Abstract
Background
Reactivation of hepatitis B virus (HBV) is a fatal complication of chemotherapy. Occult HBV infection might be reactivated in patients undergoing chemotherapy or immunosuppression. However, the mechanism of HBV reactivation induced by chemotherapy or immunosuppression remains unclear.
Material/Methods
HepG2.2.15 cells were treated with an autophagy inducer (rapamycin), an inhibitor (3-methyladenine, 3-MA), and dexamethasone. Autophagosomes were observed by a transmission electron microscope (TEM). LC3-I, LC3-II, and P62 were analyzed by western blot. HBV replicative intermediates were detected by southern blot. HBV DNA expression was quantitated with real-time polymerase chain reaction (PCR). The level of HBV surface antigen (HBsAg) in culture medium was examined by ELISA.
Results
In this study, we find that dexamethasone stimulates HBV replication and protein expression by inducing autophagy in HepG2.2.15 cells. In contrast, autophagy inhibitor (3-MA) abrogates HBsAg secretion stimulated by dexamethasone.
Conclusions
Our results suggest that dexamethasone stimulates HBV replication through autophagy. This might provide a novel insight into the mechanism of glucocorticoid-mediated HBV reactivation through autophagy, which might be a new therapeutic target.
MeSH Keywords: Autophagy, Dexamethasone, Hepatitis B virus
Background
Hepatitis B virus (HBV) infection remains a global health problem. Despite the availability of effective vaccines, almost 248 million individuals worldwide were estimated to be hepatitis B surface antigen (HBsAg) positive in 2010 [1]. Occult hepatitis B infection (OBI) is characterized by the presence of HBV DNA in the blood and/or liver of HBsAg-negative subjects [2]. Reactivation of HBV infection mostly occurs in immunosuppressed patients, in patients undergoing systemic chemotherapy for solid tumors, and in patients exposed to biological agents or high-dose corticosteroids. Nucleot(s)ide prophylaxis is highly recommended to prevent HBV reactivation in immunosuppressed settings [2,3]. Reactivation of HBV infection in HBsAg-positive or OBI patients is life-threatening [4]. It is very difficult to detect occult HBV infection in clinical work. As the sensitivity of HBV DNA detection assays is different in different regions, some OBIs cannot be detected. Therefore, it is important to understand the effects of immunosuppression on the HBV life cycle.
Previous research found that glucocorticoid increased HBsAg titer in 83% of patients with hematologic malignancies, including non-Hodgkin’s lymphoma (NHL) [5]. In clinical work, we observed that patients with NHL or immune thrombocytopenia (ITP) coexisted with HBV easily. Studies have revealed HBV infection might increase the risk of NHL, and HBV infection rate is higher in NHL patients than in healthy control patients [6]. Hepatotropic viruses were reported to be able to trigger autoimmune reactions, and about 20% of ITP patients were found to be infected with the hepatitis C virus. However, few studies have reported the relationship between HBV and ITP [7].
In this study, a retrospective analysis was conducted for 171 individuals who had been diagnosed as ITP patients from January 2009 to December 2014. The percentage of HBsAg-positive results was higher in ITP patients compared with healthy individuals (18.13% vs. 9.14%, P=0.01, Table 1). Most of the patients were treated with glucocorticoids (80.70%), such as dexamethasone and prednisone, as part of their therapies. However, the use of immunosuppressive agents might increase the risk of HBV reactivation, which could lead to acute hepatitis and even hepatic failure [8,9]. As such, it is useful to understand the mechanics of glucocorticoids in the HBV life cycle.
Table 1.
Clinical characteristics of ITP and controls.
| Variable | ITP N (%) | Controls N (%) | P-value* |
|---|---|---|---|
| Total | 171 | 186 | |
| Age; Mean ±SD | 46.09±1.40 | 43.08±0.84 | 0.06 |
| Gender | 0.08 | ||
| Male | 46 (26.90) | 66 (35.48) | |
| Female | 125 (73.10) | 120 (64.5) | |
| HBsAg+ | 31 (18.13) | 17 (9.14) | 0.01 |
| HBsAg−/HBcAb+ | 48 (28.07) | 110 (59.14) | <0.01 |
| HBsAg−/HBcAb− | 92 (53.80) | 59 (31.72) | <0.01 |
| HBsAb+ | 85 (49.71) | 134 (72.04) | <0.01 |
| HBsAb−/HBcAb+ | 23 (13.45) | 30 (16.13) | 0.47 |
| HBsAb−/HBcAb− | 63 (36.84) | 22 (11.84) | <0.01 |
| Use of glucocorticoid | 138 (80.70) |
The difference of mean ±SD between cases and controls were calculated with student’s t-test, others were analyzed by χ2 statistic.
ITP – immune thrombocytopenia; HBsAg – hepatitis B surface antigen; HBsAb – antibody to HBsAg; HBcAb – antibody to hepatitis B core antigen.
Autophagy is essential to maintain cellular homeostasis involving the degradation and elimination of long-lived proteins and organelles. Numerous studies have been carried out to identify the mechanism of glucocorticoid-induced apoptosis [10,11]. Some reported that dexamethasone could induce autophagy in lymphocyte and osteocytes [12,13]. Many others demonstrated that viral infections had complex interconnections with autophagy. Recently, it has been reported that microRNA-99 family promotes autophagy through mTOR/ULK1 signaling and thereby enhance HBV replication [14]. Another virus, Bombyx mori nuclear polyhedrosis infection induces autophagy by increasing autophagy-related genes expression, contributing to the benefit of its infection [15]. Moreover, type-III interferon treated with HCV through inhibition of the HCV-induced autophagy response [16]. Similarly, autophagy is also important for HBV replication in vivo [17]. The aim of this study was to further explore whether autophagy was involved in HBV replication in HepG2.2.15 cells undergoing dexamethasone treatment.
Material and Methods
Patients and clinical characteristics
This study analyzed retrospectively196 patients who had been diagnosed with ITP from January 2009 to December 2015 in the Second Affiliated Hospital of Chongqing Medical University. Out of those 196 patients, 25 were excluded from the study because they lacked HBV serology data, including HBsAg, hepatitis B e-antigen (HBeAg), antibody to HBsAg (HBsAb), and antibody to hepatitis B core antigen (HBcAb). Thus, in the end, 171 ITP patients were analyzed. The researchers also recruited 186 healthy age- and sex-matched individuals to participate as a control group. All had been tested for hepatitis B serology. Information about the participants’ age, gender, hepatitis B serology results, and treatment regimens was obtained by consulting clinical records.
Chemicals and antibodies
Dexamethasone, rapamycin (R8781), and 3-methyladenine (3-MA, M9281) were purchased from Sigma-Aldrich. The dexamethasone was dissolved in 100% ethanol (vehicle), and the 3-MA was dissolved in phosphate-buffered saline (PBS). Chemiluminescence reagents were obtained from Millipore. The antibodies used in experiments were anti-LC3 (L8918, Sigma), sequestosome (p62, H00008878-M01, Abnova).
Cell culture and transfection
HepG2.2.15 was a stable HBV-expressing cell line, which grew in the medium with antibiotics (G418, 500 ug/mL) at 37°C and with 5% CO2 in a humidified incubator. The pGFP-LC3 was a gift from Dr. Juan Chen (Chinese University of Hong Kong, China). Hep2.2.15 cells were transfected with pGFP-LC3 using Lipofectamine 2000 (Invitrogen).
Western blot analysis
After treatment, proteins were extracted from cells according to the instructions of a protein extraction kit (KaiJi, KGP2100, China). Equal amounts of protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies (anti-LC3, 1: 1000; anti-p62, 1: 1000) at 4°C overnight and with secondary antibodies at room temperature for 1 h. Chemiluminescence signals were detected by the Bio-Rad system and x-ray films.
Reverse transcription, real-time PCR
After transfection for 48 h, cells were collected, and total RNA was isolated by TRIzol reagent (Invitrogen). Reverse transcription was performed with PrimeScript RT reagent Kit (Takara, Japan). The forward primer used for amplification of 3.5Kb mRNA was 5′-GCCTTAGAGTCTCCTGAGCA-3′, and the reverse primer was 5′-GAGGGAGTTCTTCTTCTAGG-3′. The DNA of HBV was quantitated with the BIO-RAD CFX 96 (BIO-RAD) system. The primers used for HBV quantification were 5′-CCTAGTAGTCAGTTATGTCAAC-3′ (forward) and 5′-TCTATAA GCTGGAGTGC GA-3′ (reverse).
Southern blot analysis
Extraction of HBV replicative intermediates was performed as described by Ren et al. [18]. Briefly, DNA samples were separated on 0.9% agarose gels and transferred onto nylon membranes (Roche; Germany). After UV cross-linking and prehybridization, the membrane was hybridized with a digoxigenin-labeled HBV-specific probe generated by using a Random primed labeling kit (Roche; Germany) and then exposed to x-ray to detect the signals [19].
Transmission electron microscopy (TEM)
After treatment for 48 h, cells were washed with 1 x PBS for 3 times and collected by centrifugation. Liquid supernatant was discarded, and cells were fixed with 2% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The cells were further fixed and stained with uranyl acetate and lead citrate. An H7600 electron microscope (Hitachi, Japan) was used to observe the sections.
HBsAg detection by enzyme-linked immunosorbent assay (ELISA)
To detect HBsAg, supernatant of cell cultures examined by ELISA according to the manufacturer’s instructions (KHB, Shanghai, China). Each experiment was performed at least three times.
Statistical analyses
Chi-Square test was used to assess the differences in the distribution of categorical variables. Student’s t-test was applied to compare difference in mean of age. All data obtained from the experiment were expressed as mean values ±SD. When 2 groups were compared, unpaired Student’s t-test was used. Three groups’ means were analyzed by one-way analysis of variance (ANOVA) with a post-test Bonferroni, * P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS16.0.
Results
Prevalence of HBsAg positive in ITP patients
As shown in Table 1, there were no significant differences in proportion of male and female between cases and controls (P=0.08), nor in distribution of age (P=0.06). The percentage of HBsAg positive is 18.13 in ITP patients, which was higher than the rate of HBsAg positive in control group (P=0.01). More than 80% patients received the glucocorticoid in their treatment.
HBV replication and protein expression stimulated by dexamethasone in HepG2.2.15
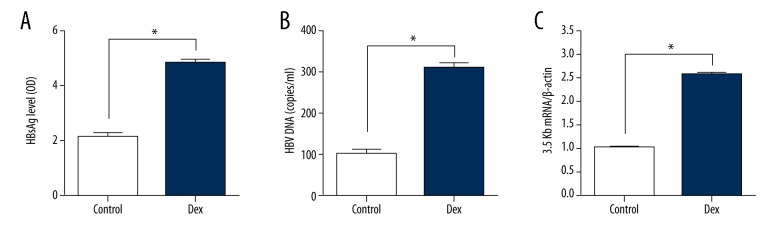
To study the effect of glucocorticoid on HBV expression, dexamethasone was used to treat with HepG2.2.15 cells. After treatment for 2 days, supernatant of the culture medium was collected to detect HBsAg level by ELISA. As shown in Figure 1, HBsAg expression was increased greater than in the control group. Additionally, we further examined HBV DNA replicative intermediates and 3.5 kb mRNA levels. Both of them increased more than 2-fold with dexamethasone (Figure 1B, 1C). These results were similar to the study of Chou et al. [20].
Figure 1.
The effect of dexamethasone (Dex) on HBV production expression. HepG2.2.15 cells were treated with DEX for 2 days. (A) HBsAg levels in culture media were detected by ELISAB. HBV intermediates were extracted and subjected to real-time PCR. (B, C) The 3.5kb mRNA levels were determined by real-time PCR. (* P<0.05)
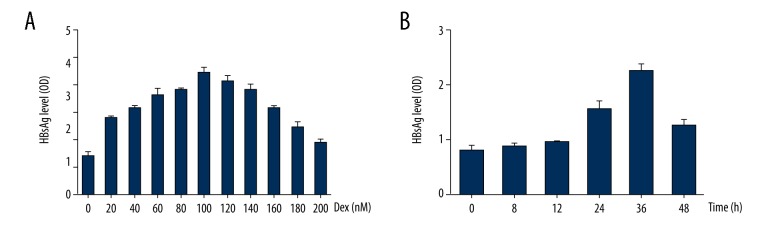
Time and dose-dependent stimulation of dexamethasone on HBsAg secretion
Intriguingly, dexamethasone induced a time and dose-dependent stimulation of HBsAg secretion in HepG2.2.15 cells. To explore the optimal concentration, cells were treated with increasing concentrations, 0, 20, 40, 60, 80, 100, 120, 140, 160, 180, 200 nM of dexamethasone. During 48 h post-treatment, cell cultures were harvested and accessed by ELISA. HBsAg levels were continuously increased in culture medium with various concentrations of dexamethasone, from 0 to 100 nM. Whereas beyond 100 nM, dexamethasone displayed inhibition of HBsAg secretion (Figure 2A). Since time for treatment is important for the efficiency of dexamethasone, we treated cells with dexamethasone for different time, 0, 8, 12, 24, 36, 48 h. It is noteworthy that there was a peak at 36 hours with 100 nM dexamethasone on HBsAg production (Figure 2B). Taken together, results suggested concentration of 100 nM and treatment time for 36 h were the optimal experimental condition.
Figure 2.
Time and dose-dependent stimulation of dexamethasone (DEX) on HBsAg secretion. (A) HepG2.2.15 cells were treated with increasing concentrations of DEX (0, 20, 40, 60, 80, 100, 120, 140, 160, 180, 200 nM). (B) HepG2.2.15 cells treated with Dex (100 nM) for different time (0, 8, 12, 24, 36, 48 h). Culture media were collected to detect HBsAg levels by ELISA.
Dexamethasone treatment increased HBsAg secretion by inducing autophagy
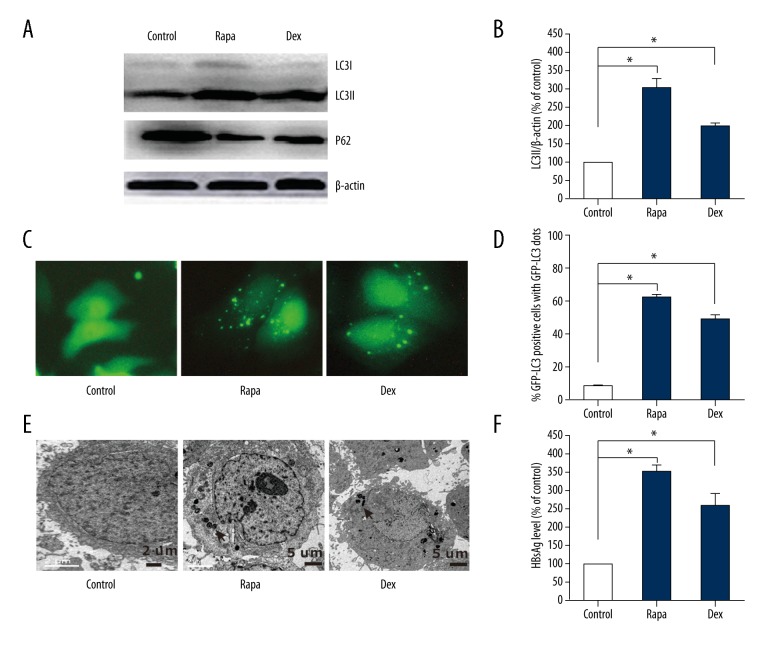
Recently, Shin et al. have reported HBV-triggered autophagy targeted TNFRSF10B death receptor 5 [21]. However, there is limited knowledge about the role of autophagy in HBV lifecycle. HepG2.2.15 cells were transfected with GFP-LC3 plasmids for 24 h, then treated with autophagy inducer rapamycin and dexamethasone respectively. Interestingly, a significant conversion from LC3-I to LC3-II was observed in cells treated with dexamethasone, which exerted similar effects with rapamycin (Figure 3A, 3B). Compared with the control group, the protein expression of p62 decreased in cells treated with dexamethasone. Cytoplasmic fluorescence punctuations (GFP-LC3) increased remarkably in cells treated with dexamethasone (Figure 3C, 3D). To further verified whether autophagy involved, TEM was used to observe autophagosomes. As expected, characteristic double-membrane autophagosomes and membrane structures were observed in cells treated with rapamycin or dexamethasone (Figure 3E). Moreover, HBsAg secretion level was increased 2-fold in supernatant of cells treated with dexamethasone and rapamycin than control group (Figure 3F). These results suggest that dexamethasone could induce autophagy, which lead to increasing HBsAg secretion in HepG2.2.15 cells.
Figure 3.
Dexamethasone (DEX) induced autophagy in HepG2.2.15 cells. HepG2.2.15 cells, which transfected with GFP-LC3 plasmids for 24 h, treated with Dex (100 nM) and autophagy inducer rapamycin (Rapa, 60 nM) respectively. After 36 h, cells were collected. (A) The conversion of LC3-I to LC3-II and p62 analyzed with western blot. (B) The LC3-II/β-actin ratios were quantified by densitometric analysis with Quantity One software (Bio-Rad). Results presented were representative of 3 independent experiments. (C, D) Representative fluorescence punctuations for autophagic dots observed in hepG2.2.15 cells transfected with GFP-LC3 plasmids for 48 h. (E) Autophagy vesicles examined by TEM. (F) HBsAg levels in culture medium of HepG2.2.15 cells were collected and detected with ELISA (* P<0.05).
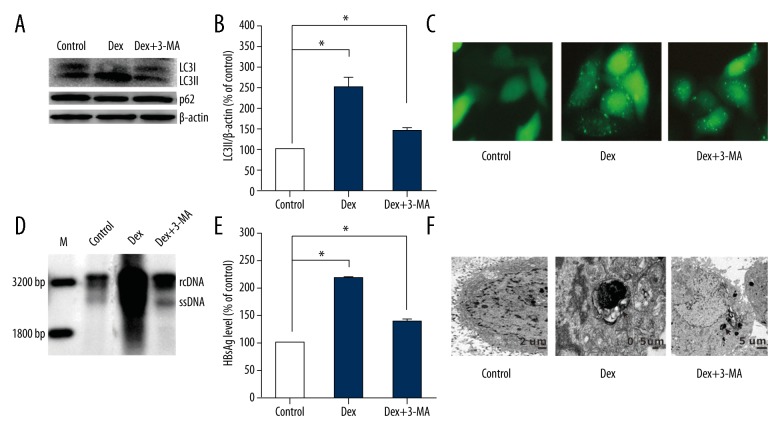
Autophagy inhibitors (3-MA) decreased HBsAg secretion in HepG2.2.15 treated with dexamethasone
We further explored whether autophagy was responsible for HBsAg secretion treated with dexamethasone. HepG2.2.15 cells were treated with autophagy inhibitor 3-MA and dexamethasone after being transfected with GFP-LC3 plasmids for 24 h. The effective inhibition of autophagy was confirmed by using western blot analysis (Figure 4A). LC3-II/actin decreased almost 50% in cells treated with 3-MA+dexamethasonethan cells treated with dexamethasone (Figure 4B). Both autophagic fluorescence study (Figure 4C) and TEM (Figure 4F) revealed autophagy was inhibited effectively by 3-MA. Meaningfully, compared with cells treated with dexamethasone, HBV replicative intermediates decreased significantly after being treated with 3-MA+dexamethasone (Figure 4D). Furthermore, compared with cells treated with dexamethasone, a reduction was observed in HBsAg secretion in cells treated 3-MA + dexamethasone (Figure 4E). Results suggest autophagy exerted vital effect in dexamethasone mediated HBsAg secretion.
Figure 4.
Autophagy inhibitors (3-MA) decreases HBsAg secretion in HepG2.2.15 treated with dexamethasone (Dex). HepG2.2.15 cells were treated with 3-MA+ Dex and Dex respectively, after transfecting with GFP-LC3 plasmids for 24 h. (A) 36 h later, cells were collected and analyzed the conversion of LC3-I to LC3-II and p62 by western blot. (B) The LC3-II/β-actin ratios were quantified by densitometric analysis with Quantity One software (Bio-Rad). Results presented were representative of 3 independent experiments. (C, F) Immunofluorescence images for autophagic dots and TEM in pGFP-LC3-transfected hepG2.2.15 cells treated with 3-MA+ Dex and Dex. (D) HBV intermediates were extracted and subjected to southern blot analysis. (E) HBsAg secreted in the supernatants were analyzed by ELISA assay.
Discussion
HBV reactivation has been reported as a severe complication of patients treated with immunosuppressive therapy or chemotherapy. In recent years, reactivation of HBV has occurred in hematologic malignancies [22], breast cancer, nasopharyngeal carcinoma, small cell lung cancer, and even in postoperative of HBV related hepatocellular carcinoma [23–26]. HBV reactivation is a well-recognized clinical issue in patients undergoing chemotherapy or immunosuppressive therapies. However, few studies emphasize the similar risk of HBV reactivation in ITP patients treated with high doses of dexamethasone (40 mg). In clinical practice, we observed a higher HBsAg(+) percentage of ITP patients (P=0.01), which was supported by our retrospective analysis results (Table1). Meanwhile, dexamethasone was widely used in this group of patients. However, the effect of dexamethasone on HBV reactivation has not been fully assessed.
Dexamethasone has a direct suppressive effect on T cell immunity function. Besides, dexamethasone stimulates a glucocorticoid-responsive element contained in HBV, which might affect expression of HBV gene [27]. In this study, our data demonstrate dexamethasone increases HBsAg secretion via inducing autophagy, whereas autophagy inhibitors (3-MA) decreases HBV replication and HBsAg secretion. In agreement with our finding, Kunanopparat et al. reported autophagy-related gene (ATG12) might play a role in HBV replication via impairing IFN pathway [28]. Our results indicate autophagy might exert important role in HBV reactivation induced by dexamethasone.
Much attention has been paid to the role of autophagy induced by glucocorticoid, in the form of dexamethasone. Dexamethasone impairs cell-cell communication in osteocytes by autophagy through inhibiting Akt-mTORC1 signaling pathway, contributing to the deleterious bone effect [29]. Others have also reported glucocorticoid resistance could be reversed by inhibition mTOR signaling pathway in pre-B ALL cells and Burkitt lymphoma cells [30,31]. In addition, dexamethasone treatment in placental cells downregulated SLC7A5 expression, which led to activation of autophagy and apoptosis via inhibition of mTOR signaling [31,32]. In summary, all of these studies demonstrated that dexamethasone could activate autophagy by regulating mTOR signaling pathway in different cells. The mechanism of autophagy induced by dexamethasone in hepatocellular carcinoma cells need to be further explored.
Conclusions
The results of our study provide a novel insight into the mechanism of glucocorticoid-mediated HBV reactivation in a mechanism of autophagy. Our study also highlights the awareness that HBV-associated antigen should be regularly tested in ITP patients. In addition, new therapeutic strategies targeting autophagy may be helpful in prevention of HBV reactivation for patients receiving glucocorticoid.
Acknowledgements
Our sincerely thanks for linguistic help from Yuanyuan Yang
Footnotes
Conflict of interest
None.
Source of support: The study was supported by research grants from National Natural Science Foundation of China (30972582)
References
- 1.Sagnelli C, Macera M, Pisaturo M, et al. Occult HBV infection in the oncohematological setting. Infection. 2016;44(5):575–82. doi: 10.1007/s15010-016-0891-1. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 3.Sagnelli E, Pisaturo M, Martini S, et al. Clinical impact of occult hepatitis B virus infection in immunosuppressed patients. World J Hepatol. 2014;6(6):384–93. doi: 10.4254/wjh.v6.i6.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49(4):652–57. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Ohtsu T, Sai T, Oka M, et al. Activation of hepatitis B virus infection by chemotherapy containing glucocorticoid in hepatitis B virus carriers with hematologic malignancies. Jpn J Clin Oncol. 1991;21(5):360–65. [PubMed] [Google Scholar]
- 6.Qi Z, Wang H, Gao G. Association of risk of non-Hodgkin’s lymphoma with hepatitis B virus infection: A meta-analysis. Int J Clin Exp Med. 2015;8(12):22167–74. [PMC free article] [PubMed] [Google Scholar]
- 7.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: Pathogenic and clinical diversity. Blood. 2009;113(26):6511–21. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindh M, Uhnoo I, Blackberg J, et al. Treatment of chronic hepatitis B infection: an update of Swedish recommendations. Scand J Infect Dis. 2008;40(6–7):436–450. doi: 10.1080/00365540802154769. [DOI] [PubMed] [Google Scholar]
- 9.Allegra A, Penna G, Alonci A, et al. Exacerbation of chronic idiopathic thrombocytopenic purpura following reactivation of an occult hepatitis B. Med Oncol. 2010;27(3):912–14. doi: 10.1007/s12032-009-9305-x. [DOI] [PubMed] [Google Scholar]
- 10.Espinasse MA, Pepin A, Virault-Rocroy P, et al. Glucocorticoid-induced leucine zipper is expressed in human neutrophils and promotes apoptosis through Mcl-1 down-regulation. J Innate Immun. 2016;8(1):81–96. doi: 10.1159/000439052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pazdrak K, Moon Y, Straub C, et al. Eosinophil resistance to glucocorticoid-induced apoptosis is mediated by the transcription factor NFIL3. Apoptosis. 2016;21(4):421–31. doi: 10.1007/s10495-016-1226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia X, Kar R, Gluhak-Heinrich J, et al. Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res. 2010;25(11):2479–88. doi: 10.1002/jbmr.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molitoris JK, McColl KS, Swerdlow S, et al. Glucocorticoid elevation of dexamethasone-induced gene 2 (Dig2/RTP801/REDD1) protein mediates autophagy in lymphocytes. J Biol Chem. 2011;286(34):30181–89. doi: 10.1074/jbc.M111.245423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Deng W, Pang J, et al. The microRNA-99 family modulates hepatitis B virus replication by promoting IGF-1R/PI3K/Akt/mTOR/ULK1 signaling-induced autophagy. Cell Microbiol. 2017;19(5) doi: 10.1111/cmi.12709. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Xiao Q, Zhou XL, et al. Bombyx mori nuclear polyhedrosis virus (BmNPV) induces host cell autophagy to benefit infection. Viruses. 2017;10(1) doi: 10.3390/v10010014. pii: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Li Y, Fang S, et al. Downregulation of autophagy-related gene ATG5 and GABARAP expression by IFN-λ1 contributes to its anti-HCV activity in human hepatoma cells. Antiviral Res. 2017;140:83–94. doi: 10.1016/j.antiviral.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Xie N, Yuan K, Zhou L, et al. PRKAA/AMPK restricts HBV replication through promotion of autophagic degradation. Autophagy. 2016;12(9):1507–20. doi: 10.1080/15548627.2016.1191857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren JH, Tao Y, Zhang ZZ, et al. Sirtuin 1 regulates hepatitis B virus transcription and replication by targeting transcription factor AP-1. J Virol. 2014;88(5):2442–51. doi: 10.1128/JVI.02861-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Q, Li W, Ren J, et al. ZEB2 inhibits HBV transcription and replication by targeting its core promoter. Oncotarget. 2016;7(13):16003–11. doi: 10.18632/oncotarget.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou CK, Wang LH, Lin HM, et al. Glucocorticoid stimulates hepatitis B viral gene expression in cultured human hepatoma cells. Hepatolog. 1992;16(1):13–18. doi: 10.1002/hep.1840160104. [DOI] [PubMed] [Google Scholar]
- 21.Shin GC, Kang HS, Lee AR, et al. Hepatitis B virus-triggered autophagy targets TNFRSF10B/death receptor 5 for degradation to limit TNFSF10/TRAIL response. Autophagy. 2016;12(12):2451–66. doi: 10.1080/15548627.2016.1239002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez SA, Perrillo RP. Hepatitis B virus reactivation in the setting of cancer chemotherapy and other immunosuppressive drug therapy. Clin Infect Dis. 2016;62( Suppl 4):S306–13. doi: 10.1093/cid/ciw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):221–44e223. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Pattullo V. Prevention of hepatitis B reactivation in the setting of immunosuppression. Clin Mol Hepatol. 2016;22(2):219–37. doi: 10.3350/cmh.2016.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin L, Wang F, Zou BW, et al. Chemotherapy-induced fatal hepatitis B virus reactivation in a small-cell lung cancer patient. Mol Clin Oncol. 2016;5(4):382–84. doi: 10.3892/mco.2016.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie ZB, Zhu SL, Peng YC, et al. Postoperative hepatitis B virus reactivation and surgery-induced immunosuppression in patients with hepatitis B-related hepatocellular carcinoma. J Surg Oncol. 2015;112(6):634–42. doi: 10.1002/jso.24044. [DOI] [PubMed] [Google Scholar]
- 27.Tur-Kaspa R, Burk RD, Shaul Y, et al. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA. 1986;83(6):1627–31. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunanopparat A, Hirankarn N, Kittigul C, et al. Autophagy machinery impaired interferon signalling pathways to benefit hepatitis B virus replication. Asian Pac J Allergy Immunol. 2016;34(1):77–85. doi: 10.12932/AP0636.34.1.2016. [DOI] [PubMed] [Google Scholar]
- 29.Gao J, Cheng TS, Qin A, et al. Glucocorticoid impairs cell-cell communication by autophagy-mediated degradation of connexin 43 in osteocytes. Oncotarget. 2016;7(19):26966–78. doi: 10.18632/oncotarget.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polak A, Kiliszek P, Sewastianik T, et al. MEK inhibition sensitizes precursor B-Cell acute lymphoblastic leukemia (B-ALL) cells to dexamethasone through modulation of mTOR activity and stimulation of autophagy. PLoS One. 2016;11(5):e0155893. doi: 10.1371/journal.pone.0155893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu L, Xie L, Zuo C, et al. Targeting mTOR/p70S6K/glycolysis signaling pathway restores glucocorticoid sensitivity to 4E-BP1 null Burkitt Lymphoma. BMC Cancer. 2015;15:529. doi: 10.1186/s12885-015-1535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He B, Zhang N, Zhao R. Dexamethasone downregulates SLC7A5 expression and promotes cell cycle arrest, autophagy and apoptosis in BeWo cells. J Cell Physiol. 2016;231(1):233–42. doi: 10.1002/jcp.25076. [DOI] [PubMed] [Google Scholar]