Abstract
Cleaning behavioral equipment between rodent subjects is important to prevent disease transmission and reduce odor cues from previous subjects. However, the reporting regarding the cleansing procedures used during such experiments is sporadic and often incomplete. In addition, some investigators are reluctant to clean devices between subjects because they are concerned that animals will react negatively to the smell of the cleansing agents. We hypothesized that mice tested on an elevated plus maze (EPM) soiled with excretions from conspecifics would test as being more stressed than mice tested on the same apparatus that was cleaned between animals. We tested the performance of C57BL/6J mice on an EPM sanitized with 3 common cleaning agents—isopropyl alcohol, chlorine dioxide, and bleach—and on an EPM soiled with rodent urine, feces, and presumably pheromones. We further tested the potentially aversive nature of the cleansing agents by using the classic light:dark box and a 2-choice light:dark box. Our data indicate that cleaning the EPM compared with leaving it soiled did not affect performance in male or female C57 mice, nor did cleaning agent choice. In addition, test subjects did not react to the presence of the cleaning agents when incorporated into the classic light:dark test. However, in the 2-choice light:dark test, mice given the option to avoid an area containing a cleaning agent showed aversion to all 3 agents, when all other conditions were equal. Given the lack of an observable effect of cleaning on EPM performance, we recommend cleaning of the EPM device between C57 mice to minimize the potential spread of disease.
Abbreviation: EPM, elevated plus maze; IPA, isopropyl alcohol
Behavior testing in mice is used for a myriad of purposes in research including, but not limited to, behavioral phenotyping of novel mutant/transgenic mice, evaluation of neurodevelopmental disorders, and as an assessment of the emotional state of the animal, often in response to external stressors or administered drugs. Common behavioral assays in rodents include the elevated zero maze, open field, light–dark exploration, and the elevated plus maze (EPM). The EPM test is one of the most common assays used in current behavioral research2,6,8,15 and is often used in the development of novel anxiolytic drugs and the investigation of the psychologic and neurochemical basis for anxiety, including generalized anxiety, phobias, and posttraumatic stress.7,9 In addition, the EPM test has been used to better understand the biologic basis of the emotional state related to learning and memory, pain, hormones, and addiction and withdrawal.7 With recent advances in gene manipulation such as the CRISPR–cas9 technique, the EPM assay has been used for screening and phenotyping transgenic and knockout mice with mutations potentially related to altered emotionality.7,15
The EPM assay requires stationary equipment that is reused to test multiple animals over an extended period of time and has led to the publication of thousands of studies (according to a PubMed search of the term ‘elevated plus maze’). Cleaning behavioral equipment between rodent subjects is important to prevent disease transmission and reduce odor cues, such as scent-marking and pheromones from previous subjects.3 However, reports regarding the cleansing procedures used during such experiments are sporadic and often incomplete. The EPM literature contains variable descriptions regarding how the maze was cleaned, ranging from complete to incomplete or nonexistent. Some authors express concern regarding odor effects and describe the cleaning methods in their publications. For example, one study23 states in the methods section that “the apparatus [EPM] was cleaned with a 5% alcohol solution before placement of animals to eliminate possible biasing effects from odor cues left by the previous subject.” Another author describes “…the maze [EPM]…[was] cleaned each time with an aqueous solution containing ethanol (20% v/v) to minimize olfactory signals between trials”.4 For both of the cited studies, the efficacy of these solutions in eliminating or reducing odors is unknown and the concentration of alcohol used in both studies4,23 was considerably lower than the standard, accepted antimicrobial concentration of 70%.
Other articles clearly state that equipment was cleaned between trials but do not provide the rationale for the cleaning. For example, “the maze was carefully cleaned with ethanol solution after each animal”20 or “between animals, the apparatus [EPM] was cleaned with 3% hydrogen peroxide and thoroughly dried”.19 Other groups acknowledge cleaning but are vague: “the maze was thoroughly cleaned with a damp cloth after each trial”10 or “the maze was cleaned between each test trial”.16 Finally, many studies that make no mention of cleaning whatsoever.12,14,17,22
The goal of this study was to determine whether the cleaning of EPM apparatus affects animal performance, because few data in this field are available. One study in mice showed decreased anxiety and neophobia in a light:dark test when tested on a soiled apparatus as compared with a clean device.13 We examined the effect of 3 common cleaning agents—isopropyl alcohol (IPA), MB10 (chlorine dioxide), and bleach—compared with a fourth condition in which the EPM was soiled with rodent urine, feces, and presumed pheromones (no sanitation). We chose these cleansing agents because they are commonly used in animal facilities, represent different methods of action, have unique odors, and are readily available. In addition, to better quantify the potential aversiveness of each agent, we tested animal performance associated with these agents in the classic light:dark box and a modified 2-choice light:dark box. We hypothesized that subjects tested on an EPM soiled with animal excretions would test as being more stressed than animals tested on the same apparatus that was cleaned between animals and that some agents would be more aversive than others. This hypothesis is supported by a previous study5 that examined the effects of sex and alarm pheromones in rodents; the authors concluded that “experimenters should assess current laboratory protocols [including] behavioral assays…to prevent pheromonal interference and stress-induced pheromonal release in their research subjects.”
Materials and Methods
Test subjects.
All mice in this study were C57BL/6J (The Jackson Laboratory, Bar Harbor, ME). EPM studies used 8-wk-old mice; 16-wk-old mice were used for the classic light:dark study, and 20-wk-old mice were used for the 2-choice light:dark study. Mice were group-housed in conventional, open-top plastic cages (Allentown, Allentown, NJ) containing 1/4-in. corncob bedding (The Andersons Lab Bedding, Maumee, OH); cotton nesting material (Ancare, Bellmore, NY) was provided in all cages. Reverse-osmosis–purified water was supplied in water bottles without restriction, and food (PicoLab 5053, LabDiet, St Louis, MO) was available at all times. This study used a 12:12-h light:dark cycle (lights on, 0600; standard overhead fluorescent fixtures); all behavior testing was performed between 0900 and 1200, the early phase of the light cycle.
EPM.
The EPM experiment consisted of 4 test groups of 20 mice each (10 males and 10 females per group). Mice were acclimated to the facility for 1 wk prior to the experiments and for 1 h in the EPM room prior to actual testing; standard overhead illumination was used in the EPM room. The maze consisted of 2 open arms (8 cm × 25 cm) intersecting with 2 closed arms (8 cm × 25 cm, with 25-cm walls); the closed arm walls of the EPM were solid black plastic, and the open arms were flat piers. The EPM was positioned 25 cm above a water bath. An overhead digital videocamera recorded all movement during the trials, with each session lasting 5 min. At the beginning of each trial, mice were placed at the intersection of the EPM arms and facing an open arm. Because recent studies demonstrated that previous EPM experience can affect subsequent trials,6,11 we used a new cohort of animals for each of the groups described.
The group design was predicated on the between-subjects cleaning condition: IPA, MB10, bleach, or unsanitized. Each cleansing agent was made fresh the morning of testing, and ATPase testing (AccuPoint Advanced ATP Hygiene Monitoring System, Neogen, Lansing, MI) was performed to ensure sufficient disinfection of the EPM. Male and female mice were tested on different days, with at least 4 d between; in addition, different cleansing agents were tested on different days, with at least 3 d between cleansers.
IPA.
Before testing began, the apparatus was sprayed with 70% IPA and wiped until no liquid was apparent on the device. We then waited 30 s after the final wipe before placing a subject in the EPM. Between test subjects, the EPM was wiped with distilled water to remove visible urine and feces, sprayed with 70% IPA, and then wiped a final time. We waited 30 s before proceeding to the next test subject.
MB10.
Before testing began, the apparatus was sprayed with MB10 solution (200 ppm chlorine dioxide; Quip Laboratories, Wilmington, DE), and then wiped down until no liquid was apparent on the device. We then waited 30 s after the final wipe before placing a subject in the EPM. Between test subjects, the apparatus was wiped with distilled water to remove visible urine and feces, sprayed with MB10, and then wiped down a final time. We waited 30 s before proceeding to the next subject.
Bleach.
We proceeded exactly as for the MB10 group, except for substituting bleach for MB10. Bleach was prepared as a dilution of a commercially available product (Chlorox, Oakland, CA), with a final concentration of 600 ppm sodium hypochlorite.
Unsanitized EPM.
Prior to testing the unsanitized (control) group, the EPM was first rendered ‘dirty’ by placing 2 compatible mice (8- to 10-wk-old C57 mice, either sex) on the EPM and allowing them to explore the maze for 5 min. This procedure was repeated until the EPM was exposed to a total of 20 mice; the device was not cleaned between mice, and none of the mice used for this step were tested in the actual EPM assay. At this point, the device was considered to be dirty, and EPM testing began as described previously; the apparatus was not cleaned between test subjects for this group.
Classic light:dark box.
A standard 2-chamber configuration was used and consisted of a clear acrylic chamber (20 × 16.5 × 18.5 cm) with overhead illumination attached to a darkened chamber (19 × 14 × 15 cm). The dark chamber initially contained absorbent material soaked with 20 mL of distilled water, which was placed behind a perforated protective shield. For the first trial (initial preference), mice were placed in the light chamber; time spent in the light chamber, time spent in the dark chamber, and the total number of entries into the dark chamber were recorded. Initial preference trials lasted 2 min per mouse. For the test trials, the water was replaced with an equal volume of one of the prepared test agents. All 3 agents were tested on 8 male C57Bl/6J mice each, with each trial lasting 2 min and administered on separate days.
Two-choice light:dark box.
The apparatus consisted of an illuminated center chamber (20.5 × 21.5 × 32 cm) attached on either side by small openings to 2 darkened chambers (12.5 × 8 × 11 cm each). Each dark chamber had a small receptacle to hold water (control) or cleanser; odors for the dark chambers were prepared by soaking absorbent material with 20 mL of cleanser (or water) and placing the moistened material in the receptacle (similar to classic light:dark test). Eight male C57BL/6J mice were tested for each agent.
The first trial determined the side preference of each mouse, to control for side bias. The mouse was placed into the illuminated center chamber for a 2-min trial, during which the time spent in each of the 3 chambers was recorded. Initially water was used in both dark chambers; for each mouse, the dark chamber in which the mouse spent most of the time was designated as the preferred chamber for that mouse. For each trial thereafter, the agent was placed in the preferred chamber, whereas water remained in the nonpreferred chamber; trials lasted 2 min per mouse. The device was cleaned with distilled water between subjects, and all agents were tested at least 24 h apart.
To control for possible side bias over multiple trials, each mouse experienced an extinction trial between agents. During these trials, water was placed in both dark chambers, but the former control chamber was blocked off, forcing the mouse to explore the chamber that previously held the cleansing agent (but that now contained water). After this extinction trial, side preference was again assessed prior to the next cleanser trial.
All procedures in this study were approved by the Rutgers IACUC and followed all relevant AAALAC guidelines.1
Data analysis.
EPM data were collected (AnyMaze, Wood Dale, IL) and included time spent in each arm, distance traveled in each arm, and the number of entries into each arm. Each measure was analyzed by using 2-way ANOVA to examine differences between conditions (agent) and sex. Additional pairwise comparisons were run to examine specific differences between groups. For the classic light:dark box study, ANOVA between conditions (agent) was performed to compare ratios of time spent in each arm and the number of entries into the light and dark boxes with data from the initial preference trial. In addition, paired-samples t tests of 2-choice light:dark data were performed to compare ratios of time spent in and entries into the water and agent chambers compared with those from control trials, in which both boxes contained water. ANOVA then was used to examine differences between conditions in the 2-choice light:dark test.
Results
EPM.
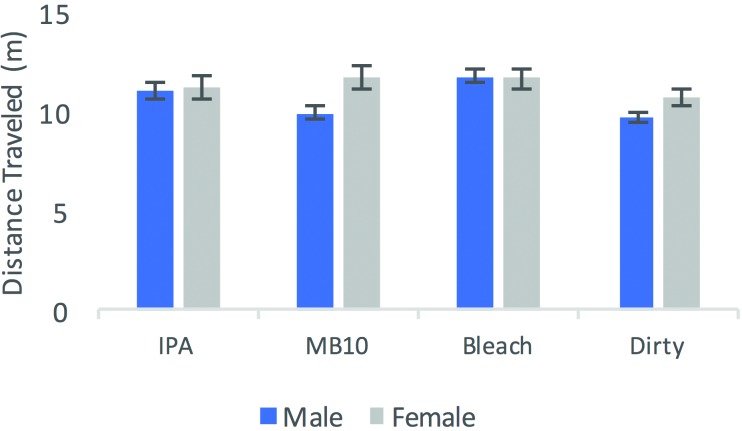
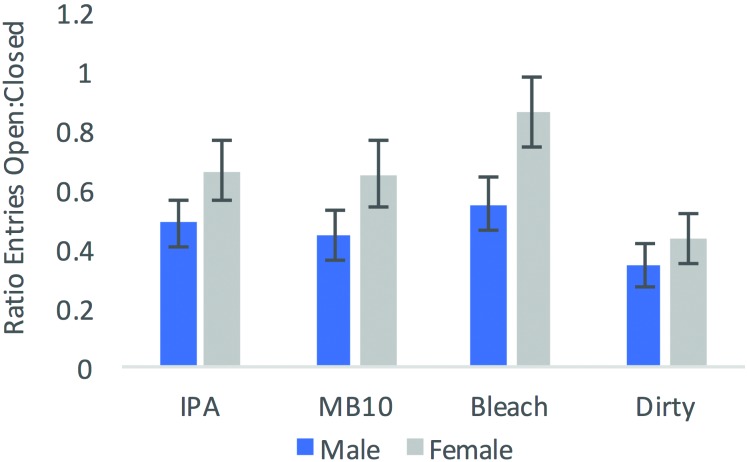
Mice (n = 20 per group) were tested under 4 conditions (IPA, bleach, MB10, and unsanitized); data were lost from one mouse (a male from the IPA group in the EPM data). Distance traveled showed no interaction between sex and cleaning agent (F3,71 = 2.216, P = 0.094; Figure 1) but revealed a sex-associated effect (F1,71 = 8.153, P = 0.053), with female mice travelling further than males (11.35 m [mean] compared with 10.70 m). In addition, group had an effect on distance traveled (F1,71 = 3.831, P = 0.013). Tukey posthoc analyses showed that bleach differed from the dirty (control) condition and thus animals traveled further in the bleach condition (mean, 11.72 m) compared with the dirty condition (10.25 m) (Figure 1). The ratio of the number of entries into the open arms over closed arms entries differed between sexes but not between conditions (F3,71 = 0.742, P = 0.530). Specifically, sex affected the ratio of entries into the open and closed arms (F1,71 = 8.169, P = 0.006), with female mice showing a higher ratio (0.87 ± 0.14; male, 0.69 ± 0.111), indicating that female mice entered the open arms more often male mice, regardless of the cleaning condition (Figure 2).
Figure 1.
Mean total distance (in meters) traveled in the EPM across sex and cleaning agent during 5-min trials. Initially each group contained 20 mice, but data from one mouse were lost. Error bars, SEM.
Figure 2.
Mean ratio of entries into the open arm divided by the number of entries into the closed arm in the EPM across sex and cleaning agent. Initially each group contained 20 mice, but data from one mouse were lost. Error bars, SEM.
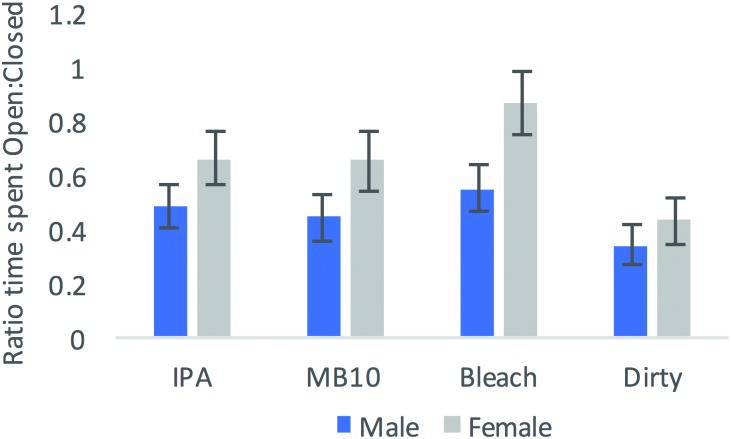
The ratio of time spent in the open relative to closed arms was evaluated by using 2-way ANOVA to assess effects of sex and condition. Results indicated a significant main effect of sex (F1,71 = 8.477, P = 0.005), with female mice having a higher overall ratio (0.65 ± 0.565; male, 0.46 ± 0.041; Figure 3), as well as a main effect of condition (F3,71 = 3.896, P = 0.012). Tukey posthoc analyses indicated a significant difference between the bleach and control conditions (P = 0.006) for the ratio of time spent in the open relative to closed arms. As noted, for all conditions, both males and females spent more time in the closed arms, but mice spent considerably more time in the closed arms when unsanitized, whereas they spent the least amount of time in the closed arms after they had been cleaned with bleach.
Figure 3.
Mean ratio of time spent in the open arm divided by the time spent in the closed arm in the EPM across sex and cleaning agent. Initially each group contained 20 mice, but data from one mouse were lost. Error bars, SEM.
Light:dark box.
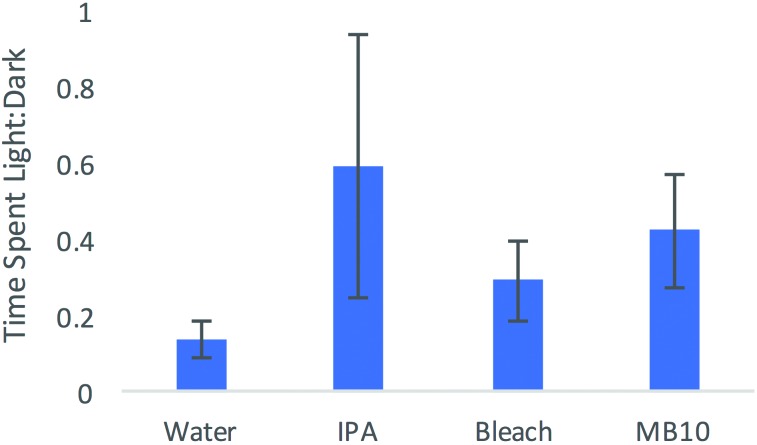
Data were reported as the ratio of time spent in the light chamber compared with the dark chamber (light: dark). ANOVA of these ratios (control ratio:condition ratio) showed no effect of cleanser on performance in the light:dark task, indicating that the time spent in the dark chamber in the presence of any cleanser did not change with regard to time spent in the dark chamber in the presence of water (F3,24 = 0.973, P = 0.42; Figure 4). This result indicates that the mice preferred the dark chamber over the light chamber despite the presence of any of the cleansers.
Figure 4.
Mean ratio of time spent in the light box divided by the time spent in the dark box during the 2-min trial. The dark box contained one of the cleaning agents. Each group contained 8 mice; error bars, SEM.
Two-choice light:dark task.
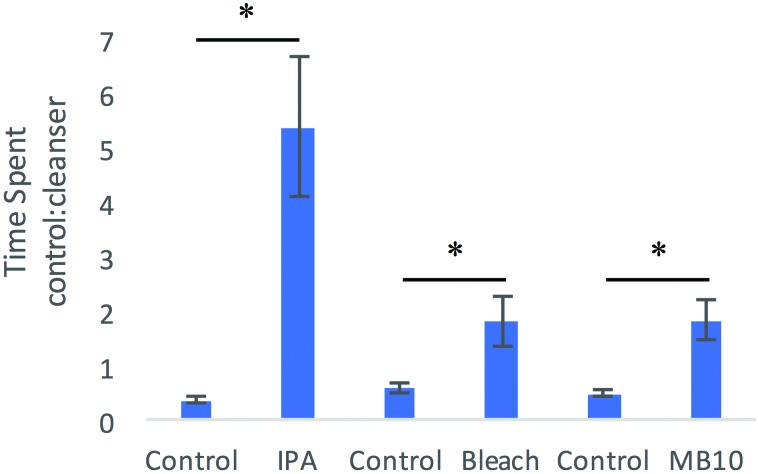
Ratios were reported as time in the chamber with the cleanser divided by time in the chamber with water. A series of paired-samples t tests were performed to examine whether the ratio of time spent in each chamber changed between control or preference trials and odor trials. Results indicated a significant difference between each cleanser and the control condition (IPA: t6 = –3.988, P = 0.008; bleach: t7 = –2.705, P = 0.030; MB10: t7 = –3.355, P = 0.012; Figure 5). When given a choice, mice preferred the dark chamber containing water over the dark chamber containing any cleanser; that is, mice still preferred the dark chamber over the light chamber, but they avoided the dark chamber when it contained a cleanser.
Figure 5.
In the 2-choice procedure, 2 dark boxes were attached to a central light box. In the control condition for each agent, water was placed in both dark boxes to establish a side preference in a 2-min trial. Data for the control condition for each agent represent the time spent in the nonpreferred dark box divided by the time spent in the preferred dark box (that is, the value will be less than 1). On the next trial for each mouse, the cleaning agent was placed in the preferred dark box and water in the less-preferred dark box, and a 2-min trial followed. Data for each agent are time spent in the dark box with water divided by the time in the dark box with the cleanser; therefore, values greater than 1 indicate the mouse preferred the dark box with the water more than the dark box with the cleanser. Each group contained 8 mice; error bars, SEM; *, significant (P < 0.05) difference between control (water) and odor trials.
In addition, mice behaved differently between agents and avoided the chamber containing IPA more than chambers containing either bleach or MB10, with no difference between bleach and MB10 (F2,22 = 7.067, P = 0.005; Figure 5). Therefore it appears that IPA was significantly more aversive than either bleach or MB10 to the test mice.
Discussion
In this study, compared with an unsanitized EPM, neither sanitization of the EPM nor cleanser choice affected the performance of male or female 8-wk-old C57BL/6J mice. This outcome is surprising, given that mice have a well-developed olfactory system and are generally quite responsive to olfactory stimuli.18 According to the literature, some investigators do not consider the potential effect of a soiled apparatus when designing and performing studies using the EPM. Investigators who do not clean the apparatus between subjects argue that cleaning introduces a new variable, namely novel odors associated with the cleaning agent, into their studies. In addition, researchers are sometimes reluctant to change their practices from a historical precedent, according to anecdotal reports we have collected.
Mice normally use urine and feces to communicate dominance and reproductive/health status,3,21 so it is logical to presume that urine and feces from previous animals affect the performance of subsequent animals in the EPM. We hypothesized that lack of cleaning would serve as an additional variable due to the persistence of pheromones and odors of urine and feces from previous trials, which might affect the outcome of the assay in a manner unrelated to the hypothesis being tested. Contrary to our original hypothesis, however, this effect did not occur in our study. In fact, mice spent the most time in the closed arms after they had been soiled. Overall, compared with males, female mice were more relaxed in the EPM regardless of its condition, consistent with the current literature. This difference was evidenced by the female mice traveling farther in the EPM, being more likely to enter the open arms, and spending more time in the open arms, regardless of experimental group.
Test subjects did not react to the presence of any of the cleansing agents when incorporated into the EPM or the classic light:dark test. However, mice showed an aversion to all 3 agents when given the option to avoid an area containing the agent when all other conditions were equal, as evidenced in the 2-choice light:dark test. The preparation of agents was identical in both the classic light:dark test and the 2-choice light:dark test, even though the outcomes of the 2 assays were different. According to the 2-choice light:dark test, IPA was significantly more aversive than either MB10 or bleach. This finding is important, given that IPA is frequently used to clean animal equipment in many laboratories.
Because the sanitization agents were aversive in the 2-choice light:dark test, it is likely they remained aversive when applied to the EPM and classic light:dark tests. Mice may have experienced 2 different, stressful events when placed in a freshly cleansed EPM—namely, the novelty of the device and the odor associated with the cleansing agent. The anxiety associated with odor may have been superseded by the anxiety of being in the device, perhaps explaining why the odor didn't influence the testing metrics when the EPM was cleaned. This hypothesis is supported by the outcome of the classic light: dark test; namely the illuminated chamber was more aversive than the smell of any of the test agents placed in the darkened chamber. This ‘dual stressor’ hypothesis could also apply to the situation where the device wasn't cleaned—the novel experience of the EPM outweighed any stress experienced by smelling pheromones, urine, or feces of previous mice.
Because we did not observe an effect of cleaning on EPM performance, we recommend cleaning of the EPM device between mice to minimize the potential spread of disease. Because IPA was the most aversive agent, investigators may want to consider avoiding this product and use MB10 or bleach as an alternative. Further experimentation is warranted for this work, given that we tested only a small subset of mice. This work should be repeated in other mice regarding strain effect (including sex and age), additional sanitation agents, other behavioral assays that might be affected by lack of sanitation prior to testing animals (for example, the effect of sanitation on the open field test), and in additional species using similar equipment, such as rats.
Acknowledgments
We acknowledge the ACLAM Foundation for their generous support of this work.
References
- 1.AAALAC International. [Internet]. 2018. AAALAC accreditation resources. [Cited 14 June 2018]. Available at: https://www.aaalac.org/accreditation/resources.cfm [Google Scholar]
- 2.Andreatini R, Bacellar LFS. 2000. Animal models: trait or state measure? The test-retest reliability of the elevated plus-maze and behavioral despair. Prog Neuropsychopharmacol Biol Psychiatry 24:549–560. 10.1016/S0278-5846(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. 2008. Scent marking as an odorant communication in mice. Neurosci Biobehav Rev 32:1236–1248. 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartolomé I, Llidó A, Darbra S, Pallarès M. 2017. Effects of neonatal and adolescent neuroactive steroid manipulation on locomotor activity induced by ethanol in male wistar rats. Behav Brain Res 330:68–74. 10.1016/j.bbr.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Bind RH, Minney SM, Rosenfeld SJ, Hallock RM. 2013. The role of pheromonal responses in rodent behavior: future directions for the development of laboratory protocols. J Am Assoc Lab Anim Sci 52:124–129. [PMC free article] [PubMed] [Google Scholar]
- 6.Campos AC, Fogaca MV, Aguiar DC, Guimaraes FS. 2013. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr 35 suppl 2:S101–S111. 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 7.Carobrez AP, Bertoglio LJ. 2005. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 y on. Neurosci Biobehav Rev 29:1193–1205. 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Crawley JN. 2007. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout Mice, 2nd ed New York (NY):John Wiley and Sons; 10.1002/0470119055 [DOI] [Google Scholar]
- 9.Dawson GR, Tricklebank MD. 1995. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16:33–36. 10.1016/S0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- 10.Daza-Losada M, Rodriquez-Arias M, Maldonado C, Aguilar MA, Guerri C, Minarro J. 2009. Acute behavioral and neurotoxic effects of MDMA plus cocaine in adolescent mice. Neurotoxicol Teratol 31:49–59. 10.1016/j.ntt.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 11.File SE, Zangrossi H, Jr, Viana M, Graeff FG. 1993. Trial 2 in the elevated plus-maze: a different form of fear? Psychopharmacology (Berl) 111:491–494. 10.1007/BF02253541. [DOI] [PubMed] [Google Scholar]
- 12.Harris EP, Allardice HA, Schenk AK, Rissman EF. 2017. Effects of maternal or paternal bisphenol a exposure on offspring behavior. Horm Behav 101:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hascoët M, Bourin M. 1998. A new approach to the light/dark test procedure in mice. Pharmacol Biochem Behav 60:645–653. 10.1016/S0091-3057(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 14.Jiao CX, Zhou H, Yang CX, Ma C, Yang YX, Mao RR, Xu L, Zhou QX. 2017. Protective efficacy of a single salvianolic acid A treatment on photothrombosis-induced sustained spatial memory impairments. Neuropsychiatr Dis Treat 13:1181–1192. 10.2147/NDT.S127094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komada M Takao K Miyakawa T. [Internet]. 2008. Elevated plus maze for mice. JoVE. 22. [Cited 13 June 2018]. Available at: http://www.jove.com/index/Details.stp?ID=1088 , doi: http://www.jove.com/index/Details.stp?ID=1088 10.3791/1088, doi: https://doi.org/10.3791/1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer AM, Larkin G, Jones A, May BJ. 2018. Behavioral animal model of the emotional response to tinnitus and hearing loss. J Assoc Res Otolaryngol 19:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Zhai J, Wang B, Fang M. 2017. Olig2 silence ameliorates cuprizone-induced schizophrenia-like symptoms in mice. Med Sci Monit 23:4834–4840. 10.12659/MSM.903842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rokni D, Hemmelder V, Kapoor V, Murthy VN. 2014. An olfactory cocktail party: figure-ground segregation of odorants in rodents. Nat Neurosci 17:1225–1232. 10.1038/nn.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouzer SK, Cole JM, Johnson JM, Varlinskaya EI, Diaz MR. 2017. Moderate maternal alcohol exposure on gestational day 12 impacts anxiety-like behavior in offspring. Front Behav Neurosci 11:1–13. 10.3389/fnbeh.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siqueira IR, Vanzella C, Bianchetti P, Rodrigues MAS, Stulp S. 2011. Anxiety-like behavior in mice exposed to tannery wastewater: the effect of photoelectrooxidation treatment. Neurotoxicol Teratol 33:481–484. 10.1016/j.ntt.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Stowers L, Cameron P, Keller JA. 2013. Ominous odors: olfactory control of instinctive fear and aggression in mice. Curr Opin Neurobiol 23:339–345. 10.1016/j.conb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue N, Huang J, Zhu X, Han Q, Wang Y, Li B, Liu Q, Wu G, Zhang U, Yu J. 2017. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation 14:1–15. 10.1186/s12974-017-0865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zager A, Brandao WN, Margatho RO, Peron JP, Tufik S, Anderson ML, Kornum BR, Palermo-Neto J. 2018. The wake-promoting drug Modafinil prevents motor impairment in sickness behavior induced by LPS in mice: role for dopaminergic D1 receptor. Prog Neuropsychopharmacol Biol Psychiatry 81:468–476. 10.1016/j.pnpbp.2017.05.003. [DOI] [PubMed] [Google Scholar]