Abstract
Cyclin-dependent kinase 1 (Cdk1) is indispensable for embryonic stem cell (ESC) maintenance and embryo development. Even though some reports have described a connection between Cdk1 and Oct4, there is no evidence that Cdk1 activity is directly linked to the ESC pluripotency transcription program. We recently reported that Aurkb/PP1-mediated Oct4 resetting is important to cell cycle maintenance and pluripotency in mouse ESCs (mESCs). In this study, we show that Cdk1 is an upstream regulator of the Oct4 phosphorylation state during cell cycle progression, and it coordinates the chromatin associated state of Oct4 for pluripotency-related gene expression within the cell cycle. Upon entry into mitosis, Aurkb in the chromosome passenger complex becomes fully activated and PP1 activity is inhibited downstream of Cdk1 activation, leading to sustaining Oct4(S229) phosphorylation and dissociation of Oct4 from chromatin during the mitotic phase. Cdk1 inhibition at the mitotic phase abnormally results in Oct4 dephosphorylation, chromosome decondensation and chromatin association of Oct4, even in replicated chromosome. Our study results suggest a molecular mechanism by which Cdk1 directly links the cell cycle to the pluripotency transcription program in mESCs.
INTRODUCTION
Embryonic stem cells (ESCs) have a very unique cell cycle pattern characterized by a very short G1 phase and a long S phase (1,2). Recent studies have shown that this unusual cell cycle pattern not only governs self-renewal and pluripotency in ESCs but also provides a window of opportunity for ESCs to differentiate into three germ layers. The onset of differentiation in human and mouse ESCs (hESCs and mESCs) occurs during the G1 phase (3–5). The S and G2 phases in hESCs establish the cells’ active roles in boosting the pluripotent state (6). Therefore, cell cycle mechanisms have been believed to have a key role in determining the fate of ESCs in differentiation.
The mammalian cell cycle in somatic cells is strictly governed by four different types of cyclin-dependent kinases (Cdks) and their binding partners, cyclins, at specific phases of the cell cycle (7). In contrast, Cdks are regulated differently through the cell cycle in ESCs. The Cdk4-cyclin D complex exhibits little activity in mESCs (8), and the Cdk4/6-cyclin D complex, which is connected with Smad transcription factors during the late G1 phase and G1/S transition, determines hESC differentiation toward to neuroectoderm (5). Cdk2 in both mESCs and hESCs has high activity throughout the cell cycle and Cdk2 knockdown in both types of ESCs leads to G1 arrest, indicating its pivotal role in the shortened G1 phase in ESCs (9,10). However, Cdk2 is unlikely to be critical for determining cell fate during ESC differentiation because cdk2-knockout embryos develop normally and cdk2-knockout mice are viable (11,12).
On the other hand, only the Cdk1/cyclin B complex is selectively activated before the mitotic phase in ESCs (13). Deficiency in Cdk1 results in embryonic lethality in the first cell divisions. Embryos lacking Cdk1 do not divide despite carrying a full complement of interphase Cdks, indicating that Cdk1 is indispensable for cell cycle progression and early embryonic development (14), and these results indicate that Cdk1 tends to be connected with ESC pluripotency programming for ESC fate decisions.
In fact, a series of studies have revealed that Cdk1 activates factors for maintenance of self-renewal in ESCs and inhibits factors that induce differentiation. Cdk1 depletion in mESCs induces reduction of pluripotency factor, Oct4, Sox2, and Nanog mRNA expressions and results in a cell shape similar to that in differentiated cells (15). Cdk1 has been found to belong to the interactome of Oct4, a major regulator of ESC pluripotency (16), and two other reports have shown that Cdk1 associates with Oct4 (17,18).
Nonetheless, although Cdk1 is a serine/threonine kinase working during the G2/M phase or the transition from the M phase to the next G1 phase, it is unclear whether Cdk1 regulates the phosphorylation status of Oct4. Cdk1, interplaying with Oct4, suppresses differentiation to trophectoderm, but Cdk1 cannot directly phosphorylate Oct4 (18). Moreover, Oct4 inhibits Cdk1 activity and delays mitotic entry in ESCs (17), describing a nontranscriptional role of Oct4 in the regulation of mitotic entry in ESCs.
We previously reported that the Aurkb-PP1 axis regulates the phosphorylation of Oct4 serine-229 residue, which is critical for Oct4-DNA binding (19). This spatiotemporal regulation of Oct4(S229) phosphorylation during cell cycle prompts Oct4 to reset pluripotency transcription on re-entry into the following G1 phase. Previous study has revealed that Cdk1 interacts with Oct4 and selectively functions as an enzyme during G2 and mitotic phase in ESCs. On that basis, we hypothesize that Cdk1 connects with Oct4 and functions through Aurkb/PP1-mediated Oct4 phosphorylation in ESCs.
In this study, we demonstrate that Cdk1 influences Aurkb and inhibits PP1 activity in early mitosis, as a result, Oct4(S229) phosphorylation is maintained at a high level during mitosis. Although mESCs entered mitosis, suppression of Cdk1 activity induced recovery of PP1 activity. Abnormal inhibition of Cdk1 in mitosis resulted in chromosome decondensation and Oct4(S229) dephosphorylation. Therefore, normal fluctuation of Cdk1 activity is necessary for regulating the phosphorylation state of Oct4. Cdk1 controls the phosphorylation-dependent chromatin-associated state of Oct4 and the transcriptional activity of Oct4 during cell cycle progression. These results provide a molecular mechanism by which cell cycle machinery dynamically modulates the pluripotency transcription program in ESCs.
MATERIALS AND METHODS
Cell culture
mESCs (E14 and ZHBTc4) in this study were cultured in feeder free condition on 0.1% gelatin (Sigma-Aldrich, St. Louis, MO, USA) coated dishes. mESCs were cultured in DMEM (Hyclone, Logan, Utah) supplemented with 15% FBS (Gibco, Grand Island, NY, USA), 2 mM l-glutamine, 55 μM β-mercaptoethanol, 1%(v/v) non-essential amino acid, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Gibco) and 1000 U/ml ESGRO LIF (Millipore, Germany). HEK293T and NIH-3T3 were maintained in DMEM (Hyclone, Logan, UT, USA) supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Gibco).
Expression plasmids and stable ESC line generation
cDNAs were synthesized from RNAs of E14 mESCs and 293T cells. Amplified ORFs using PCR were introduced into pCAG-Flag-IP vector, which was generated by inserting a Flag tag into pCAG-IP, kindly provided by Hitoshi Niwa (RIKEN, Japan). For long-term transgene expression in mESCs, expression plasmids were transfected using Lipofectamine 2000 (Invitrogen). After 48 h of transfection, selection with appropriate antibiotics (puromycin or blasticidin) was performed to determine stable integration. Antibiotics resistant cells were expanded and analyzed by western blot.
shRNA mediated knockdown
pLKO.1 lenti-viral vectors for shRNAs targeting mouse Cdk1, Aurkb, Incenp, Borealin and Survivin were purchased from Sigma-Aldrich MISSON® library. shRNA sequences used in this study are as followed. shCdk1 #1(TRCN0000274559), #3 (TRCN0000274503), shAurkb #2 (TRCN0000374361), #4 (TRCN0000321651), shINCENP #1 (TRCN0000072093), #2 (TRCN0000072094), shBorealin #1 (TRCN0000177470), #2 (TRCN0000177578), shSurvivin #3 (TRCN0000054617). For shSurvivin #18 (5′-CCGCATCTCTACATTCAAGAA-3′) (20), annealed oligomers were cloned into pLKO.1 vector. pLKO.1 puro vector was used as a control.
Mitotic arrest-release and cell cycle analysis
For G2/M phase synchronization, E14 and ZHBTc4 mESCs were treated with 100 ng/ml nocodazole (Calbiochem, Germany) for 8 h. In order to release, synchronized cells were washed three times with PBS, and incubated for the indicated time in fresh culture media. Cdk1 inhibitor, RO3306 (ALX-270-463, Enzo Life Science, Farmingdale, NY, USA) was added to the cells once they had been blocked in mitosis by nocodazole, but without removal of nocodazole (21). NIH-3T3 cells were treated with nocodazole for 16 h and harvested by mitotic shake-off. For cell cycle analysis, the collected cells at the indicated time were immediately fixed in 70% ethanol and stained with propidium iodide (PI; Sigma, P4170) for 1 h at room temperature in the dark. To determine the distribution of pH3S10-positive cells, fixed cells were incubated in pH3S10 antibody and secondary antibody (Alexa Fluor 488, Invitrogen, Carlsbad, CA, USA). Cell cycles were analyzed using FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, New Jersey). Analysis of cell cycle data was performed with BD CellQuest Pro Software.
Coimmunoprecipitation
For coimmunoprecipitation of overexpressed proteins, Flag-Oct4 and HA-Cdk1, Flag-CPCs and HA-Cdk1, Flag-PP1s and HA-Cdk1 were cotransfected into HEK293T cells. Cells were resuspended in IP150 buffer (150 mM NaCl, 25 mM Tris–HCl, pH 8.0, 0.1% NP-40, 10% glycerol, 1 mM EDTA and protease inhibitor cocktail) for 15 min ice and cell lysates were obtained by centrifugation at 15 000 rpm for 15 min. Flag-tagged proteins were immunmoprecipitated by incubating lysates with Flag antibody and protein G beads for 4 h at 4°C. Beads were washed three times with IP150 buffer and bound proteins were eluted with 5× SDS-PAGE loading buffer, separated by 10% SDS-PAGE, transferred to NC membrane and immunoblotted with HA antibody. Flag-tagged proteins expressing mESCs were washed twice with PBS and resuspended in IP150 buffer (150 mM NaCl, 25 mM Tris–HCl, pH 8.0, 0.1% NP-40, 10% glycerol, 1 mM EDTA,10 mM NaF, 200 μM Na3VO4 and protease inhibitor cocktail) or RIPA buffer (150 mM NaCl, 50 mM Tris–HCl, pH 8.0, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM EDTA, 10 mM NaF, 200 μM Na3VO4 and protease inhibitor cocktail) for 30 min ice. Cell lysates obtained by centrifugation at 15 000 rpm for 15 min were pulled down with anti-Flag beads for overnight at 4°C. Bound beads were washed three times with IP150 buffer and for bound protein elution, Flag elution solution were added to each sample. Samples were incubated with gentle shaking for 30 min at 4°C. Beads were centrifuged for 2 min at 5000 rpm and the supernatants were transferred to new tubes. Obtained elutes with 5× SDS-PAGE loading buffer were boiled for 5 min and separated by SDS-PAGE, transferred to NC membrane and immunoblotted with their respective antibodies.
Antibody for western blots
Anti-p-Oct4(S229) antibody was made by GenScript (Piscataway, NJ, USA). Cdc2 p34 (sc-54), Oct4 (sc-5279), PP1α (sc-6104) and PP1γ (sc-6108) antibodies were purchased from purchased from Santa Cruz Biotechnology; Nanog (ab14959), PP1β (ab53315), Survivin (ab469), Borealin (ab74473) were purchased from Abcam (UK); phospho PP1α(T320) (#2581), phosphor Cdc2(Y15) (#9111), INCENP (#2807) were acquired from Cell Signaling; Aurkb (611082) was purchased from BD Transduction Laboratories); β-Actin (A5441) and Flag (F3165) were from Sigma-Aldrich.
Immunofluorescence and confocal microscopy
Cells grown on coverslips were fixed in 4% (w/v) paraformaldehyde and permeabilized in 0.5% (w/v) Triton X-100 in PBS for 30 min at room temperature (RT). After permeabilization, the cells were blocked with 5% (w/v) goat serum for 30 min. Subsequently, they were incubated in primary antibody for 1 h at RT. Antibody dilutions were 1:500 for anti-Oct4 (Santa Cruz, Dallas, TX, USA, sc-5279), 1:200 for anti-p-Oct4(S229), 1:200 for Cdk1 (Santa Cruz, Dallas, TX, USA, sc-54), 1:100 for Phospho-PP1α (Thr320) (Cell Signaling, #2581). Secondary antibodies used in immunostaining were Alexa Fluor 488 or 568 (Invitrogen, Carlsbad, California). Confocal micro-images were obtained by a confocal laser scanning microscope (Leica TCS SP8).
In vitro kinase assay
(His)6-tagged PP1 proteins were expressed in bacteria and purified by using Ni-NTA agarose. For radioactive in vitro kinase assay, (His)6-PP1 and GST-Oct4 were incubated with Cdk1/CyclinB1 in kinase buffer (60 mM HEPES–NaOH pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 3 mM Na-orthovanadate, 1.2 mM DTT, 0.25 mM ATP) with 0.1 mM γ-32P-ATP (NEG002A250UC, purchased from PerkinElmer, Waltham, MA, USA) for 30 min at 30°C. Reactions were then stopped by the addition of 5× SDS-PAGE loading buffer and loaded for separation on SDS-PAGE gel. After staining with Coomassie Blue, the gels were dried and exposed to films.
AP staining
mESCs were trypsinized to a single cell and re-plated at low to medium density. On day 5, aspirate media and fix cells with 4% (w/v) paraformaldehyde for 2 min. Aspirate fixative and rinse with TBST. Fast Red Violet (FRV) solution (1.6 mg in DW 2 ml) is mixed with Naphthol AS-BI phosphate solution (4 mg in AMPD buffer 1 ml). Add enough stain solution to cover each well and incubate in dark at RT for 15min. Aspirate staining solution and rinse wells with TBST. Cover cells with PBS to prevent drying and then count the number of colonies expressing AP.
ChIP
ChIP assays were performed as described (22). For crosslinking, mESCs were treated with formaldehyde to a final concentration of 1%. Formaldehyde-treated nuclear lysates were subjected to immunoprecipitation with Oct4 antibody. Precipitated DNA fragments were amplified with primers and quantified by real-time quantitative PCR using SYBR® Green fluorescence on the CFX connect Real-time System (Bio-Rad). Values were normalized as percentage of input and presented as relative to control cells.
Quantitative realtime (qRT) PCR
Total RNAs were extracted from mESCs (E14 and ZHBTc4) with TRIzol Reagent (Invitrogen). Extracted RNAs were synthesized into cDNAs by reverse-transcription with AMV Reverse Transcriptase for RT PCR analysis. RT PCR for cDNA was performed using SYBR premix Ex Tag (Takara) and normalized to 18S rRNA. For ChIP assays, SYBR® Green qPCR mix (Finnzymes, F-410) was used and the results are normalized to 1% input chromatin on CFX Connect Real-time PCR Detection System (Bio-Rad).
Reporter gene assay
The reporter gene assay was done as described (22). Briefly, 10 copies of Oct4-responsive element (10 × Oct4 RE)-driven luciferase reporter gene was incorporated into the genome of NIH-3T3 cells by retroviral infection. To stably incorporate reporter gene into genomic DNA, cells were selected with puromycin for at least 2 weeks. These stable cells were transfected with Flag-Oct4 and luciferase activity was measured 2 days after transfection of Oct4.
Nascent RNA analysis
To prepare nascent RNA, Click-iT® Nascent RNA Capture Kit (Life Technologies, Carlsbad, CA, USA) was used according to manufacturer's instructions.
Statistics
All the data were presented as mean ± standard error from at least three independent trials. All data fitting and statistical analysis was performed using GraphPad Prism software.
RESULTS
Chromosome passenger complex is required for maintenance of ESC pluripotency and Oct4(S229) phosphorylation
We previously revealed that Aurkb activity is important for Oct4 resetting of pluripotency and cell cycle genes (19). Aurkb, together with Incenp, Survivin and Borealin, is a component of the chromosomal passenger complex (CPC) and phosphorylates several kinetochore substrates in centromeres and participates in chromosome bi-orientation at prometaphase (23–25). Thus, we wondered whether the CPC affects Oct4(S229) phosphorylation and pluripotency in mESCs. However, as there is little information on the molecular connection between CPC and Oct4 in mESCs, we first investigated the expression pattern of CPC components during mESC differentiation by analyzing previously reported RNA-sequencing data (26). We observed that the mRNA levels of CPC components fell during mESC differentiation (Supplementary Figure S1A). Further, the protein levels decreased by withdrawal of leukemia inhibitory factor (LIF) from growth media (Supplementary Figure S1B). We completely eliminated Oct4 protein from ZHBTc4 cells by 24 h treatment with doxycycline (dox) (27,28), and the results indicated that the expression patterns of CPC components in those cells are apparently independent of Oct4 transcriptional activity (Supplementary Figure S1C and D).
We then knocked down each of the CPC components by introducing small hairpin RNAs (shRNAs) into mESCs and examined their self-renewal capacity, as evidenced by alkaline phosphatase (AP) staining results. The expressions of pluripotency-related genes were reduced and lineage-specific genes expressions were considerably increased in CPC-knockdown mESCs. Knockdown of each of the CPC components affected mESC self-renewal (Supplementary Figure S2A–D). In the case of Borealin, knockdown #1 mESCs failed to survive and the number and size of AP-positive colony are too small in comparison with knockdown #2 mESCs. Mild knockdown of Borealin (knockdown #2) disturbed mESCs pluripotency and changed mESCs like differentiated cells. As Aurkb is a major catalytic subunit of CPC, we wondered whether CPC components could affect the phosphorylation state of Oct4(S229). Following treatment of E14 mESCs with nocodazole for 8 h, Oct4(S229) phosphorylation was highly accumulated at the G2/M phase. However, knockdown of each of the CPC components decreased the Oct4(S229) phosphorylation and Aurkb(T232) auto-phosphorylation levels (Supplementary Figure S2E), indicating that Aurkb, as a component of CPC, can phosphorylate Oct4(S229) at the G2/M phase.
Cdk1 is important for ESC pluripotency and preferentially interacts with Oct4 at the G2/M phase
We next wanted to determine why Aurkb, as a CPC component, specifically phosphorylates Oct4(S229) in a cell cycle-dependent manner, particularly, at the G2/M phase. It has been reported that Cdk1 solely controls the G2/M phase and promotes CPC binding to Shugoshin and chromosome bi-orientation by phosphorylating human Borealin, a CPC component (23). On that basis, we speculated that Cdk1 is a main regulator of the connection between Aurkb/CPC and Oct4 during the G2/M phase.
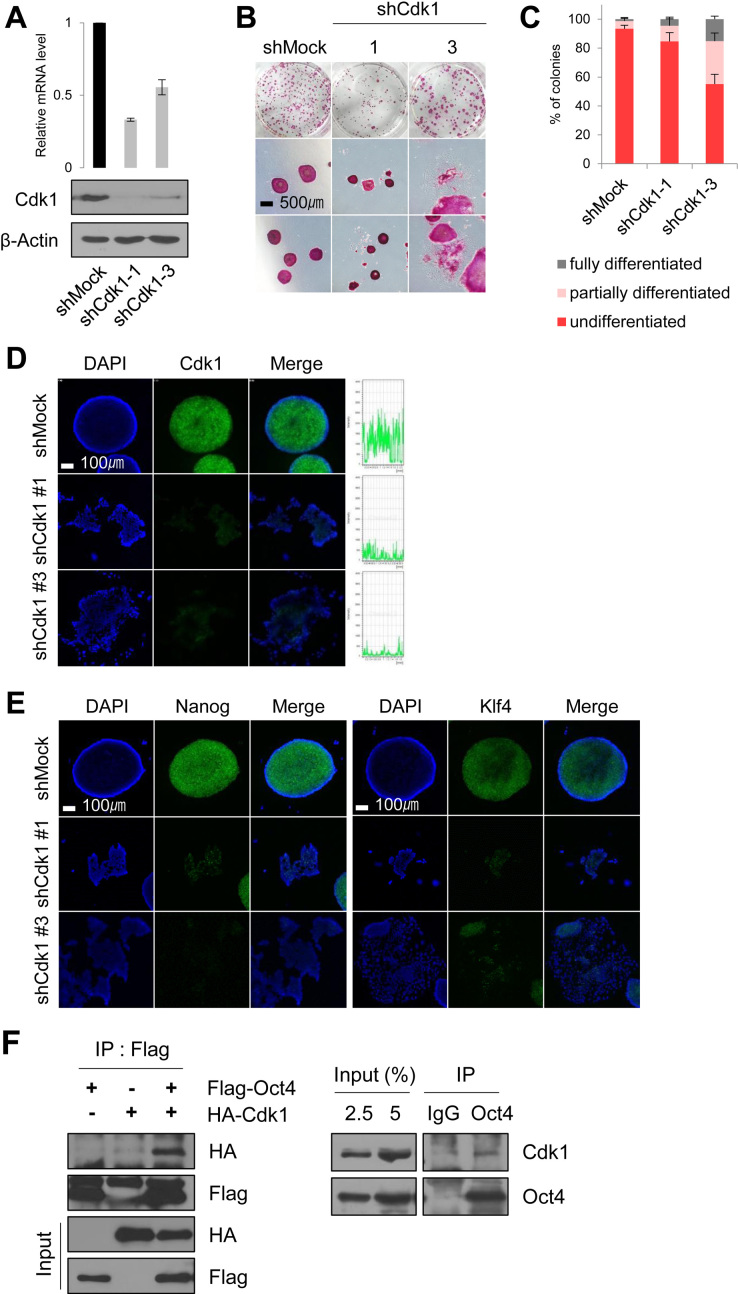
To address this question, we first knocked down Cdk1 in E14 mESCs by using a shRNA construct that targets Cdk1 (Figure 1A and Supplementary Figure S3A). Cdk1-knockdown produced greater G2/M populations in PI staining than those of control cells, as evidenced by the results of the FACS analysis (Supplementary Figure S3B). This is due to delayed mitotic entry and arrest at the G2 phase in the Cdk1-knockdown mESCs. By AP staining, the mESCs in which Cdk1 expression was severely knocked down (knockdown #1) reduced the cell proliferation and survival, while Cdk1-knockdown #3 mESCs resembled differentiated cells (Figure 1B and C). Immunofluorescence experiments also revealed that Cdk1-knockdown leads to loss of the pluripotency in mESCs (Figure 1D).
Figure 1.
Cdk1 is important for ESC pluripotency and preferentially interacts with Oct4 at the G2/M phase. (A) Protein levels and mRNA levels of Cdk1-knockdown mESCs (n = 3). (B and C) AP staining of Cdk1-knockdown mESCs. After staining to reveal AP activity, the colonies were scored and the percentages of undifferentiated, partially differentiated, and fully differentiated colonies were calculated. AP staining experiments were repeated three times (n = 3). Scale bar represents 500 μm. (D) Immunostaining of Cdk1-knockdown mESCs. Cdk1 was stained with anti-Cdk1 (green) and DNA was stained DAPI (blue). Scale bar represents 100 μm. (E) Fluorescence images of Cdk1-knockdown mESCs. Nanog and Klf4 were stained with anti-Nanog (green) and anti-Klf4 (green), respectively. DNA was stained with DAPI (blue). Scale bar represents 100 μm. (F) Flag-Oct4 and HA-Cdk1 were cotransfected into HEK293T cells. Cell lysates were immunoprecipitated with anti-Flag antibody and probed with anti-HA antibody (left). Endogenous Cdk1 was immunoprecipitated from E14 mESCs with anti-Oct4 antibody and immunoblotted with anti-Cdk1 antibody (right). (G) Changes in interaction of Cdk1 with Oct4 during cell cycle progression. Flag-Oct4-expressing ZHBTc4 mESCs were treated with nocodazole (100 ng/ml) for 8 h. At the indicated time after release into fresh media, cell cycle progression was determined by FACS analysis (right). Cell lysates were pulled down with anti-Flag beads. Bound proteins were eluted and then immunoblotted with the indicated antibodies. (A, asynchronous state; N, nocodazole treatment). (H) Changes in interaction of Oct4 with Cdk1 depending on Cyclin B knockdown. After Cyclin B knockdown, Flag-Oct4-expressing ZHBTc4 mESCs were treated with nocodazole for 8 h. Flag-Oct4 was immunoprecipitated by incubating lysates with anti-Flag beads. Following Flag elution, bound proteins were immunoblotted with the indicated antibodies. (I) Colocalization of Cdk1 and p-Oct4(S229) in mitosis-arrested mESCs was analyzed by immunostaining. mESCs were treated with nocodazole (100 ng/ml) for 8 h. Cdk1 was stained with anti-Cdk1 (green), p-Oct4(S229) was stained with anti-p-Oct4(S229) (red), and DNA was stained with DAPI (blue). Scale bar represents 5 μm. (J) Radioactive in vitro kinase assay using recombinant Cdk1 to phosphorylate GST-Oct4 wild type (WT) and S228A/S229A mutant. Autoradiogram showing incorporation of γ-32P ATP (left). For the IP kinase assay, cell lysates of nocodazole-treated E14 mESCs were immunoprecipitated with anti-CycB1 and Aurkb and subjected to an in vitro kinase assay using (His)6-PP1α and GST-Oct4 as a substrate and followed by western blotting (right).
When we quantified the mRNA expression levels of the pluripotency marker genes and the lineage-specific differentiation marker genes in Cdk1-knockdown mESCs (Supplementary Figure S3C), pluripotency-related gene expressions were considerably reduced, while those of lineage-specific genes were significantly increased, which is consistent with previous reports that Cdk1 is critical for maintenance of pluripotency in ESCs (9,15,18,29). Expression levels of Nanog and Klf4 were reduced in Cdk1-knockdown cells as assessed by immunostaining results, compared to the control cells (Figure 1E). While Oct4 depletion slightly reduced Cdk1 protein and mRNA expressions (Supplementary Figure S1C and D), Cdk1-knockdown significantly affected Oct4 expression. Therefore, Cdk1 might be an upstream modulator for Oct4-driven ESC pluripotency during cell cycle.
Even though several reports have shown the interaction between Cdk1 and Oct4 in ESCs (16–18), it is still unclear how two proteins are connected and regulated each other during cell cycle progression. Therefore, to clarify their interactions during cell cycle progression, we first investigated the interaction between Cdk1 and Oct4 by immunoprecipitation with overexpressing HA-Cdk1 and Flag-Oct4 in HEK293T cells. As expected, we detected Cdk1 in association with Oct4 after precipitation with the Flag antibody. We also found endogenous Cdk1 in Oct4 immunoprecipitations in E14 mESCs (Figure 1F), which is consistent with previous reports (16–18).
We next wondered whether Cdk1 interacts with Oct4 in a cell cycle-dependent manner and wanted to determine the cell cycle phases during which Cdk1 preferentially interacts with Oct4. Flag-Oct4-expressing ZHBTc4 mESCs were treated with nocodazole for 8 h. Under the nocodazole-treated conditions, most of mESCs were arrested at G2/M phase by evidencing that Ki-67 in mitotic cells covered condensed chromosomes (Supplementary Figure S3D) and over 85% of the cells were pH3S10-positive (Figure 1l). Nocodazole-treated ESCs were then released to continue cell cycle progression by the removal of nocodazole. Flag-Oct4 strongly interacted with endogenous Cdk1 as well as Cyclin B at the G2/M phase in Flag-Oct4-expressing ZHBTc4 mESCs, while it also associated with endogenous Cyclin A when cells were released for 4 h after nocodazole treatment (Figure 1G).
To test whether Cyclin B is necessary for the Oct4–Cdk1 interaction, we examined the in vitro interaction between Oct4 and Cdk1 using bacterially-purified recombinant Cdk1-Flag, GST-Oct4, and (His)6-Cyclin B proteins. We observed that Cdk1-Flag directly binds to GST-Oct4 regardless of (His)6-Cyclin B (Supplementary Figure S4A). However, knockdown of Cyclin B in mESCs severely reduced the Oct4–Cdk1 interactions under the nocodazole-treated conditions (Figure 1H). These results implicated that the Oct4–Cdk1 interactions might be regulated more sophisticated than we expected during cell cycle progression.
We observed that Cdk1 was mainly located in cytoplasm and Oct4 in nucleus in the asynchronous mESCs, while Cdk1 was colocated with Oct4, as a phosphorylated form, outside the condensed chromatin at the mitotic phase (Figure 1I), consistent with the above results showing that both proteins strongly interact at the G2/M phase. However, previous reports indicate that Cdk1-cyclin B translocates to the nucleus during prophase (30), and that Cdk1 and Oct4 are colocalized in the form of nuclear foci in mESCs, whereas Cdk1 is highly accumulated during late G1/S and S/G2 transitions in hESCs (9,18).
We then examined whether Cdk1 directly phosphorylates Oct4(S229) by using an in vitro32P-ATP-labeled kinase assay and western blotting with anti-p-Oct4(S229). We observed that neither recombinant Cdk1 nor the Cdk1 immunoprecipitated with Cyclin B antibody phosphorylated GST-Oct4(S229), whereas recombinant Aurkb specifically phosphorylated Oct4(S229) (Figure 1J), indicating that Cdk1 indirectly regulates Aurkb-mediated phosphorylation of Oct4(S229) at the G2/M phase rather than directly phosphorylating Oct4(S229).
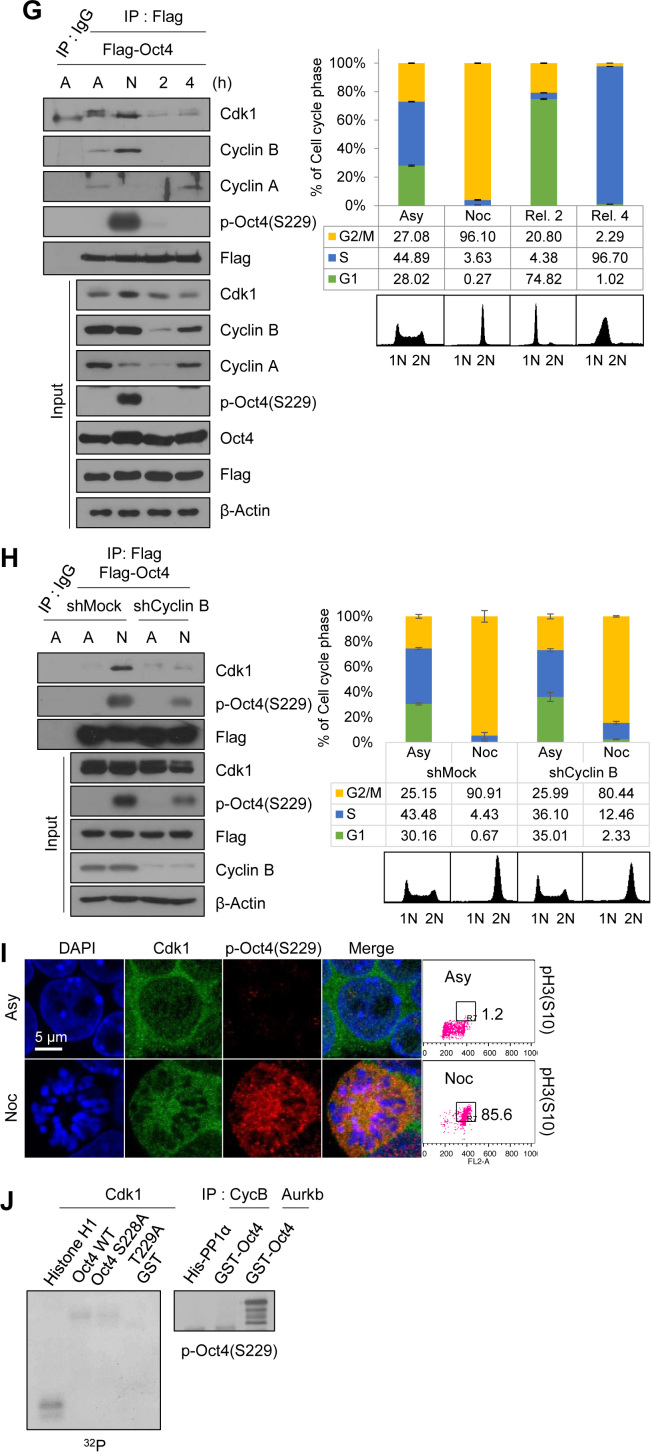
Cdk1 connects Aurkb/CPC with Oct4 phosphorylation at the G2/M phase
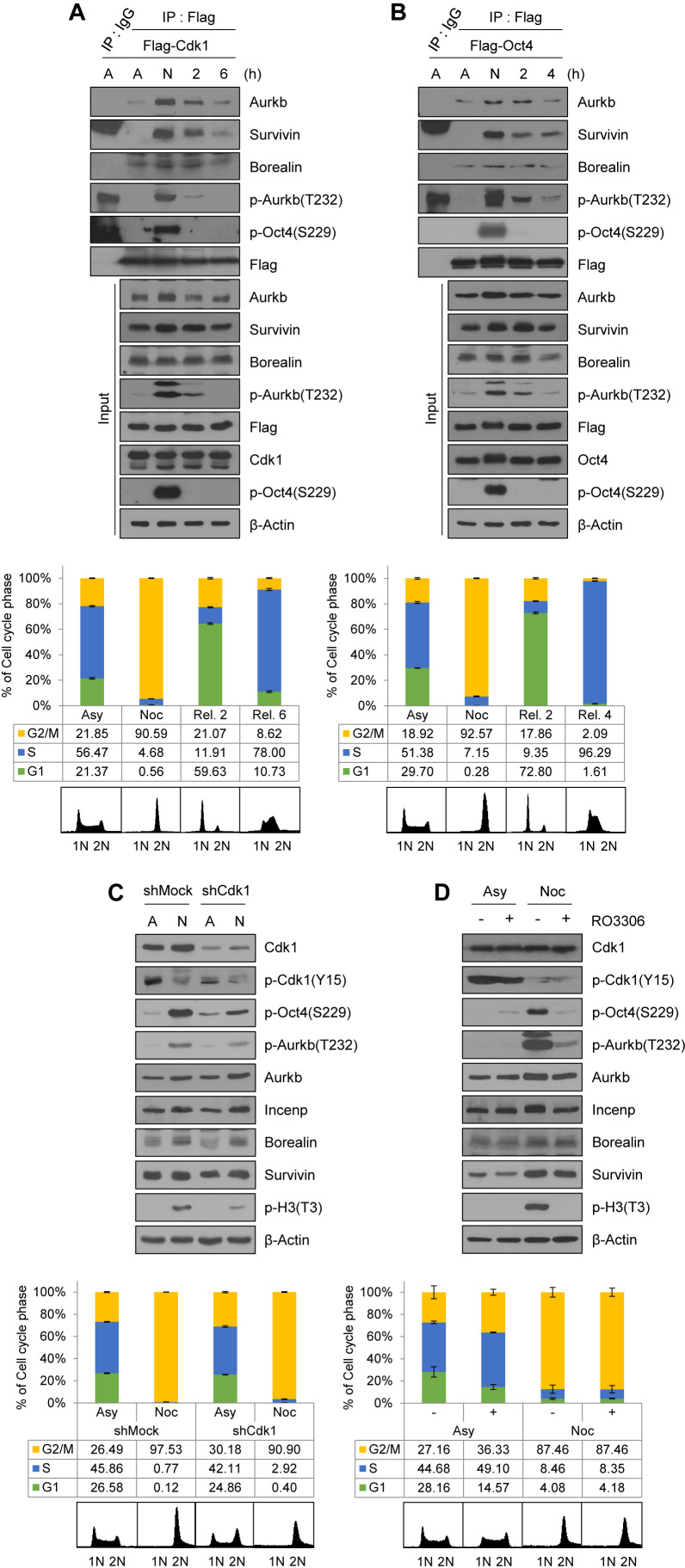
To address whether Cdk1 regulates Aurkb/CPC-mediated phosphorylation of Oct4(S229) at the G2/M phase, we examined the interaction of Cdk1 with CPC components during cell cycle progression. We first confirmed that exogenous Flag-Cdk1 co-precipitated with HA-Aurkb, HA-Incenp, and HA-Survivin in HEK293T cells and exogenous HA-Oct4 co-precipitated with each of Flag-tagged CPC components (Supplementary Figure S4B and C). Then, we synchronized Flag-Cdk1-expressing mESCs by using nocodazole. We observed that Flag-Cdk1 bound to endogenous CPC components at the G2/M phase when both Cdk1 and Aurkb were activated (Figure 2A). Interestingly, when nocodazole-arrested mESCs were released, Flag-Cdk1 was dissociated with Aurkb and Survivin; moreover, Aurkb(T232) autophosphorylation, an active marker of Aurkb, gradually decreased. Under the same conditions in which Flag-Oct4-expressing ZHBTc4 mESCs were arrested at the G2/M phase, Flag-Oct4 was strongly associated with endogenous Aurkb and Survivin, and dissociated from them when mESCs were released into the G1 phase (Figure 2B).
Figure 2.
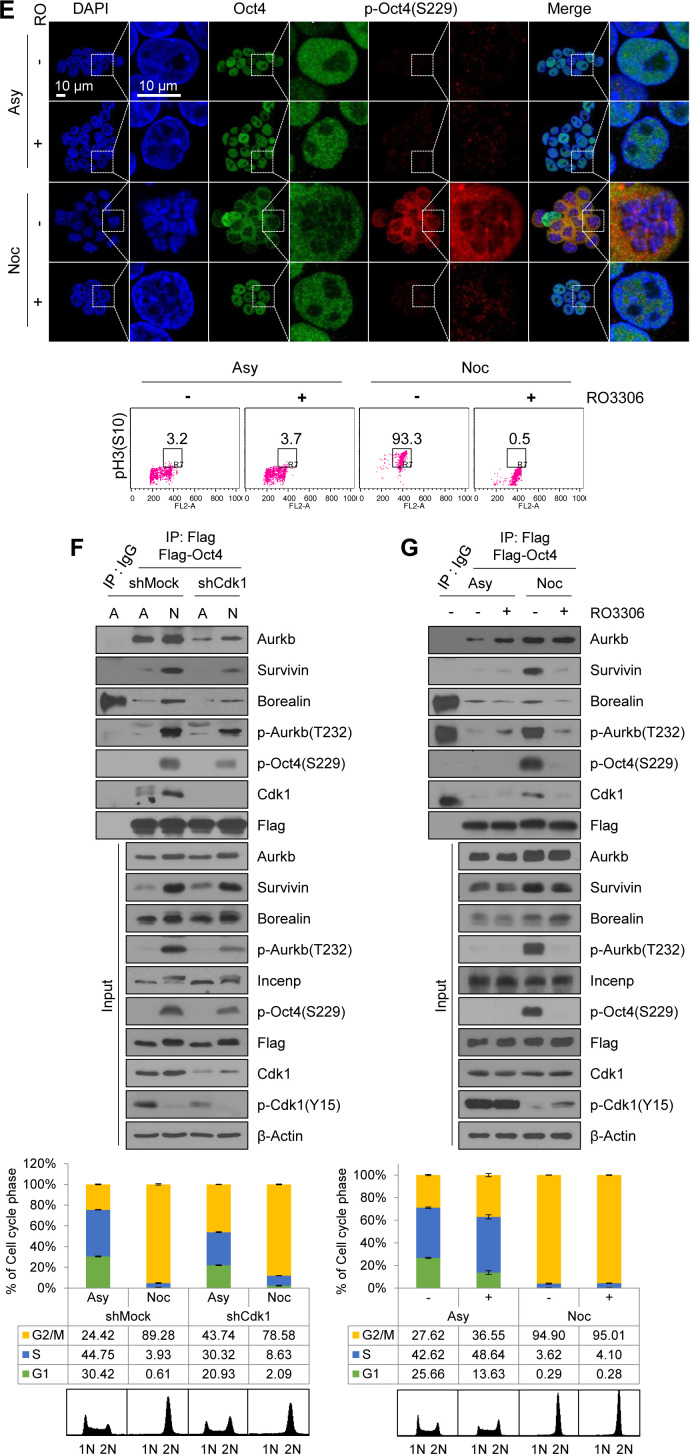
Cdk1 connects Aurkb/CPC with Oct4 phosphorylation at the G2/M phase. (A) Changes in interaction of Cdk1 with CPC during cell cycle progression. Flag-Cdk1-expressing E14 mESCs, treated with nocodazole (100 ng/mL) for 8 h, were released for 2 or 6 h. Cell lysates were pulled down with anti-Flag beads. Beads were washed three times and bound proteins were eluted with Flag peptide. Elutes were immunoblotted with the indicated antibodies. The FACS profiles of cells were shown in the below. (B) Changes in interaction of Oct4 with CPC during cell cycle progression. Flag-Oct4-expressing ZHBTc4 mESCs were arrested with nocodazole for 8 h and released into fresh media for the indicated time. Cell lysates were pulled down with anti-Flag beads. Bound proteins were eluted by using Flag peptide. Obtained samples were immunoblotted by using the indicated antibodies. (C) Cdk1-knockdown in E14 mESCs reduced the levels of p-Oct4(S229), p-Aurkb(T232), and p-H3(T3). (D and E) Nocodazole-pretreated E14 mESCs were treated with RO3306 without removal of nocodazole. Cdk1 inhibition in nocodazole-pretreated E14 mESCs reduced the p-Oct4(S229) level. Scale bar represents 10 μm. Representative FACS profiles showing the distribution of pH3(S10)-positive cells (x, DNA content; y, pH3(S10)) of E14 mESCs. Numbers indicate the percentage of pH3(S10)-positive cells in each case. (F) Changes in interaction of Oct4 with CPC depending on Cdk1-knockdown. After Cdk1-knockdown, Flag-Oct4-expressing ZHBTc4 mESCs were treated with nocodazole for 8 h. Flag-Oct4 was immunoprecipitated by incubating lysates with anti-Flag beads. Following Flag elution, bound proteins were immunoblotted with the indicated antibodies. (G) Changes in interaction of Oct4 with CPC depending on Cdk1 inhibition. Nocodazole-pretreated Flag-Oct4-expressing ZHBTc4 mESCs were treated with RO3306 without removal of nocodazole. Cell lysates were pulled down with anti-Flag beads and immunoblotted with the indicated antibodies.
To address whether Cdk1 is critical for activating Aurkb and sustaining Oct4(S229) phosphorylation at the G2/M phase in mESCs, we treated Cdk1-knockdown mESCs with nocodazole. The results showed that p-Oct4(S229) levels in Cdk1-knockdown mESCs were significantly reduced from that in wild type mESCs. In addition, auto-phosphorylated Aurkb(T232) level was decreased in nocodazole-treated Cdk1-knockdown cells (Figure 2C and Supplementary Figure S4D).
At this point, we raised the question of whether G2 arrest by Cdk1-knockdown naturally blocks the progression to mitosis so that, eventually, Oct4(S229) phosphorylation may not increase. To address this question, we investigated the role of Cdk1 on Oct4(S229) phosphorylation during mitosis by treating nocodazole-pretreated mESCs with the Cdk1 inhibitor, RO3306. We observed that Cdk1 inhibition during the mitotic phase resulted in a considerable decrease in level of Oct4(S229) phosphorylation, Aurkb autophosphorylation, and H3(T3) phosphorylation (Figure 2D and Supplementary Figure S4E). In addition, we observed similar results by using confocal microscopy (Figure 2E). When mESCs were arrested at the G2/M phase, over 90% of cells were pH3S10-positive and Ki-67 occupied around condensed chromosomes (Supplementary Figure S4F). p-Oct4(S229) was located outside the nucleus and appeared to be dissociated from chromatin in mitotic cells. Once Cdk1 was inhibited in nocodazole-pretreated mESCs, the p-Oct4(S229) level significantly decreased and condensed mitotic chromosome was decondensed. These results support the hypothesis that Cdk1 governs Aurkb/CPC activity and sustains Aurkb-mediated phosphorylation of Oct4(S229) during the G2/M phase.
Next, to verify whether interaction between CPC and Oct4 is dependent on Cdk1, we inhibited Cdk1 by using shRNAs or a chemical Cdk1 inhibitor, RO3306, and performed immunoprecipitation with Flag-Oct4 in Flag-Oct4-expressing ZHBTc4 mESCs. When we co-precipitated CPC components with Flag-Oct4 in nocodazole-treated Cdk1-knockdown mESCs, the amount of binding was reduced in the Cdk1-knockdown cells, indicating that Cdk1 mediates binding of Oct4 with CPC components at the G2/M phase (Figure 2F).
When G2/M-arrested cells were treated with a Cdk1 inhibitor, RO3306, the level of association of Oct4 with CPC components such as Survivin and Borealin, expect for Aurkb, was significantly reduced. Under the same conditions, binding of Oct4 with Cdk1 was also reduced (Figure 2G). Despite the Cdk1 inhibition, Aurkb was still associated with Oct4 (Figure 2G). The inhibition of Cdk1 by chemical inhibitor seemed to reduce the interaction of CPC components (Survivin and Borealin) with Oct4 more significantly than Cdk1-knockdown, probably due to that Cdk1-knockdown using shRNA could not completely eliminate Cdk1 protein. These results indicate that Cdk1 activity is critical for connecting Aurkb/CPC with Oct4 phosphorylation during the mitotic phase. In addition, the knockdown of Aurkb in mESCs severely reduced the Oct4–Cdk1 interaction at the G2/M phase (Supplementary Figure S4G), indicating that Oct4, Cdk1 and Aurkb are tightly connected together at G2/M phase.
Cdk1-mediated phosphorylation of PP1 sustains Oct4(S229) phosphorylation at the G2/M phase
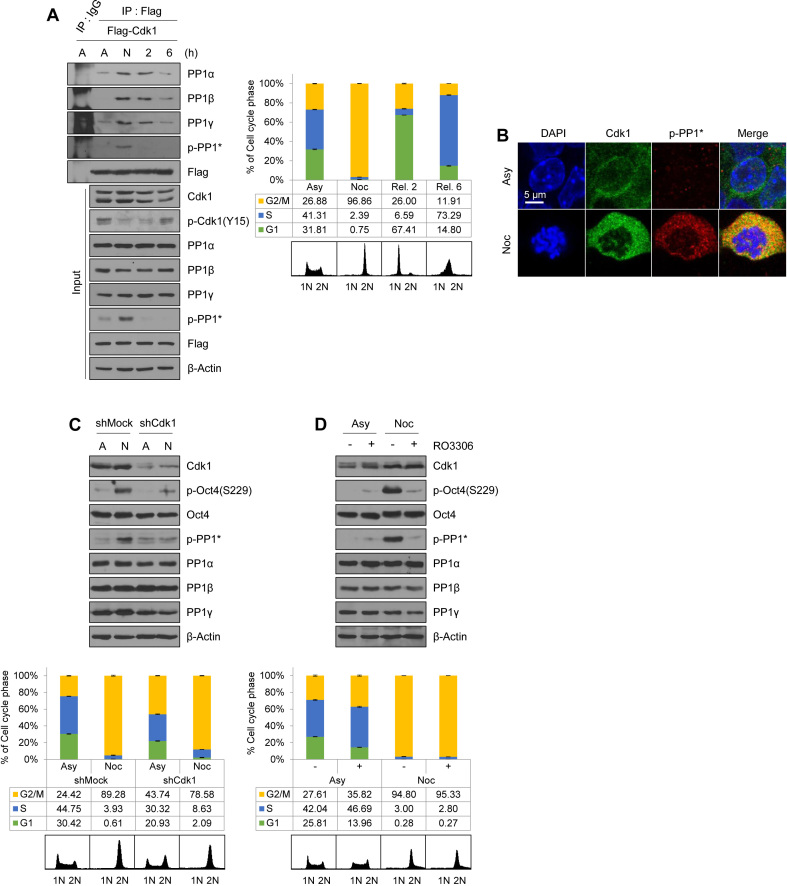
It was previously reported that PP1 antagonizes Aurkb-mediated phosphorylation of Oct4(S229) (19), and the activities of three isoforms of PP1 are inhibited by Cdk1-mediated phosphorylation of conserved threonine residues (T320 in PP1α, T316 in PP1β, T311 in PP1γ, thereafter collectively designated as p-PP1*) (24,31–34). Therefore, we wondered whether Cdk1 inhibits PP1 activity and sustains Aurkb-mediated phosphorylation of Oct4(S229) at the G2/M phase in mESCs. We observed that ectopically-expressed Cdk1 interacts with all PP1 isoforms (α, β and γ) (Supplementary Figure S5A). In addition, by using an in vitro Cdk1 kinase assay with recombinant PP1 proteins purified from Escherichia coli, we observed that Cdk1 can phosphorylate all three PP1 isoforms (Supplementary Figure S5B).
To determine whether the interaction of Cdk1 with PP1 is cell cycle dependent, we immunoprecipitated the endogenous PP1 isoforms with Flag-Cdk1 after releasing nocodazole from G2/M-arrested Flag-Cdk1-expressing E14 mESCs. Flag-Cdk1 was associated with all three PP1 isoforms (α, β and γ), especially with phosphor-PP1* after 8 h treatment with nocodazole, when the majority of cells are in the G2/M phases (Figure 3A). Once these cells were released into the next G1 phase by withdrawal of nocodazole, Flag-Cdk1 became dissociated from the PP1 isoforms. We then examined phosphor-PP1* via confocal microscopy in E14 mESCs, which revealed that p-PP1* in E14 mESCs were observed in mitotic cells (Figure 3B). As expected, the results show that Cdk1 colocalizes with p-PP1* outside the condensed chromatin at the mitotic phase. At this point, Ki-67 covered condensed chromosomes (Supplementary Figure S5C).
Figure 3.
Cdk1-mediated phosphorylation of PP1 sustains Oct4(S229) phosphorylation at the G2/M phase. (A) Changes in interaction of Cdk1 with PP1 during cell cycle progression. Flag-Cdk1-expressing E14 mESCs, treated with nocodazole for 8 h, were released into fresh media for 2 or 6 h. Flag-Cdk1 in the lysates were immunoprecipitated by using anti-Flag beads and immunoblotted with their respective antibodies. The FACS profiles of each cell sample are shown in the right panel. (B) Colocalization of Cdk1 with p-PP1* in mitosis-arrested mESCs was analyzed by immunostaining. E14 mESCs were untreated or treated with nocodazole (100 ng/mL) for 8 h. Cdk1 was stained with anti-Cdk1 (green), p-Oct4(S229) was stained with anti-p-Oct4(S229) (red), and DNA was stained with DAPI (blue). Scale bar represents 5 μm. (C) Cdk1-knockdown in mitosis reduced levels of p-Oct4(S229) and p-PP1*. (D and E) Nocodazole-pretreated E14 mESCs were treated with RO3306 without removal of nocodazole. Cdk1 inhibition in mitosis reduced levels of p-Oct4(S229) and p-PP1*. Scale bar represents 10 μm. (F) Changes in p-Oct4(S229) level depending on Cdk1-knockdown. After Cdk1-knockdown, Flag-PP1γ(WT)-expressing E14 mESCs were treated with nocodazole for 8 h. Cell lysates were incubated with anti-Flag beads and bound proteins were immunoblotted with the indicated antibodies. (G) Comparisons of p-Oct4(S229) levels in PP1γ(WT) and PP1γ(T311A)-expressing mESCs. Flag-PP1γ(WT)- and PP1γ(T311A)-expressing E14 mESCs were treated with nocodazole for 8 h and released for 2 h. Cell lysates were pulled down with anti-Flag beads. Elution samples were immunoblotted with the indicated antibodies. Compared to PP1γ(WT), PP1γ(T311A) is more weakly bound to p-Oct4(S229). Comparisons of p-Oct4(S229) levels in PP1α(WT) and PP1α(T320A) as well as in PP1β(WT) and PP1β(T316A) are shown in Supplementary Figure S6A and B.
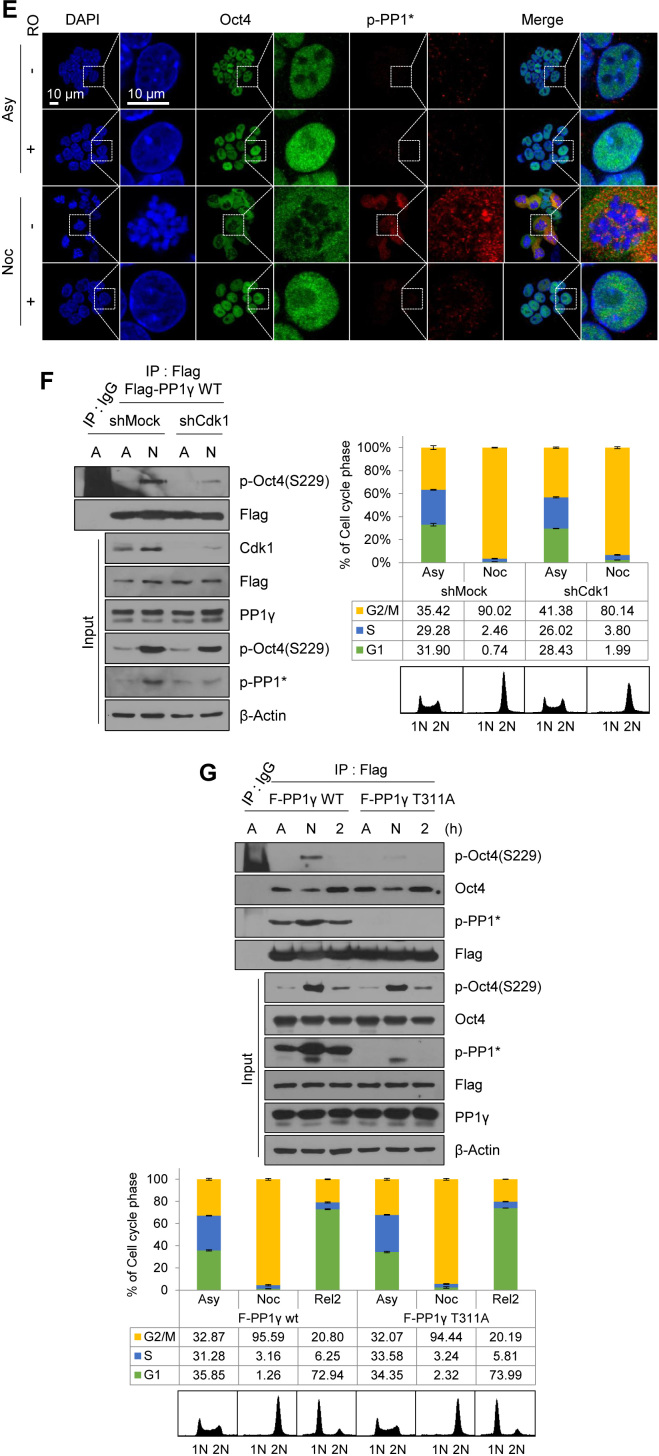
When Cdk1-knockdown cells were treated with nocodazole, p-PP1* levels significantly decreased; however, protein levels of PP1α, PP1β, and PP1γ remained unchanged (Figure 3C). Moreover, Oct4(S229) phosphorylation coexisted with phosphor-PP1* in nocodazole-treated G2/M phase mESCs. In addition, upon RO3306 treatment of nocodazole-pretreated mESCs, the phosphorylation status of both Oct4(S229) and PP1 were rapidly decreased after inhibition of Cdk1 activity for 10 min (Figure 3D and Supplementary Figure S5D). Under the same conditions, p-PP1* colocalized with Oct4 outside the condensed chromosome in mitotic cells. However, treatment of RO3306 with mitotic cells significantly reduced p-PP1* levels and induced chromosome decondensation (Figure 3E), suggesting that Cdk1 sustains p-Oct4(S229) by binding and phosphorylating PP1 in mitotic mESCs.
To further confirm that Cdk1-mediated phosphorylation of PP1 induces inactivation of PP1 and maintenance of p-Oct4(S229), we depleted Cdk1 in Flag-PP1γ (WT)-expressing E14 mESCs. After knockdown of Cdk1, we treated with nocodazole for 8 h and immunoprecipitated p-Oct4(S229) with Flag-PP1γ. As a result, binding of Flag-PP1γ to p-Oct4(S229) was reduced in Cdk1-knockdown G2/M-arrested mESCs (Figure 3F).
Next, we altered the Cdk1-mediated phosphorylation site from threonine to alanine in PP1s [PP1α(T320A), PP1β(T316A), PP1γ(T311A)] by site-directed mutagenesis and, thereby, generated isoforms of Flag-phosphor-defective-PP1-expressing E14 mESCs. After Flag-immunoprecipitation, three wild types of the PP1 isoforms exhibited strong binding to p-Oct4(S229) at the G2/M phase. In contrast, phosphor-defective mutants weakly bound to p-Oct4(S229) (Figure 3G, Supplementary Figure S6A and B), suggesting that phosphor-defective PP1 mutants are free from Cdk1 activity and, thus, dephosphorylate p-Oct4(S229) despite the onset of mitosis. Therefore, these results imply that phosphorylation and inhibition of PP1s by Cdk1 are important in sustaining the level of p-Oct4(S229).
We then generated three E14 mESCs expressing phosphor-mimetic Flag-PP1 mutants [PP1α(T320D), PP1β(T316D), PP1γ(T311D)]. Phosphor-mimetic mutants, as inactive forms, should bind to Oct4 but not strongly dephosphorylate p-Oct4(S229) in the next G1 phase. Unexpectedly, the p-Oct4(S229) levels in the E14 mESCs expressing phosphor-mimetic Flag-PP1 mutants disappeared after 2 h release (Supplementary Figure S6C–E). That result is probably due to the PP1 isoforms interacting with each other and sharing functional redundancy. Previously reported protein–protein interaction data indicate that PP1γ interacts with PP1α and PP1β in human cells (35,36). Indeed, in this study, mutants of PP1γ(T311A) and PP1γ(T311D), as well as PP1γ(WT), were able to associate with endogenous PP1β (Supplementary Figure S6F). To eliminate the redundancy among the PP1 isoforms, we simultaneously knocked down three PP1 isoforms (Supplementary Figure S6G–J). Based on AP staining results, the PP1s-knockdown mESCs appeared differentiated. In addition, re-entering G1 and p-Oct4(S229) dephosphorylation were relatively retarded upon withdrawal of nocodazole in comparison with that in wild type mESCs.
Cdk1 inhibition affects the binding of Oct4 to chromatin and its transcriptional activity
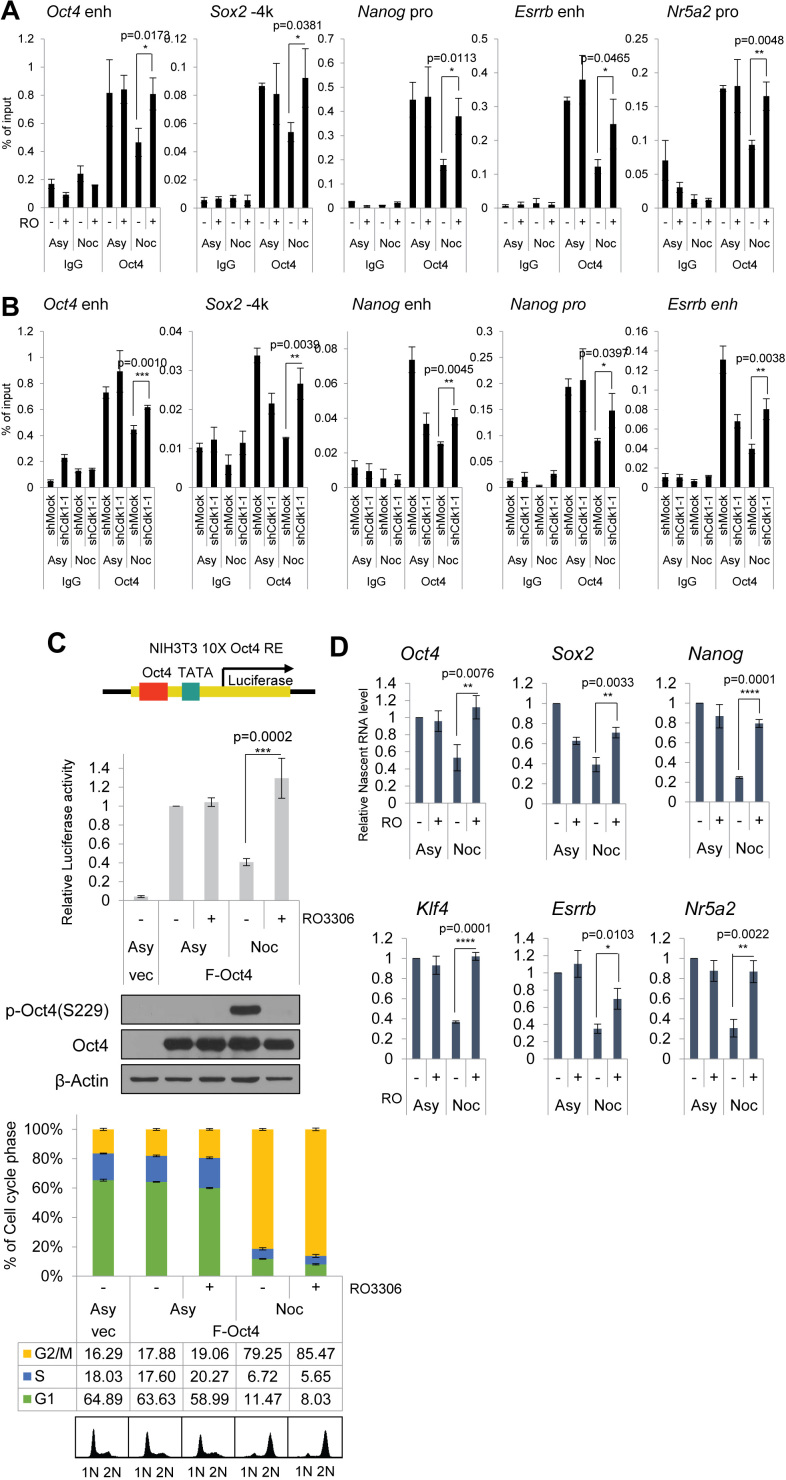
Based on the results showing that inhibition of Cdk1 at mitosis induces dephosphorylation of p-Oct4(S229), we hypothesized that Cdk1 might modulate the binding of Oct4 to chromatin for pluripotency-related gene expressions. To investigate the effect of Cdk1 on the binding of Oct4 to chromatin areas of pluripotency-related genes, we performed ChIP-qPCR assay by treating nocodazole-pretreated E14 mESC with RO3306. Binding of Oct4 to chromatin areas of pluripotency-related genes declined when cells were arrested at the G2/M phase but increased when RO3306 was added in the presence of nocodazole (Figure 4A), which is consistent with results showing that high amounts of p-Oct4(S229) that accumulate in the G2/M phase vanish quickly after treatment the RO3306 Cdk1 inhibitor (Figures 2D and 3D). Thus, inhibition of Cdk1 at the mitotic phase might force association of Oct4 on its target genes. In addition, binding of Oct4 to target chromatins was augmented in nocodazole-treated Cdk1-knockdown cells when compared to the binding of Oct4 to target genes in nocodazole-treated control cells (Figure 4B).
Figure 4.
Cdk1 inhibition affects the binding of Oct4 to chromatin and its transcriptional activity. (A) E14 mESCs arrested with nocodazole were treated with RO3306. ChIP analysis of mESCs with anti-Oct4 in regions of pluripotency-associated Oct4 target genes under the condition that Cdk1 is inhibited in mitosis. IgG was used as a control (n = 3). (B) ChIP analysis of Cdk1-knockdown mESCs with anti-Oct4 in regions of pluripotency-associated Oct4 target genes. IgG was used as a control (n = 3). (C) Measurement of Oct4-driven transcriptional activity. Ten copies of Oct4-responsive element (10 × Oct4 RE)-driven luciferase reporter gene were incorporated into the genome of NIH-3T3 cells. These stable cells were transfected with Flag-Oct4 and treated with nocodazole for 16 h. Nocodazole-treated NIH-3T3 cells were harvested by mitotic shake-off and then treated with RO3306 (n = 3). (D) Nascent RNA of pluripotency-associated Oct4 target genes from E14 mESCs were collected and analyzed by performing qRT-PCR. Levels of each nascent RNA were normalized by those in asynchronous E14 mESCs (n = 3). Data are presented as means ± SEM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
To examine how Cdk1 affects the transcriptional activity of Oct4, we transfected Flag-Oct4 into NIH-3T3 cells that stably harbored Oct4-driven luciferase reporter genes. After 48 h of transfection, we treated the cells with nocodazole for 16 h, followed by RO3306 treatment for 2 h (without removal of nocodazole). Nocodazole treatment significantly reduced Oct4 transcriptional activity. However, treatment with RO3306, a Cdk1 inhibitor, of the G2/M-arrested cells triggered recovery of Oct4 transcriptional activity (Figure 4C).
To verify that Cdk1 is important for Oct4-dependent gene expressions, we measured nascent RNA levels of pluripotency-related genes. When E14 mESCs were arrested in the G2/M phase, nascent RNA levels of a subset of Oct4-targeting pluripotency genes were significantly lower than those in asynchronous mESCs. Nascent RNA levels of some Oct4 target genes were upregulated after RO3306 was added to G2/M-arrested mESCs (Figure 4D). These results indicate that Cdk1 inhibition triggers abnormal transcription of Oct4 to target genes, even in replicated chromosome. Therefore, we suggest that Cdk1 tightly links cell cycle progression to the transcriptional activity of Oct4 to pluripotency genes.
Cdk1 inhibitor-treated mESCs in the presence of nocodazole were released for 10 h by simultaneous removal of both drugs (Supplementary Figure S7A). Intriguingly, these cells could not progress to next G1 phase after releasing cells by withdrawal of these drugs. FACS analysis with PI staining showed that cell populations with >4N DNA contents increased. These populations have larger size of cells than that of asynchronous cells (Supplementary Figure S7B and C). These polyploidy cells formed AP-positive colonies and were differentiated by removal of LIF (Supplementary Figure S7D). It will be interesting to study the ESC signatures in polyploidy cells through long-term observation.
DISCUSSION
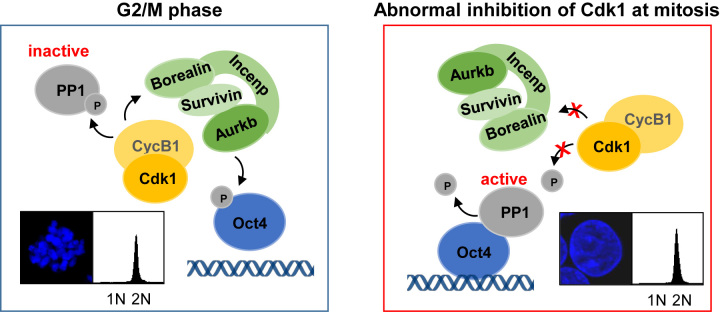
Based on the results of this study, we propose a model in which Cdk1 serves as an upstream regulator for Oct4 phosphorylation by controlling the activities of Aurkb and PP1 toward Oct4 and coordinates cell cycle progression through the expression of pluripotency genes in mESCs (Figure 5).
Figure 5.
Schematic diagram. A model describing the function of Cdk1 as an upstream regulator for Oct4 phosphorylation. Cdk1 controls the activities of Aurkb/CPC and PP1, leading to dissociation of Oct4 from chromatin during mitosis. Abnormal inhibition of Cdk1 at mitosis triggers chromosome decondensation, Oct4(S229) dephosphorylation, and evokes Oct4 transcriptional activity.
In ESCs, only Cdk1 is selectively activated during cell cycle progression, and Cdk1 knockout is lethal in mice at an early embryonic stage (13,14). Moreover, Cdk1 has been identified as an Oct4 binding partner (15,16,18), thus, Cdk1 activity is likely to be intertwined with pluripotency transcription in ESCs. Nonetheless, whether kinase activity of Cdk1 is related to transcriptional activity of Oct4 in ESCs remains to be determined. In this study, we confirmed that Cdk1 interacts with Oct4, but it does not directly phosphorylate Oct4. Intriguingly, we also found that Cdk1 specifically interacts with Oct4 and affects the phosphorylation state of Oct4(S229) at the G2/M phase (Figure 1). In vitro binding experiments showed that Cdk1 apparently interacts with Oct4 regardless of its regulatory subunit, Cyclin B (Supplementary Figure S4A), but in vivo co-immunoprecipitation experiments, Oct4 specifically associated together with Cdk1/Cyclin B at the G2/M phase, and knockdown of Cyclin B in mESCs reduced the Cdk1-Oct4 interaction at G2/M phase (Figure 1H). In fact, previous reports have shown the discrepancies that Cdk1 associates with Oct4 without Cyclin B in vitro (18), while Cdk1 did not associate with Oct4 without cyclins (17). Therefore, we thought that the Cdk1–Oct4 interactions at cellular level are presumably regulated by more complicated manners such as post-translational modifications and their binding partners, including chromosome passenger components, at the G2/M phase.
We wanted to determine why this specific interaction emerges at the G2/M phase because we recognized that this interaction correlates with results in our previous work into specific phosphorylation of Oct4(S229) at the G2/M phase (19). Therefore, we hypothesized that Cdk1 might regulate the Aurkb-mediated Oct4(S229) phosphorylation in mESCs.
To identify the mechanism by which Cdk1 regulates Oct4(S229) phosphorylation, we investigated the control of CPC by Cdk1 during G2/M phase. Cdk1 augments Aurkb activity, a central catalytic subunit of CPC, which, in turn, promotes CPC binding to Shugoshin and chromosome bi-orientation during mitosis (23). Similarly, in the present study, we showed that Oct4 strongly interacts with Cdk1 and CPC components at the G2/M phase, which is followed by Cdk1-activating Aurkb phosphorylation of Oct4(S229) (Figures 1G and 2B). In addition, we observed that the level of interaction of CPC with Oct4 was reduced when Cdk1 activity was inhibited at the G2/M phase. These results indicate that Cdk1 activity is important in the link of Aurkb/CPC with Oct4 phosphorylation. Following treatment with the Cdk1 inhibitor RO3306, Aurkb was still associated with Oct4. Thus, it appears that inhibition of Cdk1 activity does not affect the interaction of Oct4 and Aurkb, once Aurkb has interacted with Oct4 (Figure 2G).
It has been reported that Cdk1 phosphorylates PP1 and inhibits PP1 activity during early mitosis (37). As PP1 isoforms antagonize Aurkb-mediated p-Oct4(S229) during the next G1 entry (19), we examined whether Cdk1 affects PP1-mediated dephosphorylation of Oct4(S229). In mESCs, Cdk1 interacted with three isoforms of PP1 and phosphorylated them at the G2/M phase (Figure 3A). Simultaneously, Cdk1 colocalizes with phosphor-PP1* as well as p-Oct4(S229). Both p-Oct4(S229) and phosphor-PP1* decreased when Cdk1 was inhibited in G2/M-arrested mESCs, supporting the suggestion that Cdk1 modulates phosphorylation of Oct4(S229) by phosphorylating PP1 and inhibiting PP1 activity (Figure 3C and D).
To understand the effect of Cdk1 on transcriptional activity of Oct4, we used RO3306, an ATP-competitive Cdk1 inhibitor, as a specific and reversible probe (38). When we treated RO3306 to mESCs that had been nocodazole-pretreated for mitotic arrest, the condensed chromosome produced by the nocodazole treatment was markedly decondensed (Figures 2E and 3E). In addition, Cdk1 inhibition during mitosis abnormally resulted in Oct4 binding to chromatin and evoked the transcriptional activity of Oct4 even in replicated and decondensed chromosome (Figure 4A and C). Moreover, we obtained similar results in Cdk1-knockdown mESCs (Figure 4B). These findings support the suggestion that Cdk1 modulates the chromatin-associated state of Oct4 during cell cycle progression.
Previous studies have shown that transcription factors dissociate from chromatin at the onset of mitosis (39,40) due to condensed mitotic chromatin physically restraining the binding of transcription factors to promoters/enhancers at target genes. But, some transcription factors, which are particularly related with lineage-specific differentiation including Runx2 and Brd4, occupy chromatin even during mitosis (referred to as gene bookmarking) and accelerate the reactivation of lineage-specific transcription (41,42).
Recent studies reported that core transcription factors, including Oct4, in pluripotent stem cells associate with chromatin during mitosis (43–46). In term that bookmarking of transcription factors relies on the specific binding on their own DNA-sequence during mitosis, these findings still remain to be debated because Oct4 and Sox2 were reported to lose the DNA-binding ability during mitosis by Aurk-mediating phosphorylation of their DNA-binding domains, which affects the maintenance of pluripotency in ESCs (19,47).
In this work, we showed that Oct4 seems to be actively dissociated from chromatin during cell cycle progression by Cdk1-mediated phosphorylation. Our previous report showed that the S229 residue belonging to the DNA binding domain of Oct4 is critical for transcription-associated DNA binding; thus, Cdk1-mediated phosphorylation of Oct4(S229) results in active displacement of Oct4 from chromatin during mitosis. Hence, Cdk1 is proposed to be a master regulator for the Oct4 phosphorylation state at mitosis and reduces Oct4 binding to chromatin, eventually leading to reduce Oct4 transcriptional activity at mitosis. At present, although we cannot exclude the possibility that a small portion of Oct4 might be bookmarked at specific target genes because Oct4 also works as a lineage-specific transcription factor for endoderm differentiation (48,49), we are able to provide evidence that Cdk1 displaces Oct4 from chromatin at mitosis by regulating the phosphorylation state of Oct4(S229) and governs Oct4 transcription activity for ESC pluripotency.
Although Oct4 is mainly responsible for ESC pluripotency, Oct4 seems to be dissociated from chromatin during mitosis. Such dissociation might result in risky situation that ESCs lose self-renewal and pluripotency during cell cycle progression. Considering that ESCs have a great potential to differentiate into various types of lineage cells upon receipt of differentiation cues, dissociation of Oct4 from chromatin during mitosis might give rise to an ‘open window of opportunity’ for ESCs to differentiate upon re-entry into the following G1 phase.
In this study, we demonstrated that Cdk1 can regulate Oct4(S229) phosphorylation via activation of Aurkb/CPC and inactivation of PP1 during mitosis and, eventually, can have an impact on transcriptional activity of Oct4 for pluripotency-related gene expression. By identifying Cdk1 as a regulator of Oct4 phosphorylation, we have elucidated a mechanism by which Cdk1 acts as a master regulator for connecting cell cycle progression with the pluripotency transcription program in ESCs, and that mechanism might provide a small window during M/G1 transition through which ESCs can differentiate.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Research Foundation of Korea grant; Korean government (MSIP) [2012R1A3A2048767 to H.-D.Y]; Education and Research Encouragement Fund of Seoul National University Hospital. Funding for open access charge: By our research grant or by my college.
Conflict of interest statement. None declared.
REFERENCES
- 1. White J., Dalton S.. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005; 1:131–138. [DOI] [PubMed] [Google Scholar]
- 2. Kapinas K., Grandy R., Ghule P., Medina R., Becker K., Pardee A., Zaidi S.K., Lian J., Stein J., van Wijnen A. et al. The abbreviated pluripotent cell cycle. J. Cell Physiol. 2013; 228:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coronado D., Godet M., Bourillot P.Y., Tapponnier Y., Bernat A., Petit M., Afanassieff M., Markossian S., Malashicheva A., Iacone R. et al. A short G1 phase is an intrinsic determinant of naive embryonic stem cell pluripotency. Stem Cell Res. 2013; 10:118–131. [DOI] [PubMed] [Google Scholar]
- 4. Mummery C.L., van Rooijen M.A., van den Brink S.E., de Laat S.W.. Cell cycle analysis during retinoic acid induced differentiation of a human embryonal carcinoma-derived cell line. Cell Differ. 1987; 20:153–160. [DOI] [PubMed] [Google Scholar]
- 5. Pauklin S., Vallier L.. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013; 155:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzales K.A., Liang H., Lim Y.S., Chan Y.S., Yeo J.C., Tan C.P., Gao B., Le B., Tan Z.Y., Low K.Y. et al. Deterministic restriction on pluripotent state dissolution by Cell-Cycle pathways. Cell. 2015; 162:564–579. [DOI] [PubMed] [Google Scholar]
- 7. Morgan D.O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997; 13:261–291. [DOI] [PubMed] [Google Scholar]
- 8. Faast R., White J., Cartwright P., Crocker L., Sarcevic B., Dalton S.. Cdk6-cyclin D3 activity in murine ES cells is resistant to inhibition by p16(INK4a). Oncogene. 2004; 23:491–502. [DOI] [PubMed] [Google Scholar]
- 9. Neganova I., Tilgner K., Buskin A., Paraskevopoulou I., Atkinson S.P., Peberdy D., Passos J.F., Lako M.. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014; 5:e1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koledova Z., Kafkova L.R., Calabkova L., Krystof V., Dolezel P., Divoky V.. Cdk2 inhibition prolongs G1 phase progression in mouse embryonic stem cells. Stem Cells Dev. 2010; 19:181–194. [DOI] [PubMed] [Google Scholar]
- 11. Ortega S., Prieto I., Odajima J., Martin A., Dubus P., Sotillo R., Barbero J.L., Malumbres M., Barbacid M.. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 2003; 35:25–31. [DOI] [PubMed] [Google Scholar]
- 12. Berthet C., Aleem E., Coppola V., Tessarollo L., Kaldis P.. Cdk2 knockout mice are viable. Curr. Biol. 2003; 13:1775–1785. [DOI] [PubMed] [Google Scholar]
- 13. Stead E., White J., Faast R., Conn S., Goldstone S., Rathjen J., Dhingra U., Rathjen P., Walker D., Dalton S.. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002; 21:8320–8333. [DOI] [PubMed] [Google Scholar]
- 14. Santamaria D., Barriere C., Cerqueira A., Hunt S., Tardy C., Newton K., Caceres J.F., Dubus P., Malumbres M., Barbacid M.. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007; 448:811–815. [DOI] [PubMed] [Google Scholar]
- 15. Zhang W.W., Zhang X.J., Liu H.X., Chen J., Ren Y.H., Huang D.G., Zou X.H., Xiao W.. Cdk1 is required for the self-renewal of mouse embryonic stem cells. J. Cell Biochem. 2011; 112:942–948. [DOI] [PubMed] [Google Scholar]
- 16. Wang J., Rao S., Chu J., Shen X., Levasseur D.N., Theunissen T.W., Orkin S.H.. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006; 444:364–368. [DOI] [PubMed] [Google Scholar]
- 17. Zhao R., Deibler R.W., Lerou P.H., Ballabeni A., Heffner G.C., Cahan P., Unternaehrer J.J., Kirschner M.W., Daley G.Q.. A nontranscriptional role for Oct4 in the regulation of mitotic entry. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:15768–15773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L., Wang J., Hou J., Wu Z., Zhuang Y., Lu M., Zhang Y., Zhou X., Li Z., Xiao W. et al. Cdk1 interplays with Oct4 to repress differentiation of embryonic stem cells into trophectoderm. FEBS Lett. 2012; 586:4100–4107. [DOI] [PubMed] [Google Scholar]
- 19. Shin J., Kim T.W., Kim H., Kim H.J., Suh M.Y., Lee S., Lee H.T., Kwak S., Lee S.E., Lee J.H. et al. Aurkb/PP1-mediated resetting of Oct4 during the cell cycle determines the identity of embryonic stem cells. eLife. 2016; 5:e10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mull A.N., Klar A., Navara C.S.. Differential localization and high expression of SURVIVIN splice variants in human embryonic stem cells but not in differentiated cells implicate a role for SURVIVIN in pluripotency. Stem Cell Res. 2014; 12:539–549. [DOI] [PubMed] [Google Scholar]
- 21. Diaz-Rodriguez E., Pandiella A.. Multisite phosphorylation of Erk5 in mitosis. J. Cell Sci. 2010; 123:3146–3156. [DOI] [PubMed] [Google Scholar]
- 22. Jang H., Kim T.W., Yoon S., Choi S.Y., Kang T.W., Kim S.Y., Kwon Y.W., Cho E.J., Youn H.D.. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell. 2012; 11:62–74. [DOI] [PubMed] [Google Scholar]
- 23. Tsukahara T., Tanno Y., Watanabe Y.. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010; 467:719–723. [DOI] [PubMed] [Google Scholar]
- 24. Bollen M., Gerlich D.W., Lesage B.. Mitotic phosphatases: from entry guards to exit guides. Trends Cell Biol. 2009; 19:531–541. [DOI] [PubMed] [Google Scholar]
- 25. Qian J., Beullens M., Lesage B., Bollen M.. Aurora B defines its own chromosomal targeting by opposing the recruitment of the phosphatase scaffold Repo-Man. Curr. Biol. 2013; 23:1136–1143. [DOI] [PubMed] [Google Scholar]
- 26. Xiao S., Xie D., Cao X., Yu P., Xing X., Chen C.C., Musselman M., Xie M., West F.D., Lewin H.A. et al. Comparative epigenomic annotation of regulatory DNA. Cell. 2012; 149:1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J., Go Y., Kang I., Han Y.M., Kim J.. Oct-4 controls cell-cycle progression of embryonic stem cells. Biochem. J. 2010; 426:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niwa H., Miyazaki J., Smith A.G.. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000; 24:372–376. [DOI] [PubMed] [Google Scholar]
- 29. Huskey N.E., Guo T., Evason K.J., Momcilovic O., Pardo D., Creasman K.J., Judson R.L., Blelloch R., Oakes S.A., Hebrok M. et al. CDK1 inhibition targets the p53-NOXA-MCL1 axis, selectively kills embryonic stem cells, and prevents teratoma formation. Stem Cell Rep. 2015; 4:374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gavet O., Pines J.. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell Biol. 2010; 189:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dohadwala M., da Cruz e Silva E.F., Hall F.L., Williams R.T., Carbonaro-Hall D.A., Nairn A.C., Greengard P., Berndt N.. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl. Acad. Sci. U.S.A. 1994; 91:6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwon Y.G., Lee S.Y., Choi Y., Greengard P., Nairn A.C.. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc. Natl. Acad. Sci. U.S.A. 1997; 94:2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu J.Q., Guo J.Y., Tang W., Yang C.S., Freel C.D., Chen C., Nairn A.C., Kornbluth S.. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell Biol. 2009; 11:644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qian J., Beullens M., Huang J., De Munter S., Lesage B., Bollen M.. Cdk1 orders mitotic events through coordination of a chromosome-associated phosphatase switch. Nat. Commun. 2015; 6:10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hein M.Y., Hubner N.C., Poser I., Cox J., Nagaraj N., Toyoda Y., Gak I.A., Weisswange I., Mansfeld J., Buchholz F. et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell. 2015; 163:712–723. [DOI] [PubMed] [Google Scholar]
- 36. Huttlin E.L., Ting L., Bruckner R.J., Gebreab F., Gygi M.P., Szpyt J., Tam S., Zarraga G., Colby G., Baltier K. et al. The BioPlex Network: a systematic exploration of the human interactome. Cell. 2015; 162:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grallert A., Boke E., Hagting A., Hodgson B., Connolly Y., Griffiths J.R., Smith D.L., Pines J., Hagan I.M.. A PP1-PP2A phosphatase relay controls mitotic progression. Nature. 2015; 517:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vassilev L.T., Tovar C., Chen S., Knezevic D., Zhao X., Sun H., Heimbrook D.C., Chen L.. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. U.S.A. 2006; 103:10660–10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dephoure N., Zhou C., Villen J., Beausoleil S.A., Bakalarski C.E., Elledge S.J., Gygi S.P.. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 2008; 105:10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delcuve G.P., He S., Davie J.R.. Mitotic partitioning of transcription factors. J. Cell. Biochem. 2008; 105:1–8. [DOI] [PubMed] [Google Scholar]
- 41. Young D.W., Hassan M.Q., Pratap J., Galindo M., Zaidi S.K., Lee S.H., Yang X., Xie R., Javed A., Underwood J.M. et al. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature. 2007; 445:442–446. [DOI] [PubMed] [Google Scholar]
- 42. Zhao R., Nakamura T., Fu Y., Lazar Z., Spector D.L.. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat. Cell Biol. 2011; 13:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Y., Pelham-Webb B., Di Giammartino D.C., Li J., Kim D., Kita K., Saiz N., Garg V., Doane A., Giannakakou P. et al. Widespread mitotic bookmarking by histone marks and transcription factors in pluripotent stem cells. Cell Rep. 2017; 19:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teves S.S., An L., Hansen A.S., Xie L., Darzacq X., Tjian R.. A dynamic mode of mitotic bookmarking by transcription factors. eLife. 2016; 5:e22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Festuccia N., Dubois A., Vandormael-Pournin S., Gallego Tejeda E., Mouren A., Bessonnard S., Mueller F., Proux C., Cohen-Tannoudji M., Navarro P.. Mitotic binding of Esrrb marks key regulatory regions of the pluripotency network. Nat. Cell Biol. 2016; 18:1139–1148. [DOI] [PubMed] [Google Scholar]
- 46. Deluz C., Friman E.T., Strebinger D., Benke A., Raccaud M., Callegari A., Leleu M., Manley S., Suter D.M.. A role for mitotic bookmarking of SOX2 in pluripotency and differentiation. Genes Dev. 2016; 30:2538–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qi D., Wang Q., Yu M., Lan R., Li S., Lu F.. Mitotic phosphorylation of SOX2 mediated by Aurora kinase A is critical for the stem-cell like cell maintenance in PA-1 cells. Cell Cycle. 2016; 15:2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Radzisheuskaya A., Chia Gle B., dos Santos R.L., Theunissen T.W., Castro L.F., Nichols J., Silva J.C.. A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nat. Cell Biol. 2013; 15:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frum T., Halbisen M.A., Wang C., Amiri H., Robson P., Ralston A.. Oct4 cell-autonomously promotes primitive endoderm development in the mouse blastocyst. Dev. Cell. 2013; 25:610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.