Abstract
Aims
Exenatide is a glucagon-like peptide 1 (GLP-1) mimetic which induces weight loss predominantly, it is presumed, via decreased food intake. However, circulating GLP-1 is also a determinant of energy expenditure. We sought to quantify the effect of exenatide on energy expenditure (EE) and energy intake.
Materials and Methods
In this single-center, randomized double-blind placebo controlled trial, we randomized 80 healthy, non-diabetic volunteers with obesity (46 women, age: 34.4±8.7 y, body fat by DXA: 44.2±7.8%) to subcutaneous exenatide 10 μg twice daily or placebo. Subjects were admitted to our clinical research unit for measurement of 24h-EE in a whole-room indirect calorimeter and ad libitum food intake using an automated vending machine paradigm before and after randomization. Furthermore, energy expenditure and ad libitum food intake measures were repeated at 24-week after readmission for 7-day inpatient stay. Body weight was obtained weekly for up to 5 weeks and was recorded at each monthly follow up visit up to 24 weeks.
Results
Prior to randomization, participants over ate during the 3-day vending machine period in the whole study group (114.6±35.2 %), expressed as percentage of weight maintaining energy needs (WMEN) with those who were eventually randomized to exenatide overeating more (121.6±37.7 %) compared to placebo group (107.6±31.5 %). In the exenatide group, ad libitum absolute energy intake decreased by 1016.1±724.5 kcal/day (95% CI: −1250.9 to −781.2) versus a 245.1±710.5 kcal/day (95% CI: −475.4 to −14.7) decrease in placebo (Δ= −624.8 Kcal/day, p < .0001) whereas the reduction in ad libitum caloric intake relative to WMEN was a more modest 366.8±752.1 kcal/day (95% CI: −614.0 to −119.6) decrease compared to 8.0±860.1 kcal/day (95% CI: −286.8 to 270.8) reduction in placebo (Δ= −382.3 Kcal/day, p = 0.03). The decrease was uniform across all macronutrients groups.
No differences in 24hEE or substrate oxidation rates were found. In the exenatide group, body weight decreased more over the 5 weeks (β = −0.039 kg/week, p = 0.02) and was lower compared to placebo at the end of fifth week (−1.48±0.77 kg; 95% CI: −3.02 to 0.05, p = 0.06). At the 24-week follow up, there was no difference in energy intake between exenatide group and placebo group and the treatment group decreased 24-h EE more compared to placebo (β = −160.6 Kcal/day, 95% CI: −307.6 to 13.6, p = 0.03) compared to their pre-randomization measurement. However, this reduction was not present after adjustment for changes in FM and FFM (β = −87 kcal/day, p = 0.14). No difference was observed in body weight (Δ = −1.72 kg, 95% CI: −5.77 to 2.30, p = 0.39) in exenatide versus placebo over 24 weeks.
Conclusion
Compared with placebo, exenatide decreased early ad libitum energy intake but did not change 24h-EE. However, the reduction was more modest in relative versus absolute terms (i.e. below that needed for WMEN). Thus, although rate of weight change was greater in the exenatide treated subjects at 5 weeks, the absolute difference in weight was not significant. These findings indicate that although exenatide reduces food intake, it may be more beneficial in blunting overeating and thus may serve to more prevent weight regain following initial weight loss.
Clinical Trial Registration Numbers
NCT00856609; clinicaltrials.gov
Keywords: GLP-1 receptor agonists, energy expenditure, energy intake, weight loss
1. Introduction
Analogs of the incretin hormone glucagon-like peptide 1 (GLP-1) induce weight loss [1] and one such analog, liraglutide, has been FDA approved for weight loss. Exenatide is the first short acting GLP-1 agonist approved for treatment of type 2 diabetes mellitus (DM) and has also been shown to induce weight loss in patients with obesity with [2-4] and without diabetes [5, 6] with a large variability (ranging by −0.2 to −7.2 kg).
GLP-1 is an incretin hormone released from the L cells of the intestinal mucosa in response to a meal [2]. In addition to its insulinotropic action, infusion of GLP-1 suppresses short-term food intake in humans [7]. Endogenous circulating GLP-1 is positively correlated with resting energy expenditure (REE) [8], and acute infusion caused an insulin-dependent increase in EE [9]. In humans without obesity, intravenous GLP-1 infusion increases EE during hyperglycemic clamp but, after infusion of somatostatin, the insulin secretion was inhibited and, in turn, the GLP-1 effect was abolished [9]. During GLP-1 infusion increase in EE may have been caused by GLP-1-induced hyperinsulinemia due to sympathetic activation and/or glucose utilization due to activation of thermic response [10, 11]. Hwa et al [12] have shown that both central and peripheral GLP-1 administration increased oxygen consumption, an index of EE, in rats.
GLP-1 levels have also been negatively associated with respiratory quotient (RQ), a proxy for the ratio of carbohydrate to lipid oxidation, indicating that GLP-1 may be involved in shifting cell preference from carbohydrates to lipids [13, 14]. The quantification of the effect of a GLP-1 analog on energy expenditure, substrate oxidation and energy intake has not been completely elucidated. In people with DM, exenatide results in a large variability in achieved weight loss [15]. Whether inter-individual differences in energy expenditure (EE) and energy intake (EI) responses to exenatide account for this variability is unknown. The aim of this randomized, double-blinded, placebo-controlled study was to fully enumerate the effects of exenatide on 24-hour EE and EI as primary outcomes. Further, we sought to determine the association of any exenatide-induced changes with weight loss after 5-weeks of treatment. In a subset of subjects who continued on study medication for 24 weeks, we also investigated whether any changes in EE or EI were apparent with longer duration of treatment compared to placebo.
2. Methods and study design
2.1 Study design and participants
From June 2009 to December 2015, 145 adults age <55 with BMI>30 kg/m2 interested in weight loss but weight stable (variation < 2.3 kg within past 6 months) were recruited from the greater Phoenix area. Of these, 84 met initial inclusion criteria (Fig. 1) and were admitted to our clinical research unit (CRU) for at least 4 days (full inclusion/exclusion criteria, Supplementary Appendix) to perform initial tests such as oral glucose tolerance test (OGTT) after 3 days of weight maintaining diet to assess glucose regulation. Four subjects, due to personal reasons, withdrew from the study. Based on medical history, physical examination and standard laboratory tests, individuals had no evidence of medical diagnoses other than obesity and/or impaired glucose tolerance. Upon admission, subjects were initially given a weight maintaining diet (macronutrient content 20%, 30% and 50% protein, fat, and carbohydrate, respectively) with caloric content determined using unit specific equations and adjusted daily to maintain body weight at ±1% during the baseline assessments [16]. Body composition was determined by total-body dual-energy X-ray absorptiometry (DXA) (Lunar Prodigy, enCORE 2003 software version 7.53.002, GE Lunar, Madison, WI, USA). Glucose regulation was assessed by 75 g oral glucose tolerance test (OGTT) on day 4 to exclude diabetes per American Diabetes Association criteria [17]. The initial 4 days of the inpatient stay included routine laboratory testing, physical examination, medical history and 75 g oral glucose tolerance test. Subjects stayed to complete an additional 11 inpatient days for assessment of energy expenditure, substrate oxidation and ad libitum food intake (described in detail below) before and after randomization (in a double blind fashion) to placebo or exenatide (Fig. 2).
Figure 1. Consort flow diagram.
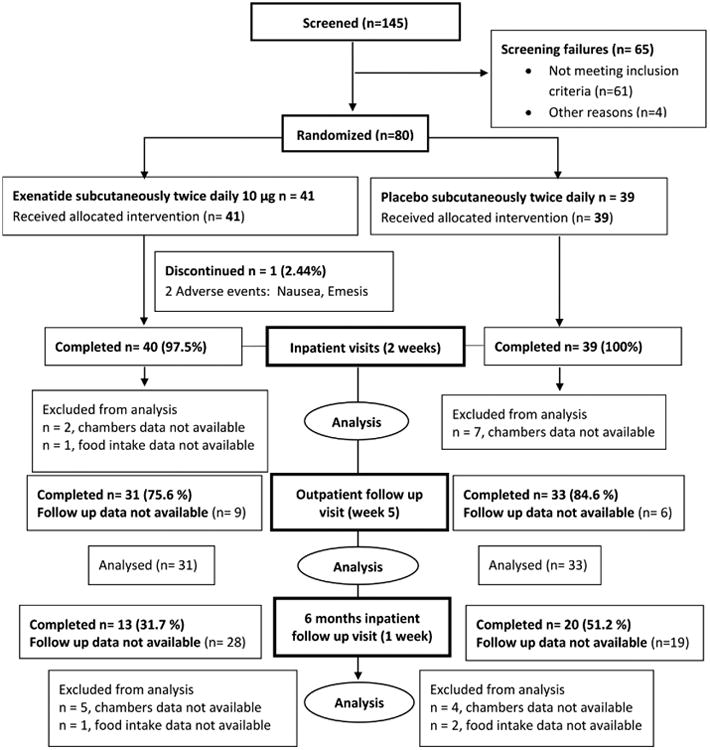
Figure 2. Study diagram of the clinical study.
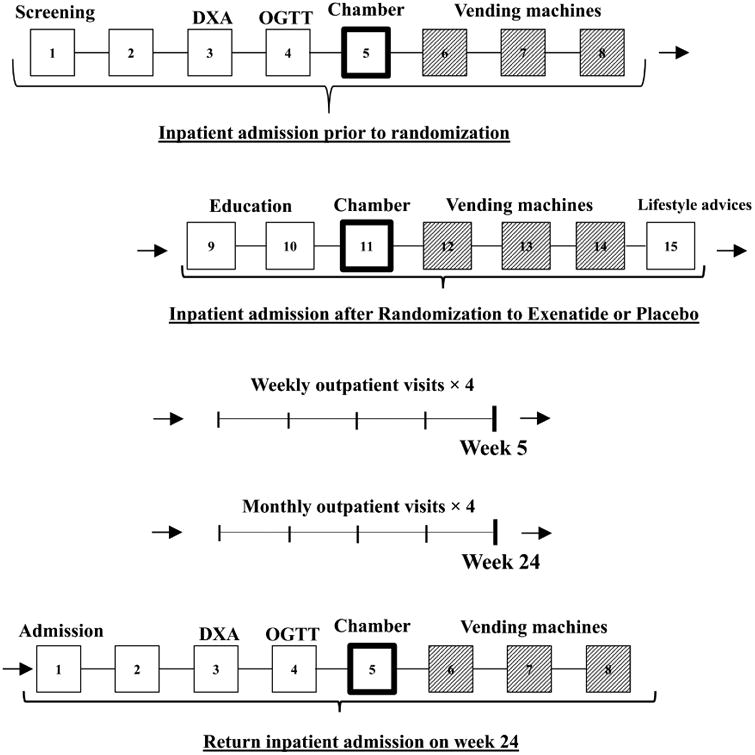
We divided the outline of the study in 2 portions (described in detail below): first inpatient stay (composed by inpatient admission prior to randomization and inpatient admission after randomization) and second (return) inpatient stay at week 24.
Inpatient Admission prior to randomization: Day 1: Screening interview including screening labs and informed consent
Day 3: DXA (dual energy X-ray absorptiometry)
Day 4: OGTT (oral glucose tolerance test)
Day 5: Whole room indirect calorimetry for the assessment of the 24h energy expenditure at baseline
Day 6-7-8: Assessment of ad libitum food intake with vending machine paradigm at baseline
Inpatient Admission after randomization: Day 9-10: Exenatide/Placebo injection education (subcutaneous)
Day 11: Whole room indirect calorimetry for the assessment of the 24h energy expenditure during Exenatide/Placebo
Day 12-13-14: Assessment of ad libitum food intake with vending machine paradigm during intervention period
Day 15: Lifestyle recommendations
After the first 2 weeks of inpatient visit, volunteers returned for weekly follow up visit up to 5 weeks, and then, for monthly follow up visits up to week 24
Return Inpatient Admission on week 24: Day 1: Screening interview including screening labs and informed consent
Day 3: DXA (dual energy X-ray absorptiometry)
Day 4: OGTT (oral glucose tolerance test)
Day 5: Whole room indirect calorimetry for the assessment of the 24h energy expenditure at baseline
Day 6-7-8: Assessment of ad libitum food intake with vending machine paradigm at baseline
All subjects were informed of the aims, nature, benefits and risks of the study prior to providing informed consent. The experimental protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The study was reviewed annually be a Data Safety and Monitoring Board for safety parameters and previously determined stopping guidelines. At each review, the DSMB approved the study to continue.
Following the initial 15 days inpatient stay subjects returned for four weekly follow-up visits (up to 5 weeks) for lifestyle counseling and measurement of weight, assessment of side effects and lab tests including amylase and lipase.
The original protocol (until 2012) followed the same initial timeline as outlined above but continued for 24-weeks of study medication. After week 5, subjects returned monthly for follow up visits up to 24-week for the same measures noted above. At week 24, subjects were readmitted for a 7-day inpatient stay while on study medication. During this admission, the OGTT, energy expenditure and ad libitum food intake measures were repeated. Given the emerging evidence of the efficacy of GLP-1 agonists for weight loss during the study period, the protocol was amended to concentrate on the mechanism of these weight loss effects. Thus, the initial inpatient admission remained the same throughout the study, but the outpatient portion was shortened to 5 weeks.
2.2 Randomization and blinding
After the baseline measurements, subjects were randomized to either exenatide or placebo using permuted block randomization with sex specific block sizes ranging from 2 to 6. Both the subjects and the study investigators were blinded to study medication allocation. The blinded, coded medication and placebo were provided from pooled supplies (Bristol Myers Squibb) to the pharmacy in identical, numbered containers and were dispensed according to the predetermined randomization scheme. The pharmacy alone had access to the randomization information. To maintain blinding and because exenatide has known side effects, all subjects were informed that they were likely to experience nausea given the results of prior studies[18] where subjects treated with either placebo or exenatide experienced nausea.
2.3 Treatment
At randomization, subjects received either exenatide 10 μg twice daily or placebo. Subjects were trained to self-administer the subcutaneously injected preparations, and performed their own injections (monitored by study staff) during their inpatient stay. Subjects were trained to use a glucometer and about sign, symptoms and treatment of hypoglycemia. Glucose measurements and side effects were reviewed by a study physician at each follow up visit. Study compliance was evaluated at outpatient visits by measuring the remaining volume of product in the cartridge. Lifestyle counseling was done by separate trained research staff who remained blinded to the individual's side effect profile. The lifestyle counseling was standardized and based on the principles of the diabetes prevention program [19] and which encourage increased physical activity and healthy eating habits.
2.4 Assessment of 24h Energy Expenditure and Macronutrient Oxidation
Twenty-four-hour energy expenditure (EE) was measured using whole-room indirect calorimetry as previously described [20] prior to and 2 days after starting study medication (n=70) and at the 6-month follow-up admission (n=24). Meals were provided at 0700, 1100, 1600, and 1900 at approximately 80% of weight maintaining diet (WMD) calories [16]. The calories provided for the first (baseline) and second chamber (post-randomization) were the same. Air temperature was maintained at approximately 23.9°±1.4° C. Spontaneous physical activity (SPA) was measured by a radar system [21]. The CO2 and O2 concentrations of the outflowing air were measured as previously described [21] and calculations of oxygen consumption and carbon dioxide production were used to calculate 24-h respiratory quotient (RQ) and EE [21]. From the 24-h RQ, carbohydrate and fat oxidation were calculated, after accounting for protein oxidation, measured from a 24-h urinary nitrogen calculation. SMR was also calculated and defined as the average energy expenditure between 2330 and 0500 while spontaneous physical activity was <1.5% [22]. Each subject remained in the chamber for 23.25 hours but all measures were extrapolated to 24 hours. AFT was calculated as the difference between EE in the inactive state and SMR[23].
2.5 Ad libitum food intake assessment using a vending machine paradigm
Ad libitum food intake (Vend) was measured over three days using an automated vending machine paradigm [24]. First, food preferences were assessed by the food selection questionnaire (FSQ)[25].The vending machines were then stocked with 40 different foods that the subject gave an intermediate hedonic rating on the FSQ (i.e. 4 to 8). Subjects then self-selected all meals and snacks from the vending machine during the 3-day time period and food choices were tracked. Unfinished items were returned to the vending machine and subsequently weighed, so the absolute exact calorie intake over the 72-hour period was calculated (The Food Processor software, ESHA Research, Salem, Oregon) and expressed as both average kilocalories consumed per day and the percentage of the weight maintenance energy needs (%WMEN) calculated as the daily average intake from the vending machine divided by the weight maintaining calories times 100. Furthermore, the caloric intake after randomization was also calculated as the change relative to WMEN, either expressed in kcal/day. Specifically, we calculated this as the difference between the daily average intake from the vending machine and the weight maintaining diet, or expressed in percentage, as the change in caloric intake relative to WMEN divided by the weight maintaining calories times 100.
Kilocalories consumed by each subject were also categorized by macronutrient content. This measure of ad libitum food intake has been found to be highly reproducible [26]. However, although this paradigm is valuable and useful during an inpatient study such as this, it likely does not reflect energy balance in a different environment.
3. Statistical analysis
Power calculations determined that 80 individuals (41 exenatide, 39 placebo) had greater than 90% power with an alpha of 0.01 to detect a clinically significant difference of 200±250 kcal/day in 24h EE and a 500±680 kcal difference in ad libitum energy intake on the vending machine between exenatide and placebo groups. All statistical analyses were performed using the procedures of SAS, version 9.2 (SAS Institute, Cary, NC). Data that were normally distributed are presented as mean±standard deviation; data that deviated from normality are presented as median with interquartile range.
Primary study outcomes were differences in energy intake and EE between placebo versus exenatide-treated subjects. Secondary outcomes included differences in macronutrient oxidation and weight. Alpha was set at 0.01 for primary outcome measures and 0.05 for secondary outcome analyses.
Differences in baseline measures between the exenatide and the placebo groups were evaluated using Student's unpaired t-test. Changes in energy intake (in absolute term and relative to WMEN), EE, and substrate oxidation between the two groups were evaluated by analysis of covariance (ANCOVA) accounting for baseline values. For EE and substrate oxidation measures, models included adjustment for baseline FFM, FM, chamber temperature, age, race and sex. Predicted values for 24-h EE at follow-up visits were calculated based on regression equations including age, sex, race, chamber temperature, FM and FFM at baseline. Predicted versus observed values were compared by Student's unpaired t-test.
Weight change at 5 weeks and 24 weeks was calculated in the two groups and compared by Student's t-test. Repeated-measures mixed models including all follow-up data were performed to compare the weight trajectories of the two groups over 5 and 24 weeks using a first-order autoregressive covariance structure. Associations of the changes in EE and EI in the exenatide group with body weight change were quantified by the Pearson's correlation coefficients. Differences in safety, tolerability and adherence between the groups were compared with Chi-squared tests.
4. Results
4.1 Participants
Screening, eligibility, and available data are reported in the CONSORT diagram (Fig. 1). Baseline characteristics of study participants are described in Table 1.
Table 1. Anthropometric and metabolic measures of the study groups at baseline.
| Whole study group (n=79) | Exenatide (n =40) | Placebo (n = 39) | p | Men (n=33) | Women (n=46) | p | |
|---|---|---|---|---|---|---|---|
| Ethnicity | 10 AA, 21 W, 10 H, 36 NA, 2 O | 1 AA, 11 W, 4 H, 22 NA, 2 O | 9 AA, 10 W, 6 H, 14 NA, 0 O | <0.0 5 | 4 AA, 9 W, 4 H, 16 NA, 0 O | 6 AA, 12 W, 6 H, 20 NA, 2 O | 0.8 |
| Sex (F/M) | 46/33 | 23/17 | 23/16 | 1.0 | 33 | 46 | |
| Age (years) | 34.4 ± 8.7 | 35.1 ± 8.4 | 33.7 ± 9.0 | 0.5 | 38.1 ± 7.7 | 31.7 ± 8.4 | <0.001 |
| Body weight (kg) | 107.1 ± 19.9 | 105.5 ± 19.2 | 108.8 ± 20.7 | 0.5 | 112.5 ± 20.7 | 103.7 ± 19.5 | 0.06 |
| BMI (Kg/m2) | 38.1 ± 6.7 | 38.0 ± 6.6 | 38.2 ± 6.8 | 0.9 | 36.5 ± 6.4 | 39.3 ± 6.7 | 0.07 |
| FFM (Kg) | 36.3 ± 12.8 | 34.6 ± 11.5 | 37.9 ± 8.7 | 0.3 | 43.6 ± 13.5 | 31.1 ± 9.4 | <0.001 |
| FM (Kg) | 29.4 ± 8.8 | 27.9 ± 8.8 | 30.8 ± 8.7 | 0.1 | 26.1 ± 7.5 | 31.7 ± 9.0 | <0.05 |
| Body fat (%) | 44.2 ± 7.8 | 44.0 ± 6.9 | 44.4 ± 8.7 | 0.8 | 37.0 ± 5.3 | 49.4 ± 4.3 | <0.001 |
| Fasting glucose (mg/dl) | 97.8 ± 8.6 | 96.9 ± 8.5 | 98.8 ± 8.8 | 0.3 | 100.7 ± 8.3 | 95.8 ± 8.4 | <0.05 |
| 2-h glucose (mg/dl) | 137.2 ± 25.6 | 139.4 ± 25.7 | 135.1 ± 25.6 | 0.5 | 137 ± 25.4 | 137.4 ± 26.0 | 0.9 |
| WMEN (Kcal/day) | 2874.8 ± 248.7 | 2874.6 ± 241.3 | 2875.0 ± 259.1 | 0.9 | 3008.1 ± 205.6 | 2743.2 ± 199.6 | <0.001 |
| Mean energy intake (kcal/day) | 3294.5 ± 1096.3 | 3495.5 ± 1169.6 | 3093.5 ± 992.3 | 0.1 | 4076.1 ± 942.3 | 2721.3 ± 814.6 | <0.001 |
| % WMEN | 114.6 ± 35.2 | 121.6 ± 37.7 | 107.6 ± 31.5 | 0.08 | 135.5 ± 33.2 | 99.2 ± 28.3 | <0.001 |
| CHO intake (kcal/day) | 1689.7 ± 544.0 | 1785.5 ± 595.0 | 1593.8 ± 476.1 | 0.1 | 2054.6 ± 504.6 | 1422.1 ± 399.4 | <0.001 |
| FAT intake (kcal/day) | 1218.1 ± 491.2 | 1305.3 ± 488.4 | 1130.9 ± 484.6 | 0.1 | 1546.6 ± 440.3 | 977.3 ± 375.6 | <0.001 |
| PRO intake (kcal/day) | 415.6 ± 150.1 | 434.2 ± 159.5 | 396.9 ± 139.6 | 0.1 | 511.6 ± 141.6 | 345.1 ± 113.3 | <0.001 |
| Soda intake (kcal/day) | 434.2 ± 159.5 | 206.5 ± 191.3 | 183.3 ± 169.1 | 0.6 | 236.08 ± 200.6 | 164.7 ± 158.2 | 0.08 |
| 24h EE (kcal/day) | 2325.9 ± 395.3 | 2319.3 ± 377.0 | 2333.8 ± 422.0 | 0.9 | 2604.9 ± 318.3 | 2104.1 ± 299.6 | <0.001 |
| RQ (ratio) | 0.86 ± 0.03 | 0.86 ± 0.03 | 0.85 ± 0.03 | 0.35 | 0.86 ± 0.04 | 0.85 ± 0.03 | 0.5 |
| Carbohydrate oxidation (kcal/day) | 1097.0 ± 338.3 | 1121.1 ± 353.5 | 1068.4 ± 322.5 | 0.5 | 1246.4 ± 343.2 | 978.3 ± 286.6 | <0.001 |
| Lipid Oxidation (kcal/day) | 823.3 ± 353.1 | 781.0 ± 330.2 | 873.5 ± 377.6 | 0.3 | 894.4 ± 438.9 | 766.8 ± 258.7 | 0.16 |
| SMR (kcal/day) | 1810.1 ± 279.7 | 1818.1 ± 277.5 | 1800.6 ± 286.5 | 0.8 | 1998.6 ± 212.5 | 1660.3 ± 233.3 | <0.001 |
| AFT (kcal/day) | 648.7 ± 203.2 | 629.9 ± 176.3 | 671.0 ± 232.0 | 0.1 | 754.5 ± 235.4 | 564.6 ± 121.8 | <0.001 |
| SPA (kcal/day) | 80.0 ± 45.1 | 80.4 ± 47.1 | 79.4 ± 43.4 | 0.8 | 96.5 ± 48.5 | 66.8 ± 38.0 | <0.001 |
Data are presented as the mean ± SD, unless otherwise indicated. AA, African American; H, Hispanic; NA, Native American; W, white: O, other
p values are for differences between exenatide vs. placebo groups, and between males and females in the whole group, as determined by Student's t-test or Chi-squared test
4.2 Ad libitum Food Intake
Prior to randomization, participants over ate during ad libitum food intake period (114.6±35.2 %, expressed as percentage of weight maintaining energy need for whole study group). By chance this was greater in the exenatide group (121.6±37.7 %) compared to placebo group (107.6±31.5 %).
At the baseline admission and following randomization, the absolute decrease in the mean 3-day ad libitum energy intake was greater in the exenatide versus the placebo group, whether expressed as mean difference in kcal/day (Δ = −624.8 Kcal/day, 95% CI: −901.8 to −347.8, exenatide: −1016.1 ± 724.5 kcal/day, placebo: −245.1 ± 710.5 kcal/day, p < 0.0001, Fig. 3A) or %WMEN (Δ= −17.6%, CI 95% −27.3 to −7.9, p = 0.0005, figure 3B). When analyzed in terms of the reduction compared to WMEN, the difference was less pronounced but still significant whether expressed as mean difference in Kcal/day (Δ= −382.3 Kcal/day, 95% CI: −725.9 to −8.4, exenatide: −366.8±752.1 kcal/day, placebo: −8.0±860.1 kcal/day, p = 0.03, figure 3C) or %WMEN (Δ= −12.8%, CI 95% −25.1 to −0.45, p = 0.04, figure 3D). The reduction in calories occurred evenly across macronutrient groups (Supplemental Figure S1, panel A). The absolute decreased energy intake in the exenatide compared to placebo group (kcal/day) was observed from day one of the ad libitum vending machine period and did not exhibit tachyphylaxis (Supplemental Figure S1, panel B). There were no differences in caloric intake from soda between the 2 groups (Δ = −35.8 kcal/day, 95% CI: −80.9 to 9.3, p = 0.1). Analyzed by sex, the reduction in food intake was greater in males (Δ = −1310.9 kcal/day, 95% CI: −1703.7 to −918.1) compared to females (Δ = −788.2 Kcal/day, 95% CI: −1061.6 to −514.8, p = 0.02) in the exenatide group. No difference was observed between men (Δ= −379.6 Kcal/day, 95% CI: −2399.0 to −664.7) and women (Δ= −1310.9 Kcal/day, 95% CI: −2399.0 to −664.7) in the placebo group (p = 0.3). Similar results were obtained when the reduction in food intake, analyzed by sex, was adjusted for FFM in the exenatide group (p = 0.01) and in the placebo group (p = 0.7).
Figure 3. Ad Libitum Food intake changes between pre and post-randomization in exenatide and placebo groups.
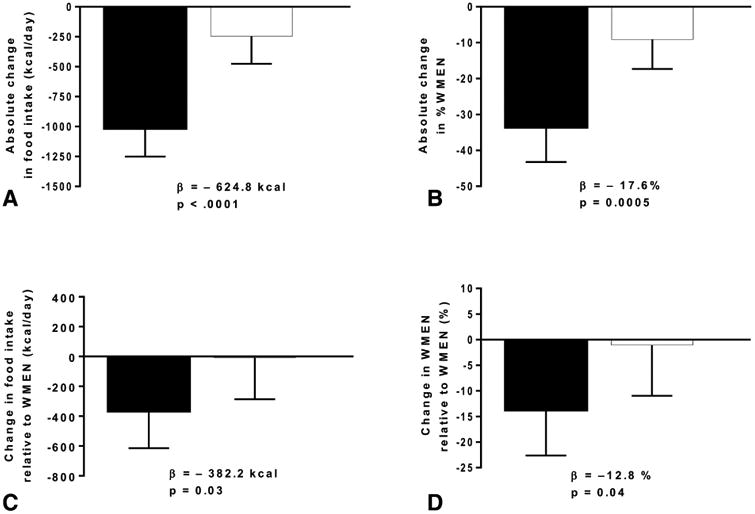
A. Mean of 3-day food intake absolute change between pre and post-randomization, expressed in Kcal/day; B. Mean of 3-day food intake, expressed as the absolute change in percentage of their weight maintaining energy need (WMEN); C. Mean of 3-day food intake change relative to the weight maintaining energy need between pre and post-randomization, expressed in Kcal/day D. Mean of 3-day food intake, expressed as the change in percentage of their WMEN relative to the weight maintaining calories.
The βs indicate the absolute values of the difference between pre and post-randomization changes in food intake between the exenatide and placebo groups.
Error bars represent the mean with 95% confidence interval. Thirty-nine individuals in each group had complete vending machine data.
At the 24-week follow up, there was no difference in EI between exenatide-treated individuals (n=12) and placebo treated individuals (n=18) (β = −379.2 Kcal/day, 95% CI: −1175.6 to 417.2, p = 0.3), nor in the overall decrease in EI in the exenatide group compared to baseline (β = −449.9 Kcal/day, 95% CI: −957.3 to 57.5, p = 0.08). Food intake reduction did not differ by sex (p = 0.9).
4.3 Energy expenditure
At the baseline admission, there was no change in 24h-EE (β = −24.1 kcal/day, 95% CI: −89.7 to 41.4, p = 0.4) (Fig.4A), RQ (β = −0.0016, 95% CI: −0.017 to 0.014, p = 0.8) (Fig.4B), AFT (β = −63.0 Kcal/day, 95% CI: −127.7 to 1.8, p = 0.06) (Fig.4C) or SMR (β = 26.7 Kcal/day, 95% CI: −23.6 to 77.1, p = 0.3) (Fig.4D) in exenatide versus placebo treated subjects. This remained so even after adjustment for FFM, FM, age, sex and race (data not shown). There was also no change in SPA (β = −6.3 kcal/day, 95% CI: −21.7 to 9.1, p = 0.4) in the exenatide treated group compared to placebo or in LIPOX and CARBOX either before or after adjustment for covariates (all p>0.7). Furthermore, no differences were found between groups when analyzed by sex (all p > 0.5).
Figure 4. Energy expenditure and RQ changes between pre and post-randomization in exenatide and placebo groups.
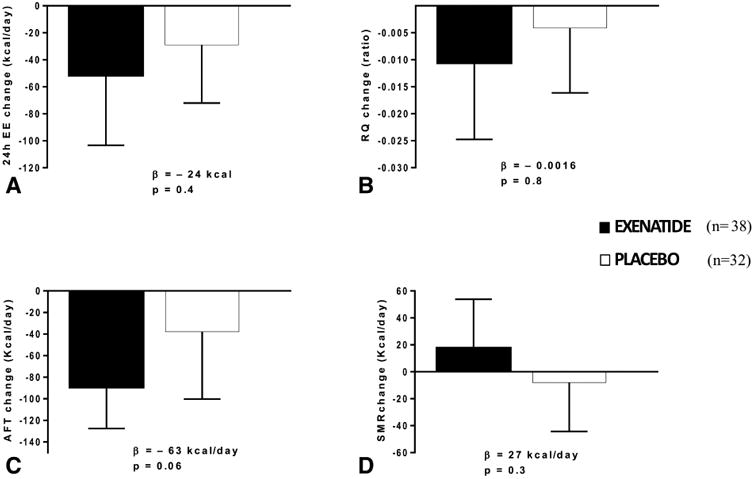
A Change in 24h-EE expressed as Kcal/day; B. Change in RQ; C. Change in AFT expressed as Kcal/day. D. Change in SMR expressed as Kcal/day.
The β indicate the absolute values of the difference between pre and post randomization measurements between the exenatide and placebo groups.
Error bars represent the mean with 95% confidence interval.
Thirty-eight participants in the exenatide group and 32 in the placebo group had complete EE data.
Compared to placebo group, subjects treated with exenatide had similar EE as assessed inside the metabolic chamber every minute over the course of 24 hrs (Δ = −0.02 kcal/min, p = 0.25) (Fig.5).
Figure 5. Post-randomization EE measures as assessed in the whole-room indirect calorimeter over 24 hours in exenatide and placebo groups.
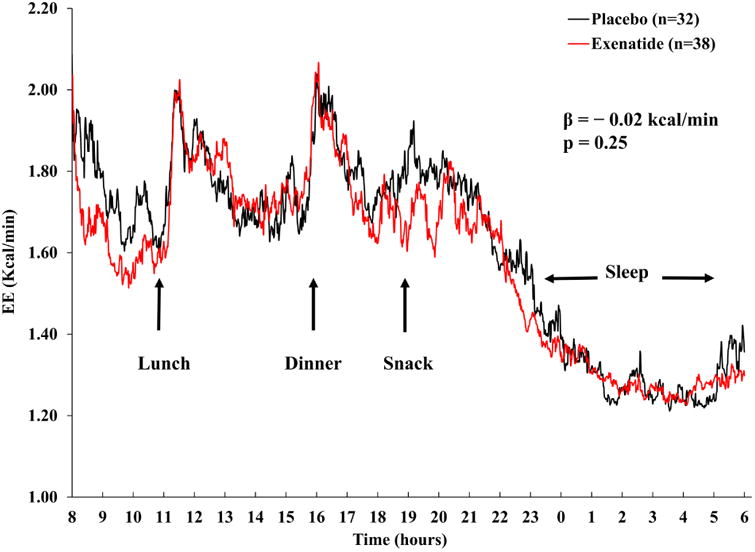
The graph shows the average EE trajectories measured every minute. The β coefficient indicates the difference between the EE trajectories of exenatide and placebo groups by mixed model analysis adjusting for age, sex, FFM, FM, physical activity and accounting for repeated measures.
At 24 weeks, 24-h EE decreased more in exenatide treated (n=8) versus placebo treated participants (n=16) (β = −160.6 Kcal/day, 95% CI: −307.6 to 13.6, p = 0.03) compared to their pre-randomization measurement. This greater decrease in 24h EE in exenatide-treated participants was still present after accounting for weight change at 24 weeks (Δ = −124.8 Kcal/day, 95% CI: −245.4 to −4.2, p = 0.04) but not after adjustment for changes in FM and FFM (β = −87 kcal/day, p = 0.14) (Fig. 6D). Furthermore, there was no difference between groups (p = 0.70) in 24-h EE residuals, i.e., observed minus predicted based on the regression equation calculated from the baseline measurements (data not shown).
Figure 6.
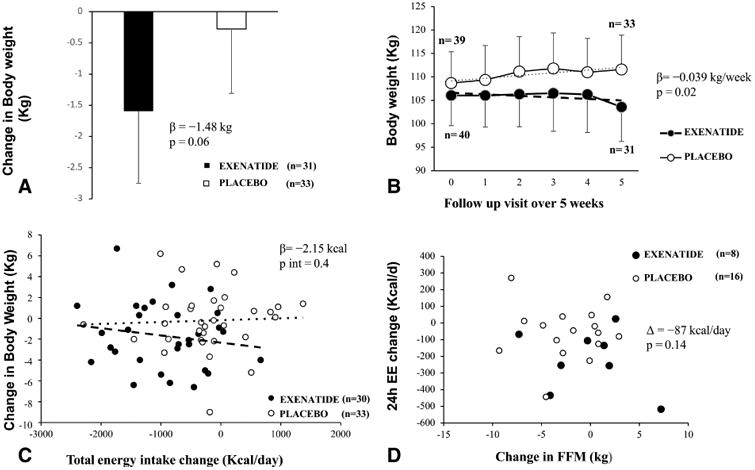
A. Change in body weight (kg) from baseline over 5 weeks. The baseline weight was the weight at the time of the DXA scan. The β is the absolute difference (Kg), between the body weight at the 5-week follow up visit and the baseline weight. B. Weight trajectory in the exenatide versus placebo groups over 5 weeks. Data represents the mean body weight at the time of the DXA scan, at the day of discharge and at each weekly follow up visit. The two fitted regression lines represent the weekly rate of weight change in the 2 groups. The β value represents the difference in the rates of body weight change between the 2 groups. Analyses used mixed models for repeated measures of change from baseline adjusted for baseline weight (as baseline comparator). C. Correlation between body weight change and total energy intake from baseline over 5 weeks. Correlation between the pre and post-randomization change in ad libitum food intake, expressed as mean Kcal/day versus weight change at 5 weeks.
- In the panel A, the sample size (Exenatide=31, Placebo= 33) indicate the numbers of volunteers who have the weight measure at 5-week follow up visit. In the panel B, some values were not analyzed due to unavailability of some body weight measurements. In the panel C, the sample size (Exenatide =30, Placebo=33) shows participants having 5-week follow up weight and completed data of food intake. In the panel D, the sample size (Exenatide =8, Placebo=16) shows participants having 24-week follow up body composition measurements and completed data of chambers.
- Error bars represent the mean with 95% confidence interval.
There were no changes in RQ (β = 0.0032, 95% CI: −0.027 to 0.033, p = 0.8), SMR (β = −111.1 Kcal/day, 95% CI: −270.4 to 48.1, p= 0.16), AFT (β = −38.2 Kcal/day, 95% CI: −162.5 to 86, p = 0.52) or SPA (β = −7.3 Kcal/day, 95% CI: −31.6 to 16.9, p = 0.53) at the 24-week follow up. Changes did not differ by sex (all p > 0.6).
During the baseline inpatient portion of the study, differences in ad libitum food intake prior to and following initiation of exenatide or placebo did not correlate with the changes in 24h-EE or related measures such as RQ, SMR, AFT and SPA (all p > 0.3) in either group.
4.4 Body weight change
There was no difference in body weight at 5 weeks between the two groups (β = −1.48 kg, 95% CI: −3.00 to 0.05, p = 0.06) (Fig.6A); however, in the repeated measures analysis of weekly body weight, the exenatide group had a greater rate of weight change over 5 weeks compared with the placebo group (β = −0.039 kg/week, p = 0.02) (Fig. 6B). The results did not change when the repeated measures analysis was not adjusted for the baseline weight (β = −0.045 kg/week, p = 0.02). Similar results (β = −0.042 kg/week, p = 0.01) were observed when only subjects on treatment (n = 64) were evaluated.
At 24 weeks, in a linear model, there was no difference in body weight (Δ = −1.72 kg, 95% CI: −5.77 to 2.30, p = 0.39) or in body composition (data not shown) in exenatide (n=13) versus placebo (n=20) treated subjects; nor was there any difference in weight trajectory using mixed models (data not shown). Weight change did not differ by sex at 5 or 24-weeks (all p > 0.4). The individual change in mean caloric intake in the exenatide group was not associated with overall weight change at 5 weeks (p = 0.4, Fig. 6C) or 24 weeks (p = 0.8). Results were similar when weight change was expressed in either absolute terms (kg) or as a percentage of baseline weight.
4.5 Safety and adverse events
Self-reported hypoglycemia and injection site reactions were more common in the exenatide group. We did not find any association between reduction in food intake and presence of nausea, defined as at least one episode, in the exenatide group (p = 0.3) or placebo group (p = 0.3). Details of the adverse events are reported in the Supplement (Supplement Table 2) and were consistent with prior reports [27]. Based on the quantification of product returned, 81 % of volunteers were assessed as having taken all the medication doses.
5. Discussion
In this double-blind placebo controlled study examining the mechanism of exenatide induced weight loss, we demonstrated that exenatide produces an initial profound, nearly 1000 kcal/day or 30%, decrease in absolute energy intake, but has no effect on EE or substrate oxidation. This effect on energy intake is non-macronutrient selective, but wanes over time as demonstrated by the less pronounced reduction at 6 months. However, when we analyzed the reduction in caloric intake relative to how many calories were calculated to maintain weight (i.e. the weight maintaining needs or WMEN), this effect was less pronounced than the absolute change. This calorie deficit was approximately 370 kcal/day. We did observe an effect of exenatide on weight loss trajectory as early as 5 weeks but absolute weight change was not different between the groups.
Previous studies have demonstrated an effect on GLP-1 agonists on energy intake, but not to the degree found in our study; nor did these studies measure ad libitum energy intake over 3 days, using our highly reproducible vending machine paradigm [26].
Early studies with exendin-4 infusions demonstrated a 19% reduction in calories consumed in participants with obesity [28]. A meta-analysis including lean and overweight subjects [29] estimated an 11.7% decrease in food intake. Using an ad libitum dinner paradigm, exenatide administration decreased food intake compared with sitagliptin in subjects with type 2 diabetes [30]. Pinelli et al [31] also demonstrated decreased food intake in lean subjects following administration of exenatide compared to placebo in a single meal test, as has another similar study [32]. In subjects with diabetes, liraglutide decreased energy intake compared to placebo by 18% [33], and a similar effect on energy intake was demonstrated in healthy individuals with obesity at doses of 1.8 mg or 3 mg[34]. We did confirm that decreased energy intake is likely the primary mechanism for weight loss with GLP-1 agonists. However, the more modest reduction in caloric intake relative to weight maintaining caloric needs indicated that at least for exenatide its effect on early weight loss is less substantial. By using a longer (3 day) ad libitum paradigm, we have been able to more precisely quantify this effect, demonstrating a larger effect on energy intake than had been reported previously and across all macronutrient groups. Furthermore, using the same paradigm, we demonstrated that this effect became less pronounced after 6 months of treatment. This may have been due to the smaller cohort or to a true waning effect of exenatide on energy intake over longer follow-up.
The effect of GLP-1 analogues on energy expenditure has been less clear. GLP-1 infusions reduced resting metabolic rate, diet induced thermogenesis and carbohydrate oxidation in two studies [35, 36], but another study found increased EE via an insulin mediated mechanism [9]. Subjects with obesity did not have an increase in total EE after 14 weeks of exenatide administration. However, our group has previously [14] found a positive association between fasting plasma GLP-1 concentrations and both resting EE and fat oxidation. Using whole room indirect calorimetry, we did not observe any effect of exenatide administration on measures of EE or substrate oxidation. We did find a greater decrease in 24h-EE in the exenatide versus placebo group at 6 months. However, this effect, although independent of weight change, was not independent of body composition change, albeit the overall sample size was small and a significant difference might have been detected in a larger cohort.
Although the point estimate indicated greater weight loss in exenatide treated subjects at 5 weeks, this did not reach statistical significance. However, more sophisticated analysis with mixed models demonstrated a greater weight loss trajectory in the exenatide treated group. A large meta-analysis including studies of participants with and without DM demonstrated that these medications do contribute to weight loss [6]. Madsbad et al [37] showed that all six GLP-1 receptor agonists, including exenatide, induced weight loss.
Per the ileal brake hypothesis, ingested nutrients trigger endogenous GLP-1 release inhibiting gastric motility [38, 39], which is considered the dominant mechanism for the decreased energy intake. However, GLP-1 receptors are also present in the brain [40], in particular in the paraventricular and arcuate nucleus, areas crucial to appetite regulation. Consistent with the effects of GLP-1 on both gastric emptying and satiety, participants not only ate less, but reported decreased hunger (Supplemental, Table 2). In mice, decrease in food intake has been reported after both central and peripheral administration of GLP-1 receptor agonists [41, 42]. In rats, GLP-1 activates brain vagal preganglionic neurons resulting in gastroinhibition[43]. In mice, radiolabeled GLP-1 analogue penetrated the blood brain barrier[44]. In humans, exenatide altered brain responses to the consumption of palatable food in diabetic patients with obesity[45]. Thus, GLP-1 analogues may have both a direct peripheral and central effect on satiety and gastric emptying.
Side effects were as expected with nausea, diarrhea, and gastroesophageal reflux as the most common but these did not differ by group (Supplement Table 2). Hypoglycemia was more common in the exenatide group (25%; vs. 7.7%, p = 0.04). None of these were serious (defined as requiring assistance). This prevalence is higher than in other studies, likely due to the immediate use of the 10 μg dose. In our data, the absence of association between presence of nausea, defined as the presence of at least one episode since randomization, and reduction in food intake suggest that nausea does not play a major role in the reduction of food intake.
The strengths of our study include the directly observed administration of the drug during the inpatient stay. Furthermore, we used objective gold standard techniques for the measurement of 24 hours energy expenditure and food intake. In addition, these measurements were made prior to and following randomization and also, in a subset of subjects, repeated at 24 weeks. Our study has several limitations. This was a small single-center study which focused on the mechanism of exenatide induced weight loss. We did have sufficient power to investigate our primary hypotheses of the effect of exenatide on energy intake and energy expenditure, but our ability to detect a difference in secondary outcomes, including weight change, was limited by our relatively small samples size, especially at the 24-week follow-up. We acknowledge also that, although the automated vending machine paradigm is highly reproducible and useful to assess food intake measurement in inpatient setting, it does not reflect energy intake during free-living conditions. In this artificial setting subjects tend to overeat resulting in a large reduction in food intake in absolute terms but less so when this is assessed by the relative decrease compared to weight maintaining energy needs. Furthermore, although subjects were monitored as inpatients and had no reported differences in nausea, we cannot exclude that milder early nausea which affected energy intake was not captured and might have affected our ad libitum energy intake results. An additional measure of energy intake after several weeks of treatment would have been helpful in this regard. In addition, although the drug administration was administered by study personnel during the inpatient stay, albeit adherence was assessed at their 5-week outpatient visit, we cannot be sure of the same degree of adherence during the outpatient portion. Lastly, although evidence indicates that exenatide 10 mcg twice daily achieves a pharmacologic steady state on day 3 of administration [46] (the same day the EE measurement was made in our study), it is not clear if this means a “metabolic” steady state was achieved in terms of fuel utilization.
6. Conclusion
In conclusion, using gold standard measurements of ad libitum energy intake and energy expenditure, exenatide, as representative of GLP-1 agonists, has an early profound and rapid effect in energy intake in absolute terms, which we were able to quantify (decreased calorie intake of over 1000 kcal/day and over a 600 kcal/day decrease compared to placebo) but no effect on energy expenditure. However, when analyzed relative to the decrease in weight maintaining energy needs, which is the caloric deficit that will truly affect weight loss, this effect is less marked (nearly 370 kcal/day below WMEN versus 1000 kcal/day as an absolute decrease). Thus, we did not observe as strong an effect on early weight loss with exenatide and this effect appeared to wane over longer term follow-up. Thus, rather than a tool for inducing early weight loss, exenatide via its effect on blunting of overeating might be more useful as a medication to prevent weight regain following initial weight loss in addition to ongoing lifestyle efforts.
Supplementary Material
Highlights.
Exenatide has an early and pronounced absolute change in daily energy intake
Decrease in food intake relative to WMEN was less pronounced than absolute reduction
Exenatide has no effect on energy expenditure
Exenatide has a modest effect on weight loss at 5 weeks
Effect on energy intake appeared to wane over long term follow-up
Acknowledgments
Medication was provided at no cost by Amylin, Lily, Bristol Myers Squibb and Astra Zeneca. The authors thank the volunteers who participated in the studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations.
A.B. and J.B. analyzed and interpreted data and wrote the manuscript. J.K. reviewed the manuscript and assisted with the interpretation of the data. M.T. designed, implemented and conducted the study. All authors critically revised the draft and approved the final manuscript. A.B. and J.B. are the guarantors of this work and A.B. has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- WMEN
weight maintaining energy need
- EE
energy expenditure
- RQ
respiratory quotient
- SMR
sleeping metabolic rate
- AFT
awake fed thermogenesis
- SPA
spontaneous physical activity
Footnotes
The authors declare no conflict of interest.
Prior Presentation: The study has not been published previously in abstract form or manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monami M, Marchionni N, Mannucci E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. European journal of endocrinology. 2009;160:909–17. doi: 10.1530/EJE-09-0101. [DOI] [PubMed] [Google Scholar]
- 2.Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clinical therapeutics. 2007;29:139–53. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 4.Nelson P, Poon T, Guan X, Schnabel C, Wintle M, Fineman M. The incretin mimetic exenatide as a monotherapy in patients with type 2 diabetes. Diabetes technology & therapeutics. 2007;9:317–26. doi: 10.1089/dia.2006.0024. [DOI] [PubMed] [Google Scholar]
- 5.Dushay J, Gao C, Gopalakrishnan GS, Crawley M, Mitten EK, Wilker E, et al. Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes. Diabetes care. 2012;35:4–11. doi: 10.2337/dc11-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical research ed) 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23:304–11. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- 8.Pannacciulli N, Bunt JC, Koska J, Bogardus C, Krakoff J. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. The American journal of clinical nutrition. 2006;84:556–60. doi: 10.1093/ajcn/84.3.556. [DOI] [PubMed] [Google Scholar]
- 9.Shalev A, Holst JJ, Keller U. Effects of glucagon-like peptide 1 (7–36 amide) on whole-body protein metabolism in healthy man. European journal of clinical investigation. 1997;27:10–6. doi: 10.1046/j.1365-2362.1997.540613.x. [DOI] [PubMed] [Google Scholar]
- 10.Vollenweider P, Tappy L, Randin D, Schneiter P, Jéquier E, Nicod P, et al. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. Journal of Clinical Investigation. 1993;92:147. doi: 10.1172/JCI116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christin L, Nacht C, Vernet O, Ravussin E, Jequier E, Acheson K. Insulin. Its role in the thermic effectof glucose. Journal of Clinical Investigation. 1986;77:1747. doi: 10.1172/JCI112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwa JJ, Ghibaudi L, Williams P, Witten MB, Tedesco R, Strader CD. Differential effects of intracerebroventricular glucagon-like peptide-1 on feeding and energy expenditure regulation. Peptides. 1998;19:869–75. doi: 10.1016/s0196-9781(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 13.Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24:288–98. doi: 10.1038/sj.ijo.0801126. [DOI] [PubMed] [Google Scholar]
- 14.Pannacciulli N, Bunt JC, Koska J, Bogardus C, Krakoff J. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. The American journal of clinical nutrition. 2006;84:556–60. doi: 10.1093/ajcn/84.3.556. [DOI] [PubMed] [Google Scholar]
- 15.Sun F, Chai S, Li L, Yu K, Yang Z, Wu S, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. Journal of diabetes research. 2015;2015 doi: 10.1155/2015/157201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott W, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. American Journal of Physiology-Endocrinology and Metabolism. 1988;255:E332–E7. doi: 10.1152/ajpendo.1988.255.3.E332. [DOI] [PubMed] [Google Scholar]
- 17.Association AD. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care. 2003;26:s5–s20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 18.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. Jama. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 19.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes, obesity & metabolism. 2006;8:436–47. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 20.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. The Journal of clinical endocrinology and metabolism. 2013;98:2791–9. doi: 10.1210/jc.2013-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. The American journal of clinical nutrition. 2007;86:625–32. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62:4043–51. doi: 10.2337/db13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rising R, Alger S, Boyce V, Seagle H, Ferraro R, Fontvieille AM, et al. Food intake measured by an automated food-selection system: relationship to energy expenditure. The American journal of clinical nutrition. 1992;55:343–9. doi: 10.1093/ajcn/55.2.343. [DOI] [PubMed] [Google Scholar]
- 25.Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiology & behavior. 1998;63:919–28. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 26.Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. The American journal of clinical nutrition. 2010;91:343–8. doi: 10.3945/ajcn.2009.28315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Drab S. Glucagon-like peptide-1 receptor agonists for type 2 diabetes: a clinical update of safety and efficacy. Current diabetes reviews. 2016;12:403–13. doi: 10.2174/1573399812666151223093841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards CMB, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. American Journal of Physiology-Endocrinology And Metabolism. 2001;281:E155–E61. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- 29.Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom P, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7−36) amide on ad libitum energy intake in humans. The Journal of Clinical Endocrinology & Metabolism. 2001;86:4382–9. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Current medical research and opinion. 2008;24:2943–52. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 31.Pinelli NR, Jantz A, Smith Z, Abouhassan A, Ayar C, Jaber NA, et al. Effect of administration time of exenatide on satiety responses, blood glucose, and adverse events in healthy volunteers. The Journal of Clinical Pharmacology. 2011;51:165–72. doi: 10.1177/0091270010367653. [DOI] [PubMed] [Google Scholar]
- 32.Acosta A, Camilleri M, Burton D, O'Neill J, Eckert D, Carlson P, et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiological reports. 2015;3:e12610. doi: 10.14814/phy2.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flint A, Kapitza C, Zdravkovic M. The once-daily human GLP-1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short-term treatment. Diabetes, Obesity and Metabolism. 2013;15:958–62. doi: 10.1111/dom.12108. [DOI] [PubMed] [Google Scholar]
- 34.Van Can J, Sloth B, Jensen C, Flint A, Blaak E, Saris W. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. International Journal of Obesity. 2014;38:784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flint A, Raben A, Rehfeld J, Holst J, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. International journal of obesity. 2000;24:288. doi: 10.1038/sj.ijo.0801126. [DOI] [PubMed] [Google Scholar]
- 36.Flint A, Raben A, Ersbøll A, Holst J, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. International journal of obesity. 2001;25:781. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 37.Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes, Obesity and Metabolism. 2015 doi: 10.1111/dom.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edholm T, Degerblad M, Grybäck P, Hilsted L, Holst JJ, Jacobsson H, et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterology & Motility. 2010;22:1191. doi: 10.1111/j.1365-2982.2010.01554.x. [DOI] [PubMed] [Google Scholar]
- 39.Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. The Journal of Clinical Endocrinology & Metabolism. 2010;95:215–21. doi: 10.1210/jc.2009-1503. [DOI] [PubMed] [Google Scholar]
- 40.Drucker D, Asa S. Glucagon gene expression in vertebrate brain. Journal of Biological Chemistry. 1988;263:13475–8. [PubMed] [Google Scholar]
- 41.Turton M, Shea D, Gunn I, Beak S. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 42.de Fonseca FR, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, et al. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism. 2000;49:709–17. doi: 10.1053/meta.2000.6251. [DOI] [PubMed] [Google Scholar]
- 43.Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagons-like peptide 1: in vitro and in vivo gastric actions. The Journal of physiology. 2009;587:4749–59. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. Journal of Molecular Neuroscience. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 45.van Bloemendaal L, Veltman DJ, Ten Kulve J, Groot PF, Ruhé HG, Barkhof F, et al. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes, Obesity and Metabolism. 2015;17:878–86. doi: 10.1111/dom.12506. [DOI] [PubMed] [Google Scholar]
- 46.Kothare PA, Soon DK, Linnebjerg H, Park S, Chan C, Yeo A, et al. Effect of Exenatide on the Steady-State Pharmacokinetics of Digoxin. The Journal of Clinical Pharmacology. 2005;45:1032–7. doi: 10.1177/0091270005278806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


