Abstract
Background:
Contradictory results exist regarding the importance of early-life exposure to phthalates for development of childhood eczema.
Objectives:
We evaluated the association between maternal urinary concentrations of phthalate metabolites between the 24th and 28th week of gestation and occurrence of eczema in their sons up to 5 y of age, according to allergic sensitization as assessed by total immunoglobulin E (IgE) in a subsample of individuals.
Methods:
Data on health outcomes and background factors were collected using five standardized annual questionnaires completed by parents at the children’s ages of 1–5 y, and their associations with phthalate metabolite urinary concentrations were assessed in 604 mother–son pairs with adjusted multiple logistic regression and Cox’s survival model. Several eczema phenotypes were considered. Atopic status was assessed at 5 y of age in 293 boys through total IgE assessment.
Results:
At 5 y of age, the prevalence of ever eczema was 30.4%. Metabolites of di-isobutyl phthalate (DiBP) and di-isononyl phthalate (DiNP) were positively associated with early-onset (0–24 mo of age) eczema (15.7%) and late-onset (24–60 mo of age) eczema (14.7%). Applying the Cox’s model showed a significant association of occurrence of eczema in the first 5 y of life with DiBP and DiNP metabolites. Among IgE-sensitized boys, metabolites of di-n-butyl phthalate (DBP) and DiBP were significantly associated with ever eczema {hazard ratio [95% confidence interval (CI): 1.10, 2.54], and (95% CI: 1.01, 3.48), , respectively}.
Conclusions:
Occurrence of eczema in early childhood may be influenced by prenatal exposure to certain phthalates in boys. Further investigations are needed to confirm this observation. https://doi.org/10.1289/EHP1829
Introduction
Phthalates are man-made chemicals widely used in our daily life [e.g., food wrappings, cosmetic products, children’s toys, cleaning products, medical devices, pharmaceuticals, polyvinyl chloride (PVC) flooring, building materials] because of their properties of transparency, durability, and flexibility (Meeker et al. 2009; Bornehag et al. 2005; Arbuckle et al. 2014). People can be exposed to phthalates through inhalation, dermal absorption (including air-to-skin transport), contact with contaminated surfaces, and use of personal care products and ingestion (including dietary ingestion and incidental ingestion) (Swan 2008). Urinary concentrations of phthalate metabolite are the most reliable measures for exposure assessment (Swan 2008). The ubiquitous presence of phthalates in the environment and the potential consequences of human exposure to phthalates have raised concerns, particularly in vulnerable populations such as pregnant women and infants. Prenatal and early life are critical in the development of the immune system; exposure to toxic pollutants during this period can result in an increased risk of adverse health outcomes later in life.
Animal as well as epidemiological studies in infants and children have found various health effects of specific phthalates, including abnormal reproductive outcomes (Meeker et al. 2009; Abdel-Maksoud et al. 2015; Niermann et al. 2015; Aydoğan Ahbab and Barlas 2015; Jurewicz and Hanke 2011), children’s neurodevelopmental and behavioral problems (Whyatt et al. 2012; Ejaredar et al. 2015; Braun et al. 2013), and asthma and allergies (Bornehag et al. 2004b; Whyatt et al. 2014; Stelmach et al. 2015; Just et al. 2012; Hsu et al. 2012), although not consistently.
The prevalence of eczema in childhood, including its allergic-related phenotype, varies globally from 3% to 37% and is steadily increasing worldwide (Asher et al. 2006; Nutten 2015; Eichenfield et al. 2014; Deckers et al. 2012). The causes of eczema and of its increased prevalence are still unclear but are likely to be multifactorial in nature and depend on both genetic and environmental factors (Nutten 2015; Pyun 2015). During in utero life, the skin of the fetus may be exposed to various products absorbed by the mothers, including phthalates given that some phthalates have been detected in amniotic fluid (Jensen et al. 2015). Later in life, children’s dermal exposure can occur through the use of emollients, personal care products, and dermal contact with plastic products, soil, and dust, which can add to the total intake of certain phthalates through other routes such as inhalation and ingestion (Wormuth et al. 2006; Overgaard et al. 2017).
Eczema pathogenesis involves immunologic dysfunction and skin barrier defects (Pyun 2015). Phthalates might influence the epidermal barrier development processes, which can take several years to occur, and this influence could be stronger in allergic individuals due to the fragility of their mucosae. Although dermal absorption of phthalates and its impact on child health is established, contradictory results exist regarding eczema development after phthalate exposure in utero (Just et al. 2012; Smit et al. 2015; Weschler et al. 2015; Gong et al. 2015; Pan et al. 2014).
The objective of the present study was to evaluate the potential associations between maternal exposure to phthalates during the second trimester (between the 24th and 28th weeks) of pregnancy and occurrence of eczema phenotypes in male children in their first 5 y of life using data collected in the French EDEN mother–child prospective cohort study. The association was explored according to atopic status as defined by total immunoglobulin E (IgE) in a subsample of boys for which this assessment was available.
Methods
Study Population and Data Collection
The population in this study is a subgroup of the French EDEN (Etude des Déterminants pré et post natals du développement de la santé de l’Enfant) mother–child prospective birth cohort restricted to boys (https://eden.vjf.inserm.fr). The mothers’ recruitment and follow-up procedures and methodological assumptions have been published in earlier studies (Heude et al. 2015; Philippat et al. 2012). Briefly, women were given an appointment with a study midwife between the 24th and 28th gestational weeks, during which an interview on lifestyle factors was conducted and biological samples were collected. The information on the mothers and their newborns, including parity, mode of delivery, birth weight, gestational age, season of birth, behavioral, and environmental data were collected by the use of questionnaires, interviews, and obstetric and pediatric records. Boys were followed from birth to 5 y of age. The present study includes all male offspring for whom maternal urine samples had been analyzed for metabolites of phthalates as a part of previous studies (Chevrier et al. 2012; Mortamais et al. 2012; Botton et al. 2016), which explains why we had only boys in the study. Restricting to one sex could be an advantage in the context where sex-specific effects of exposures are expected (Harley et al. 2013; Wolff et al. 2008); a study restricted to one sex likely has a higher statistical power than a study including both.
Urine Collection and Analysis
Women were invited for a clinical examination and to provide a first morning urine at home before the hospital study visit between the 24th and 28th gestational weeks (second trimester of the pregnancy). If forgotten, the urine sample was collected at the hospital. A polypropylene container [FP40VPS; Centre Européen de Biotechnologie SA (CEB), Angers, France] was used. The urine samples were aliquoted and then frozen at . The analyses of the samples were conducted at the National Center for Environment Health Laboratory at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia (USA) for 11 metabolites of 8 phthalates: diethyl phthalate (DEP), di-n-butyl phthalate (DBP), di-isobutyl phthalate (diBP), di(2-ethylhexyl) phthalate (DEHP), benzylbutyl phthalate (BBzP), di-isononyl phthalate (diNP), di-n-octyl phthalate (DOP), and di-isodecyl phthalate (DiDP).
The targeted phthalate metabolites were monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), mono-isobutyl phthalate (MiBP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethylhexyl) phthalate (MEHP), monobenzyl phthalate (MBzP), monocarboxy-isooctyl phthalate (MCOP), mono(3-carboxypropyl) phthalate (MCPP), and monocarboxy-isononyl phthalate (MCNP).
Quantification of the phthalate metabolites concentrations was performed using on-line solid phase extraction–high-performance liquid chromatography–electrospray ionization isotope dilution–tandem mass spectrometry (Silva et al. 2007). Creatinine concentrations were also quantified following the same analytical procedure described previously (Mortamais et al. 2012). The concentrations of metabolites below the limit of detection (LOD) were replaced by instrumental reading values or by the compound-specific lowest instrumental reading value divided by the square root of 2 when the instrumental value was missing (Hornung and Reed 1990). The vast majority of the samples/metabolites () were above LOD. Two-step standardization was performed to reduce variability in the urinary concentrations of biomarkers due to sampling conditions, as described previously (Mortamais et al. 2012). There was no significant difference in the concentrations of phthalates in the urine samples collected at homes compared with those collected in the hospital.
Eczema Phenotypes
Eczema was assessed by administration of an enriched version of the International Study of Asthma and Allergies in Childhood (ISAAC) standardized and validated questionnaire (ISAAC Steering Committee 1998; Williams et al. 1999) to the parents at the children’s ages of 1, 2, 3, 4, and 5 y by appointment. At each year of follow-up, eczema was defined as eczema diagnosed by a doctor in the last 12 mo. Ever eczema was then defined as having ever reported a diagnosis of eczema at 1, 2, 3, 4, or 5 y of age. Additionally, two eczema phenotypes were distinguished: early-onset eczema if eczema was reported on any questionnaire in the first 2 y of life, and late-onset eczema if it was reported only between 24 and 60 mo of age.
IgE and Atopic Status
Total serum IgE was assessed in 293 boys at 5 y of age by using the CAP assay according to the manufacturers’ instructions (Pharmacia CAP System™, Pharmacia and Upjohn Diagnostics AB, Uppsala, Sweden). Boys were classified as atopic if they had IgE levels of (Baiz et al. 2017).
Other Variables
Information on potential confounders related to the children’s eczema included birth weight, gestational age (two-class variable: gestational week, gestational week), season of birth, parity, number of siblings (0, 1, 2, ), exclusive breast-feeding for (yes/no), maternal age at delivery (, 25–29 y, 30–34 y, ), pre-pregnancy maternal body mass index (BMI, , 18.5–24.9, 25.0–30.0, ), maternal and paternal history of allergies, maternal and paternal educational level ( after high school, , ), household income, city of residence (Nancy/Poitiers), mode of delivery (vaginal, cesarean section), smoking during pregnancy (yes/no), maternal alcohol use during pregnancy (yes/no), maternal and paternal physician-diagnosed cases of asthma, rhinitis, eczema, and food allergies (yes/no).
Statistical Analysis
Urinary concentrations of phthalate metabolites were log-transformed because of nonnormality of the distribution. For the purpose of present analysis, MECPP, MEHHP, MEOHP, and MEHP were grouped as the sum of (DEHP being their parent compound). We calculated the molar sum of these DEHP metabolites (nanomoles per milliliter) by dividing each metabolite concentration by its molecular weight and then summing the individual concentrations.
The prevalence and the incidence of eczema were computed. We applied multiple logistic regression analysis to examine the association between each phthalate metabolite and and eczema outcomes adjusted for potential confounders. The potential confounders were determined a priori from literature review. Then we used Directed Acyclic Graph (http://www.dagitty.net/dags.html) to show the hypothesis relations between biomarkers concentration, confounders, and eczema outcomes (see Figure S1). The confounders included in our final model were parental asthma/rhinitis/eczema, maternal smoking, maternal age, maternal BMI, maternal education level, gestational age, and number of siblings. Models were additionally adjusted for recruitment center.
We also applied the Cox proportional hazard discrete time survival model to estimate the association between in utero exposure to phthalates and eczema occurrence in the first 5 y of life after adjustment for the confounding factors. Results were further stratified in order to see whether phthalate biomarkers were associated with atopic (presence of allergic sensitization according to IgE) or nonatopic (absence of allergic sensitization to IgE) eczema. We also took IgE as an additional variable to see its association with phthalate biomarkers by applying multiple logistic regression analysis. Because our statistical analysis involved multiple comparisons so that it became increasingly likely that at least one comparison would have been statistically different due to random sampling error alone, the multiple testing hypothesis was considered using the Bonferroni test. All the statistical analysis was conducted using Stata statistical software (version 14; Stata Corporation), where was considered statistically significant.
Ethics Approval
The study was approved by the relevant ethical committees (Comité Consultatif pour la Protection des Personnes dans la Recherche Biomédicale, Le Kremlin Bicêtre University Hospital, and Commission Nationale de l’Informatique et des Libertés), and all participating women gave informed written consent for their own participation, and both parents for a newborn child after delivery.
Results
Characteristics of the Population
There were 998 male offspring in the cohort, among whom 604 had phthalates assessment. The participants included in this study analysis did not differ in major demographic characteristics from the other EDEN boys (Table 1) except for maternal age. However, results did not change when adjusting for maternal age in the final model. Average maternal age was 29.9 y, 26.3% women were overweight or obese (), and 85.8% did not smoke during pregnancy. There were 24 (4.0%) boys born before the 37th week of gestation, and 15 (3.0%) had a birth weight . Ninety-five (15.7%) mothers delivered through cesarean section. Urine was reported as being collected before 0800 hours 51.3% of the time, and between 0800 and 1200 hours 24.7% of the time. We also observed that during pregnancy 14.4% of the mothers had eczema, 10.9% had asthma, and 16.7% had rhinitis.
Table 1.
Characteristics of the study population and comparison with the entire EDEN cohort of boys.
| Characteristics | All male EDEN cohort () | Present study () | p-Value | ||
|---|---|---|---|---|---|
| % | % | ||||
| Maternal age (y) | |||||
| 200 | 20.0 | 79 | 13.0 | ||
| 25–29 | 378 | 37.9 | 218 | 36.1 | |
| 30–34 | 285 | 28.6 | 205 | 34.0 | |
| 135 | 13.5 | 102 | 16.9 | 0.0006 | |
| Parity | |||||
| 0 | 436 | 43.7 | 269 | 44.5 | |
| 1 | 374 | 37.5 | 222 | 36.8 | |
| 187 | 18.7 | 112 | 18.6 | ||
| Missing | 1 | 0.1 | 1 | 0.1 | 0.9 |
| BMI () | |||||
| 92 | 9.2 | 57 | 9.4 | ||
| 18.5–24.9 | 634 | 63.6 | 379 | 62.9 | |
| 25–30 | 171 | 17.1 | 110 | 18.3 | |
| 80 | 8.0 | 49 | 8.1 | ||
| Missing | 21 | 2.1 | 9 | 1.5 | 0.8 |
| Maternal education | |||||
| after high school | 459 | 46.0 | 263 | 43.5 | |
| 219 | 21.9 | 132 | 21.9 | ||
| 297 | 29.8 | 199 | 32.9 | ||
| Missing | 23 | 2.3 | 10 | 1.7 | 0.4 |
| Active smoking (cigarettes/day) | |||||
| 0 | 827 | 82.9 | 518 | 86.0 | |
| 1–5 | 82 | 8.2 | 45 | 7.5 | |
| 86 | 8.6 | 37 | 6.2 | ||
| Missing | 3 | 0.3 | 2 | 0.3 | 0.2 |
| Passive smoking | |||||
| Yes | 278 | 27.9 | 192 | 31.8 | |
| No | 711 | 71.2 | 405 | 67.1 | |
| Missing | 9 | 0.9 | 7 | 1.1 | 0.2 |
| Alcohol during pregnancy | |||||
| Yes | 278 | 27.9 | 151 | 25.0 | |
| No | 714 | 71.5 | 450 | 74.5 | |
| Missing | 6 | 0.6 | 3 | 0.5 | 0.4 |
| Center | |||||
| Poitiers | 533 | 53.4 | 347 | 57.4 | |
| Nancy | 465 | 46.6 | 257 | 42.6 | 0.1 |
| Gestational age | |||||
| gestational week | 63 | 6.3 | 24 | 4.0 | |
| gestational week | 935 | 93.7 | 580 | 96.0 | 0.04 |
| Birth weight | |||||
| 47 | 4.7 | 15 | 2.5 | ||
| 951 | 95.3 | 589 | 97.5 | 0.02 | |
| Year of birth | |||||
| 2003–2004 | 560 | 56.1 | 330 | 54.6 | |
| 2005–2006 | 438 | 43.9 | 274 | 45.4 | 0.5 |
| Breast-feeding | |||||
| Yes | 720 | 72.2 | 432 | 71.5 | |
| No | 270 | 27.0 | 172 | 28.5 | |
| Missing | 8 | 0.8 | 0 | 0 | 0.07 |
| Employment | |||||
| Yes | 727 | 72.9 | 458 | 75.9 | |
| No | 262 | 26.2 | 142 | 23.5 | |
| Missing | 9 | 0.90 | 4 | 0.6 | 0.3 |
| Cesarean section | |||||
| Yes | 175 | 17.5 | 95 | 15.7 | |
| No | 822 | 82.4 | 509 | 84.3 | |
| Missing | 1 | 0.1 | 0 | 0 | 0.4 |
| Marital status | |||||
| Married | 534 | 53.6 | 329 | 54.5 | |
| Unmarried/single | 435 | 43.5 | 257 | 42.6 | |
| Divorced | 19 | 1.9 | 12 | 1.9 | |
| Separated | 2 | 0.2 | 2 | 0.3 | |
| Widowed | 2 | 0.2 | 1 | 0.2 | |
| Missing | 6 | 0.6 | 3 | 0.5 | 0.9 |
| Season of urine sample collection | |||||
| Winter | 262 | 26.2 | 189 | 31.3 | |
| Spring | 301 | 30.2 | 146 | 24.2 | |
| Summer | 249 | 24.9 | 129 | 21.3 | |
| Fall | 186 | 18.7 | 140 | 23.2 | 0.002 |
| Time of sampling | |||||
| 2400–0759 hours | 500 | 50.1 | 310 | 51.3 | |
| 0800–1159 hours | 272 | 27.3 | 149 | 24.7 | |
| 1200–1900 hours | 7 | 0.7 | 5 | 0.8 | |
| Missing | 219 | 21.9 | 140 | 23.2 | 0.7 |
| Maternal history of asthma | |||||
| Yes | 110 | 11.0 | 66 | 10.9 | |
| No | 884 | 88.6 | 536 | 88.7 | |
| Missing | 4 | 0.4 | 2 | 0.3 | 0.9 |
| Maternal history of eczema | |||||
| Yes | 142 | 14.2 | 87 | 14.4 | |
| No | 852 | 85.4 | 515 | 85.3 | |
| Missing | 4 | 0.4 | 2 | 0.3 | 0.9 |
| Maternal history of rhinitis | |||||
| Yes | 167 | 16.7 | 101 | 16.7 | |
| No | 827 | 82.9 | 501 | 83.0 | |
| Missing | 4 | 0.4 | 2 | 0.3 | 0.9 |
| Paternal history of asthma | |||||
| Yes | 99 | 9.9 | 58 | 9.6 | |
| No | 895 | 89.7 | 544 | 90.1 | |
| Missing | 4 | 0.4 | 2 | 0.3 | 0.9 |
| Paternal history of eczema | |||||
| Yes | 48 | 4.8 | 24 | 4.0 | |
| No | 946 | 94.8 | 578 | 95.7 | |
| Missing | 4 | 0.4 | 2 | 0.3 | 0.7 |
| Paternal history of rhinitis | |||||
| Yes | 100 | 10.0 | 60 | 9.9 | |
| No | 894 | 89.6 | 542 | 89.8 | |
| Missing | 4 | 0.4 | 2 | 0.3 | 0.3 |
Note: BMI; body mass index.
Eczema Prevalence and Incidence
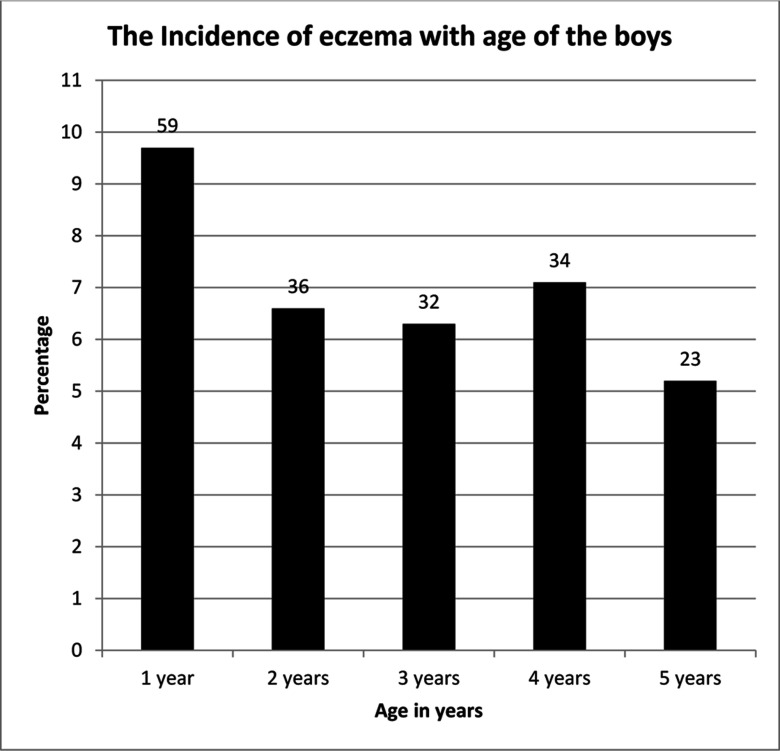
The prevalence of ever eczema was 9.7%, 15.7%, 21.0%, 26.6%, and 30.4% at 1, 2, 3, 4, and 5 y of age, respectively. The incidence decreased from 9.7% at 1 y of age to 5.2% at 5 y of age (Figure 1). The prevalence of early-onset and late-onset eczema were 15.7% and 14.7%, respectively.
Figure 1:
The incidence of eczema with age of the boys. The y-axis shows the percentage, and the numbers above each bar indicate the number of boys diagnosed at each year of age.
Prenatal Exposure to Phthalates
Phthalate metabolites were detected in the urine of more than 97% of the mothers. MEP had the highest geometric mean (), whereas MCNP had the lowest (). The LOD ranged from 0.2 to . A summary of detection frequency as well as geometric means and select percentiles of urinary concentrations of phthalate metabolites are outlined in Table 2.
Table 2.
Distribution of phthalate metabolites concentration () in maternal prenatal urine ().
| Parent compound | Abbrev | Phthalate metabolites () | Abbrev | LOD () | GM | Minimum | Percentile | Maximum | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||||||||
| Diethyl phthalate | DEP | Monoethyl phthalate | MEP | 0.6 | 100 | 110.7 | 7.9 | 21 | 51 | 96 | 195 | 714 | 4093.4 |
| Di-n-butyl phthalate | DBP | Mono-n-butyl phthalate | MBP | 0.2 | 100 | 51.6 | 2.8 | 12 | 28 | 43 | 73 | 438 | 2418.8 |
| Di-isobutyl phthalate | DiBP | Mono-isobutyl phthalate | MiBP | 0.2 | 100 | 42.7 | 1.5 | 12 | 25 | 39 | 69 | 166 | 689.9 |
| Di(2-ethylhexyl) phthalate | DEHP | Mono(2-ethyl-5-carboxypentyl) phthalate | MECPP | 0.2 | 100 | 40.5 | 2.6 | 12 | 25 | 38 | 62 | 160 | 2640.5 |
| Di(2-ethylhexyl) phthalate | DEHP | Mono(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | 0.2 | 100 | 26.9 | 1.7 | 6.8 | 16 | 27 | 44 | 103 | 2103.8 |
| Di(2-ethylhexyl) phthalate | DEHP | Mono(2-ethyl-5-oxohexyl) phthalate | MEOHP | 0.2 | 99.7 | 22.1 | 0.6 | 5.6 | 14 | 23 | 36 | 84 | 1441.4 |
| Di(2-ethylhexyl) phthalate | DEHP | Mono(2-ethylhexyl) phthalate | MEHP | 0.5 | 98 | 7.2 | 0.1 | 1.6 | 4.4 | 7.7 | 15 | 35 | 292.3 |
| Benzylbutyl phthalate | BBzP | Monobenzyl phthalate | MBzP | 0.3 | 100 | 19.7 | 1.6 | 4.5 | 11 | 18 | 33 | 104 | 970.4 |
| Di-isononyl phthalate | DiNP | Monocarboxy-isooctyl phthalate | MCOP | 0.2 | 99 | 4.1 | 0.1 | 1.2 | 2.4 | 3.9 | 6.5 | 19 | 375.3 |
| Di-n-octyl phthalate | DOP | Mono(3-carboxypropyl) phthalate | MCPP | 0.2 | 100 | 2.1 | 0.1 | 0.7 | 1.3 | 1.9 | 3.4 | 9.1 | 78.7 |
| Di-isodecyl phthalate | DiDP | Monocarboxy-isononyl phthalate | MCNP | 0.2 | 97.5 | 1.5 | 0.1 | 0.5 | 0.8 | 1.2 | 2.2 | 9.5 | 686.8 |
Note: Abbrev, abbreviation; GM, geometric mean; LOD, limit of detection.
Spearman correlation coefficients between ln-transformed phthalate metabolites urinary concentrations are shown in Table S1. The majority of biomarkers were positively correlated except for the correlation between MEP and MiBP, MECPP, MEHHP, MCOP, MCPP, or MCNP. All the other biomarkers were significantly correlated with each other (with correlation coefficients ranging from , to , ). As expected, DEHP metabolites were highly correlated with each other (, ).
Associations between Phthalates Metabolites and Eczema Phenotypes
Table 3 presents the relationship of prenatal phthalate exposure (based on phthalate metabolite urinary concentrations) to the occurrence of ever eczema outcomes in boys between 1 and 5 y of age. Based on a multiple logistic regression model that was adjusted for confounding factors, we observed an increased risk of eczema at 1 y of age in association with increased MEP concentrations [odds ratio ; 95% confidence interval (CI): 1.05, 2.73; ] and at 1, 3, 4, and 5 y of age for MiBP.
Table 3.
Associations {adjusted odds ratio and 95% confidence interval [OR (95% CI)]} between maternal urinary concentrations of phthalate metabolites and occurrence of eczema phenotypes in boys ().
| Year, eczema | MEP | MBP | MiBP | MECPP | MEHHP | MEOHP | MEHP | MBzP | MCOP | MCPP | MCNP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.69 (1.05, 2.73)* | 1.17 (0.74, 1.84) | 1.78 (0.95, 3.33)† | 1.04 (0.55, 1.98) | 1.06 (0.61, 1.85) | 1.07 (0.60, 1.92) | 1.06 (0.61, 1.83) | 1.10 (0.60, 2.00) | 1.25 (0.70, 2.20) | 1.15 (0.66, 2.00) | 0.88 (0.59, 1.32) | 1.01 (0.87, 1.18) |
| 2 | 1.08 (0.74, 1.57) | 1.12 (0.76, 1.63) | 1.15 (0.64, 2.09) | 0.94 (0.58, 1.52) | 0.94 (0.60, 1.46) | 0.97 (0.61, 1.54) | 0.88 (0.57, 1.36) | 1.14 (0.70, 1.87) | 0.85 (0.52, 1.37) | 1.18 (0.75, 1.87) | 0.84 (0.55, 1.29) | 0.98 (0.87, 1.10) |
| 3 | 1.46 (0.89, 2.40) | 1.12 (0.74, 1.69) | 1.81 (0.94, 3.49)† | 0.72 (0.38, 1.34) | 0.88 (0.50, 1.54) | 0.90 (0.50, 1.60) | 0.94 (0.61, 1.47) | 1.14 (0.70, 1.86) | 1.28 (0.79, 2.09) | 1.09 (0.64, 1.85) | 1.61 (1.00, 2.59)* | 0.96 (0.84, 1.11) |
| 4 | 1.03 (0.79, 1.33) | 1.15 (0.87, 1.85) | 1.68 (1.16, 2.45)* | 1.32 (0.96, 1.83)† | 1.29 (0.95, 1.74)† | 1.30 (0.95, 1.77)† | 1.21 (0.91, 1.61) | 1.24 (0.91, 1.69) | 1.37 (0.99, 1.89)† | 1.11 (0.79, 1.56) | 1.22 (0.95, 1.57) | 1.07 (0.99, 1.15)† |
| 5 | 1.10 (0.86, 1.42) | 1.10 (0.83, 1.47) | 1.63 (1.12, 2.36)* | 1.46 (1.04, 2.06)* | 1.32 (0.96, 1.81)† | 1.33 (0.96, 1.85)† | 1.38 (1.03, 1.85)* | 1.30 (0.96, 1.76)† | 1.60 (1.16, 2.23)* | 1.19 (0.84, 1.69) | 1.37 (1.04, 1.80)* | 1.08 (1.00, 1.18)* |
| Early-onset eczema | 1.06 (0.89, 1.25) | 1.10 (0.92, 1.32) | 1.27 (1.00, 1.72)* | 1.03 (0.77, 1.36) | 1.02 (0.79, 1.32) | 1.04 (0.80, 1.36) | 1.06 (0.85, 1.32) | 1.13 (0.88, 1.47) | 1.29 (1.04, 1.60)* | 1.18 (0.93, 1.49) | 1.08 (0.90, 1.30) | 0.98 (0.92, 1.04) |
| Late-onset eczema | 1.13 (0.90, 1.42) | 1.17 (0.96, 1.42) | 1.55 (1.10, 2.18)* | 1.34 (1.00, 1.81)* | 1.26 (0.96, 1.67)† | 1.28 (0.96, 1.70)† | 1.18 (0.92, 1.52) | 1.19 (0.90, 1.57) | 1.63 (1.20, 2.21)* | 1.14 (0.83, 1.57) | 1.29 (1.02, 1.64)* | 1.06 (0.99, 1.13)† |
Note: Models were adjusted for parental asthma/rhinitis/eczema, maternal smoking, maternal age, maternal BMI, maternal education level, gestational age, number of siblings, and recruitment center. Note: BMI, body mass index; CI, confidence interval; DEHP, di(2-ethylhexyl) phthalate; MBP, mono-n-butyl phthalate; MBzP, Monobenzyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCOP, monocarboxy-isooctyl phthalate; MCPP, mono (3-carboxypropyl) phthalate; MECPP, mono (2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono (2-ethylhexyl) phthalate; MEOHP, mono (2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate; OR, odds ratio.
,
.
MBP and MCPP did not show significant associations at any age. MEHHP and MEOHP showed borderline significance at 4 and 5 y of age, respectively; whereas MECPP was of borderline significance at 4 y of age and significant at 5 y of age. MEHP was only significant at 5 y of age [ (95% CI: 1.03, 1.85), ]. was also of borderline significance at 4 y of age and significant at 5 y of age. Interestingly, MBzP was only found to be marginally significant at 5 y [ (95% CI: 0.96, 1.76), ]. We also observed that MCOP was marginally significant at 4 y of age and significant at 5 y of age, whereas MCNP showed significant results at 3 and 5 y of age.
In both phenotypes of eczema, MiBP and MCOP were positively associated with early-onset eczema [ (95% CI: 1.00, 1.72), and (95% CI: 1.04, 1.60), , respectively] as well as late-onset eczema [ (95% CI: 1.10, 2.18), and (95% CI: 1.20, 2.21), , respectively] after adjusting for potential confounders (Table 3). Other phthalate metabolites were not significantly associated with early-onset eczema. However in the case of late-onset eczema, MECPP and MCNP were positively associated [ (95% CI: 1.00, 1.81), and (95% CI: 1.02, 1.64), , respectively], whereas the association with MEHHP, MEOHP, and were of borderline significance ().
We also applied survival analysis to compute the hazard ratio (HR) of the occurrence of eczema in the first 5 y of life with respect to exposure to phthalates (Table 4). Both MiBP [adjusted (95% CI: 1.01, 1.34), ] and MCOP [ (95% CI: 0.95, 1.25), ] were related to ever eczema after adjustment for potential confounders. No other phthalate was associated with the occurrence of eczema in this analysis.
Table 4.
Association (hazard ratio and 95% confidence interval) between maternal urinary concentrations of phthalate metabolites and occurrence of ever eczema in boys ().
| Phthalate metabolite | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HRb | 95% CI | p-Value | HR | 95% CI | p-Value | |
| MEP | 1.04 | 0.93, 1.15 | 0.47 | 1.04 | 0.94, 1.16 | 0.42 |
| MBP | 1.00 | 0.89, 1.12 | 0.96 | 1.03 | 0.92, 1.15 | 0.60 |
| MiBP | 1.16 | 1.01, 1.33* | 0.03 | 1.16 | 1.01, 1.34* | 0.03 |
| MECPP | 0.97 | 0.84, 1.12 | 0.73 | 0.98 | 0.84, 1.13 | 0.77 |
| MEHHP | 0.99 | 0.86, 1.13 | 0.90 | 0.99 | 0.88, 1.14 | 0.93 |
| MEOHP | 1.01 | 0.88, 1.15 | 0.91 | 1.01 | 0.88, 1.16 | 0.86 |
| MEHP | 0.96 | 0.86, 1.07 | 0.50 | 0.96 | 0.85, 1.08 | 0.47 |
| MBzP | 0.99 | 0.88, 1.12 | 0.99 | 1.02 | 0.90, 1.15 | 0.69 |
| MCOP | 1.04 | 0.91, 1.19 | 0.48 | 1.09 | 0.95, 1.25† | 0.05 |
| MCPP | 1.02 | 0.88, 1.17 | 0.81 | 1.05 | 0.91, 1.22 | 0.50 |
| MCNP | 1.02 | 0.90, 1.14 | 0.74 | 1.03 | 0.92, 1.17 | 0.56 |
| 0.99 | 0.96, 1.03 | 0.77 | 0.98 | 0.94, 1.03 | 0.57 | |
Note: CI, confidence interval; DEHP, di(2-ethylhexyl) phthalate; HR, hazard ratio; MBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxy-isononyl phthalate; MCOP, monocarboxy-isooctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
,
.
HR adjusted for parental asthma/rhinitis/eczema, maternal smoking, maternal age, maternal BMI, maternal education level, gestational age, number of siblings, and recruitment center.
HR according to the proportional Cox’s model.
Associations between Phthalate Metabolites and Ever Eczema According to Atopic Status
We found significant associations between total IgE and phthalate metabolites, including () and its metabolites MECPP (), MEHHP (), MEOHP (), and MEHP (). We further stratified eczema outcome by atopic status. Of the 293 boys tested for IgE, we observed that 60 (20.48%) were sensitized and 233 (79.52%) were not. Table S2 shows the associations between maternal urinary concentrations of phthalate metabolite and eczema outcomes in boys according to their atopic status. Among IgE-sensitized boys, MBP and MiBP were significantly associated with ever eczema [ (95% CI: 1.10, 2.54), and (95% CI: 1.01, 3.48), , respectively]. and MECPP, MEHHP, MEOHP, and MEHP individually were also positively associated with ever eczema in sensitized boys. MBzP was partially associated [ (95% CI: 0.98, 2.20), ]. However, the association for MEP, MCOP, MCPP, and MCNP was not significant. We did not find any association between prenatal phthalate exposure and eczema in boys without IgE sensitization. Using the multiple hypothesis testing did not modify the results except for MBP, MiBP, and MBzP, for which only a trend was observed.
Discussion
Major Findings
In this study, we report an association between maternal prenatal urinary concentration of metabolites of DiBP and DiNP and eczema development in offspring preschool boys followed-up between birth and 5 y of age. Metabolites of DiBP and DiNP were significantly associated with both phenotypes of eczema (early and late onset) (Table 4). DiBP is used in nitrocellulose plastic, nail polish, lacquer manufacturing and used with methyl methacrylate applications, whereas DiNP is used in a diverse range of industrial products (such as electrical wire and cables, flexible PVC sheeting, coated fabrics, automotive parts), building and construction, and several categories of toys (plastic books, balls, dolls, and cartoon characters) (Johns et al. 2015).
MECPP, a DEHP metabolite, and MCNP, a DiDP metabolite, were positively associated with late-onset eczema, whereas MEHHP and MEOHP, two metabolites of DEHP, were marginally associated. Our study also suggests a significant association between prenatal exposure to various phthalates and allergic eczema in boys at 5 y of age based on IgE levels. The metabolites of DBP, DiBP, and DEHP were significantly associated with ever eczema in IgE-sensitized boys, and borderline significance was associated with MBzP, the major BBzP metabolite [ (95% CI: 0.98, 2.20), ].
Comparison of Findings with Other Published Work
Several studies have reported a potential relationship between phthalates exposure and asthma (Bornehag et al. 2004b; Hsu et al. 2012; Smit et al. 2015; Ait Bamai et al. 2014) and eczema (Bornehag et al. 2004b; Stelmach et al. 2015; Just et al. 2012; Hsu et al. 2012; Smit et al. 2015; Ait Bamai et al. 2014) related symptoms in children. However, most of these studies used a case–control (Bornehag et al. 2004a, 2004b, 2005, 2006) or cross-sectional (Hsu et al. 2012; Ait Bamai et al. 2014; Hoppin et al. 2013) design or relied on phthalate levels in dust as a marker of phthalate exposure (Bornehag et al. 2005; Ait Bamai et al. 2014; Langer et al. 2014; Kolarik et al. 2008). This limits the conclusions that can be drawn because dust is not the only source of exposure to these chemicals.
In a birth cohort study in New York City where maternal urine was collected during the third trimester of pregnancy, MBzP, the major BBzP metabolite, was associated with the development of eczema by 5 y of age in 407 children (Just et al. 2012). In Sweden, an association between BBzP levels in dust and atopic dermatitis was observed in cross-sectional data (Bornehag et al. 2004b). In a cross-sectional study among 101 Taiwanese children between 3 and 9 y of age, BBzP levels in house dust were associated with eczema and asthma (Hsu et al. 2012). However, in the present study we observed only partial association between the main metabolite of BBzP and eczema at 5 y of age, even in boys with allergic eczema. In our study the metabolites of DiBP and DiNP were significantly associated with early-onset and late-onset eczema as well as ever eczema in boys. In contrast, in a birth cohort with 1,024 mother–child pairs from Greenland and Ukraine, oxidative metabolites of DiNP in maternal serum were negatively associated with current eczema as well as with ever eczema in boys and girls from Greenland (Smit et al. 2015). One limitation of that study is that it was conducted in children between 5 and 9 y of age, because approximately 40–70% of childhood eczema cases resolve by the time the children reach 6–7 y of age (Pyun 2015).
Although phthalates are not highly sensitizing, they may promote the development of allergy or eczema (Wang and Karmaus 2015). Studies on mouse models have shown that phthalates, including DEHP and DiNP at low doses, can aggravate atopic dermatitis/eczema-like skin lesions by direct or indirect activation of the immune cells, which can be responsible, at least in part, for the increase in the prevalence of atopic dermatitis/eczema (Koike et al. 2010; Sadakane et al. 2014; Yanagisawa et al. 2008). To date, a large amount of work has been done on DEHP and allergy; DEHP is banned in many industrialized countries in PVC toys that may be placed in the mouth by children of age (Sadakane et al. 2014). DiNP is a substitute for DEHP, and this might be a reason for its increasing levels with time and the adverse effects associated with DiNP.
A case–control study from Taipei, Taiwan, on the effects of phthalate exposure and filaggrin gene variants on atopic dermatitis showed that children with specific filaggrin genotypes had higher urinary concentrations of phthalates, suggesting that such genotypes may increase skin permeability and so may lead to the higher absorption of phthalates and result in increased susceptibility to atopic dermatitis in such children (Wang and Karmaus 2015). In terms of mechanisms, phthalates might impair the epidermal barrier development processes. In addition, skin barrier defects caused by filaggrin gene mutations may allow allergens or irritants to penetrate the epidermis and to interact with antigen-presenting cells, phenomena which lead to the development of atopic dermatitis (Osawa et al. 2011; van den Oord and Sheikh 2009). A Danish study in children between 3 and 5 y of age found a relationship between atopic dermatitis and MEP in urine, but only in the fourth quartile, and no association with MiBP, a DiBP metabolite, while other metabolites of DiNP were not investigated (Callesen et al. 2014). Our study shows associations between prenatal phthalate exposure and atopy as assessed by total IgE at 5 y of age based on total IgE levels; however, findings in a study of another birth cohort did not observe an association based on specific IgE levels (Gascon et al. 2015). In our study, the information on atopy was available for only 48.5% of the study population; hence the results should be interpreted with caution. Studies have suggested that two-thirds of the cases with eczema do not have measurable levels of circulating IgE antibodies (Flohr et al. 2004). In our study, about one-third (32%) of the boys had allergic eczema and two-thirds (68%) had nonallergic eczema.
Strengths and Limitations
Our study has several strengths. The population-based prospective cohort design with nutritional, environmental, biological, and social factors starting during prenatal life is a strength of our study that allowed us to control for potential confounders and to assess exposure before the health outcomes. Furthermore, to reduce subject variability, values of the exposure biomarkers were standardized for urine sampling conditions (Mortamais et al. 2012). However, we were limited to one urine sample per mother during the second trimester of pregnancy; thus we were unable to assess the variability in concentrations of each metabolite across the pregnancy. But it is also not clearly known at what point(s) in gestation the infants are at higher risk of exposure. Other studies have also used second-trimester maternal urinary concentration for biomarkers (Whyatt et al. 2014; Gascon et al. 2015; Bertelsen et al. 2013). Consistency in urinary concentrations during pregnancy has been observed for some environmental exposure biomarkers (Muckle et al. 2001; Longnecker et al. 1999), and other studies have suggested that phthalate biomarkers are relatively stable for a period of weeks to months (Hoppin et al. 2002; Hauser et al. 2004; Teitelbaum et al. 2008). Increasing the number of urine samples collected would have provided a more accurate estimate of the average exposure during the gestation period. We also adjusted for many potential confounders; however, residual confounding (if any) cannot be ruled out.
Regarding exposure assessment, it might be important to explore other techniques for assessment of the exposure, particularly those that may offer a more exhaustive and coordinated picture of the individual environment, for instance, by assessing the use of particular items in conjunction with indoor air levels and additional biomarkers of exposure.
Previous studies have reveal sex/gender-specific relationships between phthalates and health (Bertelsen et al. 2013; Vaidya and Kulkarni 2012). In our study, we considered only male offspring because the initial purpose was to see the effects of phthalates on malformations of male genitalia (Philippat et al. 2012). Although considering only males limits the generalization of our results, it is not a source of bias. From a statistical point of view, focusing on one sex is another way to increase the study accuracy when the sample size is limited.
The questionnaire data were self-reported and thus could have been subject to misclassification; in addition, recall bias was a possibility that might in part account for the high prevalence of eczema in our study population. The prevalence of early- and late-onset eczema in the present study was 15.7% and 14.7%, respectively, both of which are higher than that found (12.2%) in a cohort study from Poland in children at 2 y of age (Stelmach et al. 2015). However, a study by Augustin et al. (2015) showed a prevalence of 17.13% and 12.87% in children between 0–2 and 3–6 y of age, respectively.
Creatinine correction is most often used for urinary biomarkers of phthalates, phenols, and pesticides. There are limitations to the usage to creatinine to normalize for urine dilution; different investigators have used specific gravity as an alternative to creatinine to adjust phthalate urinary biomarkers for urine dilution (Just et al. 2012; Whyatt et al. 2014), but we did not have specific gravity measurements. Nevertheless, specific gravity is highly correlated with creatinine (Barr et al. 2005) and, consequently, we likely overcorrected for urine dilution.
The exposures we studied are relatively prevalent, and some biomarker urinary concentrations approach those with significant effects in experimental models. In a healthy cohort such as ours, effects of hormonally active environmental exposures on skin may be small, yet a continuous exposure can have adverse health effects.
A further dimension to consider in future research is multiple exposures of hormonally active agents such as these phthalates. In terms of prevention, exposure to these chemicals can be avoided if the product contents are already known or are listed on the product label; unfortunately, they are often not listed on the label because of their identification as inactive ingredients.
Conclusions
Our data provide new suggestive evidence that prenatal exposure to certain phthalates, including DiBP and DiNP, may play a role in the development of eczema in early childhood. Future research focusing on larger populations and addressing multiple exposures assessed prenatally and postnatally are required to provide more evidence on possible contributions of emerging pollutants to study the role of phthalates on eczema in children.
Supplemental Material
Acknowledgments
The EDEN Mother–Child Cohort Study Group: I. Annesi-Maesano, J. Botton, M.A. Charles, P. Dargent-Molina, B. de Lauzon-Guillain, P. Ducimetiere, M. de Agostini, B. Folifuet, A. Forhan, X. Fritel, A. Germa, V. Goua, R. Hankard, B. Heude, M. Kaminski, B. Larroque, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, R. Slama, M.J. Saurel-Cubizolles, M. Schweitzer, and O. Thiebaugeorge.
We acknowledge all funding sources for the EDEN study: Foundation for Medical Research (FRM), National Agency for Research (ANR), National Institute for Research in Public Health (IRESP: TGIR cohorte santé 2008 program), French Ministry of Health (DGS), French Ministry of Research, Inserm Bone and Joint Diseases National Research (PRO-A) and Human Nutrition National Research Programs, Paris–Sud University, Nestlé, French National Institute for Population Health Surveillance (InVS), French National Institute for Health Education (INPES), the European Union FP7 programmes (FP7/2007-2013, HELIX, ESCAPE, ENRIECO, MEDall projects), Diabetes National Research Program [through a collaboration with the French Association of Diabetic Patients (AFD)], French Agency for Environmental Health Safety (now ANSES), Mutuelle Générale de l’Education Nationale (MGEN), French National Agency for Food Security, Health and Environment-wide Associations based on Large population Surveys (HEALS), and the French-speaking association for the study of diabetes and metabolism (ALFEDIAM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Abdel-Maksoud FM, Leasor KR, Butzen K, Braden TD, Akingbemi BT. 2015. Prenatal exposures of male rats to the environmental chemicals bisphenol A and di(2-ethylhexyl) phthalate impact the sexual differentiation process. Endocrinology 156(12):4672–4683, PMID: 26372177, 10.1210/en.2015-1077. [DOI] [PubMed] [Google Scholar]
- Ait Bamai Y, Shibata E, Saito I, Araki A, Kanazawa A, Morimoto K. 2014. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci Total Environ 485–486:153–163, PMID: 24704966, 10.1016/j.scitotenv.2014.03.059. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, et al. 2014. Phthalate and bisphenol A exposure among pregnant women in Canada—results from the MIREC study. Environ Int 68:55–65, PMID: 24709781, 10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368(9537):733–743, PMID: 16935684, 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Augustin M, Radtke MA, Glaeske G, Reich K, Christophers E, Schaefer I, et al. 2015. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology (Basel) 231(1):35–40, PMID: 25966818, 10.1159/000381913. [DOI] [PubMed] [Google Scholar]
- Aydoğan Ahbab M, Barlas N. 2015. Influence of in utero di-n-hexyl phthalate and dicyclohexyl phthalate on fetal testicular development in rats. Toxicol Lett 233(2):125–137, PMID: 25637754, 10.1016/j.toxlet.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Baiz N, Macchiaverni P, Tulic MK, Rekima A, Annesi-Maesano I, Verhasselt V. 2017. Early oral exposure to house dust mite allergen through breast milk: a potential risk factor for allergic sensitization and respiratory allergies in children. J Allergy Clin Immunol 139(1):369–372.e10, PMID: 27566456, 10.1016/j.jaci.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200, PMID: 15687057, 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen RJ, Carlsen KCL, Calafat AM, Hoppin JA, Håland G, Mowinckel P, et al. 2013. Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environ Health Perspect 121(2):251–256, PMID: 23164678, 10.1289/ehp.1205256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L, Sundell J. 2005. Phthalates in indoor dust and their association with building characteristics. Environ Health Perspect 113(10):1399–1404, PMID: 16203254, 10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Sigsgaard T. 2004a. Dampness in buildings and health (DBH): report from an ongoing epidemiological investigation on the association between indoor environmental factors and health effects among children in Sweden. Indoor Air 14 (suppl 7):59–66, PMID: 15330773, 10.1111/j.1600-0668.2004.00274.x. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Sigsgaard T, Janson S. 2006. Potential self-selection bias in a nested case-control study on indoor environmental factors and their association with asthma and allergic symptoms among pre-school children. Scand J Public Health 34(5):534–543, PMID: 16990165, 10.1080/14034940600607467. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, et al. 2004b. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case–control study. Environ Health Perspect 112(14):1393–1397, PMID: 15471731, 10.1289/ehp.7187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton J, Philippat C, Calafat AM, Carles S, Charles MA, Slama R. 2016. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ Res 151:601–609, PMID: 27596487, 10.1016/j.envres.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Sathyanarayana S, Hauser R. 2013. Phthalate exposure and children’s health. Curr Opin Pediatr 25(2):247–254, PMID: 23429708, 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callesen M, Bekö G, Weschler CJ, Langer S, Brive L, Clausen G, et al. 2014. Phthalate metabolites in urine and asthma, allergic rhinoconjunctivitis and atopic dermatitis in preschool children. Int J Hyg Environ Health 217(6):645–652, PMID: 24388279, 10.1016/j.ijheh.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Chevrier C, Petit C, Philippat C, Mortamais M, Slama R, Rouget F, et al. 2012. Maternal urinary phthalates and phenols and male genital anomalies. Epidemiology 23(2):353–356, PMID: 22317818, 10.1097/EDE.0b013e318246073e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. 2012. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS One 7(7):e39803, PMID: 22808063, 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. 2014. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 70(2):338–351, PMID: 24290431, 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. 2015. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ Res 142:51–60, PMID: 26101203, 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Flohr C, Johansson SG, Wahlgren CF, Williams H. 2004. How atopic is atopic dermatitis? J Allergy Clin Immunol 114(1):150–158, PMID: 15241359, 10.1016/j.jaci.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Gascon M, Casas M, Morales E, Valvi D, Ballesteros-Gómez A, Luque N, et al. 2015. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol 135(2):370–378, PMID: 25445825, 10.1016/j.jaci.2014.09.030. [DOI] [PubMed] [Google Scholar]
- Gong M, Weschler CJ, Liu L, Shen H, Huang L, Sundell J, et al. 2015. Phthalate metabolites in urine samples from Beijing children and correlations with phthalate levels in their handwipes. Indoor Air 25(6):572–581, PMID: 25557639, 10.1111/ina.12179. [DOI] [PubMed] [Google Scholar]
- Harley KG, Schall RA, Chevrier J, Tyler K, Aguirre H, Bradman A, et al. 2013. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect 121(4):514–520, PMID: 23416456, 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. 2004. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 112(17):1734–1740, PMID: 15579421, 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heude B, Forhan A, Slama R, Douhaud L, Bedel S, Saurel-Cubizolles MJ, et al. 2015. Cohort profile: the EDEN mother–child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol, PMID: 26283636, 10.1093/ije/dyv151. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. 2002. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect 110(5):515–518, PMID: 12003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Jaramillo R, London SJ, Bertelsen RJ, Salo PM, Sandler DP, et al. 2013. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005–2006. Environ Health Perspect 121(10):1129–1134, PMID: 23799650, 10.1289/ehp.1206211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Hsu NY, Lee CC, Wang JY, Li YC, Chang HW, Chen CY, et al. 2012. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 22(3):186–199, PMID: 21995786, 10.1111/j.1600-0668.2011.00753.x. [DOI] [PubMed] [Google Scholar]
- ISAAC (International Study of Asthma and Allergies in Childhood) Steering Committee. 1998. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J 12(2):315–335, PMID: 9727780. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, Jönsson BA, Bonde JP, Hougaard DM, et al. 2015. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology 26(1):91–99, PMID: 25384265, 10.1097/EDE.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A, Meeker JD. 2015. Exposure assessment issues in epidemiology studies of phthalates. Environ Int 85:27–39, PMID: 26313703, 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. 2011. Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int J Occup Med Environ Health 24(2):115–141, PMID: 21594692, 10.2478/s13382-011-0022-2. [DOI] [PubMed] [Google Scholar]
- Just AC, Whyatt RM, Perzanowski MS, Calafat AM, Perera FP, Goldstein IF, et al. 2012. Prenatal exposure to butylbenzyl phthalate and early eczema in an urban cohort. Environ Health Perspect 120(10):1475–1480, PMID: 22732598, 10.1289/ehp.1104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike E, Yanagisawa R, Sadakane K, Inoue K, Ichinose T, Takano H. 2010. Effects of diisononyl phthalate on atopic dermatitis in vivo and immunologic responses in vitro. Environ Health Perspect 118(4):472–478, PMID: 20064775, 10.1289/ehp.0901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik B, Naydenov K, Larsson M, Bornehag CG, Sundell J. 2008. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ Health Perspect 116(1):98–103, PMID: 18197306, 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S, Bekö G, Weschler CJ, Brive LM, Toftum J, Callesen M, et al. 2014. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int J Hyg Environ Health 217(1):78–87, PMID: 23623597, 10.1016/j.ijheh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW. 1999. Serial levels of serum organochlorines during pregnancy and postpartum. Arch Environ Health 54(2):110–114, PMID: 10094288, 10.1080/00039899909602244. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH. 2009. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci 364(1526):2097–2113, PMID: 19528058, 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Chevrier C, Philippat C, Petit C, Calafat AM, Ye X, et al. 2012. Correcting for the influence of sampling conditions on biomarkers of exposure to phenols and phthalates: a 2-step standardization method based on regression residuals. Environ Health 11:29, PMID: 22537080, 10.1186/1476-069X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckle G, Ayotte P, Dewailly EE, Jacobson SW, Jacobson JL. 2001. Prenatal exposure of the northern Quebec Inuit infants to environmental contaminants. Environ Health Perspect 109(12):1291–1299, PMID: 11748038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermann S, Rattan S, Brehm E, Flaws JA. 2015. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol 53:23–32, PMID: 25765777, 10.1016/j.reprotox.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutten S. 2015. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 66(suppl 1):8–16, PMID: 25925336, 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- Osawa R, Akiyama M, Shimizu H. 2011. Filaggrin gene defects and the risk of developing allergic disorders. Allergol Int 60(1):1–9, PMID: 21173567, 10.2332/allergolint.10-RAI-0270. [DOI] [PubMed] [Google Scholar]
- Overgaard LEK, Main KM, Frederiksen H, Stender S, Szecsi PB, Williams HC, et al. 2017. Children with atopic dermatitis and frequent emollient use have increased urinary levels of low-molecular-weight phthalate metabolites and parabens. Allergy 72(11):1768–1777, PMID: 28281298, 10.1111/all.13157. [DOI] [PubMed] [Google Scholar]
- Pan TL, Wang PW, Aljuffali IA, Hung YY, Lin CF, Fang JY. 2014. Dermal toxicity elicited by phthalates: evaluation of skin absorption, immunohistology, and functional proteomics. Food Chem Toxicol 65:105–114, PMID: 24384410, 10.1016/j.fct.2013.12.033. [DOI] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. 2012. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect 120(3):464–470, PMID: 21900077, 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyun BY. 2015. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res 7(2):101–105, PMID: 25729616, 10.4168/aair.2015.7.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakane K, Ichinose T, Takano H, Yanagisawa R, Koike E. 2014. Effects of oral administration of di-(2-ethylhexyl) and diisononyl phthalates on atopic dermatitis in NC/Nga mice. Immunopharmacol Immunotoxicol 36(1):61–69, PMID: 24328677, 10.3109/08923973.2013.866678. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 860(1):106–112, PMID: 17997365, 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Smit LAM, Lenters V, Høyer BB, Lindh CH, Pedersen HS, Liermontova I, et al. 2015. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy 70(6):653–660, PMID: 25753462, 10.1111/all.12605. [DOI] [PubMed] [Google Scholar]
- Stelmach I, Majak P, Jerzynska J, Podlecka D, Stelmach W, Polańska K, et al. 2015. The effect of prenatal exposure to phthalates on food allergy and early eczema in inner-city children. Allergy Asthma Proc 36(4):e72–e78, PMID: 26108074, 10.2500/aap.2015.36.3867. [DOI] [PubMed] [Google Scholar]
- Swan SH. 2008. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 108(2):177–184, PMID: 18949837, 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 106(2):257–269, PMID: 17976571, 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Vaidya SV, Kulkarni H. 2012. Association of urinary bisphenol A concentration with allergic asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Asthma 49(8):800–806, PMID: 22957848, 10.3109/02770903.2012.721041. [DOI] [PubMed] [Google Scholar]
- van den Oord RA, Sheikh A. 2009. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ 339:b2433, PMID: 19589816, 10.1136/bmj.b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IJ, Karmaus WJ. 2015. The effect of phthalate exposure and filaggrin gene variants on atopic dermatitis. Environ Res 136:213–218, PMID: 25460639, 10.1016/j.envres.2014.09.032. [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Bekö G, Koch HM, Salthammer T, Schripp T, Toftum J, et al. 2015. Transdermal uptake of diethyl phthalate and di(n-butyl) phthalate directly from air: experimental verification. Environ Health Perspect 123(10):928–934, PMID: 25850107, 10.1289/ehp.1409151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. 2012. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect 120(2):290–295, PMID: 21893441, 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, et al. 2014. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: the Columbia Center for Children’s Environmental Health Cohort. Environ Health Perspect 122(10):1141–1146, PMID: 25230320, 10.1289/ehp.1307670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Robertson C, Stewart A, Aït-Khaled N, Anabwani G, Anderson R, et al. 1999. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol 103(1 pt 1):125–138, PMID: 9893196, 10.1016/S0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. 2008. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 116(8):1092–1097, PMID: 18709157, 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. 2006. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal 26(3):803–824, PMID: 16834635, 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa R, Takano H, Inoue K, Koike E, Sadakane K, Ichinose T. 2008. Effects of maternal exposure to di-(2-ethylhexyl) phthalate during fetal and/or neonatal periods on atopic dermatitis in male offspring. Environ Health Perspect 116(9):1136–1141, PMID: 18795153, 10.1289/ehp.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.