Abstract
Presynaptic nerve terminals release neurotransmitter synchronously, asynchronously or spontaneously. During synchronous neurotransmission release is precisely coupled to action potentials, in contrast, asynchronous release events show only loose temporal coupling to presynaptic activity whereas spontaneous neurotransmission occurs independent of presynaptic activity. The mechanisms that give rise to this diversity in neurotransmitter release modes are poorly understood. Recent studies have described several presynaptic molecular pathways controlling synaptic vesicle pool segregation and recycling, which in turn may dictate distinct modes of neurotransmitter release. In this article, we review this recent work regarding neurotransmitter release modes and their relationship to synaptic vesicle pool dynamics as well as the molecular machinery that establishes synaptic vesicle pool identity.
Introduction: Modes of release and synaptic vesicle pools
The fidelity and plasticity of information flow in neuronal networks relies not only on the speed of neurotransmission but also on its sensitivity to stimuli and changes in neuronal environment. The basic units for neurotransmission are synapses, small intercellular junctions (~1 μm2) with a narrow intersynaptic space (~15 nm spacing) that allows small neurotransmitters to rapidly diffuse away from the presynaptic terminal and bind to postsynaptic receptors, triggering a response in milliseconds [1,2]. Neurotransmitter release is initiated by action potential (AP) arrival to the axon terminal where it causes a rapid and locally concentrated rise in calcium concentration (called calcium nanodomains [3]) which in turn leads to a simultaneous, time-locked, fusion of synaptic vesicles. This synchronous mode of release constitutes the basis of fast neurotransmission. Synaptic vesicles can also continue to fuse asynchronously for tens to hundreds of milliseconds after the AP, depending on neuron type, developmental stage and prior history of activity [4–8]. The level and duration of asynchronous release is regulated by the extent of calcium entry and the buffering capacity of presynaptic terminals [3], in addition to the intrinsic molecular attributes of the neurotransmitter release machinery (see below). Spontaneous neurotransmitter release, on the other hand, is independent of neuronal activity and exhibits variable regulation by calcium [9]. These three modes of neurotransmitter release were first discovered and characterized functionally, through electrophysiological approaches, but subsequently it became evident that the molecular properties of synaptic vesicles contribute to this diversity [10].
From a functional perspective, synaptic vesicles can be classified based on their intrinsic propensity to fuse. In this context, the readily releasable pool (RRP) of vesicles are defined as the first line of vesicles that fuse in response to stimulation, i.e. these vesicles have the highest propensity to fuse [11,12]. The RRP is the only pool that has been demonstrated to have a strong function and morphology correlation, [13–16]. In sensory neuron synapses, such as the retinal or auditory ribbon synapses, the number of docked vesicles correlates with the fast, synchronous release triggered by stimulation, while vesicles unrelated to the ribbon tend to fuse spontaneously [13–16]. In small central synapses, including hippocampal synapses, the size of the RRP also corresponds to the number of docked synaptic vesicles at the active zone (AZ) and regulation of AZ size and number of docking and release sites correlates with release probability and synaptic strength [17–19], but also see [20]. The second pool of vesicles that is mobilized and fused in response to APs is the reserve pool, defined as those vesicles that can fuse and take up different probes in response to mild to sustained stimulation, replenishing the RRP and maintaining neurotransmission [21–23]. The sizes of the RRP and the reserve pool are variable among synapses, approximately they occupy ~0.5% and ~10–60% of the total pool, respectively [22,24–26]. The RRP and reserve pool together form the recycling pool of synaptic vesicles. The rest of the synaptic vesicles in the nerve terminal belong to the so called “resting” or “dormant” pool, a group of vesicles highly impervious to activity (they will only fuse after strong high frequency repetitive stimulation, possibly fuse spontaneously or never release at all) [26,27]. The functional role of such a voluminous and largely inactive pool of vesicles has been a subject of speculation for many years. Besides potential contributions to spontaneous release, additional functions may include acting as a reserve for neurotransmitter and synaptic proteins [28,29]. Unlike the RRP, the reserve and resting pools do not show a specific localization within the nerve terminal and vesicles belonging to both pools appear to be intermixed [30], although in some studies actively recycling vesicles have been reported to be distributed closer to the AZ than resting vesicles [20,31]. Moreover, vesicles that are mobilized at different stimulation intensities have been shown to fuse at different sectors of the AZ, where synaptic vesicles that respond to high frequency stimulation fuse at the edges of the AZ [32]. This finding indicates that there are certain spatiotemporal properties that are characteristic of each synaptic vesicle pool.
Molecular basis of release diversity: heterogeneity in synaptic vesicle recycling machinery
Segregation of synaptic vesicle pools appears early in neuronal development, even though the total number of synaptic vesicles is half or less than that of a fully mature synapse. At onset, nascent presynaptic terminals possess an undifferentiated total pool of synaptic vesicles capable of spontaneous recycling as well as evoked Ca2+-dependent release under strong stimulation. Fusion in response to single APs requires the appearance of the RRP during development, followed by gradual population of the reserve pool [33]. Vesicles that release in a spontaneous or evoked fashion are also separated into different pools during maturation [34]. The main molecular hallmark driving this diversity in release modes of synaptic vesicles is the particular set of fusion machinery proteins present on the vesicle membranes. For instance, the distribution of SNAREs (acronym of Soluble NSF Attachment protein Receptors) and other associated proteins composing the fusion complex among vesicles. Classical SNARE driven fusion of a synaptic vesicle involves docking and priming mediated by the vesicle SNARE synatobrevin 2 (also called vesicle-associated membrane protein 2 or VAMP2) and the plasma membrane SNAREs syntaxin 1 and synaptosomal-associated protein 25 (SNAP-25), which along with the help of the active zone matrix proteins Munc18 and Munc13 assemble and generate a fusion-ready intermediary complex, highly stable and resistant to proteases [35]. Upon AP arrival and rapid Ca2+ concentration rise in the terminal, full zippering of the SNARE complex is triggered and membranes are pulled together, catalyzing the fusion and the opening and expansion of the pore. The calcium sensor responsible for fast and cooperative Ca2+ sensing and coupling to the fusion process, thus triggering fast, reliable synchronous release, is synaptotagmin 1 (syt1) [36]. These canonical SNAREs are involved in most of evoked synchronous neurotransmission throughout the central nervous system, since deletion of any one of them leads to a drastic decrease in neurotransmission [37–39]. However, a small fraction of synaptic vesicles are still able to fuse, especially those that recycle spontaneously or asynchronously [37,39] indicating that other, non-canonical SNAREs, are involved in these different modes of release (also see [40]). Nevertheless, not all synaptic proteins show specific distribution to a particular pool. Complexins, soluble proteins that selectively bind to SNARE complexes, can differentially regulate synchronous and asynchronous release in various neuronal types, and they also clamp and regulate spontaneous release. Thus, while complexins seem to be broad molecular regulators of all modes of fusion, they can also act in a specific manner for a particular mode of release [41–44], possibly through the interaction with distinct calcium sensors.
Synaptic vesicles that fuse synchronously or asynchronously belong to the same recycling pool [45], but may have different relative content of various SNAREs and Ca2+ sensors as result of sustained activity and recycling, generating preferential release modes for each vesicle [46]. The non-canonical vesicular SNARE VAMP4 has been demonstrated to preferentially drive asynchronous fusion and recycling, independently of VAMP2 or complexin and syt1 [46]. Instead of syt1, the calcium sensor for asynchronous release has been shown to be synaptotagmin 7 (syt7) [4,47,48]. Although a molecular interaction between VAMP4 and syt7 has not been experimentally demonstrated, they both recycle with similar kinetics after strong stimulation and seem to partially reside in a plasma membrane pool [46,49–51]. SNAP-25/syt1 complex and SNAP-23/syt7 have been associated with propensity to fuse synchronously and asynchronously, respectively [49], although SNAP-25 and 23 seem to be partially interchangeable. Trafficking differences between syt1 and syt7 containing vesicles may arise from the differences in Ca2+ binding properties between the two proteins (affinity, time courses of response) [47], agreeing with the observed differential dependence of synchronous and asynchronous release on Ca2+ concentration, buffering speed and source [52–54]. Recently, it was also demonstrated that syt1 and syt7 also couple release to kinetically distinct routes of endocytosis [51], pointing out that different pools might have independent cycling pathways [55]. Nevertheless, syt1 and syt7 also have partially overlapping, redundant activity [56], supporting imaging and electrophysiological evidence that these two modes of release belong to the same, activity-dependent recycling pool [45,54].
Spontaneously recycling synaptic vesicles are also morphologically indistinguishable from vesicles fusing in response to APs, and are spatially intermixed within the presynaptic terminals [57]. Despite their similar appearance, studies employing functional loading of distinct probes support the notion that spontaneous and evoked vesicle pool identity is conserved after fusion and retrieval, and these pools do not functionally intermix [58–61]. This premise is also supported by the observation that the retrieval kinetics of individual vesicles significantly diverge depending on whether they have initially fused spontaneously or in response to APs [62]. Moreover, the probabilities of release for evoked and spontaneous neurotransmission are not correlated within single presynaptic terminals [61,63]. The spontaneous pool of vesicles recycles at rest and generally do not respond to AP stimulation, although a small proportion can traffic and release upon very intense stimulation, like an elevated (90 mM) K+ pulse. For that reason, the spontaneously recycling pool has been proposed to overlap with the resting pool of synaptic vesicles [27]. In line with this hypothesis, the non-canonical vesicular SNARE VAMP7 has been shown to be enriched in vesicles belonging to the resting pool [64] and can selectively regulate spontaneous release of neurotransmitters [65]. Additionally, a proportion of the resting synaptic vesicles also carry Vti1a, another non-canonical SNARE that directs them to a more robust spontaneous recycling [66]. Vti1a does not bind to the canonical SNARE complex proteins syntaxin 1 and SNAP-25 [67] and traffics independently of VAMP2 [66]. Instead, it was shown to interact with VAMP4, syntaxin 6 and syntaxin 16 in synaptic vesicles [68] possibly assembling a specific SNARE complex for spontaneous release. In agreement with this premise, besides its apparent role in asynchronous release, VAMP4 can also partially rescue spontaneous neurotransmission in VAMP2 deficient hippocampal neurons [46] suggesting that the division between the different pools is dynamic and depends not on one protein but on a combination of various molecular markers. The mechanisms underlying Ca2+ dependence of spontaneous release is intensely debated, but evidence from several laboratories points to a fraction of the spontaneous release being Ca2+-independent and a portion of this pool fusing through a Ca2+-dependent pathway (for detailed review on this topic see [9,69,70]). The Ca2+ source for spontaneous neurotransmission may differ compared to evoked release, as it may require release from internal stores [71]. Moreover, about half of all spontaneous fusion events have been shown to be triggered by local Ca2+ microdomains generated by stochastic openings of voltage gated Ca2+ channels [72], although this regulation seems to be present only in inhibitory synapses [69]. Additionally, extracellular Ca2+ concentration has been implicated as a trigger of spontaneous exocytosis through the activation of the Ca2+-sensing G protein–coupled receptor [73], indicating that not one but a number of Ca2+-dependent pathways regulate spontaneous neurotransmission and they might differ among different types of synapses. Potential Ca2+ sensors for the coupling of Ca2+ to spontaneous release are Doc2 and Doc2 related family of proteins, since their manipulation specifically alters spontaneous neurotransmission without affecting evoked release [74,75] Doc2 proteins possess C2 domains, similar to synaptotagmins, but they differ in Ca2+ affinity and subcellular distribution. In addition, Doc2 proteins are cytoplasmic and associate with membranes in response to Ca2+ binding [74] constituting possible switches in Ca2+ sensitivity and mobilization of the spontaneous pool of synaptic vesicles. However, it is important to note that some studies proposed that Doc2-mediated spontaneous release is Ca2+-independent [76] thus leaving the question of actual Ca2+ sensors for spontaneous neurotransmission still open.
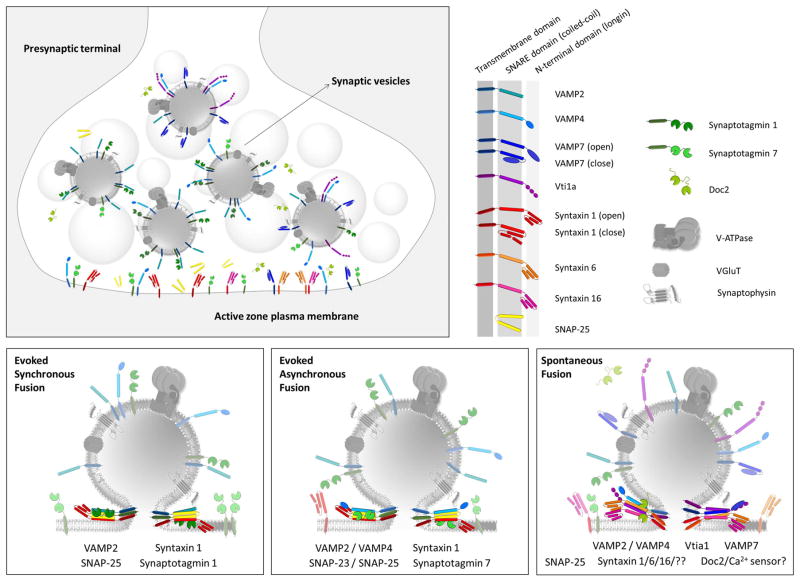
To maintain vesicle pool identities each synaptic vesicle pool needs to be segregated during recycling likely via specific endocytic routes. In this context, the canonical SNARE VAMP2 has been shown to participate in synaptic vesicle reformation (either from the presynaptic plasma membrane or synaptic endosomes) through a clathrin and AP-2 dependent pathway mediated by its interaction with the adaptor proteins CALM and AP-180 [77,78]. Proper sorting of the synaptic vesicle proteins synaptophysin-1 and SV2 is linked to VAMP2 [79] and fast exocytosis-endocytosis coupling is dependent on VAMP2 [80], indicating that trafficking properties of the recycling pool are determined by this canonical vesicular SNARE to a large extent. Interestingly, the Ca2+ sensor for synchronous release, syt1 shows a completely independent endocytic pathway [81] facilitated by another clathrin adaptor, stonin 2 [82]. The coexistence of these two parallel pathways might lead to uneven accumulation of different synaptic proteins in synaptic vesicles, generating vesicles with different copy number of syt1 for example, and thus creating vesicles with different synchronicity properties and release probabilities. Endocytic partners for the asynchronous Ca2+ sensor syt7 are still unknown, but the non-canonical SNARE for asynchronous neurotransmission, VAMP4 is retrieved to synaptic vesicles through the interaction between its di-leucine motif and the clathrin adaptor AP-1 [83] which seems to be specifically coupled to a bulk endocytosis pathway [84]. Mutations on the di-leucine motif abolish the specificity of VAMP4 dependent pathway recycling and make VAMP4 to traffic similarly to VAMP2 [46]. Remarkably, elimination of stonin 2 (syt1 endocytic partner) from synapses leads to increased bulk endocytosis [82] supporting the notion that synchronous and asynchronous release are coupled to separate endocytic routes [51]. More detailed molecular studies about exo-endocytic complexes is needed to strengthen this hypothesis. VAMP7, which labels largely the resting pool of synaptic vesicles, belongs to the longin family of SNARES (along with VAMP4 but not VAMP2), due to its longer N-terminal region [85]. This region constitutes an auto-inhibitory domain that negatively regulates SNARE complex formation and fusion [86,87] and could explain why VAMP7 containing vesicles are highly resilient to fuse. Moreover, the longing domain of VAMP7 is also necessary to its proper localization through interaction with another clathrin adaptor, AP-3 [88]. Remarkably, the vesicular glutamate transporter (VGluT) possesses numerous endocytic motifs, including several di-leucine motifs, and can interact with AP-1, AP-2 and AP-3, as well as endophilin. Each site seems to be independent and to regulate different VGluT retrieval through separate endocytic pathways [89,90] suggesting that it was evolutionarily selected to be able to traffic to all classes of synaptic vesicles and allow neurotransmitter refilling for all pools. A schematic representation of intermixing of synaptic vesicles with different molecular identities and the putative SNARE complexes mediating different modes of release is presented in Figure 1.
Figure 1.
Top left: Schematic representation of a presynaptic terminal filled with intermixed synaptic vesicles. Recent studies suggest that synaptic vesicles may contain variable combinations of SNARE proteins, as such a vesicle’s molecular composition rather than location determines its recycling trajectory.
Top right: Representation of the proteins depicted in the figure. The transmembrane, SNARE and N-terminal (longin) domains of several SNARE proteins are specified.
Bottom: Putative composition of synaptic vesicles and their interaction partners giving rise to the evoked synchronous, asynchronous and spontaneous modes of neurotransmitter release. Although, synchronous fusion requires the canonical SNARE complex composed of VAMP2, SNAP-25 and syntaxin1, vesicles giving rise to asynchronous and spontaneous modes of neurotransmitter release may use a diverse set of SNAREs.
Besides differential SNARE distribution, other molecular properties of synaptic vesicles have been proposed to be involved in their segregation into distinct pools. Actin interaction seems to be crucial for retrieval and reuse of vesicles belonging to the recycling pool [20,91,92] and association with synapsin was proposed to form a synaptic vesicle matrix that maintains the resting pool and regulates its mobility [93,94]. The active zone cytomatrix scaffold protein RIM has been shown to regulate coupling of synaptic vesicles to voltage gated Ca2+ channels and to regulate the size of the RRP via docking and also refilling steps [95,96]. Moreover, RIM and its binding proteins control fast synchronous release [97]. Evoked (synchronous as well asynchronous) release is also dynamin dependent, while fast, single vesicle, spontaneous recycling may not require dynamin [98], leaving an open question about how vesicle membranes are excised after spontaneous fusion. The evoked and spontaneous pools of synaptic vesicles have also differential, opposite membrane cholesterol requirements. Cholesterol appears to facilitate evoked fusion and retrieval of synaptic vesicles while it suppresses spontaneous synaptic vesicle recycling [99,100]. In conclusion, although several molecular markers have been implicated in determining synaptic vesicle pool segregation and maintenance (like the content of particular vesicular SNAREs), the fine tuning and richness of pool diversity seems to involve the concomitant activity of several proteins and lipids, many of them with identities and trafficking properties that remain under investigation.
Closing remarks: impact of neurotransmitter release modes on the physiology and plasticity of neuronal networks
Increasing evidence suggests that synaptic vesicle pool segregation in central synapses is dictated by mechanisms that confer specific trafficking and fusion properties to synaptic vesicles. This vesicle diversity, in turn, is critical for the maintenance of distinct forms of neurotransmitter release. Among the three forms of neurotransmission, synchronous release preserves the precise timing of presynaptic activity given its fidelity to specific activity patterns and precise timing with respect to the arrival of APs. Asynchronous release, on the other hand, complements synchronous release during repetitive activity and maintains neurotransmission strength [45]. In addition, asynchronous release is a crucial regulator of neurotransmission by influencing multiple dynamic parameters including synaptic network recovery (refractory period durations) and synchronicity, reliability and duration of the postsynaptic response, partly through its impact on inhibitory inputs [101–104]. Finally, spontaneous neurotransmitter release regulates synaptic properties including strength and plasticity independent of activity. Spontaneous events also influence dendritic arbor complexity and synapse formation during development [105,106]. Moreover, regulation of synaptic strength by spontaneous events has been implicated in the fast antidepressant effect of ketamine [107–109]. Taken together, different modes of release confer neurons a diversity of plastic responses that can be modulated and combined to transfer and integrate a wide variety of information. Understanding the molecular mechanisms underlying synaptic vesicle pool segregation will not only expand our knowledge of how complex neuronal networks function, but also generate tools to develop more specific therapeutics for neurological and neuropsychiatric disorders, including depression.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (MH066198).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goyal RK, Chaudhury A. Structure activity relationship of synaptic and junctional neurotransmission. Auton Neurosci. 2013;176:11–31. doi: 10.1016/j.autneu.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016;536:210–214. doi: 10.1038/nature19058. Using super-resolution microscopy, Tang and colleagues described a structural organization at the synapse that aligns presynaptic release sites with postsynaptic receptor clusters. They also show that this organization can be dynamically modulated and potentially couples different modes of neurotransmitter release to different postsynaptic responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Luo F, Bacaj T, Sudhof TC. Synaptotagmin-7 Is Essential for Ca2+-Triggered Delayed Asynchronous Release But Not for Ca2+-Dependent Vesicle Priming in Retinal Ribbon Synapses. J Neurosci. 2015;35:11024–11033. doi: 10.1523/JNEUROSCI.0759-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang M, Yang M, Yin L, Zhang X, Shu Y. Developmental reduction of asynchronous GABA release from neocortical fast-spiking neurons. Cereb Cortex. 2015;25:258–270. doi: 10.1093/cercor/bht236. [DOI] [PubMed] [Google Scholar]
- 6.Wen H, Linhoff MW, McGinley MJ, Li GL, Corson GM, Mandel G, Brehm P. Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13906–13911. doi: 10.1073/pnas.1008598107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirwa SS, Sastry BR. Asynchronous synaptic responses in hippocampal CA1 neurons during synaptic long-term potentiation. Neurosci Lett. 1988;89:355–360. doi: 10.1016/0304-3940(88)90552-6. [DOI] [PubMed] [Google Scholar]
- 8.Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proc Natl Acad Sci U S A. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nature reviews Neuroscience. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- 10.Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 11.Kaeser PS, Regehr WG. The readily releasable pool of synaptic vesicles. Curr Opin Neurobiol. 2017;43:63–70. doi: 10.1016/j.conb.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen JB. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch. 2004;448:347–362. doi: 10.1007/s00424-004-1247-8. [DOI] [PubMed] [Google Scholar]
- 13.Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci U S A. 2008;105:4922–4927. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snellman J, Mehta B, Babai N, Bartoletti TM, Akmentin W, Francis A, Matthews G, Thoreson W, Zenisek D. Acute destruction of the synaptic ribbon reveals a role for the ribbon in vesicle priming. Nat Neurosci. 2011;14:1135–1141. doi: 10.1038/nn.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta B, Snellman J, Chen S, Li W, Zenisek D. Synaptic ribbons influence the size and frequency of miniature-like evoked postsynaptic currents. Neuron. 2013;77:516–527. doi: 10.1016/j.neuron.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graydon CW, Cho S, Li GL, Kachar B, von Gersdorff H. Sharp Ca(2)(+) nanodomains beneath the ribbon promote highly synchronous multivesicular release at hair cell synapses. J Neurosci. 2011;31:16637–16650. doi: 10.1523/JNEUROSCI.1866-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 18.Holderith N, Lorincz A, Katona G, Rozsa B, Kulik A, Watanabe M, Nusser Z. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci. 2012;15:988–997. doi: 10.1038/nn.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel K, Muller JA, Oprisoreanu AM, Schoch S. The presynaptic active zone: A dynamic scaffold that regulates synaptic efficacy. Exp Cell Res. 2015;335:157–164. doi: 10.1016/j.yexcr.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Rey SA, Smith CA, Fowler MW, Crawford F, Burden JJ, Staras K. Ultrastructural and functional fate of recycled vesicles in hippocampal synapses. Nat Commun. 2015;6:8043. doi: 10.1038/ncomms9043. Using a combination of fluorescence and electron microscopies, Rey and colleagues show that synaptic vesicles belonging to the readily releasable pool are endocytosed in the vicinity of the active zone after fusion and randomly intermix with the total pool of vesicles. In addition, they describe how synaptic vesicles move towards the active zone after stimulation in an actin-dependent manner and how the propensity to fuse is determined by the proximity to the active zone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sara Y, Mozhayeva MG, Liu X, Kavalali ET. Fast vesicle recycling supports neurotransmission during sustained stimulation at hippocampal synapses. J Neurosci. 2002;22:1608–1617. doi: 10.1523/JNEUROSCI.22-05-01608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marra V, Burden JJ, Thorpe JR, Smith IT, Smith SL, Hausser M, Branco T, Staras K. A preferentially segregated recycling vesicle pool of limited size supports neurotransmission in native central synapses. Neuron. 2012;76:579–589. doi: 10.1016/j.neuron.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harata N, Ryan TA, Smith SJ, Buchanan J, Tsien RW. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1-43 photoconversion. Proc Natl Acad Sci U S A. 2001;98:12748–12753. doi: 10.1073/pnas.171442798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu X, Zhu Q, Sun J. Quantitative analysis of vesicle recycling at the calyx of Held synapse. Proc Natl Acad Sci U S A. 2015;112:4779–4784. doi: 10.1073/pnas.1424597112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Ge JL, Hao M, Sun ZC, Wu XS, Zhu JB, Wang W, Yao PT, Lin W, Xue L. A three-pool model dissecting readily releasable pool replenishment at the calyx of held. Sci Rep. 2015;5:9517. doi: 10.1038/srep09517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudhof TC. The synaptic vesicle cycle revisited. Neuron. 2000;28:317–320. doi: 10.1016/s0896-6273(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 27.Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denker A, Krohnert K, Buckers J, Neher E, Rizzoli SO. The reserve pool of synaptic vesicles acts as a buffer for proteins involved in synaptic vesicle recycling. Proc Natl Acad Sci U S A. 2011;108:17183–17188. doi: 10.1073/pnas.1112690108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratnayaka A, Marra V, Bush D, Burden JJ, Branco T, Staras K. Recruitment of resting vesicles into recycling pools supports NMDA receptor-dependent synaptic potentiation in cultured hippocampal neurons. J Physiol. 2012;590:1585–1597. doi: 10.1113/jphysiol.2011.226688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denker A, Krohnert K, Rizzoli SO. Revisiting synaptic vesicle pool localization in the Drosophila neuromuscular junction. J Physiol. 2009;587:2919–2926. doi: 10.1113/jphysiol.2009.170985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 32.Maschi D, Klyachko VA. Spatiotemporal Regulation of Synaptic Vesicle Fusion Sites in Central Synapses. Neuron. 2017;94:65–73e63. doi: 10.1016/j.neuron.2017.03.006. Using an innovative combination of optical, electrophysiological and computational methods, Maschi and Klyachko measured the spatiotemporal pattern of synaptic vesicle fusion, showing that release sites span the entire active zone and are reused repeatedly under mild stimulation. High frequency stimulation shifts that pattern, causing vesicle to fuse preferentially at the periphery of the active zone where probability of release site reuse is low. [DOI] [PubMed] [Google Scholar]
- 33.Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreae LC, Fredj NB, Burrone J. Independent vesicle pools underlie different modes of release during neuronal development. J Neurosci. 2012;32:1867–1874. doi: 10.1523/JNEUROSCI.5181-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Annual review of cell and developmental biology. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- 36.Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4:a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 38.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nature neuroscience. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 39.Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+-dependent and Ca2+-independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- 40.Delgado-Martinez I, Nehring RB, Sorensen JB. Differential abilities of SNAP-25 homologs to support neuronal function. J Neurosci. 2007;27:9380–9391. doi: 10.1523/JNEUROSCI.5092-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Sudhof TC. Complexin clamps asynchronous release by blocking a secondary Ca(2+) sensor via its accessory alpha helix. Neuron. 2010;68:907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao P, Yang X, Sudhof TC. Complexin activates exocytosis of distinct secretory vesicles controlled by different synaptotagmins. J Neurosci. 2013;33:1714–1727. doi: 10.1523/JNEUROSCI.4087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr Biol. 2011;21:97–105. doi: 10.1016/j.cub.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, Littleton JT. Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:18234–18245. doi: 10.1523/JNEUROSCI.3212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci. 2004;24:420–433. doi: 10.1523/JNEUROSCI.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raingo J, Khvotchev M, Liu P, Darios F, Li YC, Ramirez DM, Adachi M, Lemieux P, Toth K, Davletov B, et al. VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nature neuroscience. 2012;15:738–745. doi: 10.1038/nn.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacaj T, Wu D, Yang X, Morishita W, Zhou P, Xu W, Malenka RC, Sudhof TC. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron. 2013;80:947–959. doi: 10.1016/j.neuron.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maximov A, Lao Y, Li H, Chen X, Rizo J, Sorensen JB, Sudhof TC. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc Natl Acad Sci U S A. 2008;105:3986–3991. doi: 10.1073/pnas.0712372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber JP, Toft-Bertelsen TL, Mohrmann R, Delgado-Martinez I, Sorensen JB. Synaptotagmin-7 is an asynchronous calcium sensor for synaptic transmission in neurons expressing SNAP-23. PloS one. 2014;9:e114033. doi: 10.1371/journal.pone.0114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virmani T, Han W, Liu X, Sudhof TC, Kavalali ET. Synaptotagmin 7 splice variants differentially regulate synaptic vesicle recycling. EMBO J. 2003;22:5347–5357. doi: 10.1093/emboj/cdg514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li YC, Chanaday NL, Xu W, Kavalali ET. Synaptotagmin-1- and Synaptotagmin-7-Dependent Fusion Mechanisms Target Synaptic Vesicles to Kinetically Distinct Endocytic Pathways. Neuron. 2017;93:616–631e613. doi: 10.1016/j.neuron.2016.12.010. Li and colleagues revisited a long standing question on the role of synaptotagmin-1 in endocytosis and showed that synaptotagmin-1 slows endocytosis of single synaptic vesicles in a Ca2+-dependet manner. Moreover, they demonstrated that synchronous and asynchronous fusion processes are coupled to fast and slow endocytic pathways, respectively. The pace of these processes is determined by the levels of synaptotagmin-1 and synaptotagmin-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen H, Hubbard JM, Rakela B, Linhoff MW, Mandel G, Brehm P. Synchronous and asynchronous modes of synaptic transmission utilize different calcium sources. Elife. 2013;2:e01206. doi: 10.7554/eLife.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron. 2000;26:683–694. doi: 10.1016/s0896-6273(00)81204-0. [DOI] [PubMed] [Google Scholar]
- 54.Hagler DJ, Jr, Goda Y. Properties of synchronous and asynchronous release during pulse train depression in cultured hippocampal neurons. J Neurophysiol. 2001;85:2324–2334. doi: 10.1152/jn.2001.85.6.2324. [DOI] [PubMed] [Google Scholar]
- 55.Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and Endocytosis: Modes, Functions, and Coupling Mechanisms. Annual Review of Physiology. 2014;7676:301–331. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bacaj T, Wu D, Burre J, Malenka RC, Liu X, Sudhof TC. Synaptotagmin-1 and -7 Are Redundantly Essential for Maintaining the Capacity of the Readily-Releasable Pool of Synaptic Vesicles. PLoS Biol. 2015;13:e1002267. doi: 10.1371/journal.pbio.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 58.Afuwape OA, Wasser CR, Schikorski T, Kavalali ET. Synaptic vesicle pool-specific modification of neurotransmitter release by intravesicular free radical generation. J Physiol. 2017;595:1223–1238. doi: 10.1113/JP273115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET. Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:5378–5382. doi: 10.1523/JNEUROSCI.5234-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Virmani T, Ertunc M, Sara Y, Mozhayeva M, Kavalali ET. Phorbol esters target the activity-dependent recycling pool and spare spontaneous vesicle recycling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:10922–10929. doi: 10.1523/JNEUROSCI.3766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melom JE, Akbergenova Y, Gavornik JP, Littleton JT. Spontaneous and evoked release are independently regulated at individual active zones. J Neurosci. 2013;33:17253–17263. doi: 10.1523/JNEUROSCI.3334-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leitz J, Kavalali ET. Fast retrieval and autonomous regulation of single spontaneously recycling synaptic vesicles. Elife. 2014;3:e03658. doi: 10.7554/eLife.03658. In this study, the authors performed a detailed analysis of presynaptic boutons in the presence of tetrodotoxin, and demonstrated that synaptic vesicle proteins are retrieved swiftly after spontaneous fusion in a Ca2+-independent manner. They also showed that endocytosis of spontaneously fused vesicles occurs in a faster timescale compared to vesicles that fuse in response to activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reese AL, Kavalali ET. Single synapse evaluation of the postsynaptic NMDA receptors targeted by evoked and spontaneous neurotransmission. Elife. 2016:5. doi: 10.7554/eLife.21170. Using Ca2+ imaging techniques, the authors found that spontaneous and evoked release of neurotransmitters activate separate, non-overlaping populations of postsynaptic NMDA receptors, with negligible correlation or interaction between the two signals, even though both types of transmission can occur at the same synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hua Z, Leal-Ortiz S, Foss SM, Waites CL, Garner CC, Voglmaier SM, Edwards RH. v-SNARE composition distinguishes synaptic vesicle pools. Neuron. 2011;71:474–487. doi: 10.1016/j.neuron.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bal M, Leitz J, Reese AL, Ramirez DM, Durakoglugil M, Herz J, Monteggia LM, Kavalali ET. Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron. 2013;80:934–946. doi: 10.1016/j.neuron.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET. Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron. 2012;73:121–134. doi: 10.1016/j.neuron.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antonin W, Riedel D, von Mollard GF. The SNARE Vti1a-beta is localized to small synaptic vesicles and participates in a novel SNARE complex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:5724–5732. doi: 10.1523/JNEUROSCI.20-15-05724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreykenbohm V, Wenzel D, Antonin W, Atlachkine V, von Mollard GF. The SNAREs vti1a and vti1b have distinct localization and SNARE complex partners. European journal of cell biology. 2002;81:273–280. doi: 10.1078/0171-9335-00247. [DOI] [PubMed] [Google Scholar]
- 69.Williams CL, Smith SM. Calcium dependence of spontaneous neurotransmitter release. J Neurosci Res. 2017 doi: 10.1002/jnr.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol. 2014;76:333–363. doi: 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reese AL, Kavalali ET. Spontaneous neurotransmission signals through store-driven Ca(2+) transients to maintain synaptic homeostasis. Elife. 2015:4. doi: 10.7554/eLife.09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ermolyuk YS, Alder FG, Surges R, Pavlov IY, Timofeeva Y, Kullmann DM, Volynski KE. Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2+ channels. Nat Neurosci. 2013;16:1754–1763. doi: 10.1038/nn.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vyleta NP, Smith SM. Spontaneous glutamate release is independent of calcium influx and tonically activated by the calcium-sensing receptor. J Neurosci. 2011;31:4593–4606. doi: 10.1523/JNEUROSCI.6398-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramirez DMO, Crawford DC, Chanaday NL, Trauterman B, Monteggia LM, Kavalali ET. Loss of Doc2-Dependent Spontaneous Neurotransmission Augments Glutamatergic Synaptic Strength. J Neurosci. 2017;37:6224–6230. doi: 10.1523/JNEUROSCI.0418-17.2017. Ramirez and colleagues specifically manipulated spontaneous neurotransmission without affecting evoked transmission by reducing the levels of soluble calcium-binding double C2 domain (Doc2) proteins. In doing so, they could show that spontaneous release plays a key role regulating synaptic efficacy independently of evoked transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, Sudhof TC. Doc2 supports spontaneous synaptic transmission by a Ca(2+)-independent mechanism. Neuron. 2011;70:244–251. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koo SJ, Kochlamazashvili G, Rost B, Puchkov D, Gimber N, Lehmann M, Tadeus G, Schmoranzer J, Rosenmund C, Haucke V, et al. Vesicular Synaptobrevin/VAMP2 Levels Guarded by AP180 Control Efficient Neurotransmission. Neuron. 2015;88:330–344. doi: 10.1016/j.neuron.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 78.Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, Maritzen T, Dernedde J, Volkmer R, Oschkinat H, Haucke V. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proc Natl Acad Sci U S A. 2011;108:13540–13545. doi: 10.1073/pnas.1107067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rajappa R, Gauthier-Kemper A, Boning D, Huve J, Klingauf J. Synaptophysin 1 Clears Synaptobrevin 2 from the Presynaptic Active Zone to Prevent Short-Term Depression. Cell Rep. 2016;14:1369–1381. doi: 10.1016/j.celrep.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 80.Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 81.Pan PY, Marrs J, Ryan TA. Vesicular glutamate transporter 1 orchestrates recruitment of other synaptic vesicle cargo proteins during synaptic vesicle recycling. J Biol Chem. 2015;290:22593–22601. doi: 10.1074/jbc.M115.651711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kononenko NL, Diril MK, Puchkov D, Kintscher M, Koo SJ, Pfuhl G, Winter Y, Wienisch M, Klingauf J, Breustedt J, et al. Compromised fidelity of endocytic synaptic vesicle protein sorting in the absence of stonin 2. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E526–535. doi: 10.1073/pnas.1218432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peden AA, Park GY, Scheller RH. The Di-leucine motif of vesicle-associated membrane protein 4 is required for its localization and AP-1 binding. J Biol Chem. 2001;276:49183–49187. doi: 10.1074/jbc.M106646200. [DOI] [PubMed] [Google Scholar]
- 84.Nicholson-Fish JC, Kokotos AC, Gillingwater TH, Smillie KJ, Cousin MA. VAMP4 Is an Essential Cargo Molecule for Activity-Dependent Bulk Endocytosis. Neuron. 2015;88:973–984. doi: 10.1016/j.neuron.2015.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramirez DM, Kavalali ET. The role of non-canonical SNAREs in synaptic vesicle recycling. Cellular logistics. 2012;2:20–27. doi: 10.4161/cl.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daste F, Galli T, Tareste D. Structure and function of longin SNAREs. J Cell Sci. 2015;128:4263–4272. doi: 10.1242/jcs.178574. [DOI] [PubMed] [Google Scholar]
- 87.Vivona S, Liu CW, Strop P, Rossi V, Filippini F, Brunger AT. The longin SNARE VAMP7/TI-VAMP adopts a closed conformation. J Biol Chem. 2010;285:17965–17973. doi: 10.1074/jbc.M110.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, Daviet L, Formstecher E, Hamburger A, Filippini F, et al. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc Natl Acad Sci U S A. 2003;100:9011–9016. doi: 10.1073/pnas.1431910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voglmaier SM, Kam K, Yang H, Fortin DL, Hua ZL, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 90.Foss SM, Li H, Santos MS, Edwards RH, Voglmaier SM. Multiple dileucine-like motifs direct VGLUT1 trafficking. J Neurosci. 2013;33:10647–10660. doi: 10.1523/JNEUROSCI.5662-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu XS, Lee SH, Sheng J, Zhang Z, Zhao WD, Wang D, Jin Y, Charnay P, Ervasti JM, Wu LG. Actin Is Crucial for All Kinetically Distinguishable Forms of Endocytosis at Synapses. Neuron. 2016;92:1020–1035. doi: 10.1016/j.neuron.2016.10.014. By combining fluorescence microscopy and electrophysiological techniques at the calyx of Held and hippocampal synapses, Wu and colleagues demonstrate that fast, slow and bulk forms of endocytosis are regulated by actin mainly at two crucial steps: recycling pool mobilization and generation of membrane pits for retrieval of synaptic vesicle components. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soykan T, Kaempf N, Sakaba T, Vollweiter D, Goerdeler F, Puchkov D, Kononenko NL, Haucke V. Synaptic Vesicle Endocytosis Occurs on Multiple Timescales and Is Mediated by Formin-Dependent Actin Assembly. Neuron. 2017;93:854–866. e854. doi: 10.1016/j.neuron.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Orenbuch A, Shalev L, Marra V, Sinai I, Lavy Y, Kahn J, Burden JJ, Staras K, Gitler D. Synapsin selectively controls the mobility of resting pool vesicles at hippocampal terminals. J Neurosci. 2012;32:3969–3980. doi: 10.1523/JNEUROSCI.5058-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shupliakov O, Haucke V, Pechstein A. How synapsin I may cluster synaptic vesicles. Semin Cell Dev Biol. 2011;22:393–399. doi: 10.1016/j.semcdb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 95.Muller M, Genc O, Davis GW. RIM-binding protein links synaptic homeostasis to the stabilization and replenishment of high release probability vesicles. Neuron. 2015;85:1056–1069. doi: 10.1016/j.neuron.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han Y, Babai N, Kaeser P, Sudhof TC, Schneggenburger R. RIM1 and RIM2 redundantly determine Ca2+ channel density and readily releasable pool size at a large hindbrain synapse. J Neurophysiol. 2015;113:255–263. doi: 10.1152/jn.00488.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Acuna C, Liu X, Gonzalez A, Sudhof TC. RIM-BPs Mediate Tight Coupling of Action Potentials to Ca(2+)-Triggered Neurotransmitter Release. Neuron. 2015;87:1234–1247. doi: 10.1016/j.neuron.2015.08.027. Acuna and colleagues found that although RIM-binding proteins are not essential for neurotransmission in mammalian synapses, they are required for fast and reliable coupling of presynaptic Ca2+ channels and release sites, regulating the fidelity of evoked neurotransmission. [DOI] [PubMed] [Google Scholar]
- 98.Chung C, Barylko B, Leitz J, Liu X, Kavalali ET. Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission. J Neurosci. 2010;30:1363–1376. doi: 10.1523/JNEUROSCI.3427-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rodrigues HA, Lima RF, de Fonseca MC, Amaral EA, Martinelli PM, Naves LA, Gomez MV, Kushmerick C, Prado MA, Guatimosim C. Membrane cholesterol regulates different modes of synaptic vesicle release and retrieval at the frog neuromuscular junction. Eur J Neurosci. 2013;38:2978–2987. doi: 10.1111/ejn.12300. [DOI] [PubMed] [Google Scholar]
- 101.Iremonger KJ, Bains JS. Asynchronous presynaptic glutamate release enhances neuronal excitability during the post-spike refractory period. J Physiol. 2016;594:1005–1015. doi: 10.1113/JP271485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iremonger KJ, Bains JS. Integration of asynchronously released quanta prolongs the postsynaptic spike window. J Neurosci. 2007;27:6684–6691. doi: 10.1523/JNEUROSCI.0934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manseau F, Marinelli S, Mendez P, Schwaller B, Prince DA, Huguenard JR, Bacci A. Desynchronization of neocortical networks by asynchronous release of GABA at autaptic and synaptic contacts from fast-spiking interneurons. PLoS Biol. 2010:8. doi: 10.1371/journal.pbio.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo F, Sudhof TC. Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse. Neuron. 2017;94:826–839. e823. doi: 10.1016/j.neuron.2017.04.020. In this article, the authors describe a new and interesting role for synaptotagmin 7-mediated asynchronous release at the calyx of Held. Asynchronous release creates a long-lasting postsynaptic depolarization that results in increased sensitivity and reliability of the postsynaptic response to boost synchronous neurotransmission. [DOI] [PubMed] [Google Scholar]
- 105.Andreae LC, Burrone J. Spontaneous Neurotransmitter Release Shapes Dendritic Arbors via Long-Range Activation of NMDA Receptors. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.01.032. Andreae and Burrone demonstrate that spontaneouly released neurotransmitter can diffuse and activate NMDA receptors at long range during neuronal maturation, before synaptic contacts are formed. This activation is crucial for dendritic arbor growth, branching and maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cho RW, Buhl LK, Volfson D, Tran A, Li F, Akbergenova Y, Littleton JT. Phosphorylation of Complexin by PKA Regulates Activity-Dependent Spontaneous Neurotransmitter Release and Structural Synaptic Plasticity. Neuron. 2015;88:749–761. doi: 10.1016/j.neuron.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute Suppression of Spontaneous Neurotransmission Drives Synaptic Potentiation. Journal of Neuroscience. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crawford DC, Ramirez DM, Trauterman B, Monteggia LM, Kavalali ET. Selective molecular impairment of spontaneous neurotransmission modulates synaptic efficacy. Nat Commun. 2017;8:14436. doi: 10.1038/ncomms14436. Supporting the molecular basis of synaptic vesicle pool segregation, Crawford and colleagues were able to specifically manipulate high frequency spontaneous neurotransmission by eliminating the proteins Vti1a and VAMP7, leaving evoked activity intact. Using this approach they show that spontaneous release regulates synaptic strength, homeostasis and plasticity via the activation of NMDA receptors and the eukaryotic elongation factor 2 kinase in the postsynaptic neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]