 Organisms respond to exogenous threats and insults by a potent inflammatory and immune response, aimed at preserving tissue integrity and restoring tissue homeostasis and function.
Organisms respond to exogenous threats and insults by a potent inflammatory and immune response, aimed at preserving tissue integrity and restoring tissue homeostasis and function.
Abstract
Organisms respond to physical, chemical and biological threats by a potent inflammatory response, aimed at preserving tissue integrity and restoring tissue homeostasis and function. Systemic effects in an organism refer to an effect or phenomenon which originates at a specific point and can spread throughout the body affecting a group of organs or tissues. Ionizing radiation (IR)-induced systemic effects arise usually from a local exposure of an organ or part of the body. This stress induces a variety of responses in the irradiated cells/tissues, initiated by the DNA damage response and DNA repair (DDR/R), apoptosis or immune response, including inflammation. Activation of this IR-response (IRR) system, especially at the organism level, consists of several subsystems and exerts a variety of targeted and non-targeted effects. Based on the above, we believe that in order to understand this complex response system better one should follow a ‘holistic’ approach including all possible mechanisms and at all organization levels. In this review, we describe the current status of knowledge on the topic, as well as the key molecules and main mechanisms involved in the ‘spreading’ of the message throughout the body or cells. Last but not least, we discuss the danger-signal mediated systemic immune effects of radiotherapy for the clinical setup.
Introduction
A general, commonly accepted assumption in radiation biology has been that the nucleus is the only target of radiation, i.e. the classical target paradigm of radiation biology. In addition, it has been believed for many years that DNA damage (especially complex) occurs only in irradiated cells through direct deposition of energy or through reactive oxygen species (ROS) produced by radiolysis of water, the so called ‘indirect effect’. DNA injury was thought to be the most important biological effect of ionizing radiation (IR). This paradigm has been frequently challenged by a plethora of research findings, demonstrating that the radiation-induced effects are not solely attributed to the direct targeting of DNA or exposed cells. The findings also point to the induction of more complex effects, the so-called ‘non-targeted effects’ (NTE), in cells that do not directly interact with radiation as described above.1–7 A range of evidence that has emerged thus far supports that IR induces complex, global cellular responses, such as radiation-induced bystander effects (RIBE),4,8–11 radioadaptive response12–19 and genomic instability.3,20–25 The effects have been observed not only in irradiated, but also in non-irradiated bystander cells that receive molecular signals emitted by the irradiated cells. The main features of NTE are that direct nuclear exposure to radiation is not required for their manifestation and that they play a more important role at ‘low’ doses (<1 Gy). Damage-associated molecular patterns (DAMPs) seem to play an important role in the communication of this stress system-wide and at different organism levels from plants to humans.26,27 The spectrum of non-targeted effects still continues to broaden; currently it includes many types of exogenous and endogenous stressors that induce a systemic genotoxic response with the oxidative mechanisms holding a pivotal role.28,29
Regarding probably the most important of all the NTE, RIBE, is broadly described as the phenomenon whereby deleterious effects of radiation (chromosome aberrations, sister chromatid exchanges – SCE, micronucleation, mutations, apoptosis) are detected in cells that have not been irradiated, but are in the vicinity of those that have.30–32 RIBE show non-linear dose–response; they are frequently prominent at low doses of radiation and tend to vanish at high radiation doses. Thus they are mainly linked to low-dose radiation effects and radiation protection.33 Early evidence upholding RIBE emerged in studies performed in lymphoid cells by Murphy and Morton at the beginning of the 20th century,34 but Nagasawa and Little were the first to report this radiobiological phenomenon, in 1992.35 Their research showed a significant increase in the frequency of SCE in 30% of CHO cells analyzed, even though less than 1% of the cells’ nuclei were actually traversed by an α-particle. Subsequent studies with normal human lung fibroblasts also confirmed this finding.36 Evidence suggests that this phenomenon involves the secretion of soluble factors (e.g. cytokines and other molecules) by the irradiated cells, contributing to the upregulation of oxidative metabolism in the bystander cells.37–40 Interestingly, RIBE have been observed in a plethora of biological endpoints including DNA damage induction,41–44 micronucleation,45,46 genomic instability,20,47,48 alteration in the microRNA (miRNA) profile,47,49–52 oxidative stress53–55 and cell death or apoptosis.56,57 Such evidence of RIBE in various in vitro and in vivo systems, including human, rodents, fish and plants are summarized in Table 1.
Table 1. Key findings on the systemic effects induced by ionizing radiation of different types. In this table, we have included the biological system and the affected organs or tissues as well as the main biological molecules and mechanisms involved based on experimental evidence and current status of knowledge.
| Organism/system | Radiation type (targeted organ/tissue) | Surrounding or distant organ/tissue affected: observed effect | Molecules involved – key mechanisms proposed | Ref. | |
| Human | In vivo | ||||
| Human | Roentgen-ray therapy prior to chronic granulocytic leukemia treatment (spleen) | Bone marrow: | - Production of “clastogenic factors” in the circulating blood of exposed individuals | 244 | |
| - Decrease in bone marrow cellularity | |||||
| Human (hepatocellular carcinoma patient) | Radiotherapy prior to thoracic vertebral bone metastasis (thorax) | Liver: | - Host immune response involving cytokines (TNF-α) | 74 | |
| - Regression of hepatocellular carcinoma | |||||
| - Increased serum levels of TNF-α | |||||
| In vitro | |||||
| Non-small cell lung carcinoma cells (H1299) | ? | Non-irradiated cells treated with radiation-conditioned medium from irradiated cells: | TGF-β1–miR-21–ROS pathway | 49 | |
| - ROS level increase | |||||
| - DNA damage increase | |||||
| Human umbilical vein endothelial cells (HUVECs) | γ-Irradiation (U937 macrophage cells) | HUVECs co-cultured with γ-irradiated U937: | - p38 pathway | 56 | |
| - Induction of additional micronuclei and apoptosis | - Irradiated U937 cells release nitric oxide and thereby further triggers apoptotic and inflammatory responses in the bystander HUVECs | ||||
| - Overexpression of p38 mitogen-activated protein kinase (MAPK) | |||||
| - Increase of the contents of vascular cell adhesion molecule-1 (VCAM-1) and the activities of matrix metalloproteinase-9 (MMP-9) in HUVEC culture medium | |||||
| Neonatal human dermal fibroblasts (NHDF-Neo) | UVA, UVB, UVC (NHDF-Neo) | Non-irradiated cells co-incubated with irradiated cells in well dishes allowing diffusion of medium components between them: | - Increased levels of cellular ROS in irradiated cells may cause these bystander effects | 66 | |
| - Reduction of survival | - Increased secretion of IL-6 suggests its role as a molecular bystander signal released by irradiated cells, but mutual signaling between irradiated and bystander cells modulates this secretion | ||||
| - Increased frequency of apoptosis | |||||
| - Increased intracellular oxidation | |||||
| - Generation of proinflammatory cytokines | |||||
| - Increased levels of cellular ROS | |||||
| - Increase of IL-6 concentration in the medium (especially in UVB and UVC experiments) | |||||
| Lung adenocarcinoma cells (A549) | 6MV X-rays (A549) | - Lower clone forming, apoptosis and survival, and cell circle arrest in phase G2 in both irradiated and irradiated conditioned medium (ICM)-treated cells | - Cytokine production induces changes in the bystander cells | 67 | |
| Rodents | In vivo | ||||
| Rats (Sprague-Dawley) | 60Co γ-irradiation (lung base) | Lung apex: | - Clastogenic factor produced in the plasma following irradiation | 70 | |
| - Micronuclei induction | - Production of oxygen radicals by the induction of inflammatory cytokines (TNF-α, IL-1) | ||||
| - Partial blocking of the DNA damage in the unirradiated lung apex by superoxide dismutase | |||||
| Mice | 137Cs γ-radiation (whole body) | Spleen, bone marrow: | - Cytokine release | 68 | |
| - Macrophage and neutrophil accumulation | - Signaling pathways initiated by extensive macrophage activity | ||||
| - Increase phagocytic activity | - Communication between phagocytic cells | ||||
| Mice | 125I (whole body) | Subcutaneous tumor: | - Various signaling pathways triggered by 125I decay | 245 | |
| - Tumor growth arrest/retardation | |||||
| Mice | X-rays (whole body/half body) | Cutaneous tissues: | - Internal organ exposure | 42 | |
| - DNA double strand breaks | - DNA double strand breaks repair activity proteins | ||||
| - Upregulation of Rad51 | |||||
| Rat | X-rays (whole body/whole body without cranial exposure) | Spleen: | - miR-194 (miRNA)-regulated pathway | 50 | |
| - DNA hypomethylation | |||||
| - Altered levels of histone methylation and DNA methyltransferases | |||||
| - Upregulation of non-coding RNA molecules | |||||
| Mice | X-rays (partial body) | Skin: | - Oxidative stress metabolism | 53 | |
| - Oxidative clustered DNA lesions induction | |||||
| Mice | X-rays (whole body/cranial exposure) | Spleen: | - Cell cycle changes | 43 | |
| - DNA damage – apoptosis | - DNA repair | ||||
| - Upregulation p53 expression | |||||
| - Abnormal cellular proliferation | |||||
| - Gender specific abnormal mRNA levels | |||||
| Mice | 137Cs γ-radiation (whole body) | Haematopoietic clonogenic stem cells: | - Inflammatory mechanisms | 54 | |
| - TNF-α secretion | - Oxidative stress | ||||
| - Macrophage activation | |||||
| Mice | X-rays (whole body/whole body excluding head) | Cerebellum: | - Erroneous DSB repair or complete lack of it, leading to genetic changes | 81 | |
| - Double strand breaks | - Clastogenic factors in blood stream | ||||
| - Apoptotic cell death | |||||
| - Tumor induction | |||||
| Mice | X-rays (whole body/cranial exposure) | Spleen, skin: | - miR-194 (miRNA)-regulated pathway | 47 | |
| - Epigenetic changes: DNA hypomethylation | - Genomic instability | ||||
| - Reduction of MeCP2 (methyl-binding protein) expression | - DNA repair pathways | ||||
| Mice | 137Cs γ-radiation (whole body) | Bone marrow: | - Genetic susceptibility | 69 | |
| - Colony-forming efficiency (CFE) reduction | - Complicated signaling processes | ||||
| - Genomic instability | - Activation of cytokines | ||||
| Mice | X-rays (cerebellum) | Cerebellum: | - Gap junction intercellular communication via connexin43 (Cx43) | 63 | |
| - Upregulation of Cx43 | - Oxidative metabolism | ||||
| - Adenosine triphosphate release | |||||
| Mice (C57BL6) | 60Co γ-radiation (whole body) | Bladder: | - Intracellular calcium levels | 10 | |
| - Clonogenic death induced by the medium harvest from bladder tissues from acutely irradiated mice | - Genetic background dependent RIBE | ||||
| Mice | γ-Radiation (whole body) | Bone marrow: | - Genetic susceptibility | 71 | |
| - Fas ligand (FasL) and TNF-α activation | - Cytokine secretion | ||||
| - Inflammatory pathway of cyclooxygenase (COX-2) | |||||
| Mice | 60Co γ-irradiation (whole body) | Hematopoietic stem cells (HSCs): | - Oxidative stress metabolism | 55 | |
| - Acute cell death | |||||
| - Accelerated proliferation of the bystander HSCs | |||||
| - Increase of intracellular ROS | |||||
| Mice patched1 heterozygous (Ptch1+/–) | X-rays (partial body exposure) | Skin: | - Gap junction intercellular communication | 82 | |
| - Early responses to DNA damage | |||||
| - Apoptosis | |||||
| - Skin basal cell carcinoma | |||||
| Mice (gptdelta transgenic) | X-rays (lower abdominal region) | Lungs: | - COX-2 mediated bystander effects | 72 | |
| - Induction of COX-2 in the non-targeted bronchial epithelial cells | |||||
| - Increased levels of prostaglandin and 8-hydroxydeoxyguanosine | |||||
| - Induction of DNA DSBs | |||||
| - Apoptosis in bystander lung tissues | |||||
| In vitro | |||||
| Normal rat fibroblast cells (208F) | X-rays | Co-culture with (pre-carcinogenic) v-src-transformed rat fibroblast (208Fsrc3): | - In non-irradiated bystander cells: ER stress, cell cycle perturbation, altered interleukin signaling pathways point to fast-released molecules involved in the induction of apoptosis (IIA). | 246 | |
| - Extracellular signaling proteins (focus on TGF-β1) | |||||
| - Gene expression analysis: perturbed cell cycle related- and interleukin-related pathways | |||||
| Normal human fibroblast cells (MRC-5) | Co-culture with (pre-carcinogenic) v-src-transformed rat fibroblast (208Fsrc3): | ||||
| - Extracellular signaling proteins (focus on TGF-β1) | |||||
| Murine primary haematopoietic stem cells from CBA mice | MRC plutonium-238 α-particle source (murine cells) | Co-culture and media transfer experiments: | - Genomic instability may be significantly induced in bystander cells whether or not cells communicate during irradiation | 20 | |
| - Decrease of clonogenic survival, suggesting a major contribution of bystander cell killing | |||||
| - Appearance of delayed aberrations (genomic instability induction) | |||||
| Fish | In vivo | ||||
| Rainbow trout | X-rays | Skin, fin, kidney, spleen, and gill of unirradiated trout incubated with an irradiated one in the same container: | - The irradiated fish released factors into the water that can cause bystander responses in unexposed fish | 80 | |
| - Reduction of clonogenic survival of HPV-G reporter cells | |||||
| Rainbow trout | X-rays | Increased expression of oxidative metabolism and polarity maintenance proteins (hemopexin-like protein, Rho GDP dissociation inhibitor – RhoGDI, pyruvate dehydrogenase – PDH) in gills of nonirradiated trout placed in a container previously occupied by an irradiated one | - Protective proteomic response | 76 | |
| Zebrafish | X-rays | Skin and gill of unirradiated zebrafish incubated with an irradiated one in the same container: | - The irradiated fish released factors into the water that can cause bystander responses in unexposed fish | 78 | |
| - Reduction in HPV-G reporter cell growth of both irradiated and naive fish | |||||
| Zebrafish embryos (Danio rerio) | α-Particles | Unirradiated zebrafish embryos incubated with irradiated embryos in the same agarose plate: | - The irradiated fish released factors into the medium that can cause bystander responses in unexposed zebrafish embryos | 247 | |
| - Increase of cell death signals for both irradiated and naive embryos | |||||
| Zebrafish embryos (Danio rerio) | α-Particles | Unirradiated zebrafish embryos incubated with the irradiated ones in the same container: | - The irradiated fish released factors into the water that can cause bystander responses in unexposed fish | 77 | |
| - Decrease in apoptotic signals in both irradiated and unirradiated bystander embryos | |||||
| Partnered zebrafish embryos | High-dose X-rays | Naïve embryos partnered in the same medium with the irradiated ones: | - Bystander effect at the interorganism level. | ||
| Effect mediated by NO signalling pathways. | 79 | ||||
| - 47% increase of apoptotic signals in bystander embryos compared to control | |||||
| In vitro | |||||
| Embryonic zebrafish fibroblasts (ZF4) | Chronic low dose of 137Cs γ-rays (ZF4) | Non-irradiated cells co-cultured with irradiated cells or with irradiated culture medium: | - A soluble factor contained in the culture medium of irradiated cells is responsible of the DNA DSB appearance in non-irradiated cells, which has a molecular weight higher than 3 kDa and is inactivated by heating | 44 | |
| - DNA DSB occurrence | - Neither secretion of specific proteins, nor the oxidation of these secreted proteins may be responsible for bystander effects, although a slight increase of oxidation was noted | ||||
| - Increase in global methylation of both irradiated and bystander cells | |||||
| Plants | Arabidopsis thaliana embryos | Protons (shoot apical meristem) | Whole organism: | - Long distance effect in whole organisms | 248 |
| - Direct damage to the shoot apical meristem | |||||
| - Inhibition of root hair differentiation | |||||
| - Primary root elongation | |||||
| - Lateral root initiation | |||||
| - Decrease in the accumulation of the reporter GUS gene transcript | |||||
| Arabidopsis thaliana (intact seeds) | Heavy ions (shoot and root apical meristem) | Shoot and root apical meristem: | - Oxidative metabolism disruption and increased generation of ROS | 249 | |
| - Inhibition of postembryonic development (germination, root hair differentiation, primary root elongation, lateral root initiation and survival) of both irradiated and non-irradiated shoot apical meristem and root apical meristem cells | |||||
| Arabidopsis thaliana (whole plant) | α-Particles (whole plant) | Distal primary roots of young seedlings: | - Oxidative metabolism and ROS production | 250 | |
| - Increase in the frequency of homologous recombination (HR) in aerial plants, which occurred in every true leaf during rosette development | |||||
| - Short-term up-regulated expression of the HR-related AtRAD54 gene in non-irradiated aerial plants | |||||
| Other | Daphnia magna | Acute γ-rays | Non-exposed first-generation offspring of irradiated parents: | - Presence of transgenerational effects | 251 |
| - Compromised viability | - Detrimental effects of deleterious mutations induced in the germline of irradiated parents | ||||
| C. elegans | Proton microbeam | Apoptotic germ cell death after microbeam-localized irradiation of pharynx tissue | - CEP-1/p53-dependent germ cell death | 252 | |
| - Bystander effect mediated via MAPK pathways | |||||
While RIBE are well documented at the phenomenological level, the exact determination of the level, impact, types and especially mechanisms behind these multiparameter effects in the non-irradiated cells or tissues remains a great challenge in current radiobiology. The published results are often controversial and the mechanisms of RIBE both in vivo and in vitro still remain incompletely characterized.6 Of note is the complexity of the experimental framework and how it can be interpreted as a function of dose/radiation quality.58 It is likely that different multifactorial pathways are implicated in signaling the response from an irradiated cell to a non-irradiated cell. It is also possible that not all the types of cells will respond similarly to the signaling pathways stimulated. There is experimental evidence mainly from in vitro studies that RIBE may have at least two separate, but not necessarily mutually exclusive mechanisms for the transmission of a bystander signal: either by direct cell–cell communication via gap junctions59,60 or through the release of soluble factors into the targeted cells’ medium.61,62 The gap junction intercellular communication is mediated by a junction between the cells, which consists of many pores (connexons) and is mainly regulated by the expression and phosphorylation of connexin43 protein (Cx43).63–65 The second proposed mechanism of RIBE lies in the ability of irradiated cells to excrete intracellularly-generated low-molecular-weight factors into the culture medium. The signal inducing bystander effects have been shown to include factors such as ROS,66 cytokine(s),67–69 interleukin(s),66,70 cyclooxygenase-2 (COX-2),71–73 tumor necrosis factor α (TNFα)70,74 and transforming growth factor β1 (TGF-β1).49,73,75 The interaction of that signal with the neighboring or a distant cell can lead to variable types of cellular and sub-cellular damage.
For the study of RIBE transduction, several different approaches have been adopted based on the different cellular mechanisms proposed so far. These approaches can be classified into two main categories, in vitro or in vivo, depending on the type of study. On one hand, in vitro methods offer relative control over the parameters of the problem and a relatively simplified system to work with. On the other hand, the in vivo setup is far more realistic and interesting, since it takes into account the response of a living organism. Of note are studies such as,58 which combine in vitro experiments with mathematical modeling, in an attempt to identify how the experimental conditions perturb the system.
Typically the study of in vitro RIBE comprises three distinct methods. One approach is the transfer of medium from cultured irradiated cells to non-irradiated cells.20,44,49,67 This technique allows simultaneously the detection and analysis of secreted RIBE factors in the conditioned medium and the study of their effect on non-irradiated (bystander) cells. Another method is the in vitro co-culture,20,44,56,66 often with some kind of compartmentalization to separate the groups of irradiated and non-irradiated cells. In this case the medium is freely diffusing between compartments. It is not uncommon for both medium transfer and co-culture approaches to be investigated in parallel.20,44 The third technique uses microbeam generated charged particles to irradiate a precisely known number of cells in the population18 and study the effects on non-irradiated neighbors.
A method usually employed in fish studies in vivo is housing non-irradiated fish in containers previously occupied by irradiated fish (medium transfer analog).76 Several researchers used the same container for housing both irradiated and non-irradiated fish (co-culture analog).77–80 In rodents a quite common approach includes partial body exposure and subsequent detection of effects in the shielded (non-irradiated) area.42,43,47,50,63,72,81,82 Other quite diverse methods have also been employed and are summarized in Table 1, along with the techniques already described.
Inflammation: a self-amplifying process
It has been postulated that in the case of tissue injury or invasion from foreign to the host microorganisms an innate immune response is generated.26 Infectious inflammation in the organism is initiated by the detection of microorganism-specific Pathogen Associated Molecular Patterns (PAMPs) such as lipopolysaccharides (LPS), flagellin or peptidoglycan. The process can also be triggered by products of tissue damage, such as extra-cellular matrix degradation products. These molecules are sensed by receptors of the Pattern-Recognition Receptor (PRR) family,83,84 which encompass Toll-like receptors (TLRs), the receptor for advanced glycation end-products (RAGE) and C-type lectin receptors (CLRs), among others. These receptors are mostly expressed by various subsets of immune cells, but can also be found on other cell types such as keratinocytes85–87 and endothelial cells.88,89 Some of them, for example TLR3 and TLR9, are expressed intracellularly. TLRs detect, among others, microbacterial-derived motifs such as lipopolysaccharide (TLR2), peptidoglycan (TLR4), flagellin (TLR5), CpG rich DNA (TLR9) and double stranded RNA (TLR3). The binding of their cognate ligands triggers a signaling cascade, culminating with the activation of NF-κβ and/or the interferon responsive factors. Importantly, there is also a production of inflammatory mediators such as pro-inflammatory cytokines and chemokines, which take over the regulation of the inflammatory reaction. Indeed, inflammation involves temporal changes in the local tissue environment. Local inflammatory processes are initiated by tissue resident innate immune cells, such as macrophages and dendritic cells. Development and amplification of the inflammatory reaction depends strongly on the mobilization, recruitment and activation of additional blood-borne myeloid cells in response to soluble factors produced by these cells. In turn, this process increases the expression of adhesion molecules (or their receptors) on endothelial cells.90,91 This up-regulation flags the site of injury and allows the directional recruitment of circulating leukocytes, including monocytes and neutrophils into the affected tissue. Infiltrating monocytes then differentiate into effector macrophages and dendritic cells that secrete pro- and anti-inflammatory mediators to orchestrate the development and eventual termination of the response. Interestingly, accumulating evidence in mice and humans support the notion that, like the B and T cells of the adaptive immune system, natural killer (NK) cells can be educated during development, retain antigen-specific receptors, go through clonal expansion during infection and generate long-lived memory cells.92 Neutrophils, macrophages and dendritic cells are phagocytes that can directly engulf invading pathogens and destroy them in phagolysosomes, where they produce highly genotoxic reactive oxygen and nitrogen species. In most cases, these steps are sufficient to contain the infection. However, if this first line of defense is inefficient, dendritic cells will prompt T lymphocytes to mount an antigen specific response directed against the pathogen to facilitate its eradication. The cooperation between the innate and adaptive branches of the immune system allows efficient protection to the organism. In general, it is accepted that the innate immune response system holds no memory. But recent advances indicate that innate immune cells can retain an intrinsic memory of prior stimulation, a function until now ascribed only to antigen-specific adaptive immune cells.93 Importantly, inflammation is a self-limiting process. The recruited macrophages exhibit a high level of functional plasticity and evolve from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype that will recruit cells able to promote wound healing and stop the reaction.94,95 The profile of secreted mediators evolves during the reaction to signal its resolution.96 If for any reason the reaction does not subside, it will transition to a persistent, chronic inflammatory state. Such chronic inflammation is known to promote the development of several diseases including cancer, cardiovascular and neurodegenerative diseases.97
In recent years it has become clear that innate immune system components can also mount an inflammatory reaction to protect the organism against injuries resulting from physical trauma such as heat exposure,98 exposure to ionizing99 or UV radiation,100 ischemia reperfusion injury101 or contact with nanoparticles.102,103 This reaction was termed sterile inflammation, in contrast to the classical infectious inflammation. Several pathways can lead to the induction of a sterile inflammatory response following radiation exposure. In addition to genotoxic stress, irradiation induces tissue and cell damage, which may result in a transient alteration or even the loss of cytoplasmic membrane integrity. Thus, molecules usually expressed or found only inside the cells as adenosine triphosphate (ATP) (high-mobility group protein B1 (HMGB1), DNA, uric acid) may leak from damaged cells. They are also released from the cells as a consequence of un-programmed cell death such as necrosis. Therefore, their presence in the extra-cellular milieu denotes the occurrence of a severe stress that may endanger tissue function. Accordingly, they are referred to as “danger signals” or, by analogy with the microbial products able to induce an infectious inflammation, damage associated molecular patterns (DAMPs) as already mentioned above. The “danger theory”,104 or “injury hypothesis”,101 postulates that the immune system makes no discrimination between self/non-self immunogenic stimuli. This means that the immune system responds to tissue stress or destruction signals, regardless of their origin. In this sense, PAMPs are a subgroup of DAMPs and can be referred to as ‘exogenous DAMPs’. To further emphasize this view, DAMPs can be sensed by neighboring tissue-resident innate immune cells through some of the same PRRs that recognize and detect PAMPs. In contrast to PAMPs, which are predominantly recognized by a single receptor, DAMPs can be recognized by and activate a large panel of receptors. For example HMGB1, which is released from necrotic cells, is recognized at least by TLR2, TLR4, TLR9 and RAGE. Conversely, TLRs and RAGE can recognize many different endogenous ligands.105,106 There is a lot of promiscuity at the level of DAMP/PRR interactions. Importantly, as myeloid cells express an array of different PRRs, their response will depend on the combined activation of all the engaged PRRs. Hence, PAMPs and DAMPs will evoke qualitatively and quantitatively different responses on the same cells. This is for example illustrated by the different dynamics of secretion of inflammatory cytokines like interleukin-1β (IL-1β) and IL-6 and chemokines like IL-8 and MIP-1α following stimulation in vitro of human monocytes with HMGB1 or LPS.107 These differences may translate into the recruitment of different immune cell subsets, and therefore shape the ensuing inflammatory response. Uric acid released from dead/dying cells following exposure of mice to ionizing radiation is also a potent inducer of the inflammasome complexes (NLRs), like NLR protein family NLRP1, NLRP3, NLRP6 etc. The inflammasome is the molecular complex responsible for the processing of IL-1β before its secretion, as revealed by the proteolytic activation of caspase 1 in irradiated mice.108 The activation of one of the most well-known inflammasomes, NLRP3, has been associated with various danger signals (PAMPs, DAMPs),26 linked to innate and adaptive immune responses against dying tumor cells109 and acceleration of radiation-induced lung inflammation and fibrosis in mice.110 To this direction, it has been shown that low doses of X-rays (0.5 or 0.7 Gy) induce an anti-inflammatory phenotype of activated macrophages by lowering the amount of secreted IL-1beta in a NF-kappaB dependent manner.111 The combination of HMGB1 and uric acid crystal signaling will add another layer of complexity to the response, and all the cytokines secreted upon DAMP detection will participate in the control of the inflammatory reaction. Hence, the development of sterile inflammation will depend on the combination of initiating signals, which most probably depend on the nature of the affected tissue and cells.
Beside the release of extracellular DAMPs, cellular stress can also be detected and evoke an inflammatory response from within the cells, by the presence of nucleic acids in the cytosol, either of an endogenous or exogenous origin. Indeed, although it is commonly thought that DNA is confined to the nucleus and the mitochondria, DNA replication byproducts are present in the cytosol, where they are rapidly degraded by three prime repair exonuclease 1 (TREX1),112 which digests single- and double-stranded DNA. If the degradation is hampered, these DNA molecules can be recognized by various cytoplasmic DNA sensors such as AIM (absent in melanoma), MRE11 (meiotic recombination 11), RAD50, and DAI (DNA-dependent activator of IFN-regulatory factors). In turn, the receptors stimulate the production of cytokines like interferon gamma (IFNγ) through stimulator of interferon genes (STING) and IL-1β through inflammasome activation.113,114 Interestingly, TREX1 was shown to be less efficient on oxidized DNA, as the latter contains 8-oxoG bases which are not readily processed by this enzyme.115 Hence, after radiation exposure, especially low-dose, or in situations of oxidative stress, accumulation of nuclear or mitochondrial oxidized DNA molecules in the cytoplasm will contribute to the development of a sterile inflammatory reaction through the secretion of inflammatory cytokines. Regarding fragmented mtDNA, which may be released due to apoptotic, necrotic, and necroptotic cell death, it has been shown that it can also act as a DAMP.116 In addition, exogenous DNA can also act as a pro-inflammatory danger signal.117 Oxidized DNA released following the death of cells exposed to oxidative stress can be delivered to the endosomal compartment of phagocytes. There it will be recognized by and activate TLR9, through association with HMGB1 and recognition by RAGE.118 It is not yet known whether endogenous RNA can also act as a danger signal following IR exposure, but it was recently shown that UVB-damaged noncoding RNA can be internalized by keratinocytes and induce the secretion of inflammatory cytokines through TLR3 activation.119 Interestingly, it was shown that several DNA damaging agents, including the radiomimetic etoposide, were also able to activate interferon regulatory factor 3 (IRF3), one of the downstream effectors of TLR3 activation.120 Whether this activation requires the recognition of damaged cellular RNA is currently under investigation.
In addition to these pathways of induction via DAMP-mediated PRR activation, several lines of evidence indicate that radiation exposure can also activate inflammation more directly, through the transactivation of the transcription of pro-inflammatory genes. Radiation exposure generates DNA lesions which trigger the activation of the DNA damage response and the cell cycle checkpoints to orchestrate the cellular response and cell fate decision. The ataxia telangiectasia mutated (ATM) and p53 proteins play key roles in these events. The recruitment and activation of ATM to double strand break sites by the Mre11/Rad50/NBS1 complex initiates a complex cascade of phosphorylation that leads, among others, to the stabilization and activation of the tumor suppressor p53, which can then trans-activate the expression of its target genes. The outcome of p53 activation depends on the extent of the damage and the cellular context: the damaged cells will either make a pause in their cell cycle progression to allow DNA repair before resuming their functions or enter programmed cell death or senescence to avoid the transmission of mutation that could eventually lead to cell transformation.121 Interestingly, among the numerous genes trans-activated by p53 are several inflammatory genes or genes coding for proteins involved in the inflammatory reaction, providing a direct link between irradiation, the DNA damage response, p53 and inflammation.122 This is for example the case of the genes coding for some adhesion molecules like intercellular adhesion molecule-1 (ICAM-1) and cluster of differentiation 31 (CD31), which play a role in the marking of the inflammation site and the transmigration of myeloid cells from the circulation to the inflamed tissue.123 The induction of CD31 expression on irradiated human umbilical cord vein endothelial cells (HUVEC) results in an increased transmigration of leukocytes through HUVEC monolayers.124 ICAM-1 and CD31, but not vascular cell adhesion molecule-1 (VCAM-1) expression is induced on human dermal microvasculature endothelial cells (HDMEC) following radiation exposure. Interestingly, when stimulated with TNF-α these cells are able to express high levels of VCAM-1.125 This differential response of HDMEC to radiation and an inflammatory cytokine suggest that different subsets of leukocytes may be recruited in the inflamed tissue according to the signals received.126 This further illustrates the specificity of the different inducers of inflammation. Interestingly, it was later found that, in human primary fibroblasts, induction of ICAM-1 expression following irradiation is controlled by p53.114
Beside this role in the events controlling inflammatory cell migration, p53 was also shown to directly contribute to the ability of immune cells to sense danger signals in their environment. It has also been found that it regulates the expression of pro-inflammatory cytokines in these cells, together with NF-κB. Exposure to IR, or treatment with the genotoxic agents 5 fluorouracil or doxorubicin induced the up-regulation of TLR gene expression in human T lymphoblasts.127 This process most likely takes place through direct p53-dependent transactivation of TLR gene expression, as these genes were found to contain functional p53 responsive elements.122 This modulation was largely prevented by the pre-incubation with the p53 inhibitor pifithrin-α, and there was a large degree of inter-individual variation.127 The level of TLR protein expression was also markedly induced. Consequently, IL-1b and IL-8 transcription was enhanced in isolated T cells following stimulation with a TLR agonist. The transcriptional regulation of TLR genes after p53 stabilization or doxorubicin treatment was observed in alveolar macrophages as well, but with a different pattern: not all the TLR genes were induced, and for those that were, the level of induction was lower. Another striking difference was that alveolar macrophages, but not T lymphocytes, responded to p53 stabilization by a strong up-regulation of pro-inflammatory cytokine and chemokine genes.128 This response was also observed in monocytes and monocyte-derived macrophages, but not in neutrophils and primary macrophages. Interestingly, in monocyte-derived macrophages this response concerned only about 20% of the genes up-regulated in response to LPS, a bacterial cell major wall component used to mimic infectious inflammation, and the levels of induction of these common genes were very different.
The overall picture that emerges from these ex vivo experiments is the complexity of the regulatory networks governing the cellular responses and the onset of sterile inflammation after IR and other genotoxic agents. p53 activation will raise the sensitivity of circulating T lymphocytes or tissue macrophages to DAMPs. At the same time it exposes them to a rich cocktail of inflammatory cytokines and chemokines produced by monocytes and macrophages. This complexity is further illustrated by the gene set enrichment analysis of gene expression in whole blood irradiated ex vivo, by El-Saghire et al.129 Again, this study clearly showed the involvement of immune signaling pathways following radiation exposure. In addition, it pointed out a very interesting dose-dependent response. Exposure to low dose radiation (0.05 Gy) mainly involved immune signaling pathways, whereas the transcriptional response at higher dose (1 Gy) was dominated by the p53/DNA damage-dependent response. This dose dependency was later confirmed by PCR, ELISA and western blot analysis on isolated human monocytes irradiated ex vivo.130 More importantly, the involvement of immune signaling networks in the response of blood cells to radiation was also confirmed in vivo by the analysis of blood from radiotherapy-treated prostate cancer patients,131 who received an average total body dose per fraction of 30.97 ± 8.12 mGy. This low-dose radiation exposure was found to activate several aspects of immune signaling including TLR and cytokine signaling and interferon secretion that could result from the sensing of and reaction with DAMPs generated by stress cells, in addition to a direct effect of p53.
In any case, these studies show that radiation exposure results in the induction of numerous events related to inflammation and immune defense of the organism, including a direct induction of pro-inflammatory cytokines and chemokines by monocytes and macrophages. As the nature and relative abundance of the inflammatory mediators produced in this context are clearly different from that elicited in response to bacterial stimulus,128 the ensuing inflammatory reaction will undoubtedly be different. It could be said that the inflammatory reaction will be tailored to the nature of the initiating events, DNA damage or pathogenic infection. Importantly, these studies address only the onset of the sterile inflammatory reaction, while it is not known if and how the profile of secreted inflammatory mediators will switch from pro- to anti-inflammatory94,95 to signal the resolution and cessation of this process. This point is extremely important, as inflammation resolution is essential to ensure a return to normal homeostasis and terminate the stress response to avoid chronicity. Since for example radiation-induced fibrosis is primarily the result of an ongoing chronic inflammation,132 it might also be the case that radiation-induced sterile inflammation does not resolve at all. Thus one might speculate that one mechanism for the development of radiation-induced fibrosis is a genetic damage in the molecular pathways responsible for inflammation resolution.
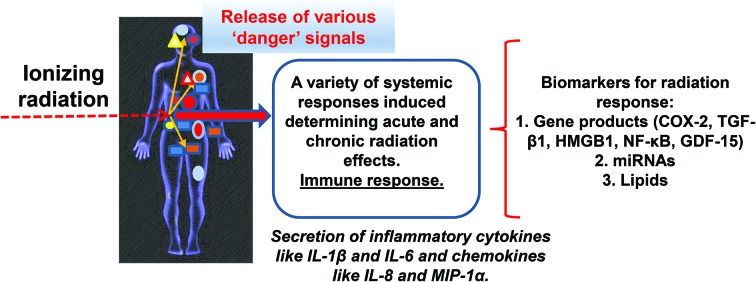
This response to local trauma is detectable at the systemic level in the blood by an increased level of circulatory cytokines and the activation of immune cells. The effects of radiation exposure are therefore amplified and become more easily measurable. Activated p53 directly induces inflammatory mediator expression in circulating myeloid cells128 and genotoxic stress-induced ATM phosphorylation modulates NF-κB activation and cytokine gene expression.133 Hence, activation of the ATM/p53 DNA damage checkpoints, together with the sensing of radiation-induced DAMPs will both contribute to the initiation of a specific inflammatory process following radiation exposure. The pivotal role of ATM as a connecting hub between DDR and immune response is further emphasized by Pateras et al.134 One aspect that has to be mentioned at this point is that certain activation markers (such as NKG2D on NK cells) are directly upregulated by cellular DNA damage in general. Accumulating evidence suggested that DDR may alert the immune system to the presence of potentially dangerous cells by upregulating the expression of ligands that can induce the activation of innate and adaptive immune cells.135,136 Therefore, we believe that we should combine biomarkers of immune activation and biomarkers of stress response to establish a specific signature of radiation exposure.
Insights from the “genomics era”
The fact that IR can influence the expression of certain genes at the mRNA level was known long before the onset of the “genomics era”; however due to technical limitations, gene expression studies were restricted to certain genes only (mostly oncogenes and tumor suppressor genes). Once large-scale gene expression arrays had become widespread, extensive gene expression profile analyses were carried out after various irradiation types, doses and dose rates in a wide range of biological systems. The analyses were performed both after in vitro and in vivo irradiation, with the scope to identify cellular mechanisms and molecular pathways responsible for the direct and/or non-targeted effects of radiation. These studies proved that transcriptional profiling is a sensitive biomarker of radiation exposure both after low and high doses.137
Genes most frequently altered by irradiation are those responsible for genotoxic and physiological stress response, including DNA damage sensing and repair as well as inflammation and immune response.138–140 While all these pathways are generally shown to be altered acutely after irradiation, expressional alterations in genes linked to DNA damage response are less characteristic at later time points. On the other hand, inflammatory responses with or without signs of a chronic oxidative stress, as well as immune response alterations, become more preponderant. In addition, different studies indicate that in vivo experiments generally show a higher level of alterations in the expression profile of genes related to inflammation and immune response compared to studies conducted in vitro, highlighting the importance of micro and macro environmental factors in modulating the direct cellular effects of IR.141
Cellular responses due to direct radiation exposure can be mediated by multiple signaling pathways initiated either directly by radiation-induced DNA damage or at the cell membrane or within the cytoplasm. These signaling pathways converge within the nucleus to the activation of a restricted number of transcription factors; the most important ones are p53, AP-1, early growth response factor 1 (Egr1) and NF-κB, as mentioned above. The interplay between the downstream signaling of these transcription factors is an important determinant of the cell fate after IR. While activation of the p53 pathway has a pro-apoptotic effect by upregulating genes directly involved in the initiation of apoptosis, NF-κB, in contrast has anti-apoptotic function by competing with p53 for p300/CREB-binding protein transcriptional coactivator complexes.142 On the other hand, NF-κB and p53 cooperate in radiation-induced cell cycle arrest. It was also shown that certain NF-κB-regulated genes (such as cyclin B1, cyclin D, and human inhibitor of apoptosis-HIAP) may play a role in p53-independent radiation resistance.138 Despite the fact that the NF-κB pathway can be activated by ROS, the regulated downstream targets depend on the factors that elicited ROS production. Thus, distinct genes are regulated by ROS produced after IR, UV, cytotoxic drugs or physiological T cell activation, indicating parallel signaling pathways working in a concerted manner in cells after NF-κB activation. The mechanism for the activation of these alternative pathways is not entirely elucidated yet. However, there is evidence indicating that different physiological or pathological stress factors activate different transcriptional regulators of NF-κB. For instance, it was shown that RELB, a modifier of NF-κB, exhibited upregulated subunit binding affinity only by IR.143,144 The upregulation was much stronger after in vivo irradiation than after in vitro irradiation.145 Many of the immune response and inflammatory genes, which are up- or down-regulated by IR, are usually NF-κB downstream target genes. For a comprehensive review of the impact of radiation on NF-κB function please see ref. 146. Similar to NF-κB, AP-1 and Egr1 are also redox-sensitive transcription factors and play a major role in the radiation-induced upregulation of inflammatory cytokine genes. The importance of NF-κB in mediating irradiation-induced inflammatory responses has been recently shown by Manna et al., who, by selectively inhibiting NF-κB activity, could successfully reverse IR-induced stress response and inflammation development by downregulating the expression of CRP (C reactive protein), MCP-1 (monocyte chemotactic protein) and iNOS2 (inducible nitric oxide synthase 2).147
Radiation-induced cytokine gene upregulation is often a biphasic process. Several inflammation-related cytokine genes are characteristically immediate early response genes that are activated within minutes to hours after irradiation. Such cytokines are G-CSF, IFNs, IL-1, IL-6, IL-8, and TNFα. Early cytokine production can be considered as a part of danger signaling in response to radiation and it is the main source of further ROS production. This secondary ROS production is in turn partly responsible for the late increase in the expression of inflammatory cytokines.105
The effect of high doses of irradiation (2 Gy or above) on gene expression profiles is relatively well characterized, although the overall majority of the studies evaluated immediate or acute effects of irradiation, while data regarding long-term expression studies are relatively rare. One category of studies focuses on gene expression profile changes of various tumor cells in order to predict sensitivity of the particular tumor to radiotherapy,141,144,148 while another category explores expression profile analysis of healthy cells in order to predict variations in individual radiosensitivity and to estimate the probability of radiation-induced side effects.149–154 The most studied cell types are peripheral blood mononuclear cells and fibroblasts;149–151 the former ones are chosen because they could reflect overall radiation damage due to their increased radiosensitivity, while the latter ones are responsible for the most frequent radiation-induced late damage and are considered a good model of senescence. Relatively few studies explore radiation-induced expression profile alterations in other tissues, despite the fact that they are also characteristic targets for radiation-induced late side effects.152–154 A common feature of these experiments is the great variability in the radiation-induced gene expression pattern not only among the different cell- or tissue types, but also among the same cells/tissues of different individuals.155 Despite this variability to radiation-response, several papers could identify a core gene set that responded in a consistent way to irradiation.137
Schmidt-Ullrich et al. were among the first groups showing that radiation influenced the expression level of certain genes involved in inflammation. They could demonstrate that both single (2–50 Gy) and fractionated irradiation (60 Gy cumulative dose) upregulated the expression of the epidermal growth factor receptor (EGFR) and transforming growth factor alpha (TGFα), while downregulated the expression of the estrogen receptor (ER) in a murine mammary carcinoma cell line.156 Khodarev et al.145 investigated dose- and time-dependent changes in the gene expression profile of the U87 glioma cell line and HEL fibroblasts after both in vitro and in vivo irradiation of nude mice transplanted with the U87 tumor cell line. By analyzing the expression characteristics of 4132 unique human genes, they could show both cell-type specific and cell-type independent changes in the expression of certain genes after irradiation. Genes responding to irradiation could be classified into nine clusters out of which the organism defense and homeostasis cluster, cell–cell interactions and cellular defense cluster as well as oxidative stress and apoptosis cluster contained several immune response and inflammation-related genes. The β2-microglobulin gene was one of the 15 genes that were equally affected by irradiation in both investigated cell types and also after in vivo irradiation. Although its upregulation was moderate-to-medium and not dose-dependent, its consistent alteration to IR most probably indicated an elevated and stabilized MHC-I complex formation, with enhanced capacity to present cellular antigens derived from IR-induced protein degradation. Another interesting observation of the above mentioned study was that certain genes showed much stronger upregulation under in vivo irradiation than after in vitro irradiation, which most probably indicated the relevance of the microenvironment in modulating IR-induced gene expression. Several of these genes were immune- and inflammation-related, such as ICAM, IL-10 receptor, and RANTES.
Expression-profile analysis of irradiated fibroblasts was evaluated by several research groups mainly with the purpose of identifying mechanisms/pathways responsible for the development of radiation-induced fibrosis and key genes that are affected in this process. Kis et al.150 evaluated radiation-induced immediate-early transcriptional alterations (2 hours after irradiation) in primary human fibroblasts and could identify 30 consensus radiation-response genes. Seven of these genes were commonly identified in other cell/tissue types. Radiation-responding genes could be classified into 9 functional clusters and the authors identified 3 genes that previously were not known to be radiation-responsive (TP53INP1, IER5 and GDF15). Interestingly the only gene directly linked to inflammation and/or immune response identified in this study was GDF-15, which is most probably due to the very early time point after irradiation when expression analysis was performed.150 In another study by Johnston et al. late gene expression profiles were analyzed in pulmonary fibroblasts derived from mouse strains sensitive and resistant to radiation-induced fibrosis. They could show the massive upregulation of a panel of chemokines and chemokine receptors in these cells 26 weeks after irradiation, proving the role of radiation-induced persistent inflammation in the development of fibrosis.149
A recent study by Aryankalayil et al. investigated radiotherapy-induced immune gene signatures in human prostate cancer cells with different p53 status.157 Their data showed that multifractionated doses could activate immune response genes more robustly than single-dose treatment, with a relatively larger number of immune genes upregulated in p53-null cells compared to wild-type or p53 mutant cells. Although both single dose (1 × 10 Gy) and multifractionated dose (10 × 1 Gy) altered DAMPs and cytokine levels, the effect was more pronounced with multifractionated treatment. Thirty-one genes were modulated more than two-fold 24 hours after multifractionated radiation, with seven genes showing strong (10–34 fold) upregulation. These genes encoded proteins involved in sensing viral/pathogen infection to trigger an immune response. Single dose irradiation induced a max 3-fold upregulation of five genes. Two genes overlapped between the two irradiation modalities, while the other 3 genes were involved in inflammatory responses by T cells and macrophages.157 This study clearly demonstrated that the different irradiation modalities and doses not only induced a different activation level of the genes, but also the pattern of activated genes and thus the initiated immune and inflammatory mechanisms differed. Very similar results were obtained by Palayoor et al. in human coronary artery endothelial cells.158 They found that multifractionated irradiation (5 × 2 Gy) led to more pronounced modulation of immune response and inflammatory genes than single dose (1 × 10 Gy) irradiation. The majority of the genes were upregulated. These genes were adhesion molecules (ICAM1, VCAM1), chemokines (CXCL10, CXCL11, CXCL12, CXCL16, CCL2, CCL5, CCL20, CCL23), chemokine receptors (CXCR4 and CXCR7), cytokines (IFNE, IFNA4, IL1A, IL1β, IL15, TGFB1, and TGFB2), IFN-induced signalling factors, diverse integrins and genes regulating the MHC-I molecules. The authors also investigated miRNAs regulating the above mentioned genes and found a strict inverse correlation between the level of mRNAs and their corresponding regulatory miRNAs.158 These observations underline the importance of miRNAs in regulating radiation-induced immune and inflammatory pathways. The importance of miRNAs in the regulation of low-dose induced inflammatory and immune responses was also shown by Luzhna et al.159 Slightly different results have been recently published by Paul et al., who showed that after ex vivo irradiation of human peripheral blood cells with doses ranging between 0.5 and 8 Gy of γ-radiation, the majority of immune-related genes were heavily down-regulated 48 hours after irradiation. The pathway most sensibly affected was NK cell-mediated immune response.160
Recently Georgakilas et al. performed a meticulous meta-analysis of all relevant literature data and identified 24 common genes altered under inflammatory conditions, immune reactions and after irradiation in both normal and cancerous cells. These gene signatures are the most probable molecular links between irradiation, inflammation and immune response. They appear to be the main inductors and maintainers of radiation induced inflammatory reactions in both healthy and tumor tissues. A relatively high number of differentially expressed genes were identified in healthy and tumor tissues linked to irradiation, inflammation and immune response. Most probably this different molecular signature is responsible for the distinct temporal evolution and long-term pathological consequences of radiation-induced inflammation in cancer versus healthy tissue.161
In contrast to high doses of irradiation, low dose effects on gene expression signatures are much less understood. Wyrobek et al. have recently performed a comparative analysis of low-dose irradiation induced gene expression in two human lymphoblastoid cell lines and their results were compared with other similar studies performed in other cell/tissue types.153 These common comparative studies led to several interesting conclusions. It was clearly shown that in human lymphoblastoid cells doses as low as 10 mGy or even below were sufficient to induce gene expression alterations with the majority of genes being upregulated. Fifty-two (52) out of the eighty-one (81) consensus genes altered after low dose irradiation were assigned to signal transduction pathways associated with immune response and cell signaling. Most of the affected signal transduction pathway genes were members of several major signaling networks such as the p38 MAPK (mitogen activated protein kinases), SAPK/JNK (stress activated protein kinases/c-Jun N-terminal kinases), JAK/STAT (Janus kinase/signal transducers and activators of transcription), JAK/AKT, IL-2, IL-4, and NF-κB. Altogether the 81 consensus genes could be classified into two major networks, broadly associated with the maintenance of cellular homeostasis, signal transduction pathways and immune response. If low dose induced expression profile analysis in lymphoblastoid cells was compared with low-dose irradiation-induced gene expression signatures in other cell/tissue types (HUVEC, primary human keratinocytes of mouse brain) several common networks could be identified, such as the MYC, FOS, TP53 and MYCN. These data suggest that functions associated with the above networks are conserved in the low-dose damage response among the different biological systems. However, a set of cell-type specific low-dose responsive genes could also be identified, which were mostly linked to lymphocyte functions. It was proven that many of the low-dose responding genes showed a non-linear dose–response relationship.153,162 A comparison of low-dose and high-dose responses indicated that although certain high-dose responsive genes such as CDKN1A, RAD54 and GADD45A were induced after low doses as well, still, most of the affected genes after low dose irradiation were different from the genes typically affected by high dose irradiation.
There are relatively few studies assessing low-dose irradiation induced gene expression alterations in vivo. Lowe et al. investigated early gene expression changes in the brain tissue after low dose irradiation and they could show a high degree of concordance in transcriptional responses of low-dose irradiated mouse brain, non-irradiated healthy human aging brain and brain tissue from patients with Alzheimer's disease. High dose irradiation, on the other hand did not show this correlation.154 Similarly, different gene expression profiles were identified in the mouse liver163 and thymus164 after low and high doses. Luzhna et al. studied low-dose irradiation effects on gene expression signatures of the mammary gland and, surprisingly, they could show that high-energy low dose X-irradiation was the only irradiation modality that led to large-scale gene expression alterations (567 genes in total). On the other hand, high energy high dose or low energy high dose irradiation had only minor effects on gene expression signatures. The majority of the altered genes could be classified into different immunological pathways such as antigen processing and presentation (CD74, CD8α, the interferon gamma inducible protein Ifi30), natural killer (NK) cell-mediated cytotoxicity pathway (CD247, ICAM1, ICAM2), B and T cell receptor signaling, chemokine signaling, and genes related to inflammation (phagocytosis, leukocyte activation and transendothelial migration). The authors concluded that the overall activation of diverse immune responses, including inflammatory pathways after low dose irradiation, might indicate anti-tumor protection and eradication of damaged cells.159 A slightly different dose–response of gene expression was observed in Balb/c mice internally contaminated with low doses of 131I. In this case gene expression was not dose-dependent but rather tissue-specific, with the exception of immune-related processes, which were equally affected by irradiation in all the studied tissues.165
GDF-15, as a potential novel biomarker of radiation exposure
GDF-15 is a divergent member of the TGF-β family also known as MIC-1 (macrophage inhibitory cytokine 1), NAG-1 (non-steroidal anti-inflammatory drug [NSAID]-activated gene-1), PLAB (placental BMP, PTGF-β or PDF) with multiple roles. The gene is a downstream target of p53 and its mRNA expression can be induced by TNF-α or IL-6. Although GDF-15 was identified many years ago, its specific receptor and signaling pathways are still uncertain. Thus its principal functions remain to be elucidated. It has been reported to interfere with inflammatory and immune reactions and to exhibit immune suppressive functions by inhibiting proliferation of peripheral blood mononuclear cells and inducing the expression of Foxp3 in CD4+and CD25+ regulatory T cells.165
GDF-15 is considered a general marker of disease, and it is associated with all-cause mortality. This was proven by two recent studies by Wiklund et al.166 and Daniels et al.167 The former ones followed more than 800 Swedish male adults, while the latter group followed community-dwelling older persons for more than 11 years. Their common conclusion was that serum GDF-15 levels predicted overall mortality irrespective of the cause of mortality and GDF-15 levels correlated with the time of death. Increased GDF-15 expression is a common feature of many cancers. Serum GDF-15 levels are frequently elevated in metastatic tumors, often in parallel with the stage and extent of disease.168,169 GDF-15 has been reported to play a similar role to TGF-β in carcinogenesis. It is antitumorigenic in the early phase of tumor development and protumorigenic later, contributing to the malignant progression of tumors and promoting the ability of tumor cells to invade their surrounding tissues. Another important disease category where GDF-15 is considered as a potential biomarker is cardiovascular diseases, especially of ischemic nature. It has been shown to be both a predictive marker, with its elevated serum levels indicating an increased risk for the onset of cardiovascular events,170 and also a prognostic marker, since its elevated serum levels constitute a bad prognostic factor for patients with chronic heart failure.171
In view of all the above data, it is not surprising that GDF-15 is modulated by radiation as well. Kis et al. were among the first to identify GDF-15 as a new radiation-consensus gene upregulated in primary human fibroblasts both after low and high doses of irradiation.150 In a later study, a dose-dependent increase in GDF-15 gene expression was shown between 0.1 and 2 Gy.172 This observation has been lately further strengthened by Chauhan et al.,173 who also showed that GDF-15 was a radiation-responsive gene in human lung fibroblasts after α-irradiation, which as a high-LET radiation is expected to induce primarily clustered DNA damage.174
Sándor et al. showed that GDF-15 was responsible for fibroblast radioresistance, since silencing the gene rendered the cells more radiation-sensitive.175 The same group investigated potential downstream targets of GDF-15 and showed that it could regulate some TP53 target genes such as TP53INP4, but it did not affect CDKN1A or GADD45A. Moreover, it was shown that one possible mechanism by which GDF-15 exerted its radioprotective effect is by abrogating the radiation induced early G2 block.
Recently it has been suggested to be a predictive marker in oral squamous cell carcinoma as a response to radiotherapy. Schiegnitz et al. demonstrated that oral squamous cell carcinoma cell lines had an increased basal level of GDF-15 compared to normal gingival cells, which grew further after irradiation, indicating its role in radioresistance.176 The authors hypothesized that the anti-apoptotic effect of GDF-15 was one possible mechanism responsible for GDF-15 mediated radioresistance. Similar findings were reported in an earlier study by Okazaki et al.177 in human colon cancer cells and by Lin et al., who identified 6 potential biomarkers suitable for predicting radiation-sensitivity of head and neck tumors, including GDF-15.178 In conclusion, we can say that GDF-15 is a promising new inflammation-related biomarker of radiation-response and radiation sensitivity of both normal and tumor cells, which was identified through expression-profile analysis in various experimental setups.
Systemic immune effects in radiotherapy
Anti-tumor therapies aim to stop the proliferation of the tumor cells, kill them and ideally induce systemic anti-tumor immunity.179 This results not only in the deletion of the primary tumor but also prevents metastasis and recurrences. An obvious prerequisite for initiating an immune response against the tumor is that the immune cells recognize the tumor cells. Only activated immune cells, which recognize the tumor as foreign, can fight the tumor.180
However, tumors can also efficiently escape immune surveillance when being established and during development. The tumor immune editing is divided into three steps.181 The first step is the elimination phase including the surveillance of the tumor by immune cells. It starts with the recruitment of innate immune cells such as natural killer (NK) cells, NKT cells, γδ T cells, macrophages and dendritic cells (DCs).182 In particular NK cells kill tumor cells independent of MHC molecules, which results in the release of tumor specific antigens. These antigens are phagocytosed and consecutively processed by DCs. The latter migrate to the nearest lymph node while presenting or cross-presenting the tumor antigen via MHC-II or MHC-I, respectively. This finally leads to the activation of naïve CD4+ and CD8+ T cells. These activated T cells migrate back and kill the tumor cells.183 This immune surveillance results in destruction of premalignant cells so that tumors do not develop. But the anti-tumor immune response might be too weak to prevent cancerogenesis. Consecutively, the system enters an equilibrium phase which can last for years. In this phase, cells and mediators of the adaptive immune system prevent the outgrowth of tumor cells but cannot destroy them completely. The main factors are CD4+ and CD8+ T cells and the cytokines INFγ and IL-2.
In the third phase, the escape phase, tumor cells begin to expand in an uncontrolled manner.184 The escape may result from the enrichment of immunosuppressive immune cells, mediators and cytokines in the tumor microenvironment. Galectin-1, indoleamine 1,2-dioxygenase (IDO), immunosuppressive cytokines such as IL-10 and TGFβ, as well as regulatory T cells (Tregs) or myeloid derived suppressor cells (MDSCs) are part of it.185 Further, secreted products of the tumor can also obviate differentiation, maturation and migration of DCs and therefore their effect or function.186 Of note is that tumor cells do not only escape from the innate and adaptive immune system, but also change processing and presentation of tumor associated antigens by altering expressions of MHC-I or HLA molecules.187–189 Additionally, vesicles with tumor antigens – tumor exosomes – are able to induce tolerance against the tumor.190 Radiation-induced systemic effects are partially mediated by such exosomes.191
Besides immune cells in the tumor microenvironment, the infiltrating ones have an impact on the prediction of radiochemotherapy treated tumors.192 Hereby the distinct phenotype of the immune cells assumes a role. Tumor associated macrophages (TAMs) e.g. are mainly “anti-inflammatory” M2 macrophages which induce TH2 (regulatory) responses through the production of prostaglandin E2, TGFβ and IL-10. In contrast, “pro-inflammatory” M1 macrophages induce TH1 (effector) responses by production of IL-12 and TNFα.193 Primary tumors with lymphogenic metastases have been demonstrated to show a significantly increased count of M2 macrophages compared to non-metastasized tumors.194 The impact of irradiation on macrophage polarization has been scarcely examined. However, hints exist that M2 macrophages are less prominent in irradiated lesions.195 Different mediators have an impact on the phenotype of macrophages. TNF-α, LPS and INFγ lead to a shift towards M1 macrophages, while IL-4, IL-10 and TGFβ favor M2 macrophages.196 Irradiation of tumors with a single dose of 2 Gy alters the phenotype of macrophages from M2 towards M1 depending on iNOS and therefore on nitric oxide production. This affects the endothelium of the vasculature and consecutively increases T cell infiltration into the tumor.197 Very recent data revealed that eosinophils also guided T cells into the tumor, which resulted in tumor eradication. Again macrophage polarization and consecutive normalization of the tumor vasculature was mandatory to foster tumor rejection.198 Inflammatory mediators originating from immune cells thus have an impact on other immune cells, the tumor vasculature, and also on the tumor itself. It has become obvious that ionizing radiation modulates inflammatory events both in benign inflammatory and in malignant diseases.161,199 But radiotherapy (RT) is especially capable of inducing immunogenic forms of tumor cell death.200
Cancer cells can be rendered visible to the immune system by standard therapies such as chemotherapy (CT) or RT, either alone or in combination with (further) immune stimulation (e.g. with hyperthermia (HT)).201 The resulting tumor microenvironment determines which immune cells get recruited and triggers the activation or suppression of DCs. Independent of the way of necrosis induction, this cell death form is generally more immunogenic compared to apoptotic cell death. Specialized phagocytes, especially macrophages, swiftly take up apoptotic cells. This process results in non- or even anti-inflammatory events. The activated macrophages secrete anti-inflammatory cytokines like IL-10 or TGFβ.202 In contrast, necrotic cells that have lost their membrane integrity have immune stimulatory potential. These dead cells release DAMPs that alert the immune system.203 Various preclinical studies have proven that DAMPs are released after combined treatment of tumor cells with RT and additional immune modulation.204–206 Irradiation is therefore one central trigger of DAMP release and consecutive immune activation.
Immunogenic cancer cell death is characterized by the release of danger signals such as HMGB1 protein, Heat shock protein (HSP) 70 and Adenosin-5′-triphosphate (ATP) as well as by the exposure of calreticulin (CRT) on the cell surface.207 RT and also cell death induction by high hydrostatic pressure for tumor vaccine generation are capable of inducing it.208,209 Of note is that danger signals are present in every cell and necessary for the survival of cells. They are physiologically located intracellularly, but act as danger signals when being released into the extracellular space. This can cause “immunotoxicity”, but is beneficial with regard to cancer cells.210
One of the most prominent danger signals is HMGB1. It is ubiquitously expressed in the nucleus of mammalian cells and highly conserved between different species. It acts as a non-histone chromatin-associated protein, it binds to DNA and facilitates the binding of transcription factors. Another function is its role in the recognition of DNA damage in the process of mismatch repair.211 Immunologically, it comes into play when cells are dying. Since necrosis leads to plasma membrane rupture, intracellular HMGB1 gets passively released,212 but inflammatory cells such as macrophages even secrete HMGB1 actively.213 HMGB1 preferentially binds to the receptor for advanced glycation end products (RAGE) and to toll-like receptors (TLRs), especially to TLR2 and TLR4.214 HMGB1 thereby represents a strong activator of DCs: it fosters antigen cross presentation by DCs and consecutive activation of naïve T cells.215 Standard tumor therapies like RT or CT have been shown to induce the release of HMGB1215,216 and RT can even enhance CT-induced immunogenic cell death when administered concomitantly.217 Radiochemotherapy often induces both apoptosis and necrosis. This mixture of dying and dead cells is especially immunogenic.204 Further, RT often primarily induces senescent tumor cells.218 The impact of the senescence-associated secretory phenotype (SASP) on tumor progression is under current intensive investigation.219
Other prominent DAMPs are HSPs, especially HSP70.220 Inside the cell, HSPs protect it from stress. They act as chaperones and thereby stabilize proteins or can ubiquitinate damaged proteins, leading to their degradation in the proteasome. Of note is that outside the cell HSPs can efficiently activate the immune system. Many HSPs chaperone tumor proteins. When HSP70 gets released, it delivers the bound antigens to antigen presenting cells (APCs). The latter internalize the HSPs and thereby also the antigens by receptor mediated endocytosis. Finally, the tumor antigens get cross-presented via MHC-I molecules and can stimulate the CD8+ T cell response (cytotoxic T cells; CTLs) in this way.221 That CD8+ T cells are important for anti-tumor responses is further underlined by the fact that radiation-induced apoptosis in these cells was demonstrated to be a predictive factor for survival in cervical carcinoma patients.222 Another immune stimulatory effect of HSPs is the enhanced secretion of pro-inflammatory cytokines by APCs like DCs following binding of HSPs to PRRs. Taken together, extracellular HSPs act as danger signals resulting in maturation and activation of APCs. But HSPs do not only act on APCs, they also stimulate and activate cells of the innate immune system, namely NK cells.223
Another DAMP is ATP, the key transporter of chemical energy. Usually, the intracellular concentration of ATP is relatively high (3–10 mM) but the extracellular concentration is pretty low (400–700 nM).224 Different sorts of stress induce the release of ATP by cells. ATP acts on purinergic receptors, especially on P2RX7, and becomes thereby a potent mediator of IL-1β and IL-18 processing and release.225 ATP also binds the P2RX7 receptor on DCs which leads to the activation of the NLPR3 inflammasome.226 Stimulation by ATP results in the aggregation of NLRP3 with the apoptosis-associated speck-like protein (ASC) and caspase-1. The mature caspase-1 consecutively cleaves pro-IL-1β and active IL-1β is released. The latter is important for priming of CD8+ T cells. Therefore, the activation of the inflammasome establishes a link between the innate and the adaptive immune system and is an important part of anticancer immunity.109 The release of ATP is stress-induced and controlled by different mechanisms. Recent studies have shown that ionizing radiation also influences its release.227
A big challenge for the future is to identify multimodal tumor therapies that lead to maximal activation of the immune system by triggering the release of distinct DAMPs.228,229 Radiotherapy is an integral part of it since it can render tumor cells immunogenic.230 Radiation-induced TNF-α release may add to radiation lethality through autocrine and paracrine mechanisms.231 Further, the death receptor Fas can be upregulated by RT232 and concomitantly tumor suppressor proteins such as p53.233 To foster immune recognition, radiation further enhances the expression of MHC-I on tumor cells as well as the surface exposure of HSP70.234,235 Radiation also impacts on the protein pool and novel peptides are made in response to gamma-irradiation.234 Since T cells especially recognize mutated tumor proteins and melanoma has many of them, this tumor entity is most accessible for additional immune therapy.236 The expression of adhesion molecules such as ICAM-1 on endothelial cells is also increased after genotoxic stress and might thereby contribute to radiation-induced inflammatory reaction of the endothelium.237
The immunogenic potential of radiotherapy is summarized in ref. 238 and distinct fractionation schemes or a single high dose application of radiation might result in different immunological outcomes.239 The DNA damage response known to arrest the cell cycle and to enhance DNA repair or to trigger apoptosis may also participate in alerting the immune system to the presence of danger coming from damaged cells.135 The modulation by radiation of immune checkpoints, namely co-stimulatory and inhibitory molecules on T cells, is under current intensive examination to pave the way for multimodal treatments consisting of radiochemotherapy plus immune therapy.240,241 The immunological effects of RT are mostly described in patients with melanoma.242 Non-redundant immune mechanisms get activated in cancer by combining RT with checkpoint inhibitors.243 This highlights the manifold immune modulatory properties of ionizing radiation that have to be utilized by finding the most beneficial combination and chronology of application with selected immune therapies.
Conclusions
Systemic effects hold a critical role in the response of all organisms in some types of stress, usually exogenous like tissue injury, infection or irradiation. In all cases, the danger signals released by the site of ‘attack’ play a role to prime the organism's defense mechanisms, as part of a general response to secure homeostasis. In the case of radiation-induced injury or tissue damage the danger signals released vary greatly. They can range from ROS, damaged DNA, RNA, ATP, heat-shock proteins, HMGB1, uric acid and different cytokines and chemokines. The NLRP3 inflammasome activation by these danger signals has been shown in several cases to hold an orchestrating role.
Based on all evidence and as summarized above and in Table 1, it becomes clear that these types of effects do exist, but a more analytical and mechanistic in-depth approach is needed. A better understanding of NTE and even more the systemic immune-mediated-effects (SIME) may have great effects on health risk assessment and radiation protection, as well as on clinical applications of IR for cancer treatment. Furthermore, the knowledge of the molecules mediating the signal and initiating the responses to radiation is considered critical for radiation protection (low doses), effective radiation therapy (immunomodulation) and minimizing radiation toxicity and adverse effects. We believe that the response to radiation and the signaling cascade and acute or most importantly chronic effects should be viewed under the prism of a holistic approach and at the whole organism level. As discussed recently by Pateras et al.,134 vital cellular danger signals stimulate the defense at the systemic (organism) level and vice versa. Disruption of DDR, DNA repair and immune response crosstalk can compromise (multi)-cellular/tissue integrity, setting the seed for cell cycle related and immune defects and consequently genomic instability.
Abbreviations
- ATP
Adenosine triphosphate
- CLRs
C-type lectin receptors
- CT
Chemotherapy
- DDR
DNA damage response
- HMGB1
High-mobility group protein B1
- ICAM-1
Intercellular adhesion molecule 1
- IFNs
Interferons
- IL-1
Interleukin 1
- IR
Ionizing radiation
- LPS
Lipopolysaccharides
- MHCI
Major histocompatibility complex class I
- NTE
Non-targeted effects
- ROS
Reactive oxygen species
- NO
Nitric oxide
- RT
Radiation therapy
- RIBE
Radiation-induced bystander effects
- DAMPs
Damage associated molecular patterns
- PAMPs
Pathogen associated molecular patterns
- PRRs
Pattern recognition receptors
- SIME
Systemic immune-mediated effects
- Tregs
Regulatory T cells
- TGF
Tumor growth factor
- TGFα
Transforming growth factor alpha
- TNF-α
Tumor necrosis factor alpha
- TLRs
Toll-like receptors
Acknowledgments
Dr Georgakilas's group (NTUA) has been supported by an EU Marie Curie Reintegration Grant MC-CIG-303514, Greek National funds through the Operational Program ‘Educational and Lifelong Learning’ of the National Strategic Reference Framework (NSRF)-Research Funding Program: THALES (Grant number MIS 379346) and COST Action CM1201 ‘Biomimetic Radical Chemistry’. This work has been funded by the European Atomic Energy Community's Seventh Framework Programme (FP7/2007-2011) under grant agreement no. 249689. BF and USG were supported by the German Federal Ministry of Education and Research (GREWIS, 02NUK017G/F). Ms Laskaratou was supported by the project “Scholarships programmes by the State Scholarships Foundation (SSF/IKY)” in the framework of the Operational Programme “Education and Lifelong Learning” resources, European Social Fund (ESF) of the National Strategic Reference Framework (2007–2013).
References
- Belyakov O. V., Malcolmson A. M., Folkard M., Prise K. M., Michael B. D. Br. J. Cancer. 2001;84:674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim M., Salomaa S., Wright E., Hildebrandt G., Belyakov O. V., Prise K. M., Little M. P. Mutat. Res. 2013;752:84–98. doi: 10.1016/j.mrrev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim M. A., Hill M. A. Radiat. Prot. Dosimetry. 2015 doi: 10.1093/rpd/ncv167. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Sowa M. B. Mutat. Res. 2007;616:159–164. doi: 10.1016/j.mrfmmm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Sowa M. B. Cancer Lett. 2015;356:17–21. doi: 10.1016/j.canlet.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Hatzi V. I., Laskaratou D. A., Mavragani I. V., Nikitaki Z., Mangelis A., Panayiotidis M. I., Pantelias G. E., Terzoudi G. I., Georgakilas A. G. Cancer Lett. 2015;356:34–42. doi: 10.1016/j.canlet.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Desouky O., Ding N., Zhou G. J. Radiat. Res. Appl. Sci. 2015;8:247–254. [Google Scholar]
- Iyer R., Lehnert B. E. Arch. Biochem. Biophys. 2000;376:14–25. doi: 10.1006/abbi.1999.1684. [DOI] [PubMed] [Google Scholar]
- Widel M. Postepy Hig. Med. Dosw. (Online) 2012;66:828–837. doi: 10.5604/17322693.1019532. [DOI] [PubMed] [Google Scholar]
- Singh H., Saroya R., Smith R. W., Mantha R., Guindon L., Mitchel R. E. J., Seymour C., Mothersill C. Dose-Response. 2011;9:225–242. doi: 10.2203/dose-response.09-062.Singh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam E. I., Little J. B. Hum. Exp. Toxicol. 2004;23:61–65. doi: 10.1191/0960327104ht418oa. [DOI] [PubMed] [Google Scholar]
- Kolesnikova I. S., Vorobtsova I. E. Radiats. Biol. Radioecol. 2011;51:542–548. [PubMed] [Google Scholar]
- Dieriks B., De Vos W., Baatout S., Van Oostveldt P. Mutat. Res. 2011;715:19–24. doi: 10.1016/j.mrfmmm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Pinto M., Azzam E. I., Howell R. W. Radiat. Res. 2010;174:216–227. doi: 10.1667/RR1866.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klammer H., Kadhim M., Iliakis G. Cancer Res. 2010;70:8498–8506. doi: 10.1158/0008-5472.CAN-10-1181. [DOI] [PubMed] [Google Scholar]
- Shankar B., Pandey R., Sainis K. Int. J. Radiat. Biol. 2006;82:537–548. doi: 10.1080/09553000600877114. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Takahashi A., Ohnishi T. Biol. Sci. Space. 2004;18:247–254. doi: 10.2187/bss.18.247. [DOI] [PubMed] [Google Scholar]
- Sawant S. G., Randers-Pehrson G., Metting N. F., Hall E. J. Radiat. Res. 2001;156:177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wolff S. Environ. Health Perspect. 1998;106:277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler D. A., Moore S. R., Macdonald D. A., Smyth S. H., Clapham P., Kadhim M. A. Mutat. Res. 2006;597:50–61. doi: 10.1016/j.mrfmmm.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Hall E. J., Hei T. K. Oncogene. 2003;22:7034–7042. doi: 10.1038/sj.onc.1206900. [DOI] [PubMed] [Google Scholar]
- Kadhim M. A., Lee R., Moore S. R., Macdonald D. A., Chapman K. L., Patel G., Prise K. M. Mutat. Res. 2010;688:91–94. doi: 10.1016/j.mrfmmm.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. F. Oncogene. 2003;22:7094–7099. doi: 10.1038/sj.onc.1206992. [DOI] [PubMed] [Google Scholar]
- Morgan W. F. Radiat. Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wright E. G. Med. Confl. Survival. 2000;16:117–130. doi: 10.1080/13623690008409501. [DOI] [PubMed] [Google Scholar]
- Heil M., Land W. G. Front. Plant Sci. 2014:5. doi: 10.3389/fpls.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakilas A. G., Pavlopoulou A., Louka M., Nikitaki Z., Vorgias C. E., Bagos P. G., Michalopoulos I. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Sprung C. N., Ivashkevich A., Forrester H. B., Redon C. E., Georgakilas A., Martin O. A. Cancer Lett. 2015;356:72–81. doi: 10.1016/j.canlet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaki S., Kotsinas A., Chronopoulos E., Kletsas D., Georgakilas A., Gorgoulis V. G. Cancer Lett. 2015;356:43–51. doi: 10.1016/j.canlet.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Mothersill C., Seymour C. Radiat. Res. 2001;155:757–765. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chai Y., Hei T. K. Acta Med. Nagasaki. 2008;53:S65–S69. [PMC free article] [PubMed] [Google Scholar]
- Chaudhry M. A. Mutat. Res. 2006;597:98–112. doi: 10.1016/j.mrfmmm.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Bair W. J. Radiat. Res. 2013;179:501–510. doi: 10.1667/RR3306.1. [DOI] [PubMed] [Google Scholar]
- Murphy J. B., Morton J. J. Proc. Natl. Acad. Sci. U. S. A. 1915;1:435–437. doi: 10.1073/pnas.1.7.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H., Little J. B. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- Deshpande A., Goodwin E. H., Bailey S. M., Marrone B., Lehnert B. E. Radiat. Res. 1996;152:552–557. [PubMed] [Google Scholar]
- Narayanan P. K., Goodwin E. H., Lehnert B. E. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Brooks A. L. Radiat. Res. 2001;5:618–627. doi: 10.1667/0033-7587(2001)156[0618:esttma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lehnert B. E., Goodwin E. H. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- Ermakov A. V., Kon'kova M. S., Kostiuk S. V., Ershova E. S., Egolina N. A., Veiko N. N. Radiats. Biol. Radioecol. 2008;48:553–564. [PubMed] [Google Scholar]
- Yang H., Asaad N., Held K. D. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- Koturbash I., Rugo R. E., Hendricks C. A., Loree J., Thibault B., Kutanzi K., Pogribny I., Yanch J. C., Engelward B. P., Kovalchuk O. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- Koturbash I., Loree J., Kutanzi K., Koganow C., Pogribny I., Kovalchuk O. Int. J. Radiat. Oncol., Biol., Phys. 2008;70:554–562. doi: 10.1016/j.ijrobp.2007.09.039. [DOI] [PubMed] [Google Scholar]
- Pereira S., Malard V., Ravanat J. L., Davin A. H., Armengaud J., Foray N., Adam-Guillermin C. PLoS One. 2014;9:e92974. doi: 10.1371/journal.pone.0092974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L., Nagasawa H., Little J. B. Radiat. Res. 2001;156:521–525. doi: 10.1667/0033-7587(2001)156[0521:hmiibc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zhou H., Randers-Pehrson G., Waldren C. A., Vannais D., Hall E. J., Hei T. K. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilnytskyy Y., Koturbash I., Kovalchuk O. Environ. Mol. Mutagen. 2009;50:105–113. doi: 10.1002/em.20440. [DOI] [PubMed] [Google Scholar]
- Lorimore S. A., Kadhim M. A., Pocock D. A., Papworth D., Stevens D. L., Goodhead D. T., Wright E. G. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5730–5733. doi: 10.1073/pnas.95.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Chen X., Tian W., Yin X., Wang J., Yang H. Br. J. Cancer. 2014;111:772–780. doi: 10.1038/bjc.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koturbash I., Boyko A., Rodriguez-Juarez R., McDonald R. J., Tryndyak V. P., Kovalchuk I., Pogribny I. P., Kovalchuk O. Carcinogenesis. 2007;28:1831–1838. doi: 10.1093/carcin/bgm053. [DOI] [PubMed] [Google Scholar]
- Koturbash I., Zemp F., Kolb B., Kovalchuk O. Mutat. Res. 2011;722:114–118. doi: 10.1016/j.mrgentox.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O., Zemp F. J., Filkowski J. N., Altamirano A. M., Dickey J. S., Jenkins-Baker G., Marino S. A., Brenner D. J., Bonner W. M., Sedelnikova O. A. Carcinogenesis. 2010;31:1882–1888. doi: 10.1093/carcin/bgq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapalle E., Wang R., Adetolu R., Tsao D., Francisco D., Sigounas G., Georgakilas A. G. Radiat. Res. 2007;167:207–216. doi: 10.1667/rr0659.1. [DOI] [PubMed] [Google Scholar]
- Lorimore S. A., Chrystal J. A., Robinson J. I., Coates P. J., Wright E. G. Cancer Res. 2008;68:8122–8126. doi: 10.1158/0008-5472.CAN-08-0698. [DOI] [PubMed] [Google Scholar]
- Shen H., Yu H., Liang P. H., Cheng H., XuFeng R., Yuan Y., Zhang P., Smith C. A., Cheng T. Blood. 2012;119:3629–3637. doi: 10.1182/blood-2011-08-373621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Liu W., Li J., Xie Y., He M., Fu J., Jin W., Shao C. Radiat. Res. 2014;182:111–121. doi: 10.1667/RR13736.1. [DOI] [PubMed] [Google Scholar]
- Belyakov O. V., Folkard M., Mothersill C., Prise K. M., Michael B. D. Radiat. Prot. Dosim. 2002;99:249–251. doi: 10.1093/oxfordjournals.rpd.a006775. [DOI] [PubMed] [Google Scholar]
- Mariotti L. G., Bertolotti A., Ranza E., Babini G., Ottolenghi A. Int. J. Radiat. Biol. 2012;88:751–762. doi: 10.3109/09553002.2012.703365. [DOI] [PubMed] [Google Scholar]
- Azzam E. I., de Toledo S. M., Gooding T., Little J. B. Radiat. Res. 1998;150:497–504. [PubMed] [Google Scholar]
- Azzam E. I., de Toledo S. M., Little J. B. Proc. Natl. Acad. Sci. U. S. A. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C., Rea D., Wright E. G., Lorimore S. A., Murphy D., Seymour C. B., O'Malley K. Carcinogenesis. 2001;22:1465–1471. doi: 10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- Little J. B. Mutat. Res. 2006;597:113–118. doi: 10.1016/j.mrfmmm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Mancuso M., Pasquali E., Leonardi S., Rebessi S., Tanori M., Giardullo P., Borra F., Pazzaglia S., Naus C. C., Di Majo V., Saran A. Oncogene. 2011;30:4601–4608. doi: 10.1038/onc.2011.176. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. J. Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe P. D., Lau A. F. Arch. Biochem. Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Widel M., Krzywon A., Gajda K., Skonieczna M., Rzeszowska-Wolny J. Free Radicals Biol. Med. 2014;68:278–287. doi: 10.1016/j.freeradbiomed.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Yang S., Xu J., Shao W., Geng C., Li J., Guo F., Miao H., Shen W., Ye T., Liu Y., Xu H., Zhang X. Cell Biochem. Biophys. 2015:1–6. doi: 10.1007/s12013-015-0555-2. [DOI] [PubMed] [Google Scholar]
- Lorimore S. A., Coates P. J., Scobie G. E., Milne G., Wright E. G. Oncogene. 2001;20:7085–7095. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- Lorimore S. A., Mukherjee D., Robinson J. I., Chrystal J. A., Wright E. G. Cancer Res. 2011;71:6485–6491. doi: 10.1158/0008-5472.CAN-11-1926. [DOI] [PubMed] [Google Scholar]
- Khan M. A., Hill R. P., Van Dyk J. Int. J. Radiat. Oncol., Biol., Phys. 1998;40:467–476. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- Rastogi S., Coates P. J., Lorimore S. A., Wright E. G. Radiat. Res. 2012;177:244–250. doi: 10.1667/rr2805.1. [DOI] [PubMed] [Google Scholar]
- Chai Y., Calaf G. M., Zhou H., Ghandhi S. A., Elliston C. D., Wen G., Nohmi T., Amundson S. A., Hei T. K. Br. J. Cancer. 2013;108:91–98. doi: 10.1038/bjc.2012.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y., Lam R. K., Calaf G. M., Zhou H., Amundson S., Hei T. K. Br. J. Cancer. 2013;108:1106–1112. doi: 10.1038/bjc.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba K., Omagari K., Nakamura T., Ikuno N., Saeki S., Matsuo I., Kinoshita H., Masuda J., Hazama H., Sakamoto I., Kohno S. Gut. 1998;43:575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme J., Bauer G. Radiat. Res. 2013;179:422–432. doi: 10.1667/RR3161.2. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Wang J., Bucking C. P., Mothersill C. E., Seymour C. B. Proteomics. 2007;7:4171–4180. doi: 10.1002/pmic.200700573. [DOI] [PubMed] [Google Scholar]
- Choi V. W. Y., Cheung A. L. Y., Cheng S. H., Yu K. N. Environ. Sci. Technol. 2012;46:11678–11683. doi: 10.1021/es301838s. [DOI] [PubMed] [Google Scholar]
- Mothersill C., Smith R. W., Agnihotri N., Seymour C. B. Environ. Sci. Technol. 2007;41:3382–3387. doi: 10.1021/es062978n. [DOI] [PubMed] [Google Scholar]
- Choi V. W., Ng C. Y., Kobayashi A., Konishi T., Suya N., Ishikawa T., Cheng S. H., Yu K. N. Environ. Sci. Technol. 2013;47:6368–6376. doi: 10.1021/es401171h. [DOI] [PubMed] [Google Scholar]
- Mothersill C., Bucking C., Smith R. W., Agnihotri N., O'Neill A., Kilemade M., Seymour C. B. Environ. Sci. Technol. 2006;40:6859–6864. doi: 10.1021/es061099y. [DOI] [PubMed] [Google Scholar]
- Mancuso M., Pasquali E., Leonardi S., Tanori M., Rebessi S., Di Majo V., Pazzaglia S., Toni M. P., Pimpinella M., Covelli V., Saran A. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M., Leonardi S., Giardullo P., Pasquali E., Tanori M., De Stefano I., Casciati A., Naus C. C., Pazzaglia S., Saran A. Int. J. Radiat. Oncol., Biol., Phys. 2013;86:993–999. doi: 10.1016/j.ijrobp.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Ovigne J. M., Powles A. V., Corcoran S., Fry L. Br. J. Dermatol. 2003;148:670–679. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- Lebre M. C., van der Aar A. M., van Baarsen L., van Capel T. M., Schuitemaker J. H., Kapsenberg M. L., de Jong E. C. J. Invest. Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- Nestle F. O., Di Meglio P., Qin J. Z., Nickoloff B. J. Nat. Rev. Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Jiang M., Ma Z., Dietze K. K., Zelinskyy G., Yang D., Dittmer U., Schlaak J. F., Roggendorf M., Lu M. J. Immunol. 2013;191:6178–6190. doi: 10.4049/jimmunol.1301262. [DOI] [PubMed] [Google Scholar]
- Krikun G., Trezza J., Shaw J., Rahman M., Guller S., Abrahams V. M., Lockwood C. J. Am. J. Reprod. Immunol. 2012;68:233–237. doi: 10.1111/j.1600-0897.2012.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Laudanna C., Kim J. Y., Constantin G., Butcher E. Immunol. Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- Sun J. C., Lanier L. L. Nat. Rev. Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. A., Elliott J. M., Keyel P. A., Yang L., Carrero J. A., Yokoyama W. M. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F. M., Henriquez F. L., Alexander J., Roberts C. W. Clin. Exp. Immunol. 2010;160:369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. D., Jiang C., Matta B., Tietzel I., Watkins S. K., Suttles J. J. Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O'Neill L. A., Perretti M., Rossi A. G., Wallace J. L. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Ding A. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Paterson H. M., Murphy T. J., Purcell E. J., Shelley O., Kriynovich S. J., Lien E., Mannick J. A., Lederer J. A. J. Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- Brickey W. J., Neuringer I. P., Walton W., Hua X., Wang E. Y., Jha S., Sempowski G. D., Yang X., Kirby S. L., Tilley S. L., Ting J. P. Int. J. Radiat. Biol. 2012;88:335–347. doi: 10.3109/09553002.2012.652723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A., Stander S., Berneburg M., Bohm M., Kulms D., van Steeg H., Grosse-Heitmeyer K., Krutmann J., Schwarz T. Nat. Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- Land W. G., Messmer K. Front. Immunol. 2012;3:349. doi: 10.3389/fimmu.2012.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B. T., Tschopp J. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Schaue D., Kachikwu E. L., McBride W. H. Radiat. Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims G. P., Rowe D. C., Rietdijk S. T., Herbst R., Coyle A. J. Annu. Rev. Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Andersson U., Wang H., Palmblad K., Aveberger A. C., Bloom O., Erlandsson-Harris H., Janson A., Kokkola R., Zhang M., Yang H., Tracey K. J. J. Exp. Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklein V. M., Osuka A., Ishikawa S., Lederer M. R., Wanke-Jellinek L., Lederer J. A. J. Immunol. 2015;194:1178–1189. doi: 10.4049/jimmunol.1303051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C., Vermaelen K., Panaretakis T., Mignot G., Ullrich E., Perfettini J. L., Schlemmer F., Tasdemir E., Uhl M., Genin P., Civas A., Ryffel B., Kanellopoulos J., Tschopp J., Andre F., Lidereau R., McLaughlin N. M., Haynes N. M., Smyth M. J., Kroemer G., Zitvogel L. Nat. Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Sohn S. H., Lee J. M., Park S., Yoo H., Kang J. W., Shin D., Jung K. H., Lee Y. S., Cho J., Bae H. Environ. Toxicol. Pharmacol. 2015;39:917–926. doi: 10.1016/j.etap.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Lodermann B., Wunderlich R., Frey S., Schorn C., Stangl S., Rodel F., Keilholz L., Fietkau R., Gaipl U. S., Frey B. Int. J. Radiat. Biol. 2012;88:727–734. doi: 10.3109/09553002.2012.689464. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Barnes D. E., Yang Y. G., Robins P. Biochem. Soc. Trans. 2009;37:535–538. doi: 10.1042/BST0370535. [DOI] [PubMed] [Google Scholar]
- Candeias S. M., Testard I. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V. G., Zacharatos P., Kotsinas A., Kletsas D., Mariatos G., Zoumpourlis V., Ryan K. M., Kittas C., Papavassiliou A. G. EMBO J. 2003;22:1567–1578. doi: 10.1093/emboj/cdg157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke N., Mertens C., Zillinger T., Wenzel J., Bald T., Zahn S., Tuting T., Hartmann G., Barchet W. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Ries M., Schuster P., Thomann S., Donhauser N., Vollmer J., Schmidt B. J. Leukocyte Biol. 2013;94:123–135. doi: 10.1189/jlb.0612278. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S. Clin. Immunol. 2012;144:32–40. doi: 10.1016/j.clim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois C. M., Jin T., Miller A. L., Bertheloot D., Nakamura H., Horvath G. L., Mian A., Jiang J., Schrum J., Bossaller L., Pelka K., Garbi N., Brewah Y., Tian J., Chang C., Chowdhury P. S., Sims G. P., Kolbeck R., Coyle A. J., Humbles A. A., Xiao T. S., Latz E. J. Exp. Med. 2013;210:2447–2463. doi: 10.1084/jem.20120201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J. J., Cowing-Zitron C., Nakatsuji T., Muehleisen B., Muto J., Borkowski A. W., Martinez L., Greidinger E. L., Yu B. D., Gallo R. L. Nat. Med. 2012;18:1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Kim T. Y., Song Y. H., Min I. M., Yim J., Kim T. K. J. Biol. Chem. 1999;274:30686–30689. doi: 10.1074/jbc.274.43.30686. [DOI] [PubMed] [Google Scholar]
- Beckerman R., Prives C. Cold Spring Harbor Perspect. Biol. 2010;2:a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez D., Shatz M., Resnick M. A. Curr. Opin. Oncol. 2013;25:85–92. doi: 10.1097/CCO.0b013e32835b6386. [DOI] [PubMed] [Google Scholar]
- Quarmby S., Kumar P., Wang J., Macro J. A., Hutchinson J. J., Hunter R. D., Kumar S. Arterioscler., Thromb., Vasc. Biol. 1999;19:588–597. doi: 10.1161/01.atv.19.3.588. [DOI] [PubMed] [Google Scholar]
- Quarmby S., Kumar P., Kumar S. Int. J. Cancer. 1999;82:385–395. doi: 10.1002/(sici)1097-0215(19990730)82:3<385::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Quarmby S., Hunter R. D., Kumar S. Anticancer Res. 2000;20:3375–3381. [PubMed] [Google Scholar]
- D'Ambrosio D., Albanesi C., Lang R., Girolomoni G., Sinigaglia F., Laudanna C. J. Immunol. 2002;169:2303–2312. doi: 10.4049/jimmunol.169.5.2303. [DOI] [PubMed] [Google Scholar]
- Menendez D., Shatz M., Azzam K., Garantziotis S., Fessler M. B., Resnick M. A. PLoS Genet. 2011;7:e1001360. doi: 10.1371/journal.pgen.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J. M., Menendez D., Bushel P. R., Shatz M., Kirk E. L., Troester M. A., Garantziotis S., Fessler M. B., Resnick M. A. Cancer Res. 2014;74:2182–2192. doi: 10.1158/0008-5472.CAN-13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saghire H., Thierens H., Monsieurs P., Michaux A., Vandevoorde C., Baatout S. Int. J. Radiat. Biol. 2013;89:628–638. doi: 10.3109/09553002.2013.782448. [DOI] [PubMed] [Google Scholar]
- El-Saghire H., Michaux A., Thierens H., Baatout S. Int. J. Mol. Med. 2013;32:1407–1414. doi: 10.3892/ijmm.2013.1514. [DOI] [PubMed] [Google Scholar]
- El-Saghire H., Vandevoorde C., Ost P., Monsieurs P., Michaux A., De Meerleer G., Baatout S., Thierens H. Int. J. Oncol. 2014;44:1073–1083. doi: 10.3892/ijo.2014.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Robbins M. E. Curr. Med. Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- Sabatel H., Di Valentin E., Gloire G., Dequiedt F., Piette J., Habraken Y. PLoS One. 2012;7:e38246. doi: 10.1371/journal.pone.0038246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateras I. S., Havaki S., Nikitopoulou X., Vougas K., Townsend P., Panayiotidis M. I., Georgakilas A. G., Gorgoulis V. G. Pharmacol. Ther. 2015 doi: 10.1016/j.pharmthera.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Gasser S., Orsulic S., Brown E. J., Raulet D. H. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriani A., Iannitto M. L., Ricci B., Fionda C., Malgarini G., Morrone S., Peruzzi G., Ricciardi M. R., Petrucci M. T., Cippitelli M., Santoni A. J. Immunol. 2014;193:950–960. doi: 10.4049/jimmunol.1400271. [DOI] [PubMed] [Google Scholar]
- Dressman H. K., Muramoto G. G., Chao N. J., Meadows S., Marshall D., Ginsburg G. S., Nevins J. R., Chute J. P. PLoS Med. 2007;4:e106. doi: 10.1371/journal.pmed.0040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson S. A., Bittner M., Fornace A. J. J. Oncogene. 2003;22:5828–5833. doi: 10.1038/sj.onc.1206681. [DOI] [PubMed] [Google Scholar]
- Jen K. Y., Cheung V. G. Genome Res. 2003;13:2092–2100. doi: 10.1101/gr.1240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A. R., Morgan W. F. Cancer Metastasis Rev. 2004;23:259–268. doi: 10.1023/B:CANC.0000031765.17886.fa. [DOI] [PubMed] [Google Scholar]
- Amundson S. A., Grace M. B., McLeland C. B., Epperly M. W., Yeager A., Zhan Q., Greenberger J. S., Fornace A. J. J. Cancer Res. 2004;64:6368–6371. doi: 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- Webster G. A., Perkins N. D. Mol. Cell. Biol. 1999;19:3485–3495. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson S. A., Bittner M., Chen Y., Trent J., Meltzer P., Fornace A. J. J. Oncogene. 1999;18:3666–3672. doi: 10.1038/sj.onc.1202676. [DOI] [PubMed] [Google Scholar]
- Park W. Y., Hwang C. I., Im C. N., Kang M. J., Woo J. H., Kim J. H., Kim Y. S., Kim J. H., Kim H., Kim K. A., Yu H. J., Lee S. J., Lee Y. S., Seo J. S. Oncogene. 2002;21:8521–8528. doi: 10.1038/sj.onc.1205977. [DOI] [PubMed] [Google Scholar]
- Khodarev N. N., Park J. O., Yu J., Gupta N., Nodzenski E., Roizman B., Weichselbaum R. R. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12665–12670. doi: 10.1073/pnas.211443698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellweg C. E. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Manna K., Das U., Das D., Kesh S. B., Khan A., Chakraborty A., Dey S. Free Radical Res. 2015;49:422–439. doi: 10.3109/10715762.2015.1016018. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Sakakura C., Miyagawa K., Kuriu Y., Kin S., Nakase Y., Hagiwara A., Mitsufuji S., Okazaki Y., Hayashizaki Y., Yamagishi H. Br. J. Cancer. 2004;91:1543–1550. doi: 10.1038/sj.bjc.6602187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C. J., Williams J. P., Okunieff P., Finkelstein J. N. Radiat. Res. 2002;157:256–265. doi: 10.1667/0033-7587(2002)157[0256:ripfeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kis E., Szatmári T., Keszei M., Farkas R., Ésik O., Lumniczky K., Falus A., Sáfrány G. Int. J. Radiat. Oncol., Biol., Phys. 2006;66:1506–1514. doi: 10.1016/j.ijrobp.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Ding L. H., Shingyoji M., Chen F., Hwang J. J., Burma S., Lee C., Cheng J. F., Chen D. J. Radiat. Res. 2005;164:17–26. doi: 10.1667/rr3354. [DOI] [PubMed] [Google Scholar]
- Goldberg Z., Schwietert C. W., Lehnert B., Stern R., Nami I. Int. J. Radiat. Oncol., Biol., Phys. 2004;58:567–574. doi: 10.1016/j.ijrobp.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Wyrobek A. J., Manohar C. F., Krishnan V. V., Nelson D. O., Furtado M. R., Bhattacharya M. S., Marchetti F., Coleman M. A. Mutat. Res. 2011;722:119–130. doi: 10.1016/j.mrgentox.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Lowe X. R., Bhattacharya S., Marchetti F., Wyrobek A. J. Radiat. Res. 2009;171:53–65. doi: 10.1667/RR1389.1. [DOI] [PubMed] [Google Scholar]
- Fält S., Holmberg K., Lambert B., Wennborg A. Carcinogenesis. 2003;24:1837–1845. doi: 10.1093/carcin/bgg134. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R. K., Valerie K., Chan W., Wazer D. E., Lin P. S. Int. J. Radiat. Biol. 1992;61:405–415. doi: 10.1080/09553009214551101. [DOI] [PubMed] [Google Scholar]
- Aryankalayil M. J., Makinde A. Y., Gameiro S. R., Hodge J. W., Rivera-Solis P. P., Palayoor S. T., Ahmed M. M., Coleman C. N. Radiat. Res. 2014;182:139–148. doi: 10.1667/RR13731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palayoor S. T., John-Aryankalayil M., Makinde A. Y., Falduto M. T., Magnuson S. R., Coleman C. N. Mol. Cancer Res. 2014;12:1002–1015. doi: 10.1158/1541-7786.MCR-13-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzhna L., Kovalchuk O. Oncoscience. 2014;1:751–762. doi: 10.18632/oncoscience.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Smilenov L. B., Amundson S. A. Radiat. Res. 2013;180:575–583. doi: 10.1667/RR13343.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakilas A. G., Pavlopoulou A., Louka M., Nikitaki Z., Vorgias C. E., Bagos P. G., Michalopoulos I. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Jin Y. W., Na Y. J., Lee Y. J., An S., Lee J. E., Jung M., Kim H., Nam S. Y., Kim C. S., Yang K. H., Kim S. U., Kim W. K., Park W. Y., Yoo K. Y., Kim J. H. Oncol. Rep. 2008;19:135–144. [PubMed] [Google Scholar]
- Uehara Y., Ito Y., Taki K., Nenoi M., Ichinohe K., Nakamura S., Tanaka S., Oghiso Y., Tanaka K., Matsumoto T., Paunesku T., Woloschak G. E., Ono T. Radiat. Res. 2010;174:611–617. doi: 10.1667/RR2195.1. [DOI] [PubMed] [Google Scholar]
- Shin S. C., Lee K. M., Kang Y. M., Kim K., Lim S. A., Yang K. H., Kim J. Y., Nam S. Y., Kim H. S. Genomics. 2011;97:358–363. doi: 10.1016/j.ygeno.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Soucek K., Slabáková E., Ovesná P., Malenovská A., Kozubík A., Hampl A. Hum. Reprod. 2010;25:2962–2971. doi: 10.1093/humrep/deq264. [DOI] [PubMed] [Google Scholar]
- Wiklund F. E., Bennet A. M., Magnusson P. K., Eriksson U. K., Lindmark F., Wu L., Yaghoutyfam N., Marquis C. P., Stattin P., Pedersen N. L., Adami H. O., Grönberg H., Breit S. N., Brown D. A. Aging Cell. 2010;9:1057–1064. doi: 10.1111/j.1474-9726.2010.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L. B., Clopton P., Laughlin G. A., Maisel A. S., Barrett-Connor E. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. J., Karan D., Johansson S. L., Lin F. F., Zeckser J., Singh A. P., Batra S. K., Lin M. F. Prostate. 2007;674:557–571. doi: 10.1002/pros.20551. [DOI] [PubMed] [Google Scholar]
- Senapati S., Rachagani S., Chaudhary K., Johansson S. L., Singh R. K., Batra S. K. Oncogene. 2010;29:1293–1302. doi: 10.1038/onc.2009.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Breit S. N., Buring J., Fairlie W. D., Bauskin A. R., Liu T., Ridker P. M. Lancet. 2002;359:2159–2163. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- Kempf T., von Haehling S., Peter T., Allhoff T., Cicoira M., Doehner W., Ponikowski P., Filippatos G. S., Rozentryt P., Drexler H., Anker S. D., Wollert K. C. J. Am. Coll. Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- Hegyesi H., Sándor N., Schilling B., Kis E., Lumniczky K. and Sáfrány G., in Radiation Damage in Biomolecular Systems, Biological and Medical Physics, Biomedical Engineering, ed. G. Garcia and M. C. Fuss, Springer, 2012, vol. Part 3, pp. 359–370. [Google Scholar]
- Chauhan V., Howland M. Toxicol. in Vitro. 2014;28:1222–1229. doi: 10.1016/j.tiv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Hada M., Georgakilas A. G. J. Radiat. Res. 2008;49:203–210. doi: 10.1269/jrr.07123. [DOI] [PubMed] [Google Scholar]
- Sándor N., Schilling-Tóth B., Kis E., Benedek A., Lumniczky K., Sáfrány G., Hegyesi H. Mutat. Res. 2015 doi: 10.1016/j.mrgentox.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Schiegnitz E., Kämmerer P. W., Rode K., Schorn T., Brieger J., Al-Nawas B. J. Oral Pathol. Med. 2015 doi: 10.1111/jop.12323. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Moon Y., Norimura T., Eling T. Radiat. Res. 2006;165:125–130. doi: 10.1667/rr3492.1. [DOI] [PubMed] [Google Scholar]
- Lin T. J., Chang J. T., Wang H. M., Chan S. H., Chiu C. C., Lin C. Y., Fan K. H., Liao C. T., Chen I. H., Liu T. Z., Li H. F., Cheng A. J. Int. J. Radiat. Oncol., Biol., Phys. 2010;78:246–256. doi: 10.1016/j.ijrobp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Gaipl U. S., Multhoff G., Scheithauer H., Lauber K., Hehlgans S., Frey B., Rodel F. Immunotherapy. 2014;6:597–610. doi: 10.2217/imt.14.38. [DOI] [PubMed] [Google Scholar]
- Kowalczyk D. W. Acta Biochim. Pol. 2002;49:295–302. [PubMed] [Google Scholar]
- Mittal D., Gubin M. M., Schreiber R. D., Smyth M. J. Curr. Opin. Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely M. D., Schreiber R. D. Ann. N. Y. Acad. Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J. F., Liu N., Candolfi M., Xiong W., Assi H., Yagiz K., Edwards M. R., Michelsen K. S., Kroeger K. M., Liu C., Muhammad A. K., Clark M. C., Arditi M., Comin-Anduix B., Ribas A., Lowenstein P. R., Castro M. G. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R., Emi M., Tanabe K. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Old L. J., Smyth M. J. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Bennaceur K., Chapman J. A., Touraine J. L., Portoukalian J. Biochim. Biophys. Acta. 2009;1795:16–24. doi: 10.1016/j.bbcan.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Lora A., Martinez M., Algarra I., Gaforio J. J., Garrido F. Int. J. Cancer. 2003;106:521–527. doi: 10.1002/ijc.11241. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Murphy S. P., Ferrone S. Hum. Immunol. 2003;64:1057–1063. doi: 10.1016/j.humimm.2003.08.357. [DOI] [PubMed] [Google Scholar]
- Wilczynski J. R., Duechler M. Arch. Immunol. Ther. Exp. 2010;58:435–448. doi: 10.1007/s00005-010-0102-1. [DOI] [PubMed] [Google Scholar]
- Valenti R., Huber V., Iero M., Filipazzi P., Parmiani G., Rivoltini L. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- Al-Mayah A., Bright S., Chapman K., Irons S., Luo P., Carter D., Goodwin E., Kadhim M. Mutat. Res. 2015;772:38–45. doi: 10.1016/j.mrfmmm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Balermpas P., Rodel F., Liberz R., Oppermann J., Wagenblast J., Ghanaati S., Harter P. N., Mittelbronn M., Weiss C., Rodel C., Fokas E. Br. J. Cancer. 2014;111:1509–1518. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C. D. Front. Immunol. 2015;6:212. doi: 10.3389/fimmu.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Buttner-Herold M., Hyckel P., Moebius P., Distel L., Ries J., Amann K., Neukam F. W., Wehrhan F. J. Craniomaxillofac. Surg. 2014;42:1087–1094. doi: 10.1016/j.jcms.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Gabriels K., Hoving S., Gijbels M. J., Pol J. F., te Poele J. A., Biessen E. A., Daemen M. J., Stewart F. A., Heeneman S. Radiother. Oncol. 2014;110:455–460. doi: 10.1016/j.radonc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Mills C. D., Kincaid K., Alt J. M., Heilman M. J., Hill A. M. J. Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Klug F., Prakash H., Huber P. E., Seibel T., Bender N., Halama N., Pfirschke C., Voss R. H., Timke C., Umansky L., Klapproth K., Schakel K., Garbi N., Jager D., Weitz J., Schmitz-Winnenthal H., Hammerling G. J., Beckhove P. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Carretero R., Sektioglu I. M., Garbi N., Salgado O. C., Beckhove P., Hammerling G. J. Nat. Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- Frey B., Hehlgans S., Rodel F., Gaipl U. S. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Lauber K., Ernst A., Orth M., Herrmann M., Belka C. Front. Oncol. 2012;2:116. doi: 10.3389/fonc.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B., Rubner Y., Kulzer L., Werthmoller N., Weiss E. M., Fietkau R., Gaipl U. S. Cancer Immunol. Immunother. 2014;63:29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll R. E., Herrmann M., Roth E. A., Stach C., Kalden J. R., Girkontaite I. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Gallucci S., Matzinger P. Curr. Opin. Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Werthmoller N., Frey B., Wunderlich R., Fietkau R., Gaipl U. S. Cell Death Dis. 2015;6:e1761. doi: 10.1038/cddis.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkopf P., Frey B., Ott O. J., Rubner Y., Multhoff G., Sauer R., Fietkau R., Gaipl U. S. Radiother. Oncol. 2011;101:109–115. doi: 10.1016/j.radonc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- Adkins I., Fucikova J., Garg A. D., Agostinis P., Spisek R. Oncoimmunology. 2014;3:e968434. doi: 10.4161/21624011.2014.968434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O., Senovilla L., Vitale I., Vacchelli E., Adjemian S., Agostinis P., Apetoh L., Aranda F., Barnaba V., Bloy N., Bracci L., Breckpot K., Brough D., Buque A., Castro M. G., Cirone M., Colombo M. I., Cremer I., Demaria S., Dini L., Eliopoulos A. G., Faggioni A., Formenti S. C., Fucikova J., Gabriele L., Gaipl U. S., Galon J., Garg A., Ghiringhelli F., Giese N. A., Guo Z. S., Hemminki A., Herrmann M., Hodge J. W., Holdenrieder S., Honeychurch J., Hu H. M., Huang X., Illidge T. M., Kono K., Korbelik M., Krysko D. V., Loi S., Lowenstein P. R., Lugli E., Ma Y., Madeo F., Manfredi A. A., Martins I., Mavilio D., Menger L., Merendino N., Michaud M., Mignot G., Mossman K. L., Multhoff G., Oehler R., Palombo F., Panaretakis T., Pol J., Proietti E., Ricci J. E., Riganti C., Rovere-Querini P., Rubartelli A., Sistigu A., Smyth M. J., Sonnemann J., Spisek R., Stagg J., Sukkurwala A. Q., Tartour E., Thorburn A., Thorne S. H., Vandenabeele P., Velotti F., Workenhe S. T., Yang H., Zong W. X., Zitvogel L., Kroemer G., Galluzzi L. Oncoimmunology. 2014;3:e955691. [Google Scholar]
- Fucikova J., Moserova I., Truxova I., Hermanova I., Vancurova I., Partlova S., Fialova A., Sojka L., Cartron P. F., Houska M., Rob L., Bartunkova J., Spisek R. Int. J. Cancer. 2014;135:1165–1177. doi: 10.1002/ijc.28766. [DOI] [PubMed] [Google Scholar]
- Weiss E. M., Frey B., Rodel F., Herrmann M., Schlucker E., Voll R. E., Fietkau R., Gaipl U. S. Ann. N. Y. Acad. Sci. 2010;1209:109–117. doi: 10.1111/j.1749-6632.2010.05743.x. [DOI] [PubMed] [Google Scholar]
- Frey B., Stache C., Rubner Y., Werthmoller N., Schulz K., Sieber R., Semrau S., Rodel F., Fietkau R., Gaipl U. S. J. Immunotoxicol. 2012;9:301–313. doi: 10.3109/1547691X.2012.693547. [DOI] [PubMed] [Google Scholar]
- Yuan F., Gu L., Guo S., Wang C., Li G. M. J. Biol. Chem. 2004;279:20935–20940. doi: 10.1074/jbc.M401931200. [DOI] [PubMed] [Google Scholar]
- Rovere-Querini P., Capobianco A., Scaffidi P., Valentinis B., Catalanotti F., Giazzon M., Dumitriu I. E., Muller S., Iannacone M., Traversari C., Bianchi M. E., Manfredi A. A. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldi T., Talamo F., Scaffidi P., Ferrera D., Porto A., Bachi A., Rubartelli A., Agresti A., Bianchi M. E. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Svetkauskaite D., He Q., Kim J. Y., Strassheim D., Ishizaka A., Abraham E. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M. C., Ullrich E., Saulnier P., Yang H., Amigorena S., Ryffel B., Barrat F. J., Saftig P., Levi F., Lidereau R., Nogues C., Mira J. P., Chompret A., Joulin V., Clavel-Chapelon F., Bourhis J., Andre F., Delaloge S., Tursz T., Kroemer G., Zitvogel L. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Dong Xda E., Ito N., Lotze M. T., Demarco R. A., Popovic P., Shand S. H., Watkins S., Winikoff S., Brown C. K., Bartlett D. L., Zeh 3rd H. J. J. Immunother. 2007;30:596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- Golden E. B., Frances D., Pellicciotta I., Demaria S., Helen Barcellos-Hoff M., Formenti S. C. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A., Anders H., Kapfhammer H., Orth M., Hennel R., Seidl K., Winssinger N., Belka C., Unkel S., Lauber K. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Coppe J. P., Desprez P. Y., Krtolica A., Campisi J. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radons J., Multhoff G. Exerc. Immunol. Rev. 2005;11:17–33. [PubMed] [Google Scholar]
- Arnold-Schild D., Hanau D., Spehner D., Schmid C., Rammensee H. G., de la Salle H., Schild H. J. Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- Ordonez R., Henriquez-Hernandez L. A., Federico M., Valenciano A., Pinar B., Lloret M., Bordon E., Rodriguez-Gallego C., Lara P. C. Strahlenther. Onkol. 2014;190:210–216. doi: 10.1007/s00066-013-0488-x. [DOI] [PubMed] [Google Scholar]
- Multhoff G. Int. J. Hyperthermia. 2002;18:576–585. doi: 10.1080/0265673021000017109. [DOI] [PubMed] [Google Scholar]
- Coade S. B., Pearson J. D. Circ. Res. 1989;65:531–537. doi: 10.1161/01.res.65.3.531. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. Trends Pharmacol. Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Aymeric L., Apetoh L., Ghiringhelli F., Tesniere A., Martins I., Kroemer G., Smyth M. J., Zitvogel L. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Tsukimoto M., Takenouchi T., Harada H., Suzuki A., Sato M., Kitani H., Kojima S. Biochim. Biophys. Acta. 2010;1800:40–46. doi: 10.1016/j.bbagen.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Frey B., Rubner Y., Wunderlich R., Weiss E. M., Pockley A. G., Fietkau R., Gaipl U. S. Curr. Med. Chem. 2012;19:1751–1764. doi: 10.2174/092986712800099811. [DOI] [PubMed] [Google Scholar]
- Scheithauer H., Belka C., Lauber K., Gaipl U. S. Radiat. Oncol. 2014;9:185. doi: 10.1186/1748-717X-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S., Formenti S. C. Int. J. Radiat. Biol. 2007;83:819–825. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]
- Hallahan D. E., Spriggs D. R., Beckett M. A., Kufe D. W., Weichselbaum R. R. Proc. Natl. Acad. Sci. U. S. A. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M., Abrams S. I., Coleman C. N., Camphausen K., Schlom J., Hodge J. W. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- Sheard M. A., Vojtesek B., Janakova L., Kovarik J., Zaloudik J. Int. J. Cancer. 1997;73:757–762. doi: 10.1002/(sici)1097-0215(19971127)73:5<757::aid-ijc24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Reits E. A., Hodge J. W., Herberts C. A., Groothuis T. A., Chakraborty M., Wansley E. K., Camphausen K., Luiten R. M., de Ru A. H., Neijssen J., Griekspoor A., Mesman E., Verreck F. A., Spits H., Schlom J., van Veelen P., Neefjes J. J. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastpar R., Gehrmann M., Bausero M. A., Asea A., Gross C., Schroeder J. A., Multhoff G. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov L. B., Nik-Zainal S., Wedge D. C., Aparicio S. A., Behjati S., Biankin A. V., Bignell G. R., Bolli N., Borg A., Borresen-Dale A. L., Boyault S., Burkhardt B., Butler A. P., Caldas C., Davies H. R., Desmedt C., Eils R., Eyfjord J. E., Foekens J. A., Greaves M., Hosoda F., Hutter B., Ilicic T., Imbeaud S., Imielinski M., Jager N., Jones D. T., Jones D., Knappskog S., Kool M., Lakhani S. R., Lopez-Otin C., Martin S., Munshi N. C., Nakamura H., Northcott P. A., Pajic M., Papaemmanuil E., Paradiso A., Pearson J. V., Puente X. S., Raine K., Ramakrishna M., Richardson A. L., Richter J., Rosenstiel P., Schlesner M., Schumacher T. N., Span P. N., Teague J. W., Totoki Y., Tutt A. N., Valdes-Mas R., van Buuren M. M., van 't Veer L., Vincent-Salomon A., Waddell N., Yates L. R., Zucman-Rossi J., Futreal P. A., McDermott U., Lichter P., Meyerson M., Grimmond S. M., Siebert R., Campo E., Shibata T., Pfister S. M., Campbell P. J., Stratton M. R. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler M. H., Squiban C., van der Meeren A., Bertho J. M., Vandamme M., Mouthon M. A. Int. J. Radiat. Biol. 1997;72:201–209. doi: 10.1080/095530097143428. [DOI] [PubMed] [Google Scholar]
- Hodge J. W., Ardiani A., Farsaci B., Kwilas A. R., Gameiro S. R. Semin. Oncol. 2012;39:323–339. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulzer L., Rubner Y., Deloch L., Allgauer A., Frey B., Fietkau R., Dorrie J., Schaft N., Gaipl U. S. J. Immunotoxicol. 2014;11:328–336. doi: 10.3109/1547691X.2014.880533. [DOI] [PubMed] [Google Scholar]
- Bernstein M. B., Garnett C. T., Zhang H., Velcich A., Wattenberg M. M., Gameiro S. R., Kalnicki S., Hodge J. W., Guha C. Cancer Biother. Radiopharm. 2014;29:153–161. doi: 10.1089/cbr.2013.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R. R., Fu Y. X. J. Clin. Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker C. A., Postow M. A. Int. J. Radiat. Oncol., Biol., Phys. 2014;88:986–997. doi: 10.1016/j.ijrobp.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman-Saint Victor C., Rech A. J., Maity A., Rengan R., Pauken K. E., Stelekati E., Benci J. L., Xu B., Dada H., Odorizzi P. M., Herati R. S., Mansfield K. D., Patsch D., Amaravadi R. K., Schuchter L. M., Ishwaran H., Mick R., Pryma D. A., Xu X., Feldman M. D., Gangadhar T. C., Hahn S. M., Wherry E. J., Vonderheide R. H., Minn A. J. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons W. B., Watkins C. H., Pease G. L., Childs D. S. Cancer. 1954;7:179–189. doi: 10.1002/1097-0142(195401)7:1<179::aid-cncr2820070120>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Xue L. Y., Butler N. J., Makrigiorgos G. M., Adelstein S. J., Kassis A. I. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13765–13770. doi: 10.1073/pnas.182209699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babini G., Bellinzona V. E., Morini J., Baiocco G., Mariotti L., Unger K., Ottolenghi A. Radiat. Prot. Dosimetry. 2015 doi: 10.1093/rpd/ncv133. [DOI] [PubMed] [Google Scholar]
- Yum E. H. W., Choi V. W. Y., Nikezic D., Li V. W. T., Cheng S. H., Yu K. N. Radiat. Meas. 2009;44:1077–1080. [Google Scholar]
- Yang G., Wu L., Chen L., Pei B., Wang Y., Zhan F., Wu Y., Yu Z. Radiat. Res. 2007;167:298–305. doi: 10.1667/RR0710.1. [DOI] [PubMed] [Google Scholar]
- Yang G., Mei T., Yuan H., Zhang W., Chen L., Xue J., Wu L., Wang Y. Radiat. Res. 2008;170:372–380. doi: 10.1667/RR1324.1. [DOI] [PubMed] [Google Scholar]
- Li F., Liu P., Wang T., Bian P., Wu Y., Wu L., Yu Z. Radiat. Res. 2010;174:228–237. doi: 10.1667/RR2052.1. [DOI] [PubMed] [Google Scholar]
- Sarapultseva E. I., Gorski A. I. Dose-Response. 2013;11:460–468. doi: 10.2203/dose-response.12-033.Sarapultseva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Sun J., Bian P., Chen L., Zhan F., Wang J., Xu A., Wang Y., Hei T. K., Wu L. Radiat. Res. 2013;180:268–275. doi: 10.1667/RR3218.1. [DOI] [PubMed] [Google Scholar]


