Abstract
Incomplete transformations from ferromagnetic to charge ordered states in manganite perovskites lead to phase-separated microstructures showing colossal magnetoresistances. However, it is unclear whether electronic matter can show spontaneous separation into multiple phases distinct from the high temperature state. Here we show that paramagnetic CaFe3O5 undergoes separation into two phases with different electronic and spin orders below their joint magnetic transition at 302 K. One phase is charge, orbital and trimeron ordered similar to the ground state of magnetite, Fe3O4, while the other has Fe2+/Fe3+charge averaging. Lattice symmetry is unchanged but differing strains from the electronic orders probably drive the phase separation. Complex low symmetry materials like CaFe3O5 where charge can be redistributed between distinct cation sites offer possibilities for the generation and control of electronic phase separated nanostructures.
Electronic phase separation is an important feature of many correlated perovskite compounds but hasn’t been seen in other complex oxides with similar physical behaviour such as magnetite. Hong et al. find phase separation between a magnetite-like charge ordered phase and a charge averaged phase in CaFe3O5.
Introduction
Manganites such as La0.7Ca0.3MnO3 and magnetite, Fe3O4, share similar physics as both have a spin-polarised conducting state near ambient temperature due to double exchange between ferromagnetically aligned Mn3+ and Mn4+ or Fe2+ and Fe3+ spins, respectively. Both undergo charge ordering on cooling, which for magnetite is accompanied by a lattice distortion below the much-studied Verwey transition at 125 K1. This was recently found to result from a complex ordering of Fe2+/Fe3+ charge states, Fe2+ orbitals, and three-Fe trimeron groups2. However, long range phase segregation is observed below charge ordering transitions in many manganites3–5 and related perovskites but has not been found in magnetite or related ferrite spinels.
Here, following recent studies of spin and charge ordering in non-spinel MFen–1On+1 (n ≥ 4) ferrites with M = Fe6,7, Mn8,9 and Ca10,11, we investigate n = 4 CaFe3O5 and discover long range electronic phase separation between a magnetite-like charge ordered phase and a charge averaged phase.
Results
Preparation and characterisation of CaFe3O5
CaFe3O5 was synthesised and characterised as described in Methods. The orthorhombic crystal structure consists of FeO6 octahedra sharing edges to form infinite chains parallel to the a-axis and ribbons of three octahedra parallel to the bc-plane as shown in Fig. 1a. Magnetisation measurements (Fig. 1b) reveal a sharp magnetic ordering transition at TM = 302 K and a small net magnetisation of 0.05 µB per CaFe3O5 unit with a moderate coercivity of 0.3 T at 2 K. Ceramic pellets of CaFe3O5 are semiconducting with a small magnetoresistance of −1% at 200 K (Fig. 1c), likely reflecting the small net magnetisation of the sample.
Fig. 1.
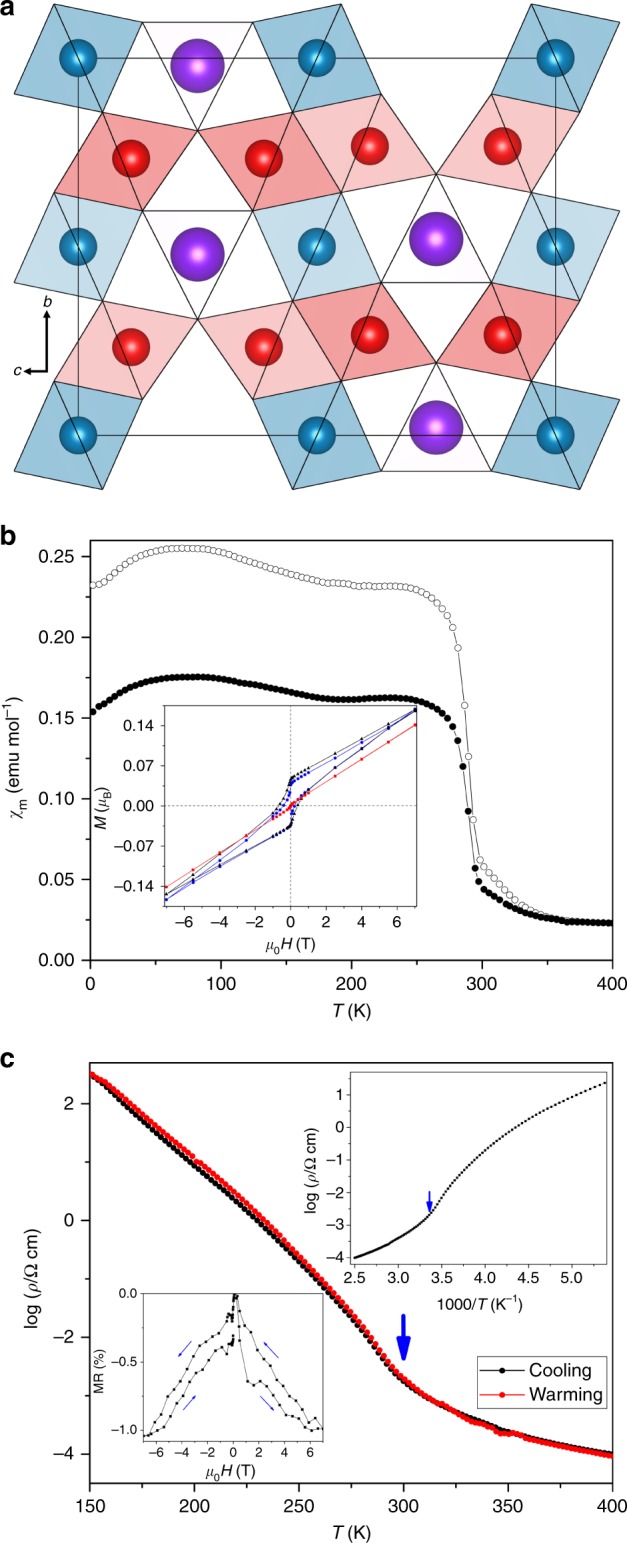
Crystal structure and magnetisation studies of CaFe3O5. a Polyhedral projection of the CaFe3O5 structure showing FeO6 octahedra in red/blue for symmetry inequivalent Fe1/Fe2 sites, and Ca within trigonal prismatic tunnels in purple. b ZFC (closed symbols) and FC (open symbols) magnetic susceptibilities for CaFe3O5, with insert showing magnetisation-field measurements at 2, 230 and 300 K (blue, black and red points respectively). c Log10 plot of the electrical resistivity of a sintered pellet of CaFe3O5 against temperature on cooling and warming, with the discontinuity at 302 K marked. Inset in the top right shows the plot against reciprocal temperature with a change in activation energy from 0.61 eV below TM to 0.26 eV above the transition. Inset in lower left displays the magnetoresistance MR at 200 K
Evidence for electronic phase separation in CaFe3O5
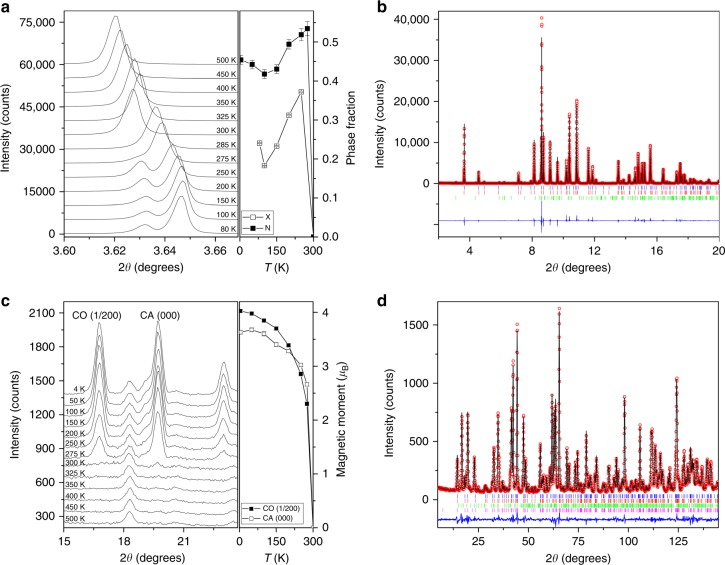
A single high temperature (HT) crystalline phase of CaFe3O5 is observed above TM = 302 K, but both powder synchrotron X-ray (Fig. 2a, b) and neutron diffraction data (Fig. 2c, d) reveal long range phase separation as diffraction peaks broaden or split into two components below the magnetic ordering transition as shown in Fig. 2a, c. The shift of the X-ray (002) peak to lower 2θ at 300 K evidences a small bulk lattice distortion due to spin ordering before separation into two phases at lower temperatures. Both low temperature phases have the same Cmcm space group symmetry as the HT parent. Crystallographic results are shown in Supplementary Tables 1–5. Magnetic neutron diffraction peaks observed below TM (Supplementary Fig. 1) show that one phase has magnetic propagation vector (½ 0 0) while the other has (0 0 0) (Fig. 2c), and a good fit to the data (Fig. 2d) was obtained using the magnetic structure models shown in Fig. 3. No additional broadening of magnetic peaks was observed showing that both magnetic orders have correlation lengths of at least ~200 nm. Both phases have collinear antiferromagnetic orders with moments parallel to the c-axis, but an important difference is that the (½ 0 0) phase has ferromagnetic (FM) alignment of spins within the three-atom ribbons parallel to the bc-plane, while the (0 0 0) has FM spin chains parallel to the a-axis. Although both orders are antiferromagnetic, the observation of a small net magnetisation below TM suggests that one or both of the spin structures are canted. The fractions of the two phases are found to be different in the synchrotron and neutron diffraction experiments (Fig. 2a), consistent with a strain-driven phase separation being dependent on the thermal history of the sample.
Fig. 2.
Electronic phase separation of CaFe3O5. a Evolution of the X-ray (002) peak which shifts to lower 2θ when cooled to 300 K, just below the TM = 302 K transition, with separation into two components at lower temperatures. The phase fractions of the charge averaged (CA) phase, obtained from synchrotron (X) and neutron (N) diffraction data are shown in the right-hand panel. Error bars are the estimated standard deviations calculated during profile fitting. b Rietveld fit to synchrotron powder diffraction profiles for CaFe3O5 at 80 K (R-factors Rp = 9.35 %, Rwp = 11.0 %), with blue and red tick marks indicating the two low temperature phases and green marks 2.3% of Ca2Fe2O5 impurity. c The appearance of the magnetic reflections with propagation vectors of (000) for the CO phase and (½00) for the charge averaged (CA) phase below 302 K and the temperature evolution of their magnetic moments. d The Rietveld fit to neutron powder diffraction profiles for CaFe3O5 at 4 K (R-factors Rp = 6.14 %, Rwp = 7.28 %), with structural phases indicated with blue and red tick marks. The green and pink tick marks represent the magnetic phases with propagation vectors of (½00) and (000)
Fig. 3.
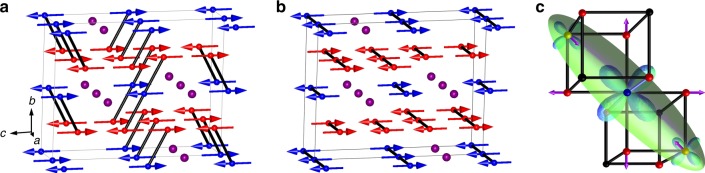
Magnetic structures for the two phases of CaFe3O5. Fe1/Fe2 spins are shown in red/blue and Ca ions in purple. a Magnetic structure of the charge ordered (CO) phase CaFe3O5 with propagation vector (½ 0 0), with lines showing ferromagnetic order within trimerons. b Magnetic structure of the charge averaged (CA) phase, with (0 0 0) propagation and lines showing the ferromagnetic chain parallel to the x axis. c A trimeron unit as found in the CO structure (a) with bonding electron density represented as an ellipsoid. The size of the t2g orbitals approximates the atomic populations. The atomic displacement arrows indicate the elongation of the four Fe–O bonds perpendicular to the Jahn–Teller contracted axis and the shortening of the cation–cation distances due to weak Fe–Fe bonding interactions
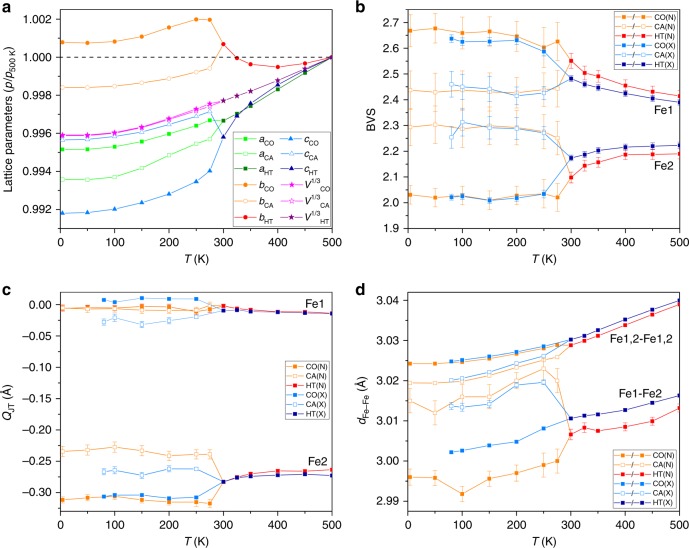
Thermal evolution of lattice parameters from neutron and X-ray fits are, respectively, shown in Fig. 4a and Supplementary Fig. 2, and variations of internal structural quantities from both studies are shown in Fig. 4b–d to emphasise the reproducibility of discovered structural differences between the two phases. Two inequivalent iron sites Fe1 and Fe2 are present in a 2:1 ratio in CaFe3O5, and in the HT phase they have respective Bond Valence Sums (BVSs) of 2.43 and 2.22, equivalent to formal charges of +2.75 and +2.50 when renormalized to the average of +2.67. Differing charge redistributions occur below the 302 K magnetic and phase segregation transition (Fig. 4b). In the phase with magnetic propagation vector (½ 0 0), the Fe1 and Fe2 BVSs diverge to very different values of 2.67(6) and 2.03(4) at 4 K, consistent with charge order (CO) of Fe3+ and Fe2+ respectively. The coexisting phase with (0 0 0) magnetic order shows the opposite behaviour as the Fe1 and Fe2 BVSs converge to similar values of 2.44(8) and 2.29(7) at 4 K showing that the electronic states at the two sites are not significantly different, hence this phase is charge averaged (CA).
Fig. 4.
Crystal structure parameters of CaFe3O5. a Changes in the lattice parameters relative to 500 K values obtained from PND (a500 K = 3.03896(1), b500 K = 10.01355(5) and c500 K = 12.67039(7) Å). Temperature dependence of the b BVS and c the amplitude of Jahn–Teller distortion (QJT) of the FeO6 octahedra for the two sites in the high temperature (HT), charge ordered (CO) and charge averaged (CA) phases, obtained from neutron (N) and synchrotron X-ray (X) diffraction data. d The evolution of the Fe1-Fe2 bonding distance with temperature, with the lattice parameter (Fe1,2-Fe1,2) as reference. Error bars are the estimated standard deviations calculated during profile fitting
Degenerate 3d6 Fe2+ states are subject to Jahn–Teller distortion which leads to tetragonal octahedral compression in magnetite2, corresponding to negative values of the QJT distortion parameter. The Fe2 site in CaFe3O5 has a large negative QJT value due to intrinsic distortions within the HT structure (Fig. 4c), but below TM = 302 K QJT becomes more negative for the CO phase, consistent with localisation of Fe2+ states, while the magnitude of QJT decreases in keeping with the observed increase in Fe2 site valence for the CA phase. Jahn–Teller distortion at the Fe2 site in the CO phase leads to order of the t2g orbital with minority spin electron density directed towards the two neighbouring Fe1 sites. Hence this localised orbital has the correct orientation to form a trimeron, a linear unit of three Fe ions formed by delocalisation of the minority spin electron in the ordered orbital of the central Fe2+ ion2, as shown in Fig. 3c. Trimeron formation requires FM alignment of the three magnetic moments, Fe3+–Fe2+–Fe3+ CO, orbital order at the central Fe2+ in the plane of the three atoms, and shortening of the Fe–Fe distances within the trimeron2. All these conditions are observed in the Fe1–Fe2–Fe1 ribbons in the CO phase with shortening of Fe1–Fe2 distances below TM observed in Fig. 4d, but none are fulfilled in the CA structure, so the observed spin orders and structural distortions in CaFe3O5 demonstrate that trimeron order is observed only in the CO phase. However, a slight shortening of Fe–Fe distances in the FM chains parallel to the a-axis is observed in the CA phase (Fig. 4d), consistent with a weak bonding effect from extended band states of the minority spin electrons. Fe1–Fe1 and Fe2–Fe2 distances are constrained to be equal (to a/2) and this is the likely driver for the observed averaging of charge states between the two sites in the CA phase so that their minority spin populations become more equal.
The electronic orders in the two phases of CaFe3O5 do not change the structural symmetry, but they lead to different cell distortions (Fig. 4a) as trimeron formation shortens the c-axis parameter in the CO phase, with a and b expanding to compensate, while the a-axis shortening in the CA phase leads to expansion of c. Although the overall cell volumes for the two components remain identical within error at low temperatures, the differing spontaneous strains from these lattice distortions are the likely driver for the long range separation as proposed for perovskite manganites12–14. Short range phase and strain fluctuations in the HT phase are evidenced indirectly below 350 K through divergence of zero field- and field- cooled susceptibilities in Fig. 1b, and changes in the slopes of b and c lattice parameters in Fig. 4a.
Charge ordering in one of the two low temperature phases is clearly a key driver for the long range electronic phase separation in CaFe3O5 and perovskite type oxides15 although in CaFe3O5 this leads to the formation of orbital molecules16, more complex electronic objects than simple localised-charge ions. However, phase separation in CaFe3O5 occurs without a change of lattice symmetry in either component, demonstrating that strain variations within a given lattice are sufficient to drive long range segregation. Band structure calculations (shown in Supplementary Fig. 4 and Supplementary Table 6) confirm that these distortions are sufficient to stabilise CO in one structure but not the other for a realistic value of the Hubbard U-parameter. Phase separation in manganites usually results from an incomplete phase transition where a metallic HT FM phase is partially transformed to a charge ordered insulator, and the high and low temperature phases coexist to base temperature. A notable example is Nd0.5Sr0.5MnO3 which on cooling was observed to order as a single FM phase at 250 K, then to phase separate into a mixture of FM and A-type antiferromagnetic (AFM-A) phases below 220 K, and finally to undergo a further phase separation into a mixture of FM, AFM-A and charge ordered AFM-CE phases below 150 K, with all three phases extant down to the lowest measured temperature of 15 K17. CaFe3O5 undergoes a genuine electronic phase separation in the sense that both of the two low temperature CA and CO phases are electronically and magnetically distinct from the HT paramagnetic state, and so is analogous to e.g. the separation of a fluid into a liquid and gas below a critical transition. Both low temperature CaFe3O5 phases are magnetically ordered and it is remarkable that they appear to share a common magnetic ordering temperature as shown in Fig. 2c although the spin-spin exchange interactions within the two magnetic structures will not be identical.
Another important difference is that phase separation in manganites is associated with substantial intrinsic disorder due to cation mixing, and a recent study has demonstrated that chemical ordering of cations in (La1−yPry)1−xCaxMnO3 suppresses long range phase separation18. CaFe3O5 is in principle a stoichiometric material although small strain variations due to the 4% substitution of Fe for Ca observed in our polycrystalline sample may tip the local balance between the energies of the two ground states leading to phase coexistence. The resulting disorder in magnetic interactions could also be important in stabilising the phase coexistence in a Griffiths-type model19. All Mn sites are electronically equivalent in the HT aristotype manganite perovskite structure but CaFe3O5 has a further electronic degree of freedom as charge can be redistributed between structurally inequivalent Fe1 and Fe2 sites, leading to the observed extremes of charge ordering (CO) in one phase and charge averaging (CA) in the other. This charge redistribution mechanism is akin to electronic separation models originally proposed for manganites20,21, although couplings of both the charge redistribution degree of freedom and trimeron formation associated with charge and orbital ordering to the lattice appear to be important factors that drive phase segregation in CaFe3O5.
Discussion
In conclusion, the present study demonstrates that phase separation of a single HT paramagnetic state into two distinct low temperature phases with different long range spin and electronic orders occurs in CaFe3O5 when cooled below 302 K. Although both phases are antiferromagnetic overall, the formation of different FM units within them is coupled to the electronic orders; one phase has full Fe2+/Fe3+ CO associated with trimeron formation, but CA stabilises FM chains in the other phase. Weak Fe–Fe bonding driven by the FM orders introduces different lattice strains into the two phases although no change of structural symmetry occurs, and strain variations within the polycrystalline sample tip the local balance between the energies of the two phases leading to phase coexistence. CaFe3O5 thus links the trimeron-ordering of magnetite to the microstructural physics of perovskite manganites. CaFe3O5 also demonstrates new possibilities for more complex ‘electronically soft matter’22 than in perovskites where orbital molecule formation and redistribution of charge between distinct cation sites offer additional degrees of freedom for generation and potential control of electronic phase-segregated nanostructures.
Methods
Sample preparation and characterisation
Polycrystalline CaFe3O5 was prepared from stoichiometric quantities of CaFe2O4, Fe2O3, and Fe powders pressed into pellets, sealed in evacuated quartz tubes, and heated at 1100 °C for 12 h. (The CaFe2O4 was synthesised at ambient pressure using the ceramic technique outlined by Wan et al23. where CaCO3 and Fe2O3 powders were ground together in 1:1 ratio, pressed into pellets, heated at 850 °C for 4 h, reground and repelleted, and finally reheated at 1100 °C for 12 h.) Thermogravimetric analysis heating the sample in air at 10 °C min−1 to 900 °C, as shown in Supplementary Fig. 3, gave a mass increase of 2.789%, in agreement with the calculated value of 2.781% for oxidation of CaFe3O5. Powder X-ray diffraction confirmed that CaFe3O5 adopts an orthorhombic Sr2Tl2O5 type structure with space group Cmcm as reported previously10.
Magnetic and electrical measurements
Magnetic measurements were carried out with a Quantum Design MPMS XL SQUID magnetometer. Magnetic susceptibility was recorded in zero field cooled (ZFC) and field cooled (FC) conditions between 2 and 400 K with an applied magnetic field of 1000 Oe. Hysteresis loops were also measured at 2, 230 and 300 K. Electrical resistivity measurements were carried out with a Quantum Design PPMS, between 180 and 400 K. Magnetoresistance hysteresis loops were also measured at 200 K.
Powder synchrotron X-ray and neutron diffraction studies
High resolution powder X-ray diffraction data were collected at the ID22 beamline of the ESRF with incident wavelength 0.3999 Å. The powder was packed into a glass capillary with an outer diameter of 0.7 mm and spun during data acquisition with temperatures from 80 to 500 K controlled using an Oxford Cryostream system. High resolution neutron diffraction data were collected at the D2B beamline of the ILL with incident wavelength 1.5940 Å. 5 g of powder samples were packed into a vanadium can and diffraction patterns were collected at temperatures from 4 to 500 K. Crystal and magnetic structures of CaFe3O5 were Rietveld-fitted using the FullProf Suite24. A small amount of Fe at the Ca site was found from both synchrotron X-ray [4.4(3)% Fe] and neutron [4.0(8)% Fe] refinements of cation site occupancies at 500 K. Crystal structure refinements of the two phases formed below 302 K in CaFe3O5 was possible with both the synchrotron X-ray and neutron powder diffraction data except at temperatures just below the transition (275 and 285 K). Magnetic irreducible representation analysis was carried out using BasIrReps. Bond valence sums for each iron site were calculated using a standard method with linear interpolation to estimate mixed charge states between Fe2+ and Fe3+25,26. The crystal structure projection in Fig. 1a was generated using VESTA27 and the magnetic structures in Fig. 3a, b were made using FullProf Studio.
Electronic structure calculations
DFT+U electronic structure calculations were performed using CASTEP28 (v16 and 17) utilising planewaves (650 eV cutoff) and on-the-fly pseudopotentials, within the PBE approximation to exchange and correlation. Band structures (shown in Supplementary Fig. 4 and Supplementary Table 6) predict metallic charge averaged ground states for both the refined CO and CA structures at small values of the Hubbard U energy, and insulating charge ordered states for both phases at large U. However, values in the range 2.0 < U < 4.0 eV simultaneously predict the charge averaged state for the CA structure and the charge ordered state for the CO structure, and hence confirm that this range is realistic for Fe oxides.
Data availability
Data that support the findings of this study have been deposited at 10.7488/ds/2378.
Electronic supplementary material
Acknowledgements
The authors acknowledge financial support from European Research Council (ERC), Engineering and Physical Sciences Research Council (EPSRC) and Science and Technology Facilities Council (STFC). The authors also thank Andy Fitch for help at ESRF and Gary Nichol, Graham McNally, Alex Browne and Giuditta Perversi for local support.
Author contributions
K.H.H. and J.P.A. designed the concept for this study. K.H.H., A.M.A.-L., and C.R. performed the experimental work and data analysis, and J.C. performed the band structure calculations. K.H.H. and J.P.A. wrote the manuscript with inputs from all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/28/2018
The original version of this Article contained an error in the third sentence of the legend of Fig. 2, which incorrectly read ‘The phase fractions of the charge ordered (CO) phase, obtained from synchrotron (X) and neutron (N) diffraction data are shown in the right-hand panel.’ The correct version states ‘charge averaged (CA)’ in place of ‘charge ordered (CO)’. This has been corrected in both the PDF and HTML versions of the Article.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05363-6.
References
- 1.Verwey EJW. Electronic conduction of magnetite (Fe3O4) and its transition point at low temperatures. Nature. 1939;144:327–328. doi: 10.1038/144327b0. [DOI] [Google Scholar]
- 2.Senn MS, Wright JP, Attfield JP. Charge order and three-site distortions in the Verwey structure of magnetite. Nature. 2012;481:173–176. doi: 10.1038/nature10704. [DOI] [PubMed] [Google Scholar]
- 3.Dagotto E, Hotta T, Moreo A. colossal magnetoresistant materials: the key role of phase separation. Phys. Rep. 2001;344:1–153. doi: 10.1016/S0370-1573(00)00121-6. [DOI] [Google Scholar]
- 4.Uehara M, Mori S, Chen CH, Cheong SW. Percolative phase separation underlies colossal magnetoresistance in mixed-valent manganites. Nature. 1999;399:560–563. doi: 10.1038/21142. [DOI] [Google Scholar]
- 5.Tokura, Y. Critical features of colossal magnetoresistive manganites. Rep. Prog. Phys. 69, 797–851 (2006).
- 6.Lavina B, et al. Discovery of the recoverable high-pressure iron oxide Fe4O5. Proc. Natl Acad. Sci. U. S. A. 2011;108:17281–5. doi: 10.1073/pnas.1107573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovsyannikov SV, et al. Charge-ordering transition in iron oxide Fe4O5 involving competing dimer and trimer formation. Nat. Chem. 2016;8:501–508. doi: 10.1038/nchem.2478. [DOI] [PubMed] [Google Scholar]
- 8.Hong KH, McNally GM, Coduri M, Attfield JP. Synthesis, crystal structure, and magnetic properties of MnFe3O5. Z. für Anorg. und Allg. Chem. 2016;642:1355–1358. doi: 10.1002/zaac.201600365. [DOI] [Google Scholar]
- 9.Hong KH, Arevalo-Lopez AM, Coduri M, McNally GM, Attfield JP. Cation, magnetic, and charge ordering in MnFe3O5. J. Mater. Chem. C. 2018;6:3271–3275. doi: 10.1039/C8TC00053K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evrard O, et al. Mise en évidence de CaFe4O6 et détermination des structures cristallines des ferrites de calcium CaFe2+nO4+n (n =1, 2, 3): nouvel exemple d’intercroissanc. J. Solid State Chem. 1980;35:112–119. doi: 10.1016/0022-4596(80)90471-5. [DOI] [Google Scholar]
- 11.Delacotte C, et al. Structural transition at 360 K in the CaFe5O7 ferrite: toward a new charge ordering distribution. Inorg. Chem. 2014;53:10171–10177. doi: 10.1021/ic5011456. [DOI] [PubMed] [Google Scholar]
- 12.Ahn KH, Lookman T, Bishop AR. Strain-induced metal-insulator phase coexistence in perovskite manganites. Nature. 2004;428:401–404. doi: 10.1038/nature02364. [DOI] [PubMed] [Google Scholar]
- 13.Burgy J, Moreo A, Dagotto E. Relevance of cooperative lattice effects and stress fields in phase-separation theories for CMR manganites. Phys. Rev. Lett. 2004;92:3–6. doi: 10.1103/PhysRevLett.92.097202. [DOI] [PubMed] [Google Scholar]
- 14.Guzmán-Verri, G. G., Brierley, R. T., Littlewood, P. B. Elastic interactions and control of the Mott transition. Preprint at arXiv:1701.02318 (2017).
- 15.Shenoy VB, Rao CNR. Electronic phase separation and other novel phenomena and properties exhibited by mixed-valent rare-earth manganites and related materials. Philos. Trans. R. Soc. A. 2008;366:63–82. doi: 10.1098/rsta.2007.2140. [DOI] [PubMed] [Google Scholar]
- 16.Attfield JP. Orbital molecules in electronic materials. APL Mater. 2015;3:1–7. doi: 10.1063/1.4913736. [DOI] [Google Scholar]
- 17.Woodward PM, Cox DE, Vogt T, Rao CNR, Cheetham AK. Effect of compositional fluctuations on the phase transitions in (Nd1/2Sr1/2)MnO3. Chem. Mater. 1999;11:3528–3538. doi: 10.1021/cm990281d. [DOI] [Google Scholar]
- 18.Zhu Y, et al. Chemical ordering suppresses large-scale electronic phase separation in doped manganites. Nat. Commun. 2016;7:1–6. doi: 10.1038/ncomms11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magen C, et al. Observation of a Griffiths-like phase in the magnetocaloric compound Tb5Si2Ge2. Phys. Rev. Lett. 2006;96:167201. doi: 10.1103/PhysRevLett.96.167201. [DOI] [PubMed] [Google Scholar]
- 20.Yunoki S, et al. Phase separation in electronic models for manganites. Phys. Rev. Lett. 1998;80:845–848. doi: 10.1103/PhysRevLett.80.845. [DOI] [Google Scholar]
- 21.Moreo A, Yunoki S, Dagotto E. Phase separation scenario for manganese oxides and related materials. Science. 1999;283:2034–2040. doi: 10.1126/science.283.5410.2034. [DOI] [PubMed] [Google Scholar]
- 22.Milward GC, Calderon MJ, Littlewood PB. Electronically soft phases in manganites. Nature. 2005;433:607–610. doi: 10.1038/nature03300. [DOI] [PubMed] [Google Scholar]
- 23.Wan R, Jia C, Zhang W. Preparation and photoelectric properties of p-CaFe2O4/n-WO3 composites. J. Alloy Compd. 2012;544:1–5. doi: 10.1016/j.jallcom.2012.07.134. [DOI] [Google Scholar]
- 24.Rodriguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B. 1993;192:55–69. doi: 10.1016/0921-4526(93)90108-I. [DOI] [Google Scholar]
- 25.Attfield JP. Charge ordering in transition metal oxides. Solid State Sci. 2006;8:861–867. doi: 10.1016/j.solidstatesciences.2005.02.011. [DOI] [Google Scholar]
- 26.Brown, I. D. VALENCE: a program for calculating bond valences. J. Appl. Crystallogr. 29, 479–480 (1996).
- 27.Momma K, Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011;44:1272–1276. doi: 10.1107/S0021889811038970. [DOI] [Google Scholar]
- 28.Clark SJ, et al. First principles methods using CASTEP. Z. Krist. 2005;220:567–570. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study have been deposited at 10.7488/ds/2378.