Abstract
Rhus verniciflua is commonly known as a lacquer tree in Korea. The bark of R. verniciflua has been used as an immunostimulator in traditional medicine, but also causes allergic dermatitis due to urushiol derivatives. For the development of active natural resources with less toxicity, the antibacterial activity of various parts of R. verniciflua such as bark, lignum, leaves and fruit, together with chemical composition, were investigated. Among the various parts of R. verniciflua, lignum showed the most potent antibacterial activity against fish pathogenic bacteria such as Edwardsiella tarda, Vibrio anguillarum and Streptococcus iniae. Measurement of total phenolic content and flavonoid content clearly showed a high content of phenolic and flavonoids in lignum among the various parts of R. verniciflua. Further analysis showed a close correlation between antibacterial activity and phenolic content. In addition, methyl gallate and fustin, the major constituents of bark and lignum, showed antibacterial activity, which suggested phenolic constituents as active constituents. The content of urushiols, however, was highest in bark, but there was a trace amount in lignum. LC-MS-MS and PCA analysis showed good discrimination with the difference of phenolic composition in various parts of R. verniciflua. Taken together, phenolic compounds are responsible for the antibacterial activity of R. verniciflua. The lignum of R. verniciflua contains high content of phenolic compounds with less urushiols, which suggests efficient antibacterial activity with less toxicity. Therefore, the lignum of R. verniciflua is suggested as a good source for antibacterial material to use against fish bacterial diseases.
Introduction
Fish is rich in nutrients including protein, vitamins, minerals and polyunsaturated fatty acids. Due to the high consumption of fish, aquaculture is a major global industry that has developed rapidly in a short period of time. However, industrial aquaculture is susceptible to diverse infections caused by overcrowded rearing and excessive feeding for mass production within an intensive aquaculture system. The infection of fish with bacteria or viruses results in serious economic losses due to its high mortality. Moreover, increased outbreaks of disease require more use of antibiotics and chemicals, which gives rise to resistance to antibiotics and food safety issues. Therefore, the demand for antibiotics with strong potency and less toxicity has risen sharply, and natural products have been suggested as alternatives to chemical antibiotics [1–3]. Natural products are considered to be safe for both fish and humans and exert little resistance to bacteria [4]. In addition, natural products contain various constituents with different skeletons such as flavonoids, terpenoids, xanthones, alkaloids and polysaccharides. The diversity of structures enables a wide range of pharmacological effects including antioxidant, anticancer, anti-inflammatory and neuroprotective activity, and also contributes synergic activities [5–6]. Recently, several natural substances such as bee venom, essential oil and phenolic compounds have been reported to have antimicrobial agents [7–9].
Rhus verniciflua Stokes (Anacardiaceae) is a plant native to East Asian countries, including Korea. It is also known as a lacquer tree and is used in traditional herbal medicine. The bark of this tree has been used as an immunostimulant in folk medicine and various biological activities including antioxidant, anticancer, anti-inflammatory and antimicrobial effects have been reported [10–13]. In our previous study, extracts and fractions of the bark of R. verniciflua showed significant antibacterial activity against fish pathogen bacteria such as Edwardsiella tarda and Vibrio anguillarum. The bark of R. verniciflua and its flavonoids also have antiviral activities against fish pathogenic viruses [14–15]. Therefore, R. verniciflua is suggested to have potential as antimicrobial therapeutics against fish infectious diseases. However, the bark of R. verniciflua is consumed at a high price due to small supply. In addition, its use has been limited due to the presence of allergic components, urushiols, in the bark. Other parts of this plant are also consumed as food ingredients or alcoholic beverages in traditional use; however, few investigations have been carried out regarding the composition and biological activity of various parts of R. verniciflua. In particular, no studies have been conducted to evaluate the antibacterial activity of each part of R. verniciflua against fish pathogens. Generally, different parts of plants contain different types of constituents that contribute diverse biological activities. Therefore, we compared the antibacterial activities of various parts of R. verniciflua such as bark, lignum, leaves and fruit (Fig 1) for development as an alternative to antibiotics for fish pathogens. The effects of phenolic contents and its major compounds on antibacterial activity were also investigated.
Fig 1. Representative photographs of bark, lignum, leaves and fruit of Rhus verniciflua.
Materials and methods
Plant materials
The bark, lignum and leaves of R. verniciflua were collected from four different regions in Korea, such as Wonju, Okcheon, and Buyeo. No specific permissions were required for access because these locations were privately owned and the field studies did not involve endangered or protected species.
The materials were air-dried at room temperature for 2 weeks and pulverized and extracted with MeOH using sonic apparatus for 2 hrs. Voucher specimens were deposited in a specimen room of the herbarium of the College of Pharmacy at Chungbuk National University.
Measurement of antibacterial activity
Bacteria and culture conditions
Streptococcus iniae KCTC 3657, Vibrio anguillarum KCTC 2711 and Edwardsiella tarda KCTC 12267 were purchased from the Korean Collection for Type Cultures (Daejeon, Korea). For antimicrobial susceptibility tests, strains were cultured on Brain Heart Infusion Agar (BHIA) in an incubator at 25 °t for 24 h. Bacterial colonies taken directly from BHIA plates were incubated in Brain Heart Infusion Broth (BHIB) at 25 ° for 24 h. From this culture, a suspension equivalent to a 0.5 McFarland standard in BHIB was prepared.
Disc diffusion assay
The extracts of different parts from RVS were tested with a disc diffusion assay [15]. Bacterial suspensions with a turbidity equivalent to 0.5 McFarland standard were prepared as described above. The bacterial inocula of 100 μl (108 CFU/ml) were seeded into BHI agar using a disposable spreader. After drying, filter paper discs (6 mm in diameter) impregnated with the extracts (2 mg/disc) were placed on test bacteria-inocula plates. Commercially available discs (Oxoid Ltd., Basingstoke, Hampshire, UK) containing 30 μg oxytetracycline each were used as a positive control. Plates were incubated at 25 °5 for 48 h and the clear zones including the diameter of the disc (mm) were measured with a digital caliper.
Microdilution method—Minimum inhibitory concentration (MIC)
The compounds were serially diluted with BHIB in a 96-well plate. An equal volume of bacterial suspension (1x106 CFU/ml) was added to the wells to give a final volume of 200 μl. The plate was incubated at 25°. for 24 h. Appropriate controls included the solvent used to dissolve the samples, broth alone and the antibiotics amoxicillin and oxytetracycline as positive controls. The lowest concentration of samples that visibly inhibited bacterial growth was considered the minimum inhibitory concentration (MIC). Each assay was repeated three times.
Determination of total flavonoid content
An aluminum chloride colorimetric assay was employed for the measurement of the total flavonoid content in the samples. Briefly, samples were prepared in a 96-well plate and 5% NaNO3 was added to the reaction mixture. The reaction mixture was kept in incubation for 5 min, and 10% AlCl3 was added. After incubation with gentle shaking, 1 N NaOH and H2O was added to the reaction plate. Absorbance at 510 nm was measured with a microplate reader. The total flavonoid content of each sample was expressed as catechin equivalent (CE) using catechin as a standard.
Determination of total phenolic content
A Folin-Ciocalteu assay was employed for the determination of the total phenolic content. The reaction was started with the addition of Folin-Ciocalteu’s phenol reagent to the 96-well plate containing the test samples. The reaction mixture was incubated for 5 min with gentle shaking, and then 7% Na2CO3 was added to the reaction mixture. The reaction mixture was kept in a dark condition at room temperature for reaction. After 90 min of incubation, the absorbance was measured at 630 nm with a microplate reader. The total phenolic content in each sample was expressed as gallic acid equivalent (GAE) using gallic acid as a standard.
LC-Q-TOF MS/MS analysis
LC-Q-TOF MS/MS analysis was performed on an Agilent 1260 series system (Agilent, Santa Clara, CA, USA) connected to an Agilent 6530 Q-TOF mass spectrometer (Agilent, Santa Clara, CA, USA). The HPLC system was equipped with an auto-sampler, binary pump, degasser, and diode array detector. UV spectra was monitored at 220, 254, and 300 nm. The mass spectrometer was equipped with electrospray ionization (ESI) in the negative mode. The MS and MS/MS spectra were obtained with a mass range of m/z 50–1700. The collision energy for MS/MS fragmentation was set at 10, 20, 30, and 40 V. MassHunter Workstation software LC/MS Data Acquisition for 6530 series Q-TOF (version B.05.00) was applied to adjust all the acquisition parameters. The chromatographic separation of the sample was performed on a Shiseido CapCell PAK C18 column (5μm, 4.6 mm I.D. × 150 mm) with a C18 guard column (4.00 × 3.00 mm; Phenomenex, USA). The mobile phase consisted of water containing 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B). The gradient elution was 0–5 min, 5% B, 5–30 min, 5–95% B. The injection volume was 5 μl, and the flow rate was 0.6 ml/min. Principal component analysis (PCA) and statistical analysis were performed to evaluate the differences between samples using Mass Profiler Professional (MPP).
Isolation of the compounds
Compounds 8 and 14, major compounds from bark and lignum, were isolated as previously reported [15]. The leaves of R. verniciflua (60 g) were extracted twice with 100% MeOH, which yielded the methanol extract (10.1 g). The methanol extract was suspended in H2O and partitioned successively with n-hexane, CH2Cl2, EtOAc, and n-BuOH. The EtOAc fraction of the leaves (RVLE, 1.2 g) was subjected to Sephadex LH-20 and eluted with n-hexane:CH2Cl2:MeOH (5:5:1) to give nine subfractions (RVLE1- RVLE9). The RVLE6 was subjected to medium-pressure liquid chromatography (MPLC) over silica gel and eluted with a mixture of CH2Cl2-MeOH to give five subfractions (RVLE6A- RVLE6E). Compound 15 (12.0 mg) was obtained from RVLE6D by semi-preparative HPLC eluting with acetonitrile-water (27:73).
Results and discussion
Preparation of samples of different parts of R. verniciflua
Four parts of R. verniciflua, bark, lignum, leaves and fruit, were collected from different regions of Korea, including Wonju, Okcheon and Boeun (Fig 1). For the comparison of chemical patterns and biological activity, each part was extracted with 80% MeOH, respectively.
Antibacterial activity against fish pathogens
The antibacterial activity against fish pathogens was determined using the disc diffusion method. E. tarda, V. anguillarum and S. iniae were selected as fish pathogenic bacteria and antibacterial activity was assessed by measuring the diameter of the inhibition zone formed around paper discs containing an extract from each part of R. verniciflua (2 mg/disc).
As shown in Table 1, the antibacterial activity was differed substantially depending on the plant part and bacteria tested. Among four different parts, the lignum of R. verniciflua showed the strongest activity against all bacteria tested, with inhibition zone diameters ranging from 10.38 to 11.09 mm. Fruit and bark showed similar antibacterial potency against V. anguillarum and S. iniae, with inhibition zone diameters ranging from 8.62 to 8.93 mm; however, only bark showed antibacterial activity against E. tarda. Leaves showed only weak antibacterial activity against V. anguillarum and S. iniae.
Table 1. Antibacterial activity of bark, lignum, leaves and fruit of R. verniciflua against E. tarda, V. anguillarum and S. iniae.
| Samples (2 mg/disc) |
Clear zone (mm) | ||
|---|---|---|---|
| E. tarda | V. anguillarum | S. iniae | |
| Bark | 7.39 ± 0.10 | 8.64 ± 1.36 | 8.93 ± 1.15 |
| Lignum | 10.38 ± 0.70 | 10.50 ± 0.46 | 11.09 ± 0.56 |
| Leaf | No Effect | 6.78 ± 0.26 | 7.68 ± 0.72 |
| Fruit | No Effect | 8.62 ± 0.82 | 8.50 ± 1.10 |
| Positive control (OTC) a) | 20.03 ± 0.68 | 20.23 ± 0.50 | 19.64 ± 0.42 |
a) Oxytetracycline was used as the positive control
Total phenolic and flavonoid contents
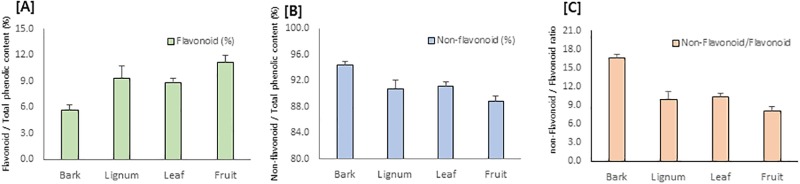
R. verniciflua has been reported to contain various polyphenols, including flavonoids and phenolic compounds, and they contribute to its diverse biological activity [16–19]. Therefore, the total phenolic content of each part was analyzed. Consistent with previous studies [10–12], R. verniciflua is rich in phenolic compounds. The amount of total phenolic compounds, however, differs depending on the plant part and ranges from 68.9 to 363.6 mg GAE/g extract. Among the parts of R. verniciflua, lignum contains the highest amounts of phenolic constituents, followed by bark, leaves and fruit (Table 2). Lignum extract contains up to 363.6 mg GAE/g, which is more than twice that of leaves and bark. Total flavonoid content and non-flavonoid content also showed a similar pattern, which is the most abundant in lignum. Interestingly, however, the ratio of non-flavonoid and flavonoid showed a differential pattern. Bark contains relatively high non-flavonoid content, with a non-flavonoid/flavonoid ratio of 16.4, whereas other parts contain relatively high flavonoid content with a non-flavonoid/flavonoid ratio of 8.1 to 10.9 (Fig 2). Taken together, not only the total amount of phenolic and flavonoid content, but also the composition of each constituent differs depending on the part of R. verniciflua.
Table 2. Total phenolic, flavonoid and non-flavonoid contents in the extracts of bark, lignum, leaves and fruit of R. verniciflua.
| Parts of R. verniciflua | ||||
|---|---|---|---|---|
| Bark | Lignum | Leaf | Fruit | |
| Total phenolic | 151.7 ± 38.3 | 363.6 ± 80.6 | 127.7 ± 7.7 | 68.9 ± 7.7 |
| Flavonoid | 8.6 ± 1.9 | 35.0 ± 12.1 | 11.2 ± 0.2 | 7.7 ± 1.5 |
| Non-flavonoid | 143.1 ± 36.3 | 328.6 ± 68.6 | 120.2 ± 12.4 | 61.1 ± 6.2 |
Fig 2. Ratio of flavonoid, non-flavonoid and non-flavonoid/flavonoid of extracts of bark, lignum, leaves and fruit of R. verniciflua.
Correlation between biological activity and phenolic content
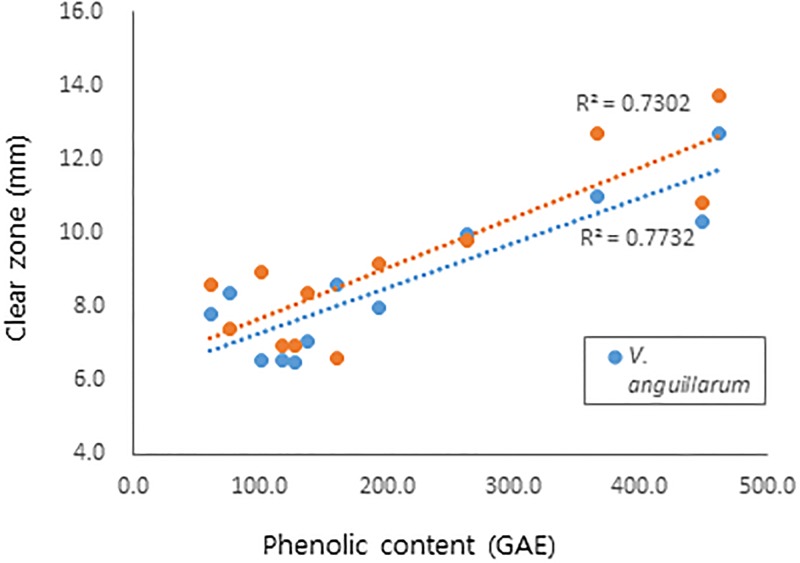
Polyphenols are known to exert diverse biological activities, including antioxidant and antibacterial activities [19–22]. Therefore, the effect of total polyphenol content on antibacterial and antioxidant activity was analyzed. As shown in Fig 3, antibacterial activities against V. anguillarum and S. iniae showed a correlation with total phenolic content, with R2 of 0.7732 and 0.7302, respectively. These results suggest phenolic constituents as an active ingredient of the antibacterial activity of R. verniciflua.
Fig 3. Correlation between antibacterial activity and phenolic contents of R. verniciflua.
Chemical profiles of bark, lignum, leaves and fruit
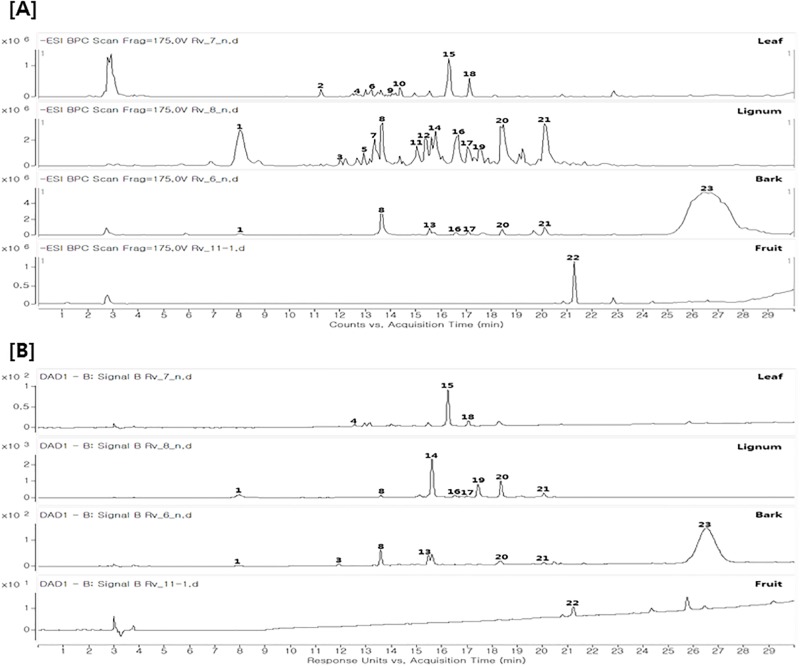
The chemical profiles of bark, stem, leaves and fruit of R. verniciflua were first analyzed using LC-MS/MS analysis and a database (Fig 4). Twenty-three compounds were detected in R. verniciflua (S1 and S2 Figs) and 13 compounds were tentatively identified based on retention times, UV spectra, and MS data (accurate mass, MS/MS fragments) (Table 3). As expected, phenolic compounds including flavonoids are a major group in R. verniciflua. However, the chemical profiles of each part were totally different.
Fig 4. HPLC chromatogram of bark, lignum, leaves and fruit of R. verniciflua.
(A) Mass chromatogram (negative mode), (B) UV chromatogram (254 nm).
Table 3. Compounds identified in the extract of bark, lignum, leaves and fruit of R. verniciflua by LC-MS/MS analysis.
| Peak No. | Compounds identification | tR (mins) |
observed m/z |
calculated m/z |
Molecular formula [M-H]- |
MS/MS fragments (m/z) |
UV (λmax, nm) |
Detected parts a) |
|---|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 8.0320 | 169.0153 | 169.0142 | C7H5O5 | 125 [M-CO2-H]- | 271 | BK, LG |
| 2 | Unidentified | 11.2310 | 315.1107 | 315.1085 | C14H19O8 | 153 [M-C6H10O5-H]- | LF | |
| 3 | Dihydroxybenzoic acid | 11.9810 | 153.0202 | 153.0193 | C7H5O4 | 109 [M-CO2-H]- | 217, 261, 297 | B, L |
| 4 | Unidentified | 12.6050 | 297.0636 | 297.0616 | C13H12O8 | 135 [M-C6H10O5-H]- | 291, 325 | LF |
| 5 | Unidentified | 12.9230 | 579.1552 | 579.1567 | C23H31O17 | 137 [M-442-H]- | 280 | LG |
| 6 | Unidentified | 13.2300 | 353.0905 | 353.0878 | C16H17O9 | 191 [M-C6H10O5-H]- | 294, 325 | LF |
| 7 | Unidentified | 13.3590 | 579.1548 | 579.1567 | C23H31O17 | 137 [M-442-H]- | 281 | LG |
| 8 | 4-methyl gallate | 13.6510 | 183.0309 | 183.0299 | C8H7O5 | 124 [M-C2H3O2-H]- | 275 | BK, LG, LF |
| 9 | Unidentified | 14.0420 | 297.0636 | 297.0616 | C13H12O8 | 135 [M-C6H10O5-H]- | 316 | LF |
| 10 | Unidentified | 14.4170 | 337.0957 | 337.0929 | C16H17O8 | 191 [M-C6H10O4-H]- | 313 | LF |
| 11 | Unidentified | 15.0460 | 287.0582 | 287.0561 | C15H11O6 | 109 [M-178-H]- | LG | |
| 12 | Unidentified | 15.3580 | 607.1866 | 607.1880 | C25H35O17 | 271 [M-336-H]- | 280 | LG |
| 13 | Pentagalloyl glucose | 15.5510 | 939.1188 | 939.1103 | C41H31O26 | 770 [M-C7H5O5-H]- | 279 | BK, LG |
| 14 | Fustin | 15.7950 | 287.0585 | 287.0561 | C15H11O6 | 109 [M-C9H6O4-H]- | 218, 231, 279 | BK, LG |
| 15 | Quercitrin | 16.2910 | 447.0964 | 447.0933 | C21H19O11 | 301 [M-C6H10O4-H]- | 258, 351 | LF |
| 16 | Taxifolin | 16.6700 | 303.0533 | 303.0510 | C15H11O7 | 285 [M-H2O-H]- | 290 | BK, LG |
| 17 | Garbanzol | 17.0440 | 271.0635 | 271.0612 | C15H11O5 | 243 [M-CO-H]- | 213, 276, 311 | BK, LG |
| 18 | Kaempferol-3-O-rhamnoside | 17.1650 | 431.1017 | 431.0984 | C21H19O10 | 285 [M-C6H10O4-H]- | 262, 344 | LF |
| 19 | Fisetin | 17.5440 | 285.0427 | 285.0405 | C15H9O6 | 135 [M-C8H6O3-H]- | 316, 359 | BK, LG |
| 20 | Sulfuretin | 18.4810 | 269.0477 | 269.0455 | C15H9O5 | 133 [M-C8H8O2-H]- | 257, 269 | BK, LG |
| 21 | Butein | 20.1050 | 271.0634 | 271.0612 | C15H11O5 | 135 [M-C8H8O2-H]- | 261, 380 | BK, LG |
| 22 | Unidentified | 21.2620 | 541.1168 | 541.1140 | C30H21O10 | 311 [M-230-H]- | 225 | FR |
| 23 | Urushiol (3-pentadecyl catechol, double bond = 3) |
26.7350 | 313.2198 | 313.2173 | C21H29O2 | 122 [M-C14H23-H]- | 225 | BK |
a) BK, bark; LG, lignum; LF, leaf; FR, fruit
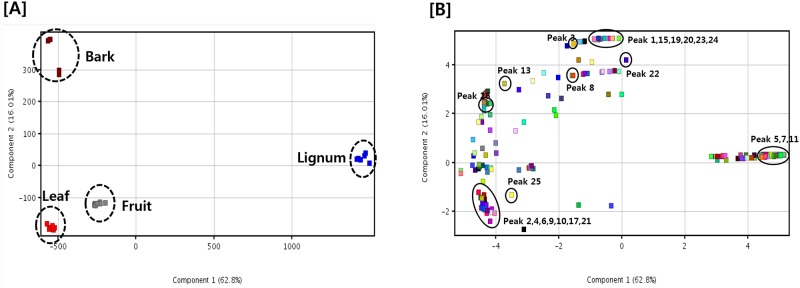
To better analyze and visualize the similarities and differences among each part, multivariate data analyses were employed. Principal component analysis (PCA) was first applied to classify the patterns of each part. PCA is an unsupervised clustering process for identifying patterns by reduction of the number of dimensions. It is widely used for the classification of various samples with respect to designated criteria [23, 24]. As shown in Fig 5, discrimination between each part was well performed. The PCA score plot discriminated lignum from other parts, with separate clustering on the positive score value of PC1, whereas other parts were positioned on the negative side of PC1 (Fig 5). Further analysis using the corresponding loading plot of PC1 suggested the most discriminatory constituents in lignum as peaks 5, 7 and 11, which contained high amounts in lignum compared to other parts. Score plot and corresponding loading plot also showed that leaves was separated from other parts by peaks 2, 4, 6, 9, 10, 17 and 21.
Fig 5. PCA results of the extracts of bark, lignum, leaves and fruit of R. verniciflua.
(A) Score plot and (B) loading plot.
Identification of major constituents of bark, lignum, leaves and fruit
Comparison of the HPLC chromatogram of R. verniciflua showed differences depending on the part. HPLC analysis also suggested the presence of major characteristic compounds in each part of R. verniciflua. For the verification of its major characteristic compounds and evaluation of antibacterial activity, further isolation was conducted using chromatographic techniques. The structures of compounds were identified by spectroscopic analysis including NMR data and UV spectrum, and by the comparison of references [15, 25].
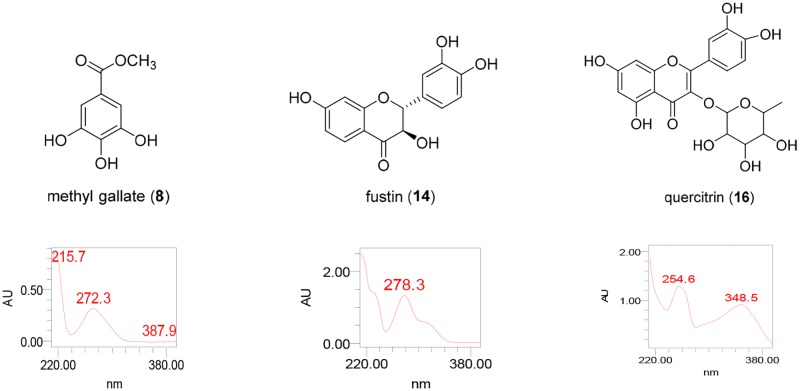
The major constituents of bark, lignum and leaves were identified as methyl gallate (8), fustin (14) and quercitrin (15) (Fig 6), respectively, which was consistent with LS-MS/MS analysis. These three major constituents are phenolic compounds, but they can be divided into further subtypes. Fustin (14) and quercitrin (15) are flavonoids, whereas methyl gallate (8) is a simple phenolic compound. These results are consistent with Fig 3C, which supported the differential composition of phenolic content in each part of R. verniciflua. The major constituent of fruit, however, could not be identified in our present study.
Fig 6. Chemical structures of methyl gallate (8), fustin (14) and quercitrin (15), major constituents of bark, lignum and leaves, respectively.
Evaluation of antibacterial of major constituents of bark, stem and leaves
Next, the antibacterial activity of the major constituents of bark, lignum and leaves was tested (Table 4). All three major constituents are phenolic compounds, but the activities were quite different. Methyl gallate (8) showed the most potent antibacterial activity. Fustin (14) also showed mild antibacterial activity against E. tarda; however, quercitrin (15) showed only weak activity against V. anguillarum and S. iniae. Considering the biological activity of the extract and major constituents of each part of R. verniciflua, they showed different patterns. The lignum of R. verniciflua was the most active among the parts, but methyl gallate (8), the major constituent of bark, was more potent than fustin (14), and that of lignum. Although the antibacterial activity of the major constituent of lignum is less potent than that of bark, the phenolic content in lignum is much higher than in bark. From these results, we suppose that a high content of phenolic compounds with moderate activity might contribute to the potent antibacterial activity of lignum compared to other parts.
Table 4. Antibacterial activity of major constituents of bark, lignum and leaves of R. verniciflua against E. tarda, V. anguillarum and S. iniae.
| Compound | Antibacterial activity (MIC, μg/ml) | ||
|---|---|---|---|
| E. tarda | V. anguillarum | S. iniae | |
| Methyl gallate (8) | 31.25 | 2000 | > 2000 |
| Fustin (14) | 1000 | 1000 | 1000 |
| Quercitrin (15) | > 2000 | 2000 | 2000 |
| Positive control (OTC) a) | 0.5 | 0.25 | 0.25 |
a) Oxytetracycline was used as the positive control
Comparison of biological activity and chemical constituents of bark, stem, leaves and fruit
A phytochemical investigation of four different parts of R. verniciflua revealed that phenolic constituents are major compounds of R. verniciflua. The amount of total phenolic compounds was highest in lignum, followed by bark, leaves and fruit. Interestingly, the bark contains a relatively high portion of non-flavonoids compared to other parts, as derived from a high ratio of non-flavonoid/flavonoid (Fig 3). The chemical profiles of bark, stem, leaves and fruit of R. verniciflua analyzed using LC-MS/MS and database analysis consistently suggested phenolic compounds as major constituents of this plant. Further purification yielded major compounds such as methyl gallate (8) from bark, fustin (14) from lignum and quercitrin (15) from leaves. All three major constituents belong to phenolic compounds, and further can be divided into methyl gallate (8) as a simple phenolic compound and fustin (14) and quercitrin (15) as flavonoids, which is consistent with our analysis.
Concerning antibacterial activity, lignum showed the most potent activity, followed by bark, leaves and fruit, which is the same order as phenolic contents. Further analysis revealed a positive correlation between antibacterial activity and phenolic content of each part of R. verniciflua. Among the major constituents, methyl gallate (8) from bark was the most effective, followed by fustin (14) from lignum. Therefore, phenolic compounds are responsible for the biological activity of each part of R. verniciflua; however, the type of constituent and potency of its biological activity are quite different depending on the part. In addition, fruit has less phenolic compound contents compared to bark and leaves, but showed similar or better activity. Previous studies reported that glycoproteins are active constituents of the fruit of R. verniciflua [26, 27]. In our present study, few compounds were detected in our LC-MS-MS analysis of the fruit of R. verniciflua, and their structures could not be identified. Taken together, the presence of other types of constituents in the fruit of R. verniciflua were suggested, which needs to be clarified by further study.
The similarities and differences between each part of R. verniciflua were also analyzed by PCA in the present study. The PCA score plot showed good discrimination between parts. Lignum was discriminated from other parts with the positive score values of PC1 and peaks 5, 7, and 11 are suggested as discriminant compounds. Bark also formed a cluster with the positive score values of PC2. Moreover, urshiol, an allergic component, is one of the discriminant constituents in bark, and is contained in high amounts in bark compared to lignum, leaves and fruit. These results suggested that, contrary to bark, other parts can be developed for functional products with fewer side effects.
Comparative analysis of different parts of plants showed differential composition and biological activity depending on the part [28, 29]. In the case of Salvia miltiorrhiza, tanshinones and phenolic acids are abundant in roots, whereas flavonoids and triterpenes are abundant in stems and leaves. The composition of flowers of S. milriorrhiza was quite dependent on growth stage. Different parts of plants and plant waste materials have been developed as alternatives for drug development with better efficacy and economic advantages. However, information about the constituents and biological activity of different plant resources is quite limited. Our present study clearly showed the differences in chemical composition and antibacterial activity between each part of R. verniciflua, which was supported by PCA analysis. We further characterized the major constituents of bark, lignum, leaves of R. verniciflua and their antibacterial activity. Therefore, our present study provided a basis for the use of other parts of R. verniciflua. In particular, lignum showed strong antibacterial activity against E. tarda, V. anguillarum and S. iniae and high phenolic contents and less urshiol compared to bark, which can increase biological activity and reduce allergic toxicity. Therefore, we carefully suggest that the lignum of R. verniciflua can be a good candidate for the development of antibacterial agents against fish diseases.
Conclusions
We investigated the chemical composition and antibacterial activity of different parts of R. verniciflua, which were bark, lignum, leaves and fruit. Among them, lignum showed strong antibacterial activity against E. tarda, V. anguillarum and S. iniae and phenolic contents were suggested as active constituents. The similarities and differences among each part were analyzed by PCA and good discrimination between each part was observed in the PCA score plot. Lignum formed a uniform cluster in the PCA score plot and the loading plot showed that lignum contains little content of urshiol, an allergic component of this plant. Therefore, the lignum of R. verniciflua can be a good candidate for the development of antibacterial agents against fish pathogens.
Supporting information
(PPTX)
(PPTX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Ministry of Oceans and Fisheries (https://www.mof.go.kr) grant no. 20160024 to MKL. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crane M, Hyatt A. Viruses of fish: an overview of significant pathogens. Viruses 2011; 3: 2025–2046. 10.3390/v3112025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizunoe S, Yamasaki T, Tokimatsu I, Matsunaga N, Kushima H, Hashinaga K, et al. A case of empyema caused by Edwardsiella tarda. J Infect. 2006; 53: e255–e258. 10.1016/j.jinf.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Guijarro JA, Cascales D, García-Torrico AI, García-Domínguez M, Méndez J. Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Front Microbiol. 2015; 6: 700 10.3389/fmicb.2015.00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emeca U, Iloegbunam NG, Gbekele-Oluwa AR, Bola M. Natural products and aquaculture development. J Pharm Biol Sci. 2014; 9: 70–82. [Google Scholar]
- 5.Ulrich-Merzenich G, Panek D, Zeitler H, Vetter H, Wagner H. Drug development from natural products: Exploiting synergistic effects. Indian J Exp Biol. 2010; 48: 208–219. [PubMed] [Google Scholar]
- 6.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004; 67: 2141–2153. 10.1021/np040106y [DOI] [PubMed] [Google Scholar]
- 7.Han SM, Lee KG, Park KK. Antimicrobial activity of honeybee venom against fish pathogenic bacteria. J Fish Pathol. 2011; 24: 113–120. [Google Scholar]
- 8.Park JW, Wendt M, Heo GJ. Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria. Lab Anim Res. 2016; 32: 87–90. 10.5625/lar.2016.32.2.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim JW, Kim NY, Jung SH, Kang SY. The simultaneous quantification of antibacterial compounds against fish pathogens in combined extract of Angelica gigas and Aetemisia iwayomogi using LC-MS/MS. Planta Med. 2016; 81: S1–S381. [Google Scholar]
- 10.Kim K, Kwon Y, Chun W, Kim T, Sun J, Yu C, et al. Rhus verniciflua Stokes flavonoids extract have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010; 120: 539–543. [Google Scholar]
- 11.Kim KH, Moon E, Choi SU, Kim SY, Lee KR. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor anti-inflammatory activities. Phytochemistry. 2013; 92: 113–121. 10.1016/j.phytochem.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 12.Park KY, Jung GO, Lee KT, Choi J, Choi JY, Kim GT, et al. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J. Ethnopharmacol. 2004; 90: 73–79. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lee H, Lee HJ, Choi WC, Yoon SW, Ko SG. Rhus verniciflua Stokes prevents cisplatin-induced cytotoxicity and reactive oxygen species production in MDCK-I renal cells and intact mice. Phytomedicine 2009; 16: 188–197. 10.1016/j.phymed.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 14.Kang SY. The antimicrobial compound of Rhus verniciflua barks against fish pathogenic Gram-negative bacteria, Edwardsiella tarda and Vibrio anguillarum. J. Fish Pathol. 2005; 18: 227–237. [Google Scholar]
- 15.Kang SY, Kang JY, Oh MJ. Antiviral activities of flavonoids isolated from the bark of Rhus verniciflua Stokes against fish pathogenic viruses in vitro. J Microbiol. 2012; 50: 293–300. 10.1007/s12275-012-2068-7 [DOI] [PubMed] [Google Scholar]
- 16.Kim KH, Moon E, Choi SU, Kim SY, Lee KR. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor and anti-inflammatory activities. Phytochemistry. 2013, 92:113–21. 10.1016/j.phytochem.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 17.Jeong GS, Lee DS, Song MY, Park BH, Kang DG, Lee HS, et al. Butein from Rhus verniciflua protects pancreatic β cells against cytokine-induced toxicity mediated by inhibition of nitric oxide formation. Biol Pharm Bull. 2011; 34: 97–102 [DOI] [PubMed] [Google Scholar]
- 18.Kim SA, Kim SH, Kim IS, Lee D, Dong MS, Na CS, et al. Simultaneous determination of bioactive phenolic compounds in the stem extract of Rhus verniciflua stokes by high performance liquid chromatography. Food Chem. 2013; 141:3813–3819. 10.1016/j.foodchem.2013.06.068 [DOI] [PubMed] [Google Scholar]
- 19.Jung CH, Kim JH, Hong MH, Seog HM, Oh SH, Lee PJ, et al. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-kB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2007; 110: 490–497. 10.1016/j.jep.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 20.Ahmad A, Tandon S, Xuan TD, Nooreen Z. A Review on phytoconstituents and biological activities of Cuscuta species. Biomed Pharmacother. 2017; 92: 772–795. 10.1016/j.biopha.2017.05.124 [DOI] [PubMed] [Google Scholar]
- 21.Calzada F, Juárez T, García-Hernández N, Valdes M, Ávila O, Mulia LY, et al. Antiprotozoal, antibacterial and antidiarrheal properties from the flowers of Chiranthodendron pentadactylon and isolated flavonoids. Pharmacogn Mag. 2017; 13:240–244. 10.4103/0973-1296.204564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmasri WA, Zhu R, Peng W, Al-Hariri M, Kobeissy F, Tran P, et al. Multitargeted flavonoid inhibition of the pathogenic bacterium Staphylococcus aureus: A Proteomic characterization. J Proteome Res. 2017; 16: 2579–2586. 10.1021/acs.jproteome.7b00137 [DOI] [PubMed] [Google Scholar]
- 23.Farag MA, Ali SE, Hodaya RH, El-Seedi HR, Sultani HN, Laub A, et al. Phytochemical profiles and antimicrobial activities of Allium cepa Red cv. and A. sativum subjected to different drying methods: A comparative MS-based metabolomics. Molecules. 2017; 22: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziółkowska A, Wąsowicz E, Jeleń HH. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016; 213:714–720. 10.1016/j.foodchem.2016.06.120 [DOI] [PubMed] [Google Scholar]
- 25.Yang NY, Tao WW, Duan JA. Antithrombotic flavonoids from the faeces of Trogopterus xanthipes. Nat Prod Res. 2010; 24: 1843–1849. 10.1080/14786419.2010.482057 [DOI] [PubMed] [Google Scholar]
- 26.Oh PS, Lee SJ, Lim KT. Glycoprotein isolated from Rhus verniciflua Stokes inhibits inflammation-related protein and nitric oxide production in LPS-stimulated RAW 264.7 cells. Biol Pharm Bull. 2007; 30:111–116. [DOI] [PubMed] [Google Scholar]
- 27.Ko JH, Lee SJ, Lim KT. 36 kDa glycoprotein isolated from Rhus verniciflua Stokes fruit has a protective activity to glucose/glucose oxidase-induced apoptosis in NIH/3T3 cells. Toxicol In Vitro. 2005; 19: 353–363. 10.1016/j.tiv.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 28.Agu KC, Okolie PN. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci Nutr. 2017; 5: 1029–1036. 10.1002/fsn3.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng H, Su S, Xiang X, Sha X, Zhu Z, Wang Y, et al. Comparative analysis of the major chemical constituents in Salvia miltiorrhiza roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS and HPLC-ELSD methods. Molecules. 2017; 22: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX)
(PPTX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.