Abstract
Background
Although it is well established that heavy alcohol consumption increases the risk of hypertension, the risk associated with low levels of alcohol intake in men and women is unclear.
Methods and Results
We searched Medline and Embase for original cohort studies on the association between average alcohol consumption and incidence of hypertension in people without hypertension. Random‐effects meta‐analyses and metaregressions were conducted. Data from 20 articles with 361 254 participants (125 907 men and 235 347 women) and 90 160 incident cases of hypertension (32 426 men and 57 734 women) were included. In people drinking 1 to 2 drinks/day (12 g of pure ethanol per drink), incidence of hypertension differed between men and women (relative riskwomen vs men=0.79; 95% confidence interval, 0.67–0.93). In men, the risk for hypertension in comparison with abstainers was relative risk=1.19 (1.07–1.31; I2=59%), 1.51 (1.30–1.76), and 1.74 (1.35–2.24) for consumption of 1 to 2, 3 to 4, and 5 or more standard drinks per day, respectively. In women, there was no increased risk for 1 to 2 drinks/day (relative risk=0.94; 0.88–1.01; I2=73%), and an increased risk for consumption beyond this level (relative risk=1.42; 1.22–1.66).
Conclusions
Any alcohol consumption was associated with an increase in the risk for hypertension in men. In women, there was no risk increase for consumption of 1 to 2 drinks/day and an increased risk for higher consumption levels. We did not find evidence for a protective effect of alcohol consumption in women, contrary to earlier meta‐analyses.
Keywords: alcohol, cohort studies, hypertension, meta‐analysis, systematic review
Subject Categories: Epidemiology, Risk Factors, Hypertension
Clinical Perspective
What Is New?
This is the first meta‐analysis based on high‐quality cohort studies on the relationship between different levels of alcohol consumption and risk for incident hypertension.
We investigated the risk for hypertension separately for men and women in people who did not have hypertension at baseline.
The risk for hypertension in former drinkers was similar to that of lifetime abstainers.
We found that, compared with nondrinkers, the risk for hypertension was increased at all levels of alcohol consumption in men. Contrary to earlier meta‐analyses, we did not find a protective effect of low levels of alcohol consumption in women.
What Are the Clinical Implications?
The findings support sex‐specific drinking guidelines with regard to risk for hypertension. These guidelines may be revised to indicate the increased risk for any alcohol consumption in men.
Alcohol consumption should be assessed at the primary care level whenever there is elevated blood pressure.
Changing clinical practice promises to reduce substantial mortality and burden of disease associated with both alcohol consumption and hypertension.
Introduction
Hypertension (raised blood pressure [BP], >140 mm Hg systolic BP, and/or >90 mm Hg diastolic BP) ranks as the third‐most important risk factor for global burden of disease,1 responsible for considerable and increasing noncommunicable diseases burden and mortality.1, 2 This condition affects more than 1 billion people worldwide with a global prevalence of close to 20%. Despite decreases in raised BP mainly in higher income countries, in part attributed to improved detection and treatment,3 global prevalence of hypertension has been increasing and is predicted to further increase in the next decade.1, 2 In 2015, hypertension was responsible for 10.7 (95% confidence interval [CI], 9.6–11.8) million deaths and 211.8 (95% CI, 192.7–231.1) million disability‐adjusted life years globally.1
Hypertension is largely a by‐product of modern lifestyle factors such as lack of physical activity,4 unhealthy diet (in particular, salt intake4), or consumption of alcohol.5 In fact, some guidelines for clinical management, including those from the National Institute for Health and Care Excellence, stipulate that all patients undergoing assessment or treatment for hypertension should receive initial and periodic lifestyle advice, which includes ascertaining their level of alcohol consumption and encouraging a reduced intake if they drink hazardously or heavily.6, 7 The American Heart Association guidelines for the prevention and treatment of high BP recommend limiting daily alcohol intake to 2 or less drinks for men and 1 or less drinks for women.8
The relationship between alcohol consumption and hypertension was first reported by Lian9 researching French soldiers serving in World War II. He found a dose‐response association with a 4‐fold increase between drinkers with the lowest (up to 2 L of wine per day) and highest (>3 L of wine per day plus aperitifs) levels of consumption. Numerous studies since then have confirmed the association between heavy drinking and development of hypertension.10 However, the association between light‐to‐moderate drinking and hypertension is still disputed,11, 12 despite a number of meta‐analyses13, 14 and countless reviews (overview of recent reviews15). The association may also depend on sex, which could be related to differential alcohol metabolism16 or drinking patterns.17
In part because there have been a number of studies since the last systematic review including a meta‐analysis,14 and in part because the techniques for conducting meta‐analyses have expanded considerably in recent years,18 we conducted a systematic review and meta‐analysis with the explicit aim to restricting our review to studies above a high‐quality threshold and to explore potential influencing factors by stratification by sex and metaregression. This review intends not only to produce improved sex‐specific estimates for comparative risk assessments within the Global Burden of Disease studies19 and for the Global Status Reports of the World Health Organization,20 but also provide much needed evidence for hypertension‐specific drinking guidelines.
Methods
All data are from publicly available sources.
Search Strategy and Selection Criteria
Following the meta‐analysis of observational studies in epidemiology checklist,21 we conducted a systematic electronic literature search using Medline and Embase from inception to April 3, 2017 for keywords and MeSH terms relating to alcohol consumption, hypertension, and observational studies (Table S1). Additionally, we searched reference lists of identified articles and published meta‐analyses and reviews. Inclusion criteria were as follows:
Full‐text article with original cohort data (including nested case‐control studies) examining the association between total alcohol consumption and incidence of hypertension.
Participants with hypertension at baseline were excluded.
Analyses were adjusted or matched for age at baseline.
Incidence for at least 2 quantitatively defined categories of average alcohol consumption in addition to nondrinkers, or incidence for former drinkers in relation to lifetime abstainers were reported.
Results were sex specific.
For a continuous nonlinear dose‐response meta‐analysis, results for at least 3 drinking groups in addition to nondrinkers had to be reported. We did not apply language restrictions. Authors were contacted for clarification and missing data. Two reviewers independently excluded articles based on title and abstract or full text, and abstracted the data. Any discrepancies were resolved in consultation with a third reviewer.
Data Extraction
From all relevant articles, we extracted authors’ names, year of publication, country, calendar year(s) of baseline examination, follow‐up period, setting of the study, assessment of hypertension status, age (mean or median) at baseline, sex, number of observed incident hypertension cases among participants by drinking group, number of total participants by drinking group, specific adjustment or stratification for potential confounders, and adjusted measures of effect (relative risks [RRs], odds ratios, and hazard ratios) and their confidence intervals or standard errors. Risk estimates by sex and race/ethnicity were treated as independent samples. As a result, multiple articles and estimates from the same study22, 23, 24 were included, but each case of incident hypertension was used only once in each of the analyses conducted. If necessary, effect sizes within studies were recalculated to contrast alcohol consumption categories against nondrinkers.25 Because incidence of hypertension was not rare, we transformed odds ratios to RRs based on the formula described by Zhang and Yu.26 Hazard ratios and RRs were treated as equivalent measures of risk.
Exposure and Outcome Assessment
Consolidating exposure measures across primary studies involved a 2‐step process. First, among drinkers, we converted reported alcohol intake categories in primary studies into an average of pure alcohol in g/d using the midpoints (mean or median) of reported drinking group categories. For open‐ended categories, we added three quarters of the second‐highest category's range to the lower limit of the open‐ended category of alcohol intake if the mean was not reported. Standard drinks vary by country, with 1 standard drink containing ≈8 to 14 g of pure alcohol.27 We used reported conversion factors when standard drinks were the unit of measurement to convert all measures to g/d. Then, for reporting of our analyses, we considered categories with a mean of up to 12 g of pure ethanol as 1 standard drink for a global representation. Qualitative descriptions, such as “social” or “frequent” drinkers with no clear total alcohol intake in g/d, were excluded.
Because of the changing definitions of hypertension over time, we defined hypertension status at baseline and incident cases of hypertension as defined in the primary studies (typically assessed as taking antihypertensive medications or as mean systolic BP at baseline >140 mm Hg).
Quality Assessment
Most quality scores are tailored for meta‐analyses of randomized trials of interventions,28, 29, 30 and many criteria do not apply to epidemiological studies examined in this study. Additionally, quality score use in meta‐analyses remains controversial.31, 32, 33 As a result, study quality was incorporated by including quality components, such as study design, measurement of alcohol consumption and hypertension, adjustment for age, and sex‐specific RRs, in the inclusion and exclusion criteria and further by investigating potential heterogeneity in metaregression models and several subgroup analyses. We used the most adjusted RR reported and the most comprehensive data available for each analysis, and gave priority to estimates where lifetime abstainers were used as the risk reference group.
In a formal risk of bias analysis, we used the Cochrane risk of bias tool for nonrandomized studies (ROBINS‐I)34 to assess risk of bias in primary studies. We rated the evidence for the association between alcohol consumption and incidence of hypertension based on the Grades of Recommendation, Assessment, Development and Evaluation system.35
Statistical Analyses
In analyses using standard drinks as the exposure measure, RRs were pooled with inverse‐variance weighting using DerSimonian‐Laird random‐effect models to allow for between‐study heterogeneity.36 Small‐study bias was examined using Egger's regression‐based test.37 Variation in the effect size because of heterogeneity between‐studies was quantified using the I2 statistic.38 Between‐study heterogeneity was investigated with random‐effects metaregressions.39
Using studies that reported data for 4 or more alcohol intake groups, we conducted 2‐stage restricted cubic spline regression in multivariate metaregression models taking into account the variance‐covariance matrix for risk estimates derived from 1 reference group40, 41 to calculate continuous nonlinear dose‐response curves for total alcohol consumption (g/d) in relation to abstainers. All meta‐analytical analyses were conducted on the natural log scale of the RRs (and hazard ratios) in Stata statistical software version 14.2 (Stata LP, College Station, TX).
Results
Literature Search and Study Characteristics
Of 3771 identified references, 465 were reviewed in full text. In total, data from 20 reports from 18 studies were used in the analysis (Figure 1). Nine reports were from the United States,22, 23, 24, 42, 43, 44, 45, 46, 47 4 from Japan,48, 49, 50, 51 2 from China,52, 53 and 1 each from Germany,54 South Korea,55 Finland,56 Turkey,57 and Thailand58 (Table). Several reports from the Nurses’ Health Study42, 44, 45, 46 were included, but any 1 case of incident hypertension was included only once in any particular analysis. Overall, data from 361 254 participants (125 907 men and 235 347 women) and 90 160 incident cases of hypertension (32 426 men and 57 734 women) were analyzed. Mean age at baseline among men ranged from 25 to 57 years with a weighted mean of 47.1 years (median=50 years), with mean follow‐up duration of 5.3 years (median=4; range, 3.9–20.0). In women, mean age ranged from 25 to 60 years with a weighted mean of 46.7 years (median=54), with mean follow‐up duration of 7.3 years (median=4; range, 3.9–20.0). Most studies were well adjusted for potential confounders; 1 study was adjusted only for age.55
Figure 1.
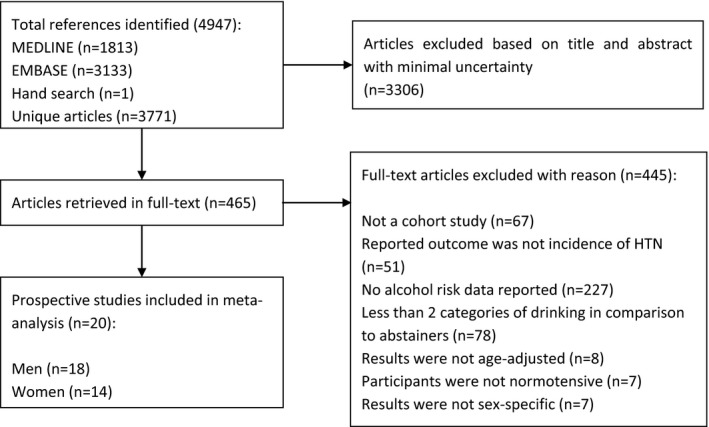
Flowchart of study selection.
Table 1.
Characteristics of 20 Cohort Studies Investigating Sex‐Specific Incidence of Hypertension by Alcohol Intake in People Without Hypertension at Baseline, 1989–2017
| Reference | Baseline Year(s), Setting | Baseline Hypertension Status, Sex, Age (y), Country | Design, Cases (No.), Participants (No.), Follow‐up Time (y) | Alcohol Assessment at Baseline | Assessment of Hypertension During Follow‐up | Adjustments |
|---|---|---|---|---|---|---|
| Ascherio et al, 199642 |
1984. White female nurses from the NHS I (Nurses’ Health Study). Baseline exclusions: pregnant for at least 6 months, use of antihypertensive drugs, on a special diet, high BP (140/90 mm Hg), MI, coronary artery surgery, stroke, angina pectoris, diabetes mellitus, and all cancers except nonmelanoma skin cancer |
Normotensive, W, 38 to 63, USA |
Cohort, 2526, 41 541, 4 |
Lifetime abstainers, current drinkers: (0.1–9, 10–19, 20–29, ≥30) g/d | Self‐reported physician diagnosed hypertension (140/90 mm Hg), confirmed by review of medical record in a subsample n=100 | Age, BMI |
| Bae and Ahn, 200255 |
1992. Healthy Korean men from the Seoul Cohort Study, and beneficiaries of the Korea Medical Insurance Corporation (KMIC) |
Normotensive, M, 40 to 59, South Korea |
Nested case control, 236, 1116, 4 |
Current abstainers, current drinkers: (1–70, 71–280, 281–560, >560) g/wk | Review of medical records through the hospital survey, use of antihypertensive drugs, self‐reporting on telephone, and clinical assessment of hypertension (140/90 mm Hg). JNC VI criteria for hypertension were used. | Frequency matched on age |
| Bai et al, 201753 |
2000. CHNS (China Health and Nutrition Survey). A multistage random cluster sampling in Heilongjiang, Liaoning, Jiangsu, Shandong, Henan, Hubei, Hunan, Guizhou, and Guangxi. Baseline exclusions: <18 or >60 years age, missing data on BP, hypertension at baseline, taking antihypertensive medication, existing diagnosis of diabetes mellitus, MI, stroke |
Normotensive, M,W, 18 to 60, China |
Cohort, 1147, 2751, 11 |
Lifetime abstainers, former drinkers, current drinkers (0.1–10.0, 10.1–25.0, >25.0) g/d | Having an average SBP⩾140 mm Hg, an average DBP ≥90 mm Hg, currently undergoing treatment with an antihypertensive medication, or having received a previous diagnosis by a physician | Age, income, employment status, education, province, urban or rural, DASH score, physical activity, BMI, smoking |
| Banda et al, 201043 |
1974–2003. Predominantly white males from the ACLA (Aerobics Center Longitudinal Study), well‐educated, middle and upper socioeconomic class, free of CVD, cancer, and hypertension at baseline |
Normotensive, M, 44 (20–82), USA |
Cohort, 1959, 14 568, 10.7 |
Current abstainers, current drinkers: (1–14, >14) drinks/week | Self‐reported physician diagnosed hypertension (140/90 mm Hg) through health survey | Age (single year), examination year, survey response pattern, resting SBP and DBP, diabetes mellitus, and family history of hypertension, BMI, smoking, physical activity, and cardiorespiratory fitness |
| Diederichs and Neuhauser, 201754 |
1998. Adult population from the GNHIES (German National Health Interview and Examination Survey), free of hypertension at baseline |
Normotensive, M,W, 18 to 79, Germany |
Cohort, 585, 2231, 11.9 |
Men: Current abstainers, current drinkers: (<20, ≥20) g/d Women: Current abstainers, current drinkers: (<10, ≥10) g/d |
Clinical assessment of hypertension (140/90 mm Hg), by taking average of the last 2 of 3 BP readings, each 3 minutes apart, after an initial rest of 5 minutes | Age, socioeconomic status, SBP, DBP, BMI, diabetes mellitus, hyperlipidemia, smoking, physical activity, community size, regions, health insurance |
| Forman et al, 200944 |
1991. Female nurses from the NHS II (Nurses’ Health Study), with normal BP (≤120/80 mm Hg) and free of diabetes mellitus, CVD, or cancer at baseline |
Normotensive, W, 36, USA |
Cohort, 10 152, 83 882, 14 |
Current abstainers, current drinkers: (0.1–5, 5.1–10, 10.1–15, 15.1–29.9, ≥30) g/d | Self‐reported hypertension (140/90 mm Hg) confirmed by medical record review in a subsample n=147 | Age, race, family history of hypertension, use of oral contraceptive pills, smoking status, quintile of DASH score, vigorous exercise, BMI, supplemental folic acid intake, frequency of acetaminophen use, frequency of NSAID use, frequency of aspirin use |
| Fuchs et al, 200123 |
1988. Black and white adults from the ARIC (Atherosclerosis Risk in Communities) Study, free of hypertension and CHD at baseline and alive throughout the follow‐up |
Normotensive, M,W, 45 to 64, USA |
Cohort, 1243, 8334, 6 |
Current abstainers, current drinkers: (1–209, ≥210) g/wk | Clinical assessment of hypertension (140/90 mm Hg) by taking the average of the second and third reading after 5 minutes of rest |
Age, BMI, education, physical activity, and diabetes mellitus. Stratified by race |
| Halanych et al, 201022 |
1985. Young black and white men and women from the CARDIA (Coronary Artery Risk Development in Young Adults) Study, free of hypertension at baseline |
Normotensive, M,W, 24.8, USA |
Cohort, 1022, 4711, 20 |
Men: Never drinkers, former drinkers, current drinkers: (0–7, 7–14, >14) drinks/week. Women: Never drinkers, former drinkers, current drinkers: (0–4, 4–7, >7) drinks/week |
Clinical assessment of hypertension (140/90 mm Hg) as the mean of the second and third BP measurements, or use of antihypertensive drugs |
Age, family history of hypertension, BMI (continuous), smoking status, race, sex, education, income, difficulty paying for basics, and difficulty paying for medical care. Stratified by race |
| Nakanishi et al, 200148 |
1990. Japanese male office workers from the Takenaka Corporation in Osaka, free of hypertension at baseline |
Normotensive, M, 45.7, Japan |
Cohort, 458, 1130, 9 |
Current abstainers, current drinkers: (0.1–22.9, 23–45.9, 46–68.9, ≥69) g/d | Clinical assessment of hypertension (140/90 mm Hg) after a 5‐minute rest, and/or receipt of antihypertensive medications | Age, BMI, cigarette smoking, total cholesterol level, triglyceride level, and fasting plasma glucose level at study entry |
| Nakanishi et al, 200249 |
1996. Japanese male office workers, free of hypertension at baseline |
Normotensive, M, 23 to 59, Japan |
Cohort, 964, 3784, 4 |
Current abstainers, current drinkers: (<12, 12–22, 23–45, ≥46) g/d | Clinical assessment of hypertension (140/90 mm Hg) after a 5‐minute rest, in a seated position, or self‐report of antihypertensive medication use on an annual survey | Age, BMI, family history of hypertension, cigarette smoking, total cholesterol level, triglyceride level, fasting plasma glucose level |
| Niskanen et al, 200456 |
1987–1989. General population from the Kuopio Ischemic Heart Disease Risk Factor Study, free of hypertension and diabetes mellitus at baseline |
Normotensive, M, 51, Finland |
Cohort, 124, 379, 11 |
Current abstainers, current drinkers: (1–83, ≥84) g/wk | Clinical assessment of hypertension (140/90 mm Hg) by taking the average of 2 BP readings while sitting with a 5‐minute interval of rest in between | Age, smoking, socioeconomic status, leisure‐time physical activity, CVD, dietary factors (saturated fat, sodium, potassium, fruits, vegetables), baseline SBP, waist girth, concentrations of insulin, glucose, HDL cholesterol, changes in waist girth, smoking, alcohol intake during follow‐up |
| Ohmori et al, 200250 |
1978. Subrural Japanese men from the Hisayama Study, with normal BP and free from CVD at baseline |
Normotensive, M, 53, Japan |
Cohort, 101, 433, 10 |
Never drinkers, former drinkers, current drinkers: (<23, 23–45, ≥46) g/d | Clinical assessment of hypertension (140/90 mm Hg) on at least 2 occasions in different examinations | Age, BMI |
| Okubo et al, 201451 |
1993–2004. General Japanese population from the IPHS (Ibarakai Prefectural Health Study) underwent community‐based health checkups, free of hypertension, history of heart disease or stroke at baseline. Those who had stopped drinking alcohol were also excluded. |
Normotensive, M,W, 56.9, Japan |
Cohort, 45 428, 115 736, 3.9 (1–18) |
Current abstainers, current drinkers: (1.0–19.9, 20.0–39.9, 40.0–59.9, ≥60) g/d | Clinical assessment of hypertension (140/90 mm Hg) by taking a BP measurement after 5 minutes of rest by a trained nurse |
Age, BMI, SBP, cholesterol, HDL‐cholesterol level, triglyceride level (log), antidyslipidemic medication use, blood glucose level, anti–diabetes mellitus medication use, smoking status. Stratified by age |
| Onat et al, 200857 |
1997. General population from the Turkish Adult Risk Factor Study, free of hypertension at baseline |
Normotensive, M,W, 47.6, Turkey |
Cohort, 645, 2683, 9 |
Current abstainers, current drinkers: (1–3, >3) drinks/day | Clinical assessment of hypertension (140/90 mm Hg), while sitting, average of 2 readings, at least 3 minutes apart | Age, physical activity, smoking status, lipid‐lowering therapy, hormone replacement therapy (only in women) |
| Peng et al, 201352 |
2006. Current and retired coal mine workers from the Kailuan study, free of hypertension, stroke, transient ischemia attack, MI, and cancer (except nonmelanoma skin cancer) at baseline |
Normotensive, M, 49.9, China |
Cohort, 9151, 32 389, 4 |
Current abstainers, current drinkers: (1–24, 25–49, 50–99, 100–149, ≥150) g/d | Cases had to meet 2 of the 3 criteria: self‐report of newly diagnosed hypertension; self‐report of newly initiated antihypertensive treatment; on‐site measured SBP at least 140 mm Hg and DBP at least 90 mm Hg, or either of them, then confirmed by at least 2 follow‐up BP measurements | Age, exercise, smoking status, type of work (mental or physical), salt intake, BMI, history of high cholesterol, history of diabetes mellitus |
| Sesso et al, 200847 |
1992. Male physicians (age, 40–84) from the PHS (Physicians’ Health Study) and female health professionals (age, ≥45) from the WHS (Women's Health Study), who were postmenopausal or not intending to become pregnant. All participants were also free of hypertension, stroke, MI, transient ischemic attack, and cancer (except nonmelanoma skin cancer) at baseline |
Normotensive, M,W, 40 to 84 (PHS), ≥45 (WHS), USA |
Cohort, 14 692, 42 303, 10.9 (WHS) and 21.8 (PHS) |
Men: Rarely or never drinkers, current drinkers: (1–3) drinks/mo (1, 2–4, 5–6) drinks/wk (1, ≥2) drinks/day Women: Rarely or never drinkers, current drinkers: (1–3) drinks/mo (1, 2–4, 5–6) drinks/wk (1, 2–3, ≥4) drinks/d |
Self‐reported hypertension (140/90 mm Hg), not necessarily physician diagnosed, and use of antihypertensive drugs | Age, exercise, parental history of MI, aspirin use, carotene, vitamin E treatment, postmenopausal status, smoking status, hormone replacement therapy, BMI, history of high cholesterol, history of diabetes mellitus |
| Thawornchaisit et al, 201358 |
2005. University students from the TCS (Thai Cohort Study), free of hypertension at baseline |
Normotensive, M,W, 31, Thailand |
Cohort, 578, not reported, 4 |
Never drinkers, former drinkers | Self‐reported physician diagnosed hypertension | Age, marital status, education, income, BMI category, underlying diseases, personal behaviors |
| Wang et al, 201124 |
1994–1998. Postmenopausal black and white women from the Women's Health Initiative Observational Study |
Normotensive, W, 60.8, USA |
Nested case control, 800, 1600, 5.9 |
Never drinkers, former drinkers, current drinkers: (<1, 1–7, ≥7) drinks/week | Clinical assessment of hypertension (140/90 mm Hg), after 5 minutes of rest, and mean of 2 readings 30 seconds apart, or self‐report of use of antihypertensive drugs on an annual questionnaire | Individually matched on age, ethnicity, clinical center, and time of enrollment |
| Witteman et al, 1989,45 199046 |
1980. Female nurses from the NHS I, free of antihypertensive medication, pregnancy in the last 6 months, high BP, MI, angina pectoris, diabetes mellitus, all cancers except nonmelanoma skin cancer, and any special diet at baseline |
Normotensive, W, 34 to 59, USA |
Cohort, 3275, 58 218, 4 |
Current abstainer, current drinkers: (0.1–9, 10–19, 20–29, ≥30) g/d. Stratified by age46 |
Self‐reported physician diagnosed hypertension (140/90 mm Hg) |
Age, Quetelet's index, and intakes of calcium, magnesium, potassium, and fiber. Age‐stratified data46 were adjusted for Quetlet's index. |
BMI indicates body mass index; BP, blood pressure; CHD, congestive heart disease; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; JNC VI, sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; M, men; M,W, men and women stratified; MI, myocardial infarction; NSAID, nonsteroidal anti‐inflammatory drug; SBP, systolic blood pressure; W, women.
Meta‐Analyses
The pooled RR among former drinkers22, 24, 50, 53, 58 in comparison with lifetime abstainers was 1.03 (95% CI, 0.89–1.20), with virtually no differences between men and women (Figure S1). Any alcohol consumption increased the risk for hypertension compared with abstainers in men (Figure 2 and Figure S2). In women, there was no observed risk increase for consumption of 1 or 2 drinks/day in comparison with abstainers, and an increased risk beyond this level with a pooled RR=1.42 (95% CI, 1.22–1.66; I2=88%) for consumption of 3 or more drinks per day (Figure 3 and Figure S3). Because we included 2 studies from the Nurses’ Health Study42, 45 with different follow‐up periods of the same participants, we ran a sensitivity analysis including only 1.42 The results compared to Figure 3 were almost identical (1–2 drinks/day: pooled RR=0.95; 95% CI, 0.88–1.03). Different adjustment for potential confounders in regression models in primary studies resulted in little changes in RRs for different levels of alcohol consumption. In men, data for alcohol consumption beyond 75 g/d were only available from Asian countries (Figure 4). There were no data for women consuming more than 75 g/d (Figure 5). Two studies53, 55 were judged to be of serious risk of bias, 1 of low risk, and 16 of moderate risk of bias mainly because of the observational study design and 1‐time measurement of alcohol consumption (Table S2). Thirteen studies used clinical measurements of BP to determine incidence of hypertension.22, 23, 24, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 Similar relationships were found when we excluded studies with potential serious risk of bias and that relied on self‐reported incidence of hypertension (Figures S4 and S5).
Figure 2.
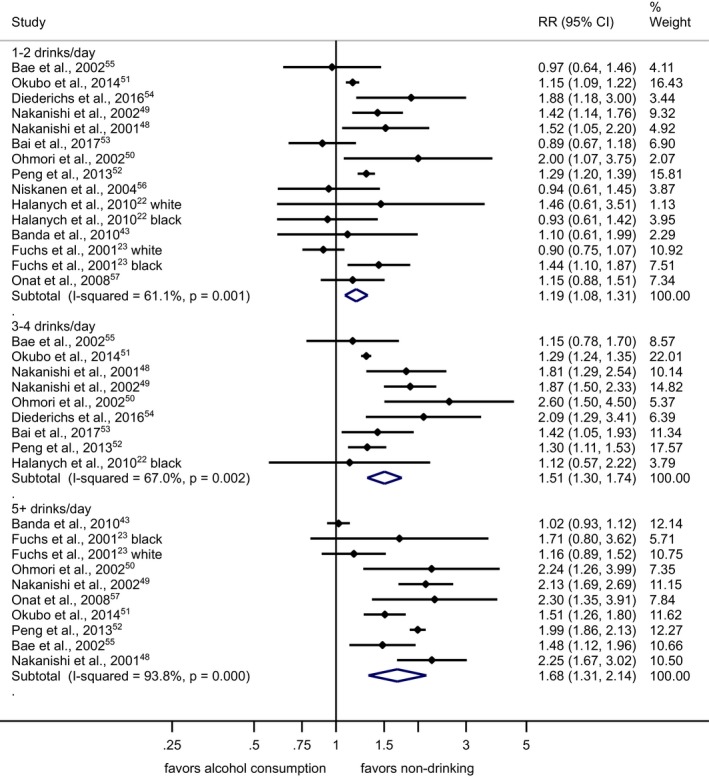
Incidence of hypertension in men by alcohol intake at baseline in standard drinks compared with abstainers in cohort studies, 1989–2017. 1 standard drink=12 g of pure ethanol per day. CI indicates confidence interval; RR, relative risk.
Figure 3.
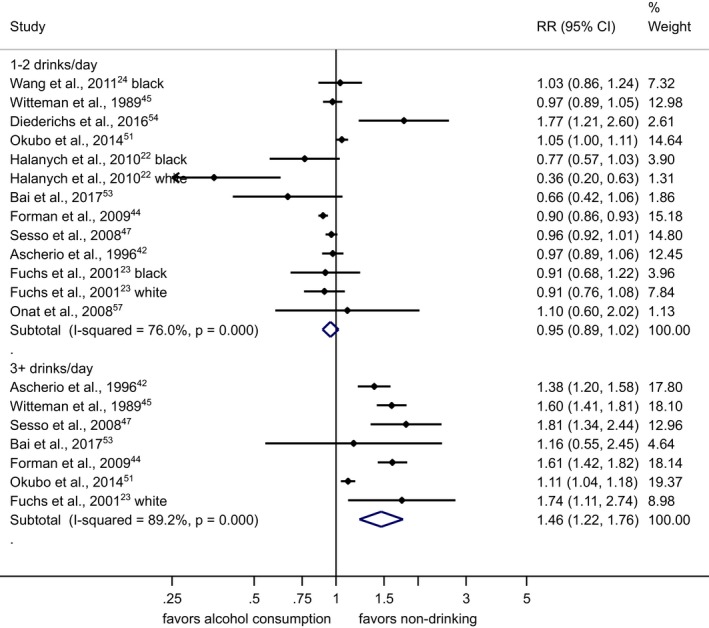
Incidence of hypertension in women by alcohol intake at baseline in standard drinks compared with abstainers in cohort studies, 1989–2017. 1 standard drink=12 g of pure ethanol per day. CI indicates confidence interval; RR, relative risk.
Figure 4.
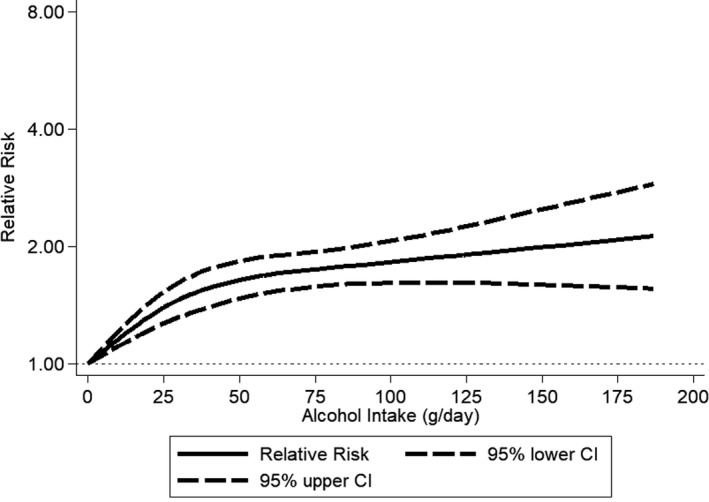
Incidence of hypertension in men by alcohol intake at baseline compared with abstainers using restricted cubic spline metaregression, 1989–2017, n=8 studies with at least 4 alcohol intake groups relative risk on the log scale. CI indicates confidence interval.
Figure 5.
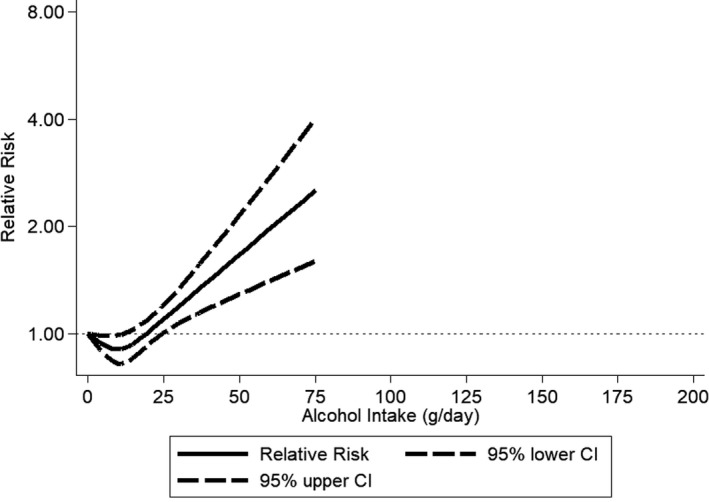
Incidence of hypertension in women by alcohol intake at baseline compared with abstainers using restricted cubic spline metaregression, 1989–2017, n=10 studies with at least 4 alcohol intake groups relative risk on the log scale. CI indicates confidence interval.
Heterogeneity was high in most analyses, and we conducted metaregressions to investigate potential sources of heterogeneity in drinkers of 1 to 2 drinks/day. The difference between men and women was statistically significant (RRwomen vs men=0.79; 95% CI, 0.67–0.95; P=0.012; proportion of heterogeneity explained: 69%). There was no significant difference between studies from Asian and non‐Asian countries in men (RRnon‐Asian vs Asian=0.93; 95% CI, 0.71–1.21; P=0.55) or women (RRnon‐Asian vs Asian=0.92; 95% CI, 0.76–1.11; P=0.33). Three studies from the United States presented results stratified by race (black versus white). In men, people of white origin had nominally higher risk for incidence of hypertension compared with people of black origin (RRBlack vs White=0.63; 95% CI, 0.08–4.93; P=0.44), whereas in women the opposite was observed (RRBlack vs White=1.31; 95% CI, 0.66–2.63; P=0.38). However, the number of incident hypertension cases was low and CIs were wide, indicating low statistical power to detect significant differences. Three studies presented results by age groups.46, 49, 51 There was no difference in incidence of hypertension by age group (per linear increase in 4 age categories; men: RR=0.95; 95% CI, 0.84–1.09; P=0.43; women: RR=0.99; 95% CI, 0.86–1.13; P=0.83); however, statistical power was also low.
We found no evidence for small‐study bias in men or women consuming 1 to 2 drinks/day in visual inspection of funnel plots (Figures S6 and S7) or using Egger's test (P=0.50 and 0.38, respectively). Leaving each trial out of the analysis 1 at a time revealed no meaningful differences in effects (Figures S8 and S9).
Given the observational nature of the studies included, we rated the evidence for a causal effect of high alcohol consumption (3 or more drinks/day) on incidence of hypertension as moderate. However, evidence from randomized controlled trials59 support a causal effect with higher confidence. Regarding alcohol consumption of 1 to 2 drinks/day, our findings indicate effect modification by sex and no protective association. Evidence from randomized controlled trials for this level of alcohol consumption is limited and thus we judge the quality of the evidence as moderate.
Discussion
In high‐quality cohort studies, we found that the association between average alcohol consumption of 1 to 2 drinks/day and risk of hypertension was modified by sex, with men showing an increased risk, whereas women showed no different risk compared with abstainers. Alcohol intake beyond 2 drinks/day was consistently associated with increased incidence of hypertension in both men and women.
Before discussing these results and their implications, we would like to point out some limitations. Conclusions of every meta‐analysis are determined by the quality of the original studies. This meta‐analysis is based on cohort studies, and thus this study type does not allow conclusions about causality.60 However, as indicated above, analogue dose‐response relationships for alcohol reduction on reduction of BP and hypertension based on trial data point to a causal effect of level of alcohol consumption on risk of hypertension,59 and this reasoning is corroborated by plausible biological pathways.10 Second, all alcohol assessments were based on subjective measurements, which may entail bias.61 However, most reviews come to the conclusion that subjective measurement of alcohol is reliable,62 even though there is some bias of underestimating true consumption, for example, sales data show higher consumption compared to survey data.61, 63 Thus, although the overall dose‐response relationship would not be affected, there may be some misestimation of the RRs of the levels of drinking (eg, previous work64). Finally, although patterns of drinking, in particular irregular heavy drinking occasions, have been shown to impact on BP and risk of hypertension,65 we could not find enough cohort studies meeting our inclusion criteria to quantify this effect by meta‐analysis.
Despite these limitations, the results are consistent in showing a dose‐response relationship between level of consumption and risk of hypertension based on observational data, corroborated by randomized controlled trials. For men, there seems to be no lower threshold, whereas for women, the dose‐response seems to emerge only beyond 2 drinks a day. For both sexes, no protective effect could be found (and was not expected given the biological pathways10). What could explain the differences between men and women? One explanation could be the difference in heavy drinking occasions within an overall average intake of alcohol of less than 2 drinks. An average of 2 drinks/day could be achieved by actually drinking 2 drinks every day, or by drinking 7 drinks each on Saturday and Sunday. The latter has different effects on blood pressure66 and thus on risk of hypertension. Further research (both observational and experimental) is necessary, however, to ascertain the effects of pattern of drinking (including peak blood alcohol level) on hypertension and thus the repeated plea to include more measures on patterns of drinking into all epidemiological work.67, 68
Implications
Clinicians are faced with a dilemma. On the one side, low‐level drinking has been associated with less risk for ischemic heart disease69, 70; on the other side, the risk for hypertension seems increased, at least in men. In order to side with caution, patients should be advised to drink as little as possible for many reasons, including increased risk of cancer and injury, to name a few,15 and the risk of hypertension should be added to the list of diseases where no alcohol consumption is safe. This may require a change in drinking advice in current guidelines for prevention and treatment of hypertension, which state that men should limit their alcohol intake to 2 drinks or less, a level which we found to be associated with increased risk for hypertension. Additional research with stronger, more‐experimental study design may help in answering outstanding questions on cardiovascular risk from low levels of drinking.
Other implications of this research are clear: Alcohol consumption should be assessed at the primary care level whenever there is elevated BP.71 Unfortunately, despite some guidelines recommending such an approach, it is rarely followed in clinical practice (for the example of the 6 largest European Union countries72). Efforts should be made to change clinical practice, given that alcohol‐induced hypertension is both preventable and reversible,10, 59 and there are effective and cost‐effective interventions to reduce alcohol consumption level in primary care.73, 74 Changing clinical practice promises to reduce substantial mortality and burden of disease associated with both alcohol consumption and hypertension.10, 75
Sources of Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) of the National Institutes of Health under Award Number R21AA023521 to Roerecke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor of the study (NIAAA) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors collected the data and had full access to all of the data in the study. The authors also had final responsibility for the decision to submit the study results for publication.
Disclosures
Roerecke and Rehm report grants from the National Institutes of Health (NIH), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), during the conduct of the study. Rehm reports grants and honoraria from Lundbeck outside of this work (modest relationship). The remaining authors have no disclosures to report.
Supporting information
Table S1. Search Strategy for Medline(R) (1946–Most Recent) and Embase (Embase+Embase Classic)
Table S2. Risk of Bias in Nonrandomized Studies—of Interventions (ROBINS‐I) Assessment Tool, Modified Version
Figure S1. Incidence of hypertension in former drinkers compared with lifetime abstainers at baseline by sex, 1989–2017.
Figure S2. Incidence of hypertension in men by alcohol intake in standard drinks at baseline compared with abstainers, all studies, 1989–2017.
Figure S3. Incidence of hypertension in women by alcohol intake in standard drinks at baseline compared with abstainers, all studies, 1989–2017.
Figure S4. Incidence of hypertension in men by alcohol intake in standard drinks at baseline compared with abstainers in cohort studies with clinical measurement of blood pressure and low or moderate risk of bias, 1989–2017.
Figure S5. Incidence of hypertension in women by alcohol intake in standard drinks at baseline compared with abstainers in cohort studies with clinical measurement of blood pressure and low or moderate risk of bias, 1989–2017.
Figure S6. Funnel plot for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in men, 1989–2017.
Figure S7. Funnel plot for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in women, 1989–2017.
Figure S8. Influence of omitting a single study for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in men, 1989–2017.
Figure S9. Influence of omitting a single study for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in women, 1989–2017.
(J Am Heart Assoc. 2018;7:e008202 DOI: 10.1161/JAHA.117.008202.)
References
- 1. GBD 2015 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . A global brief on hypertension. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 3. NCD Risk Factor Collaboration . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017;389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fodor JG, Whitmore B, Leenen F, Larochelle P. Lifestyle modifications to prevent and control hypertension. 5. Recommendations on dietary salt. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ. 1999;160(9 Suppl):S29–S34. [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell NR, Ashley MJ, Carruthers SG, Lacourcière Y, McKay DW. Lifestyle modifications to prevent and control hypertension. 3. Recommendations on alcohol consumption. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ. 1999;160(9 Suppl):S13–S20. [PMC free article] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 7. National Clinical Guideline Centre . Hypertension: the Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. London: Royal College of Physicians (UK)—National Clinical Guideline Centre; 2011. [PubMed] [Google Scholar]
- 8. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017. Nov 13. pii: HYP.0000000000000066. DOI: 10.1161/hyp.0000000000000066. [Epub ahead of print] [DOI] [Google Scholar]
- 9. Lian C. Alcoholism: cause of arterial hypertension. Bull Acad Med. 1915;74:525–528. [Google Scholar]
- 10. Puddey IB, Zilkens RR, Beilin LJ. Alcohol, blood pressure and hypertension In: Preedy VR, Watson RR, eds. Comprehensive Handbook of Alcohol Related Pathology. Oxford, UK: Elsevier Academic; 2005:607–626. [Google Scholar]
- 11. Klatsky AL, Gunderson E. Alcohol and hypertension In: Mohler ER, Townsend RR, eds. Advanced Therapy in Hypertension and Vascular Disease. Hamilton, ON, Canada: BC Decker; 2006:108–117. [Google Scholar]
- 12. Klatsky AL, Gundersen E, Kipp H, Udaltsova N, Friedman GD. Higher prevalence of systemic HTN among moderate alcohol drinkers: exploring the role of under‐reporting. J Stud Alcohol. 2006;67:421–428. [DOI] [PubMed] [Google Scholar]
- 13. Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol and hypertension: gender differences in dose‐response relationships determined through systematic review and meta‐analysis. Addiction. 2009;104:1981–1990. [DOI] [PubMed] [Google Scholar]
- 14. Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta‐analysis. J Clin Hypertens. 2012;14:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rehm J, Gmel GE Sr, Gmel G, Hasan OSM, Imtiaz S, Popova S, Probst C, Roerecke M, Room R, Samokhvalov AV, Shield KD, Shuper PA. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. 2017;112:968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first‐pass metabolism. N Engl J Med. 1990;322:95–99. [DOI] [PubMed] [Google Scholar]
- 17. Seppa K, Laippala P, Sillanaukee P. Drinking pattern and blood pressure. Am J Hypertens. 1994;7:249–254. [DOI] [PubMed] [Google Scholar]
- 18. Palmer TM, Sterne JAC. Meta‐Analysis in Stata: an Updated Collection from the Stata Journal. 2nd ed College Station, TX: StataCorp LP; 2016. [Google Scholar]
- 19. Institute for Health Metrics and Evaluation . Global Burden of Disease (GBD). Seattle, WA: Institute for Health Metrics and Evaluation; 2013.
- 20. World Health Organization . Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. Halanych JH, Safford MM, Kertesz SG, Pletcher MJ, Kim YI, Person SD, Lewis CE, Kiefe CI. Alcohol consumption in young adults and incident hypertension: 20‐year follow‐up from the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2010;171:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: the Atherosclerosis Risk in Communities Study. Hypertension. 2001;37:1242–1250. [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Manson JE, Gaziano JM, Liu S, Cochrane B, Cook NR, Ridker PM, Rifai N, Sesso HD. Circulating inflammatory and endothelial markers and risk of hypertension in white and black postmenopausal women. Clin Chem. 2011;57:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta‐analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954–970. [DOI] [PubMed] [Google Scholar]
- 26. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization . International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 28. Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. [DOI] [PubMed] [Google Scholar]
- 29. Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbe KA. Incorporating variations in the quality of individual randomized trials into meta‐analysis. J Clin Epidemiol. 1992;45:255–265. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses? Lancet. 1998;352:609–613. [DOI] [PubMed] [Google Scholar]
- 31. Herbison P, Hay‐Smith J, Gillespie WJ. Adjustment of meta‐analyses on the basis of quality scores should be abandoned. J Clin Epidemiol. 2006;59:1249–1256. [DOI] [PubMed] [Google Scholar]
- 32. Greenland S, O'Rourke K. On the bias produced by quality scores in meta‐analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463–471. [DOI] [PubMed] [Google Scholar]
- 33. Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010;63:1061–1070. [DOI] [PubMed] [Google Scholar]
- 34. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck‐Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 37. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Higgins J, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 39. Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. [DOI] [PubMed] [Google Scholar]
- 40. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose‐response data. Stata J. 2006;6:40–57. [Google Scholar]
- 41. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ascherio A, Hennekens C, Willett WC, Sacks F, Rosner B, Manson J, Witteman J, Stampfer MJ. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension. 1996;27:1065–1072. [DOI] [PubMed] [Google Scholar]
- 43. Banda JA, Clouston K, Sui X, Hooker SP, Lee CD, Blair SN. Protective health factors and incident hypertension in men. Am J Hypertens. 2010;23:599–605. [DOI] [PubMed] [Google Scholar]
- 44. Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Witteman JC, Willett WC, Stampfer MJ, Colditz GA, Sacks FM, Speizer FE, Rosner B, Hennekens CH. A prospective study of nutritional factors and hypertension among US women. Circulation. 1989;80:1320–1327. [DOI] [PubMed] [Google Scholar]
- 46. Witteman JC, Willett WC, Stampfer MJ, Colditz GA, Kok FJ, Sacks FM, Speizer FE, Rosner B, Hennekens CH. Relation of moderate alcohol consumption and risk of systemic hypertension in women. Am J Cardiol. 1990;65:633–637. [DOI] [PubMed] [Google Scholar]
- 47. Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension. 2008;51:1080–1087. [DOI] [PubMed] [Google Scholar]
- 48. Nakanishi N, Yoshida H, Nakamura K, Suzuki K, Tatara K. Alcohol consumption and risk for hypertension in middle‐aged Japanese men. J Hypertens. 2001;19:851–855. [DOI] [PubMed] [Google Scholar]
- 49. Nakanishi N, Makino K, Nishina K, Suzuki K, Tatara K. Relationship of light to moderate alcohol consumption and risk of hypertension in Japanese male office workers. Alcohol Clin Exp Res. 2002;26:988–994. [DOI] [PubMed] [Google Scholar]
- 50. Ohmori S, Kiyohara Y, Kato I, Kubo M, Tanizaki Y, Iwamoto H, Nakayama K, Abe I, Fujishima M. Alcohol intake and future incidence of hypertension in a general Japanese population: the Hisayama study. Alcohol Clin Exp Res. 2002;26:1010–1016. [DOI] [PubMed] [Google Scholar]
- 51. Okubo Y, Sairenchi T, Irie F, Yamagishi K, Iso H, Watanabe H, Muto T, Tanaka K, Ota H. Association of alcohol consumption with incident hypertension among middle‐aged and older Japanese population: the Ibarakai Prefectural Health Study (IPHS). Hypertension. 2014;63:41–47. [DOI] [PubMed] [Google Scholar]
- 52. Peng M, Wu S, Jiang X, Jin C, Zhang W. Long‐term alcohol consumption is an independent risk factor of hypertension development in northern China: evidence from Kailuan study. J Hypertens. 2013;31:2342–2347. [DOI] [PubMed] [Google Scholar]
- 53. Bai G, Zhang J, Zhao C, Wang Y, Qi Y, Zhang B. Adherence to a healthy lifestyle and a DASH‐style diet and risk of hypertension in Chinese individuals. Hypertens Res. 2017;40:196–202. [DOI] [PubMed] [Google Scholar]
- 54. Diederichs C, Neuhauser H. The incidence of hypertension and its risk factors in the German adult population: results from the German National Health Interview and Examination Survey 1998 and the German Health Interview and Examination Survey for Adults 2008–2011. J Hypertens. 2017;35:250–258. [DOI] [PubMed] [Google Scholar]
- 55. Bae JM, Ahn YO. A nested case‐control study on the high‐normal blood pressure as a risk factor of hypertension in Korean middle‐aged men. J Korean Med Sci. 2002;17:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, Tuomainen TP, Salonen R, Salonen JT. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44:859–865. [DOI] [PubMed] [Google Scholar]
- 57. Onat A, Hergenc G, Dursunoglu D, Ordu S, Can G, Bulur S, Yuksel H. Associations of alcohol consumption with blood pressure, lipoproteins, and subclinical inflammation among Turks. Alcohol. 2008;42:593–601. [DOI] [PubMed] [Google Scholar]
- 58. Thawornchaisit P, de Looze F, Reid CM, Seubsman SA, Sleigh AC. Health risk factors and the incidence of hypertension: 4‐year prospective findings from a national cohort of 60 569 Thai Open University students. BMJ Open. 2013;3:e002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OSM, Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta‐analysis. Lancet Public Health. 2017;2:e108–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 61. Gmel G, Rehm J. Measuring alcohol consumption. Contemp Drug Probl. 2004;31:467–540. [Google Scholar]
- 62. Del Boca FK, Darkes J. The validity of self‐reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98:1–12. [DOI] [PubMed] [Google Scholar]
- 63. Rehm J, Kehoe T, Gmel G, Stinson F, Grant B, Gmel G. Statistical modeling of volume of alcohol exposure for epidemiological studies of population health: the example of the US. Popul Health Metr. 2010;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Britton A, O'Neill D, Bell S. Underestimating the alcohol content of a glass of wine: the implications for estimates of mortality risk. Alcohol Alcohol. 2016;51:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rakic V, Puddey IB, Burke V, Dimmitt SB, Beilin LJ. Influence of pattern of alcohol intake on blood pressure in regular drinkers: a controlled trial. J Hypertens. 1998;16:165–174. [DOI] [PubMed] [Google Scholar]
- 67. Rehm J, Ashley MJ, Room R, Single E, Bondy S, Ferrence R, Giesbrecht N. On the emerging paradigm of drinking patterns and their social and health consequences. Addiction. 1996;91:1615–1621. [PubMed] [Google Scholar]
- 68. Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta‐analyses and a systematic review and meta‐analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta‐analysis. Addiction. 2012;107:1246–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta‐analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rehm J, Anderson P, Prieto JAA, Armstrong I, Aubin HJ, Bachmann M, Bastus NB, Brotons C, Burton R, Cardoso M, Colom J, Duprez D, Gmel G, Gual A, Kraus L, Kreutz R, Liira H, Manthey J, Møller L, Okruhlica Ľ, Roerecke M, Scafato E, Schulte B, Segura‐Garcia L, Shield KD, Sierra C, Vyshinskiy K, Wojnar M, Zarco J. Towards new recommendations to reduce the burden of alcohol‐induced hypertension in the European Union. BMC Med. 2017;15:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rehm J, Prieto JA, Beier M, Duhot D, Rossi A, Schulte B, Zarco J, Aubin HJ, Bachmann M, Grimm C, Kraus L, Manthey J, Scafato E, Gual A. The role of alcohol in the management of hypertension in patients in European primary health care practices—a survey in the largest European Union countries. BMC Fam Pract. 2016;17:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Angus C, Latimer N, Preston L, Li J, Purshouse R. What are the implications for policy makers? A systematic review of the cost‐effectiveness of screening and brief interventions for alcohol misuse in primary care. Front Psychiatry. 2014;5:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, Heather N, Saunders J, Burnand B. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007;18:CD004148. [DOI] [PubMed] [Google Scholar]
- 75. Rehm J, Gmel G, Sierra C, Gual A. Reduction of mortality following better detection of hypertension and alcohol problems in primary health care in Spain. Adicciones. 2018;30:9–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy for Medline(R) (1946–Most Recent) and Embase (Embase+Embase Classic)
Table S2. Risk of Bias in Nonrandomized Studies—of Interventions (ROBINS‐I) Assessment Tool, Modified Version
Figure S1. Incidence of hypertension in former drinkers compared with lifetime abstainers at baseline by sex, 1989–2017.
Figure S2. Incidence of hypertension in men by alcohol intake in standard drinks at baseline compared with abstainers, all studies, 1989–2017.
Figure S3. Incidence of hypertension in women by alcohol intake in standard drinks at baseline compared with abstainers, all studies, 1989–2017.
Figure S4. Incidence of hypertension in men by alcohol intake in standard drinks at baseline compared with abstainers in cohort studies with clinical measurement of blood pressure and low or moderate risk of bias, 1989–2017.
Figure S5. Incidence of hypertension in women by alcohol intake in standard drinks at baseline compared with abstainers in cohort studies with clinical measurement of blood pressure and low or moderate risk of bias, 1989–2017.
Figure S6. Funnel plot for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in men, 1989–2017.
Figure S7. Funnel plot for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in women, 1989–2017.
Figure S8. Influence of omitting a single study for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in men, 1989–2017.
Figure S9. Influence of omitting a single study for 1 to 2 drinks/day alcohol intake at baseline compared with abstainers in women, 1989–2017.


