 This study evaluated the impact of resveratrol (RSV) and pterostilbene (PT) on the aqueous extract of smokeless tobacco (AEST) induced cardiovascular aberrations in estrogen deficient female Sprague-Dawley rats.
This study evaluated the impact of resveratrol (RSV) and pterostilbene (PT) on the aqueous extract of smokeless tobacco (AEST) induced cardiovascular aberrations in estrogen deficient female Sprague-Dawley rats.
Abstract
This study evaluated the impact of resveratrol (RSV) and pterostilbene (PT) on the aqueous extract of smokeless tobacco (AEST) induced cardiovascular aberrations in estrogen deficient female Sprague-Dawley rats. Exposure to 4-vinylcyclohexene diepoxide (VCD) (80 mg kg–1, i.p.) for 30 days induces estrogen deficiency. The rats were administered AEST alone or AEST along with resveratrol and/or pterostilbene. Several markers of cardiovascular health were estimated to evaluate the repercussion of the exposures. RSV and PT per se and in combination significantly reversed the derangements caused by AEST. RSV decreased the atherogenic index and systolic blood pressure and normalized ECG. RSV and PT treatment markedly decreased aortic collagen, cardiac-carbonylated proteins, serum creatine-kinase, cholesterol, LDH, LDL, VLDL, CRP and TNF-α levels. Conversely, they increased serum nitrate–nitrite and HDL levels. The drugs improved the gene expression of SIRT1, PGC-1α, PPAR-α, TFAM, NRF-1 and mtDNA in the cardiac tissue. However, the expression of SIRT1 was not modified by PT. These favorable effects were comparable to those of estradiol therapy. Histopathological outcomes also corroborated these benefits. Thus, resveratrol and pterostilbene abrogated the deleterious effects of AEST on cardiovascular parameters in estrogen deficient female rats.
Introduction
It has been projected that by the year 2030, tobacco use in the developing world is expected to lead to 7 million deaths annually.1 Smokeless tobacco is believed to be a significant contributor to excess mortality. The relationship between smokeless tobacco consumption and occurrence of adverse cardiovascular events like myocardial infarction, stroke, and ischemic heart disease has been well established.2 Smoking and tobacco use pose a serious health risk in women. It has been reported that women who smoke have an accelerated ovarian aging leading to early menopausal transition.3,4 It is known that post-menopausal women are predisposed to cardiovascular risks due to an estrogen deficient state. Hence, it was imperative to postulate that post-menopausal women consuming smokeless tobacco will be at a higher risk of cardiovascular complications.
The North American Menopause Society (NAMS) has proposed that the number of postmenopausal women will increase to 1.1 billion worldwide by 2025.5 Menopause associated changes in hormone production affect various parts of the body, in particular the cardiovascular system, bones and the urogenital system,6 which is mainly linked to estrogen deficiency.7 Hormone replacement therapy (HRT) is an option for alleviating the majority of symptoms of menopause. However, clinical studies and meta-analysis have indicated increased risk of stroke, breast and endometrial hyperplasia with HRT, thus limiting its use.8
Mitochondrial dysfunction has been at the centre stage in cardiovascular anomalies. Studies in rodents,9,10 dogs11 and humans12 suggest that a reduced number of mitochondria and mitochondrial DNA (mtDNA) with down-regulation of the mitochondrial master regulator PGC-1α acts as a prelude to prospective adverse cardiac events.9,10,13,14 Induction of mitochondrial biogenesis by the activation of nutritional sensors, sirtuin-1 (SIRT 1) and AMP-Activated Protein Kinase (AMPK), through peroxisome proliferator activated receptor-γ coactivator-1α (PGC-1α) has recently emerged as an important pathway to achieve caloric restriction and exercise mimetic health benefits.15–17 The peroxisome proliferator-activated receptor-α (PPAR-α), nuclear respiratory factors (NRF 1 and NRF 2) and mitochondrial transcription factor A (TFAM) are other known intricate players of this pathway.18,19
Resveratrol and pterostilbene are stilbene derivatives which have been extensively explored for their health benefits.20,21 Numerous studies have highlighted the positive implications of resveratrol on mitochondrial biogenesis.22–25 Resveratrol activates SIRT 1 and PGC-1α and contributes to improved mitochondrial function26 and offers intrinsic protective potential to multiple targets related to cardiovascular diseases.27,28 Pterostilbene, a remarkable structural relative of resveratrol with improved bioavailability has been shown to play a beneficial role in optimizing heart function.29 This lies in its ability to activate PPAR-α, furnishing cardiovascular benefits through lipid homeostasis and oxidative metabolism30,31 and promoting normal growth of vascular smooth muscle cells.32
The current investigation was planned to evaluate the influence of oral exposure to the aqueous extract of tobacco leaves on several markers of cardiovascular health and mitochondrial biogenesis in 4-vinylcyclohexene diepoxide (VCD) induced estrogen deficient female rats. Furthermore, we explored the impact of resveratrol and pterostilbene in this milieu. Studies were directed to unraveling the impact on genes involved in mitochondrial biogenesis and on other cardiovascular markers.
Materials and methods
Drugs and chemicals
Resveratrol and pterostilbene were purchased from Nanjing Zelang Medical Technology Co., Ltd, Nanjing, China. Nicotine was purchased from Pravinchandra Ltd, Mumbai, India. 4-Vinylcyclohexene diepoxide (VCD) and 17β-estradiol were purchased from Sigma Chemicals, St Louis, MO, USA. All other reagents used in the study were of analytical grade.
Preparation of aqueous extract of smokeless tobacco (AEST)
The aqueous extract of smokeless tobacco (AEST) was prepared as described in the literature.33 Tobacco leaves were bought from local traders and authenticated by Dr Ganesh Iyer, Ramnarain Ruia College, Mumbai. The tobacco leaves were finely powdered and 20 g of powder was dissolved in 50 mL of phosphate buffer solution (pH 7.4) and incubated at 37 °C for 30 min with thorough shaking. The dissolved contents were filtered twice through filter paper and quickly frozen at –80 °C before lyophilization. The dried yield was found to be 3% w/w. The nicotine content was estimated using HPLC. The nicotine content in AEST by HPLC was found to be 9.077% w/w. The required amount of lyophilized extract was freshly reconstituted in distilled water for oral administration in rats.
Procurement and housing of animals
22 days old female Sprague Dawley rats were procured from Piramal Healthcare Ltd, Goregaon, Mumbai, India. They were housed in polypropylene cages with wire mesh top and husk bedding. The temperature and relative humidity of the experiment room were maintained at 25 °C ± 2 °C and 65 ± 5%, respectively. The animals were subjected to 12 : 12 h light and dark cycles (8 a.m. to 8 p.m.). They were fed with soya free standard rat chow (Pranav Agro Industries Ltd, Sangli, Maharashtra, India) and fresh drinking water (Brihanmumbai Corporation, Mumbai, India) ad libitum throughout the study. The composition of the soya free rat chow was as follows: carbohydrates (52%), crude protein (16.65%), crude fat (3.72%), crude fibre (5.5%), calcium (1.82%) and phosphorus (0.8%). The energy provided by chow was 3020 kcal kg–1. All the experimental procedures used in this study were approved by the Institutional Animal Ethics Committee (IAEC) of Bombay College of Pharmacy (protocol no. CPCSEA-BCP/2013-10 dated 26 July 2013) and carried out in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines for the care of laboratory animals.
Experimental protocol
After one week of acclimatization, the 28 days old female Sprague Dawley rats were randomized into eleven groups.
Group 1 (vehicle control): rats were administered sesame oil intraperitoneally for 30 days;
Group 2 (VCD): rats were administered 4-vinylcyclohexene diepoxide (VCD) (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days;
Group 3 (AEST): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + aqueous extract of smokeless tobacco (100 mg kg–1 day–1, p.o.) for 60 days;
Group 4 (ET): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + 17β-estradiol (0.05 mg kg–1 day–1, s.c.) three times a week for 8 weeks;
Group 5 (RSV 5): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + resveratrol (5 mg kg–1 day–1, p.o.) for 60 days;
Group 6 (RSV 20): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + resveratrol (20 mg kg–1 day–1, p.o.) for 60 days;
Group 7 (RSV 40): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + resveratrol (40 mg kg–1 day–1, p.o.) for 60 days;
Group 8 (PT 5): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + pterostilbene (5 mg kg–1 day–1, p.o.) for 60 days;
Group 9 (PT 20): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + pterostilbene (20 mg kg–1 day–1, p.o.) for 60 days;
Group 10 (PT 40): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + pterostilbene (40 mg kg–1 day–1, p.o.) for 60 days;
Group 11 (CT: combination therapy) (RSV 20 + PT 20): rats were administered VCD (80 mg kg–1 day–1, i.p. in sesame oil) for 30 days + AEST (100 mg kg–1 day–1, p.o.) for 60 days + resveratrol (20 mg kg–1 day–1, p.o.) for 60 days + pterostilbene (20 mg kg–1 day–1, p.o.) for 60 days.
Non-invasive blood pressure measurement
Systolic blood pressure was measured once in two weeks during the duration of the study. The rats were trained for at least one week until the BP was steadily recorded with minimal stress and restrain. Measurements were done in the morning in the pre-warmed, restrained rats by tail cuff plethysmography using a Power Lab/4sp AD Instrument, Australia. The first few data captures were discarded and the means of five or six subsequent measurements were recorded.
ECG recording and heart rate recording
On the 59th day, ECG and heart rate were recorded under ketamine and xylazine anesthesia. Needle electrodes (Lab chart 7 Pro, ADI Instruments, Australia), i.e. positive, negative and reference, were placed beneath the skin of the 4 limbs of the animals near the paws. For each ECG tracing the heart rate, P–R interval, R–R interval, QT interval, QRS interval and P wave duration were measured.
Euthanasia, blood and tissue collection
On the 61st day, rats were euthanized using dry CO2; blood was collected from the abdominal aorta. Blood samples were kept in an ice bath for 1 h and then centrifuged at 3000g for 15 min at 4 °C. Serum was aliquoted and stored at –20 °C for the biochemical estimations. The heart, aorta and ovaries were dissected and weighed for further analysis.
Biochemical markers of myocardial injury in serum
Estimation of total cholesterol (CHL), triglycerides (TG), creatine kinase (CK-MB and CK-NAC), lactate dehydrogenase (LDH), and high density lipoproteins (HDL) in serum was done using a commercial diagnostic kit (Erba Diagnostics, Mannheim, Germany). The low density lipoproteins (LDL) and very low density lipoproteins (VLDL) were calculated using Friedewald's formula.40 Non-HDL cholesterol was calculated as the difference between CHL and HDL. The serum nitrite–nitrate levels were also estimated by a modified cadmium reduction method as previously described.41 The atherogenic index was also calculated as described previously.42
Determination of serum inflammatory markers
The C reactive protein (CRP) in serum was estimated by a turbidimetric immunoassay using a commercially available kit (Tulip Diagnostics Pvt Ltd, Verna, Goa, India). The tumor necrosis factor-α (TNF-α) in serum was estimated by the enzyme linked immunosorbent assay using a commercially available kit (Abcam, USA) as per the manufacturer's instructions. All the samples were analyzed in duplicate.
Measurement of aortic collagen
For the estimation of aortic collagen, 30 mg of aortic tissue was hydrolyzed in 2 mL of 6 N HCl in sealed ampoules at 120 °C for 2 h. After the hydrolysis of tissue, the tubes were opened and contents were decanted into a measuring cylinder. Tubes were washed with water and combined with a hydrolysate to make up the final volume of 20 mL, 2 drops of 0.002% methyl indicator was added and the mixture was titrated with 2.5 N NaOH (red to yellow). The concentration of hydroxyproline was estimated using standard hydroxyproline solutions at 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μg mL–1.43Collagen concentration (mg of collagen per 100 mg of tissue) = 7.25 × hydroxyproline concentration (mg)
Protein carbonyl assay of heart tissue
The protein carbonyl content of heart tissue was estimated by using the method previously discussed.44 Briefly, the heart tissue homogenate was incubated with 2,4-dinitrophenylhydrazine (DNPH) at room temperature for 60 min, subsequently samples were precipitated with 20% trichloroacetic acid followed by centrifugation and washing of pellets with an ethanol–ethyl acetate solution (1 : 1) in order to remove excess DNPH. The pellets were then dissolved in guanidine hydrochloride solution (6 mol L–1) and the absorbance at 360 nm was used to calculate the protein carbonyl content of the heart tissue. The Lowry method was used to assay the protein content.45
Serum estradiol determination
Serum estradiol levels were determined using a radioimmunoassay kit obtained from AMP Diagnostics GmbH, Obrigheim, Germany. Additions were made as per the kit manufacturer's specifications and the resultant radioactivity was measured using a γ-counter (Pla Electro Appliances Pvt Ltd, Mumbai, India).
Total RNA extraction
To study the alterations in the expression of genes involved in the energy metabolism, quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) based studies were carried out. Total RNA was isolated from the dissected heart tissues (approximately 70–80 mg) by using a SV total mRNA extraction kit (Promega Z3100, Madison, WI, USA) as per the manufacturer's instructions. The yield of total RNA obtained was determined by a Nanodrop spectrophotometer (NanoDrop ND-1000, Montchanin, DE, USA). The quality and purity of RNA were estimated by spectrophotometry from the relative absorbances at 230, 260 and 280 nm (i.e., A260/A280 and A260/A230).
Quantitative real-time PCR analysis
The forward and reverse primers for SIRT1, PGC-1α, PPAR-α, TFAM, NRF-1, mitochondrial D-loop, nuclear DNA (18S DNA) and mitochondrial ribosomal protein, 18S (house-keeping gene), were commercially synthesized (Integrated DNA Technologies, Leuven, Belgium); the sequences are listed in Table 1. One-step quantitative real-time PCR was performed using a One Step GoTaq® qPCR Master mix (Promega, Madison, WI, USA) on a 7500 real-time PCR System (Applied Biosystems, CA, USA). The real-time PCR reactions (25 μL) contained 0.2 μM forward primer, 0.2 μM reverse primer, 114 ng μL–1 of sample mRNA, 0.5 μL of 1× RT mix and 12.5 μL of 1× Master mix and nuclease free water. The reaction conditions were as follows: initial 2 min (50 °C), denaturation at 95 °C (10 min), followed by 50 cycles of denaturation at 95 °C (15 s), annealing at 60 °C (30 s) and extension at 60 °C (30 s). The level of each target gene was normalized to 18S, which was performed in parallel. The results were expressed as fold change of the threshold cycle (Ct) relative to the control group using the 2–ΔΔCt method.46 The cardiac mitochondrial count was determined as the ratio of the mitochondrial DNA, D-loop, relative to the non-mitochondrial nuclear DNA, 18S DNA.
Table 1. Primer sequences used for quantitative real-time PCR.
| Primer | Sense primer | Antisense primer |
| SIRT 1 | 5′-GAC GAC GAG GGC GAG GAG-3′ | 5′-ACA GGA GGT TGT CTC GGT AGC-3′ |
| PGC-1α | 5′-CCA AAG CTGA AGC CCT CTT GC-3′ | 5′-GTT TAG TCT TCC TTT CCT CGT GTC C-3′ |
| PPAR-α | 5′-GAG AAA GCA AAA CTG AAA GCA GAG A-3′ | 5′-GAA GGG CGG GTT ATT GCT G-3′ |
| TFAM | 5′-CAC GAG CCC TGG AGT ACC C-3′ | 5′-CCA CAT TCC CCG GAA CAG C-3′ |
| NRF-1 | 5′-GCT CTT TGA GAC CCT GCT TTC-3′ | 5′-GTG GAG TTG AGT ATG TCC GAG T-3′ |
| D-loop | 5′-GGT TCT TAC TTC AGG GCC ATC A-3′ | 5′-GAT TAG ACC CGT TAC CAT CGA GAT-3′ |
| 18S DNA | 5′-GCG ATG CGG CGG CGT TAT-3′ | 5′-AGA CTT TGG TTT CCC GGA AGC-3′ |
| 18S | 5′-CAT CGA GCA GGT CTG TTC CC-3′ | 5′-TAG ATT GGC TTG ACG GAC TTG G-3′ |
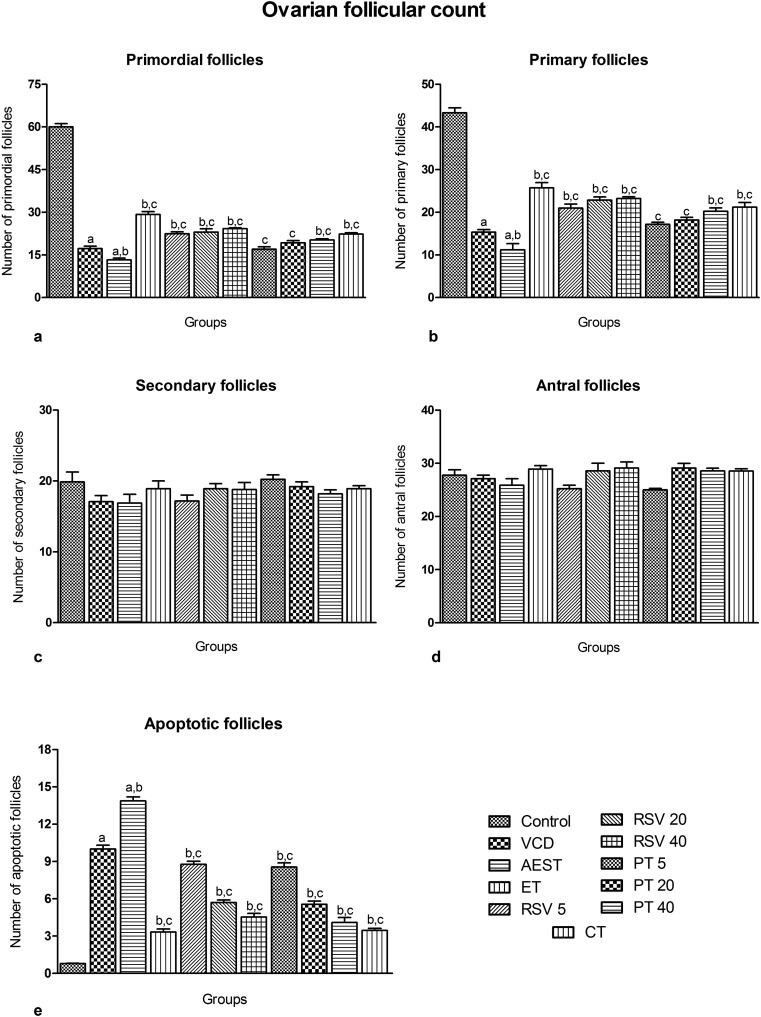
Ovarian follicular count
The ovaries were removed, trimmed of fat, weighed and processed for ovarian follicle counts. The ovaries were sectioned serially (4 to 5 μm). In every 40th section, preantral follicles (containing an oocyte nucleus) were classified as primordial (oocytes surrounded by a single layer of flattened granulose cells), primary (oocytes surrounded by a single layer of cuboidal granulose cells) or secondary (oocytes surrounded by two or more layers of cuboidal granulose cells) and counted. The total number of sections per ovary varied from 15 to 19, depending on the difference in ovarian size and the presence (or absence) of corpora lutea.47
Histopathological evaluations of heart tissue
The heart specimens were dehydrated with increasing grades of the alcohol and cleared with xylene followed by embedding in paraffin. Thereafter the processed tissue were sectioned (at 5 μm) and taken on clean glass slides, stained with hematoxylin and eosin and observed under a microscope at different magnifications.
Statistical analysis
All values are expressed as means ± SEM. Statistical analyses were performed using GraphPad Prism 5 software for Windows (GraphPad Software, San Diego, CA, USA). The differences between the groups were analyzed with one-way analysis of variance, followed by post hoc analysis with the Newman–Keuls test. For noninvasive blood pressure measurements, two-way analysis of variance (with time and treatment as variables) followed by post hoc analysis with Bonferroni's test were used. Statistical significance was considered at P < 0.05.
Results
Non-invasive blood pressure measurement
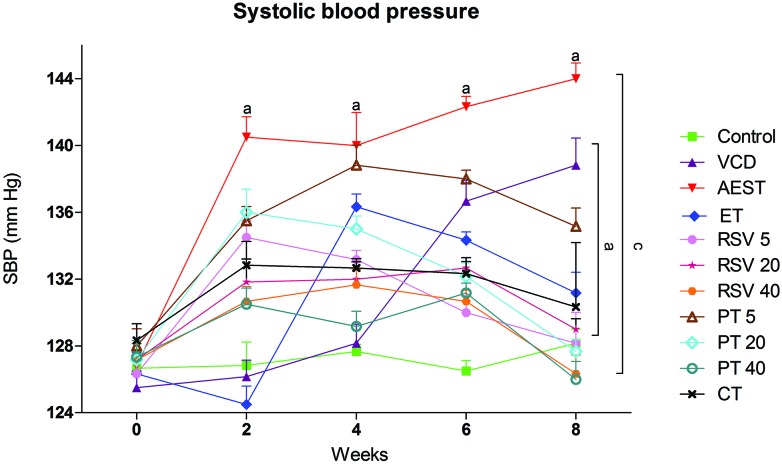
At the beginning of the experimental period, rats from all the groups showed normal systolic blood pressure (SBP). At the end of the 2nd week, administration of AEST (100 mg kg–1) and VCD (80 mg kg–1) led to a significant rise in SBP when compared with control rats. This rise in SBP was sustained until the 8th week. Treatment with 17β-estradiol (0.05 mg kg–1), resveratrol (5 mg kg–1, 20 mg kg–1 and 40 mg kg–1), and pterostilbene (20 mg kg–1, 40 mg kg–1) per se and in combination (RSV 20 + PT 20 mg kg–1) significantly prevented the rise in SBP. However, a low dose of pterostilbene was found to be sub-effective in abolishing the rise in SBP (Fig. 1).
Fig. 1. Effect of resveratrol and pterostilbene on systolic blood pressure in AEST administered rats. Values are means ± SEM (n = 6 for all groups). Statistical analysis was performed by using two-way analysis of variance (with time and treatment as variables), followed by Bonferroni's test as a post hoc analysis to identify significant differences among the groups. VCD, 4-vinylcyclohexene diepoxide; AEST, aqueous extract of smokeless tobacco; ET, 17β-estradiol; RSV 5, resveratrol 5 mg kg–1; RSV 20, resveratrol 20 mg kg–1; RSV 40, resveratrol 40 mg kg–1; PT 5, pterostilbene 5 mg kg–1; PT 20, pterostilbene 20 mg kg–1; PT 40, pterostilbene 40 mg kg–1; CT, combination therapy (resveratrol 20 mg kg–1 and pterostilbene 20 mg kg–1). aP < 0.05 when compared to the vehicle control group; cP < 0.05 when compared with the AEST group.
ECG and heart rate measurements
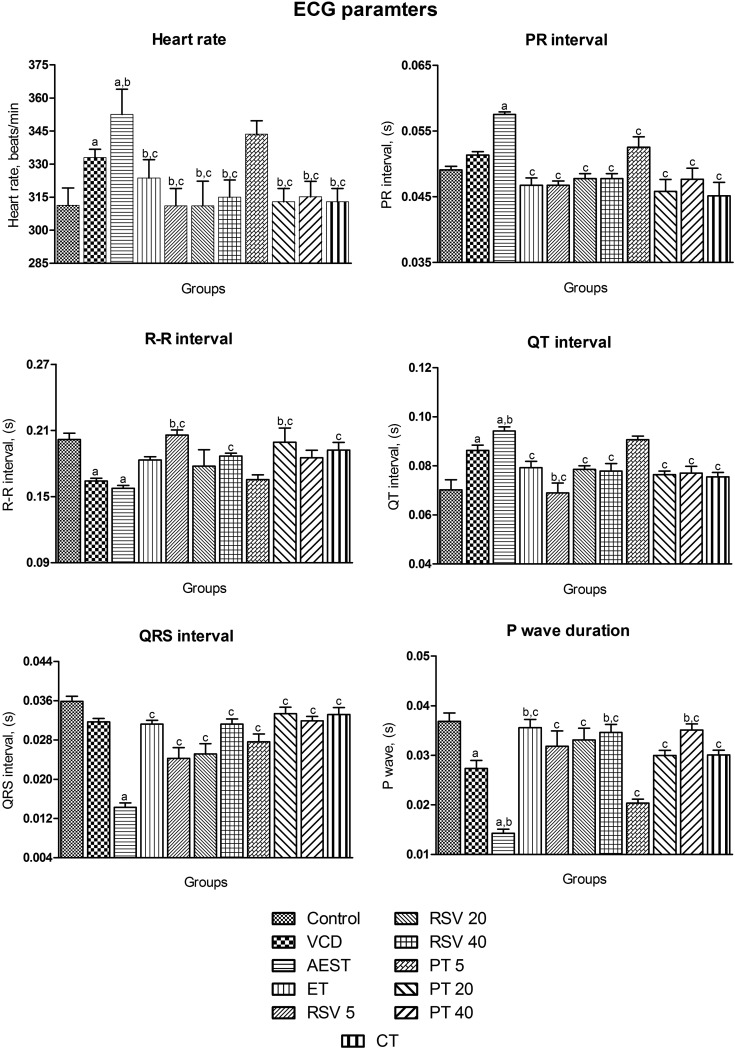
The estrogen deficiency state induced by VCD and AEST resulted in a significant increase in heart rate [F10,65 = 20.6, p < 0.05], an increase in the PR interval [F10,65 = 8.81, p < 0.05], prolongation of the QT interval [F10,65 = 9.41, p < 0.05] and a significant reduction in the RR interval [F10,65 = 4.82, p < 0.05], QRS interval [F10,65 = 19.6, p < 0.05], and P wave duration [F10,65 = 17.3, p < 0.05] when compared to control rats. These results clearly depict that the AEST exposure further worsens the cardiac parameters in estrogen deficient rats. Rats treated with 17β-estradiol, resveratrol and pterostilbene per se and in combination had shown normal ECG patterns by abating the detrimental repercussion induced by estrogen deficiency and AEST administration. However, a low dose of pterostilbene (5 mg kg–1) fails to restore estrogen deficiency and AEST induced alterations in the heart rate, QT and RR interval. In addition, resveratrol (20 mg kg–1) and pterostilbene (40 mg kg–1) showed no effect on the RR interval (Fig. 2).
Fig. 2. Effect of resveratrol and pterostilbene on ECG and heart rate in AEST administered rats. Values are means ± SEM (n = 6 for all groups). Statistical analysis was performed by using one-way analysis of variance (ANOVA), followed by the Newman–Keuls test as a post hoc analysis to identify significant differences among the groups. VCD, 4-vinylcyclohexene diepoxide; AEST, aqueous extract of smokeless tobacco; ET, 17β-estradiol; RSV 5, resveratrol 5 mg kg–1; RSV 20, resveratrol 20 mg kg–1; RSV 40, resveratrol 40 mg kg–1; PT 5, pterostilbene 5 mg kg–1; PT 20, pterostilbene 20 mg kg–1; PT 40, pterostilbene 40 mg kg–1; CT, combination therapy (resveratrol 20 mg kg–1 and pterostilbene 20 mg kg–1). aP < 0.05 when compared to the vehicle control group; bP < 0.05 when compared with the VCD group; cP < 0.05 when compared with the AEST group.
Biochemical markers of myocardial injury in serum
Compared to the control animals, AEST administered estrogen deficient rats demonstrated a significant increase in the serum circulating levels of TG [F10,65 = 5.23, p < 0.05], CHL [F10,65 = 19.03, p < 0.05], VLDL [F10,65 = 5.07, p < 0.05], LDL [F10,65 = 20.8, p < 0.05], non-HDL [F10,65 = 25.44, p < 0.05], CK-MB [F10,65 = 32.02, p < 0.05], CK-NAC [F10,65 = 7.59, p < 0.05], LDH [F10,65 = 13.2, p < 0.05], notably increased heart weight [F10,65 = 5.23, p < 0.05] and atherogenic indices [F10,65 = 10.01, p < 0.05], whereas serum HDL [F10,65 = 8.75, p < 0.05] and nitrate–nitrite levels [F10,65 = 44.5, p < 0.05] were significantly reduced. These perturbations in the cardiac injury markers were restored to normal by 17β-estradiol, resveratrol, and pterostilbene per se and in combination. However, pterostilbene (5 mg kg–1) did not significantly lower the TG, VLDL, HDL and CK-MB levels (Table 2).
Table 2. Effect of resveratrol and pterostilbene on markers of myocardial injury in AEST administered rats.
| Parameters | Control | VCD | AEST | ET | RSV 5 | RSV 20 | RSV 40 | PT 5 | PT 20 | PT 40 | CT |
| Heart weight (g) | 0.803 ± 0.03 | 0.771 ± 0.01 | 1.053 ± 0.08a,b | 0.811 ± 0.02c | 0.811 ± 0.03c | 0.843 ± 0.04c | 0.840 ± 0.02c | 0.812 ± 0.04c | 0.769 ± 0.03c | 0.795 ± 0.02c | 0.771 ± 0.02c |
| Serum TG (mg dL–1) | 54.43 ± 5.18 | 73.88 ± 2.46 | 85.55 ± 3.99a | 54.61 ± 5.18c | 58.70 ± 7.82c | 45.90 ± 4.71c | 57.30 ± 3.04c | 73.95 ± 10.1 | 52.87 ± 4.02c | 54.66 ± 3.54c | 52.79 ± 2.21c |
| Serum CHL (mg dL–1) | 65.74 ± 3.39 | 96.61 ± 2.18a | 150.1 ± 13.4a,b | 69.11 ± 2.29b,c | 84.10 ± 7.15c | 67.85 ± 3.94b,c | 67.53 ± 6.79b,c | 85.10 ± 2.98c | 64.65 ± 5.23b,c | 44.12 ± 8.36b,c | 62.33 ± 4.79b,c |
| Serum VLDL (mg dL–1) | 11.22 ± 1.14 | 14.78 ± 0.49 | 17.11 ± 0.79a | 10.92 ± 1.04c | 11.74 ± 1.56c | 9.180 ± 0.94c | 11.46 ± 0.61c | 14.79 ± 2.01 | 10.57 ± 0.80c | 10.93 ± 0.71c | 10.56 ± 0.44c |
| Serum LDL (mg dL–1) | 7.801 ± 4.28 | 54.90 ± 5.81a | 115.1 ± 15.3a,b | 8.787 ± 3.96b,c | 27.08 ± 9.44b,c | 2.218 ± 4.91b,c | 16.03 ± 6.12b,c | 40.08 ± 6.59c | 16.69 ± 8.15b,c | 1.392 ± 5.20b,c | 1.711 ± 4.39b,c |
| Serum non-HDL (mg dL–1) | 21.35 ± 3.85 | 69.68 ± 6.05a | 132.1 ± 15.4a,b | 19.71 ± 3.75b,c | 38.82 ± 9.23b,c | 11.40 ± 4.39b,c | 27.49 ± 5.85b,c | 54.87 ± 5.05c | 27.2 ± 7.63b,c | 12.33 ± 4.58b,c | 11.73 ± 4.03b,c |
| Serum HDL (mg dL–1) | 44.39 ± 1.19 | 26.93 ± 4.71a | 18.01 ± 3.63a | 49.40 ± 4.66b,c | 45.18 ± 3.98b,c | 56.45 ± 3.11b,c | 40.04 ± 2.47c | 30.23 ± 4.56 | 37.39 ± 3.67b,c | 51.77 ± 7.20b,c | 55.60 ± 3.87b,c |
| Serum CK-MB (IU L–1) | 47.48 ± 1.73 | 92.45 ± 3.69a | 143.3 ± 12.8a,b | 70.73 ± 4.43b,c | 65.77 ± 2.41b,c | 55.52 ± 3.18b,c | 54.66 ± 2.62b,c | 79.19 ± 5.73c | 49.05 ± 5.29b,c | 56.24 ± 2.88b,c | 32.88 ± 3.55b,c |
| Serum CK-NAC (IU L–1) | 103.4 ± 9.19 | 123.1 ± 6.18 | 281.4 ± 39.1a | 122.8 ± 16.7c | 172.5 ± 29.7c | 116.7 ± 20.7c | 111.8 ± 17.9c | 187.5 ± 24.9c | 132.7 ± 10.2c | 106.8 ± 10.1c | 83.38 ± 9.82c |
| Serum LDH (IU L–1) | 173.3 ± 8.31 | 737.1 ± 120a | 997.1 ± 69.1a,b | 383.2 ± 97.9b,c | 605.4 ± 81.2b,c | 425.6 ± 49.1b,c | 373.2 ± 46.1b,c | 733.8 ± 116c | 428 ± 39.7b,c | 222 ± 16.7b,c | 200.6 ± 29.0b,c |
| Serum nitrite–nitrate (mmol L–1) | 49.49 ± 0.68 | 40.29 ± 0.90a | 35.70 ± 0.42a,b | 50.81 ± 0.89b,c | 43.14 ± 0.91b,c | 44.72 ± 0.75b,c | 47.94 ± 0.87b,c | 40.99 ± 0.75c | 44.89 ± 0.60b,c | 49.11 ± 0.39b,c | 50.39 ± 0.61b,c |
| Atherogenic index | 0.488 ± 0.09 | 3.844 ± 1.59 | 9.525 ± 2.19a | 0.446 ± 0.11c | 1.019 ± 0.40c | 0.215 ± 0.10c | 0.691 ± 0.15c | 2.484 ± 0.95c | 0.863 ± 0.30c | 0.298 ± 0.14c | 0.234 ± 0.10c |
| Serum estradiol (pg mL–1) | 34.29 ± 1.10 | 16.56 ± 1.14a | 12.62 ± 0.98a | 51.21 ± 3.31c | 14.43 ± 0.99 | 18.93 ± 0.37c | 22.51 ± 0.61b,c | 13.17 ± 1.29 | 17.03 ± 0.56 | 20.84 ± 0.62c | 24.31 ± 1.03b,c |
Serum inflammatory markers
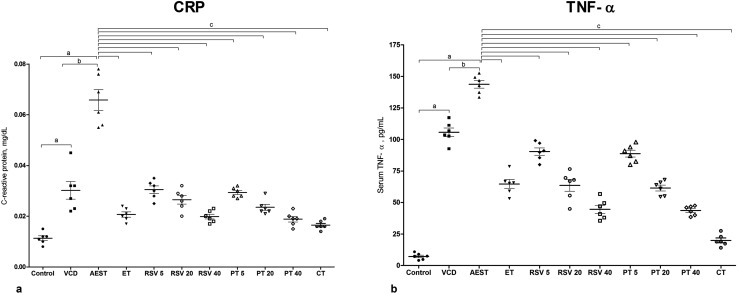
Compared to the control animals (0.011 mg dL–1 and 7.263 pg mL–1), AEST administered estrogen deficient rats demonstrated a significant increase in the serum circulating levels of CRP (0.065 mg dL–1) [F10,65 = 56.3, p < 0.05] and TNF-α (7.263 pg mL–1) [F10,65 = 180, p < 0.05], respectively. These perturbations in the serum inflammatory markers were significantly restored to normal levels by 17β-estradiol, resveratrol, and pterostilbene per se and combination treatments (Fig. 3a and b).
Fig. 3. Effect of resveratrol and pterostilbene on serum (a) CRP levels and (b) TNF-α levels in AEST administered rats. Values are means ± SEM (n = 6 for all groups, in duplicate). Statistical analysis was performed by using one-way analysis of variance (ANOVA), followed by the Newman–Keuls test as a post hoc analysis to identify significant differences among the groups. VCD, 4-vinylcyclohexene diepoxide; AEST, aqueous extract of smokeless tobacco; ET, 17β-estradiol; RSV 5, resveratrol 5 mg kg–1; RSV 20, resveratrol 20 mg kg–1; RSV 40, resveratrol 40 mg kg–1; PT 5, pterostilbene 5 mg kg–1; PT 20, pterostilbene 20 mg kg–1; PT 40, pterostilbene 40 mg kg–1; CT, combination therapy (resveratrol 20 mg kg–1 and pterostilbene 20 mg kg–1). aP < 0.05 when compared to the vehicle control group; bP < 0.05 when compared with the VCD group; cP < 0.05 when compared with the AEST group.
Serum estradiol levels
Upon VCD administration, serum estradiol levels decreased to 16.56 pg mL–1, which further declined to 12.62 pg mL–1 upon 60 days of AEST administration. The average serum estradiol level of control rats was found to be 34.29 pg mL–1. Treatment with 17β-estradiol, resveratrol and pterostilbene (40 mg kg–1) and combination therapy for 8 weeks significantly raised the serum estradiol level to 51.21, 18.93, 22.51, 20.84, and 24.31 pg mL–1, respectively. However, resveratrol (5 mg kg–1) and pterostilbene (5 mg kg–1 and 20 mg kg–1) failed to show any significant impact on serum estradiol levels (Table 2).
Aortic collagen content
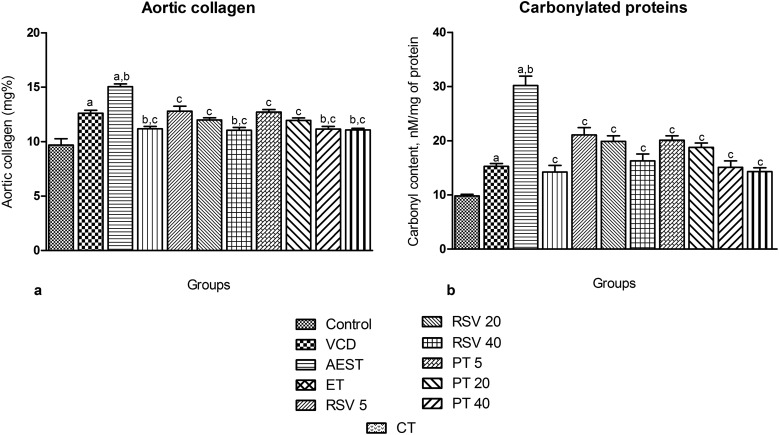
Stiffness of aorta is due to collagen and its uncontrolled accumulation may elicit atherosclerosis risk.48 Estrogen deficiency induced by VCD increased the aortic collagen levels to 12.61 mg%, which was further raised to 15.05 mg% by AEST administration for 60 days. Treatment with 17β-estradiol, resveratrol, pterostilbene per se and combination therapy significantly attenuated the AEST induced increase in aortic collagen to 11.08, 12.79, 11.99, 11.04, 12.72, 11.95, 11.16, and 11.07 mg%, respectively, thereby reversing the risk of atherosclerosis (Fig. 4a).
Fig. 4. Effect of resveratrol and pterostilbene on (a) aortic collagen levels and (b) heart protein carbonyl content in AEST administered rats. Values are means ± SEM (n = 6 for all groups). Statistical analysis was performed by using one-way analysis of variance (ANOVA), followed by the Newman–Keuls test as a post hoc analysis to identify significant differences among the groups. VCD, 4-vinylcyclohexene diepoxide; AEST, aqueous extract of smokeless tobacco; ET, 17β-estradiol; RSV 5, resveratrol 5 mg kg–1; RSV 20, resveratrol 20 mg kg–1; RSV 40, resveratrol 40 mg kg–1; PT 5, pterostilbene 5 mg kg–1; PT 20, pterostilbene 20 mg kg–1; PT 40, pterostilbene 40 mg kg–1; CT, combination therapy (resveratrol 20 mg kg–1 and pterostilbene 20 mg kg–1). aP < 0.05 when compared to the vehicle control group; bP < 0.05 when compared with the VCD group; cP < 0.05 when compared with the AEST group.
Protein carbonyl content of heart tissue
The protein carbonyl content in heart tissue was increased in VCD exposed estrogen deficient rats (VCD 15.28 mM mg–1 of protein vs. control 9.821 mM mg–1 of protein), which was further significantly elevated upon 60 days of AEST exposure (30.21 mM mg–1 of protein), indicating cardiac oxidative stress. Therapy with 17β-estradiol, resveratrol, and pterostilbene per se and in combination significantly decreased the protein carbonyl levels of heart tissue (14.22, 21.09, 19.89, 16.68, 20.09, 18.76, 15.09, and 14.29 mM mg–1 of protein, respectively), thereby blunting the VCD and smokeless tobacco administration induced increase in the protein carbonyl levels in heart tissue (Fig. 4b).
Quantitative real-time PCR analysis
Estrogen deficiency induced by VCD and oral administration of AEST for 60 days resulted in a decreased gene expression of SIRT1 [F10,43 = 92.7, p < 0.05], PGC-1α [F10,43 = 19.8, p < 0.05], PPAR-α [F10,43 = 68.4, p < 0.05], NRF-1 [F10,43 = 59.08, p < 0.05], and TFAM [F10,43 = 22.9, p < 0.05] and the mitochondrial content of the cardiac tissue. The cardiac mRNA expression of SIRT1, PGC-1α, PPAR-α, NRF-1, and TFAM (Table 3) and mitochondrial content (Table 3) were significantly increased in the 17β-estradiol, resveratrol and pterostilbene per se and combination treatment groups. However, pterostilbene at all doses fails to modify the SIRT1 gene expression. In addition, a low dose of pterostilbene (5 mg kg–1) failed to induce PGC-1α mRNA expression in the cardiac tissue, which indicates a decrease in mitochondrial content.
Table 3. Effect of resveratrol and pterostilbene on the gene expressions and mitochondrial content of heart tissue in AEST administered rats.
| Heart mRNA levels (arbitrary units) | |||||||||||
| Parameters | Control | VCD | AEST | ET | RSV 5 | RSV 20 | RSV 40 | PT 5 | PT 20 | PT 40 | CT |
| SIRT 1 | 1.00 ± 0.07 | 0.55 ± 0.03a | 0.37 ± 0.01a | 0.93 ± 0.02b,c | 1.32 ± 0.19b,c | 1.44 ± 0.08b,c | 4.23 ± 0.29b,c | 0.45 ± 0.05 | 0.43 ± 0.07 | 0.48 ± 0.09 | 0.90 ± 0.03c |
| PGC-1α | 1.00 ± 0.02 | 0.56 ± 0.05 | 0.45 ± 0.02a | 0.97 ± 0.01c | 0.91 ± 0.03c | 0.98 ± 0.04c | 1.06 ± 0.07c | 0.76 ± 0.05 | 0.94 ± 0.11c | 1.17 ± 0.10c | 2.44 ± 0.33c |
| PPAR-α | 1.00 ± 0.06 | 0.52 ± 0.03 | 0.41 ± 0.03 | 1.08 ± 0.08 | 1.33 ± 0.02 | 1.57 ± 0.11 | 3.01 ± 0.25b,c | 1.74 ± 0.10 | 2.64 ± 0.23b,c | 7.47 ± 0.66b,c | 10.2 ± 0.99b,c |
| NRF-1 | 1.00 ± 0.03 | 0.76 ± 0.04 | 0.32 ± 0.04 | 8.91 ± 0.96b,c | 2.36 ± 0.15b,c | 4.38 ± 0.51b,c | 6.24 ± 0.26b,c | 2.80 ± 0.27b,c | 5.67 ± 0.15b,c | 5.96 ± 0.37b,c | 7.48 ± 0.21b,c |
| TFAM | 1.00 ± 0.04 | 0.63 ± 0.06 | 0.41 ± 0.03a | 0.95 ± 0.02c | 0.82 ± 0.02 | 0.85 ± 0.04 | 1.07 ± 0.06c | 0.79 ± 0.05 | 1.18 ± 0.22c | 1.34 ± 0.22c | 2.70 ± 0.23c |
| Mitochondrial content (nDNA/mtDNA) | 1.00 ± 0.07 | 0.86 ± 0.04 | 0.61 ± 0.04a | 0.93 ± 0.02c | 0.84 ± 0.02c | 0.95 ± 0.02c | 1.19 ± 0.09c | 0.75 ± 0.02 | 0.93 ± 0.02c | 1.13 ± 0.06c | 1.44 ± 0.05c |
Ovarian follicular count
Administration of VCD for 30 days caused a 71.31% and 64.53% loss of primordial and primary follicles, respectively (17.21 ± 3.33 and 15.33 ± 2.33, respectively), as compared to control animals (60.00 ± 4.57 and 43.32 ± 4.44, respectively). This atretic loss of primordial and primary ovarian follicles was further increased to 77.95% and 74.08% upon 60 days of AEST administration (13.23 ± 2.44 and 11.20 ± 5.44, respectively), suggesting its ovatoxic potential. Treatments with 17β-estradiol, resveratrol, and pterostilbene per se and in combination significantly countered this primordial and primary follicular loss by AEST (Fig. 5).
Fig. 5. Effect of resveratrol and pterostilbene on the ovarian follicular count in AEST administered rats. Values are means ± SEM (n = 15 sections per ovary). Statistical analysis was performed by using one-way analysis of variance (ANOVA), followed by the Newman–Keuls test as a post hoc analysis to identify significant differences among the groups. VCD, 4-vinylcyclohexene diepoxide; AEST, aqueous extract of smokeless tobacco; ET, 17β-estradiol; RSV 5, resveratrol 5 mg kg–1; RSV 20, resveratrol 20 mg kg–1; RSV 40, resveratrol 40 mg kg–1; PT 5, pterostilbene 5 mg kg–1; PT 20, pterostilbene 20 mg kg–1; PT 40, pterostilbene 40 mg kg–1; CT, combination therapy (resveratrol 20 mg kg–1 and pterostilbene 20 mg kg–1). aP < 0.05 when compared to the vehicle control group; bP < 0.05 when compared with the VCD group; cP < 0.05 when compared with the AEST group.
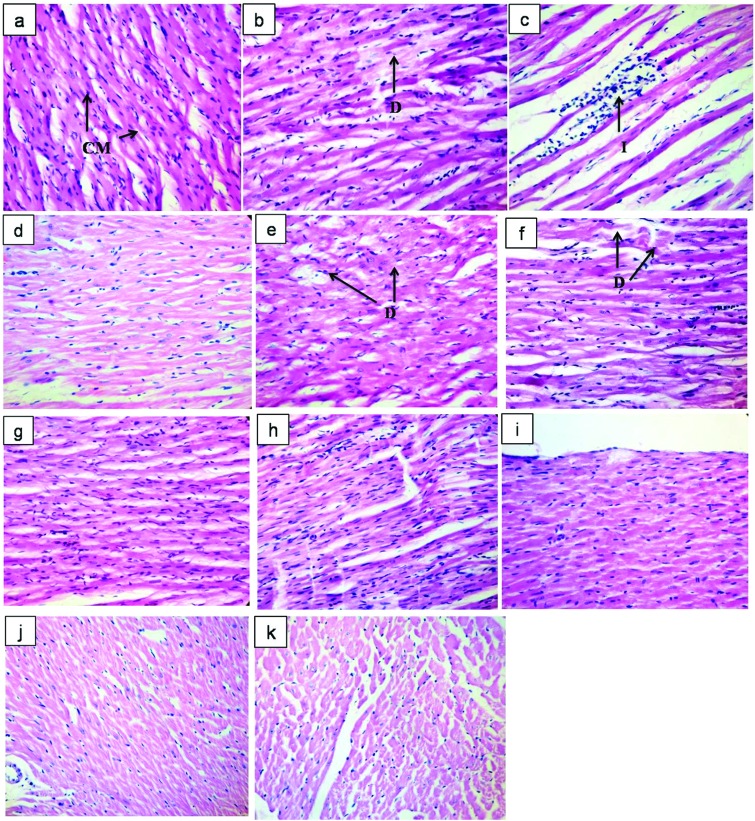
Histology of heart
The histological sections of the heart tissue of rats treated with VCD (80 mg kg–1) for 30 days displayed mild to moderate focal degeneration of cardiac muscle cells with a loss of cardiac muscle striations. Administration of AEST (100 mg kg–1) to estrogen deficient female rats for 60 days resulted in severe focal infiltration of leukocytes with a reduced density of cardiac muscle cells in contrast to the control hearts. Rats treated with 17β-estradiol, resveratrol, and pterostilbene per se and combination therapy showed no signs of cardiac damage. However, groups treated with a low dose of resveratrol (5 mg kg–1) and pterostilbene (5 mg kg–1) showed mild degeneration of cardiac muscles (Fig. 6).
Fig. 6. Impact of resveratrol and pterostilbene on the histopathological features of heart tissue in the AEST administered rats. Representative photomicrographs of haematoxylin and eosin (H and E) stained sections of cardiac tissue: (a) vehicle control showing healthy cardiac myocytes, suggesting normal cardiac physiology including structure and function; (b) VCD (80 mg kg–1, i.p.) administered rats showed moderate degenerative changes in cardiac myocytes; (c) AEST (100 mg kg–1 p.o.) administration causes inflammatory changes with decreasing density of cardiac myocytes; (e) RSV 5 (5 mg kg–1, p.o.), (h) PT 5 (5 mg kg–1 p.o.) treated rats showed mild degeneration of cardiac muscle cells; importantly, (d) ET (0.05 mg kg–1, s.c.), (f) RSV 20 (20 mg kg–1, p.o.), (g) RSV 40 (40 mg kg–1, p.o.), (i) PT 20 (20 mg kg–1, p.o.), (j) PT 40 (40 mg kg–1, p.o.) and (k) CT (RSV 20 + PT 20 mg kg–1, p.o.) treatment showed no signs of cardiac damage [H and E ×400]. Abbreviations: CM: cardiac myocytes; D: degenerative change in cardiac myocytes; I: infiltration of leucocytes.
Discussion
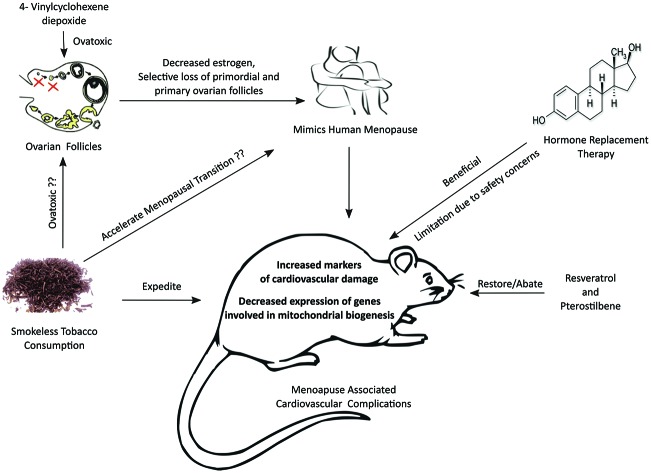
The post-menopausal period in women lasts for one-third to half of their life time, which spurs cardiovascular diseases.49 Smokeless tobacco use amongst women is of serious health concern and has been linked with significant cardiovascular adversity.50 This dual onslaught involving physiological depletion (estrogen deficiency) and external addiction to smokeless tobacco may perpetuate cardiovascular ill health. Herein, we tried to address this adverse synergism in 4-vinylcyclohexene diepoxide (VCD) induced estrogen deficient states in rats administered with the aqueous extract of smokeless tobacco (AEST). Furthermore, we explored the impact of resveratrol and pterostilbene per se and in combination in this milieu.
VCD was used to induce estrogen deficiency in female rats. In women, most primordial and primary ovarian follicles are naturally degenerated through an apoptotic process of atresia.51 VCD induced estrogen deficiency mimics the human menopause in atretic loss, acyclicity and final hormone levels.51–53 Moreover, the utility of this model in cardiovascular research has been highlighted.54 We found a temporal decrease in serum estradiol levels and selective lessening of primordial and primary ovarian follicles and an increase in apoptotic (atretic) follicles in VCD treated rats, creating a menopause like condition. This decrease in serum estradiol levels, primordial and primary ovarian follicles and rise in apoptotic follicles was further aggravated by 60-day AEST administration. The noticeable decline in serum estradiol levels by AEST can be ascribed to stimulation of the 2-hydroxylation pathway of estrogen metabolism by tobacco involving cytochrome P450 isoenzymes CYP1A1 and CYP1A2.35,55 In the current study, treatment with 17β-estradiol (0.05 mg kg–1), resveratrol (20 mg kg–1 and 40 mg kg–1), pterostilbene (40 mg kg–1) and their combination (RSV 20 mg kg–1 + PT 20 mg kg–1) significantly increased the serum estradiol levels and protected the primordial and primary ovarian follicles from atretic loss as indicated by a decrease in apoptotic follicles.
The ECG evaluation shows that estrogen deficiency induced an increase in heart rate, PR interval and prolongation of the QT interval with a reduction in the RR interval, QRS interval and P wave duration. We have observed a rise in SBP after two weeks of AEST administration that was sustained for the entire duration of the study, which can be linked to a terminal rise in aortic collagen content. Furthermore, the AEST exposure worsens these electrocardiographic outcomes in estrogen deficient rats. Previous findings suggest that resveratrol and pterostilbene mediate their vasoprotective role by inducing nitric oxide synthase through ER-α and promoting normal growth of vascular smooth muscle cells, respectively.32,35 Moreover, increased serum nitrate–nitrite levels upon resveratrol and pterostilbene treatment contribute to this vasoprotective mechanism. Importantly, a recent clinical trial in female dominant patients reveals the blood pressure lowering potential of pterostilbene.56 Pterostilbene showed better oral bioavailability and higher distribution to heart tissue; this signifies its therapeutic benefits in cardiovascular disease.57,58 In coherence with past findings, rats treated with 17β-estradiol, resveratrol, and pterostilbene per se and combination therapy significantly prevented the rise in SBP and showed normal ECG patterns by abating the detrimental repercussion induced by estrogen deficiency and AEST exposure. However, a low dose of pterostilbene fails to restore the alteration in SBP, heart rate, QT and RR interval. In addition, resveratrol (20 mg kg–1) and pterostilbene (40 mg kg–1) showed no effect on the RR interval.
Before menopause, women are protected from the cardiovascular complications due to the profound beneficial implications of estrogen on lipid metabolism. Estrogen plays a vital role in limiting the liver fat accumulation and promotes lipogenesis. Human studies and knockout rodent models have established the link between the global loss of estrogen signalling, increased hepatic fat accumulation and defective lipogenesis.59–61 In the present investigation, estrogen deficiency leads to a significant increase in serum CHL, LDL and non-HDL levels and a non-significant rise in TG and LDL levels, whereas HDL levels were significantly decreased. These adverse repercussions are expedited upon AEST administration, which is in agreement with previous reports.2 The increase in the atherosclerotic risk factor was reflected by the increase in the atherogenic index. These perturbations in the lipid profile and atherogenic index were restored to normal levels by 17β-estradiol, resveratrol, and pterostilbene per se and combination therapy. Moreover, a low dose of pterostilbene significantly lowered CHL, LDL and non-HDL levels without affecting other parameters. This lipid lowering impact of resveratrol and pterostilbene is possibly mediated by decreasing HMG CoA reductase activity and by PPAR-α agonism, respectively.31,62
Assessment of the serum circulating levels of CK-MB, CK-NAC and LDH offers a powerful and sensitive early indicator of growing myocardial adversities.63 In this study, AEST exposure in estrogen deficient states dramatically increases the serum CK-MB, CK-NAC and LDH levels, proving its deleterious effect on cardiovascular health. Recent studies have reported that the acute resveratrol and blueberry administration countered increased serum creatine kinase (CK-MB and CK-NAC) and LDH levels.64,65 Resonating with the above studies these perturbations of AEST were significantly reduced by 17β-estradiol, resveratrol, and pterostilbene per se and combination therapy. However, pterostilbene at low dose fails to prevent the increase in CK-MB levels induced by AEST.
In the current study, the serum levels of pro-inflammatory cytokines TNF-α and the inflammatory marker CRP were increased in VCD induced estrogen deficient states. Further increase of these inflammatory markers was seen in AEST administered rats. Treatment with 17β-estradiol, resveratrol, and pterostilbene per se and in combination significantly decreased these serum inflammatory marker levels, thereby abating the VCD and smokeless tobacco administration induced inflammatory changes. The above result shows that the cardioprotective roles of resveratrol and pterostilbene were partly related to their potent anti-inflammatory properties.29,66
Protein carbonylation is an irreversible, non-enzymatic modification of proteins by reactive oxygen species. It is a direct marker for oxidative insults by ROS, reflecting cellular damage of multiple forms.67 The estrogen deficient states induced by VCD significantly raise the protein carbonyl content in heart tissue as compared to the control. These levels were further exacerbated upon AEST exposure, reflecting oxidative stress induced myocardial damage. Treatment with 17β-estradiol, resveratrol, and pterostilbene per se and in combination significantly decreased the carbonyl protein levels in the heart, thereby abating the VCD and smokeless tobacco administration induced oxidative insults. This beneficial potential of resveratrol and pterostilbene is amenable to their potent antioxidant properties.29
Aortic collagen increases the extracellular matrix, which may lead to chronic hypertension. In this study, consistent with our pervious findings, the estrogen deficiency state leads to a significant rise in aortic collagen levels.35 The AEST administration exhilarates this rise, triggering further aortic stiffness. Treatment with 17β-estradiol, resveratrol, and pterostilbene per se and in combination significantly decreased the aortic collagen, offering improved vascular elasticity and helping to normalise the blood pressure.
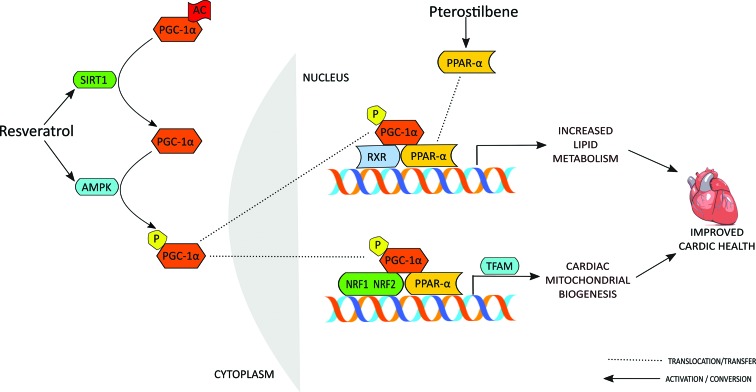
In the recent past, research on SIRT1 and AMPK was progressive owing to their undeniable role in energy sensing and subsequent propelling of PGC-1α, as a key regulator of mitochondrial biogenesis and function. Upon activation, PGC-1α in concert with other coactivators such as PPAR-α, NRFs (nuclear respiratory factor) etc. orchestrate the mitochondrial biogenesis involving mitochondrial transcription factor A (TFAM).68 In the present investigation, we encountered defective cardiac mitochondrial biogenesis in AEST administered estrogen deficient rats as reflected by down-regulation of SIRT1, PGC-1α, PPAR-α, NRF-1, and TFAM and decreased cardiac mitochondrial content. A recent study by Rimbaud et al. (2011) demonstrated that resveratrol treatment upregulates the PGC-1α, NRF-2 and TFAM gene expressions but fails to show any change in the expression and activity of SIRT 1 in cardiac tissue.69 On the contrary, our finding reflects significant upregulation of SIRT 1, PGC-1α, PPAR-α, NRF-1 and TFAM on oral treatment with resveratrol for 60 days with an eventual rise in the cardiac mitochondrial content. A close structural relative of resveratrol, pterostilbene, is not much different in this notion, showing an increase in the expression of PGC-1α, PPAR-α, NRF-1, and TFAM and countering estrogen deficiency and AEST exposure induced mitochondrial meltdown. However, unlike resveratrol, pterostilbene does not modify the cardiac SIRT 1 gene expression, which may suggest its beneficiary role through AMPK and PPAR-α (Fig. 7).30,31,70 In addition, we found that a higher dose of pterostilbene is required in favouring these cardiac benefits as a lower dose was found to be sub-effective. Estradiol administration is known to induce mitochondrial biogenesis by upregulating PGC-1α, NRF-1 and TFAM mRNA levels as has been previously reported.71,72 We found that treatment with 17β-estradiol, being a counterpart of endogenous estrogen, significantly upregulates the aforementioned regulators of mitochondrial biogenesis. However, the contradictory cardiac events of estrogen replacement therapy in post-menopausal women may raise questions against these benefits, which needs further due diligence.
Fig. 7. Schematic description of resveratrol and pterostilbene targets in mediating mitochondrial biogenesis in cardiac tissue. Resveratrol activates SIRT1 and AMPK, leading to deacetylation and phosphorylation of PGC-1α. Activated PGC-1α binds to nDNA as a complex with nuclear receptors (NRFs, PPAR-α etc.) and other co-activators, fostering the TFAM transcription. Transcribed TFAM reaches the mitochondria, binds to mtDNA and initiates mtDNA gene expression, which leads to diverse cardiac benefits through augmenting mitochondrial oxidative metabolism and biogenesis. Alongside, in the nucleus, PGC-1α also increases the basal transcription of specific target genes of lipid metabolism, thereby regulating lipid homeostasis and oxidative metabolism. Pterostilbene activates PPAR-α, which subsequently binds with NRF 1 and NRF 2 in conjugation with its co-activator PGC-1α to switch on the genes of mitochondrial fatty acid oxidation. SIRT1, silent information regulator two type 1 or sirtuin; AMPK, AMP-activated protein kinase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; nDNA: nuclear DNA; NRF-1: nuclear respiratory factor-1; NRF-2: nuclear respiratory factor-2; mtDNA: mitochondrial DNA PPAR-α, peroxisome proliferator-activated receptor-α; TFAM: mitochondrial transcription factor A; RXR, retinoid X receptors; AC, acetyl group; P, phosphate group.
Lastly, pathological outcome paints a similar picture as AEST administration in estrogen deficient states aggravated the loss of cardiac muscle cells, inflammation and focal degeneration. These derogatory pathological changes were reversed by 17β-estradiol, resveratrol, and pterostilbene per se and combination therapy. These results were in resonance with the biochemical estimations indicating cardiac health. However, in low doses, resveratrol and pterostilbene treated rats expressed mild degeneration of cardiac cells.
Conclusion
In conclusion, the investigation substantiates and generates compelling preclinical evidence on the detrimental outcomes of smokeless tobacco on cardiovascular parameters in experimental menopausal states in female rats. The study projects that resveratrol and pterostilbene improve the cardiovascular health by upregulating the genes of energy metabolism, thus enhancing mitochondrial biogenesis. Since cardiovascular diseases involve a cluster of anomalies, targeting at multiple points may be prudent for better disease control. In agreement, the syndicate of resveratrol and pterostilbene showed promising results in abating the detrimental cardiovascular outcomes of smokeless tobacco in estrogen deficient female rats. This provides an alternative to HRT which needs to be explored in clinical settings.
Conflict of interest statement
The authors declare that there is no conflict of interest associated with this manuscript.
Abbreviations
- RSV
Resveratrol
- PT
Pterostilbene
- ET
17β-Estradiol therapy
- VCD
4-Vinylcyclohexene diepoxide
- AEST
Aqueous extract of smokeless tobacco
- SBP
Systolic blood pressure
- ECG
Electrocardiography
- TG
Triglycerides
- CHL
Cholesterol
- LDL
Low density lipoprotein
- VLDL
Very low density lipoprotein
- HDL
High density lipoprotein
- CK-MB
Creatinine kinase muscle brain
- CK-NAC
Creatinine kinase N-acetylcysteine
- LDH
Lactate dehydrogenase
- CRP
C-reactive protein
- TNF-α
Tumor necrosis factor-α
- SIRT1
Sirtuin-1
- PGC-1α
Peroxisome proliferator-activated receptor-γ coactivator-1α
- PPAR-α
Peroxisome proliferator activated receptor-α
- TFAM
Mitochondrial transcription factor-A
- NRF-1
Nuclear respiratory factor-1
- 18S
18S ribosomal RNA
- mtDNA
Mitochondrial DNA
- CVD
Cardiovascular disease
- NAMS
North American Menopause Society
- HRT
Hormone replacement therapy
- AMPK
AMP-activated protein kinase
Acknowledgments
This work was supported by a major research project from University Grants Commission, New Delhi, India. We thank Dr S. S. Sachdev, Dr N. Sivaprasad, Mrs Jayula Sarnaik and Dr Vijay Kadwad of BRIT, India for their help in carrying out the RIA of estradiol.
References
- Öberg M., Jaakkola M. S., Woodward A., Peruga A., Prüss-Ustün A. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- Piano M. R., Benowitz N. L., FitzGerald G. A., Corbridge S., Heath J., Hahn E., Pechacek T. F., Howard G. Circulation. 2010;122:1520–1544. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- McKinlay S. M., Bifano N. L., McKinlay J. B. Ann. Intern. Med. 1985;103:350–356. doi: 10.7326/0003-4819-103-3-350. [DOI] [PubMed] [Google Scholar]
- Guida M., Zullo F., Buonomo B., Marra M., Palatucci V., Pascale R., Visconti F., Guerra G., Spinelli M., Sardo A. D. S. Transl. Med. UniSa. 2012;3:25. [PMC free article] [PubMed] [Google Scholar]
- Shifren J. L., Gass M. L. Menopause. 2014;21:1038–1062. doi: 10.1097/GME.0000000000000319. [DOI] [PubMed] [Google Scholar]
- Wend K., Wend P., Krum S. A. Front. Endocrinol. 2012;3:19. doi: 10.3389/fendo.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules M. R., Sherman S., Parrott E., Rebar R., Santoro N., Utian W., Woods N. J. Women's Health Gender-Based Med. 2001;10:843–848. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- Sood R., Faubion S. S., Kuhle C. L., Thielen J. M., Shuster L. T. Int. J. Women's Health. 2014;6:47. doi: 10.2147/IJWH.S38342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier A., Fortin D., Delomenie C., Momken I., Veksler V., Ventura-Clapier R. J. Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H., Schubert C., Jaekel J., Fliegner D., Penkalla A., Tiemann K., Stypmann J., Roepcke S., Brokat S., Mahmoodzadeh S. J. Mol. Med. 2008;86:1013–1024. doi: 10.1007/s00109-008-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-García J., Goldenthal M. J., Damle S., Pi Y., Moe G. W. J. Card. Failure. 2009;15:700–708. doi: 10.1016/j.cardfail.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Sebastiani M., Giordano C., Nediani C., Travaglini C., Borchi E., Zani M., Feccia M., Mancini M., Petrozza V., Cossarizza A. J. Am. Coll. Cardiol. 2007;50:1362–1369. doi: 10.1016/j.jacc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Sun C. K., Chang L. T., Sheu J. J., Wang C. Y., Youssef A. A., Wu C. J., Chua S., Yip H. K. Int. Heart J. 2007;48:533–546. doi: 10.1536/ihj.48.533. [DOI] [PubMed] [Google Scholar]
- Watson P. A., Reusch J. E., McCune S. A., Leinwand L. A., Luckey S. W., Konhilas J. P., Brown D. A., Chicco A. J., Sparagna G. C., Long C. S. Am. J. Physiol.: Heart Circ. Physiol. 2007;293:H246–H259. doi: 10.1152/ajpheart.00734.2006. [DOI] [PubMed] [Google Scholar]
- Cantó C., Auwerx J. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C., Auwerx J. Physiology. 2011;26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R., Garnier A., Veksler V. Cardiovasc. Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- Brenmoehl J., Hoeflich A. Mitochondrion. 2013;13:755–761. doi: 10.1016/j.mito.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C. Biochim. Biophys. Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalgol B., Batirel S., Taga Y., Ozer N. K. Front. Pharmacol. 2012;3:141. doi: 10.3389/fphar.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F. Z., Markus M. A., Morris B. J. Int. J. Biochem. Cell Biol. 2009;41:2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Csiszar A., Labinskyy N., Pinto J. T., Ballabh P., Zhang H., Losonczy G., Pearson K., De Cabo R., Pacher P., Zhang C. Am. J. Physiol.: Heart Circ. Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala A., Tauriainen E., Siltanen A., Shi J., Merasto S., Louhelainen M., Martonen E., Finckenberg P., Muller D. N., Mervaala E. Blood Pressure. 2010;19:196–205. doi: 10.3109/08037051.2010.481808. [DOI] [PubMed] [Google Scholar]
- Menzies K. J., Singh K., Saleem A., Hood D. A. J. Biol. Chem. 2013;288:6968–6979. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Crandall J. P., Barzilai N. Diabetes. 2013;62:1022–1023. doi: 10.2337/db12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J. A., Sinclair D. A. Nat. Rev. Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Lecour S. Eur. Heart J. 2007;28:1683–1693. doi: 10.1093/eurheartj/ehm149. [DOI] [PubMed] [Google Scholar]
- McFadden D. Oxid. Med. Cell. Longevity. 2013;2013:1–5. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilde A., Van Bilsen M. Acta Physiol. Scand. 2003;178:425–434. doi: 10.1046/j.1365-201X.2003.01161.x. [DOI] [PubMed] [Google Scholar]
- Fruchart J. C., Staels B. and Duriez P., in Atherosclerosis, Hypertension and Diabetes, Springer, 2003, pp. 3–16. [Google Scholar]
- Park E. S., Lim Y., Hong J. T., Yoo H. S., Lee C. K., Pyo M. Y., Yun Y. P. Vasc. Pharmacol. 2010;53:61–67. doi: 10.1016/j.vph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Lam E., Kelley E., Martin S., Buettner G. Tob. Induced Dis. 2003;1:1–5. doi: 10.1186/1617-9625-1-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avti P. K., Kumar S., Pathak C. M., Vaiphei K., Khanduja K. L. Toxicol. Sci. 2006;89:547–553. doi: 10.1093/toxsci/kfj041. [DOI] [PubMed] [Google Scholar]
- Majumdar A. S., Joshi P. A., Giri P. R. Menopause. 2013;20:869–876. doi: 10.1097/GME.0b013e31827fdda4. [DOI] [PubMed] [Google Scholar]
- Kappeler C. J., Hoyer P. B. Syst. Biol. Reprod. Med. 2012;58:57–62. doi: 10.3109/19396368.2011.648820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Dudley J. I., Das D. K. Dose–Response. 2010;8:478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Zorita S., Fernández-Quintela A., Lasa A., Aguirre L., Rimando A. M., Portillo M. P. J. Agric. Food Chem. 2014;62:8371–8378. doi: 10.1021/jf501318b. [DOI] [PubMed] [Google Scholar]
- Chung M. T., Cheng P. Y., Lam K. K., Chen S. Y., Ting Y. F., Yen M. H., Lee Y. M. Menopause. 2010;17:127–134. doi: 10.1097/gme.0b013e3181b4c4ac. [DOI] [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I., Fredrickson D. S. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Cortas N. K., Wakid N. W. Clin. Chem. 1990;36:1440–1443. [PubMed] [Google Scholar]
- Muruganandan S., Srinivasan K., Gupta S., Gupta P., Lal J. J. Ethnopharmacol. 2005;97:497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Woessner J. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Yan L. J. Curr. Protoc. Protein Sci. 2009;14:28. doi: 10.1002/0471140864.ps1404s55. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Borman S., Christian P., Sipes I., Hoyer P. Toxicol. Appl. Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Rekhter M. D. Cardiovasc. Res. 1999;41:376–384. doi: 10.1016/s0008-6363(98)00321-6. [DOI] [PubMed] [Google Scholar]
- Rosano G., Vitale C., Marazzi G., Volterrani M. Climacteric. 2007;10:19–24. doi: 10.1080/13697130601114917. [DOI] [PubMed] [Google Scholar]
- Gupta R., Gupta N., Khedar R. Indian Heart J. 2013;65:369–377. doi: 10.1016/j.ihj.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. P., Devine P. J., Dyer C. A., Hoyer P. B. Biol. Reprod. 2004;71:130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- Lohff J. C., Christian P. J., Marion S. L., Arrandale A., Hoyer P. B. Comp. Med. 2005;55:523–527. [PubMed] [Google Scholar]
- Hoyer P. B., Sipes I. G. Annu. Rev. Pharmacol. Toxicol. 1996;36:307–331. doi: 10.1146/annurev.pa.36.040196.001515. [DOI] [PubMed] [Google Scholar]
- Williams J. K. Arterioscler., Thromb., Vasc. Biol. 2005;25:1765–1766. doi: 10.1161/01.ATV.0000175757.28698.c2. [DOI] [PubMed] [Google Scholar]
- Kapoor D., Jones T. Eur. J. Endocrinol. 2005;152:491–499. doi: 10.1530/eje.1.01867. [DOI] [PubMed] [Google Scholar]
- Riche D. M., Deschamp D., Griswold M. E., McEwen C. L., Riche K. D., Sherman J. J., Wofford M. R. Hypertension. 2012;60:A617. [Google Scholar]
- Choo Q. Y., Yeo S. C. M., Ho P. C., Tanaka Y., Lin H. S. J. Funct. Foods. 2014;11:352–362. [Google Scholar]
- Yeo S. C. M., Ho P. C., Lin H. S. Mol. Nutr. Food Res. 2013;57:1015–1025. doi: 10.1002/mnfr.201200651. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G., Gao H., Ahrén B., Zierath J., Galuska D., Steiler T., Dahlman-Wright K., Nilsson S., Gustafsson J.-Å., Efendic S. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Ribas V., Nguyen M. A., Henstridge D. C., Nguyen A.-K., Beaven S. W., Watt M. J., Hevener A. L. Am. J. Physiol.: Endocrinol. Metab. 2010;298:E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. E., Thorburn A. W., Britt K. L., Hewitt K. N., Wreford N. G., Proietto J., Oz O. K., Leury B. J., Robertson K. M., Yao S. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I. J., Ahn J. Y., Kim S., Choi M. S., Ha T. Y. Biochem. Biophys. Res. Commun. 2008;367:190–194. doi: 10.1016/j.bbrc.2007.12.140. [DOI] [PubMed] [Google Scholar]
- Howard-Alpe G., Sear J., Foex P. Br. J. Anaesth. 2006;97:758–769. doi: 10.1093/bja/ael303. [DOI] [PubMed] [Google Scholar]
- Zhang W., Guo C., Gao R., Ge M., Zhu Y., Zhang Z. J. Evidence–Based Complementary Altern. Med. 2013;2013:1–8. doi: 10.1155/2013/407839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman H. F. Int. J. Med. Med. Sci. 2014;47:1437–1446. [Google Scholar]
- Das S., Das D. K. Inflammation Allergy: Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Aldini G., Carini M., Colombo R., Rossi R., Milzani A. J. Cell. Mol. Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C., Vega R. B., Kelly D. P. Trends Endocrinol. Metabo. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbaud S., Ruiz M., Piquereau J., Mateo P., Fortin D., Veksler V., Garnier A., Ventura-Clapier R. PLoS One. 2011;6:e26391. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V. C. H., Tsai Y. C., Lin J. N., Fan L. L., Pan M. H., Ho C. T., Wu J. Y., Way T. D. J. Agric. Food Chem. 2012;60:6399–6407. doi: 10.1021/jf301499e. [DOI] [PubMed] [Google Scholar]
- Mattingly K. A., Ivanova M. M., Riggs K. A., Wickramasinghe N. S., Barch M. J., Klinge C. M. Mol. Endocrinol. 2008;22:609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capllonch-Amer G., Sbert-Roig M., Galmés-Pascual B. M., Proenza A. M., Lladó I., Gianotti M., García-Palmer F. J. J. Endocrinol. 2014;221:391–403. doi: 10.1530/JOE-14-0008. [DOI] [PubMed] [Google Scholar]