 The objective of this study is to determine testicular pathological damage and explore its molecular mechanisms after di-2-ethylhexyl phthalate (DEHP) treatment.
The objective of this study is to determine testicular pathological damage and explore its molecular mechanisms after di-2-ethylhexyl phthalate (DEHP) treatment.
Abstract
The objective of this study is to determine testicular pathological damage and explore its molecular mechanisms after di-2-ethylhexyl phthalate (DEHP) treatment. A total of 40 healthy 5-week-old male Sprague-Dawley rats were randomly divided into four groups, which received intragastric administration of 0 mg kg–1, 100 mg kg–1, 500 mg kg–1 and 1500 mg kg–1 DEHP for six continuous weeks. After DEHP treatment, the testes wet weight and testes coefficient were calculated, the histopathological changes of the testes were examined by HE staining and the testicular ultrastructure was examined by transmission electron microscopy. The gene expression levels were analyzed by quantitative RT-PCR and the protein expression levels were analyzed by western blotting. Both 500 mg kg–1 and 1500 mg kg–1 DEPH treatments decreased the wet weight of the testes and testes coefficient, due to vacuoles in Sertoli cells, broken mitochondrial ridges, and degranulation. Quantitative RT-PCR showed that the relative gene expression levels of steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD) increased in the 100 mg kg–1, 500 mg kg–1, and 1500 mg kg–1 DEHP groups, respectively. Additionally, 17β-hydroxysteroid dehydrogenase (17β-HSD) expression levels were increased in the 1500 mg kg–1 DEHP treatment group. Gonadotropin-releasing hormone (GnRH) expression levels were decreased with 500 mg kg–1 and 1500 mg kg–1 DEHP treatments. DEHP induced serious pathological damage and ultrastructure changes in rat testes, caused endocrine disorders, interfered with the synthesis of male hormones, and ultimately led to male reproductive system dysfunction.
1. Introduction
Phthalates or phthalic esters (PAEs) are esters of phthalic acid, a family of industrial chemicals used widely in products such as plasticizers; they impart flexibility, transparency, durability, and longevity to polyvinyl chloride (PVC) products.1 PVC products contain PAEs which are widely used in building materials, children's toys, food packages, medical devices and cosmetics.2 More than 18 billion pounds of PAEs are used worldwide each year.3 When added into PVC products, PAEs are not covalently bound and therefore can easily contaminate the atmosphere, water, and soil. This has resulted in animal and human PAE exposure. PAEs can be absorbed into the human body through the mouth, the skin, and the respiratory tract, causing disruption to the endocrine system.4,5 One study performed over 25 years has shown that some PAEs are transformed to monoesters with an ester side chain of between four to six carbons, including di-2-ethylhexyl phthalate (DEHP), benzyl butyl phthalate (BBP), and dibutyl phthalate (DBP), that may induce testicular injury.
Among the PAEs, DEHP is one of the most commonly used phthalate plasticizers, possibly leading to reproductive and developmental toxicities.3,6 DEHP has been found in amniotic fluid, placenta, and other rat tissues, suggesting that DEHP may play a role during certain biological processes.7 Studies have indicated that the exposure of DEHP ranges between 3 and 30 mg kg–1 day–1; higher exposure may occur in some special medical conditions, such as through parenteral nutrition or neonatal transfusion, in which the DEHP content may be up to 20 mg kg–1 day–1.8 For example, cancer patients have exposure to DEHP after receiving the previously mentioned types of interventions.9
Studies have demonstrated that DEHP, one of the most widely applied PAEs, is metabolized into mono-(2-ethylhexyl) phthalate (MEHP), which is considered a testicular toxicant.10,11 Human epidemiologic studies have pointed out associations between DEHP exposure and various adverse reproductive effects, including testicular dysgenesis syndrome,12 the inhibition of testosterone production,13 and impaired reproductive development and function.14–16 Zhang et al. indicated that treatment with 150 mg kg–1 DEHP caused testicular injury, demonstrated by the impairment of the seminiferous epithelium and its cell layers.17 However, mechanisms of the testicular toxicity of DEHP are not fully understood. Studies indicated that DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development associated with the rat family.13 Previous studies mainly focused on the effects of DEHP on testosterone rather than the systematic study of the testicular hormones related to the endocrine system.13,18,19 This study was performed to explore the effects of DEHP exposure on testicular histological changes and the ultrastructure, and the possible involvement of hormone-regulated genes related to DEHP treatment toxicity.
2. Materials and methods
2.1. Chemicals
DEHP was purchased from Tokyo Chemical Industry Corporation (Tokyo, Japan). Corn oil was bought from Yihai Kerry Food Marketing Co., Ltd (Shanghai, China). TRIzol reagent was purchased from Invitrogen-Life Technologies (Gaithersburg, Maryland, USA). The mouse antibodies included anti-GAPDH, mouse anti-β-actin, mouse anti-3β-HSD, and rabbit anti-GnRHR. The mouse anti-17β-HSD and mouse anti-StAR were purchased from Santa Cruz Biotech (Santa Cruz, California, USA).
2.2. Animal treatment
A total of 40 healthy 5-week-old male Sprague-Dawley rats were supplied by the Guangdong Provincial Medical Experimental Animal Center. The rats were kept in cages under a 12 : 12 h light–dark cycle; the light was on from 7 : 00 AM to 7 : 00 PM with controlled ambient temperature (18–25 °C) and humidity (40–50%). The rats were randomly divided into four groups with 10 rats in each group. The DEHP doses were 0 mg kg–1 in the control group (corn oil), 100 mg kg–1 in the low-dose group, 500 mg kg–1 in the medium-dose group, and 1500 mg kg–1 in the high-dose group. The rats were exposed to DEHP via gavage administration for a period of six weeks; DEHP administration was once a day, five times a week. All experiments were performed in compliance with the relevant guidelines of China National Animal Epidemic Prevention Law; all experiments followed institutional guidelines; all experiments were carried out in accordance with a protocol approved by the Institutional Ethical Committee (IEC) of Shenzhen Center for Disease Control and Prevention with the permission no. IEC/3-4/5.1-5.8.
2.3. Hematoxylin and eosin (HE) staining
The testicular tissues from the rats were fixed with formalin for 24 h and embedded overnight with paraffin. The paraffin sections for HE staining were prepared. Following hematoxylin solution (ZSGB-BIO, Beijing, China) staining for 5 min, the sections were stained in aqueous eosin solution for 1 min. The testicular sections were treated with xylene three times and then mounted.
2.4. Transmission electron microscopy (TEM)
Ultrathin sections of the testicular tissue were analyzed using TEM as previously reported.20 Briefly, the rats were decapitated after DEHP treatment. The testicular tissue was rapidly removed and fixed in 2.5% glutaraldehyde for 2 hours, and the samples were dehydrated in an ethanol series and embedded in epoxy resin. After that, the samples were sent to the Key Laboratory of Modern Molecular Biology of Shenzhen Center for Disease Control and Prevention for electron microscopy preparation.
2.5. RNA isolation and reverse transcription
RNA isolation and quantitative PCR of the testicular tissue were performed as previously reported.21 Briefly, the rats were decapitated and the testicular tissue was removed. After testicular tissue homogenization, the samples were subjected to RNA isolation using TRIzol and chloroform, respectively. After isolation, the quantity and quality of the RNA were measured with a NanoDrop ND-1000 (NanoDrop Technologies, Inc. Wilmington, New Jersey). The RNA content was obtained by measuring the absorbance at 260 nm, and the quantity was assessed by measuring the A260/A280 ratio, which should be between 1.8 and 2.0, and the A260/A230 ratio. One microgram of total RNA was reverse transcribed in a volume of 20 μL containing 10 mM dNTPs, 0.1 M dithiothereitol, 200 units of SuperScript II, 20 units of RNase inhibitor, 1 μL of random primer, and 2 μL of reverse transcriptase buffer at 42 °C for 1 h, followed by incubation at 70 °C for 10 min.
2.6. Quantitative PCR (Q-PCR)
We measured the samples by Q-PCR using a SYBR® Advantage® Q-PCR Premix (Takara Bio-Company, Dalian, China). PCR primers were StAR-F (CAAA CTCAC GTGGC TGCTCAGTA) and StAR-R (GCAAGTGGCTGG CGAA CTCTATT), 3β-HSD-F (TGAAGGAGGTCAGGGTC) and 3β-HSD-R (CACTGGGTGTCAA GAATGT), and 17β-HSD-F (CACTGCAA CATTACCTCCGTAGTCA) and 17β-HSD-R (CTATACAGAGG CCAGG GACGAAC). The reaction was performed in a 25 μL volume with 12.5 μL Q-PCR premix, 1 μL primers (10 μM), and 2 μL of DNA templates at 80 ng μL–1 to 1 pg μL–1. The Q-PCR amplification was performed as follows: initial denaturation at 95 °C for 5 min, 40 cycles of 95 °C for 5 s, 54 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. The expression of the housekeeping gene GAPDH was used to normalize the samples in this experiment. All samples were run in triplicate, and the standard curves were represented by plotting the Ct value versus the logarithm of the gene number.
2.7. Western blot analysis
The testicular tissue was rapidly removed and homogenized at 4 °C in 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 2 mM benzamidine, and 1 mM phenylmethylsulfonyl fluoride after the rats were decapitated. The lysis was mixed with a loading buffer containing 200 mM Tris-HCl, pH 7.6, 8% SDS, 40% glycerol, and 40 mM DTT, boiled for 5 min, and then centrifuged at 12 000g for 30 min at room temperature. After this the concentration of proteins was measured using a Pierce BCA kit (Perbio Science, Tattenhall, UK), the proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a poly (vinylidene) fluoride (PVDF) membrane. The PVDF membrane was blocked with 5% skimmed milk for 2 h at room temperature and incubated with primary antibodies at 4 °C overnight. The following primary antibodies were diluted in TBS containing 0.05% Tween-20 (TBST): mouse anti-GAPDH (1 : 2000), mouse anti-β-actin (1 : 2000), mouse anti-3β-HSD (1 : 400), mouse anti-17β-HSD (1 : 1000), mouse anti-StAR (1 : 1000), and rabbit anti-GnRHR (1 : 400). The membranes were then incubated with anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase (1 : 5000) for 1 h at room temperature and visualized with an ImageQuant™ RT ECL System (GE Healthcare). The relative levels of the proteins were measured using ImageJ 1.43U software (National Institutes of Health, Bethesda, Maryland, USA).
2.8. Statistical analysis
The values were expressed as mean ± SEM. All the experimental data were processed by one-way ANOVA (LSD) of all groups using the software package SPSS, version 14.0. Significant differences were regarded at P < 0.05.
3. Results
3.1. Effects of DEHP on body weight and the weight coefficients of testes
The effects of the administration of DEHP on the body weight and the weight coefficients of the testes are summarized in Tables 1 and 2. After DEHP exposure, all surviving rats had normal clinical signs. No differences were found in the initial and final body weight in each group. Treatment with 100 mg kg–1 and 500 mg kg–1 DEHP did not affect the weight coefficients of the testes compared with the control group. However, treatment with 1500 mg kg–1 DEHP significantly decreased the weight coefficients of the testes compared with the control group.
Table 1. The effects of DEHP on body weight (g). Mean ± SEM (n = 10).
| Group | Body weight (g) (pre-treatment) | Body weight (g) (after treatment) | Body weight change (g) |
| Control | 59.10 ± 3.21 | 355.07 ± 12.56 | 295.97 ± 9.59 |
| DEHP (100 mg kg–1) | 61.82 ± 2.75 | 387.91 ± 7.63 | 326.08 ± 10.34 |
| DEHP (500 mg kg–1) | 60.57 ± 3.23 | 377.70 ± 19.86 | 317.12 ± 22.15 |
| DEHP (1500 mg kg–1) | 56.87 ± 7.50 | 375.12 ± 11.16 | 318.24 ± 13.77 |
Table 2. The effects of DEHP on the weight coefficients of the testes. Values are expressed as the mean ± SEM (n = 10). *Significant differences of the 1500 mg kg–1 DEHP-treated group compared to the control group (0 mg kg–1 DEHP) at P < 0.05.
| Group | Body weight (g) | Testicular weight (g) | Weight coefficients of testis (%) |
| Control | 355.07 ± 12.56 | 3.46 ± 0.28 | 0.97 ± 0.07 |
| DEHP (100 mg kg–1) | 387.91 ± 7.63 | 3.49 ± 0.48 | 0.90 ± 0.13 |
| DEHP (500 mg kg–1) | 377.70 ± 19.86 | 3.47 ± 0.04 | 0.92 ± 0.06 |
| DEHP (1500 mg kg–1) | 375.12 ± 11.16 | 1.47 ± 0.46 | 0.39 ± 0.12* |
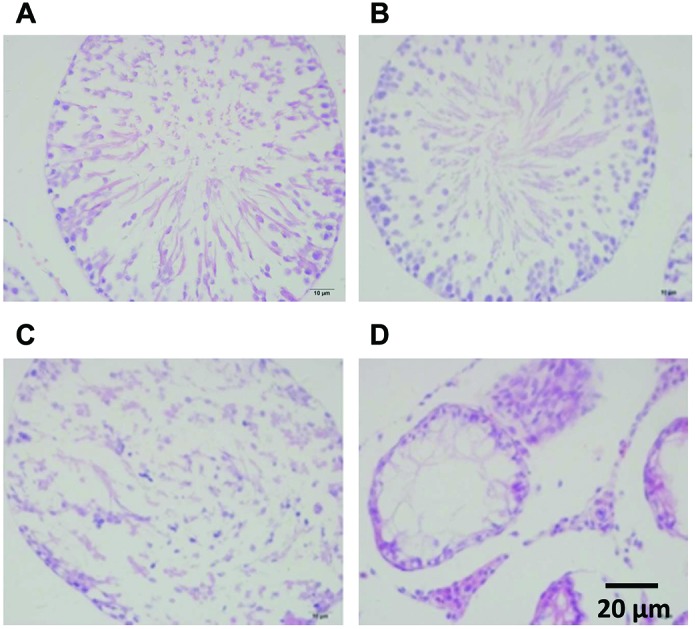
3.2. Effects of DEHP on histological changes of testis after DEHP treatment
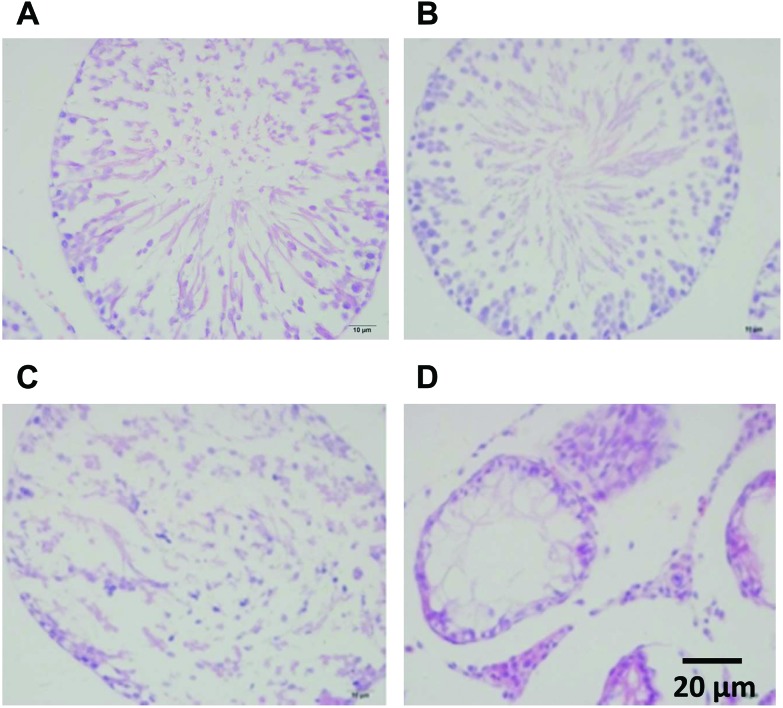
No detectable histological damage was detected in the testes of rats in the control group (Fig. 1A). There was slight damage to the testes with Sertoli cells detachment after treatment with 100 mg kg–1 DEHP (Fig. 1B). The spermatocytes were decreased and there was an abnormal arrangement with the detached Sertoli cells after treatment with 500 mg kg–1 DEHP (Fig. 1C). Moreover, atrophic seminiferous tubules, a loss of different stages of spermatogenic cells, vacuoles of the Sertoli cells and abnormal proliferation of Leydig cells were found in the group of 1500 mg kg–1 DEHP compared with the control group (Fig. 1D).
Fig. 1. A photomicrograph of a paraffin section in testes HE staining. A: Testis of the control group showing no detectable histological damage. B: Testes of the 100 mg kg–1 DEHP group showing a slight damage of the Sertoli cell detachment. C: Testes of the 500 mg kg–1 DEHP group showing decreased spermatocytes and the abnormal arrangement of the Sertoli cells. D: Testes of the 1500 mg kg–1 DEHP group showing a loss of different stages of the spermatogenic cells and the abnormal proliferation of Leydig cells.
3.3. Ultrastructural observations of testis after DEHP treatment
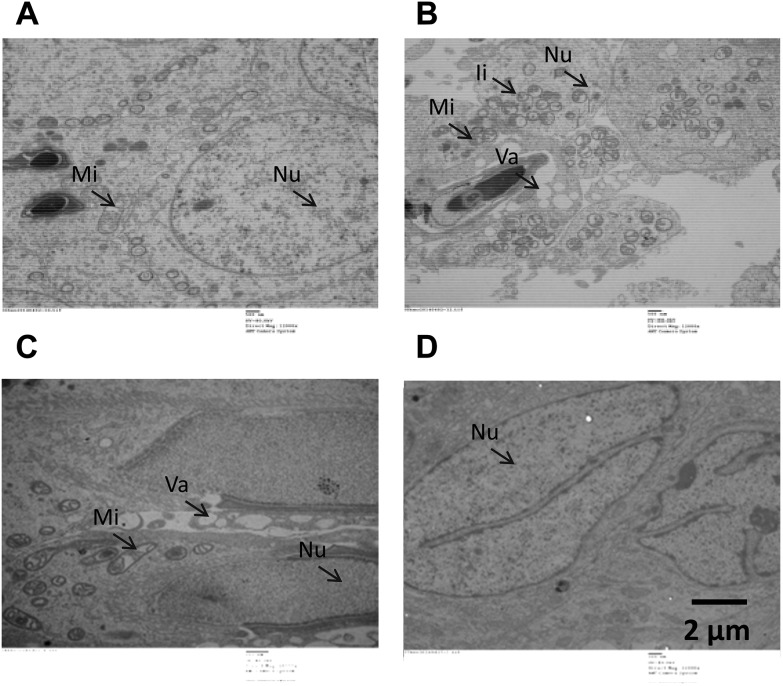
The results obtained from transmission electron microscopy confirmed the HE staining observations. In the control group, we found that the ultrastructure of secondary spermatocytes and spermatoblasts was normal, displaying a smooth cell membrane, uniformity of the nuclear membrane, abundant euchromatin, round or short columnar mitochondria with tubulovesicular shapes, as well as a normal microstructure in the sperm head, sperm acrosomal cap, and tail (Fig. 2A). In the group of 100 mg kg–1 DEHP, intranuclear inclusions occurred in the spermatogenic cells’ nuclei, vacuoles were increased in the cytoplasm, and the mitochondria were mildly swollen, with broken crests and degranulation; the spermatocyte head contained inclusions and an abnormal mitochondrial sheath of the sperm cells was found (Fig. 2B). In the group of 500 mg kg–1 DEHP, numerous vacuoles occurred in the sperm nucleus. An incomplete cell membrane as well as a reduction of mitochondria and vacuoles were found in the spermatogonium after treatment with 500 mg kg–1 DEHP. Meanwhile, the abnormal development of secondary spermatocytes and spermatoblasts occurred with a decreased electron density in the head of the sperm. Moreover, the reduction of mitochondria, medullary and cavitation changes, as well as microtubule structural changes did not occur in the sperm tail in the secondary spermatocyte and spermatoblasts (Fig. 2C). We found some injuries caused by 1500 mg kg–1 DEHP, including a disorder of the cell structure, the loss of spermatoblasts, an increased nuclear volume, depressions in deformed nuclei, an increased nucleus/cytoplasm ratio, and the existence of cleaved notching in the rough nuclear membrane, with nuclear heterochromatin binding under the nuclear envelope. Moreover, 1500 mg kg–1 DEHP could induce the increase of the mitochondria in various forms, decreased mitochondrial cristae and organelles, and apomorphosis and necrosis (Fig. 2D).
Fig. 2. The results obtained from transmission electron microscopy revealed the testicular ultrastructure. A: Testis of the control group showing no detectable damage. B: Testis of the 100 mg kg–1 DEHP group showing intranuclear inclusions and vacuoles. C: Testis of the 500 mg kg–1 DEHP group showing the abnormal development of secondary spermatocytes and spermatoblasts. D: Testis of the 1500 mg kg–1 DEHP group showing disorder of the cell structure and the decrease of mitochondrial cristae and organelles. Nu: Nucleus; Mi: mitochondria; Ii: intranuclear inclusions; Va: vacuoles.
3.4. Effects of DEHP on the changes of hormone-regulated genes
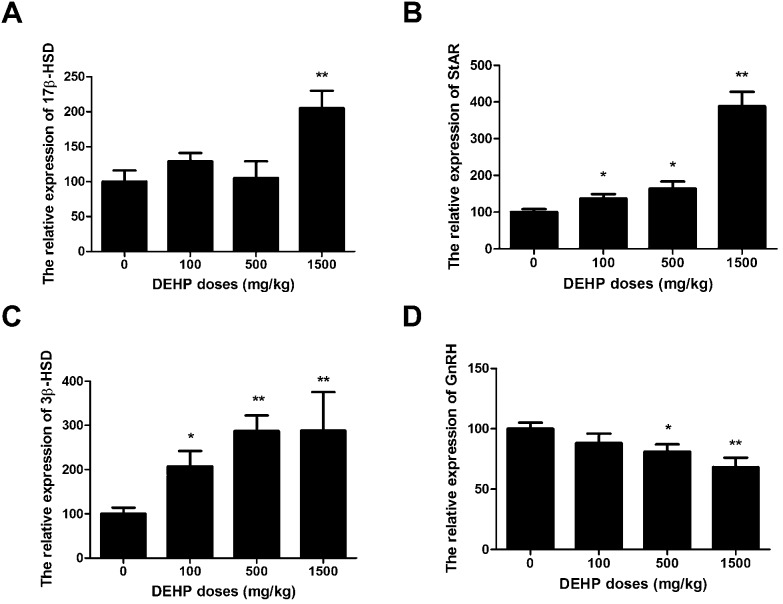
The expressions of StAR, 3β-HSD, 17β-HSD, and GnRH genes are shown in Fig. 3. In this study, DEHP (100 mg kg–1, 500 mg kg–1, and 1500 mg kg–1) significantly increased the expression of the StAR gene compared to the control group in a dose-dependent manner (DEHP significantly increased the StAR gene levels by 40%, 60%, and 380% in the 100 mg kg–1, 500 mg kg–1 and 1500 mg kg–1 groups, respectively (Fig. 3A)). DEHP significantly increased the 3β-HSD gene levels by 105%, 270%, and 290% in the 100 mg kg–1, 500 mg kg–1, and 1500 mg kg–1 groups, respectively (Fig. 3B). In the 1500 mg kg–1 DEHP group, the 17β-HSD gene expression levels were increased by 130% with no significant change compared to the control group (Fig. 3C). In comparison, the GnRH gene levels were reduced by 30% or 40% in the 500 mg kg–1 and 1500 mg kg–1 groups compared to the control group (Fig. 3D).
Fig. 3. Effects of DEHP on the mRNA expression of hormone-regulated genes. The values are expressed as mean ± SEM (n = 5). A: Testicular 17β-HSD relative mRNA expression. B: Testicular StAR relative mRNA expression. C: Testicular 3β-HSD relative mRNA expression. D: Testicular GnRH relative mRNA expression. *, **Significant differences compared to the control group (0 mg kg–1 DEHP) at P < 0.05 and P < 0.01, respectively.
3.5. Effects of DEHP on protein expression
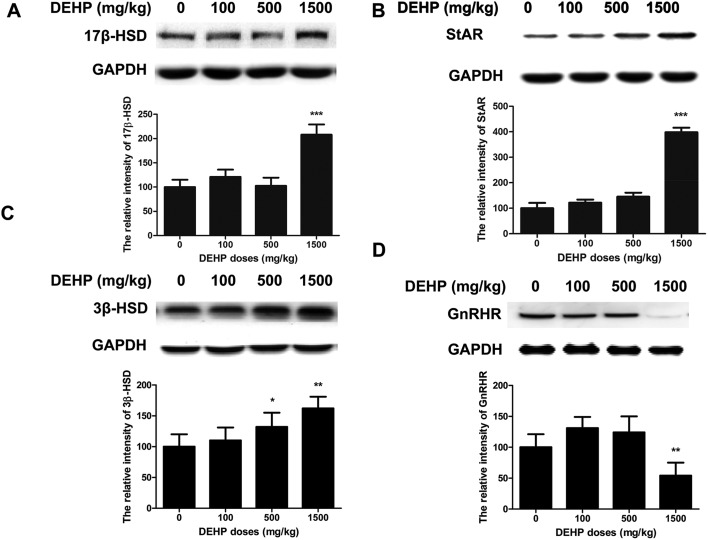
We measured the expression of 17β-HSD, StAR, 3β-HSD, and GnRHR in rat testis after DEHP treatment. The levels of 17β-HSD and StAR were increased about two or four-fold in the 1500 mg kg–1 group compared to the control group. However, this protein level did not significantly change in the 100 mg kg–1 and 500 mg kg–1 groups compared to the control group (Fig. 4A and B). We found that the levels of 3β-HSD were significantly increased by 30% or 70% compared to the control group (Fig. 4C). However, the GnRHR level was significantly decreased by 40% in the 1500 mg kg–1 group compared to the control group (Fig. 4D).
Fig. 4. Effects of DEHP on the expression of hormone-related proteins. The values are expressed as mean ± SEM (n = 5). A: Western-blot analysis showed the levels of 17β-HSD. B: Western-blot analysis showed the change of StAR. C: Western-blot analysis showed the levels of 3β-HSD. D: Western-blot analysis showed the change of GnRHR. *, **Significant differences compared to the control group (0 mg kg–1 DEHP) at P < 0.05 and P < 0.01, respectively.
4. Discussion
DEHP exposure has attracted attention due to its widespread direct and indirect exposure in the environment.22 Previous studies have shown that DEHP could impair male reproductive health.23,24 However, most of the previous studies related to DEHP on male reproductive health focused only on studying the testes.23 In this study, we studied both the testicular anatomy and the endocrine system. The endocrine system is a vitally important regulating system, characterized by various hormone regulatory processes. Complementary to the nervous system, it regulates the growth and metabolism of organisms, maintains the stability of the internal environment, and affects organisms’ behaviors and reproductive ability. In males, the disturbance of the endocrine system can cause hypogonadism and premature senility. The results of hematoxylin and eosin (HE) staining and transmission electron microscopy (TEM) show that DEHP (1500 mg kg–1) induced the formation of vacuoles in the Sertoli cells. In fact, the Sertoli cells play a key role in the reproductive system of male animals, which could suggest that impairment of the Sertoli cells induced by DEHP may be one of the important indices. In this study, DEHP induced the abnormal proliferation of Leydig cells, which pointed out that the DEHP may affect the enzyme function relative to the Leydig cell differentiation, and then impair male reproductive function. Ultrastructural observations have revealed that DEHP induced the impairment of different stages of spermatogenic cells in seminiferous tubules. Thus, we suspected that DEHP could interfere with the formation of sperms, and then impair the reproductive function of male animals.
Androgens control the development and maintenance of male characteristics by binding to androgen receptors; they are involved in the activity of the primary male sex organs and the development of male secondary sex characteristics.25 The Leydig cells produce androgen, which is found adjacent to the seminiferous tubules in the testicle.26 Testosterone is a primary and well-known androgen,27 composed of 19-carbon steroids synthesized in the zona reticularis.28 At least 90% of testosterone is synthesized from the Leydig cells, which requires a series of enzymatic reactions,29 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β-hydroxysteroid dehydrogenase (17β-HSD), and steroidogenic acute regulatory protein (StAR) play a vital role in these reactions.30–32 The steroid hormone synthesis results in StAR expression in the kidney and gonadal tissue during testosterone production.33 The StAR can transfer the cholesterol in the Leydig cells from the outside of the mitochondria into the mitochondria; it is considered to be the rate-limiting step in the biosynthesis of testosterone.34,35 However, the expression of StAR may be affected by the stress state.36 The 3β-HSD is mainly responsible for transferring pregnenolone to progesterone.37 Progesterone has been catalyzed by a series of enzymes to produce testosterone.38 Note that 17β-HSD plays a key role in the last step of the synthesis of testosterone, specifically the catalysis of androstenedione into testosterone.39 Among these three proteins, the formation and function of StAR and 3β-HSD were regulated by steroidogenic factor 1 (SF1).40,41 In this study, the upregulation of StAR and 3β-HSD might be caused by exposure to DEHP, resulting in the activation of the MAPK signaling pathway, which is upstream of SF1.42 After that, the expression levels of StAR and 3β-HSD were upregulated. However, the results showed that the gene and protein expression levels of 17β-HSD were upregulated, suspecting that it may be the biological response of the exposure to DEHP. Although the levels of gene expression, including StAR, 3β-HSD and GnRHR, were not completely consistent with the protein content at all doses, the expression trend in the 1500 mg kg–1 group was the same. We speculated that it may be due to the post-transcriptional modification. More specifically, excess expression of some enzymes aims to meet the requirements of the testosterone levels in the body, whereas the specific mechanism related to this process requires further study. The gonadotropin-releasing hormone (GnRH) can be selectively stimulated to produce the follicle-stimulating hormone (FSH) and luteotropic hormone (LH),43 and both hormones play a vital role in testosterone production and sperm formation.44,45 The results have shown that GnRH gene expression was down-regulated after DEHP exposure. We suspected that DEHP could impair the reproductive system of male animals via the expression of LH and FSH.
In conclusion, the results of this study show that DEHP exposure can regulate the expression of proteins related to hormone genes, interfere with male sex hormone metabolism, and lead to reproductive dysfunction in male rats. Thus, we suspect that DEHP has a close relationship with reproductive toxicity and further research is desirable.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This project was supported in part by the grants from the basic research programs of Shenzhen Science and Technology Committee to Dr Xinyun Xu (JCYJ20150402102135509 and JCYJ20170306160553495).
References
- Cirillo T., Latini G., Castaldi M. A., Dipaola L., Fasano E., Esposito F., Scognamiglio G., Francesco F. D., Cobellis L. J. Agric. Food Chem. 2015;63:3303–3310. doi: 10.1021/jf505563k. [DOI] [PubMed] [Google Scholar]
- Guo Y., Kannan K. Environ. Sci. Technol. 2013;47:14442–14449. doi: 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- Crinnion W. J. Altern. Med. Rev. 2010;15:190–196. [PubMed] [Google Scholar]
- Abdel-Kawi S. H., Hashem K. S., Abd-Allah S. Food Chem. Toxicol. 2016;90:64–75. doi: 10.1016/j.fct.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Autian J. Environ. Health Perspect. 1973;4:3–26. doi: 10.1289/ehp.73043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. Environ. Health Perspect. 1995;103:582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Agrawal S., Cook T. J., Knipp G. T. Placenta. 2008;29:962–969. doi: 10.1016/j.placenta.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culty M., Thuillier R., Li W., Wang Y., Martinez-Arguelles D. B., Benjamin C. G., Triantafilou K. M., Zirkin B. R., Papadopoulos V. Biol. Reprod. 2008;78:1018–1028. doi: 10.1095/biolreprod.107.065649. [DOI] [PubMed] [Google Scholar]
- Takeshita A., Inagaki K., Igarashi-Migitaka J., Ozawa Y., Koibuchi N. J. Endocrinol. 2006;190:897–902. doi: 10.1677/joe.1.06664. [DOI] [PubMed] [Google Scholar]
- Habert R., Muczynski V., Lehraiki A., Lambrot R., Lecureuil C., Levacher C., Coffigny H., Pairault C., Moison D., Frydman R., Rouiller-Fabre V. Folia Histochem. Cytobiol. 2009;47:S67–S74. doi: 10.2478/v10042-009-0056-5. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Fertil. Steril. 2008;89:e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Stenz L., Escoffier J., Rahban R., Nef S., Paoloni-Giacobino A. PLoS One. 2017;12:e0170441. doi: 10.1371/journal.pone.0170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega N. C., Howdeshell K. L., Furr J., Lambright C. R., Wilson V. S., Gray Jr. L. E. Toxicol. Sci. 2009;111:163–178. doi: 10.1093/toxsci/kfp129. [DOI] [PubMed] [Google Scholar]
- Halden R. U. Annu. Rev. Public Health. 2010;31:179–194. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- Main K. M., Mortensen G. K., Kaleva M. M., Boisen K. A., Damgaard I. N., Chellakooty M., Schmidt I. M., Suomi A. M., Virtanen H. E., Petersen D. V., Andersson A. M., Toppari J., Skakkebaek N. E. Environ. Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H. Environ. Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. D., Li H. C., Chong T., Gao M., Yin J., Fu D. L., Deng Q., Wang Z. M. BioMed Res. Int. 2014;2014:598630. doi: 10.1155/2014/598630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshan M., Hatef A., Socha M., Milla S., Butts I. A., Carnevali O., Rodina M., Sokolowska-Mikolajczyk M., Fontaine P., Linhart O., Alavi S. M. Aquat. Toxicol. 2015;163:16–26. doi: 10.1016/j.aquatox.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Ha M., Guan X., Wei L., Li P., Yang M., Liu C. Sci. Total Environ. 2016;563–564:566–575. doi: 10.1016/j.scitotenv.2016.04.145. [DOI] [PubMed] [Google Scholar]
- Han Z., Yan Q., Ge W., Liu Z. G., Gurunathan S., De Felici M., Shen W., Zhang X. F. Int. J. Nanomed. 2016;11:5187–5203. doi: 10.2147/IJN.S111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Huang C. L., Vonk L. A., Lu Z. F., Bank R. A., Helder M. N., Doulabi B. Z. Bone Joint Res. 2016;5:560–568. doi: 10.1302/2046-3758.511.BJR-2016-0033.R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. M., Mason J. I., Sharpe R. M. Endocr. Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Fisher J. S. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Cattley R. C., Foster P. M. Toxicol. Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Hayes T. B., Anderson L. L., Beasley V. R., de Solla S. R., Iguchi T., Ingraham H., Kestemont P., Kniewald J., Kniewald Z., Langlois V. S., Luque E. H., McCoy K. A., Munoz-de-Toro M., Oka T., Oliveira C. A., Orton F., Ruby S., Suzawa M., Tavera-Mendoza L. E., Trudeau V. L., Victor-Costa A. B., Willingham E. J. Steroid Biochem. Mol. Biol. 2011;127:64–73. doi: 10.1016/j.jsbmb.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. S., Nanjappa M. K., Ko C., Prins G. S., Hess R. A. Physiol. Rev. 2017;97:995–1043. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J., Le Q., Goodyer C., Gelfand M., Trifiro M., LeBlanc A. J. Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Ghayee H. K., Auchus R. J. Rev. Endocr. Metab. Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Akingbemi B. T., Sottas C. M., Koulova A. I., Klinefelter G. R., Hardy M. P. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Homma H., Lee J. A., Imai K. FEBS Lett. 1999;444:160–164. doi: 10.1016/s0014-5793(99)00045-9. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Baker P. J., Heikkila M., Vainio S., McMahon A. P. Endocrinology. 2000;141:2631–2637. doi: 10.1210/endo.141.7.7545. [DOI] [PubMed] [Google Scholar]
- Wiebe J. P. Endocrinology. 1976;98:505–513. doi: 10.1210/endo-98-2-505. [DOI] [PubMed] [Google Scholar]
- Clark B. J., Soo S. C., Caron K. M., Ikeda Y., Parker K. L., Stocco D. M. Mol. Endocrinol. 1995;9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- Stocco D. M. Proc. Soc. Exp. Biol. Med. 1998;217:123–129. doi: 10.3181/00379727-217-44214. [DOI] [PubMed] [Google Scholar]
- Stocco D. M. Annu. Rev. Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Artemenko I. P., Zhao D., Hales D. B., Hales K. H., Jefcoate C. R. J. Biol. Chem. 2001;276:46583–46596. doi: 10.1074/jbc.M107815200. [DOI] [PubMed] [Google Scholar]
- Pasqualini J. R. J. Steroid Biochem. Mol. Biol. 2005;97:401–415. doi: 10.1016/j.jsbmb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Penning T. M., Burczynski M. E., Jez J. M., Hung C. F., Lin H. K., Ma H., Moore M., Palackal N., Ratnam K. Biochem. J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F., Luu-The V., Lin S. X., Simard J., Labrie C. Trends Endocrinol. Metab. 2000;11:421–427. doi: 10.1016/s1043-2760(00)00342-8. [DOI] [PubMed] [Google Scholar]
- Jordan B. K., Shen J. H., Olaso R., Ingraham H. A., Vilain E. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10866–10871. doi: 10.1073/pnas.1834480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrenberg U., Prange-Kiel J., Rune G. M. J. Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Mizusaki H., Shimono A., Mukai T., Okumura K., Abe K., Shimada K., Morohashi K. Genes Cells. 2005;10:421–434. doi: 10.1111/j.1365-2443.2005.00844.x. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Crowley Jr. W. F. Annu. Rev. Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- Dierich A., Sairam M. R., Monaco L., Fimia G. M., Gansmuller A., LeMeur M., Sassone-Corsi P. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. T., Scutt D., Wilson J., Lewis-Jones D. I. Hum. Reprod. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]