Abstract
The AP-1 complex is essential for membrane protein traffic via its role in the pinching-off and sorting of secretory vesicles (SVs) from the trans-Golgi and/or endosomes. While its essentiality is undisputed in metazoa, its role in simpler eukaryotes seems less clear. Here, we dissect the role of AP-1 in the filamentous fungus Aspergillus nidulans and show that it is absolutely essential for growth due to its role in clathrin-dependent maintenance of polar traffic of specific membrane cargoes toward the apex of growing hyphae. We provide evidence that AP-1 is involved in both anterograde sorting of RabERab11-labeled SVs and RabA/BRab5-dependent endosome recycling. Additionally, AP-1 is shown to be critical for microtubule and septin organization, further rationalizing its essentiality in cells that face the challenge of cytoskeleton-dependent polarized cargo traffic. This work also opens a novel issue on how nonpolar cargoes, such as transporters, are sorted to the eukaryotic plasma membrane.
Keywords: fungi, traffic, secretion, Rab GTPases, transport, microtubules
ALL eukaryotic cells face the challenge of topological sorting of their biomolecules to their proper subcellular destinations. In particular, newly synthesized membrane proteins, which are translationally translocated into the membrane of the endoplasmic reticulum (ER), follow complex, dynamic, and often overlapping, trafficking routes, embedded in the lipid bilayer of “secretory” vesicles, to be sorted to their final target membrane (Feyder et al. 2015; Viotti 2016). In vesicular membrane trafficking, the nature of the protein cargo and relevant adaptor proteins play central roles in deciding the routes followed and the final destination of cargoes. Despite the emerging evidence of alternative, or nonconventional, trafficking routes, cargo passage through a continuously maturing early-to-late Golgi is considered to be part of the major mechanism and the most critical step in membrane protein sorting. Following exit from the trans-Golgi network (TGN, also known as late-Golgi), cargoes packed in distinct vesicles travel to their final destination, which, in most cases, is the plasma membrane or the vacuole. This anterograde vesicular movement can be direct or via the endosomal compartment, and, in any case, assisted by motor proteins and the cytoskeleton (Bard and Malhotra 2006; Cai et al. 2007; Anitei and Hoflack 2011; Hunt and Stephens 2011; Guo et al. 2014). Membrane protein cargoes at the level of late Golgi can also follow the opposite route, getting sorted into retrograde vesicles, recycling back to an earlier compartment. Acquiring a “ticket” for a specific route implicates adaptors and accessory proteins, several of which are also associated with clathrin (Nakatsu and Ohno 2003; Robinson 2004, 2015).
Apart from the COPI and COPII vesicle coat adaptors that mediate traffic between the ER and the early Golgi compartment (Lee et al. 2004; Zanetti et al. 2011), of particular importance are the heterotetrameric AP (formally named after assembly polypeptide and later as adaptor protein) complexes, comprising of two large subunits (also called adaptins; β-adaptin and γ- or α-adaptin), together with a medium-sized (μ) and a small (σ) subunit (Robinson 2004, 2015). Other adaptors, some of which display similarity to AP subunits, such as the GGAs, epsin-related proteins, or components of the exomer and retromer complexes, are also critical for the sorting of specific cargoes (Bonifacino 2004, 2014; Robinson 2015; Spang 2015; Anton et al. 2018). Importantly, the various cargo sorting routes often overlap, and might share common adaptors (Hoya et al. 2017). Among the major AP complexes (Bonifacino 2014; Nakatsu et al. 2014), AP-1 and AP-2, which in most cells work to propel vesicle formation through recruitment of clathrin, are the most critical for cell homeostasis and function (Robinson 2004, 2015). In brief, AP-2 is involved in vesicle budding for protein endocytosis from the PM, whereas AP-1 is involved in vesicle pinching-off from the TGN and/or endosomal compartments, although in the latter case it is still under debate whether secretory vesicles (SVs) derive from the TGN, from the endosome, or from both (Nakatsu et al. 2014; Robinson 2015). AP-1 was also shown to be responsible for retrograde transport from early endosomes (EEs) in both yeast and mammalian cells, but also to guide recycling pathways from the endosome to the plasma membrane in yeast (Spang 2015). The undisputed essentiality of AP-1 and AP-2 in mammalian cells is, however, less obvious in simple unicellular eukaryotes, such as the yeasts Saccharomyces cerevisiae or Schizosaccharomyces pombe, where null mutants in the genes encoding AP subunits are viable, with only relatively minor growth or morphological defects (Phan et al. 1994; Huang et al. 1999; Meyer et al. 2000; Valdivia et al. 2002; Ma et al. 2009; Yu et al. 2013; Arcones et al. 2016). In sharp contrast, the growth of AP-1 and AP-2 null mutants in the filamentous fungus Aspergillus nidulans is severely arrested after spore germination (Martzoukou et al. 2017, and results presented therein), reflecting blocks in essential cellular processes, probably similar to mammalian cells.
In recent years, A. nidulans has emerged as a powerful system for studying membrane cargo traffic (Momany 2002; Taheri-Talesh et al. 2008; Steinberg et al. 2017). This is due not only to its powerful classical and reverse genetic tools, but also to its specific cellular characteristics and manner of growth. A. nidulans is made of long cellular compartments (hyphae), characterized by polarized growth, in a process starting with an initial establishment of a growth site, followed by polarity maintenance and cell extension through the regulated continuous supply of vesicles toward the apex. A vesicle sorting terminal at the hyphal apex, termed Spitzenkörper (Spk), is thought to generate an exocytosis gradient, which, when coupled with endocytosis from a specific hotspot behind the site of growth, termed endocytic collar, is able to sustain apical growth (Peñalva 2015; Schultzhaus and Shaw 2015; Pantazopoulou 2016; Steinberg et al. 2017). Apical trafficking of cargoes, traveling from the ER through the different stages of early (cis−) and late (trans−) Golgi toward their final destination, and apical cargo endocytosis/recycling, are essential for growth, as null mutations blocking either Golgi function, microtubule (MT) organization, or apical cargo recycling are lethal or severely deleterious (Fischer et al. 2008; Takeshita and Fischer 2011; Peñalva 2015; Pantazopoulou 2016; Steinberg et al. 2017). Curiously, the role of AP complexes in A. nidulans or any other filamentous fungus, has not been studied, with the exception of our recent work on AP-2 (Martzoukou et al. 2017). In the latter study, we showed that the AP-2 of A. nidulans has a rather surprising clathrin-independent essential role in polarity maintenance and growth, related to the endocytosis of specific polarized cargoes involved in apical lipid and cell wall composition maintenance. This was in line with the observation that AP-2 β subunit (β2) lacks the ability to bind clathrin, which itself has been shown to be essential for the endocytosis of distinct cargoes, as, for example, various transporters (Martzoukou et al. 2017; Schultzhaus et al. 2017). In the current study, we focus on the role of the AP-1 complex in cargo trafficking in A. nidulans. We provide evidence that AP-1 is essential for fungal polar growth via its dynamic role in post-Golgi secretory vesicle polar sorting, proper MT organization, and endosome recycling.
Materials and Methods
Media, strains, growth conditions, and transformation
Standard complete and minimal media for A. nidulans were used (details in FGSC, http://www.fgsc.net.). Media and chemical reagents were obtained from Sigma-Aldrich (Life Science Chemilab SA, Hellas) or AppliChem (Bioline Scientific SA, Hellas). Glucose 0.1–1% (w/v) was used as a carbon source. NaNO3 and NH4+ (ammonium tartrate dibasic) were used as nitrogen sources at 10 mM. Thiamine hydrochloride (thi) was used at a final concentration of 10 μM. Transformation was performed as described previously in Koukaki et al. (2003), using an nkuA DNA helicase deficient (TNO2A7; Nayak et al. 2006) recipient strain or derivatives for “in locus” integrations of gene fusions, or deletion cassettes by the Aspergillus fumigatus markers orotidine-5′-phosphate-decarboxylase (AFpyrG, Afu2g0836), GTP-cyclohydrolase II (AFriboB, Afu1g13300), and a pyridoxine biosynthesis gene (AFpyroA, Afu5g08090), resulting in complementation of auxotrophies for uracil/uridine (pyrG89), riboflavin (riboB2), or pyridoxine (pyroA4), respectively. Transformants were verified by PCR and Southern analysis. Combinations of mutations and tagged strains with fluorescent epitopes were generated by standard genetic crossing. The Escherichia coli strain used was DΗ5α. A. nidulans strains used in this study are listed in Supplemental Material, Table S1 (see also File S7).
Nucleic acid manipulations and plasmid constructions
Genomic DNA was extracted from A. nidulans as described in FGSC (http://www.fgsc.net). Plasmid preparation and DNA gel extraction were performed using the Nucleospin Plasmid kit and the Nucleospin Extract II kit (Macherey-Nagel, Lab Supplies Scientific SA, Hellas). Restriction enzymes were from Takara Bio or Minotech (Lab Supplies Scientific SA, Hellas). DNA sequences were determined by Eurofins-Genomics (Vienna, Austria). Southern blot analysis using specific gene probes was performed as described in Sambrook et al. (1989), using [32P]-dCTP labeled molecules prepared by a random hexanucleotide primer kit and purified on MicroSpin S-200 HR columns (Roche Diagnostics, Hellas). Labeled [32P]-dCTP (3000 Ci mmol−1) was purchased from the Institute of Isotops Co. Ltd, Miklós, Hungary. Conventional PCR reactions, high fidelity amplifications, and site-directed mutagenesis were performed using KAPA Taq DNA, and Kapa HiFi polymerases (Kapa Biosystems, Roche Diagnostics, Hellas). Gene fusion cassettes were generated by one-step ligations or sequential cloning of the relevant fragments in the plasmids pBluescript SKII or pGEM-T using oligonucleotides carrying additional restriction sites. These plasmids were used as templates to amplify the relevant linear cassettes by PCR. For ap1β site directed mutations, the relevant gene was cloned in the pBS-argB plasmid (Vlanti and Diallinas 2008). For primers, see Table S2.
Protein extraction and western blots
Cultures for total protein extraction were grown in minimal media supplemented with NaNO3 or NH4+ at 25°. Total protein extraction was performed as previously described (Papadaki et al. 2017). Total proteins (30–50 μg estimated by Bradford assays) were separated in a polyacrylamide gel (8–10% w/v) onto PVDF membranes (Macherey-Nagel, Lab Supplies Scientific SA, Hellas). Immunodetection was performed with a primary anti-FLAG M2 monoclonal antibody (Sigma-Aldrich), an anti-actin monoclonal (C4) antibody (MP Biomedicals Europe) and a secondary HRP-linked antibody (Cell Signaling Technology Inc, Bioline Scientific SA, Hellas). Blots were developed using the LumiSensor Chemiluminescent HRP Substrate kit (Genscript USA, Lab Supplies Scientific SA, Hellas) and SuperRX Fuji medical X-Ray films (FujiFILM Europe).
Microscopy and statistical analysis
Samples for wide-field epifluorescence microscopy were prepared as previously described (Martzoukou et al. 2017). Germlings were incubated in sterile 35 mm μ-dishes, high glass bottom (ibidi, Germany) in liquid minimal media for 16–22 hr at 25°. Benomyl, Latrunculin B, Brefeldin A, and Calcofluor white were used at final concentrations of 2.5, 100, 100 μg ml−1, 0.001% (w/v), respectively. FM4-64 and CMAC staining was according to Peñalva (2005) and Evangelinos et al. (2016), respectively. Images were obtained using an inverted Zeiss Axio Observer Z1 with appropriate filters (motorized turret with position change <200 ms, according to the manufacturer) and an Axio Cam HR R3 camera. Contrast adjustment, area selection, and color combining were made using the Zen lite 2012 software. Sum Intensity Projections of selected frames were created using the “Z project” command of ImageJ software. ImageJ Plot profile was used for measurements of fluorescence intensity (https://imagej.nih.gov/ij/). For quantifying colocalization, Pearson’s correlation coefficient (PCC) above thresholds, for a selected region of interest (ROI) was calculated using the ICY colocalization studio plugin (pixel-based method) (http://icy.bioimageanalysis.org/). One sample t-test was performed to test the significance of differences in PCCs, using Graphpad Prism software. Confidence interval (C.I.) was set to 95%. For quantifying dot density in Figure 6, ROIs were selected using the Area Selection tool and the Spot Detector plugin of ICY. Tukey’s multiple comparison test was performed (one-way ANOVA) using Graphpad Prism software for statistical analyses. C.I. was set to 95%. Scale bars were added using the FigureJ plugin of ImageJ software. Images were further processed and annotated in Adobe Photoshop CS4 Extended version 11.0.2.
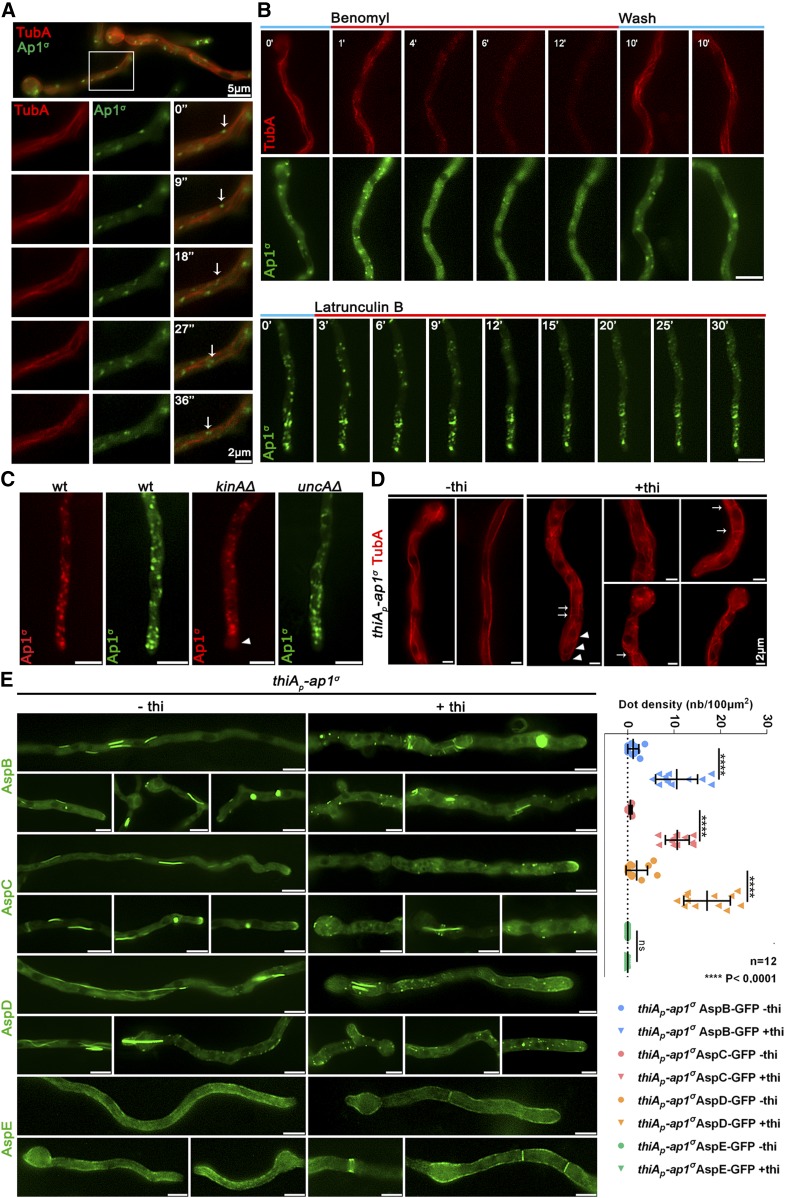
Figure 6.
AP-1 associates with the cytoskeleton and affects septin organization. (A) Relative Ap1σ-GFP and mCherry-TubA (α-tubulin) subcellular localization. Notice Ap1σ fluorescent foci decorating dynamically TubA-labeled MTs, as highlighted with arrows in the selected time-lapse images on the lower panels (see also File S5). Hyphal apex is at the right side of the image series. (B) Time course of treatment of strains expressing Ap1σ and TubA with the anti-MT drug Benomyl (upper panels). Notice that Benomyl elicits an almost complete, but reversible, disassociation of Ap1σ and TubA, resulting in diffuse cytoplasmic florescent signals. In contrast, treatment with the anti-actin drug Latrunculin B does not elicit a significant change in the polar distribution of Ap1σ (lower panels). Hyphal apex is at the lower side of the image series. (C) Subcellular localization of AP-1 in wt and in strains lacking the kinesins KinA and UncA, respectively. Notice the absence of apical labeling of AP-1 in the kinAΔ strain, indicated with an arrowhead. Hyphal apex is at the lower side of the image series. (D) Subcellular organization of the MT network, as revealed by TubA-labeling, in a strain carrying a thiamine-repressible thiAp-ap1σ allele, observed under conditions of expression (−thi) or repression (+thi) of ap1σ. Note that the absence of Ap1σ leads to a less orientated network, bearing vertical (arrows) and curved MTs (arrowheads), and, in some cases, the appearance of bright cortical spots (two to seven puncta/hypha, usually exhibiting perinuclear localization). (E) Subcellular localization of GFP-tagged versions of septins AspB, AspC, AspD, and AspE in a strain carrying a thiamine-repressible thiAp-ap1σ allele, observed under conditions of expression (−thi) or repression (+thi) of ap1σ. Note that when ap1σ is repressed, AspB, AspC, and AspD form less higher order structures (HOS) such as filaments or bars (ap1+: 1.58 HOS/hypha, n = 87, ap1−: 0.96 HOS/hypha, n = 103) and instead label more cortical spots (see right panel for quantification), some of which appear as opposite pairs at both sides of the plasma membrane, resembling septum formation initiation areas. In contrast, AspE localization remains apparently unaffected under ap1σ repression conditions. Hyphal apex is at the right side of the image series. Unless otherwise stated, Bar, 5 μm.
Data availability
Strains constructed in this work are available upon request. Supplementary material has been uploaded to figshare. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6619922.
Results
The AP-1 complex localizes polarly in distinct cytoplasmic structures and is essential for growth
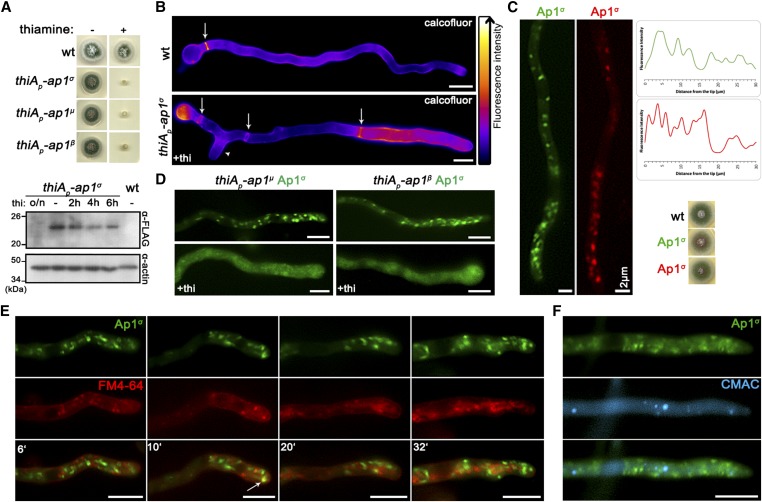
In A. nidulans, the AP-1 adaptor complex is encoded by the genes AN7682 (ap-1σ), AN4207 (ap-1γ), AN3029 (ap-1β), and AN8795 (ap-1μ). In a previous study, a knockout of the gene encoding the AP-1 σ subunit proved lethal, therefore we employed a conditional knock-down strain (Martzoukou et al. 2017), using the thiamine-repressible promoter thiAp (Apostolaki et al. 2012). The phenotypic analysis of this strain showed that repression of ap1σ results in severely retarded colony growth, reflected at the microscopic level in wider and shorter hyphae with increased numbers of side branches and septa. Figure 1, A and B highlights these results, further showing that thiAp-dependent full repression of not only ap1σ, but also ap1β and ap1μ, results in lack of growth. Notably, besides increased numbers of side branches and septa, staining level and cortical localization of calcofluor white are modified upon repression of AP-1σ, suggesting altered chitin deposition (Figure 1B). Given that the genetic disruption of three AP-1 subunits appears to affect growth in A. nidulans similarly, and also that the inactivation of any subunit has been reported to disrupt the full complex in other organisms (Robinson 2015 and refs therein), the AP-1σ subunit was chosen to further investigate the role of the AP-1 complex in intracellular cargo trafficking pathways. Figure 1C shows that expression of functional GFP- or mRFP-tagged AP-1σ has distinct localization in cytoplasmic puncta, the motility of which resembles a Brownian motion, being more abundant in the apical region of hyphae and apparently absent from the Spk. The distinct localization of AP-1σ, which resembles the distribution of Golgi markers (see below), is lost and replaced by a fluorescent cytoplasmic haze when the β or μ subunits are knocked-down (Figure 1D). Noticeably, the majority of these foci are not stained by FM4-64 or CMAC (Figure 1, E and F), strongly suggesting that they are distinct from endosomes and vacuoles.
Figure 1.
The AP-1 complex localizes in distinct polarly distributed structures and is essential for growth. (A) Upper panel: Growth of isogenic strains carrying thi-repressible alleles of ap1σ, ap1μ, and ap1β (thiAp-ap1σ, thiAp-ap1μ, and thiAp-ap1β) compared to wild-type (wt) in the absence (−) or presence (+) of thi. Lower panel: Western blot analysis comparing protein levels of FLAG-Ap1σ in the absence (0 hr) or presence of thi, added for 2, 4, 6, or 16 hr (overnight culture, o/n). wt is a standard wild-type strain (untagged ap1σ) which is included as a control for the specificity of the α-FLAG antibody. Equal loading is indicated by actin levels. (B) Microscopic morphology of hyphae in a strain repressed for ap1σ expression (+thi, lower panel) compared to wt (upper panel) stained with calcofluor white. Septal rings and side branches are indicated by arrows and arrowheads. Notice the differences in the calcofluor deposition at the hyphal head, tip, and the sub apical segment (Lookup table [LUT] fire [ImageJ, National Institutes of Health]). (C) Subcellular localization of Ap1σ-GFP and Ap1σ-mRFP in isogenic strains and relative quantitative analysis of fluorescence intensity (right upper panel), highlighting the polar distribution of Ap1σ. Growth tests showing that the tagged versions of Ap1σ are functional (right lower panel). (D) Subcellular localization of Ap1σ-GFP in isogenic strains carrying thi-repressible alleles of ap1μ (left panels) or ap1β (right panels) in the absence (upper panels) or presence of thi (+thi, o/n). Note that repression of expression of either the μ or the β subunit leads to diffuse cytoplasmic fluorescence of Ap1σ. (E) Subcellular localization of Ap1σ-GFP in the presence of FM4-64, which labels dynamically endocytic steps (PM, EEs, late endosomes/vacuoles). Notice that Ap1σ-GFP structures do not colocalize with FM4-64, except a few cases observed in the subapical region (indicated with an arrow at the 10 min picture). (F) Subcellular localization of Ap1σ-GFP in the presence of the vacuolar stain 7-amino-4-chloromethylcoumarin (Blue CMAC). No Ap1σ-GFP/CMAC colocalization is observed. Unless otherwise stated, Bar, 5 μm. Except (C) where the hyphal apex is at the lower side, in all other cases the hyphal apex is at the right side of the image series.
Knockdown of AP-1 affects the localization of polarly localized cargoes
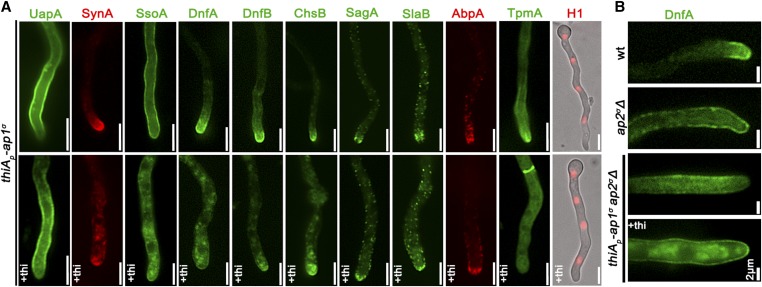
As mentioned in the Introduction, polarized growth of fungal hyphae is sustained by the continuous delivery of cargo-containing SVs toward the hyphal apex, and accumulation at the Spk before fusion with the plasma membrane (PM). Once localized in the PM at the hyphal apex, several cargoes diffuse laterally and become recycled through the actin-patch-enriched subapical regions of the endocytic collar, balancing exocytosis with endocytosis (Harris et al. 2005; Steinberg 2007; Berepiki et al. 2011; Takeshita et al. 2014; Peñalva et al. 2017; Steinberg et al. 2017; Zhou et al. 2018). In order to study the potential implications of the AP-1 complex in these processes, we monitored the localization of specific established apical and collar markers in conditions where the ap-1σ expression has been fully repressed. These markers include the secretory v-SNARE SynA and t-SNARE SsoA (Taheri-Talesh et al. 2008), the phospholipid flippases DnfA and DnfB that partially localize in the Spk (Schultzhaus et al. 2015), the class III chitin synthase ChsB known to play a key role in hyphal tip growth and cell wall integrity maintenance (Yanai et al. 1994; Takeshita et al. 2015), the tropomyosin TpmA decorating actin at the Spk and on actin cables at the hyphal tip (Taheri-Talesh et al. 2008), and, finally, the endocytic patch specific marker AbpA marking the sites of actin polymerization (Araujo-Bazán et al. 2008), along with the endocytic markers SlaB and SagA (Araujo-Bazán et al. 2008; Hervás-Aguilar and Peñalva 2010; Karachaliou et al. 2013). Additionally, we also tested the localization of the UapA xanthine-uric acid transporter, which our previous work suggested is not affected by the loss of function of the AP-1 complex (Martzoukou et al. 2017).
Figure 2 and Figure S1 highlight our results, which show that the localization of all markers tested is affected in the absence of AP-1σ, with the only clear exception being the plasma membrane transporter UapA. Additionally, the general positioning of nuclei also appears unaffected as indicated by labeled histone 1 (Nayak et al. 2010). Of the markers tested, SynA, DnfA, DnfB, and ChsB significantly lose their polar distribution (Figure 2 and Figure S1A) and do not seem to properly reach the Spk, concomitant with their increased presence in distinct, rather static, cytoplasmic puncta of various sizes. The nonpolar distribution of SsoA is generally conserved, but its cortical positioning is reduced and replaced by numerous cytoplasmic puncta. The cytoplasmic puncta that appear under AP-1σ depletion show very minor cargo-dependent colocalization with vacuoles stained with CMAC, mostly evident with chitin synthase ChsB (Figure S1B; see also later). Finally, collar-associated markers (SagA, SlaB, and AbpA) appear “moved” in an acropetal manner toward the hyphal tip. TpmA has also lost its proper localization at the hyphal tip, suggesting defective stabilization of actin filaments at the level of the Spk (Bergs et al. 2016). For relative quantification of fluorescence intensity see also Figure S1A.
Figure 2.
Lack of expression of AP-1 affects the topology of polar cargoes. (A) Comparison of the cellular localization of specific GFP- or mRFP/mCherry-tagged protein cargoes under conditions where ap1σ is expressed (upper panel) or fully repressed by thi (lower panel, +thi). The cargoes tested are the UapA transporter, the SNAREs SynA and SsoA, phospholipid flippases DnfA and DnfB, chitin synthase ChsB, endocytic markers SagA and SlaB, the actin-polymerization marker AbpA, tropomyosin TpmA, and histone H1 (i.e., nuclei). Notice that when ap1σ is fully repressed polar apical cargoes are depolarized and mark numerous relatively static cytoplasmic puncta. Hyphal apex is at the lower side of the image series. (B) Localization of DnfA-GFP in strains carrying the ap2σΔ null allele, or the ap2σΔ null allele together with the repressible thiAp-ap1σ allele, or an isogenic wild-type control (wt: ap2σ+ ap1σ+). Note that loss of polar distribution due to defective apical endocytosis observed in the ap2σΔ strains (Martzoukou et al. 2017) persists when AP-1σ is also repressed, indicating that, in the latter case, the majority of accumulating internal structures are due to problematic exocytosis of DnfA. Hyphal apex at the right side of the image series. Unless otherwise stated, Bar, 5 μm.
Previous studies have shown that mutations preventing endocytosis of polar markers result in a uniform rather than polarized distribution of cargoes at the PM (Schultzhaus et al. 2015; Schultzhaus and Shaw 2016; Martzoukou et al. 2017). In contrast, when exocytosis is impaired due to the absence of clathrin, several cargoes show predominantly noncortical cytoplasmic localization (Martzoukou et al. 2017). Thus, our present observations strongly suggest that secretion and/or recycling is the process blocked in the absence of the AP-1 complex, while endocytosis remains functional. The latter is further supported by the fact that repression of AP-1 in the absence of a functional AP-2 complex, results in significant apparent cortical retention of specific cargoes, such as DnfA, despite the concurrent subapical accumulation of cytoplasmic DnfA-labeled structures (Figure 2B), which notably do not colocalize with endocytic membranes stained by FM4-64 (Figure S2).
AP-1 associates transiently with the trans-golgi
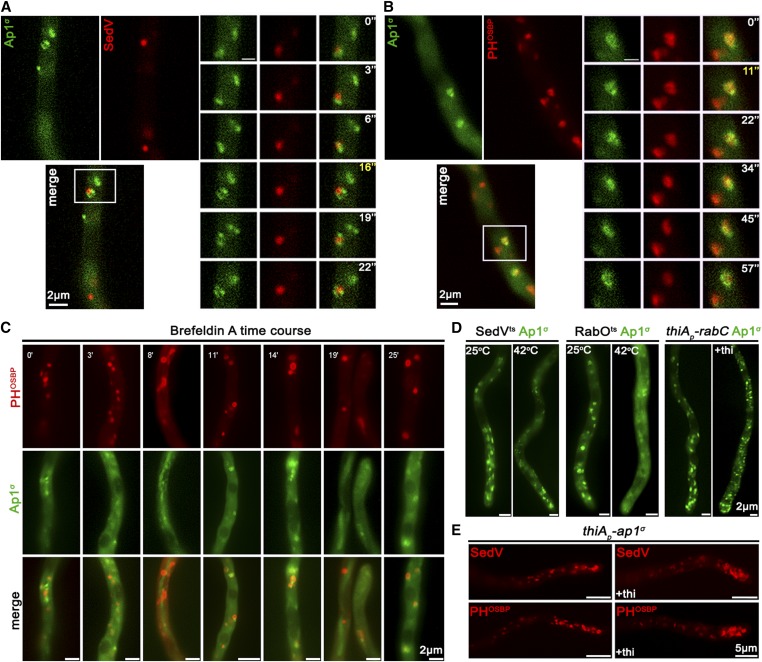
In A. nidulans, the process of maturation of Golgi has been extensively studied (Peñalva 2010; Pantazopoulou 2016; Steinberg et al. 2017). The markers syntaxin SedVSed5 and the human oxysterol-binding protein PH domain (PHOSBP) are well-established markers to follow the dynamics of early/cis-Golgi (Pinar et al. 2013) and late/trans-Golgi compartments (Pantazopoulou and Peñalva 2009), respectively. Here we examined the possible association of the AP-1 complex with Golgi compartments using these markers. Figure 3A shows that AP-1σ exhibits no colocalization, despite some topological proximity, with the early-Golgi, although in some cases it orbits around SedV marker (see also File S1). In contrast, most AP-1σ labeled structures show a significant degree of apparent association with PHOSBP, which suggests that AP-1 partially colocalizes with the late-Golgi (Figure 3B, see also File S2). Notably, the degree of association of AP-1 with PHOSBP has a transient character, as seen by the apparent progressive loss of colocalization. The increased association of AP-1σ with late-Golgi is further supported by the effect of Brefeldin A, which leads to transient Golgi collapse in aggregated bodies, several of which included the AP-1 marker (Figure 3C). Thermosensitive mutations in SedV (SedV-R258G) or the regulatory GTPase RabORab1 (RabO-A136D) are known to lead to early-Golgi, or both early- and late-Golgi, disorganization upon shift to the restrictive temperature (Pinar et al. 2013). These mutations led to AP-1σ subcellular distribution modification, further supporting the association of AP-1 with late-, but not with early-Golgi. In particular, in SedV-R258G, AP-1σ localization was less affected, whereas in RabO-A136D AP-1σ fluorescence was significantly delocalized from distinct puncta to a cytoplasmic haze (Figure 3D). Finally, knockdown of RabCRab6, another small GTPase responsible for Golgi network organization, also results in smaller AP-1σ foci (Figure 3D), resembling the fragmented Golgi equivalents observed for PHOSBP in a rabCΔ genetic background (Pantazopoulou and Peñalva 2011). Importantly, knockdown of AP-1 had a moderate but detectable effect on the overall picture of early- or late-Golgi markers, which in this case seem relocated in the subapical region of the hypha, thus showing increased polarization (Figure 3E and Figure S3). The significance of this observation is discussed later.
Figure 3.
AP-1 associates transiently with the late-Golgi. (A and B) Subcellular localization of Ap1σ-GFP relative to cis- (SedV-mCherry) and trans-Golgi (PHOSBP-mRFP) markers. Notice that Ap1σ colocalizes significantly with the trans-Golgi marker PHOSBP (n = 7; PCC = 0.66, P < 0.0001), but not with the cis-Golgi marker SedV (n = 5; PCC = 0.35, P < 0.01). This colocalization is dynamic and transient, as shown in selected time-lapse images on the right panels (for relevant videos, see also File S1 and File S2). (C) Subcellular localization of Ap1σ and PHOSBP in the presence of the inhibitor Brefeldin A, showing that a fraction of Brefeldin bodies (i.e., collapsed Golgi membranes) includes both markers, further supporting a transient AP-1/late Golgi association. (D) Subcellular localization of Ap1σ in SedVts or RabOts thermosensitive mutants or a strain carrying a repressible rabC allele. These strains are used as tools for transiently blocking proper Golgi function. Notice that at the restrictive temperature (42°), Ap1σ fluorescence becomes increasingly diffuse, mostly in the RabOts mutant, whereas, under RabC-repressed conditions, small Ap1σ-labeled puncta increase in number. These results are compatible with the notion that AP-1 proper localization necessitates wild-type Golgi dynamics. (E) Distribution of early- and late-Golgi markers SedV and PHOSBP relative to ap1σ expression or repression (+thi). Notice the effect of accumulation of Golgi toward the hyphal apex under repressed conditions. Image series in (A–C) present subapical regions of hyphae, whereas in (D) the hyphal apex is at the lower image side and in (E) at the right image side.
AP-1 associates with clathrin via specific C-terminal motifs
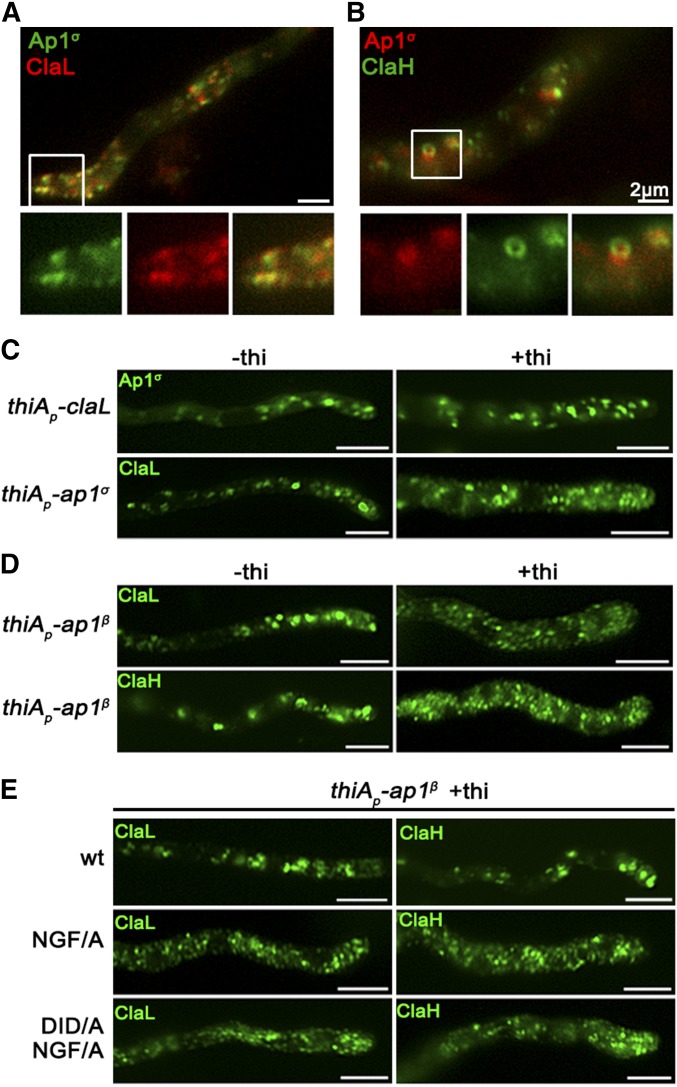
AP-1 and AP-2 association with clathrin is considered as a key interaction mediating the recognition of cargo prior to clathrin cage assembly in metazoa (Robinson 2015). Clathrin-binding motifs, or boxes, have been identified in the hinge regions of the β subunits (Dell’Angelica et al. 1998). In A. nidulans, clathrin light and heavy chains have been recently visualized (Martzoukou et al. 2017; Schultzhaus et al. 2017) and shown to dynamically decorate the late-Golgi, also coalescing after Brefeldin A treatment (Schultzhaus et al. 2017). Given that AP-1 was shown here to associate with late-Golgi, we tested whether it also associates with clathrin light and/or heavy chains, despite having a truncated C-terminal region (Martzoukou et al. 2017). Figure 4, A and B suggests a high degree of colocalization of AP-1σ with both clathrin light chain, ClaL, and heavy chain, ClaH. In the case of ClaL, foci are often detected comigrating with AP-1σ, which, once formed, move to all dimensions coherently (see also File S3). In the case of ClaH, “horseshoe”-like structures appear to coalesce predominantly, which again are characterized by coherent movement with AP-1σ (see also File S4).
Figure 4.
C-terminal motifs in AP-1β are essential for wild-type clathrin localization. (A and B) Subcellular localization of Ap1σ relative to that of clathrin light (ClaL) and heavy (ClaH) chains. Notice the significant colocalization of AP-1 with both clathrin chains (claL: n = 9; PCC = 0.78, P < 0.0001) (claH: n = 5; PCC = 0.76, P < 0.0001), also highlighted by the comigration of the two markers in the relevant videos (File S3 and File S4, see also Figure S4). Hyphal apex is at the left side of (A), whereas in (B) a subapical hyphal region is presented. (C and D) Subcellular distribution of Ap1σ and clathrin light chain ClaL under conditions where claL or ap1σ are expressed/repressed, respectively (−thi/+thi). Note that repression of ClaL expression has no significant effect on Ap1σ-GFP localization, whereas repression of Ap1σ expression leads to more diffuse ClaL fluorescence with parallel appearance of increased numbers of cytoplasmic puncta. A similar picture is obtained when clathrin light and heavy chain localization are monitored under conditions of expression/repression of ap1β. These results are compatible with the idea that clathrin localization is dependent on the presence of AP-1, but not vice versa. Hyphal apex is at the right side of the image series. (E) Effect of Ap1β C-terminal mutations, modifying putative clathrin-binding motifs (709NGF/A711 and 632DID/A634), on ClaL and ClaH distribution. Notice that replacement of 709NGF711, and to a lesser extent, of 632DID634, by alanines, leads to modification of clathrin subcellular localization, practically identical to the picture observed in (D) when Ap1β expression is fully repressed. Hyphal apex is at the right side of the image series. Unless otherwise stated, Bar, 5 μm.
We also followed the localization of clathrin in the absence of AP-1, and vice versa, the localization of AP-1 in the absence of clathrin. Results in Figure 4C (upper panel) show that repression of ClaL expression does not affect the wild-type localization of AP-1σ. In contrast, repression of AP-1σ leads to a prominent increase in rather static, ClaL-containing, cytoplasmic puncta (Figure 4C, lower panel). This suggests that AP-1 functions upstream from ClaL, in line with the established role of AP-1 in clathrin recruitment after cargo binding at late-Golgi or endosomal membranes.
Since our results supported a physical and/or functional association of AP-1 with clathrin, we addressed how this could be achieved given that the A. nidulans AP-1β, which is the subunit that binds clathrin in metazoa and yeast (Gallusser and Kirchhausen 1993), lacks canonical clathrin binding domains in its C-terminal region, but still possesses putative clathrin boxes (630LLDID634 and 707LLNGF711). Noticeably, these motifs resemble the LLDLF or LLDFD sequences, found at the extreme C-terminus of yeast AP-1β, which have been shown to interact with clathrin (Yeung and Payne 2001). First, we showed that total repression of AP-1β expression leads to a prominent increase in static ClaL or ClaH puncta, compatible with altered clathrin localization (Figure 4D). Then, we asked whether the putative clathrin boxes in AP-1β play a role in the proper localization of clathrin. To do so, the 707LLNGF711 or/and 630LLDID634 motifs of AP-1β were mutated in a genetic background that also possesses a wild-type ap-1β allele expressed via the repressible thiAp promoter. These strains allowed us to follow the localization of clathrin (claL or claH) when wild-type or mutant versions of ap-1β were expressed. Figure 4E shows that mutations in 707LLNGF711, and to a much lesser extent 630LLDID634, lead to modification of clathrin localization, similar to the picture obtained under total repression of AP-1β. Notably, the mutated versions of AP-1β partially restore the growth defects of repressed AP-1β (Figure S5). This suggests that interaction with clathrin via these boxes is not the primary determinant for the essentiality of AP-1 in fungal growth.
AP-1 associates with RabERab11-labeled SVs
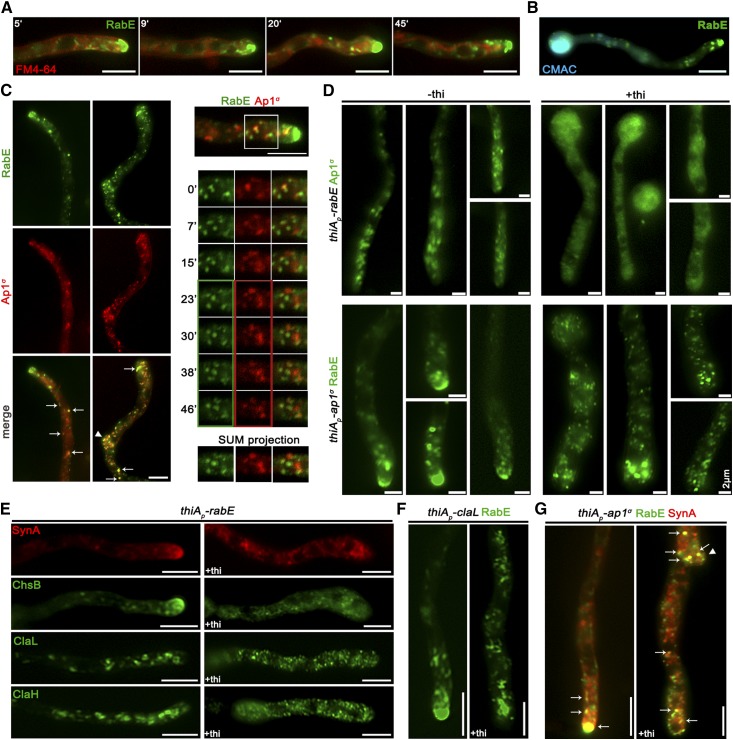
The results obtained thus far suggested that the AP-1 complex is involved in post-Golgi anterograde trafficking of SVs. In A. nidulans, such vesicles deriving from the late-Golgi, traffic along MT tracks toward regulated discharge at the apical plasma membrane level (Berepiki et al. 2011; Peñalva et al. 2017; Steinberg et al. 2017; Zhou et al. 2018). The small GTPase RabERab11, which is recruited along with its regulators, precedes and very probably mediates late-Golgi exit of SVs toward the hyphal tip, and plays a pivotal role in these early processes (Pantazopoulou et al. 2014; Pinar et al. 2015; Peñalva et al. 2017). Post-Golgi RabE labeled structures, including the Spk, do not colocalize with endosomes stained by FM4-64 or late-endosome/vacuoles stained by CMAC (Figure 5, A and B). In contrast, they show a significant degree of association with AP-1σ, suggesting that the majority of these vesicles are coated by AP-1 (Figure 5C). This is particularly prominent on foci of subapical regions (Figure 5C, left panels) and also at sites of newly emerging branches (Figure 5C, middle panels).
Figure 5.
AP-1 associates with RabERab11-labeled SVs. (A and B) Time course of RabE-GFP localization in the presence of FM4-64 (see also Figure S6A) or CMAC, indicating the nonendocytic character for RabE-labeled structures. Hyphal apex is at the right side of the image series. (C) Left panel: subcellular localization of Ap1σ-mRFP and RabE-GFP, showing significant colocalization in several fluorescent cytoplasmic puncta (arrows) throughout the hyphae but more prominent at subapical regions and sites of branch emergence (arrowheads) (n = 5; PCC = 0.72, P < 0.0001). Notice that colocalization is apparently excluded at the level of Spk, where RabE is prominent, whereas Ap1σ is not. Hyphal apex is at the upper side of the image series. Right panel: localization of Ap1σ–mRFP and RabE-GFP in a selected area sliced in time frames. Colored boxes indicate the slices used for SUM projection. Hyphal apex is at the right side of the image series. (D) Subcellular localization of Ap1σ-GFP or RabE-GFP in strains carrying thiamine-repressible thiAp-rabE or thiAp-ap1σ alleles respectively, observed under conditions of expression (−thi) or repression (+thi). Note that, in the absence of rabE expression, Ap1σ-labeled fluorescence appears as a cytoplasmic haze rather than distinct puncta (upper panels), while in the absence of ap1σ expression, RabE fluorescence disappears from the Spk, and is associated with numerous scattered bright puncta along the hypha (lower panels − 43.24% uniform distribution, and intensity of puncta, 37.84% more than two brighter puncta close to the apex are observed, 18.9% one brighter mislocalized punctum at the apex is observed, n = 37). Hyphal apex is at the lower side of the image series. (E) Subcellular localization of SynA, ChsB, ClaL, and ClaH in strains carrying the thiamine-repressible thiAp-rabE allele, observed under conditions of expression (−thi) or repression (+thi) of rabE. Note that, in all cases, the wild-type distribution of fluorescence is severely affected, resulting in loss of polarized structures and appearance of an increased number of scattered bright foci, the latter being more evident in ClaL and ClaH. Hyphal apex is at the right side of the image series. (F) Localization of RabE-GFP in a strain carrying a thiamine-repressible thiAp-claL allele, observed under conditions of expression (−thi) or repression (+thi) of claL. Notice the disappearance of RabE from the Spk and its association with numerous scattered bright clusters along the hypha—a picture similar to that obtained in absence of ap1σ expression in (D). Hyphal apex is at the lower side of the image series. (G) Colocalization analysis of SynA and RabE in a strain carrying a thiamine-repressible thiAp-ap1σ allele (see also Figure S6B). Note that, when Ap1σ is expressed, SynA and RabE colocalize intensively at the Spk but also elsewhere along the hypha, whereas when Ap1σ expression is repressed (+thi), both fluorescent signals disappear from the Spk and appear mostly in numerous scattered and rather immotile puncta, several of which show double fluorescence. Hyphal apex is at the lower side of the image series. Unless otherwise stated, Bar, 5 μm.
We further examined the association of AP-1 and RabE by following their localization in relevant knockdown mutants. Given that the knockout of RabE proved lethal (results not shown), we monitored AP-1 localization in a knockdown strain where rabE expression can be totally repressed via the thiAp promoter. Similarly, we followed RabE localization in an analogous AP-1 knockdown mutant. Figure 5D (upper panel) shows that, when RabE is fully repressed, AP-1 fluorescence appears mostly as a cytoplasmic haze, suggesting that AP-1 acts downstream of RabE. In contrast, when AP-1 is repressed, RabE does not reach the Spk, while most fluorescence dissolves into scattered static puncta (Figure 5D, lower panel). This strongly suggests that polar secretion of RabE and apparently of SVs is blocked.
Furthermore, upon repression of rabE, crucial apical markers like SynA or ChsB lose their polar distribution, failing to reach their proper destination at the cell cortex (Figure 5E, upper panels). This inhibition of targeting appears more dramatic than the one observed when AP-1 is repressed (see Figure 2), thus indicating the existence of possible alternative RabE-dependent, but AP-1 independent routes. In addition, clathrin labeled structures also lose their wild-type distribution under rabE repression conditions, resulting in scattered small puncta (Figure 5E, lower panels), resembling the phenotype observed for ClaL in the absence of a functional AP-1 complex (see Figure 4, C and D). Similar polar localization defects are observed in RabE-labeled SVs in strains repressed for clathrin light chain, suggesting that the majority of SVs requires a clathrin coat to reach the Spk (Figure 5F). Given the fact that, unlike RabE, neither AP-1σ nor clathrin appear to occupy the Spk, it seems that SVs are uncoated from AP-1 and clathrin prior to their localization in Spk, and thus before actin-dependent localization at the apical PM.
We also tested the relative localization of an apical marker (SynA) and RabE in a genetic background where Ap1σ expression can be repressed. When Ap1σ is expressed, SynA and RabE colocalize significantly, mostly evident in the Spk, whereas when Ap1σ is repressed, colocalization persists but shows a more dispersed pattern and is practically absent from the Spk (Figure 5G and Figure S6B). This strongly suggests the AP-1 is essential for anterograde movement of post-Golgi vesicles.
AP-1 associates with the MT cytoskeleton
Previous studies have shown that RabE-labeled SVs utilize MT tracks and kinesin-1 for their anterograde traffic, and, when present at the Spk, use myosin-5 and actin cables to be delivered at the apical PM or eventually move back in retrograde direction powered by dynein motors (Zhang et al. 2011; Egan et al. 2012; Peñalva et al. 2017; Steinberg et al. 2017; Zhou et al. 2018). Here, we examined the possible association of AP-1 with specific dynamic elements of the cytoskeleton involved in cargo traffic. Figure 6A shows that AP-1 puncta decorate MTs labeled by alpha-tubulin, TubA. Notably, the path of motile AP-1 puncta is, in most cases, dictated by the direction of the MTs (see also File S5). The association with the MT network is further supported by the effect of the anti-MT drug Benomyl, which results in an almost complete, but reversible, disassembly of MTs with a parallel increase in Ap1σ-GFP labeled cytoplasmic haze (Figure 6B, upper panel, mostly evident at 4–6 min). In contrast, inhibition of F-actin dynamics via Latrunculin B treatment shows that actin depolymerization does not lead to detectable modification of AP-1 localization (Figure 6B lower panel). This result is in agreement with the observation that AP-1 is excluded from the actin polymerization area.
Kinesins are motor proteins involved in the transport of SVs, EEs, organelles, and also mRNA and dynein motors (Steinberg 2011; Baumann et al. 2012; Egan et al. 2012; Salogiannis and Reck-Peterson 2017). Based on previous results showing that kinesin-1 KinA (Konzack et al. 2005; Zekert and Fischer 2009) is the main motor responsible for anterograde traffic of RabE-labeled SVs, whereas kinesin-3 UncA has no significant role in SV secretion (Peñalva et al. 2017), we tested whether KinA and UncA are involved in powering the motility of AP-1 on MTs. The use of strains carrying deletions of KinA and UncA showed that the motility of AP-1 on MTs is principally powered by KinA, the absence of which leads to a redistribution and apparent “stalling” of Ap1σ-labeled foci at subapical regions, excluding localization at the hyphal tip area (Figure 6C). This picture is practically identical with the localization of apical cargoes, such as ChsB, in the absence of KinA (Takeshita et al. 2015). In the case of UncA, the Ap1σ-labeled foci appear to be largely unaffected; however, a more prominent localization at the level of Spk, and also rather lateral accumulation of relative foci is observed (55.2% of n = 25 hyphae) (Figure 6C). These results suggest that UncA might have auxiliary roles in the anterograde traffic of Ap1-labeled SVs.
The functional association of AP-1 with the cytoskeleton was also investigated by following the appearance of MTs in a strain lacking AP-1. Figure 6D shows that repression of AP-1 expression led to prominent changes in the MT network, as monitored by mCherry-TubA fluorescence. These include more curved MTs toward the apex, distinct bright spots at the periphery of the hyphal head, and increased cross sections throughout the hypha, all together suggesting a possible continuous polymerization at the plus end and a problematic interaction with actin through cell-end markers (Takeshita et al. 2013, 2014; Zhou et al. 2018).
AP-1 is critical for septin organization
Given the role of AP-1 in MT organization, we also studied its role in septin localization. Septins are less well characterized GTP-binding proteins, which form hetero-polymers associating into higher order structures, and are thought to play a central role in the spatial regulation and coordination of the actin and MT networks in most eukaryotes (Mostowy and Cossart 2012; Spiliotis 2018). In A. nidulans, five septins have been under investigation, the four core septins AspA–D, which form hetero-polymers appearing in various shapes, including spots, rings, and filaments, and a fifth septin of currently unknown function, AspE, not involved in the hetero-polymer and appearing as dense cortical spots at the proximity of the plasma membrane (Hernández-Rodríguez and Momany 2012; Hernández-Rodríguez et al. 2014; Momany and Talbot 2017). Figure 6E shows that upon AP-1 repression, hetero-polymer forming core septins AspB, AspC, and AspD appear less in the form of filamentous structures, while distinct bright cortical spots tend to accumulate at the hyphal periphery, several of which possibly mark positions of new septa, in agreement with increased numbers of septa observed in the absence of AP-1. Interestingly, AspE, appears largely unaffected with the exception of the more frequent appearance of septa. All the above observations are in agreement with many other previously described phenotypes associating with AP-1 repression and suggest an implication of AP-1 in the processes regulating septin polymer formation. Noticeably, proper endosomal trafficking of septins at growth poles is necessary for growth in Ustilago maydis (Baumann et al. 2014; Zander et al. 2016).
AP-1 is involved in endosomal function
The AP-1 complex has also been implicated in anterograde and retrograde traffic between endosomal compartments and the plasma membrane (Robinson 2004, 2015). However, the existence of a relative sorting or recycling endosome, originating from EEs, has not been shown formally in filamentous fungi (Steinberg et al. 2017), with the exception of a recently characterized endosome to TGN recycling route for chitin synthase ChsB (Hernández-González et al. 2018). Major determinants of EE identity are the Rab5 GTPases (Nielsen et al. 1999). The A. nidulans Rab5 paralogues RabA and RabB both localize to EEs moving on MT tracks, with RabB appearing also in relatively static late endosomes. Importantly, the RabA and RabB markers do not colocalize with RabE, which confirms that motile, anterograde-moving, SVs, and motile endosomes are distinct entities (Pantazopoulou et al. 2014). Here, we investigated whether AP-1 associates with Rab5 endosomes.
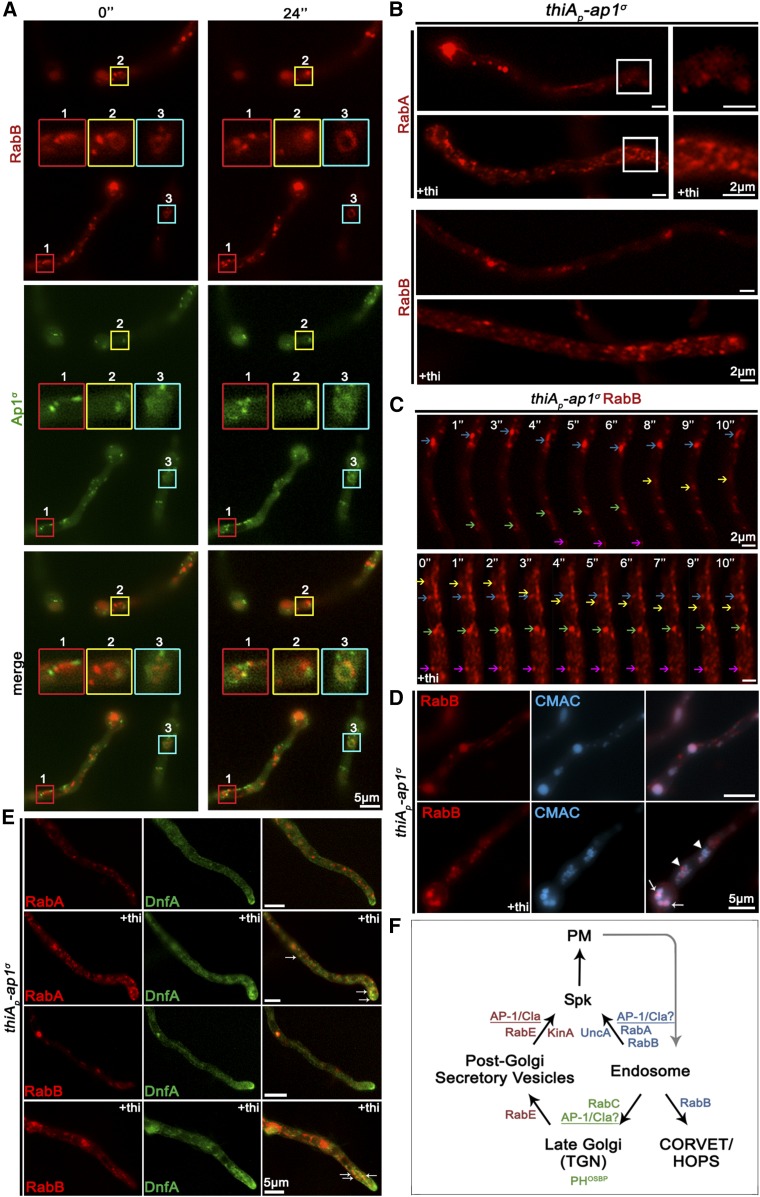
Figure 7A shows that AP-1 exhibits a degree of transient comigration with RabB (see also File S6). The coalescence of fluorescence is mostly observed in ring-like structures, which tend to accumulate and convert to more compact forms, suggesting an involvement of AP-1 in recycling, without excluding an additional involvement in vacuolar degradation. Importantly, knockdown of AP-1 led to increased numbers of both RabA and RabB-labeled endosomes (Figure 7B), the majority of which are immotile. In fact, the motile subpopulation of endosomes appears unaffected (Figure 7C). In the absence of AP-1, several distinct RabB foci were also stained by CMAC (Figure 7D), indicating that they are mini-vacuoles, resembling the phenotype of RabA/B in the absence of RabSRab7, a mediator of vacuolar degradation (Abenza et al. 2012). Limited colocalization of RabA/B upon AP-1 depletion is also observed with DnfA (Figure 7E). The evidence presented above and the partial colocalization of the chitin synthase ChsB with CMAC shown (see Figure S1), strongly support that AP-1 is involved in endosome function, affecting direct recycling to the PM or retrograde sorting to the TGN or both.
Figure 7.
AP-1 is involved in endosome recycling. (A) Images showing the relative localization of Ap1σ and the endosomal marker RabB. Selected areas in small color boxes are magnified, marked with the same border coloration and positioned at the center of the relevant subpanel. Notice the dynamic association of AP-1 with RabB (see also File S6). (B) Subcellular localization of RabA (upper panels) and RabB (lower panels) in a strain carrying a thi-repressible thiAp-ap1σ allele, observed under conditions of expression (−thi) or repression (+thi) of ap1σ. Notice the increased numbers and clustering of both endosomal markers in rather immotile puncta when AP-1 expression is repressed. RabA endosomal subpopulation motility for n = 5 hyphae is 0.91 ± 0.11 static and 2.8 ± 0.49 mobile endosomes per 10 μm hyphal length in wild-type and 3.03 ± 0.10 static and 3.15 ± 0.31 motile endosomes per 10 μm hyphal length in AP-1 depleted conditions. In the case of RabB and for n = 7, endosomal subpopulations motility data are 1.41 ± 0.36 static and 2.76 ± 0.26 mobile endosomes per 10 μm hyphal length in wild-type and 3.16 ± 0.99 static and 2.72 ± 0.63 motile endosomes per 10 μm hyphal length in AP-1 depleted conditions. The increase of immotile endosomes in AP-1 depleted conditions compared to wt is statistically significant (P < 0.001 for RabA and P < 0.0001 for RabB). The motile subpopulation appears to be unaffected and shows no statistically significant difference. Hyphal apex is at the right side of the image series. (C) Selected time-lapse images of RabB in a strain carrying a thi-repressible thiAp-ap1σ allele, showing that the immotile RabB foci increase in number when ap1σ is repressed (+thi). However, faster trafficking endosomes can still be observed, in both retrograde and anterograde direction. Colored arrows are used to point out specific endosomal dots at 1 sec intervals. Subapical hyphal regions are presented in the image series. (D) Expression of RabB in a strain carrying a thi-repressible thiAp-ap1σ allele, stained with CMAC. Note that when ap1σ expression is repressed (+thi), most immotile RabB puncta are stained with CMAC. Note foci that colocalize with CMAC (arrow) and others that do not (arrowheads). Hyphal head is at the bottom left side of the image series. (E) Localization of endosomal markers RabA/B with the phospholipid flippase DnfA, in a strain carrying a thi-repressible thiAp-ap1σ allele, observed under conditions of expression (−thi) or repression (+thi) of ap1σ. Colocalization in both cases occurs only in a few spots (arrows) dispersed mostly subapically, being, however, more prominent in the case of RabA. Hyphal apex is at the bottom right side of the image series. (F) Working model summarizing major findings on the role of the AP-1 complex. Unless otherwise stated, Bar, 5 μm.
Discussion
We have previously shown that the AP-2 complex of A. nidulans and probably other higher fungi have a clathrin-independent role in the endocytosis of cargoes necessary for apical recycling of plasma membrane and cell wall components, and thus for fungal polar growth maintenance. This was rather unexpected due to the generally accepted view that AP-2 functions uniquely as a cargo-clathrin adaptor, but also due to its compromised role in the growth of unicellular fungi. Thus, it seems that sorting and trafficking mechanisms are genetically and/or physiologically adaptable in order to meet the specific growth or homeostatic strategies different cells face. In the present work, we functionally analyzed the AP-1 complex of A. nidulans, as a prototypic example of a simple eukaryote that exhibits continuous polar growth, and showed that AP-1 is indeed essential for cell survival and growth, in a way similar to metazoan cells (Bonifacino 2014) and probably plants (Robinson and Pimpl 2014). To our knowledge, no previous study has addressed the role of the AP-1 in filamentous fungi.
In yeasts, which do not maintain polar growth and where the MT cytoskeleton is not critical for cargo traffic, AP-1 null mutants are viable, showing relatively moderate growth defects, which, in some cases, are associated with problematic traffic of specific cargoes, such as chitin synthase Chs3 (Valdivia et al. 2002; Ma et al. 2009; Yu et al. 2013; Arcones et al. 2016). Yeast AP-1 null mutants also have minor defects in lipid PtdIns(3,5)P2-dependent processes, and show reduced ability to traffic ubiquitylated cargoes to the vacuolar lumen (Phelan et al. 2006). In fact, in budding yeast, AP-1 seems to be involved in a bidirectional cycling route between the TGN and endosomes (Hirst et al. 2012; Robinson 2015). Notably, in S. cerevisiae, there are two forms of AP-1 that share the same large (Apl2 and Apl4) and small (Aps1) subunits, but distinct medium subunits (Apm1 or Apm2) that seem to confer differential cargo recognition and sorting (Valdivia et al. 2002; Renard et al. 2010; Whitfield et al. 2016). Additionally, in fission yeast, the AP-1 complex seems to co-operate with the exomer, a nonessential, fungal-specific heterotetrameric complex assembled at the trans-Golgi network, for the delivery of a distinct set of proteins to the plasma membrane (Hoya et al. 2017; Anton et al. 2018).
In contrast to yeasts, repression of AP-1 expression in A. nidulans leads to lack of growth, which is related to its inability to maintain apical sorting of all polar cargoes tested, including those necessary for plasma membrane and cell wall biosynthesis. Thus, not only the growth phenotype, but also several underlying cellular defects in AP-1 null mutants resemble those obtained previously with AP-2 loss-of-function mutants (Martzoukou et al. 2017). This is in perfect agreement with the notion that growth of filamentous fungi, unlike yeasts, requires polar apical exocytosis combined with subapical endocytosis and recycling to the apex of specific cargoes related to plasma membrane and cell wall modification (Taheri-Talesh et al. 2008; Peñalva 2010; Shaw et al. 2011). Overall, the results presented herein emphasize important differences in membrane trafficking mechanisms employed by yeasts and filamentous fungi, the latter proving a unique genetic and cellular system to dissect cargo sorting in cells characterized by membrane polarity.
Interestingly, despite the similarity in AP-2 and AP-1 phenotypic growth defects, AP-2 has been shown to act independently of clathrin at the PM, while AP-1 is shown here to associate and function with clathrin at several post-Golgi membrane trafficking steps. The similarity of effects caused by null mutations in AP-1 and clathrin chains, concerning RabERab11-labeled secretory vesicle anterograde traffic and RabA/BRab5-labeled endosome recycling, constitutes strong evidence that AP-1 function is clathrin-dependent. Interestingly, however, the β subunit of AP-1 of A. nidulans and all higher fungi lacks the C-terminal appendage domain that contributes to clathrin-binding (Martzoukou et al. 2017). Here, we identified specific short motifs in the C-terminal region of AP-1β that proved critical for proper clathrin subcellular localization and AP-1 function. These (LLNGF and LLDID) resemble motifs shown previously to bind clathrin in yeast (Yeung and Payne 2001). Thus, in contrast to the fact that clathrin is dispensable for the function of AP-2 in polar cargo endocytosis, it is essential for AP-1-driven polar exocytosis.
A novel point of this work concerns the interaction of AP-1 with RabERab11. To our knowledge, such an interaction has been described only in a single report in mammalian cells (Parmar and Duncan 2016). In this case, Rab11 and AP-1 colocalize with the reptilian reovirus p14 FAST protein at the TGN. In metazoa, Rab11 acts as a molecular switch essential for building the necessary molecular machinery for membrane cargo trafficking to the cell surface via its localization and action at the trans-Golgi network, post-Golgi vesicles and specialized recycling endosomes (Welz et al. 2014). In A. nidulans, the Rab11 homolog RabE has been previously shown to mark similar subcellular compartments (e.g., late-Golgi and SVs) and to be involved in anterograde moving of cargoes to the Spk and eventually to the apical PM. Notably, however, RabE does not colocalize with RabA/BRab5-labeled endosomes. The present work strongly suggests that AP-1 and clathrin are sequentially recruited on cargoes, after RabE-dependent maturation of late-Golgi membranes to pre-SVs, and that secreted cargoes travel embedded within AP-1/clathrin-coated vesicle carriers on MT (see later) to the Spk. At the Spk, AP-1/clathrin coat is most likely released, but RabE remains until the involvement of actin in the last step of fusion with the apical PM.
The impressive similarity of the A. nidulans trafficking mechanisms with those of higher organisms is also reflected in the absolute need for proper MT cytoskeleton organization and dynamics (Fischer et al. 2008; Takeshita et al. 2014). We showed that AP-1 is essential for MT organization and associates with MTs, mainly via KinA. Thus, a specific kinesin motor provides the molecular link between cargo/AP-1/clathrin complexes and cytoskeletal tracks. This is very similar to what has been found in mammalian epithelial cells, where the molecular motor kinesin KIF13A connects AP-1 coated SVs containing mannose-6-phosphate receptor to MT tracks, and thus mediates their transfer from the TGN to the plasma membrane (Nakagawa et al. 2000). Similarly, in HeLa cells another motor protein kinesin, KIF5, links TGN-derived endosomal vesicles via a direct interaction with Gadkin, a γ-BAR membrane accessory protein of the AP-1 complex, with the MT cytoskeleton (Schmidt et al. 2009). Thus, tripartite complexes, including transmembrane cargoes, coat adaptors, and motor kinesins, seem to constitute an evolutionary conserved molecular machinery for membrane protein subcellular transport in eukaryotes.
One simple explanation for the essentiality of AP-1 in proper MT organization would be that, in its absence, membrane-associated polarity markers, such as Rho GTPases TeaA or TeaR, which are necessary for MT attachment to actin (Fischer et al. 2008; Takeshita and Fischer 2011; Takeshita et al. 2013; Takeshita 2018), are not sorted correctly in the apex of growing hyphae. Lack of such cell-end markers is known to result in curved or zigzagged organization of MTs and less straight hyphae, compatible with the picture we obtained in the AP-1 null mutant. Importantly, we further supported the essential role of AP-1 in MT organization and function by showing the dramatic effect of the absence of AP-1 on the subcellular organization of septins, proteins that play fundamental roles in the ability of diverse fungi to undergo shape changes and organize the cytoskeleton for polar growth (Momany and Talbot 2017; Zhang et al. 2017).
Another notable finding of this work concerns the association of AP-1 with endosomal routes that are distinct from those of RabE-labeled SVs. Thus, it seems that the combined action of genetically and cellularly distinct pathways serves the polar distribution of specific cargoes. In A. nidulans, EEs marked by the homologs of the Rab5 family (RabA and RabB) are generated via endocytosis and are easily distinguishable due to their high and long-distance bidirectional motility (Abenza et al. 2009; Steinberg 2014). A fraction of EEs matures to less motile late endosomes or multi-vesicular bodies (concurrent with increased replacing of RabA/B with RabSRab7), which eventually fuse with vacuoles for cargo degradation (Abenza et al. 2010, 2012; Steinberg 2014). Another fraction of EEs, mostly the one localized at the subapical collar region of hyphae where very active endocytosis takes place, apparently recycles back to the Spk, and, from there, vesicular cargoes reach the PM (Steinberg 2014). Whether this takes place directly or via retrograde transport to the late-Golgi and anterograde transport in SVs, is not clear and might well depend on the nature of the cargoes studied. In a recent study in this context, it was shown that the chitin synthase ChsB segregates into “recycling” domains in sorting endosomes to be delivered to the TGN before being sorted back to the Spk via RabERab11 SVs (Hernández-González et al. 2018). This leaves open the issue whether there is bona fidae direct recycling endosome in fungi. Here, we showed that lack of AP-1 leads to a dramatic increase in nonmotile RabA or RabB endosomes, which might reflect either handicapped immotile endosomes and/or enhanced maturation to MVB/late endosomes. In any case, our results show that AP-1 has a critical role in the fueling endosomal cargoes to the PM, either directly or via the TGN. Similarly, lack of AP-1 function in mammalian cells leads to problematic maturation of EEs, associated with aberrant delivery of synaptic cargoes (Candiello et al. 2016). Establishing the essential role of AP-1 in polar secretion of specific cargoes in A. nidulans (for a schematic view of our findings see Figure 7F and Figure 8), which will probably hold true for other filamentous fungi, also opens a novel little-studied issue. How specific nonpolar cargoes are sorted to the plasma membrane? For example, here and previously, we showed that AP-1 and AP-2 complexes are redundant for the proper subcellular expression of transporters that are homogenously present in the PM of growing hyphae, and which do not show any indication of polar localization. A critical question to answer is which route(s) and mechanism(s) transporters, and possibly other nonpolar transmembrane cargoes (i.e., channels and receptors), use for their sorting, endocytosis, or recycling. This question also concerns metazoan and plant cells, where nonpolar sorting remains largely understudied. Finally, under the light of previous results obtained in yeast, metazoa, or plants, our present work highlights the importance of using different model organisms to address common but evolutionary adaptable mechanisms for membrane cargo traffic in eukaryotes.
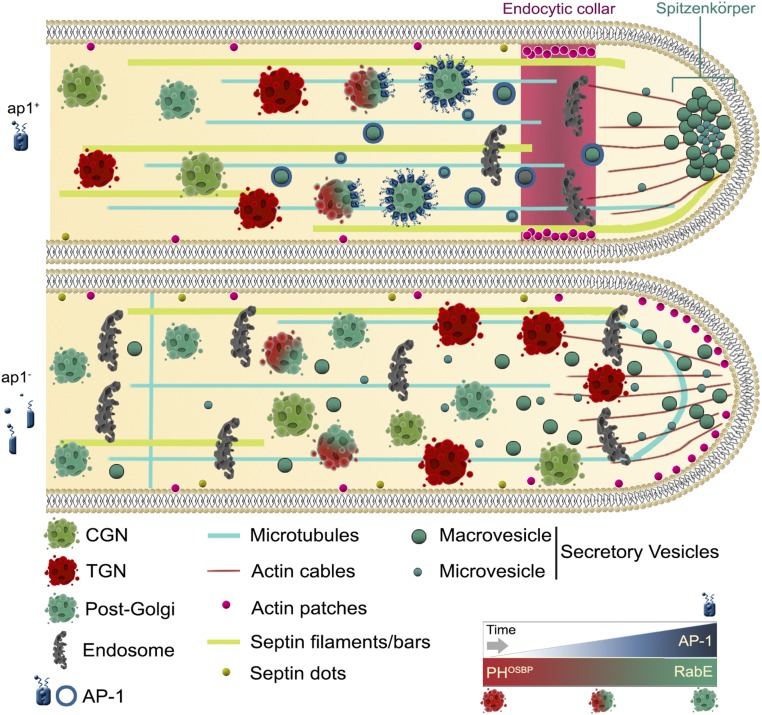
Figure 8.
Highly speculative scheme on the role of AP-1 in A. nidulans hyphal tip growth based on the herein described microscopic observation of fluorescent tagged protein markers. In an ap1+ background the late-Golgi progressively turns into post-Golgi RabE-containing SVs coated by AP-1 (also depicted in the lower right panel). In the absence of a functional AP-1 complex (ap1−), apparent accumulation of Golgi toward the fungal apex, mislocalization of endocytic collar associated actin patches toward the tip, failure of accumulation of SVs at the level of Spk, septin, and MT disorganization, and an overall enrichment in “sorting” endosomal and/or vacuolar structures are observed.
Acknowledgments
We thank Reinhard Fischer for the tagged tubulin, chitin synthase and kinesins strains, Michele Momany for the tagged septins strains and Spyros Efthimiopoulos for the Anti-FLAG M2 antibody. The current research was supported by the Fondation Santé, to which we are grateful. O.M. is supported through the Action “Doctorate Scholarships Programs by the State Scholarships Foundation” by the Greek State Scholarships Foundation (IKY) and the European Social Fund (ESF), in the framework of the Operational Program “Education and Life Long Learning” within the NSRF 2014-2020 of the ESF.
Author contributions: O.M., Data curation, Software, Formal analysis, Investigation, Methodology, Conceptualization; G.D., Conceptualization, Resources, Formal analysis, Funding acquisition, Validation, Visualization, Manuscript Writing, Project administration; S.A., Data curation, Investigation, Methodology, Conceptualization; Manuscript Writing.
Footnotes
Communicating editor: M. Rose
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6619922.
Literature Cited
- Abenza J. F., Pantazopoulou A., Rodríguez J. M., Galindo A., Peñalva M. A., 2009. Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic 10: 57–75. 10.1111/j.1600-0854.2008.00848.x [DOI] [PubMed] [Google Scholar]
- Abenza J. F., Galindo A., Pantazopoulou A., Gil C., de los Ríos V., et al. , 2010. Aspergillus RabB Rab5 integrates acquisition of degradative identity with the long distance movement of early endosomes. Mol. Biol. Cell 21: 2756–2769. 10.1091/mbc.e10-02-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza J. F., Galindo A., Pinar M., Pantazopoulou A., de los Ríos V., et al. , 2012. Endosomal maturation by Rab conversion in Aspergillus nidulans is coupled to dynein-mediated basipetal movement. Mol. Biol. Cell 23: 1889–1901. 10.1091/mbc.e11-11-0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitei M., Hoflack B., 2011. Exit from the trans-Golgi network: from molecules to mechanisms. Curr. Opin. Cell Biol. 23: 443–451. 10.1016/j.ceb.2011.03.013 [DOI] [PubMed] [Google Scholar]
- Anton C., Taubas J. V., Roncero C., 2018. The functional specialization of exomer as a cargo adaptor during the evolution of fungi. Genetics 208: 1483–1498. 10.1534/genetics.118.300767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolaki A., Harispe L., Calcagno-Pizarelli A. M., Vangelatos I., Sophianopoulou V., et al. , 2012. Aspergillus nidulans CkiA is an essential casein kinase I required for delivery of amino acid transporters to the plasma membrane. Mol. Microbiol. 84: 530–549. 10.1111/j.1365-2958.2012.08042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo-Bazán L., Peñalva M. A., Espeso E. A., 2008. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol. Microbiol. 67: 891–905. 10.1111/j.1365-2958.2007.06102.x [DOI] [PubMed] [Google Scholar]
- Arcones I., Sacristán C., Roncero C., 2016. Maintaining protein homeostasis: early and late endosomal dual recycling for the maintenance of intracellular pools of the plasma membrane protein Chs3. Mol. Biol. Cell 27: 4021–4032. 10.1091/mbc.e16-04-0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F., Malhotra V., 2006. The formation of TGN-to-plasma-membrane transport carriers. Annu. Rev. Cell Dev. Biol. 22: 439–455. 10.1146/annurev.cellbio.21.012704.133126 [DOI] [PubMed] [Google Scholar]
- Baumann S., Pohlmann T., Jungbluth M., Brachmann A., Feldbrügge M., 2012. Kinesin-3 and dynein mediate microtubule-dependent co-transport of mRNPs and endosomes. J. Cell Sci. 125: 2740–2752. 10.1242/jcs.101212 [DOI] [PubMed] [Google Scholar]
- Baumann S., König J., Koepke J., Feldbrügge M., 2014. Endosomal transport of septin mRNA and protein indicates local translation on endosomes and is required for correct septin filamentation. EMBO Rep. 15: 94–102. 10.1002/embr.201338037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berepiki A., Lichius A., Read N. D., 2011. Actin organization and dynamics in filamentous fungi. Nat. Rev. Microbiol. 9: 876–887. 10.1038/nrmicro2666 [DOI] [PubMed] [Google Scholar]
- Bergs A., Ishitsuka Y., Evangelinos M., Nienhaus G. U., Takeshita N., 2016. Dynamics of actin cables in polarized growth of the filamentous fungus Aspergillus nidulans. Front. Microbiol. 7: 682 10.3389/fmicb.2016.00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., 2004. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 5: 23–32. 10.1038/nrm1279 [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., 2014. Adaptor proteins involved in polarized sorting. J. Cell Biol. 204: 7–17. 10.1083/jcb.201310021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Reinisch K., Ferro-Novick S., 2007. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell 12: 671–682. 10.1016/j.devcel.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Candiello E., Kratzke M., Wenzel D., Cassel D., Schu P., 2016. AP-1/σ1A and AP-1/σ1 Badaptor-proteins differentially regulate neuronal early endosome maturation via the Rab5/Vps34-pathway. Sci. Rep. 6: 29950 10.1038/srep29950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica E. C., Klumperman J., Stoorvogel W., Bonifacino J. S., 1998. Association of the AP-3 adaptor complex with clathrin. Science 280: 431–434. 10.1126/science.280.5362.431 [DOI] [PubMed] [Google Scholar]
- Egan M. J., Tan K., Reck-Peterson S. L., 2012. Lis1 is an initiation factor for dynein-driven organelle transport. J. Cell Biol. 197: 971–982. 10.1083/jcb.201112101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelinos M., Martzoukou O., Chorozian K., Amillis S., Diallinas G., 2016. BsdA(Bsd2)-dependent vacuolar turnover of a misfolded version of the UapA transporter along the secretory pathway: prominent role of selective autophagy. Mol. Microbiol. 100: 893–911. 10.1111/mmi.13358 [DOI] [PubMed] [Google Scholar]
- Feyder S., De Craene J. O., Bär S., Bertazzi D. L., Friant S., 2015. Membrane trafficking in the yeast Saccharomyces cerevisiae model. Int. J. Mol. Sci. 16: 1509–1525. 10.3390/ijms16011509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R., Zekert N., Takeshita N., 2008. Polarized growth in fungi-interplay between the cytoskeleton, positional markers and membrane domains. Mol. Microbiol. 68: 813–826. 10.1111/j.1365-2958.2008.06193.x [DOI] [PubMed] [Google Scholar]
- Gallusser A., Kirchhausen T., 1993. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 2: 5237–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Sirkis D. W., Schekman R., 2014. Protein sorting at the trans-Golgi network. Annu. Rev. Cell Dev. Biol. 30: 169–206. 10.1146/annurev-cellbio-100913-013012 [DOI] [PubMed] [Google Scholar]
- Harris S. D., Read N. D., Roberson R. W., Shaw B., Seiler S., et al. , 2005. Polarisome meets Spitzenkörper: microscopy, genetics, and genomics converge. Eukaryot. Cell 4: 225–229. 10.1128/EC.4.2.225-229.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-González M., Bravo-Plaza I., Pinar M., de Los Ríos V., Arst H. N., Jr., et al. , 2018. Endocytic recycling via the TGN underlies the polarized hyphal mode of life. PLoS Genet. 14: e1007291 10.1371/journal.pgen.1007291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Rodríguez Y., Momany M., 2012. Posttranslational modifications and assembly of septin heteropolymers and higher-order structures. Curr. Opin. Microbiol. 15: 660–668. 10.1016/j.mib.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Hernández-Rodríguez Y., Masuo S., Johnson D., Orlando R., Smith A., et al. , 2014. Couto-Rodriguez M, Momany M. Distinct septin heteropolymers co-exist during multicellular development in the filamentous fungus Aspergillus nidulans. PLoS One 9: e92819 10.1371/journal.pone.0092819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervás-Aguilar A., Peñalva M. A., 2010. Endocytic machinery protein SlaB is dispensable for polarity establishment but necessary for polarity maintenance in hyphal tip cells of Aspergillus nidulans. Eukaryot. Cell 9: 1504–1518. 10.1128/EC.00119-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Borner G. H., Antrobus R., Peden A. A., Hodson N. A., et al. , 2012. Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr. Biol. 22: 1711–1716. 10.1016/j.cub.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoya M., Yanguas F., Moro S., Prescianotto-Baschong C., Doncel C., et al. , 2017. Traffic through the trans-golgi network and the endosomal system requires collaboration between exomer and clathrin adaptors in fission yeast. Genetics 205: 673–690. 10.1534/genetics.116.193458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. M., D’Hondt K., Riezman H., Lemmon S. K., 1999. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 18: 3897–3908. 10.1093/emboj/18.14.3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. D., Stephens D. J., 2011. The role of motor proteins in endosomal sorting. Biochem. Soc. Trans. 39: 1179–1184. 10.1042/BST0391179 [DOI] [PubMed] [Google Scholar]
- Karachaliou M., Amillis S., Evangelinos M., Kokotos A. C., Yalelis V., et al. , 2013. The arrestin-like protein ArtA is essential for ubiquitination and endocytosis of the UapA transporter in response to both broad-range and specific signals. Mol. Microbiol. 88: 301–317. 10.1111/mmi.12184 [DOI] [PubMed] [Google Scholar]
- Konzack S., Rischitor P. E., Enke C., Fischer R., 2005. The role of the kinesin motor KipA in microtubule organization and polarized growth of Aspergillus nidulans. Mol. Biol. Cell 16: 497–506. 10.1091/mbc.e04-02-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukaki M., Giannoutsou E., Karagouni A., Diallinas G., 2003. A novel improved method for Aspergillus nidulans transformation. J. Microbiol. Methods 55: 687–695. 10.1016/S0167-7012(03)00208-2 [DOI] [PubMed] [Google Scholar]
- Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R., 2004. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20: 87–123. 10.1146/annurev.cellbio.20.010403.105307 [DOI] [PubMed] [Google Scholar]
- Ma Y., Takeuchi M., Sugiura R., Sio S. O., Kuno T., 2009. Deletion mutants of AP-1 adaptin subunits display distinct phenotypes in fission yeast. Genes Cells 14: 1015–1028. 10.1111/j.1365-2443.2009.01327.x [DOI] [PubMed] [Google Scholar]
- Martzoukou O., Amillis S., Zervakou A., Christoforidis S., Diallinas G., 2017. The AP-2 complex has a specialized clathrin-independent role in apical endocytosis and polar growth in fungi. eLife 6: e20083 10.7554/eLife.20083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C., Zizioli D., Lausmann S., Eskelinen E. L., Hamann J., et al. , 2000. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19: 2193–2203. 10.1093/emboj/19.10.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momany M., 2002. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr. Opin. Microbiol. 5: 580–585. 10.1016/S1369-5274(02)00368-5 [DOI] [PubMed] [Google Scholar]
- Momany M., Talbot N. J., 2017. Septins focus cellular growth for host infection by pathogenic fungi. Front. Cell Dev. Biol. 5: 33 10.3389/fcell.2017.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S., Cossart P., 2012. Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 13: 183–194. 10.1038/nrm3284 [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Setou M., Seog D., Ogasawara K., Dohmae N., et al. , 2000. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell 103: 569–581. 10.1016/S0092-8674(00)00161-6 [DOI] [PubMed] [Google Scholar]
- Nakatsu F., Ohno H., 2003. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct. Funct. 28: 419–429. 10.1247/csf.28.419 [DOI] [PubMed] [Google Scholar]
- Nakatsu F., Hase K., Ohno H., 2014. The role of the clathrin adaptor AP-1: polarized sorting and beyond. Membranes (Basel) 4: 747–763. 10.3390/membranes4040747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak T., Szewczyk E., Oakley C. E., Osmani A., Ukil L., et al. , 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172: 1557–1566. 10.1534/genetics.105.052563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak T., Edgerton-Morgan H., Horio T., Xiong Y., De Souza C. P., et al. , 2010. Gamma-tubulin regulates the anaphase-promoting complex/cyclosome during interphase. J. Cell Biol. 190: 317–330. 10.1083/jcb.201002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E., Severin F., Backer J. M., Hyman A. A., Zerial M., 1999. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1: 376–382. 10.1038/14075 [DOI] [PubMed] [Google Scholar]
- Pantazopoulou A., 2016. The Golgi apparatus: insights from filamentous fungi. Mycologia 108: 603–622. 10.3852/15-309 [DOI] [PubMed] [Google Scholar]
- Pantazopoulou A., Peñalva M. A., 2009. Organization and dynamics of the Aspergillus nidulans Golgi during apical extension and mitosis. Mol. Biol. Cell 20: 4335–4347. 10.1091/mbc.e09-03-0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulou A., Peñalva M. A., 2011. Characterization of Aspergillus nidulans RabC/Rab6. Traffic 12: 386–406. 10.1111/j.1600-0854.2011.01164.x [DOI] [PubMed] [Google Scholar]
- Pantazopoulou A., Pinar M., Xiang X., Peñalva M. A., 2014. Maturation of late Golgi cisternae into RabE(RAB11) exocytic post-Golgi carriers visualized in vivo. Mol. Biol. Cell 25: 2428–2443. 10.1091/mbc.e14-02-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadaki G. F., Amillis S., Diallinas G., 2017. Substrate specificity of the FurE transporter is determined by cytoplasmic terminal domain interactions. Genetics 207: 1387–1400. 10.1534/genetics.117.300327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H. B., Duncan R., 2016. A novel tribasic Golgi export signal directs cargo protein interaction with activated Rab11 and AP-1-dependent Golgi-plasma membrane trafficking. Mol. Biol. Cell 27: 1320–1331. 10.1091/mbc.e15-12-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva M. A., 2005. Tracing the endocytic pathway of Aspergillus nidulans with FM4–64. Fungal Genet. Biol. 42: 963–975. 10.1016/j.fgb.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Peñalva M. Á., 2010. Endocytosis in filamentous fungi: cinderella gets her reward. Curr. Opin. Microbiol. 13: 684–692. 10.1016/j.mib.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., 2015. A lipid-managing program maintains a stout Spitzenkörper. Mol. Microbiol. 97: 1–6. 10.1111/mmi.13044 [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., Zhang J., Xiang X., Pantazopoulou A., 2017. Transport of fungal RAB11 secretory vesicles involves myosin-5, dynein/dynactin/p25, and kinesin-1 and is independent of kinesin-3. Mol. Biol. Cell 28: 947–961. 10.1091/mbc.e16-08-0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan H. L., Finlay J. A., Chu D. S., Tan P. K., Kirchhausen T., et al. , 1994. The Saccharomyces cerevisiae APS1 gene encodes a homolog of the small subunit of the mammalian clathrin AP-1 complex: evidence for functional interaction with clathrin at the Golgi complex. EMBO J. 13: 1706–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan J. P., Millson S. H., Parker P. J., Piper P. W., Cooke F. T., 2006. Fab1p and AP-1 are required for trafficking of endogenously ubiquitylated cargoes to the vacuole lumen in S. cerevisiae. J. Cell Sci. 119: 4225–4234. 10.1242/jcs.03188 [DOI] [PubMed] [Google Scholar]
- Pinar M., Pantazopoulou A., Arst H. N., Jr., Peñalva M. A., 2013. Acute inactivation of the Aspergillus nidulans Golgi membrane fusion machinery: correlation of apical extension arrest and tip swelling with cisternal disorganization. Mol. Microbiol. 89: 228–248. 10.1111/mmi.12280 [DOI] [PubMed] [Google Scholar]
- Pinar M., Arst H. N., Jr., Pantazopoulou A., Tagua V. G., de los Ríos V., et al. , 2015. TRAPPII regulates exocytic Golgi exit by mediating nucleotide exchange on the Ypt31 ortholog RabERAB11. Proc. Natl. Acad. Sci. USA 112: 4346–4351. 10.1073/pnas.1419168112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard H. F., Demaegd D., Guerriat B., Morsomme P., 2010. Efficient ER exit and vacuole targeting of yeast Sna2p require two tyrosine-based sorting motifs. Traffic 11: 931–946. 10.1111/j.1600-0854.2010.01070.x [DOI] [PubMed] [Google Scholar]
- Robinson D. G., Pimpl P., 2014. Clathrin and post-Golgi trafficking: a very complicated issue. Trends Plant Sci. 19: 134–139. 10.1016/j.tplants.2013.10.008 [DOI] [PubMed] [Google Scholar]
- Robinson M. S., 2004. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14: 167–174. 10.1016/j.tcb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Robinson M. S., 2015. Forty years of clathrin-coated vesicles. Traffic 16: 1210–1238. 10.1111/tra.12335 [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E. F. Fritsch, and T. Maniatis, 1989 Molecular Cloning: A Laboratory Manual, Vol. 9, Ed. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Salogiannis J., Reck-Peterson S. L., 2017. Hitchhiking: a non-canonical mode of microtubule-based transport. Trends Cell Biol. 27: 141–150. 10.1016/j.tcb.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. R., Maritzen T., Kukhtina V., Higman V. A., Doglio L., et al. , 2009. Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc. Natl. Acad. Sci. USA 106: 15344–15349. 10.1073/pnas.0904268106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzhaus Z., Shaw B. D., 2015. Endocytosis and exocytosis in hyphal growth. Fungal Biol. Rev. 29: 43–53. 10.1016/j.fbr.2015.04.002 [DOI] [Google Scholar]
- Schultzhaus Z., Shaw B. D., 2016. The flippase DnfB is cargo of fimbrin-associated endocytosis in Aspergillus nidulans, and likely recycles through the late Golgi. Commun. Integr. Biol. 9: e1141843 10.1080/19420889.2016.1141843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzhaus Z., Yan H., Shaw B. D., 2015. Aspergillus nidulans flippase DnfA is cargo of the endocytic collar and plays complementary roles in growth and phosphatidylserine asymmetry with another flippase, DnfB. Mol. Microbiol. 97: 18–32. 10.1111/mmi.13019 [DOI] [PubMed] [Google Scholar]
- Schultzhaus Z., Johnson T. B., Shaw B. D., 2017. Clathrin localization and dynamics in Aspergillus nidulans. Mol. Microbiol. 103: 299–318. 10.1111/mmi.13557 [DOI] [PubMed] [Google Scholar]
- Shaw B. D., Chung D. W., Wang C. L., Quintanilla L. A., Upadhyay S., 2011. A role for endocytic recycling in hyphal growth. Fungal Biol. 115: 541–546. 10.1016/j.funbio.2011.02.010 [DOI] [PubMed] [Google Scholar]
- Spang A., 2015. The road not taken: less traveled roads from the TGN to the plasma membrane. Membranes (Basel) 5: 84–98. 10.3390/membranes5010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis E. T., 2018. Spatial effects - site-specific regulation of actin and microtubule organization by septin GTPases. J. Cell Sci. 131: jcs207555 10.1242/jcs.207555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G., 2007. Hyphal growth: a tale of motors, lipids, and the Spitzenkörper. Eukaryot. Cell 6: 351–360. 10.1128/EC.00381-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G., 2011 Motors in fungal morphogenesis: cooperation versus competition. Curr. Opin. Microbiol. 14: 660–667. 10.1016/j.mib.2011.09.013 [DOI] [PubMed] [Google Scholar]
- Steinberg G., 2014. Endocytosis and early endosome motility in filamentous fungi. Curr. Opin. Microbiol. 20: 10–18. 10.1016/j.mib.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G., Peñalva M. A., Riquelme M., Wösten H. A., Harris S. D., 2017. Cell Biology of Hyphal Growth. Microbiol. Spectr. 5: 231–265. 10.1128/microbiolspec.FUNK-0034-2016 [DOI] [PubMed] [Google Scholar]
- Taheri-Talesh N. T., Horio L., Araujo-Bazán X., Dou E. A., Espeso E. A., et al. , 2008. The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell 19: 1439–1449. 10.1091/mbc.e07-05-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita N., 2018. Oscillatory fungal cell growth. Fungal Genet. Biol. 110: 10–14. 10.1016/j.fgb.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Takeshita N., Fischer R., 2011. On the role of microtubules, cell end markers, and septal microtubule organizing centres on site selection for polar growth in Aspergillus nidulans. Fungal Biol. 115: 506–517. 10.1016/j.funbio.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Takeshita N., Mania D., Herrero S., Ishitsuka Y., Nienhaus G. U., et al. , 2013. The cell-end marker TeaA and the microtubule polymerase AlpA contribute to microtubule guidance at the hyphal tip cortex of Aspergillus nidulans to provide polarity maintenance. J. Cell Sci. 126: 5400–5411. 10.1242/jcs.129841 [DOI] [PubMed] [Google Scholar]
- Takeshita N., Manck R., Grün N., de Vega S. H., Fischer R., 2014. Interdependence of the actin and the microtubule cytoskeleton during fungal growth. Curr. Opin. Microbiol. 20: 34–41. 10.1016/j.mib.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Takeshita N., Wernet V., Tsuizaki M., Grün N., Hoshi H. O., et al. , 2015. Transportation of Aspergillus nidulans class III and V chitin synthases to the Hyphal tips depends on conventional kinesin. PLoS One 10: e0125937 10.1371/journal.pone.0125937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia R. H., Baggott D., Chuang J. S., Schekman R. W., 2002. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2: 283–294. 10.1016/S1534-5807(02)00127-2 [DOI] [PubMed] [Google Scholar]
- Viotti C., 2016. ER to golgi-dependent protein secretion: the conventional pathway. Methods Mol. Biol. 1459: 3–29. 10.1007/978-1-4939-3804-9_1 [DOI] [PubMed] [Google Scholar]
- Vlanti A., Diallinas G., 2008. The Aspergillus nidulans FcyB cytosine-purine scavenger is highly expressed during germination and in reproductive compartments and is downregulated by endocytosis. Mol. Microbiol. 68: 959–977. 10.1111/j.1365-2958.2008.06198.x [DOI] [PubMed] [Google Scholar]
- Welz T., Wellbourne-Wood J., Kerkhoff E., 2014. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 24: 407–415. 10.1016/j.tcb.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Whitfield S. T., Burston H. E., Bean B. D., Raghuram N., Maldonado-Báez L., et al. , 2016. The alternate AP-1 adaptor subunit Apm2 interacts with the Mil1 regulatory protein and confers differential cargo sorting. Mol. Biol. Cell 27: 588–598. 10.1091/mbc.e15-09-0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai K., Kojima N., Takaya N., Horiuchi H., Ohta A., et al. , 1994. Isolation and characterization of two chitin synthase genes from Aspergillus nidulans. Biosci. Biotechnol. Biochem. 58: 1828–1835. 10.1271/bbb.58.1828 [DOI] [PubMed] [Google Scholar]
- Yeung B. G., Payne G. S., 2001. Clathrin interactions with C-terminal regions of the yeast AP-1 beta and gamma subunits are important for AP-1 association with clathrin coats. Traffic 2: 565–576. 10.1034/j.1600-0854.2001.20806.x [DOI] [PubMed] [Google Scholar]
- Yu Y., Li C., Kita A., Katayama Y., Kubouchi K., et al. , 2013. Sip1, an AP-1 accessory protein in fission yeast, is required for localization of Rho3 GTPase. PLoS One 8: e68488 10.1371/journal.pone.0068488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander S., Baumann S., Weidtkamp-Peters S., Feldbrügge M., 2016. Endosomal assembly and transport of heteromeric septin complexes promote septin cytoskeleton formation. J. Cell Sci. 129: 2778–2792. 10.1242/jcs.182824 [DOI] [PubMed] [Google Scholar]
- Zanetti G., Pahuja K. B., Studer S., Shim S., Schekman R., 2011. COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 14: 20–28 (erratum: Nat. Cell Biol. 14: 221) 10.1038/ncb2390 [DOI] [PubMed] [Google Scholar]
- Zekert N., Fischer R., 2009. The Aspergillus nidulans kinesin-3 UncA motor moves vesicles along a subpopulation of microtubules. Mol. Biol. Cell 20: 673–684. 10.1091/mbc.e08-07-0685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yao X., Fischer L., Abenza J. F., Peñalva M. A., et al. , 2011. The p25 subunit of the dynactin complex is required for dynein-early endosome interaction. J. Cell Biol. 193: 1245–1255. 10.1083/jcb.201011022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gao T., Shao W., Zheng Z., Zhou M., et al. , 2017. The septins FaCdc3 and FaCdc12 are required for cytokinesis and affect asexual and sexual development, lipid metabolism and virulence in Fusarium asiaticum. Mol. Plant Pathol. 18: 1282–1294. 10.1111/mpp.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Evangelinos M., Wernet V., Eckert A. F., Ishitsuka Y., et al. , 2018. Superresolution and pulse-chase imaging reveal the role of vesicle transport in polar growth of fungal cells. Sci. Adv. 4: e1701798 10.1126/sciadv.1701798 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains constructed in this work are available upon request. Supplementary material has been uploaded to figshare. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6619922.