Abstract
Postpartum depression is one of the most frequent complications of childbirth affecting approximately 500,000 women annually (prevalence 10 – 15%). Despite the documented adverse outcomes for mother and child, there remains a great need to develop prospective approaches to identify women at risk. This review examines some of the best characterized molecular and clinical risk factors for PPD. We illustrate that this is a growing literature but there remains a lack of reliable molecular predictors for PPD. Current best predictors are clinical assessments for psychiatric history and adverse life events, highlighting the need for increased depression screening across the perinatal period.
Introduction/Background
Postpartum depression (PPD) is a perinatal form of major depressive disorder (MDD) and affects approximately 500,000 women annually in the US (prevalence 10–15%)1. PPD is one of the most frequent complications of childbirth2 and is associated with many adverse outcomes for both mother and offspring including, maternal mortality and morbidity1, increased risk for infanticide3, poorer maternal-infant attachment and impaired parenting behaviors2. There is a great need for prospective approaches that identify women at risk for PPD. This is a growing field of research that seeks to predict those women at risk for PPD so that prevention and early detection are possible.
The purpose of this review is to examine some of the best characterized molecular and clinical risk factors for PPD (Table 1). These risk factors are divided into two categories: inherent or pre-pregnancy risk factors and perinatal risk factors. It is important to recognize that there is no single predictor of PPD that currently exists. What we present here are the risk factors that represent the largest increases in the odds for developing PPD, measured using prospective studies.
Table 1.
Reported Risk Factors for PPD. Evidence level indicates if there are positive associations with PPD, mixed associations, or null findings (no associations).
| Inherent / Pre-pregnancy Risk Factors | Evidence Level |
|---|---|
| Genetics | Positive |
| Epigenetics | Mixed |
| Neuroactive Molecules | Mixed |
| Psychiatric History | Positive |
| Other Health History | Mixed |
| Adverse Life Events | Positive |
| Social Support | Positive |
| Substance Use | Mixed |
| Perinatal Risk Factors | |
| Demographic Information | Mixed |
| Fertility History | Null |
| Nutrition | Mixed |
| Obstetrical Outcomes | Mixed |
Inherent / Pre-pregnancy Risk Factors
In this section, we highlight an individual’s putative risk factors that exist prior to pregnancy. These areas of risk are important to note because they can significantly contribute to an individual’s risk and may not be assessed during routine screening for PPD.
Genetics
DNA provides every individual with a baseline risk of disease. It is the unique combination of all genetic variants that predisposes an individual to any given disorder. The heritability of PPD is approximately 50%4 which means that half of the phenotypic variation seen in PPD is due to genetic variation. This is higher than the heritability of MDD (32%) suggesting that PPD may be a more homogenous subtype of depression. These recent heritability findings represent a leap in our understanding of the genetics of PPD. However, our ability to use patient DNA sequences to estimate individual risk for PPD is not currently feasible. Much of the published research into the genetic associations with PPD focuses on candidate genes. While the evidence may appear convincing for any one study, candidate genes overall have shown they are not a reliable mechanism to predict PPD risk. First, candidate gene studies often rely on small sample sizes and are not able to be replicated. Second, they often do not account for population differences in genetic variation. Using the commonly studied BDNF Val66Met polymorphism as an example, those of European ancestry are homozygous for the G allele 60% of the time. This is compared to those of African ancestry who are homozygous for the G allele more than 80% of the time. These drastic changes may appear as significant differences between PPD cases and controls when they are actually just measures of population structure. Candidate gene studies are typically not able to account for these changes in genetic ancestry. There has been a single study that looks at the effect of genetic ancestry on predictors of PPD5. They showed that genetic ancestry was not a predictor of PPD and did not modify the predictive value of other risk factors. Lastly, candidate gene studies, by definition, do not take into account the variation of individuals across the genome. No single gene or variant works alone in terms of biological function. These reasons are perhaps why no candidate gene has been shown to explain the variation associated with PPD or any other psychiatric disorder. Current genomics approaches, such as genetic risk score prediction, use variation across the genome to provide risk estimates for various psychiatric disorders. However, genetic risk scores for PPD have not yet been established and are an area of current research. This method has allowed for initial investigations into the genetic overlap between PPD and other mood disorders6. Ultimately, the goal of genetic risk scores is to provide a clinical risk assessment for PPD based on an individual’s unique genetic background. However, this goal is not immediately feasible and is reliant on very large sample sizes.
Epigenetics
Epigenetics refers to changes in gene function that do not alter the DNA sequence itself. The PPD literature focuses mainly on DNA methylation, which is the addition of methyl groups to the DNA that regulate gene transcription and cell specificity. This type of epigenetic modification is of particular interest in psychiatry because DNA methylation is altered by stress, medication and reproductive hormones. The identification of DNA methylation biomarkers has been particularly successful in PPD. Using blood drawn during at any point during pregnancy, DNA methylation at two genomic locations, along with complete blood count data, was able to prospectively predict PPD case status (determined using clinician diagnosis) with 96% accuracy (area under the receiver operating characteristic curve, AUC = 0.96)7. This prediction is irrespective of maternal antenatal depression status. These biomarkers were first identified in a relatively small sample of women (n = 52), but have been since replicated in two additional cohorts with 81% accuracy (AUC = 0.81)8. Additionally, DNA methylation at the oxytocin receptor gene (OXTR) was found to reflect changes in inflammatory cell types and can be used as a proxy for complete blood count data in the PPD prediction model9. These epigenetic biomarkers are promising and would require a more large-scale clinical trial before implementation in the clinic. Though the literature on PPD-related epigenetics is currently small, it shows promise as a way to develop a clinically meaningful biomarker.
Neuroactive Molecules
Much of the biological literature associated with PPD focuses on the association with neuroactive molecules, such as hormones and neuropeptides. The recent literature shows a focus on reproductive hormones, BDNF10, γ-aminobutyric acid (GABA)11, and ghrelin12. When examining these studies through the lens of predicting PPD, it is important to note that these are reporting on cross-sectional associations and should not be interpreted as predictive without more longitudinal follow-up. However, there are several longitudinal studies examining the predictive ability of neuroactive molecules that we discuss next:
Allopregnanolone
A metabolite of progesterone, allopregnanolone is a positive allosteric modulator of the GABA-A receptor. Recent work has shown positive treatment effects in clinical trials as a novel treatment for PPD using a proprietary intravenous formulation of allopregnanolone, brexanolone13. However, its potential as a predictor of PPD onset has not been completely evaluated. A single exploratory study has been published looking at plasma allopregnanalone in the second and third trimesters as a predictor of PPD14. Their relatively small sample size of 60 women found that lower second trimester allopregnanalone increased the odds of developing subsequent PPD. More work will need to be done to replicate and refine the predictive ability of antepartum allopregnanalone, but this work is encouraging and aligns with the recent therapeutic findings.
Beta-endorphin
Beta-endorphin is an endogenous opioid neuropeptide created by the metabolism of pro-opiomelanocortin (POMC). It has associated function within the HPA-axis making it an attractive candidate for association with PPD. In a sample of 307 pregnant women, Yim et al. took blood samples at multiple time points during gestation and a nine-week postpartum visit15. Upon analyses, beta-endorphin levels and depression status (using EPDS score > 10) at 25 weeks’ gestation predicted PPD status at the nine-week postpartum visit. While other antepartum timepoints were assessed, only the 25-week assessment was significantly predictive of future PPD. Compared to other biomarkers in this review, this predictive model only requires a single assessment and biological sampling to determine case status. However, this finding needs to be replicated before implementing in clinical practice.
Cortisol
The stress hormone cortisol has been widely investigated in psychiatric disorder etiology. However, longitudinal studies into the role of cortisol with PPD onset have yielded largely null results. The single study which identified predictive value for cortisol also examined the role of several inflammatory molecules across seven time points16. The final predictive model requires measures of salivary cortisol and plasma-derived inflammatory markers (IL8 and IL10) at day 14 postpartum to predict PPD through six-months postpartum. These constitute multiple measures from multiple tissues, making the clinical implementation challenging, but not impossible. However, one limitation of this study is that it will miss any PPD onset that occurs within the first two weeks following childbirth. However, this interesting study should be compared to the other studies17,18 that did not find predictive power in cortisol measurements. The lack of other positive findings may be a result of differing sample sources, diagnostic definitions, and collection times.
CRH
Similar to cortisol, corticotropin-releasing hormone (CRH) is investigated in psychiatric disorders for its role in HPA-axis function and stress response. During pregnancy, the placenta produces CRH, which is followed by an abrupt drop post-delivery mirroring the milieu of reproductive hormones. Unlike reproductive hormone levels which have been shown not to be predictive of PPD, CRH has shown positive results. Work by Yim et al.19 and Iliadis et al.20 independently employed longitudinal studies during pregnancy and into the postpartum period. Yim et al. used week 25 CRH to predict PPD at 4 weeks postpartum using an EPDS threshold of ≥ 10 to define PPD case status. Using a similar experimental model, Iliadis et al. used week 17 CRH to predict PPD at 6 weeks postpartum defining PPD cases with a more stringent EPDS threshold of ≥ 13. While these findings are similar and point to the same outcome of CRH prediction of PPD, the differences may be driven by small differences in study design, most notably follow-up period and EPDS thresholds for PPD case status. A follow-up study, or possibly a re-analysis, could harmonize these results. However, another large study found no association between pregnancy levels of CRH and subsequent PPD onset21. This may also be an artifact of differences in study design with the null findings using less specific windows for sampling during pregnancy (< 20 weeks and 24–29 weeks), EPDS thresholds (≥ 12), and PPD follow-up (12 weeks and one year postpartum). This work is supported by another study by Hahn-Holbrook et al. which found greater increases between 29 weeks and 37 weeks’ gestation in CRH are associated with later PPD symptoms22. Despite these three studies highlighting CRH as a predictor of PPD, work by Glynn and Sandman did not find CRH to be predictive of PPD in a similar longitudinal sampling of CRH and mood across pregnancy and up to 6 months postpartum23. Overall, CRH may be a viable biomarker in the future if more work is done to replicate previous findings using consensus sampling and measurement protocols.
Oxytocin
The oxytocin signaling network has been of great interest to perinatal mental health researchers for many years due to its role in bonding and mother-infant interactions. Recent work has begun to identify the trajectories of oxytocin through pregnancy and into the postpartum period, with a focus on how PPD onset potentially alters these trajectories. The first of these studies came in 2011 when Skrundz et al. measured plasma oxytocin levels during the third trimester of pregnancy in 74 women24. Depression status was assessed at baseline and two weeks postpartum. They found that plasma oxytocin levels during the third trimester can predict PPD symptoms at two weeks postpartum. Moreover, their findings showed that those with EPDS scores ≥ 10 at baseline were characterized by lower oxytocin concentrations. These initial findings were followed up in two studies in 2016. First, Jobst et al. evaluated 100 women for depression symptoms and plasma oxytocin levels at two time points during pregnancy (35 and 38 weeks) and three times postpartum (2 days, 7 weeks, 6 months)25. With multiple time points they showed plasma oxytocin levels increased from week 35 of pregnancy to 6 months postpartum in all women. However, those women who developed PPD showed a characteristic drop in oxytocin levels from week 38 of gestation to two days after delivery, where those without PPD continuously increase. This finding supports work by Skrundz et al. proposing that oxytocin may be a predictor of PPD in the immediate postpartum period (within two weeks). This window may be critical for prediction because when measuring PPD status at six weeks postpartum, Massey et al. were unable to find differences in third trimester oxytocin levels alone that predict PPD status26. However, using past history of MDD in their prediction model shows an interaction with oxytocin levels to significantly predict PPD symptom severity. Briefly, they found that higher levels of oxytocin predicted PPD severity in women with a history of MDD, but not in women without such history. Overall, these three studies show that the transition from gestation to postpartum may be a critical window for oxytocin prediction of PPD. In addition, oxytocin trajectory may be altered by previous MDD history, which should be accounted for in future studies evaluating the predictive ability of serum oxytocin as a biomarker for PPD.
Thyroid Function
During pregnancy several thyroid hormone levels are altered and have been investigated in relation to perinatal mood previously27. Tests that measure various aspects of thyroid function are readily available and widely used, specifically measuring thyroid stimulating hormone (TSH), thyroxine (T4), and triiodothyronine (T3). Additionally, thyroid peroxidase (TPO) and thyroid binding globulin (TBG) are other indicators of thyroid function. But when examining the role of thyroid hormones in predicting PPD, timing is key given thyroid function is reacting to the constant changes in other hormones across pregnancy. Albacar et al. performed some of the first investigations into whether thyroid function can prospectively predict PPD28. In a large cohort (n = 1053), they were not able to identify any indications that thyroid function at two days after birth could predict future depression at 8 or 32 weeks postpartum. To illustrate the issue of timing, Sylven et al. found that TSH levels at childbirth over the clinical threshold of 4.0 mU/L was associated with increased risk for PPD (EPDS ≥ 12) at six-months postpartum (OR 11.30, 95% CI 1.93–66.11)29. This work by Sylven et al examined 227 women, of which 26 (11.5%) developed PPD and 21 had TSH over the clinical threshold. While this is an encouraging finding, it is a small sample that requires replication in larger cohorts with more women with PPD. Predictors assayed at the time of childbirth are less practical than those drawn earlier in pregnancy, when there is ample opportunity for prevention and intervention. Groer and Vaughan found that TPO-positive women measured between 16 and 25 weeks gestation were more likely to have PPD symptoms six months after delivery30. In a complimentary study, Wesseloo et al. recently found that screening positive for TPO-antibodies at 10–12 weeks gestation was associated with a nearly four-fold increased risk (adjusted OR 3.8; 95% CI 1.3–11.6) for first-onset depression at four months postpartum31. Taken together, this work suggests that recognizing the presence of thyroid-related immune markers may also predict PPD. Increased TSH indicates hypothyroidism while TPO-positive tests suggest autoimmune disorders. If these findings are replicated, it could implicate thyroid dysfunction as a mechanism contributing to PPD, in addition to providing a prospective biomarker for PPD.
Inflammatory Markers
MDD, including PPD, has been associated with increased inflammation suggesting a shared molecular mechanism for mood disorders and inflammation processes. Identifying this mechanism is a relatively difficult task given the degree of cell-specificity and number of markers that indicate immune activation. Two studies suggest that inflammatory markers can be more than just associated with PPD and prospectively predict PPD. First, Krause et al. found that regulatory T cells were increased 34–38 weeks prenatally in mothers who develop PPD and are able to significantly predict PPD onset32. Second, Liu et al. demonstrate that two inflammatory markers, interleukin-6 (IL-6) and high-sensitivity C-reactive protein (Hs-CRP), each are independently capable of predicting PPD using serum at delivery33. IL-6 and Hs-CRP had an accuracy of 86% (AUC = 0.86, 95% CI: 0.80 – 0.92) and 83% (AUC = 0.83, 95% CI: 0.78 – 0.89), respectively in their study of 296 women. However, several other studies with larger sample sizes have reported lack of evidence supporting inflammatory markers as PPD biomarkers34. These studies reporting null findings used a wide range of sample sizes and racial/ethnic diversity, as well as looking across several types of inflammatory markers. In one study of note, their molecular predictor did not perform better than using previous MDD diagnoses as a predictor34. This illustrates that while associations may be significant, the ability of inflammatory markers to predict PPD require much more investigation.
Psychiatric History
Perhaps the current greatest predictor of PPD is the assessment of psychiatric disorders both prior to and during pregnancy. The literature has many examples that illustrate this using both retrospective and prospective measures. Practically, this abundance of information can be distilled into one take-home message: it is imperative to screen patients for psychiatric history to better evaluate PPD risk.
The literature strongly shows that various lifetime histories of MDD5,25,35,36, anxiety5, PPD35,37, PMS/PMDD37, other mood disorders38, personality disorders, or any psychiatric disorders37,39–41 all significantly predict increase risk for PPD onset. Moreover, there is a wealth of literature showing that antenatal MDD25,37,42–44, anxiety5, or other psychiatric disorder40 also significantly increase risk for PPD. Lastly, there is a great amount of power in self-reported family history of MDD45, family history of bipolar disorder, and any psychiatric illness39,46. Unlike many other predictors of PPD outlined in this review, assessments of psychiatric history are validated repeatedly. There is a high degree of confidence in generating risk prediction models using this data for several reasons. First, the literature represents findings that are globally recognized, encompassing women from all genetic and cultural backgrounds. Second, many of these studies used multifactorial methods to look at the effect of psychiatric history on PPD onset, with most studies finding psychiatric history as the most significant predictor associated with prospective case status. For many non-psychiatry clinicians, screening for psychiatric history, either through diagnostic interview, chart review, or self-report, is often missed or neglected. However, this small addition to screening can improve quality of care for PPD which is currently underdiagnosed and under-treated.
Adverse Life Events
If we consider a history of psychiatric disorders as the best current predictor for PPD, adverse life events may account for the next largest source of variation associated with the disorder. Many studies find a dose-response between adverse life events and risk for PPD5. These environmental risk factors will contribute to an individual’s existing vulnerability to PPD. Many studies report cumulative measures of adverse life events5, also referred to stressful life events36,47. These reports often use summary measures to capture cumulative life events and their effect on PPD risk. Additionally, researchers have attempted to tease out specific adverse life events that contribute increased risk.
Evidence has shown in multiple populations that physical, psychological, or sexual abuse37,39–41,45,48 significantly increases a woman’s risk to PPD. There is a single report that shows lifetime trauma exposure is not associated with PPD49 identified among the many positive associations. Also, given the critical role partners play during the perinatal period, intimate partner violence (IPV) is especially relevant to PPD risk36,43,44,50–52. It should be noted that one study did not identify IPV as a significant risk factor for PPD53, though this could be due to their assessment at one month postpartum. Overall, IPV, as well as more general forms of physical, psychological, or sexual abuse, has been shown to increase PPD risk whether it occurs pre- or perinatally.
Perhaps often overlooked adverse life events in PPD research are issues of immigration and discrimination. These are important topics to cover because they represent chronic stressors compared to singular adverse life events. Albeit small, the literature shows that both immigration43,54 and discrimination35 increase PPD risk. Issues of immigration and discrimination may account for the variation in prevalence of PPD across racial/ethnic groups, especially where increased prevalence is observed among immigrants or other groups experiencing discrimination. Overall, the current literature shows specific adverse life events, such as IPV, childhood trauma, or military deployment, increase risk for PPD. This increased risk for PPD is also observed for many other forms of adverse life events not reported in this review, including divorce, financial hardship, death of a loved one, natural disasters, and mass conflict. However, careful assessment of cumulative life events provides greater power for assessing an individual’s risk for PPD.
Perinatal Risk Factors
During pregnancy and delivery, the mother and baby are carefully monitored for many medical outcomes. With this comes a lot of information to make sure developmental trajectories are met, including variables that may aid in assessing risk for PPD. This section reviews risk factors that may be identified during perinatal care visits and delivery.
Demographic Information
The most widely study risk factors for PPD are derived from demographic information. In many cases, this data is accessible from population level data collected a large number of people at one time, making demographic data easily accessible to investigate associations with PPD. Some studies interrogate a wide range of demographic variables and report no associations with PPD risk42. There are also many demographic factors reported in the literature with mixed results.
Maternal age
Much of the literature implicating age as a risk factor for PPD does so in very general terms55. Other studies show maternal age of PPD onset appears to have a U-shaped curve of increased risk as age increases. That is, risk for PPD is higher under the age of ~2438,45,48,51,52,56, decreasing between the ages of 24 – 3557, and increasing again over the age of 3545. These age specific changes in PPD risk may reflect periods of stress and degrees of social support. However, there are also multiple studies that do not find such associations between age and PPD risk41,50,54,58.
Race
The topic of race and ethnicity in the area of biomedical research is a complicated one. There are studies that find associations with PPD risk and race59, and those that do not5,50,54,58,60. Discrepancies here may be due to the fact that race/ethnicity may be proxies for environmental rather than biological factors. Race is typically associated with one’s skin color whereas ethnicity correlates with language. The preferred measure to examine the biological relatedness between individuals is genetic ancestry. There has been a single study investigating the role of genetic ancestry with PPD risk and found no association5. This study also found that self-reported race/ethnicity did not correlate well with genetic ancestry. Other studies have identified socioeconomic status, community of residence and immigrant status as moderating factors for discrepancies between PPD risk and race60. Additionally, differences observed in race may be attributable to discrimination61 (see Adverse Life Events). Though these issues of racial disparities are best summed up by the findings of Ertel et al. which states: “Black and Hispanic depressed mothers were more likely to experience multiple adversities and less likely to receive services than white depressed mothers.”47
Socioeconomic status
Socioeconomic status (SES) refers to a summary measure that measures a person’s economic and social position relative to others. Many individual factors contribute to the measurement of SES, including education, income, employment, and insurance status. Overall, SES has been reported as a significant contributor to PPD risk41,48. Specifically, it is low SES that seems to contribute the greatest risk to PPD39,60,62. Indicators of low SES in the United States are qualifying for the WIC Program (Special Supplemental Food Program for Women, Infants, and Children) and SNAP (Supplemental Nutrition Assistance Program). As a potential surrogate marker of low SES, women who reported being on WIC51,63 and SNAP64 have shown increased PPD risk. However, it is important to note that not every study finds SES as a risk factor for PPD42.
Educational attainment is one contributing factor in determining SES. There are many examples in the literature that show lower levels of education are associated with increased risk for PPD45,47,51,52,55,56,59,62. This may be an effect of lower literacy, which also has been reported to increase risk41. In contrast, there are also findings of increased levels of education increasing PPD risk38. The differences in associations should also be noted with the negative findings observed between level of education and risk for PPD50,54,58.
One of the main drivers of SES is income. Low household income increases the odds of developing PPD36,47,51,55,56,62, though not all studies find this association58. Just as individual income is negatively associated with PPD risk, so is domestic economic performance65. Employment status is a more direct influence on an individual’s income. Therefore, it makes sense that employment status also has been associated with PPD risk44,56,62, though not always50.
Obstetrical Outcomes
Preterm Birth
Preterm neonates (born prior to 37 completed weeks’ gestation) account for 70% of neonatal morbidity, 50% of infant hospitalization costs, and carry a 40× increased risk of neonatal death compared to their term counterparts.66 Surviving neonates also carry a disproportionate share of lifelong complications, including cerebral palsy, respiratory illness, blindness, and deafness.67 Preterm birth is triggered by multiple mechanisms, including hormonal mediation, inflammation and infection, and genetic factors, converging in a final common pathway of delivery earlier than 37 weeks.68
Despite the multiple underlying etiologies, there are clear links between stress, depression, and preterm birth. Traditionally, studies evaluating the association between depression and preterm birth in particular have been challenging to interpret due to potential confounding effects of antidepressant use. A recent systematic review of 14 studies including 25,663 women found that those with untreated depression during pregnancy have a significantly elevated risk for preterm birth (aOR 1.56, 95% CI 1.25–1.94) compared with women without depression.69 Further, there was a trend towards an even greater preterm birth risk in women with severe depression.69 Another study of 2,208 women showed that women with major depressive disorder and post-traumatic stress disorder have a >4 fold increased risk for preterm birth (aOR 4.08, 95% CI 1.3–13.2); this risk is greater than - and independent of - antidepressant use and not a function of acute mood or anxiety symptoms.70 Cumulative psychosocial stress was also found to be a significant risk factor for preterm delivery <37 weeks’ gestation among 3,021 women in Canada (OR 1.73, 95% CI 1.07–2.81). Importantly, in this cohort, preterm birth risk was highest among those with low levels of social support or optimism.71 Higher general levels of stress72 and higher perceived racial discrimination73 – which may be linked with depression – have also been associated with elevated risk for spontaneous preterm birth.
Similarly, as expected, preterm birth is associated with an elevated risk for PPD in multiple studies, including population based studies, with the magnitude of the effect ranging from OR 1.35–1.7474–76. One systematic review found rates of PPD as high as 40% among women who delivered preterm77. However, studies in this area should be interpreted with caution because many do not control for depression and other psychiatric illnesses during pregnancy.
Putative Molecular Mechanisms for PPD
When we examine the risk factors that contribute most to risk for PPD, it seems to strongly support a gene-environment interaction model. Genetic studies show there is a large heritable component to PPD (~50%). An individual’s genes are deterministic for developing PPD, but it provides an inherent level of risk that is ever-present. This level of risk is only exacerbated by environmental factors that significantly increase risk: previous psychiatric history, adverse life events, decreased socioeconomic status, and negative obstetrical outcomes. What these environmental risk factors have in common is they increase stress levels and alter the HPA-axis. This is significant because increased levels of stress only exacerbate underlying genetic levels of risk. In summary, manifestation of PPD is a result of inherent genetic risk plus environmental insults reaching a threshold level of risk.
Clinical Implications
Biomarkers for PPD show a lot of promise but none are ready for clinical use yet. Work in genetics and epigenetics show a lot of promise for personal risk prediction. There are several hormonal and neurosteroids that are predictive in preliminary studies, but require further replication for clinical use. While there is not an established prediction method for PPD, the literature shows there are practical steps a provider can take to assess who is at increased risk for PPD. In 2016, the U.S. Preventive Services Task Force released recommendations for increased depression screening during the perinatal period78. This is to monitor mood symptoms in mothers at multiple time points through pregnancy since they increase risk for subsequent PPD. However, we recommend performing assessments for psychiatric history and adverse life events at a single time point during routine perinatal care, while also monitoring for adverse obstetrical outcomes as they may occur. These single assessments can take place at any time prior to childbirth to assess prospective risk for PPD. Figure 1 illustrates the recommended assessments and the timeframes when they should take place. The addition of these short assessments will allow providers a better evaluation of an individual’s risk for PPD. Additionally, monitoring of any adverse obstetrical outcomes is critical as this also increases the odds of developing PPD. Future work will need to be done to formalize this process and evaluate metrics for efficacy, though this should not prevent clinicians from employing these methods. Ongoing research will hopefully replicate some of the biomarker findings and lead to novel ways of predicting those at risk to prevent unnecessary suffering for women, their children and families.
Figure 1.
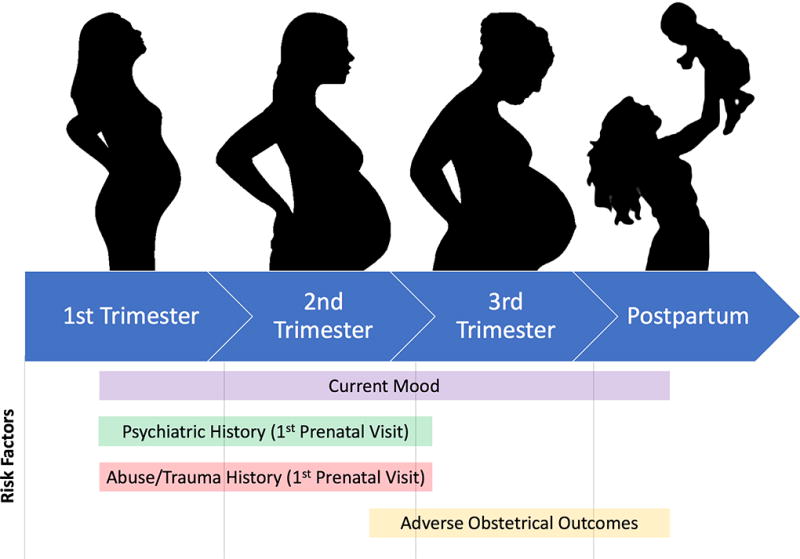
Recommended Assessments During the Perinatal Period.
Acknowledgments
This work was supported by the National Institutes of Mental Health: JG grant 4T32MH093315-05 and SMB grant R01MH104468
References
- 1.Gavin NI, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 2.Flynn HA, Davis M, Marcus SM, Cunningham R, Blow FC. Rates of maternal depression in pediatric emergency department and relationship to child service utilization. Gen Hosp Psychiatry. 2004;26:316–22. doi: 10.1016/j.genhosppsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8:77–87. doi: 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- 4.Viktorin A, et al. Heritability of Perinatal Depression and Genetic Overlap With Nonperinatal Depression. Am J Psychiatry. 2016;173:158–65. doi: 10.1176/appi.ajp.2015.15010085. [DOI] [PubMed] [Google Scholar]
- 5.Guintivano J, et al. Adverse life events, psychiatric history, and biological predictors of postpartum depression in an ethnically diverse sample of postpartum women. Psychol Med. 2017:1–14. doi: 10.1017/S0033291717002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne EM, et al. Applying polygenic risk scores to postpartum depression. Arch Womens Ment Health. 2014 doi: 10.1007/s00737-014-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guintivano J, Arad M, Gould TD, Payne JL, Kaminsky ZA. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry. 2014;19:560–7. doi: 10.1038/mp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborne L, et al. Replication of Epigenetic Postpartum Depression Biomarkers and Variation with Hormone Levels. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmel M, et al. Oxytocin receptor DNA methylation in postpartum depression. Psychoneuroendocrinology. 2016;69:150–60. doi: 10.1016/j.psyneuen.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinheiro RT, et al. Brain-derived neurotrophic factor levels in women with postpartum affective disorder and suicidality. Neurochem Res. 2012;37:2229–34. doi: 10.1007/s11064-012-0851-9. [DOI] [PubMed] [Google Scholar]
- 11.Deligiannidis KM, et al. Peripartum neuroactive steroid and gamma-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology. 2016;70:98–107. doi: 10.1016/j.psyneuen.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker JH, Pedersen C, Leserman J, Brownley KA. Active ghrelin and the postpartum. Archives of Women's Mental Health. 2016;19:515–520. doi: 10.1007/s00737-015-0578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanes S, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390:480–489. doi: 10.1016/S0140-6736(17)31264-3. [DOI] [PubMed] [Google Scholar]
- 14.Osborne LM, et al. Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology. 2017;79:116–121. doi: 10.1016/j.psyneuen.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yim IS, et al. Prenatal beta-endorphin as an early predictor of postpartum depressive symptoms in euthymic women. J Affect Disord. 2010;125:128–33. doi: 10.1016/j.jad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corwin EJ, et al. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun. 2015;49:86–93. doi: 10.1016/j.bbi.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okun ML, et al. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130:378–84. doi: 10.1016/j.jad.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iliadis SI, et al. Prenatal and Postpartum Evening Salivary Cortisol Levels in Association with Peripartum Depressive Symptoms. PLoS One. 2015;10:e0135471. doi: 10.1371/journal.pone.0135471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yim IS, et al. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–9. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliadis SI, et al. MID-PREGNANCY CORTICOTROPIN-RELEASING HORMONE LEVELS IN ASSOCIATION WITH POSTPARTUM DEPRESSIVE SYMPTOMS. Depression and Anxiety. 2016;33:1023–1030. doi: 10.1002/da.22529. [DOI] [PubMed] [Google Scholar]
- 21.Meltzer-Brody S, et al. Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD) J Clin Endocrinol Metab. 2011;96:E40–7. doi: 10.1210/jc.2010-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn-Holbrook J, Schetter CD, Arora C, Hobel CJ. Placental Corticotropin-Releasing Hormone Mediates the Association Between Prenatal Social Support and Postpartum Depression. Clin Psychol Sci. 2013;1:253–264. doi: 10.1177/2167702612470646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014;76:355–62. doi: 10.1097/PSY.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 24.Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–93. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobst A, et al. Oxytocin course over pregnancy and postpartum period and the association with postpartum depressive symptoms. Arch Womens Ment Health. 2016;19:571–9. doi: 10.1007/s00737-016-0644-2. [DOI] [PubMed] [Google Scholar]
- 26.Massey SH, Schuette SA, Pournajafi-Nazarloo H, Wisner KL, Carter CS. Interaction of oxytocin level and past depression may predict postpartum depressive symptom severity. Arch Womens Ment Health. 2016;19:799–808. doi: 10.1007/s00737-016-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szpunar MJ, Parry BL. A systematic review of cortisol, thyroid-stimulating hormone, and prolactin in peripartum women with major depression. Archives of Women's Mental Health. 2017:1–13. doi: 10.1007/s00737-017-0787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albacar G, et al. Thyroid function 48h after delivery as a marker for subsequent postpartum depression. Psychoneuroendocrinology. 2010;35:738–42. doi: 10.1016/j.psyneuen.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Sylven SM, et al. Thyroid function tests at delivery and risk for postpartum depressive symptoms. Psychoneuroendocrinology. 2013;38:1007–13. doi: 10.1016/j.psyneuen.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Groer MW, Vaughan JH. Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum. J Obstet Gynecol Neonatal Nurs. 2013;42:E26–32. doi: 10.1111/j.1552-6909.2012.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wesseloo R, Kamperman AM, Bergink V, Pop VJM. Thyroid peroxidase antibodies during early gestation and the subsequent risk of first-onset postpartum depression: A prospective cohort study. J Affect Disord. 2018;225:399–403. doi: 10.1016/j.jad.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 32.Krause D, et al. Prenatal immunologic predictors of postpartum depressive symptoms: a prospective study for potential diagnostic markers. Eur Arch Psychiatry Clin Neurosci. 2014;264:615–24. doi: 10.1007/s00406-014-0494-8. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Zhang Y, Gao Y, Zhang Z. Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Res. 2016;243:43–8. doi: 10.1016/j.psychres.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Brann E, et al. Inflammatory markers in late pregnancy in association with postpartum depression-A nested case-control study. Psychoneuroendocrinology. 2017;79:146–159. doi: 10.1016/j.psyneuen.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Dennis CL, et al. Determinants of comorbid depression and anxiety postnatally: A longitudinal cohort study of Chinese-Canadian women. Journal of Affective Disorders. 2017;227:24–30. doi: 10.1016/j.jad.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 36.Dennis CL, Heaman M, Vigod S. Epidemiology of postpartum depressive symptoms among Canadian women: regional and national results from a cross-sectional survey. Can J Psychiatry. 2012;57:537–46. doi: 10.1177/070674371205700904. [DOI] [PubMed] [Google Scholar]
- 37.Turkcapar AF, et al. Sociodemographic and clinical features of postpartum depression among Turkish women: a prospective study. BMC Pregnancy Childbirth. 2015;15:108. doi: 10.1186/s12884-015-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viguera AC, et al. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. Am J Psychiatry. 2011;168:1179–85. doi: 10.1176/appi.ajp.2011.11010148. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed HM, Alalaf SK, Al-Tawil NG. Screening for postpartum depression using Kurdish version of Edinburgh postnatal depression scale. Arch Gynecol Obstet. 2012;285:1249–55. doi: 10.1007/s00404-011-2165-6. [DOI] [PubMed] [Google Scholar]
- 40.Bayrampour H, Tomfohr L, Tough S. Trajectories of Perinatal Depressive and Anxiety Symptoms in a Community Cohort. J Clin Psychiatry. 2016;77:e1467–e1473. doi: 10.4088/JCP.15m10176. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh A, Goswami S. Evaluation of post partum depression in a tertiary hospital. Journal of Obstetrics and Gynecology of India. 2011;61:528–530. doi: 10.1007/s13224-011-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batmaz G, Dane B, Sarioglu A, Kayaoglu Z, Dane C. Can we predict postpartum depression in pregnant women? Clin Exp Obstet Gynecol. 2015;42:605–9. [PubMed] [Google Scholar]
- 43.Gaillard A, Le Strat Y, Mandelbrot L, Keita H, Dubertret C. Predictors of postpartum depression: prospective study of 264 women followed during pregnancy and postpartum. Psychiatry Res. 2014;215:341–6. doi: 10.1016/j.psychres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Woolhouse H, Gartland D, Hegarty K, Donath S, Brown SJ. Depressive symptoms and intimate partner violence in the 12 months after childbirth: a prospective pregnancy cohort study. BJOG. 2012;119:315–23. doi: 10.1111/j.1471-0528.2011.03219.x. [DOI] [PubMed] [Google Scholar]
- 45.Savarimuthu RJ, et al. Post-partum depression in the community: a qualitative study from rural South India. Int J Soc Psychiatry. 2010;56:94–102. doi: 10.1177/0020764008097756. [DOI] [PubMed] [Google Scholar]
- 46.Bauer A, et al. Familiality of psychiatric disorders and risk of postpartum psychiatric episodes: A population-based cohort study. American Journal of Psychiatry. 2018 doi: 10.1176/appi.ajp.2018.17111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ertel KA, Rich-Edwards JW, Koenen KC. Maternal depression in the United States: Nationally representative rates and risks. Journal of Women's Health. 2011;20:1609–1617. doi: 10.1089/jwh.2010.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidebottom AC, Hellerstedt WL, Harrison PA, Hennrikus D. An examination of prenatal and postpartum depressive symptoms among women served by urban community health centers. Arch Womens Ment Health. 2014;17:27–40. doi: 10.1007/s00737-013-0378-3. [DOI] [PubMed] [Google Scholar]
- 49.Robertson-Blackmore E, et al. Antecedent trauma exposure and risk of depression in the perinatal period. J Clin Psychiatry. 2013;74:e942–8. doi: 10.4088/JCP.13m08364. [DOI] [PubMed] [Google Scholar]
- 50.Cerulli C, Talbot NL, Tang W, Chaudron LH. Co-occurring intimate partner violence and mental health diagnoses in perinatal women. J Womens Health (Larchmt) 2011;20:1797–803. doi: 10.1089/jwh.2010.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pooler J, Perry DF, Ghandour RM. Prevalence and risk factors for postpartum depressive symptoms among women enrolled in WIC. Matern Child Health J. 2013;17:1969–80. doi: 10.1007/s10995-013-1224-y. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease, C. & Prevention. Prevalence of self-reported postpartum depressive symptoms-17 states, 2004–2005. MMWR Morb Mortal Wkly Rep. 2008;57:361–6. [PubMed] [Google Scholar]
- 53.Kita S, Haruna M, Matsuzaki M, Kamibeppu K. Associations between intimate partner violence (IPV) during pregnancy, mother-to-infant bonding failure, and postnatal depressive symptoms. Archives of Women's Mental Health. 2016;19:623–634. doi: 10.1007/s00737-016-0603-y. [DOI] [PubMed] [Google Scholar]
- 54.Eastwood JG, Phung H, Barnett B. Postnatal depression and socio-demographic risk: factors associated with Edinburgh Depression Scale scores in a metropolitan area of New South Wales, Australia. Aust N Z J Psychiatry. 2011;45:1040–6. doi: 10.3109/00048674.2011.619160. [DOI] [PubMed] [Google Scholar]
- 55.Choi SY, Kim EJ, Ryu E, Chang KO, Park MN. Postpartum depression and parental self-efficacy: a comparison of native Korean and Vietnamese immigrant mothers in Korea. J Transcult Nurs. 2012;23:181–7. doi: 10.1177/1043659611434057. [DOI] [PubMed] [Google Scholar]
- 56.Lara MA, et al. Prevalence and incidence of perinatal depression and depressive symptoms among Mexican women. J Affect Disord. 2015;175:18–24. doi: 10.1016/j.jad.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 57.Rincon-Pabon D, Ramirez-Velez R. Postnatal depression in Colombian women: secondary analysis of the 2010 Colombian Demographic and Health Survey. Rev Salud Publica (Bogota) 2014;16:534–46. [PubMed] [Google Scholar]
- 58.Sutan R, et al. Psychosocial impact of mothers with perinatal loss and its contributing factors: An insight. Journal of Zhejiang University: Science B. 2010;11:209–217. doi: 10.1631/jzus.B0900245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wisner KL, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490–8. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dolbier CL, et al. Relationships of race and socioeconomic status to postpartum depressive symptoms in rural African American and non-Hispanic white women. Matern Child Health J. 2013;17:1277–87. doi: 10.1007/s10995-012-1123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du Preez A, Conroy S, Pawlby S, Moran P, Pariante CM. Differential effects of ethnic density on the risk of postnatal depression and personality dysfunction. Br J Psychiatry. 2016;208:49–55. doi: 10.1192/bjp.bp.114.148874. [DOI] [PubMed] [Google Scholar]
- 62.Goyal D, Gay C, Lee KA. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Womens Health Issues. 2010;20:96–104. doi: 10.1016/j.whi.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayes DK, Ta VM, Hurwitz EL, Mitchell-Box KM, Fuddy LJ. Disparities in self-reported postpartum depression among Asian, Hawaiian, and Pacific Islander Women in Hawaii: Pregnancy Risk Assessment Monitoring System (PRAMS), 2004–2007. Matern Child Health J. 2010;14:765–73. doi: 10.1007/s10995-009-0504-z. [DOI] [PubMed] [Google Scholar]
- 64.Munger AL, Hofferth SL, Grutzmacher SK. The Role of the Supplemental Nutrition Assistance Program in the Relationship Between Food Insecurity and Probability of Maternal Depression. Journal of Hunger and Environmental Nutrition. 2016;11:147–161. doi: 10.1080/19320248.2015.1045672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang FW, et al. The relationship between economic conditions and postpartum depression in Taiwan: A nationwide population-based study. Journal of Affective Disorders. 2016;204:174–179. doi: 10.1016/j.jad.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 66.Russell RB, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 67.Manuck TA, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215:103 e1–103 e14. doi: 10.1016/j.ajog.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kramer MS, et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;206:108–12. doi: 10.1016/j.ajog.2011.10.864. [DOI] [PubMed] [Google Scholar]
- 69.Jarde A, et al. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2016;73:826–37. doi: 10.1001/jamapsychiatry.2016.0934. [DOI] [PubMed] [Google Scholar]
- 70.Yonkers KA, et al. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry. 2014;71:897–904. doi: 10.1001/jamapsychiatry.2014.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonald SW, Kingston D, Bayrampour H, Dolan SM, Tough SC. Cumulative psychosocial stress, coping resources, and preterm birth. Arch Womens Ment Health. 2014;17:559–68. doi: 10.1007/s00737-014-0436-5. [DOI] [PubMed] [Google Scholar]
- 72.Dole N, et al. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 73.Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychol. 2008;27:194–203. doi: 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ihongbe TO, Masho SW. Do Successive Preterm Births Increase the Risk of Postpartum Depressive Symptoms? J Pregnancy. 2017;2017:4148136. doi: 10.1155/2017/4148136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cruise SM, Layte R, Stevenson M, O'Reilly D. Prevalence and factors associated with depression and depression-related healthcare access in mothers of 9-month-old infants in the Republic of Ireland. Epidemiology and Psychiatric Sciences. 2017:1–11. doi: 10.1017/S2045796017000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Helle N, et al. Very low birth-weight as a risk factor for postpartum depression four to six weeks postbirth in mothers and fathers: Cross-sectional results from a controlled multicentre cohort study. J Affect Disord. 2015;180:154–61. doi: 10.1016/j.jad.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Vigod SN, Villegas L, Dennis CL, Ross LE. Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: a systematic review. BJOG. 2010;117:540–50. doi: 10.1111/j.1471-0528.2009.02493.x. [DOI] [PubMed] [Google Scholar]
- 78.Siu AL, et al. Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380–7. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]


