Abstract
The 2014 Surgeon General’s Report on smoking and health concluded that changing cigarette designs have caused an increase in lung adenocarcinomas, implicating cigarette filter ventilation that lowers smoking machine tar yields. The Food and Drug Administration (FDA) now has the authority to regulate cigarette design if doing so would improve public health. To support a potential regulatory action, two weight-of-evidence reviews were applied for causally relating filter ventilation to lung adenocarcinoma. Published scientific literature (3284 citations) and internal tobacco company documents contributed to causation analysis evidence blocks and the identification of research gaps. Filter ventilation was adopted in the mid-1960s and was initially equated with making a cigarette safer. Since then, lung adenocarcinoma rates paradoxically increased relative to other lung cancer subtypes. Filter ventilation 1) alters tobacco combustion, increasing smoke toxicants; 2) allows for elasticity of use so that smokers inhale more smoke to maintain their nicotine intake; and 3) causes a false perception of lower health risk from “lighter” smoke. Seemingly not supportive of a causal relationship is that human exposure biomarker studies indicate no reduction in exposure, but these do not measure exposure in the lung or utilize known biomarkers of harm. Altered puffing and inhalation may make smoke available to lung cells prone to adenocarcinomas. The analysis strongly suggests that filter ventilation has contributed to the rise in lung adenocarcinomas among smokers. Thus, the FDA should consider regulating its use, up to and including a ban. Herein, we propose a research agenda to support such an effort.
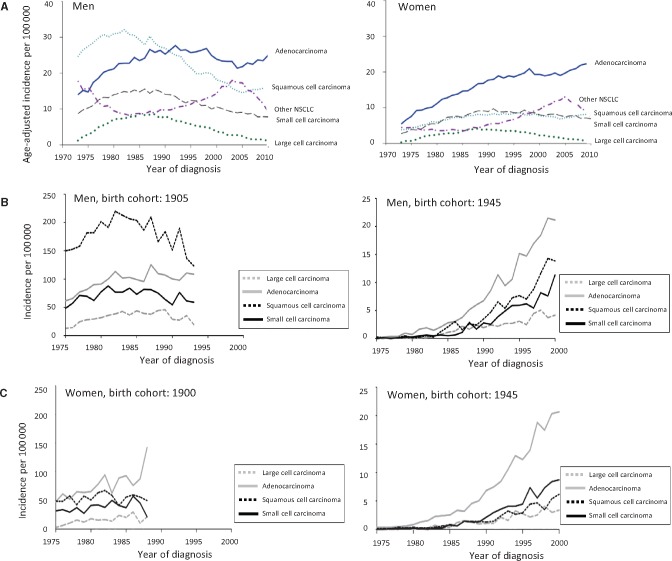
Cigarette smoke is the major cause of lung cancer, containing numerous carcinogens, mutagens, and other toxicants (1–3). When the incidence of lung cancer began to rapidly increase in the 1950s through the 1970s, squamous cell lung cancers were the most common sub type for men, but these decreased over the next 40 years with the decreasing smoking prevalence (1,4–10). However, the incidence of lung adenocarcinomas did not similarly decrease for men and women, exceeding squamous cell cancers in about 1990 and currently comprising about 60% of non–small cell lung cancers (Figure 1A). In 2014, the Surgeon General’s Report (SGR) on the Health Consequences of Smoking concluded: “The evidence is sufficient to conclude that the increased risk of adenocarcinoma of the lung in smokers results from changes in the design and composition of cigarettes since the 1950s” (1). It was observed that the changes in lung cancer over time followed a birth cohort effect in men, when successive generations of smokers transitioned from the use of non-filtered cigarettes to cigarettes of lower smoking machine tar yields (Figure 1B). A less obvious effect is seen for women because they generally started smoking later in the century and so mostly smoked only cigarettes with lower tar yields (Figure 1,A and C). It was further noted that the magnitude of lung cancer risk among smokers had increased over time; for example, in the American Cancer Society Cancer Prevention Studies, an almost two fold increase in risk for smoking men, and a 10-fold increase in risk for smoking women, from the 1960s to the 1980s was observed (11). Concurrently, the relative risks for adenocarcinomas increased from 4.6 (95% confidence interval [CI] = 1.7 to 12.6) to 19.0 (95% CI = 8.3 to 47.7) in men and 1.5 (95% CI = 0.3 to 7.7) to 8.1 (95% CI = 4.5 to 14.6) in women, while the risks of other lung cancer subtypes did not increase (6). Other studies similarly report increased rates and risks (6,12–14). Thus, there was a paradoxical increase for lung adenocarcinomas while squamous cell cancers decreased with decreased smoking rates.
Figure 1.
Trends of incidence of lung cancer among US men and women and from various birth cohorts. Adapted from the 2014 Surgeon General’s Report (1). (A–C) Graphs present trends in age-standardized incidence rates in the United States from 1973 to 2010 for lung cancer for men (A, left) and women (A, right) and histologic type of lung cancers using data from the National Cancer Institute's Surveillance, Epidemiology, and End Results program. Among men, there has been a shift in the histology patterns, with an increase of adenocarcinomas over squamous cell carcinoma (B); similar trends are seen for women (C). Graphs present trends in incidence rates of lung cancer in the United States for 1905 and 1945 from birth cohorts of men (B) and for 1900 and 1945 from birth cohorts of women (C) and histologic type of lung cancers. NSCLC = non–small cell carcinoma.
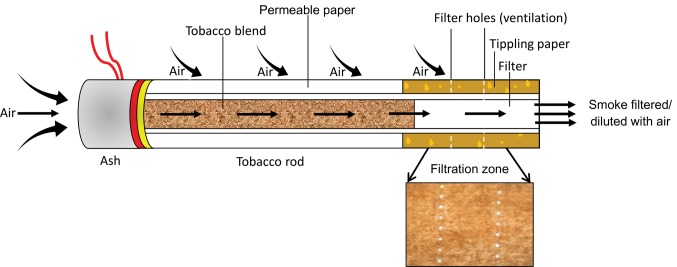
Beginning in the 1950s, the concept was developed that lower smoking machine tar yields equate to reduced smoking-related risks. This led to the cigarette industry to progressively lower tar yields in different ways, beginning with the placement of filters on cigarettes (15–18). This was then followed by the use of less tobacco in cigarettes of the same length, use of reconstituted and expanded tobaccos, increasing cigarette paper porosity, and the placement of ventilation holes in the filter to dilute the smoke (Figure 2) (19). The 2014 SGR indicated two reasons that lowering tar yields could have increased the risk of lung adenocarcinomas: the use of filter ventilation holes and increasing amounts of tobacco-specific nitrosamines (TSNAs) in tobacco (1). Filter ventilation became the critical way for cigarettes with similar designs to have lower smoking machine tar yields, and cigarettes were marketed as “regulars” (a few “regulars” remained with 0% ventilation) and “lights” in the 1970s, and “ultralights” in the 1990s (20–23). The public health community believed that smoking cigarettes with lower smoking machine tar yields was preferable for smokers who would not quit (24–27). Lower tar yield cigarettes became the preferred choice of many smokers who perceived them to be a lower health risk because of health messaging. This perception was reinforced by the sensation of reduced harshness when smoking due to the mixing of air and smoke and reduced resistance to draw when puffing the cigarette (16,28,29). While today many countries such as the United States, Canada, and the European Union, have banned the use of “light” and “ultralight” cigarette descriptors because of the evidence that these are not safer cigarettes, filter ventilation continues to be used in almost all commercial cigarettes (16,30). In some jurisdictions, including the European Union, a maximum machine-measured tar yield is mandated for all cigarettes, attributable to the belief that lower tar yields lead to safer cigarettes, which happens to be achieved primarily through the use of filter ventilation (31–33).
Figure 2.
The modern cigarette. An adapted depicted modern cigarette as to elucidate mechanisms in and around the burning cigarette by Richard R. Baker in 1982 (https://industrydocuments.library.ucsf.edu/tobacco/docs/#id=knyy0131) (1).
There have been previous calls for regulating cigarette filter ventilation because of smokers’ misperceptions that lower-tar cigarettes would cause less disease (34,35). Under the 2009 Family Smoking Prevention and Tobacco Control Act (TCA), the FDA has the authority to regulate tobacco products and issue “product standards” when there is sufficient evidence that a standard would be “appropriate for the protection of public health.” The purpose of this review is to provide a weight-of-evidence review using causation criteria linking filter ventilation to an increasing risk of lung adenocarcinoma expanding the analysis to include chemistry and toxicology studies, human clinical trials, and epidemiologic studies of smoking behavior and lung cancer risk. This review will use two methods and a consensus-building process to evaluate the evidence for the causal relationship of filter ventilation to increasing risk of lung adenocarcinomas in order to provide a scientific evidence base for the regulation of filter ventilation. One method has been traditionally used for the assessment of smoking-related health risks, and the second method is more recently being advocated for the use in regulatory decision-making because of its transparency and identification of inconsistent data and data gaps (1,24,36–40).
Methods
This review is an evidence-based causation analysis, which uses a weight-of-evidence review of published scientific literature and internal tobacco company documents (experimental and human studies) to provide a comprehensive overview of filter ventilation in relation to lung adenocarcinoma. After grouping disparate types of evidence, reviews were conducted within topics. Scientific publications were identified through PubMed using the following search terms: lights, ultralights, tar, cigarettes, filter ventilation, air dilution, adenocarcinoma, Ames, tumorigenicity, nitrosamines, polycyclic aromatic hydrocarbons, inhalation, puff topography, mutagenicity, smoking machine, compensation, smoking behavior, and chemical yields. Using cigarette and any of the above terms yielded 81 382 publications, which were then narrowed to 3284 based on studies that considered tar yields (Supplementary Table 1, available online). It should be noted that a causation analysis considers all available relevant studies and does not exclude any based on some particular design or feature; rather, it weights highly the most relevant and best studies. Additionally, the online Tobacco Documents Bibliography of internal tobacco company documents archived by the library of the University of California, San Francisco’s Center for Knowledge Management was searched using search strings similar to those above (41). This database includes numerous duplicates, and there are numerous unique documents providing duplicative data and methods (eg, draft reports, partial reports, interim reports, final reports, and summaries). Thus, reporting the number of documents that were considered is not informative.
Causality Assessment
Two weight-of-evidence methodologies were utilized, as described in the Supplementary Methods (available online). The first is detailed in the 2004 SGR, derived from the method applied in the 1964 SGR and subsequently used for the later reports (1,24,36). This approach is consistent with an “evidence-based causation methodology” that recognizes the importance of human data, challenges in extrapolating laboratory toxicology data to human risk, use of laboratory toxicology data to support conclusions based on mechanisms, and an integration of data ranging from the laboratory to epidemiology (37). The causation criteria, briefly, include the consideration of 1) consistency as applied to both experimental and human studies; 2) strength of association that considers the magnitude of the effect (eg, reported risk); 3) dose-response relationship; 4) specificity of the effect vs other contributing causes; 5) coherence between laboratory and human data; 6) interventions that provide direct experimental evidence using laboratory and human studies; 7) plausibility; and 8) analogy to other known causes. While there is no particular guidance on how to weight disparate pieces of evidence, this review classifies categories of data on a scale of 0 to 3, where consistency and interventions were given a threefold weight compared with other criteria, dose-response and biological plausibility were given a two fold weight, and the others were the comparator at a one fold weight (coherence, plausibility, and analogy), so that the maximum score was 45 (Supplementary Table 2, available online). The authors evaluated the different types of evidence presented herein during numerous meetings and phone calls to reach a consensus for the various weights and rankings.
The second approach uses a mode of action (MOA), and human relevance framework for weighing the evidence has been applied (Supplementary Table 3, available online), which has evolved for risk assessment purposes from causation criteria developed in the 1960s by Sir Austin Bradford Hill (38), where more recent considerations now are applied in the context of modern scientific principles and specific defining questions (39,40). This second framework is used to enhance transparency and determine if similar conclusions result using different approaches. The MOA links the exposure of a cell to a substance that reflects the outcome of interest, for example, increasing cancer risk, and does this in the context of human relevance. This framework explicitly considers the consistency of the data from disparate types of studies, outcomes based on a sequence of events, and biological evidence of pathways that can be interrupted so that risk is not considered inevitable, and it necessitates the consideration of the dose-response relationship. Inconsistencies and data gaps are identified using a standardized template.
Results
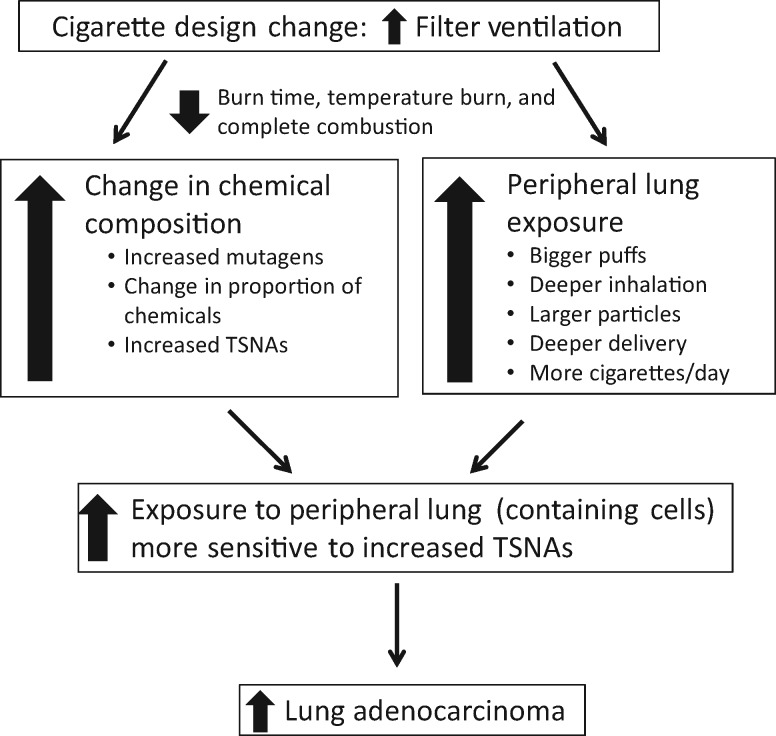
The published scientific literature and unpublished internal tobacco company research can be grouped by laboratory experimental data (ie, impact of filter ventilation on smoke chemistry yields, in vitro mutagenicity, and in vivo animal studies) and human studies (ie, smoking behavior, exposure including observational studies and clinical trials, and long-term epidemiology). Figure 3 provides the framework for the relationship of filter ventilation to the increased risk of adenocarcinomas.
Figure 3.
The framework for the relationship of filter ventilation to the increased rate of adenocarcinoma. The placement and increase of filter ventilation lead to higher levels of mutagens and carcinogens, compensation with the greater depth of inhalation, and deposition of smoke that increases exposures to in the peripheral portion of the lungs. Thus, smokers who smoke low-tar cigarettes have developed a greater risk for adenocarcinoma of the lung. TSNAs = tobacco-specific nitrosamines.
Smoking Machines and Toxicant Delivery
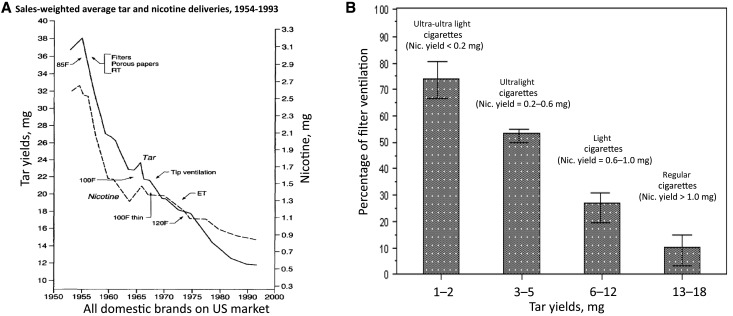
Smoking machines are used to generate smoke in a standardized manner for laboratory testing and were thought to be useful for comparing cigarette smoke tar yields (and constituents) in the context of relative disease risks (42–45). When cigarettes with increasing filter ventilation are smoked on a smoking machine, tar yields are lowered because the filter ventilation holes allow for the smoke to be diluted with air. Machine-measured cigarette tar yields generally decreased from an estimated average of 38 mg in 1954 to 12 mg in 1997 (Figure 4A) (46). Figure 4B shows when filter ventilation was adopted compared with other design changes that also were intended to lower tar yields (23). Filter ventilation was used in about 7% of marketed cigarettes by the end of the 1960s, but rapidly increased to 94% to 100% by 1982 (35, 47). Today, the percent age of filter ventilation used in commercial cigarettes ranges from 0% to 83%, although most smokers choose cigarettes that have 10% to 20% ventilation (10–15 mg tar yield). A small number of smokers prefer cigarettes with greater than 40% ventilation (1–6 mg tar yields) (23,30,47–49). Until 2008, US cigarettes were branded by the industry as “regulars” or “full-flavor” (>15 mg tar), “lights” (6–15 mg tar), and “ultralights” (<6 mg tar) depending on the machine-rated tar yield, in large part determined by filter ventilation (Figure 4B) (19,47,50).
Figure 4.
Sales-weighted average tar and nicotine deliveries, 1954 to 1993, and percentage of filter ventilation of cigarettes based on tar yields using the Federal Trade Commission. A) Tar and nicotine as measured by a smoking machine. Source: Hoffmann D, Djordjevic MV, Hoffman I. The changing cigarette. Prev Med. 1997;26(4):427–434. (19). B) Adapted from: Centers for Disease Control and Prevention. Filter ventilation levels in selected U.S. cigarettes, 1997. MMWR Morb Mortal Wkly Rep., 1997;46(44):1043–1047 (22). Bars represent 95% confidential interval. Percentage of filter ventilation is the percentage of a standard puff (two second duration, 35 mL), that is, air taken into puff through the filter vents. A cigarette with no filter ventilation would produce a puff undiluted by air from filter vents; a cigarette with 80% filter ventilation would produce a puff that is 80% air from vents and 20% smoke undiluted by air from vents. Descriptors are no longer allowed by law because they are misleading and because the classification and nicotine yields vary by definition in the literature. ET = expanded tobacco; F = filter; Nic. = nicotine; RT = reconstituted tobacco.
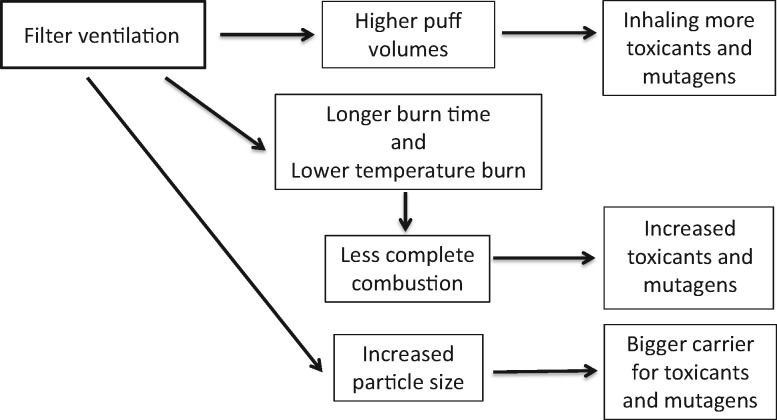
While the application of filter ventilation can result in lower machine-measured tar yields, the composition of the smoke changes increases tobacco toxicant yields and adverse biological effects as follows (Figure 5) (51–55):
Figure 5.
The relationship of filter ventilation to changes in chemical yields and toxicity. With increased filter ventilation, smokers take higher puff volumes and inhale more toxicants and mutagens, tobacco burns longer and at a reduced temperature that increases incomplete combustion with increased toxicants and mutagens, and increased particle size becomes a bigger carrier for toxicants and mutagens.
As filter ventilation increases, the cigarette is burned down less rapidly on the smoking machine, and there are more puffs per cigarette (54–59).
As the tobacco rod burns down less rapidly, there is more time for the coal to smolder and form more toxic constituents (54,55,57).
With increased ventilation in the range of most commercial cigarettes, there is decreased air flow through the burning coal tip and lower coal temperatures, resulting in more incomplete combustion and toxic constituents (60–66). An important publication for the chemical yields of two commercial cigarettes that differ by the amount of filter ventilation shows that most toxic constituents are statistically significantly increased (67). The analysis of this study was done with smoke constituent yields on a per-mg-of-nicotine basis, mimicking smoke intake for a smoker adjusting their smoking behavior to compensate for lower nicotine delivery. Among the increased toxicants was (N-nitrosomethylamino)-1-(3-pyridyl)-1-butanone (NNK), a potent lung carcinogen, in agreement with other published studies (57,64,68–72). Blocking ventilation holes decreases NNK levels (73).
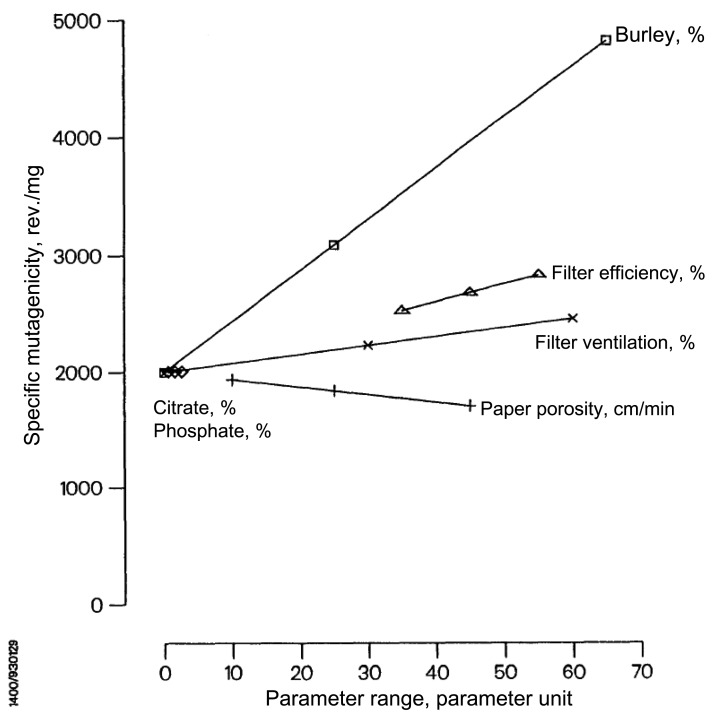
Increasing filter ventilation increases cigarette smoke mutagenicity as measured by the Salmonella Reverse Mutation Assay (Ames test), which is a highly replicated and extensively used assay for the screening of mutagenic potential (74–78) (Supplementary Figure 1, available online) (79). Filter ventilation increases mutagenicity across the full range of cigarette ventilation (69,74,80). An internal tobacco company study assessed six different design parameters to model the contribution of various design changes, including ventilation to mutagenicity, using 30 different research cigarettes (81). Filter ventilation statistically significantly increased the mutagenicity of tar independent of other cigarette designs and tobacco formulations (Figure 6) (81).
Increased filter ventilation increases particle size in the smoke due to increased water content, condensation, and coagulation as the smoke passes through the tobacco rod (Supplementary Table 4, available online) (54,82–88). This is due to the slower burn down of the cigarette and increased residence time of the smoke, allowing for the particles to absorb more water and constituent gases.
Figure 6.
The increase in mutagenicity per % increase in ventilation, which was statistically significant. Source: Mutagenicity of the mainstream smoke condensate of 30 research cigarettes with differences in 6 parameters,” 1993. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=thcc0126 (81).
In summary, the consistency and biological plausibility resulting from changes in combustion provide mechanistic support for a causal relationship between filter ventilation and the increased risk of lung adenocarcinoma, owing to a dose-response relationship between ventilation and increased toxicant yield. This applies to the full range of filter ventilation, including highly ventilated cigarettes with the lowest machine yields that have been previously marketed as “ultralights.”
Filter Ventilation Provides No Benefits to Smokers
Filter ventilation allows for the cigarette yields to be “elastic” when smoked by smokers. Increased puffing intensity resulted in a nonlinear increase in the concentration of tar and nicotine yields because ventilation and tar reduction depend on how fast and large the puff is (89,90). Varying puffing intensity allows smokers of ventilated cigarettes to titrate the nicotine yield, maintain their desired blood nicotine levels, and optimize nicotine reward (34,91). This process, known as “compensation,” is accomplished by individual puffing styles (puff topography) of harder or longer puffs, or by blocking the ventilation holes with one’s fingers (either consciously or unconsciously; most smokers are unaware of ventilation holes) (34,42,92–101). In 2006, a federal court decision determined that that the reporting of machine-rated tar and nicotine yields was “totally unreliable for measuring the actual nicotine and tar any real life smoker would absorb because it did not take into account the phenomenon of smoker compensation” (102). In November 2008, the FTC took action that prompted the removal of nicotine and tar listings from cigarette packs and ads. As of June 2010, the TCA prohibited the use of explicit or implicit descriptors on tobacco packaging or in advertising that convey messages of reduced risk or exposure, specifically including terms like “light,” “mild,” and “low” (103). Nonetheless, there has been no action to regulate filter ventilation, and ventilation holes in cigarette filters remain today on most cigarettes.
Replicating Human Puff Profiles on Smoking Machines
Academic studies replicating human smoking behavior on smoking machines demonstrated how none of the standardized smoking machine puffing regimens accurately predict exposure to smokers (42,104,105). For example, Djordjevic and colleagues found that smokers of 8 to 9 mg machine-rated tar yield cigarettes, when compared with 15 mg cigarette smokers, had a 2.5-fold higher intake of tar, nicotine, and TSNAs compared with the machine yield (104). Hammond and coworkers compared nicotine yields using different machine smoking regimens with actual exposure for smokers using salivary cotinine levels, showing that these are unrelated (105). While there are no published studies replicating human smoking behavior on a smoking machine by levels of ventilation, the Philip Morris Tobacco Company conducted several studies (cross-sectional and clinical trials) in the 1970s recording puffing behaviors by smokers and programming a smoking machine to replicate their profiles (106–111). They showed that filter ventilation resulted in a compensatory response by the smoker such that standard machine yields grossly underpredicted actual exposure and that compensation led to similar exposures from cigarettes with different ventilation levels.
Clinical Trials
The most direct evidence for determining a smoker’s exposure from filters with different yield elasticity comes from clinical trials of smokers who switched to cigarettes with different levels of filter ventilation. These studies encompass a range of designs including smoking one or several cigarettes in a laboratory, confining smokers to an inpatient setting so that smoking is directly monitored, to longer-term switching trials in the naturalistic setting. Among these, longer-duration studies and those in the naturalistic setting are more informative because they allow smokers to adjust to taste and other cigarette characteristics and to compensate for changes in nicotine yields in the “real world.” Also, studies with control groups (ie, smokers who continue to smoke their usual brand) are more informative as smokers may alter their behavior simply because they are in a study about smoking (112–119). The largest study conducted to date, which used commercial cigarettes that differ little except by filter ventilation, was conducted by Philip Morris (113). It studied 225 smokers of low-ventilation cigarettes (∼10%) who switched to higher-ventilated cigarettes (∼17% or ∼47%) or unswitched (controls). The smokers were first confined to an inpatient facility for eight days and had restrictions based on how much they could smoke per day. Then, they continued in a 24-week naturalistic environment study. Compared with the controls, there was no statistical reduction for switching to the approximately 17% cigarettes, and while there was some statistical reduction for some biomarkers when switching to the approximately 47% cigarette, it was much less than the expected reduction based on decreased tar yields. One limitation for this study is a high dropout rate at 24 weeks, although there are similar results for interval follow-ups. Other studies also indicate little to no difference in actual exposure reduction by filter ventilation or other methods to reduce exposures with ventilated cigarettes (eg, reduction in cigarettes per day) (89,112,113,116–118,120–122). These clinical studies are summarized in Supplementary Table 5 (available online).
Cross-Sectional Studies
These studies of general population smokers assess puff topography and exposure biomarkers at a single point in time. While clinical trials for cigarettes with different tar yields provide direct evidence for the effects of filter ventilation on exposure, there also are observational cross-sectional studies that provide corroborative data, although of lesser weight. A major limitation of these studies is that they provide little information about cause and effect because of inherent bias and confounding (eg is an observed difference in a biomarker level due to the cigarette design or due to self-selection by a smoker with innate characteristics). They also do not solely assess the effect of filter ventilation because marketed cigarettes differ by other characteristics. Nevertheless, consistent with the clinical trials, these studies (Supplementary Table 6, available online) demonstrate that exposure biomarkers are not statistically reduced when smoking cigarettes with differing tar yields and filter ventilation, except for perhaps some comparisons of the most extreme differences in tar yields (25,89,104,123–150). The largest study to date (n = 3600), also conducted by Philip Morris, was specifically designed to assess biomarker exposures by tar yields. This study showed few differences in biomarkers based on tar yields, and statistical differences were reported only for the most extreme comparisons of tar yields (137,144,151). Tar yield was substantially less of a predictor for nicotine exposure compared with number of cigarettes per day, nicotine dependence, and puff topography. Other studies, albeit smaller, show similar results (25,138,139,143,144). Separately, while machine-measured tar and nicotine levels have decreased over time, serial cross-sectional data over 25 years from the National Health and Nutrition Examination Survey (NHANES) demonstrate little overall change in daily nicotine intake among smokers, with cotinine per cigarette increasing by 42% over that time (152).
In summary, the consistency of the human clinical trials and cross-sectional studies demonstrates that lower machine tar yields do not predict lower exposures determined by biomarkers of exposure. And actually, puff volumes increase for smokers of cigarettes with more ventilation, suggesting greater exposures in the lung. Reported results in cross-sectional studies of lower biomarkers for smokers of cigarettes with the most ventilation may be due to the characteristics of the smokers choosing these cigarettes rather than the tar yields affected by ventilation (153). It can be noted that these studies do not support a causal relationship for filter ventilation and lung adenocarcinoma because they do not show increased levels of blood and urinary biomarkers. However, the above studies are somewhat limited in study design, do not measure exposure at the lung level, do not include validated biomarkers of harm, and the urine and blood studies might not be a surrogate for changes in lung exposure because of rapid absorption of carcinogens through the lung.
Effects of Filter Ventilation on Consumer Perception and Response
As early as 1955, filter ventilation was recognized by the tobacco industry to produce a smoke that is less strong, harsh, and irritating (58,154–159). This led smokers to believe that they are smoking a product that is less harmful (156,157,160). Most smokers are unaware of the presence of filter ventilation leading to this effect (161,162), although some might subconsciously partially block the holes with their fingers (23,163). These perceptions were reinforced by implicit and explicit advertising claims about safer cigarettes (20,23,34,101,162,164–181). Although tar yield descriptors are currently prohibited, the messaging remains because the coloring and packaging has not changed (180,181), and smokers retain their misperceptions about health effects based on the character and sensory effects of the smoke (20,23,34,101,162,164–179). Thus, an added adverse impact of filter ventilation is the fostering of a false belief that a lower-tar cigarette is a healthier cigarette.
Filter Ventilation, Inhalation, and Smoke Distribution in the Lung
The process of inhalation is separate from puffing for most smokers and is a multistep process of mouth-holding followed by inhalation (182–186). Filter ventilation allows smokers to have higher puff volumes and to take more frequent puffs (42), making more toxicants available to be inhaled to deeper parts of the lungs and allowing for greater retention of nicotine and toxic chemicals (42,182–184,187). To date, there are inconsistent results as to whether cigarettes with different tar yields directly influence inhalation, separate from allowing for more smoke to enter the lungs because of larger puff volumes, although the consensus within the academic community is that the depth of inhalation increases with greater filter ventilation (1,42,97,116,117,147,188–200). There is no validated method to assess inhalation for smoking, and the inconsistencies may relate to variations in methodologies, use of unnatural environments (eg, use of smoke chambers, constricting bands around the chest, and radiotracer studies), small study size, and inadequate study design (eg, single use or limited use). Importantly, many smoke constituents, such as nicotine, are rapidly absorbed through the lungs so that biomarker studies of the urine and blood might not reflect local lung exposures, and there is evidence for differential retention of particulate matter constituents such as TSNAs (97,184,186,201). Several studies have indicated that 95% to 100% of inhaled nicotine is retained, but only 60% to 97% of particulate constituents (186,201). Smoke reaching the most distal parts of the lung, where air flow decreases, allows for easier sedimentation of the particles. Also, particles may grow in size and water content in the lungs, allowing for more deposition and retention of particles with higher amounts of smoke toxicants due to filter ventilation (186,199,202). To validate this in humans, smoke distribution and retention would need to be directly measured, but these methodologies do not exist. There is some data for smoke distribution using experimental animal studies and modeling, but these are not developed based on actual smoking behavior data, which likely underestimate deposition (199,203). These models also do not account for flow of the gas phase chemicals or account for changes in filter ventilation.
In summary, there is conflicting data to conclude that filter ventilation increases depth of inhalation. Furthermore, how particles distribute in the lung generally is unclear, and this has not been studied with respect to filter ventilation specifically. However, a logical inference is that smokers with larger puff volumes due to cigarette elasticity will make more smoke available to travel deeper into the lungs. Thus, greater depth of inhalation or a change in particle size do not necessarily need to occur to affect risk because more smoke is inhaled either way. The assumption of greater lung exposure to tobacco toxicants leading to an increased risk for lung adenocarcinomas due to filter ventilation is may not be in conflict with clinical trials and cross-sectional biomarker studies using blood and urine biomarkers because these studies do not provide information about lung exposure, distribution, or other local effects in the lung. Small differences in exposure that are distributed widely in the body may not be measurable and subject to numerous factors related to innate characteristics of the smoker and rapid transfer from the lungs to the blood stream. However, we postulate that small differences in exposure concentrated on a per-puff basis might have a large impact localized in the lungs.
Different Sensitivity of Distal Airway Lung Cells Leading to the Development of Different Types of Lung Cancer
Experimental studies and limited human evidence indicate that the distal airways of the lung contain cells prone to the development of adenocarcinoma and that these regions may be more sensitive to TSNAs. It should be noted that most lung carcinogenesis studies in experimental animals focus on TSNAs and polycyclic aromatic hydrocarbons (PAHs) and are limited both in number and type of animal models; other smoke toxicants increased by filter ventilation also may contribute. Experimental animal studies indicate that there are generally three types of epithelial cells in the lung, namely type I pneumocytes, type II pneumocytes primarily located in the alveolar space (more distal airways—probably the progenitors of type I pneumocytes) and Clara cells that are nonciliated and located in the terminal bronchioles (now known as club cells or bronchiolar cells and located in the more proximal region of the lung) (204,205). Although not well studied, there is evidence that type II pneumocytes are cells involved in inflammatory reactions (206), provide an inflammatory signal to recruit granulocytes and cause inflammation (207), and develop into adenocarcinoma (207–209), while the Clara cell lineage secretes anti-inflammatory proteins and reduced with smoking, and may be the precursor to both squamous cell lung cancer and adenocarcinomas (206,211,212). These cell types also have different carcinogen-metabolizing capacities (205,206,212–214). For example, more proximally located Clara cells have a greater ability to metabolize the carcinogen benzo(a)pyrene than type II pneumocytes, but the opposite occurs for TSNAs, although both cell types metabolize both carcinogens (205,215–217). Further, suggestive experimental animal studies indicate that NNK induces peripheral lung adenomas (219,220), while PAHs are more likely to induce central squamous cell tumors, although not exclusively (220–227). While there is some evidence that the Clara cells have more DNA damage than type II pneumocytes following exposure to NNK, the alveolar regions have more cell proliferation and tumors (215,223).
There are two prospective human studies assessing TSNA exposure and lung cancer risk (228–230). Neither considers filter ventilation in its analysis, but each provides important support for the relationship of TSNAs and lung cancer risk. In a case-control study nested within the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Church et al. reported that a 1 standard deviation increase in urinary total NNAL (a metabolite of NNK) was associated with a 57% (95% CI = 8% to 128%) increased risk of lung cancer (229). When analyzed by lung cancer histology, the association of urinary NNAL with the risk of lung adenocarcinoma was statistically significant, but the results for other lung histologies combined were elevated, but not statistically significant. The second study, using the Shanghai Cohort Study and the Singapore Chinese Study, also showed an overall increased lung cancer risk with higher levels of NNK exposure (230,231); data for specific cancer subtypes were not provided because histological confirmation was not done for many subjects.
In summary, experimental animal studies indicate that the distal airways may be more sensitive to NNK than the proximal regions of the lung. Limited human cohort studies identify NNK as contributing to lung cancer risk, particularly for adenocarcinomas. Given that filter ventilation increases NNK (as do other factors) and that larger puffs of smoke with higher NNK levels can reach the distal airways, along with other toxicants, these studies add to the biological plausibility for a relationship of filter ventilation to increased lung adenocarcinoma.
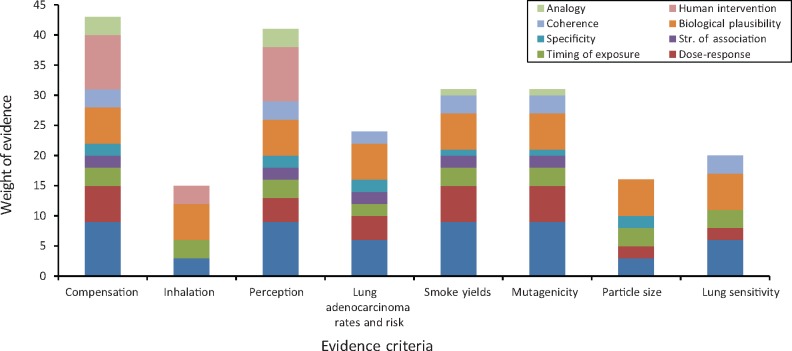
Weight of Evidence Review and Causation Analysis
This weight of evidence review broadly uses three groups of evidence, namely laboratory experimental data, human smoking behavior studies, and the epidemiology of lung cancer. Table 1 and Figure 7 summarize our weight-of-evidence review in terms of the consistency of evidence, evidence of dose-response, temporality of exposure, strength of association, specificity of the evidence, and other causal criteria as they relate to the relationship of filter ventilation causing an increased risk of lung adenocarcinoma. In this analysis, human studies are given the greatest weight (clinical trials > cross-sectional studies; prospective studies with lung cancer outcomes for variation in ventilation would be given the greatest weight, but none directly exist), while experimental animal and toxicology studies are useful to support mechanistic associations because the direct extrapolation from the laboratory to human risk is not possible.
Table 1.
Causation analysis for filter ventilation leading to lung adenocarcinomas
| Criteria | Human smoking behavior: compensation (puff topography and/or biomarker studies), increased, inhalation, and perception* | |
|---|---|---|
| Consistency | Compensation: +++ | Both clinical trials and cross-sectional studies demonstrate compensation, although human biomarker studies using urine and blood assays indicate that circulating exposures may not be increased |
| Inhalation: + | Available studies use methodologies that are not validated, and methodologies were used differently across studies, precluding an assessment of consistency | |
| Perception: +++ | Consumer perception studies indicate that the feelings of “lighter smoke” provide a sense of health benefit | |
| Dose response | Compensation: +++ | Increasing filter ventilation increases compensatory response |
| Inhalation: IA | Insufficient study of increasing ventilation on inhalation parameters | |
| Perception: ++ | Consumer perception studies indicate that the feelings of “lighter smoke” provide a sense of health benefit, but assessing degree of response based on amount of ventilation has received limited study | |
| Timing of exposure | Compensation: +++ | Filter ventilation precedes the effect in experimental and observational studies |
| Inhalation: +++ | Filter ventilation precedes the effect in experimental and observational studies | |
| Perception: +++ | Filter ventilation precedes the effect in experimental and observational studies | |
| Strength of association | Compensation: ++ | Degree of compensation commensurate with dose response |
| Inhalation: IA | Inadequate study using methods that are not validated or consistent across studies | |
| Perception: ++ | Magnitude of effect less clear based on degree of filter ventilation | |
| Specificity | Compensation: ++ | Filter ventilation causes compensation, but other cigarette design changes also may cause compensation; filter ventilation is the principal way of reducing smoking machine tar yields for conventional cigarettes |
| Inhalation: IA | Inadequate study using methods that are not validated or consistent across studies and, other factors that affect nicotine yields may affect inhalation | |
| Perception: ++ | “Lighter” smoke leads to false perceptions of health benefits, but other branding could cause misperceptions | |
| Biological plausibility | Compensation: +++ | Reduction in nicotine yield by filter ventilation causes smokers to compensate their puffing to address nicotine dependence |
| Inhalation: +++ | Reduction in nicotine yield by filter ventilation causes smokers to compensate their inhalation to address nicotine dependence | |
| Perception: +++ | “Lighter” smoke reduces sensory effects, leading to false perception of health benefit | |
| Coherence | Compensation: +++ | Experimental laboratory methods consistent with human studies (trials and cross-sectional studies), although other methods to lower nicotine yields may also affect compensation; human biomarker studies using urine and blood assays indicate that circulating exposures may not be increased |
| Inhalation: IA | Laboratory studies cannot assess this; inadequate study of filter ventilation for experimental human and observational studies | |
| Perception: +++ | Experimental human studies consistent with cross-sectional studies | |
| Human interventions | Compensation: +++ | Clinical trials demonstrate compensation |
| Inhalation: + | Inconsistent results for human clinical trials | |
| Perception: +++ | Clinical trials (human laboratory studies) demonstrate the sensation of a “lighter” smoke, leading to false perceptions of a health benefit | |
| Analogy | Compensation: +++ | Other methods to lower nicotine yields may affect compensation |
| Inhalation: IA | There has been inadequate study of various cigarette designs that may affect inhalation | |
| Perception: +++ | Flavors and branding/marketing with claims for health benefits also lead to a false perception of a health benefit | |
| Increased lung adenocarcinoma risk and rates | ||
| Consistency | ++ | Numerous ecological and observational cohort studies are consistent for lower tar yields, leading to increased lung adenocarcinoma, but the direct assessment of filter ventilation on risk has not been done, nor is it feasible |
| Dose response | ++ | Lower tar yield cigarettes lead to increased risk and rates of lung adenocarcinomas, but there has been insufficient study and consistency by levels of filter ventilation, eg, risk across the full spectrum of tar and nicotine yields, including “ultralight” cigarettes |
| Timing of exposure | ++ | Rise in adenocarcinomas coincident with use of filter ventilation, but latency suggests that additional factors may contribute |
| Strength of association | ++ | Large rates and risk changes with use of cigarettes manufactured after the introduction of filter ventilation |
| Specificity | ++ | Other cigarette design changes such as increased nitrates and changes in tobacco blend also likely contribute |
| Biological plausibility | +++ | Changes in the way tobacco burns and smoke yields are consistent with the effects on location and cell sensitivity for lung adenocarcinomas |
| Coherence | ++ | Experimental animal studies indicate that NNK causes lung adenocarcinomas; other design changes increasing NNK could also cause increased lung adenocarcinoma risk |
| Human interventions | IA | Not feasible to test |
| Analogy | IA | Nitrates and tobacco blends could alter NNK yields, which cannot be separately tested from filter ventilation given that both design features changed simultaneously |
| Experimental data: increased chemical yields including NNK, increased mutagenicity, change in particle size, and sensitivity to NNK for distal lung cells | ||
| Consistency | Smoke constituent yields: +++ | Multiple published and unpublished studies demonstrate the effect for increasing chemical yields per mg/tar and per mg/nicotine, including TSNAs |
| Mutagenicity: +++ | Multiple published and unpublished studies demonstrate this adverse effect; tumorigenicity effects have not been studied | |
| Particle size: + | Multiple studies indicate an effect on particle size, including increased water content; how this affects smoke distribution in the lung is unclear | |
| Distal airway lung sensitivity: + | Epidemiology studies show increased risks and rates for adenocarcinoma with lowering tar yields over time, and adenocarcinomas occur more frequently in the lung periphery, where experimental studies show that the lung cells are more sensitive to NNK | |
| Dose-response | Smoke chemical yields: +++ | Numerous studies show that increasing levels of filter ventilation cause a dose-response increase in chemical yields, including NNK, per mg of tar or nicotine |
| Mutagenicity: +++ | Numerous studies show increasing levels of filter ventilation cause a dose-response increase in Ames mutagenicity | |
| Particle size: + | Increased ventilation increases particle size and water content, although how this affects lung distribution is unclear mechanistically | |
| Distal airway lung sensitivity: + | This has not been directly studied, although there is a dose-response effect for NNK and lung adenocarcinoma in experimental animal studies | |
| Timing of exposure | Smoke chemical yields: +++ | Laboratory experimental studies directly assess exposures leading to outcomes |
| Mutagenicity: +++ | Laboratory experimental studies directly assess exposures leading to outcomes | |
| Particle size: +++ | Laboratory experimental studies directly assess exposures leading to outcomes | |
| Distal airway lung sensitivity: +++ | Laboratory experimental studies directly assess exposures leading to outcomes | |
| Strength of association | Smoke chemical yields: ++ | Increased chemical yields cannot be extrapolated to human risk |
| Mutagenicity: ++ | Increased mutagenicity cannot be extrapolated to human risk | |
| Particle size: IA | There has been inadequate study for understanding the magnitude of effect on particle size and smoke distribution, so that extrapolation to human risk is not possible | |
| Distal airway lung sensitivity: IA | There has been inadequate study for understanding the magnitude of effect on lung cell sensitivity, so that extrapolation to human risk is not possible | |
| Specificity | Smoke chemical yields: + | Other cigarette design parameters affect smoke chemistries, and some chemical constituents can be directly transferred from tobacco |
| Mutagenicity: + | Other cigarette design parameters affect Ames mutagenicity | |
| Particle size: ++ | Other cigarette design parameters affect particle size and lung distribution | |
| Distal airway lung sensitivity: IA | Other cigarette design parameters affect lung sensitivity, such as those that also increase NNK | |
| Biological plausibility | Smoke chemical yields: +++ | Changes in the way the tobacco column is burned increase incomplete combustion and burn time |
| Mutagenicity: +++ | Changes in the way the tobacco column is burned increase incomplete combustion and burn time, affecting yields that increase mutagenicity | |
| Particle size: +++ | Changes in the way the tobacco column is burned increase chemical constituents in the particles and water content | |
| Distal airway lung sensitivity: +++ | Peripheral lung cells in experimental animals metabolize NNK differently and have related carcinogenic effects | |
| Coherence | Smoke chemical yields: +++ | Multiple experimental studies demonstrate the effect on how tobacco is burned and increased per-nicotine yields of many constituents including NNK |
| Mutagenicity: +++ | Multiple experimental studies demonstrate the effect on how tobacco is burned and increased levels of some constituents' formation of chemicals that can increase mutagenicity | |
| Particle size: IA | Other cigarette designs affecting particle size have not been adequately studied | |
| Distal airway lung sensitivity: +++ | Multiple experimental studies indicate the metabolizing effects and the induction of tumors by NNK | |
| Human interventions | Not applicable | |
| Analogy | Smoke chemical yields: + | Other design changes, such as filter efficiency, can affect chemical yields, but this also is affected by filter ventilation |
| Mutagenicity: + | Other design changes, such as filter efficiency, can affect mutagenicity, but this also is affected by filter ventilation | |
| Particle size: IA | Inadequate study | |
| Distal airway lung sensitivity: IA | Inadequate study | |
Grading of evidence based on scientific judgment: no relationship (0), limited evidence (+), strongly suggestive evidence ( ++), sufficient evidence ( +++), or inadequate study (IA). NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; TSNAs = tobacco-specific nitrosamines.
Figure 7.
Evidence blocks for causation analysis. Grading of evidence showing the confidence in the weighting, from 0 to 45, based on scientific judgment: no relationship (0), limited evidence (+), strongly suggestive evidence ( ++), sufficient evidence ( +++), or inadequate study (IA); the taller the block, the higher the level of evidence. Both IA and no relationship are treated as 0 and do not appear in blocks. Some criteria are weighted more heavily than others, as follows: Consistency and human intervention are adjusted for the greatest weight (factor of 3), dose response and biological plausibility are adjusted for a medium weight (factor of 2) and the others are unadjusted.
A mode of action and human relevance framework also was applied, which is summarized in Supplementary Table 3 (available online). In addition to what is identified for Table 1 and Figure 7, this framework also identifies what data may be inconsistent with a causal relationship and also what data are missing, for example a future research agenda.
Consistency
There is consistency within evidence categories among the experimental data, human behavior studies, and lung cancer epidemiology (with the exception of filter ventilation affecting inhalation and smoke distribution). Numerous studies from the tobacco industry and academia indicate that filter ventilation, in spite of decreasing tar yields using standardized smoking machine methods on a per-cigarette basis, increases the generation of smoke toxicants, carcinogens, and mutagens on a per-mg-of-tar-and-nicotine basis. Smoking behavior and exposures are clearly affected by smoking machine nicotine yields, such that smokers of low–nicotine yield cigarettes demonstrate an increase in puffing behavior due to the elasticity of the cigarette filter ventilation. This is borne out by both clinical trials and cross-sectional studies (see Supplementary Tables 5 and 6, available online). The effects of filter ventilation on depth of smoke inhalation are less clear for consistency or show no effect. However, the methodologies to assess depth of inhalation and particle deposition are not well developed, largely rely on methods that have not been validated, use statistical modeling that also is not validated, and do not consider gases and inhaling more smoke. Studies of smoke particle distribution consistently show increased size with ventilation (see Supplementary Table 4, available online). It should be noted that increased depth of inhalation may not be required to alter regional distribution and adenocarcinoma risk because smokers either way are increasing the amount of smoke inhaled into the lungs because of larger puff volumes.
The research data indicating the shift from squamous cell cancers to adenocarcinomas have been replicated among studies, concurrent in time with lowering tar yields and the use of filter ventilation. Other data indicate that lung cancer risk from smoking more modern cigarettes has increased over time, by considering birth cohorts of men separately from women. It is not possible to directly assess the impact of cigarette design on lung cancer risk because almost all cigarettes on the market simultaneously decreased tar yields and increased filter ventilation, although, limited prospective data associate an increase in TSNA exposure with adenocarcinomas. It is not possible at this time to assess lung cancer risk from the highest ventilated filters (“ultralights”) because these were only introduced to the marketplace in the late 1990s, so that there has not been enough latency to observe a change in risk, and relatively few smokers smoke these cigarettes.
Dose Response
There is consistency among experimental studies for increasing filter ventilation, resulting in increased toxicant yields and mutagens. Increasing filter ventilation affects smoking behavior and increases puff volumes, but the effects on inhalation are less clear and not studied based on levels of ventilation. Human studies of smoking behavior do not show increased biomarker levels with lower-tar cigarettes, and some biomarkers may decrease, but these do not assess regional lung exposure. Temporal trends of decreasing tar yields and decreasing cigarette smoking rates, concurrent with increasing risks based on birth cohorts that progressively imitate the use of ventilated filter cigarettes, are consistent with a dose-response effect, although studies directly assessing filter ventilation or risks by tar yields are not available.
Biological Plausibility and Coherence
These two criteria are met given the full range of studies from the laboratory to population-level surveillance, which provides important scientific support, although there is some uncertainty relating to human biomarker exposure studies. How filter ventilation increases tobacco toxicant yield, mutagenicity, and particle size is understood. The elasticity of filter ventilation allows for increasing puffing behavior, allowing for more of the toxicants to enter the lungs, which then exposes distal airway lung cells that are more sensitive to NNK and the development of adenocarcinomas. Coherence comes from experimental studies of specific tobacco toxicants and animal tumorigenesis, as well as mutagenicity and other cell culture studies. Human studies using biomarkers of exposure showing similar levels of exposure for smokers of cigarettes with different degrees of ventilation present some uncertainty, and while these studies are consistent with each other, they present an argument against coherence. The human studies indicate, however, that there is no difference rather than a beneficial effect. As noted above, the human studies using urine and blood biomarkers may not reflect exposures at the target organ level (ie the lung) where lung adenocarcinomas occur, and they do not use validated biomarkers of harm. Thus, this area is an important research gap to address.
Specificity
While there is highly suggestive evidence to conclude that filter ventilation has increased the rates of lung adenocarcinoma, there are other potential causes. As noted in the 2014 SGR, in addition to filter ventilation, there is suggestive evidence that increased levels of TSNAs over time also could explain the increased adenocarcinoma risks. However, one mechanism does not preclude the other, and both may be contributing (232); filter ventilation further increases NNK levels on a per-mg-of-tar-and-nicotine basis. Higher levels of NNK and other TSNAs in cigarette smoke can be driven by their increases in tobacco filler as a result of changes in tobacco blend content (eg increasing burley tobacco content), increase in nitrate content, and changes in microbial contamination (16,1,46,231–236). While most NNK yields in smoke happen as a direct transfer from tobacco, additional amounts may also be formed during tobacco burning, with nitrate-rich tobaccos potentially generating higher levels of NNK (238). While filter ventilation influences NNK levels less than changing tobacco leaf blends filter ventilation also increases other toxicant exposures.
Other Criteria
Several other causation criteria are met, although the emphasis of these is less, such as timing of exposure, where all the clinical trials and experimental studies demonstrate effects after exposure (or lack of effects); strength of association, which is inferred in some cases because the totality of the data indicates significant strength to cause a measurable change in adenocarcinoma rates and risks; and analogy, such as experimental animal studies using specific smoke constituents, such as NNK.
Discussion
This weight of evidence review and causation analysis strongly suggests that the inclusion of ventilation in cigarette filters has contributed to increased lung adenocarcinomas among smokers. There are some uncertainty and research gaps as noted below, including the potential lack of coherence between mechanistic smoking machine yields and human exposure biomarker studies (the machine studies indicate the potential for increased exposure while the human studies indicate no difference). Thus, it should not be concluded that there is sufficient evidence for causality, rather that this review leads to a conclusion that the data is highly suggestive. Importantly, the weight of evidence does not indicate a public health benefit for the inclusion of filter ventilation. The smoke from cigarettes with ventilated filters provides false perceptions to the smoker of reduced harmfulness. Filter ventilation affects how the tobacco burns, smoking behavior, and how the lung is exposed to carcinogens, so that it plausibly contributes to the increased adenocarcinomas by a cigarette that the smoker falsely believes is less harmful. Epidemiologic data provide indirect evidence for filter ventilation as a contributing factor to the increased lung adenocarcinoma rates and risks.
The FDA has the regulatory authority to issue cigarette “product standards” (Section 907(a))(4), regulating the “construction,” “components,” or “properties” of tobacco products. To do this, the FDA must have evidence that a product standard would be “appropriate for the protection of public health.” Based on the findings from this weight-of-evidence review, we would recommend that the FDA ask cigarette manufacturers to provide clear and convincing evidence that there is a public health benefit gained by filter ventilation in filter design and that the benefits outweigh any health risks. Absent such clear and convincing evidence from any source, the FDA should consider adopting a standard to prohibit filter ventilation. Given that there are cigarettes with 0% ventilation already on the market in the United States and elsewhere, the tobacco industry can feasibly implement this change (72,238–240).
While there may be other cigarette design features that have contributed to the risk of smoking and the rise of adenocarcinomas compared with squamous cell cancers (232), it is our belief, based on the evidence reviewed herein, that filter ventilation has contributed to at least some of the increased risk. It should be noted that an FDA action regulating filter ventilation would not imply that filter ventilation is the only or most important cigarette design to impact lung cancer risk, and a filter ventilation standard could be adopted alone or in conjunction with other product standards, for example addressing NNK exposure or other aspects of cigarette design that contribute to addiction and disease risk. If the FDA prohibits filter ventilation, it may issue complementary regulations that restrict other design methods that reduce exposures, for example using higher amounts of expanded tobaccos, decreasing rod length, using tobacco strains and curing methods to reduce TSNA formation, and using highly activated carbon filters, so long as the FDA has concluded that these other regulations would not adversely affect smoking behavior (16,242,243).
Using the SEER 9 database, we calculated the yearly age-adjusted incidence rates for adenocarcinoma, squamous cell carcinoma, and total lung cancer cases for men between 1975 and 2012. Using the CDC WONDER Population Projections (http://wonder.cdc.gov/population.html), and we computed the number of new adenocarcinomas and squamous cell cases in the overall US male population for the years 2008 to 2012. We found an excess of 32 400 adenocarcinoma cases when compared with squamous cell carcinomas (data not shown). While this may not be solely attributable to filter ventilation, this represents an adverse public health impact. Equally important, there is no existing evidence that filter ventilation reduces lung cancer risk or has any other beneficial health effect that would argue against regulation.
There are important research gaps that have been identified, including the reconciliation for coherence of human biomarker studies showing no increased exposure for smokers using cigarettes with higher degrees of ventilation and patterns of inhalation and smoke distribution in the lung. These would need to be addressed by lung biomarker studies, including biomarkers of harm, for example, using bronchoscopy to collect biospecimens as smokers switch from ventilated cigarettes to unventilated cigarettes. Importantly, prior to the regulation of filter ventilation, the FDA also will need to assess possible unintended effects of regulating filter ventilation, including a ban, for example increasing smoking initiation, delaying cessation due to perceptions that these are safer cigarettes, and that these would not likely outweigh the benefits. To date, there are no studies on the impact of removing filter ventilation on smoking behavior and perceptions, the addictiveness of unventilated cigarettes, and the resultant exposures and toxicity. This and other data gaps are indicated in Supplementary Table 3 (available online). If ventilation were removed from cigarette filters, we expect three possible results: 1) that toxic exposure will be decreased because the cigarette delivery is no longer elastic, limiting the ability of the smoker to compensate with larger puff volumes; 2) the greater amount of nicotine in smoke will result in the smoker decreasing the number of cigarettes per day and less smoke will enter the lungs; and 3) some smokers may quit smoking or transition to alternative nicotine delivery systems such as electronic cigarettes or nicotine replacement therapy because of the harshness of the cigarette smoke and perceptions of a more harmful smoke. To assess this, a combination of human and experimental animal studies could be conducted in the context of the conceptual framework for tobacco product evaluation (244). For example, clinical trials could assess smokers switching to filtered cigarettes without ventilation and with different packaging and study smoking topography, inhalation depth, and biomarkers of nicotine exposure and smoke toxicants. Because no single biomarker is available that can be used alone to predict the reduction in harm from smoking (ventilated vs nonventilated cigarettes), a panel of biomarkers of exposure to carcinogens and lung toxicants, markers of oxidative damage and inflammation should be measured in lung, blood, or/and urine. The studies also should assess smokers’ perceptions, appeal, and transition to alternate products, for example electronic cigarettes and nicotine replacement therapy (behavioral economics and abuse liability). Studies would need to be done in ways to assess differing effects by race and ethnicity, gender, age, and vulnerable populations to inform the potential population-level effects. Experimental animal studies that allow for manipulations in both adolescent and adult rodents would parallel the human trials to provide evidence for the impact on smoking initiation. Another research agenda item could focus on the impact of filter ventilation on the risk of other diseases (eg chronic obstructive pulmonary disease), given shared etiologies due to tobacco toxicants. Such research would provide additional support for an FDA regulatory action.
In conclusion, the use of ventilation in the filters of cigarettes has failed to make cigarettes safer, and more than likely has made them more harmful. There is no demonstrated public health benefit, and smokers perceive these as less harmful, which in turn encourages smoking and causes harm. The FDA now has the authority to require the elimination of filter ventilation because ventilation does not serve any public health purpose and instead provides a false promise of reduced risk. This single action for banning filter ventilation by the FDA is scientifically justified and within its mandate to improve the public health. Based on these weight-of-evidence reviews, the FDA should embark on a regulatory process of data evaluation and consider regulation(s) for the use of ventilation in filters, up to and including a ban on their use.
Funding
Research reported in this publication was supported by grant number P50CA180908 from the National Cancer Institute of the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Notes
Dr. Cummings has received grant funding from the Pfizer, Inc. to study the impact of a hospital-based tobacco cessation intervention and Drs. Shields, Benowitz, Brasky, and Cummings have served as consultants and expert witnesses in litigation against tobacco companies. The other authors declare no conflict of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the writing of the review, data collection or analysis, conclusions, or decision to submit the review for publication. The authors appreciate the input and advice about this manuscript from Drs. William Farone (Applied Power Concepts, Inc., Anaheim, CA) and Irina Stepanov (University of Minnesota, Minneapolis, MN).
Supplementary Material
References
- 1.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- 2.IARC. Tobacco Smoke and Involuntary Smoking IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Geneva: Who Press; 2004:83. [PMC free article] [PubMed]
- 3. Borgerding MF, Bodnar JA, Wingate DE. The 1999 Massachusetts benchmark study; final report. 2000. Brown and Williamson Records. https://industrydocuments.library.ucsf.edu/docs/#id=rfdx0057.
- 4. Travis WD, Travis LB, Devesa SS.. Lung cancer. Cancer. 1995;75(suppl 1):191–202. [DOI] [PubMed] [Google Scholar]
- 5. Perng DW, Perng RP, Kuo BI, et al. The variation of cell type distribution in lung cancer: A study of 10 910 cases at a medical center in Taiwan between 1970 and 1993. Jpn J Clin Oncol. 1996;264:229–233. [DOI] [PubMed] [Google Scholar]
- 6. Thun MJ, Lally CA, Flannery JT, et al. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;8921:1580–1586. [DOI] [PubMed] [Google Scholar]
- 7. Charloux A, Quoix E, Wolkove N, et al. The increasing incidence of lung adenocarcinoma: Reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol. 1997;261:14–23. [DOI] [PubMed] [Google Scholar]
- 8. Percy C, Sobin L.. Surveillance, Epidemiology, and End Results lung cancer data applied to the World Health Organization's classifications of lung tumors. J Natl Cancer Inst. 1983;704:663–666. [PubMed] [Google Scholar]
- 9. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin. 2015;651:5–29. [DOI] [PubMed] [Google Scholar]
- 10. Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;3684:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thun MJ, Heath CW Jr.. Changes in mortality from smoking in two American Cancer Society prospective studies since 1959. Prev Med. 1997;264:422–426. [DOI] [PubMed] [Google Scholar]
- 12. Burns DM, Anderson CM, Gray N.. Has the lung cancer risk from smoking increased over the last fifty years? Cancer Causes Control. 2011;223:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burns DM, Anderson CM, Gray N.. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control. 2011;221:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;3287455:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monograph 7: The FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of U.S. cigarettes: Report of the NCI Expert Committee. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1996.
- 16. Monograph 13: Risks Associated with Smoking Cigarettes with Low Tar Machine-Measured Yields of Tar and Nicotine Bethesda: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2001.
- 17.Resnik F, Wakeham H, Wickham JE. Special report no. 223; summary of cigarette trends for 640000. 1964. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=mkkg0114.
- 18. Hoffmann D, Hoffmann I.. The changing cigarette, 1950-1995. J Toxicol Environ Health. 1997;504:307–364. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Prev Med. 1997;26(4):427–434. [DOI] [PubMed]
- 20. O'Connor RJ, Hammond D, McNeill A, et al. How do different cigarette design features influence the standard tar yields of popular cigarette brands sold in different countries? Tob Control. 2008;17 (suppl 1):i1–5. [DOI] [PubMed] [Google Scholar]
- 21. Stephens WE. Dependence of tar, nicotine and carbon monoxide yields on physical parameters: Implications for exposure, emissions control and monitoring. Tob Control. 2007;163:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Filter ventilation levels in selected U.S. cigarettes, 1997. MMWR Morb Mortal Wkly Rep.1997;46(44):1043–1047. [PubMed]
- 23. Kozlowski LT, Mehta NY, Sweeney CT, et al. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;74:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- 25. Jarvis MJ, Boreham R, Primatesta P, et al. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: Evidence from a representative population survey. J Natl Cancer Inst. 2001;932:134–138. [DOI] [PubMed] [Google Scholar]
- 26. Stratton K, Shetty P, Wallace R, et al. Clearing the smoke: The science base for tobacco harm reduction—executive summary. Tob Control. 2001;102:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. SACTob Recommendation On Health Claims Derived From ISO/FTC Method to Measure Cigarette Yield Geneva: WHO Scientific Advisory Committee on Tobacco Product Regulation; 2013.
- 28. Pollay RW, Dewhirst T.. The dark side of marketing seemingly “Light” cigarettes: Successful images and failed fact. Tob Control. 2002;11 (suppl 1):I18–I31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufman N, Nichter M. The marketing of tobacco to women: Global perspectives. In: Samet J, Yoon SY. Gender, Women and the Tobacco Epidemic: Challenges for the 21st Century Geneva: World Health Organization; 2001.
- 30. Pazo DY, Moliere F, Sampson MM, et al. Mainstream smoke levels of volatile organic compounds in 50 U.S. domestic cigarette brands smoked with the ISO and Canadian intense protocols. Nicotine Tob Res. 2016;189:1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mutti S, Hammond D, Borland R, et al. Beyond light and mild: Cigarette brand descriptors and perceptions of risk in the International Tobacco Control (ITC) Four Country Survey. Addiction. 2011;1066:1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gan Q, Lu W, Xu J, et al. Chinese ‘low-tar’ cigarettes do not deliver lower levels of nicotine and carcinogens. Tob Control. 2010;195:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mir H, Buchanan D, Gilmore A, et al. Cigarette pack labelling in 12 countries at different levels of economic development. J Public Health Policy. 2011;322:146–164. [DOI] [PubMed] [Google Scholar]
- 34. Kozlowski LT, O'Connor RJ.. Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control. 2002;11 (suppl 1):I40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kozlowski LT, O'Connor RJ, Giovino GA, et al. Maximum yields might improve public health--if filter vents were banned: A lesson from the history of vented filters. Tob Control. 2006;153:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Public Health Service. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. United States. Public Health Service. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Public Health Service Publication No. 1103. Rockville, MD Office of the Surgeon Genereal; 1964.
- 37. Hoffmann S, Hartung T, Stephens M.. Evidence-based toxicology. Adv Exp Med Biol. 2016;856:231–241. [DOI] [PubMed] [Google Scholar]
- 38. Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 39. Meek ME, Palermo CM, Bachman AN, et al. Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 2014;346:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Becker RA, Ankley GT, Edwards SW, et al. Increasing scientific confidence in adverse outcome pathways: Application of tailored Bradford-Hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol. 2015;723:514–537. [DOI] [PubMed] [Google Scholar]
- 41.The UCSF Tobacco Documents Bibliography, UCSF Library and Center for Knowledge Management. https://industrydocuments.library.ucsf.edu/tobacco/
- 42. Marian C, O'Connor RJ, Djordjevic MV, et al. Reconciling human smoking behavior and machine smoking patterns: Implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev. 2009;1812:3305–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baker RR, Massey ED, Smith G.. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem Toxicol. 2004;42 (suppl):S53–S83. [DOI] [PubMed] [Google Scholar]
- 44. Bialous SA, Yach D.. Whose standard is it, anyway? How the tobacco industry determines the International Organization for Standardization (ISO) standards for tobacco and tobacco products. Tob Control. 2001;102:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peeler CL, Butters GR.. Re: It's time for a change: Cigarette smokers deserve meaningful information about their cigarettes. J Natl Cancer Inst. 2000;9210:842–843. [DOI] [PubMed] [Google Scholar]
- 46. Hoffmann D, Djordjevic MV, Hoffmann I.. The changing cigarette. Prev Med. 1997;264:427–434. [DOI] [PubMed] [Google Scholar]
- 47.Filter ventilation levels in selected U.S. cigarettes, 1997. MMWR Morb Mortal Wkly Rep 1997;46(44):1043–1047. [PubMed]
- 48. Maxwell JC Jr. Historical sales trends in the cigarette industry: A statistical summary covering 69 years (1925-93). Richmond, VA: Wheat, First Securities, Inc.; 1994.
- 49. Reed MB, Anderson CM, Burns DM.. The temporal relationship between advertising and sales of low-tar cigarettes. Tob Control. 2006;156:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tar and nicotine deliveries. Sales weighted average basis. US Food and Drug Administration 1987. RJ Reynolds Records. https://industrydocuments.library.ucsf.edu/tobacco/docs/#id=lfbn0100.
- 51.Morie G, Baggett MS. Effect of filter ventilation on some physical and chemical properties of cigarette smoke. Tennessee Eastman Company (for RJ Reynolds). RJ Reynolds Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xlyv0140.
- 52. Altizer C, Bolanowski GJ,, Christopher RW, Goodwin CR Ventilation seminar. Philip Morris 1983. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=pxkv0119.
- 53. Yu T. In vitro biological activity of cigarette smoke condensates from experimental cigarettes -- a summary. Philip Morris 1984. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=qjyh0120.
- 54. Blakley RL. Facts, figures, and figuring on why air dilution increases ames TA98 specific (rev/mg) biological activity. RJ Reynolds 1990. RJ Reynolds Records. https://industrydocuments.library.ucsf.edu/docs/#id=qfll0097.
- 55. Baker RP. The effect of ventilation on cigarette combustion mechanics. RJ Reynolds Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=mmyg0083.
- 56.British American Tobacco Co Ltd. Bench level conference on cigarette ventilation. 1978. British American Tobacco Records. https://industrydocuments.library.ucsf.edu/docs/#id=kynl0203.
- 57.Heat treatment of tobacco. 1991. RJ Reynolds Records. https://industrydocuments.library.ucsf.edu/docs/#id=mnmw0084.
- 58. Leahy RJ. Ventilated cigarettes. 1955. Philip Morris Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=ftwh0045.
- 59. Lydia J, Holt LWR. The effect of ventilation on burn rates. 1994. Philip Morris Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=fmdy0002.
- 60. Bates C, McNeill A, Jarvis M, et al. The future of tobacco product regulation and labelling in Europe: Implications for the forthcoming European Union directive. Tob Control. 1999;82:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raker RR. Mechanisms of smoke formation and delivery. 1978. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=mghc0151.
- 62.Barker RR. Tobacco combustion. 1982. https://industrydocuments.library.ucsf.edu/docs/#id=knyy0131.
- 63.Cigarette smoke. 1978. Lorillard Records. https://industrydocuments.library.ucsf.edu/docs/#id=tgfc0116.
- 64. Adam T, McAughey J, Mocker C, et al. Influence of filter ventilation on the chemical composition of cigarette mainstream smoke. Anal Chim Acta. 2010;6571:36–44. [DOI] [PubMed] [Google Scholar]
- 65. Ferris R. The effect of ventilation on smoke deliveries. 1982. British American Tobacco Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=fkyf0202.
- 66.Filter ventilation systems. British American Tobacco Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=pnvj0208.
- 67. Harris JE. Incomplete compensation does not imply reduced harm: Yields of 40 smoke toxicants per milligram nicotine in regular filter versus low-tar cigarettes in the 1999 Massachusetts Benchmark Study. Nicotine Tob Res. 2004;65:797–807. [DOI] [PubMed] [Google Scholar]
- 68. Patskan GJ, Podraza KF, Meurrens K, et al. Toxicological comparisons of three styles of a commercial U.S. cigarette (Marlboro with the 1R4F reference cigarette). Inhal Toxicol. 2008;207:695–721. [DOI] [PubMed] [Google Scholar]
- 69. Roemer E, Stabbert R, Rustemeier K, et al. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;1951:31–52. [DOI] [PubMed] [Google Scholar]
- 70. Morton MJ, Laffoon SW.. Cigarette smoke chemistry market maps under Massachusetts Department of Public Health smoking conditions. Regul Toxicol Pharmacol. 2008;511:1–30. [DOI] [PubMed] [Google Scholar]
- 71. Swauger JE, Steichen TJ, Murphy PA, et al. An analysis of the mainstream smoke chemistry of samples of the U.S. cigarette market acquired between 1995 and 2000. Regul Toxicol Pharmacol. 2002;35(2 pt 1):142–156. [DOI] [PubMed] [Google Scholar]
- 72. Counts ME, Morton MJ, Laffoon SW, et al. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;413:185–227. [DOI] [PubMed] [Google Scholar]
- 73. Bodnar JA, Morgan WT, Murphy PA, et al. Mainstream smoke chemistry analysis of samples from the 2009 US cigarette market. Regul Toxicol Pharmacol. 2012;641:35–42. [DOI] [PubMed] [Google Scholar]
- 74. Johnson MD, Schilz J, Djordjevic MV, et al. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol Biomarkers Prev. 2009;1812:3263–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Crooks I, Scott K, Dalrymple A, et al. The combination of two novel tobacco blends and filter technologies to reduce the in vitro genotoxicity and cytotoxicity of prototype cigarettes. Regul Toxicol Pharmacol. 2015;713:507–514. [DOI] [PubMed] [Google Scholar]
- 76. Scott K, Saul J, Crooks I, et al. The resolving power of in vitro genotoxicity assays for cigarette smoke particulate matter. Toxicol In Vitro. 2013;274:1312–1319. [DOI] [PubMed] [Google Scholar]
- 77. Booker J, Gannon WF, Kuhn WF, Minor M, Neal WK, Pages RH. Biological effects of smoke the bioactivity of 1r1 and doad whole smoke condensates in the salmonella typhimurium mutation assay. 1975. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=hphf0130.
- 78. Roemer E, Ottmueller TH, Zenzen V, et al. Cytotoxicity, mutagenicity, and tumorigenicity of mainstream smoke from three reference cigarettes machine-smoked to the same yields of total particulate matter per cigarette. Food Chem Toxicol. 2009;478:1810–1808. [DOI] [PubMed] [Google Scholar]
- 79. Rice WY, Sensabaugh AJ, Ridings HT, Raker ML. Project ran i. Impact of smoking conditions and cigarette configuration on specific ames activity of CSC. 1986. RJ Reynolds Records. https://industrydocuments.library.ucsf.edu/docs/#id=pzgv0079.
- 80.A MH, T F. E7 - in vitro mutagenicity of mainstream smoke condensate of 30 research cigarettes with differences in 6 parameter. 1995. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=hrxb0096.
- 81.Mutagenicity of mainstream smoke condensate of 30 research cigarettes with differences in 6 parameters. 1993. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=thcc0126.
- 82. Kane DB, Asgharian B, Price OT, et al. Effect of smoking parameters on the particle size distribution and predicted airway deposition of mainstream cigarette smoke. Inhal Toxicol. 2010;223:199–209. [DOI] [PubMed] [Google Scholar]
- 83. McCusker K, Hiller FC, Wilson JD, et al. Aerodynamic sizing of tobacco smoke particulate from commercial cigarettes. Arch Environ Health. 1983;384:215–218. [DOI] [PubMed] [Google Scholar]
- 84. Ingebrethsen BK. Evolution of the particle size distribution of mainstream cigarette smoke during a puff. Aerosol Sci Technol. 1986;5:423–433. [Google Scholar]
- 85. Creamer RM. The effects of puff velocity, rod lengths and filters on smoke formation and particle size. Philip Morris Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hqwh0045.
- 86. MacRae DD. R107; the physical and chemical nature of tobacco smoke. 1990. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=mycc0096.
- 87. Batuke F-R. Measurement of smoke particle characteristics as influenced by filter ventilation. 1990. Brown and Williamson Records. https://industrydocuments.library.ucsf.edu/docs/#id=yzhj0141.
- 88. Wayne GF, Connolly GN, Henningfield JE, et al. Tobacco industry research and efforts to manipulate smoke particle size: implications for product regulation. Nicotine Tob Res. 2008;104:613–625. [DOI] [PubMed] [Google Scholar]
- 89. Hammond D, Fong GT, Cummings KM, et al. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;146:1370–1375. [DOI] [PubMed] [Google Scholar]
- 90. Battig K, Buzzi R, Nil R.. Smoke yield of cigarettes and puffing behavior in men and women. Psychopharmacology (Berl). 1982;762:139–148. [DOI] [PubMed] [Google Scholar]
- 91. Anderson SJ, Ling PM, Glantz SA.. Implications of the federal court order banning the terms “light” and “mild”: what difference could it make? Tob Control. 2007;164:275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl). 1999;1451:1–20. [DOI] [PubMed] [Google Scholar]
- 93. Ryan FJ, Hancock B. Puffing behavior on high and low delivery cigarettes. 1973. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=jkxb0040.
- 94.Technical report on “‘tar’” and nicotine. 196. 6. RJ Reynolds Records. https://industrydocuments.library.ucsf.edu/docs/#id=tscn0101.
- 95. Graham S, Crouch S, Levin ML, et al. Variations in Amounts of Tobacco Tar Retrieved from Selected Models of Smoking Behavior Simulated by Smoking Machine. Cancer Res. 1963;23:1025–30. [PubMed] [Google Scholar]
- 96. Kozlowski LT, Pope MA, Lux JE.. Prevalence of the misuse of ultra-low-tar cigarettes by blocking filter vents. Am J Public Health. 1988;786:694–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nil R, Buzzi R, Battig K.. Effects of different cigarette smoke yields on puffing and inhalation: is the measurement of inhalation volumes relevant for smoke absorption? Pharmacol Biochem Behav. 1986;243:587–595. [DOI] [PubMed] [Google Scholar]
- 98. Woodman G, Newman SP, Pavia D, et al. Response and acclimatisation of symptomless smokers on changing to a low tar, low nicotine cigarette. Thorax. 1987;425:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lombardo T, Davis CJ, Prue DM.. When low tar cigarettes yield high tar: cigarette filter ventilation hole blocking and its detection. Addict Behav. 1983;81:67–69. [DOI] [PubMed] [Google Scholar]
- 100. Kozlowski LT, Frecker RC, Khouw V, et al. The misuse of ‘less-hazardous’ cigarettes and its detection: hole-blocking of ventilated filters. Am J Public Health. 1980;7011:1202–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sweeney CT, Kozlowski LT.. Blocking filter vents increases carbon monoxide levels from ultralight, but not light cigarettes. Pharmacol Biochem Behav. 1998;593:767–773. [DOI] [PubMed] [Google Scholar]
- 102.United States v. Philip Morris, Inc. D.D.C. 2006)(Appeal Pending) For Detailed Findings Regarding the Tobacco Industry’s Decades Long Fraudulent and Deceptive Marketing of “Low-Tar” and “Low Nicotine” Cigarettes. Washington, DC: United States District Court; 2006; 449F(suppl 2d 1):430–561.
- 103.US Food and Drug Administration. Family Smoking Prevention And Tobacco Control Act. Public law 111-31 [H.R. 1256]; 2009.
- 104. Djordjevic MV, Stellman SD, Zang E.. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;922:106–111. [DOI] [PubMed] [Google Scholar]
- 105. Hammond D, Fong GT, Cummings KM, et al. Cigarette yields and human exposure: a comparison of alternative testing regimens. Cancer Epidemiol Biomarkers Prev. 2006;158:1495–1501. [DOI] [PubMed] [Google Scholar]
- 106. Kelley M GP, Kelley Mf Jr, Meyer Lf. Reduced CO Marlboro 85 versus Marlboro 85 special report. 2012. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=pgdv0184.
- 107. Wakeham HD. Some unexpected observations on tar and nicotine and smoker behavior. 1974. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=ztmw0040.
- 108. Goodman BL. Changes in smoker profiles with changes in nicotine and tar deliveries, both on and off smoking profile recorders. 1977. Filter Ventilation and Design MSA Collection. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=qpwh0045.
- 109. Goodman BL. Report title: The influence of dilution on smoker parameters. 1975. https://www.industrydocumentslibrary.ucsf.edu/docs/#id=tlfh0045.
- 110.Marlboro-marlboro lights study delivery data. 1975. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=mghh0045.
- 111. Goodman B. Memo from B. Goodman to l.F. Meyer re: Effect of reduced dilution on tar delivery to a smoker. 2003. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=ngdv0184.
- 112. Benowitz NL, Jacob P 3rd, Yu L, et al. Reduced tar, nicotine, and carbon monoxide exposure while smoking ultralow- but not low-yield cigarettes. JAMA. 1986;2562:241–246. [PubMed] [Google Scholar]
- 113. Mendes P, Kapur S, Wang J, et al. A randomized, controlled exposure study in adult smokers of full flavor Marlboro cigarettes switching to Marlboro Lights or Marlboro Ultra Lights cigarettes. Regul Toxicol Pharmacol. 2008;513:295–305. [DOI] [PubMed] [Google Scholar]
- 114. Hatsukami DK, Hanson K, Briggs A, et al. Clinical trials methods for evaluation of potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;1812:3143–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Frost C, Fullerton FM, Stephen AM, et al. The tar reduction study: randomised trial of the effect of cigarette tar yield reduction on compensatory smoking. Thorax. 1995;5010:1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Robinson JC, Young JC, Rickert WS, et al. A comparative study of the amount of smoke absorbed from low yield (‘less hazardous’) cigarettes. Part 2: Invasive measures. Br J Addict. 1983;781:79–87. [DOI] [PubMed] [Google Scholar]
- 117. Robinson JC, Young JC, Rickert WS.. A comparative study of the amount of smoke absorbed from low yield (‘less hazardous’) cigarettes. Part 1: Non-invasive measures. Br J Addict. 1982;774:383–97. [DOI] [PubMed] [Google Scholar]
- 118. Zacny JP, Stitzer ML.. Cigarette brand-switching: effects on smoke exposure and smoking behavior. J Pharmacol Exp Ther. 1988;2462:619–27. [PubMed] [Google Scholar]
- 119. Rees VW, Wayne GF, Connolly GN.. Puffing style and human exposure minimally altered by switching to a carbon-filtered cigarette. Cancer Epidemiol Biomarkers Prev. 2008;1711:2995–3003. [DOI] [PubMed] [Google Scholar]
- 120. Benowitz NL, Jacob P 3rd, Kozlowski LT, et al. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;31521:1310–3. [DOI] [PubMed] [Google Scholar]
- 121. Benowitz NL, Jacob P 3rd, Bernert JT, et al. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;146:1376–83. [DOI] [PubMed] [Google Scholar]
- 122. McKinney DL, Frost-Pineda K, Oldham MJ, et al. Cigarettes with different nicotine levels affect sensory perception and levels of biomarkers of exposure in adult smokers. Nicotine Tob Res. 2014;167:948–60. [DOI] [PubMed] [Google Scholar]
- 123.Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. Smoking and Tobacco Control Monograph No. 13. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2001.
- 124. Bernert JT, Jain RB, Pirkle JL, et al. Urinary tobacco-specific nitrosamines and 4-aminobiphenyl hemoglobin adducts measured in smokers of either regular or light cigarettes. Nicotine Tob Res. 2005;75:729–38. [DOI] [PubMed] [Google Scholar]
- 125. Scherer G, Engl J, Urban M, et al. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;472:171–83. [DOI] [PubMed] [Google Scholar]
- 126. Woodward M, Tunstall-Pedoe H.. Self-titration of nicotine: evidence from the Scottish Heart Health Study. Addiction. 1993;886:821–30. [DOI] [PubMed] [Google Scholar]
- 127. Coultas DB, Stidley CA, Samet JM.. Cigarette yields of tar and nicotine and markers of exposure to tobacco smoke. Am Rev Respir Dis. 1993;1482:435–40. [DOI] [PubMed] [Google Scholar]
- 128. Gori GB, Lynch CJ.. Analytical cigarette yields as predictors of smoke bioavailability. Regul Toxicol Pharmacol. 1985;53:314–26. [DOI] [PubMed] [Google Scholar]
- 129. Russell MA, Sutton SR, Iyer R, et al. Long-term switching to low-tar low-nicotine cigarettes. Br J Addict. 1982;772:145–58. [DOI] [PubMed] [Google Scholar]
- 130. Nakazawa A, Shigeta M, Ozasa K.. Smoking cigarettes of low nicotine yield does not reduce nicotine intake as expected: a study of nicotine dependency in Japanese males. BMC Public Health. 2004;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Andersson G, Vala EK, Curvall M.. The influence of cigarette consumption and smoking machine yields of tar and nicotine on the nicotine uptake and oral mucosal lesions in smokers. J Oral Pathol Med. 1997;263:117–23. [DOI] [PubMed] [Google Scholar]
- 132. Blackford AL, Yang G, Hernandez-Avila M, et al. Cotinine concentration in smokers from different countries: relationship with amount smoked and cigarette type. Cancer Epidemiol Biomarkers Prev. 2006;1510:1799–804. [DOI] [PubMed] [Google Scholar]
- 133. Woodward M, Tunstall-Pedoe H.. Do smokers of lower tar cigarettes consume lower amounts of smoke components? Results from the Scottish Heart Health Study. Br J Addict. 1992;876:921–8. [DOI] [PubMed] [Google Scholar]
- 134. Melikian AA, Djordjevic MV, Chen S, et al. Effect of delivered dosage of cigarette smoke toxins on the levels of urinary biomarkers of exposure. Cancer Epidemiol Biomarkers Prev. 2007;167:1408–15. [DOI] [PubMed] [Google Scholar]
- 135. Xia Y, Bernert JT, Jain RB, et al. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007-2008. Biomarkers. 2011;162:112–9. [DOI] [PubMed] [Google Scholar]
- 136. Morin A, Shepperd CJ, Eldridge AC, et al. Estimation and correlation of cigarette smoke exposure in Canadian smokers as determined by filter analysis and biomarkers of exposure. Regul Toxicol Pharmacol. 2011;61(suppl 3):S3–12. [DOI] [PubMed] [Google Scholar]
- 137. Mendes Pm, Liang Q, Frost-Pineda K, Munjal S, Walk RA, Roethig HJ.. The relationship between smoking machine derived tar yields and biomarkers of exposure in adult cigarette smokers in the US. Regul Toxicol Pharmacol. 2009;551:17–27. [DOI] [PubMed] [Google Scholar]
- 138. Hofer I, Nil R, Wyss F, et al. The contributions of cigarette yield, consumption, inhalation and puffing behaviour to the prediction of smoke exposure. Clin Investig. 1992;70(3-4):343–51. [DOI] [PubMed] [Google Scholar]
- 139. Russell MA, Jarvis M, Iyer R, et al. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br Med J. 1980;2806219:972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rosa M, Pacifici R, Altieri I, et al. How the steady-state cotinine concentration in cigarette smokers is directly related to nicotine intake. Clin Pharmacol Ther. 1992;523:324–9. [DOI] [PubMed] [Google Scholar]
- 141. Shepperd CJ, Eldridge AC, Mariner DC, et al. A study to estimate and correlate cigarette smoke exposure in smokers in Germany as determined by filter analysis and biomarkers of exposure. Regul Toxicol Pharmacol. 2009;551:97–109. [DOI] [PubMed] [Google Scholar]
- 142. Bridges RB, Humble JW, Turbek JA, et al. Smoking history, cigarette yield and smoking behavior as determinants of smoke exposure. Eur J Respir Dis (suppl). 1986;146:129–37. [PubMed] [Google Scholar]
- 143. Bridges RB, Combs JG, Humble JW, et al. Population characteristics and cigarette yield as determinants of smoke exposure. Pharmacol Biochem Behav. 1990;371:17–28. [DOI] [PubMed] [Google Scholar]
- 144. Muhammad-Kah RS, Hayden AD, Liang Q, et al. The relationship between nicotine dependence scores and biomarkers of exposure in adult cigarette smokers. Regul Toxicol Pharmacol. 2011;601:79–83. [DOI] [PubMed] [Google Scholar]
- 145. Byrd GD, Robinson JH, Caldwell WS, et al. Comparison of measured and FTC-predicted nicotine uptake in smokers. Psychopharmacology (Berl). 1995;1222:95–103. [DOI] [PubMed] [Google Scholar]
- 146. Byrd GD, Davis RA, Caldwell WS, et al. A further study of FTC yield and nicotine absorption in smokers. Psychopharmacology (Berl). 1998;1394:291–299. [DOI] [PubMed] [Google Scholar]
- 147. Hee J, Callais F, Momas I, et al. Smokers' behaviour and exposure according to cigarette yield and smoking experience. Pharmacol Biochem Behav. 1995;521:195–203. [DOI] [PubMed] [Google Scholar]
- 148. Russell MA, Jarvis MJ, Feyerabend C, et al. Reduction of tar, nicotine and carbon monoxide intake in low tar smokers. J Epidemiol Community Health. 1986;401:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ebert RV, McNabb ME, McCusker KT, et al. Amount of nicotine and carbon monoxide inhaled by smokers of low-tar, low-nicotine cigarettes. JAMA. 1983;25020:2840–2842. [PubMed] [Google Scholar]
- 150. Rickert WS, Robinson JC.. Estimating the hazards of less hazardous cigarettes. II. Study of cigarette yields of nicotine, carbon monoxide, and hydrogen cyanide in relation to levels of cotinine, carboxyhemoglobin, and thiocyanate in smokers. J Toxicol Environ Health. 1981;7(3-4):391–403. [DOI] [PubMed] [Google Scholar]
- 151. Kinser Rd LQ, Nelson Bl, Roethig Hj, Walk Ra, Zedler Bk. Exposure to nicotine and carbon monoxide in a population of U.S. adult cigarette smokers. 2008. Philip Morris Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=gkcg0189.
- 152. Jarvis MJ, Giovino GA, O'Connor RJ, et al. Variation in nicotine intake among U.S. cigarette smokers during the past 25 years: evidence from NHANES surveys. Nicotine Tob Res. 2014;1612:1620–1628. [DOI] [PubMed] [Google Scholar]
- 153. Redner R, White TJ, Bunn JY, et al. Use of high-nicotine/tar-yield (full-flavor) cigarettes and risk for nicotine dependence in nationally representative samples of US smokers. nicotine Tob Res. 2016;186:1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ferris R. The phenomenon of lights. 1994. British American Tobacco Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=gmbx0209.
- 155.Dupuis RN. Untitled correspondence regarding: product advantages for ventilated cigarettes recently discovered. 1956. Philip Morris Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=yjhv0184.
- 156. Roper E. A study of cigarette smokers' habits and attitudes in 600000 volume 2. 1960. Philip Morris Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=ttck0112.
- 157.Harding BC, Hardwick M. The effect of ventilation variability and style on consumers' perception of quality. 1983. British American Tobacco Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=sjgb0201.
- 158. Crellin R. The role of smoking behavioural studies in ultra low tar product development. 1994. British American Tobacco Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xmdl0213.
- 159. Irwin D. Ultra low tar smokers - what do they seek from their brands? Are they getting it? 1994. British American Tobacco Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hlbx0209.
- 160.Resources GKM. Smokers' reactions to an ultra light brand extension for marlboro a qualitative study (three focused group interviews). 1979. Philip Morris Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=pkmj0114.
- 161. Kozlowski LT, Goldberg ME, Yost BA, et al. Smokers are unaware of the filter vents now on most cigarettes: results of a national survey. Tob Control. 1996;54:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cummings KM, Hyland A, Bansal MA, et al. What do Marlboro Lights smokers know about low-tar cigarettes? Nicotine Tob Res. 2004;6 (suppl 3):S323–S332. [DOI] [PubMed] [Google Scholar]
- 163. Sandford RA. Internal Memorandum to E.E. Kohnhorst. Research Development and Engineering (June 28, 1985), Minnesota Trial Exhibit 13250 1985.
- 164. Bansal-Travers M, Cummings KM, Hyland A, et al. Educating smokers about their cigarettes and nicotine medications. Health Educ Res. 2010;254:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bansal-Travers M, Hammond D, Smith P, et al. The impact of cigarette pack design, descriptors, and warning labels on risk perception in the U.S. Am J Prev Med. 2011;406:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Borland R, Yong HH, King B, et al. Use of and beliefs about light cigarettes in four countries: findings from the International Tobacco Control Policy Evaluation Survey. Nicotine Tob Res. 2004;6 (suppl 3):S311–S321. [DOI] [PubMed] [Google Scholar]
- 167. Cummings KM, Hyland A, Giovino GA, et al. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res. 2004;6 (suppl 3):S333–S340. [DOI] [PubMed] [Google Scholar]
- 168. Etter JF, Kozlowski LT, Perneger TV.. What smokers believe about light and ultralight cigarettes. Prev Med. 2003;361:92–98. [DOI] [PubMed] [Google Scholar]
- 169. Kozlowski LT, Goldberg ME, Yost BA.. Measuring smokers' perceptions of the health risks from smoking light cigarettes. Am J Public Health. 2000;908:1318–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kozlowski LT, Goldberg ME, Yost BA, et al. Smokers' misperceptions of light and ultra-light cigarettes may keep them smoking. Am J Prev Med. 1998;151:9–16. [DOI] [PubMed] [Google Scholar]
- 171. Kozlowski LT, Pillitteri JL.. Beliefs about “Light” and “Ultra Light” cigarettes and efforts to change those beliefs: an overview of early efforts and published research. Tob Control. 2001;10 (suppl 1):i12-i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kropp RY, Halpern-Felsher BL.. Adolescents' beliefs about the risks involved in smoking “light” cigarettes. Pediatrics. 2004;1144:e445–e451. [DOI] [PubMed] [Google Scholar]
- 173. Borland R, Fong GT, Yong HH, et al. What happened to smokers' beliefs about light cigarettes when “light/mild” brand descriptors were banned in the UK? Findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2008;174:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yong HH, Borland R, Cummings KM, et al. Impact of the removal of misleading terms on cigarette pack on smokers' beliefs about ‘light/mild' cigarettes: cross-country comparisons. Addiction. 2011;10612:2204–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Bansal-Travers M, O'Connor R, Fix BV, et al. What do cigarette pack colors communicate to smokers in the U.S.? Am J Prev Med. 2011;406:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. King B, Borland R.. What was “light” and “mild” is now “smooth” and “fine”: new labelling of Australian cigarettes. Tob Control. 2005;143:214–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. King B, Borland R.. The “low-tar” strategy and the changing construction of Australian cigarettes. Nicotine Tob Res. 2004;61:85–94. [DOI] [PubMed] [Google Scholar]
- 178. O'Connor RJ, Wilkins KJ, Caruso RV, et al. Cigarette characteristic and emission variations across high-, middle- and low-income countries. Public Health. 2010;12412:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Sweeney CT, Kozlowski LT, Parsa P.. Effect of filter vent blocking on carbon monoxide exposure from selected lower tar cigarette brands. Pharmacol Biochem Behav. 1999;631:167–173. [DOI] [PubMed] [Google Scholar]
- 180. Yong HH, Borland R, Cummings KM, et al. US Smokers' Beliefs, Experiences and Perceptions of Different Cigarette Variants Before and After the FSPTCA Ban on Misleading Descriptors Such as “Light,” “Mild,” or “Low.” Nicotine Tob Res 2016; 10.1093/ntr/ntw107. [DOI] [PMC free article] [PubMed]
- 181. O'Connor RJ, Bansal-Travers M, Cummings KM, et al. Filter presence and tipping paper color influence consumer perceptions of cigarettes. BMC Public Health. 2015;15:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Guillerm R, Radziszewski E. Analysis of smoking pattern including intake of carbon monoxide and influences of changes in cigarette design. In: Thornton RE, ed. Smoking Behaviour. Edinburgh: Churchill Livingstone; 1978. British American Tobacco Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=ppgm0178.
- 183. Dixon M, Derrick M.J. The monitoring of puffing and inhalating patterns of consumers of middle and low tar delivery cigarettes. 1986. British American Tobacco Records. https://industrydocuments.library.ucsf.edu/tobacco/docs/#id=hpwm0192.
- 184. Zacny JP, Stitzer ML, Brown FJ, et al. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther. 1987;2402:554–564. [PubMed] [Google Scholar]
- 185. Zheng H, Liu Y, Huang T, Fang Z, Li G, He S., Vas CÜY, Dickens CJ, Prasad K. Evelopment and characterisation of a smoking behaviour measurement system. Beiträge zur Tabakforschung International. 2015;265:219–231. [Google Scholar]
- 186. Baker RR, Dixon M.. The retention of tobacco smoke constituents in the human respiratory tract. Inhal Toxicol. 2006;184:255–294. [DOI] [PubMed] [Google Scholar]
- 187.Frost BE, Mariner DC, Sinclair NM. Factors relating to nicotine physio-chemistry and retention in human smokers. Paper presented at the Meeting of the Smoke and Technology Groups, CORESTA Congress; Brighton, UK; 1988:211–218.
- 188. Tobin MJ, Sackner MA.. Monitoring smoking patterns of low and high tar cigarettes with inductive plethysmography. Am Rev Respir Dis. 1982;1262:258–264. [DOI] [PubMed] [Google Scholar]
- 189. Tobin MJ, Chadha TS, Jenouri G, et al. Breathing patterns. 2. Diseased subjects. Chest. 1983;843:286–294. [DOI] [PubMed] [Google Scholar]
- 190. Tobin MJ, Jenouri G, Sackner MA.. Subjective and objective measurement of cigarette smoke inhalation. Chest. 1982;826:696–700. [DOI] [PubMed] [Google Scholar]
- 191. Appleton S, Liu J, Lipowicz PJ, et al. Effect of cigarette design on biomarkers of exposure, puffing topography and respiratory parameters. Inhal Toxicol. 2015;273:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. McBride MJ, Guyatt AR, Kirkham AJ, et al. Assessment of smoking behaviour and ventilation with cigarettes of differing nicotine yields. Clin Sci (Lond). 1984;676:619–631. [DOI] [PubMed] [Google Scholar]
- 193. St Charles FK, Krautter GR, Mariner DC.. Post-puff respiration measures on smokers of different tar yield cigarettes. Inhal Toxicol. 2009;218:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guillerm R RE, Ann Tabac Etudes. A new method of analyzing the act of smoking. 1975. RJ Reynolds Records. https://industrydocuments.library.ucsf.edu/docs/#id=znvl0089.
- 195.Mcaughey Jj PJ, Black A,Knight Da. Tar retention relative to other biomarkers in cigarette smokers switching to products with lower tar yields. AEA Technology. 1990. RJ Reynolds Records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=kmhp0013.
- 196. Pritchard JN, Mcaughey J, Black A Changes in smoke retention with decreasing tar deliveries. Am Rev Resp Dis Supp. 1990;141.
- 197. Pritchard JN, Mcaughey J, Black A. A technique for radio-labelling tar particulate material in mainstream cigarette smoke. J Aerosol Sci. 1988;19:715–724. [Google Scholar]
- 198. Pritchard JN, Mcaughey J, Black A. Dosimetric equivalence of tar deposition in rodents and man. Exp Pathol. 1989;37:95–97. [DOI] [PubMed] [Google Scholar]
- 199. Gower S, Hammond D.. CSP deposition to the alveolar region of the lung: implications of cigarette design. Risk Anal. 2007;276:1519–1533. [DOI] [PubMed] [Google Scholar]
- 200. Ingebrethsen BJ. The physical properties of mainstream cigarette smoke and their relationship to deposition in the respiratory tract. In: Extrapolation of Dosimetric Relationships for Inhaled Particles and Gases. New York: Academic Press; 1989: 125–141.
- 201. Feng S, Plunkett SE, Lam K, et al. A new method for estimating the retention of selected smoke constituents in the respiratory tract of smokers during cigarette smoking. Inhal Toxicol. 2007;192:169–179. [DOI] [PubMed] [Google Scholar]
- 202. Hiller FC, Anderson PJ, Mazumder MK.. Deposition of sidestream cigarette smoke in the human respiratory tract. II. Deposition of ultrafine smoke particles. Toxicol Lett. 1987;351:95–99. [DOI] [PubMed] [Google Scholar]
- 203. Hollander W, Stober W.. Aerosols of smoke, respiratory physiology and deposition. Arch Toxicol Suppl. 1986;9:74–87. [DOI] [PubMed] [Google Scholar]
- 204. Devereux TR. Alveolar type II and Clara cells: isolation and xenobiotic metabolism. Environ Health Perspect. 1984;56:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Rokicki W, Rokicki M, Wojtacha J, Dżeljijli A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir Torakochirurgia Pol. 2016;131:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Saffiotti U. Alveolar type II cells at the crossroad of inflammation, fibrogenesis, and neoplasia. Am J Pathol. 1996;1495:1423–1426. [PMC free article] [PubMed] [Google Scholar]
- 207. Fagerstrom KO. Effects of a nicotine-enriched cigarette on nicotine titration, daily cigarette consumption, and levels of carbon monoxide, cotinine, and nicotine. Psychopharmacology (Berl). 1982;772:164–167. [DOI] [PubMed] [Google Scholar]
- 208. Tsutahara S, Shijubo N, Hirasawa M, et al. Lung adenocarcinoma with type II pneumocyte characteristics. Eur Respir J. 1993;61:135–137. [PubMed] [Google Scholar]
- 209. Hachiya T, Honda T, Kubo K, et al. Expression patterns of type II pneumocyte apical surface glycoconjugates in lung adenocarcinoma cells. Virchows Arch. 1999;4341:63–639. [DOI] [PubMed] [Google Scholar]
- 210. Stenhouse G, Fyfe N, King G, et al. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol. 2004;574:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Barth PJ, Koch S, Muller B, et al. Proliferation and number of Clara cell 10-kDa protein (CC10)-reactive epithelial cells and basal cells in normal, hyperplastic and metaplastic bronchial mucosa. Virchows Arch. 2000;4376:648–655. [DOI] [PubMed] [Google Scholar]
- 212. Sutherland KD, Berns A.. Cell of origin of lung cancer. Mol Oncol. 2010;45:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zhu LR, Thomas PE, Lu G, et al. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab Dispos. 2006;3410:1672–1676. [DOI] [PubMed] [Google Scholar]
- 214. Castell JV, Donato MT, Gomez-Lechon MJ.. Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach. Exp Toxicol Pathol. 2005;57 (suppl 1):189–204. [DOI] [PubMed] [Google Scholar]
- 215. Belinsky SA, Devereux TR, White CM, et al. Role of Clara cells and type II cells in the development of pulmonary tumors in rats and mice following exposure to a tobacco-specific nitrosamine. Exp Lung Res. 1991;172:263–278. [DOI] [PubMed] [Google Scholar]
- 216. Barhoumi R, Mouneimne Y, Chapkin RS, et al. Effects of fatty acids on benzo[a]pyrene uptake and metabolism in human lung adenocarcinoma A549 cells. PLoS One. 2014;93:e90908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Smerdova L, Neca J, Svobodova J, et al. Inflammatory mediators accelerate metabolism of benzo[a]pyrene in rat alveolar type II cells: the role of enhanced cytochrome P450 1B1 expression. Toxicology. 2013;3141:30–38. [DOI] [PubMed] [Google Scholar]
- 218. Smith GB, Castonguay A, Donnelly PJ, et al. Biotransformation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in freshly isolated human lung cells. Carcinogenesis. 1999;209:1809–1818. [DOI] [PubMed] [Google Scholar]
- 219. Hoffmann D, Rivenson A, Hecht SS.. The biological significance of tobacco-specific N-nitrosamines: smoking and adenocarcinoma of the lung. Crit Rev Toxicol. 1996;262:199–211. [DOI] [PubMed] [Google Scholar]
- 220. Hoffmann D, Rivenson A, Amin S, et al. Dose-response study of the carcinogenicity of tobacco-specific N-nitrosamines in F344 rats. J Cancer Res Clin Oncol. 1984;1081:81–86. [DOI] [PubMed] [Google Scholar]
- 221. Yan Y, Wang Y, Tan Q, et al. Efficacy of deguelin and silibinin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2005;712:1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Gunning WT, Kramer PM, Lubet RA, et al. Chemoprevention of benzo(a)pyrene-induced lung tumors in mice by the farnesyltransferase inhibitor R115777. Clin Cancer Res. 2003;95:1927–1930. [PubMed] [Google Scholar]
- 223. Belinsky SA, Devereux TR, Foley JF, et al. Role of the alveolar type II cell in the development and progression of pulmonary tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the A/J mouse. Cancer Res. 1992;5211:3164–3173. [PubMed] [Google Scholar]
- 224. Montesano R, Saffiotti U, Ferrero A, et al. Synergistic effects of benzo(alpha)pyrene and diethylnitrosamine on respiratory carcinogenesis in hamsters. J Natl Cancer Inst. 1974;535:1395–1397. [DOI] [PubMed] [Google Scholar]
- 225. Rehm S, Lijinsky W.. Squamous metaplasia of bronchiolar cell-derived adenocarcinoma induced by N-nitrosomethyl-n-heptylamine in Syrian hamsters. Vet Pathol. 1994;315:561–571. [DOI] [PubMed] [Google Scholar]
- 226. Deutsch-Wenzel RP, Brune H, Grimmer G.. Experimental studies on the carcinogenicity of five nitrogen containing polycyclic aromatic compounds directly injected into rat lungs. Cancer Lett. 1983;201:97–101. [DOI] [PubMed] [Google Scholar]
- 227. Harris CC, Kaufman DG, Sporn MB, et al. Ultrastructural effects of N-methyl-N-nitrosourea on the tracheobronchial epithelium of the Syrian golden hamster. Int J Cancer. 1973;121:259–269. [DOI] [PubMed] [Google Scholar]
- 228. Saffiotti U, Montesano R, Sellakumar AR, et al. Respiratory tract carcinogenesis induced in hamsters by different dose levels of benzo-(a)pyrene and ferric oxide. J Natl Cancer Inst. 1972;494:1199–1204. [PubMed] [Google Scholar]
- 229. Church TR, Anderson KE, Caporaso NE, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;181:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yuan JM, Koh WP, Murphy SE, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;697:2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Yuan JM, Gao YT, Murphy SE, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;7121:6749–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Hecht SS. It is time to regulate carcinogenic tobacco-specific nitrosamines in cigarette tobacco. Cancer Prev Res (Phila). 2014;77:639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Ding YS, Zhang L, Jain RB, et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev. 2008;1712:3366–3371. [DOI] [PubMed] [Google Scholar]
- 234. Fischer S, Spiegelhalder B, Preussmann R.. Tobacco-specific nitrosamines in mainstream smoke of West German cigarettes—tar alone is not a sufficient index for the carcinogenic potential of cigarette smoke. Carcinogenesis. 1989;101:169–173. [DOI] [PubMed] [Google Scholar]
- 235. Adams JD, Lee SJ, Vinchkoski N, et al. On the formation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone during smoking. Cancer Lett. 1983;173:339–346. [DOI] [PubMed] [Google Scholar]
- 236. Andersen RA, Kemp TR.. Accumulation of 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone in alkaloid genotypes of burley tobacco during postharvest processing: comparisons with N'-nitrosonornicotine and probable nitrosamine precursors. Cancer Res. 1985;45(11 Pt 1):5287–5293. [PubMed] [Google Scholar]
- 237. Rickert WS, Joza PJ, Sharifi M, et al. Reductions in the tobacco specific nitrosamine (TSNA) content of tobaccos taken from commercial Canadian cigarettes and corresponding reductions in TSNA deliveries in mainstream smoke from such cigarettes. Regul Toxicol Pharmacol. 2008;513:306–310. [DOI] [PubMed] [Google Scholar]
- 238. Hoffmann D, Dong M, Hecht SS.. Origin in tobacco smoke of N'-nitrosonornicotine, a tobacco-specific carcinogen. J Natl Cancer Inst. 1977;586:1841–1844. [DOI] [PubMed] [Google Scholar]
- 239.K P. Basic principles of cigarette design and function. 2001. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=nrhc0150.
- 240.Marlboro 80 HP. 2009. Philip Morris Records. https://industrydocuments.library.ucsf.edu/docs/#id=zhjf0189.
- 241. Borgerding MF, Bodnar J A, Wingate D E. The 1999 massachusetts benchmark study; final report. 2000. Brown and Williamson Records.
- 242. Marcilla A., Gómez-Siurana A., Berenguer D., et al. Reduction of tobacco smoke components yield in commercial cigarette brands by addition of HUSY, NaY and Al-MCM-41 to the cigarette rod. Toxicol Rep. 2014; 2:152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Liu C, DeGrandpre Y, Porter A, et al. The use of a novel tobacco treatment process to reduce toxicant yields in cigarette smoke. Food Chem Toxicol. 2011;499:1904–1917. [DOI] [PubMed] [Google Scholar]
- 244. Berman ML, Connolly G, Cummings KM, et al. Providing a Science Base for the Evaluation of Tobacco Products. Tob Regul Sci. 2015;11:76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.