Abstract
Objectives
Extremely low gestational age neonates with chronic lung disease requiring oxygen therapy frequently experience fluctuations in arterial oxygen saturation or intermittent hypoxia (IH). These infants are at risk for multi-organ developmental delay, reduced growth, and short stature. The growth hormone (GH)/insulin-like growth factor-I (IGF-1) system, an important hormonal regulator of lipid and carbohydrate metabolism, promotes neonatal growth and development. We tested the hypothesis that increasing episodes of IH delay neonatal growth by influencing the GH/IGF-I axis.
Design
Newborn rats were exposed to 2, 4, 6, 8, 10, or 12 hypoxic episodes (12% O2) during hyperoxia (50% O2) from P0-P7, P0-P14 (IH), or allowed to recover from P7-P21 or P14-P21 (IHR) in room air (RA). RA littermates at P7, P14, and P21 served as RA controls; and groups exposed to hyperoxia only (50% O2) served as zero IH controls. Histopathology of the liver; hepatic levels of GH, GHBP, IGF-I, IGFBP-3, and leptin; and immunoreactivities of GH, GHR, IGF-I and IGF-IR were determined.
Results
Pathological findings of the liver, including cellular swelling, steatosis, necrosis and focal sinusoid congestion were seen in IH, and was particularly severe in the P7 animals. Hepatic GH levels were significantly suppressed in the IH groups exposed to 6–12 hypoxic episodes per day and were not normalized during IHR. Deficits in the GH levels were associated with reduced body length and increase body weight during IHR suggesting increased adiposity and catchup fat. Catchup fat was also associated with elevations in GHBP, IGF-I, leptin.
Conclusions
IH significantly impairs hepatic GH/IGF-1 signaling during the first few weeks of life, which is likely responsible for hepatic GH resistance, increased body fat, and hepatic steatosis. These hormonal perturbations may contribute to long-term organ and body growth impairment, and metabolic dysfunction in preterm infants experiencing frequent IH and/or apneic episodes.
Keywords: Growth hormone, Insulin-Like Growth Factor-I, Intermittent Hypoxia, Somatic Growth
INTRODUCTION
Preterm birth, defined as birth before 37 weeks gestation, is a major cause of death and significant morbidities in children [1,2]. Global studies show that approximately 10% of all newborns are born premature [3,4], with substantial developmental disorders and chronic diseases occurring in later life [5–7]. Surviving preterm neonates with immature respiratory control mechanisms experience frequent arterial oxygen desaturations, intermittent hypoxia (IH), apnea of prematurity (AOP) with bradycardia, or tissue injuries associated with recovery/re-oxygenation in hyperoxia or room air [8]. Recurrent IH episodes increases reactive oxygen species (ROS) and oxidative stress, which are implicated in the development of major neonatal co-morbidities which are often the cause of feeding intolerance, poor growth and nutrition, long-term cardiovascular health, and metabolic syndrome in adult life [9–11].
The growth hormone (GH)/insulin-like growth factor (IGF)-I system, which consists of GH, GH receptor (GHR), IGF-I, IGF-II, IGF-I receptor (IGF-IR), and IGF binding proteins (IGFBPs 1–6), plays a critical role in fetal and postnatal growth and development. In the fetus, GH levels are high, but it plays a minimal role in fetal growth. This is due to low fetal levels of GH receptor (GHR) and GH binding protein (GHBP), suggesting that fetal growth is independent of GH [12]. Instead, fetal GH regulates carbohydrate metabolism. Immediately after birth, GH levels rapidly decline possibly due to postnatal activation of the GHR and GHBPs [13]. GH is produced by the anterior pituitary gland and is an important regulator of linear growth, metabolism, and body composition from childhood to adult life [14]. It travels through the circulation to target cells and tissues that express its receptor [15]. Although the liver is the major target organ for GH where it has plays a key role in lipid metabolism and glucose homeostasis [16], it regulates cell growth and metabolism in various non-hepatic tissues. For example, in adipose tissue GH stimulates lipolysis; in skeletal muscle it promotes growth, maintenance, repair and regeneration; in bone it promotes longitudinal growth [15]; and the GH/IGF system is involved in renal development and nephron size [17].
GH promotes postnatal somatic growth by inducing the production of IGF-I in the liver [18] and in contrast to GH, is the main regulator of fetal and postnatal growth. IGF-1, a 70-amino acid polypeptide hormone, accounts for about 75% of all circulating IGFs [19], and together with its receptor, is expressed in almost all tissues for autocrine/paracrine purposes [20]. IGF-I expression in the liver markedly exceeds that in any other tissue, and most circulating IGF-1 originates from liver [21]. Regardless, studies have shown that liver-derived IGF-I is not required for postnatal growth, suggesting that local production of IGF-I may be more important than liver-derived circulating IGF-I for body growth [22].
IGF-1 availability is tightly regulated by its binding proteins (IGFBPs), which increase IGF-1 half-life from minutes to hours, and shuttles IGF-I to specific target tissues [23]. IGF-1 deficiency is associated to GH resistance, and its replacement therapy restores altered GH/IGF-1 axis by reducing circulating GH levels [19]. IGF-I is present in high concentrations in serum, and is mostly protein bound [24]. Approximately 90% of IGF-1 is bound to IGFBP-3, the primary hepatic-derived IGFBP [25], which serves as its major constitutive binding protein [26,27]. In mice, 70–80% of IGF-I exists in the circulation as ternary complex consisting of IGF-I, IGFBP-3, and the liver-derived acid-labile subunit (ALS). This ternary complex has a relatively long half-life of 10–16 hours [28,29], and may be regulated by GH [30]. In rats, the fetal serum profile, characterized by high IGF-II and IGFBP-2, is replaced around the third week of life by the adult-type profile of high IGF-I and IGFBP-3, with a dramatic reduction in IGF-II and IGFBP-2 [31]. Studies by Ibañez de Cáceres et al. [32] showed that administration of recombinant human GH to rats resulted in improved body weight gain and dose-dependent elevations in serum GHBP-3 compared to other IGFBPs, demonstrating a GH association/dependence of IGFBP-3. The smaller binary complexes of IGF-I and serum IGFBPs (mainly IGFBP-3) comprise 15–20% of the circulating pool, and the remainder (free IGF-I) comprise <5% with an extremely short half-life [33]. Leptin is a hormone produced by adipocytes that regulates metabolism and appetite. It regulates food intake and body weight and its levels are high in obesity [34]. In mice, leptin deficiency and lack of functional leptin receptors were associated with decreased muscle and bone mass, as well as IGF-1 deficiency, and leptin treatment stimulated the GH/IGF-I axis [35].
The liver is a prominent organ in neonates. It constitutes approximately 5% of body weight at birth (compared to 2% in adults) with a large volume (per unit of body weight) of 48 mL/Kg [36]. Premature neonates have compromised serum IGF-1 levels which contribute in part to poor postnatal growth [37,38]. Intrinsically low levels of IGF-I combined with supplemental oxygen and IH predisposes the preterm liver to increased production of reactive oxygen species (ROS) and oxidative stress. Propagation of ROS that target the liver due to its high lipid content, will result in lipid peroxidation [39–41]. Studies have shown that IH significantly influences the modulation of hepatic GH and IGF-I [42,43]. These alterations can have a negative long-term impact on growth and carbohydrate metabolism. Furthermore, reductions in GH and IGF-I can further propagate ROS accumulation in the liver, particularly in transfused preterm infants with iron deficiency and compromised antioxidant systems. Studies have shown that free iron, via the Fenton reaction reacts with H2O2 to induce lipid peroxidation, an effect that can be ameliorated with GH and IGF-I [44,45]. We therefore conducted a series of experiments to understand the role of increasing IH episodes on hepatic GH and IGF-I levels. We hypothesized that there is a critical number of IH episodes which will induce hepatocyte loss, and alter hepatic growth factors. These IH-induced changes will have permanent implications on growth despite recovery and re-oxygenation in room air. To test our hypothesis, we examined GH, GHBP (the soluble form of GHR), and IGF-I, as well as weight accretion in response to increasing IH episodes and during recovery from IH (IHR) in room air.
MATERIAL AND METHODS
This study was approved by the State University of New York, Downstate Medical Center Animal Care and Use Committee, Brooklyn, NY. Animals were cared for and handled according to the United States Department of Agriculture (USDA) guidelines and National Institutes of Health guide for the Care and Use of Laboratory Animals. Euthanasia was carried out according to the American Veterinary Medical Association Panel for Euthanasia guidelines.
Experimental Design
Certified infection-free, timed-pregnant Sprague Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) at 17 days gestation. The animals were housed in an animal facility with a 12-hour-day/12-hour-night cycle and provided standard laboratory diet and water ad libitum until delivery. Within 2–3 hours of birth, newborn pups delivered on the same day were pooled and randomly assigned to expanded litters of 18 pups/litter (9 males and 9 females). Gender was determined by the anogenital distance. The expanded litter size was used to simulate relative postnatal malnutrition of critically ill ELGANs. Each pup was weighed and measured for linear growth (crown to rump length in centimeters). A total of 31 groups of 18 rat pups (9 males and 9 females) were studied according to the experimental design previously published [46–49]. The groups are described as follows: 1) Groups 1 to 6 were exposed to 2, 4, 6, 8, 10 or 12 IH cycling episodes from P0 to P7; 2) Groups 7 to 12 were exposed to 2, 4, 6, 8, 10 or 12 IH cycling episodes from P0 to P14; 3) Groups 13 to 18 were exposed to 2, 4, 6, 8, 10 or 12 IH cycling episodes from P0 to P7, followed by re-oxygenation in room air (RA) for 14 days from P7 to P21; 4) Groups 18 to 24 were exposed to 2, 4, 6, 8, 10 or 12 IH cycling episodes from P0 to P14, followed by re-oxygenation in RA for 7 days from P14 to P21; 5) Groups 25 to 28 were exposed to hyperoxia (Hx) consisting of 50% O2 only for 7 days, 14 days, 7 days with 14 days of re-oxygenation in RA, or 14 days with 7 days of re-oxygenation in RA. These groups served as “0” IH such that the range of IH episodes was 0 to 12; and 6) Groups 29–31 were littermates raised in RA from birth to P7, P14, or P21 with all conditions identical except for atmospheric oxygen and served as RA controls. Animals were weighed at birth (P0) prior to placement in the various oxygen environments, and at P7, P14 and P21. Body length (crown to rump length, cm) was determined simultaneously. Percentage changes in body weight and body length were calculated as weight or length at the end of the experiment (P7, P14 or P21) minus weight or length at birth (P0) divided by the weight or length at birth x 100.
Intermittent Hypoxia (IH) Cycling
The IH cycles consisted of hyperoxia (50% O2)/hypoxia (12% O2) in stepwise increments of brief (1-minute), hypoxia (12%) clusters (3 clusters) during 50% O2 for a total of 2, 4, 6, 8, 10 or 12 episodes/day. This clustering design has been shown to produce severe oxidative stress in neonatal rats [46–49]. There were two IH groups corresponding to 7-day (P0-P7), and 14-day (P0-P14). To determine immediate effects, animals were euthanized on P7 or P14. To determine the effects of IHR, all animals were euthanized on P21 after either 14 days of recovery in RA following 7-day IH (P7-P21), or 7 days of recovery in RA following 14-day IH (P14-P21).
Sample Collection & Processing
Liver samples (n=8/group, approximately 200 mg) were excised, rinsed in ice-cold phosphate buffered saline (PBS) to remove blood elements, and placed in sterile Lysing Matrix D 2.0 mL tubes containing 1.4 mm ceramic spheres (MP Biomedicals, Santa Ana, CA, USA) and 1.5 mL phosphate buffered saline (PBS) prior to snap-freezing in liquid nitrogen. Samples were stored at −80°C until analysis. All samples were analyzed on the same day. On the day of analysis the tubes were placed in a high-speed FastPrep-24 instrument (MP Biomedicals, Santa Ana, CA, USA), which utilizes a unique, optimized motion to efficiently homogenize biological samples within 40 seconds via a multidirectional simultaneous beating of the Lysing Matrix ceramic beads on the tissue. The homogenates were then centrifuged at 4°C at 10,000 rpm for 20 minutes. The supernatant was filtered and the filtrate was used for the assays. An aliquot of the filtrate (10 μL) was used to determine the total cellular protein levels in each sample. A total of 8 samples per group were analyzed (4 males and 4 females).
Assay of Serum GH
Due to the small serum volume, only serum GH levels were determined. Serum GH levels were determined using commercially available quantitative sandwich enzyme-linked immunosorbent assay (ELISA) kits purchased from MyBiosource (San Diego, CA, USA), according to the manufacturer’s protocol. Briefly, 50 μL samples or standards were added to appropriate wells with 100 μL HRP-conjugate reagent. The plates were covered and incubated at 37°C for 60 minutes, washed 4 times with appropriate washing buffers using an automatic plate washer (BioTek, Winooski, VT). After the final wash the plates were blotted on absorbent paper. Fifty μL chromogen solution A and 50 μL chromogen solution B were added to each well and incubated for 15 minutes at 37°C, following which 50 μL stop solution was added. The plates were read at 450 nm using a BioTek plate reader. The concentration of GH, GHBP and IGFBP-3 in the samples was calculated based on the standard curve generated by the standards in each plate following subtraction of the optical density of the blank/control samples.
Assay of Liver GH, GHBP and IGFBP-3
GH, GHBP and IGFBP-3 levels in the liver homogenates were determined using commercially-available rat GH, GHBP, and IGFBP-3 ELISA kits purchased from MyBiosource, according to the manufacturer’s protocol. Details of the assay are as described for serum GH. Data were standardized using total cellular protein levels.
Assay of Liver IGF-I and Leptin
IGF-I and leptin levels the liver homogenates were determined using commercially-available rat IGF-I and leptin Quantikine mouse/rat IGF-I and leptin ELISA kits purchased from R&D Systems (Minneapolis, MN, USA), according to the manufacturer’s protocol. The immunoassays were 4.5-hour solid phase ELISAs. IGF-I circulates primarily as a ternary complex with IGFBP-3 and ALS [28,29]. Some IGF-I is present in binary complexes with other IGFBPs, but the ternary complexes are restricted to the vasculature. Although this assay was designed by the manufacturer to eliminate interference by other factors present in biological samples, and specificity assays showed no cross reactivity or interference with binding proteins, no validation assays were conducted under hypoxic conditions to confirm that the assay measures only free IGF-I. The tissue homogenates were processed and stored as described above. For IGF-I, samples and reagents were at room temperature prior to assay. Fifty μL calibrator diluent RDS-38 was added to each well followed by 50 μL standard, control or sample in appropriate wells. The plate was covered and incubated at room temperature for 2 hours in on an orbital microplate shaker set at 500 rpm. After incubation, the wells were washed 5 times with 400 μL wash buffer and blot dried on absorbent paper. Mouse/rat IGF-I conjugate (100 μL) was added to each well and the plate was covered and incubated at room temperature for an additional 2 hours on an orbital microplate shaker set at 500 rpm. The wash was repeated and 100 μL substrate solution was added followed by a 30-minute incubation at room temperature in the dark. Stop solution (100 μL) was added and the optical density was determined using a BioTek plate reader set at 450 nm. The concentration of IGF-I in the samples was calculated based on the standard curve generated by the standards in each plate. The assay was linear with a standard curve ranging from 0 to 2000 pg/mL. The % variation for the intra- and inter-assay precision were calculated as <10%. The average IGF-I recovery was 98% (range: 82–108%). Details of the leptin assay are as described above for IGF-I. The assay was linear with a standard curve ranging from 0 to 4000 pg/mL. The % variation for the intra- and inter-assay precision were calculated as <8%. The average leptin recovery was 114% (range: 103–125%). Data were standardized using total cellular protein levels.
Total Cellular Protein Assay
On the day of the assay, liver homogenates were assayed for total protein levels using the dye-binding Bio-Rad protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard.
H & E Staining
Liver biopsies were fixed in 10% neutral buffered formalin (NBF) and sent to Histowiz. Inc. (Brooklyn, NY, USA) for processing, sectioning and H&E staining. Images were captured at 40X magnification using an Olympus BX53 microscope, DP72 digital camera, and CellSens imaging software (Olympus, Center Valley, PA, USA), attached to a Dell Precision T3500 computer (Dell, Round Rock, TX, USA). Unstained sections were used for immunofluorescence assays.
Immunofluorescence Staining
Unstained sections were de-paraffinized and rehydrated with xylenes and ethanol. The slides were placed in 10 mM sodium citrate buffer, pH 6.0 and heated at 95–100°C for 20 minutes to unmask the antigens. Following several washes, IF staining was conducted using primary antibodies immunoreactive with GH, GHR, IGF-I and IGF-IR (Santa Cruz Biotechnology, Inc. Dallas, TX, USA) and Alexa Fluor fluorescent secondary antibodies (Life Technologies Grand Island, NY, USA). All IF protocols were conducted according to the manufacturer’s protocols. IF sections were imaged at 40X magnification using an Olympus BX53 microscope, DP72 digital camera, and CellSens imaging software (Olympus, Center Valley, PA, USA), attached to a Dell Precision T3500 computer (Dell, Round Rock, TX, USA).
Statistical Analyses
Data were analyzed in two ways: 1) comparison of 50% O2 and IH groups to RA for each IH increment; and 2) comparison of IH to IHR. For comparison among the oxygen groups, a test for normality was first conducted using the Bartlett’s test. Normally distributed data was analyzed using two-way analysis of variance (ANOVA) with Bonferroni post-hoc tests. Non-normally distributed data was analyzed using Kruskall Wallis test with Dunn’s multiple comparison test. For comparisons between IH and IHR, unpaired t-tests were performed following Levene’s test for equality of variances. Normally distributed data was analyzed using Student t-test, and non-normally distributed data was analyzed using the Mann-Witney-U test. Data are presented as mean±SEM (n-8 samples per group) and a p-value of <0.05 was considered as statistically significant, using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) and graphs were prepared using GraphPad Prizm (version 5.0, GraphPad, San Diego, CA, USA).
RESULTS
Effect of IH on Somatic Growth
Percentage changes in body weight and length are presented in Table 1. Data showed that both Hx and IH significantly decreased body length accretion. Body weight increased with 7 days of Hx and decreased only in group exposed to 12 IH episodes. Liver to body weight ratios declined in all Hx and IH groups. Animals allowed to recover for 14 days in RA following 7 days of IH demonstrated significant catchup growth, but linear growth remained reduced. Liver to body weight ratios were elevated only in the group recovering from Hx. Body weight changes in the 14-day IH groups declined only in the group exposed to 2 IH episodes, but linear growth was reduced in all IH groups. No significant changes in liver to body weigh ratios were detected. In contrast, animals recovering from 14 days of Hx and IH at P21 also exhibited significant weight accretion with reductions in linear growth in the groups that were exposed to 6–12 IH episodes. However, no significant changes in liver to body weigh ratios were detected.
Table 1.
Somatic Growth
| RA (21%) | Hyperoxia (50% O2) | 2 IH cycles/day | 4 IH cycles/day | 6 IH cycles/day | 8 IH cycles/day | 10 IH cycles/day | 12 IH cycles/day | |
|---|---|---|---|---|---|---|---|---|
| P7 IH: | ||||||||
| Δ Body Wt. | 76.0±4.4 | 94.4±5.2* | 87.2±5.1 | 90.4±5.4 | 67.5±4.5 | 66.9±3.0 | 66.7±3.6 | 52.3±4.3** |
| Δ Body Lth. | 44.1±1.5 | 22.9±1.5** | 21.2±1.1** | 32.1 ±1.5** | 17.1±0.86** | 22.6±1.6** | 10.0±1.3** | 13.7±2.3** |
| Liver/Body Wt. Ratio | 0.033±0.002 | 0.028±0.001** | 0.03±0.001** | 0.031±0.001** | 0.029±0.0006** | 0.028±0.003** | 0.03±0.0008** | 0.03±0.001** |
| P21 (7-Day IH): | ||||||||
| Δ Body Wt. | 317.4±17.4 | 493.3±20.2** | 429.9±12.8** | 508.9±20.7** | 411.6±13.4** | 328.1±13.3 | 419.5±13.3** | 403.3±13.9** |
| Δ Body Lth. | 94.8±4.0 | 91.4±2.6 | 81.1±1.7** | 85.9±2.1 | 81.2±1.6** | 83.2±3.8* | 85.5±2.2* | 81.6±3.3* |
| Liver/Body Wt. Ratio | 0.038±0.002 | 0.045±0.002* | 0.036±0.001 | 0.04±0.002 | 0.04±0.002 | 0.035±0.001 | 0.039±0.0009 | 0.037±0.002 |
| P14 (IH): | ||||||||
| Δ Body Wt. | 216.0±8.6 | 241.1±13.4 | 172.0±10.2* | 237.6±8.2 | 228.8±14.6 | 198.9±6.6 | 210.7±6.1 | 200.5±7.6 |
| Δ Body Lth. | 72.0±2.2 | 62.0±2.7 | 44.4±0.94* | 53.3±1.9** | 43.5±2.0** | 45.8±1.4** | 56.4±3.6** | 44.8±2.1** |
| Liver/Body Wt. Ratio | 0.026±0.001 | 0.028±0.001 | 0.03±0.002 | 0.029±0.001 | 0.031±0.002 | 0.029±0.002 | 0.028±0.0009 | 0.029±0.001 |
| P21 (14-Day IH): | ||||||||
| Δ Body Wt. | 317.4±17.4 | 493.9±17.6** | 417.9±19.4** | 418.2±20.7** | 339.0±15.2 | 402.1±10.9** | 415.7±15.8** | 365.1±11.7 |
| Δ Body Lth. | 94.8±4.0 | 99.1±1.4 | 90.3±2.5 | 97.6±2.4 | 63.9±3.3** | 72.3±2.2** | 64.7±4.0** | 65.5±2.3** |
| Liver/Body Wt. Ratio | 0.038±0.002 | 0.04±0.002 | 0.043±0.002 | 0.04±0.003 | 0.039±0.001 | 0.038±0.0009 | 0.039±0.001 | 0.037±0.001 |
Data are mean±SD (*p<0.05, **p<0.01 vs. 21%, n=18/group). Δ (% change calculated as weight (or length) at P7, P14 or P21 minus weight (or length at birth/weight or length at birth x 100); Wt. (weight); Lth. (Length); P21 (7-day IH): animals were exposed to IH for 7 days and recovered in RA (IHR) for 14 days; P21 (14-day IH): animals were exposed to IH for 14 days and recovered in RA (IHR) for 7 days.
Effect of IH on Serum GH
Serum GH levels in response to increasing IH episodes are presented in Figure 1. Groups exposed to IH from P0 to P7 and groups recovering from IH in RA (IHR) from P7 to P21 are presented in Figure 1A. Groups exposed to IH from P0 to P14 and groups recovering from IH in RA (IHR) from P14 to P21 are presented in Figure 1B. The data showed that generally, GH levels are higher at P21 than P7 (Figure 1A). This difference was abated in the 50% O2 (zero IH) group and groups exposed to 2 and 4 IH episodes. Six IH episodes per day resulted in suppression of circulating GH at P7 and this response remained sustained during IHR at P21. In contrast, serum GH levels were significantly higher in the 8 IHR group and lower in the 12 IHR group at P21. Longer exposures to 50% O2 decreased serum GH levels during IHR at P21 (Figure 1B). This suppressive effect during IHR was also noted with 6–12 IH episodes at P21.
Figure 1.
Effects of incremental IH episodes on serum GH levels in neonatal rats at P7 (7D-IH), P14 (14D-IH), or P21-IHR. Animals recovering from 7D-IH were placed in RA from P7-P21 (Figure 1A) and animals recovering from 14D-IH were placed in RA from P14-P21 (Figure 1B). Animals randomized to IH were exposed to brief clustered IH episodes during 50% O2. Animals randomized to hyperoxia were exposed to 50% O2 only and served as zero (0) IH. RA controls remained in atmospheric O2. Data are presented as mean±SEM (n=8 samples/group).
Effect of IH on Liver GH
GH originates from the anterior pituitary gland. Its major target is the liver where it binds GHR to induce IGF-I and promote growth. Although the liver does not secrete GH, we measured the amount of GH that was available in the liver homogenates at each incremental IH episode (Figures 2A and 2B). The data showed that in the RA groups, GH in the liver was higher in the P21 than P7 and P14 groups. Exposure to Hx for 7 days decreased GH and the levels did not rebound during recovery (Figure 2A). Exposure to Hx for 14 days did not reduce GH levels compared to RA, but during IHR, the levels declined significantly (Figure 2B). Exposure to 2 IH episodes for 14 days resulted in higher GH levels and these levels remained sustained during IHR. In contrast, there was a progressive decline in GH levels in the 6–12 IH groups and the levels did not rebound during IHR (Figure 2B). GH expression in selected liver samples from 14-day old rats exposed to 14 days of IH (upper panels) and 21-day old rats exposed to 14 days of IH and 7 days of IHR (lower panel) is presented in Figure 3. Consistent with ELISA data, hepatic GH immunoreactivity declined in all IH and IHR samples.
Figure 2.
Effects of incremental IH episodes on GH (A, B) and GHBP (C, D) levels in the liver of neonatal rats at P7 (7D-IH), P14 (14D-IH), or P21-IHR. Groups are as described in Figure 1. Data are presented as mean±SEM (n=8 samples/group).
Figure 3.
Representative immunofluorescence stain of GH liver sections from 14-day old and 21-day old rats exposed to 14 days of IH and 7 days of IHR, and exposed to 4, 8, and 12 IH episodes per day compared to RA. Images are 40X magnification. Scare bar represents 20 μm.
Effect of IH on Liver GHBP and GHR
GHBP is the soluble form of GHR that is formed by alternative splicing of GHR (rodents), and proteolytic cleavage of the extracellular domain of GHR (humans) with translational levels paralleling GHR status. Increased GHBP cleavage and circulating levels are positively associated with body fat content. GHBP in the liver homogenates and GHR immunoreactivity are presented in Figure 2C and 2D, respectively. Data showed that GHBP levels increased during 7-day IH with 8–12 episodes/day and during IHR from 2–8 IH episodes, but declined in the groups recovering from 10 and 12 IH episodes (Figure 2C). This pattern was also evident in the 14-day IH groups (Figure 2D). GHR expression in selected liver samples from 14-day old rats exposed to 14 days of IH (upper panels) and 21-day old rats exposed to 14 days of IH and 7 days of IHR (lower panel) is presented in Figure 4. Increased GHR expression was noted during IH and IHR, particularly in the group exposed to 8 IH episodes.
Figure 4.
Representative immunofluorescence stain of GHR in liver sections from 14-day old and 21-day old rats exposed to 14 days of IH and 7 days of IHR, and exposed to 4, 8, and 12 IH episodes per day compared to RA. Images are 40X magnification. Scare bar represents 20 μm.
Effect of IH on Liver IGF-I, IGFBP-3 and IGF-IR
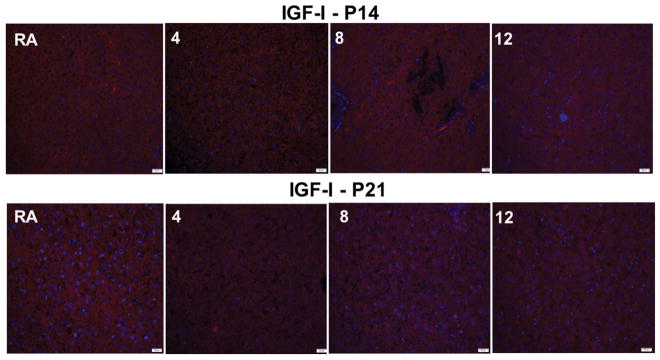
IGF-I and IGFBP-3 levels in the liver homogenates are presented in Figure 5; and IGF-I and IGF-IR immunoreactivities are presented in Figures 6 and 7 respectively. Data showed that IGF-I levels declined during 7 days of Hx and increased during re-oxygenation. Exposure to 8 IH episodes resulted in suppressed IGF-I with no rebound during IHR. A similar response was noted with 12 IH episodes (Figure 5A). Similarly, exposure to 14 days of Hx lowered IGF-I levels, but there was no rebound during re-oxygenation (Figure 5B). IGF-I levels increased during IHR following all IH exposures (Figure 5B). IGFBP-3 also increased during IHR following 2–8 IH episodes, but remained suppressed during IH and IHR in the groups exposed to 12 IH episodes (Figure 5C). Similar IHR increases were observed in the groups exposed to Hx and 2 IH episodes for 14 days, but not in the groups exposed to 6–12 IH episodes (Figure 5D). IGF-I immunoreactivity in the liver did not appreciably change in the groups exposed to 14 days of IH and 7 days of IHR (Figure 6). In contrast, there was a reduction in IGF-IR immunoreactivity (Figure 7).
Figure 5.
Effects of incremental IH episodes on IGF-I (A, B) and IGFBP-3 (C, D) levels in the liver of neonatal rats at P7 (7D-IH), P14 (14D-IH), or P21-IHR. Animals recovering from 7D-IH were placed in RA from P7-P21 and animals recovering from 14D-IH were placed in RA from P14-P21. Animals randomized to IH were exposed to brief clustered IH episodes during 50% O2. Animals randomized to hyperoxia were exposed to 50% O2 only. RA controls remained in atmospheric O2. Data are presented as mean±SEM (n=8 samples/group).
Figure 6.
Representative immunofluorescence stain of IGF-I liver sections from 14-day old and 21-day old rats exposed to 14 days of IH and 7 days of IHR, and exposed to 4, 8, and 12 IH episodes per day compared to RA. Images are 40X magnification. Scare bar represents 20 μm.
Figure 7.
Representative immunofluorescence stain of IGF-IR in liver sections from 14-day old and 21-day old rats exposed to 14 days of IH and 7 days of IHR, and exposed to 4, 8, and 12 IH episodes per day compared to RA. Images are 40X magnification. Scare bar represents 20 μm.
Effect of IH on Liver Leptin
Leptin levels in the liver are presented in Figure 8. Data showed that leptin levels normally increased at P14 and P21 in the RA groups, declined in the group recovering from Hx and elevated in the groups recovering from 2, 4, 8, and 10 IH episodes following 7 days of IH (Figure 8A). In the groups exposed to 14 days of IH, leptin levels also increased in the IHR groups exposed to 2 and 8 IH episodes, but remained suppressed in the groups exposed to 10 and 12 IH episodes (Figure 8B).
Figure 8.
Effects of incremental IH episodes on leptin levels in the liver of neonatal rats at P7 (7D-IH), P14 (14D-IH), or P21-IHR. Animals recovering from 7D-IH were placed in RA from P7-P21 and animals recovering from 14D-IH were placed in RA from P14-P21. Animals randomized to IH were exposed to brief clustered IH episodes during 50% O2. Animals randomized to hyperoxia were exposed to 50% O2 only. RA controls remained in atmospheric O2. Data are presented as mean±SEM (n=8 samples/group).
Effect of IH on Liver Histopathology
Figure 9 represents histopathology of the liver in response to IH. Representative samples from the RA groups are presented in the upper panel and representative samples from the groups exposed to IH and IHR are presented in the lower panel. In the 7-day IH group, there was increased cellular swelling, steatosis, necrosis and focal sinusoid congestion starting as early as 2 IH episodes per day. More severe apoptosis occurred with 6 IH episodes per day. Samples from animals exposed to 14 days of IH episodes exhibited less severe damage than that seen in the 7-day group, but necrosis and steatosis persisted in the groups exposed to 6–10 IH episodes. Exposure to 12 IH episodes showed evidence of blood sinusoid dilation and hemorrhage. Samples from animals exposed to 7 days of IH with 14 days of IHR showed no major evidence of necrosis, but there was evidence of cytoplasmic vacuolization, pervasive steatosis, blood sinusoid dilation and hemorrhage, particularly in the 12 IH/IHR group. Samples from animals exposed to 14 days of IH with 7 days of IHR also showed no major evidence of necrosis, but there was hepatocyte swelling, focal sinusoid congestion, and cytoplasmic vacuolization.
Figure 9.
Representative H & E stain of liver sections from RA controls at P7, P14 and P21 (upper panel), and P7, P14, P21 (7 days of IH with 14 days of RA recovery), and P21 (14 days of IH with 7 days of RA recovery) for 6–12 IH episodes per day. Images are 40X magnification. Scare bar represents 20 μm.
DISCUSSION
The overarching goal of the present investigation was to examine the effects of increasing IH episodes on somatic growth and factors that modulate early postnatal growth and development, and to establish whether recovery from IH causes catchup growth via rebound elevations in these growth factors. We, and others have previously established that IH causes oxidative stress in the liver leading to lipid peroxidation and damage [46,50–52]. It is also well known that IH-induced oxidative stress causes hapatosteatosis and metabolic dysfunction [53–55]. However, gaps in the knowledge remain regarding the number of IH episodes that may initiate these adverse responses, as well as the number that renders the liver incapable of recovery from the injury. By knowing this information, strategic pharmacologic interventions can be implemented. The results obtained from this study demonstrate that the liver is highly responsive to neonatal IH and susceptible to IH-induced damage which can develop even when the liver weight is spared. In the neonatal rat model, IH-induced hepatic compromise of GH/IGF-I axis is evidenced by the reductions in circulating and hepatic GH content, when exposed to as few as 4–6 IH episodes per day, suggesting that the neonatal pituitary cannot tolerate more than 2–4 IH episodes per day. IH also induced moderate deficits in weight gain with a greater magnitude of deficits in body length. This has major implications for nutritionally-deprived preterm neonates who experience IH episodes and AOP in the order of several hundreds over the first weeks of life [8]. Although the rats exhibited some catchup-up fat associated weight gain during the recovery/re-oxygenation period, their body length remained compromised. The similar discrepancy between the catch-up fat and body lengthening has been shown in low birth weight infants [56,57] and rats [58]. This catch-up fat phenomenon has been shown to be associated with metabolic dysfunction [59] and obesity [60] during adult life, and liver steatosis [61]. Our findings provide evidence that curtailing IH in preterm infants may not only have a significant impact on both growth and development, but also exert long-lasting adverse metabolic effects.
Adenohypophysis producing GH stimulates postnatal growth in various tissues with the liver being a major target. Several clinical studies have demonstrated prematurity is associated with growth factors deficiency, and GH supplementation has shown a beneficial, growth-promoting effect in both the short-and long-term [62–64]. However, during the perinatal period, GH does not appear to play a role in growth and IGF-I secretion [65,66], but may play a more important role in regulation of glucose metabolism. Our findings of IH-induced GH reductions is corroborated by previous studies which showed that IH suppresses the GH release and reduces GH mRNA expression in the anterior pituitary resulting in the low circulating GH and tissue distribution [67,68]. Low GH distribution to the liver will result in low IGF-I secretion and reduced growth. This was evidenced by the sustained shorter body length of the rats during IHR, further proving that increased body fat was responsible for the higher body weight. GHBP is produced in many tissues by either alternative GHR mRNA splicing (rodents) or cleavage from the extracellular domain of growth hormone receptor (humans). The liver is the major source for GHBP and its levels roughly parallel GHR expression, thus reflecting GHR status [69]. GHBP binds to about half of available GH. It prolongs GH half-life, and competes with GHR for binding of GH, and is associated with obesity [69,70]. Growth hormone deficiency is also associated with obesity [71]. It is interesting to note that despite the low GH levels, GHBP levels remained high, which may have contributed to metabolic dysregulation and the catchup fat phenomenon.
IGF-1 secretion is regulated by GH [72]. IGF-I and its binding proteins are more important regulators of neonatal growth than other growth factors [73]. The deficit of IGF-I during the perinatal period could increase the risk of major neonatal morbidities [74]. IGF-1 is protective against hypoxic injury by suppressing apoptosis [75]. In our study, IGF-I levels were higher in all IHR groups exposed to 14 days of IH, concurrent with higher body weight and deficits in body length within the groups, implicating IGF-I in the catchup fat phenomenon. IGF-I elevation in the presence of low GH suggest a GH-independent release of IGF-I. These high levels may exert a negative feedback on GH release, and play a key role in GH suppression in the catchup fat phenomenon. Leptin is secreted by adipocytes and plays important roles in energy expenditure and insulin sensitivity. It is also induced by hypoxia and is involved in the adaptive response to hypoxic ischemia [76,77]. This was corroborated in our study. Although the liver does not produce leptin under normal conditions, activated hepatic stellate cells can produce leptin as found in hepatic steatosis. Leptin prevents hepatic steatosis in animal models by interfering both lipid and glucose metabolism. In our study, IH did induce steatosis with concurrence of elevation of leptin levels. This is likely due to the effects of oxidation of fatty acids within hepatocytes which is a major source of ROS [78,79]. Inter-species differences in circulating GH profiles have been observed in mammals [80]. In males, GH secretion occurs nocturnally in humans and in 3–4 hour intervals in rodents; while in females, rats have residual GH levels between periods of GH secretion which are absent in humans and mice [81]. While these studies provide important information regarding the role of neonatal IH and IHR on growth and growth factors, there are limitations including species differences which should be considered when extrapolating rodent responses to humans. Furthermore, although the genders were studied at the same maturational age, differences between genders were not assessed given the sexual dimorphism of GH. Finally, although validation assays by the manufacturer showed not interference with binding proteins, similar validation assays were not conducted under hypoxia conditions to confirm no interference with IGFBP-1. Hence, interference by increases in IGFBP-1 in response to hypoxia may potentially lead to underestimates in IGF-I concentrations in the liver homogenates.
In conclusion, we have shown that IH can induce catchup fat and overweight with sustained deficits in body length, particularly during the recovery/re-oxygenation period. These effects are associated with altered distribution of GH to the liver, GH resistance, and elevations in hepatic GHBP, IGF-I, and leptin levels, as well as histopathological evidence of hepatic steatosis and damage. These findings suggest that long-term accumulative IH events may have a negative impact on growth and metabolic homeostasis in preterm infants who experience frequent arterial oxygen desaturations and apneas during oxygen therapy. Strict monitoring and curtailing IH episodes may decrease oxidative stress and ROS production, maintain normal GH/IGF-I pathway, and prevent hepatic injury and long-term IH-induced metabolic disorders.
Highlights.
Intermittent hypoxia (IH) damages hepatocytes and result in hepatic steatosis.
IH-induced hepatic damage is associated with alterations in growth factors.
IH-induced alterations in growth factors caused growth deficits and catchup fat.
Curtailing IH in preterm neonates may improve growth and long-term sequelae.
Acknowledgments
Financial support: This work was made possible through the Eunice Kennedy Shriver National Institute of Child Health & Human Development Grant # U54HD071594.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang HH, Larson J, Blencowe H, Spong CY, Simpson JL, Lawn LE. Preterm births in countries with a very high human development index – authors’ reply. Lancet. 2013;381:1356–1357. doi: 10.1016/S0140-6736(13)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 3.Lawn JE, Davidge R, Paul VK, et al. Born Too Soon: Care for the preterm baby. Reprod Health. 2013;10:S5. doi: 10.1186/1742-4755-10-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Dai Q, Xu Y, et al. Time trends and risk factor associated with premature birth and infants deaths due to prematurity in Hubei Province, China from 2001 to 2012. BMC Pregnancy Childbirth. 2015;15:329. doi: 10.1186/s12884-015-0767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The global burden of preterm birth. Lancet. 2009;374:1214. doi: 10.1016/S0140-6736(09)61762-1. No authors listed. [DOI] [PubMed] [Google Scholar]
- 6.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marconi AM. 37% of child survivors of intrauterine or neonatal insults experience at least one long-term sequela, the most common being neurodevelopmental delay. Evid Based Nurs. 2013;16:75–76. doi: 10.1136/eb-2012-100701. [DOI] [PubMed] [Google Scholar]
- 8.Martin RJ, Wang K, Köroğlu O, Di Fiore J, Kc P. Intermittent Hypoxic Episodes in Preterm Infants: Do They Matter? Neonatology. 2011;100:303–310. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farahani R, Kanaan A, Gavrialov O, et al. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol. 2008;43:20–28. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- 10.Pozo ME, Cave A, Köroğlu OA, et al. Effect of postnatal intermittent hypoxic on growth and cardiovascular regulation of rat pups. Neonatology. 2012;102:107–113. doi: 10.1159/000338096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrone S, Santacroce A, Picardi A, Buonocore G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J Clin Pediatr. 2016;5:172–181. doi: 10.5409/wjcp.v5.i2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada I, Tsutsumi O, Momoeda M, et al. Comparative concentrations of growth hormone-binding protein in maternal circulation, fetal circulation, and amniotic fluid. Endocr J. 1997;44:111–116. doi: 10.1507/endocrj.44.111. [DOI] [PubMed] [Google Scholar]
- 13.Tiong TS, Herington AC. Ontogeny of messenger RNA for the rat growth hormone receptor and serum binding protein. Mol Cell Endocrinol. 1992;83:133–141. doi: 10.1016/0303-7207(92)90154-x. [DOI] [PubMed] [Google Scholar]
- 14.Kargi AY, Merriam GR. Diagnosis and treatment of growth hormone deficiency in adults. Nat Rev Endocrinol. 2013;9:335–345. doi: 10.1038/nrendo.2013.77. [DOI] [PubMed] [Google Scholar]
- 15.Chia DJ. Minireview: Mechanisms of growth hormone-mediated gene regulation. Mol Endocrinol. 2014;28:1012–1025. doi: 10.1210/me.2014-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijayakumar A, Yakar S, LeRoith D. The intricate role of growth hormone in metabolism. Front Endocrinol (Lausanne) 2011;2:32. doi: 10.3389/fendo.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De La Puenta A, Goya L, Ramos S, Martín MA, Alvarez C, Escrivá F, Pascual-Leone AM. Effects of experimental diabetes on renal IGF/IGFBP system during neonatal period in the rat. Am J Physiol Renal Physiol. 2000;279:F1067–F1076. doi: 10.1152/ajprenal.2000.279.6.F1067. [DOI] [PubMed] [Google Scholar]
- 18.Cornblath M, Parker ML, Reisner SH, Forbes AE, Daughaday WH. Secretion and metabolism of growth hormone in premature and full-term infants. J Clin Endocrinol. 1965;25:209–218. doi: 10.1210/jcem-25-2-209. [DOI] [PubMed] [Google Scholar]
- 19.Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. 2016;14:3. doi: 10.1186/s12967-015-0762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Ercole AJ, Applewhite GT, Underwood LE. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980;75:315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- 21.Murphy LJ, Bell GI, Friesen HG. Tissue distribution of insulin-like growth factor I and II messenger ribonucleic acid in the adult rat. Endocrinology. 1987;120:1279–1282. doi: 10.1210/endo-120-4-1279. [DOI] [PubMed] [Google Scholar]
- 22.Sjögren K, Liu JL, Blad K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 24.Maki RG. Small is beautiful: insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28:4985–4995. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 26.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–31. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 27.Elijah IE, Branski LK, Finnerty CC, Herndon DN. The GH/IGF-I system in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:759–767. doi: 10.1016/j.beem.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlsson C, Mohan S, Sjögren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuemmerie JF. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol Metab Clin North Am. 2012;41:409–23. doi: 10.1016/j.ecl.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binoux M. GH, IGFs, IGF binding protein-3 and acid-labile subunit: what is the pecking order? Eur J Endocrinol. 1997;137:605–609. doi: 10.1530/eje.0.1370605. [DOI] [PubMed] [Google Scholar]
- 31.Donovan SM, Oh Y, Pham H, Rosenfeld RG. Ontogeny of serum insulin-like growth factor binding proteins in the rat. Endocrinology. 1989;125:2621–7. doi: 10.1210/endo-125-5-2621. [DOI] [PubMed] [Google Scholar]
- 32.Ibañez De Cáceres I, Villanúa MA, Soto L, Martin AI, López-Calderón AA. IGF-I and IGF-I-binding proteins in rats with adjuvant-induced arthritis given recombinant human growth hormone. J Endocrinol. 2000;165:537–44. doi: 10.1677/joe.0.1650537. [DOI] [PubMed] [Google Scholar]
- 33.LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev. 2008;5:739–743. [PubMed] [Google Scholar]
- 34.Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64:157–65. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 35.Hamrick MW, Dukes A, Arounleut P, et al. The adipokine leptin mediates muscle- and liver-derived IGF-1 in aged mice. Exp Gerontol. 2015;70:92–6. doi: 10.1016/j.exger.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak DA, et al. Disorders of the liver and biliary system relevant to clinical practice. In: McMillan JA, editor. Osk’s Pediatrics Principles and Practice. 3. Chapter 369. Philadelphia: Lipincott, Williams and Wilkins; 1999. pp. 1714–1737. [Google Scholar]
- 37.Hellström A, Ley D, Hansen-Pupp I, et al. Insulin- like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016;105:576–586. doi: 10.1111/apa.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkes CP, Grimberg A. Measuring Growth Hormone and Insulin-like Growth Factor-I in Infants: What is Normal? Pediatr Endocrinol Rev. 2013;11:126–146. [PMC free article] [PubMed] [Google Scholar]
- 39.Feng SZ, Tian JL, Zhang Q, et al. An experimental research on chronic intermittent hypoxia leading to liver injury. Sleep Breath. 2011;15:493–502. doi: 10.1007/s11325-010-0370-3. [DOI] [PubMed] [Google Scholar]
- 40.Jun J, Savransky V, Nanayakkara A, et al. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–R1281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savransky V, Bevans S, Nanayakkara A, et al. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871–G877. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 42.Chen XQ, Xu NY, Du JZ, Wang Y, Duan C. Corticotropin-releasing factor receptor subtype 1 and somatostatin modulating hypoxia-caused downregulated mRNA of pituitary growth hormone and upregulated mRNA of hepatic insulin-like growth factor-I of rats. Mol Cell Endocrinol. 2005;242:50–58. doi: 10.1016/j.mce.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Xu NY, Chen XQ, Du JZ, Wang TY, Duan C. Intermittent hypoxia causes a suppressed pituitary growth hormone through somatostatin. Neuro Endocrinol Lett. 2004;25:361–367. [PubMed] [Google Scholar]
- 44.Kokoszko A, Dabrowski J, Lewiński A, Karbownik-Lewińska M. Protective effects of GH and IGF-I against iron-induced lipid peroxidation in vivo. Exp Toxicol Pathol. 2008;60:453–458. doi: 10.1016/j.etp.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Youn YK, Suh GJ, Jung SE, Oh SK, Demling R. Recombinant human growth hormone decreases lung and liver tissue lipid peroxidation and increases antioxidant activity after thermal injury in rats. J Burn Care Rehabil. 1998;19:542–548. doi: 10.1097/00004630-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Cai CL, Aranda JV, Valencia GB, Xu J, Beharry KD. Chronic Intermittent Hypoxia Causes Lipid Peroxidation and Altered Phase 1 Drug Metabolizing Enzymes in the Neonatal Rat Liver. Reactive Oxygen Species. 2017;3:218–236. [PMC free article] [PubMed] [Google Scholar]
- 47.Aranda JV, Cai CL, Ahmad T, et al. Pharmacologic synergism of ocular ketorolac and systemic caffeine citrate in rat oxygen-induced retinopathy. Pediatr Res. 2016;80:554–565. doi: 10.1038/pr.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu C, Beharry KD, Shen X, et al. Proteomic profiling of the retinas in a neonatal rat model of oxygen-induced retinopathy with a reproducible ion-current-based MS1 approach. J Proteome Res. 2015;14:2109–2120. doi: 10.1021/pr501238m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beharry KD, Cai CL, Sharma P, et al. Hydrogen peroxide accumulation in the choroid during intermittent hypoxia increases risk of severe oxygen-induced retinopathy in neonatal rats. Invest Ophthalmol Vis Sci. 2013;54:7644–7657. doi: 10.1167/iovs.13-13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 1985;102:557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 51.Savransky V, Nanayakkara A, Vivero AJ, et al. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007–1013. doi: 10.1002/hep.21593. [DOI] [PubMed] [Google Scholar]
- 52.Feng SZ, Tian JL, Zhang Q, et al. An experimental research on chronic intermittent hypoxia leading to liver injury. Sleep Breath. 2011;15:493–502. doi: 10.1007/s11325-010-0370-3. [DOI] [PubMed] [Google Scholar]
- 53.Briançon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7:25. doi: 10.1186/s13098-015-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olea E, Agapito MT, Gallego-Martin T, et al. Intermittent hypoxia and diet-induced obesity: effects on oxidative status, sympathetic tone, plasma glucose and insulin levels, and arterial pressure. J Appl Physiol (1985) 2014;117:706–719. doi: 10.1152/japplphysiol.00454.2014. [DOI] [PubMed] [Google Scholar]
- 55.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 2011;19:2167–2174. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monset-Couchard M, de Bethmann O. Catch-up growth in 166 small-for-gestational age premature infants weighing less than 1, 000 g at birth. Biol Neonate. 2000;78:161–167. doi: 10.1159/000014265. [DOI] [PubMed] [Google Scholar]
- 57.Cho WK, Suh BK. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr. 2016;59:1–7. doi: 10.3345/kjp.2016.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marcelino H, Veyrat-Durebex C, Summermatter S, et al. A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth: a Randle cycle favoring fat storage. Diabetes. 2013;62:362–72. doi: 10.2337/db12-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nobili V, Alisi A, Panera N, Agostoni C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten-year systematic review. Pediatr Endocrinol Rev. 2008;6:241–247. [PubMed] [Google Scholar]
- 60.Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr Diab Rep. 2013;13:27–33. doi: 10.1007/s11892-012-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faienza MF, Brunetti G, Ventura A, et al. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: influence of rapid weight catch-up growth. Horm Res Paediatr. 2013;79:103–109. doi: 10.1159/000347217. [DOI] [PubMed] [Google Scholar]
- 62.Smeets CC, Codd V, Denniff M, Samani NJ, Hokken-Koelega AC. Effects of size at birth, childhood growth patterns and growth hormone treatment on leukocyte telomere length. PLoS One. 2017;12:e0171825. doi: 10.1371/journal.pone.0171825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Labarta JI, Ruiz JA, Molina I, De Arriba A, Mayayo E, Longás AF. Growth and growth hormone treatment in short stature children born small for gestational age. Pediatr Endocrinol Rev. 2009;6:350–357. [PubMed] [Google Scholar]
- 64.Kovács GT, Oh J, Kovács JB, et al. Growth promoting effects of growth hormone and IGF-I are additive in experimental uremia. Kidney Int. 1996;49:1413–21. doi: 10.1038/ki.1996.199. [DOI] [PubMed] [Google Scholar]
- 65.Wollmann HA. Growth hormone and growth factors during perinatal life. Horm Res. 2000;53:50–54. doi: 10.1159/000053205. [DOI] [PubMed] [Google Scholar]
- 66.Skalkidou A, Petridou E, Papathoma E, Salvanos H, Trichopoulos D. Growth velocity during the first postnatal week of life is linked to a spurt of IGF-I effect. Paediatr Perinat Epidemiol. 2003;17:281–286. doi: 10.1046/j.1365-3016.2003.00494.x. [DOI] [PubMed] [Google Scholar]
- 67.Xu NY, Chen XQ, Du JZ, Wang TY, Duan C. Intermittent hypoxia causes a suppressed pituitary growth hormone through somatostatin. Neuro Endocrinol Lett. 2004;25:361–367. [PubMed] [Google Scholar]
- 68.Nair D, Ramesh V, Li RC, Schally AV, Gozal D. Growth hormone releasing hormone (GHRH) signaling modulates intermittent hypoxia- induced oxidative stress and cognitive deficits in mouse. J Neurochem. 2013;127:531–540. doi: 10.1111/jnc.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baumann G. Growth hormone binding protein. J Pediatr Endocrinol Metab. 2001;14:355–375. doi: 10.1515/jpem.2001.14.4.355. [DOI] [PubMed] [Google Scholar]
- 70.Baumann G. Growth hormone binding protein. The soluble growth hormone receptor. Minerva Endocrinol. 2002;27:265–76. [PubMed] [Google Scholar]
- 71.Lewitt MS. The role of the growth hormone/insulin-like growth factor system in visceral adiposity. Biochem Insights. 2017;10:1178626417703995. doi: 10.1177/1178626417703995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oberbauer AM. The regulation of IGF-1 gene transcription and splicing during development and aging. Front Endocrinol (Lausanne) 2013;4:39. doi: 10.3389/fendo.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skalkidou A, Petridou E, Papathoma E, Salvanos H, Trichopoulos D. Growth velocity during the first postnatal week of life is linked to a spurt of IGF-I effect. Paediatr Perinat Epidemiol. 2003;17:281–286. doi: 10.1046/j.1365-3016.2003.00494.x. [DOI] [PubMed] [Google Scholar]
- 74.Hellström A, Engström E, Hård AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 75.Seigel GM, Chiu L, Paxhia A. Inhibition of neuroretinal cell death by insulin-like growth factor-1 and its analogs. Mol Vis. 2000;6:157–163. [PubMed] [Google Scholar]
- 76.Nüsken E, Herrmann Y, Wohlfarth M, et al. Strong hypoxia reduces leptin synthesis in purified primary human trophoblasts. Placenta. 2015;36:427–32. doi: 10.1016/j.placenta.2015.01.191. [DOI] [PubMed] [Google Scholar]
- 77.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Path. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Netzer N, Gatterer H, Faulhaber M, Burtscher M, Pramsohler S, Pesta D. Hypoxia, Oxidative Stress and Fat. Biomolecules. 2015;5:1143–1150. doi: 10.3390/biom5021143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chatterjee S, Ganini D, Tokar EJ, et al. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J Hepatol. 2013;58:778–784. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vijayakumar A, Novosyadlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res. 2010;20:1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shapiro BH, Agrawal AK, Pampori NA. Gender Differences in Drug Metabolism Regulated by Growth Hormone. Int J Biochem Cell Biol. 1995;27:9–20. doi: 10.1016/1357-2725(94)00056-5. [DOI] [PubMed] [Google Scholar]