Abstract
Background Few studies have evaluated whether histopathologic lesions on kidney biopsy provide prognostic information beyond clinical and laboratory data.
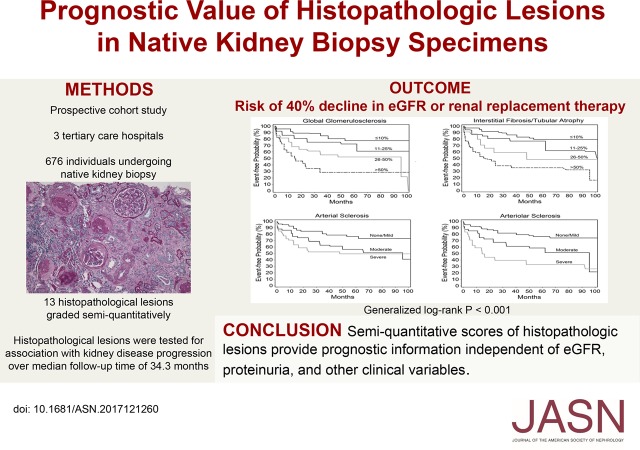
Methods We enrolled 676 individuals undergoing native kidney biopsy at three tertiary care hospitals into a prospective, observational cohort study. Biopsy specimens were adjudicated for semiquantitative scores in 13 categories of histopathology by two experienced renal pathologists. Proportional hazards models tested the association between histopathologic lesions and risk of kidney disease progression (≥40% eGFR decline or RRT).
Results Mean baseline eGFR was 57.5±36.0 ml/min per 1.73 m2. During follow-up (median, 34.3 months), 199 individuals suffered kidney disease progression. After adjustment for demographics, clinicopathologic diagnosis, and laboratory values, the following lesions (hazard ratio; 95% confidence interval) were independently associated with progression: inflammation in nonfibrosed interstitium (0.52; 0.32 to 0.83), moderate and severe versus minimal interstitial fibrosis/tubular atrophy (2.14; 1.24 to 3.69 and 3.42; 1.99 to 5.87, respectively), moderate and severe versus minimal global glomerulosclerosis (2.17; 1.36 to 3.45 and 3.31; 2.04 to 5.38, respectively), moderate and severe versus minimal arterial sclerosis (1.78; 1.15 to 2.74 and 1.64; 1.04 to 2.60, respectively), and moderate and severe versus minimal arteriolar sclerosis (1.63; 1.08 to 2.46 and 2.33; 1.42 to 3.83, respectively). An 11-point chronicity score derived from semiquantitative assessments of chronic lesions independently associated with higher risk of kidney disease progression (hazard ratio per one-point increase, 1.19; 95% confidence interval, 1.12 to 1.27).
Conclusions Across a diverse group of kidney diseases, histopathologic lesions on kidney biopsy provide prognostic information, even after adjustment for proteinuria and eGFR.
Keywords: chronic kidney disease, renal biopsy, histopathology, ESRD
Tens of thousands of native kidney biopsies are performed annually across the world for the evaluation and management of a variety of forms of kidney disease.1,2 Information from pathologic examination of biopsy tissue is used for diagnosis as well as prognosis.3–5 Despite the importance of the kidney biopsy in the care of patients with acute kidney diseases and CKDs,6 there are no widely used standardized guidelines for structuring and reporting the results of kidney biopsy pathologic examination. Standardized scoring systems have been developed for specific kidney diseases but not as a global scoring or reporting system.7–15 A recent expert opinion–based proposal to score and grade chronic changes on the kidney biopsy specimen included four lesions and a global chronicity score and highlighted the need for studies to examine the prognostic significance of each variable.16
It is often claimed that chronic tubulointerstitial lesions are the most important prognostic indicators from kidney biopsies.17–21 Despite this widespread belief, the underlying evidence base is relatively thin and derives from small numbers of patients with specific forms of kidney diseases without adequate adjustment for confounding variables. Few studies have tested the prognostic value of histopathologic findings across a diverse set of kidney diseases with adjustment for clinical variables, such as demographics, clinicopathologic diagnosis, and laboratory variables.22,23 To address this gap in our understanding of the prognostic significance of kidney histopathology, we conducted a prospective cohort study, in which biopsies were re-read by two study pathologists who were unaware of the original clinical or pathologic diagnosis. Each biopsy was reviewed under light microscopy jointly and graded semiquantitatively for acute and chronic lesions in the glomerular, tubulointerstitial, and vascular compartments. We followed patients prospectively and tested whether individual histopathologic lesions predicted the subsequent development of kidney disease progression (≥40% decline in eGFR or the need for RRT).
Methods
Study Population
The Boston Kidney Biopsy Cohort (BKBC) is a prospective, observational cohort study of patients undergoing native kidney biopsy at three tertiary care hospitals in Boston, Massachusetts. This study includes adult patients (≥18 years of age) who underwent a clinically indicated native kidney biopsy between September 2006 and June 2016. Patients provided blood and urine samples on the day of kidney biopsy. Exclusion criteria included the inability to provide consent, enrollment in competing studies, severe anemia, and pregnancy. All participants provided written consent. The study protocol was approved by the Partners Human Research Committee (the Brigham and Women’s Hospital Institutional Review Board), and is in accordance with the principles of the Declaration of Helsinki.
Evaluation of Histopathology
All biopsies were adjudicated under light microscopy by two renal pathologists, who provided semiquantitative scores of kidney inflammation, fibrosis, vascular sclerosis, and tubular injury. The scoring system for individual histopathologic lesions was developed by the pathologists for use in this study on the basis of their review of the literature and clinical experience.12,14,24–30 Formal criteria were not used for individual lesions. In joint pathology review sessions, the pathologists reviewed the light microscopy slides alongside one another and verbally suggested their score for individual histopathologic lesion severity in the presence of A.S. and/or S.S.W. If they did not initially agree, the case was discussed in more detail to obtain consensus on individual histopathologic lesion severity. Of the 13 categories used, all were scored during study sessions except for the grades of segmental and global glomerulosclerosis, which were taken from the biopsy report, because they were each calculated as a percentage of the total number of glomeruli. Global glomerulosclerosis was further graded as “age related” or “pathologic” on the basis of the number of globally sclerosed glomeruli divided by the total number of glomeruli as done by Hommos et al.31 The study pathologists reviewed the slides without knowledge of the original biopsy diagnosis or biopsy report, typically several months after the biopsy procedure and original pathology report. Not all lesions were able to be evaluated on all biopsies, but this amounted to <5% missing data. We reviewed all patients’ charts alongside the final histopathologic diagnosis to identify the primary and any secondary clinicopathologic diagnoses.
Exposure and Outcomes
The primary exposures were semiquantitative measures of 13 separate histopathologic lesions: mesangial expansion, global glomerulosclerosis, segmental glomerulosclerosis, endocapillary glomerular inflammation, extracapillary cellular crescents, fibrinoid necrosis, fibrocellular crescents, interstitial fibrosis and tubular atrophy (IFTA), inflammation in the nonfibrosed interstitium, inflammation in the fibrosed interstitium, acute tubular injury (ATI), arterial sclerosis, and arteriolar sclerosis. We calculated a kidney biopsy chronicity score on the basis of the proposal by Sethi et al.16 for standardized semiquantitative grading of chronic changes of glomerulosclerosis (zero to three), interstitial fibrosis (zero to three), tubular atrophy (zero to three), and arteriolosclerosis (zero or one). Because the total renal chronicity score by Sethi et al.16 adds the score for interstitial fibrosis and the score for tubular atrophy separately, we weighted our IFTA scores by a factor of two. We also converted our semiquantitative arterial sclerosis score to a binary score in keeping with the proposal by Sethi et al.16
The primary outcome was kidney disease progression defined as ≥40% eGFR decline from baseline or the need for RRT (dialysis or kidney transplantation). eGFR during follow up was obtained from the electronic medical record (EMR). The secondary outcome was the need for RRT. RRT status was confirmed by reviewing the EMR and linkage with the US Renal Data System database. Mortality status was confirmed with the Social Security Death Index. Participants were followed up until the occurrence of death, voluntary study withdrawal, loss to follow-up, or June 30, 2017.
Covariates
We collected patient information at the biopsy visit, including demographics, detailed medical history, medication lists, and pertinent laboratory data. Study data were collected and managed using REDCap electronic data capture tools hosted at Partners HealthCare.32 We obtained serum creatinine (SCr) from the EMR on the day of biopsy. In participants for whom this was unavailable (n=117), we measured SCr in individuals with available blood samples collected on the day of biopsy (n=82). If no samples were available (n=35), we used the most recent SCr values available in the EMR from the date of kidney biopsy up to 3 months before biopsy. We obtained spot urine protein-to-creatinine ratio or urine albumin-to-creatinine ratio from the date of kidney biopsy to 3 months before biopsy from the EMR. If a participant did not have any of these values, we measured urine albumin-to-creatinine ratio from urine collected on the day of the kidney biopsy (n=54). SCr and urinary creatinine were measured using a Jaffe-based method, and urine albumin was measured by an immunoturbidometric method. We used the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation to calculate the eGFR.33
Statistical Analyses
Descriptive statistics were summarized as mean±SD or median (interquartile range) for continuous variables, and frequency distribution is presented with percentages for categorical variables. For skewed data distributions, we performed natural logarithmic transformation as appropriate. We assessed for associations between categorical variables and two-group comparisons using chi-squared tests. For evaluations between continuous variables and multiple group comparisons, we used ANOVA (for normally distributed variables) and Kruskal–Wallis tests (for non-normally distributed variables). We used Spearman correlation coefficients to determine the associations between continuous variables and histopathologic lesion scores. We calculated the weighted κ-statistic to evaluate the reproducibility of histopathologic scores.
For the primary outcome (time to either ≥40% decline in eGFR or starting RRT), we treated the data as interval censored, because the exact date of the event may not be known (e.g., the exact time to ≥40% decline in eGFR could have occurred any time in the interval between two successive laboratory measurements). We evaluated the association between individual histopathologic lesions and subsequent kidney disease progression using a nonparametric survival function for interval-censored data with differences assessed by the generalized log rank test.34,35 We modeled the data using Cox proportional hazards (PH) regression models that accommodated interval-censored times to event.36,37 Cox PH models were used for the secondary outcome of time to RRT. All PH models were fit first without adjustment and then stratified by site with multivariable adjustment for covariates, including age, sex, race, baseline eGFR, natural log-transformed proteinuria, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, and primary clinicopathologic diagnostic category of kidney disease.
To test the predictive models, we calculated c statistics for the outcome of RRT using a base model (including age, sex, log-transformed proteinuria, and baseline eGFR) and the model further including the chronicity score.38,39 Results from subdistribution hazards models that acknowledged the competing risk of death were performed as a sensitivity analysis for the outcome of RRT, but they were qualitatively unchanged; therefore, they are not reported.40 We confirmed no violations of the PH assumption using the Kolmogorov-type supremum test, and the functional forms of the covariates were assessed by checking martingale residuals. All statistical tests were two sided, and P values <0.05 were considered significant. Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Results
Study Population Characteristics
Patient characteristics in the BKBC at the time of kidney biopsy are presented in Table 1. The mean age was 52.2±16.5 years old, 52.5% were women, and 63.8% were white. The mean eGFR was 57.5±36.0 ml/min per 1.73 m2, and median proteinuria was 1.74 g/g creatinine [interquartile range, 0.43–4.38 g/g creatinine]. The most common reasons for kidney biopsy were proteinuria, abnormal eGFR, and hematuria. Supplemental Figure 1 shows a flow diagram for individuals enrolled in the BKBC.
Table 1.
Baseline characteristics of the Boston Kidney Biopsy Cohort
| Characteristics | n=676 |
|---|---|
| Age, yr | 52.2±16.5 |
| Women, % | 52.5 |
| Race, % | |
| White | 63.8 |
| Black | 20.6 |
| Other | 15.7 |
| eGFR, ml/min per 1.73 m2 | 57.5±36.0 |
| Serum creatinine, mg/dl | 1.41 [0.94–2.24] |
| Proteinuria, g/g creatinine | 1.74 [0.43–4.38] |
| No. of glomeruli on biopsy | 31 [20–43] |
| Reason for biopsy,a % | |
| Proteinuria | 59.2 |
| Hematuria | 27.4 |
| Nephrotic syndrome | 14.5 |
| Nephritic syndrome | 2.5 |
| Abnormal eGFR | 53.5 |
| Primary clinicopathologic diagnosis, % | |
| Proliferative GN | 30.2 |
| Nonproliferative glomerulopathies | 18.2 |
| Advanced glomerulosclerosis | 11.5 |
| Diabetic nephropathy | 11.1 |
| Vascular | 8.0 |
| Tubulointerstitial | 7.3 |
| Paraprotein | 7.3 |
| Other | 6.5 |
| Comorbid conditions, % | |
| Diabetes mellitus | 22.6 |
| Hypertension | 49.3 |
| SLE | 14.8 |
| Hepatitis C | 2.5 |
| Malignancy | 13.0 |
| Medications, % | |
| ACEi/ARB | 45.9 |
| Mineralocorticoid receptor antagonists | 1.9 |
| Calcium channel blockers | 23.4 |
| β-Blockers | 29.4 |
| Immunosuppression | 17.8 |
| Corticosteroids | 17.6 |
| Clinical site, % | |
| Site 1 | 57.8 |
| Site 2 | 32.0 |
| Site 3 | 10.2 |
Data presented as percentages, mean±SD, and median [interquartile range]. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Percentages do not add to 100, because there may have been multiple reasons for biopsy.
Histopathologic and Clinicopathologic Classification
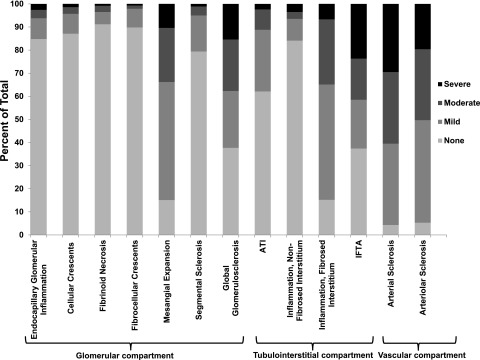
All biopsies were reviewed under light microscopy during study sessions with two experienced renal pathologists who jointly adjudicated each biopsy semiquantitatively for acute and chronic lesions as shown in Supplemental Table 1. Figure 1 shows the breakdown of the semiquantitative scores for histopathologic lesions. To test the reproducibility of the adjudicated scores, we had study pathologists rescore 26 selected kidney biopsies several months after their initial review. Weighted κ-statistics (95% CI) were 0.92 (0.77–1.00) for endocapillary glomerular inflammation, 1.00 (1.00–1.00) for cellular crescents, 0.72 (0.56–0.89) for fibrinoid necrosis, 0.65 (0.02–1.00) for fibrocellular crescents, 0.54 (0.12–0.69) for mesangial expansion, 0.67 (0.45–0.89) for ATI, 0.46 (0.16–0.76) for inflammation in the nonfibrosed interstitium, 0.52 (0.27–0.78) for inflammation in the fibrosed interstitium, 0.72 (0.52–0.93) for IFTA, 0.64 (0.41–0.87) for arterial sclerosis, and 0.66 (0.44–0.87) for arteriolar sclerosis. Weighted κ-statistics for global and segmental glomerulosclerosis were not performed, because these lesions were not readjudicated but rather, were abstracted from the biopsy report.
Figure 1.
Distribution of semiquantitative severity scores for each of the adjudicated 13 histopathological lesions in the Boston Kidney Biopsy Cohort. The histopathologic categories were graded by percentage of renal cortical volume or affected glomeruli: none, ≤10%; mild, 11%–25%; moderate, 26%–50%; and severe, >50%. ATI, acute tubular injury; IFTA, interstitial fibrosis and tubular atrophy.
Patients were classified into eight primary clinicopathologic diagnostic categories using histopathologic information and review of the medical record. All primary clinicopathologic diagnoses included within each category are presented in Table 2.
Table 2.
Primary clinicopathologic diagnoses
| Proliferative GN, n=204 | Nonproliferative Glomerulopathies, n=123 | Paraprotein, n=49 | DN, n=75 | Vascular, n=54 | Tubulointerstitial, n=49 | Advanced, n=78 | Other, n=44 |
|---|---|---|---|---|---|---|---|
| IgA nephropathy (73) | Membranous nephropathy (47) | AL amyloid (21) | DN (75) | Vascular sclerosis (37) | AIN (14) | Secondary | TBM (15) |
| Lupus nephritis | Minimal change disease (24) | MIDD (15) | TMA (12) | ATN (14) | FSGS (40) | Normal (15) | |
| Class 2 (8) | Idiopathic FSGS (15) | AA amyloid (5) | APLS (2) | Chronic/active IN (6) | Advanced chronic changes (37) | Lymphoma infiltration (7) | |
| Class 3 (A or A/C) (14) | Lupus nephritis | Cast nephropathy (4) | Scleroderma (1) | Oxalate nephropathy (6) | Adenovirus (1) | Mild IFTA (7) | |
| Class 3+5 (A or A/C) (11) | Class 2+5 (1) | Immunotactoid GN (1) | CNI toxicity (1) | CIN (3) | |||
| Class 4 (A or A/C) (23) | Class 3 (C) (1) | Light-chain crystal tubulopathy (1) | Cholesterol emboli (1) | Granulomatous IN (3) | |||
| Class 4+5 (A or A/C) (9) | Class 4 (C) (5) | Light-chain proteinuria (1) | Phosphate nephropathy (2) | ||||
| Class 4+6 (A or A/C) (1) | Class 3 (C) +5 (1) | Multiple myeloma (1) | Tenofovir nephrotoxicity (1) | ||||
| ANCA (27) | Class 4 (C) +5 (1) | ||||||
| Immune complex GN (14) | Class 5 (23) | ||||||
| Postinfectious GN (3) | Class 6 (1) | ||||||
| Cryoglobulinemia (4) | Collapsing glomerulopathy (3) | ||||||
| PGNMID (2) | C1q nephropathy (1) | ||||||
| C3 GN (4) | Idiopathic nodular glomerulosclerosis (1) | ||||||
| HIV immune complex GN (1) | |||||||
| Fibrillary GN (3) | |||||||
| Hereditary nephritis (1) | |||||||
| Membranoproliferative GN (2) | |||||||
| Crescentic GN (3) |
Actual number of patients (n) in each category. DN, diabetic nephropathy; AL, amyloid light chain; AIN, acute interstitial nephritis; TBM, thin basement membrane; MIDD, monoclonal immunoglobulin deposition disease; TMA, thrombotic microangiopathy; ATN, acute tubular necrosis; AA, serum amyloid A protein; APLS, antiphospholipid syndrome; IN, interstitial nephritis; IFTA, interstitial fibrosis and tubular atrophy; CNI, calcineurin inhibitor toxicity; CIN, chronic interstitial nephritis; PGNMID, proliferative GN with monoclonal immunoglobulin deposits.
Correlations with Histopathology
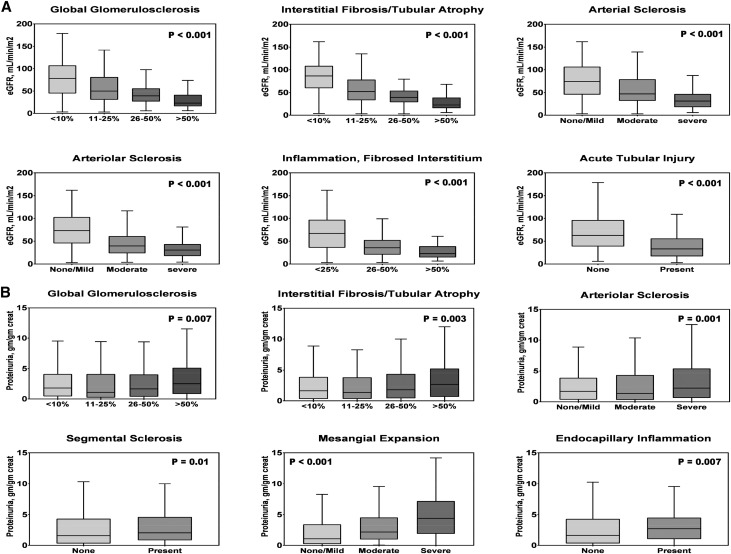
Supplemental Figure 2 shows the distribution of eGFR and proteinuria categories in all patients with biopsies. The histopathologic lesions that most strongly correlated with eGFR were IFTA (Rs=−0.62, P<0.001), arteriolar sclerosis (Rs=−0.48, P<0.001), inflammation in the fibrosed interstitium (Rs=−0.47, P<0.001), global glomerulosclerosis (Rs=−0.46, P<0.001), arterial sclerosis (Rs=−0.44, P<0.001), and ATI (Rs=−0.40, P<0.001) (Supplemental Table 2). The histopathologic lesions that most strongly correlated with proteinuria were mesangial expansion (Rs=0.29, P<0.001), IFTA (Rs=0.13, P<0.001), endocapillary glomerular inflammation (Rs=0.11, P=0.004), segmental sclerosis (Rs=0.11, P<0.01), arteriolar sclerosis (Rs=0.09, P=0.02), inflammation in the fibrosed interstitium (Rs=0.09, P=0.03), and ATI (Rs=0.08, P=0.03) (Supplemental Table 2). Figure 2 shows boxplots between grades of histopathologic lesions that were most significantly associated with eGFR and proteinuria.
Figure 2.
Histopathologic lesions are significantly associated with eGFR and proteinuria. Boxplots show significant differences between grades of adjudicated histopathologic lesions and (A) eGFR or (B) proteinuria.
We found a number of significant correlations among histopathologic lesions of glomerular, tubulointerstitial, and vascular injury (Supplemental Table 3). IFTA moderately correlated with global glomerulosclerosis (Rs=0.64, P<0.001), inflammation in the fibrosed interstitium (Rs=0.61, P<0.001), arteriolar sclerosis (Rs=0.59, P<0.001), and arterial sclerosis (Rs=0.52, P<0.001). Crescents correlated with necrosis (Rs=0.69, P<0.001) and fibrocellular crescents (Rs=0.60, P<0.001).
Prognostic Significance of Histopathologic Lesions
During a median follow-up time of 34.3 months, 199 individuals experienced subsequent kidney disease progression, and 111 individuals were started on RRT. Table 3 shows the unadjusted and multivariable-adjusted associations between each individual histopathologic lesion and kidney disease progression. Results for progression to RRT were similar (Supplemental Table 4).
Table 3.
Individual histopathologic lesions and the risk of kidney disease progression
| Histopathologic Lesion | N | Events per 100 person-yra | Model 1 HR [95% CI] | Model 2 HR [95% CI] | Model 3 HR [95% CI] |
|---|---|---|---|---|---|
| Glomerular compartment | |||||
| Endocapillary glomerular inflammation | |||||
| Absent | 569 | 13.0 | — | — | — |
| Present | 101 | 10.6 | 0.91 [0.61 to 1.35] | 1.22 [0.74 to 2.02] | 1.33 [0.81 to 2.20] |
| Cellular crescents | |||||
| Absent | 586 | 13.1 | — | — | — |
| Present | 85 | 9.0 | 0.72 [0.45 to 1.15] | 0.99 [0.56 to 1.78] | 1.02 [0.54 to 1.95] |
| Fibrinoid necrosis | |||||
| Absent | 611 | 13.3 | — | — | — |
| Present | 60 | 5.9 | 0.47 [0.24 to 0.92] | 0.57 [0.28 to 1.16] | 0.61 [0.29 to 1.27] |
| Fibrocellular crescents | |||||
| Absent | 602 | 12.9 | — | — | — |
| Present | 68 | 9.8 | 0.84 [0.52 to 1.36] | 1.18 [0.68 to 2.06] | 1.08 [0.59 to 1.96] |
| Mesangial expansion | |||||
| None or mild | 438 | 8.9 | — | — | — |
| Moderate | 155 | 15.1 | 1.59 [1.13 to 2.23] | 1.35 [0.92 to 1.98] | 1.25 [0.85 to 1.83] |
| Severe | 69 | 38.1 | 3.64 [2.52 to 5.25] | 1.56 [0.92 to 2.64] | 1.21 [0.71 to 2.07] |
| Segmental sclerosis | |||||
| Absent | 532 | 12.0 | — | — | — |
| Present | 138 | 14.8 | 1.19 [0.85 to 1.66] | 1.17 [0.82 to 1.67] | 1.09 [0.71 to 1.69] |
| Global glomerulosclerosis | |||||
| Minimal (≤10%) | 255 | 5.1 | — | — | — |
| Mild (11%–25%) | 164 | 9.8 | 1.76 [1.11 to 2.79] | 1.62 [1.00 to 2.61] | 1.54 [0.95 to 2.48] |
| Moderate (26%–50%) | 149 | 18.1 | 3.31 [2.18 to 5.02] | 2.63 [1.66 to 4.14] | 2.17 [1.36 to 3.45] |
| Severe (>50%) | 104 | 39.1 | 6.30 [4.18 to 9.48] | 4.61 [2.91 to 7.32] | 3.31 [2.04 to 5.38] |
| Tubulointerstitial compartment | |||||
| Acute tubular injury | |||||
| Absent | 412 | 10.4 | — | — | — |
| Present | 254 | 16.8 | 1.47 [1.11 to 1.95] | 1.25 [0.92 to 1.70] | 0.95 [0.68 to 1.33] |
| Inflammation, nonfibrosed interstitium | |||||
| Absent | 560 | 13.1 | — | — | — |
| Present | 104 | 10.0 | 0.79 [0.53 to 1.20] | 0.68 [0.43 to 1.08] | 0.52 [0.32 to 0.83] |
| Inflammation, fibrosed interstitium | |||||
| None or mild | 436 | 9.5 | — | — | — |
| Moderate | 187 | 18.7 | 1.84 [1.36 to 2.48] | 1.47 [1.07 to 2.02] | 1.09 [0.79 to 1.50] |
| Severe | 45 | 28.3 | 2.51 [1.53 to 4.10] | 2.52 [1.48 to 4.27] | 1.74 [0.99 to 3.06] |
| Interstitial fibrosis/tubular atrophy | |||||
| Minimal (≤10%) | 249 | 4.3 | — | — | — |
| Mild (11%–25%) | 142 | 8.6 | 1.85 [1.13 to 3.05] | 1.63 [0.96 to 2.75] | 1.34 [0.78 to 2.31] |
| Moderate (26%–50%) | 118 | 18.2 | 3.71 [2.34 to 5.88] | 2.99 [1.83 to 4.90] | 2.14 [1.24 to 3.69] |
| Severe (>50%) | 158 | 38.3 | 7.15 [4.73 to 10.8] | 5.07 [3.17 to 8.09] | 3.42 [1.99 to 5.87] |
| Vascular compartment | |||||
| Arterial sclerosis | |||||
| None or mild | 287 | 6.3 | — | — | — |
| Moderate | 204 | 14.2 | 2.18 [1.49 to 3.19] | 1.97 [1.18 to 3.28] | 1.78 [1.15 to 2.74] |
| Severe | 194 | 22.8 | 3.18 [2.20 to 4.61] | 2.13 [1.25 to 3.64] | 1.64 [1.04 to 2.60] |
| Arteriolar sclerosis | |||||
| None or mild | 331 | 6.0 | — | — | — |
| Moderate | 204 | 15.5 | 2.31 [1.62 to 3.29] | 2.08 [1.41 to 3.08] | 1.63 [1.08 to 2.46] |
| Severe | 131 | 36.7 | 4.90 [3.44 to 7.00] | 3.06 [1.93 to 4.84] | 2.33 [1.42 to 3.83] |
Model 1 is unadjusted. Model 2 is stratified by site and adjusted for age, sex, race, log-transformed proteinuria, primary clinicopathologic diagnosis, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker medication use. Model 3 includes Model 2 and further adjusts for eGFR. HR, hazard ratio; 95% CI, 95% confidence interval; —, reference group.
Approximate events per 100 person-years. If an event occurred, the time used is one half of the interval width plus all of the time before the interval as the approximate exposure time (the exact time an event occurred is not known if a ≥40% decline in eGFR occurred).
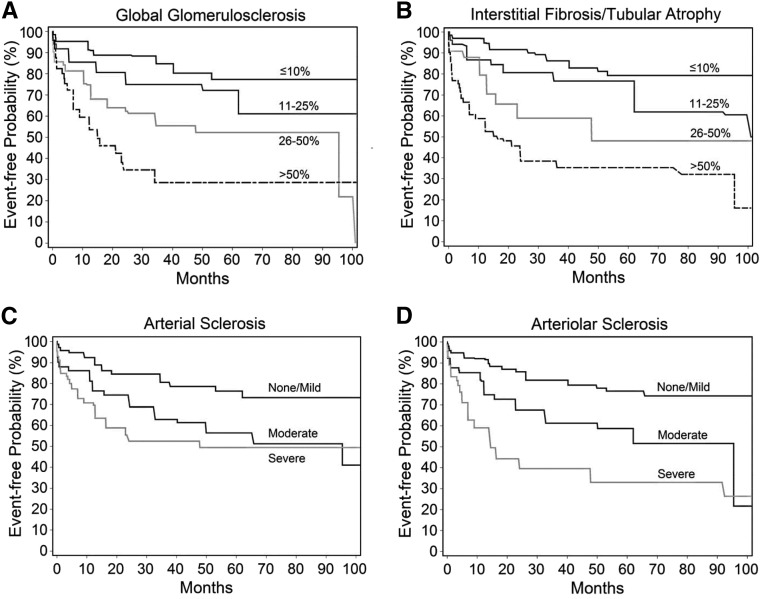
Glomerular Lesions
Higher degrees of global glomerulosclerosis were associated with an increased risk of kidney disease progression (Figure 3A) (generalized log rank P<0.001). The association between global glomerulosclerosis and kidney disease progression was confounded by eGFR. In a fully multivariable-adjusted clinical model including log-transformed proteinuria and eGFR, 26%–50% and >50% versus ≤10% global glomerulosclerosis were associated with more than two- and more than threefold increased risks of kidney function loss, respectively (Table 3). After accounting for age-related or no global glomerulosclerosis as previously described,31,41 global glomerulosclerosis deemed pathologic (abnormal for age) was associated with a higher risk of kidney disease progression after multivariable adjustment (hazard ratio, 1.87; 95% confidence interval, 1.29 to 2.73). Age-related global glomerulosclerosis was not associated with an increased risk of kidney disease progression compared with no global glomerulosclerosis after multivariable adjustment (hazard ratio, 0.97; 95% confidence interval, 0.51 to 1.81). In multivariable-adjusted models, pathologic global glomerulosclerosis with involvement of 26%–50% and >50% glomeruli, compared with no or age-related global glomerulosclerosis, remained associated with an increased risk of kidney disease progression (Supplemental Table 5).
Figure 3.
Chronic histopathologic lesions are associated with kidney disease progression. (A) Global glomerulosclerosis. (B) Interstitial fibrosis/tubular atrophy. (C) Arterial sclerosis. (D) Arteriolar sclerosis. Generalized log rank P<0.001 for all lesions.
Tubulointerstitial Lesions
Increasing severity of IFTA was associated with subsequent loss of kidney function (Figure 3B) (generalized log rank P<0.001). The association was strongly confounded by eGFR. In a fully multivariable-adjusted model including log-transformed proteinuria and eGFR, 26%–50% and >50% IFTA versus ≤10% IFTA were associated with a 2.1- and nearly 3.5-fold increased risks of kidney disease progression, respectively (Table 3). Severe inflammation in the fibrosed interstitium was associated with 1.7-fold increased risk of kidney disease progression that did not reach statistical significance after multivariable adjustment, but it was significant for the outcome of RRT (Supplemental Table 4). Inflammation in the nonfibrosed interstitium (present versus absent) was associated with a lower rather than a higher risk of kidney disease progression in the fully adjusted model (Table 3).
Vascular Lesions
Because of the low number of scores of “none” for both arterial and arteriolar lesions, “none” and “mild” were collapsed into a single category for analyses. The severity of arterial sclerosis and arteriolar sclerosis were both associated with future kidney disease progression (Figure 3, C and D) (generalized log rank P<0.001). The association of arterial and arteriolar sclerosis with subsequent kidney disease progression was confounded by eGFR. Moderate arterial sclerosis and severe arterial sclerosis (versus none/mild) remained associated with nearly 1.8- and 1.7-fold increased risks of kidney disease progression after multivariable adjustment, respectively (Table 3). Moderate arteriolar sclerosis and severe arteriolar sclerosis (versus none/mild) were associated with 1.6- and 2.3-fold increased risks of kidney disease progression after multivariable adjustment, respectively (Table 3).
Kidney Biopsy Chronicity Score
Sethi et al.16 proposed a standardized grading of chronic changes in native kidney biopsy specimens on the basis of the following four variables: interstitial fibrosis, tubular atrophy, arteriosclerosis (without mention of arterioles), and glomerulosclerosis—all of which were found in our study to be independently associated with kidney disease progression. Results on the association between the chronicity score and kidney disease progression are shown in Table 4. Results for the outcome of RRT were similar and are shown in Supplemental Table 6. We calculated total renal chronicity scores for each individual in this study on the basis of the proposal by Sethi et al.16 and tested the performance of the score for predicting 5-year risk of RRT; we compared the score with the Kidney Failure Risk Equation (KFRE), which includes age, race, sex, proteinuria, and eGFR.42,43 The c statistic of the model with only the chronicity score was 0.84 (95% confidence interval, 0.80 to 0.88). The c statistic of the model with KFRE variables was 0.85 (95% confidence interval, 0.81 to 0.89). The c statistic of the model with both the chronicity score and the KFRE variables increased to 0.87 (95% confidence interval, 0.83 to 0.90; P=0.13 for comparison with the c statistic of the KFRE model).
Table 4.
Risk of kidney disease progression using the kidney biopsy chronicity score
| Chronicity Scorea | N | Events per 100 person-yr | Model 1 HR [95% CI] | Model 2 HR [95% CI] | Model 3 HR [95% CI] |
|---|---|---|---|---|---|
| Per one-point change | 654 | 12.1 | 1.28 [1.22 to 1.33] | 1.23 [1.17 to 1.30] | 1.19 [1.12 to 1.27] |
| Minimal chronic changes (0–1) | 199 | 3.6 | — | — | — |
| Mild chronic changes (2–4) | 168 | 8.1 | 2.02 [1.17 to 3.48] | 1.72 [0.97 to 3.06] | 1.53 [0.85 to 2.75] |
| Moderate chronic changes (5–7) | 146 | 17.3 | 4.24 [2.55 to 7.04] | 3.81 [2.20 to 6.59] | 2.92 [1.61 to 5.31] |
| Severe chronic changes (≥8) | 141 | 39.6 | 8.67 [5.35 to 14.1] | 6.09 [3.51 to 10.6] | 4.42 [2.37 to 8.22] |
Shown are HRs (95% CIs) per one-point increase in the score (range from zero to ten) or compared to the reference group of minimal chronic changes. Model 1 is unadjusted. Model 2 is Model 1 stratified by site and adjusted for age, sex, race, log-transformed proteinuria, primary clinicopathologic diagnosis, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker medication use. Model 3 includes Model 2 and adjusts for eGFR. HR, hazard ratio; 95% CI, 95% confidence interval; —, reference group.
Chronicity score is calculated by adding scores of global glomerulosclerosis (zero to three), interstitial fibrosis and tubular atrophy (zero to three), and arterial sclerosis (zero to one). Interstitial fibrosis and tubular atrophy are counted twice, because interstitial fibrosis and tubular atrophy are scored separately in the proposal by Sethi et al.16 Arterial sclerosis is given a score of zero for none/mild and one for moderate/severe lesions.
Discussion
In this prospective study of adjudicated histopathologic lesions across a diverse set of kidney diseases, we found that several histopathologic lesions were independent predictors of kidney disease progression. The main findings were that IFTA, arterial and arteriolar sclerosis, and global glomerulosclerosis were each associated with kidney disease progression even after adjusting for a number of clinical predictors, including eGFR, proteinuria, and clinicopathologic diagnosis. A previously proposed chronicity score from the sum of semiquantitative grading of chronic lesions was also independently associated with kidney disease progression but did not change risk prediction appreciably as judged by the change in c statistic. The final findings were that the presence of inflammation in the fibrosed interstitium and the presence of inflammation in nonfibrosed interstitium were associated with higher and lower risk of subsequent kidney disease progression, respectively; the latter was presumably due to the treatable nature of such lesions.
Kidney biopsies provide diagnostic and also prognostic information.3–6 A key strength of our study is the inclusion of diverse forms of kidney disease and multivariable adjustment for clinicopathologic diagnosis as well as demographics and laboratory values. Our findings extend and validate findings from the published literature dating back to the 1960s.17,44,45
It is commonly stated that interstitial fibrosis is the histopathologic lesion most strongly associated with a higher risk of kidney failure.18,19,22,46 The evidence base for this assertion, however, derives from a number of studies restricted to specific etiologies47–50 and studies that did not adjust comprehensively for known confounders, such as clinicopathologic diagnosis, eGFR, and proteinuria.20,51,52 Our findings confirm the importance of IFTA and expand on the importance of interstitial inflammation,53 global glomerulosclerosis, arterial sclerosis, and arteriolar sclerosis. Our finding that kidney disease progression is more common in those with global glomerulosclerosis exceeding the expected degree on the basis of age is consistent with the findings of Hommos et al.31 and support the need to take into account age-related pathologic changes.
A number of studies have developed scoring systems for renal pathology. Examples include activity and chronicity indices in lupus nephritis,50 the Oxford classification in IgA nephropathy,13,14 the Nephrotic Syndrome Study Network digital pathology scoring system,54 and prior suggestions for global chronicity scores.20,49,55 More recently, Sethi et al.16 suggested that biopsies should include semiquantitative scores for the degree of glomerulosclerosis, interstitial fibrosis, tubular atrophy, and arteriosclerosis along with an overall chronicity score equal to the sum of the individual scores. Our findings provide empirical evidence in support of such a scoring system. The proposed scoring system by Sethi et al.16 did not include arteriolar sclerosis, but our study provides evidence for the prognostic importance of arteriolar sclerosis for kidney disease progression. The degrees of arterial and arteriolar lesions, although correlated, can diverge from one another on the basis of our finding of a modest correlation between the two scores (R=0.68). These findings suggest that nephropathologists should score arterial and arteriolar lesions separately because they both associate with kidney disease progression and may represent different pathologic processes. Given the importance of arterial and arteriolar lesions, greater standardization by pathologists—in both terminology and grading criteria—would be desirable.
eGFR and proteinuria are used by clinicians for prognostic assessment, but they do not capture the full extent of kidney pathology. Our results of independent associations between histopathology and kidney disease progression reflect the inadequacy of SCr and proteinuria as risk markers and point toward well described hypotheses on the causal associations between histopathologic lesions and progression of kidney disease. Global glomerulosclerosis and IFTA, for example, may denote focal nephron loss and adaptive responses of surviving nephrons that ultimately prove to be detrimental to kidney function.56,57 Arterial and arteriolar lesions could be a reflection (or cause) of systemic hypertension and failure of glomerular autoregulation.58–60 Whether chronic lesions from kidney biopsy findings can direct specific treatments to modify disease progression (for example, antifibrotic agents for those with excessive IFTA) is an area in need of more research and clinical trials. It is tempting to speculate that kidney biopsies could provide value in patients who do not currently undergo kidney biopsy, such as individuals with presumed hypertension-associated CKD and presumed diabetic nephropathy. Indeed, the Kidney Precision Medicine Project launched by the National Institute of Diabetes, Digestive and Kidney Diseases is conducting multicenter studies to expand the role for kidney biopsies and their molecular interrogation in individuals with common forms of CKD and AKI.
Several limitations should be noted in our study. We did not include readjudication of electron microscopy or immunofluorescence results primarily due to logistic constraints. We also did not perform quantitative morphometry or quantify features, such as podocyte number or peritubular capillary density, which are important in the pathogenesis and progression of various kidney diseases. We did not take into account therapy administered after the biopsy, which could alter an individual’s trajectory to the outcome, particularly in individuals requiring immunosuppressive therapy. The two renal pathologists did not use formalized definitions and criteria for each grading category; this is not a source of bias for our findings, however, because they were completely blinded to clinical variables, reason for biopsy, and original diagnosis. The relatively low κ-statistics for certain lesions could have biased results toward the null hypothesis of no association between a given lesion and the outcomes tested. Although our cohort was diverse (from three separate academic medical centers) and large relative to other biopsy cohorts with adjudicated histopathologic scores, it includes only individuals who underwent biopsies for specific clinical indications with diseases that are not necessarily reflective of the majority of patients with CKD and patients with AKI. The number of biopsies from individual disease categories was also small, particularly for rare forms of kidney disease. Our readjudication process provided internally consistent scoring for biopsies; however, it is not necessarily generalizable, because we relied on two expert renal pathologists who convened to score biopsies together in study sessions. Further development of standardized criteria for scoring lesions, as has been done by the Banff group for transplant biopsies, would be important to allow adoption by other centers and enhance generalizability. The development of image analysis algorithms using digital pathology could also be focused on lesions that provide prognostic information, such as we have described.
In conclusion, greater severity of lesions on native kidney biopsies—independent of clinical factors, including clinicopathologic diagnosis, eGFR, and proteinuria—associates with a greater risk of kidney disease progression across a variety of kidney disease etiologies. A kidney biopsy chronicity score also provides additional prognostic value above routine clinical variables. Our findings highlight the prognostic value of careful review and semiquantitative scoring of the kidney biopsy specimen.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the members of the laboratory of S.S.W. for their invaluable assistance in the Boston Kidney Biopsy Cohort.
This study is supported by National Institutes of Health (NIH) grant R01DK093574 (to S.S.W.). A.S. was supported by NIH grant F32DK11106. S.S.W. is also supported by NIH grants U01DK085660, U01DK104308, and UG3DK114915.
This work was conducted with support from Harvard Catalyst. The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Part of this work was presented as a poster at the 2016 American Society of Nephrology Scientific Session on November 19 in Chicago, Illinois.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121260/-/DCSupplemental.
References
- 1.Fiorentino M, Bolignano D, Tesar V, Pisano A, Van Biesen W, D’Arrigo G, et al.; ERA-EDTA Immunonephrology Working Group : Renal biopsy in 2015--from epidemiology to evidence-based indications. Am J Nephrol 43: 1–19, 2016 [DOI] [PubMed] [Google Scholar]
- 2.O’Shaughnessy MM, Hogan SL, Thompson BD, Coppo R, Fogo AB, Jennette JC: Glomerular disease frequencies by race, sex and region: Results from the International Kidney Biopsy Survey [published online ahead of print July 2, 2017]. Nephrol Dial Transplant doi: 10.1093/ndt/gfx189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards NT, Greaves I, Lee SJ, Howie AJ, Adu D, Michael J: Increased prevalence of renal biopsy findings other than diabetic glomerulopathy in type II diabetes mellitus. Nephrol Dial Transplant 7: 397–399, 1992 [PubMed] [Google Scholar]
- 4.Gambara V, Mecca G, Remuzzi G, Bertani T: Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol 3: 1458–1466, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Mazzucco G, Bertani T, Fortunato M, Bernardi M, Leutner M, Boldorini R, et al.: Different patterns of renal damage in type 2 diabetes mellitus: A multicentric study on 393 biopsies. Am J Kidney Dis 39: 713–720, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Richards NT, Darby S, Howie AJ, Adu D, Michael J: Knowledge of renal histology alters patient management in over 40% of cases. Nephrol Dial Transplant 9: 1255–1259, 1994 [PubMed] [Google Scholar]
- 7.Zweiman B, Kornblum J, Cornog J, Hildreth EA: The prognosis of lupus nephritis. Role of clinical-pathologic correlations. Ann Intern Med 69: 441–462, 1968 [DOI] [PubMed] [Google Scholar]
- 8.Baldwin DS, Lowenstein J, Rothfield NF, Gallo G, McCluskey RT: The clinical course of the proliferative and membranous forms of lupus nephritis. Ann Intern Med 73: 929–942, 1970 [DOI] [PubMed] [Google Scholar]
- 9.Cheatum DE, Hurd ER, Strunk SW, Ziff M: Renal histology and clinical course of systemic lupus erythematosus. A prospective study. Arthritis Rheum 16: 670–676, 1973 [DOI] [PubMed] [Google Scholar]
- 10.Baldwin DS, Gluck MC, Lowenstein J, Gallo GR: Lupus nephritis. Clinical course as related to morphologic forms and their transitions. Am J Med 62: 12–30, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Appel GB, Silva FG, Pirani CL, Meltzer JI, Estes D: Renal involvement in systemic lupud erythematosus (SLE): A study of 56 patients emphasizing histologic classification. Medicine (Baltimore) 57: 371–410, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al.: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al.; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al.; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Sethi S, Haas M, Markowitz GS, D’Agati VD, Rennke HG, Jennette JC, et al.: Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 27: 1278–1287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi S, D’Agati VD, Nast CC, Fogo AB, De Vriese AS, Markowitz GS, et al.: A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 91: 787–789, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Risdon RA, Sloper JC, De Wardener HE: Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968 [DOI] [PubMed] [Google Scholar]
- 18.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Nath KA: The tubulointerstitium in progressive renal disease. Kidney Int 54: 992–994, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Howie AJ, Ferreira MA, Adu D: Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant 16: 1163–1169, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J: Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl 99: S82–S86, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Mariani LH, Martini S, Barisoni L, Canetta PA, Troost JP, Hodgin JB, et al.: Interstitial fibrosis scored on whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies. Nephrol Dial Transplant 33: 310–318, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menn-Josephy H, Lee CS, Nolin A, Christov M, Rybin DV, Weinberg JM, et al.: Renal interstitial fibrosis: An imperfect predictor of kidney disease progression in some patient cohorts. Am J Nephrol 44: 289–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al.; Renal Pathology Society : Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC: Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al.: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, et al.: Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 22: 176–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al.: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Bajema IM, Hagen EC, Hansen BE, Hermans J, Noël LH, Waldherr R, et al.: The renal histopathology in systemic vasculitis: An international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant 11: 1989–1995, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M, et al.: Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int 93: 1175–1182, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wellner JA, Zhan Y: A hybrid algorithm for computation of the nonparametric maximum likelihood estimator from censored data. J Am Stat Assoc 92: 945–959, 1997 [Google Scholar]
- 35.Zhao Q, Sun J: Generalized log-rank test for mixed interval-censored failure time data. Stat Med 23: 1621–1629, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sun J: A non-parametric test for interval-censored failure time data with application to AIDS studies. Stat Med 15: 1387–1395, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Sun J: Interval censoring. Stat Methods Med Res 19: 53–70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pencina MJ, D’Agostino RB: Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med 23: 2109–2123, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 41.Kremers WK, Denic A, Lieske JC, Alexander MP, Kaushik V, Elsherbiny HE, et al.: Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: The Aging Kidney Anatomy study. Nephrol Dial Transplant 30: 2034–2039, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al.: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, et al.; CKD Prognosis Consortium : Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. JAMA 315: 164–174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Striker GE, Schainuck LI, Cutler RE, Benditt EP: Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum Pathol 1: 615–630, 1970 [DOI] [PubMed] [Google Scholar]
- 45.Schainuck LI, Striker GE, Cutler RE, Benditt EP: Structural-functional correlations in renal disease. II. The correlations. Hum Pathol 1: 631–641, 1970 [DOI] [PubMed] [Google Scholar]
- 46.Serón D, Alexopoulos E, Raftery MJ, Hartley B, Cameron JS: Number of interstitial capillary cross-sections assessed by monoclonal antibodies: Relation to interstitial damage. Nephrol Dial Transplant 5: 889–893, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, et al.: Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol 5: 425–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford SL, Polkinghorne KR, Longano A, Dowling J, Dayan S, Kerr PG, et al.: Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis 63: 227–235, 2014 [DOI] [PubMed] [Google Scholar]
- 49.An Y, Xu F, Le W, Ge Y, Zhou M, Chen H, et al.: Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant 30: 257–266, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Yu F, Wu LH, Tan Y, Li LH, Wang CL, Wang WK, et al.: Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int 77: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Bohle A, Mackensen-Haen S, von Gise H, Grund KE, Wehrmann M, Batz C, et al.: The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract 186: 135–144, 1990 [DOI] [PubMed] [Google Scholar]
- 52.Bohle A, Wehrmann M, Bogenschütz O, Batz C, Müller CA, Müller GA: The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract 187: 251–259, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, et al.; DeKAF Investigators : Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am J Transplant 10: 2066–2073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, et al.: Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 29: 671–684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.To KF, Choi PC, Szeto CC, Li PK, Tang NL, Leung CB, et al.: Outcome of IgA nephropathy in adults graded by chronic histological lesions. Am J Kidney Dis 35: 392–400, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM: Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. Am J Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 57.Fogo AB: Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 22: 2011–2022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bidani AK, Griffin KA: Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Hill GS, Heudes D, Jacquot C, Gauthier E, Bariéty J: Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int 69: 823–831, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Tracy RE: Renal vasculature in essential hypertension: A review of some contrarian evidence. Contrib Nephrol 169: 327–336, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.