As a global regulator, RpoN controls a wide range of biological pathways, including virulence in P. aeruginosa PAO1. This work shows that RpoN plays critical and global roles in the regulation of bacterial pathogenicity and fitness. ChIP-seq provided a useful database to characterize additional functions and targets of RpoN in the future. The functional characterization of RpoN-mediated regulation will improve the current understanding of the regulatory network of quorum sensing and virulence in P. aeruginosa and other bacteria.
KEYWORDS: Pseudomonas aeruginosa, quorum-sensing system, T6SS
ABSTRACT
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen of humans, particularly those with cystic fibrosis. As a global regulator, RpoN controls a group of virulence-related factors and quorum-sensing (QS) genes in P. aeruginosa. To gain further insights into the direct targets of RpoN in vivo, the present study focused on identifying the direct targets of RpoN regulation in QS and the type VI secretion system (T6SS). We performed chromatin immunoprecipitation coupled with high-throughput sequencing (ChIP-seq) that identified 1,068 binding sites of RpoN, mostly including metabolic genes, a group of genes in QS (lasI, rhlI, and pqsR) and the T6SS (hcpA and hcpB). The direct targets of RpoN have been verified by electrophoretic mobility shifts assays (EMSA), lux reporter assay, reverse transcription-quantitative PCR, and phenotypic detection. The ΔrpoN::Tc mutant resulted in the reduced production of pyocyanin, motility, and proteolytic activity. However, the production of rhamnolipids and biofilm formation were higher in the ΔrpoN::Tc mutant than in the wild type. In summary, the results indicated that RpoN had direct and profound effects on QS and the T6SS.
IMPORTANCE As a global regulator, RpoN controls a wide range of biological pathways, including virulence in P. aeruginosa PAO1. This work shows that RpoN plays critical and global roles in the regulation of bacterial pathogenicity and fitness. ChIP-seq provided a useful database to characterize additional functions and targets of RpoN in the future. The functional characterization of RpoN-mediated regulation will improve the current understanding of the regulatory network of quorum sensing and virulence in P. aeruginosa and other bacteria.
INTRODUCTION
Pseudomonas aeruginosa is an important Gram-negative opportunistic pathogen that leads to chronic lung infection, pulmonary inflammation, and soft-tissue and other types of infections in susceptible hosts (1), accounting for 10% of all hospital-acquired infections (2). Chronic infection and pulmonary inflammation associated with P. aeruginosa are the main factors of mortality in patients with cystic fibrosis (CF) (1). P. aeruginosa produces many virulence-associated factors, such as flagella, type IV pili, exopolysaccharide alginate, lipopolysaccharide, toxins, pyocyanin, proteases, lipases, and rhamnolipids (3, 4), half of which are strictly controlled by quorum-sensing (QS) systems. P. aeruginosa has one of the most intricate QS systems of all bacterial species, which includes las and rhl systems, based on N-acyl-homoserine lactones (AHLs) (5, 6), and the pqs system, based on 2-alkyl-4-quinolone (AQ) (7). Among these systems, a group of key transcriptional regulators, such as VqsM (8), VqsR (9), QscR (10), RpoN, GacA, Vfr, CdpR (11), and RsaL (12), form a hierarchical regulatory network (13). In addition, a fourth QS signal, 2-(2-hydroxylphenyl)-thiazole-4-carbaldehyde (IQS), regulates the las system and phosphate stress response to the pqs and rhl systems (14).
As one of the key regulators in P. aeruginosa virulence networks, RpoN (sigma factor σ54) has been well studied for 30 years. RpoN plays differential roles in the pathogenicity of the PA14 strain in different hosts, including plants, nematodes, insects, and mice (15). RpoN regulates a wide range of biological pathways, including flagellum synthesis, metabolism, and antibiotic resistance. RpoN affects the expression of nitrogen-regulated genes (ntrBC, glnA, glnK-amtB, nirBD, nasA, nasST and nosRZDFYL) at the transcription level (16). A previous microarray study in the PAK strain also shows that RpoN regulates 62 genes, including a group of flagellum-related genes (fleSR, fliEFGHIJ, flhA, flhF, fleN, flgA, fliLMNOPQR, and flhB) (17).
RpoN regulates the metabolism of a wide range of metabolites. In Pseudomonas putida, RpoN affects the utilization of nitrate, urea, and uncharged amino acids as nitrogen sources, as well as lysine, C4-dicarboxylates (succinate and fumarate), and alpha-ketoglutarate as carbon sources (18, 19). Additionally, the expression of oprE is controlled by RpoN under aerobic conditions (20). RpoN also regulates the susceptibility to tobramycin (21), quinolones, and carbapenems (22, 23) in P. aeruginosa.
A group of the enhancer-binding proteins (EBPs) interact with RpoN to activate the transcription of target genes (see Table S1 in the supplemental material). For example, DdaR responds to aromatic amino acids (24). RpoN regulates the glyoxylate pathway and ethanolamine catabolism via EatR (25). (R)-3-Hydroxybutyrate induces the expression of bdhA, which is dependent on RpoN and HbcR (26). GcsR, a TyrR-like EBP, controls the glycine cleavage system and pyocyanin biosynthesis in P. aeruginosa (27, 28). MifR interacts with RpoN to induce the transcription of PA5530 by sensing extracellular C5-dicarboxylates (29).
The expression of approximately 20% of the genome is regulated by RpoN in P. aeruginosa strains (30). A recent study revealed regulons of all sigma factors, indicating that RpoN plays a dominant role in sigma factor networks (31). RpoN also has profound effects on QS systems (32–34), the type VI secretion system (T6SS) (35), and virulence (15). RpoN plays different roles in regulating QS in various P. aeruginosa strains. For instance, the QS system in the PAO1 strain is negatively regulated by RpoN (33). However, RpoN positively regulates the expression of rhlI in the PA01UNSW strain (34) and positively controls virulence factors via PqsR in PAO1 (32). RpoN also negatively affects the levels of SadB, which contributes to biofilm formation in PA14 (36).
The T6SS is a contact-dependent bacteriophage-like secretion machinery that directly translocates bacterial toxins into other bacteria as well as eukaryotic cells (37). The T6SS has been found in many important Gram-negative pathogens, such as P. aeruginosa (38), avian pathogenic Escherichia coli (39), Burkholderia thailandensis (40), Vibrio cholerae (41), and Serratia marcescens (42). Three T6SS loci (H1-, H2-, and H3-T6SS) have been identified in P. aeruginosa (38). Transcriptomics analysis revealed that las-QS and rhl-QS systems regulate HSI-2 (43), but the specific regulation mechanism is still not fully characterized. RpoN induces the left H3-T6SS (driven bylip3) but inhibits the right H3-T6SS (driven by hsiB3), as well as H2-T6SS, in PAO1 (35), indicating that RpoN regulates P. aeruginosa T6SS gene expression in a divergent manner. However, the molecular regulatory mechanism of RpoN on the T6SS needs further examination.
In summary, RpoN displays complicated regulatory roles in different strains and diverse hosts. The present study focused on identifying the direct targets of RpoN in QS and the T6SS. Chromatin immunoprecipitation sequencing (ChIP-seq) and transcriptome sequencing (RNA-seq) verified five direct targets of RpoN via biochemical and genetic approaches. These RpoN targets are associated with various virulence factors, including pyocyanin, rhamnolipid, swarming motility, proteolytic activity, and PQS. RpoN directly regulated QS by binding to the promoters of lasI, rhlI, and pqsR. RpoN also controlled the transcription of the T6SS genes hcpA and hcpB. Overall, the results of the present study improved the current understanding of RpoN-mediated regulation in P. aeruginosa.
RESULTS
ChIP-seq analysis revealed the direct RpoN targets, including QS and T6SS genes, in the P. aeruginosa genome.
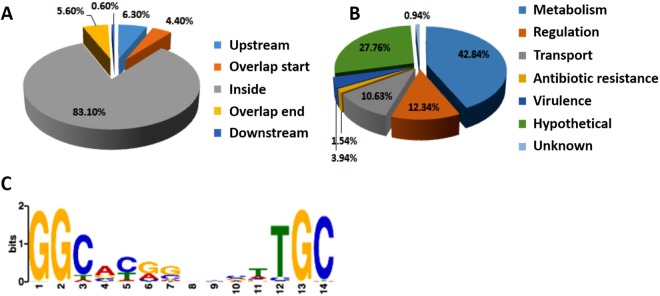
To identify targets of RpoN in vivo, a global ChIP-seq assay was performed in the P. aeruginosa PAO1 strain overexpressing vesicular stomatitis viral glycoprotein (VSV)-tagged, full-length rpoN in the pAK1900 plasmid. The wild-type PAO1 strain containing an empty pAK1900 vector was used as the control. Each experiment was repeated twice. The VSV-tagged RpoN could fully complement the reduced production of pyocyanin and proteolytic activity in the rpoN insertion-deletion mutant, indicating that the VSV tag does not interfere with the function of RpoN (see Fig. S1A and B in the supplemental material). A total of 1,068 enriched loci containing specific RpoN-binding peaks (Table S4) were identified with significant enrichments compared with the control strain carrying empty pAK1900 (P value of e−5). Notably, 10.7% of all peaks were in the promoter or intergenic regions (Fig. 1A). These genes were equipped with a variety of physiological functions, including antibiotic resistance (1.5%), regulation (12.3%), virulence (3.9%), transportation (10.6%), metabolism (42.8%), and hypothetical proteins (27.7%) (Fig. 1B). Notably, RpoN directly bound to the promoter regions of several transcriptional regulator genes, including PA0243, aguR, argR, fleQ, PA2704, PA3249, PA3782, and PA4021. More than 40% of the targets were metabolic genes involved in multiple pathways, including translation and the tricarboxylic acid cycle. In addition, the genes involved in flagellar biosynthesis (such as flgF, pilG, flgB, flgJ, flgK, fliC, fliE, fliO, and fliR) and catabolism genes, gbt (encoding glycine betaine transmethylase), PA4616 (encoding a probable C4-dicarboxylate-binding protein), and aroP1 (encoding aromatic amino acid transport protein AroP1), were also present in the ChIP-seq data. Overall, these target genes indicated that RpoN is closely associated with major metabolic pathways and virulence factors. RpoN-bound QS genes include hacB (encoding acylhomoserine lactone acylase B), pmpR (encoding pqsR-mediated PQS regulator), and pvdQ (encoding 3-oxo-C12-homoserine lactone acylase) (Table S4). Additionally, 11 other genes, hcpA, hcpB, vgrG2b, fha2, tssF1, tssl1, icmF2, hsiA3, clpV3, hsiG3, and tsi2, are associated with the T6SS (Table S4). Taken together, the ChIP-seq data suggested the direct regulation of RpoN on las and pqs systems in QS as well as the T6SS.
FIG 1.
ChIP-seq assay revealed in vivo binding sites of RpoN in the P. aeruginosa genome. (A) Pie chart of 1,068 RpoN-binding peaks. (B) Pie chart displaying the percentage of RpoN targets with functional categories defined in the Pseudomonas database (http://pseudomonas.com). (C) Most significant motif derived from ChIP-seq binding sequence returned by the MEME tool. The height of each letter represents the relative frequency of each base at different positions in the consensus sequence.
In addition, the ChIP-seq results confirmed the motif of RpoN. By using the MEME suite, a 14-bp RpoN-binding sequence, GG(C/T/A)(A/G/C)(C/T/A)(G/A/C)(G/C)N3(T/A)(T/C)GC, was confirmed (Fig. 1C), which is consistent with the previously identified motif (31, 44).
Transcriptomics profiling of the rpoN deletion strain revealed its regulation in QS and the T6SS.
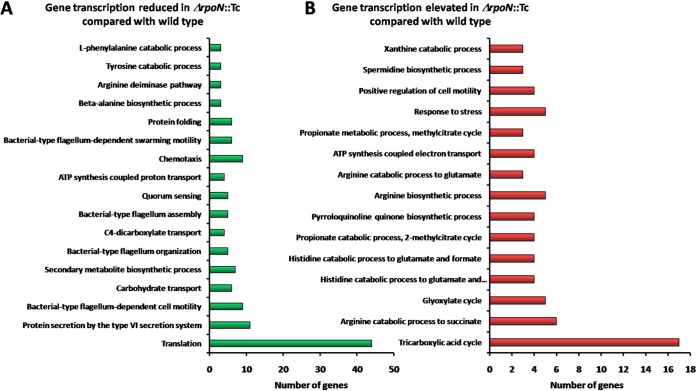
To explore the biological roles of RpoN in P. aeruginosa, an rpoN insertion-deletion mutant carrying a tetracycline resistance marker (ΔrpoN::Tc) was constructed with homologous recombination technology as described in a previous study (45). Given that many virulence genes were highly expressed in the mid-log phase in a previous study on CdpR (11), AlgR (46), and VqsM (8), we performed RNA-seq analysis in the mid-log phase to compare the transcriptome of the ΔrpoN::Tc strain with that of the wild-type PAO1 strain. We measured the expression of rpoN by reverse transcription-quantitative PCR (RT-qPCR) in wild-type strain PAO1 (carrying empty pAK1900), the ΔrpoN::Tc strain (carrying empty pAK1900), and the complementary strain ΔrpoN::Tc (carrying pAK1900-rpoN). We could not detect the expression of rpoN in the ΔrpoN::Tc strain, which would be well complemented by pAK1900-rpoN (Fig. S1C). Each sample was repeated twice. A total of 324 genes with increased transcriptional levels (≥2-fold) and 390 genes with decreased transcription levels (≥2-fold) (Table S2) were identified in the ΔrpoN::Tc strain versus the wild-type PAO1 strain. These 714 genes represent 12.8% of all genes in the P. aeruginosa PAO1 genome. Notably, 71% of these genes encode hypothetical proteins of unknown functions. Among the annotated genes (29% of 714), 64.3% of these genes were downregulated and 35.7% of these genes were upregulated in the ΔrpoN::Tc strain. The downregulated genes are involved in translation, bacterial-type flagellum organization, assembly and motility, protein secretion in the T6SS, chemotaxis, protein folding, secondary metabolite biosynthetic process, and quorum sensing (Fig. 2A). The upregulated genes primarily belong to the tricarboxylic acid cycle, arginine catabolic and biosynthetic process, glyoxylate cycle, histidine catabolic process, and response to stress (Fig. 2B).
FIG 2.
Functional profiling of the RNA-seq in P. aeruginosa. DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/home.jsp) was used to categorize members with gene transcription reduced in the ΔrpoN::Tc strain compared with the wild type (A) and gene transcription elevated in the ΔrpoN::Tc strain compared with the wild type (B). The enrichment of specific gene classes is displayed. The P values of the enriched categories are provided in Table S4.
In particular, a series of QS genes (lasI, lasB, pqsA, pqsH, pqsR, pqsB, pqsC, pqsD, pqsE, pqsL, and phnA) and T6SS genes (hcpA, hcpB, hcp1, clpV1, and vgrG1) were highlighted in the RNA-seq data, and all of these genes were downregulated in the RpoN mutant (Table S5). Many of these genes (such as lasI, pqsR, hcpA, and hcpB) were also identified in the ChIP-seq and previous data (31), prompting us to test the RpoN-mediated regulation of these genes.
To further verify the RNA-seq data on these selected QS or T6SS genes, we performed RT-qPCR to examine their transcriptional expression in the ΔrpoN::Tc strain and the wild-type PAO1 strain, and 16S rRNA was used as an internal control. The transcription levels of these targets were verified in the ΔrpoN::Tc strain and wild-typePAO1 strain (see Fig. 3D, 4D, and 6C). In summary, the RNA-seq data showed that RpoN regulates hundreds of genes involved in various metabolic pathways, flagellar motility, and virulence factors (especially QS and T6SS), providing a useful database for further characterization of RpoN regulatory networks.
FIG 3.
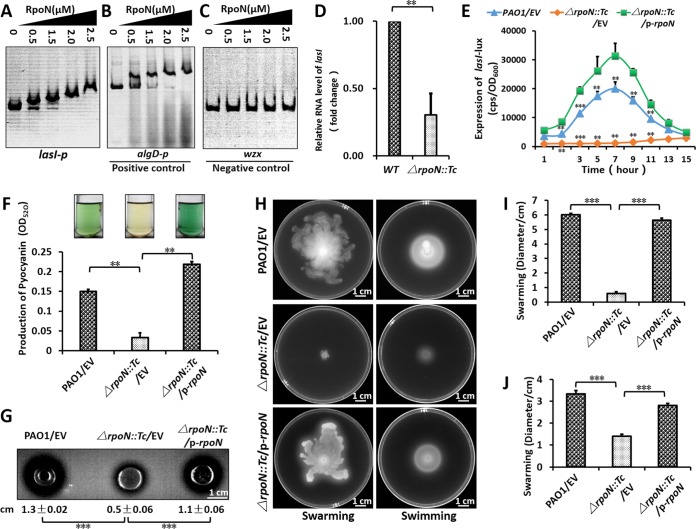
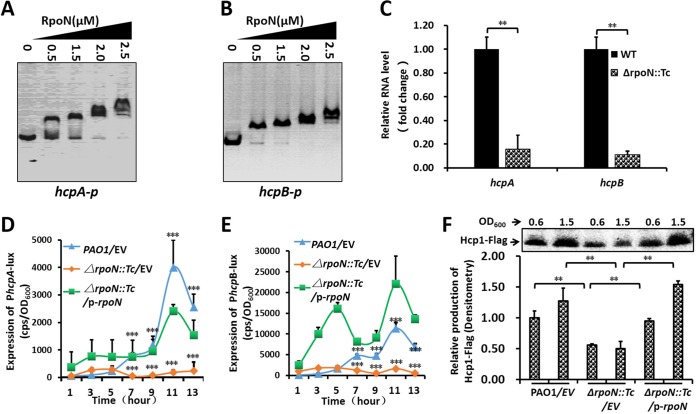
RpoN positively regulated the las-QS system by lasI. (A to C) EMSA showed that RpoN directly bound to the rsaL-lasI intergenic region (A) and the promoter of algD (B) but did not bind to the promoter of wzx (C). PCR products containing the lasI, algD, and wzx promoter regions were added to the reaction mixtures at 30 ng for each well. RpoN protein was added to reaction buffer in lanes with 0, 0.5, 1.25, 2.0, and 2.5 μM. (D) The relative expression of lasI was downregulated in the rpoN mutant. The relative gene expression level in the wild-type PAO1 was set to 1, and the other values were adjusted accordingly. (E) Expression of lasI-lux in PAO1 and its derivatives. Bacteria were grown in LB at 37°C for 12 h with shaking (220 rpm), and then the lasI-lux activity was measured. CPS (counts per second) values represent relative promoter-lux activities. All experiments were independently repeated at least three times, and the data shown represent comparable results. Values represent means ± standard errors of the means (SEM). (F) P. aeruginosa PAO1 and its derivatives were grown in LB medium at 37°C for 16 h with shaking (220 rpm); the presence of the blue-green pigment indicates pyocyanin production. (G) Proteolytic activity of three P. aeruginosa strains on milk plates. (H) Effect of RpoN mutation on motility. Overnight cultures were spotted onto swarming plates (2-μl aliquots), and the plates were incubated at 37°C. The images were captured after 20 h of growth. The experiments were repeated at least three times, and similar results were observed. (I and J) The quantitative analysis (diameter of motility locus) of swarming (I) and swimming (J). P < 0.01 (**) and P < 0.001 (***) compared to the wild-type or complemented strain by Student's t test. Results represent means ± standard deviations (SD), and data are representative of at least three independent experiments. EV represents empty vector pAK1900; p-RpoN represents pAK1900-rpoN.
FIG 4.
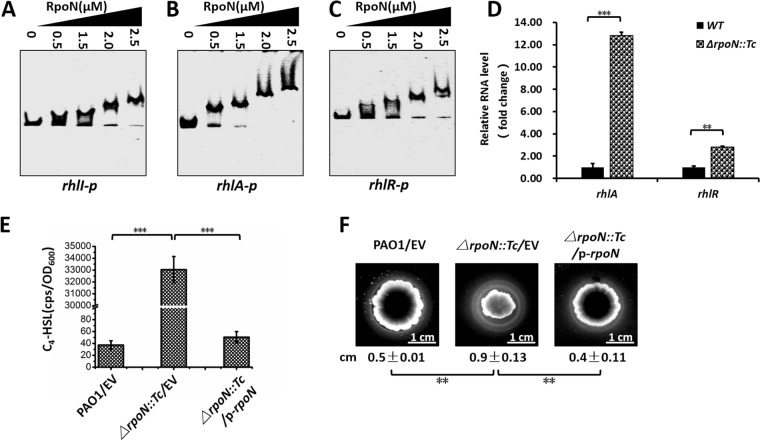
RpoN negatively regulated the rhl-QS system. (A to C) EMSA showed that RpoN directly bound to the rhlI (A), rhlA (B), and rhlR (C) promoter regions. PCR products containing the rhlI, rhlA, and rhlR promoter regions were added to the reaction mixtures at 30 ng in each well. RpoN protein was added to reaction buffer in lanes at 0, 0.5, 1.25, 2.0, and 2.5 μM. (D) The relative expression of rhlA and rhlR upregulated in ΔrpoN::Tc strain. The relative gene expression level in wild-type PAO1 was set to 1, and the other values were adjusted accordingly. (E) Relative amount of C4-HSL measured by pKD-rhlI plus pMCSG19-rhlR in the DH5α system. E. coli DH5α containing pKD-rhlI and pMCSG19-rhlR plasmids, PAO1, and its derivatives were grown in LB medium at 37°C for 12 h with shaking (220 rpm). The E. coli DH5α culture, PAO1, and its derivative cultures then were mixed at 37°C for 4 h with shaking (220 rpm). The mixed cultures were subsequently measured for their relative C4-HSL contents. Plasmid pKD-rhlI carries the C4-HSL-responsive rhlI promoter fused to luxCDABE, so CPS values become an indirect measure of supernatant C4-HSL. (F) Bacterial strains were inoculated onto cetyltrimethylammonium bromide (CTAB) plates and incubated at 37°C for 24 h and then for 36 h at room temperature. The presence of a halo surrounding the colonies indicates the production of rhamnolipids. P < 0.01 (**) and P < 0.001 (***) compared to the wild-type or complemented strain by Student's t test. Results represent means ± SD, and data are representative of at least three independent experiments.
FIG 6.
RpoN positively regulated hcpA and hcpB in T6SS. (A and B) EMSA showed that RpoN directly bound to hcpA (A) and hcpB (B) promoter regions. PCR products containing the hcpA and hcpB promoter regions were added to the reaction mixtures at 30 ng for each well. RpoN protein was added to reaction buffer in lanes at 0, 0.5, 1.25, 2.0, and 2.5 μM. (C) The relative expression of hcpA and hcpB downregulated in the RpoN mutant. The relative gene expression level in the wild-type PAO1 was set to 1, and the other values were adjusted accordingly. (D and E) Expression of hcpA-lux (D) and hcpB-lux (E) in PAO1 and its derivatives. Bacteria were grown in LB 37°C for 12 h with shaking (220 rpm), and then the promoter-lux activity was measured. (F) The expression of Hcp1 was downregulated in the ΔrpoN::Tc strain. The relative Hcp1-Flag expression level in wild-type PAO1 (OD600) was set to 1, and the other values were adjusted accordingly. P < 0.01 (**) and P < 0.001 (***) compared to the wild-type or complemented strain by Student's t test. Results represent means ± SD, all experiments were independently repeated at least three times, and the data shown represent comparable results. EV, empty vector.
RpoN positively controlled the las system in P. aeruginosa QS.
To determine the direct link between RpoN and virulence genes, lasR and lasI were identified in the present ChIP-seq results and those of a previous study (31). Since RpoN is able to bind promoter DNA in the absence of core RNA polymerase in E. coli, we performed electrophoretic mobility shifts assays (EMSA) without a core RNA polymerase (RNAP) (47). We first purified RpoN protein (Fig. S2A) with a 6× His tag EMSA. The results verified that RpoN directly bound to the lasI-rsaL intergenic region (Fig. 3A). The positive-control algD (encoding GDP-mannose 6-dehydrogenase AlgD) (34, 44) was bound by RpoN (Fig. 3B), while the promoter of wzx, a negative control, was not bound by RpoN at the same concentration (Fig. 3C). We also expressed recombinant RpoF (FliA) with a 6× His tag as a control for RpoN specificity (Fig. S2B), which demonstrated that RpoN and RpoF could specifically bind to the promoters of lasI and fliC, respectively (Fig. S2C and E) (48). As a negative control, RpoF could not bind to QS genes (lasI, rhlR, and pqsR) and a T6SS gene (hcpA) (Fig. S2D and F to H) at the same protein concentration.
RT-qPCR showed that the expression of lasI in the ΔrpoN::Tc strain was compromised compared with that of the wild-type PAO1 strain, indicating that RpoN regulates lasI (Fig. 3D). We then measured the promoter activities of lasR and lasI by constructing their promoter-lux fusions in the pMS402 plasmid. The expression of the lasI promoter-lux fusion in ΔrpoN::Tc was significantly lower than that in other strains (Fig. 3E), while the expression of the lasR promoter-lux fusion had no significant difference in the tested strains (Fig. S3). We next examined pyocyanin production, swimming motility, and proteolytic activity in the wild-type PAO1 strain, the ΔrpoN::Tc strain, and the complemented (ΔrpoN::Tc/p-rpoN) strain. Pyocyanin production, proteolytic activity, and motility were all significantly compromised in the ΔrpoN::Tc strain compared to those in the other two strains (Fig. 3F to I). Taken together, these results indicate that RpoN positively regulated the las-QS system by directly binding to the promoter of lasI to control virulence.
RpoN repressed the rhl-QS system and the production of rhamnolipids.
We also attempted to verify the ChIP-seq data on rhl genes in vitro by EMSA. The results showed that RpoN directly bound to the promoters of rhlI, rhlA, and rhlR (Fig. 4A to C), which are all key genes in rhl-QS. We then identified similar RpoN-binding motifs in these promoters (Fig. S4). We further performed RT-qPCR, which showed that the expression of rhlA and rhlR was higher in the ΔrpoN::Tc strain than in the wild-type strain (Fig. 4D).
We also measured the level of N-butyryl-l-homoserine lactone (C4-HSL) in these three strains using an E. coli DH5a strain carrying pKD-rhlI-lux and pMCSG19-rhlR in LB (Luria-Bertani) medium. The luminescence value (counts per second [CPS]) represented the promoter activity of rhlI, which encodes the biosynthesis of autoinducer synthesis protein RhlI. We found that the ΔrpoN::Tc strain produced excessive C4-HSL compared with the wild-type strain and the RpoN-complemented (ΔrpoN::Tc/p-rpoN) strain (Fig. 4E), confirming the negative regulatory role of RpoN in the rhl-QS system.
Strictly regulated by the rhl system, rhamnolipids are glycolipidic biosurfactants with different structures and roles in P. aeruginosa (49, 50). Rhamnolipids are also important immune modulators and virulence determinants in P. aeruginosa pathogenicity (49, 51).We tested the production of rhamnolipids by measuring the size of the transparent zones around the colonies by using cetyltrimethylammonium bromide (CTAB) plates, which showed that the production of rhamnolipids was significantly higher in the ΔRpoN strain than in the wild-type PAO1 strain and the complemented (ΔrpoN::Tc/p-rpoN) strain (Fig. 4F). Taking these results together, we concluded that RpoN represses the biosynthesis of rhamnolipids and C4-HSL via controlling the expression of rhl genes, thus regulating the rhl-QS system in P. aeruginosa.
RpoN positively regulated the pqs-QS system and the biosynthesis of PQS.
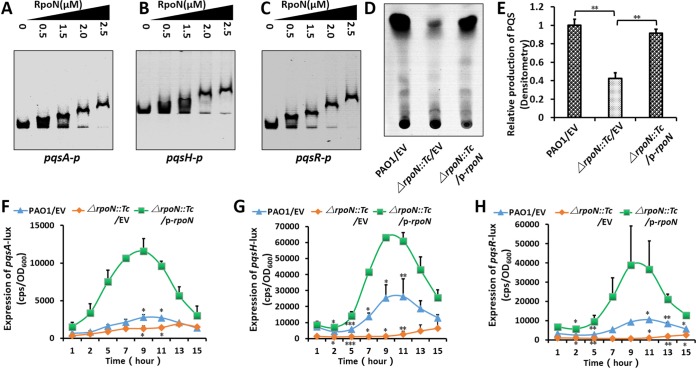
Interestingly, three pqs genes were targets of RpoN. EMSA results showed that RpoN directly bound to the promoters of pqsA, pqsH, and pqsR (Fig. 5A to C), suggesting the direct regulation of the pqs-QS system through RpoN. In addition, we localized RpoN motifs in the promoters of pqsA and pqsR by EMSA using an array of promoter deletions (Fig. S5 and S6).
FIG 5.
RpoN positively regulated the pqs-QS system. (A to C) EMSA showed that RpoN directly bound to pqsA (A), pqsH (B), and pqsR (C) promoter regions. PCR products containing the pqsA, pqsH, and pqsR promoter regions were added to the reaction mixtures at 30 ng for each well. RpoN protein was added to reaction buffer in lanes at 0, 0.5, 1.25, 2.0, 2.5 μM. (D) The production of PQS was decreased in the ΔrpoN::Tc strain. Lanes 1 to 3 represent wild-type PAO1, the ΔRpoN strain, and the ΔRpoN complemented strain, respectively. (E) The relative quantitative analysis (percentage of value for grey-shaded bar) of PQS was also compared among three strains. (F to H) Expression of pqsA-lux (F), pqsH-lux (G), and pqsR-lux (H) in PAO1 and its derivatives. Bacteria were grown in LB at 37°C for 12 h with shaking (220 rpm), and then pqsA-lux, pqsH-lux, and pqsR-lux activity was measured. P < 0.05 (*) and P < 0.01 (**) compared to the wild-type or complemented strain by Student's t test. Results represent means ± SD, all experiments were independently repeated at least three times, and the data shown represent comparable results. EV, empty vector.
This result prompted us to measure the production of PQS by thin-layer chromatography (TLC). As expected, PQS production was compromised in the ΔrpoN::Tc strain compared with that in the wild-type strain and the complemented (ΔrpoN::Tc/p-rpoN) strain (Fig. 5D and E). We next examined the activity of pqsA, pqsH, and pqsR promoter-lux fusions in LB broth, which showed that their activities in the ΔrpoN::Tc strain were all significantly lower than that in the wild-type PAO1 strain and the complemented (ΔrpoN::Tc/p-rpoN) strain (Fig. 5F to H). Collectively, RpoN directly regulated pqsA, pqsH, and pqsR to promote the production of PQS, thus controlling the pqs-QS system in P. aeruginosa.
RpoN positively controlled the T6SS by targeting hcpA and hcpB.
The T6SS protects P. aeruginosa by directly translocating toxins to its neighboring bacteria and secreting virulence effectors into the host cells. The T6SS machinery contains two parts, a bacteriophage-like structure and a membrane anchor, to penetrate target cell membranes and secrete effector proteins (37, 52). P. aeruginosa encodes three T6SS Hcp secretion islands (H1, H2, and H3), with different roles in interactions between these bacteria and other organisms (53). The T6SS is regulated at several levels by diverse mechanisms in P. aeruginosa.
In the present study, we selected hcpA and hcpB as the targets of RpoN to control the T6SS. RpoN directly bound to the promoter regions of hcpA and hcpB (Fig. 6A and B), which encode Hcp1-family T6SS effectors. We then searched for RpoN motifs in the hcpA and hcpB promoters by EMSA using different lengths of promoter deletions. As a consequence, we confirmed the specific RpoN motifs in both promoters (Fig. S7 and S8), with GC being less conserved than the consensus motifs in other RpoN targets. These RT-qPCR results showed that the transcription of hcpA and hcpB was significantly lower in the ΔrpoN::Tc strain than that in the wild-type strain (Fig. 6C). The expression of hcpA and hcpB promoter-lux fusion in the ΔrpoN::Tc strain was lower than that in the wild-type strain and the complemented (ΔrpoN::Tc/p-rpoN) strain (Fig. 6D and E).
The promoter and coding region of hcp1 was tagged with FLAG and integrated into the genomes of the wild-type and ΔrpoN::Tc strains by pUC18-mini-Tn7T-Gm. The expression of Hcp1 was visualized by Western blotting with an anti-FLAG antibody, which showed that the expression of Hcp1 was significantly lower in the ΔrpoN::Tc mutant than in wild-type PAO1(pAK1900) and its complemented (ΔrpoN::Tc/p-rpoN) strain (Fig. 6F). The insertions at attTn7 were assessed by colony PCR with the primers PTn7L/PglmS-up and PTn7R/PglmS-down, which produced 287-bp and 272-bp products, respectively (Fig. S9A). Either untagged or FLAG-tagged Hcp1 fully restored the production of pyocyanin and proteolytic activity in the ΔrpoN::Tc strain (Fig. S9B and C), indicating that the FLAG tag did not interfere with the function of RpoN. In summary, we demonstrated that RpoN positively mediates the T6SS via controlling the expression of hcpA and hcpB, indicating new directions in investigating RpoN functions in the future.
RpoN affected biofilm formation.
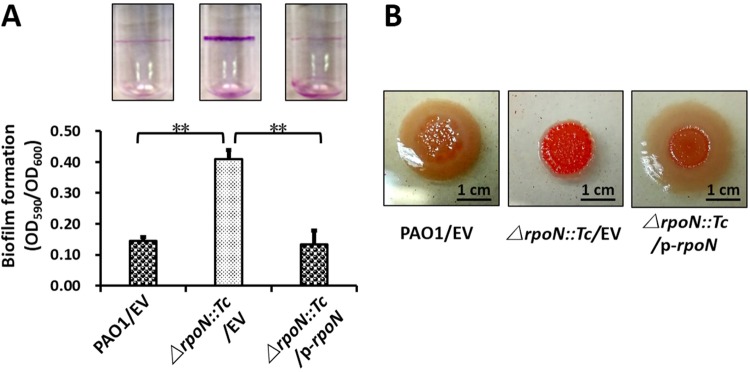
As mentioned above, RpoN directly regulated QS systems, which are key determinants of the virulence-related phenotypes in P. aeruginosa. Biofilm formation is a sophisticated regulatory procedure regulated by a variety of regulators in P. aeruginosa, particularly QS systems. Since RpoN strictly regulates the QS system in the present study, we hypothesized that RpoN controls biofilms in P. aeruginosa. Biofilm production was detected in polystyrene tubes with crystal violet staining. After incubation at 30°C for 24 h, the ΔrpoN::Tc strain produced more biofilm than either the wild-type strain or the complemented strain, which had more than a 2-fold difference after dissolving the remaining crystal violet with 95% ethanol (Fig. 7A). The exopolysaccharides (EPS) stained by Congo red were elevated in the rpoN::Tc mutant compared with that in the wild-type strain and the complemented strain, suggesting the negative regulation of RpoN to biofilm EPS. In addition, the colony morphology of the ΔrpoN::Tc strain displayed a deep red color on the Congo red M9 agar plate, but the wild-type and the complemented (ΔrpoN::Tc/p-rpoN) strains displayed a yellow color (Fig. 7B), indicating that the ΔrpoN::Tc strain produced more EPS. Collectively, we verified that RpoN negatively regulates biofilm formation in the P. aeruginosa PAO1 strain.
FIG 7.
RpoN affected biofilm formation. (A) Quantification of CV staining of biofilm grown in borosilicate tubes at 24 h after standing incubation at 30°C. Photos of the tubes from the binding assay were taken. (B) The deletion of rpoN changed the production of exopolysaccharides (EPS) in P. aeruginosa. Different levels of red of colony morphology on the Congo red plate represent the relative amounts of EPS. Results represent means ± SEM, and data are representative of three independent experiments.
DISCUSSION
A previous microarray study shows that 62 genes are regulated by RpoN in the PAK strain (17). Among these genes, 36 were identified in the present RNA-seq data (see Fig. S10A in the supplemental material). Flagellar biogenesis in P. aeruginosa is strictly controlled by a four-tiered transcriptional regulatory circuit, coregulated by FleQ, FleR, and RpoN. The results of the present study revealed that RpoN regulates additional biological pathways, including quorum sensing, T6SS, chemotaxis, protein folding, secondary metabolite biosynthetic process, and those for many hypothetical genes.
Although most of the results obtained in the present study have been previously demonstrated by ChIP-seq and RNA-seq in the P. aeruginosa PA14 strain (31), there are significant differences between the present study and previous reports. (i) The two regulons are different; (ii) PAO1 is the model strain in the present study, while the previous studies examined PA14; and (iii) more importantly, the present study provided significant biochemical and genetic evidence (such as EMSA, RT-qPCR, Lux reporter assay, and multiple phenotypes) to confirm the direct regulation of RpoN on QS (las, rhl, and pqs) and the T6SS. Additionally, we identified several new direct targets of RpoN, such as lasI, rhlR, rhlI, pqsR, pqsA, hcpA, and hcpB. We compared the present data in PAO1 to previous results in PA14 (31). There are 26 shared genes whose promoters bind to RpoN in PAO1 (Fig. S10B), which indicated that RpoN had these same targets in both PAO1 and PA14 strains. RNA-seq data in PA14 shared 81 genes with PAO1 according to the present results (Fig. S10C), and 55.4% of these genes were downregulated (Fig. 2A) while 44.6% of these genes were upregulated in theΔrpoN::Tc mutant (Fig. 2B). Moreover, the present ChIP-seq and RNA-seq data shared 130 genes in the PAO1 strain (Fig. S10D). However, the culture condition and sampling times of these two studies are different (mid-log phase in LB for PAO1 versus early stationary phase and nitrogen limitation for PA14), which might lead to the differences in the results (31). We compared our present RNA-seq data with the previous results in PA14. A group of genes associated with flagella (flhA and flgB), rhl-QS (rhlA and rhlR), and las-QS (lasI) shared similar patterns under both conditions. On the other hand, pqs-QS genes (pqsR, pqsA, and pqsE) and nitrogen-related genes (glnA and glnK) are differentially regulated by RpoN under two conditions. To test the possible importance of sampling times, we used RT-qPCR to examine the 15 genes in the present RNA-seq data in both growth phases, and the results suggested that RpoN-dependent regulation has a stronger effect in the stationary phase than in the mid-log phase. For example, the expression of the 11 genes (flgB, PA1093, PA1096, pilA, crcZ, hslU, PA1041, pqqD, PA4387, mmsA, and rhlA) was higher in the stationary phase than in the mid-log phase. Additionally, PA1657, PA3908, dnaJ, and rhlR showed different regulatory directions between the mid-log and stationary phases (Fig. S11). Transcription factors, particularly sigma factors, should mostly bind to promoter regions. We found that the majority of RpoN-binding peaks were located in coding regions of the genome, which is similar to the results of previous studies on CdpR (11), AlgR (46), and VqsM (8), where the coding regions accounted for 71%, 55%, and 50% of all peaks, respectively. We showed direct binding between these transcription factors and peaks in coding regions, suggesting that these proteins have additional complicated regulatory functions in recognizing coding regions.
Previous studies have shown that RpoN positively regulates the expression of RhlI in PA01UNSW and PqsR in PAO1 (32, 34). Previous ChIP-seq analysis in PA14 also identified a group of QS genes (31). A recent study showed that the expression of RpoN* (RpoN molecular roadblock, binds to RpoN motif) reduced the production of protease, pyocyanin, pyoverdine, rhamnolipid, and biofilm (54). In contrast, Heurlier et al. showed that in the ΔrpoN::Tc mutant, the expression of lasR and lasI was higher at an early growth stage (33). The network of RpoN-dependent regulation in P. aeruginosa is complicated. The differences among individual studies may result from differences in culture conditions or the source of the strains. Direct biochemical evidence of RpoN-mediated regulation on QS is still largely elusive. In the present study, we found that RpoN directly bound and regulated key genes in three major QS systems (las, rhl, and pqs) (Fig. 3), providing the direct targets of RpoN on QS. In addition, RpoN regulates the left H3-T6SS (driven by lip3) and the right H3-T6SS (driven by hsiB3) (35). In a previous study, we found that RpoN directly bound to the T6SS effector genes hcpA and hcpB (Fig. 6).
In the present EMSAs, multiple shifts suggest multiple binding sites of RpoN. RpoN binds to cognate promoters containing a (GG}N10(GC) motif, such as algD (34, 44), which was used as a positive control (Fig. 3B). We also demonstrated that pqsA and pqsR contain similar motifs, which was also verified by EMSA using the upstream regions without the motif (Fig. S5 and S6). The (GG)/(GC) sequences in the RpoN motif were variable compared to those initially determined by a previous study (55), which explains the less conserved GC sequences in the promoters of hcpA and hcpB (Fig. S7 and S8). The binding sites inside transcriptional start sites (TSS) normally lead to negative regulation, while the binding sites outside TSS typically result in positive regulation (56).
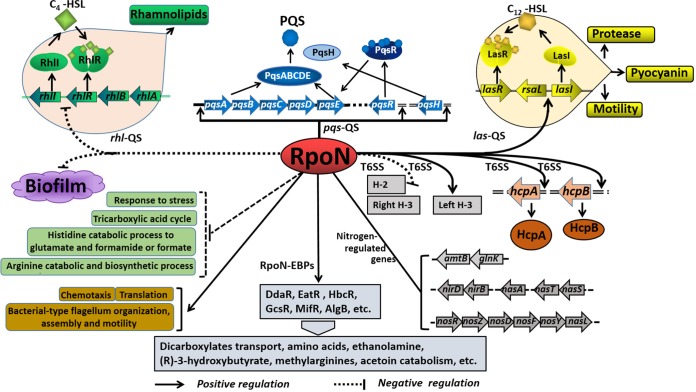
In the present study, we uncovered the RpoN-controlled pathways in the QS system, the T6SS, and biofilm biosynthesis in P. aeruginosa. As shown in Fig. 8, RpoN directly controls QS (las, rhl, and pqs), T6SS, and other virulence factors. Moreover, the deletion of rpoN leads to significantly compromised pyocyanin production, motility, and proteolytic activity (Fig. 3F to J), as well as the level of the signal molecule PQS (Fig. 5D and E). However, the production of both rhamnolipids and biofilm in the ΔrpoN::Tc strain was higher than that in the wild-type and complemented strains (Fig. 4F and 7A). A group of major metabolic pathways, such as those for the tricarboxylic acid cycle, arginine catabolic and biosynthetic processes, and histidine catabolism to glutamate and formamide or formate are all upregulated in the ΔrpoN::Tc strain (Fig. 2A). Other pathways, such as those for translation, flagellum synthesis, and motility, are downregulated in the ΔrpoN::Tc strain (Fig. 2B).
FIG 8.
Schematic of the proposed RpoN regulatory mechanism on QS, T6SS, and major metabolic pathways. The potential regulatory pathways and interplays of RpoN are proposed according to our observations and previous studies. RpoN affects the expression of nitrogen-regulated genes (glnK-amtB, nirBD, nasA, nasST, and nosRZDFYL) (16) and regulates dicarboxylates transport, amino acid catabolism, ethanolamine catabolism, (R)-3-hydroxybutyrate, methylarginines, and acetoin catabolism (24–29). The typical QS systems, including the las, rhl, and pqs systems and their interactions, were summarized based on previous reports (8). In the present study, we showed that RpoN directly bound to the lasI and pqsR promoter regions and positively regulated the expression of las and pqs QS systems, while it negatively regulated the rhl QS system and biofilm formation. Moreover, RpoN directly bound to the hcpA and hcpB promoter regions. In addition, RpoN regulated the major metabolic pathways in P. aeruginosa.
Taken together, the present results showed that RpoN directly regulates QS and T6SS by directly binding to promoters of lasI, rhlI, rhlA, rhlR, pqsA, pqsH, pqsR, hcpA, and hcpB. Previous studies show that RpoN affects the expression of nitrogen-regulated genes (glnK-amtB, nirBD, nasA, nasST, and nosRZDFYL) (16) and interacts with EBPs to regulate diverse metabolic pathways, including glycine catabolism, (R)-3-hydroxybutyrate catabolism, ethanolamine metabolism, methylarginine metabolism, flagellum biosynthesis, dicarboxylate transport, aromatic amino acid catabolism, and acetoin catabolism (24–29) (Fig. 8). Altogether, as a global regulator, RpoN controls a large group of metabolic pathways and pathogenicity, which provides additional cues to the treatment of infections caused by P. aeruginosa in the future.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in the present study are shown in Table S2 in the supplemental material. The P. aeruginosa PAO1 strain and its derivatives were grown at 37°C in LB (Luria-Bertani) broth with shaking at 220 rpm or on LB solid agar plates. The following concentrations of antibiotics were used: for P. aeruginosa, gentamicin at 50 μg/ml in Pseudomonas isolation agar (PIA) or 30 μg/ml in LB, tetracycline at 300 μg/ml in PIA or 50 μg/ml in LB, and carbenicillin at 200 μg/ml in PIA or 100 μg/ml in LB; for Escherichia coli, tetracycline at 10 μg/ml, gentamicin at 10 μg/ml, kanamycin at 50 μg/ml, and ampicillin at 100 μg/ml. All experiments were done in the biosafety level 2 laboratory at Nankai University.
ChIP-seq analysis.
The chromatin immunoprecipitation (ChIP) procedures were modified from a previous study (57). Wild-type PAO1 strain with pAK1900 or pAK1900-rpoN-VSV (vesicular stomatitis viral glycoprotein) was cultured with LB broth at 37°C with shaking at an optical density at 600 nm (OD600) of 0.6. We cross-linked the samples with 1% formaldehyde for 10 min. Subsequently, 125 mM glycine was mixed in the culture to stop the cross-linking. Samples were centrifuged (12,000 rpm, 4°C) and then washed three times with a Tris buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl). The resulting pellets were resuspended in 500 μl IP buffer (50 mM HEPES-KOH, pH 7.5, 0.1% SDS, 1% Triton X-100, 150 mM NaCl, mini-protease inhibitor cocktail [Roche], 1 mM EDTA, 0.1% sodium deoxycholate). The DNA was broken into pieces (100 to 300 bp) with an ultrasonic processor. Before saving the supernatant, the chromatins were centrifuged (12,000 rpm, 4°C). We next added 50 μl agarose-conjugated anti-VSV antibodies to the samples and then treated them with protein A beads (General Electric). As previous procedures described (57), we washed, reverse cross-linked, and purified the ChIP DNA. To construct the DNA fragment library with a NEXTflex ChIP-Seq kit (Bio Scientific), agarose gel was used to cut DNA fragments between 150 and 250 bp. We used a HiSeq 2000 system (Illumina) to sequence the libraries and mapped the reads to the P. aeruginosa PAO1 genome via TopHat (version 2.0.0) (58). MACS software (version 2.0.0) was used to call the peaks (59). Each sample in the ChIP-seq assay was repeated twice.
RNA-seq analysis.
To test the effect of RpoN on the transcriptome, 2-ml samples of mid-log-growth-phase (OD600 of 0.6) bacterial cultures (PAO1 and ΔrpoN::Tc strains) were collected by centrifugation (12,000 rpm, 4°C). An RNeasy minikit (Qiagen) was used for RNA purification and was treated by DNase I (NEB) subsequently. After using the MICROBExpress kit (Ambion) to remove RNA, the cDNA library was made by following the instructions for the TruSeq RNA sample preparation kit (Illumina). A HiSeq 2000 system (Illumina) was used for sequencing. Using TopHat (version 2.0.0) with two mismatches allowed (58), the RNA-seq reads were mapped to the P. aeruginosa genome. Using Cuffdiff software (version 2.0.0), differential expression analysis was performed. All differentially transcribed genes were examined for GO enrichment analyses using DAVID (60). All data sets were analyzed with Cytoscape with the Enrichment Map plug-in (61). Each sample in the RNA-seq assay was examined twice.
Expression and purification of RpoN protein.
The open reading frame (ORF) of rpoN from P. aeruginosa PAO1 genomic DNA was amplified and inserted into pMCSG19 (62) (NdeI/BamHI) to generate pMCSG19-rpoN. The vector pET28a-rpoF was generated by cloning rpoF into pET28a (BamHI/HindIII) to express RpoF protein by a pair of primers, rpoF-ORF-F/rpoF-ORF-R (Table S3). The plasmids were transformed into BL21 Star(DE3). pMCSG19-rpoN needs a pRK1037 plasmid (63) in BL21. Briefly, a single colony was inoculated into 10 ml sterilized LB broth (50 μg/ml kanamycin for pET28a-rpoF and 50 μg/ml kanamycin plus 100 μg/ml ampicillin for pMCSG19-rpoN) for 12 h. We then transferred the culture into 1 liter of the same medium as that described above, and the cells were grown at 37°C and 220 rpm to an OD600 of 0.6. Isopropyl-β-d-1-thiogalactopyranoside (IPTG; 0.5 mM) was added for inducing protein expression at 16°C for 16 h. The bacteria were collected after incubation at 4°C and 7,000 rpm for 5 min. The pellet was suspended in 20 ml binding buffer (500 mM NaCl, 25 mM Tris-HCl, pH 7.4, 5% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride [PMSF]). The bacteria were lysed by using sonication at 6-s intervals and centrifuged at 4°C (12,000 rpm, 30 min). The supernatant was filtered (0.45-μm-pore-size filter), and the filtrate was added to a nickel-nitrilotriacetic acid (Ni-NTA) column (Qiagen) which had been balanced with binding buffer before using. After the Ni-NTA column was washed three times with binding buffer, the column was eluted with a 2-ml gradient of 60 mM, 100 mM, 200 mM, 300 mM, and 500 mM imidazole prepared in binding buffer. Fractions from 100 mM to 500 mM were collected, SDS-PAGE was performed to validate the molecular weight of the target protein, and then fractions from 150 mM to 250 mM were pooled for EMSA.
EMSA.
The 40-ng samples of different DNA probes (primers are listed in Table S3) were mixed with various amounts of RpoN proteins in 20 μl of the sample buffer (50 mM HEPES, pH 7.9, 0.1 mM EDTA, 100 mM NaCl, 2.8% polyethylene glycol 8000, 100 mM KCl, 1.0 mM dithiothreitol, 10 mM MgCl2, 5% glycerol, and 100 μg/ml bovine serum albumin). To analyze the samples, 6% polyacrylamide gel (containing 10% glycerol) electrophoresis in 0.5× Tris-borate-EDTA (TBE) buffer was used at 90 V for 120 min after reaction for 20 min at room temperature. The gels were stained by gel stain (TransGen Biotech, China) at room temperature for 5 min and photographed by using a gel imaging system (Beijing Liuyi, China).
Construction of P. aeruginosa ΔrpoN::Tc mutant.
A SacB-based strategy was used for rpoN gene replacement as described in a previous study (45). To construct the ΔrpoN::Tc mutant (ΔrpoN), an upstream fragment (1,951 bp) and a downstream fragment (1,971 bp) of RpoN were amplified by PCRs using primers pEX-rpoN-Knockout-Up-F/rpoN-Knockout-Up-R (BamHI/XbaI) and pEX-rpoN-down-F/pEX-rpoN-down-R (XbaI/HindIII), respectively (Table S3). The two fragments were cloned into pEX18Ap (BamHI/HindIII), generating pEX18Ap-rpoN. A 1.19-kb tetracycline resistance fragment from mini-CTX-lacZ (XbaI) was cloned into pEX18Ap-rpoN, generating pEX-Tc-rpoN. The finished pEX18Ap-Tc-rpoN vector was electroporated into strain PAO1 with tetracycline resistance. Positive colonies were screened for carbenicillin sensitivity, tetracycline resistance, and loss of sucrose sensitivity (10%). The ΔrpoN::Tc mutant was checked by PCR with the primers pAK1900-rpoN-F/pAK1900-rpoN-R and rpoN-verification-F/rpoN-verification-F, respectively (Table S3).
Construction of plasmids and integration strains for Western blotting.
Plasmid pAK1900-rpoN (p-rpoN) was constructed by amplifying rpoN promoter-ORF fragments with the primers pAK1900-rpoN-F/pAK1900-rpoN-R (Table S3) by PCR. The PCR fragments were cloned into pAK1900 (BamHI/HindIII) (64). All constructs were verified by sequencing. The pMS402 plasmid was used to generate promoter-lux fusions or promoter-ORF-lux fusions as described in a previous study (65). The promoter region or promoter-ORF was generated by using the primers (Table S3) pKD-hcpA-F/R and pKD-hcpB-F/R. These promoters or promoter-ORF regions were cloned into the XhoI/BamHI sites upstream of the lux genes in pMS402. The resultant vector was electroporated into the PAO1, ΔrpoN::Tc, and complemented (ΔrpoN::Tc/p-rpoN) strains by electroporation. Cloned promoter or promoter-ORF vectors were sequenced.
The Tn7T-Gm-hcp1-FLAG fragment was integrated into the genome of these 3 strains by following previously reported procedures (66). The integration vector pUC18-mini-Tn7T-Gm-hcp1-FLAG and the helper vector pTNS3 were electrotransformed and selected on PIA plates with 50 μg/ml gentamicin. Verification of insertions at the attTn7 locus was done by colony PCR with the primers PTn7L/PglmS-up and PTn7R/PglmS-down (66).
Luminescence screening assays.
The procedures of the lux reporter assay were described in our previous study (67). Briefly, overnight cultures were diluted to an OD600 of 0.2 for 2 h. The cultures were transferred into a black 96-well plate with a transparent bottom. Fresh cultures (5 μl) were transferred into 95 μl LB broth. Promoter activities were measured every 2 h for 16 h. Bacterial growth was measured by OD600 in a Synergy 2 plate reader (BioTek) at the same time.
Motility assay.
The motility assay was performed as described in a previous study (68). Swimming medium consisted of 0.4% agar supplemented with 1% tryptone and 0.5% NaCl, 0.8% biotech nutrient broth, and 0.5% glucose. The medium used for the swarming motility assay consisted of 0.5% agar, 8 g/liter nutrient broth mix, and 5 g/liter glucose. Overnight LB cultures were inoculated on swarming plates as 2.5-μl aliquots. Swarming plates were incubated for 16 h at 37°C and then incubated at room temperature for an additional 20 h. Finally, the diameter of the motility trace was measured. Photographs were taken by using the Bio-Rad imaging system.
Proteolytic activity assay.
Proteolytic activity was performed as described previously (69). Briefly, 2-μl aliquots of overnight cultures were spotted on LB agar plates containing 2.0% (wt/vol) skim milk (Sangon) and 100 μg/ml carbenicillin. The plates were incubated at 37°C for 12 h. Proteolytic activity was determined as zones of clearing circles around the bacterial colonies.
Western blot analysis.
Overnight cultures of the tested strains were transformed into the same fresh LB medium with gentamicin at 30 μg/ml and carbenicillin at 100 μg/ml to an OD600 of 0.02 and cultivated for an additional time to an OD600 of 0.6 and 1.5, respectively. Cultures (100 μl) were centrifuged and the supernatant was discarded. The pellets were washed in 10 μl PBS. Bacterial cells were loaded and separated by 15% SDS-PAGE. The proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Science) and hybridized with a mouse monoclonal FLAG antibody (1:10,000 dilutions; Aogma) and peroxidase-conjugated Affinipure goat anti-mouse IgG (H+L) (Proteintech). The signal was detected by an eECL Western blot kit (Cwbiotech). Photographs were taken by using the Bio-Rad imaging system. The signal intensity was measured by Quantity One software (Bio-Rad). The relative level of Hcp1-Flag expression in the wild-type PAO1 (OD600) was set to 1, and the other values were calculated accordingly.
Congo red assay and biofilm formation assay.
Congo red assay was performed based on a previous study (70), with minor modifications to measure the production of exopolysaccharide. M9-based 1% agar plates contained 0.05% (vol/vol) glutamate (nitrogen source), Congo red (80 μg/ml), 2 mM MgSO4, and 0.2% (vol/vol) glucose. The overnight culture was diluted to an OD600 of 0.001 in liquid M9 medium, and 2 μl of the diluted culture was spotted onto the surface of the Congo red plates and cultivated at 30°C. Colony staining was photographed after 2 to 3 days.
Biofilm formation was detected with a modified method as previously reported (68). Biofilm formation was performed in 10-ml borosilicate tubes. Briefly, overnight cultures were transferred into LB broth (1:100 dilutions) supplemented with 100 μg/ml carbenicillin and cultured statically at 30°C for 16 h. Crystal violet (0.1%) was used to stain biofilm adhered to the tubes, and unbound dye was washed with distilled water. Quantification of biofilm formation was carried out in transparent 24-well polystyrene plates. Overnight cultures were transferred into LB broth (1:100 dilutions) supplemented with 100 μg/ml carbenicillin and cultured statically to an OD600 of 0.01. The plates were cultured statically at 30°C for 16 h and the OD600 was measured. Each well was stained by 0.1% crystal violet for 15 min. Wells were washed three times gently with sterilized water, and the residual crystal violet was dissolved in 1 ml of 95% ethanol with shaking. A volume of 100 μl of this eluate was transferred to a new transparent 96-well plate to measure the absorbance at 590 nm. The ratio (OD590/OD600) represents the final biofilm production.
Measurement of pyocyanin production.
Overnight bacterial culture supernatants were used to extract pyocyanin and to measure its production according to previous methods (71, 72). Briefly, 3 ml chloroform was added to 5 ml supernatant and fully mixed. The chloroform layer was transferred to a new tube before mixing with 1 ml 0.2 M HCl. The tubes were centrifuged at 12,000 rpm for 10 min. One hundred microliters of the top layer was transferred to a new transparent polystyrene 96-well plate. Finally, the OD520 was measured by using a Synergy 2 plate reader (BioTek).
Detection of rhamnolipid production.
Rhamnolipid production was detected by a previous method (73). Briefly, 2-μl samples of different overnight cultures were inoculated on M9-based agar plates plus 0.2% (vol/vol) glucose, 0.0005% (vol/vol) methylene blue, 0.05% (vol/vol) glutamate (nitrogen source), 2 mM (vol/vol) MgSO4, and 0.02% cetyltrimethylammonium bromide (CTAB). After incubating at 37°C for 24 h, the plates were transferred to room temperature for at least 72 h. A blue halo that appeared around the colony represented the production of rhamnolipids. Photographs were taken by using the Bio-Rad imaging system.
Bioassay of C4-HSL activity.
Procedures for bioassay of C4-HSL activity were modified from those previously reported (74). We measured C4-HSL activity with E. coli DH5a carrying pKD-rhlI-lux and pMCSG19-rhlR. The reporter strain was cultured in LB broth (50 μg/ml kanamycin and 100 μg/ml ampicillin) at 37°C for 10 h and diluted to an OD600 of 0.05 in fresh LB (kanamycin and ampicillin). PAO1(pAK1900), ΔrpoN::Tc(pAK1900), and ΔrpoN::Tc(pAK1900-rpoN) strains were cultured in LB broth (100 μg/ml carbenicillin) at 37°C for 10 h and diluted to an OD600 of 0.05 in fresh LB (carbenicillin). Diluted reporter culture (100 μl) was mixed with PAO1(pAK1900), ΔrpoN::Tc(pAK1900), and ΔrpoN::Tc(pAK1900-rpoN) cultures in the same volume. Luminescence was measured after 4 h at 37°C with shaking by using a Synergy 2 plate reader (BioTek).
Quantification of PQS.
PQS production was quantified with a method described previously (75). Overnight cultures were transferred into fresh LB broth (1:100 dilutions) and cultured at 37°C for 12 h. Acidified ethyl acetate (1 ml) was added to 500 μl of each culture and vigorously mixed by using a vortex vibration meter for 2 min. The mixture then was centrifuged at 12,000 rpm for 10 min, and the top layer was transferred to a new tube and volatilized completely. Fifty microliters of a mixture of acidified ethyl acetate and acetonitrile (1:1, vol/vol) was used to dissolve the solute. To separate the extracts described above, 5-μl extracts were loaded and 17:2:1 methylene chloride-acetonitrile-dioxane acted as a solution. Finally, the plate was illuminated with UV light. The signal intensity was measured by Quantity One software (Bio-Rad). The relative production level of PQS in wild-type PAO1 was set to 1, and the other values were calculated accordingly.
RT-qPCR.
For real-time quantitative PCR (RT-qPCR), all strains were cultured at 37°C overnight in LB, diluted 100-fold in fresh 20 ml LB in 50-ml conical flasks, and cultured with shaking at 220 rpm at 37°C until an OD600 of 0.6 was reached. To harvest the bacteria, the cultures were centrifuged and the pellets were dissolved by lysozyme. RNA purification was performed by using the GeneJET RNA purification kit (Thermo Fisher). RNA concentration was measured by UV light at 260 nM. cDNA synthesis was performed by using a FastKing RT kit (Tiangen Biotech) with 1.2 μg total RNAs in each reaction mix. The 96-well RT-qPCR plate was prepared by following the manufacturer's instructions. One hundred nanograms of each sample was added into each well. Each reaction was performed in triplicate in 20-μl reaction volumes with 16S rRNA as a control. For each reaction, 200 nM primers (Table S3) were used for RT-qPCR. The reactions were run at 42°C for 15 min, 95°C for 3 min, and kept at 4°C until used. RT-qPCR next was conducted using SYBR green (Tiangen Biotech) with an ABI 7500 real-time PCR system. The reaction procedure includes 15 min at 95°C, with 40 cycles of 10 s at 95°C and 32 s at 60°C. The fold change represents relative expression levels of mRNA, which can be estimated by the values of 2−ΔΔCT. All reactions were conducted with three repeats.
Statistical analyses.
Two-tailed Student's t tests were performed using Microsoft Office Excel 2010. Results represent means ± SD. All experiments were repeated at least three times.
Accession number(s).
The ChIP-seq data sets have been deposited in the National Center for Biotechnology Information (NCBI) database under accession number GSE110522, and the RNA-seq data sets have been deposited in the NCBI database under accession number GSE110522.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31670127 to X.D.), Tianjin Natural Science Foundation (17JCYBJC23800), and Health and Medical Research Fund of Hong Kong (17160022).
X.D., X.S., and H.L. designed the study and wrote the paper. X.S. performed experiments, analyzed data, and generated figures. X.Z. constructed the ΔrpoN::Tc mutant and complemented vector. Y.Z. and M.Z. performed RT-qPCR. P.Y., J.Y., Y.X., T.Z., W.W., and S.C. helped to edit the paper and provided input into the experiments. All authors reviewed the results and approved the final version of the manuscript.
We declare no financial relationships with any organizations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00205-18.
REFERENCES
- 1.Deretic V, Schurr MJ, Yu H. 1995. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol 3:351. doi: 10.1016/S0966-842X(00)88974-X. [DOI] [PubMed] [Google Scholar]
- 2.Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster M, Greenberg EP. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J Med Microbiol 296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Smith RS, Iglewski BH. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol 6:56–60. doi: 10.1016/S1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 5.Pearson JP, Passador L, Iglewski BH, Greenberg EP. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science (New York, NY) 260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 7.Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang H, Deng X, Li X, Ye Y, Wu M. 2014. Molecular mechanisms of master regulator VqsM mediating quorum-sensing and antibiotic resistance in Pseudomonas aeruginosa. Nucleic Acids Res 42:10307–10320. doi: 10.1093/nar/gku586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang H, Deng X, Ji Q, Sun F, Shen T, He C. 2012. The Pseudomonas aeruginosa global regulator VqsR directly inhibits QscR to control quorum-sensing and virulence gene expression. J Bacteriol 194:3098–3108. doi: 10.1128/JB.06679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledgham F, Ventre I, Soscia C, Foglino M, Sturgis JN, Lazdunski A. 2003. Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR. Mol Microbiol 48:199–210. doi: 10.1046/j.1365-2958.2003.03423.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Yu X, Zhu M, Kang H, Ma J, Wu M, Gan J, Deng X, Liang H. 2016. Structural and molecular mechanism of CdpR involved in quorum-sensing and bacterial virulence in Pseudomonas aeruginosa. PLoS Biol 14:e1002449. doi: 10.1371/journal.pbio.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang H, Gan J, Zhao J, Kong W, Zhang J, Zhu M, Li F, Song Y, Qin J, Liang H. 2017. Crystal structure of Pseudomonas aeruginosa RsaL bound to promoter DNA reaffirms its role as a global regulator involved in quorum-sensing. Nucleic Acids Res 45:699–710. doi: 10.1093/nar/gkw954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coggan KA, Wolfgang MC. 2012. Global regulatory pathways and cross-talk control pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr Issues Mol Biol 14:47. [PubMed] [Google Scholar]
- 14.Lee J, Wu J, Deng Y, Wang J, Wang C, Wang J, Chang C, Dong Y, Williams P, Zhang LH. 2013. A cell-cell communication signal integrates quorum sensing and stress response. Nat Chem Biol 9:339. doi: 10.1038/nchembio.1225. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickson EL, Plotnikova J, Mahajan-Miklos S, Rahme LG, Ausubel FM. 2001. Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice. J Bacteriol 183:7126–7134. doi: 10.1128/JB.183.24.7126-7134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt TP, Magasanik B. 1985. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci U S A 82:8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta N, Wolfgang MC, Goodman AL, Arora SK, Jyot J, Lory S, Ramphal R. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol Microbiol 50:809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 18.Kohler T, Harayama S, Ramos JL, Timmis KN. 1989. Involvement of Pseudomonas putida RpoN sigma factor in regulation of various metabolic functions. J Bacteriol 171:4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera MC, Duque E, Rodriguez-Herva JJ, Fernandez-Escamilla AM, Ramos JL. 2010. Identification and characterization of the PhhR regulon in Pseudomonas putida. Environ Microbiol 12:1427–1438. [DOI] [PubMed] [Google Scholar]
- 20.Yamano Y, Nishikawa T, Komatsu Y. 1998. Involvement of the RpoN protein in the transcription of the oprE gene in Pseudomonas aeruginosa. FEMS Microbiol Lett 162:31–37. doi: 10.1111/j.1574-6968.1998.tb12975.x. [DOI] [PubMed] [Google Scholar]
- 21.Viducic D, Murakami K, Amoh T, Ono T, Miyake Y. 2017. RpoN Promotes Pseudomonas aeruginosa survival in the presence of tobramycin. Front Microbiol 8:839. doi: 10.3389/fmicb.2017.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viducic D, Ono T, Murakami K, Katakami M, Susilowati H, Miyake Y. 2007. rpoN gene of Pseudomonas aeruginosa alters its susceptibility to quinolones and carbapenems. Antimicrob Agents Chemother 51:1455–1462. doi: 10.1128/AAC.00348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viducic D, Murakami K, Amoh T, Ono T, Miyake Y. 2016. RpoN modulates carbapenem tolerance in Pseudomonas aeruginosa through Pseudomonas quinolone signal and PqsE. Antimicrob Agents Chemother 60:5752–5764. doi: 10.1128/AAC.00260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundgren BR, Bailey FJ, Moley G, Nomura CT. 2017. DdaR (PA1196) regulates expression of dimethylarginine dimethylaminohydrolase for the metabolism of methylarginines in Pseudomonas aeruginosa PAO1. J Bacteriol 199:e00001-. doi: 10.1128/JB.00001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundgren BR, Sarwar Z, Pinto A, Ganley JG, Nomura CT. 2016. Ethanolamine catabolism in Pseudomonas aeruginosa PAO1 is regulated by the enhancer-binding protein EatR (PA4021) and the alternative sigma factor RpoN. J Bacteriol 198:2318–2329. doi: 10.1128/JB.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundgren BR, Harris JR, Sarwar Z, Scheel RA, Nomura CT. 2015. The metabolism of (R)-3-hydroxybutyrate is regulated by the enhancer-binding protein PA2005 and the alternative sigma factor RpoN in Pseudomonas aeruginosa PAO1. Microbiology 161:2232–2242. doi: 10.1099/mic.0.000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarwar Z, Lundgren BR, Grassa MT, Wang MX, Gribble M, Moffat JF, Nomura CT. 2016. GcsR, a TyrR-like enhancer-binding protein, regulates expression of the glycine cleavage system in Pseudomonas aeruginosa PAO1. mSphere 1:e00020-16. doi: 10.1128/mSphere.00020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundgren BR, Thornton W, Dornan MH, Villegas-Penaranda LR, Boddy CN, Nomura CT. 2013. Gene PA2449 is essential for glycine metabolism and pyocyanin biosynthesis in Pseudomonas aeruginosa PAO1. J Bacteriol 195:2087–2100. doi: 10.1128/JB.02205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundgren BR, Villegas-Penaranda LR, Harris JR, Mottern AM, Dunn DM, Boddy CN, Nomura CT. 2014. Genetic analysis of the assimilation of C5-dicarboxylic acids in Pseudomonas aeruginosa PAO1. J Bacteriol 196:2543–2551. doi: 10.1128/JB.01615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damron FH, Owings JP, Okkotsu Y, Varga JJ, Schurr JR, Goldberg JB, Schurr MJ, Yu HD. 2012. Analysis of the Pseudomonas aeruginosa regulon controlled by the sensor kinase KinB and sigma factor RpoN. J Bacteriol 194:1317–1330. doi: 10.1128/JB.06105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz S, Eckweiler D, Bielecka A, Nicolai T, Franke R, Dötsch A, Hornischer K, Bruchmann S, Düvel J, Häussler S. 2015. Elucidation of sigma factor-associated networks in Pseudomonas aeruginosa reveals a modular architecture with limited and function-specific crosstalk. PLoS Pathog 11:e1004744. doi: 10.1371/journal.ppat.1004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai Z, Liu Y, Chen Y, Yam J, Chew S, Chua S, Wang K, Givskov M, Yang L. 2015. RpoN regulates virulence factors of Pseudomonas aeruginosa via modulating the PqsR quorum sensing regulator. Int J Mol Sci 16:28311–28319. doi: 10.3390/ijms161226103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heurlier K, Denervaud V, Pessi G, Reimmann C, Haas D. 2003. Negative control of quorum sensing by RpoN (sigma54) in Pseudomonas aeruginosa PAO1. J Bacteriol 185:2227–2235. doi: 10.1128/JB.185.7.2227-2235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson LS, Webb JS, Rice SA, Kjelleberg S. 2003. The alternative sigma factor RpoN regulates the quorum sensing gene rhlI in Pseudomonas aeruginosa. FEMS Microbiol Lett 220:187–195. doi: 10.1016/S0378-1097(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 35.Sana TG, Soscia C, Tonglet CM, Garvis S, Bleves S. 2013. Divergent control of two type VI secretion systems by RpoN in Pseudomonas aeruginosa. PLoS One 8:e76030. doi: 10.1371/journal.pone.0076030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caiazza NC, O'Toole GA. 2004. SadB is required for the transition from reversible to irreversible attachment during biofilm formation by Pseudomonas aeruginosa PA14. J Bacteriol 186:4476–4485. doi: 10.1128/JB.186.14.4476-4485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonemann G, Mogk PAA. 2010. Tubules and donuts: a type VI secretion story. Mol Microbiol 76:815–821. doi: 10.1111/j.1365-2958.2010.07171.x. [DOI] [PubMed] [Google Scholar]
- 38.Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordonez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science (New York, NY) 312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Pace F, Nakazato G, Pacheco A, de Paiva JB, Sperandio V, da Silveira WD. 2010. The type VI secretion system plays a role in type 1 fimbria expression and pathogenesis of an avian pathogenic Escherichia coli strain. Infect Immun 78:4990–4998. doi: 10.1128/IAI.00531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD, Rohmer L, Tolker-Nielsen T, Skerrett SJ, Mougous JD. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog 6:e1001068. doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murdoch SL, Trunk K, English G, Fritsch MJ, Pourkarimi E, Coulthurst SJ. 2011. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J Bacteriol 193:6057–6069. doi: 10.1128/JB.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savioz A, Zimmermann A, Haas D. 1993. Pseudomonas aeruginosa promoters which contain a conserved GG-N10-GC motif but appear to be RpoN-independent. Mol Genet Genomics 238:74–80. [DOI] [PubMed] [Google Scholar]
- 45.Schweizer HP, Hoang TT. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15–22. doi: 10.1016/0378-1119(95)00055-B. [DOI] [PubMed] [Google Scholar]
- 46.Kong W, Zhao J, Kang H, Zhu M, Zhou T, Deng X, Liang H. 2015. ChIP-seq reveals the global regulator AlgR mediating cyclic di-GMP synthesis in Pseudomonas aeruginosa. Nucleic Acids Res 43:8268–8282. doi: 10.1093/nar/gkv747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cannon W, Claverie-Martin F, Austin S, Buck M. 1993. Core RNA polymerase assists binding of the transcription factor sigma 54 to promoter DNA. Mol Microbiol 8:287–298. doi: 10.1111/j.1365-2958.1993.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 48.Totten PA, Lory S. 1990. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol 172:7188–7199. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdel-Mawgoud AM, Lepine F, Deziel E. 2010. Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochsner UA, Koch AK, Fiechter A, Reiser J. 1994. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 176:2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Gennip M, Christensen LD, Alhede M, Phipps R, Jensen PO, Christophersen L, Pamp SJ, Moser C, Mikkelsen PJ, Koh AY, Tolker-Nielsen T, Pier GB, Hoiby N, Givskov M, Bjarnsholt T. 2009. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 117:537–546. doi: 10.1111/j.1600-0463.2009.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cascales E, Cambillau C. 2012. Structural biology of type VI secretion systems. Philos Trans R Soc Lond B Biol Sci 367:1102–1111. doi: 10.1098/rstb.2011.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang F, Waterfield NR, Yang J, Yang G, Jin Q. 2014. A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 15:600–610. doi: 10.1016/j.chom.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 54.Lloyd MG, Lundgren BR, Hall CW, Gagnon LB, Mah TF, Moffat JF, Nomura CT. 2017. Targeting the alternative sigma factor RpoN to combat virulence in Pseudomonas aeruginosa. Sci Rep 7:12615. doi: 10.1038/s41598-017-12667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrios H, Valderrama B, Morett E. 1999. Compilation and analysis of sigma(54)-dependent promoter sequences. Nucleic Acids Res 27:4305–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bijlsma JJ, Groisman EA. 2003. Making informed decisions: regulatory interactions between two-component systems. Trends Microbiol 11:359–366. [DOI] [PubMed] [Google Scholar]
- 57.Blasco B, Chen JM, Hartkoorn R, Sala C, Uplekar S, Rougemont J, Pojer F, Cole ST. 2012. Virulence regulator EspR of Mycobacterium tuberculosis is a nucleoid-associated protein. PLoS Pathog 8:e1002621. doi: 10.1371/journal.ppat.1002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trapnell C, Pachter L, Salzberg SL. 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 61.Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S, Pico AR, Bader GD, Ideker T. 2012. A travel guide to Cytoscape plugins. Nat Methods 9:1069–1076. doi: 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stols L, Gu M, Dieckman L, Raffen R, Collart FR, Donnelly MI. 2002. A new vector for high-throughput, ligation-independent cloning encoding a tobacco etch virus protease cleavage site. Protein Expr Purif 25:8–15. doi: 10.1006/prep.2001.1603. [DOI] [PubMed] [Google Scholar]
- 63.Nallamsetty S, Kapust RB, Tozser J, Cherry S, Tropea JE, Copeland TD, Waugh DS. 2004. Efficient site-specific processing of fusion proteins by tobacco vein mottling virus protease in vivo and in vitro. Protein Expr Purif 38:108–115. doi: 10.1016/j.pep.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Jansons I, Touchie G, Sharp R, Almquist K, Farinha MA, Lam JS, Kropinski AM. 1994. Deletion and transposon mutagenesis and sequence analysis of the pRO1600 OriR region found in the broad-host-range plasmids of the pQF series. Plasmid 31:265–274. doi: 10.1006/plas.1994.1028. [DOI] [PubMed] [Google Scholar]
- 65.Duan K, Dammel C, Stein J, Rabin H, Surette MG. 2003. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- 66.Choi KH, Schweizer HP. 2006. Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 67.Liang H, Li L, Dong Z, Surette MG, Duan K. 2008. The YebC family protein PA0964 negatively regulates the Pseudomonas aeruginosa quinolone signal system and pyocyanin production. J Bacteriol 190:6217–6227. doi: 10.1128/JB.00428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 69.Wretlind B, Pavlovskis OR. 1984. Genetic mapping and characterization of Pseudomonas aeruginosa mutants defective in the formation of extracellular proteins. J Bacteriol 158:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bordi C, Lamy MC, Ventre I, Termine E, Hachani A, Fillet S, Roche B, Bleves S, Mejean V, Lazdunski A, Filloux A. 2010. Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol Microbiol 76:1427–1443. doi: 10.1111/j.1365-2958.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurachi M. 1958. Studies on the biosynthesis of pyocyanine. Isolation and determination of pyocyanine. Chem Res Kyoto Univ Bull Inst 36:163–173. [Google Scholar]
- 72.Essar DW, Eberly L, Hadero A, Crawford IP. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Köhler T, Van DC, Curty LK, Hamzehpour MM, Pechere JC. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J Bacteriol 183:5213. doi: 10.1128/JB.183.18.5213-5222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oinuma K, Greenberg EP. 2011. Acyl-homoserine lactone binding to and stability of the orphan Pseudomonas aeruginosa quorum-sensing signal receptor QscR. J Bacteriol 193:421–428. doi: 10.1128/JB.01041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collier DN, Anderson L, McKnight SL, Noah TL, Knowles M, Boucher R, Schwab U, Gilligan P, Pesci EC. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol Lett 215:41–46. doi: 10.1111/j.1574-6968.2002.tb11367.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.