Abstract
Background
Preterm birth is linked to cardiovascular risks and diseases. Endothelial progenitor cells play a critical role in vascular development and repair. Cord blood endothelial progenitor cells of preterm‐born infants, especially endothelial colony‐forming cells (ECFC), show enhanced susceptibility to prematurity‐related pro‐oxidant stress. Whether ECFC dysfunction is present in adulthood following preterm birth is unknown.
Methods and Results
This cross‐sectional observational study includes 55 preterm‐born (≤29 gestational weeks) young adults (18–29 years old, 38% male) and 55 sex‐ and age‐matched full‐term controls. ECFC were isolated from peripheral blood; cell proliferative and vascular cord formation capacities were assessed in vitro. Daytime systolic blood pressure was higher, whereas glucose tolerance and body mass index were lower in preterm‐born subjects. ECFC colonies grew in culture for 62% of full‐term‐ and 58% of preterm‐born participants. Preterm‐born participants have formed ECFC colonies later in culture and have reduced proliferation compared with controls. Only in preterm‐born individuals, we observed that the later the ECFC colony grows in culture, the worse was overall ECFC function. In addition, in preterms, elevated systolic blood pressure significantly correlated with reduced ECFC proliferation (rS=−0.463; P=0.030) and numbers of branches formed on matrigel (rS=−0.443; P=0.039). In preterm‐born subjects, bronchopulmonary dysplasia was associated with impaired ECFC function, whereas exposure to antenatal steroids related to better ECFC function.
Conclusions
This study is the first to examine ECFC in preterm‐born adults and to demonstrate ECFC dysfunction compared with full‐term controls. In the preterm‐born group, ECFC dysfunction was associated with bronchopulmonary dysplasia, the major prematurity‐related neonatal morbidity, and with increased systolic blood pressure into adulthood.
Keywords: bronchopulmonary dysplasia, cardiovascular disease risk factors, endothelial progenitor cells, hypertension, pregnancy and postpartum, preterm birth
Subject Categories: High Blood Pressure, Translational Studies, Angiogenesis, Endothelium/Vascular Type/Nitric Oxide, Cell Biology/Structural Biology
Clinical Perspective
What Is New?
This study is the first to examine endothelial colony‐forming cells (ECFC) in preterm‐born adults and to demonstrate ECFC dysfunction in preterm‐born subjects compared with full‐term adults.
What Are the Clinical Implications?
This study demonstrates that preterm‐born adults have dysfunctional ECFC compared with full‐term controls.
ECFC dysfunction in preterm‐born subjects was linked to elevated systolic blood pressure and major prematurity‐related morbidity as newborns such as bronchopulmonary dysplasia.
The first generations of adults born very preterm are reaching an age at which the incidence of cardiovascular diseases rises; it is therefore important to determine whether ECFC can serve as a biomarker for cardiovascular risk and to monitor novel therapies, including for vascular repair.
Introduction
Ten percent of current‐day children and young adults were born preterm (<37 weeks gestational age) (March of Dimes http://www.marchofdimes.com/mission/globalpreterm.html). Mounting evidence supports the adverse effect of preterm birth, and more so very preterm birth (<32 weeks gestational age), on cardiovascular health.1, 2 Individuals born preterm display both structural and functional cardiovascular alterations, which include higher blood pressure,2 microvasculature rarefaction,3, 4 increased vascular resistance,5 as well as changes in heart shape and function,6, 7, 8 which are independent harbingers of hypertension and cardiovascular disease.9, 10 Given that rates of preterm birth have increased along with improved survival, it is expected that the absolute number of adults born preterm, and therefore at risk of developing cardiovascular disease, will escalate in the future. Understanding the mechanisms underlying long‐term cardiovascular changes after preterm birth is crucial to allow for intervention before, or early in the process of, overt cardiovascular disease with therapies targeted to their specific physiology.
Endothelial progenitor cells (EPC) are key to cardiovascular development and integrity.11 These bone marrow–derived cells proliferate and differentiate into mature endothelial cells that contribute to blood vessel formation and growth.11 They also play an important role during vascular repair and regeneration by homing to sites of tissue injury and activating the migration and proliferation of in situ endothelial cells.12, 13 These processes are critical during both organogenesis and postnatal growth and development. However, preterm birth is associated with perinatal conditions that are deleterious for organ development, and often inherently prooxidative (including the major rise in po 2 occurring during the transition from fetal to extrauterine life) and proinflammatory in nature.14, 15 Importantly, studies have shown that EPC of preterm infants, more specifically the subpopulation of endothelial colony‐forming cells (ECFC), have an increased vulnerability to hyperoxia‐induced oxidative stress resulting in cell dysfunction.16, 17 ECFC, although highly present in the cord blood of newborn infants, are only found in very low quantities in the peripheral circulation of adults.12, 18, 19 When primary ECFC are successfully expanded from cord or peripheral blood mononuclear cells (PBMC), they form cobblestone‐patterned colonies that are visible after 7 to 25 days in culture; accordingly ECFC are also named late‐outgrowth EPC.12, 13 These cells are capable of self‐renewal, high proliferation rates, as well as in vitro and in vivo angiogenesis.13, 19 To our knowledge, no study has examined ECFC from preterm‐born subjects beyond infancy.
We therefore hypothesized that very preterm birth (≤29 weeks of gestation) is associated with peripheral blood ECFC dysfunction in early adulthood. This study specifically aimed to (1) compare ECFC function of young adults born very preterm with full‐term controls; (2) examine the relationship between ECFC function and adult cardiovascular measures; and (3) examine ECFC function in the preterm group in relation to perinatal factors.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure after other ongoing analyses of this cohort will be completed.
Study Design and Population
This cross‐sectional observational study enrolled 55 young adults aged 18 to 29 years that were born preterm at ≤29 gestational weeks between 1987 and 1997, and 55 controls born at term (37–42 gestational weeks) matched for age and sex (23±2 years, 38% male in both groups). Former preterm infants admitted to the neonatal intensive care unit of Sainte‐Justine University Hospital (Centre Hospitalier Universitaire Sainte‐Justine, Montreal, Quebec, Canada) were traced from lists of patients from the neonatal follow‐up clinic, and 42 participants were recruited. An additional 13 preterm participants were recruited from advertisement targeted to patients born prematurely at any neonatal intensive care units in the province of Quebec. Gestational age was calculated from the date of last menstrual period or by obstetric ultrasound at <24 weeks. We excluded participants currently pregnant or with severe neurosensory deficit preventing test completion. Compared with all eligible preterms born at Centre Hospitalier Universitaire Sainte‐Justine, the participants of the current study were similar in gestational age, birth weight, sex, incidence of small for gestational age, bronchopulmonary dysplasia (BPD), severe brain injury, and retinopathy of prematurity. This study was approved by the institutional human research ethics review board (Comité d’Éthique de la Recherche du Centre Hospitalier Universitaire Sainte‐Justine) and informed written consent was obtained from all participants.
Perinatal Data
Perinatal data were collected from medical charts for all preterm‐born participants. BPD, the most frequent major neonatal morbidity associated with very preterm birth, was defined as supplemental oxygen requirements at 36 weeks of postmenstrual age.20 For full‐term controls, we used medical charts, their immunization booklet (which contains birth information), or maternal recall in cases where no records were available. Participants completed questionnaires on lifestyle habits including smoking and medical history.
Blood Sampling, Cell Isolation, and Culture
ECFC were isolated from venous peripheral blood following a protocol adapted from Martin‐Ramirez et al.21 A total volume of 24 mL was collected per individual, and PBMC were separated using Ficoll‐Paque PLUS (GE Healthcare Life Sciences, Pittsburgh, PA) density gradient following 30 minutes 300g centrifugation at room temperature. PBMC were further washed twice with Dulbecco's phosphate‐buffered saline (Gibco by Life Technologies, Burlington, ON, Canada) and subsequently plated on collagen I‐coated (Corning, Corning, NY) 25 cm2 tissue culture–treated Falcon flasks (Thermo Fisher Scientific, Waltham, MA) at a density of 5.0×106 cells/flask. Cultured cells were maintained at 37°C, 21% O2 and 5% CO2, using complete endothelial cell growth medium‐2 (Lonza, Basel, Switzerland) supplemented with 1% penicillin/streptomycin (Gibco by Life Technologies) and 10% fetal bovine serum. Media was changed every 2 or 3 days, and cells were maintained for up to 30 days in culture. PBMC cultures were observed daily from days 7 to 30 to determine the first day of cobblestone‐patterned ECFC colony formation. ECFC colonies were then passaged and further expanded under similar conditions. ECFC function was assessed using cells from the second passage.
ECFC Phenotype and Functional Assessments
ECFC phenotype was examined in 3 samples to confirm ECFC clonogenic potential and the expression of classic ECFC surface markers including CD31 and CD34 (Figure). Paraformaldehyde‐fixed cells were co‐immunostained with anti‐CD31 (Abcam, ab76533), anti‐CD34 (Abcam, ab185732), and 4′,6‐diamidino‐2‐phenylindole (DAPI) to mark cell nuclei, and imaged using a fluorescence microscope (Leica), as shown in Figure A. In addition, ECFC clonogenic capacity was tested by diluting 104 ECFC to obtain 5 to 10 individual cells per 1 mL of endothelial cell growth medium‐2. Cells were plated in collagen‐coated (Corning) 24‐wells plates (Thermo Fisher Scientific) and observed using a phase contrast microscope (Leica) after 6, 48, and 96 hours for ECFC colony growth.
Figure 1.
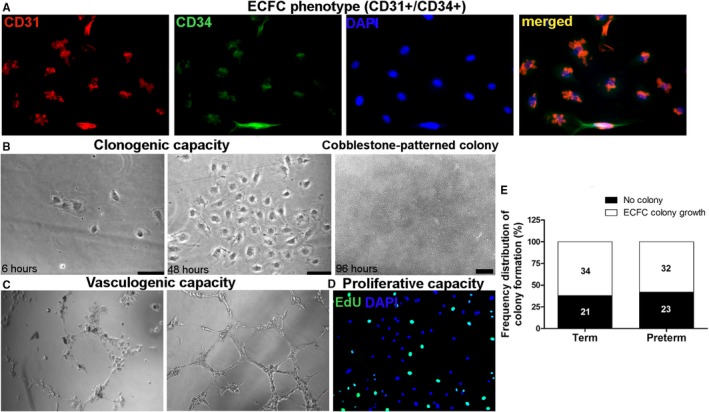
Endothelial colony‐forming cell (ECFC) phenotype, functional characteristics, and frequency distribution of time until ECFC colony formation in full‐term and preterm groups. A, ECFC phenotype shown by cells expressing CD31 (AlexaFluor 555, red), CD34 (AlexaFluor 488, green), DAPI (blue), and all merged. B, Representative images of ECFC clonogenic capacity and cobblestone‐patterned colony formation after 6 and 48 hours, and later cobblestone‐patterned colony formation while in culture; phase contrast imaging using 20× (50‐μm scale) and 5× objectives. C, ECFC vasculogenic capacity demonstrated by branches and tube formation on Matrigel, phase contrast imaging using 10× objective. D, ECFC proliferation by DNA EdU incorporation (AlexaFluor 488, green) and nonproliferative ECFCs marked with DAPI (blue), 10× objective. E, Frequency distribution of ECFC colony growth grouped as ECFC colony growth (from 7 to 30 days after plating PBMC) and no colony growth observed, in full‐term controls and preterm‐born subjects. The number of subjects in each category is shown in the histogram bars. DAPI indicates 4′,6‐diamidino‐2‐phenylindole; EdU, 5‐ethynyl‐2′‐deoxyuridine; PBMC, peripheral blood mononuclear cells.
In all subjects for whom ECFC colonies grew, ECFC function was determined by cellular proliferation rate, as well as by the in vitro capacity to form branches or closed tubes on Matrigel (Corning), which are hallmarks of in vitro vascular cord formation and vasculogenic capacity.
Proliferation rate was assessed through the quantification of 5‐ethynyl‐2′‐deoxyuridine (EdU) cellular incorporation using the Click‐iT EdU Alexa Fluor 488 Imaging kit (Thermo Fisher Scientific). ECFCs (4.0×104) were plated on collagen‐coated flasks of 1.86 cm2 surface area, maintained with complete endothelial cell growth medium‐2 and grown for 24 hours. Cells were then incubated with 10 μmol/L EdU in complete endothelial cell growth medium‐2 (Lonza) for 4.5 hours at 37°C, then fixed with 3.7% formaldehyde in PBS, permeabilized with 0.5% Triton X‐100 in PBS, and stained according to kit instructions. Assays were performed in triplicates and cell images were obtained using a fluorescence microscope (Leica) and a 10× objective. The number of cells under proliferation and incorporating EdU into their DNA, stained positive for EdU (AlexaFluor 488), were counted in 3 to 5 full pictures per assay, as well as the total number of cells with nuclei stained with DAPI; the percentage of proliferating cells (a measure of proliferation rate) was then calculated.
In vitro vascular cord formation was assessed by plating 1.5×104 cells on 50 μL of growth factor reduced basement membrane matrix (Matrigel) in a 96‐well plate. Cells were imaged using a phase contrast microscope (Leica) and 10× objective after 6 hours of incubation at 37°C. Assays were performed in triplicates, and the number of closed tubes and branches formed (not necessarily forming closed tubes) were quantified in 3 to 6 random images per participant using ImageJ software (National Institutes of Health). All ECFC functional assessments were performed blinded to the participants’ status.
Clinical Assessment of Cardiovascular Risk Factors
Anthropometric measures included weight, height, and calculation of body mass index. Blood glucose levels were measured after 12‐hour fasting, and again at 2 hours following a 75‐g oral glucose tolerance test. Peak aerobic exercise capacity (peak VO2) was assessed in 43 subjects per group on a cycle ergometer (Corrival, Lode, Netherlands) using a ramped exercise protocol at room air with patients breathing through a facemask connected to a gas analysis system (Jaeger CareFusion, Yorba Linda, CA). Peak VO2 was indexed to lean body mass determined by dual‐energy X‐ray absorptiometry (GE Healthcare, Chicago, IL).
Blood Pressure Measurements
Brachial blood pressure was measured with subjects in a sitting position using an automated oscillometric device (DINAMAP model DPC300M‐CF; GE Medical Systems Information Technologies Inc., Milwaukee, WI) in both arms and the average value was recorded. Participants also received an appropriately sized (nondominant arm) 24‐hour ambulatory blood pressure measurement (ABPM) device (model 902007; Spacelabs Medical Inc., Redmond, WA) that recorded blood pressure every 15 minutes for a 24‐hour period. For this analysis we only considered awake (daytime) blood pressure recordings since many participants removed the device, especially to sleep. Thirty‐six preterm and 33 full‐term had daytime ABPM recordings available for analyses. There was no difference in the clinical characteristics of study participants born full‐term and preterm with and without daytime ABPM (Table S1).
Echocardiography
Echocardiographic imaging was performed by trained technicians blinded to prematurity status using a GE Vivid E9 ultrasound system, and the images were analyzed offline to measure left ventricular mass index and ejection fraction22, 23 with EchoPac PC software (both GE Healthcare, Horten, Norway). The mean of triplicate measurements were utilized for analysis.
Statistical Analyses
Baseline characteristics of the term and preterm groups were compared using independent t test for continuous variables and a χ2 test or Fisher exact tests for categorical variables. Between‐group comparisons for ECFC functional data were performed using independent‐samples Mann–Whitney U test, given the lack of correlation within pairs despite study design (Cohen's kappa for ECFC growth within pairs=0.017, P=0.902). Rho Spearman correlation analyses were conducted to assess the relationship between ECFC function and cardiovascular parameters with 95% confidence intervals estimated by Bootstrap analysis. Finally, independent‐samples Mann–Whitney U test was performed to examine ECFC function among participants born preterm in relation to perinatal characteristics. All analyses were conducted using SPSS 21 (IBM, North Castle, NY). P<0.05 were considered statistically significant.
Results
Population Description
Perinatal characteristics, anthropometric measures, and cardiovascular risk factors for the preterm‐ and full‐term‐born participants are presented in Table 1. Daytime ABPM systolic blood pressures and 2‐hour glycemia were significantly higher, whereas body mass index was significantly lower in the preterm group.
Table 1.
Perinatal and Adult Characteristics of Young Adults Born Full‐Term and Preterm
| Clinical Characteristics | Term (n=55) | Preterm (n=55) | P Value |
|---|---|---|---|
| Perinatal characteristics | |||
| Gestational age, wks | 39.4±1.3 | 27.2±1.3 | |
| Birth weight, g | 3384±385 | 993±226 | |
| Small for gestational age, n (%)a | 3 (6) | 2 (4) | 0.647 |
| Hypertensive complications of pregnancy, n (%) | 3 (6) | 8 (15) | 0.112 |
| Antenatal corticosteroids, n/N (%) | N/A | 21/52 (40) | |
| Bronchopulmonary dysplasia, n/N (%)b | N/A | 15/54 (28) | |
| Adult characteristics | |||
| Age, y | 23±2 | 23±2 | 0.389 |
| Male sex, n (%) | 21 (38) | 21 (38) | 1.00 |
| Height, cm | 169±8 | 166±9 | 0.059 |
| Weight, kg | 69±15 | 61±12 | 0.001 |
| BMI, kg/m2 | 24±4 | 22±3 | 0.006 |
| Peak VO2, mL/min per kg of lean body massc | 33±9 | 32±8 | 0.660 |
| Smoking, n (%) | 9 (16) | 12 (22) | 0.471 |
| 2‐h glucose, mmol/L | 6.3±1.3 | 7.0±1.4 | 0.006 |
| Resting SBP, mm Hg | 115±10 | 118±10 | 0.199 |
| Resting DBP, mm Hg | 66±8 | 68±7 | 0.104 |
| Daytime ABPM SBP, mm Hgd | 120±7 | 124±10 | 0.049 |
| Daytime ABPM DBP, mm Hgd | 72±5 | 74±6 | 0.098 |
| Left ventricular mass index, g/m2 | 63±13 | 62±13 | 0.509 |
| Left ventricular ejection fraction, % | 62±5 | 64±6 | 0.130 |
Data are mean±SD unless stated otherwise. Independent t test or χ2 test. BMI indicates body mass index; DBP, diastolic blood pressure; N/A, not applicable; SBP, systolic blood pressure.
Birth weight <10th percentile for gestational age.
Supplemental oxygen requirement at 36 wks post menstrual age.
VO2, peak oxygen consumption measured in a subgroup of 43 participants per group.
ABPM (ambulatory blood pressure monitoring) determined in a subgroup of 33 term and 36 preterm subjects.
In Vitro ECFC Characteristics in Young Adults Born Preterm Versus Full Term
Peripheral blood ECFC phenotype and functional characteristics, including in vitro clonogenic, vasculogenic, and proliferative capacities, are illustrated in Figure. Cobblestone‐patterned ECFC colonies grew in 34/55 (62%) of full‐term‐ and 32/55 (58%) of preterm‐born participants’ PBMC after a maximum of 30 days in culture (Figure E).
Perinatal and adult characteristics from individuals for whom ECFC colonies grew are shown in Table S2.
Table 2 shows comparisons of all ECFC functional parameters between term and preterm groups. The time for ECFC growth in culture before the first colony was observed was significantly longer in the preterm subjects, and the proliferation rate was significantly reduced in young adults born preterm compared with term controls (Table 2). However, significant between‐group differences in number of closed tubes and number of branches formed were not identified.
Table 2.
ECFC Characteristics in Young Adults Born Full‐Term Versus Preterm
| ECFC Characteristics | Term (N) | Preterm (N) | P Value | ||
|---|---|---|---|---|---|
| Time to first ECFC colony formation, days, median (IQR) | 34 | 12 (11–16) | 32 | 15 (12–19) | 0.028a |
| Proliferation rate, % of total cells, median (IQR) | 31 | 27 (17–35) | 31 | 20 (15–27) | 0.042a |
| Closed tubes formed, n per 1.5×104 ECFC, median (IQR) | 31 | 8 (1–15) | 31 | 5 (1–10) | 0.126 |
| Branches formed, n per 1.5×104 ECFC, median (IQR) | 31 | 32 (16–38) | 31 | 25 (14–35) | 0.310 |
Data assessed by independent samples Mann–Whitney U test. ECFC indicates endothelial colony‐forming cells; IQR, interquartile range.
P<0.05.
In the preterm group, we observed a correlation between the number of days to form the first ECFC colony and ECFC proliferative and vascular cord formation capacity (Table 3). The longer the onset of first ECFC colony formation, the lower the proliferation rate and the number of closed tube and branch formation were, which was not changed when stratifying samples by sex. This relationship was not observed in the term group (Table 3).
Table 3.
Association Between the Number of Days to First ECFC Colony Growth and ECFC Proliferative and Vasculogenic Capacity in Young Adults Born Full‐Term and Preterm
| Rho Spearman Correlations (95% Confidence Interval) | |||
|---|---|---|---|
| Proliferation Ratea | Closed Tubes Formedb | Branches Formedb | |
| Days to grow the first ECFC colony | |||
| Term | −0.24 (−0.55, 0.13) | −0.24 (−0.60, 0.18) | −0.19 (−0.53, 0.19) |
| Preterm | −0.53 (−0.81, −0.17)c | −0.45 (−0.80, −0.06)c | −0.40 (−0.71, −0.05)c |
ECFC indicates endothelial colony‐forming cells.
% of total cells.
n per 1.5×104 ECFC. Rho Spearman correlations.
P<0.05.
Relationship Between ECFC Function and Cardiovascular Risk Factors in Young Adults Born Preterm Versus Full‐Term
We further investigated whether ECFC characteristics correlated with adult cardiovascular risk factors known to be associated with very preterm birth, in particular, blood pressure2, 24 and left ventricular mass index.6 Table 4 shows the relationship between ECFC function and daytime ABPM systolic blood pressure and left ventricular mass index in preterm and term groups. In preterm participants, there was an inverse association between the proliferation rate and the number of branches formed on matrigel with systolic blood pressure values. In the full‐term participants, no significant correlation was detected between ECFC functional characteristics and systolic blood pressure.
Table 4.
Correlational Analyses of ECFC Characteristics and Cardiovascular Risk Factors in Young Adults Born Full‐Term and Preterm
| ECFC Characteristics | Rho Spearman Correlation Coefficients (95% Confidence Interval) | |||
|---|---|---|---|---|
| Daytime ABPM SBP, mm Hg | LV Mass Index, g/m2 | |||
| Term | n | n | ||
| Days to first ECFC colony | 20 | −0.25 (−0.68, 0.18) | 30 | −0.34 (−0.66, 0.07) |
| Proliferation ratea | 20 | 0.30 (−0.25, 0.71) | 30 | 0.41 (0.05, 0.67)b , c |
| Closed tubes formedc | 20 | 0.19 (−0.29, 0.57) | 30 | 0.11 (−0.18, 0.39) |
| Branches formedc | 20 | 0.24 (−0.18, 0.59) | 30 | 0.26 (0.01, 0.49) |
| Preterm | ||||
| Days to first ECFC colony | 22 | 0.248 (−0.219, 0.622) | 30 | 0.198 (−0.165, 0.536) |
| Proliferation ratea | 22 | −0.46 (−0.76, −0.04)b | 30 | −0.202 (−0.583, 0.193) |
| Closed tubes formedc | 22 | −0.28 (−0.65, 0.25) | 30 | −0.05 (−0.42, 0.30) |
| Branches formedc | 22 | −0.44 (−0.77, 0.04)b | 30 | −0.16 (−0.49, 0.22) |
ABPM indicates ambulatory blood pressure monitoring; ECFC, endothelial colony‐forming cells; LV, left ventricular; SBP, systolic blood pressure.
% of total cells.
P<0.05.
n per 1.5×104 ECFC. Rho Spearman correlations.
ECFC functional characteristics did not correlate with left ventricular mass index in the preterm group (Table 4). However, only in term‐born participants, enhanced ECFC proliferation rate was associated with higher left ventricular mass index. After additional analyses performed a posteriori to explore whether the result could be confounded by ejection fraction and maximum oxygen consumption (VO2), as proxy measures of physical fitness, this relationship was no longer significant (rS=0.325, P=0.140).
Relationship Between ECFC Function and Perinatal Factors in Young Adults Born Preterm
Colonies grew in 6/15 (40%) preterm individuals with BPD versus 25/39 (64%) individuals without, which was not statistically significantly different. Similarly, colonies grew in 13/21 (62%) preterm‐born participants exposed to antenatal steroids versus 17/31 (55%) individuals without, also not significantly different.
Young adults with BPD had overall impaired ECFC function as shown by a lower ECFC proliferation rate, lower number of closed tubes and of branches formed (Table 5). Moreover, exposure to antenatal steroids was associated with a shorter time to grow the first ECFC colony, and an increase in the number of branches formed (Table 5).
Table 5.
ECFC Function Among Participants Born Preterm in Relation to Perinatal Factors
| Neonatal Characteristics | ECFC Function, Median (IQR) | ||||
|---|---|---|---|---|---|
| N | Days to First ECFC Colony | Proliferation Ratea | Closed Tubes Formedb | Branches Formedb | |
| Male | 13 | 17.0 (12.0;19.5) | 16.5 (9.2; 26.0) | 2.8 (0.1; 6.5) | 22.0 (8.5; 30.5) |
| Female | 18 | 14.5 (11.8; 16.8) | 22.5 (16.3; 27.4) | 5.4 (3.0; 15.0) | 27.3 (19.1; 35.5) |
| Antenatal steroids | 12 | 12.5 (10.2; 15.5) | 23.8 (16.1; 32.8) | 6.4 (3.6; 15.7) | 33.8 (23.8; 37.0) |
| No antenatal steroids | 17 | 16.0 (13.0; 19.5)§ | 18.0 (9.2; 24.6) | 4.4 (0.1; 9.0) | 20.7 (5.3; 28.2)§ |
| BPD | 6 | 19.5 (13.5; 21.2) | 12.0 (0.0; 16.8) | 2.0 (0.0; 4.7)§ | 12.8 (2.4; 25.5)§ |
| No BPD | 24 | 14.0 (11.2; 16.8) | 22.5 (16.1; 28.0)∥ | 5.2 (2.2; 13.7)∥ | 27.3 (19.6; 35.7)∥ |
Data assessed by independent samples Mann–Whitney U test. BPD indicates bronchopulmonary dysplasia; ECFC, endothelial colony‐forming cells; IQR, interquartile range.
% of total cells.
n per 1.5×104 ECFC.
§ P<0.05 vs antenatal steroids group and ∥ P<0.05 vs BPD group.
Discussion
This study showed that ECFC from young adults born very preterm were less proliferative and grew colonies significantly more slowly compared with controls born full term. More specifically, in preterm subjects only, the later the time to grow an ECFC colony at first isolation step, the worse the overall ECFC function. Additionally, in preterm, but not in term subjects, impaired ECFC proliferation and vasculogenic capacity, indicated by reduction of the numbers of branches formed on matrigel, were associated with higher systolic blood pressure into adulthood. This study also finds that young adults born preterm who had BPD, the most common major complication of preterm birth characterized by impaired/interrupted pulmonary tissue and vascular development,25, 26 displayed poorer ECFC function. Conversely, exposure to antenatal steroids, which are administered to enhance fetal lung maturation and are associated with reduced neonatal morbidity and mortality,27 was associated with faster ECFC growth and enhanced in vitro vasculogenic capacity in early adulthood.
Reduced EPC counts and/or impaired ECFC function have been proposed as markers of cardiovascular and metabolic disease risks.28, 29 Studies have reported an inverse correlation between the number of other types of EPC such as the colony‐forming units (or myeloid angiogenic cells) or overall EPC counts (circulating CD34+KDR+ cells), and hypertension, diabetes mellitus, combined Framingham risk factor score, as well as cardiovascular events and death.30, 31, 32 A significant relationship between colony‐forming units and EPC counts (CD133(+) or CD34(+)/KDR(+)) and function, and vascular endothelial function was also reported in patients with cardiovascular diseases.30, 31, 33 The literature presents different EPC subtypes classified according to specific cellular characteristics and function. The myeloid‐like colony‐forming units type of EPC, also called early outgrowth EPC, can form colonies within 4 days after PBMC culture and last no longer than 7 to 9 days in culture.12, 13, 34 These cells and colonies cannot be expanded and have no specific growing pattern, which also clearly differentiate them from the ECFC subtype.12, 13 ECFC, also called late outgrowth EPC, can form well‐defined cobblestone‐patterned colonies from days 7 to 30 in culture.13, 19 Unlike hematopoietic progenitor cells, ECFC do not express CD133 and CD45.19, 35 However, the presence of CD34 and CD31 markers added to their clonogenic and vasculogenic potential confers to these cells a progenitor and endothelial phenotype.19 They additionally present remarkable self‐renewable and expansion capacities in vitro, which also confer to this subtype of EPC higher angiogenic and regenerative potentials.12, 13, 36
Prematurity is now a well‐recognized risk factor for hypertension and cardiovascular disease.2, 37, 38 The differences we observed in blood pressure values between preterm and term participants are consistent with reported data.2, 39 To our knowledge, no previous study has examined any sort of EPC in adults born preterm. Our findings show that the in vitro angiogenic function of the ECFC subtype was impaired in preterm versus full‐term subjects, suggesting a long‐term impact of preterm birth on this cell function. During fetal and neonatal development, ECFC actively participate in vasculogenesis and angiogenesis.12, 13, 18 The function of circulating ECFC into adulthood is still not fully understood in humans. Experimental studies have indicated that circulating or tissue resident ECFC may contribute to maintaining the vascular tree or to actively form new local microvessels postnatally or during regenerative processes.36, 40, 41 These findings suggest that ECFC may play a role in the maintenance of microvasculature and potentially stimulate angiogenesis postnatally. Interestingly, microvasculature rarefaction, considered as an important element in the pathogenesis of increased peripheral resistance and hypertension, was previously reported in young adults born prematurely.3, 4 This phenomenon was more marked among those with higher blood pressure and an enhanced antiangiogenic state. Additionally, in a ventilatory assessment study, Lovering et al42 made the interesting observation that leg fatigue significantly contributed to the limited exercise capacity of young adults born preterm. While leg discomfort was unexplained in Lovering's study, reduced microvascular growth was postulated,43 considering that skeletal muscle capillary rarefaction is present in rats exposed to neonatal hyperoxia.44 Microvascular density was not assessed in our study.
In the current study, we also found that ECFC dysfunction was related to higher blood pressure in preterm subjects. The relationship between hypertension and EPC in general, and ECFC in particular, was previously reported, although results are still controversial in showing a positive correlation between blood pressure and ECFC dysfunction.45 In addition, the mechanisms linking hypertension and ECFC dysfunction are still unknown. Beyond the hypothesis of a cause/effect relationship between ECFC dysfunction and elevated blood pressure, ECFC dysfunction and hypertension may result from common physiopathological pathways, such as the activation of the autonomic nervous system, renin–angiotensin system, and inflammation,45 which in turn were all previously shown to be altered in preterm‐born subjects or in experimental models of preterm birth.46, 47, 48, 49, 50, 51, 52, 53 These observations suggest that both ECFC dysfunction and elevated blood pressure could be long‐term consequences of very preterm birth. Whether ECFC dysfunction is a cause or a consequence, or alternatively a co‐occurrence of high blood pressure remains to be determined.
Furthermore, the adverse and premature exposure of preterm neonates to the extrauterine environment, resultant morbidities, and/or an intrinsic susceptibility to those neonatal exposures are all possible mechanistic avenues that can contribute to the observed ECFC dysfunction in adulthood. In the cord blood of preterm infants, EPC counts have been shown to be either similar to or increased versus full‐term infants, but studies of ECFC subtype proliferation and vessel formation have yielded contradictory results.54 Cord blood ECFC from preterm infants were shown to be particularly vulnerable to oxidant conditions (such as hyperoxia) with drastically impaired growth potential, disrupted nitric oxide/vascular endothelial growth factor signaling, accelerated cell senescence, as well as increased oxidative stress status.16, 17, 55 In addition, human fetal lung ECFC and newborn rat ECFC function are also impaired after high oxygen exposure (mimicking preterm birth conditions).36 EPC dysfunction in preterm cord blood, particularly the ECFC, has been associated with the development of BPD.56, 57 BPD is characterized by disrupted alveolar and lung vasculogenesis, and is considered an early manifestation of an impaired angiogenic capacity in preterm newborns.26 Adult mice exposed to neonatal hyperoxia show reduced bone marrow–derived proangiogenic cell numbers and vasculogenic function, and reduced postischemic muscle neovascularization.58 Interestingly, our findings show that individuals born preterm and who developed BPD are also those with more significant alterations in ECFC function into adulthood. On the other hand, exposure to antenatal steroids in preterm‐born subjects had a positive impact on ECFC function into adulthood. This positive relationship can in turn reflect a beneficial effect of accelerated intrauterine maturation of preterm babies stimulated by steroidal treatment or to the prevention of more severe neonatal complications in newborns exposed to this treatment.27 Alternatively, one can also postulate that epigenetic changes could result from perinatal stressors associated with very preterm birth and may contribute to the establishment of ECFC dysfunction. Key pro‐angiogenic pathways including vascular endothelial growth factor receptors and Notch expression in ECFC are susceptible to epigenetic histone modifications, which were shown to remarkably blunt in vitro ECFC vasculogenic capacity.59, 60 Together, our current study and others suggest that the function of adult EPCs, particularly ECFCs, may be determined by perinatal factors; however, the mechanisms by which such ECFC dysfunction can persist beyond birth and into adulthood remain to be studied.
Importantly, treatment with ECFC from cord blood of human full‐term infants promoted lung vascular growth and reversed experimental BPD in rodents.36 Thus, as cell therapy and regenerative medicine studies are being designed for prematurely born neonates and for adults with cardiovascular diseases, it is important to determine the mechanisms of inherent or programmed impaired function of ECFC for preterm‐born subjects in order to determine the potential cellular role during cardiovascular disease development or in vascular regenerative therapies.
The main strengths of this study are the relatively large sample size, the homogeneity of the study groups, including the narrow gestational age in the preterm group, detailed functional characterization of proliferative and angiogenic cellular properties, and the extensive perinatal and adult clinical data. However, the evaluation of the impact of individual perinatal factors such as being small for gestational age and pregnancy‐induced hypertension, which have been previously linked to both adult cardiovascular health and EPCs, was limited because of the small number of preterm individuals with these conditions and the numbers with ECFC growth. Our incidence of ECFC colony growth was ≈60%, which is consistent with values reported.61 In addition, classical phenotypic characteristics of ECFC including clonogenic potential and formation of cobblestone‐patterned colonies, as well as in vitro vascular cord formation capacity and expression of CD31 and CD34, were observed and confirm the progenitor and endothelial nature of ECFC isolated from peripheral blood. Finally, only a subgroup of our participants completed the 24‐hour ABPM assessment; however, this noncompletion was not related to ECFC characteristics, therefore limiting the risk of selection bias.
Perspectives
We have shown that adults born very preterm have impaired ECFC compared with term controls. Furthermore, in preterm adults, ECFC dysfunction was associated with elevated systolic blood pressure beyond classical cardiovascular risk factors known to be associated with preterm birth. ECFC dysfunction was more pronounced in preterm adults who had BPD as neonates and was relatively prevented in preterm subjects exposed to antenatal steroids. The pathways linking deleterious conditions associated with preterm birth and impaired ECFC function in adults and whether ECFC dysfunction contributes to vascular alterations in preterm‐born subjects, particularly in those with severe neonatal morbidities, remain to be explored. It also remains to be established whether ECFC function could be considered as a potential biomarker to identify among young adults born very preterm those carrying higher risk to develop cardiovascular diseases including hypertension, stroke, and heart failure. This is important to determine because the first generations of young adults born very preterm are now reaching an age at which the incidence of cardiovascular diseases rises, and there is a definite need to accelerate the search for vascular repair therapies.
Sources of Funding
This work was supported by Canadian Institutes of Health Research (CIHR) MOP 133572 to Nuyt and Luu. Merck Sharp & Dhome Corp.—Université de Montréal Fund award to Nuyt, Luu, and Rivard. Canadian Foundation for Innovation grant to Nuyt. Fonds de recherche du Québec—Santé (FRQ‐S) salary award to Luu. FRQ‐S—Société Québécoise d'Hypertension Artérielle Jacques de Champlain fellowship award to Bertagnolli. National Health and Medical Research Council (NHMRC) of Australia CJ Martin Early Career Fellowship to Sutherland.
Disclosures
None.
Supporting information
Table S1. Clinical Characteristics of Study Participants Born Full‐Term and Preterm With and Without Daytime (Awake) Ambulatory Blood Pressure Monitoring (ABPM)
Table S2. Clinical and Perinatal Characteristics of Study Participants Born Full‐Term and Preterm With ECFC Colony Growth
Acknowledgments
We would like to thank Ines Boufaied, Valerie Orlando, and Rong Wu for their technical support, as well as the Sainte‐Justine University Hospital Research Center research nurses, the participants, and many of their parents for their involvement.
(J Am Heart Assoc. 2018;7:e009720 DOI: 10.1161/JAHA.118.009720.)
This work was presented at the Council on Hypertension Scientific Sessions, September 14 to 16, 2016, in Orlando, FL. Dr Bertagnolli received the Hypertension Early Career Award.
References
- 1. Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA. 2011;306:1233–1240. [DOI] [PubMed] [Google Scholar]
- 2. Hovi P, Vohr B, Ment LR, Doyle LW, McGarvey L, Morrison KM, Evensen KA, van der Pal S, Grunau RE; Collaboration AABPI , Brubakk AM, Andersson S, Saigal S, Kajantie E. Blood pressure in young adults born at very low birth weight: adults born preterm international collaboration. Hypertension. 2016;68:880–887. [DOI] [PubMed] [Google Scholar]
- 3. Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P. Elevated blood pressure in preterm‐born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614. [DOI] [PubMed] [Google Scholar]
- 4. Bonamy AK, Martin H, Jorneskog G, Norman M. Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med. 2007;262:635–642. [DOI] [PubMed] [Google Scholar]
- 5. Boardman H, Birse K, Davis EF, Whitworth P, Aggarwal V, Lewandowski AJ, Leeson P. Comprehensive multi‐modality assessment of regional and global arterial structure and function in adults born preterm. Hypertens Res. 2016;39:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, McCormick K, Wilkinson AR, Singhal A, Lucas A, Smith NP, Neubauer S, Leeson P. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127:197–206. [DOI] [PubMed] [Google Scholar]
- 7. Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, Lucas A, Neubauer S, McCormick K, Leeson P. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713–720. [DOI] [PubMed] [Google Scholar]
- 8. Kowalski RR, Beare R, Doyle LW, Smolich JJ, Cheung MM; Victorian Infant Collaborative Study G . Elevated blood pressure with reduced left ventricular and aortic dimensions in adolescents born extremely preterm. J Pediatr. 2016;172:75–80.e2 [DOI] [PubMed] [Google Scholar]
- 9. Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. [DOI] [PubMed] [Google Scholar]
- 10. Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. [DOI] [PubMed] [Google Scholar]
- 12. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. [DOI] [PubMed] [Google Scholar]
- 13. Prasain N, Meador JL, Yoder MC. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp. 2012;13:3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Comporti M, Signorini C, Leoncini S, Buonocore G, Rossi V, Ciccoli L. Plasma F2‐isoprostanes are elevated in newborns and inversely correlated to gestational age. Free Radic Biol Med. 2004;37:724–732. [DOI] [PubMed] [Google Scholar]
- 15. Sutherland MR, Bertagnolli M, Lukaszewski MA, Huyard F, Yzydorczyk C, Luu TM, Nuyt AM. Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension. 2014;63:12–18. [DOI] [PubMed] [Google Scholar]
- 16. Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V. Endothelial colony‐forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujinaga H, Baker CD, Ryan SL, Markham NE, Seedorf GJ, Balasubramaniam V, Abman SH. Hyperoxia disrupts vascular endothelial growth factor‐nitric oxide signaling and decreases growth of endothelial colony‐forming cells from preterm infants. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1160–L1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, Goebel WS, Yoder MC, Haneline LS, Ingram DA. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res. 2008;64:68–73. [DOI] [PubMed] [Google Scholar]
- 19. Mund JA, Estes ML, Yoder MC, Ingram DA Jr, Case J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Synnes A, Luu TM, Moddemann D, Church P, Lee D, Vincer M, Ballantyne M, Majnemer A, Creighton D, Yang J, Sauve R, Saigal S, Shah P, Lee SK; Canadian Neonatal N and the Canadian Neonatal Follow‐Up N . Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. 2017;102:F235–F243. [DOI] [PubMed] [Google Scholar]
- 21. Martin‐Ramirez J, Hofman M, van den Biggelaar M, Hebbel RP, Voorberg J. Establishment of outgrowth endothelial cells from peripheral blood. Nat Protoc. 2012;7:1709–1715. [DOI] [PubMed] [Google Scholar]
- 22. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 23. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. [DOI] [PubMed] [Google Scholar]
- 24. Sipola‐Leppänen M, Karvonen R, Tikanmäki M, Matinolli H‐M, Martikainen S, Pesonen A‐K, Räikkönen K, Järvelin M‐R, Hovi P, Eriksson JG, Vääräsmäki M, Kajantie E. Ambulatory blood pressure and its variability in adults born preterm. Hypertension. 2015;65:615–621. [DOI] [PubMed] [Google Scholar]
- 25. Day CL, Ryan RM. Bronchopulmonary dysplasia: new becomes old again!. Pediatr Res. 2017;81:210–213. [DOI] [PubMed] [Google Scholar]
- 26. Strueby L, Thebaud B. Advances in bronchopulmonary dysplasia. Expert Rev Respir Med. 2014;8:327–338. [DOI] [PubMed] [Google Scholar]
- 27. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meneveau N, Deschaseaux F, Seronde MF, Chopard R, Schiele F, Jehl J, Tiberghien P, Bassand JP, Kantelip JP, Davani S. Presence of endothelial colony‐forming cells is associated with reduced microvascular obstruction limiting infarct size and left ventricular remodelling in patients with acute myocardial infarction. Basic Res Cardiol. 2011;106:1397–1410. [DOI] [PubMed] [Google Scholar]
- 29. Sobrino T, Hurtado O, Moro MA, Rodriguez‐Yanez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Davalos A, Lizasoain I, Castillo J. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–2764. [DOI] [PubMed] [Google Scholar]
- 30. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 31. Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102:565–571. [DOI] [PubMed] [Google Scholar]
- 32. MacEneaney OJ, DeSouza CA, Weil BR, Kushner EJ, Van Guilder GP, Mestek ML, Greiner JJ, Stauffer BL. Prehypertension and endothelial progenitor cell function. J Hum Hypertens. 2011;25:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oliveras A, de la Sierra A, Martinez‐Estrada OM, Larrousse M, Vazquez S, Soler MJ, Zuasti M, Vila JS, Reina M, Roca‐Cusachs A, Lloveras J. Putative endothelial progenitor cells are associated with flow‐mediated dilation in refractory hypertensives. Blood Press. 2008;17:298–305. [DOI] [PubMed] [Google Scholar]
- 34. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR‐2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. [DOI] [PubMed] [Google Scholar]
- 36. Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, O'Reilly M, Ohls RK, McConaghy S, Eaton F, Zhong S, Yoder M, Thebaud B. Existence, functional impairment, and lung repair potential of endothelial colony‐forming cells in oxygen‐induced arrested alveolar growth. Circulation. 2014;129:2144–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raju TNK, Buist AS, Blaisdell CJ, Moxey‐Mims M, Saigal S. Adults born preterm: a review of general health and system‐specific outcomes. Acta Paediatr. 2017;106:1409–1437. [DOI] [PubMed] [Google Scholar]
- 38. Luu TM, Katz SL, Leeson P, Thebaud B, Nuyt AM. Preterm birth: risk factor for early‐onset chronic diseases. CMAJ. 2016;188:736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta‐analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SH, Lee JH, Han YS, Ryu JM, Yoon YM, Han HJ. Hypoxia accelerates vascular repair of endothelial colony‐forming cells on ischemic injury via STAT3‐BCL3 axis. Stem Cell Res Ther. 2015;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsukada S, Kwon SM, Matsuda T, Jung SY, Lee JH, Lee SH, Masuda H, Asahara T. Identification of mouse colony‐forming endothelial progenitor cells for postnatal neovascularization: a novel insight highlighted by new mouse colony‐forming assay. Stem Cell Res Ther. 2013;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lovering AT, Elliott JE, Laurie SS, Beasley KM, Gust CE, Mangum TS, Gladstone IM, Duke JW. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann Am Thorac Soc. 2014;11:1528–1537. [DOI] [PubMed] [Google Scholar]
- 43. O'Donnell DE. Adult survivors of preterm birth. What spirometry conceals, exercise tests reveal. Ann Am Thorac Soc. 2014;11:1606–1607. [DOI] [PubMed] [Google Scholar]
- 44. Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting Shun Y, Wolff J, Deschepper C, Touyz RM, Lelievre‐Pegorier M, Nuyt AM. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension. 2008;52:889–895. [DOI] [PubMed] [Google Scholar]
- 45. Luo S, Xia W, Chen C, Robinson EA, Tao J. Endothelial progenitor cells and hypertension: current concepts and future implications. Clin Sci. 2016;130:2029–2042. [DOI] [PubMed] [Google Scholar]
- 46. Raaijmakers A, Zhang ZY, Claessens J, Cauwenberghs N, van Tienoven TP, Wei FF, Jacobs L, Levtchenko E, Pauwels S, Kuznetsova T, Allegaert K, Staessen JA. Does extremely low birth weight predispose to low‐renin hypertension? Hypertension. 2017;69:443–449. [DOI] [PubMed] [Google Scholar]
- 47. South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Snively BM, Shaltout HA, Rose JC, O'Shea TM, Washburn LK. Antenatal corticosteroids and the renin‐angiotensin‐aldosterone system in adolescents born preterm. Pediatr Res. 2017;81:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bertagnolli M, Dios A, Beland‐Bonenfant S, Gascon G, Sutherland M, Lukaszewski MA, Cloutier A, Paradis P, Schiffrin EL, Nuyt AM. Activation of the cardiac renin‐angiotensin system in High Oxygen‐Exposed Newborn Rats: Angiotensin Receptor Blockade Prevents the developmental programming of cardiac dysfunction. Hypertension. 2016;67:774–782. [DOI] [PubMed] [Google Scholar]
- 49. Bertagnolli M, Huyard F, Cloutier A, Anstey Z, Huot‐Marchand JE, Fallaha C, Paradis P, Schiffrin EL, Deblois D, Nuyt AM. Transient neonatal high oxygen exposure leads to early adult cardiac dysfunction, remodeling, and activation of the renin‐angiotensin system. Hypertension. 2014;63:143–150. [DOI] [PubMed] [Google Scholar]
- 50. Fyfe KL, Odoi A, Yiallourou SR, Wong FY, Walker AM, Horne RS. Preterm infants exhibit greater variability in cerebrovascular control than term infants. Sleep. 2015;38:1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berry M, Jaquiery A, Oliver M, Harding J, Bloomfield F. Preterm birth has sex‐specific effects on autonomic modulation of heart rate variability in adult sheep. PLoS One. 2013;8:e85468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev. 2013;89:145–152. [DOI] [PubMed] [Google Scholar]
- 53. O'Reilly MA, Marr SH, Yee M, McGrath‐Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bertagnolli M, Nuyt AM, Thebaud B, Luu TM. Endothelial progenitor cells as prognostic markers of preterm birth‐associated complications. Stem Cells Transl Med. 2017;6:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vassallo PF, Simoncini S, Ligi I, Chateau AL, Bachelier R, Robert S, Morere J, Fernandez S, Guillet B, Marcelli M, Tellier E, Pascal A, Simeoni U, Anfosso F, Magdinier F, Dignat‐George F, Sabatier F. Accelerated senescence of cord blood endothelial progenitor cells in premature neonates is driven by SIRT1 decreased expression. Blood. 2014;123:2116–2126. [DOI] [PubMed] [Google Scholar]
- 56. Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan SL, Abman SH. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, Ferrari G, Bonetti E, Chiesa G, de Silvestri A, Spinillo A, Rosti V, Stronati M. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180:540–546. [DOI] [PubMed] [Google Scholar]
- 58. Mathieu R, Dussault S, Desjarlais M, Rivard F, Dhahri W, Cloutier A, Nuyt AM , Rivard A. Neonatal exposure to high oxygen levels leads to impaired ischemia‐induced neovascularization in adulthood. Sci Rep. 2017;7:14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fraineau S, Palii CG, McNeill B, Ritso M, Shelley WC, Prasain N, Chu A, Vion E, Rieck K, Nilufar S, Perkins TJ, Rudnicki MA, Allan DS, Yoder MC, Suuronen EJ, Brand M. Epigenetic activation of pro‐angiogenic signaling pathways in human endothelial progenitors increases vasculogenesis. Stem Cell Reports. 2017;9:1573–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tang LL, Zhang LY, Lao LJ, Hu QY, Gu WZ, Fu LC, Du LZ. Epigenetics of Notch1 regulation in pulmonary microvascular rarefaction following extrauterine growth restriction. Respir Res. 2015;16:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, Randi AM, Barnes PJ. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Characteristics of Study Participants Born Full‐Term and Preterm With and Without Daytime (Awake) Ambulatory Blood Pressure Monitoring (ABPM)
Table S2. Clinical and Perinatal Characteristics of Study Participants Born Full‐Term and Preterm With ECFC Colony Growth


