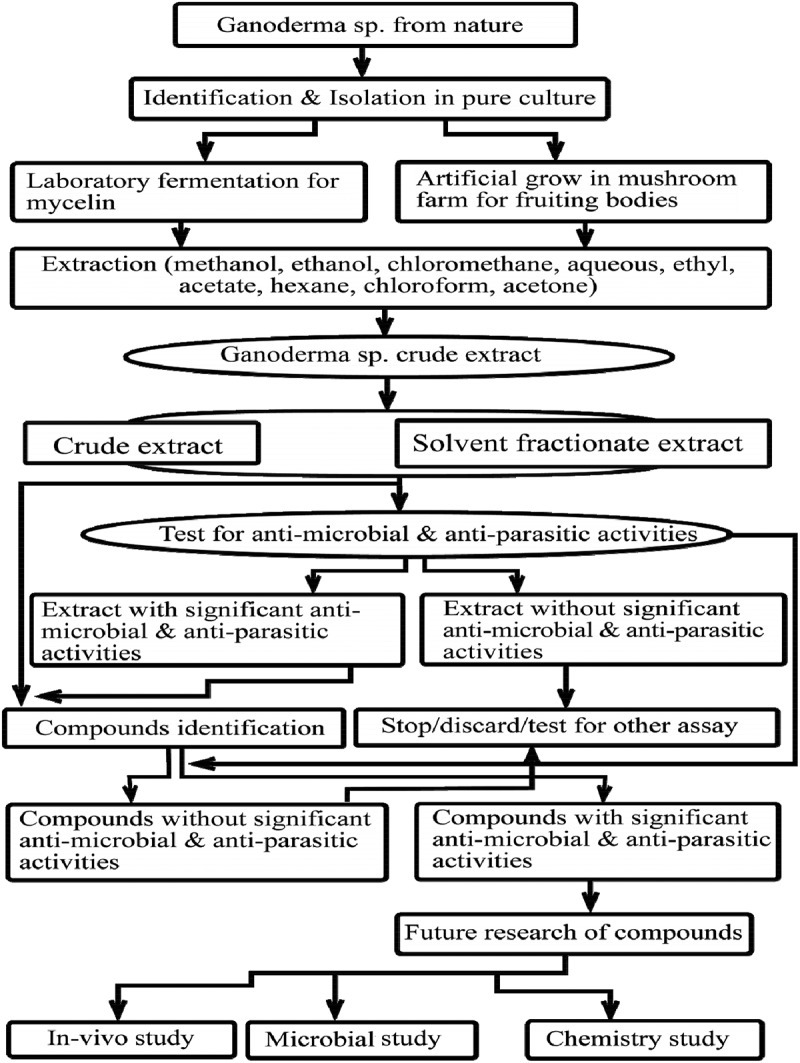
Flowchart of isolation of antimicrobial and anti-parasitic compounds from Ganoderma sp.
4. Antibacterial activities of compounds and extracts of Ganoderma sp
Currently bioassay-guided antibiotics identification, TLC and chromatography bio-autography are used to track antibacterial ingredients from the extract (Huie & Di 2004). Minimum inhibitory concentration (MIC) and 50% inhibitory concentration (IC50) values are used to determine the potency of antibacterial agents. Our literatures review showed that MeOH and EtOH are good solvents for the extraction of antibacterial compounds of interest rather than other organic solvents; however, the parts of Ganoderma sp. used and the tested bacterial strains may be the limiting factors in choosing the solvent. Most studies that use alcoholic solvents for extraction showed very low MIC (Li et al. 2012; Shang et al. 2013; Cilerdzic et al. 2016). Several studies on the fruiting bodies of Ganoderma sp. reveal that the compounds have the inhibitory ability to the different types of Gram positive bacteria (GPB), Gram negative bacteria (GNB) including the mycobacteria (Al-Fatimi et al. 2005; Isaka et al. 2016).
Colossolactone E (6) and 23-hydroxycolossolactone E (53), two colossolactones-triterpenes, were active against Bacillus subtilis and Pseudomonas syringae. However, the researcher did not determine the MIC and MBC of compounds against this bacterium (Ofodile et al. 2011). Moreover, two hydroquinones, ganomycins A (27) and B (28), were found to be the most effective to inhibit the bacterium. The MIC values of compounds 27 and 28 were 25 µg/ml against Staphylococcus aureus and 2.5 µg/ml against Micrococcus flavus, respectively, taking positive control ampicillin (MIC = 0.05 µg/ml and 0.25 µg/ml for S. aureus and M. flavus, respectively). In addition, in agar diffusion assay Zone of Inhibition (ZOI) 15–25 mm/100 µg/paper disk was found for GPB such as B. subtilis, S. aureus and M. flavus. However, P. aeruginosa, Candida albicans and C. maltose at 100 µg/paper disk did not respond to these compounds (Mothana et al. 2000). In a work performed by Isaka et al. (2016), EtOAc and MeOH extract of Ganoderma sp. BCC 16,642 isolated different compounds astraodoric acid C (50), ganorbiformin F (72), ganoderic acid TR (34), ganoderic acid T (73), ganoderic acid S (18), lanostanoid, ((22S,24E)-3β,15α,22-triacetoxylanosta-8,24-dien-26-oic acid (45), (24E)-3β-acetoxy-7α-hydroxylanosta-8,24-dien-26-oic acid (44), (24E)-3β,15α-diacetoxy-7α-hydroxylanosta-8,24-dien-26-oic acid (43), (22S,24E)-7α-hydroxy-3β,15α,22-triacetoxylanosta-8,24-dien-26-oic acid (42), (22S,24E)-3β,22-diacetoxy-7α-methoxylanosta-8,24-dien-26-oic acid (46), (22S,24E)-7α-methoxy-3β,15α,22-triacetoxylanosta-8,24-dien-26-oic acid (47), (22S,24E)-3β,22-diacetoxylanosta-7,9(11),24-trien-26-oic acid (41), which were observed to be active against the Tubercular bacilli with the MIC value in the range of 0.781–50 µg/ml. In another study, steroidal compounds like ergosta-5,7,22-trien-3β-yl acetate (11), ergosta-5,7,22-dien-3β-yl acetate (70), ergosta-7,22-dien-3-one (15), ergosta-7,22-dien-3β-ol (13), ergosta-5,7,22-trien-3β-ol (12) and ganodermadiol (20) were found to be effective against S. aureus and B. subtilis with MIC value of 2.5–5 mg/ml (Vazirian et al. 2014). Ethanolic and EtOAc extract compounds 12β-acetoxy-3β, 7β-dihydroxy-11, 15, 23-trioxolanost-8-en-26-oic acid butyl ester (71) from fruiting bodies of G. lucidium showed significant inhibition against S. aureus and B. subtilis with MIC values of 68.5 µM and 123.8 µM, respectively (positive control ampicillin = 4.1 µM and 19.3 µM, resp.) (Liu et al. 2014).
Literatures reveal most of the antibacterial tests are performed on crude extract with significant effective results rather than pure compounds (Sa-Ard et al. 2015; Zengin et al. 2015; Cilerdzic et al. 2016). In addition, scanty information is available on the in vivo model test of effective compounds; we noticed only compounds (27) and 28 have been tested the in vivo model of the Methicillin-resistant Staphylococcus aureus (MRSA)-infected mouse (Mikolasch et al. 2016).
5. Antifungal activities of compounds and extracts of Ganoderma sp
An antifungal protein – ganodermin – isolated from the fruiting bodies of G. lucidium inhibits the growth of Botrytis cinerea, Fusarium oxysporum and Physalo sporapiricola with an IC50 value of 15.2 mM, 12.4 mM and 18.1 mM, respectively (Wang & Ng 2006). Terpeneoids like applanoxidic acids A (1), C (2) and F (3) isolated from G. annulare inhibit the growth of the fungi Microsporum cannis and Trichophyton mentagrophytes at concentrations of 500–1000 µg/ml (Smania et al. 2003).
In another study, researchers synthesised the complexes of polysaccharide with different rare earth metal (RE–CGAP (RE: La, Eu and Yb)) and evaluated their efficacy against fungi and reported that rare earth carboxymethylated G. Applanatum polysaccharide (RE-CGAP) complexes with antifungal activities with EC50 value of 1.01–28.48 mg/ml (>100 mg/ml not included) (Sun et al. 2014). The details of the antifungal action of Ganoderma sp. are demonstrated in Table 2.
Table 2.
Illustration of antifungal activities of Ganoderma sp. parts, products and compounds.
| Ganodermasp. | Extraction Solvent | Parts/products/compounds | Tested Fungal strains | Method | Antifungal Concentration/ZOI/MIC/MFC/EC50 value | References |
|---|---|---|---|---|---|---|
| G. colossus | MeOH | Fruiting bodies |
C. maltosa | Agar Diffusion Assay | 8mm/2mg/disc-ZOI | (Al-Fatimi et al. 2005) |
| G. applanatum | MeOH& H2O | NG | C. albicans and C. parasilopsis | Broth Micro dilution | 1.25 & 2.5mg/ml-Antifungal activity | (Zengin et al. 2015) |
| G. resinaceum | MeOH& H2O | NG | C. albicans and C. parasilopsis | Broth Micro dilution | 1.25 & 2.5mg/ml-Antifungal activity | (Zengin et al. 2015) |
| G. lucidium | 96%EtOH | Fruiting bodies | Acremonium strictumBEOFB10m, A. glaucusBEOFB21m, A. flavusBEOFB22m, A.fumigatusBEOFB23m, A.nidulansBEOFB24m, A.nigerBEOFB25m, A. terreusBEOFB26m, T. virideBEOFB61m | Disc-diffusion & Micro dilution | 0.5-308mg/ml-MIC; 1.0–4.0mg/ml-MFC | (Ćilerdžić et al. 2014) |
| G.lucidium | MeOH | Fruiting bodies | A. fumigatus(human isolate), A. versicolor(ATCC 11730), A. zochraceus(ATCC 12066), A. niger(ATCC 6275), T. viride(IAMz5061), P. funiculosum(ATCC 36839), P. ochrochloron(ATCC 9112)and P. verrucosum var. cyclopium (food isolate) | Micro dilution | 0.005–1.5mg/ml-MIC; 0.1–4.5mg/ml-MFC | (Heleno et al. 2013) |
| G.lucidium | EtOH& chemical synthesis | RE–CGAP(RE: La, EuandYb) | V. mali, F. oxysporum, G. graminis, C. gloeosporioides, A. brassicae | Disc diffusion | 1.85–568.30mg/ml-EC50 | (Sun et al. 2014) |
| G.lucidium | dH2O | Ganodermin | Botrytis cinerea, F. oxysporum and Physalo sporapiricola | Paper Disks | 8.1–12.4mM-Antifungal | (Wang & Ng 2006) |
| G.annulare | NG | Applanoxidic acids A(1), C(2) & F(3) | M. cannis & T. mentagrophytes | Micro dilution | 500 to 1000mg/ml-Antifungal | (Smania et al. 2003) |
| G. applanatum | 96%EtOH | Mycelia | Acremonium strictum, A. glaucus, A. flavus, A. fumigatus, A. nidulans, A. niger, A. terreus, T. viride | Colorimetric | 1.00–2.00mg/ml-MIC; 1.17–4.00mg/ml-MFC | (Cilerdzic et al. 2016) |
| G. carnosum | 96%EtOH | Mycelia | Acremonium strictum, A. glaucus, A. flavus, A. fumigatus, A. nidulans, A. niger, A. terreus, T. viride | Colorimetric | 0.83–2.00mg/ml- MIC; 2.00–3.33mg/ml-MFC | (Cilerdzic et al. 2016) |
| G. lucidum | 96%EtOH | Mycelia | Acremonium strictum, A. glaucus, A. flavus, A. fumigatus, A. nidulans, A. niger, A. terreus, T.viride | Colorimetric | 0.50–2.00mg/ml- MIC; 1.17–4.00mg/ml-MFC | (Cilerdzic et al. 2016) |
MeOH: Methanol; EtOH: Ethanol; dH2O: Distilled water; NG: Data Not Given; ZOI: Zone Of Inhibition; MIC: Minimum Inhibitory Concentration; MFC: Minimum Fungicidal Concentration; EC50: Concentration; RE–CGAP: Rare Earth-CarboxymethylatedGanodermaapplanatum Polysaccharide; μM: Micro Mole; mg/ml: milligram/millilitre.
6. Antiviral activities of compounds and extracts from Ganoderma sp
It is interesting to note that the majority of antiviral investigations on Ganoderma sp. have been performed from fruiting body against the protease enzyme of HIV virus. The compounds ganoderiol F (22) and ganodermanontriol (23) were found to be active as anti-HIV-1 agents with an inhibitory concentration of 7.8 µg/ml. In addition, in the same experiment ganoderiol B (51), ganoderiol A (21), ganoderic acid A (76), ganoderic acid B (77), ganoderic acid C1 (78) and ganoderic acid H (79) were found to be moderate in their efficacy (El-Mekkawy et al. 1998; El Dine et al. 2008). Colossolactone types of triterpenoids such as colossolactone V (10), colossolactone VII (8), colossolactone VIII (7), schisanlactone A (33), colossolactone G (5) and colossolactone A (9) were isolated from the chloroform extract from G. lucidium with and IC50 value of 5–39 μg/ml (El Dine et al. 2008). Similarly in Sato et al. (2009), isolated lanostane-type triterpenoids-ganoderiol F (22), ganoderic acid GS-2 (48) and 20-hydroxylucidenic acid N (74), 20(21)-dehydrolucidenic acid N (39) from CHCl3 extract of the fruiting body of G. sinense and demonstrated the anti-HIV-1 protease activity with IC50 values of 20–40 μM (El Dine et al. 2008; Sato et al. 2009). Compounds from the CHCl3 extract of the fruiting bodies of G. colossum, farnesyl hydroquinone, ganomycin I (29) and ganomycin B (28), competitively inhibit the active site of HIV-1 protease enzyme with IC50 values of 7.5 and 1.0 μg/ml, respectively (El Dine et al. 2008).
Ganoderma pfeifferi triterpenes, ganodermadiol (20), lucidadiol (30) and applanoxidic acid G (4), were active against influenza virus type A with ED50 of greater than 0.22 mM, 0.22 mM and 0.19 mM, respectively (MothanaRa et al. 2003). Similarly others triterpenes such as ganoderone C (26) (IC50: 2.6 µg/ml), lucialdehyde B (31) (IC50:3.0 µg/ml) and ergosta-7, 22-dien-3α-ol (14) (IC50: 0.78 µg/ml) inhibited the growth of Madin-Darby canine kidney (MDCK) cells infected with influenza virus (Niedermeyer et al. 2005). Herpes simplex virus were inhibited by triterpenes such as compound (20) (ED50: 0.068 mM), ganoderone A (25) (IC50:0.075 µg/ml), (31) (IC50:0.03 µg/ml) and compound 14 (IC50: 0.03 µg/ml), whereas compounds 21 and 51 were less effective in comparison (MothanaRa et al. 2003; Niedermeyer et al. 2005). G. lucidium triterpenes lanosta-7, 9 (11), 24-trien-3-one, 15; 26-dihydroxy (GLTA) (40) and ganoderic acid Y (19) possess inhibitory action towards enterovirus 71 with IC50 value of 0.16–4 μg/ml (Zhang et al. 2015). The details of the antiviral activities of Ganoderma sp. have been illustrated in Table 3.
Table 3.
Illustration of antiviral activities of Ganoderma sp. parts, products and compounds.
| Ganodermasp. | Tested Viral strains | Extraction Solvent | Parts/products/compounds | Method | IC50 (≤50µM)/EC50/ED50 value | References |
|---|---|---|---|---|---|---|
| G. sinense | HIV 1(HIV-1 protease) | CHCl3 | Ganoderic acid GS-2(48), 20-hydroxylucidenic acid N(74), 20(21)-dehydrolucidenic acid N(39) & ganoderiol F(22) | In vitro (Enzymatic) | 20 – 40µM | (Sato et al. 2009) |
| G. colossum | HIV 1(HIV-1 protease) | CHCl3 | Colossolactone V(10), Colossolactone VII(8), Colossolactone VIII(7), Schisanlactone A(33), Colossolactone G(5), Colossolactone A(9) | In vitro (Enzymatic) | 5-39μg/mL | (El Dine et al. 2008) |
| G. colossum | HIV 1(HIV-1 protease) | CHCl3 | Ganomycin I(29) &Ganomycin B(28) | In vitro (Enzymatic) | 7.5 and 1.0 μg/mL | (El Dine et al. 2009) |
| G.lucidium | HIV 1(HIV-1 protease) | MeOH | Ganoderiol F(21) &Ganodermanontriol(23) | In vitro (Enzymatic) | 7.8μg/mL | (El-Mekkawy et al. 1998) |
| G. lucidum | Herpes Simplex Virus types 1 (HSV-1) and 2 (HSV-2), Influenza A virus (Flu A) and Vesicular Stomatitis Virus (VSV) Indiana and New Jersey strains | H2O &MeOH | Carpophores | Cytopathic Effect (CPE) Inhibition Assay & Plaque Reduction Assay | 68-1790μg/mL-EC50 | (Eo et al. 2000) |
| G. lucidum | HSV-1 and HSV-2 | H2O/EtOH | Acidic protein bound polysaccharide |
Plaque Reduction Assay | (Eo et al. 2000) | |
| G. lucidum | Oral Human Papillomavirus (HPV) | NG | Fruiting bodies | In vivo (Human) | 87% clearance of virus | (Donatini 2014) |
| G. lucidum | Newcastle Disease Virus(anti-neuraminidase) | MeOH, EtOAc & Butanol | In vitro | Virus dilution ratio(1:16, 1:16, 1:32) | (Shamaki et al. 2014) | |
| G. lucidum | Epstein-Barr Virus |
MeOH | Fruiting bodies* | In vitro | 96–100% at 1 103 mol ratio/TPA | (Iwatsuki et al. 2003) |
| G. lucidum | Hepatitis B virus | NG | mycelia | In vitro (HepG2 cells) | IRA(HBsAg, HBeAg) up to 100% | (Y. Li et al. 2006) |
| G. lucidum | Hepatitis B | H2Oand CHCl3 | mycelia(Ganoderic acid) | In vitro (HepG2215) | Inhibition of production of HBV surface antigen and HBVe at 8μg/mL | (Y.-Q. Li & Wang 2006) |
| G. pfeifferi | Influenza virus type A and HSV type 1 | NG | Ganodermadiol(20), lucidadiol (30) & applanoxidic acid G(4) | Dye Uptake Assay | Influenza ED50(0.19–0.22mmol/l); HSV 1(0.068 mmol/l for ganodermadiol) | (MothanaRa et al. 2003) |
| G. pfeifferi | HSV type 1 | CH2Cl2 | Ganoderone A(25), Lucialdehyde B(31), Ergosta-7,22-dien-3α-ol(14), Ganoderol A(21) & Ganoderol B(51) | In vitro (Vero cells) | 0.03–0.75μg/mL(IC50) | (Niedermeyer et al. 2005) |
| G. pfeifferi | Influenza virus type A | CH2Cl2 | Ganoderone C(26), Lucialdehyde B(31) & Ergosta-7,22-dien-3α-ol(14) | In vitro (MDCK cells)) | 0.78–2.6μg/mL(IC50) | (Niedermeyer et al. 2005) |
| G. lucidum | Enterovirus 71 | NG | Lanosta-7,9(11),24-trien-3- one,15;26-dihydroxy (GLTA)(40), Ganoderic acid Y(19) |
In vitro (Human Rhabdomyosarcoma) | 0.16 to 4 μg/ml(IC50) | (W. Zhang et al. 2014) |
MeOH: Methanol; EtOH Ethanol; H2O: water; NG: Data Not Given; IC50: half-maximal Inhibitory Concentration; EC50: half-maximal Effective Concentration; ED50: median effective dose; μM: Micro Mole; mg/m: -Milligram/Millilitre; μg/ml: Microgram/millilitre; *(Lucidenic acid P(58), Methyl lucidenate P(59), Methyl lucidenate Q(60), Lucidenic acid A(61), Methyl lucidenate A(62), Lucidenic acid C(63), Lucidenic acid D2(64), Methyl lucidenate D2(65), Lucidenic acid E2(66), Methyl lucidenate E2(67), Methyl lucidenate F(68), Methyl lucidenate L(69), Ganoderic acid E(54), Ganoderic acid F(57), Methyl ganoderate F(56), Ganoderic acid T-Q(54)).
7. Anti-parasitic activities of compounds and extracts from Ganoderma sp
Nortriterpenes-ganoboninketals A-C (15–17) obtained from the biochemical analysis of the fruiting bodies of G. boninense were found to possess anti-parasitic activity against P. falciparum with IC50 values of 4.0, 7.9 and 1.7 μM, respectively (Adams et al. 2010; Ma et al. 2014). Similarly three triterpenes – schisanlactone B (32), ganodermalactone F (24) and colossolactone E (6) – isolated with EtOAc and MeOH from Ganoderma sp. KM01 are active against P. falciparum in the range 6.0−10.0 μM (Lakornwong et al. 2014a). In addition, G. lucidium terepenes – ganoderic acid DM (35), ganoderic acid TR1 (52), ganoderic aldehyde TR (37), ganoderic acid S (18), ganodermanondiol (38) and ganofuran B (49) – isolated from EtOAc inhibit P. falciparum with IC50 value of range 6.0–20 μM (Adams et al. 2010). In a recent study, Zhao et al. found lectin to be active against the plant nematodes Heterodera glycines and Ditylenchus dipsaci, though their potency was not significant to be used practically (Zhao et al. 2009) .
8. Conclusion and future perspective
Ganoderma sp. has been used for treatment in various diseases over a long period (Paterson 2006). Our review clearly showed that compounds from Ganoderma sp., under the extensive in vivo and pharmacological research, can be used in various microorganisms and parasitic diseases. However, the in vivo experiment and pharmacological research of the identified compounds are very limited. Therefore, future work should be focused on in vivo and pharmacological assays of known compounds, especially Ganoderma terpenes that have antimicrobial and anti-parasitic properties. A better understanding of the antimicrobial and anti-parasitic compounds from Ganoderma sp. is crucial for identifying the potential side effects and trace out the new host target and molecular mechanisms, which will provide evidence to further clinical applications of these compounds.
Although extensive researches have been carried out on Ganoderma sp., most of the studies were concentrated on few species, G. lucidum for instance. Researchers must need to pay more attention to closely related species based on the phylogenic analysis though numerous challenges including genetic analysis, biosynthetic metabolism, separation, isolation and identification may be encountered. In addition, due to the rapid emergence of drug resistance in microorganisms and parasites, fewer options have been left for the treatment of diseases caused by microorganism and parasites. To fight back this problem, further research should be focused on this field for all the identified compounds and the unidentified compounds, which are on the way to be identified. Our review revealed numerous extracts of Ganoderma sp. exhibit the inhibition to microorganisms including parasites, indicating that Ganoderma sp. in particular still seem to possess opportunities for new drug lead compounds.
Scanty literatures are found on the assay of identified compounds for animal and plants pathogens including parasites, indicating that this area of research for the Ganoderma sp. compounds is overlooked. Also, our current experience on a literatures review of Ganoderma sp. compounds, more than 430 compounds identified (Baby et al. 2015; Rai et al. 2015), most of the compounds have not been performed on the antimicrobial and anti-parasite assay. Therefore, further studies need to be carried out in order to explore this concealed area.
No doubt, it is evident that Ganoderma sp. is going to serve as one of the potential sources of novel antibiotics and anti-parasitic drugs in the near future. To reach the apex and specificity of effective antimicrobial and anti-parasite activity, cooperative investigations need to be carried out in the areas of genomic, bioinformatics, chemistry and pharmacology. Moreover, strategies to evoke the sleeping gene clusters linked for the production of bioactive compounds and its regulation need to be adopted.
Acknowledgements
We would like to express sincere gratitude to the laboratory members of State Key Laboratory of Mycology, Chinese Academy of Sciences, and we would like to thank the reviewers in advance for their comments that will help improve an earlier version of the manuscript.
Funding Statement
This work was supported financially by the National Nature Science Foundation (numbers 21472233 and 81673334) and the Youth Innovation Promotion Association of Chinese Academy of Sciences (number 2014074).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adams M, Christen M, Plitzko I, Zimmermann S, Brun R, Kaiser M, Hamburger M.. 2010. Anti plasmodial Lanostanes from the Ganoderma lucidum Mushroom. J Nat Prod. 73:897–900. [DOI] [PubMed] [Google Scholar]
- Al-Fatimi M, Wurster M, Kreisel H, Lindequist U.. 2005. Antimicrobial, cytotoxic and antioxidant activity of selected basidiomycetes from Yemen. Pharmazie. 60:776–780. [PubMed] [Google Scholar]
- Baby S, Johnson AJ, Govindan B. 2015. Secondary metabolites from Ganoderma. Phytochem. 114:66–101. [DOI] [PubMed] [Google Scholar]
- Chuang CM, Wang HE, Chang CH, Peng CC, Ker YB, Lai JE, Chen KC, Peng RY. 2013. Sacchachitin, a novel chitin-polysaccharide conjugate macromolecule present in Ganoderma lucidum: purification, composition, and properties. Pharm Biol. 51:84–95. [DOI] [PubMed] [Google Scholar]
- Ćilerdžić J, Vukojević J, Stajić M, Stanojković T, Glamočlija J. 2014. Biological activity of Ganoderma lucidum basidiocarps cultivated on alternative and commercial substrate. J Ethnopharmacol. 155:312–319. [DOI] [PubMed] [Google Scholar]
- Cilerdzic J, Stajic M, Vukojevic J. 2016. Potential of submergedly cultivated mycelia of Ganoderma spp. as antioxidant and antimicrobial agents. Curr Pharm Biotechnol. 17:275–282. [DOI] [PubMed] [Google Scholar]
- Donatini B. 2014. Control of Oral Human Papillomavirus (HPV) by medicinal mushrooms, trametes versicolor and Ganoderma lucidum: A preliminary clinical trial. Int J Med Mucshrooms. 16:497-498. [DOI] [PubMed] [Google Scholar]
- El Dine RS, El Halawany AM, Ma C-M, Hattori M. 2008. Anti-HIV1 Protease Activity of Lanostane Triterpenes from the Vietnamese Mushroom Ganoderma colossum . J Nat Prod. 71:1022–1026. [DOI] [PubMed] [Google Scholar]
- El Dine RS, El Halawany AM, Ma C-M, Hattori M. 2009. Inhibition of the Dimerization and Active Site of HIV-1 Protease by Secondary Metabolites from the Vietnamese Mushroom Ganoderma colossum . J Nat Prod. 72:2019–2023. [DOI] [PubMed] [Google Scholar]
- El-Mekkawy S, Meselhy MR, Nakamura N, Tezuka Y, Hattori M, Kakiuchi N, Shimotohno K, Kawahata T, Otake T. 1998. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum . Phytochem. 49:1651–1657. [DOI] [PubMed] [Google Scholar]
- Eo SK, Kim YS, Lee CK, Han SS. 2000. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J Ethnopharmacol. 72:475–481. [DOI] [PubMed] [Google Scholar]
- Ferreira ICFR, Heleno SA, Reis FS, Stojkovic D, Queiroz MJRP, Vasconcelos MH, Sokovic M. 2015. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochem. 114:38–55. [DOI] [PubMed] [Google Scholar]
- Heleno SA, Ferreira IC, Esteves AP, Ćirić A, Glamočlija J, Martins A, Soković M, Queiroz MJR. 2013. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chemtoxicol. 58:95–100. [DOI] [PubMed] [Google Scholar]
- Hill RA, Connolly JD. 2013. Triterpenoids. Nat Prod Rep 30:1028–1065. Available from: http://www.speciesfungorum.org/ [DOI] [PubMed] [Google Scholar]
- Huie CW, Di X. 2004. Chromatographic and electrophoretic methods for Lingzhi pharmacologically active components. J Chromatogr B Analyt Technol Biomed Life Sci. 812:241–257. [DOI] [PubMed] [Google Scholar]
- Isaka M, Chinthanom P, Sappan M, Danwisetkanjana K, Boonpratuang T, Choeyklin R. 2016. Antitubercular Lanostane Triterpenes from Cultures of the Basidiomycete Ganoderma sp. BCC 16642. J Nat Prod. 79:161–169. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Akihisa T, Tokuda H, Ukiya M, Oshikubo M, Kimura Y, Asano T, Nomura A, Nishino H. 2003. Lucidenic Acids P and Q, Methyl Lucidenate P, and Other Triterpenoids from the Fungus Ganoderma lucidum and Their inhibitory effects on epstein−barr virus activation. J Nat Prod. 66:1582–1585. [DOI] [PubMed] [Google Scholar]
- Karwa A, Rai M. 2012. Naturally occurring medicinal mushroom-derived antimicrobials: A case-study using lingzhi or reishi Ganoderma Iucidum (W. Curt.: fr.) P. Karst. (Higher Basidiomycetes). Int J Med Mushrooms.14:481. [DOI] [PubMed] [Google Scholar]
- Lakornwong W, Kanokmedhakul K, Kanokmedhakul S, Kongsaeree P, Prabpai S, Sibounnavong P, Soytong K. 2014. Triterpene Lactones from Cultures of Ganoderma sp. KM01. J Nat Prod. 77:1545–1553. [DOI] [PubMed] [Google Scholar]
- Li WJ, Nie SP, Liu XZ, Zhang H, Yang Y, Yu Q, Xie MY. 2012. Antimicrobial properties, antioxidant activity and cytotoxicity of ethanol-soluble acidic components from Ganoderma atrum . Food Chem Toxicol. 50:689–694. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang Y, Fang L, Zhang Z, Jin J, Zhang K. 2006. Anti-hepatitis activities in the broth of Ganoderma lucidum supplemented with a Chinese herbal medicine. Am J Chin Med. 34:341–349. [DOI] [PubMed] [Google Scholar]
- Li YQ, Wang SF. 2006. Anti-hepatitis B activities of ganoderic acid from Ganoderma lucidum . Biotechnol Lett. 28:837–841. [DOI] [PubMed] [Google Scholar]
- Liu DZ, Zhu YQ, Li XF, Shan WG, Gao PF. 2014. New triterpenoids from the fruiting bodies of Ganoderma lucidum and their bioactivities. ChemBiodivers. 11:982–986. [DOI] [PubMed] [Google Scholar]
- Ma K, Ren JW, Han JJ, Bao L, Li L, Yao YJ, Sun C, Zhou B, Liu HW. 2014. Ganoboninketals A–C, antiplasmodial 3,4-seco-27-norlanostane triterpenes from Ganoderma boninense Pat. J Nat Prod. 77:1847–1852. [DOI] [PubMed] [Google Scholar]
- Mikolasch A, Hildebrandt O, Schlüter R, Hammer E, Witt S, Lindequist U. 2016. Targeted synthesis of novel β-lactam antibiotics by laccase-catalyzed reaction of aromatic substrates selected by pre-testing for their antimicrobial and cytotoxic activity. Appl Microbiol Biotechnol. 100:4885–4899. [DOI] [PubMed] [Google Scholar]
- Mothana RAA, Jansen R, Jülich WD, Lindequist U. 2000. Ganomycins A and B, New antimicrobial farnesyl hydroquinones from the basidiomycete Ganoderma pfeifferi . J Nat Prod. 63:416–418. [DOI] [PubMed] [Google Scholar]
- MothanaRa A, Awadh Ali NA, Jansen R, Wegner U, Mentel R, Lindequist U. 2003. Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi . Fitoterapia. 74:177–180. [DOI] [PubMed] [Google Scholar]
- Niedermeyer THJ, Lindequist U, Mentel R, Gördes D, Schmidt E, Thurow K, Lalk M. 2005. Antiviral terpenoid constituents of Ganoderma pfeifferi . J Nat Prod. 68:1728–1731. [DOI] [PubMed] [Google Scholar]
- Ofodile LN, Ogbe AO, Oladipupo O. 2011. Effect of the mycelial culture of Ganoderma lucidum on human pathogenic bacteria. Int J Biol. 3: 111-114. [Google Scholar]
- Ofodile LN, Uma NU, Kokubun T, Grayer RJ, Ogundipe OT, Simmonds MSJ. 2005. Antimicrobial activity of some Ganoderma species from Nigeria. Phytother Res. 19:310–313. [DOI] [PubMed] [Google Scholar]
- Oluba OM, Olusola AO, Fagbohunka BS Onyeneke E. 2012. (w.curt.:fr.)p.karst.(higher basidiomycetes), in plasmodium berghei-infected mice. antimalarial And Hepatoprotective Effects Of Crude Ethanolic Extract Of lingzhi Or Reishi Medicinal mushroom, Ganoderma Lucidum. 14:459-466. [DOI] [PubMed] [Google Scholar]
- Paterson RRM. 2006. Ganoderma – A therapeutic fungal biofactory In: Phytochem. 67: 1985–2001. [DOI] [PubMed] [Google Scholar]
- Rai MK, Gaikwad S, Nagaonkar D, Dos Santos CA. 2015. Current advances in the antimicrobial potential of species of genus ganoderma (Higher basidiomycetes) against human pathogenic microorganisms (Review). Int J Med Mucshrooms. 17: 921-932. [DOI] [PubMed] [Google Scholar]
- Sa-Ard P, Sarnthima R, Khammuang S, Kanchanarach W. 2015. Antioxidant, antibacterial and DNA protective activities of protein extracts from Ganoderma lucidum . J Food Sci Technol. 52:2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanodiya BS, Thakur GS, Baghel RK, Prasad G, Bisen PS. 2009. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 10:717–742. [DOI] [PubMed] [Google Scholar]
- Sato N, Zhang Q, Ma C-M, Hattori M. 2009. Anti-human immunodeficiency virus-1 protease activity of new lanostane-type triterpenoids from Ganoderma sinense. Chem Pharm Bull. 57:1076–1080. [DOI] [PubMed] [Google Scholar]
- Shamaki BU, Sandabe UK, Ogbe AO, Abdulrahman FI, El-Yuguda A-D. 2014. Methanolic soluble fractions of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Higher basidiomycetes) Extract inhibit neuraminidase activity in newcastle disease virus (LaSota). Int J Med Mushrooms. 16: 579-583. [DOI] [PubMed] [Google Scholar]
- Shang X, Tan Q, Liu R, Yu K, Li P, Zhao G-P. 2013. In vitro anti-Helicobacter pylori effects of medicinal mushroom extracts, with special emphasis on the Lion’s Mane mushroom, Hericium erinaceus (higher Basidiomycetes). Int J Med Mushrooms. 15: 165-174. [DOI] [PubMed] [Google Scholar]
- Smania EFA, DelleMonache F, Smania A, Yunes RA, Cuneo RS. 2003. Antifungal activity of sterols and triterpenes isolated from Ganoderma annulare . Fitoterapia. 74:375–377. [DOI] [PubMed] [Google Scholar]
- Sun X, Jin X, Pan W, Wang J. 2014. Syntheses of new rare earth complexes with carboxymethylated polysaccharides and evaluation of their in vitro antifungal activities. Carbohydr Polym. 113:194–199. [DOI] [PubMed] [Google Scholar]
- Vazirian M, Faramarzi MA, Ebrahimi SES, Esfahani HRM, Samadi N, Hosseini SA, Asghari A, Manayi A, Mousazadeh SA, Asef MR, et al. 2014. Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds. Int J Med Mushrooms. 16: 77-84. [DOI] [PubMed] [Google Scholar]
- Wang H, Ng TB. 2006. Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom Ganoderma lucidum . Peptides. 27:27–30. [DOI] [PubMed] [Google Scholar]
- Xia Q, Zhang H, Sun X, Zhao H, Wu L, Zhu D, Yang G, Shao Y, Zhang X, Mao X, et al. 2014. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 19:17478–17535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Eo SK, Kim YS, Lee CK, Han SS. 1994. Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch Pharm Res. 17:438–442. [DOI] [PubMed] [Google Scholar]
- Zengin G, Sarikurkcu C, Gunes E, Uysal A, Ceylan R, Uysal S, Gungor H, Aktumsek A. 2015. Two Ganoderma species: profiling of phenolic compounds by HPLC–DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 6:2794–2802. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Wang YG, Ma QY, Huang SZ, Hu LL, Dai HF, Yu ZF, Zhao YX. 2015. Three new lanostanoids from the mushroom Ganoderma tropicum . Molecules. 20:3281–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Tao J, Yang X, Yang Z, Zhang L, Liu H, Wu K, Wu J. 2014. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem Biophys Res Commun. 449:307–312. [DOI] [PubMed] [Google Scholar]
- Zhao S, Guo YX, Liu QH, Wang HX, Ng TB. 2009. Lectins but not antifungal proteins exhibit anti-nematode activity. Environ Toxicol Pharmacol. 28:265–268. [DOI] [PubMed] [Google Scholar]


