Abstract
Women with larger personal social networks have better breast cancer survival and a lower risk of mortality. However, little work has examined the mechanisms through which social networks influence breast cancer outcomes and cancer outcomes more generally, potentially limiting the development of feasible, clinically effective interventions. In fact, much of the emphasis in cancer research regarding the influence of social relationships on cancer outcomes has focused on the benefits of the provision of social support to patients, especially through peer support groups, and only more recently through patient navigation. Though critically important, there are other ways through which social relationships might influence outcomes, around which interventions might be developed. In addition to social support, these include social resources, social norms, social contagion, social roles, and social burdens and obligations. This narrative review addresses how social networks may influence cancer outcomes and discusses potential strategies for improving outcomes given these relationships. The paper (a) describes background and limitations of previous research, (b) outlines terms and provides a conceptual model that describes interrelationships between social networks and relevant variables and their hypothesized influence on cancer outcomes, (c) clarifies social and psychosocial mechanisms through which social networks affect downstream factors, (d) describes downstream behavioral, treatment, and physiological factors through which these subsequently influence recurrence and mortality, and (e) describes needed research and potential opportunities to enhance translation. Though most literature in this area pertains to breast cancer, this review has substantial relevance for cancer outcomes generally. Further clarification and research regarding potential mechanisms are needed to translate epidemiological findings on social networks into clinical and community strategies to improve cancer outcomes.
Keywords: Social networks, Social support, Cancer outcomes, Conceptual model
Social networks may influence cancer mortality. As part of a conceptual model, we discuss research, potential opportunities, and strategies to enhance translation of findings.
Implications
Practice: Peer support groups, patient navigation, and acute and social services have been predominant strategies to augment support in cancer patients though other potential strategies could include caregiver training in late-stage patients, family and community social network interventions, patient communication and relationship skill interventions, and online support resources.
Policy: While it is important to augment social support to cancer patients, policymakers should also consider the many resources social networks bring that transcend social support; such resources may provide clues regarding the types of strategies that could be leveraged to improve cancer survival.
Research: This paper provides a conceptual model describing mechanisms through which social networks influence cancer outcomes though further clarification and research will be needed to translate epidemiological findings into clinical and community strategies to improve outcomes.
INTRODUCTION
Women with larger personal social networks, defined as the web of social relationships that surround an individual [1], have often been shown to have better breast cancer survival [2–6] and larger social networks have been associated with lower mortality generally [7]. However, exceedingly little work has examined the mechanisms through which social networks influence breast cancer outcomes and cancer outcomes more generally, inhibiting the development of feasible, clinically effective interventions [8–12].
This narrative review addresses how social networks may influence cancer outcomes and discusses potential strategies for improving outcomes given these relationships. Though the review references breast cancer extensively given that the bulk of the literature in this area focuses on this cancer, the model has considerable relevance for cancer outcomes generally.
It is divided into five parts. The first section describes background and limitations of previous research. The second section begins with a conceptual model that describes interrelationships between social networks and relevant variables and their hypothesized influence on cancer outcomes. In that section, I define relevant terms and describe advances afforded by the model. In the third section, I clarify social and psychosocial mechanisms through which social networks affect downstream factors. In the fourth section, I describe downstream behavioral, treatment, and physiological factors through which social relationships influence recurrence and mortality. The fifth section describes needed research and potential points of leverage, that is, opportunities and strategies, limitations of those, and alternative potential strategies.
BACKGROUND AND LIMITATIONS OF PREVIOUS RESEARCH
Substantial interest in the area of social support and cancer survival emerged with a paper published by Spiegel et al. [13] in which the authors reported that a peer emotional social support intervention, designed to improve quality of life (QoL) in metastatic breast cancer patients, was related to two-fold higher survival compared with the controls (36.6 vs. 18.9 months). This was an incidental finding; the study was not powered to examine survival, and only 11 participants remained in the control group by study end. Goodwin et al. [9] attempted to replicate this intervention. They powered the study to examine survival, and Spiegel was a consultant involved in developing the intervention. However, in 2001, in the New England Journal of Medicine, Goodwin et al. reported no effect of the social support intervention on survival. Spiegel et al. [8] also reported no benefit of another, more recent peer support intervention on survival though results suggested a possible benefit in the small subset of triple-negative metastatic breast cancer patients.
Social support interventions have focused primarily on providing informational and emotional support [4, 14], and these have been conducted in women with metastatic breast cancer. Though peer support interventions are generally, though not always [11, 12], related to improvements in QoL, most trials have failed to show improvements in survival [8–10]. A meta-analysis of previous trials further corroborates the lack of significant survival benefit [15].
Clinical trials are generally considered the gold standard in research, superior to results obtained from observational studies. However, there are well-known limitations of clinical trials, which could negate the influence of an intervention even when there is a true causal effect. An intervention may be of insufficient duration, or support from strangers could be less efficacious than support from people already known. The study population may be inappropriate. Most previous trials have been in metastatic patients in whom it may be difficult to extend life though opportunities might exist in evaluating the benefit of emotional support in earlier stage patients. There are several types of support including emotional, tangible/instrumental, appraisal, and informational support, positive interaction, and affection [1, 16], and potentially others including spiritual support. Types of support relevant to survival may include any or all of these; emotional support itself may be insufficient to affect survival. Moreover, there is a multiplicity of social and psychosocial mechanisms through which social networks may influence or be leveraged to improve survival. As a result, implications regarding the influence of social support on breast cancer survival based on previous trials are unclear.
By contrast, observational data strongly suggest a potential role of naturally occurring social relationships on breast cancer-specific survival [4, 17]. In 2,835 postmenopausal breast cancer survivors in the Nurses’ Health Study (NHS), Kroenke et al., found that socially isolated women, that is, women with small networks, assessed prior to diagnosis were twice as likely to die of breast cancer than were socially integrated women [4]. In a more recent study, in 9,267 women from the After Breast Cancer Pooling Project (ABCPP), socially isolated women with small networks had higher risks of recurrence (HR = 1.43, 95% CI: 1.15–1.77), breast cancer- specific mortality (HR = 1.64, 95% CI: 1.33–2.03), and total mortality (HR = 1.69, 95% CI: 1.43–1.99), compared with socially integrated women [17]. A recent meta-analysis corroborates these findings [18].
Observational studies have substantial limitations, most notably that those with more salutary social networks generally also have other characteristics or behaviors that are related to survival. Women with larger networks tend to have greater physical activity and a lower prevalence of smoking, obesity, and alcohol overconsumption [19]. However, in analyses of social network size and breast cancer survival, adjustment for lifestyle factors has not explained and in fact has only slightly attenuated associations [4, 17]. It is possible that there are confounding factors, for example, personality factors such as extraversion and conscientiousness, which could influence a person’s social networks and their health behaviors. However, previous research shows modest or weak relationships between personality and health behaviors [20–22] and no association between personality factors and breast cancer outcomes [23, 24]. Indeed, the magnitude of the association between social networks and breast cancer survival has been larger than associations of either lifestyle factors or personality factors and breast cancer survival. Some scientists might disregard observational findings given findings from trials. But, given the multiplicity of different types of social support as well as other mechanisms through which social relationships may influence breast cancer survival, disparate findings should instead signal the need for additional research to develop and evaluate other strategies. To date, few other types of social interventions have been conducted. Current efforts are underway to evaluate the influence of support provided, for example, by peer navigators, individuals who have previously undergone diagnosis and treatment of breast cancer, on QoL [25] in cancer patients and on timeliness of cancer care [26, 27]. Considerable recent research has evaluated the influence of patient navigation. However, further observational studies are needed to inform possible intervention strategies. The development of a conceptual model may help to inform these strategies.
TERMS, CONCEPTUAL MODEL, AND THE IMPORTANCE OF SOCIAL NETWORK MEASURES
Defining and distinguishing terms
Social network terms described in this paper are found in Table 1. Previous studies have often equated social terms including social networks, social ties, social function, social well-being, and social support. Though these may be correlated, they are not synonymous. Other terms including social regulation, social norms, and social contagion, have not been explored in the context of cancer survivorship research.
Table 1.
Social network terms
| Social network terms | Definition |
|---|---|
| General terms | |
| Social networks | The web of social relationships that surround an individual |
| Social ties | Members of a social network |
| Sociocentric social networks | A social network comprised of a web of social ties in which the complete set of ties in a group is known |
| Egocentric social networks | A personal network approach that studies ties from the reference point of the patient. A social network is comprised of a web of ties (egos and alters) with the patient (ego) at the center of the network. |
| Ego | The central person in an egocentric social network |
| Alters | Members within a social network |
| Structural social support | Often measured in terms of social ties and is conceptualized as the social support that derives from the social network |
| Strong ties | Close friends and family |
| Weak ties | Acquaintances or ties to acquaintances |
| Structural dimensions of social networks | |
| Strength of ties | Represents the strength of relationships and is a function of the amount of time a person has known an alter and the closeness/intensity of the relationship |
| Diversity | Represents the number of different types of ties in a person’s network. |
| Multiplexity | Refers to overlap in relationships |
| Density | The extent to which the ties are directly connected to, that is, know and associate with, one another |
| Network | Refers to the ties that connect a specific set of alters |
| Degree | Quantifies the connections an individual has in a network; akin to social network size |
| Psychosocial mechanisms of social networks | |
| Social support (or functional social support) | The perception and reality of the exchange of assistance through social relationships |
| Social strain | The negative aspects of social relationships that result from stressful relationships or the burdens that arise from social obligations |
| Social regulations | Restrictions designed to discourage harmful behavior or encourage socially desirable behavior |
| Social norms | Rules of behavior considered acceptable in a group or society |
| Social contagion | The spread of ideas, attitudes, moods, or behaviors in a group |
| Social roles | The roles an individual fills (e.g., parent, employee, volunteer, spouse) |
Social networks are defined as the web of social relationships that surround an individual [1]. A social network is comprised of a web of social ties (egos and alters). Social networks and social ties are structural factors, that is, economic, social, policy, organizational or other aspects of the environment that serve as barriers to or facilitators of behaviors and other predictors of risk, and have been called structural social supports. Social support, the perception and reality of the exchange of assistance through social relationships, is also called functional social support. Thus, the individuals make up the structure and the assistance provided the function. However, the term “structural social support” is problematic; while social relationships can and do provide social support, this terminology disregards the negative aspects of social relationships, that is, social strain, as a result of stressful relationships or the burdens that arise from the obligations of social relationships. The quality of social relationships is affected by both support and strain, and the influence of both positive and negative relationship factors on health should be considered in studies of cancer patients. Social well-being and social function, also sometimes used interchangeably with social support, do not describe the support provided in relationships but rather refer to QoL outcomes—feelings of satisfaction with social relationships or the ability to maintain social obligations. Social regulations are restrictions designed to discourage harmful behavior or encourage socially desirable behavior. Social norms are the rules of behavior considered acceptable in a group or society, and social contagion is the spread of ideas, attitudes, moods, or behaviors in a group. Social roles refer to the roles an individual fills (e.g., parent, employee, volunteer, spouse). In most cancer research, investigators explore the influence of strong ties, that is, close friends and family, on outcomes. However, weak ties or ties to acquaintances [28] could also have an important role in cancer outcomes.
Conceptual model
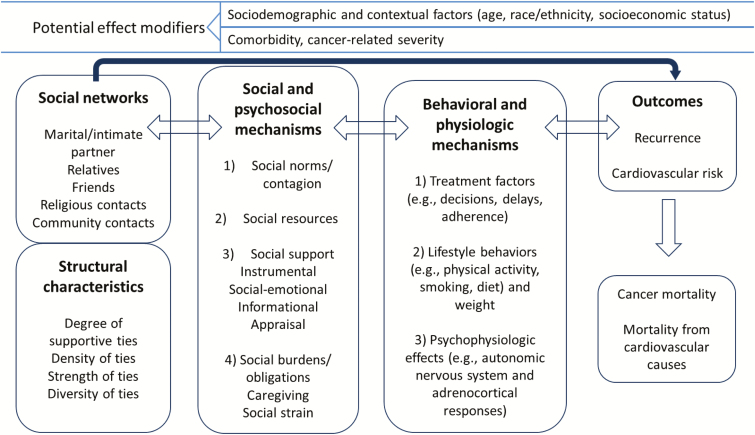
The conceptual model presented below (Fig. 1) was informed in part by an influential model of social networks and health developed by Berkman and Glass [1]. However, the model presented here focuses specifically on cancer outcomes and is informed by major current topics in the cancer literature. While the broad social context is highly relevant, this model eliminates explicit reference to embeddedness within social-structural conditions, to focus attention on social networks and because social network characteristics reflect that embeddedness. Moreover, by necessity, studies of cancer survivorship focus on individual cancer patients and survivors. While the measures of interest are not limited to the individual level, the model centers around the individual. The model also emphasizes the need for direct measures of social networks and their structural characteristics. Clarification is provided in Fig. 1 regarding interrelationships between these variables as well as how social relationships influence cancer outcomes.
Fig 1.
Social networks and mechanisms through which social relationships influence cancer survival
In this conceptual model, social networks influence cancer outcomes through several social and psychosocial pathways including social support, social regulation/norms, social roles, social burdens, and institutional social resources. Through these pathways, social networks influence downstream factors including treatment and lifestyle factors as well as psychophysiologic pathways, which in turn influence recurrence, cancer mortality, and mortality from other causes. These relationships are further modified by social contextual factors and factors related to disease severity. Understanding how social networks influence cancer treatment and the ability to identify patients at risk are critical to being able to devise effective social strategies.
Advances afforded by this conceptual model
Useful conceptual models are sufficiently broad to stimulate thinking and apply to a variety of situations but also point to the need to consider ideas that might be overlooked. This model motivates consideration of the following: (a) the structural, not just the individual, effects of social networks on cancer outcomes, (b) the context in which social networks exert their influence leading to potentially positive and negative effects, and (c) psychosocial mechanisms other than social support through which social networks exert influences. It also points to the need to consider behavioral and biological mechanisms and the need to consider associations in different cancers.
Need to examine structural, not just individual, effects of social networks
In cancer research to date, approaches to examining the influence of social networks have employed individual-level data including epidemiologic social network indexes including information on numbers of (close) friends and relatives, religious and community participation, marital status and work status, and measures of social support. These have provided useful information, and the approach is appropriate given the types of data that typically exist in cohort studies (a limited set of social ties) or cancer registries (usually marital status only). However, the social environment is not optimally characterized only by measures of individual social ties.
The influence of social networks on health outcomes is also a function of characteristics or the structure of the network and of actors’ positions in the network. In sociocentric social networks, the complete set of ties in a group is known (e.g., friendship ties in a school classroom). When resources constrain the ability to evaluate the universe of ties or when it is more advantageous to understand the ties of a specific individual, researchers can collect data on egocentric social networks. In this personal network approach [29], the egocentric approach studies ties from the reference point of the patient.
In current work, colleagues and I are beginning to explore potential insights that may be developed from egocentric social network methods. In egocentric data, social networks are naturally occurring groups within which members (‘‘alters’’) may influence each other’s behaviors. A ‘‘network’’ formally refers to the ties that connect a specific set of alters [30, 31]. Social network analysis is an effort to describe the social environment by quantifying social relationships among this group of alters [32]. Using social network data collection and analysis, several new dimensions can be characterized and analyzed; these enable examination of the influence of social networks beyond size and individual ties.
Among these, degree quantifies the connections an individual has, akin to social network size. Because of the potentially substantial burden on study participants, researchers using egocentric social network data collection generally limit the number of alters. In this case, the degree or number of supportive ties, or alternatively, the degree of strained ties, may be useful measures of the quality of the network. The strength of a patient’s ties represents the strength of relationships and is a function of the amount of time a person has known an alter in combination with the closeness and intensity of the relationship. Diversity of ties represents the number of different types of ties in a person’s network. If a patient has a large network that consists primarily of family members, the influences on health may differ from those who have a greater number of different types of ties including people they know through work, volunteering, community participation, and religious participation. Multiplexity refers to overlap in relationships and it may be more or less advantageous to have persons in one’s network assuming multiple roles. Density is the extent to which the ties are directly connected to, that is, know and associate with, one another. Greater network density, occurring when the ties mentioned by the patient also know each other well, could help with the coordination of patient care in seriously ill patients. Alternatively, a denser network may limit the influx of new information influencing decisions about or persistence with treatment. Given that caregiving is predominantly provided by women, a higher proportion of female network members could potentially translate to better health outcomes. Higher bridging potential of a participant in a network may afford greater access to resources to otherwise disconnected networks, but it can also reflect greater burden on an actor as that person navigates, connects, and meets the needs of actors from separate networks, with possible positive or negative effects on a patient. Social networks can also include weak ties, that is, acquaintances, that connect patients with opportunities, experts, and information. To our knowledge, no research has examined sociocentric social networks and cancer outcomes though work in egocentric social network data collection may provide new insights about the nature of the impact of social networks on cancer survivorship.
Researchers should consider the context of social relationships
Effects may differ by sociodemographic factors.
It is important to consider that while social network measures measure an individual’s immediate social environment, they also capture information about the macro-level social environment. Thus, it is also important to consider the social context of people’s relationships. Most studies of social networks and health outcomes report findings from predominantly white populations [7]. However, in recent work in the ABCPP, the largest observational study of social networks and breast cancer mortality to date, Kroenke and colleagues found that associations with mortality outcomes depended considerably on sociodemographic factors [33]. Specifically, associations between social ties and breast cancer mortality differed by race, age, and country of origin. Family and friendship ties were critical predictors of the outcome in the subgroup of African-American, Asian, and Hispanic women. A partner or spouse was predictive in older white women. Community ties predicted lower breast cancer mortality in older white women and in Asian women. There were no ties that predicted outcomes in younger white women. The social networks of different race/ethnic groups may differ substantially due to cultural factors and socioeconomic structures since education, income, and race determine access to healthful networks. Moreover, the ability to leverage assistance in relationships from naturally occurring networks may be strongly shaped by sociodemographic characteristics. Most cancer cohorts have extensive cancer data but limited social data. Because of this, a common approach to examining the social environment has been to look at effects of social networks in conjunction with or stratified by sociodemographic factors. To capture the influence of social networks on health, collecting information about context (e.g., egos’ and alters’ race/ethnicity, socioeconomic status, supportiveness, strain, health beliefs) is important. Fortunately, collection and analyses of these data are facilitated by processes in egocentric social network data collection.
Effects may differ by treatment severity.
Cancer stage may also modify effects of social relationships on outcomes. In the ABCPP, higher breast cancer-specific and overall mortality were apparent in socially isolated women with stage I and II, but not in those with stage III and IV, breast cancer. In the Pathways study, affection predicted better QoL in early-stage breast cancer patients but worse QoL in late-stage patients [34]. In contrast, tangible support was related to better QoL in late, but not early stage, patients. This may be related to the differing needs of cancer patients by stage as well as the abilities within naturally occurring social networks to meet those needs.
Researchers should consider psychosocial mechanisms beyond social support
Many investigators have collected information on socially supportive relationships to attempt to understand the influence of social relationships on health outcomes. This is an important start. However, the exploration of the influence of social relationships on cancer outcomes should not be limited to the study of supportive relationships and should include examination of other psychosocial mechanisms such as social norms, social resources, social roles, and social burdens. In fact, previous research in cancer almost uniformly presumes benefits in social support and social relationships, though this idea has never been explicitly stated. This means that research should reflect the potential negative aspects of social relationship on outcomes. A Women’s Health Initiative study showed among those with caregiving obligations or highly strained relationships that a larger network of relatives was related to poorer survival after a diagnosis of breast cancer [35]. In addition, a study in the Life After Cancer Epidemiology (LACE) cohort showed that women with small but supportive relationships had no higher mortality than women with large networks though women with small, unsupportive networks did have higher mortality [36].
This approach may help address criticisms regarding replication of findings and ensuring scientific rigor, particularly in regard to social relationships and their complex influence on health. It may also help to devise approaches to translate findings to cancer care. In the cancer literature, associations between social relationships and cancer outcomes have been somewhat mixed, even within studies such as the recent study in the ABCPP [33]. Understanding the context of relationships, including their positive and negative aspects, may help to explain these “mixed” findings, which may simply reflect the complexity of social relationships. They may also facilitate a clear-eyed view of what is feasible and necessary given the context of people’s relationships. For example, a patient with few supportive social ties might benefit from peer emotional support groups. However, a patient with supportive relationships who provides caregiving to others might better be helped through provision of services to dependents. At a minimum, it is important to collect information on the quality of relationships. However, the collection of data on context also extends to understanding the sociocultural and socioeconomic contexts of patients’ social networks.
Researchers should consider associations in varied cancers
The literature on social networks and cancer has focused largely on breast cancer but work is needed to evaluate relationships with other types of cancer.
PSYCHOSOCIAL MECHANISMS THROUGH WHICH SOCIAL NETWORKS OPERATE
Little is known about how social networks influence cancer prognosis, and as indicated, the primary focus in cancer research has been on evaluating the influence of social support, critically important but insufficient to describe the influence of social relationships on cancer outcomes. Other relevant social and psychosocial mechanisms include access to social structures and resources, social roles, social regulation and relatedly social norms and social “contagion,” social strain, and social/caregiving roles.
Social support
One of the primary ways social networks are hypothesized to influence cancer outcomes is through provision of social support. In addition to tangible, emotional, informational, and appraisal support [1], Sherbourne and Stewart identified affectionate support and “positive social interaction” in patients with chronic illness [16]. Tangible support includes rides to the hospital, trips to the pharmacy, or provision of healthy meals [37, 38]. Network members may provide informational support through referrals to physicians and clinics, alternative types of treatment, or may buffer stress [39] through provision of emotional support, “positive interaction,” or other types of support. Affection includes hugs and other signs of tenderness, warmth, and caring. Positive interaction is defined as the availability of someone with whom to have fun and get one’s mind off things for a while and was the most predictive type of support in a Pathways study of social support and QoL [34]. In addition to emotional and social dimensions of QoL, higher “positive interaction” predicted better physical QoL; it was related to significantly lower levels of pain, less need for bed rest, and higher levels of energy [34]. Among patients who require caregiving, their caregivers’ social networks may also exert indirect effects on patient outcomes through levels of caregivers’ perceived social support [40].
Social resources (social capital, social structures, resources)
Though social structures provide the context in which social networks operate, being a part of a social network can afford opportunities to connect to resources available within (the structure of) a network. This overlaps with the concepts of social support and social capital [41], the latter leading to benefits to an individual even without individual connection to actors within a network (e.g. social capital and the trust within a community it affords making the community a safer place to live). However, it is distinguished from these concepts to the degree that the resources derive from connections to others based on position within a specific network. A person from one’s social network may facilitate referrals and connections to highly experienced oncologists or doctors in larger clinics or comprehensive cancer centers, those who can help navigate issues with health insurance, referrals to scientists and groups conducting clinical trials, those who have experienced cancer, others with financial resources to help, or other people in the network with access to institutions that can increase access to resources, information, or opportunities to bring about change (e.g., increasing political will to fund cancer research grants, develop philanthropic organizations, build educational organizations, or organize fund-raising walks and other activities). Thus, depending on network structure [28], cancer patients may have different levels of access to resources, referrals, advice, opportunities to participate in clinical trials, and knowledge about the side effects and outcomes of treatment. These then lead to differences in decisions about treatment, influencing prognosis. Thus, beyond direct provision of resources, the network brings with it access to other people and to the social institutions, power, and resources that go along with knowing and being able to access others in the network.
Social regulation, social norms, and “contagion”
Social relationships and interactions influence behaviors and health outcomes by “contagion” [42, 43] through norms [1, 43], peer modeling [44], and social regulation. Identification with social network members may increase the likelihood of adopting behaviors [45, 46] through influences on shared behaviors and norms. One’s health-related behaviors including smoking, diet, alcohol intake, and obesity status may influence the behaviors of others in a network. It is unknown whether attitudes around adjuvant endocrine therapy (AET) could influence others’ decisions to start or persist with AET, though it is an intriguing question given low levels of adherence currently reported in breast cancer patients [47–52].
Social and caregiving roles
Social networks may also bring costs or burdens that can adversely influence health [35]. Larger social networks may increase caregiving obligations since women comprise 75% of informal caregivers [53, 54]. While potentially rewarding, caregiving can be physically and emotionally demanding, leading to poorer self-care and worse health outcomes [55–60]. However, the “mission” African-American women reported in caring for others in one study promoted continuation with treatment [61].
Social strain and stress
Though social isolation is considered to be stressful, and that is the major underlying hypothesis of most research on social isolation and physiological factors, social relationships can also be stressful. Family communication issues can also lead to higher levels of stress. Social relationships can be burdensome, strained, or even abusive. Though being married has often been associated with better health and lower mortality, marital quality may modify the influence on health, especially in women.
DOWNSTREAM BEHAVIORAL AND PHYSIOLOGICAL MECHANISMS
Social networks influence cancer outcomes through several downstream factors including behavioral, treatment, and physiologic factors.
Behavioral
Social network members may influence lifestyle through different types of social support [16]. Social norms and identification with social network members increase the likelihood of adopting lifestyle behaviors similar to those members [45, 46]. Low social network diversity, defined as the variety of social ties (connections) or roles, has been related to alcohol dependency, smoking, low levels of physical activity [62, 63], and poorer health generally [64]. Norms have been shown to influence screening [65]. Social relationships may also adversely affect health-related outcomes through relationship obligations or social strain, and related to this, higher levels of psychological distress and reduced motivation for self-care. Lifestyle risk factors, including poor diet quality [66], physical inactivity [67], smoking [68], and related to these, obesity [69] have each been associated with poorer cancer-specific and overall survival and may be mechanisms through which social relationships influence cancer outcomes.
Treatment
Social relationships may influence delays in diagnosis and treatment and affect treatment decisions. Greater social integration has been related to a higher likelihood of mammography [70], though not in all studies [71]. Perceptions that screening is normative, encouragement by family/friends, and religious support are associated with screening adherence [65, 72–74]. Women’s social relationships (e.g., spouse, family, friends) influence choice of mastectomy or lumpectomy [75], whether to pursue chemotherapy [76], and other treatment decisions [76]. Supportive relationships have been related to higher treatment adherence in people with coronary heart disease, hypertension, emerging diabetes [77–82], and other conditions [83]. As previously indicated, the “mission” women feel in caring for others may promote continuation with treatment [61]. However, strained family relationships may lower treatment adherence, mediated by risky health behaviors [84] or reduced self-management of disease [85]. Caregiving burdens may reduce adherence [86]. A lack of support or social resources may lead to treatment delays which are associated with poorer cancer outcomes [87]; delays as short as 3–6 months from diagnosis to the start of treatment decreased 5-year breast cancer survival rates by 12% [87]. These may also alter treatment decisions (e.g., radiotherapy [88, 89], chemotherapy [90]), which may influence survival. A substantial literature shows that suboptimal treatment jeopardizes breast cancer survival.
Physiological
Recent reviews by Hinzey et al. [91] and Lutgendorf and Andersen [92] have described possible biological mechanisms by which social relationships might have direct effects on cancer survival. In the context of the “hallmarks of cancer” [93, 94], those are described by the effects of social relationships on physiologic changes that underlie and determine cancer progression.
In particular, social isolation, considered a chronic psychological stressor, is well known to activate the hypothalamic-pituitary axis resulting in the secretion of catecholamines (stress hormones), which have downstream effects on tumorigenic markers through adrenergic pathways. Biomarkers involved in cancer progression include interleukins (IL), vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), prolactin, and tumor necrosis factor (TNF)-α. Stress hormones such as norepinephrine have been shown to cause upregulation of cytokines such as IL-6 and IL-8, which are proangiogenic and support tumor progression by regulating hallmarks of cancer [93, 94]. IL-6 also protects cancer cells from therapy- induced DNA damage, oxidative stress, and apoptosis. Hypothalamic–pituitary–adrenal-axis activation has also been related to higher VEGF expression; overexpression of VEGF enables a tumor to develop the vascular architecture needed to grow and metastasize. VEGF is higher in cancer patients [95]; numerous studies show decreased overall and disease-free survival in tumors overexpressing VEGF, and elevated VEGF-A has been related to poor breast [96, 97] and colorectal [98] cancer prognosis. Psychological stress has also been related to elevated MMPs, which are enzymes that degrade the extracellular matrix and promote carcinogenesis through tissue remodeling, cell proliferation, apoptosis, angiogenesis, and metastasis. Prior research has also found caregiving stress to be related to elevated levels of IL-6[99] and to prolactin [100], important in cancer progression by inhibiting apoptosis, increasing cell proliferation, and enhancing cell migration in certain cancer cell lines, as well as inducing malignant transformation in human immortalized normal epithelial cells. TNF-α is a cell signaling protein (cytokine) involved in systemic inflammation and is also strongly implicated in cancer prognosis [101]. Measures of psychological stress have been related to higher levels of TNF-α, IL-6, VEGF, and prolactin in healthy populations and in breast and ovarian cancer patients [92].
In cancer patients, social isolation has also been related to higher levels of VEGF, IL-6, and TNF-α, factors that support angiogenesis and further growth of a tumor [101, 102]. Normally, there is inhibition of unchecked cell growth. However, social isolation is related to inactivation of tumor suppressor genes. A lack of social support has also been related to elevated levels of MMPs, enzymes which stimulate invasion into the cell matrix leading to metastasis of the tumor. Social support has been linked to greater natural killer cell activity in ovarian cancer patients [103]. Each of these biological markers has been related to cancer survival.
NEEDED RESEARCH AND OPPORTUNITIES FOR TRANSLATION TO CLINICAL CARE
Relatively few strategies to leverage social relationships to improve cancer survival have been developed or tested. A possible presumption to date is that social networks are clinically irrelevant because changes in the social environment may require nonclinical strategies. And yet, advances could be made at individual, clinical, and community levels. To date, in the clinical setting, strategies have focused on providing social support through peer groups or through peer navigators or by meeting acute needs that might otherwise usually be provided by personal supports (e.g., rides to the doctor). This next section details strategies for augmenting support to patients focusing on potential clinical strategies including caregiver training, patient (peer or nurse) navigation, provision of acute (social) services, social support in early-stage patients, resources available in religious and community groups, online resources, relationship skills improvement, family interventions to improve medication adherence, and other social resources and opportunities including social network behavioral interventions [104, 105]. It will be important to evaluate these strategies in continued research; their benefits cannot be presumed. Effective clinical strategies will capitalize on information about the specific types of resources afforded by social networks within specific populations of cancer patients.
Patient navigation: Nurse navigator and peer navigators
An important direction in research over the past several years has been the area of patient navigation. Patient navigation programs were initially developed to assist breast cancer patients, particularly minority and low income women, with navigating the complex network of cancer diagnosis and treatment services with the goal of reducing barriers to earlier diagnosis and treatment. Some address QoL and psychosocial outcomes [106]. Patient navigators were originally envisioned as members of the community they were serving, that is, cancer patients or peer navigators. However, navigators include cancer survivors, nurses, social workers, other health professionals, or lay/community health workers [107]. The role of the navigator varies between programs but usually involves coordination of care and encouraging patients to pursue continued care, if needed [106]. Navigators may also provide health education about breast cancer and its treatment, emotional support, informational support, assessment of barriers, and assistance with and resources related to financial and health insurance issues, transportation services, child care, and translation.
Despite the rapid proliferation of patient navigation programs for patients with cancer, particularly for breast cancer, research on the efficacy of these programs has been limited. A recent systematic review found that while patient navigation programs improved surveillance and mammography rates, there are few high quality studies of the effect on treatment outcomes [108]. Of seven studies that looked at timeliness of treatment initiation, all found that women who received navigation initiated treatment earlier, but only two demonstrated statistically significant differences [108]. One study found that that receiving patient navigation was associated with higher rates of receiving AET among women with hormone receptor-positive breast cancer though assistance from a navigator was not associated with rates of chemotherapy or radiation therapy [109]. Two studies found improvements in psychosocial measures after receiving navigation. However, neither included a control group [110, 111]. A third study found that women with suspicious mammogram results randomized to patient navigation had lower mean anxiety scores and higher satisfaction with care compared with usual care controls one month after final resolution of mammogram results [112]. No studies have examined associations with survival; such research is greatly needed.
Peer social support interventions in early-stage patients
Given that patient needs differ by stage, needs for and the influence of social support may also differ by stage. Though peer support group interventions have been associated with modest psychosocial benefits [9, 113, 114], as indicated, there is little evidence that peer social support interventions improve cancer survival in metastatic patients even when conducted by well-trained experts. Corroborating this was the aforementioned finding from the ABCPP in which associations showing potentially salutary effects of larger social networks on breast cancer mortality were apparent only in early-, not late, stage patients. To influence survival, social interventions might target patients with earlier stage cancer, a strategy which has not been tested in research. Another potential opportunity, investigators might consider the possible merits of augmenting types of social support other than emotional support to influence patient outcomes.
Acute services and social services
Given barriers to and a lack of supportive care, social workers may be called on to communicate with patients about social resources to meet acute needs during treatment. Delays in treatment may be related to the inability to get a ride to the hospital in a timely manner, or opting against treatment may be related to the anticipated difficulties in meeting one’s own needs or in coping with the combined demands of treatment and work and caregiving responsibilities. A person’s ability to complete treatment with chemotherapy, for example, could be influenced by a lack of help in managing day-to-day responsibilities, which may lead to the desire to end treatment early; acute services could promote treatment completion. Kaiser Permanente, for example, is piloting strategies to provide low-cost meals during acute illness, and social workers are connecting patients with financial need to resources to help pay for care. The Road to Recovery program, offered by the American Cancer Society, provides rides to cancer patients to treatment appointments. There are also numerous educational and emotional support online resources. Among these and partnering with the American Cancer Society, “I Can Cope” is an online cancer education and support program for people facing cancer and the caregivers supporting them. Each of these support resources merits evaluation.
Caregiver training in late-stage patients
Nonsignificant associations in late-stage cancer patients in the ABCPP also suggested that resources provided within naturally occurring networks may not be well matched to the needs of those with late-stage cancer [115]. Managing relationships with family and friends providing caregiving may be difficult when both patients and caregivers are coping with feelings of high distress [116] and expectations regarding needs differ [117]. As mentioned, in the Pathways Study, tangible support was important to QoL but only in women with late, not early, stage breast cancer [36]. Specific training may be needed to help assist late-stage cancer patients to improve QoL. Deserving mention are the frequent economic sacrifices made by caregivers to provide support and care; consideration should be given to providing resources or paid support for otherwise unpaid, “informal” caregivers and evaluating the effects on those receiving caregiving.
Interventions involving naturally occurring social network members
There has been limited exploration of the ways through which naturally occurring networks affect cancer survival and thus no exploration of ways to clinically address the needs met by patients’ personal social networks. Relatedly, there has been no research to evaluate or test how network characteristics might be influenced to alter survival.
Family interventions
However, opportunities potentially exist to involve family members in interventions with cancer survivors. Relevant here, family interventions have been used to improve lifestyle factors in persons with other medical conditions. The British Family Heart Study [118] showed that counseling about diet and exercise delivered to both marital partners reduced the number of cardiovascular risk factors for both partners over time compared with a protocol that only addressed the patient. Also, a major study of a family intervention for patients with hypertension demonstrated that a single home visit to develop a customized plan for families to assist with medication and lifestyle change resulted in reduced blood pressure and patient mortality and increased cost savings, relative to control families [119, 120]. Though unknown, it is possible that such strategies could be applied to populations of cancer survivors with similar issues with medication adherence and lifestyle. In addition, in non-cancer populations, social network behavioral interventions are being used to reduce risky behaviors or to prompt solicitation of support [104, 105]. The translation to cancer populations is yet unclear though insights about cancer patients’ social networks may help patients to request the assistance they need and set boundaries for relationships that might compromise their care or well-being.
Religious and community groups
Churches are settings that have been used to promote screening [121–123] particularly in African-Americans. Also in the community setting, peer network strategies are being tested [124–126] to improve support in African-American breast cancer patients. In the future, the development of community cancer volunteer networks could also more widely help provide critical support to survivors with limited social networks. Considerably more work in this area is warranted.
Communication and relationship skill interventions
Little considered, interventions to improve relationship skills (e.g., social sensitivity, communication) within existing relationships could also have beneficial influences on cancer health outcomes. Research has demonstrated the difficulties to families when a member is diagnosed with cancer, particularly in those families with communication problems prior to diagnosis [127–130]. Considerable emphasis in the literature has also focused on patient-physician communication and most particularly physician communication skills [131–137]. Emphasis might also be devoted to empowering patients through improvements in patient communication skills to facilitate interactions with the many clinicians with whom they interface.
Online resources
The proliferation of disease-specific online support groups, both self-directed and professionally led, has resulted in additional resources for cancer patients. The literature on online peer social support is, however, nascent and the effects of online peer support networks on cancer outcomes are unknown. There is also no published research regarding the influence of online networks on cancer treatment, a major predictor of survival. Initial research has focused on general reasons for use and level of engagement [138–141]. Breast cancer patients seek online peers for information (91.3%) and symptom management (69.6%) and less so for emotional support (47.8%) [142]. In an online network of prostate cancer patients, patients also asked for treatment recommendations (66%) [143]. In another online network forum, among breast cancer patients taking aromatase inhibitors (AIs), 12.8% mentioned discontinuing AIs, and another 28.1% mentioned switching AIs [138]. Online participation could have ramifications for treatment decision-making and survival since patients use the Internet to help make decisions about type of surgery [144], and social norms influence cancer patients’ decisions about chemotherapy [145], but this is untested in research.
Online groups may also be useful for those who are unable to access in-person groups, for example, those who live in rural areas, have mobility limitations, or have rare cancers or subtypes. To date, limited evidence exists regarding the use and efficacy of online support groups, and results regarding their influence on QoL, the major focus of research conducted to date, are mixed [146–151]. The Comprehensive Health Enhancement Support System (CHESS) and related trials, for example, may help to address questions regarding the impact of online resources on cancer outcomes in the future [152–154].
Crowdfunding sites are also intriguing in their potential to assist patients and families after a cancer diagnosis though this may ultimately reach only a select set of patients. Regardless, considerable work will be needed to be able to characterize the influence of online support on survival.
Implications for future research
The conceptual model outlined here may motivate research examining structural aspects of social networks, as well as multiple and potentially competing psychosocial and downstream mechanisms of cancer outcomes. This model also has considerable relevance for health disparities in cancer outcomes. More research must be done to understand the influence of social networks on cancer outcomes. Though ongoing research is being undertaken to improve outcomes in cancer patients, other clinically relevant hypotheses are suggested by this model, which may also point to new areas of research.
Hypothesis (Hyp) 1. Patients with large, diverse social networks may have better cancer outcomes generally.
As mentioned, most prior work has been in breast cancer patients though these relationships should be examined in other cancers as well. And as indicated, work will be needed to understand the structural aspects including the positive and negative aspects of social networks on cancer outcomes.
Hyp 2. Certain cancer patients may have healthier behaviors if they have smaller, less diverse social networks, leading to better cancer survival.
This model has substantial relevance for work in health disparities. In the general research literature, larger, more diverse social networks are related to lower mortality and healthier behaviors. However, these may not always lead to better outcomes such as when social networks lead to unhealthy behaviors. For example, immigrants from countries with healthier lifestyle patterns who live in higher co-ethnic neighborhoods and have social networks with higher proportions of recent immigrants, may have healthier behaviors, leading to better cancer outcomes. Understanding these relationships may help clarify social factors that may enhance or impede individual-level clinical interventions and recommendations.
Hyp 3. Social networks may not lead to better cancer outcomes in late-stage patients due to a mismatch between caregiver knowledge and resources and patient needs.
Many people depend on close friends and relatives to provide care in the case of cancer, but many of these people do not have the training or resources to take on this care, particularly for patients with late-stage cancer. This has broader clinical and policy implications both with regard to concerns about medical care being handed off to non-clinicians without training as well as the ethics of unpaid caregiving in the USA in which substantial resources go to unproven medical interventions, but few resources are provided to caregivers. Research is needed to understand the implications of this on cancer outcomes.
Hyp 4. The structural characteristics of social networks may influence treatment management.
Network density may have a role in the optimization of treatment if it enables better coordination of care. Alternatively, highly dense networks may limit influx of relevant information. Research is needed to explore the influence of structural characteristics on cancer and outcomes.
Hyp 5. Social norms matter in decision-making about cancer treatment where treatment options are not clear cut.
In settings where norms may influence behaviors as is possible in an unmoderated online network, the attitudes and beliefs of certain participants may influence the attitudes and beliefs of others. Research is needed to explore social norms as a conduit to decision-making about treatment particularly when treatment options are not predetermined.
These are a few examples of hypotheses that derive from the conceptual model. Thus, this model provides the basis for these and other questions about social networks that are relevant to cancer patients and survivors.
CONCLUSION
Ultimately, it is important to consider both macro- and micro-level processes of how social networks influence outcomes. There is a variety of potential interventions, with direct influences on social relationships or indirect influences on the resources they provide, some strategies which are currently in use and some the effects of which are unknown, that may serve to improve cancer outcomes. The relevance and potential of each of these types of strategies will be informed by knowledge of mechanisms. The size and quality of one’s social networks are also influenced by the broader social environment as well as individual-level factors such as quality of relationships. The context in which patient social networks operate also influence the resources accessible to patients. Considerable work has been done in the area of patient navigation. Other social strategies have received much less attention. Further work will be needed to identify and test new strategies to leverage social relationships to improve cancer survival.
Acknowledgements
The findings of this review article have not been previously published and the manuscript has not simultaneously been submitted elsewhere. The writing of this article was funded by NCI Grant #K07CA187403 (PI: Kroenke).
Compliance with Ethical Standards
Conflict of Interest The author has no conflicts of interest.
Primary Data No primary data were used in writing this review article.
Ethical Approval This article does not contain any studies with human participants or animals performed by the author.
Informed Consent For this type of study, formal consent is not required.
References
- 1. Berkman L, Glass TA. Social integration, social networks, social support, and health. In: Berkman L, Kawachi I, eds. Social epidemiology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 2. Beasley JM, Newcomb PA, Trentham-Dietz A, et al. . Social networks and survival after breast cancer diagnosis. j Cancer Surviv. 2010;4(4):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou AF, Stewart SL, Wild RC, Bloom JR. Social support and survival in young women with breast carcinoma. Psychooncology. 2012;21(2):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. j Clin Oncol. 2006;24(7):1105–1111. [DOI] [PubMed] [Google Scholar]
- 5. Reynolds P, Boyd PT, Blacklow RS, et al. . The relationship between social ties and survival among black and white breast cancer patients. National Cancer Institute Black/White Cancer Survival Study Group. Cancer Epidemiol Biomarkers Prev. 1994;3(3):253–259. [PubMed] [Google Scholar]
- 6. Waxler-Morrison N, Hislop TG, Mears B, Kan L. Effects of social relationships on survival for women with breast cancer: a prospective study. Soc Sci Med. 1991;33(2):177–183. [DOI] [PubMed] [Google Scholar]
- 7. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. Plos Med. 2010;7(7):e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spiegel D, Butler LD, Giese-Davis J, et al. . Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer. 2007;110(5):1130–1138. [DOI] [PubMed] [Google Scholar]
- 9. Goodwin PJ, Leszcz M, Ennis M, et al. . The effect of group psychosocial support on survival in metastatic breast cancer. n Engl j Med. 2001;345(24):1719–1726. [DOI] [PubMed] [Google Scholar]
- 10. Gellert GA, Maxwell RM, Siegel BS. Survival of breast cancer patients receiving adjunctive psychosocial support therapy: a 10-year follow-up study. j Clin Oncol. 1993;11(1):66–69. [DOI] [PubMed] [Google Scholar]
- 11. Cousson-Gélie F, Bruchon-Schweitzer M, Atzeni T, Houede N. Evaluation of a psychosocial intervention on social support, perceived control, coping strategies, emotional distress, and quality of life of breast cancer patients. Psychol Rep. 2011;108(3):923–942. [DOI] [PubMed] [Google Scholar]
- 12. Helgeson VS, Cohen S, Schulz R, Yasko J. Group support interventions for women with breast cancer: who benefits from what?Health Psychol. 2000;19(2):107–114. [DOI] [PubMed] [Google Scholar]
- 13. Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2(8668):888–891. [DOI] [PubMed] [Google Scholar]
- 14. Phillips KA, Osborne RH, Giles GG, et al. . Psychosocial factors and survival of young women with breast cancer: a population-based prospective cohort study. j Clin Oncol. 2008;26(28):4666–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smedslund G, Ringdal GI. Meta-analysis of the effects of psychosocial interventions on survival time in cancer patients. j Psychosom Res. 2004;57(2):123–131; discussion 133–135. [DOI] [PubMed] [Google Scholar]
- 16. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. [DOI] [PubMed] [Google Scholar]
- 17. Kroenke CH, Michael YL, Poole EM, et al. . Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer. 2017;123(7):1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol Hematol. 2010;75(2):122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kroenke CH, Michael YL, Shu XO, et al. . Post-diagnosis social networks, and lifestyle and treatment factors in the After Breast Cancer Pooling Project. Psychooncology. 2017;26(4):544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hakulinen C, Elovainio M, Batty GD, Virtanen M, Kivimäki M, Jokela M. Personality and alcohol consumption: pooled analysis of 72,949 adults from eight cohort studies. Drug Alcohol Depend. 2015;151:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hakulinen C, Hintsanen M, Munafò MR, et al. . Personality and smoking: individual-participant meta-analysis of nine cohort studies. Addiction. 2015;110(11):1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jokela M, Hintsanen M, Hakulinen C, et al. . Association of personality with the development and persistence of obesity: a meta-analysis based on individual-participant data. Obes Rev. 2013;14(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Minami Y, Hosokawa T, Nakaya N, et al. . Personality and breast cancer risk and survival: the Miyagi cohort study. Breast Cancer Res Treat. 2015;150(3):675–684. [DOI] [PubMed] [Google Scholar]
- 24. Jokela M, Batty GD, Hintsa T, Elovainio M, Hakulinen C, Kivimäki M. Is personality associated with cancer incidence and mortality? An individual-participant meta-analysis of 2156 incident cancer cases among 42,843 men and women. Br j Cancer. 2014;110(7):1820–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krok-Schoen JL, Oliveri JM, Paskett ED. Cancer care delivery and women’s health: the role of patient navigation. Front Oncol. 2016;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freund KM, Battaglia TA, Calhoun E, et al. . Impact of patient navigation on timely cancer care: the Patient Navigation Research Program. J Natl Cancer Inst. 2014;106 (6):dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Battaglia TA, Darnell JS, Ko N, et al. . The impact of patient navigation on the delivery of diagnostic breast cancer care in the National Patient Navigation Research Program: a prospective meta-analysis. Breast Cancer Res Treat. 2016;158(3):523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Granovetter M. The strength of weak ties. Am. J. Sociol. 1973;78(6):1360–1380. [Google Scholar]
- 29. McCarty C. Measuring structure in personal networks. JoSS. 2002;3(1). Available at http://www.cmu.edu/joss/content/articles/volume3/McCarty.html. Accessibility verified December 21, 2017. [Google Scholar]
- 30. Mitchell JC. The concept and use of social networks. In: Mitchell JC, ed. Social Networks in Urban Situations. Manchester: Manchester University Press; 1969:1–50. [Google Scholar]
- 31. Scott J. Social Network Analysis: A Handbook. London: Sage Publications; 1991. [Google Scholar]
- 32. Wasserman S, Faust K.. Social Network Analysis: Methods and Applications. New York: Cambridge University Press; 1994. [Google Scholar]
- 33. Kroenke CH, Michael YL, Poole EM, et al. . Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer. 2017;123(7):1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kroenke CH, Kwan ML, Neugut AI, et al. . Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Res Treat. 2013;139(2):515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroenke CH, Michael Y, Tindle H, et al. . Social networks, social support and burden in relationships, and mortality after breast cancer diagnosis. Breast Cancer Res Treat. 2012;133(1):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kroenke CH, Quesenberry C, Kwan ML, Sweeney C, Castillo A, Caan BJ. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) study. Breast Cancer Res Treat. 2013;137(1):261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hirschman KB, Bourjolly JN. How do tangible supports impact the breast cancer experience?Soc Work Health Care. 2005;41(1):17–32. [DOI] [PubMed] [Google Scholar]
- 38. Woloshin S, Schwartz LM, Tosteson AN, et al. . Perceived adequacy of tangible social support and health outcomes in patients with coronary artery disease. j Gen Intern Med. 1997;12(10):613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- 40. Kelley DE, Lewis MA, Southwell BG. Perceived support from a caregiver’s social ties predicts subsequent care-recipient health. Prev Med Rep. 2017;8:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moore S, Daniel M, Paquet C, Dubé L, Gauvin L. Association of individual network social capital with abdominal adiposity, overweight and obesity. j Public Health (Oxf). 2009;31(1):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valente TW. Social Networks and Health: Models, Methods, and Applications. New York, NY: Oxford University Press; 2009. [Google Scholar]
- 43. Smith K, Christakis N. Social networks and health. Annu. Rev. Sociol. 2008;34:405–429. [Google Scholar]
- 44. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 45. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. n Engl j Med. 2007;357(4):370–379. [DOI] [PubMed] [Google Scholar]
- 46. Pachucki MA, Jacques PF, Christakis NA. Social network concordance in food choice among spouses, friends, and siblings. Am j Public Health. 2011;101(11):2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCowan C, Shearer J, Donnan PT, et al. . Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br j Cancer. 2008;99(11):1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(21):1543–1549. [DOI] [PubMed] [Google Scholar]
- 50. Geiger AM, Thwin SS, Lash TL, et al. . Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109(5):966–974. [DOI] [PubMed] [Google Scholar]
- 51. Yood MU, Owusu C, Buist DS, et al. . Mortality impact of less-than-standard therapy in older breast cancer patients. j Am Coll Surg. 2008;206(1):66–75. [DOI] [PubMed] [Google Scholar]
- 52. Bryant J, Fisher B, Dignam J. Duration of adjuvant tamoxifen therapy. J Natl Cancer Inst Monogr. 2001;(30):56–61. [DOI] [PubMed] [Google Scholar]
- 53. Arno PS. The Economic Value of Informal Caregiving, U.S., 2000. Florida: American Association for Geriatric Psychiatry; 2002. [Google Scholar]
- 54. Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Aff (Millwood). 1999;18(2):182–188. [DOI] [PubMed] [Google Scholar]
- 55. Kiecolt-Glaser JK, Glaser R, Shuttleworth EC, Dyer CS, Ogrocki P, Speicher CE. Chronic stress and immunity in family caregivers of Alzheimer’s disease victims. Psychosom Med. 1987;49(5):523–535. [DOI] [PubMed] [Google Scholar]
- 56. Cannuscio CC, Jones C, Kawachi I, Colditz GA, Berkman L, Rimm E. Reverberations of family illness: a longitudinal assessment of informal caregiving and mental health status in the Nurses’ Health Study. Am j Public Health. 2002;92(8):1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee S, Kawachi I, Grodstein F. Does caregiving stress affect cognitive function in older women?j Nerv Ment Dis. 2004;192(1):51–57. [DOI] [PubMed] [Google Scholar]
- 58. Lee S, Colditz G, Berkman L, Kawachi I. Caregiving to children and grandchildren and risk of coronary heart disease in women. Am j Public Health. 2003;93(11):1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. Am j Prev Med. 2003;24(2):113–119. [DOI] [PubMed] [Google Scholar]
- 60. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282(23):2215–2219. [DOI] [PubMed] [Google Scholar]
- 61. Gates MF, Lackey NR, Brown G. Caring demands and delay in seeking care in African American women newly diagnosed with breast cancer: an ethnographic, photographic study. Oncol Nurs Forum. 2001;28(3):529–537. [PubMed] [Google Scholar]
- 62. Mowbray O, Quinn A, Cranford JA. Social networks and alcohol use disorders: findings from a nationally representative sample. Am j Drug Alcohol Abuse. 2014;40(3):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr. Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–1944. [PubMed] [Google Scholar]
- 64. Barefoot JC, Grønbaek M, Jensen G, Schnohr P, Prescott E. Social network diversity and risks of ischemic heart disease and total mortality: findings from the Copenhagen City Heart Study. Am j Epidemiol. 2005;161(10):960–967. [DOI] [PubMed] [Google Scholar]
- 65. Allen JD, Stoddard AM, Sorensen G. Do social network characteristics predict mammography screening practices?Health Educ Behav. 2008;35(6):763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kroenke CH, Fung TT, Hu FB, Holmes MD. Dietary patterns and survival after breast cancer diagnosis. j Clin Oncol. 2005;23(36): 9295–9303. [DOI] [PubMed] [Google Scholar]
- 67. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. [DOI] [PubMed] [Google Scholar]
- 68. Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA. Smoking and survival after breast cancer diagnosis. Int j Cancer. 2007;120(12):2672–2677. [DOI] [PubMed] [Google Scholar]
- 69. Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. j Clin Oncol. 2005;23(7):1370–1378. [DOI] [PubMed] [Google Scholar]
- 70. Suarez L, Lloyd L, Weiss N, Rainbolt T, Pulley L. Effect of social networks on cancer-screening behavior of older Mexican-American women. j Natl Cancer Inst. 1994;86(10):775–779. [DOI] [PubMed] [Google Scholar]
- 71. Suarez L, Ramirez AG, Villarreal R, et al. . Social networks and cancer screening in four U.S. Hispanic groups. Am j Prev Med. 2000;19(1):47–52. [DOI] [PubMed] [Google Scholar]
- 72. Allen JD, Sorensen G, Stoddard AM, Peterson KE, Colditz G. The relationship between social network characteristics and breast cancer screening practices among employed women. Ann Behav Med. 1999;21(3):193–200. [DOI] [PubMed] [Google Scholar]
- 73. Fowler BA. Social processes used by African American women in making decisions about mammography screening. j Nurs Scholarsh. 2006;38(3):247–254. [DOI] [PubMed] [Google Scholar]
- 74. Fowler BA. The influence of social support relationships on mammography screening in African-American women. j Natl Black Nurses Assoc. 2007;18(1):21–29. [PubMed] [Google Scholar]
- 75. Hawley ST, Griggs JJ, Hamilton AS, et al. . Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. j Natl Cancer Inst. 2009;101(19):1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shelton RC, Clarke Hillyer G, Hershman DL, et al. . Interpersonal influences and attitudes about adjuvant therapy treatment decisions among non-metastatic breast cancer patients: an examination of differences by age and race/ethnicity in the BQUAL study. Breast Cancer Res Treat. 2013;137(3):817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: epidemiologic evidence and implications for treatment. Psychosom Med. 2005;67(6):869–878. [DOI] [PubMed] [Google Scholar]
- 78. Molloy GJ, Perkins-Porras L, Bhattacharyya MR, Strike PC, Steptoe A. Practical support predicts medication adherence and attendance at cardiac rehabilitation following acute coronary syndrome. j Psychosom Res. 2008;65(6):581–586. [DOI] [PubMed] [Google Scholar]
- 79. Burroughs TE, Harris MA, Pontious SL, Santiago JV. Research on social support in adolescents with IDDM: a critical review. Diabetes Educ. 1997;23(4):438–448. [DOI] [PubMed] [Google Scholar]
- 80. Stanton AL. Determinants of adherence to medical regimens by hypertensive patients. j Behav Med. 1987;10(4):377–394. [DOI] [PubMed] [Google Scholar]
- 81. Doherty WJ, Schrott HG, Metcalf L, Iasiello-Vailas L. Effect of spouse support and health beliefs on medication adherence. j Fam Pract. 1983;17(5):837–841. [PubMed] [Google Scholar]
- 82. Pyatak EA, Florindez D, Weigensberg MJ. Adherence decision making in the everyday lives of emerging adults with type 1 diabetes. Patient Prefer Adherence. 2013;7:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23(2):207–218. [DOI] [PubMed] [Google Scholar]
- 84. Wickrama KAS, Lorenz FO, Conger RD, Elder GH. Marital quality and physical illness: a latent growth curve analysis. J. Marriage Fam. 1997;59(1):143–155. [Google Scholar]
- 85. Trevino DB, Young EH, Groff J, Jono RT. The association between marital adjustment and compliance with antihypertension regimens. j Am Board Fam Pract. 1990;3 (1):17–25. [PubMed] [Google Scholar]
- 86. Mellins CA, Brackis-Cott E, Dolezal C, Abrams EJ. The role of psychosocial and family factors in adherence to antiretroviral treatment in human immunodeficiency virus-infected children. Pediatr Infect Dis j. 2004;23(11):1035–1041. [DOI] [PubMed] [Google Scholar]
- 87. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. [DOI] [PubMed] [Google Scholar]
- 88. Blamey RW, Bates T, Chetty U, et al. . Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur j Cancer. 2013;49(10):2294–2302. [DOI] [PubMed] [Google Scholar]
- 89. Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119(7):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van der Sangen MJ, van de Wiel FM, Poortmans PM, et al. . Are breast conservation and mastectomy equally effective in the treatment of young women with early breast cancer? Long-term results of a population-based cohort of 1,451 patients aged ≤ 40 years. Breast Cancer Res Treat. 2011;127(1):207–215. [DOI] [PubMed] [Google Scholar]
- 91. Hinzey A, Gaudier-Diaz MM, Lustberg MB, DeVries AC. Breast cancer and social environment: getting by with a little help from our friends. Breast Cancer Res. 2016;18(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lutgendorf SK, Andersen BL. Biobehavioral approaches to cancer progression and survival: mechanisms and interventions. Am Psychol. 2015;70(2):186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 94. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 95. Heer K, Kumar H, Read JR, Fox JN, Monson JR, Kerin MJ. Serum vascular endothelial growth factor in breast cancer: its relation with cancer type and estrogen receptor status. Clin Cancer Res. 2001;7(11):3491–3494. [PubMed] [Google Scholar]
- 96. Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist. 2000;5(Suppl 1):37–44. [DOI] [PubMed] [Google Scholar]
- 97. Liu Y, Tamimi RM, Collins LC, et al. . The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: results from the Nurses’ Health Study. Breast Cancer Res Treat. 2011;129(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bendardaf R, Buhmeida A, Hilska M, et al. . VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res. 2008;28(6B):3865–3870. [PubMed] [Google Scholar]
- 99. Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. j Gerontol a Biol Sci Med Sci. 1999;54(9):M434–M439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kroenke CH, Hankinson SE, Schernhammer ES, Colditz GA, Kawachi I, Holmes MD. Caregiving stress, endogenous sex steroid hormone levels, and breast cancer incidence. Am j Epidemiol. 2004;159(11):1019–1027. [DOI] [PubMed] [Google Scholar]
- 101. Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–i108. [DOI] [PubMed] [Google Scholar]
- 102. Guthrie GJ, Roxburgh CS, Horgan PG, McMillan DC. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer?Cancer Treat Rev. 2013;39(1):89–96. [DOI] [PubMed] [Google Scholar]
- 103. Lutgendorf SK, Sood AK, Anderson B, et al. . Social support, psychological distress, and natural killer cell activity in ovarian cancer. j Clin Oncol. 2005;23(28):7105–7113. [DOI] [PubMed] [Google Scholar]
- 104. Kennedy A, Vassilev I, James E, Rogers A. Implementing a social network intervention designed to enhance and diversify support for people with long-term conditions. A qualitative study. Implement Sci. 2016;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kennedy DP, Hunter SB, Chan Osilla K, Maksabedian E, Golinelli D, Tucker JS. A computer-assisted motivational social network intervention to reduce alcohol, drug and HIV risk behaviors among Housing First residents. Addict Sci Clin Pract. 2016;11(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Robinson-White S, Conroy B, Slavish KH, Rosenzweig M. Patient navigation in breast cancer: a systematic review. Cancer Nurs. 2010;33(2):127–140. [DOI] [PubMed] [Google Scholar]
- 107. Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61(4):237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Baik SH, Gallo LC, Wells KJ. Patient navigation in breast cancer treatment and survivorship: a systematic review. J Clin Oncol. 2016;34(30):3686–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ko NY, Darnell JS, Calhoun E, et al. . Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. j Clin Oncol. 2014;32(25):2758–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Giese-Davis J, Bliss-Isberg C, Carson K, et al. . The effect of peer counseling on quality of life following diagnosis of breast cancer: an observational study. Psychooncology. 2006;15(11):1014–1022. [DOI] [PubMed] [Google Scholar]
- 111. Madore S, Kilbourn K, Valverde P, Borrayo E, Raich P. Feasibility of a psychosocial and patient navigation intervention to improve access to treatment among underserved breast cancer patients. Support Care Cancer. 2014;22(8):2085–2093. [DOI] [PubMed] [Google Scholar]
- 112. Ferrante JM, Chen PH, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. J Urban Health. 2008;85(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Classen C, Butler LD, Koopman C, et al. . Supportive-expressive group therapy and distress in patients with metastatic breast cancer: a randomized clinical intervention trial. Arch Gen Psychiatry. 2001;58(5):494–501. [DOI] [PubMed] [Google Scholar]
- 114. Kissane DW, Grabsch B, Clarke DM, et al. . Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16(4):277–286. [DOI] [PubMed] [Google Scholar]
- 115. Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA Cancer J Clin. 2001;51(4):213–231. [DOI] [PubMed] [Google Scholar]
- 116. Given B, Wyatt G, Given C, et al. . Burden and depression among caregivers of patients with cancer at the end of life. Oncol Nurs Forum. 2004;31(6):1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Beatty L, Oxlad M, Koczwara B, Wade TD. The psychosocial concerns and needs of women recently diagnosed with breast cancer: a qualitative study of patient, nurse and volunteer perspectives. Health Expect. 2008;11(4):331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Family Heart Study Group. Randomised controlled trial evaluating cardiovascular screening and intervention in general practice: principal results of British family heart study. Family Heart Study Group. BMJ. 1994;308(6924):313–320. [PMC free article] [PubMed] [Google Scholar]
- 119. Cantor JC, Morisky DE, Green LW, Levine DM, Salkever DS. Cost-effectiveness of educational interventions to improve patient outcomes in blood pressure control. Prev Med. 1985;14(6):782–800. [DOI] [PubMed] [Google Scholar]
- 120. Morisky DE, Levine DM, Green LW, Shapiro S, Russell RP, Smith CR. Five-year blood pressure control and mortality following health education for hypertensive patients. Am j Public Health. 1983;73(2):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Drake BF, Shelton RC, Gilligan T, Allen JD. A church-based intervention to promote informed decision making for prostate cancer screening among African American men. j Natl Med Assoc. 2010;102(3):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Husaini BA, Reece MC, Emerson JS, Scales S, Hull PC, Levine RS. A church-based program on prostate cancer screening for African American men: reducing health disparities. Ethn Dis. 2008;18(2 Suppl 2):S2–179–84. [PubMed] [Google Scholar]
- 123. Husaini BA, Sherkat DE, Levine R, et al. . The effect of a church-based breast cancer screening education program on mammography rates among African-American women. j Natl Med Assoc. 2002;94(2):100–106. [PMC free article] [PubMed] [Google Scholar]
- 124. Ashing-Giwa K, Tapp C, Rosales M, et al. . Peer-based models of supportive care: the impact of peer support groups in African American breast cancer survivors. Oncol Nurs Forum. 2012;39(6):585–591. [DOI] [PubMed] [Google Scholar]
- 125. Hanson LC, Armstrong TD, Green MA, et al. . Circles of care: development and initial evaluation of a peer support model for African Americans with advanced cancer. Health Educ Behav. 2013;40(5):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hanson LC, Green MA, Hayes M, et al. . Circles of care: Implementation and evaluation of support teams for African Americans with cancer. Health Educ Behav. 2014;41(3):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nissen KG, Trevino K, Lange T, Prigerson HG. Family relationships and psychosocial dysfunction among family caregivers of patients with advanced cancer. j Pain Symptom Manage. 2016;52(6):841–849.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Traa MJ, De Vries J, Bodenmann G, Den Oudsten BL. Dyadic coping and relationship functioning in couples coping with cancer: a systematic review. Br j Health Psychol. 2015;20(1):85–114. [DOI] [PubMed] [Google Scholar]
- 129. Regan TW, Lambert SD, Girgis A, Kelly B, Kayser K, Turner J. Do couple-based interventions make a difference for couples affected by cancer? A systematic review. bmc Cancer. 2012;12:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kim Y, Carver CS. Frequency and difficulty in caregiving among spouses of individuals with cancer: effects of adult attachment and gender. Psychooncology. 2007;16(8):714–723. [DOI] [PubMed] [Google Scholar]
- 131. Diefenbach M, Turner G, Carpenter KM, et al. . Cancer and patient-physician communication. j Health Commun. 2009;14(Suppl 1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ernstmann N, Weissbach L, Herden J, Winter N, Ansmann L. Patient-physician communication and health-related quality of life of patients with localised prostate cancer undergoing radical prostatectomy a longitudinal multilevel analysis. bju Int. 2017;119(3):396–405. [DOI] [PubMed] [Google Scholar]
- 133. Lee S, Chen L, Ma GX, Fang CY. What is lacking in patient-physician communication: perspectives from Asian American breast cancer patients and oncologists. J Behav Health. 2012;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Royak-Schaler R, Passmore SR, Gadalla S, et al. . Exploring patient-physician communication in breast cancer care for African American women following primary treatment. Oncol Nurs Forum. 2008;35(5):836–843. [DOI] [PubMed] [Google Scholar]
- 135. Piette JD, Heisler M, Krein S, Kerr EA. The role of patient-physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165(15):1749–1755. [DOI] [PubMed] [Google Scholar]
- 136. Razavi D, Merckaert I, Marchal S, et al. . How to optimize physicians’ communication skills in cancer care: results of a randomized study assessing the usefulness of posttraining consolidation workshops. j Clin Oncol. 2003;21(16):3141–3149. [DOI] [PubMed] [Google Scholar]
- 137. Zachariae R, Pedersen CG, Jensen AB, Ehrnrooth E, Rossen PB, von der Maase H. Association of perceived physician communication style with patient satisfaction, distress, cancer-related self-efficacy, and perceived control over the disease. Br j Cancer. 2003;88(5):658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mao JJ, Chung A, Benton A, et al. . Online discussion of drug side effects and discontinuation among breast cancer survivors. Pharmacoepidemiol Drug Saf. 2013;22(3):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lepore SJ, Buzaglo JS, Lieberman MA, Golant M, Davey A. Standard versus prosocial online support groups for distressed breast cancer survivors: a randomized controlled trial. bmc Cancer. 2011;11:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bender JL, Jimenez-Marroquin MC, Ferris LE, Katz J, Jadad AR. Online communities for breast cancer survivors: a review and analysis of their characteristics and levels of use. Support Care Cancer. 2013;21(5):1253–1263. [DOI] [PubMed] [Google Scholar]
- 141. Klemm P. Effects of online support group format (moderated vs peer-led) on depressive symptoms and extent of participation in women with breast cancer. Comput Inform Nurs. 2012;30(1):9–18. [DOI] [PubMed] [Google Scholar]
- 142. Bender JL, Katz J, Ferris LE, Jadad AR. What is the role of online support from the perspective of facilitators of face-to-face support groups? A multi-method study of the use of breast cancer online communities. Patient Educ Couns. 2013;93(3):472–479. [DOI] [PubMed] [Google Scholar]
- 143. Huber J, Ihrig A, Peters T, et al. . Decision-making in localized prostate cancer: lessons learned from an online support group. bju Int. 2011;107(10):1570–1575. [DOI] [PubMed] [Google Scholar]
- 144. Couper MP, Singer E, Levin CA, Fowler FJ Jr, Fagerlin A, Zikmund-Fisher BJ. Use of the Internet and ratings of information sources for medical decisions: results from the DECISIONS survey. Med Decis Making. 2010;30(5 Suppl):106S–114S. [DOI] [PubMed] [Google Scholar]
- 145. Zikmund-Fisher BJ, Windschitl PD, Exe N, Ubel PA. ‘I’ll do what they did”: social norm information and cancer treatment decisions. Patient Educ Couns. 2011;85(2):225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gustafson DH, Hawkins R, McTavish F, et al. . Internet-based interactive support for cancer patients: are integrated systems better?J. Commun. 2008;58(2):238–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Høybye MT, Dalton SO, Deltour I, Bidstrup P, Frederiksen K, Johansen C. Effect of Internet peer-support groups on psychosocial adjustment to cancer: a randomised study. Br J Cancer. 2010;102(9):1348–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lieberman MA, Golant M, Giese-Davis J, et al. . Electronic support groups for breast carcinoma. Cancer. 2003;97(4):920–925. [DOI] [PubMed] [Google Scholar]
- 149. Lieberman MA, Goldstein BA. Self-help on-line: an outcome evaluation of breast cancer bulletin boards. J. Health Psychol. 2005;10(6):855–862. [DOI] [PubMed] [Google Scholar]
- 150. Salzer MS, Palmer SC, Kaplan K, et al. . A randomized, controlled study of Internet peer-to-peer interactions among women newly diagnosed with breast cancer. Psychooncology. 2010;19(4):441–446. [DOI] [PubMed] [Google Scholar]
- 151. Winzelberg AJ, Classen C, Alpers GW, et al. . Evaluation of an internet support group for women with primary breast cancer. Cancer. 2003;97(5):1164–1173. [DOI] [PubMed] [Google Scholar]
- 152. Gustafson DH, DuBenske LL, Namkoong K, et al. . An eHealth system supporting palliative care for patients with non-small cell lung cancer: a randomized trial. Cancer. 2013;119(9):1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Baker TB, Hawkins R, Pingree S, et al. . Optimizing eHealth breast cancer interventions: which types of eHealth services are effective?Transl Behav Med. 2011;1(1):134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gustafson DH, McTavish FM, Stengle W, et al. . Use and impact of eHealth system by low-income women with breast cancer. j Health Commun. 2005;10(Suppl 1):195–218. [DOI] [PubMed] [Google Scholar]