Glyceryl trinitrate administration causes prolonged mechanical allodynia in rodents, which correlates temporally with delayed migraine attacks in patients. Marone et al. show that the allodynia is mediated by TRPA1 activation in cell bodies of trigeminal neurons and ensuing oxidative stress. This neuronal pathway may be of relevance to migraine-like headaches.
Keywords: migraine, oxidative stress, ion channels, trigeminal headache: experimental models
Abstract
Glyceryl trinitrate is administered as a provocative test for migraine pain. Glyceryl trinitrate causes prolonged mechanical allodynia in rodents, which temporally correlates with delayed glyceryl trinitrate-evoked migraine attacks in patients. However, the underlying mechanism of the allodynia evoked by glyceryl trinitrate is unknown. The proalgesic transient receptor potential ankyrin 1 (TRPA1) channel, expressed by trigeminal nociceptors, is sensitive to oxidative stress and is targeted by nitric oxide or its by-products. Herein, we explored the role of TRPA1 in glyceryl trinitrate-evoked allodynia. Systemic administration of glyceryl trinitrate elicited in the mouse periorbital area an early and transient vasodilatation and a delayed and prolonged mechanical allodynia. The systemic, intrathecal or local administration of selective enzyme inhibitors revealed that nitric oxide, liberated from the parent drug by aldehyde dehydrogenase 2 (ALDH2), initiates but does not maintain allodynia. The central and the final phases of allodynia were respectively associated with generation of reactive oxygen and carbonyl species within the trigeminal ganglion. Allodynia was absent in TRPA1-deficient mice and was reversed by TRPA1 antagonists. Knockdown of neuronal TRPA1 by intrathecally administered antisense oligonucleotide and selective deletion of TRPA1 from sensory neurons in Advillin-Cre; Trpa1fl/fl mice revealed that nitric oxide-dependent oxidative and carbonylic stress generation is due to TRPA1 stimulation, and resultant NADPH oxidase 1 (NOX1) and NOX2 activation in the soma of trigeminal ganglion neurons. Early periorbital vasodilatation evoked by glyceryl trinitrate was attenuated by ALDH2 inhibition but was unaffected by TRPA1 blockade. Antagonists of the calcitonin gene-related peptide receptor did not affect the vasodilatation but partially inhibited allodynia. Thus, although both periorbital allodynia and vasodilatation evoked by glyceryl trinitrate are initiated by nitric oxide, they are temporally and mechanistically distinct. While vasodilatation is due to a direct nitric oxide action in the vascular smooth muscle, allodynia is a neuronal phenomenon mediated by TRPA1 activation and ensuing oxidative stress. The autocrine pathway, sustained by TRPA1 and NOX1/2 within neuronal cell bodies of trigeminal ganglia, may sensitize meningeal nociceptors and second order trigeminal neurons to elicit periorbital allodynia, and could be of relevance for migraine-like headaches evoked by glyceryl trinitrate in humans.
Introduction
Occupational exposure to, or treatment with, organic nitrates has long been known to provoke headaches (Trainor and Jones, 1966; Thadani and Rodgers, 2006). These observations have led to the clinical use of glyceryl trinitrate (GTN) as a reliable provocation test for migraine attacks (Sicuteri et al., 1987; Iversen et al., 1989; Thomsen et al., 1994; Olesen, 2008). In most subjects, including healthy controls, GTN administration causes a mild headache that develops rapidly and is short-lived. However, after a remarkable time lag (hours) from GTN exposure, migraineurs develop severe headaches that fulfil the criteria of a typical migraine attack (Sicuteri et al., 1987; Iversen et al., 1989; Thomsen et al., 1994; Olesen, 2008). This ability of GTN to provoke migraine is temporally dissociated from the immediate and short-lived (<10 min) release of nitric oxide (NO) (Persson et al., 1994) and the consequent cGMP-dependent vascular responses (Guo et al., 2008). Thus, while vasodilatation by GTN/NO might account for the early dull headache experienced by most subjects (Iversen and Olesen, 1996), it cannot explain the delayed headache symptoms observed in migraineurs (Olesen, 2008).
Several mechanisms have been proposed to explain GTN-evoked headaches, including degranulation of meningeal mast cells (Reuter et al., 2001; Ferrari et al., 2016), phosphorylation of extracellular signal-regulated kinase (ERK) in meningeal arteries (Zhang et al., 2013), delayed meningeal inflammation sustained by induction of NO synthase and prolonged NO generation, and the release of calcitonin gene-related peptide (CGRP) (Strecker et al., 2002; Ramachandran et al., 2014), a primary migraine neuropeptide (Ho et al., 2010; Edvinsson, 2015). GTN administration to rodents and humans produces a delayed and prolonged (hours) hyperalgesia that temporally correlates with GTN-induced migraine-like attacks in humans (Thomsen et al., 1996; Tassorelli et al., 2003; Bates et al., 2010; Ferrari et al., 2016). However, the molecular processes responsible for this delayed hyperalgesia in rodents and humans are unknown.
Transient receptor potential (TRP) ion channels expressed by nociceptors play a major role in pain (Nilius and Szallasi, 2014). NO can target the TRP ankyrin 1 (TRPA1) channel (Miyamoto et al., 2009), which is abundantly expressed by a specific nociceptor subpopulation (Nassini et al., 2014). TRPA1-positive sensory neurons express additional pain-inducing TRPs, including the vanilloid 1 (TRPV1) and vanilloid 4 (TRPV4) channels, as well as the neuropeptides CGRP, substance P (SP), and neurokinin A (NKA), which mediate neurogenic inflammation (Nassini et al., 2014).
TRPA1 is uniquely sensitive to an unprecedented series of oxidants and electrophiles, including reactive oxygen, nitrogen and carbonylic species, thereby sensing oxidative/nitrative/carbonylic stress (Trevisani et al., 2007; Andersson et al., 2008; Materazzi et al., 2008; Taylor-Clark et al., 2009). TRPA1 is considered a major pain transducer since TRPA1 inhibition attenuates different types of neuropathic (Trevisan et al., 2016) and inflammatory (McNamara et al., 2007; Trevisan et al., 2014) pain. TRPA1 may also contribute to migraine, since triggers of headache attacks can target the channel in peptidergic nociceptors (Kunkler et al., 2011; Shatillo et al., 2013; Denner et al., 2017; Borkum, 2018). Furthermore, drugs (Andersson et al., 2011; Nassini et al., 2015) or herbal preparations (Benemei et al., 2017) used for migraine treatment inhibit or desensitize TRPA1.
As previously reported in rats (Di et al., 2016; Kopruszinski et al., 2017) and mice (Farkas et al., 2016) with systemic administration of NO donors, herein we show that GTN produces an early (0–10 min) and transient vasodilation and a delayed and prolonged (0.5–8 h) mechanical allodynia in the cutaneous periorbital area in mice. We found that allodynia, but not vasodilation, is prevented by genetic deletion or pharmacological blockade of TRPA1. NO, generated from GTN by aldehyde dehydrogenase 2 (ALDH2), is required to initiate the entire process, but is not sufficient for its maintenance. TRPA1- and NADPH oxidase (NOX)-dependent reactive oxygen species generation from cell bodies of trigeminal ganglion neurons maintains the first phase of sustained allodynia. Reactive oxygen species-dependent peroxidation of membrane phospholipids generates reactive carbonylic species, including 4-hydroxynonenal (4-HNE), which activates TRPA1 to promote the continuation and final phase of allodynia. CGRP released from cutaneous nerve fibres contributes to the allodynia. The autocrine function of nociceptor TRPA1 that, by generating oxidative stress, sustains GTN-induced allodynia, highlights the unforeseen role of the channel in producing, and possibly sensing, oxidative stress in cell bodies of primary sensory neurons, thereby contributing to periorbital allodynia.
Materials and methods
Animals
In vivo experiments were in accordance with the European Union Directive 2010/63/EU guidelines, the Italian legislation (DLgs 26/2014), and the University of Florence research permit #194/2015-PR. The following mouse strains were used: C57BL/6 (male, 20–25 g, 5–6 weeks; Envigo); littermate wild-type (Trpa1+/+) and TRPA1-deficient (Trpa−/−) mice (25–30 g, 5–8 weeks) (Kwan et al., 2006); wild-type (Trpv4+/+) and TRPV4-deficient (Trpv4−/−) mice (25–30 g, 5–8 weeks) (Liedtke and Friedman, 2003); and TRPV1-deficient mice (Trpv1−/−; B6.129X1-Trpv1tm1Jul/J) backcrossed with C57BL/6 mice (Trpv1+/+) for at least 10 generations (Jackson Laboratories, 25–30 g, 5–8 weeks). To selectively delete the Trpa1 gene in primary sensory neurons, 129S-Trpa1tm2Kykw/J mice (floxed TRPA1, Trpa1fl/fl, Stock No: 008649; Jackson Laboratories), which possess loxP sites on either side of the S5/S6 transmembrane domains of the Trpa1 gene, were crossed with hemizygous Advillin-Cre male mice (Zurborg et al., 2011; Guan et al., 2016). The progeny was genotyped by standard PCR for Trpa1 (PCR Protocol 008650, Jackson Laboratories; www.jax.org) and Advillin-Cre (Guan et al., 2016). Mice negative for Advillin-Cre (Adv-Cre−; Trpa1fl/fl) were used as control. Successful Advillin-Cre driven deletion of TRPA1 mRNA was confirmed by RT-qPCR (Zappia et al., 2017). Mice were housed in a temperature- and humidity-controlled vivarium (12-h dark/light cycle, free access to food and water, 10 animals per cage). Mice were acclimatized in a quiet, temperature-controlled room (20–22°C) for 1 h before behavioural studies between 9 am and 5 pm. A randomization procedure (http://www.randomizer.org/) was used to allocate animals to treatments. Investigators were blinded to genotype and drug treatments. For logistical reasons up to 18 mice could be studied on any one day. In all cases, control and experimental groups were studied on the same day, and experiments were replicated on different days to generate results from the required number of mice. Animals were anaesthetized with intraperitoneal (i.p.) ketamine (90 mg/kg) and xylazine (3 mg/kg) and euthanized with inhaled CO2 plus 10–50% O2.
Based on our experience in similar experimental settings and on data published by others, we anticipated a standardized difference of 1.8 standard deviations (SDs) between wild-type and Trpa1−/−animals 1 h after exposure to GTN and control treatments. Using G*Power (v3.1) (Faul et al., 2009), we determined that six animals per group were the minimum necessary to detect the size effect in a post hoc t-test with type 1 and 2 error rates of 5 and 20%, respectively. Initial data acquisition then showed that the effect size in some comparisons remained meaningful but smaller than anticipated, ∼1.6 SDs. Sample sizes of subsequent experiments were increased in order to maintain power at 80%.
Reagents
Glyceryl trinitrate (GTN 50 mg/50 ml, Bioindustria L.I.M. S.p.A.) or its vehicle (5% glucose and 1.5% propylene glycol in sterile water) were used. HC-030031 [2-(1,3-dimethyl-2, 6-dioxo-1,2,3,6-tetrahydro-7 H-purin-7-yl)-N-(4-isopropylphenyl) acetamide] was synthesized as previously described (Materazzi et al., 2008). If not otherwise indicated, reagents were obtained from Sigma-Aldrich. The vehicle, except for GTN or where expressly indicated, was 4% dimethyl sulfoxide (DMSO) plus 4% Tween 80 in isotonic saline, NaCl 0.9%.
Behavioural tests
Mechanical allodynia
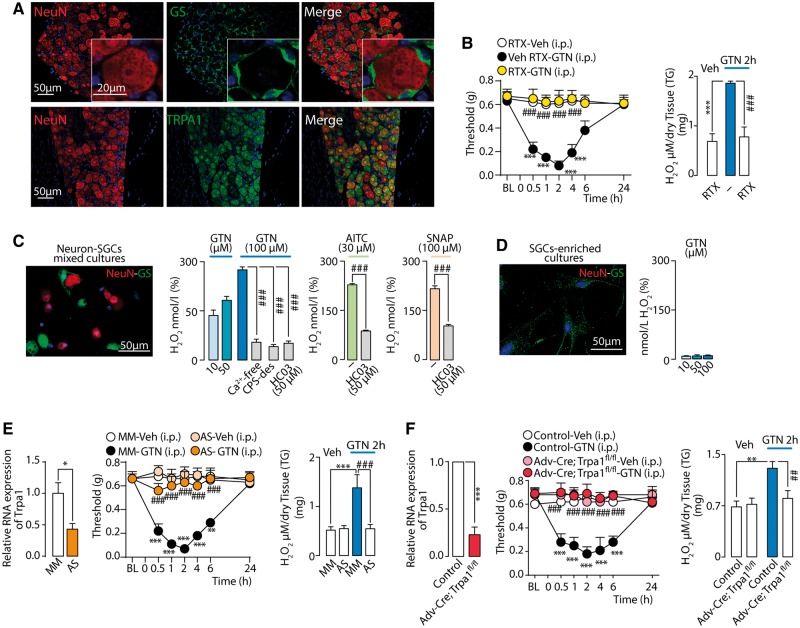
Mechanical allodynia was evaluated by applying von Frey filaments to the periorbital region over the rostral portion of the eye (periorbital mechanical allodynia, PMA) (Elliott et al., 2012) or to the posterior hind paw of mice, before (basal threshold) and 24 h after GTN (1, 5 and 10 mg/kg) or vehicle. Some C57BL/6, Trpa1+/+ and Trpa1−/− mice were injected [10 µl/site, subcutaneously (s.c.)] with allyl isothiocyanate (AITC, 10 nmol in 2.5% DMSO in 0.9% NaCl), GTN (10 µg/site), S-nitroso-N-acetylpenicillamine (SNAP, 40 µg/site in 2.5% DMSO in 0.9% NaCl), CGRP (0.5–5 µg/site in 0.9% NaCl) or their vehicles, and PMA was assessed before and 6 h after treatment. Additional C57BL/6 mice received resiniferatoxin (50 μg/kg, s.c.) or its vehicle (10% ethanol and 10% Tween 80 in 0.9% NaCl) (Pecze et al., 2009). Seven days after resiniferatoxin, when responses (the eye wiping test, see below) to capsaicin were abolished, GTN (10 mg/kg, i.p.)-evoked PMA and H2O2 levels were evaluated.
TRPA1 antisense (5′-TATCGCTCCACATTGCTAC-3′) or mismatch control (5′-ATTCGCCTCACATTGTCAC-3′) oligonucleotide (TRPA1 antisense or mismatch oligonucleotide) were administered to C57BL/6 mice by intrathecal injection (5 nmol/5 μl) (Bonet et al., 2013) for four consecutive days. On Day 5, the efficiency of TRPA1 silencing was tested by eye wiping responses to AITC and the Trpa1 mRNA content in trigeminal ganglion neurons, and PMA evoked by GTN (10 mg/kg, i.p.) and H2O2 levels were evaluated. Adv-Cre+; Trpa1fl/fl and Adv-Cre−; Trpa1fl/fl received GTN (10 mg/kg, i.p.) or its vehicle and PMA and H2O2 levels in trigeminal ganglion neurons (collected 2 h after GTN/vehicle) were evaluated.
The mechanical threshold was calculated by the up-and-down paradigm (Dixon, 1980). All the drugs, at the maximum used doses, did not evoke any direct nociceptive/allodynic responses or locomotor impairment. Doses and routes of administration of drugs and their targets are reported in Supplementary Table 1. Times of administration are reported in boxes placed above graphs.
Eye wiping test
The number of eye wiping movements, following the instillation of eye drops of capsaicin (1 nmol/5 µl), AITC (10 nmol/5 µl) or vehicle (2% and 4% DMSO, respectively) to the conjunctiva, was recorded for a 10-min time period (Nassini et al., 2012).
Cell culture and isolation of primary sensory neurons
HEK293 cells (American Type Culture Collection; ATCC® CRL-1573™), cultured according to the manufacturer’s instructions, were transiently transfected with the cDNAs (1 µg) codifying for wild-type (hTRPA1-HEK293) or mutant 3C/K-Q human TRPA1 (C619S, C639S, C663S, K708Q; 3C/K-Q hTRPA1-HEK293) (Hinman et al., 2006) using the jetPRIME® transfection reagent (Polyplus-transfection® SA) according to the manufacturer’s protocol. Primary trigeminal ganglion neurons were isolated from C57BL/6 or Trpa1+/+ and Trpa1−/− mice, and cultured as previously described (Nassini et al., 2012). To obtain trigeminal neuronal and satellite glial cell (SGC) mixed cultures or SGC-enriched cultures, the protocol reported previously was used (Chung et al., 2015).
Calcium imaging assay
Intracellular calcium mobilization, [Ca2+]i, was measured in transfected HEK293 cells and in trigeminal ganglion neurons, as reported previously (Nassini et al., 2012). Trigeminal ganglion neurons were challenged with GTN (10–300 μM), SNAP (30 μM), AITC (10 μM) or their respective vehicle. Capsaicin (0.1 μM) was used to identify capsaicin-sensitive neurons. Some experiments were performed in the presence of HC-030031 (50 µM), capsazepine (10 µM), HC-067047 (30 µM), carboxy-PTIO (cPTIO, 100 µM) and disulfiram (1 µM). Buffer solution containing 0.5% DMSO was used as vehicle for SNAP and all inhibitors/scavenger. Wild-type or mutant hTRPA1-HEK293 cells were challenged with GTN (100 μM), menthol (100 μM) or their vehicle.
H2O2 assay
H2O2 was determined by using the Amplex Red® assay (Invitrogen), in C57BL/6 mice, in trigeminal ganglion neurons before or after (0.5–6 h) GTN (10 mg/kg, i.p.), and 2 h after GTN/vehicle in mice pretreated (0.5 h before GTN) with cPTIO (0.6 mg/kg, i.p.) and disulfiram (100 mg/kg, i.p.) or treated (1 h after GTN) with HC-030031 (100 mg/kg, i.p.) and alpha lipoic acid (100 mg/kg i.p.), or desensitized with resiniferatoxin or silenced with TRPA1 antisense or mismatch oligonucleotide; in dorsal horn of brain stem before and after (1 and 2 h, respectively) GTN. In Trpa1+/+ and Trpa1−/−, and in Adv-Cre; Trpa1fl/fl and Adv-Cre−; Trpa1fl/fl mice H2O2 was 2 h after GTN.
Increase in H2O2 release was quantified in trigeminal neuron-SGCs mixed and SGC-enriched primary culture after challenge with GTN (10, 50 and 100 µM), AITC (30 µM), SNAP (100 µM) or their vehicle (0.3% DMSO for AITC and SNAP) in the presence of HC-030031 (50 µM) or its vehicle (0.5% DMSO in Krebs-Ringer phosphate buffer), or in a Ca2+-free Krebs-Ringer phosphate (KRP) buffer containing EDTA (1 mM) or after a pre-exposure to a high concentration of capsaicin (10 µM, 20 min) (Holzer, 1991). The detailed method is reported in the Supplementary material.
Immunofluorescence
Anaesthetized C57BL/6 and Trpa1+/+ and Trpa1−/− mice treated with GTN (10 mg/kg, i.p.) or its vehicle were transcardially perfused with PBS, followed by 4% paraformaldehyde. Trigeminal ganglion neurons and brainstem were removed, postfixed for 24 h, and paraffin embedded or cryoprotected (4°C, overnight) in 30% sucrose. Cryosections (10 µm) of brainstem and formalin fixed paraffin-embedded sections (5 µm) of trigeminal ganglion neurons were incubated with the following primary antibodies: 4-HNE (ab48506, 1:40, HNEJ-2, Abcam), TRPA1 (ab58844, 1:400, Abcam), NOX1 (SAB2501686, 1:250), NOX2 (ab80897, 1:200, Abcam), NOX4 (ab109225, 1:200, Abcam), glutamine synthetase (G2781, 1:400) (1 h, room temperature) diluted in phosphate-buffered saline (PBS) and 2.5% normal goat serum (NGS). Sections were then incubated with fluorescent secondary antibodies: polyclonal Alexa Fluor® 488, and polyclonal Alexa Fluor® 594 (1:600, Invitrogen) (2 h, room temperature) and coverslipped. The analysis of negative controls (non-immune serum) was simultaneously performed to exclude the presence of non-specific immunofluorescent staining, cross-immunostaining, or fluorescence bleed-through. Tissues were visualized, and digital images were captured using an Olympus BX51 (Olympus srl). 4-HNE staining in trigeminal ganglion was evaluated as the fluorescence intensity measured by an image processing software (ImageJ 1.32 J, National Institutes of Health). Data are expressed as mean fluorescence intensity (% of basal).
4-HNE staining was determined in trigeminal ganglion neurons collected from C57BL/6 mice before (basal level) or after (0.5, 1, 2, 3, 4, 6 h) GTN (10 mg/kg, i.p.) and 4 h after GTN/vehicle administration in C57BL/6 mice pretreated with cPTIO (0.6 mg/kg, i.p. given 0.5 h before GTN) or N-acetyl-l-cysteine (NAC, 250 mg/kg, i.p., given 0.5 h before GTN) or treated with HC-030031 or alpha lipoic acid (both 100 mg/kg i.p., given 3 h after GTN) and in Trpa1+/+ and Trpa1−/− mice, and in dorsal horn of brain stem collected from C57BL/6 mice before and after (1 and 2 h) GTN.
Mouse trigeminal neuron-SGC mixed and SGC-enriched cultures were cultured for 2–3 days, fixed in ice-cold methanol/acetone (5 min, 20°C), washed with PBS and blocked with NGS (10%) (1 h, room temperature). The cells were then incubated with the primary antibodies (NeuN, 1:600, and glutamine synthetase, 1:300) (1 h, room temperature), with fluorescent secondary antibodies (1:600, polyclonal Alexa Fluor® 488, and polyclonal Alexa Fluor® 594, Invitrogen) (2 h, room temperature), mounted and digital images were captured using an Olympus BX51.
Proximity ligation assay
Co-localization of TRPA1 and NOX2 in mouse trigeminal ganglion was obtained using an in situ PLA detection kit (Duolink, Olink Biosciences Inc.) as previously described (Sullivan et al., 2015). In trigeminal ganglion sections (5 µm) fluorescence images were obtained using Olympus BX51 and a 100× oil immersion objective. Negative control was performed by omitting primary antibodies or proximity ligation assay (PLA) probes.
Real-time PCR
RNA was extracted from trigeminal ganglion of C57BL/6 mice after TRPA1 antisense or mismatch oligonucleotide (i.th.) and of Adv-Cre; Trpa1fl/fl and Adv-Cre−; Trpa1fl/fl. The standard TRIzol® extraction method was used. Detailed methods are reported in the Supplementary material.
Blood flow experiments
Cutaneous blood flow was assessed using a laser Doppler flowmeter (Perimed Instruments) in anaesthetized in C57BL/6, Trpa1+/+ and Trpa1−/− mice. Cutaneous blood flow was monitored by a probe (cutaneous type) fixed to the shaved periorbital area, before and after the systemic administration of GTN (10 mg/kg, i.p.) or its vehicle. Before (0.5 h) GTN injection, mice were treated with intraperitoneal HC-030031, disulfiram (both, 100 mg/kg) and BIBN4096BS (1 mg/kg) or their vehicles and cutaneous blood flow was monitored for at least 0.5 h. Baseline blood flow was calculated by the mean flow value measured during a 5-min period before the stimulus. The increase in cutaneous blood flow was calculated as the percentage change over the baseline.
Statistical analysis
Statistical analysis was performed by the two-tailed Student’s t-test for comparisons between two groups. A one-way ANOVA followed by the post hoc Bonferroni’s test was used for comparisons of multiple groups. For behavioural experiments with repeated measures, the two-way mixed model ANOVA followed by the post hoc Bonferroni’s test was used. P < 0.05 was considered statistically significant (GraphPad Prism version 5.00). The statistical tests and the exact F- and P-values for each experiment are reported in Supplementary Table 2.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
GTN evokes NO-mediated TRPA1-independent vasodilatation and TRPA1-dependent allodynia
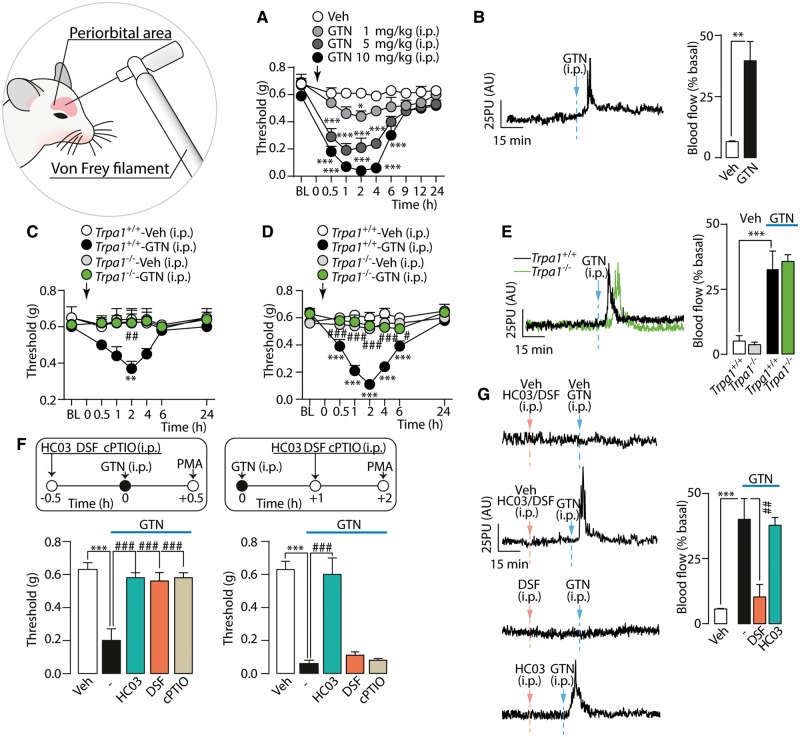
Administration of GTN (1–10 mg/kg, i.p.) to C57BL/6 mice induced a dose-dependent and prolonged PMA (Fig. 1A). GTN (10 mg/kg, i.p.) also produced an early and transient (0–10 min) cutaneous increase in blood flow in the periorbital skin (Fig. 1B). GTN (1 and 10 mg/kg, Fig. 1C and D, respectively) evoked PMA in Trpa1+/+ mice, whereas Trpa1−/− mice were fully protected. However, the increase in cutaneous blood flow evoked by GTN (10 mg/kg) in Trpa1+/+ mice was maintained in Trpa1−/− mice (Fig. 1E). Genetic deletion of TRPV1 or TRPV4 channels did not affect GTN-evoked PMA (Supplementary Fig. 1A and B). Systemic GTN (10 mg/kg) also induced sustained mechanical allodynia in the hind paw of C57BL/6 and Trpa1+/+, but not Trpa1−/− mice (Supplementary Fig. 1C and D). This response, which indicates a general proalgesic action of GTN, was not further investigated.
Figure 1.
GTN induces PMA via a TRPA1-dependent mechanism. (A) Dose- and time-dependent PMA evoked by GTN in C57BL/6 mice. (B) Representative traces and pooled data of the increases in periorbital skin blood flow evoked by GTN (10 mg/kg). PMA evoked by (C) low (1 mg/kg, i.p.) and (D) high (10 mg/kg, i.p.) dose of GTN, in Trpa1+/+ and Trpa1−/− mice. (E) Representative traces and pooled data of the increases in periorbital skin blood flow evoked by GTN (10 mg/kg) in Trpa1+/+ and Trpa1−/− mice. (F) Pretreatment with systemic (i.p.) HC-030031 (HC03; 100 mg/kg), disulfiram (DSF, 100 mg/kg) or cPTIO (0.6 mg/kg) abates PMA measured 0.5 h after GTN (10 mg/kg). HC03 but not DSF and cPTIO (given after GTN) reduces PMA measured 2 h after GTN. (G) Representative traces and pooled data of the increases in periorbital skin blood flow evoked by GTN (10 mg/kg). DSF but not HC03, both given before GTN abolishes the increase in blood flow induced by GTN. BL = baseline mechanical threshold; Veh = the vehicle of GTN. Dash (‐) indicates combined vehicles of treatments. Arrows indicate times of drug administration. AU = arbitrary units; PU = perfusion units. Error bars indicate mean ± SEM, 6–8 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle, Trpa1+/+-vehicle and #P < 0.05, ##P < 0.01, ###P < 0.001 versus Trpa1+/+-GTN or GTN; one-way or two-way ANOVA with Bonferroni post hoc correction, and Student’s t-test.
The TRPA1 antagonist, HC-030031, given (i.p.) 0.5 h before and 1, 3, 4 and 5 h after GTN, transiently and completely reversed PMA at all time points (Fig. 1F and Supplementary Fig. 1E). HC-030031 (1 h after GTN) reversed PMA induced by the lowest dose of GTN (1 mg/kg) (Supplementary Fig. 1F). Another channel antagonist, A967079, given 0.5 h before and 1 h after GTN (10 mg/kg) also reversed allodynia (Supplementary Fig. 1G). Antagonists of TRPV1 (capsazepine) and TRPV4 (HC-067047), administered before GTN did not affect GTN-induced PMA (Supplementary Fig. 1H and I).
Mitochondrial ALDH2 is known to generate NO from GTN (Beretta et al., 2008). To determine the contribution of NO to GTN-evoked PMA, mice were treated with the ALDH2 inhibitor, disulfiram, or the specific NO scavenger, cPTIO. Both disulfiram and cPTIO, when administered (i.p.) 0.5 h before, but not 1 h after, GTN, attenuated GTN-evoked PMA (Fig. 1F). The ability of disulfiram and cPTIO to prevent PMA if given before GTN indicates that NO is needed to initiate GTN-evoked PMA. However, failure of disulfiram and cPTIO to reverse established PMA suggests that additional mechanisms are required to sustain PMA. Pretreatment with disulfiram, but not with HC-030031 (both i.p., 0.5 h before GTN), attenuated the increase in periorbital blood flow evoked by GTN (10 mg/kg) (Fig. 1G).
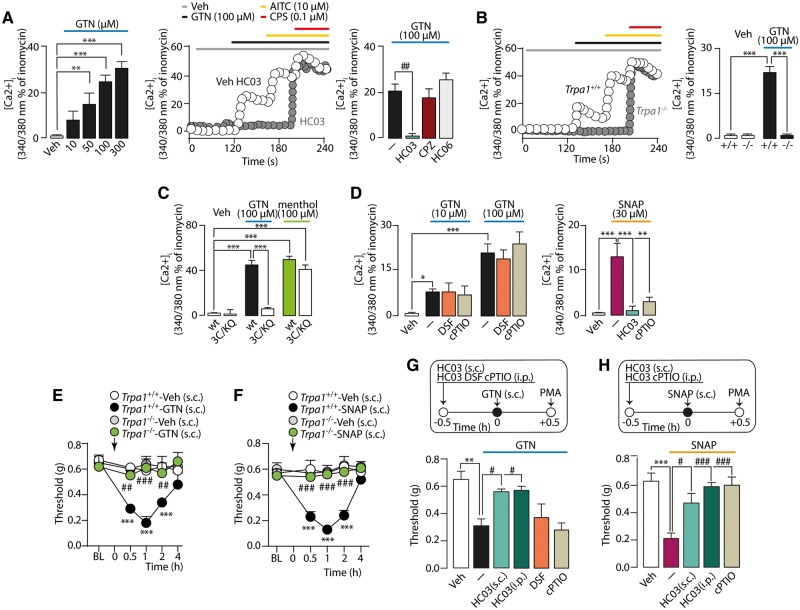
NO, but not GTN, directly targets TRPA1
To determine whether TRPA1 is directly gated by GTN and/or NO, responses to different NO donors were investigated in vitro. GTN caused a concentration-dependent increase in [Ca2+]i in trigeminal ganglion neurons from C57BL/6 and Trpa1+/+, but not from Trpa1−/− mice (Fig. 2A and B). The TRPA1 antagonist (HC-030031), but not the TRPV1 or TRPV4 antagonists (capsazepine or HC-067047, respectively), abolished responses (Fig. 2A). Key intracellular cysteine and lysine residues of TRPA1 interact with oxidants and electrophilic agents (Hinman et al., 2006). Whereas GTN increased [Ca2+]i in hTRPA1-HEK293, HEK293 cells expressing a mutant 3C/K-Q/hTRPA1 were unresponsive (Fig. 2C). The NO scavenger (cPTIO) or ALDH2 inhibitor (disulfiram) did not affect GTN signals in neurons from C57BL/6 mice (Fig. 2D). In contrast, the HC-030031-dependent Ca2+-response to a different NO donor, SNAP, was inhibited in the presence of cPTIO (Fig. 2D). Thus, GTN activates TRPA1 by targeting specific cysteine and lysine residues. However, conversely to SNAP, the in vitro action of GTN is not mediated through NO release.
Figure 2.
GTN-evoked PMA is mediated by NO. (A) Ca2+-response to GTN in mouse trigeminal ganglion neurons, which also respond to AITC or capsaicin (CPS), is attenuated by HC-030031 (HC03), but not by HC-067047 (HC06, TRPV4 antagonist) or capsazepine (CPZ, TRPV1 antagonist), and (B) abated by TRPA1 deletion. (C) Ca2+-response to GTN or menthol in HEK293-cells expressing wild-type (wt) or mutant (3C/K-Q) human TRPA1. (D) GTN-evoked Ca2+-response in trigeminal ganglion neurons is unaffected by disulfiram (DSF) or cPTIO while SNAP-evoked Ca2+-response is abated by cPTIO and HC03. Veh = the vehicle of GTN. Dash (‐) indicates combined vehicles of treatments. Error bars indicate mean ± SEM of n > 15 neurons or 50 cells (A–D). *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA with Bonferroni post hoc correction. (E and G) Local (10 µl s.c.) GTN (10 µg) evokes a time-dependent PMA that is abated in Trpa1−/− mice and by the pretreatment with local (s.c.) and systemic (i.p.) HC03 (100 µg and 100 mg/kg, respectively), but not with systemic DSF (100 mg/kg, i.p.) and cPTIO (0.6 mg/kg, i.p.). (F and H) Local (10 µl s.c.) SNAP (40 µg) induces a time-dependent PMA that is abated in Trpa1−/− mice and prevented by pretreatment with local (s.c.) and systemic (i.p.) HC03 (100 µg and 100 mg/kg, respectively) and systemic cPTIO (0.6 mg/kg, i.p.). BL = baseline mechanical threshold; Veh = the vehicle of GTN. Dash (‐) indicates combined vehicles of treatments. Arrows indicate time of drug administration. Error bars indicate mean ± SEM, 6–8 mice per group. **P < 0.01, ***P < 0.001 versus Trpa1+/+-Veh, vehicle and #P < 0.05, ##P < 0.01, ###P < 0.001 versus Trpa1+/+-GTN, GTN, Trpa1+/+-SNAP or SNA, one-way or two-way ANOVA with Bonferroni post hoc correction.
Results obtained in vivo by local administration of NO donors recapitulated the in vitro findings. Injection (s.c.) in the periorbital area of the TRPA1 agonist, AITC, evoked PMA that was reversed by local (s.c.) pretreatment with HC-030031 (Supplementary Fig. 1J). Local (s.c.) NO donors, GTN or SNAP, induced PMA in Trpa1+/+, but not Trpa1−/− mice (Fig. 2E and F). Both local (s.c.) or systemic (i.p.) TRPA1 antagonism by HC-030031 reversed PMA evoked by local GTN or SNAP (Fig. 2G and H). Systemic (i.p.) disulfiram or cPTIO did not affect PMA evoked by local (s.c.) GTN (Fig. 2G). However, cPTIO (i.p.) attenuated PMA evoked by local (s.c.) SNAP (Fig. 2H). Thus, GTN evokes allodynia by mechanisms that depend from the route of administration. Local GTN directly regulates TRPA1 gating, whereas systemic GTN indirectly regulates TRPA1 by a process that involves ALDH2-mediated liberation of NO.
Oxidative stress and TRPA1 sustain GTN-evoked mechanical allodynia
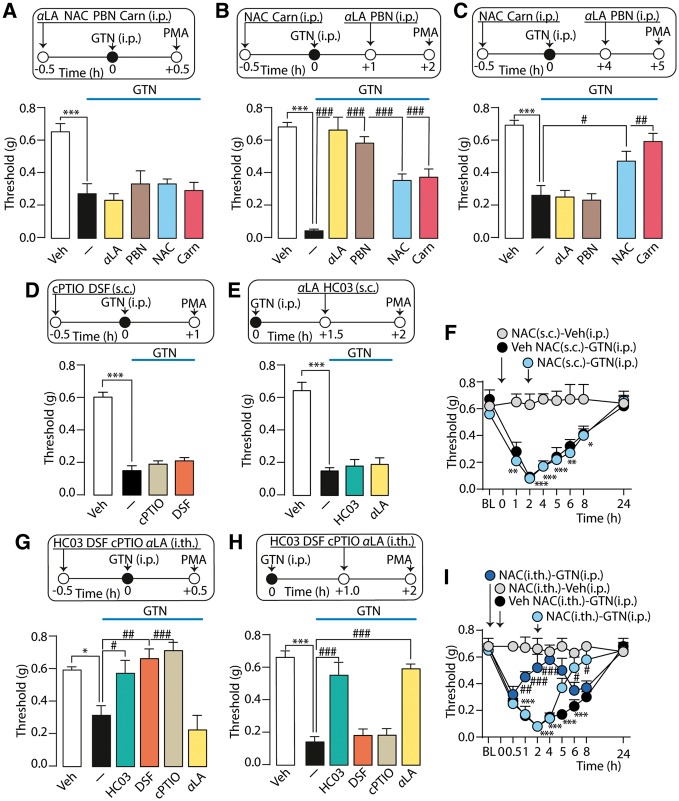
The NO scavenger (cPTIO) or ALDH2 inhibitor (disulfiram) prevented GTN-evoked allodynia when administered before GTN, but were ineffective when given after. Thus, NO is necessary to initiate the hypersensitivity condition, but is not sufficient for its maintenance. Since GTN stimulates oxidative stress (Wenzl et al., 2009), we hypothesized that NO, liberated from GTN, initiates the process that subsequently sustains TRPA1-dependent PMA via reactive oxygen species generation. Two different reactive oxygen species scavengers, alpha lipoic acid and N-tert-butyl-α-phenylnitrone (PBN), reversed PMA only when given (i.p.) 1 h after, but not 0.5 h before GTN (Fig. 3A and B).
Figure 3.
Prolonged PMA induced by GTN is mediated by oxidative stress. (A) PMA evoked by GTN (10 mg/kg) in C57BL/6 mice is not affected by the alpha lipoic acid (αLA, 100 mg/kg), NAC (250 mg/kg), PBN (100 mg/kg) or l-carnosine (Carn, 200 mg/kg) (all pre-GTN). (B and C) Alpha lipoic acid and PBN (all post-GTN) reduce PMA measured at 2 h not at 5 h. Pretreatment with NAC and Carn reach the maximum effect in the reduction of PMA, 5 h after GTN. (D) PMA evoked by systemic (i.p.) GTN (10 mg/kg) is unaffected by local (10 µl, s.c.) cPTIO (60 µg) or disulfiram (DSF; 10 µg) (all pre-GTN), and by (E) HC-030031 (HC03; 50 µg) and alpha lipoic acid (αLA, 5 µg) (all post-GTN). (F) Local (10 µl, s.c.) administration of NAC (20 µg, post-GTN) does not affect GTN-evoked PMA. (G) PMA evoked by systemic (i.p.) GTN (10 mg/kg) is reversed by intrathecal (5 µl, i.th.) HC03 (10 µg), DSF (5 µg) and cPTIO (30 µg) but not with alpha lipoic acid (10 µg) (all pre-GTN). (H) Intrathecal (i.th.) HC03 (10 µg) and alpha lipoic acid (10 µg), but not DSF (5 µg) and cPTIO (30 µg), (all post-GTN) reduce PMA measured 2 h after GTN. (I) Intrathecal (i.th.) NAC (50 µg) (pre- or post-GTN) reduces GTN-evoked PMA. BL = baseline mechanical threshold; Veh = the vehicle of GTN. Dash (‐) indicates combined vehicles of treatments. Arrows indicate time of drug administration. Error bars indicate mean ± SEM, 6–9 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle, NAC-Veh. #P < 0.05, ##P < 0.01, ###P < 0.001 versus GTN, vehicle NAC-GTN; one-way or two-way ANOVA with Bonferroni post hoc correction.
GTN-evoked PMA persisted for ∼8 h, but alpha lipoic acid or PBN were unable to reverse PMA 5 h post-GTN (Fig. 3C), indicating that mediators other than reactive oxygen species are required to sustain hypersensitivity beyond 5 h. 4-HNE is a major electrophilic aldehyde that is generated by free radical attack of polyunsaturated fatty acids (Dalle-Donne et al., 2006), and is a TRPA1 agonist (Trevisani et al., 2007). In contrast to reactive oxygen species, which are short-lived, the biological activity of 4-HNE may last for hours (Brame et al., 1999). NAC and l-carnosine efficiently scavenge α,β-unsaturated aldehydes, including 4-HNE. NAC or l-carnosine (i.p.), administered before GTN, did not attenuate the first phase of GTN-evoked PMA (2 h), but strongly inhibited later phases (5 h) (Fig. 3B and C), indicating that carbonylic derivatives more stable than reactive oxygen species sustain the final phase of PMA.
GTN does not produce periorbital allodynia by a local mechanism
Local injections of the TRPA1 agonist, AITC, and the NO donors, GTN or SNAP, caused TRPA1-dependent PMA, suggesting the involvement of TRPA1 on cutaneous nerve terminals. However, PMA evoked by systemic GTN was unaffected by local treatment with disulfiram, cPTIO, HC-030031, alpha lipoic acid and NAC (Fig. 3D–F), excluding that systemic GTN activates TRPA1 on cutaneous afferent nerve fibres to induce PMA. Centrally administered TRPA1 antagonists, HC-030031 or A967079 (pre- or post-GTN, i.th.), attenuated PMA-evoked by systemic GTN (Fig. 3G and H and Supplementary Fig. 1K). Disulfiram or cPTIO (pre-, but not post-GTN), alpha lipoic acid (post-, but not pre-GTN) and NAC (pre- or post-GTN) (all i.th.) (Fig. 3G–I) also attenuated PMA.
GTN/NO targets TRPA1 in the soma of trigeminal ganglion neurons to generate oxidative stress
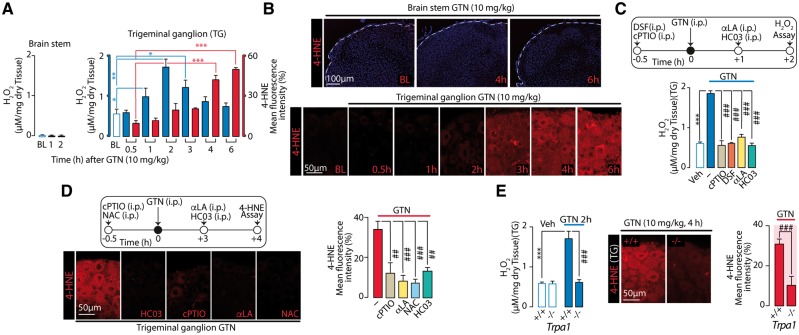
Failure of subcutaneous and ability of intrathecal drugs to attenuate GTN-evoked PMA suggested the involvement of central anatomical areas, including the terminals in the dorsal horn of the brainstem and nociceptor cell bodies in the trigeminal ganglion. To determine whether systemic GTN induces oxidative stress in these locations, we measured two markers of oxidative and carbonylic stress, H2O2 and 4-HNE, respectively. GTN, which failed to increase H2O2 and 4-HNE in brainstem, caused a rapid and transient increase in H2O2 (1–3 h) and a gradual and sustained increase in 4-HNE (4–6 h) in the trigeminal ganglion neurons (Fig. 4A and B). cPTIO and disulfiram (both pre-GTN) and alpha lipoic acid (post-GTN) blunted the H2O2 increase (at 2 h) (Fig. 4C). cPTIO and NAC (pre-GTN), and alpha lipoic acid (post-GTN) blunted the 4-HNE signal (at 4 h) (Fig. 4D). Unexpectedly, antagonism (HC-030031) or genetic deletion of TRPA1 also attenuated GTN-induced increases in H2O2 or 4-HNE (Fig. 4 C–E). These data suggest that oxidative stress generation, which seems to mediate GTN-evoked PMA, is initiated by NO-induced activation of TRPA1 within the trigeminal ganglion.
Figure 4.
GTN generates oxidative stress in trigeminal ganglion via NO and TRPA1. (A and B) Systemic (i.p.) GTN increases H2O2 levels and 4-HNE staining in trigeminal ganglion, but not in brain stem of C57BL/6 mice. (C) Two hours after GTN the increase in H2O2 in trigeminal ganglion neurons, is abolished by systemic (i.p.) cPTIO (0.6 mg/kg) and disulfiram (DSF, 100 mg/kg) (all pre-GTN) or alpha lipoic acid (αLA) or HC-030031 (HC03, both 100 mg/kg) (all post-GTN). (D) Representative images and pooled data of 4-HNE staining in trigeminal ganglion neurons, 4 h after GTN (10 mg/kg) administration in C57BL/6 mice treated systemically (i.p.) with cPTIO (0.6 mg/kg) or NAC (250 mg/kg) (all pre-GTN), or with HC03 and alpha lipoic acid (both, 100 mg/kg) (all post-GTN). (E) Systemic (i.p.) GTN (10 mg/kg) increases H2O2 levels and 4-HNE staining in trigeminal ganglion neurons from Trpa1+/+, but not Trpa1−/− mice. BL = baseline level of H2O2 or 4-HNE; Veh = the vehicle of GTN. Dash (‐) indicates combined vehicles of treatments. Error bars indicate mean ± SEM, 4–6 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001; ###P < 0.001; one-way ANOVA with Bonferroni post hoc correction and Student’s t-test.
Sensory neurons and SGCs are present in the trigeminal ganglion (Fig. 5A). To determine which cell type generates GTN/NO-evoked oxidative stress, C57BL/6 mice were treated with resiniferatoxin, which is known to defunctionalize TRPV1-expressing neurons (Pecze et al., 2009), which co-express TRPA1 (Karai et al., 2004; De Logu et al., 2017). Treatment with resiniferatoxin, which suppressed eye wiping evoked by TRPV1 and TRPA1 agonists (capsaicin and AITC, respectively) (Supplementary Fig. 1L), attenuated both GTN-evoked PMA and H2O2 generation (Fig. 5B), supporting a role of TRPV1/TRPA1-positive neurons in these responses. GTN stimulated release of H2O2 from mixed cultures of trigeminal ganglion neurons/SGCs (Fig. 5C), but not from primary cultures of isolated SGCs (Fig. 5D). Removal of extracellular Ca2+ or pre-exposure to a high capsaicin concentration that, similar to resiniferatoxin, desensitizes TRPV1/TRPA1-positive neurons (Nassini et al., 2015), attenuated GTN-evoked increase in H2O2 in mixed cultures of trigeminal ganglion neurons/SGCs (Fig. 5C). GTN, AITC and SNAP increased H2O2 release from trigeminal ganglion neurons/SGCs mixed cultures in a HC-030031-dependent manner (Fig. 5C).
Figure 5.
GTN targets neuronal TRPA1 to generate periorbital oxidative stress and PMA. (A) Neurons (identified by neuronal nuclei, NeuN), SGCs (identified by glutamine synthetase, GS) and TRPA1 staining in trigeminal ganglion. (B) Neuronal defunctionalization with resiniferatoxin prevents systemic (i.p.) GTN-evoked PMA and H2O2 increase in trigeminal ganglion neurons of C57BL/6 mice. (C) H2O2 release elicited by GTN from trigeminal ganglion neurons-SGCs mixed cultures (see staining for NeuN/GS) is inhibited by extracellular Ca2+ removal (Ca2+-free), pre-exposure to capsaicin (CPS-des) or HC-030031 (HC03), which also inhibits H2O2 release elicited by AITC or SNAP. (D) In SGC-enriched cultures (see staining for GS, but not NeuN) GTN does not release H2O2. (E) Intrathecal TRPA1 antisense oligonucleotide inhibits TRPA1 mRNA expression, systemic (i.p.) GTN-evoked PMA and H2O2 increase in trigeminal ganglion neurons. (F) TRPA1 mRNA expression in trigeminal ganglion neurons from control and Advillin-Cre; Trpa1fl/fl (Adv-Cre; Trpa1fl/fl). Systemic (i.p.) GTN-evoked PMA and H2O2 increase in trigeminal ganglion neurons are reduced in Adv-Cre; Trpa1fl/fl mice. BL = baseline mechanical threshold; Veh = the vehicle of GTN. Dash (‐) indicates vehicles of treatments. Error bars indicate mean ± SEM, 6–8 mice per group or 2–7 replicates from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus resiniferatoxin-vehicle (RTX-Veh), mismatch oligonucleotide-vehicle (MM-Veh) and control-Veh; ##P < 0.01, ###P < 0.001 versus vehicle resiniferatoxin-GTN, MM-GTN, control-GTN, GTN, AITC or SNAP, one-way or two-way ANOVA with Bonferroni post hoc correction and Student’s t-test.
Intrathecal administration of TRPA1 antisense oligonucleotide downregulated Trpa1 mRNA expression in trigeminal ganglion neurons (Fig. 5E) and reduced AITC-evoked eye wiping (Supplementary Fig. 1M). TRPA1 antisense oligonucleotide attenuated GTN-evoked PMA and H2O2 increase in trigeminal ganglion neurons (Fig. 5E). Further evidence for the role of neuronal TRPA1 in GTN-evoked oxidative stress and allodynia was obtained by studying Advillin-Cre+; Trpa1fl/fl mice, which exhibited reduced TRPA1 mRNA in trigeminal ganglion neurons (Fig. 5F) and TRPA1-mediated eye wiping evoked by AITC (Supplementary Fig. 1M). GTN failed to evoke PMA or H2O2 generation in trigeminal ganglion neurons in Advillin-Cre+; Trpa1fl/fl mice (Fig. 5F), thus supporting that GTN/NO initiates a TRPA1-dependent and oxidative stress-mediated mechanism that perpetuates nociceptor activation by an autocrine pathway that is confined to the trigeminal ganglion.
TRPA1 and NOXs in the soma of trigeminal ganglion nociceptors maintain GTN-evoked allodynia
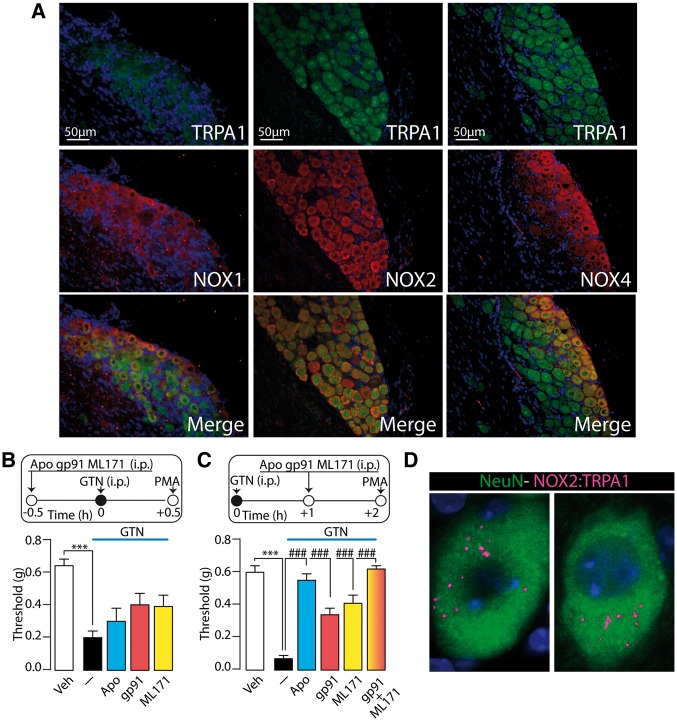
To explore the mechanism by which oxidative stress sustains GTN-evoked allodynia within trigeminal ganglion neurons we localized immunoreactivity for NOX1, NOX2, and NOX4 in mouse trigeminal ganglion neurons but not in SGCs (Supplementary Fig. 2A), confirming previous studies (Bedard and Krause, 2007). Immunoreactivities for the three NOX isoforms colocalized with that for TRPA1 (Fig. 6A). The non-selective NOX inhibitor, apocynin (i.p., post- but not pre-GTN) reversed GTN-evoked allodynia (Fig. 6B and C). Selective NOX2 (gp91ds-tat) or NOX1 (ML171) inhibitors (i.p., post- but not pre-GTN) attenuated, and their combination abolished, GTN-evoked PMA (Fig. 6B and C). The NOX4 inhibitor GKT137831 reduced, and the combination of GKT137831 and gp91ds-tat abolished, GTN-evoked allodynia (Supplementary Fig. 2B and C). However, since GKT137831 also inhibits NOX1, the role of NOX4 remains uncertain. Furthermore, a proximity ligation assay showed that NOX2 and TRPA1 are closely located in trigeminal ganglion neuronal cell bodies (Fig. 6D), suggesting that their interaction could underlie efficient reactive oxygen species release. Thus, the soma of TRPA1-expressing trigeminal ganglion neurons possesses the biochemical machinery required to initiate and sustain oxidative stress evoked by GTN.
Figure 6.
GTN evokes PMA via NADPH oxidase dependent mechanism. (A) Representative images of TRPA1, NOX1, NOX2 and NOX4 staining in mouse trigeminal ganglion. (B) The unselective NOX inhibitor, apocynin (APO; 100 mg/kg), the selective (C) NOX2 (gp91ds-tat peptide, gp91; 10 mg/kg) or the selective NOX1 (ML171; 60 mg/kg) (i.p., all pre-GTN) do not affect PMA evoked by systemic (i.p.) GTN (10 mg/kg). (C) APO (100 mg/kg), gp91 (10 mg/kg) or ML171 (60 mg/kg) (i.p., all post-GTN) partially reduce PMA. The combination of gp91 and ML171 (i.p., post-GTN) reverses PMA measured 2 h after GTN. (D) In situ proximity ligation assays (PLAs) for TRPA1: NOX2 in mouse trigeminal ganglion labelled with NeuN. Veh = the vehicle of GTN. Dash (‐) indicates vehicles of treatments. Error bars indicate mean ± SEM, 7–8 mice per group. ***P < 0.001 versus vehicle. ###P < 0.001 versus GTN; one-way ANOVA with Bonferroni post hoc correction.
CGRP contributes only in part to GTN-evoked allodynia
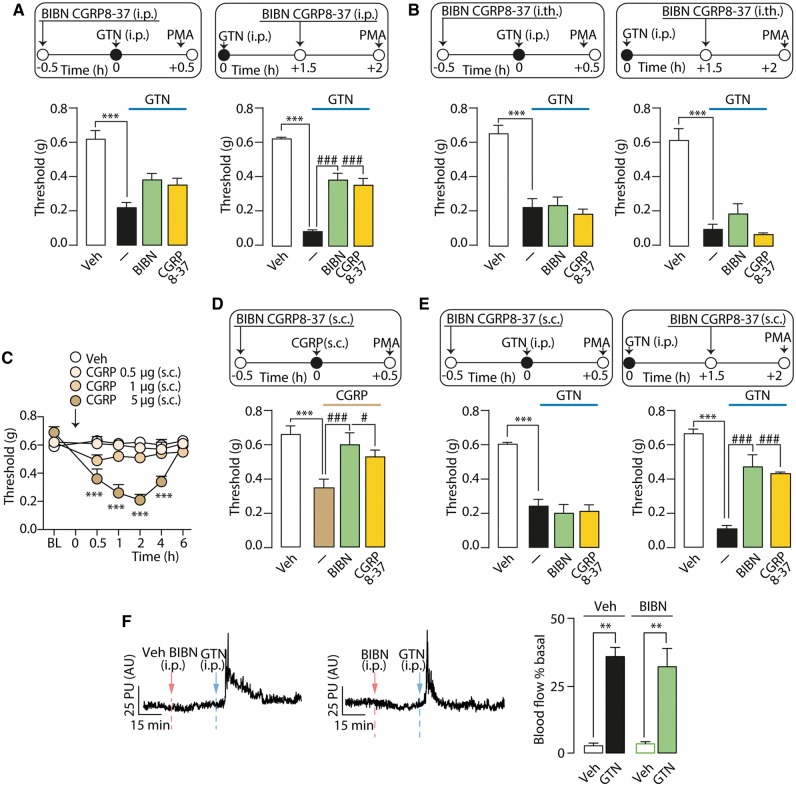
As CGRP is a key mediator of migraine headaches (Ho et al., 2010; Edvinsson, 2015) the contribution of CGRP to GTN-induced vasodilation and PMA was explored. Two different CGRP receptor antagonists, CGRP8-37 and BIBN4096BS (olcegepant) given (i.p.) after, but not before GTN, partially inhibited PMA (Fig. 7A). CGRP8-37 or BIBN4096BS administered centrally (i.th.) either before or after GTN did not affect GTN-evoked PMA (Fig. 7B). Local CGRP (0.5–5 µg/10 µl s.c.) in the periorbital area induced a dose-dependent and sustained (4 h) PMA (Fig. 7C). Local (s.c.) pretreatment with CGRP8-37 or BIBN4096BS prevented CGRP-induced PMA (Fig. 7D). Local administration of CGRP8-37 or BIBN4096BS after, but not before GTN, partially attenuated PMA in a manner similar to that produced by their systemic administration (Fig. 7E). GTN-evoked increase in cutaneous blood flow was unaffected by the pretreatment with BIBN4096BS (Fig. 7F). Thus, the early vasodilation evoked by GTN is unrelated to CGRP.
Figure 7.
CGRP released from periorbital nerve terminals contributes to GTN evoked PMA. (A) PMA evoked by systemic (i.p.) GTN (10 mg/kg) is partially reduced by i.p. administration of CGRP8-37 (4 µmol/kg) or BIBN4096BS (BIBN; 1 mg/kg), when given post-, but not pre-GTN. (B) Intrathecal (i.th.) administration of CGRP8-37 (5 nmol) or BIBN (1 µg) pre- and post-GTN (10 mg/kg) does not affect GTN-evoked PMA. (C) Local (s.c.) CGRP (0.5-5 µg) evokes a dose- and time-dependent PMA. (D) Pretreatment with local (s.c.) BIBN (4 nmol) or CGRP8-37 (10 nmol) prevents CGRP (5 µg)-evoked PMA. (E) Local (s.c.) CGRP8-37 (10 nmol) or BIBN (4 nmol) reduce PMA when given post-, but not pre-GTN (i.p., 10 mg/kg). BL = baseline mechanical threshold; Veh = the vehicle of GTN or CGRP. Dash (‐) indicates vehicles of treatments. Data are presented as mean ± SEM of 6–8 mice per group. ***P < 0.001 versus Veh. #P < 0.05, ###P < 0.001 versus GTN or CGRP; one-way and two-way ANOVA with Bonferroni post hoc correction. (F) Representative traces and pooled data of the increases in periorbital skin blood flow evoked by GTN (i.p., 10 mg/kg). Pretreatment with systemic (i.p.) BIBN (1 mg/kg) does not affect the early increase in blood flow. Veh = the vehicle of GTN. Data are presented as mean ± SEM of 6–7 mice per group. **P < 0.01 versus vehicle; one-way ANOVA with Bonferroni post hoc correction.
Discussion
We report that the delayed and prolonged GTN-evoked PMA in mice is entirely TRPA1-dependent. Two chemically unrelated TRPA1 antagonists, but not TRPV1 or TRPV4 antagonists, completely reversed PMA. Furthermore, deletion of Trpa1, but not Trpv1 or Trpv4, prevented the development of PMA. A recent report that TRPA1 antagonism attenuates GTN potentiation of formalin-evoked periorbital allodynia in rats is in line with our findings (Demartini et al., 2017). We exposed mice to a dose of GTN (10 mg/kg) that has been used by others (Tassorelli et al., 2003; Bates et al., 2010; Farkas et al., 2016), but which exceeds the dose used in humans (∼40 µg/kg, i.v.) (Iversen and Olesen, 1996; Olesen, 2008). However, genetic deletion or pharmacological blockade of TRPA1 also suppressed PMA evoked by a low dose of GTN (1 mg/kg). The observation that, after correction for the mouse to man conversion factor (Reagan-Shaw et al., 2008), this dose is only 2-fold higher than the human dose, supports the translational relevance of the role of TRPA1 in GTN-evoked allodynia in mice.
NO activation of TRPA1, either directly, or indirectly via its by-products, through nitrosylation of cysteinyl residues, is an important posttranslational mechanism of channel regulation (Miyamoto et al., 2009). NO donors activate TRPA1 in cultured trigeminal ganglion neurons and hTRPA1-HEK293 cells by distinct mechanisms. As Ca2+ responses by SNAP, but not those by GTN, were inhibited by the NO scavenger, cPTIO, NO does not seem required for TRPA1 activation by GTN. It is possible that under in vitro conditions NO is released with insufficient velocity or in insufficient amounts to elicit TRPA1 gating, while the channel is engaged directly by GTN which binds to the same key cysteine/lysine residues required for channel activation by electrophilic and oxidant molecules (Hinman et al., 2006; Kozai et al., 2014).
In vivo GTN induces PMA via diverse anatomical pathways, mechanisms and time courses that depend from the route of administration. As the ALDH2 inhibitor, disulfiram, and the NO scavenger, cPTIO, blocked PMA elicited by systemic, but not local administration of GTN, local GTN likely causes allodynia by targeting TRPA1 by a direct NO-independent mechanism, whereas systemic GTN by an indirect, NO dependent pathway. As a consequence, the ability of NO released from GTN to target TRPA1 requires that the conversion occurs distant (Beretta et al., 2008) from the site where NO engages the channel. Evidence that NO must have spent some time in the extracellular environment before leading to S-nitrosothiol formation (Zhang and Hogg, 2004) supports this explanation. Furthermore, the observation that cPTIO and disulfiram prevent allodynia when administered before, but not after systemic GTN implies that NO is initially necessary, but is not subsequently sufficient, to sustain the allodynia. Attenuation by two different TRPA1 antagonists, HC-030031 or A967079, of GTN-evoked allodynia was a transient phenomenon, probably because of the limited half-lives of the antagonists in mice. These findings suggest that to maintain allodynia, TRPA1 must be engaged continuously by one or more mediators generated by GTN/NO and whose identity and source are unknown.
GTN generates oxidative stress (Wenzl et al., 2009). The observation that pretreatment with the reactive oxygen species scavengers, alpha lipoic acid or PBN, did not prevent GTN-evoked PMA, but attenuated allodynia from for 3–4 h after GTN, indicates that reactive oxygen species do not initiate the response, but mediate the ensuing phase. However, additional TRPA1 agonists must contribute to the final phase of PMA, when antioxidants were ineffective. Carbonylic by-products of oxidative stress, including 4-HNE, have half-lives longer than reactive oxygen species (Brame et al., 1999), and are known to target TRPA1 (Trevisani et al., 2007). PMA attenuation by NAC and l-carnosine, which efficiently quenches aldehydes, indicates that 4-HNE and/or related aldehydes engage TRPA1 to mediate the terminal phase of PMA, from 3–4 to 8 h after GTN.
Failure of local and efficacy of intrathecal antioxidants and TRPA1 antagonists to block PMA indicate that systemic GTN/NO does not target TRPA1 on cutaneous terminals of nociceptor and suggests the involvement of TRPA1 in a central site, such as the soma and central terminals in the brainstem of trigeminal ganglion neurons. Assessment of GTN-induced oxidative stress showed no change in the dorsal brain stem, whereas a marked increase in H2O2 and 4-HNE levels were found in trigeminal ganglion neurons. Remarkably, the time course of H2O2 formation (1–3 h) paralleled the ability of reactive oxygen species scavengers to attenuate PMA, and the time course of increased 4-HNE staining (3–6 h) paralleled the ability of aldehyde scavengers to inhibit PMA.
Primary sensory neurons and associated SGCs are most abundant cell types in trigeminal ganglion. The observation that resiniferatoxin, which defunctionalizes TRPV1/TRPA1 expressing nociceptors (Pecze et al., 2009), attenuated GTN-evoked H2O2 generation in vivo, suggests that trigeminal ganglion neurons rather than SGCs generate oxidative stress. As pharmacological blockade of TRPA1, global TRPA1 deletion, selective deletion of TRPA1 in peripheral sensory neurons and TRPA1 knockdown all abrogated GTN-evoked oxidative stress and PMA, TRPA1 expressed by trigeminal ganglion sensory neurons seems to be a critical step for PMA. Although various TRP channels can promote reactive oxygen species release (Shimizu et al., 2014), to our knowledge this is the first evidence that TRPA1 expressed by cell bodies of primary sensory neurons increase the tissue burden of oxidative stress. Sensory neurons express several NOX isoforms that may mediate the GTN/NO/TRPA1 signal (Bedard and Krause, 2007). Trigeminal ganglion neurons, but not SGCs, co-express TRPA1 and NOX1, NOX2 and NOX4, and the NOX inhibitor apocynin reverses GTN-evoked mechanical allodynia, thus suggesting that NOXs expressed by trigeminal ganglion neurons and located downstream from TRPA1 generate the pro-allodynic oxidative burden. NOX1 and NOX2 isoforms provide a major contribution, since the combination of selective NOX1 and NOX2 inhibitors afforded complete inhibition of allodynia. Notably, similar to alpha lipoic acid, the capacity of NOX inhibitors to suppress allodynia faded with time and was absent when the drugs were administered 3–4 h after GTN.
Several findings of the current study support the hypothesis that TRPA1/NOXs, reactive oxygen species and aldehydes sustain GTN-evoked PMA by an action that is confined to trigeminal ganglion neurons. First, GTN induced reactive oxygen species/4-HNE in trigeminal ganglion but not the brainstem. In particular, failure to identify any increase in HNE staining in any specific brainstem area supports the hypothesis that oxidative stress is not generated at this level. Second, intrathecal administration of TRPA1 channel antagonists or antisense oligonucleotides attenuated this response. Third, both GTN-evoked PMA and reactive oxygen species/4-HNE generation were attenuated by resiniferatoxin, which selectively desensitizes TRPV1-expressing primary sensory neurons, a population that encompasses the TRPA1-positive subpopulation (Karai et al., 2004; De Logu et al., 2017). Finally, similar attenuation was found in mice expressing Cre-recombinase from the locus of the primary sensory neuron-specific gene Advillin (Hasegawa et al., 2007; Zurborg et al., 2011; Guan et al., 2016), resulting in a selective Trpa1 mRNA attenuation in nociceptors. Although our findings suggest a key role for TRPA1 in trigeminal ganglion neurons, the present study did not systematically investigate the possibility that TRPA1 activation and oxidative stress in the CNS also contribute to GTN-evoked PMA. The ability of centrally administered NO donors to affect the function of neurons of the spinal trigeminal nucleus (Koulchitsky et al., 2004, 2009) suggests that GTN can also act centrally. Furthermore, the cell bodies of trigeminal ganglion neurons that mediate allodynia via TRPA1/NOX/oxidative stress may belong to meningeal nociceptors, which are known to contribute to the sensitisation process observed after exposure to inflammatory mediators or GTN (Strassman et al., 1996; Levy et al., 2004; Zhang et al., 2013).
The beneficial action of CGRP/CGRP receptor blockade by small molecules or monoclonal antibodies indicate that CGRP is a major mediator of migraine headaches (Ho et al., 2010; Edvinsson, 2015). The observation that two different CGRP receptor antagonists, when administered by systemic or local routes, but not central administration, reduced GTN-evoked PMA suggests that CGRP acts at a peripheral site. One possible explanation is that excitation of the NO/TRPA1/NOX pathway in the soma of trigeminal ganglion neurons generates antidromic action potentials, which ultimately invade cutaneous nerve terminals to promote local CGRP release. The observation that a CGRP monoclonal antibody, which does not cross the blood–brain barrier and should act peripherally, attenuates PMA-induced by sodium nitroprusside plus sumatriptan in rats (Kopruszinski et al., 2017), supports the existence of a peripheral mechanism. However, further studies are required to examine the effects of GTN on antidromic transmission in trigeminal ganglion neurons. Our findings that CGRP provides a limited and delayed contribution to the allodynia may explain the failure of BIBN4096BS to reduce GNT-evoked migraine-like pain in patients (Tvedskov et al., 2010). Notably, we report that the early periorbital vasodilation elicited by GTN is entirely due to ALDH2-dependent NO formation, but neither TRPA1 nor CGRP are involved. Thus, vasodilatation and allodynia are temporally and mechanistically distinct. While vasodilatation is probably due to a direct NO action in the vascular smooth muscle, allodynia is a neuronal phenomenon mediated by TRPA1 activation and the ensuing oxidative stress.
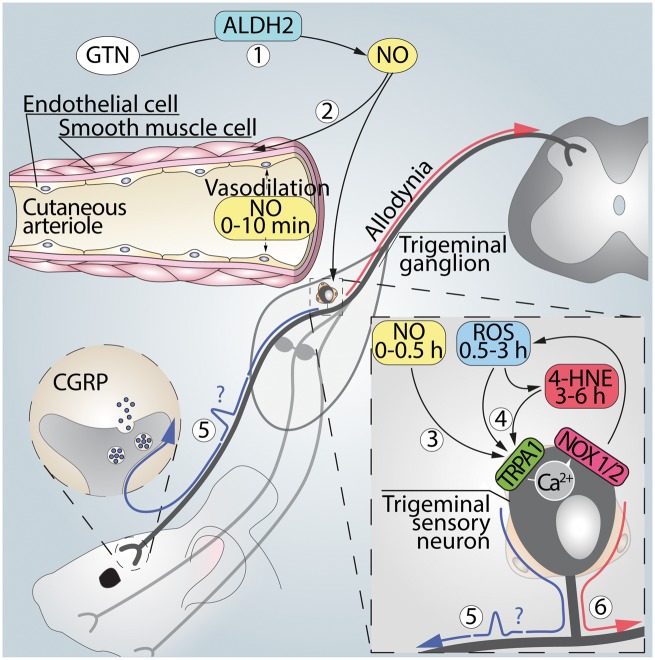
Our present results suggest that systemic GTN is converted by ALDH2 to NO that causes a transient vasodilatation unrelated to TRPA1 and most probably due to a direct action of the gaseous mediator on smooth muscle guanylyl cyclase (Fig. 8). NO and/or its by-products also target TRPA1/NOXs in the soma of trigeminal ganglion neurons to increase reactive oxygen species/reactive carbonylic species, thereby sustaining mechanical allodynia. In this view, the channel that triggers the oxidative burst is expressed by the same sensory neurons that potentially mediate the allodynia. Thus, it is not possible to discriminate whether TRPA1, in addition to generating the proalgesic oxidative stress, is also the final mediator of the hypersensitivity. Nevertheless, since TRPA1 is a major sensor of oxidative and carbonylic stress (Trevisani et al., 2007; Andersson et al., 2008; Materazzi et al., 2008), it is possible that reactive oxygen species/4-HNE, generated by TRPA1 activated by GTN/NO, target the same neuronal channel to signal allodynia. TRPA1 antagonists, which are currently in clinical development, or established anti-migraine drugs, which have recently been identified as selective channel inhibitors (Bigal et al., 2002; Nassini et al., 2015), may be used to test whether a mechanism similar to the present one mediates the delayed attack evoked by GTN in migraineurs.
Figure 8.
Signalling pathway that mediates TRPA1-dependent PMA induced by GTN. (1) Systemic GTN is converted to NO by ALDH2 at sites distant to the trigeminal ganglion. (2) NO produces via TRPA1- and CGRP-independent mechanisms a rapid and transient increase in periorbital blood flow. (3) NO also targets TRPA1 in the soma of trigeminal ganglion neurons to evoke a Ca2+-dependent NOX1/NOX2 activation, thereby increasing reactive oxygen (H2O2) and carbonylic (4-HNE) species. (4) H2O2 and 4-HNE by an apparently autocrine pathway in trigeminal ganglion neurons sustain periorbital mechanical allodynia. (5) Activation of the NO/TRPA1/NOX pathway in the soma of trigeminal ganglion neurons could possibly (?) generate antidromically-propagated action potentials, which ultimately invade cutaneous nerve terminals to promote local CGRP release that partially contributes to PMA. Glyceryl trinitrate administration causes prolonged mechanical allodynia in rodents, which correlates temporally with delayed migraine attacks in patients. Marone et al. show that the allodynia is mediated by TRPA1 activation in cell bodies of trigeminal neurons and ensuing oxidative stress. This neuronal pathway may be of relevance to migraine-like headaches.
A limitation of our study is that GTN-evoked PMA in naïve mice may not fully replicate migraine in patients. However, periorbital allodynia has been successfully used in rodents to identify neural pathways that are relevant for migraine pathophysiology, such as those responsible for headache triggered by overuse of medications (De Felice et al., 2010; Kopruszinski et al., 2017). In addition, GTN provocation studies report that healthy volunteers experience both an early headache during GTN infusion (Iversen et al., 1989), and also a delayed headache, although usually milder than in migraineurs (Sicuteri et al., 1987; Olesen et al., 1993; Perrotta et al., 2011). Moreover, cutaneous allodynia, which is usually localized to the temporal and periocular areas (Drummond, 1987) occurs in ∼80% of migraine attacks and can be detected also in extracephalic areas (Burstein et al., 2000). The report that GTN evokes a series of typical premonitory symptoms (Afridi et al., 2004), in addition to headaches, strengthens the value of studying allodynia as part of the various symptoms of the migraine attack. Such sensitization, deriving from activation of meningeal nociceptors by locally-released inflammatory mediators (Strassman et al., 1996), may lead to sensitization of central trigeminal neurons that receive convergent input from the dura and skin (Burstein et al., 1998). Endogenous mediators and exogenous chemicals, including GTN, elicit delayed sensitization that, via dynamic changes in meningeal arteries (Zhang et al., 2013) and central second order trigeminovascular neurons (Burstein et al., 1998), may be observed in periorbital skin and other cutaneous areas. Our data suggest that the TRPA1/NOX pathway in the soma of trigeminal neurons, in addition to the previously reported peripheral or central sites, contributes to GTN-evoked allodynia.
Supplementary Material
Acknowledgements
We thank Alyn H. Morice (University of Hull, UK) for the hTRPA1-HEK293 cells, David Julius (University of California, San Francisco, USA) for the human TRPA1 wild-type and human TRPA1 mutant (C619S, C639S, C663S, K708Q) cDNAs, Allan I. Basbaum (University of California, San Francisco, USA) for Advillin-Cre mice and Delia Preti (University of Ferrara, Italy) for providing HC-030031. We also thank Mary K. Lokken for her expert English revision.
Glossary
Abbreviations
- 4-HNE
4-hydroxynonenal
- AITC
allyl isothiocyanate
- CGRP
calcitonin gene-related peptide
- GTN
glyceryl trinitrate
- NAC
N-acetyl-l-cysteine
- PMA
periorbital mechanical allodynia
- SGC
satellite glial cell
- SNAP
S-nitroso-N-acetylpenicillamine
Funding
This work was supported by grants from Istituto Toscano Tumori (ITT), grant 2014; Regione Toscana, grant Nutraceuticals 2014, ‘POFCADT’; Ministry for University and Scientific Research (MiUR) Rome, Italy Grants PRIN 201532AHAE_003 (to P.G.); and NIH NS102722 and DE026806 (to N.W.B.).
Supplementary material
Supplementary material is available at Brain online.
References
- Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain 2004; 110: 675–80. [DOI] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 2008; 28: 2485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A et al. . TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat Commun 2011; 2: 551. [DOI] [PubMed] [Google Scholar]
- Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI et al. . Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 2010; 30: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of reactive oxygen species-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245–313. [DOI] [PubMed] [Google Scholar]
- Benemei S, De Logu F, Li Puma S, Marone IM, Coppi E, Ugolini F. et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br J Pharmacol 2017; 16: 13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta M, Gruber K, Kollau A, Russwurm M, Koesling D, Goessler W et al. . Bioactivation of nitroglycerin by purified mitochondrial and cytosolic aldehyde dehydrogenases. J Biol Chem 2008; 283: 17873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Bordini CA, Tepper SJ, Speciali JG. Intravenous dipyrone in the acute treatment of migraine without aura and migraine with aura: a randomized, double blind, placebo controlled study. Headache 2002; 42: 862–71. [DOI] [PubMed] [Google Scholar]
- Bonet IJ, Fischer L, Parada CA, Tambeli CH. The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia. Neuropharmacology 2013; 65: 206–12. [DOI] [PubMed] [Google Scholar]
- Borkum JM. The Migraine Attack as a Homeostatic, Neuroprotective Response to Brain Oxidative Stress: preliminary Evidence for a Theory. Headache 2018; 58: 118–35. [DOI] [PubMed] [Google Scholar]
- Brame CJ, Salomon RG, Morrow JD, Roberts LJ. 2nd Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem 1999; 274: 13139–46. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 1998; 79: 964–82. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol 2000; 47: 614–24. [PubMed] [Google Scholar]
- Chung MK, Asgar J, Lee J, Shim MS, Dumler C, Ro JY. The role of TRPM2 in hydrogen peroxide-induced expression of inflammatory cytokine and chemokine in rat trigeminal ganglia. Neuroscience 2015; 297: 160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 2006; 10: 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I et al. . Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann Neurol 2010; 67: 325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Logu F, Nassini R, Materazzi S, Carvalho Goncalves M, Nosi D, Rossi Degl’Innocenti D et al. . Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun 2017; 8: 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demartini C, Tassorelli C, Zanaboni AM, Tonsi G, Francesconi O, Nativi C et al. . The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: evaluation in an animal model. J Headache Pain 2017; 18: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner AC, Vogler B, Messlinger K, De Col R. Role of transient receptor potential ankyrin 1 receptors in rodent models of meningeal nociception - Experiments in vitro. Eur J Pain 2017; 21: 843–54. [DOI] [PubMed] [Google Scholar]
- Di W, Shi X, Lv H, Liu J, Zhang H, Li Z et al. . Activation of the nuclear factor E2-related factor 2/anitioxidant response element alleviates the nitroglycerin-induced hyperalgesia in rats. J Headache Pain 2016; 17: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–62. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Scalp tenderness and sensitivity to pain in migraine and tension headache. Headache 1987; 27: 45–50. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. The Journey to Establish CGRP as a Migraine Target: a Retrospective View. Headache 2015; 55: 1249–55. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Oshinsky ML, Amenta PS, Awe OO, Jallo JI. Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache 2012; 52: 966–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas S, Bolcskei K, Markovics A, Varga A, Kis-Varga A, Kormos V et al. . Utility of different outcome measures for the nitroglycerin model of migraine in mice. J Pharmacol Toxicol Methods 2016; 77: 33–44. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41: 1149–60. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Levine JD, Green PG. Mechanisms mediating nitroglycerin-induced delayed-onset hyperalgesia in the rat. Neuroscience 2016; 317: 121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S et al. . Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016; 19: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Chen XP, Guo X, Chen L, Li D, Peng J et al. . Evidence for involvement of calcitonin gene-related peptide in nitroglycerin response and association with mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism. J Am Coll Cardiol 2008; 52: 953–60. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han BX, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci 2007; 27: 14404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 2006; 103: 19564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010; 6: 573–82. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 1991; 43: 143–201. [PubMed] [Google Scholar]
- Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain 1989; 38: 17–24. [DOI] [PubMed] [Google Scholar]
- Iversen HK, Olesen J. Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs. Cephalalgia 1996; 16: 412–8. [DOI] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M et al. . Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 2004; 113: 1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopruszinski CM, Xie JY, Eyde NM, Remeniuk B, Walter S, Stratton J et al. . Prevention of stress- or nitric oxide donor-induced medication overuse headache by a calcitonin gene-related peptide antibody in rodents. Cephalalgia 2017; 37: 560–70. [DOI] [PubMed] [Google Scholar]
- Koulchitsky S, Fischer MJ, De Col R, Schlechtweg PM, Messlinger K. Biphasic response to nitric oxide of spinal trigeminal neurons with meningeal input in rat–possible implications for the pathophysiology of headaches. J Neurophysiol 2004; 92: 1320–8. [DOI] [PubMed] [Google Scholar]
- Koulchitsky S, Fischer MJ, Messlinger K. Calcitonin gene-related peptide receptor inhibition reduces neuronal activity induced by prolonged increase in nitric oxide in the rat spinal trigeminal nucleus. Cephalalgia 2009; 29: 408–17. [DOI] [PubMed] [Google Scholar]
- Kozai D, Kabasawa Y, Ebert M, Kiyonaka S, Firman Otani Y, Numata T et al. . Transnitrosylation directs TRPA1 selectivity in N-nitrosamine activators. Mol Pharmacol 2014; 85: 175–85. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011; 152: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ et al. . TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50: 277–89. [DOI] [PubMed] [Google Scholar]
- Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A 2004; 101: 4274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4(−/−) mice. Proceedings of the National Academy of Sciences of the United States of America 2003; 100: 13698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M et al. . Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 2008; 105: 12045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M et al. . TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A 2007; 104: 13525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One 2009; 4: e7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G et al. . The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012; 135: 376–90. [DOI] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol 2014; 167: 1–43. [DOI] [PubMed] [Google Scholar]
- Nassini R, Fusi C, Materazzi S, Coppi E, Tuccinardi T, Marone IM et al. . The TRPA1 channel mediates the analgesic action of dipyrone and pyrazolone derivatives. Br J Pharmacol 2015; 172: 3397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Szallasi A. Transient Receptor Potential Channels as Drug Targets: from the Science of Basic Research to the Art of Medicine. Pharmacological Reviews 2014; 66: 676–814. [DOI] [PubMed] [Google Scholar]
- Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport 1993; 4: 1027–30. [DOI] [PubMed] [Google Scholar]
- Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther 2008; 120: 157–71. [DOI] [PubMed] [Google Scholar]
- Pecze L, Pelsoczi P, Kecskes M, Winter Z, Papp A, Kaszas K et al. . Resiniferatoxin mediated ablation of TRPV1+ neurons removes TRPA1 as well. Can J Neurol Sci 2009; 36: 234–41. [DOI] [PubMed] [Google Scholar]
- Perrotta A, Serrao M, Tassorelli C, Arce-Leal N, Guaschino E, Sances G et al. . Oral nitric-oxide donor glyceryl-trinitrate induces sensitization in spinal cord pain processing in migraineurs: a double-blind, placebo-controlled, cross-over study. Eur J Pain 2011; 15: 482–90. [DOI] [PubMed] [Google Scholar]
- Persson MG, Agvald P, Gustafsson LE. Detection of nitric oxide in exhaled air during administration of nitroglycerin in vivo. Br J Pharmacol 1994; 111: 825–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Bhatt DK, Ploug KB, Hay-Schmidt A, Jansen-Olesen I, Gupta S et al. . Nitric oxide synthase, calcitonin gene-related peptide and NK-1 receptor mechanisms are involved in GTN-induced neuronal activation. Cephalalgia 2014; 34: 136–47. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb J 2008; 22: 659–61. [DOI] [PubMed] [Google Scholar]
- Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R et al. . Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain 2001; 124: 2490–502. [DOI] [PubMed] [Google Scholar]
- Shatillo A, Koroleva K, Giniatullina R, Naumenko N, Slastnikova AA, Aliev RR et al. . Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience 2013; 253: 341–9. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Takahashi N, Mori Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). Handb Exp Pharmacol 2014; 223: 767–94. [DOI] [PubMed] [Google Scholar]
- Sicuteri F, Del Bene E, Poggioni M, Bonazzi A. Unmasking latent dysnociception in healthy subjects. Headache 1987; 27: 180–5. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996; 384: 560–4. [DOI] [PubMed] [Google Scholar]
- Strecker T, Dux M, Messlinger K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J Vasc Res 2002; 39: 489–96. [DOI] [PubMed] [Google Scholar]
- Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W et al. . Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 2015; 8: ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G. Nitroglycerin induces hyperalgesia in rats–a time-course study. Eur J Pharmacol 2003; 464: 159–62. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol 2009; 75: 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadani U, Rodgers T. Side effects of using nitrates to treat angina. Expert Opin Drug Saf 2006; 5: 667–74. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol 1994; 1: 73–80. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Brennum J, Iversen HK, Olesen J. Effect of a nitric oxide donor (glyceryl trinitrate) on nociceptive thresholds in man. Cephalalgia 1996; 16: 169–74. [DOI] [PubMed] [Google Scholar]
- Trainor DC, Jones RC. Headaches in explosive magazine workers. Arch Environ Health 1966; 12: 231–4. [DOI] [PubMed] [Google Scholar]
- Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Silva CR et al. . TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic Biol Med 2014; 72: 200–9. [DOI] [PubMed] [Google Scholar]
- Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C et al. . TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 2016; 139: 1361–77. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B et al. . 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 2007; 104: 13519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvedskov JF, Tfelt-Hansen P, Petersen KA, Jensen LT, Olesen J. CGRP receptor antagonist olcegepant (BIBN4096BS) does not prevent glyceryl trinitrate-induced migraine. Cephalalgia 2010; 30: 1346–53. [DOI] [PubMed] [Google Scholar]
- Wenzl MV, Beretta M, Gorren AC, Zeller A, Baral PK, Gruber K et al. . Role of the general base Glu-268 in nitroglycerin bioactivation and superoxide formation by aldehyde dehydrogenase-2. J Biol Chem 2009; 284: 19878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia KJ, O’Hara CL, Moehring F, Kwan KY, Stucky CL. Sensory Neuron-Specific Deletion of TRPA1 Results in Mechanical Cutaneous Sensory Deficits. eNeuro 2017; 4: 0069–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kainz V, Zhao J, Strassman AM, Levy D. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Ann Neurol 2013; 73: 741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hogg N. Formation and stability of S-nitrosothiols in RAW 264.7 cells. Am J Physiol Lung Cell Mol Physiol 2004; 287: L467–74. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Piszczek A, Martinez C, Hublitz P, Al Banchaabouchi M, Moreira P et al. . Generation and characterization of an Advillin-Cre driver mouse line. Mol Pain 2011; 7: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.