Abstract
Objective
To evaluate the association between furosemide exposure and patent ductus arteriosus (PDA) in a large, contemporary cohort of hospitalized very low birth weight (VLBW) infants.
Study design
Using the Pediatrix Medical Group Clinical Data Warehouse, we identified all inborn VLBW infants <37 weeks’ gestation discharged from the neonatal intensive care unit (NICU) after the first postnatal week from 2011 to 2015. We defined PDA as any medical (ibuprofen or indomethacin) or surgical PDA therapy. We collected data up to the day of PDA treatment or postnatal day 18 for infants not diagnosed with PDA. We performed multivariable logistic regression to evaluate the association between PDA and exposure to furosemide.
Results
We included 43,576 infants from 337 NICUs, of whom 6675 (15%) underwent PDA treatment. Infants with PDA were more premature and more often exposed to mechanical ventilation and inotropes. Furosemide was prescribed to 4055 (9%) infants. On multivariable regression, exposure to furosemide was associated with decreased odds of PDA treatment (odds ratio [OR]=0.72; 95% confidence interval [CI] 0.65, 0.79). Increasing percentage of days with furosemide exposure was not associated with PDA treatment (OR=1.01; 95% CI 0.97, 1.06).
Conclusions
Furosemide exposure was not associated with increased odds of PDA treatment in hospitalized VLBW infants. Further studies are needed to characterize the efficacy and safety of furosemide in premature infants.
Keywords: furosemide exposure, patent ductus arteriosus, very low birth weight infants
An estimated 15 million infants are born prematurely in the United States each year.1 Although the survival rate for premature infants born <28 weeks has increased, these infants continue to experience significant morbidity.1 One such morbidity is persistence of a hemodynamically significant patent ductus arteriosus (PDA).2 The ductus arteriosus is a normal structure in fetal life and is necessary to divert oxygenated right ventricular blood towards the systemic circulation.3 During the transition to extrauterine life, a complex and still incompletely understood mechanism involving increasing oxygenation of neonatal blood, pressure changes due to removal of the low resistance placenta, and a decrease in prostaglandin serum concentrations lead to closure of the PDA.4 Persistence of the PDA is associated with the development of multiple short- and long-term morbidities including bronchopulmonary dysplasia (BPD).4
To prevent or treat complications associated with prematurity, infants are exposed to multiple drugs despite an incompletely understood risk-benefit relationship.5,6 One example is furosemide, which is administered to as many as 50% of hospitalized premature infants.6 Potential benefits of furosemide include improved lung function from a reduction in pulmonary edema, which may occur in the setting of PDA. Nevertheless, furosemide also stimulates the renal production of prostaglandin E2 (PGE2), which may inhibit ductal closure5; this was first observed in a randomized controlled trial of premature infants <2500 g birth weight with respiratory distress syndrome. Infants in this trial exposed to furosemide were twice as likely to suffer from persistent PDA compared with those receiving chlorothiazide (55% vs 24%, P < .02)7; however, the relevance of this study to current practice is limited given the changes in management of premature infants that have occurred over the last 30 years.
More recent studies, including a Cochrane review of infants treated with indomethacin, found no increased failure of PDA closure in the subgroup of infants exposed to furosemide, although in these cases, the diagnosis of PDA had already been made.8,9 Whether furosemide use is associated with PDA in premature infants remains unknown. We aimed to characterize the relationship between furosemide use and PDA in a large contemporary cohort of hospitalized very low birth weight (VLBW, <1500 g birth weight) infants. We hypothesized that furosemide exposure would not be associated with increased incidence of PDA therapy.
Methods
We used a clinical database of infants discharged from one of 337 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group from 2011 to 2015. De-identified data were obtained from an electronic medical record database that prospectively captures information including clinician-generated admission history, daily progress notes, discharge summaries, medications with limited dosing information, and diagnoses.10 We included inborn infants <37 weeks gestational age (GA) with birth weight (BW) <1500 g. We excluded infants with congenital heart disease other than isolated PDA, those with any exposure to prostaglandin therapy during the first 120 days of life, and those who died or were discharged before postnatal day 7. We collected demographic data including sex, BW, and GA, as well as the Apgar score at 5 minutes of life.
Our primary endpoint was PDA treatment as a surrogate marker for hemodynamically significant PDA and the main predictor of interest was exposure to furosemide prior to the first day of PDA treatment or during the evaluable period in infants without PDA treatment. We defined PDA treatment as the receipt of indomethacin or ibuprofen, or surgical intervention from postnatal day 2 to postnatal day 60. We defined prophylactic indomethacin as any exposure to indomethacin in the first 2 days of life (day 0 or 1). We defined an evaluable period during which we collected data on furosemide exposure, exposure to other diuretics, prenatal steroid exposure, inotrope exposure, and use of mechanical ventilation. For infants with PDA treatment, we defined the evaluable period from postnatal day 0 (day of birth) to the first day of PDA treatment. For those without PDA treatment, we defined the evaluable period from postnatal day 0 to postnatal day 18. This cut-off was chosen because ≥90% of PDA were treated before postnatal day 18, thereby providing comparable evaluation periods. We used two definitions of furosemide exposure: 1) any exposure to furosemide during the evaluable period (binary variable); and 2) a percent of evaluable days with exposure to furosemide, defined as a continuous variable subsequently categorized into 10% increments.
Statistical Analyses
The unit of observation for this analysis was the infant. We used standard summary statistics including counts (percentages) and medians (25th, 75th percentiles) to describe all categorical and continuous study variables, respectively. We compared the distribution of study variables between infants with and without PDA treatment, and infants with and without furosemide exposure using Chi-square, Fisher exact, and Wilcoxon rank sum tests, as appropriate. We also conducted secondary analyses stratified by GA and BW. We performed mixed multivariable logistic regression to evaluate the adjusted odds of PDA treatment in infants exposed to furosemide prior to diagnosis, controlling for GA, small for gestational age status, sex, prenatal steroids, APGAR scores at 5 minutes, discharge year, exposure to inotropes, mechanical ventilation and other diuretics, and discharge year. We also controlled for site as a fixed effect. We fit separate regressions for each definition of furosemide exposure with the same covariates and performed a sensitivity analysis in the subset of infants <1000 g BW. We performed two further sensitivity analyses: first, we repeated the regression after extending the evaluable period for furosemide exposure to postnatal day 60; second, to evaluate the effect of fluid management on PDA treatment, we included the percent weight change in the first seven days of life, a surrogate marker of fluid balance used in prior studies, as a covariate in the model.11,12 We used standard tests and graphing techniques to evaluate all model assumptions, and conducted all analyses using Stata 14.1 (Stata Corps, College Station, TX) with a p<0.05 considered statistically significant. The study was approved by the Duke Institutional Review Board.
Results
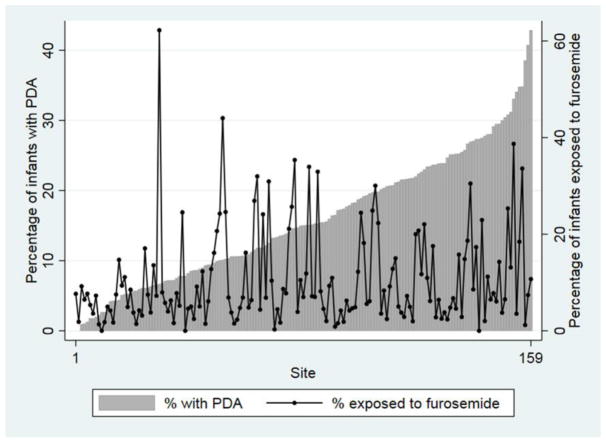
Our cohort included 43,576 infants; of these, 6675 (15%) underwent treatment for PDA and 4055 (9%) were exposed to furosemide. The median (25th–75th percentile) BW and GA of all infants were 1120 g (860, 1330) and 29 weeks (27, 31). The median postnatal age at PDA treatment was 4 days (3, 9). In unadjusted analyses, infants with PDA had a lower BW and GA compared with those not treated for a PDA, 820 g (660, 1030) vs. 1170 g (930, 1350), p<0.01; 26 weeks (24, 28) vs. 29 weeks (27, 31), p<0.01. The majority of infants with PDA treatment received mechanical ventilation, and more than one-third were exposed to inotropes (Table I). Both furosemide use and PDA treatment varied by site, without obvious clear correlation (Figure).
Table I.
Characteristics of infants with and without treated PDA
| PDA | No PDA | |
|---|---|---|
| N=6675 (%) | N=36,901 (%) | |
| Birth weight (g) | ||
| <501 | 310 (5) | 534 (1) |
| 501–750 | 2358 (35) | 4025 (11) |
| 751–1000 | 2201 (33) | 7141 (19) |
| 1001–1499 | 1806 (27) | 25,201 (68) |
| Gestational age (weeks) | ||
| 22–24 | 560 (8) | 758 (2) |
| 25–26 | 3385 (50) | 5984 (16) |
| 27–29 | 2307 (35) | 14,166 (38) |
| 30–32 | 393 (6) | 12,839 (35) |
| 33–36 | 30 (1) | 3154 (9) |
| APGAR score at 5 minutes | ||
| 0–3 | 506 (8) | 1286 (4) |
| 4–6 | 1544 (24) | 5131 (14) |
| 7–10 | 4475 (69) | 29,753 (82) |
| Male | 3480 (52) | 18,059 (49) |
| SGA | 1124 (17) | 9897 (27) |
| Antenatal steroids | 5656 (85) | 31,610 (86) |
| Mechanical ventilation | 5649 (85) | 16,765 (45) |
| Inotropes | 1911 (29) | 4289 (12) |
| Other diuretics | 214 (0.6) | 79 (1) |
APGAR, Activity/muscle tone, Pulse, Grimace, Appearance/color, Respirations; PDA, patent ductus arteriosus; SGA, small for gestational age
Figure 1. Furosemide use and PDA treatment.
Furosemide use and PDA treatment by Pediatrix site with >50 infant discharges.
PDA, patent ductus arteriosus
Infants exposed to furosemide had a lower BW and GA compared with those who did not receive furosemide, 830 g (660, 1070) vs. 1150 g (890, 1340), p<0.02; 26 weeks (25, 28) vs. 29 weeks (27, 31), p<0.02. Infants exposed to furosemide also had lower Apgar scores and were more likely to be small for gestational age, male, and exposed to mechanical ventilation, inotropes, and antenatal steroids (Table II). Median postnatal age at furosemide exposure was 17 days (9, 30) for infants treated for PDA, and 24 days (13, 40) for infants not treated for PDA. Compared with infants without PDA treatment, infants with PDA treatment were more likely to be exposed to furosemide, 956/6675 (14.3%) vs. 3099/36,901 (8.4%), p<0.01. Infants with PDA treatment also had more days exposed to furosemide prior to treatment than infants without PDA treatment, 2 days (1, 4) vs. 2 days (1, 3), p<0.01, and had a higher percentage of evaluable days exposed to furosemide, 13.3% (7.1, 23.5) vs. 10.5% (5.3, 15.8), p<0.01.
Table II.
Characteristics of infants with and without exposure to furosemide before PDA treatment or day 18
| Furosemide exposure | No furosemide exposure | |
|---|---|---|
| N=4055 (%) | N=39,521 (%) | |
| Birth weight (g) | ||
| <501 | 217 (5) | 627 (2) |
| 501–750 | 1357 (33) | 5026 (13) |
| 751–1000 | 1231 (30) | 8111 (21) |
| 1001–1499 | 1250 (31) | 25,757 (65) |
| Gestational age (weeks) | ||
| 22–24 | 313 (8) | 1005 (3) |
| 25–26 | 1869 (46) | 7500 (19) |
| 27–29 | 1332 (33) | 15,141 (38) |
| 30–32 | 461 (11) | 12,771 (32) |
| 33–36 | 80 (2) | 3104 (8) |
| APGAR score at 5 minutes | ||
| 0–3 | 364 (9) | 1428 (4) |
| 4–6 | 1010 (25) | 5665 (15) |
| 7–10 | 2604 (65) | 31,624 (82) |
| Male | 2211 (55) | 19,328 (49) |
| SGA | 934 (23) | 10,087 (26) |
| Antenatal steroids | 3381 (83) | 33,885 (86) |
| Mechanical ventilation | 3494 (86) | 18,920 (48) |
| Inotropes | 1539 (38) | 4661 (12) |
| Other diuretics | 217 (5) | 76 (0.2) |
APGAR, Activity/muscle tone, Pulse, Grimace, Appearance/color, Respirations; PDA, patent ductus arteriosus; SGA, small for gestational age
On multivariable analysis, exposure to furosemide was associated with decreased odds of PDA treatment (odds ratio [OR]=0.72; 95% confidence interval [CI] 0.65, 0.79). The association was more pronounced among infants <1000 g (OR=0.65; 95% CI 0.58, 0.73). This association was more pronounced when the evaluable period for furosemide exposure was extended up to 60 days of life (OR=0.24, 95% CI 0.22, 0.26). There was no association between odds of PDA treatment and each 10% increase in the percentage of evaluable days with exposure to furosemide (OR=1.01; 95% CI 0.97, 1.06), except in the subset of infants <1000 g where the odds of PDA treatment decreased with each 10% increase in the percentage of evaluable days exposed to furosemide (OR=0.93; 95% CI 0.89, 0.98) (Table III). In a sensitivity analysis including weight change in the first 7 days of life as a covariate in the model, furosemide exposure remained associated with decreased odds of PDA treatment (OR=0.65, 95% CI 0.58, 0.72).
Table III.
Adjusted* OR (95% CI) of treated PDA diagnosis
| All infants | Infants <1000 g BW | |
|---|---|---|
| Any furosemide exposure | 0.72 (0.65, 0.79) | 0.65 (0.58, 0.73) |
| 10% increase in evaluable days exposed to furosemide | 1.01 (0.97, 1.06) | 0.93 (0.89, 0.98) |
APGAR, Activity/muscle tone, Pulse, Grimace, Appearance/color, Respirations; BW, birth weight; CI, confidence interval; OR, odds ratio; PDA, patent ductus arteriosus
Variables adjusted for include: APGAR scores at 5 minutes, gestational age, sex, small for gestational age status, prenatal steroid exposure, exposure to other diuretics, exposure to inotropes, exposure to mechanical ventilation, discharge year, and random effects for site.
Discussion
After adjusting for clinically relevant covariates, any furosemide exposure was associated with decreased odds of receiving medical or surgical PDA therapy while the percentage of days with furosemide exposure was not associated with PDA treatment. In this study, we found that 15% of infants <37 weeks GA and <1500g BW received medical or surgical PDA therapy. Infants with PDA therapy were more likely to be exposed to furosemide and were more likely to be exposed for longer periods of time. This finding differs from a previous randomized controlled trial where premature infants with respiratory distress syndrome who received furosemide were more frequently diagnosed with a PDA compared with infants treated with chlorothiazide.7
Prenatally, patency of the ductus arteriosus is maintained by circulating prostaglandins, most potently PGE2, synthesized in utero by the ductal wall and placenta.7,13 Immature ductal tissue is most sensitive to PGE2-induced smooth muscle relaxation, and the sensitivity decreases as the fetus nears term.4 After birth, PGE2 synthesis is reduced by increased arterial oxygen tension and removal of the placental circulation, causing constriction of the ductus arteriosus.13 In addition to effects of PG, pressure changes in the pulmonary and systemic circulation mediate ductal closure. When the neonate inhales oxygen, the lung expands, which decreases pulmonary vascular resistance. Concomitantly, the low resistance placental circulation is removed from the systemic circulation, increasing systemic vascular resistance.14 This establishes a pressure gradient between the pulmonary and systemic circulation and reverses flow through the ductus. With flow reversal, there is increased turbulence of flow and shear-stress which releases vasoconstrictive factors, promoting closure.14 Furosemide may affect both of these ductal closure mechanisms in an opposing manner.
Furosemide is hypothesized to inhibit ductal closure through a complex prostaglandin-mediated process.4 The drug stimulates the release of renal PGE2 from the thick ascending limb of the loop of Henle into the systemic circulation in a dose-dependent manner. Circulating renal PGE2 mimics the effects of PGE2 synthesized by the ductal wall, and promotes ductal relaxation.7,15 In a randomized prospective trial comparing furosemide to chlorothiazide in premature infants, urinary excretion of PGE2 more than doubled in the first 5 days of life in infants randomized to receive furosemide, and no change was seen in infants randomized to chlorothiazide.7 The authors postulated that stimulation of renal PGE2 production explained the almost two-fold higher prevalence of a clinically diagnosed PDA in infants treated with furosemide compared with chlorothiazide, even though rates of surgical ligation did not differ between both groups. To our knowledge, no subsequent studies have confirmed the association between furosemide use and renal prostaglandin production, but multiple studies have since failed to demonstrate an increased association between furosemide exposure and the occurrence of PDA.8,9,16,17 These studies include a randomized controlled trial of preterm infants determined to be high-risk for PDA, which compared indomethacin for PDA closure followed by furosemide vs. indomethacin alone, and found no difference between groups in failure of PDA closure diagnosed by echocardiogram.8 In another randomized controlled trial of 57 premature infants ≤ 2000 g birth weight with respiratory distress syndrome receiving furosemide or placebo in the first 8 hours of life, the incidence of clinically diagnosed PDA did not differ between both groups.16 Results were similar in a randomized controlled trial of 39 neonates with respiratory distress syndrome receiving furosemide or placebo in the first 24 hours of life, with no significant differences observed in the diagnosis of PDA between both groups. Importantly in this study, PDAs were diagnosed by echocardiography using protocol-specific criteria and blinded investigators.17 None of these trials explored the potential mechanistic underpinnings of their observations, but multiple physiologic alterations associated with furosemide use may result in ductal closure.4,18,19
Furosemide is often given to preterm infants shortly after birth to promote diuresis and achieve a negative fluid balance.5,20 Through its diuretic effect, furosemide improves compliance of the premature neonatal lung, resulting in improved arterial oxygenation which may inhibit PGE2 production in ductal tissues.21 Furosemide may also aid in decreasing pulmonary vascular resistance by improving lung function and further increase the pressure gradient between the pulmonary and systemic circulation, promoting PDA closure.21 As a result of its influence on multiple mechanisms associated with ductal closure, the interaction between furosemide and ductal closure is complex. In our cohort, duration of exposure did not appear to affect overall odds of PDA therapy based on length of exposure, although any exposure favored closure in the VLBW cohort. This may be due to timing of exposure or a dose-dependence, with more limited furosemide exposure only affecting pulmonary hemodynamics, although longer exposure leads to changes promoting ductal patency, such as increasing levels of prostaglandins. However, in our extremely low birth weight cohort, any exposure and length of exposure appeared to favor ductal closure. A larger sample size and more precise information on furosemide indication, drug efficacy in individual infants, and other clinical variables associated with PDA closure would likely be needed to further explain this discrepancy.
The strengths of our study include its large sample size and contemporary diverse study population from academic and community NICUs across North America. This study is limited by the retrospective nature of the data. In addition, we cannot comment on causality due to the absence of information about PGE2 levels (not routinely measured in clinical practice), indication for furosemide usage, or changes in levels of pulmonary edema or fluid balance (not available in the data source). However, in the sensitivity analysis, we attempted to account for fluid balance as percent of weight gain which does show an association with PDA. We defined PDA based on the need for medical or surgical intervention due to the lack of other diagnostic data such as ductal or cardiac chamber size by echocardiography, plasma brain natriuretic peptide levels, or illness severity as caused by the PDA.2,22,23 Consequently, the day of PDA treatment may not be the day of PDA diagnosis. Our definition of PDA may underestimate the true prevalence of a hemodynamically significant PDA, and may have caused some bias by which infants receiving furosemide may have been perceived as failing conservative management, leading to higher rates of medical or surgical closure attempts. This is further complicated by the lack of consensus on the benefit of PDA treatment, most recently noted by the Etude Epidémiologique sur les Petits Ages Gestationnels 2 (EPIPAGE 2) study among others, which may bias how clinicians ultimately manage infants with PDA. This is especially true when the potential benefits are being weighed against the potential side effects of furosemide in infants, including ototoxicity, nephrocalcinosis, and electrolyte imbalances.24 This assumption appears supported by the significant morbidity of infants with PDA in our cohort, including frequent exposure to mechanical ventilation and inotropes, and a high rate of BPD. Nonetheless, this bias should have resulted in an increased risk of PDA in the furosemide group, but we found the opposite result. Despite the retrospective nature of this study, we were able to adjust our analysis for multiple important covariates. To minimize bias associated with different evaluation periods, infants without PDAs were only evaluated for furosemide use up to day 18. This definition may cause us to underestimate the true prevalence of furosemide exposure in infants without PDA, which may occur at a later age. Our conclusions are limited to hospitalized VLBW infants, a group we chose because it represents infants with the highest PDA prevalence, as well as the highest risk of associated morbidity and mortality. Currently, there are no usage guidelines for furosemide or other PDA therapies, which could introduce significant variability in management, although we attempted to mitigate this by controlling for site practices.
In conclusion, our study did not find increased odds of receiving medical or surgical PDA therapy in infants exposed to furosemide. Despite its limitations, this finding is important to clinicians, given that an adequately powered prospective randomized placebo-controlled trial of furosemide in this population would be challenging to conduct. Even when interpreted cautiously, our study results support the lack of association between furosemide exposure and PDA observed in prior trials. A comprehensive, ideally prospective evaluation of the efficacy and safety of furosemide in premature infants, including its effects on multiple clinical outcomes, is warranted.
Acknowledgments
We acknowledge the Duke Pediatrics Resident Research Grant and the Duke Pediatric Research Scholars Program for.
CP Hornik: Dr. Hornik receives salary support for research from National Institute for Child Health and Human Development (NICHD; 1K23HD090239 and HHSN275201000003I PI: Benjamin)
E.T. received funding from the Duke Pediatric Resident Research Grant. R.G. receives salary support for research from the National Institutes of Health training (5T32HD043728–10 and 5T32HD043029–13). M.L. is supported by the U.S. government for his work in pediatric and neonatal clinical pharmacology (HHSN267200700051C [Principal Investigator: Benjamin]), the National Institute for Child Health and Human Development (NICHD) (K23 HD068497), and the National Heart, Lung, and Blood Institute (R34 HL124038). P.S. receives salary support for research from the NIH (NIH-1R21HD080606–01A1) and the NICHD (HHSN275201000003I). The other authors declare no conflicts of interest.
Abbreviations
- APGAR
Activity/muscle tone, Pulse, Grimace, Appearance/color, Respirations
- BPD
bronchopulmonary dysplasia
- BW
birth weight
- CI
confidence interval
- GA
gestational age
- NICU
neonatal intensive care units
- OR
odds ratio
- PDA
patent ductus arteriosus
- PGE2
prostaglandin E2
- VLBW
very low birth weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates 1993–2012. JAMA. 2015;314:1039–51. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNamara PJ, Sehgal A. Towards rational management of the patent ductus arteriosus; the need for disease staging. Arch Dis Child Fetal Neonatal Ed. 2007;92:F424–7. doi: 10.1136/adc.2007.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermes-DeSantis ER, Clyman R. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006;26:S14–8. doi: 10.1038/sj.jp.7211465. discussion S22–3. [DOI] [PubMed] [Google Scholar]
- 4.Dice JE, Bhatia J. Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther. 2007;12:138–46. doi: 10.5863/1551-6776-12.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughon MM, Chantala K, Aliaga A, Herrin AH, Hornik CP, Hughes R, et al. Diuretic exposure in premature infants from 1997 to 2011. Am J Perinatol. 2015;32:49–56. doi: 10.1055/s-0034-1373845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Smith P. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31:811–22. doi: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green TP, Thompson TR, Johnson DE, Lock J. Furosemide promotes patent ductus arteriosus in premature infants with the respiratory distress syndrome. N Engl J Med. 1983;308:743–8. doi: 10.1056/NEJM198303313081303. [DOI] [PubMed] [Google Scholar]
- 8.Lee BS, Byun SY, Chung ML, Chang JY, Kim HY, Kim EA, et al. Effect of furosemide on ductal closure and renal function in indomethacin-treated preterm infants during the early neonatal period. Neonatology. 2010;98:191–9. doi: 10.1159/000289206. [DOI] [PubMed] [Google Scholar]
- 9.Brion LP, Campbell D. Furosemide in indomethacin-treated infants - systematic review and meta-analysis. Pediatr Nephrol. 1998;13:212–8. doi: 10.1007/s004670050595. [DOI] [PubMed] [Google Scholar]
- 10.Spitzer AR, Ellsbury DL, Handler D, Clark R. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system - tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Pandey V, Kumar D, Vijayaraghavan P, Chaturvedi T, Raina R. Non-dialytic management of acute kidney injury in newborns. J Renal Inj Prev. 2017;6:1–11. doi: 10.15171/jrip.2017.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Askenazi D, Saeidi B, Koralkar R, Ambalavanan N, Griffin RL. Acute Changes in fluid status affect the incidence, associative clinical outcomes, and urine biomarker performance in premature infants with acute kidney injury. Pediatr Nephrol. 2016;31:843–51. doi: 10.1007/s00467-015-3258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olley PM, Coceani F. Prostaglandin and the ductus arteriosus. Ann Rev Med. 1981;32:375–85. doi: 10.1146/annurev.me.32.020181.002111. [DOI] [PubMed] [Google Scholar]
- 14.Crossley KJ, Allison BJ, Polglase GR, Morley CJ, Davis PG, Hooper S. Dynamic changes in the direction of blood flow through the ductus arteriosus at birth. J Physiol. 2009;587:4695–703. doi: 10.1113/jphysiol.2009.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyanoshita A, Terada M, Endou H. Furosemide directly stimulates prostaglandin E2 production in the thick ascending limb of Henle’s loop. J Pharmacol Exp Ther. 1989;251:1155–9. [PubMed] [Google Scholar]
- 16.Yeh TF, Shibli A, Leu ST, Raval D, Pildes R. Early furosemide therapy in premature infants (less than or equal to 2000 gm) with respiratory distress syndrome: a randomized controlled trial. J Pediatr. 1984;105:603–9. doi: 10.1016/s0022-3476(84)80431-x. [DOI] [PubMed] [Google Scholar]
- 17.Belik J, Spitzer AR, Clark BJ, Gewitz MH, Fox W. Effect of early furosemide administration in neonates with respiratory distress syndrome. Pediatr Pulmonol. 1987;3:219–25. doi: 10.1002/ppul.1950030405. [DOI] [PubMed] [Google Scholar]
- 18.Vanhaesebrouch S, Zonnenberg I, Vandervoort P, Bruneel E, Van Hoestenberghe M, Theyskens C. Conservative treatment for patent ductus arteriosus in the preterm. Arch Dis Child Fetal Neonatal Ed. 2007;92:F244–7. doi: 10.1136/adc.2006.104596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laughon MM, Simmons MA, Bose C. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated? Curr Opin Pediatr. 2004;16:146–51. doi: 10.1097/00008480-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Segar J. Neonatal diuretic therapy: furosemide, thiazides, and spironolactone. Clin Perinatol. 2012;39:209–20. doi: 10.1016/j.clp.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Pacifici G. Clinical pharmacology of furosemide in neonates: a review. Pharmaceuticals (Basel) 2013;6:1094–129. doi: 10.3390/ph6091094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arlettaz R. Echocardiographic evaluation of patent ductus arteriosus in preterm infants. Front Pediatr. 2017;5:147. doi: 10.3389/fped.2017.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni M, Gokulakrishnan G, Price J, Fernandes CJ, Leeflang M, Pammi M. Diagnosing significant PDA using natriuretic peptides in preterm neonates: a systemic review. Pediatrics. 2015;135:e510–25. doi: 10.1542/peds.2014-1995. [DOI] [PubMed] [Google Scholar]
- 24.Roze JC, Cambonie G, Marchand-Martin L, Gournay V, Durrmeyer X, Durox M, et al. Association Between Early Screening for Patent Ductus Arteriosus and In-Hospital Mortality Among Extremely Preterm Infants. JAMA. 2015;313:2441–8. doi: 10.1001/jama.2015.6734. [DOI] [PubMed] [Google Scholar]