A potential new treatment for AML
Keywords: antibody therapy, fratricide, tumor immunotherapy
Abstract
Acute myeloid leukemia (AML) remains a significant health problem, with poor outcomes despite chemotherapy and bone marrow transplants. Although one form of AML, acute promyelocytic leukemia (APL), is successfully treated with all-trans retinoic acid (ATRA), this drug is seemingly ineffective against all other forms of AML. Here, we show that ATRA up-regulates CD38 expression on AML blasts to sufficient levels that promote antibody-mediated fratricide following the addition of anti-CD38 daratumumab (DARA). The combination of ATRA plus DARA induced Fc-dependent conjugate formation and cytotoxicity among AML blasts in vitro. Combination treatment also led to reduction in tumor volume and resulted in increased overall survival in murine engraftment models of AML. These results suggest that, although ATRA does not induce differentiation of non-APL, it may be effective as a therapy in conjunction with DARA.
Introduction
Acute myeloid leukemia (AML) is characterized by disruption in cellular differentiation and enhanced proliferation of early hematopoietic stem cells with subsequent accumulation of immature myeloid blasts in the bone marrow. Over 20000 people are estimated to be diagnosed with AML in the USA each year, and this number is expected to grow as the population ages (1). Aggressive chemotherapy followed by allogeneic stem cell transplant is the standard approach for most patients with AML but is ultimately often ineffective. In addition, targeted therapy has only recently shown success, in part because AML is likely multiple diseases to the recognition that AML is likely multiple diseases with driving gene translocations or gene mutations involved in differentiation and proliferation (2, 3). These factors, together with the older age of onset, result in a 40% survival rate for patients under the age of 60 (1, 4) and 26.6% overall (5).
The major breakthrough for AML treatment within the last two decades has been the application of retinoic acid for patients with acute promyelocytic leukemia (APL) (4). APL is characterized by a t(15; 17) chromosomal translocation resulting in a fusion protein of retinoic acid receptor (RAR) and promyelocytic leukemia gene, and all-trans retinoic acid (ATRA) leads to terminal differentiation of the myeloid blasts (6). However, while ATRA combined with chemotherapy or arsenic trioxide results in cure of the great majority of patients with APL, it has failed to elicit the same differentiating effect in other subtypes of AML (7).
Daratumumab (DARA) is a monoclonal IgG1κ antibody against CD38, a transmembrane glycoprotein with both receptor and enzymatic functions expressed on hematopoietic cells. It was approved by the FDA in 2015 for the treatment of multiple myeloma (8). While the CD38 antigen is only expressed on a subset of AML patients (and therefore represents a less optimal target for antibody therapy), it has been found to be up-regulated by ATRA on a variety of oncogenic cells including APL (9) and multiple myeloma (10). Given the survival benefit of antibody therapy in other hematologic malignancies including ALL, CLL, NHL, and multiple myeloma, significant interest in developing antibody therapeutics for AML exists. Antibodies directed at CD33 or CD123 have moved forward with modest success and minimal single-agent activity. As the effectiveness of antibody therapy is in part related to density of antigen on tumor cells, we hypothesized that efforts to up-regulate targetable antigens on these leukemia cells would offer therapeutic advantage.
Herein, on the basis of the experience of modulating CD38 in multiple myeloma, we assessed the effects of ATRA on non-APL AML cells and found that it led to significant up-regulation of CD38 and enhanced DARA-mediated AML cell fratricide in vitro. Additionally, using a murine engraftment model of AML, we found that ATRA plus DARA significantly increased overall survival time compared to either agent alone. These results support future trials combining ATRA with DARA in AML.
Methods
Reagents
ATRA was purchased from Sigma-Aldrich (St Louis, MO, USA); DARA was supplied from commercial sources (the Ohio State University, Columbus, OH, USA). AM580 was purchased from Abcam (Cambridge, MA, USA).
Cell culture
The AML cell lines used for this study were MV4-11, OCI-AML3, MOLM-13 and U937. Cells were purchased from American Type Culture Collection (ATCC) and cultured in RPMI medium 1640 (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone Laboratories, Grand Island, NY, USA), 2 mM l-glutamine (Invitrogen, Grand Island, NY, USA) and 56 U ml−1/56 µg ml−1 penicillin/streptomycin (Invitrogen) at 37°C in 5% CO2. Aphaeretic white blood cells from AML patients were obtained under written informed consent in accordance to protocol approved by the institutional review board of the Ohio State University. Samples were stored in liquid nitrogen in 20% FBS and 10% dimethyl sulfoxide prior to use. Upon thawing, cells were cultured in RPMI medium 1640 (Gibco) supplemented with 20% FBS, 2 mM l-glutamine (Invitrogen) and 56 U ml−1/56 µg ml−1 penicillin/streptomycin (Invitrogen) at 37°C in 5% CO2.
Quantitative real-time polymerase chain reaction
RNA was extracted using the Total RNA Purification Plus Kit (Norgen Biotek Corp., Ontario, Canada). RNA was reverse-transcribed before being quantified by quantitative (qPCR) using Power SYBR® Green Master Mix (Applied Biosystems, Grand Island, NY, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene for normalizing target genes. Primers used were: CD38 (forward 5′-GCT CAA TGG ATC CCG CAG T-3′ and reverse 5′-TCC TGG CAR AAG TCT CTG G-3′) and GAPDH (forward 5′-ATT CCC TGG ATT GTG AAA TAG TC-3′ and reverse 5′-ATT AAA GTC ACC GCC TTC TGT AG-3′). Data are presented as relative copy number, calculated as 2−ΔCt × 100, where ΔCt is the Ct(target) − Ct(GAPDH).
Flow cytometry
Cells (1 × 106 ml−1) were incubated with 1 µg anti-human CD38 antibody conjugated to fluorescein isothiocyanate (FITC) (BioLegend, San Diego, CA, USA: Cat #303503) or FITC-conjugated isotype (BioLegend: Cat #400108) for 30 min at 4°C preceding two washes with fluorescence activated cell sorting (FACS) buffer [phosphate-buffered saline (PBS), 0.09% sodium azide, 10% FBS]. Samples were analyzed using an LSRII flow cytometer (BD Bioscience, San Jose, CA, USA) and FlowJo software (FLOWJO, LLC, Ashland, OR, USA).
Solid tumor murine model
All animal experiments were carried out in full accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the Ohio State University. Non-obese diabetic severe combined immunodeficient-IL2Rγ−/− (NSG) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and bred in a university vivarium under the direction of Dr Adrienne Dorrance. MV4-11 cells (2.5 × 106) in PBS were injected subcutaneously into the flanks of twenty-five 3-month-old female mice. Tumors were allowed to grow for 10 days before the mice were randomly sorted into four groups: untreated, ATRA (10 mg kg−1 mouse), DARA (1 µg g−1 mouse) and ATRA + DARA. Intra-peritoneal injections of ATRA were administered four times a week (Monday, Tuesday, Thursday, Friday) via corn-oil vehicle; DARA in PBS vehicle was administered via intra-peritoneal injection twice a week (Tuesday, Friday). Treatment was carried out for 18 days, with tumor volume measured in a blinded fashion.
Leukemic murine model
For the leukemic model, 3.0 × 105 spleen cells from MV4-11-engrafted mice were injected intravenously into 75 NSG mice (male and female; 2–5 months old) and allowed to engraft for 1 week before commencing treatment. Grouping, dosing, scheduling and mode of injection of ATRA and DARA were identical to the solid tumor model. Early removal criteria consisted of 20% weight loss, paralysis or inability to stand, uncontrolled shivering or unwillingness to eat or drink. Animal studies were approved by the Institutional Laboratory Animal Care and Use Committee at the Ohio State University.
Conjugate formation assay
MV4-11 cells were plated at 1 × 106 cells ml−1 and treated with or without 1 µM ATRA and/or 20 µg ml−1 DARA for 24 h at 37°C in 5% CO2. Cells were centrifuged at 200 × g for 10 min, culture media were removed and the cells were washed with PBS before being fixed in 4% paraformaldehyde for 10 min at 21°C. Rhodamine Phalloidin F-actin stain (Cytoskeleton, Inc., Denver, CO, USA) was diluted to 100 nM per manufacturer’s instruction; 200 µl were added to each sample before incubation for 30 min at 21°C in the dark. Samples were then washed three times with PBS and then counted in a blinded fashion via fluorescence microscopy. Conjugation index is defined as the number of cells with at least one conjugate per 100 cells.
Lactate dehydrogenase assay
MV4-11 cells were plated at 5 × 105 cells ml−1 and treated with 1 µM ATRA and/or 20 µg ml−1 DARA. At 24-h intervals, supernatants were removed and used for a CytoTox96® Non-Radio Cytotoxicity Assay (Promega, Madison, WI, USA) according to manufacturer’s instruction. Percent cytotoxicity was defined as [Experimental lactate dehydrogenase (LDH) release OD490 /Maximum LDH release OD490] × 100 and was normalized to untreated UT or ATRA alone for all samples.
Trypan blue exclusion assay
Trypan blue (Sigma-Aldrich) was used to stain cells according to manufacturer instructions. Cells were counted on a Luna Dual Fluorescence Cell Counter (Logos Biosystems Inc., Annandale, VA, USA).
Statistical analysis
For all analyses: *P ≤ 0.05, α = 0.05; **P ≤ 0.01, α = 0.01; ***P ≤ 0.001, α = 0.001. For the experiments with repeated measures, data were analyzed by mixed-effect models, and the time or dose dependencies were analyzed by trend tests. A paired t-test was used for qPCR and flow cytometry analysis of CD38 expression. For the experiments involving multiple independent groups, data were analyzed by analysis of variance followed by pairwise comparisons. Tumor volumes in the solid tumor murine model were adjusted from their baseline and a mixed-effect model incorporating repeated measures was used to test the difference in tumor growth trends and tumor volumes at the last 2 days; an interaction contrast was used to test the synergy of the two drugs. Mantel–Cox log-rank tests were used to analyze the survival functions derived from the tail-vein murine model. The difference in survival probabilities among groups were analyzed by log-rank tests. The synergy between ATRA and DARA was tested by interaction contrasts. Multiplicity was adjusted by Holm’s method. Data analyses were performed by using SAS 9.4 (SAS 9.4, Inc., Cary, NC, USA).
Results and discussion
ATRA up-regulates CD38 in AML cells
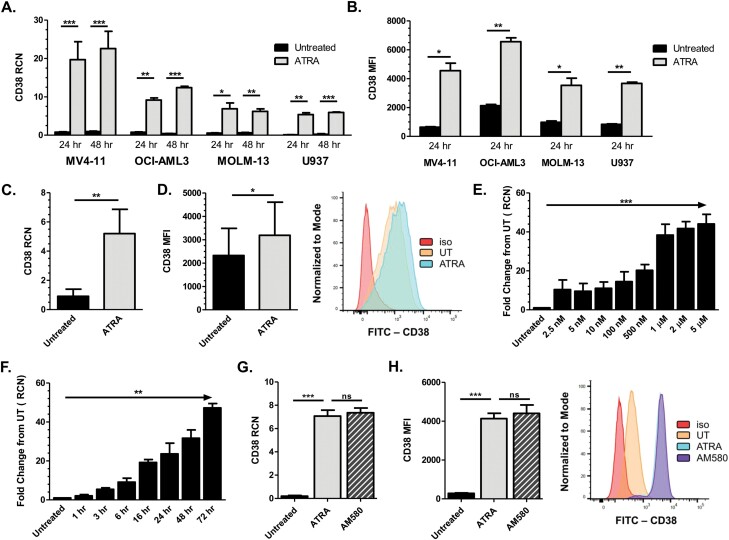
Recent work by Yoshida et al. showed that ATRA up-regulated CD38 expression in KG-1 and U937 AML cell lines, and in selected primary AML samples (11). To further confirm this effect of ATRA, we treated AML cell lines and primary patient samples with 1 µM ATRA for 24 and 48 h, then measured CD38 mRNA expression via qPCR. Results showed that for both time points, ATRA significantly up-regulated CD38 in all cell lines and primary samples (Fig. 1A and C, respectively; patient cytogenetic and mutational status are included in Supplementary Table 1). To verify that this increase in transcript correlated with an increase in surface antigen expression, samples were similarly treated with ATRA and analyzed for CD38 via flow cytometry. Results showed significant up-regulation in surface expression of CD38 after 24 h of ATRA treatment in all four AML cell lines (Fig. 1B), as well as in all nine patient samples (Fig. 1D; average increase of 94.4%). To further corroborate the increased CD38 protein, we also performed Western blotting on lysates from ATRA-treated MV4-11 cells and saw substantial increases in CD38 (data not shown).
Fig. 1.
ATRA up-regulates CD38 in AML cells. (A) MV4-11 (n = 5 separate experiments), OCI-AML3, MOLM-13 and U937 cells (n = 3 separate experiments each) were treated with 1 µM ATRA for 24 and 48 h (ATRA added every 24 h). CD38 transcript was measured by qPCR. (B) AML cell lines MV4-11, OCI-AML3, MOLM-13 and U937 (n = 3 separate experiments each) were treated with 1 µM ATRA for 24 h and analyzed for CD38 surface protein expression by flow cytometry. (C, D) Primary AML patient apheresis samples were treated with 1 µM ATRA for 24 h and CD38 transcript level (C; n = 10 donors) and surface protein expression (D; n = 9 donors) were measured; representative histogram for the flow cytometry is shown in (D). (E) MV4-11 cells (n = 3 separate experiments) were treated with concentrations of ATRA ranging from 0 to 5 µM and CD38 transcripts were measured after 24 h by qPCR. (F) MV4-11 cells (n = 3 separate experiments) were treated with 1 µM ATRA for 1–72 h and CD38 transcripts were measured by qPCR. (G, H) MV4-11 cells (n = 3 separate experiments) were treated with 10 nM ATRA or AM580 for 24 h; CD38 levels were measured by qPCR (G) and flow cytometry (H; representative histogram shown). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
In order to explore dose–response relationships, we then treated MV4-11 cells for 24 h with concentrations of ATRA ranging from 0 to 5 µM and measured CD38 via qPCR. Although some increase in expression could be seen with as little as 2.5 nM, 1 µM of ATRA led to optimal induction (Fig. 1E). Next, we treated MV4-11 cells with 1 µM ATRA for time points between 0 and 72 h and found that CD38 mRNA increased in as early as 3 h with continued rise through the 72-h time point observed (Fig. 1F). These results suggest that ATRA can significantly increase mRNA and surface expression of CD38 antigen on AML cells.
It has been previously shown that RARα, when bound to its ligand ATRA, associates with CD38 and induces transcription of the gene (12). Using AM580, a selective agonist for RARalpha, we tested whether direct activation of the transcription factor was sufficient to elicit the potent up-regulation of CD38 that we observed with ATRA. Fig. 1(G) shows CD38 mRNA levels in MV4-11 cells treated with 10 nM of either ATRA or AM580 after 24 h. Transcript levels, while significantly higher than the untreated cells, showed no difference between ATRA and AM580. Flow cytometry was utilized to assess surface protein levels in identical conditions (Fig. 1H); again no difference between ATRA- and AM580-treated samples was observed. These data suggest that direct activation of RARα is sufficient to up-regulate CD38 expression in AML blasts.
ATRA triggers DARA-mediated immune conjugate formation and killing in vitro
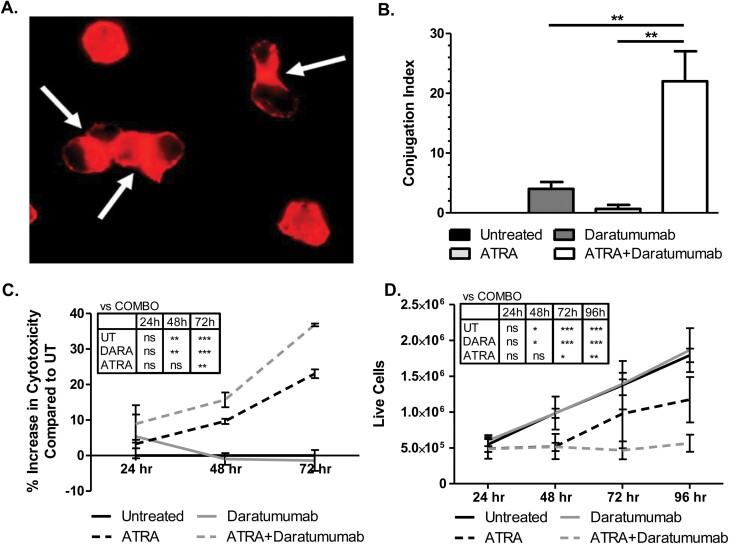
Since ATRA up-regulates surface expression of CD38, we next tested whether the combination of ATRA and anti-CD38 antibody DARA would enhance cytotoxicity of AML cells. One of DARA’s mechanisms of tumor elimination is antibody-dependent cellular cytotoxicity by immune cells (10), and we have previously found that AML blasts were capable of targeting one another in an antibody-dependent manner (13). To test if ATRA with DARA could elicit this self-targeting, or fratricide, we treated cells for 24 h with each as a single agent or in combination, then stained for actin using rhodamine phalloidin fluorescent dye. Results showed that combination treatment led to significant increases in cell-to-cell conjugate formation versus single-agent treatment (Fig. 2A and B).
Fig. 2.
ATRA triggers DARA-mediated immune conjugate formation and killing in vitro. (A, B) MV4-11 cells were incubated with or without 1 µM ATRA and with or without 20 µg ml−1 DARA for 24 h, after which samples were fixed and stained with rhodamine phalloidin actin dye. Conjugate formation between the cells was measured via fluorescence microscopy in a blinded fashion (A) and graphed in (B), where conjugation index = (# cells conjugated to at least one additional cell/# total cells) (n = 3 separate experiments). (C, D) MV4-11 cells were incubated with or without 1 µM ATRA and with or without 20 µg ml−1 DARA for 24, 48, 72 (and 96) h (drugs re-added every 24 h). (C) Cytotoxicity was analyzed via LDH assay. (D) Cell viability was measured via Trypan blue exclusion (n = 3 separate experiments). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Following this, MV4-11 cells were treated with single or dual agents and tested for antibody-mediated cellular cytotoxicity over the course of 72 h via LDH assay. Results showed that the combination treatment led to a significant increase in percent cytotoxicity compared to untreated and DARA alone at 48 and 72 h, and a significant increase from ATRA alone at 72 h (Fig. 2C). To confirm this finding, we performed a Trypan blue exclusion assay, which also showed a significant decrease in live cells for combination-treated samples compared to all control groups by 72 h post-treatment (Fig. 2D). ATRA as a single agent also led to cytotoxicity, albeit to a lesser extent. This is consistent with previous findings that ATRA could induce cell death in AML cell lines, reportedly due to Bcl-2 down-regulation, loss of mitochondrial membrane potential (ΔΨm) and cytochrome c efflux (14).
To confirm that this fratricide was mediated by Fcγ receptors, we generated F(ab)’2 fragments of DARA via pepsin digestion [the ability of this F(ab)’2 fragment to bind to CD38 was confirmed via flow cytometry, data not shown]. Pepsin cleaves the Fc portion of antibodies resulting in a F(ab)’2 molecule that maintains antigen affinity and the ability to confer direct effects, but cannot interact with FcγR. The conjugate formation and fratricide experiments were repeated and, as expected, there was complete ablation of conjugate formation when the F(ab)’2 was used in place of whole antibody (Supplementary Figure 1A). This corresponded to a significant decrease in cytotoxicity at 72 h with the F(ab)’2 fragment compared to whole antibody (Supplementary Figure 1B). These results suggest that combined ATRA plus DARA treatment induces fratricide through Fcγ receptor engagement.
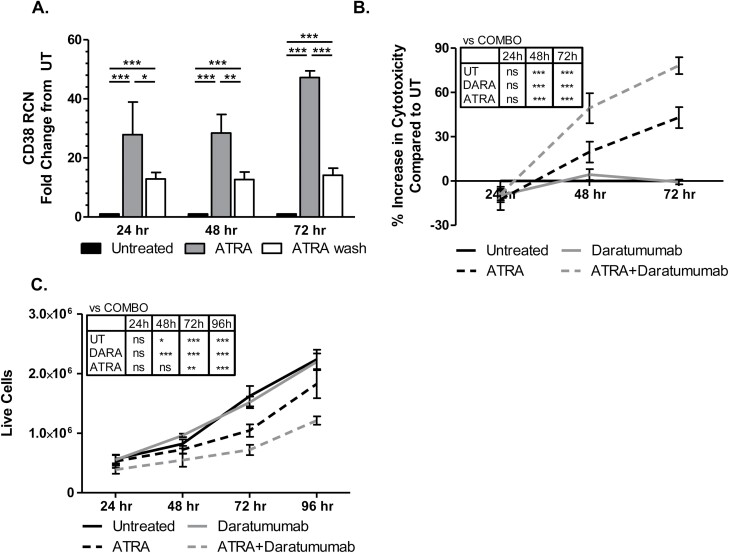
Single-dose ATRA elicits CD38 up-regulation and confers DARA activity
The above experiments utilized treatment regimens which provided a relatively constant source of ATRA, so we next sought to determine whether short-term exposure to the drug would elicit similar effects on CD38 expression and antibody-mediated fratricide. We treated MV4-11 cells with ATRA for 45 min and then washed it away in order to simulate the reported plasma half-life of ATRA in vivo (15). CD38 transcript was measured 24, 48 and 72 h after treatment via qPCR and, while expression was markedly lower than non-washout controls, the single dose led to an ~12-fold increase in CD38 transcript that held steady throughout the 72-h time course (Fig. 3A). Additionally, when combined with DARA, a single dose of ATRA retained its killing potential through 72 h as measured by LDH release (Fig. 3B). Similarly, live cell counts were reduced through 96 h (Fig. 3C). These results suggest that CD38 expression on AML blasts can be modulated by short-term exposure to ATRA.
Fig. 3.
Single-dose ATRA elicits CD38 up-regulation and confers DARA activity. (A) MV4-11 cells were incubated with 1 µM ATRA for 45 min before the drug was washed off. Cells were collected at 24, 48 and 72 h after which CD38 expression was measured via qPCR (n = 3 separate experiments). (B, C) MV4-11 cells were treated with or without a 45-min dose of 1 µM ATRA and with or without 20 µg ml−1 DARA every 24 h for up to 96 h. (B) LDH assays were run at 24, 48 and 72 h to determine % cytotoxicity; (C) live cells were counted using Trypan blue exclusion (n = 3 separate experiments). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
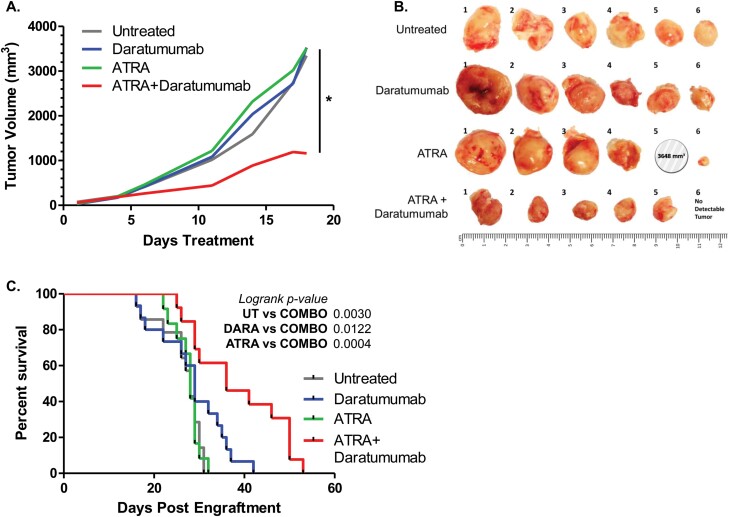
ATRA synergizes with DARA to impede AML tumor growth and extend survival in vivo
Given the effectiveness of the combination of ATRA and DARA in vitro, we next wanted to determine its efficacy in vivo. To test this, we injected MV4-11 cells subcutaneously into the right flanks of NSG mice to generate solid tumors and then treated mice with ATRA, DARA, or both agents. As shown in Fig. 4(A), ATRA and DARA each individually had no effect on the rate of tumor growth or final tumor volume after 18 days treatment. However, the combination of ATRA plus DARA synergized to significantly reduce tumor growth as well as final tumor volume (Fig. 4B).
Fig. 4.
ATRA synergizes with DARA to impede AML tumor growth. (A, B) NSG mice were subcutaneously injected with 2.5 × 106 MV4-11 cells and treated with vehicle (untreated), ATRA, DARA or the combination of ATRA and Daratumumab (n = 6 per group). (A) Tumor growth rate was compared between the combination of ATRA + DARA and vehicle control (untreated), ATRA alone and DARA alone (P values: <0.0001, <0.0001 and 0.0048, respectively). An interaction test for synergistic effects between ATRA and DARA was also done (P = 0.0364, denoted by vertical line and asterisk). (B) Photographs of excised tumors. Note: Mouse #5 in the ATRA group died of disease prior to study conclusion; tumor size was recorded at the time of death (day 17). (C) NSG mice were intravenously injected with 3.0 × 105 spleen cells from MV4-11-engrafted mice and treated with vehicle (untreated), ATRA, Daratumumab or the combination of ATRA and Daratumumab (n = 18 mice per group). Survival was compared between ATRA + Daratumumab group versus untreated, ATRA and Daratumumab groups, respectively (log-rank P values were 0.0030, 0.0004 and 0.0122, respectively). *P ≤ 0.05.
Next, we tested the effect of combination treatment on survival in a disseminated AML mouse model. MV4-11 cells were injected intravenously into NSG mice and treated as above in the solid tumor experiment. As shown in Fig. 4C, survival was significantly increased in the combination-treated group compared to the single-drug groups. Hence, although seen as largely ineffective as a differentiation agent for non-APL AML, ATRA appears to be a strong enhancer of anti-CD38 antibody therapy for AML in vivo.
In this study, we demonstrate that the combination of ATRA and DARA has potent anti-leukemic effects, mediated through CD38 up-regulation and antibody-induced fratricide, in vitro and in vivo. Indeed, within the murine models, combination treatment led to reduced tumor size and growth rate and a 46.6% increase in mean survival time.
Attempts to replicate the efficacy of ATRA treatment in APL for other AML subtypes have seen limited success over the past decades. While a few studies suggest that ATRA in combination with low- to standard-dose chemotherapy improves the complete remission rate and/or event-free and overall survival in non-APL AML patients (16, 17), these results have not been replicated in subsequent studies (18–21). One study of note, the UK Medical Research Council trial in 1075 patients failed to demonstrate any benefit of adding ATRA to standard-dose chemotherapy in patients overall as well as in distinct genotypic subpopulations (FLT3/ITD, NPM1, CEBPA and MN1) (7). A variety of mechanisms have been proposed for the observed resistance of non-APL AML to ATRA. These include expression of the dominant co-repressor of RAR signaling PRAME (22), expression of the retinoid metabolizer CYP26 in the bone marrow microenvironment and overexpression of RAR-complex cofactor and transcriptional repressor MN1. CYP26, a retinoid metabolizer, in the bone marrow microenvironment (23) and overexpression of RAR complex cofactor and transcriptional repressor MN1 (24). Other mechanisms likely exist as well.
Because ATRA led to robust CD38 up-regulation in our studies despite showing no effects on differentiation, its use, when in combination with DARA, may elicit a response in ATRA-resistant AML. In patient samples analyzed by qPCR, expression of CD38 was consistently up-regulated by ATRA treatment, even though the same treatment failed to elicit significant up-regulation of the differentiation marker CD11b (data not shown), which is readily observed in ATRA-treated APL blasts (25). It has been suggested that the CD38 promoter is highly sensitive to ATRA, even at doses far below those traditionally used for in vivo treatments (26). Hence, it is plausible that the threshold for CD38 up-regulation by ATRA is lower than that for differentiation, such that CD38 can still be induced in non-APL AML. Data from the Cancer Genome Atlas (27) show that baseline CD38 expression is variable among the numerous listed AML subgroups. We were unable to test all such subgroups with respect to ATRA and CD38, but in our largely representative group, we observed an ATRA-mediated increase in CD38 within each sample. This suggests that ATRA may be effective at up-regulating CD38 for many, if not most or all, subgroups of AML.
Contrary to the cytotoxicity that ATRA as a single agent displayed in vitro, the drug on its own ultimately conferred no advantage in vivo. One explanation would be that the murine engraftment models we used are known to be aggressive, such that only the stronger combination treatment might have been sufficient to elicit anti-tumor effects. Alternatively, it could suggest that the resistance mechanisms observed in non-APL AML are at least partially operative in our murine models. However, the significant survival advantage resulting from the combination treatment of ATRA and DARA suggests that ATRA does make the AML blasts more targetable by anti-CD38 DARA.
As such, this likely exploits the direct effects of ATRA upon CD38 transcription, permitting greater quantities of CD38 to be available for binding by DARA. There is a binding site for the canonical ATRA receptor, the transcription factor RARα, in the first intron of the CD38 gene. Binding to this retinoic acid response element, or RARE, results in gene expression (12). The requirement of RARα for CD38 expression was demonstrated by Mehta et al., where they silenced RARα in vitro and in vivo using an AML cell line, HL-60 and transgenic mice expressing a RARα antisense construct. This silencing led to essentially a full suppression of retinoid-induced CD38 expression (28). Another study showed that retroviral transduction of RARα into HL-60 cells genetically deleted of RARalpha completely restored sensitivity knock-out HL-60 cells completely restored sensitivity to ATRA-induced CD38 up-regulation (29). Our work with ATRA in conjunction with the RARα-selective agonist AM580 suggests that the canonical pathway can account for CD38 up-regulation in AML blasts, which would be in agreement with these earlier studies.
It is important to note that because DARA is specific for human CD38 and in order to avoid rejection of engrafted human cells, all in vivo experiments were carried out in immune-compromised mice. The NSG mice used for these experiments lack mature B cells, T cells, NK cells and complement. Additionally, there are defects reported in dendritic cells and macrophages (30, 31). This permitted us to test the interactions between the human myeloid blasts themselves, as responses from the host immune system were highly unlikely. This blast-against-blast activity, fratricide, can explain the results found, both in vivo and in vitro. Indeed, the experiments utilizing the F(ab)’2 fragment of DARA suggests that the main anti-leukemic mechanism we observed was Fc-dependent fratricide. And, while fratricide alone conferred potent anti-leukemic effects in our study, addition of other arms of innate and adaptive immunity would likely drive the effects of the combination therapy to greater levels. Indeed, a previous study of ATRA and DARA in multiple myeloma demonstrated that ATRA down-regulated complement-inhibitory proteins CD55 and CD59, effectively increasing cellular complement-dependent cytotoxicity (10). Additionally, DARA has been shown to modulate adaptive immune functions by reducing regulatory T cells while robustly increasing T helper and cytotoxic T-cell counts (32). Further studies would be required to determine whether these mechanisms occur in AML.
Clinically, ATRA treatment in APL is reportedly well tolerated. Approximately 25% of APL patients treated with ATRA develop retinoic acid syndrome (RAS) characterized by cardiorespiratory distress; in the majority of cases, treatment can continue under the addition of high-dose steroids (33, 34). Yet because RAS is characterized by tissue infiltration by maturing myeloid cells (35), occurrence may be avoided when treating differentiation-resistant non-APL AML. Similarly, DARA, approved for the treatment of multiple myeloma, has a favorable safety profile with manageable toxicities (36).
In conclusion, to our knowledge, these studies are the first to describe that although ATRA itself fails to effectively differentiate non-APL AML cells, it potently up-regulates the surface expression of CD38 and, when in combination with DARA, may be an efficacious therapy against non-APL disease. Through the activation of Fc-mediated antibody-dependent blast fratricide, this combination induces anti-leukemic effects in vitro and effectively retards tumor growth and extends overall survival time in vivo. In light of favorable safety profiles and previous FDA approval for each drug, these findings warrant the further study of ATRA and DARA as a potential treatment for AML.
Funding
This work was supported in part by National Institutes of Health (NIH) P01-CA095426 (S.T., J.C.B.); NIH R01 CA162411 (S.T., J.C.B.); NIH R01 CA203584 (S.T., J.P.B.); the Ohio State University Comprehensive Cancer Center P30 CA016058; the Ohio State University Comprehensive Cancer Center Leukemia Research Program Seed Award ELP171 (S.V., J.P.B., S.T.).
Conflicts of interest statement: The authors declared no conflicts of interest.
Supplementary Material
References
- 1. Stein, E. M. and Tallman, M. S. 2016. Emerging therapeutic drugs for AML. Blood 127:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rowe, J. M. 2016. AML in 2016: where we are now?Best Pract. Res. Clin. Haematol. 29:315. [DOI] [PubMed] [Google Scholar]
- 3. Estey, E., Levine, R. L. and Löwenberg, B. 2015. Current challenges in clinical development of ‘targeted therapies’: the case of acute myeloid leukemia. Blood 125:2461. [DOI] [PubMed] [Google Scholar]
- 4. Dombret, H. and Gardin, C. 2016. An update of current treatments for adult acute myeloid leukemia. Blood 127:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howlader, N., Noone, A. M., Krapcho, M.et al. . 2016. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute, Bethesda, MD. [Google Scholar]
- 6. Johnson, D. E. and Redner, R. L. 2015. An ATRActive future for differentiation therapy in AML. Blood Rev. 29:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burnett, A. K.,, Hills, R. K.,, Green C., et al. 2010. The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and CEBPA. Blood 115:948. [DOI] [PubMed] [Google Scholar]
- 8. Landgren, O. and Iskander, K. 2017. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J. Intern. Med. 281:365. [DOI] [PubMed] [Google Scholar]
- 9. Malavasi, F., Funaro, A., Roggero, S., Horenstein, A., Calosso, L. and Mehta, K. 1994. Human CD38: a glycoprotein in search of a function. Immunol. Today 15:95. [DOI] [PubMed] [Google Scholar]
- 10. Nijhof, I. S., Groen, R. W., Lokhorst, H. M.et al. . 2015. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 29:2039. [DOI] [PubMed] [Google Scholar]
- 11. Yoshida, T., Mihara, K., Takei, Y.et al. . 2016. All-trans retinoic acid enhances cytotoxic effect of T cells with an anti-CD38 chimeric antigen receptor in acute myeloid leukemia. Clin. Transl. Immunology 5:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kishimoto, H.,, Hoshino, S.,, Ohori, M., et al. 1998. Molecular mechanism of human CD38 gene expression by retinoic acid. Identification of retinoic acid response element in the first intron. J. Biol. Chem. 273:15429. [DOI] [PubMed] [Google Scholar]
- 13. Fatehchand, K.,, McMichael, E. L.,, Reader, B. F., et al. 2016. Interferon-γ promotes antibody-mediated fratricide of acute myeloid leukemia cells. J. Biol. Chem. 291:25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng, A., Mäntymaa, P., Säily, M., Siitonen, T., Savolainen, E. R. and Koistinen, P. 1999. An association between mitochondrial function and all-trans retinoic acid-induced apoptosis in acute myeloblastic leukaemia cells. Br. J. Haematol. 105:215. [PubMed] [Google Scholar]
- 15. Adamson, P. C. 1994. Clinical and pharmacokinetic studies of all-trans-retinoic acid in pediatric patients with cancer. Leukemia 8:1813. [PubMed] [Google Scholar]
- 16. Venditti, A.,, Stasi, R.,, Del Poeta, G., et al. 1995. All-trans retinoic acid and low-dose cytosine arabinoside for the treatment of ‘poor prognosis’ acute myeloid leukemia. Leukemia 9:1121. [PubMed] [Google Scholar]
- 17. Schlenk, R. F.,, Fröhling, S.,, Hartmann, F., et al. ; AML Study Group Ulm . 2004. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia 18:1798. [DOI] [PubMed] [Google Scholar]
- 18. Estey, E. H.,, Thall, P. F.,, Pierce, S., et al. 1999. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/- all-trans retinoic acid +/- granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood 93:2478. [PubMed] [Google Scholar]
- 19. Milligan, D. W., Wheatley, K., Littlewood, T., Craig, J. I. and Burnett, A. K.; NCRI Haematological Oncology Clinical Studies Group . 2006. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 107:4614. [DOI] [PubMed] [Google Scholar]
- 20. Burnett, A. K.,, Milligan, D.,, Prentice, A. G., et al. 2007. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 109:1114. [DOI] [PubMed] [Google Scholar]
- 21. Belhabri, A.,, Thomas, X.,, Wattel, E., et al. 2002. All trans retinoic acid in combination with intermediate-dose cytarabine and idarubicin in patients with relapsed or refractory non promyelocytic acute myeloid leukemia: a phase II randomized trial. Hematol. J. 3:49. [DOI] [PubMed] [Google Scholar]
- 22. Bullinger, L.,, Schlenk, R. F.,, Götz, M., et al. 2013. PRAME-induced inhibition of retinoic acid receptor signaling-mediated differentiation—a possible target for ATRA response in AML without t(15;17). Clin. Cancer Res. 19:2562. [DOI] [PubMed] [Google Scholar]
- 23. Su, M.,, Alonso, S.,, Jones, J. W., et al. 2015. All-trans retinoic acid activity in acute myeloid leukemia: role of cytochrome p450 enzyme expression by the microenvironment. PLoS One 10:e0127790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heuser, M.,, Argiropoulos, B.,, Kuchenbauer, F., et al. 2007. MN1 overexpression induces acute myeloid leukemia in mice and predicts ATRA resistance in patients with AML. Blood 110:1639. [DOI] [PubMed] [Google Scholar]
- 25. De Marchis, M. L., Ballarino, M., Salvatori, B., Puzzolo, M. C., Bozzoni, I. and Fatica, A. 2009. A new molecular network comprising PU.1, interferon regulatory factor proteins and miR-342 stimulates ATRA-mediated granulocytic differentiation of acute promyelocytic leukemia cells. Leukemia 23:856. [DOI] [PubMed] [Google Scholar]
- 26. Chillemi, A.,, Zaccarello, G.,, Quarona, V., et al. 2013. Anti-CD38 antibody therapy: windows of opportunity yielded by the functional characteristics of the target molecule. Mol. Med. 19:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ley, T. J.,, Miller, C.,, Ding, L., et al. ; Cancer Genome Atlas Research Network . 2013. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368:2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta, K., McQueen, T., Manshouri, T., Andreeff, M., Collins, S. and Albitar, M. 1997. Involvement of retinoic acid receptor-alpha-mediated signaling pathway in induction of CD38 cell-surface antigen. Blood 89:3607. [PubMed] [Google Scholar]
- 29. Drach, J.,, McQueen, T.,, Engel, H., et al. 1994. Retinoic acid-induced expression of CD38 antigen in myeloid cells is mediated through retinoic acid receptor-alpha. Cancer Res. 54:1746. [PubMed] [Google Scholar]
- 30. Ishikawa, F.,, Yasukawa, M.,, Lyons, B., et al. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shultz, L. D.,, Lyons, B. L.,, Burzenski, L. M., et al. 2005. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174:6477. [DOI] [PubMed] [Google Scholar]
- 32. Krejcik, J.,, Casneuf, T.,, Nijhof, I. S., et al. 2016. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang, M. E.,, Ye, Y. C.,, Chen, S. R., et al. 1988. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 72:567. [PubMed] [Google Scholar]
- 34. Patatanian, E. and Thompson, D. F. 2008. Retinoic acid syndrome: a review. J. Clin. Pharm. Ther. 33:331. [DOI] [PubMed] [Google Scholar]
- 35. Tallman, M. S. 2002. Retinoic acid syndrome: a problem of the past?Leukemia 16:160. [DOI] [PubMed] [Google Scholar]
- 36. Lokhorst, H. M.,, Plesner, T.,, Laubach, J. P., et al. 2015. Targeting CD38 with Daratumumab monotherapy in multiple myeloma. N. Engl. J. Med. 373:1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.