Abstract
Introduction
Although bariatric surgery seems to increase spontaneous fertility by improving ovulatory function in young women, its impact on ovarian reserve remains largely unknown.
Objective
To evaluate changes in serum anti-Mullerian hormone (AMH) levels in reproductive-age severely obese women after bariatric surgery (BS).
Methods
AMH levels were measured retrospectively in 39 women (mean age 34.6 ± 1.1 years, range 18–45) that underwent a sleeve gastrectomy or Roux-en-Y gastric bypass (RYGB) at baseline, and 6 and 12 months after BS. Metabolic and micronutrient status, including fasting plasma insulin and glucose, HOMA-IR, leptin, adiponectin, calcium, albumin, transthyretin, ferritin, vitamins (B9, B12, B1, A, E, D), zinc, and selenium, were assessed in all patients before and 1 year after BS.
Results
Of the patients, 79% had class-3 obesity. At 6 and 12 months, mean total weight losses (TWL) were 26 and 30%; mean excess weight losses (EWL) were 61.7 and 70.2%. Compared to baseline, AMH levels significantly decreased by 18% at 6 months, and 32% at 12 months post-operatively (p = 0.010 and p = 0.001, respectively). There was no correlation between AMH variation and changes in metabolic parameters or micronutrient levels. Remarkably, changes in AMH levels did not differ between sleeve and RYGB patients and were not correlated with EWL.
Conclusion
This pilot study shows a drastic reduction in AMH levels at 1 year after BS in reproductive-age severely obese women, which was not related to weight loss: this suggests a negative impact of BS on ovarian reserve, at least in the short term.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1196-3) contains supplementary material, which is available to authorized users.
Keywords: Anti-Mullerian hormone, Bariatric surgery, Obesity, Fertility, Ovarian reserve
Introduction
The prevalence of obesity has increased over the last two decades and is a worldwide health problem [1]. It is well known that obesity is a major prognostic factor for fertility. It is mainly caused by ovulation dysfunction. The impact of obesity on ovarian reserve and notably on AMH levels is still debated and contradictory data have been reported in the scientific literature. In some studies, AMH was negatively related to BMI, but age was probably a confounding factor [2, 3]. Obesity is associated with a higher risk of obstetrical complications such as gestational diabetes, hypertension, preeclampsia, and preterm birth [4]. Obesity-associated gonadal dysfunction has been recently found to be more prevalent than thought in severely obese patients (36% in women, 64% in men) that have undergone bariatric surgery (BS) [5, 6]. BS is the most efficient therapy for severe obesity, which has beneficial effects on metabolic parameters and obstetrical as well as perinatal complications [7]. It benefits fertility [8]; however, research on ovarian reserve is scant. Previous studies report decreases in AMH concentrations at 3 and 6 months post-BS [9, 10]. Yet, data are lacking on whether this variation is caused by a transitional phase of malnutrition and metabolic change or whether there is a real impact of BS on ovarian reserve. Although BS does appear to increase spontaneous fertility in young women by improving ovulatory function [5, 11], its impact on assisted reproduction and ovarian reserve should be evaluated.
Anti-Mullerian hormone (AMH) is a glycoprotein produced by the granulosa cells from primary to pre-antral follicles, with very low expression in larger antral follicles. AMH appears to reflect the primordial follicle reserve [12]. It is a poor marker of spontaneous pregnancy in patients without a history of infertility [13], but currently remains the best surrogate marker for estimating ovarian reserve. Serum AMH levels differ only subtly throughout the menstrual cycle, which is a major advantage over other markers of fertility [14]. Therefore, AMH level is used worldwide to evaluate ovarian reserve in reproductive medicine and to predict ovarian response to stimulation in in vitro fecundation [12].
Our objective was to analyze variations in AMH levels at 6 months and 1 year post-BS, and, secondly, to evaluate its relationship to metabolic parameters and nutritional status.
Materials and methods
Patients
We conducted a retrospective study using the medical records of women of reproductive age (18–45 years) that had undergone BS and had been previously included in other studies in the endocrinology, metabolic diseases and nutrition departments of the university hospitals of La Timone and North in Marseille (France) between 2008 and 2013. All women were eligible for BS by sleeve gastrectomy or a Roux-en-Y gastric bypass (RYGB) [15] (i.e., body-mass index (BMI) was ≥ 40 or ≥ 35 kg/m2 with at least one obesity-related co-morbidity) and previous failed adequate conservative treatment during at least 6 to 12 months. We retrospectively studied 39 patients. Non-inclusion criteria included women with ovarian surgery or patients undergoing menopause.
Data retrieved from files allowed us to define patients with polycystic ovarian syndrome (PCOS), based on Rotterdam criteria [16].
Study design and follow-up
Patients underwent at least three medical consultations: before and at 6 and 12 months after surgery. During the medical consultations, anthropometric parameters, such as weight, BMI, excess weight, fat mass (using dual energy X-ray absorptiometry (GE Lunar)), and comorbidities, were recorded. Percentages of total and excess weight loss (%TWL and %EWL, respectively) were calculated as follows: %EWL = (weight loss/excess weight) × 100 with excess weight defined as: actual weight − calculated weight to attain a BMI of 25 kg/m2. During each visit, complete laboratory tests after an overnight fast were performed.
Comorbidities, ethnicity, and hormonal contraceptive use were noted at baseline or retrieved in medical records. Diabetes, dyslipidemia, and arterial hypertension were diagnosed according to current international guidelines [16, 17]. The evolution of comorbidities was recorded post-bariatric surgery every 3 months. According to ADA guidelines, complete diabetes remission was defined as fasting blood glucose < 5.6 mmol/L and HbA1c < 6% without treatment 1 year after BS [18] .
Blood samples were analyzed for serum-AMH level, blood count, fasting plasma glucose, insulin, leptin, adiponectin, serum-albumin, transthyretin, serum-ferritin, vitamin B9, vitamin B12, vitamin B1, vitamin A, vitamin E, vitamin D, zinc, and selenium.
HOMA-IR was calculated as follows = insulin (μUI/L) × fasting glucose (nmol/L)/22.5. Nutritional deficiencies, when detected, were treated with appropriate supplementation. All patients, whatever the bariatric procedure, received a multivitamin supplementation over a 1-year period after BS. As recommended for patients submitted to RYGB, we prescribed long-term iron, calcium, vitamin D, and vitamin B12 supplements, along with multivitamins.
Biochemical analyses
AMH assessment was performed by electrochemiluminescence (Cobas Roche). The repeatability coefficients of variation for the assay were 2.70, 0.89, and 0.76% for 0.10, 20, and 90 pmol/L, respectively. Adiponectin (Quantikine ELISA, R&D Systems) and leptin (Human Leptin ELISA, BioVendor) were assessed using an Elisa kit. Albumin was measured with a Green Bromocresol technique (Cobas 6000 Roche). Transthyretin was assessed by immunoturbidimetry (BN Prospec Siemens). Ferritin, 25-(OH)-vitamin-D3, and vitamins B9 and B12 were measured by chemiluminescent assays (Advia Centaur Siemens and Cobas 6000 Roche, respectively). Vitamins B1, B6, A, and E were assessed by high-performance liquid chromatography. Zinc and selenium were measured by atomic-absorption spectrophotometry. Biological nutritional deficiency was defined as a value below the lower threshold identified by the manufacturer of the corresponding assay. Vitamin D deficiency was defined as a serum concentration < 75 nmol/L.
Statistical analyses
All data were analyzed using GraphPad Prism 6 for Windows. Descriptive statistics were expressed as means and standard errors of the mean (SEM) for continuous variables, and as percentages for categorical variables. Correlations were analyzed using Pearson’s test or Spearman’s rho correlation when at least one of the variables did not follow a Gaussian distribution. A Student’s t test was used to compare continuous variables at different time points when data followed a Gaussian distribution, and a paired Wilcoxon test was used when the distribution was non-Gaussian. To test the effects of different variables independently of a covariate, multiple regression modeling was conducted using Statview Version 5.0. A p value of < 0.05 was considered statistically significant.
Results
Patients’ characteristics at baseline
We retrospectively studied 39 severely obese women of reproductive age (see supplementary fig. 1 for age distribution). The majority of women included were Caucasian (85%). Among these, 34 had complete follow-up data at 6 months, and 28 women had data at 12 months after BS. The patients’ characteristics are summarized in Table 1. The mean age of the study population was 34 ± 1.1 years and 79% of patients had class-3 obesity, of which 18% had a BMI > 50 kg/m2 (n = 7). Twenty-three patients underwent sleeve gastrectomy and 16 patients underwent a RYGB. One third of patients were nulliparous. Only 6 of 39 patients had PCOS. Twelve patients were active smoker. Twenty-nine patients had hormonal contraception (9 with hormonal intra-uterin devices, 16 with oral progestin contraception, and 4 with estroprogestin contraception), seven patients had no contraception and used barrier method, two patients had a copper intra-uterine system, and one patient underwent a tubal ligation. Five patients were diagnosed with type 2 diabetes pre-operatively and all of them were treated with metformin before BS, 2 were treated with bi or tri-oral antidiabetic therapy and 2 with insulin. Four patients had a history of infertility with assisted reproductive technology (ART) attempts: two for endometriosis, one for impaired ovarian reserve, and one for a male infertility.
Table 1.
Baseline characteristics of the study population (n = 39)
| Age (years) | 34.6 ± 1.1 |
| Weight (kg) | 122.5 ± 2.6 |
| BMI (kg/m2) | 45.4 ± 1.0 |
| Obesity class (n (%)) | |
| Class 2 | 8 (20%) |
| Class 3 | 31 (80%) |
| BMI > 50 kg/m2 | 7 (18%) |
| Comorbidities and risk factors (n (%)) | |
| Type-2 diabetes | 5 (13%) |
| Arterial hypertension | 3 (8%) |
| Dyslipidemia | 5 (13%) |
| Active smoker | 12 (31%) |
| Ethnicity (n (%)) | |
| Caucasian | 33 (85%) |
| Others | 6 (15%) |
| Parity (n (%)) | |
| 0 | 13 (33%) |
| 1–2 | 20 (51%) |
| > 2 | 5 (12%) |
| Undetermined | 1 (3%) |
| Polycystic ovary syndrome (PCOS) (n (%)) | 6 (15%) |
| Use of contraception and type (n (%)) | |
| No contraception | 7 (18%) |
| Hormonal contraception | 29 (74%) |
| Copper IUD | 2 (5%) |
| Tubal ligation | 1 (3%) |
| Type of surgery (n (%)) | |
| Sleeve gastrectomy | 23 (59%) |
| Roux-en-Y gastric bypass | 16 (41%) |
Mean ± SEM, BMI body-mass index, IUD intra-uterine device
At baseline, mean AMH was 13.45 ± 1.90 pmol/L for all patients: 9.88 ± 1.47 pmol/L in the non-PCOS group, and 33.04 ± 3.43 pmol/L in the PCOS group.
Age was negatively correlated with AMH level at baseline (p < 0.001). In contrast, no relationship was found between AMH at baseline and BMI. We found a positive correlation between HOMA-IR and initial AMH levels (r = 0.37, p = 0.024), but this correlation no longer existed after exclusion of PCOS patients. No correlation was found between AMH concentration and the number of nutritional deficiencies, or with each nutritional parameter, or with leptin or adiponectin concentrations.
At baseline, we found no difference in AMH levels between Caucasian women and other ethnic groups (13.6 mmol/L vs 12.6 mmol/L, respectively, p = 0.98).
At baseline, mean AMH concentration did not differ significantly between smokers and non-smokers (15.2 mmol/L vs 13.1 mmol/L, p = 0.72), suggesting that tobacco was not a confounding factor.
Evolution after BS (Table 2)
Table 2.
Anthropometric and biologic parameters at baseline, and at 6 and 12 months post-bariatric surgery
| M0 (n = 39) | M6 (n = 34) | M12 (n = 28) | |
|---|---|---|---|
| Body-mass index (kg/m2) | 45.4 ± 1.0 | 33.6 ± 0.8†† | 31.4 ± 0.9## |
| Excess weight loss (%) | 61.7 ± 3.0 | 70.2 ± 5.0 | |
| Fat-mass DEXA (%) | 53.5 ± 0.6 | 44.9 ± 1.2†† | 41.0 ± 1.5## |
| Nutritional parameters | |||
| Albumin (g/L) (n > 35 g/L) |
37.5 ± 0.5 | 37.4 ± 0.6 | 37.0 ± 0.3 |
| Transthyretin (g/L) (n > 0.21 g/L) |
0.241 ± 0.009 | 0.198 ± 0.007†† | 0.222 ± 0.008 |
| Vitamin D (nmol/L) (n > 75.0 nmol/L) |
49.2 ± 3.5 | 63.7 ± 4.8† | 64.7 ± 6.5# |
| Vitamin B9 (μg/L) (n > 4.6 μg/L) |
6.2 ± 0.5 | 9.9 ± 1.1† | 10.4 ± 1.2# |
| Vitamin B12 (ng/L) (n > 191 ng/L) |
428.0 ± 25.3 | 359.4 ± 26.4† | 351.7 ± 24.2# |
| Ferritin (μg/L) (n > 10 μg/L) |
58.3 ± 7.5 | 84.3 ± 13.2 | 44.2 ± 9.4# |
| Vitamin A (mg/L) (n > 0.41 mg/L) |
0.61 ± 0.03 | 0.49 ± 0.02†† | 0.47 ± 0.02# |
| Vitamin E (mg/L) (n > 8 mg/L) |
10.7 ± 0.3 | 11.1 ± 0.4 | 12.9 ± 2.7 |
| Selenium (μg/L) (n > 60 μg/L) |
61.9 ± 3.4 | 47.0 ± 2.8†† | 51.9 ± 1.8# |
| Zinc (μg/dL) (n > 60 μg/dL) |
72.6 ± 2.6 | 69.2 ± 1.8 | 71.9 ± 1.7 |
| Vitamin B1 (nmol/L) (n > 67 nmol/L) |
143.0 ± 8.9 | 124.9 ± 8.7 | 146.0 ± 6.0 |
| Nutritional deficiencies (n (%)) | |||
| 0–1 | 23 (58) | 7 (20) | 8 (28) |
| 2–3 | 12 (30) | 17 (50) | 15 (53) |
| ≥ 4 | 4 (12) | 10 (30) | 5 (17) |
| Metabolic parameters | |||
| HOMA-IR | 5.3 ± 0.7 | 1.5 ± 0.2†† | 1.5 ± 0.1## |
| Leptin (ng/mL) | 112.6 ± 7.2 | 47.0 ± 4.8†† | 44.3 ± 5.4## |
| Adiponectin (μg/mL) | 5.2 ± 0.4 | 8.6 ± 1.0†† | 9.1 ± 0.8## |
| AMH (pmol/L) | 13.4 ± 1.9 | 11.2 ± 2.3† | 10.5 ± 2.4# |
DEXA dual energy X-ray absorptiometry
††p = <0.001 M0–M6 comparison
†p < 0.05 M0–M6 comparison
##p < 0.001 M0–M12 comparison
#p < 0.05 M0–M12 comparison
At 6 and 12 months, mean %TWL were 26% (33.3 ± 1.3 kg) and 30% (37.1 ± 2.5 kg), respectively, while mean %EWL were 61.7 and 70.2%, respectively. At 12 months, anti-hypertensive treatments were stopped for all patients. Similarly, 2 of 5 patients with dyslipidemia had no more lipid lowering therapy. Five patients were diagnosed type 2 diabetes pre-operatively. At 12 months, two had complete remission of their diabetes, and 3 were still treated with metformin but glycemic control improved for all patients. There was no early or late surgical complication for all the women included in the study.
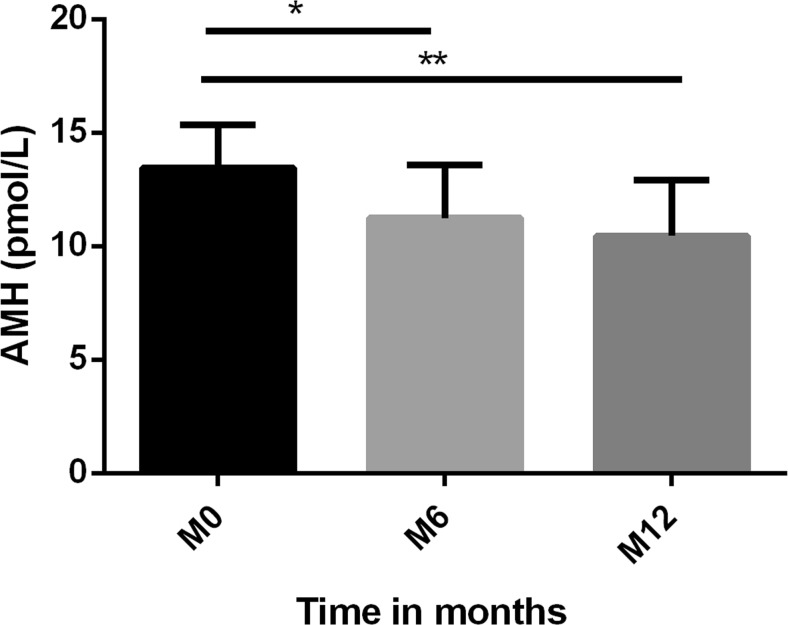
Mean AMH concentration decreased significantly by 18% at 6 months, and by 35% at 12 months compared to concentrations before surgery (p = 0.010 and p = 0.001, respectively) (Fig. 1).
Fig. 1.
Mean concentrations of anti-Mullerian hormone (AMH) (pmol/L) at month M0, M6, and M12. * p < 0.05 compared to baseline, ** p < 0.01 compared to baseline
Even after excluding the six patients with PCOS, there was a significant drop in AMH concentrations (22.1% at 6 months and 38.3% at 12 months, p = 0.002 and p = 0.002, respectively).
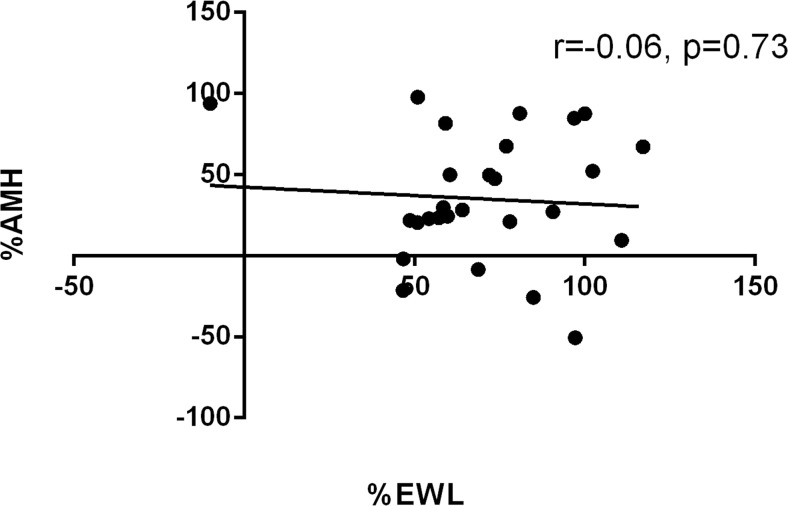
A change in AMH was not correlated with %EWL (Fig. 2). Likewise, AMH variation did not differ between the two types of bariatric surgery (i.e., − 33% and − 36% at 12 months post-RYGP and sleeve gastrectomy, respectively). The variation in AMH concentration was not associated with changes in nutritional parameters. Moreover, there was no difference in AMH change between patients with 0–1 or 2–3 nutritional deficiencies (p = 0.179 at 6 months, and p = 0.497 at 12 months), or even those with more than 4 deficiencies (p = 0.578 at 6 and p = 0.822 at 12 months). Similarly, there was no difference between patients in the lowest tertile of albumin and those with mild (p = 0.785 at 6 months, and p = 0.173 at 12 months) or within the highest tertile (p = 0.113 at 6 months, and p = 0.766 at 12 months).
Fig. 2.
Variations between M0 and M12 for percentages of excess weight loss (EWL) and levels of anti-Mullerian hormone (AMH). X axis represents percentage of EWL; Y axis represent percentage of variation in AMH
We did not find a correlation between fat-mass loss and AMH decrease at 6 or 12 months (p = 0.293 and p = 0.420, respectively). No correlation was found between leptin, adiponectin, and AMH variations throughout the study period.
We did not find any correlation between age and AMH decrease at 6 and 12 months after BS (p = 0.09 and p = 0.115 respectively).
Discussion
In this preliminary study, where we investigated a marker for ovarian reserve in young obese women post-bariatric surgery, we found a significant decrease in AMH levels at 6 and 12 months post-surgery. These results suggest possible impairment of the ovarian reserve in young, non-PCOS, obese women, and highlight the need for long-term studies to determine the pattern of AMH changes over time with weight stabilization. Indeed, BS induces intense caloric restriction with drastic weight and fat-mass loss. This hyper-catabolic state is associated with a significant decrease in insulin resistance, liver fat, inflammatory mediators (IL-6, TNF-α), and androgen excess [19–21].
Many studies have shown that weight loss at post-BS may correct ovulatory dysfunction [5, 22], as a weight loss of > 5 BMI points was associated with an OR of 20.2 for successful pregnancy [23]. Moreover, a meta-analysis showed resolution of hirsutism in 53%, menstrual dysfunction in 96%, and PCOS in 96% of women that had BS [5]. So, AMH decrease in patients with PCOS is possibly a sign of clinical improvement [24]. A recent study by Milone et al. suggested improved assisted reproductive technology (ART) outcomes after BS in terms of oocyte number and quality and found no significant post-operative variations in AMH levels [25]. However, inversely, some studies have reported decreased AMH concentrations after bariatric surgery. Indeed, Mehri et al. reported a 24% decrease in AMH concentration in 16 women aged < 35 years at 3 months post-BS [9]. Similarly, Bhandari et al. reported a 36% decrease in AMH concentration in women without PCOS at 6 months post-BS [10]. In a recent study including 18 patients without PCOS and that underwent BS, Chiofalo et al. reported significant variation in AMH at 1 year post-surgery [26]. In accordance with these studies, we found a 38% decrease in AMH concentration at 1 year post-BS in non-PCOS patients.
How BS can impact on gonadal function and AMH levels has not yet been fully elucidated. A hypothesis is that reproductive function is tightly regulated by nutritional status [6, 27]. Some studies suggest significant associations between nutritional factors and ovarian reserve or timing of menopause [28], but these studies often use other markers for ovarian reserve, like the concentration of follicle-stimulating hormone. Only an association between AMH and serum vitamin D has been investigated [29–31]: the first review reported a positive correlation between concentrations of serum vitamin D and AMH, whereas other studies found no link between vitamin D and AMH. In accordance with these results, we found no correlation in our study between these parameters. Similarly, we did not find any correlation between changes in BMI or EWL and changes in AMH, despite an average decrease of 14 points in BMI at 12 months [10, 26].
We also looked for how much AMH changed between patients that had fewer or more nutritional deficiencies or patients that had low or high albumin concentration: we found no differences. This could suggest that a decreased AMH level is not related to nutritional deficiency or to severe malnutrition, and that other mechanisms apart from caloric restriction are implicated. However, this result should be interpreted with caution as our patients received systematic supplementation and their nutritional status was closely monitored.
As metabolic disorders, such as insulin resistance and the influence of leptin, affect female reproduction [32], one could argue that rapid improvement in adipose-tissue dysfunction and adipokines excess could impact on gonadal function. Indeed, obese women have higher circulating levels of leptin, which could lead to chronic downregulation of the leptin receptor in the hypothalamus, and thus disrupt the hypothalamic-pituitary-ovarian axes [33]. Besides, leptin expression has been observed in human granulosa cells [32]. Higher leptin-serum levels in obese women correlate with higher levels of leptin in follicular fluid. In vitro studies have confirmed that leptin affects steroidogenic pathways in granulosa cells, decreasing estrogen and progesterone production [6, 32]. Moreover, when studying a granulosa-cell model exposed to leptin, Merhi et al. showed a decrease in AMH and AMH-receptor-II mRNA expression, whereas adiponectin treatment had no effect [34]. However, in accordance with the literature, we found no correlation between serum-leptin and serum-AMH levels [35]. Thus, we studied the impact of insulin-resistance parameters, such as HOMA-IR, adiponectin, and changes in leptin levels on changes in AMH, and found no correlations. Further large studies are nevertheless needed to confirm these findings.
One other supposition is that lipo-soluble toxic and endocrine disruptors present in adipose tissue could be released during rapid weight loss and impact on AMH secretion by granulosa cells [36, 37]. Indeed, several studies have identified a significant serum increase in persistent organic pollutants (POPs) including biphenyls polychlorinated (PCBs) at 6 and 12 months post-operatively [36, 38]. In humans, POPs are stored primarily in adipose tissue but can be released in the circulation after drastic weight loss. Interestingly, POP content in adipose tissue and serum correlated with biological markers of obesity-related dysfunctions such as liver toxicity markers or lipid parameters independently of age and BMI. [37].
Similarly, obesity has a negative impact on spermatogenesis that is multifactorial. Indeed, an alteration of the hypothalamic-pituitary axis, a decrease in the secretion of testosterone, and hyperoestrogeny have been observed [39]. In addition, it has been recently reported that epigenetic modifications of spermatozoids (DNA methylation, histone localization, expression profile of noncoding RNA) may affect not only reproductive functions but also epigenetic profile (notably metabolic) of transmitted descendants [39]. Donkin et al. reported in their study changes in epigenetic profile of gametes after bariatric surgery with a remodeling of sperm DNA methylation [40]. However after bariatric surgery, the results of drastic weight loss on spermatogenesis are contradictory [41, 42]. It has been described in some cases an alteration of spermatic parameters. Studies to better understand the impact of bariatric surgery on spermatogenesis and investigations to identify the gametic epigenetic variation with weight loss are awaiting.
It is important to note that the decrease in AMH level is not synonymous with a reduction in fertility [13] or a decrease in ovarian reserve, but may be caused by damage to in-growth follicles exposed to post-operative stress. A longer follow-up is essential to conclude the impact of BS on ovarian reserve.
We chose to evaluate AMH levels because AMH is the first biological marker of the ovarian reserve to change with advanced age, demonstrating longitudinal decline over time compared with serum-FSH, inhibin B, or estradiol. Moreover, AMH secretion is correlated with antral follicle counts [12, 43]. Its variation throughout the menstrual cycle and inter-cycles is small; it thus represents a unique marker for ovarian reserve, regardless of menstrual timing [14].
Our study has several limitations, such as its retrospective design and the small population size. We assumed that PCOS patients were a specific population with a high risk of impairment to ovarian quality and function; recent studies have focused on this particular population [10, 26]. However, exclusion of PCOS patients did not significantly change our results. Similarly, comparisons with a control group would have been interesting in order to unmask the effect of time on ovarian reserve. However, the reported physiological decrease of AMH in women during 1 year is not as high as in year 1 post-BS [44, 45]. Moreover, the age distribution of our patients was wide (Supplementary fig. 1), but it is important to distinguish patients under 35 years old from older patients. Indeed, age remains the main prognostic factor of women fertility. Due to the decrease in ovarian reserve with age, in vitro fecundation (IVF) results fall after 35 years [46].
In conclusion, despite a growing body of evidence suggesting the efficacy of BS on female fertility by restoring spontaneous ovulation, its impact in pre-existing situations of decreased ovarian reserve remains controversial. This study points to a possible negative impact of BS on ovarian reserve. It would be interesting to conduct a prospective evaluation on fertility by studying AMH levels, the pituitary–gonadal axis, and antral follicular counts in older pre-menopausal obese women undergoing BS. Bariatric surgery is the most efficient therapy for weight loss but if it does have a deleterious impact on ovarian reserve, we need to propose a fertility-preservation program to pre-menopausal women before BS.
Electronic supplementary material
Age distribution in the study population (JPG 83 kb)
Compliance with ethical standards
Formal consent is not required for this type of retrospective study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1196-3) contains supplementary material, which is available to authorized users.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NME, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DFJ, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SEAH, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KMV, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJC, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang XR, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJL, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardi LA, Carnethon MR, de Chavez PJ, Ikhena DE, Neff LM, Baird DD, Marsh EE. Relationship between obesity and anti-Müllerian hormone in reproductive-aged African American women. Obes Silver Spring Md. 2017;25:229–235. doi: 10.1002/oby.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefebvre T, Dumont A, Pigny P, Dewailly D. Effect of obesity and its related metabolic factors on serum anti-Müllerian hormone concentrations in women with and without polycystic ovaries. Reprod BioMed Online. 2017;35:325–330. doi: 10.1016/j.rbmo.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of the American Society for Reproductive Medicine Obesity and reproduction: a committee opinion. Fertil Steril. 2015;104:1116–1126. doi: 10.1016/j.fertnstert.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update. 2017;23:390–408. doi: 10.1093/humupd/dmx012. [DOI] [PubMed] [Google Scholar]
- 6.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. 2017;107:840–847. doi: 10.1016/j.fertnstert.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Galazis N, Docheva N, Simillis C, Nicolaides KH. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;181:45–53. doi: 10.1016/j.ejogrb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Milone M, Placido GD, Musella M, Fernandez LMS, Fernandez LVS, Campana G, et al. Incidence of successful pregnancy after weight loss interventions in infertile women: a systematic review and meta-analysis of the literature. Obes Surg. 2015;26:443–451. doi: 10.1007/s11695-015-1998-7. [DOI] [PubMed] [Google Scholar]
- 9.Merhi ZO, Minkoff H, Feldman J, Macura J, Rodriguez C, Seifer DB. Relationship of bariatric surgery to Müllerian-inhibiting substance levels. Fertil Steril. 2008;90:221–224. doi: 10.1016/j.fertnstert.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 10.Bhandari S, Ganguly I, Bhandari M, Agarwal P, Singh A, Gupta N, et al. Effect of sleeve gastrectomy bariatric surgery-induced weight loss on serum AMH levels in reproductive aged women. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol 2016;32:799-802. [DOI] [PubMed]
- 11.Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, Zeitlian G, Hickmon C, Feng S, Santoro N. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril. 2009;92:1410–1415. doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, la Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, Wallace WH, Anderson RA. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 13.Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, Baird DD. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318:1367–1376. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, Perkins NJ, Sjaarda L, Weck J, Mumford SL. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29:1764–1772. doi: 10.1093/humrep/deu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres A, Weiner R, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24:42–55. doi: 10.1007/s11695-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 16.WHO | Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia [Internet]. WHO. [cited 2017 Jul 25]. Available from: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/
- 17.de 1’Afssaps R. Prise en charge thérapeutique du patient dyslipidémique. 2005. 2017 [cited 2017 Jul 25]; Available from: http://www.jle.com/fr/revues/met/e-docs/prise_en_charge_therapeutique_du_patient_dyslipidemique._mars_2005__266098/article.phtml
- 18.Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–2135. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.João Cabrera E, Valezi AC, Delfino VDA, Lavado EL, Barbosa DS. Reduction in plasma levels of inflammatory and oxidative stress indicators after Roux-en-Y gastric bypass. Obes Surg. 2010;20:42–49. doi: 10.1007/s11695-009-9988-2. [DOI] [PubMed] [Google Scholar]
- 20.Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, Baldi S, Nannipieri M, Rebelos E, Anselmino M, Muscelli E, Ferrannini E. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093–2102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 21.Mingrone G, Cummings DE. Changes of insulin sensitivity and secretion after bariatric/metabolic surgery. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2016;12:1199–1205. doi: 10.1016/j.soard.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Legro RS. Effects of obesity treatment on female reproduction: results do not match expectations. Fertil Steril. 2017;107:860–867. doi: 10.1016/j.fertnstert.2017.02.109. [DOI] [PubMed] [Google Scholar]
- 23.Musella M, Milone M, Bellini M, Sosa Fernandez LM, Leongito M, Milone F. Effect of bariatric surgery on obesity-related infertility. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2012;8:445–449. doi: 10.1016/j.soard.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Reinehr T, Kulle A, Rothermel J, Knop C, Lass N, Bosse C, Holterhus PM. Weight loss in obese girls with polycystic ovarian syndrome is associated with a decrease in anti-Muellerian hormone concentrations. Clin Endocrinol. 2017;87:185–193. doi: 10.1111/cen.13358. [DOI] [PubMed] [Google Scholar]
- 25.Milone M, Sosa Fernandez LM, Sosa Fernandez LV, Manigrasso M, Elmore U, De Palma GD, et al. Does bariatric surgery improve assisted reproductive technology outcomes in obese infertile women? Obes Surg. 2017;27:2106–2112. doi: 10.1007/s11695-017-2614-9. [DOI] [PubMed] [Google Scholar]
- 26.Chiofalo F, Ciuoli C, Formichi C, Selmi F, Forleo R, Neri O, Vuolo G, Paffetti P, Pacini F. Bariatric surgery reduces serum anti-mullerian hormone levels in obese women with and without polycystic ovarian syndrome. Obes Surg. 2017;27:1750–1754. doi: 10.1007/s11695-016-2528-y. [DOI] [PubMed] [Google Scholar]
- 27.Cetin I, Berti C, Calabrese S. Role of micronutrients in the periconceptional period. Hum Reprod Update. 2010;16:80–95. doi: 10.1093/humupd/dmp025. [DOI] [PubMed] [Google Scholar]
- 28.Moslehi N, Mirmiran P, Tehrani FR, Azizi F. Current evidence on associations of nutritional factors with ovarian reserve and timing of menopause: a systematic review. Adv Nutr. 2017;8(4):597–612. doi: 10.3945/an.116.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102:460–468.e3. doi: 10.1016/j.fertnstert.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Chang EM, Kim YS, Won HJ, Yoon TK, Lee WS. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J Clin Endocrinol Metab. 2014;99:2526–2532. doi: 10.1210/jc.2013-3873. [DOI] [PubMed] [Google Scholar]
- 31.Pearce K, Gleeson K, Tremellen K. Serum anti-Mullerian hormone production is not correlated with seasonal fluctuations of vitamin D status in ovulatory or PCOS women. Hum Reprod. 2015;30:2171–2177. doi: 10.1093/humrep/dev167. [DOI] [PubMed] [Google Scholar]
- 32.Comninos AN, Jayasena CN, Dhillo WS. The relationship between gut and adipose hormones, and reproduction. Hum Reprod Update. 2014;20:153–174. doi: 10.1093/humupd/dmt033. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005;130:583–597. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- 34.Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S, Jindal S. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod Oxf Engl. 2013;28:1661–1669. doi: 10.1093/humrep/det072. [DOI] [PubMed] [Google Scholar]
- 35.Olszanecka-Glinianowicz M, Madej P, Owczarek A, Chudek J, Skałba P. Circulating anti-Müllerian hormone levels in relation to nutritional status and selected adipokines levels in polycystic ovary syndrome. Clin Endocrinol. 2015;83:98–104. doi: 10.1111/cen.12687. [DOI] [PubMed] [Google Scholar]
- 36.Dirinck E, Dirtu AC, Jorens PG, Malarvannan G, Covaci A, Gaal V, et al. Pivotal role for the visceral fat compartment in the release of persistent organic pollutants during weight loss. J Clin Endocrinol Metab. 2015;100:4463–4471. doi: 10.1210/jc.2015-2571. [DOI] [PubMed] [Google Scholar]
- 37.Kim M-J, Marchand P, Henegar C, Antignac J-P, Alili R, Poitou C, Bouillot JL, Basdevant A, le Bizec B, Barouki R, Clément K. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect. 2011;119:377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dirinck EL, Dirtu AC, Govindan M, Covaci A, Jorens PG, Gaal LFV. Endocrine-disrupting polychlorinated biphenyls in metabolically healthy and unhealthy obese subjects before and after weight loss: difference at the start but not at the finish. Am J Clin Nutr. 2016;103(4):989–998. doi: 10.3945/ajcn.115.119081. [DOI] [PubMed] [Google Scholar]
- 39.Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertil Steril. 2017;107:848–859. doi: 10.1016/j.fertnstert.2017.02.115. [DOI] [PubMed] [Google Scholar]
- 40.Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, Mortensen B, Appel EVR, Jørgensen N, Kristiansen VB, Hansen T, Workman CT, Zierath JR, Barrès R. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23:369–378. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Sermondade N, Massin N, Boitrelle F, Pfeffer J, Eustache F, Sifer C, Czernichow S, Lévy R. Sperm parameters and male fertility after bariatric surgery: three case series. Reprod BioMed Online. 2012;24:206–210. doi: 10.1016/j.rbmo.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Reis LO, Dias FGF. Male fertility, obesity, and bariatric surgery. Reprod Sci Thousand Oaks Calif. 2012;19:778–785. doi: 10.1177/1933719112440053. [DOI] [PubMed] [Google Scholar]
- 43.Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21:698–710. doi: 10.1093/humupd/dmu062. [DOI] [PubMed] [Google Scholar]
- 44.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seifer DB, Baker VL, Leader B. Age-specific serum anti-Müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–750. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Broekmans FJ, Soules MR, Ovarian Aging FBC. Mechanisms and clinical consequences. Endocr Rev. 2008;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Age distribution in the study population (JPG 83 kb)