Abstract
The transition from adolescent to adult cognition and emotional control requires neurodevelopmental maturation likely involving intrinsic functional networks (IFNs). Normal neurodevelopment may be vulnerable to disruption from environmental insult such as alcohol consumption commonly initiated during adolescence. To test potential disruption to IFN maturation, we used resting-state functional magnetic resonance imaging (rs-fMRI) in 581 no-to-low alcohol-consuming and 117 moderate-to-high-drinking youth. Functional seed-to-voxel connectivity analysis assessed age, sex, and moderate alcohol drinking on default-mode, executive-control, salience, reward, and emotion networks and tested cognitive and motor coordination correlates of network connectivity. Among no-to-low alcohol-consuming adolescents, executive-control frontolimbicstriatal connectivity was stronger in older than younger adolescents, particularly boys, and predicted better ability in balance, memory, and impulse control. Connectivity patterns in moderate-to-high-drinking youth were tested mainly in late adolescence when drinking was initiated. Implicated was the emotion network with attenuated connectivity to default-mode network regions. Our cross-sectional rs-fMRI findings from this large cohort of adolescents show sexual dimorphism in connectivity and suggest neurodevelopmental rewiring toward stronger and spatially more distributed executive-control networking in older than younger adolescents. Functional network rewiring in moderate-to-high-drinking adolescents may impede maturation of affective and self-reflection systems and obscure maturation of complex social and emotional behaviors.
Keywords: alcohol, emotion, executive control, functional MRI, puberty, resting state connectivity
Introduction
Neurodevelopment of brain structure continues throughout adolescence (Kipke 1999; Sowell et al. 2004; Giedd et al. 2014; Hutchison and Morton 2015; Pfefferbaum et al. 2015) with some changes involving expansion, such as growth of white matter enabling increased connectivity and processing efficiency (Yakovlev and Lecours 1967; Richardson 1982; Lebel and Beaulieu 2011; Avants et al. 2015). Others are regressive, such as reductions in regional gray matter (Gogtay et al. 2004; Lebel and Beaulieu 2011; Raznahan et al. 2011; Klein et al. 2014) reflecting pruning of unused synapses and neurons also toward enhancing efficiency (Feinberg et al. 1990; Huttenlocher and Dabholkar 1997; Chugani 1998). These complementary processes are marked by heterochronicity, that is, they develop at different times, and underlie neural rewiring of large-scale cortical and subcortical networks by forming complex fiber connections (Sowell et al. 2004; Lenroot et al. 2007). This structural ontogeny dynamically alters brain function to support the increasingly sophisticated cognitive abilities, motor performance, self-regulation, and reward-focused processing during adolescent development (Crone et al. 2009; Smith et al. 2013). Intrinsic networks underlying selective brain functions can be detected and tracked with whole brain, resting-state functional magnetic resonance imaging (rs-fMRI), which relates functional connectivity to synchronous fluctuations in blood oxygen level-dependent (BOLD) signals among brain regions (Biswal et al. 1995; Shulman et al. 1997). Complementing recent findings based on adolescence rs-fMRI (Fair et al. 2007, 2008, 2009; Jolles et al. 2011; Avants et al. 2015), the present study used a large sample of adolescents to investigate the effects of age, sex (Alarcon et al. 2015; Satterthwaite et al. 2015), and alcohol consumption (Cservenka et al. 2015a) on the development of intrinsic functional networks (IFNs) (Vanderwal et al. 2013) associated with cognitive and emotional functioning.
Several IFNs have been previously described (for a review, Yeo et al. 2011; Vanderwal et al. 2013) with surprising consistency despite differences in applied analysis techniques (e.g., Van Dijk et al. 2010; Erhardt et al. 2011; Raichle 2011; Calhoun and Allen 2013; Posse et al. 2013). Of the 5 IFNs selected here, the default mode network (DMN) is the most researched at rest and is associated with self-referential thought, introspection, memory, and future-related processing (Buckner et al. 2008; Habas et al. 2009; Raichle 2009). Selective cognitive functioning is commonly linked to regions connected by the salience network (SAN) and executive control network (ECN). SAN enables switching between networks by directing attention to salient and conceptually relevant interoceptive or external stimuli (Seeley et al. 2007; Menon and Uddin 2010); the ECN subserves attention, cognitive control, response selection, and working memory (Dosenbach et al. 2006; Seeley et al. 2007; Sridharan et al. 2008). Emotional functioning links to regions connected by the remaining 2 IFNs, where the emotion network (EMN) contributes to regulating and modulating emotional responses (Wrase et al. 2003; Perlman and Pelphrey 2011), and the reward network (RWN) is associated with positive and negative reinforcement (Courtney et al. 2013; Müller-Oehring et al. 2015), thus posited to play a critical role in the development and maintenance of addiction (Koob and Volkow 2010).
Findings on brain maturation of IFNs indicate shifts from spatially local to more widely distributed connectivity (Fair et al. 2007, 2008, 2009; Kelly et al. 2009; Jolles et al. 2011). Converging evidence depicts stronger functional coupling among key nodes of the DMN (see also Fair et al. 2008), SAN, and ECN in older adolescents than preadolescents and has been related to structural fiber connectivity (Uddin et al. 2011). Contributing to variance in functional connectivity maturation (Avants et al. 2015) is sexual dimorphism in pubertal development, with an average lag of 2 years in boys relative to girls, contributing further to heterogeneity in development over age (Kipke 1999). Neural network modifications during adolescence are modulated by experience and learning (Braun and Bogerts 2000) in interaction with sex hormones, heredity (Arain et al. 2013), and experience with alcohol and drug use (e.g., Bava et al. 2010; Cservenka et al. 2015b; Weissman et al. 2015). A further complication to normal developmental trajectories may arise from nonlinear and nonparallel maturation processes of distinctive functional networks that may underlie hypersensitivity to emotion-laden stimuli during adolescence (Strakowski et al. 2011). This discoordination in development occurs when the self-reflection and executive control systems of the adolescent brain have not matured adequately to compensate for heightened emotion and reward system responses (Smith et al. 2013) and may differ substantially between boys and girls, given their different rates of pubertal maturation. Adolescence also defines a time of vulnerability to the effects of alcohol (e.g., Bava et al. 2010; Cservenka et al. 2015b), potentially jeopardizing IFN maturation and the adolescent's capability to regulate temptation and avert risky behavior. Whether excessive use of alcohol and drugs attenuates or accelerates functional neuromaturation, and whether functional networks based in cortical and subcortical regions are similarly affected between sexes remains in question.
To study developmental differences in brain functional networks across the adolescent age range, identify sex-linked differences, and measure the influence of a history of adolescent alcohol consumption on IFN development, we examined rs-fMRI in 698 adolescents (age 12–21 years) from the multisite National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) study (Brown et al. 2015). This cohort comprised 2 groups: 581 who met criteria for no-to-low alcohol or drug exposure and 117 recruits who exceeded NCANDA alcohol or drug use history criteria at recruitment but met all other eligibility criteria (Brown et al. 2015). The rs-fMRI was collected on 3 T General Electric (GE) and Siemens MRI scanners at 5 sites in the continental United States of America. Similar to other multisite studies (Friedman et al. 2008; Greve et al. 2011; Saad et al. 2013; Yan et al. 2013; Zuo et al. 2014; Jovicich et al. 2015), we identified significant signal differences attributable to the 2 scanner types. A novel data-driven matching algorithm mitigated those differences while preserving the effect size of our analysis. Neuropsychological tests (Sullivan et al. 2016) of attention, abstraction, general ability, working and episodic memory, balance, and impulse control tested for behavioral ramifications of observed functional network connectivity.
We tested the following hypotheses: (1) connectivity of 5 IFNs (DMN, SAN, ECN, EMN, and RWN) would be stronger and spatially more distributed in older than younger adolescents; (2) these developmental differences would occur at a younger age in girls than boys; (3) functional connectivity maturation would be attenuated in adolescents with alcohol and drug histories of exceeding study criteria; and (4) stronger functional connectivity would be predictive of better cognitive or motor test performance.
Material and Methods
Participants
The 698 participants (Table 1) were age from 12 to 21 years and recruited by the 5 NCANDA collection sites. The sample included 581 adolescents meeting the basic alcohol and drug use criteria for no/low-drinking use in the NCANDA study (Brown et al. 2015) and 117 adolescents exceeding these entry criteria. The criteria were staggered by age; for alcohol histories, for example, adolescents should have never consumed more than 5 (ages from 12 to 15), 11 (age 16), 23 (age 17), or 51 (ages from 18 to 21) drinks in their lifetime. Also, girls should never have exceeded more than 3 drinks per occasion, and boys no more than 3 (up to age 14), 4 (ages from 14 to 19), or 5 (ages from 20 to 21) drinks per occasion. Youth who exceeded drinking thresholds were allowed to exceed marijuana and nicotine exposure criteria but required to meet all other eligibility criteria. Of the exceeds-criteria group, 9 met criteria for DSM-IV (American-Psychiatric-Association 2000) alcohol abuse; 1 met criteria for alcohol dependence. The MRI report created by a clinical neuroradiologist was negative for all 698 youth (for clinical summaries, Pfefferbaum et al. 2015). As required, consent (for majors) and assent (for minors) were obtained according to the Declaration of Helsinki. Participants were characterized by age, sex, pubertal stage using the self-assessment Pubertal Development Scale (PDS) (Petersen et al. 1988), and socioeconomic status (Akshoomoff et al. 2014) and measured the highest education of either parent (Akshoomoff et al. 2014; Pfefferbaum et al. 2015). The data of all samples were archived as a data release (VERSION: NCANDA_DATA_0015) using the software Scalable Informatics for Biomedical Imaging Studies (https://sibis.sri.com) (Rohlfing et al. 2014; Nichols and Pohl 2015; Pfefferbaum et al. 2015).
Table 1.
Demographic characteristics of adolescent study groups for those meeting no/low alcohol use history (AUH) criteria and those exceeding criteria for at-risk alcohol consumption: n = subject count; mean ± standard deviation (SD) (range); significant P-values are marked in italic
| No/low AUH | Matched groups | Difference between matched groups; P = |
|||
|---|---|---|---|---|---|
| Exceeds-criteria group | Matched no/low AUH subgroup | ||||
| n | Total | 581 | 117 | 117 | |
| Girls/boys | 306/275 | 62/55 | 62/55 | 1.0a | |
| GE/Siemens | 385/196 | 80/37 | 72/45 | 0.27a | |
| Age (years) | 15.9 ± 2.3 (12–21.9) | 18.6 ± 1.9 (13–1.9) | 18.4 ± 1.8 (13–21.9) | 0.39 | |
| PDSb | 3.2 ± 0.7 (1–4) | 3.7 ± 0.4 (1.8–4) | 3.6 ± 0.4 (2.2–4) | 0.28 | |
| Alcohol use | Days lifetime | 1.1 ± 4.2 | 51.6 ± 75.8 | 3.1 ± 7.2 | <0.001 |
| Days past year | 0.0±2.9 | 23.7 ± 31.9 | 1.8 ± 4.9 | <0.001 | |
| Max drinksc | 0.4±0.9 | 7.6 ± 4.7 | 0.8 ± 1.2 | <0.001 | |
| Binges past year | 0 | 12.2 ± 12.2 | 0 | 0.004 | |
| Marijuana use | Days lifetime | 0.6 ± 2.5 | 10.8 ± 17.7 | 1.0 ± 3.9 | 0.004 |
| Days past year | 0.3 ± 1.6 | 7.5 ± 16.0 | 0.6 ± 2.5 | 0.015 | |
| Parental education (years) | 16.9 ± 2.4 (6–20) | 17.4 ± 2 (12–20) | 17.0 ± 2 (11–20) | 0.19 | |
| Highest grade | 9.2 ± 2.4 (5–15) | 11.9 ± 1.9 (6–16) | 11.8 ± 1.9 (7–15) | 0.66 | |
| WRATd | Reading | 116 ± 17 (80–145) | 113 ± 14 (85–145) | 114 ± 14 (84–145) | 0.83 |
| Writing | 112 ± 16 (66–145) | 113 ± 14 (72–143) | 113 ± 167 (75–145) | 0.73 | |
aChi-square test.
bScore ranges between 1 = “puberty not started” and 4 = “puberty completed.”
cMaximum number of drinks at one occasion in the past year.
dWide Range Achievement Test (WRAT): standard scores are reported with an expected mean ± SD of 100 ± 15.
Neuropsychological Tests
Adolescents took a test battery of traditional and computer-based neuropsychological tests (Gur et al. 2010; Gur et al. 2012). Composite performance accuracy scores were expressed as standardized Z-scores for 7 functional domains (abstraction, attention, emotion, episodic memory, working memory, balance, and general ability). In addition, the Delay Discounting task (Bickel et al. 2007; Stanger et al. 2011; Stanger et al. 2013) examined reward seeking, decision-making, and impulsive behavior. Descriptions of the tests are in Sullivan et al. (2016) and summarized in the Supplemental Material.
Acquisition and Processing of MRI
This section summarizes the acquisition and analysis of MRI detailed in the supplement. MRI data were acquired using 3 T GE Discovery MR750 at 3 sites (180 from University of California-San Diego, 132 from SRI International, and 153 from Duke University) and 3 T Siemens TIM TRIO scanners at 2 sites (99 from University of Pittsburgh and 134 from Oregon Health & Sciences University) using a standardized protocol for rs-fMRI (10 min scan duration), participants kept eyes open while viewing a gray screen.
From rs-fMRI data, model-driven seed-to-voxel correlation maps (Biswal et al. 1995; Greicius et al. 2003; Fox et al. 2005) were generated using modified version of the resting state-specific analysis pipeline of Nipype (Gorgolewski et al. 2011) in combination with following publicly available, common tools: FSL (Jenkinson et al. 2012), Numpy (www.numpy.org/), CMTK (Rohlfing et al. 2003), and ConnToolbox (Whitfield-Gabrieli and Nieto-Castanon 2012). The processing included standard motion correction via FSL MCFLIRT (Jenkinson et al. 2002). Based on the estimated motion, outlier frames (displacement < 0.3 mm/TR) in individual BOLD time series were detected using Nipype RapidArt (Chai et al. 2012). For the 782 baseline rs-fMRI scans of the NCANDA study, the mean number of outliers was 17.8 and maximum number of outliers was 225 frames. We omitted scans from further analysis whose remaining scan time of good, usable frames was <7.8 min, which resulted in n = 84 subjects being excluded. We choose this conservative threshold given the lack of consensus in the literature on motion thresholds for scan exclusion (Power et al. 2010). For the remaining 698 subjects, NiPype applied the standard scrubbing approach regressing out outlier frames. As mentioned, additional processing steps are outlined in the supplement such as the estimation and correction of physiological noise via NiPype's implementation of Behzadi et al. (2007).
We chose seed-based correlation analysis, which is a model-based approach that uses prior knowledge (e.g., Raichle 2011), because it has advantages over independent component analysis (ICA) for detecting small signal changes (Posse et al. 2013). As such the seed-based approach may be especially sensitive for detecting age- and sex-related changes in the maturation of functional networking and perturbations in connectivity associated with alcohol consumption during adolescence. Furthermore, the interpretation of the results of seed-based correlation analysis is hypothesis-driven and prospective (Cole et al. 2014), whereas data-driven approaches, such as ICA, require user interaction to interpret resulting maps and make implicit assumptions about parameter settings that are often not well understood (Posse et al. 2013). We selected the seed regions for each network according to Raichle (2011) (posterior cingulate cortex [PCC] for the DMN, superior frontal gyrus [SFG] for the ECN, anterior cingulate cortex [ACC] for the SAN) and Müller-Oehring et al. (2015) (amygdala for the EMN [Phan et al. 2002], nucleus accumbens [NAcc] for the RWN [Neto et al. 2008; Demos et al. 2012; Müller-Oehring et al. 2015]). Note, each selected seed region is a main node in its respective IFN independent of analysis technique (e.g., Van Dijk et al. 2010; Erhardt et al. 2011). For example, we chose the ACC as seed, because it is a principal node in the SAN (e.g., Seeley et al. 2007; Raichle 2011). Consistent with this functional character of the ACC, we found it connected with regions (e.g., insula) that have also been previously identified as principal nodes of the SAN, and further by ICA-based analysis techniques (e.g., Seeley et al. 2007; Menon and Uddin 2010).
To account for site differences, we note that all NCANDA collection sites strictly followed the same data acquisition protocol with the exception of the MRI scanner used for acquiring imaging data. The protocol was exactly the same for the 3 GE sites as well as for the 2 Siemens sites. All data were checked for compliance to those protocols, which included reviewing phantom scans acquired on the same day as rs-fMRI scans. Modeling site differences was thus equivalent to accounting for differences between the acquisition of the 2 scanner types, which functional seed-to-voxel connectivity analysis confirmed by revealing significant differences between timeline correlation maps from GE and Siemens scanners for each seeded network (see Supplemental Figure). To overcome those differences, we developed a novel algorithm that matched all the correlation maps inferred from Siemens images to those generated from GE images. For each seed region and image location, the algorithm first generated a histogram of the correlation values inferred from data acquired with the same manufacturer. It then matched the Siemens histogram to the GE histogram. Finally, the Siemens correlation maps were modified according to the matching thus resulting in a histogram of correlation values that equaled those of the GE histogram (see Supplement). Application of our data-driven approach removed manufacturer effects at the single-subject level. This correction enabled group analysis using statistical models typically applied to single scanner type studies but did not induce spurious findings, rendering group effects spatially consistent and statistically stronger compared with the findings on uncorrected data (see Supplement Figure).
Using ConnToolbox, group analysis statistical threshold for peak levels for overall IFN seed-to-voxel correlation maps in no/low criteria adolescents (n = 581) with age as covariate were set at t = 6.0 (family-wise error [FWE]-corrected for multiple comparisons). Group analysis of sex (boys and girls) and age effects (continuous variable for boys and girls) in “no/low criteria” adolescents was set at P value = 0.001 (t = 3.1) and correction for multiple comparisons required a cluster size of k = 350 contiguous voxels for sex, k = 293 contiguous voxels for age effects, and k = 287 continuous voxels for age-by-sex interactions, where clusters were computed via AFNI 3dclustSim V.16.1.15 (addressing the concerns of Eklund et al. 2016). For analysis of the effect of alcohol use history (AUH) on IFN, a matched-group analysis compared 117 exceeds-criteria adolescents to a subgroup of 117 no/low-drinking adolescents matched to the exceeds group on age, sex, education, ethnicity, and ratio between scanner types (Siemens, GE). Also for the matched subgroups analysis, the statistical threshold for peak levels was set to P = 0.001 (t = 3.13) requiring a cluster size of k = 291 contiguous voxels to pass the multiple comparisons correction test.
Brain–Behavior Correlations
To test our directional hypothesis, where greater functional connectivity would correlate with better test performance, we correlated the corrected correlation maps of each seed region with neuropsychological test scores (Pearson correlation; SPSS software package). Family-wise Bonferroni correction for 8 neuropsychological test comparisons required p ≤ 0.0125.
Results
Default Mode Network
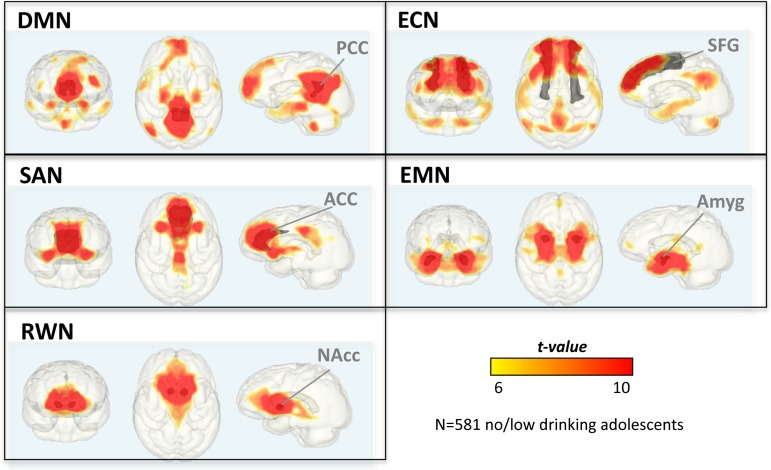
DMN maps revealed temporally synchronous voxel clusters that involved expected structures: PCC and precuneus, medial prefrontal cortex and ACC, and bilateral lateral parietal cortices. Synchronous activation also occurred between the PCC seed and limbic areas (hippocampus, amygdala, and insula), striatum (putamen and caudate), thalamus, and cerebellar areas (Table 2a and Fig. 1).
Table 2.
IFNs in adolescence
| a. DMN (age as covariate) | ||
|---|---|---|
| PCC—connected nodes | t | k |
| L.+R. PCC/MCC, precuneus, calcarine, medial occipital, L. cerebellum, L.+R. thalamus, L. hippocampus, amygdala | 46.57 | 10 609 |
| L.+R. medial prefrontal, ACC, L. superior frontal | 12.90 | 4682 |
| L. lateral parietal | 16.17 | 848 |
| R. hippocampus, amygdala | 10.20 | 597 |
| L. lateral temporal, temporal pole | 8.36 | 575 |
| L.+R. inferior cerebellum, vermis | 10.71 | 475 |
| R. lateral temporal | 8.17 | 271 |
| R. lateral parietal | 9.00 | 242 |
| R. superior frontal | 7.85 | 113 |
| Sex | ||
| Girls > boys | ||
| L.+R. PCC, precuneus | 5.70 | 589 |
| R. midbrain, hippocampus | 5.16 | 567 |
| L.+R. MCC, post- and precentral | 4.71 | 377 |
| Age correlation (boys and girls) | ||
| Negative correlation | ||
| L. hippocampus, amygdala | 3.99 | 411 |
| R. hippocampus, amygdala | 4.29 | 301 |
| Boys: negative correlation | ||
| L. hippocampus, amygdala | 4.49 | 425 |
| AUH: Matched groups | ||
| No suprathreshold cluster |
| b. ECN (age as co-variate) | ||
|---|---|---|
| SFG—connected nodes | t | k |
| L.+R. Lateral and medial frontal and prefrontal, ACC | 16.00 | 12 780 |
| L.+R. middle and PCC, precuneus | 10.13 | 1895 |
| L. lateral superior, middle, and inferior temporal | 8.74 | 1339 |
| L. lateral inferior parietal, supramarginal gyrus, TPO junction | 12.82 | 922 |
| R. superior and inferior cerebellum | 9.07 | 918 |
| R. lateral superior, middle, and inferior temporal | 7.78 | 848 |
| L. superior and inferior cerebellum | 11.20 | 721 |
| R. lateral inferior parietal lobe | 9.43 | 378 |
| Sex | ||
| Girls > boys | ||
| L.+R. medial prefrontal, ACC, superior frontal | 4.65 | 1342 |
| Age correlation (boys and girls) | ||
| Positive correlation | ||
| R. insula, inf. lateral frontal, hippocampus, amygdala, striatum, thalamus | 5.01 | 2932 |
| L. hippocampus, amygdala, striatum, parietal, occipital, posterior precuneus | 4.77 | 965 |
| R. inferior parietal lobe, superior occipital | 4.26 | 401 |
| L insula, inf. lateral frontal, sup. temporal | 3.92 | 300 |
| Boys: positive age correlation | ||
| R. insula, inf. lateral frontal, hippocampus, amygdala, striatum, thalamus | 5.16 | 2232 |
| L. thalamus, caudate tail | 4.18 | 575 |
| L. striatum (caudate head, putamen), inferior lateral frontal | 4.44 | 544 |
| AUH: Matched groups | ||
| No suprathreshold cluster |
| c. SAN (age as co-variate) | ||
|---|---|---|
| ACC—connected nodes | t | k |
| L.+R. ACC, medial prefrontal, inferior frontal triangular, anterior insula, striatum, thalamus, globus pallidus | 29.30 | 11 203 |
| L.+R. middle and PCC, anterior, middle, and posterior precuneus | 12.50 | 1791 |
| Sex | ||
| Boys > girls | ||
| R. superior temporal, temporal pole, inferior frontal, orbitofrontal | 5.63 | 1108 |
| L. angular, supramarginal | 4.56 | 602 |
| L. superior temporal, temporal pole, inferior frontal, orbitofrontal, insula | 4.56 | 503 |
| Girls > boys | ||
| L.+R. ACC | 5.64 | 1276 |
| c. SAN | ||
| Age correlation (boys and girls) | ||
| No suprathreshold cluster | ||
| Boys: negative correlation | ||
| R. extrastriate cortex: inferior occipital (lingual gyrus) | 4.70 | 558 |
| Girls: positive correlation | ||
| R. prefrontal cortex | 4.56 | 350 |
| Boys > Girls | ||
| R. extrastriate cortex: inferior occipital (lingual gyrus) | 5.03 | 714 |
| L. extrastriate cortex: inferior occipital (lingual gyrus) | 4.26 | 295 |
| AUH: Matched groups | ||
| No suprathreshold cluster |
| d. EMN (age as covariate) | ||
|---|---|---|
| Amygdala—connected nodes | t | k |
| L.+R. lateral superior, middle, and inferior temporal, parahippocampus, hippocampus, insula, striatum, globus pallidus, thalamus, midbrain, cerebellum | 36.67 | 14 380 |
| L. lateral frontal, insula | 7.03 | 235 |
| L.+R. medial prefrontal | 6.88 | 144 |
| L.+R. PCC, posterior precuneus | 6.81 | 144 |
| Sex | ||
| Girls > boys | ||
| L.+R. posterior and middle precuneus, PCC | 5.28 | 1489 |
| R. inferior and superior parietal lobe | 4.50 | 605 |
| L. supramarginal gyrus, temporo-parietal junction | 4.40 | 405 |
| Age correlation (boys and girls) | ||
| Negative correlation | ||
| L.+R. superior and inferior cerebellum, vermis, hippocampus, parahippocampus | 4.95 | 1608 |
| Boys:negative correlation | ||
| L.+R. superior and inferior cerebellum, vermis | 3.70 | 501 |
| AUH: Matched groups | ||
| No/low > exceeds-criteria | ||
| L+R anterior and posterior precuneus, PCC, middle cingulate cortex | 4.24 | 366 |
| e. RWN (age as covariate) | ||
|---|---|---|
| NAcc—connected nodes | ||
| L.+R. striatum (caudate, putamen), globus pallidus, anterior insula | 46.23 | 11 153 |
| L.+R. amygdala, hippocampus, midbrain | ||
| L.+R. subgenual cortex, orbitofrontal, rectus, ACC, medial prefrontal | ||
| Sex | ||
| Girls > boys | ||
| L.+R. NAcc, septum | 5.76 | 925 |
| Age correlation (boys and girls) | ||
| No suprathreshold cluster | ||
| AUH: Matched groups | ||
| No suprathreshold cluster | ||
Seed-to-voxel connectivity: ANCOVA with sex (boys, girls) and age (continuous variable modeled for boys and girls) in “no/low criteria” adolescents (n = 581). Effect of AUH for matched groups (n = 234): comparing n = 117 “exceeds-criteria” and n = 117 “no/low criteria” adolescents. The p-threshold for the whole brain network connectivity (age as covariate) was set at a peak t-value = 6.00 (FWE-corrected) and a cluster p-threshold = 0.05 FWE-corrected for multiple comparisons. For group, sex, and age effects, the threshold was set at a peakP value = 0.001 (t = 3.1) and cluster size FWE-corrected for multiple comparisons requiring k = 350 voxels for sex, k = 293 voxels for age, and k = 291 voxels AUH effects (AFNI 3dclustSim; https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html).
Figure 1.
IFNs of 581 non/low-drinking adolescents depicting seed-to-voxel connectivity maps of the DMN, ECN, SAN, EMN, and RWN; thresholded at t = 6. Network seeds are depicted in gray: PCC, SFG, ACC, amygdala (Amyg), and NAcc.
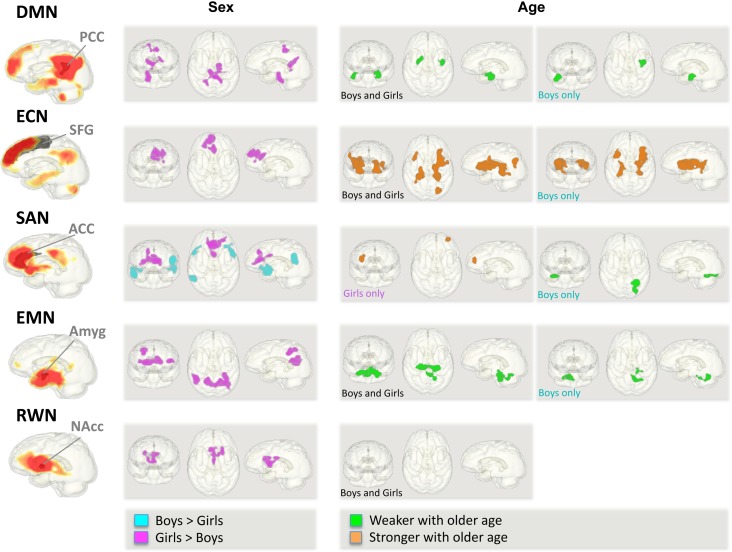
Effects of Sex and Age
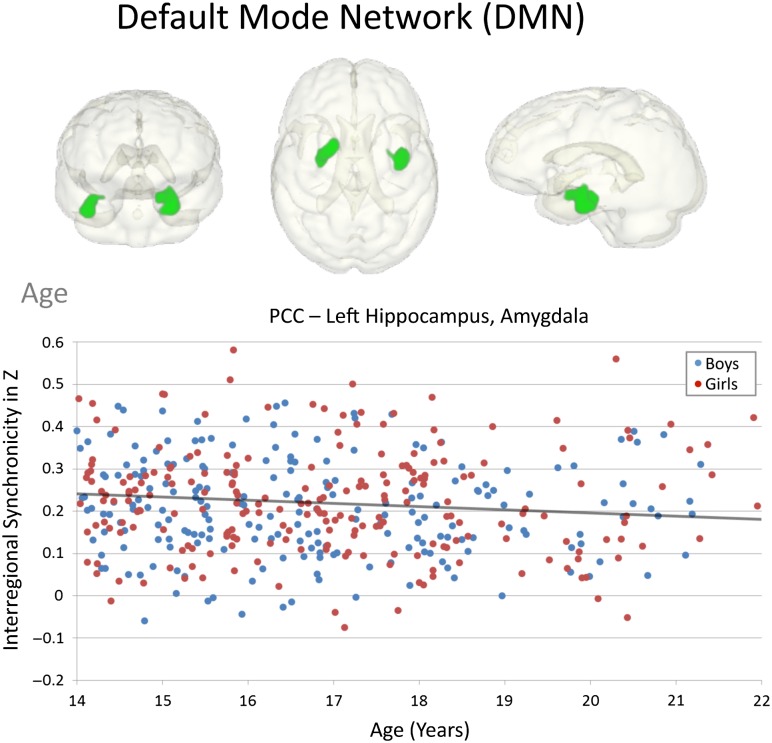
Connectivity strength between the sexes differed (Fig. 2). Girls showed more PCC–medial parietal and limbic connectivity than boys involving precuneus, middle cingulate, hippocampal, and midbrain regions. Regarding age, PCC connectivity was weaker with older adolescent age to bilateral hippocampal-amygdala regions. Although this PCC-limbic age-related weakening in intrinsic functional connectivity was specifically observed in boys (Fig. 3), it did not significantly differ between sexes (no age-by-sex interaction).
Figure 2.
Effects of sex and age during adolescence on functional connectivity of 5 IFNs. Left panel: sex effects comparing 275 boys and 306 girls. Right panels: age effects in 581 adolescents with no/low alcohol use overall and for boys and girls separately.
Figure 3.
Effects of age during adolescence on DMN connectivity. Graph depicting the correlation between age and PCC-connected limbic (hippocampus, amygdala) regions for the group of 581 adolescents with no/low alcohol use.
Effects of AUH
No significant effect was observed for AUH in adolescents for the DMN.
Executive Control Network
Adolescents in the total no/low-drinking group showed synchronous activation levels between the SFG seed and lateral and medial frontal regions, insula, striatum, medial parietal regions, bilateral temporal cortices, and cerebellum (Table 2b and Fig. 1).
Effects of Sex and Age
Girls showed stronger functional connectivity between the SFG and medial prefrontal and superior frontal regions than boys (Fig. 2). Stronger SFG connectivity to insular, fronto-limbic-striatal, and parieto-occipital regions occurred with older age (Fig. 2). Specifically, boys showed stronger SFG–insular/fronto-limbic and thalamo-striatal connectivity with older age, although not significantly different from girls on direct comparison.
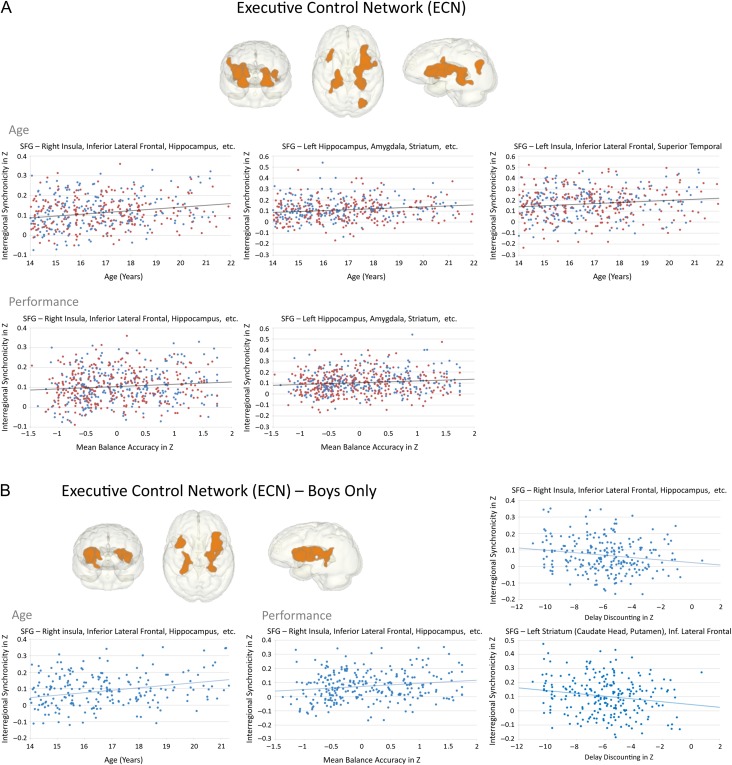
Age-related strengthening of ECN connectivity had behavioral implications, where stronger SFG–limbic (anterior insula-striatum) connectivity correlated with better performance on tests of balance and general ability. Specifically, age-related strengthening of frontotemporal connectivity in boys correlated with better episodic memory and strengthening of fronto-limbic connectivity with delay discounting performance showing greater control for greater reward (Table 3 and Fig. 4A,B).
Table 3.
Pearson correlations between composite neuropsychological test scores and regional seed-to-voxel cluster connectivity of IFNs in adolescents
| Age | All | Boys | Girls | ||||
|---|---|---|---|---|---|---|---|
| Voxel cluster | Performance | r | P | r | P | r | P |
| ECN (SFG seed) | |||||||
| R insula/frontolimbicstriatal | Balance | 0.106 | 0.012 | 0.155 | 0.011 | 0.056 | ns |
| L limbic-striatal, parieto-occipital | Balance | 0.117 | 0.005 | 0.155 | 0.011 | 0.072 | ns |
| L insula/inf. frontal/sup. temporal | Episodic memory | 0.041 | ns | 0.161 | 0.008 | −0.051 | ns |
| R insula/frontolimbicstriatal (boys) | Balance | 0.097 | 0.021 | 0.164 | 0.007 | 0.030 | ns |
| Delay discountinga | −0.085 | ns | −0.169 | 0.010 | 0.003 | ns | |
| L striatum, inf. lat. frontal (boys) | Delay discountinga | −0.085 | 0.051 | −0.175 | 0.007 | −0.011 | ns |
| EMN (Amygdala seed) | |||||||
| L+R cerebellum (boys) | Emotion | −0.125 | 0.003 | −0.172 | 0.004 | −0.072 | ns |
| Sex | Boys | Girls | Group difference | ||||
|---|---|---|---|---|---|---|---|
| Voxel cluster | Performance | r | P | r | P | Z | P |
| SAN (ACC seed) | |||||||
| L+R within ACC (girls > boys) | general ability | 0.03 | ns | −0.163 | 0.004 | −2.3 | 0.0214 |
Family-wise Bonferroni correction for 8 neuropsychological test comparisons required P ≤ 0.0125 (marked in bold), with a directional hypothesis, where greater age-related functional connectivity would correlate with better test performance.
aDelay discounting: negative scores represent better performance, that is, to withhold responses (larger delay) for greater monetary reward.
Figure 4.
Effects of age during adolescence on ECN connectivity. (A) In 581 adolescents with no/low alcohol use. Upper panels: graphs depicting the correlation between age and SFG-connected regions; Lower panels: correlations between performance (balance, memory, emotion, and general ability accuracy scores) and age-related ECN connectivity regions. (B). In 275 boys with no/low alcohol use. Left panel: graph depicting the correlation between age and SFG-limbic connectivity; Right panels: graphs depicting the correlation between performance (delay discounting, balance accuracy) and SFG-limbic connectivity.
Effects of AUH
The matched-subgroup analysis did not reveal any significant differences in SFG functional connectivity. Exploratory analysis tested for age-related SFG strengthening in connectivity in the exceeds sample and the matched subsample of adolescents with no/low AUH. No strengthening of ECN connectivity with older age was observed in the exceeds group, but still observed in the subsample of matched no/low-drinking adolescents for SFG–insula connectivity even at this older adolescent age range (SFG–R insula/hippocampus/amygdala/striatum r = 0.34, P < 0.0001; SFG–L insula/inf frontal/sup temporal r = 0.264, P = 0.002) (significant group difference for age-related ECN connectivity: Z = 1.73, P = 0.042; Z = 2.04, P = 0.021).
Salience Network
The ACC was functionally connected to a widespread system of cortical and subcortical regions (Table 2c and Fig. 1). Namely, ACC activation was highly synchronous with (1) other frontal regions including medial prefrontal, inferior frontal, orbitofrontal cortices; (2) striato-thalamic regions including caudate, putamen, pallidum, and thalamus; (3) limbic regions including amygdala, hippocampus, and insula; and (4) posterior cortices including medial parietal (precuneus, cuneus, PCC, and middle cingulate) and occipital regions (calcarine and lingual gyri).
Effects of Sex and Age
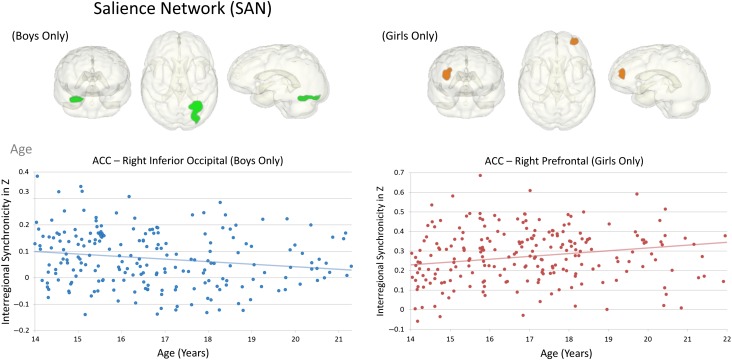
Whereas boys showed stronger ACC connectivity to temporo-parietal, inferior frontal, and orbitofrontal regions, girls showed stronger connectivity to voxels neighboring the ACC seed (Fig. 2) that was related to general ability (Table 3). With older age, girls showed stronger ACC-prefrontal cortical connectivity, whereas boys showed weaker ACC-occipital (extrastriate, lingual gyrus) connectivity extending into cerebellar regions (Fig. 5). A significant age-by-sex interaction supported a sexual dimorphism in the maturation of ACC functional connectivity where boys specifically, and in contrast to girls, showed weaker ACC-extrastriate cortical connectivity with older age.
Figure 5.
Effects of age during adolescence on SAN connectivity. Left panel: graph depicting the correlation between age and ACC-occipital connectivity in 275 boys with no/low alcohol use; Right panel: graph depicting the correlation between age and ACC-prefrontal connectivity in 306 girls with no/low alcohol use.
Effects of AUH
No significant group differences in the SAN emerged for the matched subgroups of adolescents.
Emotion Network
EMN maps revealed amygdala-seeded functional connectivity in limbic areas including hippocampus, temporal lobes and insula and in reward-related midbrain-striatal and thalamic regions. Furthermore, the amygdala was functionally connected to medial and inferior frontal and orbitofrontal cortices, sensorimotor and premotor cortices, and cerebellar regions (Table 2d and Fig. 1).
Effects of Sex and Age
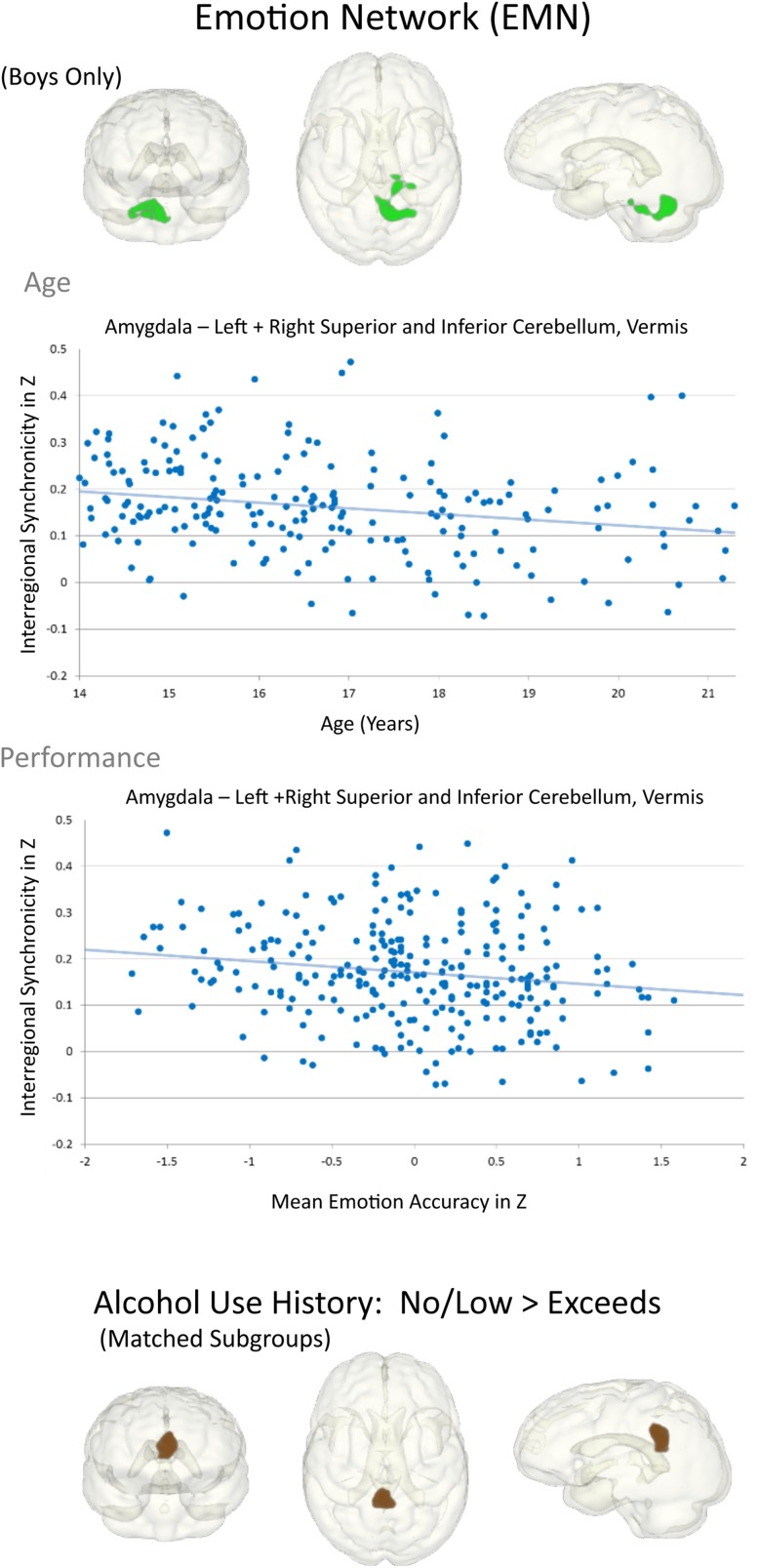
Girls exhibited stronger amygdala activation synchrony with medial occipito-parietal and lateral temporo-parietal activity than boys (Table 2d and Fig. 2, left). Amygdala functional connectivity was less robust with older age to hippocampal, parahippocampal, and cerebellar regions (Fig. 2). Although this weakening in EMN connectivity was observed over the whole no/low-drinking group (girls and boys), boys specifically showed less synchronous amygdala-bilateral cerebellar activity with older age that was furthermore associated with better attention and emotion (Table 3 and Fig. 6).
Figure 6.
Effects of age and AUH during adolescence on EMN connectivity. Graphs depicting the correlation between amygdala-cerebellar connectivity and age (upper panel), and between age-related amygdala-cerebellar connectivity and performance (emotion accuracy) (lower panel) in boys with no/low alcohol use. Lower 3D glass brains depict the PCC/precuneus region that was weaker connected in 117 adolescents exceeding alcohol use criteria than in the matched subgroup of 117 adolescents with no/low alcohol use.
Effects of AUH
In the matched subgroup analysis, weaker amygdala synchrony with medial parietal activity involving PCC, middle cingulate cortex, and anterior and posterior precuneus was observed in the exceeds criteria relative to the no/low-drinking group (Fig. 6). This weaker amydala–precuneus/PCC connectivity in the exceeds group did not significantly correlate with composite NP scores after correction for multiple comparisons. Testing the relevance for neurodevelopment, an exploratory analyses found that weaker amydala–precuneus/PCC connectivity was related to higher puberty scores in the full no/low AUH group (r = –0.134, P = 0.001; ρ = −142, P = 0.001). In addition, for regions exhibiting age-related DMN–EMN desynchonization, the variability in their connectivity strength within the exceeds group, even at an older age, correlated with episodic memory performance (DMN: PCC–right hippocampus/amygdala: r = –0.252, P = 0.003; ρ = –0.220, P = 0.009; EMN: amygdala–cerebellum: r = –0.214, P = 0.011; ρ = –0.177, P = 0.029, n = 116).
Reward Network
The NAcc-seeded RWN maps showed functional connectivity with the striatum, pallidum, anterior insula, and subgenual cortex in addition to hippocampus, amygdala, thalamus, and superior cerebellar regions. NAcc–cortical synchrony was further observed with inferior and orbitofrontal, medial prefrontal, and occipito-parietal regions (Table 2e and Fig. 2).
Effects of Sex and Age
Girls exhibited stronger NAcc functional connectivity than boys to adjacent seed voxels involving the septum (Fig. 2). No significant age-related differences in functional RWN connectivity were observed.
Effects of AUH
No significant effect of AUH on NAcc connectivity was observed in our matched subgroup sample (Table 2).
Discussion
Using rs-fMRI acquired in 698 adolescents, we identified that age, sex, and a history of alcohol use can modulate functional connectivity in the 3 cortically based networks (DMN, ECN, and SAN) and 2 subcortically based intrinsic networks (EMN and RWN) examined. The overarching pattern observed in these cross-sectional rs-fMRI data was that heterochronicity related to age and sex characterizes neurodevelopmental differences in regional functional network connectivity. Further, in the EMN, this normal pattern is vulnerable to disruption in adolescents with a history of moderate drinking. Critically, the strength of local functional connectivity was related to cognitive and motor performance. These observations and those that follow provide support for our study hypotheses.
Modulation of Network Connectivity by Age and Sex
We hypothesized that connectivity of IFNs in older adolescents would be stronger and spatially more distributed than in younger adolescents; this prediction held for the ECN. For the SAN, this pattern was modulated by sex differences consistent with Satterthwaite et al. (2015) in that girls showed stronger connectivity to regions more proximal to the seed than boys, a pattern observed in all IFNs except the EMN, whereas boys showed a spatially more distributed SAN connectivity. Age analyses conducted for boys and girls indicated that the observed connectivity in DMN, ECN, and EMN were driven by the boys’ activation synchrony. Thus, we observed sexual dimorphism in the patterns of age-related differences in connectivity strength.
Greater SFG–fronto-limbic and parieto-occpital synchrony in the ECN occurred with older age and was expressed differently in boys from girls, that is, boys but not girls showed this age-related strengthening in SFG–thalamo-limbic-striatal functional connectivity.
Similar to structural brain development (Sowell et al. 2004; Lenroot et al. 2007; Uddin et al. 2011), IFN maturation during adolescence is marked by heterochronicity in its development of neurofunctional architecture. Stronger and more distributed network connectivity occurred with older age for the ECN, but not the DMN and EMN, possibly indicative of later maturation of the ECN than the DMN. In support of this possibility, another study reported that default mode regions, specifically the medial prefrontal, posterior cingulate, and lateral parietal cortices, are only sparsely functionally connected at early school age (7–9 years old) but strongly connected in adults (Fair et al. 2008). Thus, even our youngest adolescents, 12-year olds, might have already undergone significant maturational change toward an adult-like DMN connectivity. Indeed, limbic nodes were found to be loosely integrated in the DMN in adults (Ward et al. 2014; Müller-Oehring et al. 2015), and reduction in EMN limbic–cerebellar connectivity (perhaps “functional pruning”) during adolescence could serve to promote connectivity between main nodes of the DMN and EMN with maturity (Greicius et al. 2003; Fox et al. 2005; Fransson 2005; Fair et al. 2008).
The hippocampal and parahippocampal regions in older than younger adolescents were more strongly connected with the SFG seed of the ECN, suggesting “functional rewiring” of limbic connections from parieto-hippocampal (DMN) to fronto-hippocampal (ECN) networking as adolescence progressed. This shift could enable maturation from an introspective and self-referential network to a supervisory cognitive control, planning, and execution system. With the hippocampus being a node of the DMN, such cortico-limbic rewiring may be a key to behavioral change associated with maturity, that is, the ability to master emotions, pause for planning, and exhibit cognitive control. Our finding that stronger ECN connectivity to parietal, insular, and striatal regions was associated with better performance in general ability, attention, and delay discounting with older age further supports the concept that age-related “rewiring” of the functional architecture is shaped to facilitate cognition.
We found weaker neurofunctional connectivity between the EMN amygdala seed and cerebellar regions marking a novel developmental theme. The cerebellum plays a role in a various nonmotor functions that include learning, memory, and affective-behavioral modulation (Ito 1993; Ramnani et al. 2006; Strick et al. 2009; Balsters et al. 2010). With learning through practice and experience, cortico-limbic-cerebellar circuitry enables storing of the most efficient representations of behavior in frontal motor cortices (Ito 1993; Koziol et al. 2012) with reduced participation of the cerebellum as the task is mastered. Although cerebro-cerebellar functional circuitry in children closely resembles that of adults (Power et al. 2010), our finding of weaker amygdala-cerebellar connectivity with older age may mark a point in adolescent development signifying pruning of cerebellar-instructed limbic-connected learning capabilities (Koziol et al. 2010) potentially helpful to establish cortical anticipatory skill learning mechanisms in the matured brain (Saling and Phillips 2007; Imamizu and Kawato 2009). This dynamic interplay comports with the experience that complex skill learning to perfection is challenging after adolescence.
Together, the herein observed synchronization of limbic networks with frontal ECN regions while, at the same time, limbic connectivity weakens to DMN and cerebellar regions, is supportive of the concept that the prefrontal cortex gradually assumes responsibility for various cognitive processes during adolescent neurodevelopment that were initially performed by subcortical and limbic structures (e.g., Rubia et al. 2000; Yurgelun-Todd and Killgore 2006; Kesek et al. 2009).
Modulation of Network Connectivity with AUH
The effect of AUH was observed mainly in the EMN with weaker EMN–DMN connectivity. Similarly, developmental differences indicated attenuation of medial parieto–limbic DMN–EMN connectivity strength. In addition, strengthening of ECN connectivity was observed even at an older age in the matched group of no/low-drinking adolescents, but was absent in those with alcohol use histories. Together, these coincidences raise the possibility that moderate-to-high alcohol use even in later adolescence can retard maturational changes.
Limitations of these findings must be acknowledged with respect to the relatively low use of alcohol and drugs in most of the adolescents in the exceeds-criteria group. Further, none of the participants were recruited from treatment programs and only 10 of the 117 in that group met minimal diagnostic criteria for alcohol use disorder. Whether the apparent effects of alcohol drinking or other substance use are pre-existing or causative await longitudinal study. Although seed-based techniques are sensitive to the choice of the seed region (Posse et al. 2013; Cole et al. 2014), IFNs are highly consistent between analysis techniques (e.g., Van Dijk et al. 2010; Erhardt et al. 2011; Raichle 2011; Calhoun and Allen 2013; Posse et al. 2013). Here, seed-based analyses enabled the detection of small signal changes in voxel clusters (Posse et al. 2013) supporting the concept that functional connectedness is flexible to change (Sridharan et al. 2008) specifically with development (e.g., Supekar et al. 2010; Di Martino et al. 2014).
Implications for Normal Neurodevelopment of Functional Architecture
This cross-sectional analysis of rs-fMRI data from a large cohort of youth spanning the adolescent age range revealed heterochronicity of neurodevelopmental patterns in rewiring 5 selected IFNs toward maturity. Normal developmental patterns differed in adolescents with moderate-to-heavy AUH on the EMN with limbic-cortico-cerebellar rewiring that may impede maturation of affective and self-reflection systems underwriting the ability to regulate emotions and exhibit cognitive control.
Notes
Conflict of Interest: None declared.
Supplementary Material
Supplementary material can be found at Cerebral Cortex online.
Funding
The U.S. National Institute on Alcohol Abuse and Alcoholism with cofunding from the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Health Office of the Director, the National Institute of Child Health and Human Development, and the Office of the Director, National Institutes Of Health (NCANDA grant numbers: AA021697 [A.P. and K.M.P.], AA021697-04S1 [K.M.P.], AA021695 [S.A.B. and S.F.T.], AA021692 [S.F.T.], AA021696 [I.M.C. and F.C.B.], AA021681 [M.D.D.B.], AA021690 [D.B.C.], AA021691 [B.N.N.], and AA017168 [E.V.S.]).
Supplementary Material
References
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, Amaral DG, Casey BJ, Ernst TM, Frazier JA, et al. . 2014. The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology. 28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. 2015. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 115:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American-Psychiatric-Association. 2000. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Press. [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S. 2013. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 9:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Duda JT, Kilroy E, Krasileva K, Jann K, Kandel BT, Tustison NJ, Yan L, Jog M, Smith R, et al. . 2015. The pediatric template of brain perfusion. Sci Data. 2:150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Cussans E, Diedrichsen J, Phillips KA, Preuss TM, Rilling JK, Ramnani N. 2010. Evolution of the cerebellar cortex: the selective expansion of prefrontal-projecting cerebellar lobules. Neuroimage. 49:2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. 2010. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 1327:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. 2007. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 90 (Suppl 1):S85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34:537–541. [DOI] [PubMed] [Google Scholar]
- Braun K, Bogerts B. 2000. Juvenile experience and learning modulate the functional maturation of the brain: relevance for the genesis and therapy of mental disorders. Psychother Psychosom Med Psychol. 50:420–427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, et al. . 2015. The National Consortium on Alcohol and Neuro Development in Adolescence (NCANDA): a multi-site study of adolescent development and substance use. J Stud Alcohol Drugs. 76:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Allen E. 2013. Extracting intrinsic functional networks with feature-based group independent component analysis. Psychometrika. 78:243–259. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. 2012. Anticorrelations in resting state networks without global signal regression. Neuroimage. 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT. 1998. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 27:184–188. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. 2014. Intrinsic and task-evoked network architectures of the human brain. Neuron. 83:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA. 2013. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol. 18:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. 2009. Neurocognitive development of relational reasoning. Dev Sci. 12:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ. 2015. a. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci. 16:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Stein H, Wilson AC, Nagel BJ. 2015. b. Neurobiological phenotypes of familial chronic pain in adolescence: a pilot fMRI study. J Pain. 16:913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. 2012. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 32:5549–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, Craddock RC, Luna B, Leventhal BL, Zuo XN, et al. . 2014. Unraveling the miswired connectome: a developmental perspective. Neuron. 83:1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. 2006. A core system for the implementation of task sets. Neuron. 50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. 2011. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 32:2075–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. 2008. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 105:4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. 2007. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 104:13507–13512.17679691 [Google Scholar]
- Feinberg I, Thode HC, Chugani HT, March JD. 1990. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 142:149–161. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. 2005. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Stern H, Brown GG, Mathalon DH, Turner J, Glover GH, Gollub RL, Lauriello J, Lim KO, Cannon T, et al. . 2008. Test-retest and between-site reliability in a multicenter fMRI study. Hum Brain Mapp. 29:958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. 2014. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 40:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, et al. . 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS. 2011. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Mueller BA, Liu T, Turner JA, Voyvodic J, Yetter E, Diaz M, McCarthy G, Wallace S, Roach BJ, et al. . 2011. A novel method for quantifying scanner instability in fMRI. Magn Reson Med. 65:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, et al. . 2012. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 26:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. 2010. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 187:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. 2009. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Morton JB. 2015. Tracking the brain's functional coupling dynamics over development. J Neurosci. 35:6849–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Kawato M. 2009. Brain mechanisms for predictive control by switching internal models: implications for higher-order cognitive functions. Psychol Res. 73:527–544. [DOI] [PubMed] [Google Scholar]
- Ito M. 1993. Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci. 16:448–450. discussion 453–444. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. 2012. FSL. NeuroImage. 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SA. 2011. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 21:385–391. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Minati L, Marizzoni M, Marchitelli R, Sala-Llonch R, Bartres-Faz D, Arnold J, Benninghoff J, Fiedler U, Roccatagliata L, et al. . 2015. Longitudinal reproducibility of default-mode network connectivity in healthy elderly participants: a multicentric resting-state fMRI study. Neuroimage. 124:442–454. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. 2009. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 19:640–657. [DOI] [PubMed] [Google Scholar]
- Kesek A Zelazo PD, Lewis MD. 2009. The development of executive cognitive function and emotion regulation in adolescence. Adolescent emotional development and the emergence of depressive disorders. Allen NB, Sheeber LB. Cambridge, Cambridge University Press. [Google Scholar]
- Kipke MD, editor.. 1999. Adolescent Development and the Biology of Puberty: Summary of a Workshop on New Research. National Academy Press, NW, Washington, DC 20418. [PubMed] [Google Scholar]
- Klein D, Rotarska-Jagiela A, Genc E, Sritharan S, Mohr H, Roux F, Han CE, Kaiser M, Singer W, Uhlhaas PJ. 2014. Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One. 9:e84914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. 2010. Neurocircuitry of addiction. Neuropsychopharmacology. 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Budding DE, Chidekel D. 2010. Adaptation, expertise, and giftedness: towards an understanding of cortical, subcortical, and cerebellar network contributions. Cerebellum. 9:499–529. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding DE, Chidekel D. 2012. From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum. 11:505–525. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. . 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. 2015. The resting brain of alcoholics. Cereb Cortex. 25:4155–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto LL, Oliveira E, Correia F, Ferreira AG. 2008. The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation. 11:13–22. [DOI] [PubMed] [Google Scholar]
- Nichols BN, Pohl KM. 2015. Neuroinformatics software applications supporting electronic data capture, management, and sharing for the neuroimaging community. Neuropsychol Rev. 25:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. 2011. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol. 108:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. 1988. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 17:117–133. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nolan Nichols B, Brown SA, Tapert SF, Cummins K, et al. . 2015. Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. 2002. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 16:331–348. [DOI] [PubMed] [Google Scholar]
- Posse S, Ackley E, Mutihac R, Zhang T, Hummatov R, Akhtari M, Chohan M, Fisch B, Yonas H. 2013. High-speed real-time resting-state FMRI using multi-slab echo-volumar imaging. Front Hum Neurosci. 7:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. 2010. The development of human functional brain networks. Neuron. 67:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2009. A paradigm shift in functional brain imaging. J Neurosci. 29:12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2011. The restless brain. Brain Connect. 1:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JL, Rudebeck P, Ciccarelli O, Richter W, Thompson AJ, et al. . 2006. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from Macaque monkeys and humans. Cereb Cortex. 16:811–818. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. 2011. How does your cortex grow? J Neurosci. 31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson EP., Jr 1982. Myelination in the human central nervous system In: Haymaker W, Adams RD, editors.. Histology and histopathology of the nervous system. Springfield, IL: Thomas; p. 146–173. [Google Scholar]
- Rohlfing T, Cummins K, Henthorn T, Chu W, Nichols BN. 2014. N-CANDA data integration: anatomy of an asynchronous infrastructure for multi-site, multi-instrument longitudinal data capture. J Am Med Inform Assoc. 21:758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T, Maurer CR Jr., Bluemke DA, Jacobs MA. 2003. Volume-preserving nonrigid registration of MR breast images using free-form deformation with an incompressibility constraint. IEEE Trans Med Imaging. 22:730–741. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. 2000. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 24:13–19. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, Cox RW. 2013. Correcting brain-wide correlation differences in resting-state FMRI. Brain Connect. 3:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling LL, Phillips JG. 2007. Automatic behaviour: efficient not mindless. Brain Res Bull. 73:1–20. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, et al. . 2015. Linked sex differences in cognition and functional connectivity in youth. Cereb Cortex. 25:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. 1997. Common blood flow changes across visual tasks. II. Decreases in cerebral cortex. J Cogn Neurosci. 9:648–663. [DOI] [PubMed] [Google Scholar]
- Smith AR, Chein J, Steinberg L. 2013. Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Horm Behav. 64:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Bickel WK. 2013. A developmental perspective on neuroeconomic mechanisms of contingency management. Psychol Addict Behav. 27:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. 2011. Delay discounting predicts adolescent substance abuse treatment outcome. Exp Clin Psychopharmacol. 20:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, Lee JH, Welge JA, DelBello MP, Fleck DE, et al. . 2011. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 69:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. 2009. Cerebellum and nonmotor function. Annu Rev Neurosci. 32:413–434. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, Cummins K, Thompson WK, Colrain IM, Baker FC, et al. . 2016. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 30:449–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. 2010. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 52:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. 2011. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 31:18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T, Kelly C, Castellanos FX. 2013. Of bandwagons and bathwater: the value of resting state functional magnetic resonance imaging for child psychiatric research. J Am Acad Child Adolesc Psychiatry. 52:562–565. [DOI] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. 2014. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 35:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Schriber RA, Fassbender C, Atherton O, Krafft C, Robins RW, Hastings PD, Guyer AE. 2015. Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Dev Cogn Neurosci. 16:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2:125–141. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, Braus DF, Heinz A. 2003. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neurosci Lett. 348:41–45. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A-R. 1967. The myelogenetic cycles of regional maturation of the brain In: Minkowski A, editor.. Regional development of the brain in early life. Oxford: Blackwell Scientific Publications; p. 3–70. [Google Scholar]
- Yan CG, Craddock RC, Zuo XN, Zang YF, Milham MP. 2013. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuroimage. 80:246–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. . 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. 2006. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci Lett. 406:194–199. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Anderson JS, Bellec P, Birn RM, Biswal BB, Blautzik J, Breitner JC, Buckner RL, Calhoun VD, Castellanos FX, et al. . 2014. An open science resource for establishing reliability and reproducibility in functional connectomics. Sci Data. 1:140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.