Abstract
Phenotypic plasticity results in a diversity of phenotypes from a single genotype in response to environmental cues. To understand the molecular basis of phenotypic plasticity, studies have focused on differential gene expression levels between environmentally determined phenotypes. The extent of alternative splicing differences among environmentally determined phenotypes has largely been understudied. Here, we study alternative splicing differences among plastically produced morphs of the pea aphid using RNA-sequence data. Pea aphids express two separate polyphenisms (plasticity with discrete phenotypes): a wing polyphenism consisting of winged and wingless females and a reproduction polyphenism consisting of asexual and sexual females. We find that pea aphids alternatively splice 34% of their genes, a high percentage for invertebrates. We also find that there is extensive use of differential spliced events between genetically identical, polyphenic females. These differentially spliced events are enriched for exon skipping and mutually exclusive exon events that maintain the open reading frame, suggesting that polyphenic morphs use alternative splicing to produce phenotype-biased proteins. Many genes that are differentially spliced between polyphenic morphs have putative functions associated with their respective phenotypes. We find that the majority of differentially spliced genes is not differentially expressed genes. Our results provide a rich candidate gene list for future functional studies that would not have been previously considered based solely on gene expression studies, such as ensconsin in the reproductive polyphenism, and CAKI in the wing polyphenism. Overall, this study suggests an important role for alternative splicing in the expression of environmentally determined phenotypes.
Keywords: phenotypic plasticity, alternative splicing, polyphenism, Acyrthosiphon pisum, pea aphid
Introduction
Many organisms can express several possible phenotypes based on environmental conditions (Bradshaw 1965; West-Eberhard 2003). This phenomenon, termed phenotypic plasticity, creates phenotypic diversity without any underlying genetic diversity (Simpson et al. 2011). Plastically produced phenotypes can be continuous, such as when abdominal pigmentation in Drosophila varies in response to temperature (David et al. 1990). Alternatively, they can be discrete, termed polyphenism, such as when queen and worker female honey bees are produced depending on whether or not they receive royal jelly as a larvae (Weaver 1957). The discrete morphs of polyphenic species often differ by multiple characters, such as behavioral, physiological, and morphological traits. For this reason, the molecular basis of polyphenic morph expression is complex.
Differences in gene expression between polyphenic morphs have been explored in several species, with studies often finding hundreds to thousands of genes differing between phenotypes (Brisson et al. 2007; Le Trionnaire et al. 2009; Chen et al. 2012; Jiang et al. 2012; Long et al. 2013; Daniels et al. 2014; Liu, Zheng, et al. 2014). These studies have greatly advanced our understanding of the molecular functions that underlie polyphenic traits by identifying candidate genes and signaling pathways. However, these gene expression differences, although large, are not the sole molecular determinants of trait differences between polyphenic morphs. Multiple levels of regulation and modifications likely occur after transcription.
Alternative splicing has been proposed as a posttranscriptional mediator of polyphenic morph differences (Marden 2008), but few studies have addressed this empirically. Alternative splicing changes the final mRNA sequence by removing portions of exons or adding portions of introns, resulting in multiple mRNA isoforms from a single gene (Matlin et al. 2005). Genome-wide studies of splicing and plasticity have largely been confined to two general domains. First, alternative splicing changes have been identified in response to environmental stressors, but not between alternative morphs. These studies, performed in Drosophila (Jakšić and Schlötterer 2016), zebrafish (Long et al. 2013), and Arabidopsis (James et al. 2012), have revealed that alternative splicing is, indeed, environmentally responsive. Second, alternative splicing has been considered with respect to polyphenic morphs, but often secondarily. For example, a cursory examination of alternative splicing between locust morphs was performed on a genome-wide scale as part of the locust genome paper (Wang et al. 2014). Alternative splicing was also considered globally in caste-polyphenic ants and honey bees, but largely in the context of how it was potentially affected by DNA methylation (Cingolani et al. 2013; Li-Byarlay et al. 2013). Thus, the extent and identity of alternative splicing differences among polyphenic morphs is not well explored. To address this knowledge gap, here we have used the pea aphid (Acyrthosiphon pisum) as a model system (Brisson and Stern 2006; Grantham et al. 2015) for exploring morph-biased alternative splicing patterns. This system is advantageous for studying phenotypically plastic traits because it produces genetically identical offspring with very different phenotypes based on environmental conditions.
The pea aphid expresses two distinct polyphenisms with separate environmental triggers (Sutherland 1969a; Via 1992). The first is the wing polyphenism. During the summer, pea aphid females reproduce parthenogenetically, creating clonal daughters that are genetically identical, but can be phenotypically distinct winged or wingless adults (Blackman et al. 1987a). Winged daughters are produced when pea aphid mothers experience high population density, deficient nutrition, and/or predation (Sutherland 1969a, 1969b; Purandare, Tenhumberg, et al. 2014). Wingless females are produced in low population density environmental conditions. Winged females have fully developed wings and wing musculature, have expanded sensory systems, weaker immune system and are more restless (Parker et al. 2017). Wingless females lack all wing musculature, have higher fecundity, and are more sedentary (Blackman et al. 1987a).
The second pea aphid polyphenism relates to the female reproductive mode. In the fall, as the photoperiod shortens and the temperature decreases, egg-laying sexual females and males are produced. Males differ from females, genetically, by the loss of one X chromosome (Blackman et al. 1987b). Males are also wing dimorphic, but their dimorphism is not environmentally determined. Rather, it is determined by a single locus on the X (Caillaud et al. 2002; Braendle et al. 2005; Li et al. 2017). Sexual pea aphid females are always wingless. Sexual and asexual females are genetically identical, but differ reproductively: Sexual females produce haploid eggs from meiosis, whereas asexual females produce live juveniles from modified mitotic divisions (Blackman et al. 1987b; Miura et al. 2003). Therefore, their ovaries differ in whether they contain developing eggs or embryos. These two polyphenisms, the wing polyphenism and the reproduction polyphenism, are both complex phenotypes that are associated with hundreds to thousands of gene expression differences (Brisson et al. 2007; Le Trionnaire et al. 2009; Purandare, Bickel, et al. 2014; Vellichirammal et al. 2016), yet differences in splicing between the morphs have not been investigated.
Here, we characterize alternative splicing across different pea aphid morph transcriptomes using mRNA sequencing data. We identify hundreds of genes that show significant differential splicing between polyphenic morphs. We find that many differentially spliced genes (DSGs) are not differentially expressed genes (DEGs). Furthermore, many of the DSGs have annotations that are associated with the phenotypic traits that differ between morphs. This is one of the first in-depth study of genome-wide alternative splicing expression in a polyphenic system (see Price et al. 2018, which came out while this paper was in review). Our results show that differential alternative splicing diversifies the transcriptome in a manner unique from gene expression differences across polyphenic morphs.
Results and Discussion
Morphs Exhibit Alternative Splicing
We identified alternative splicing events in four different adult pea aphid samples (wingless males, sexual females, winged asexual females, and wingless asexual females) and two embryonic samples (female embryos destined to be winged or wingless) for a total of six phenotypes (see supplementary table S1, Supplementary Material online). Our focus was primarily on exploring the expression differences of alternative splicing events between polyphenic morphs (asexual vs. sexual females [the reproductive polyphenism comparison]; winged vs. wingless females [the wing polyphenism comparison]), but we also compared the genetically different sexual females and males (XX vs. XO; the sexual morph comparison) since males and females are known to differ extensively in alternative splicing in other species (Blekhman et al. 2010; Graveley et al. 2011; Gibilisco et al. 2016). The use of adult and embryonic samples allowed us to expand our study to differences between maintenance of the polyphenic morphs (adults) compared with the development of the polyphenic morphs (the embryonic wing polyphenism comparison).
We discovered 4,870 alternatively spliced genes from 14,228 expressed genes, resulting in 34.2% of the expressed genes exhibiting alternative splicing in one or more of the six phenotypes (see supplementary table S2, Supplementary Material online). This is similar to a previous estimate of pea aphid alternatively spliced genes, 37.2%, from a study that used expressed sequence tags to calculate the prevalence of alternative splicing across multiple eukaryotes (Chen et al. 2014). Both estimates agree that pea aphids alternatively splice ∼10% more of their expressed genes than the average of 24.6% of alternatively spliced genes found across 10 other invertebrates (Chen et al. 2014). The other invertebrates with similar levels of alternative splicing are Drosophila melanogaster (34.8%), Nematostella vectensis (32.2%), and Schistosoma mansoni (45.8%). Interestingly, both N. vectensis and S. mansoni express different intraspecific reproductive phenotypes (N. vectensis plastically, and S. mansoni during different stages of their life cycle); they reproduce sexually and asexually (Combes 2001; Reitzel et al. 2007). High levels of alternative splicing in these two species and in pea aphids raises the intriguing possibility that different reproductive phenotypes present across the life history of a species results in a high number of alternatively spliced genes. As more genomes become available, the association between increased alternative splicing and the expression of different phenotypes can be further explored.
DSCAM and slowpoke (slo, or KCNMA1 in humans) are some of the most well-known examples of genes expressing many isoforms (Lagrutta et al. 1994; Schmucker et al. 2000). As proof of principle, we examined alternative splicing patterns of these two genes in our data. We found that among all pea aphid genes, slowpoke has the greatest number of splicing events across adult phenotypes (fig. 1A) and DSCAM has the greatest number of splice variants in embryos (fig. 1B). Slo is a calcium- and voltage-activated potassium channel gene, with splicing events that change the requirements for opening the channel and that change the closing speed of the channel (Lagrutta et al. 1994; Miranda-Rottmann et al. 2010). The pea aphid slo gene has 27 annotated exons with an average of 20 splice sites per morph. Alternative splicing of DSCAM is well studied in D. melanogaster, where four clusters containing many exons are alternatively spliced to produce an estimated 38,016 possible isoforms (Schmucker et al. 2000). DSCAM has 86 exons in pea aphids with 131 observed splice variants in embryos. Similar to D. melanogaster, most of the pea aphid embryonic splicing of DSCAM that we identified is the result of retaining one exon from three different exon clusters.
Fig. 1.
Slowpoke and DSCAM are highly spliced in pea aphids, similar to other species. (A) slowpoke exhibits 20 alternative splice sites on average per pea aphid adult morph and (B) DSCAM has 131 detected alternative splice events in embryos. For clarity, not all splice events are shown. All functional domains are shown in grey above the gene models.
We categorized observed pea aphid alternative splicing events as one of five splice types. Skipped exons (SE) are whole exons that are included or excluded in the final transcript. Alternative 5′ and 3′ splice sites (A5′SS and A3′SS) make exons longer or shorter on either the 5′ or 3′ end of the intron, respectively. Mutually exclusive exons (MXE) are similar to SE, but with two exons where one is kept whereas the other is skipped. A retained intron (RI) is when the entire intron is retained making two exons into one large exon. Each type of alternative splicing can occur multiple times within a single gene, and a single gene can exhibit more than one type.
There is a potential risk that some of the observed splicing is nonfunctional “noise”. There are three hypothesized reasons an organism makes multiple mRNA isoforms: 1) functionally, to produce different proteins (Graveley 2001), 2) functionally, to posttranscriptionally regulate gene expression by the nonsense-mediated mRNA decay (NMD) pathway (Lewis et al. 2003; Lareau et al. 2007), and 3) nonfunctionally, with the isoforms being the result of an error-prone splicing process (Tress et al. 2007; Melamud and Moult 2009; Pickrell et al. 2010). The first and second reasons produce biologically relevant isoforms. All three occur (Lareau and Brenner 2015; Weatheritt et al. 2016), but spliceosome mistakes tend to be lowly expressed (Sorek et al. 2004; Melamud and Moult 2009; Tress et al. 2017).
To remove low abundance splicing events likely to be splicing mistakes, we required a splice site to have a percent spliced in (PSI) value of >10%, a cutoff used by other studies (Wang et al. 2008; Shapiro et al. 2011; He et al. 2015). After filtering, we identified 6,491 alternative splice sites on average per phenotype. Across all the phenotypes measured, 29% of genes were still alternatively spliced, indicating that the majority of alternatively spliced isoforms we identified before filtering were moderately to highly expressed. We further examined the effects of using the 10% PSI filter with the expectation that if we were retaining functional splice variants, we should see preferential retention of SE and MXE splice types postfiltering. SE and MXE are much more likely to translate into functional proteins than any of the other types of splice events (Weatheritt et al. 2016). This filter removed 41–48% of the splice sites found in the six phenotypes. The phenotypes were similar in the number and proportion of each splice type identified. However, which splice types were retained after filtering differed. Consistent with the idea that we preferentially filtered out nonfunctional variants, 82% of MXE and 59% of SE remained postfilter, compared with only 49% of A3′SS and 48% of A5′SS events. RI events were retained 57% of the time, but only comprise 6% of the total splice sites identified. Males exhibited the largest number of postfiltering alternative splice sites, whereas sexual females exhibited the smallest (table 1). Adult males alternatively spliced the largest number of genes, whereas polyphenic females (sexual, winged asexual, and wingless asexual) spliced the largest proportion of their expressed genes (table 1). Strong sex-biased alternative splicing differences have been previously found in D. melanogaster where males express more genes than females, but females use more alternative splicing (Brown et al. 2014; Gibilisco et al. 2016). This may be a general trend in insects that the sexes diversify their proteome in different ways; males by expressing more genes and females by expressing more alternative splicing variants.
Table 1.
Alternative Splice Sites and Genes Detected per Pea Aphid Morph Transcriptome with a Minimum Minor Isoform Expression of 10%.
| Morph | Alternative Splice Sites Detected | Genes with Alternative Splice Sites Detected | Expressed Genes | % of Expressed Genes with Alternative Splicing |
|---|---|---|---|---|
| Wingless adult males | 7,040 | 3,260 | 13,322 | 24% |
| Wingless adult sexual females | 6,018 | 2,941 | 10,858 | 27% |
| Winged adult asexual females | 6,828 | 3,196 | 11,245 | 28% |
| Wingless adult asexual females | 6,119 | 2,920 | 10,884 | 27% |
| Winged-destined asexual embryos | 6,484 | 3,046 | 12,208 | 25% |
| Wingless-destined asexual embryos | 6,455 | 3,050 | 12,193 | 25% |
| All morphs | 10,187 | 4,169 | 14,228 | 29% |
To confirm that the splicing differences between phenotypes were not an artifact of sequencing coverage (male samples exhibited the highest sequencing coverage; see supplementary table S1, Supplementary Material online), we randomly reduced each library to 20× coverage and reanalyzed for splicing events. The reduced set identified 7,827 unique splice events across all phenotypes compared with 13,558 unique splice events in the original analysis (see supplementary table S2, Supplementary Material online). We found that the reduced data set was generally a subset of the original splice events; 95% of the reduced set splice sites were identical to the full data set. Additionally, the percentage of each splice event per phenotype was largely maintained between the full and reduced data sets (see supplementary table S3, Supplementary Material online).
Because we used whole body sampling for this study, we likely excluded some tissue-specific splice variants by using a 10% PSI filter. Tissue-specific splicing may occur at levels <10% of the highly expressed isoform when observations are made from the whole body (Wang et al. 2008; Brown et al. 2014). Our postfiltering splicing counts (table 1) are, therefore, likely an underestimate of the true numbers of expressed isoforms.
Polyphenic Morphs Differentially Splice Hundreds of Genes
Just as genes can be differentially expressed between morphs, splice events can be differentially expressed between morphs. When a splice event is differentially expressed between morphs (called differentially spliced) it is included at a different rate between the morphs. For example, morph A includes exon 3 in 80% of its isoforms for gene X, but morph B only includes exon 3 in 10% of its isoforms for gene X. Differentially spliced events are the most likely alternative splicing events to contribute to phenotypic differences between the morphs, such as behavior and physiology, because they are morph biased. They are also more likely to be functional: Previous studies have found that alternative splicing that differs between tissues, physiological transitions, and developmental time points is often functional and conserved between species (Kalsotra and Cooper 2011; Lees et al. 2015). Differentially spliced events are therefore excellent candidates for isoforms that underlie the intraspecific phenotypic differences between polyphenic morphs.
To identify differentially spliced events and which genes they occur in, we performed pairwise comparisons of the genetically different wingless males and wingless sexual females (the sexual morph comparison) and the two polyphenic morph pairs: Adult wingless sexual compared with wingless asexual females (the reproductive polyphenism) and adult and embryonic winged compared with wingless asexual females (the wing polyphenism at two different developmental stages). We found that the sexual morph comparison had a large number of differentially spliced sites (1,013 sites from 561 genes). The reproductive polyphenism comparison also revealed a large number of differences: 933 sites from 536 genes, a remarkably high number considering that these females are genetically identical. This high number rivals the number of differences seen in the comparison of the males to sexual females, which are known, at least in other species, to express many splicing differences (Blekhman et al. 2010; Brown et al. 2014; Gibilisco et al. 2016). Winged and wingless adult females differentially expressed 332 splice sites from 230 genes. Winged versus wingless-destined embryos exhibited the fewest differences, with only 13 differentially spliced sites from 11 genes. All five splice types were differentially used in some morphs. However, we found SE and MXE events were differentially spliced more than expected in all adult morphs comparisons (fig. 2). Additionally, there were significantly fewer A3′SS, A5′SS, and RI events differentially spliced than expected in the adult morphs (fig. 2). It should be noted that the expected number of significant splice events per type are not independent measures. If one splice type is highly overrepresented as significantly differentially spliced, then another splice type must be underrepresented.
Fig. 2.
Skipped exon (SE) and mutually exclusive exon (MXE) events are differentially spliced more frequently than expected. Shown is the observed number of each splice type (black) and the expected number of splice sites (grey) by type. The expected value was calculated as ([splice type events * significant splice events]/expressed spliced events). If the observed was significantly greater that the expected, the FDR is black; it’s grey if the observed was less than the expected. However, the five splice types are not independent in this test; if one group is significantly overrepresented than another must be underrepresented. Fisher’s Exact *FDR < 0.05; **FDR < 0.01; ***FDR < 0.001.
We explored whether these putatively functional splice differences were being used to produce diverse proteins or to posttranscriptionally regulate gene expression. In other species, SE and MXE events are more likely to produce functional proteins because they maintain an open reading frame (ORF); they are also more likely to be engaged by a ribosome and thus translated (Weatheritt et al. 2016). Most frame shifts cause a downstream premature terminal codon and a truncated mRNA, which signals degradation of the transcript by NMD (Lewis et al. 2003; Lareau and Brenner 2015). We found that SE and MXE events in the pea aphid maintained the ORF of differentially spliced sites in adult comparisons more than the other splice types (table 2). Larger portions of the other three splice types (A3′SS, A5′SS, and RI) introduced shifts in the ORF, suggesting that they are being used to regulate mRNA transcript abundance by coupling alternative splicing with the NMD pathway. However, these three splice types (A3′SS, A5′SS, and RI) also constitute a much smaller percentage of the differentially spliced types in all the adult comparisons, indicating a reduced use of NMD in regulating differential splicing (table 2). The enrichment of SE and MXE events as differentially spliced and the evidence that these two splice types more frequently maintain the ORF indicate that differential splicing is being used to functionally diversify the proteome between adult polyphenic morphs.
Table 2.
Differential Splicing in the Pea Aphid is Enriched for SE and MXE Events that are More Likely to Maintain the Open Reading Frame in Adult Comparisons.
| Morph Comparison | Splice Type | Differentially Spliced Sites | % Differentially Spliced Sites | % Splice Sites with the ORF Maintaineda |
|---|---|---|---|---|
| Sexual morph comparison | MXE | 143 | 14% | 65% |
| SE | 557 | 55% | 51% | |
| A3′SS | 127 | 13% | 32% | |
| A5′SS | 153 | 15% | 37% | |
| RI | 33 | 3% | 45% | |
| Reproductive polyphenism comparison | MXE | 177 | 19% | 62% |
| SE | 478 | 51% | 52% | |
| A3′SS | 87 | 9% | 46% | |
| A5′SS | 164 | 18% | 38% | |
| RI | 27 | 3% | 33% | |
| Wing polyphenism comparison | MXE | 54 | 16% | 61% |
| SE | 155 | 47% | 42% | |
| A3′SS | 45 | 14% | 44% | |
| A5′SS | 69 | 21% | 38% | |
| RI | 9 | 3% | 22% | |
| Embryonic wing polyphenism comparison | MXE | 4 | 31% | 50% |
| SE | 0 | 0% | – | |
| A3′SS | 3 | 23% | 67% | |
| A5′SS | 6 | 46% | 33% | |
| RI | 0 | 0% | – |
Disrupting the open reading frame (ORF) likely causes downstream premature stop codons. We measured the number of differentially spliced sites that are divisible by three (for SE and RI), if switching between exons changed the reading frame (for MXE), or if the long and short forms of the exon maintained the same reading frame (for A3′SS and A5′SS), as a proxy for identifying splice sites that maintain the (ORF) between morphs.
DSGs Are Often Not DEGs
We hypothesized that the set of DSGs and DEGs would be different, because this has generally been true for other systems (reviewed in Kalsotra and Cooper 2011). Overall, we found that the set of DEGs largely does not overlap with the set of DSGs (fig. 3A and B). Thus, as hypothesized, our set of DSGs is a unique set of genes that would not necessarily be considered for their roles in maintaining morph differences if only gene expression level measures were used.
Fig. 3.

Most differentially spliced genes (DSGs) are not differentially expressed genes (DEGs) between pea aphid morph comparisons. (A) Graph shows the magnitude of gene expression differences (log2) and alternative splicing inclusion differences (ΔPSI) from each respective comparison for all significantly (FDR < 0.05) DSGs (blue) and DEGs (yellow). Purple indicates genes that were both differentially spliced and differentially expressed. If more than one significantly different splice event occurred in a gene, the largest ΔPSI is shown. (B) The total number of each type of gene: DEG (yellow), DSG (blue) and genes that are both DEG and DSG (purple).
Despite this overall trend of different sets of DSGs and DEGs, we found that the relationship between DSGs and DEGs is complex; it depended on the morphs compared and the level of gene expression in the morphs. The ratio of the total number of DSGs to DEGs in any comparison was consistently around 10% (range: 7.5–15.5%). In contrast, the number of genes that were both differentially spliced and differentially expressed varied broadly (9% overlap in the embryonic wing polyphenism comparison to 56% overlap in the sexual polyphenism comparison) between comparisons (fig. 3B). DEGs and DSGs overlapped less than expected in the sexual morph comparison (hypergeometric test, P = 5.3 × 10−12, RF = 0.7; an RF > 1 is more overlap than expected and an RF < 1 is less overlap than expected), the overlap was not significant in the reproductive polyphenism comparison (hypergeometric test, P = 0.5, RF = 1.0), and the overlap was greater than expected in the adult wing polyphenism (hypergeometric test, P = 0.003, RF = 1.5). DSGs and DEGs overlapped by only a single gene in the embryonic wing polyphenism comparison; this was not significant. The amount of overlap between DSGs and DEGs depended on the minimum read count used to define a splice junction and expressed gene (see supplementary fig. S1, Supplementary Material online), but in all cases there were sets of DSGs that were not differentially expressed and vice versa (compare fig. 3B with supplementary fig. S2, Supplementary Material online).
While the specific genes may not largely overlap between DSGs and DEGs, their functions may be synonymous. Comparing gene ontology (GO) enrichment between DSGs and DEGs, we found similar results as above: DSGs and DEGs were enriched for different GO terms in the sexual morph comparison, the GO terms overlapped somewhat in the reproductive polyphenism comparison, and the GO terms overlapped the most in the adult wing polyphenism comparison (see supplementary table S4, Supplementary Material online). A caveat to comparing GO terms between DSGs and DEGs is gene length bias. We found that our DSGs were longer than our DEGs in the adult comparisons (see supplementary fig. S3A, Supplementary Material online; Wilcoxon rank sum test; wing polyphenism comparison, P = 0.008; reproductive polyphenism comparison, P = 2.6 × 10−5; sexual morph comparison, P = 2.2 × 10−16). This bias towards longer genes in DSGs likely reduces the measureable overlap between GO categories of DEGs and DSGs.
To further examine differences between differential gene expression and differential splicing and their relationships to polyphenic morphs, we used a principle component analysis (PCA). The resulting PCA plots are strikingly different when using the two different data types (fig. 4), suggesting that alternative splicing and differential gene expression both contribute to phenotypic differences, but they do so in distinct ways. For example, for adult phenotypes, alternative splicing PC1 differentiates sexual females from asexual females and males, whereas the gene expression PC1 does not differentiate adults. The gene expression PC2 largely separates the adult phenotypes and groups the asexual females closely with the asexual embryos. PC2 calculated from alternative splicing data separates the polyphenic morphs (sexual females from the asexual female morphs [winged and wingless], and the winged females from the wingless females), but does not separate males from asexual females. Including more genes as expressed by lowering the minimum read count and cpm did not demonstrably change the alternative splicing PCA plots, but did marginally change how adults were differentiated in the gene expression PCA (compare fig. 4 with supplementary fig. S4, Supplementary Material online). Specifically, males were more strongly differentiated from females, and the female phenotypes were less differentiated from each other. This indicates that fairly lowly expressed genes, or possibly tissue-specific genes such as testis-specific genes, contribute to sexual morph differences but not as much to polyphenic differences.
Fig. 4.
Principle component analysis (PCA) of alternative splicing (left) and gene expression (right) separate the morphs differently. The relationship between morphs and alternative splicing (left) versus gene expression (right) were visualized by PCA, which explain similar amounts of variation, but separate the morphs differently along the first two principle component axes. Points are individual RNA-Seq experiments and ellipses represent the 95% confidence interval for a morph.
Genotypic differences were also apparent on the PCA. Regardless of the filter level, genotype I18 separated from the other two genotypes (BK11 and F1) in three of the adult phenotypes in the gene expression PCAs (see supplementary fig. S4, Supplementary Material online: genotypes labeled in grey). Separation of a single genotype in the alternative splicing PCAs depends on the phenotype; I18 in males, BK11 in winged females and F1 in sexual females separated from the other two respective genotypes (see supplementary fig. S4, Supplementary Material online). In contrast, the embryonic samples cluster tightly, even though the single genotype used in these samples is highly plastic (see Materials and Methods).
Plotting just the adult polyphenic females by PCA further emphasizes that patterns of alternative splicing distinguish the adult female polyphenic morphs differently than gene expression patterns (see supplementary fig. S5, Supplementary Material online). PC1 of the female-only, alternative splicing PCA separates all three female phenotypes, but PC1 of gene expression only separates sexual from asexual females. PC2 is needed to distinguish winged and wingless females in the gene expression PCA. Thus, alternative female phenotypes are defined both by splicing and by gene expression differences.
DSGs Are Related to Polyphenic Phenotypes
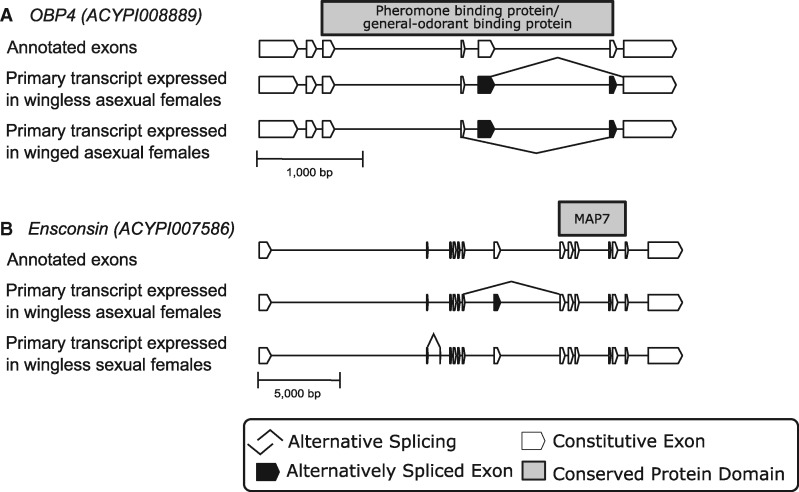
We found that the putative functions of many of the DSGs can be related to the phenotypes of the polyphenic morphs. We identified a list of highlighted candidate DSGs for future functional analyses in the adult wing and reproductive polyphenism comparisons that have GO terms that we deemed to be related to specific phenotypic traits of each morph. Winged and wingless adult females differ morphologically (presence or absence of wing structures and additional sensory organs), physiologically (metabolic output to flight muscles or ovaries), and behaviorally (agitated or sedentary; Dixon 1985). Using terms related to wings, sensory systems, metabolic processes, and locomotor behavior, we found 32 DSGs between winged and wingless females (see supplementary table S5, Supplementary Material online). Some genes are both differentially spliced and expressed. For example, three of the 15 odorant binding proteins (OBPs) in the pea aphid genome (Zhou et al. 2010) are differentially expressed and alternatively spliced between winged and wingless females (OBP1, OBP3, and OBP4); all three genes have significantly higher expression in wingless females. OBPs are important to odorant reception in chemosensory pathways because they transport hydrophobic odorant molecules to odorant receptors (Leal 2013). The ecological differences between winged and wingless females result in divergent chemosensory needs, so it’s possible that these differences are related to their ecological specializations. Exons 5 and 6 are mutually exclusive in both OBP1 and OBP4 (fig. 5A shows splicing in OBP4), with exon 5 more frequently included in winged females and exon 6 in wingless females. For OBP3, the second exon is skipped in a larger fraction of isoforms in winged than wingless females. So, whereas wingless females have higher expression levels of these three OBPs, winged females express larger fractions of alternatively spliced isoforms.
Fig. 5.
Examples of differentially spliced genes between polyphenic adult females with putative functions related to phenotype differences. (A) Odorant binding protein 4 (OBP4) has a differentially expressed MXE event where exon 5 is included more frequently in winged females and exon 6 is included more frequently in wingless females. (B) Ensconsin is a microtubule-associated gene that has two SE events that differ in expression between sexual and wingless asexual females.
Many genes, however, are differentially spliced but not differentially expressed. They would not have been implicated in morph-biased processes if transcript abundance, alone, were compared. For example, CAKI (also known as CASK or Camguk) is not differentially expressed between winged and wingless females, but wingless females include an unannotated exon between exons one and two more than winged females. Drosophila melanogaster expresses multiple isoforms of CAKI that influence locomotor behavior, in particular walking speed (Martin and Ollo 1996; Slawson et al. 2011), so the observed splicing difference in pea aphids may impact locomotion. The pea aphid homolog of another important locomotor-related gene, troponin-t, which regulates muscle contraction (Gomes et al. 2002), has a differentially spliced MXE event between winged and wingless female pea aphids. In dragonfly males, a trade-off in courtship dance investment between environmentally determined male morphs is associated with alternative isoforms of troponin-t (Fitzhugh and Marden 1997; Marden et al. 1999).
Sexual and asexual females are oviparous or viviparous, respectively (Blackman et al. 1987b; Miura et al. 2003). Using annotation terms related to mitosis/meiosis, embryonic development, oocytes, and oogenesis, we found 41 genes among the 536 genes with differential splicing between sexual and asexual females (see supplementary table S6, Supplementary Material online). Many of these DSGs are associated with the microtubules that establish axis polarity in the oocyte. For example, one of the most DSG is ensconsin, a microtubule-associated gene that is expressed in the oocyte. Ensconsin is a critical cofactor of kinesin-1, which transports oskar along ensconsin microtubules (Sung et al. 2008; Barlan et al. 2013). The ensconsin gene contains two, highly expressed SE events, one in the asexual females and one in sexual females. Both maintain the ORF and do not disrupt the gene’s conserved MAP7 domain (fig. 5B). The splice variants of ensconsin between these two female morphs suggest viviparous and oviparous embryonic development requires different maternally expressed isoforms. We did not include sexual female embryos in our comparisons, thus we cannot conclude if the same splice patterns exist between sexual and asexual female embryos. However, we did note that these two SE events are expressed in our asexual embryos (both winged and wingless destined) and exhibit a similar pattern to asexual females, with primary expression of the short isoform for both variants in the asexual embryos. Future functional studies are needed to assess the effect of splicing differences on the production or development of sexually and asexually produced offspring.
Conclusion
Here, we have shown that alternative splicing is used to diversify the proteome between polyphenic morphs: We identified many differences between morphs, and demonstrated that the majority of them are likely to be functional isoforms. We have also shown that the genes that are differentially spliced are not the same as those that are differentially expressed, highlighting that alternative splicing is likely important in the maintenance of polyphenic morph differences. Interestingly, we observed that many of the DSGs have putative biological functions that overlap with those of DEGs. In other words, although alternatively spliced genes are different than DEGs in identity, their functions may largely overlap. This may indicate that constraints on transcript abundance of a particular gene in a process may be overcome by differentially splicing of the gene, and vice versa (Marden 2008). We conclude that alternative splicing is an understudied, potentially important molecular mechanism underlying the expression of the morphological, behavioral, and physiological differences between plastically produced morphs.
Materials and Methods
The pea aphid samples used in this study are from four different genotypes: Three genotypes were used as three biological replicates for adult samples (Purandare, Bickel, et al. 2014) and four biological replicates of a single genotype for embryo samples. All samples were collected as whole bodies. The adult samples (wingless males, wingless sexual females, winged asexual females, and wingless asexual females) consisted of three biological replicates of 30 aphids each from three genotypes: F1, I18, and BK11 (see supplementary table S1, Supplementary Material online). The embryonic samples (winged-destined and wingless-destined) consisted of four biological replicates of 15 embryos each from a single genotype, BK10. The embryonic genotype was chosen because it was previously found to consistently produce high levels of winged or wingless offspring depending on the maternal environment (called MA2 in Grantham et al. 2016).
RNA-Sequencing Libraries
RNA-sequencing libraries were obtained from sequence read archives (BioProject accession no. PRJNA244726; Purandare, Tenhumberg, et al. 2014) for the following adult morphs: Wingless males, sexual females, winged asexual females, and wingless asexual females.
Stage 18 embryonic samples were collected for this study from wingless females producing winged-destined or wingless-destined embryos (BioProject accession no. PRJNA445883). During development, aphids undergo 20 embryonic stages. Wing determination occurs embryonically, and stage 18 embryos are the most likely to be in the key stage of wing morph determination (Miura et al. 2003; Ishikawa and Miura 2013). Most females produce a mix of winged and wingless offspring phenotypes, but produce the same percentage of offspring phenotypes over multiple days (Grantham et al. 2016). We used the following protocol to determine the percentage of offspring phenotypes being produced by each female: 10 adult female pea aphids were crowded in a 35 mm Petri dish with a moist filter to prevent desiccation. After 24 h, the females were moved to Vicia faba plants individually for 24 h to produce offspring. The females were removed from the plants and all embryos were dissected from each female and stored at −80° in TRIzol (Life Technologies). The percentage of winged offspring produced by each female was measured from the offspring that were produced on V. faba during the 24 h after crowding, but before dissection. Embryos from females producing 0% winged offspring were labeled wingless-destined offspring, whereas embryos from females producing ≥70% winged offspring were labeled winged-destined embryos. One stage 18 embryo was collected from each female until 15 embryos of each phenotype were collected per replicate. A total of four replicates per phenotype (winged-destined and wingless-destined) were collected from 120 adult females.
RNA was extracted using TRIzol. The product was cleaned and DNA was digested using the Zymo clean and concentrator kit (Zymo Inc.) with the in-tube DNase treatment. Libraries were prepared using the TruSeq Stranded mRNA Library Prep Kit (Illumina). Libraries were sequenced on an Illumina HiSeq 2500 (University of Rochester Genomics Research Center).
Data Analyses
To identify alternative splicing and gene expression differences, the following workflow and parameters were used and are summarized in supplementary fig. S6, Supplementary Material online. All mRNA-seq libraries were quality trimmed with trimmomatic version 0.32 (parameters: SE -phred33 SLIDINGWINDOW: 4: 20 TRAILING: 13 LEADING: 13 ILLUMINACLIPTruSeq3-SE.fa: 2: 30: 10 MINLEN: 15; Bolger et al. 2014). The alternative splicing program used, rMATS, requires all mRNA fastq reads to be equal length in each comparison. Therefore, each fastq library was filtered and trimmed to five bp of the shortest read length in each comparison (see supplementary table S1, Supplementary Material online) prior to submitting libraries to rMATS [version 3.2.5, Shen et al. 2014] for assessment of alternative splicing. Additionally, to assess the degree of 5′ degradation on transcripts we used the geneBody_coverage.py program within the package RSeQC (Wang et al. 2012). The embryo libraries were largely unaffected by 5′ degradation (see supplementary fig. S7A, Supplementary Material online). The adult libraries did exhibit some degradation. However, the 5′ degradation noted in adults was equal between the morphs and therefore should not affect the calling of differentially expressed splice events (see supplementary fig. S7B–D, Supplementary Material online). For example, in the female reproduction comparison, there are two libraries that clearly suffer from 5′ degradation, but one is a sexual female library (F1) and the other is an asexual female library (I18).
The rMATS program consists of three steps to assess alternative splicing: 1) mRNA reads are mapped to the genome by the RNA-seq aligner, STAR (Dobin et al. 2013), 2) annotated and novel splice junctions are assessed for alternative splicing per mRNA fastq library, and 3) differential splicing is tested between replicated samples at each identified splice site.
Using the output from first two steps of the rMATS program, we measured alternative splicing for each phenotype. All mRNA libraries (trimmed and filtered to 45 bp) were submitted to rMATS (parameters: -t single -len 45 -a 5 -novelSS 1). The consensus gene list (ACYPI OGS v2.1b) and genome assembly version 2 in scaffolds (aphidbase.com) were used for identifying splice sites and mapping reads to the genome, respectively (see supplementary table S7, Supplementary Material online). Transcriptome coverage can influence the detection of alternative splice variants. To assess the influence of sequencing depth between libraries, we randomly reduced each mRNA library to 20× coverage (18 million reads per library) of the transcriptome using seqtk and reanalyzed the number of detected splice variants. We required a minimum read count of 20 reads to support both the long and short splice junctions for the full (all sequencing data) and reduced (20× coverage) splice sets (Brooks et al. 2015).
To assess differential splicing between morphs we used the following pairwise comparisons: The sexual morph comparison (wingless males vs. wingless sexual females), the reproductive polyphenism comparison (wingless asexual females vs. wingless sexual females), the wing polyphenism comparison (winged vs. wingless asexual females) and lastly, the embryonic wing polyphenism comparison (winged-destined vs. wingless-destined embryonic asexual females). Sexual females are strictly wingless, so we only compared them to wingless morphs (wingless males or wingless asexual females). mRNA fastq libraries (trimmed and filtered to -5 bp of the sequenced read length) of each pairwise comparison were assessed by rMATS (parameters: -t single –len, see supplementary table S1, Supplementary Material online, -a 5 -novelSS 1) to test for significant differential splicing between morphs (see supplementary table S8, Supplementary Material online). As the read length and sequencing depth differed between mRNA libraries, we tested the correlation of read length (correlation = 0.51, P = 0.30) and sequencing depth (correlation = -0.47, P = 0.34) to the number of splice junctions identified on average per phenotype and found they did not significantly correlate (see supplementary table S1, Supplementary Material online).
rMats outputs the proportion of the long to short isoform of each splice site per mRNA library as PSI. This means that a splice site in a single morph with PSI of 1 or 0 is not alternatively spliced; only the long or short isoform is expressed, respectively. To compare splicing between phenotypes, an inclusion difference (ΔPSI) is reported. The inclusion difference is the PSI difference between the morphs (average PSI of Morph 1 replicates—average PSI of Morph 2 replicates); it ranges from -1 (the longer isoform is only expressed in one morph) to 1 (the longer isoform is only expressed in the other morph). A ΔPSI of zero means the alternative splicing pattern does not differ between the morphs compared (i.e., the proportion of long to short isoforms for that splice is the same between morphs). Sites were said to be differentially spliced if an FDR ≤ 0.05 was obtained for a splice site.
The first step of rMATS is to use the RNA-seq aligner, STAR, to map mRNA reads to the genome. From each pairwise comparison, we used the mapped mRNA reads per library to find all expressed genes per morph and all DEGs. This ensured the same mRNA alignment was used for measuring gene expression and alternative splicing differences between morphs. The featureCount program from the Rsubread package version 1.24.2 was used to collate raw counts per gene (Liao et al. 2013) using the consensus gene list (ACYPI OGS v2.1b). The ability to detect splicing events is limited by sufficient sequencing coverage over splice junctions. We required a total of 20 reads to support the inclusion and exclusion junctions in each comparison (Brooks et al. 2015). We found this 20 read junction coverage correlated with ∼1 read count per million reads (cpm) on a gene. Few to no splicing events could be detected below 1 cpm in a library on a gene. We therefore used this as our cutoff for calling a gene expressed. This was necessary so that we could compare gene expression to alternative splicing differences in our comparisons. To check the influence of the read count filter we reduced it to 5, 10, and 15 read counts across each splice junction with a 0.1 cpm for gene expression (see supplementary fig. S1, Supplementary Material online). Differential gene expression was assessed with the edgeR package in R version 3.1.0 (Robinson et al. 2010; R Core Team 2015). GO enrichment analyses (see supplementary table S4, Supplementary Material online) were conducted in DAVID version 6.8 (Huang et al. 2009a, 2009b). To address gene length bias in GO enrichment tests we compared gene lengths between DSGs and DEGs using a Wilcoxon rank sum test in R. Author-identified GO terms related to known polyphenic traits were used to organize candidate DSGs between the reproductive and wing polyphenism comparisons.
Frame shifts due to alternative splicing likely introduce downstream premature stop codons resulting in degradation of the transcript by the NMD pathway. While rMATS outperforms other alternative splicing detection methods on real data, it does not assemble splice variants into complete transcripts (Liu, Loraine, et al. 2014). As we do not know the transcript sequence we cannot predict premature stop codons or the reading frame, but frame shifts can be predicted. A frame shift from alternative splicing causes the sequence after the splice site to be read in a different frame. So a splice site must maintain the same “position” in the reading frame for the next exon. For example, an exon that is 91 nucleotides long consists of 30 codons and 1 additional base, which is part of the either the codon up or downstream of the exon. The alternative splice site must maintain that 1/3 of the codon to avoid disrupting the reading frame. If an alternative 3′ splice site occurs and makes that exon 60 bases longer (now a 151 base exon) the reading frame would be maintained for the next exon (50 codons and 1/3 codon). However, if the A3′SS were 58 bases (49 codons and 2/3 of a codon) it would shift the reading frame for the next exon and the rest of the mRNA. We measured how often a frame shift was introduced in differentially spliced events by measuring if the alternative form was divisible by three (for SE and RI), if switching between exons changed the reading frame (for MXE), or if the long and short forms of the exon maintained the same reading frame (for A3′SS and A5′SS). Because we cannot assemble the entire transcript, it is possible that a second, downstream alternative splice site in the same isoform could restore the reading frame.
Gene Models
Gene model images were generated from Splicegrapher 0.2.2 using the plotter.py script (Rogers et al. 2012) for the annotated gene model. We identified conserved domains with the NCBI Conserved Domain Database (Marchler-Bauer et al. 2015).
Statistics
Hypergeometric and Fisher’s Exact tests were performed in R version 3.2.5 (R Core Team 2015). When conducting multiple tests, a Benjamini–Hochberg (FDR) P-value correction was applied in R. The representation factor (RF) compares the observed number of overlapping genes to expected number of overlapping genes ([DSGs * DEGs]/expressed genes); if RF > 1, there is more overlap than expected and if RF < 1, there is less overlap than expected. A Fisher’s Exact test was performed for each splice type category per pairwise comparison using 2 × 2 contingency tables (e.g., # of SE events; # of nonSEs events by # of significant splice events; # of nonsignificant splice events). The expected number of differentially spliced sites was calculated as the expected number of significant splice events of each type ([splice type * significant splice events]/expressed spliced events).
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank Jack Werren and Ben Parker for their critical reading of an earlier version of this paper and two anonymous reviewers for their helpful comments. This research was supported by award R01GM116867 from the National Institute of General Medical Sciences to J.A.B.
References
- Barlan K, Lu W, Gelfand VI.. 2013. The microtubule-binding protein ensconsin is an essential cofactor of kinesin-1. Curr Biol. 234:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman RL, Minks AK, Harrewijn P.. 1987a. Aphids: their biology, natural enemies and control, world crop pests. Amsterdam (The Netherlands: ): Elsevier Science Publishers [Google Scholar]
- Blackman RL, Minks AK, Harrewijn P.. 1987b. In: Minks AK, Harrewijn P, editors. Reproduction, cytogenetics and development. 2nd ed Amsterdam (The Netherlands: ): Elsevier Science Publishers [Google Scholar]
- Blekhman R, Marioni JC, Zumbo P, Stephens M, Gilad Y.. 2010. Sex-specific and lineage-specific alternative splicing in primates. Genome Res. 202:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B.. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 3015:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. Adv Genet. 13:115–155. [Google Scholar]
- Braendle C, Caillaud MC, Stern DL.. 2005. Genetic mapping of aphicarus – a sex-linked locus controlling a wing polymorphism in the pea aphid (Acyrthosiphon pisum). Heredity (Edinb) 944:435–442. [DOI] [PubMed] [Google Scholar]
- Brisson JA, Davis GK, Stern DL.. 2007. Common genome-wide patterns of transcript accumulation underlying the wing polyphenism and polymorphism in the pea aphid (Acyrthosiphon pisum). Evol Dev. 94:338–346. [DOI] [PubMed] [Google Scholar]
- Brisson JA, Stern DL.. 2006. The pea aphid, Acyrthosiphon pisum: an emerging genomic model system for ecological, developmental and evolutionary studies. Bioessays 287:747–755. [DOI] [PubMed] [Google Scholar]
- Brooks AN, Duff MO, May G, Yang L, Bolisetty M, Landolin J, Wan K, Sandler J, Booth BW, Celniker SE.. 2015. Regulation of alternative splicing in Drosophila by 56 RNA binding proteins. Genome Res. 2511:1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JB, Boley N, Eisman R, May GE, Stoiber MH, Duff MO, Booth BW, Wen J, Park S, Suzuki AM.. 2014. Diversity and dynamics of the Drosophila transcriptome. Nature 5127515:393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud MC, Boutin M, Braendle C, Simon JC.. 2002. A-linked locus controls wing polymorphism in males of the pea aphid, Acyrthosiphon pisum (Harris). Heredity (Edinb) 895:346–352. [DOI] [PubMed] [Google Scholar]
- Chen L, Bush SJ, Tovar-corona JM, Castillo-morales A, Urrutia AO.. 2014. Correcting for differential transcript coverage reveals a strong relationship between alternative splicing and organism complexity. Mol Biol Evol. 316:1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hu Y, Zheng H, Cao L, Niu D, Yu D, Sun Y, Hu S, Hu F.. 2012. Transcriptome comparison between honey bee queen- and worker-destined larvae. Insect Biochem Mol Biol. 429:665–673. [DOI] [PubMed] [Google Scholar]
- Cingolani P, Cao X, Khetani RS, Chen C-C, Coon M, Sammak A, Bollig-Fischer A, Land S, Huang Y, Hudson ME, et al. 2013. Intronic non-CG DNA hydroxymethylation and alternative mRNA splicing in honey bees. BMC Genomics. 14:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes C. 2001. Parasitism: the ecology and evolution of intimate interactions. Chicago: University of Chicago Press. [Google Scholar]
- Daniels EV, Murad R, Mortazavi A, Reed RD.. 2014. Extensive transcriptional response associated with seasonal plasticity of butterfly wing patterns. Mol Ecol. 2324:6123–6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JR, Capy P, Gauthier J-P.. 1990. Abdominal pigmentation and growth temperature in Drosophila melanogaster: similarities and differences in the norms of reaction of successive segments. J Evol Biol. 3(5–6):429–445. [Google Scholar]
- Dixon AFG. 1985. Aphid ecology. New York: Blackie; Distributed in the U.S.A. by Chapman and Hall [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR.. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 291:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzhugh G, Marden J.. 1997. Maturational changes in troponin T expression, Ca2+-sensitivity and twitch contraction kinetics in dragonfly flight muscle. J Exp Biol. 200(Pt 10):1473–1482. [DOI] [PubMed] [Google Scholar]
- Gibilisco L, Zhou Q, Mahajan S, Bachtrog D.. 2016. Alternative splicing within and between drosophila species, sexes, tissues, and developmental stages. PLOS Genet. 1212:e1006464.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AV, Potter JD, Szczesna-Cordary D.. 2002. The role of troponins in muscle contraction. IUBMB Life (Int Union Biochem Mol Biol Life). 546:323–333. [DOI] [PubMed] [Google Scholar]
- Grantham M, Brisson JA, Tagu D, Trionnaire GL.. 2015. Integrative genomic approaches to studying epigenetic mechanisms of phenotypic plasticity in the aphid. Short Views Insect Genomics Proteomics. 3:75–93. [Google Scholar]
- Grantham ME, Antonio CJ, O'Neil BR, Zhan YX, Brisson JA.. 2016. A case for a joint strategy of diversified bet hedging and plasticity in the pea aphid wing polyphenism. Biol Lett. 1210:20160654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR. 2001. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 172:100–107. [DOI] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, et al. 2011. The developmental transcriptome of Drosophila melanogaster. Nature 4717339:473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Liu Q, Zheng L, Cui Y, Shen Z, Zheng L.. 2015. RNA-Seq analysis of rice roots reveals the involvement of post-transcriptional regulation in response to cadmium stress. Front Plant Sci. 6:1136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA.. 2009a. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 41:44–57. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA.. 2009b. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 371:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Miura T.. 2013. Transduction of high-density signals across generations in aphid wing polyphenism. Physiol Entomol. 382:150–156. [Google Scholar]
- Jakšić AM, Schlötterer C.. 2016. The interplay of temperature and genotype on patterns of alternative splicing in Drosophila melanogaster. Genetics 116:192310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AB, Syed NH, Bordage S, Marshall J, Nimmo GA, Jenkins GI, Herzyk P, Brown JWS, Nimmo HG.. 2012. Alternative splicing mediates responses of the Arabidopsis circadian clock to temperature changes. Plant Cell. 243:961–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Yang M, Guo W, Wang X, Kang L.. 2012. Large-scale transcriptome analysis of retroelements in the migratory locust, Locusta migratoria. PLoS ONE. 77:e40532.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsotra A, Cooper TA.. 2011. Functional consequences of developmentally regulated alternative splicing. Nat Rev Genet. 1210:715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrutta A, Shen KZ, North RA, Adelman JP.. 1994. Functional differences among alternatively spliced variants of slowpoke, a Drosophila calcium-activated potassium channel. J Biol Chem. 26932:20347–20351. [PubMed] [Google Scholar]
- Lareau LF, Brenner SE.. 2015. Regulation of splicing factors by alternative splicing and NMD is conserved between kingdoms yet evolutionarily flexible. Mol Biol Evol. 324:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lareau LF, Brooks AN, Soergel DAW, Meng Q, Brenner SE.. 2007. The coupling of alternative splicing and nonsense-mediated mRNA decay In: Blencowe BJ, Graveley BR, editors. Alternative splicing in the postgenomic era. New York: Springer. [Google Scholar]
- Leal WS. 2013. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 58:373–391. [DOI] [PubMed] [Google Scholar]
- Lees JG, Ranea JA, Orengo CA.. 2015. Identifying and characterising key alternative splicing events in Drosophila development. BMC Genomics. 16608:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Green RE, Brenner SE.. 2003. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc Natl Acad Sci U S A. 1001:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Byarlay H, Li Y, Stroud H, Feng S, Newman TC, Kaneda M, Hou KK, Worley KC, Elsik CG, Wickline SA.. 2013. RNA interference knockdown of DNA methyl-transferase 3 affects gene alternative splicing in the honey bee. Proc Natl Acad Sci U S A. 11031:12750–12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Bickel RD, Parker BJ, Vellichirammal NN, Grantham ME, Simon J-C, Stern DL, Brisson JA.. 2017. Unravelling the genomic basis and evolution of the pea aphid male wing dimorphism. bioRxiv. 156133:1–15. [Google Scholar]
- Liao Y, Smyth GK, Shi W.. 2013. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 4110:e108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LJ, Zheng HY, Jiang F, Guo W, Zhou ST.. 2014. Comparative transcriptional analysis of asexual and sexual morphs reveals possible mechanisms in reproductive polyphenism of the cotton aphid. PLOS ONE. 96:e99506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Loraine AE, Dickerson JA.. 2014. Comparisons of computational methods for differential alternative splicing detection using RNA-seq in plant systems. BMC Bioinformatics. 15:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Song G, Yan J, He X, Li Q, Cui Z, Wootton R, Lopez-Olmeda J, Sanchez-Vazquez F, Brett J, et al. 2013. Transcriptomic characterization of cold acclimation in larval zebrafish. BMC Genomics. 141:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, et al. 2015. CDD: nCBI’s conserved domain database. Nucleic Acids Res. 43(D1):D222–D226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JH. 2008. Quantitative and evolutionary biology of alternative splicing: how changing the mix of alternative transcripts affects phenotypic plasticity and reaction norms. Heredity (Edinb) 1002:111–120. [DOI] [PubMed] [Google Scholar]
- Marden JH, Fitzhugh GH, Wolf MR, Arnold KD, Rowan B.. 1999. Alternative splicing, muscle calcium sensitivity, and the modulation of dragonfly flight performance. Proc Natl Acad Sci U S A. 9626:15304–15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JR, Ollo R.. 1996. A new Drosophila Ca2+/calmodulin-dependent protein kinase (Caki) is localized in the central nervous system and implicated in walking speed. EMBO J. 158:1865–1876. [PMC free article] [PubMed] [Google Scholar]
- Matlin AJ, Clark F, Smith CWJ.. 2005. Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol. 65:386–398. [DOI] [PubMed] [Google Scholar]
- Melamud E, Moult J.. 2009. Stochastic noise in splicing machinery. Nucleic Acids Res. 3714:4873–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Rottmann S, Kozlov AS, Hudspeth AJ.. 2010. Highly specific alternative splicing of transcripts encoding BK channels in the chicken’s cochlea is a minor determinant of the tonotopic gradient. Mol Cell Biol. 3014:3646–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Braendle C, Shingleton A, Sisk G, Kambhampati S, Stern DL.. 2003. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: aphidoidea). J Exp Zool B Mol Dev Evol. 295B1:59–81. [DOI] [PubMed] [Google Scholar]
- Parker BJ, Barribeau SM, Laughton AM, Griffin LH, Gerardo NM.. 2017. Life-history strategy determines constraints on immune function. J Anim Ecol. 863:473–483. [DOI] [PubMed] [Google Scholar]
- Pickrell JK, Pai AA, Gilad Y, Pritchard JK, Pfiffner J.. 2010. Noisy splicing drives mRNA isoform diversity in human cells. PLoS Genet. 612:e1001236.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Harrison MC, Hammond RL, Adams S, Gutierrez–Marcos JF, Mallon EB.. 2018. Alternative splicing associated with phenotypic plasticity in the bumble bee Bombus terrestris. Mol Ecol. 27:1036–1043. [DOI] [PubMed] [Google Scholar]
- Purandare SR, Bickel RD, Jaquiery J, Rispe C, Brisson JA.. 2014. Accelerated evolution of morph-biased genes in pea aphids. Mol Biol Evol. 318:2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purandare SR, Tenhumberg B, Brisson JA.. 2014. Comparison of the wing polyphenic response of pea aphids (Acyrthosiphon pisum) to crowding and predator cues. Ecol Entomol. 392:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing.
- Reitzel AM, Burton PM, Krone C, Finnerty JR.. 2007. Comparison of developmental trajectories in the starlet sea anemone Nematostella vectensis: embryogenesis, regeneration, and two forms of asexual fission. Invertebr Biol. 1262:99–112. [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK.. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 261:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MF, Thomas J, Reddy AS, Ben-Hur A.. 2012. SpliceGrapher: detecting patterns of alternative splicing from RNA-Seq data in the context of gene models and EST data. Genome Biol. 131:R4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL.. 2000. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 1016:671–684. [DOI] [PubMed] [Google Scholar]
- Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB.. 2011. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet. 78:e1002218.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Park JW, Lu Z, Lin L, Henry MD, Wu YN, Zhou Q, Xing Y.. 2014. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci U S A. 11151:E5593–E5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Sword GA, Lo N.. 2011. Polyphenism in Insects. Curr Biol. 2118:R738–R749. [DOI] [PubMed] [Google Scholar]
- Slawson JB, Kuklin EA, Ejima A, Mukherjee K, Ostrovsky L, Griffith LC.. 2011. Central regulation of locomotor behavior of Drosophila melanogaster depends on a CASK isoform containing CaMK-like and L27 domains. Genetics 1871:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Shamir R, Ast G.. 2004. How prevalent is functional alternative splicing in the human genome? Trends Genet. 202:68–71. [DOI] [PubMed] [Google Scholar]
- Sung HH, Telley IA, Papadaki P, Ephrussi A, Surrey T, Rørth P.. 2008. Drosophila ensconsin promotes productive recruitment of kinesin-1 to microtubules. Dev Cell. 156:866–876. [DOI] [PubMed] [Google Scholar]
- Sutherland ORW. 1969a. The role of crowding in the production of winged forms by two strains of the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 158:1385–1410. [Google Scholar]
- Sutherland ORW. 1969b. The role of the host plant in the production of winged forms by two strains of the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 1511:2179–2201. [Google Scholar]
- Tress ML, Abascal F, Valencia A.. 2017. Alternative splicing may not be the key to proteome complexity. [DOI] [PMC free article] [PubMed]
- Tress ML, Martelli PL, Frankish A, Reeves GA, Wesselink JJ, Yeats C, Olason PI, Albrecht M, Hegyi H, Giorgetti A, et al. 2007. The implications of alternative splicing in the ENCODE protein complement. Proc Natl Acad Sci U S A. 10413:5495–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Trionnaire G, Francis F, Jaubert-Possamai S, Bonhomme J, De Pauw E, Gauthier JP, Haubruge E, Legeai F, Prunier-Leterme N, Simon JC, et al. 2009. Transcriptomic and proteomic analyses of seasonal photoperiodism in the pea aphid. BMC Genomics. 10:456.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellichirammal NN, Madayiputhiya N, Brisson JA.. 2016. The genomewide transcriptional response underlying the pea aphid wing polyphenism. Mol Ecol. 2517:4146–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via S. 1992. Inducing the sexual forms and hatching the eggs of pea aphids. Entomol Exp Appl. 652:119–127. [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB.. 2008. Alternative isoform regulation in human tissue transcriptomes. Nature 4567221:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang S, Li W.. 2012. RSeQC: quality control of RNA-seq experiments. Bioinformatics 2816:2184–2185. [DOI] [PubMed] [Google Scholar]
- Wang X, Fang X, Yang P, Jiang X, Jiang F, Zhao D, Li B, Cui F, Wei J, Ma C, et al. 2014. The locust genome provides insight into swarm formation and long-distance flight. Nat Commun. 5:2957.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatheritt RJ, Sterne-Weiler T, Blencowe BJ.. 2016. The ribosome-engaged landscape of alternative splicing. Nat Struct Mol Biol. 2312:1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver N. 1957. Effects of larval age on dimorphic differentiation of the female honey bee. Ann Entomol Soc Am. 503:283. [Google Scholar]
- West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford. New York: Oxford University Press [Google Scholar]
- Zhou J-J, Vieira FG, He X-L, Smadja C, Liu R, Rozas J, Field LM.. 2010. Genome annotation and comparative analyses of the odorant-binding proteins and chemosensory proteins in the pea aphid Acyrthosiphon pisum. Insect Mol Biol. 19:113–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.