Abstract
Background/objectives
Ghrelin, a stomach-derived hormone implicated in numerous behaviors including feeding, reward, stress, and addictive behaviors, acts through binding to the growth hormone secretagogue receptor (GHSR). Here, we present the development, verification and initial characterization of a novel GHSR knockout (KO) Wistar rat model created with CRISPR genome editing.
Methods
Using CRISPR/Cas9, we developed a GHSR knockout (KO) in a Wistar background. Loss of GHSR mRNA expression was histologically verified using RNAscope in wild-type WT (n = 2) and KO (n = 2) rats. We tested the effects of intraperitoneal acyl-ghrelin administration on food consumption and plasma growth hormone (GH) concentrations in WT (n = 8) and KO (n = 8) rats. We also analyzed locomotion, food consumption, and body fat composition in these animals. Body weight was monitored from early development to adulthood.
Results
The RNAscope analysis revealed an abundance of GHSR mRNA expression in the hypothalamus, midbrain, and hippocampus in WTs, and no observed probe binding in KOs. Ghrelin administration increased plasma GH levels (p = 0.0067) and food consumption (p = 0.0448) in WT rats but not KOs. KO rats consumed less food overall at basal conditions and weighed significantly less compared with WTs throughout development (p = 0.0001). Compared with WTs, KOs presented higher concentrations of brown adipose tissue (BAT) (p = 0.0322).
Conclusions
We have verified GHSR deletion in our KO model using histological, physiological, neuroendocrinological and behavioral measures. Our findings indicate that GHSR deletion in rats is not only associated with a lack of response to ghrelin, but also associated with decreases in daily food consumption and body growth, and increases in BAT. This GHSR KO Wistar rat model provides a novel tool for studying the role of the ghrelin system in obesity and in a wide range of medical and neuropsychiatric disorders.
Keywords: ghrelin, GHS-R1a, knockout, wild-type, transgenic rat, food intake, CRISPR, GHSR
Introduction
The 28-amino acid peptide hormone ghrelin is synthesized primarily by endocrine cells in the stomach, where pre-pro-ghrelin is transcribed from the GHRL gene, translated and proteolytically cleaved into ghrelin (1). The ghrelin-O-acyltransferase (GOAT) enzyme is responsible for adhering an octanyl group onto the third serine acid of unacetylated ghrelin to yield acyl-ghrelin. Acyl-ghrelin is commonly considered the “active” form (2, 3), and henceforth simply referred as “ghrelin.”
The growth hormone secretagogue receptor 1a (GHS-R1a) was discovered first as a key receptor in regulating growth hormone (GH) release (4). A few years later, its endogenous ligand was identified from the rat stomach and given its key role as a GH-releasing peptide, it was named ghrelin (1). The surprising discovery that ghrelin is mainly produced by gastric endocrine cells led to research on its physiological role in regulating food intake, energy metabolism and glucose homeostasis (5). As of now, ghrelin’s orexigenic effects (increased appetite, increased food intake, and decreased latency to meal initiation) have been extensively described in both rodent and human subjects (6–10). Blood concentrations of ghrelin are characterized by pre-prandial increases and post-prandial decreases in both rodents and humans (11, 12). Ghrelin binding to GHS-R1a additionally appears to be important for metabolic activity, with a variety of functional roles in energy metabolism and homeostasis (13). Indeed, soon after its initial discovery, it was reported that once daily ghrelin administration over two weeks led to increased body weight and adiposity in mice, and altered carbohydrate-fat utilization compared to saline-treated animals (7).
Ghrelin is the only known endogenous ligand for the G-protein coupled GHS-R1a (4), which originates from the GHSR gene and which is expressed both centrally and peripherally (14). Indeed, the functions and effects of acyl-ghrelin have been attributed to its selective binding to GHS-R1a (1). Unacetylated ghrelin binds to GHS-R1a with approximately 1000-fold lower affinity than acyl-ghrelin, its potential functional role is unclear and its putative receptor is currently unknown (14). An additional receptor, GHS-R1b, is synthesized from the GHSR gene (15). Although the exact function of GHS-R1b is not fully understood and no endogenous ligand has been identified, there is preliminary evidence suggesting that GHS-R1b may form heterodimers with GHS-R1a to act as a negative feedback for acyl-ghrelin signaling (16) and that GHS-R1b determines the ability of GHS-R1a to form oligomeric complexes with other receptors (17).
Ghrelin neurons, expressing the GHS-R1a, are found throughout the central nervous system (18). There is a high level of expression of GHS-R1a in nuclei of the hypothalamus, where GHS-R1a activation via acyl-ghrelin binding has been implicated in the control of homeostatic eating (9, 19). Additionally, GHS-R1as are expressed in brain regions associated with memory, reward, and stress, including the hippocampus, midbrain, and amygdala (18). GHS-R1a are also expressed in the periphery, where the receptor is found in the stomach, intestine and numerous other regions (20). Indeed, ghrelin signaling via GHS-R1a is thought to include both central orexigenic (NPY/AgRP) and anorexigenic (POMC/CART) pathways as well as peripheral mechanisms via the vagus and solitary tract nucleus (14).
In recent years, transgenic mouse lines with mutations in the ghrelin system have been developed. Ghrelin peptide, GHSR and GOAT knockout (KO) mice are available, and these models have been substantially studied in terms of appetite, food intake, energy expenditure, metabolism, and reward processing (21–26). Ghrelin peptide-deficient mice, however, display limited physiological and behavioral modifications compared with wild type, with no differences in body weight, body composition, or food consumption (23, 24). In contrast, GHSR KO mice have reduced body weight, altered metabolism, and decreased food intake compared with wild type mice (23). One possible explanation for the discrepancy between the effects of the deletion of the ghrelin peptide and the receptor is the ghrelin receptor’s high level of constitutive activity in the absence of ghrelin peptide binding (27, 28). In addition to GHSR KO mice, there is currently an available GHSR KO rat line, developed from a Fawn Hooded Hypertensive strain (FHH; (29, 30)). The FHH rats are not susceptible to over eating or obesity, and are not commonly used to study other compulsivity-related behaviors such as addiction (30), making isolation of ghrelin’s role in behavior difficult. Nonetheless, the GHSR KO FHH line may still serve an important role in the ghrelin field, given that they show reduced intake of palatable, high-calorie food (30) and diminished development of cocaine-induced locomotor sensitization relative to wild type rats (31, 32).
Critically, ghrelin has been recently shown to play a role in compulsive-like and other maladaptive behaviors (e.g., addiction, eating disorders; for reviews: see ((33–37)). It is crucial for future investigations of these disorders to have effective KO models available rat models may have advantages over mice for several aspects of behavioral pathologies including addiction and eating disorders (for reviews: see (38–40)). This includes rats’ increased range and complexity of behavior compared to mice, as well as rats’ larger body size, which allows greater ease for several common neuroscientific methods including neurosurgery, catheter implantation, and brain imaging (40).
In this study, we describe the development, verification, and initial characterization of a novel GHSR KO Wistar rat generated using CRISPR/Cas9 technology to delete the first exon of GHSR. The ghrelin receptor was chosen in consideration of its high constitutive activity (14, 27), and given the growing interest in GHS-R1a as a novel pharmacological target for the treatment of a variety of medical and neuropsychiatric disorders. In situ hybridization (RNAscope) was used to verify GHSR mRNA expression in several brain regions. GH release and meal initiation were measured after ghrelin challenge to verify a loss-of-function resulting from ghrelin receptor knockout. Further, differences between homozygous GHSR KO and WT rats were analyzed to evaluate the effects of GHSR deletion on body composition, food intake, and locomotion.
Methods
Generation of the GHSR KO Wistar rats
The genomic sequence of the rat GHSR gene was screened for nickase-compatible gRNA pairs that flank the first exon (Figure 1A). Two pairs of gRNAs (Figure 1B) were synthesized and transcribed using Guide-IT synthesis kit (Clontech, Mountain View, CA), and injected into Wistar rat embryos along with mRNA encoding Cas9 nickase (Tri-Link, San Diego, CA). Injected embryos were implanted into pseudopregnant females and brought to term. The tissue from pups was genotyped by polymerase chain reaction (PCR). Genomic DNA was prepared from tail biopsy using Macherey-Nagel TissueSpin purification columns (Takara, Shiga, Japan). Primers flanking the gRNA target sequences (Forward 5′ CCTAGGCTTCTCACTTCTCCCTTTC, Reverse 5′ GCAGAGAAAGACAGAGACTTGAGAGAC) were used to amplify the region corresponding to exon 1 in the wild-type allele (1063 nucleotides) with OneTaq polymerase (New England Biolabs, Ipswitch, MA) for two-step PCR with an extension time of 60 sec. Twelve deletion alleles were identified out of the 57 animals that were genotyped. The largest of which was designated “T-1M” (843 bp deleted) representing a removal of the entire first exon (Figure 1A). The selected mutant GHSR was designated “W-Ghsrem1Ottc” and registered with the Rat Genome Database (#12879386) and deposited at the Rat Resource and Research Center (RRRC#827; University of Missouri, Columbia MO). Herein, “W-Ghsrem1Ottc” rats are referred to as “GHSR KO” rats. Animals were bred with WT Wistar rats from Charles River Laboratories for 4–5 generations then heterozygous animals were crossed to generate homozygous animals for experiments.
Figure 1. Sequences for gRNAs and Genomic structure of GHSR knockout allele.

(A) gRNA sequences used to target GHSR gene, with the RNAscope GHSR mRNA probe specified. (B) The coding region for GHSR consists of two exons and spans 3.1 kilobases. Four gRNAs, one “upstream” pair and one “downstream” pair were chosen to flank the first exon of GHSR in rats. The GHSR(KO) allele (T-1M) removes 846 nucleotides and encompasses exon 1. Arrows indicate relative locations of Forward and Reverse genotyping primers (sequence provided in text). GHSR: growth hormone secretagogue receptor; KO: knockout; WT: wild type.
Animals
For this initial characterization of the GHSR KO model, male rats were used. A critical point of our verification of the model is an orexigenic response to systemic ghrelin administration, and estrous cycling interferes with female rats’ appetitive responses to ghrelin (41). All rats were maintained in a temperature-controlled colony with water and food available ad libitum, except when specified. Body weight was monitored weekly during early development and experimental phases. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program, Baltimore, MD. For all experiments, similar past work was used to determine appropriate sample sizes to detect group differences. All behavioral and pharmacological tests, as well as carcass analysis, were performed using two consistent groups of WT (n = 8) and KO (n = 8) rats.
RNAscope analysis for GHSR expression in rat brain
Two WT and two homozygous GHSR KO rats, 8 weeks old, were transcardially perfused using 0.9% saline followed by 4% paraformaldehyde. The brains were kept in 30% sucrose for three days at 4°C then frozen on dry ice. Using a Leica cryostat, 12 μm coronal sections containing the prefrontal cortex, dorsal striatum, hypothalamus, hippocampus, and midbrain (VTA) were prepared. Cryosections were cut directly onto Superfrost Plus Microscope Slides (Fisher Scientific, Hampton, NH) and stored at −80 °C until in situ hybridization processing. In situ hybridization analysis of GHSR mRNA was performed using RNAscope 2.5 High Definition Detection Reagent-BROWN kit (Advanced Cell Diagnostics, Newark, CA) according to the user manual. Slides were thawed to −20 °C for 20 min and washed with 1× PBS for 5 min. Sections were pretreated with H2O2 for 10 min at room temperature followed by boiling in 1× Target Retrieval solution for 5 min. Slides were washed with ultrapure water, 100% ethanol and dried for 1 min at room temperature (RT). Dried sections were incubated for 30 min at 40°C in Protease Plus then rinsed twice with distilled H2O. The target probe specific for rat GHSR mRNA was applied to the brain sections and incubated at 40°C for 2 h in the HybEZ™ oven from Advanced Cell Diagnostics. The GHSR-O1 probe target region corresponds to bases 2–742 of rat GHSR mRNA (NM_032075.3) and this target sequence is absent in the T1-M mutation (Figure 1A). BLAST analysis indicates that the probe is specific to GHSR and sequence alignment shows 96% and 90% sequence identity with mouse and human GHSR mRNA, respectively. Sections were incubated with preamplifier and amplifier probes by applying AMP1 (40°C for 30 min), AMP2 (40°C for 15 min), AMP3 (40°C for 30 min), AMP4 (40°C for 15 min), AMP5 (RT for 30 min), and AMP6 (RT for 15 min). Between each step, sections were washed twice with 1×Wash buffer for 2 min at room temperature. To visualize the bound probe, a mixture of DAB A and DAB B solution was added to slides and incubated for 10 min. A counter stain of 50% Hematoxylin staining solution was added for 2 min, followed by 2-min ethanol rinses (70%, 95% and 95%) to dehydrate the slides. After a 5-min xylene wash, samples were air dried and mounted. Photomicrographs were captured using an OLYMPUS MVPLAPO 0.63× for low magnification and an OLYMPUS BX61 60× oil to take high magnification images. Acquisition settings were kept constant across samples.
Ghrelin challenge
Rat acyl-ghrelin (AnaSpec, Fremont, CA) was dissolved in 0.9% saline to concentrations of 4.5 nmol/kg (for GH measurement) and of 3 nmol/kg and 6 nmol/kg (for meal initiation test) for intraperitoneal (IP) injections with 1 ml/kg volume.
Growth Hormone
WT (n = 8) and KO (n = 8) rats were challenged with acyl-ghrelin to induce GH release. Rats received counterbalanced IP injections of 0 nmol (saline) or 4.5 nmol/kg ghrelin over two days, with three days of washout in between. The 4.5 nmol/kg dose was chosen because it has been previously shown to stimulate GH release (30). Tail blood was collected 15 min post IP injection. Blood was collected in EDTA coated tubes and stored on ice until centrifuged at 4000 RPM for 15 min. The supernatant from each sample was then stored at −80 °C until they were assayed. Plasma GH concentrations were analyzed using a commercial rat/mouse GH ELISA kit (EMD Millipore, Billerica, MA) and assayed in duplicate according to the manufacturer’s instructions. The intra- and inter-assay variations for rat GH were less than 5%. GH concentrations were interpolated using the 5-parameter logic equation from standard curve running for each plate.
Blood Glucose Concentrations
Concurrently with the ghrelin challenge for GH, blood glucose concentrations were also measured from tail blood 15 min after the IP injection of counterbalanced saline and 4.5 nmol/kg ghrelin. Glucose concentrations were analyzed with a commercially available Aimstrip Plus (Germaine Laboratories, San Antonio, TX) blood glucose monitor.
Meal Initiation Test
WT (n = 8) and KO (n = 8) rats were challenged with acyl-ghrelin to induce changes in meal initiation over three injection days with an ascending dose design. The 3 and 6 nmol/kg doses were chosen based on previous work demonstrating these doses generate an orexigenic response (6). On the first injection day, each rat received an IP injection of vehicle saline, and rats were immediately placed in their cages with pre-weighed food and water. Food and water consumption were measured at 1, 2, and 6 h post injection. On the second injection day, the procedure was repeated with an IP injection of 3 nmol/kg acyl-ghrelin, and on the third, an IP injection of 6 nmol/kg acyl-ghrelin. One washout day was allowed between each injection day.
Open Field
The apparatus made with white wood was a 100 cm × 100 cm square with 40 cm high walls. The center was designated as a 80 cm × 80 cm square, with all other area designated as the periphery. Rats (without ghrelin injection; WT: n = 8, KO: n = 8) were placed in the center square and their movement was recorded for 5 min. The videos were analyzed for distance moved in the periphery and the center using Ethovision tracking software.
Activity Chamber Locomotion
Rats were habituated to activity chambers (Med Associates, St. Albans, VT) equipped with infrared beams for twelve hours the day before the test. On testing day, rats were placed in the chambers the onset of the dark cycle (19:00). Locomotion was measured by crossover beam breaks for 12 h. Rats had continuous access to food and water throughout both habituation and testing.
Carcass Analysis
Rats (WT: = 8, KO: n =8) were fasted for 23 h before euthanasia (42) and carcass analysis. Experimenters were blind to the genotype of the rats throughout carcass analysis procedures. The rats were first weighed, and then deeply anesthetized with isoflurane and decapitated. Brains, livers, and the left adrenal glands were extracted and weighed. Adipose tissues including visceral fat pads (gonadal, inguinal) and intrascapular brown adipose tissue (BAT) were dissected and weighed. The carcass analysis was adapted from a previously published protocol (43).
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). The level of significance for all statistical tests was established at α = 0.05. Food and water consumption at 1, 2, and 6 h after the ghrelin challenge were analyzed with three independent two-way analyses of variance (ANOVA) with group (WT vs. KO) as the between-subjects factor and dose as the within-subjects factor. Two-way ANOVAs were used to assess locomotor activity, pre-experimental phase body weight, and experimental period body weight, with group as the between-subjects factor and time as the within-subjects factor. Growth hormone concentrations after the ghrelin challenge were analyzed using two-way ANOVA with group as the between-subjects factor and drug treatment as the within-subjects factor. The Holms-Sidak test was used for post hoc analysis, where appropriate. Paired-sample t-tests were used to compare weight differences for the carcass analysis and group differences in open field locomotion. No animals were excluded from the analysis. GraphPad Prism 7.01 was used for statistical analysis.
Results
RNAscope Verification of Knockout of the GHSR
In situ hybridization demonstrated GHSR mRNA expression in the midbrain, hypothalamus and hippocampus but not in the striatum or prefrontal cortex of WT rats (Figure 2). In GHSR KO rats, no GHSR mRNA signal was detected in any of these brain regions (Figure 2).
Figure 2. GHSR mRNA expression.

WT rats exhibit GHSR mRNA expression in midbrain, hypothalamus, hippocampus but no detectable expression in dorsal striatum and prefrontal cortex. In GHSR KO rats, no detectable mRNA expression was found in any brain region examined. Low magnification scale bar = 1 mm, High magnification scale bar = 20 μm. GHSR: growth hormone secretagogue receptor; KO: knockout; WT: wild type.
Growth Hormone
There was a significant interaction between ghrelin dose and genotype (F1,14 = 10.12, p < 0.01) on GH concentrations. Post hoc analyses confirmed that the IP ghrelin challenge, as compared to vehicle, increased blood concentrations of GH in the WT (p = 0.001) but not in the KO rats (Figure 3).
Figure 3. Blood GH concentrations after IP ghrelin challenge.

GH concentrations (mean + SEM) in GHSR KO (purple; n = 8) and WT (blue; n = 8) rats 15 min after IP injections of saline or acyl-ghrelin (4.5 nmol/kg). In the WT group, IP ghrelin administration increased blood GH concentrations compared to vehicle (p = 0.001). There was no effect of IP ghrelin on blood GH concentrations in the GHSR KO group. The GHSR KO group had lower concentrations of GH compared to the WT group regardless of vehicle or ghrelin treatment (p < 0.01). GH: growth hormone; GHSR: growth hormone secretagogue receptor; IP: intraperitoneal; KO: knockout; WT: wild type.
Blood Glucose Concentrations
There was no significant effect of IP ghrelin administration on blood glucose concentrations in either WT (vehicle: 103.8 ± 2.6 mg/dL; ghrelin: 105.4 ± 3 mg/dL) or KO (vehicle: 99.5 ± 3.6 mg/dL; ghrelin: 103.8 ± 1.4 mg/dL) groups, and there was no statistical difference in glucose concentrations between the two groups.
Meal Initiation Test
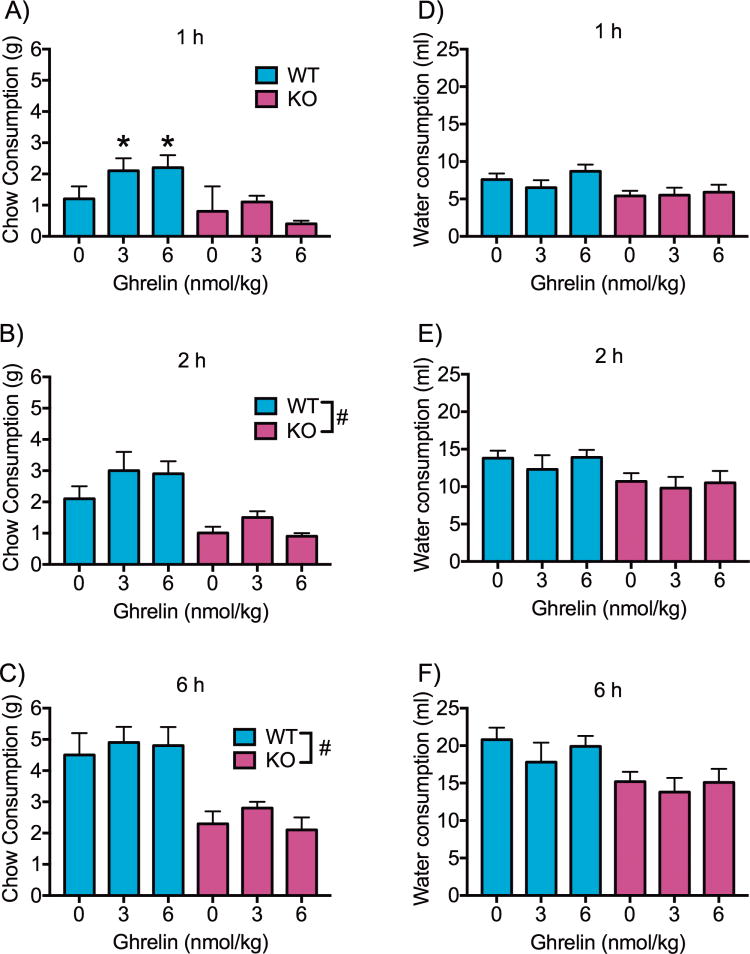
There was a significant interaction between ghrelin dose and genotype (F2,28 = 3.476, p < 0.05) at 1 h post ghrelin administration. Post hoc analyses showed a significant increase in food consumption relative to vehicle found in WT rats following administration of 3 and 6 nmol ghrelin (p’s < 0.05). However, ghrelin had no effect on food consumption in KO rats (Figure 4A). A main effect of genotype was found at 2 h (F1,14 = 11.94, p < 0.01) and 6 h (F1,14 = 15.82, p < 0.01), with KO rats eating significantly less regardless of drug treatment (Figure 4B–C). There was no effect of ghrelin or genotype on water consumption at 1, 2 or 6 h (Figure 4D–F).
Figure 4. Food and water intake after IP ghrelin challenge.
Food intake (mean + SEM) in GHSR KO (purple; n = 8) and WT (blue; n = 8) rats after IP injections of saline or acyl-ghrelin (3 nmol/kg or 6 nmol/kg) measured at 1 (A), 2 (B) and 6 h (C) post injection. In the WT group, both doses of ghrelin increased food consumption at the 1 h time point compared to vehicle (p < 0.05). There was no effect of IP ghrelin administration on food consumption in the GHSR KO group. Across time points, the GHSR KO group consumed less food than the WT regardless of vehicle or ghrelin treatment (p < 0.05, overall genotype effect; p < 0.05, different chow consumption from 0 nmol ghrelin (saline)). There was no effect of IP ghrelin administration on water intake at 1 (D), 2 (E) or 6 h (F) post injection, and there was no difference in water intake between the GHSR KO and WT groups. GHSR: growth hormone secretagogue receptor; IP: intraperitoneal; KO: knockout; SEM: standard error of the mean; WT: wild type.
Activity Chamber Locomotion
There was no difference in locomotor activity between the GHSR KO and WT groups, as measured by crossover beam breaks in an activity chamber (Figure 5A).
Figure 5. Locomotion and anxiety-like behavior.
(A) There was no difference between locomotor activity as measured by crossover beam breaks over 12 h in activity chambers (mean ± SEM) between GHSR KO (purple; n = 8) and WT rats (blue; n = 8). (B) There was no difference in distance moved in either the center or the periphery of the open field (mean ± SEM) between GHSR KO (purple; n = 8) and WT rats (blue; n = 8). GHSR: growth hormone secretagogue receptor; KO: knockout; WT: wild type.
Open Field
There was no difference between GHSR KO and WT rats for distance moved in either the center or the periphery of the open field (Figure 5B).
Body Weight
Body weight was tracked from 5 to 8 weeks of age, as well as during the experimental phase from 21 to 34 weeks of age. KO rats weighed significantly less than WT controls (Figure 6A). There was a significant effect of genotype on body weight over the 5–8 weeks of age period (F1,14 = 5.099, p < 0.05) and during the experimental phase (F1,14 = 5.217, p < 0.05).
Figure 6. Body weight, BAT, gonadal fat, and inguinal fat.
(A) Body weight (mean ± SEM) of GHSR KO (purple; n = 8) and WT (blue; n = 8) rats in the juvenile/early adulthood stage of development and during the experimental phase. GHSR KO rats weighed significantly less than WT controls in both stages (p < 0.05, overall genotype effect; see main text for details). (B) GHSR KO rats (purple; n = 8) had significantly more BAT as a percentage of body weight than GHSR WT (blue; n = 8). (C) There was no difference between concentrations of gonadal fat as a percentage of body weight between GHSR KO (purple; n = 8) and WT rats (blue; n = 8). (D) There was no difference between concentrations of inguinal fat as a percentage of body weight between GHSR KO (purple; n = 8) and WT rats (blue; n = 8). GHSR: growth hormone secretagogue receptor; KO: knockout; WT: wild type; BAT: brown adipose tissue.
Carcass Analysis
As compared to the WT group, KO rats had significantly lower body weights (WT: 743.8 ± 23.5 g; KO: 652.4 ± 30.4 g; t14 = 2.482, p < 0.05) and significantly higher percent brown adipose tissue (BAT; t14 = 2.368, p < 0.05; Figure 6B). All other body composition measures were not significantly different, including gonadal fat (Figure 6C), inguinal fat (Figure 6D), brain (WT: 2.28 ± 0.08 g; 0.32 ± 0.01 percent body weight; KO: 2.16 ± 0.04 g; 0.35± 0.01 percent body weight), and adrenal gland (WT: 0.03 ± 0.005 g; 0.004 ± 0.001 percent body weight; KO: 0.04 ± 0.01 g; 0.006 ± 0.001 percent body weight). Although a significant group difference was found for liver weights (WT: 14.76 ± 0.55 g; KO: 12.46 ± 0.39 g; t14 =3.435, p < 0.01), the difference was not statistically significant when liver weight was expressed as a percentage of total body weight (WT: 2.04 ± 0.05%; KO: 1.99 ± 0.06).
Discussion
The present results represent a neuroanatomical, neuroendocrine and initial behavioral characterization of a novel Wistar rat line with CRISP/Cas9-targeted deletion resulting in knockout of the GHSR exon 1 (Figure 1). CRISP/Cas9 deletion of the GHSR resulted in a Wistar rat that did not express detectable GHSR mRNA in several brain regions (Figure 2) where it is normally expressed (hypothalamus, hippocampus, and midbrain). The KO rats were totally insensitive to the GH secreting (Figure 3) and meal initiating/orexigenic (Figure 4) effects of a systemic acyl-ghrelin administration. The KO exhibited a phenotype of chronic reduced body weight (Figure 6A) and blunted food consumption (see vehicle condition over 6 h in Figure 4). Both WT and KO rats displayed similar levels of locomotor activity in activity chambers (Figure 5A) and in the the center or the periphery of the open field (Figure 5B). Moreover, WT and KO rats did not differ for gross body composition except for higher levels of BAT observed in KO rats (Figure 6). The present findings from this novel GHSR KO rat adds to the existing literature by confirming the functional role of the ghrelin receptor in the orexigenic effects of ghrelin, and will be useful as a research tool for examining the role of ghrelin signaling in feeding, metabolism, stress, memory, and in disorders of excessive and compulsive behavior.
A significant strength of our GHSR KO rat model is the Wistar background. We chose Wistar rats for several reasons. First, Wistar rats rapidly gain weight, as shown by our WT rats reaching over 600 g by 6 months of age on a laboratory chow diet (Figure 6A). Second, Wistar rats fed high fat and sugar diets gain significant weight compared with animals fed standard chow, and are commonly used as models of obesity (44–46). Third, Wistar rats are the basis for models of susceptibility to obesity and metabolic disease, including the Wistar fatty rat and the Wistar Ottawa Karlsburg W rat (47–49). Fourth, Wistar rats are also commonly used to study drug addiction, eating and other compulsive-like behaviors (50–52). An important limitation of the work conducted until now with this model is that it was limited to male rats. Future studies should seek to explore this further in characterizing female GHSR KO rats, possibly through pharmacological control of estrous or through the utilization of ovariectomized females.
In the previously published GHSR KO rat on the FHH background, the KO was the result of a C>T transition mutation creating a stop codon that produced a protein truncated at the C-terminus by 21 amino acids (30). Although > 90% of the protein was still produced, the KO rats exhibited a loss of GHSR function. In the current approach, we targeted the entire first exon to ensure a truncated form of the receptor is not produced, as verified by RNAscope in situ hybridization showing a lack of GHSR mRNA expression. We also observed a phenotype consistent with loss of GHSR function. It is important to point out that this is a model of global GHSR KO and although we used brain tissue only to verify the lack of GHS mRNA expression, obviously there is no reason to assume that the deletion was limited to the brain. As a matter of fact, it is conceivable that the physiological and/or behavioral phenotype we observed was at least partially due to the loss of peripheral ghrelin signaling related to the role of GHS-R1a in the viscera, specifically expressed in vagal afferents. Future work should continue to parse out the mechanisms underlying this phenotype, including differentiation between central and peripheral ghrelin pathways.
Further supporting the contribution of both central and peripheral loss of GHS-R1a in our model, a particularly intriguing result from the current study is the finding that GHSR KO rats had increased BAT as a percentage of total body weight compared to WT animals. BAT is primarily responsible for energy expenditure through thermogenesis (53), and both obese humans and rodents have decreased BAT activity (54). The decreased body weight of the ghrelin receptor KO rats compared with the WT group may be not only due to reduction in food consumption, but also by increased thermal energy output due to higher BAT content. Our results are consistent with a previous study using transgenic rats expressing an antisense GHSR mRNA under the control of the promoter for tyrosine hydroxylase (selectively attenuating GHSR protein expression in the arcuate nucleus) (55); this transgenic manipulation resulted in higher BAT weight (56). Furthermore, a clinical study with 18 healthy men indicated that cold-induced BAT activation was significantly associated with lower serum ghrelin concentrations (57). Mechanistically, previous work shows that not only do GHSR KO mice have increased energy expenditure, but also ablation of GHSR attenuates age-associated decline in thermogenesis; the BAT of older GHSR KO mice reveals enhanced thermogenic capacity, suggesting a shift in adiposity balance from fat storage to thermogenesis, possibly via peripheral and/or central mechanisms (56, 58–60). Future studies will need to investigate the nuances of potential body composition and metabolic differences between GHSR KO and WT rats to shed light on the potential specific mechanisms linking the BAT and the ghrelin system.
The presented GHSR KO model is particularly useful because rats are commonly used in behavioral neuroscience research, and therefore provides a critical tool for continued investigations of the ghrelin system. The present GHSR KO rat model has the potential to facilitate our understanding of the role of the ghrelin system in a wide range of diseases, including medical disorders that represent leading causes of mortality and morbidity worldwide. This includes metabolic disruptions and obesity, which have long been a focus of ghrelin related research. In addition, this transgenic rat is essential to increasing our burgeoning knowledge of the ghrelin system’s role in disorders of acute and/or chronic stress and in addictive, eating and other compulsive-like behaviors. In conclusion, this novel GHSR KO rat model provides an important, verified tool for further research into the ghrelin system in biomedical research.
Acknowledgments
This work was supported by the National Institute on Drug Abuse Intramural Research Program (LJZ, BJT, CTR, YJZ, ZBY, ELG, GFK, LFV, BKH, LL), the National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research (LJZ, YJZ, MH, LL), and the National Institute of Mental Health Intramural Research Program (JP).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105(17):6320–5. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science (New York, NY) 1996;273(5277):974–7. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 5.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab. 2015;4(6):437–60. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141(11):4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 7.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. American journal of physiology Endocrinology and metabolism. 2004;287(2):E297–304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- 9.Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R353–R8. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- 10.Schele E, Bake T, Rabasa C, Dickson SL. Centrally Administered Ghrelin Acutely Influences Food Choice in Rodents. PLoS One. 2016;11(2):e0149456. doi: 10.1371/journal.pone.0149456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 12.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147(1):23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- 13.Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31(1):44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Howick K, Griffin BT, Cryan JF, Schellekens H. From Belly to Brain: Targeting the Ghrelin Receptor in Appetite and Food Intake Regulation. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersenn S, Rasch AC, Penshorn M, Beil FU, Schulte HM. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology. 2001;142(6):2649–59. doi: 10.1210/endo.142.6.8184. [DOI] [PubMed] [Google Scholar]
- 16.Chow KB, Sun J, Chu KM, Tai Cheung W, Cheng CH, Wise H. The truncated ghrelin receptor polypeptide (GHS-R1b) is localized in the endoplasmic reticulum where it forms heterodimers with ghrelin receptors (GHS-R1a) to attenuate their cell surface expression. Molecular and cellular endocrinology. 2012;348(1):247–54. doi: 10.1016/j.mce.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Navarro G, Aguinaga D, Angelats E, Medrano M, Moreno E, Mallol J, et al. A Significant Role of the Truncated Ghrelin Receptor GHS-R1b in Ghrelin-induced Signaling in Neurons. The Journal of biological chemistry. 2016;291(25):13048–62. doi: 10.1074/jbc.M116.715144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. The Journal of comparative neurology. 2006;494(3):528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melis MR, Mascia MS, Succu S, Torsello A, Muller EE, Deghenghi R, et al. Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neurosci Lett. 2002;329(3):339–43. doi: 10.1016/s0304-3940(02)00673-0. [DOI] [PubMed] [Google Scholar]
- 20.Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, et al. Growth hormone secretagogue binding sites in peripheral human tissues. The Journal of clinical endocrinology and metabolism. 2000;85(10):3803–7. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- 21.Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11318–23. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107(16):7467–72. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albarran-Zeckler RG, Sun Y, Smith RG. Physiological roles revealed by ghrelin and ghrelin receptor deficient mice. Peptides. 2011;32(11):2229–35. doi: 10.1016/j.peptides.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang K, Zmuda E, Sleeman MW. Physiological role of ghrelin as revealed by the ghrelin and GOAT knockout mice. Peptides. 2011;32(11):2236–41. doi: 10.1016/j.peptides.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A, Touzani K, Ackroff K. Ghrelin signaling is not essential for sugar or fat conditioned flavor preferences in mice. Physiol Behav. 2015;149:14–22. doi: 10.1016/j.physbeh.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kouno T, Akiyama N, Fujieda K, Nanchi I, Okuda T, Iwasaki T, et al. Reduced intake of carbohydrate prevents the development of obesity and impaired glucose metabolism in ghrelin O-acyltransferase knockout mice. Peptides. 2016;86:145–52. doi: 10.1016/j.peptides.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Mear Y, Enjalbert A, Thirion S. GHS-R1a constitutive activity and its physiological relevance. Front Neurosci. 2013;7:87. doi: 10.3389/fnins.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol Endocrinol. 2003;17(11):2201–10. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 29.Bulbul M, Babygirija R, Zheng J, Ludwig K, Xu H, Lazar J, et al. Food intake and interdigestive gastrointestinal motility in ghrelin receptor mutant rats. Journal of gastroenterology. 2011;46(4):469–78. doi: 10.1007/s00535-010-0366-6. [DOI] [PubMed] [Google Scholar]
- 30.MacKay H, Charbonneau VR, St-Onge V, Murray E, Watts A, Wellman MK, et al. Rats with a truncated ghrelin receptor (GHSR) do not respond to ghrelin, and show reduced intake of palatable, high-calorie food. Physiol Behav. 2016;163:88–96. doi: 10.1016/j.physbeh.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 31.Clifford PS, Rodriguez J, Schul D, Hughes S, Kniffin T, Hart N, et al. Attenuation of cocaine-induced locomotor sensitization in rats sustaining genetic or pharmacologic antagonism of ghrelin receptors. Addict Biol. 2012;17(6):956–63. doi: 10.1111/j.1369-1600.2011.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifford S, Zeckler RA, Buckman S, Thompson J, Hart N, Wellman PJ, et al. Impact of food restriction and cocaine on locomotion in ghrelin- and ghrelin-receptor knockout mice. Addict Biol. 2011;16(3):386–92. doi: 10.1111/j.1369-1600.2010.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panagopoulos VN, Ralevski E. The role of ghrelin in addiction: a review. Psychopharmacology (Berl) 2014;231(14):2725–40. doi: 10.1007/s00213-014-3640-0. [DOI] [PubMed] [Google Scholar]
- 34.Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. Ghrelin’s Role in the Hypothalamic-Pituitary-Adrenal Axis Stress Response: Implications for Mood Disorders. Biol Psychiatry. 2015;78(1):19–27. doi: 10.1016/j.biopsych.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Wittekind DA, Kluge M. Ghrelin in psychiatric disorders - A review. Psychoneuroendocrinology. 2015;52:176–94. doi: 10.1016/j.psyneuen.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Leggio L. Role of the ghrelin system in alcoholism: Acting on the growth hormone secretagogue receptor to treat alcohol-related diseases. Drug news & perspectives. 2010;23(3):157–66. doi: 10.1358/dnp.2010.23.3.1429490. [DOI] [PubMed] [Google Scholar]
- 37.Zallar LJ, Farokhnia M, Tunstall BJ, Vendruscolo LF, Leggio L. The Role of the Ghrelin System in Drug Addiction. International review of neurobiology. 2017;136:89–119. doi: 10.1016/bs.irn.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, et al. Rats are the smart choice: Rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology. 2014;76(Pt B):250–8. doi: 10.1016/j.neuropharm.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vengeliene V. The role of ghrelin in drug and natural reward. Addict Biol. 2013;18(6):897–900. doi: 10.1111/adb.12114. [DOI] [PubMed] [Google Scholar]
- 40.Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9(10):1079–87. doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56(4):1051–8. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein JL, Zhao TJ, Li RL, Sherbet DP, Liang G, Brown MS. Surviving starvation: essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol. 2011;76:121–7. doi: 10.1101/sqb.2011.76.010447. [DOI] [PubMed] [Google Scholar]
- 43.Mann A, Thompson A, Robbins N, Blomkalns AL. Localization, identification, and excision of murine adipose depots. Journal of visualized experiments : JoVE. 2014;(94) doi: 10.3791/52174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azzout-Marniche D, Chalvon-Demersay T, Pimentel G, Chaumontet C, Nadkarni NA, Piedcoq J, et al. Obesity-prone high-fat-fed rats reduce caloric intake and adiposity and gain more fat-free mass when allowed to self-select protein from carbohydrate:fat intake. Am J Physiol Regul Integr Comp Physiol. 2016;310(11):R1169–76. doi: 10.1152/ajpregu.00391.2015. [DOI] [PubMed] [Google Scholar]
- 45.Lima ML, Leite LH, Gioda CR, Leme FO, Couto CA, Coimbra CC, et al. A Novel Wistar Rat Model of Obesity-Related Nonalcoholic Fatty Liver Disease Induced by Sucrose-Rich Diet. Journal of diabetes research. 2016;2016:9127076. doi: 10.1155/2016/9127076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aslani S, Vieira N, Marques F, Costa PS, Sousa N, Palha JA. The effect of high-fat diet on rat’s mood, feeding behavior and response to stress. Translational psychiatry. 2015;5:e684. doi: 10.1038/tp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikeda H, Shino A, Matsuo T, Iwatsuka H, Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty) Diabetes. 1981;30(12):1045–50. doi: 10.2337/diab.30.12.1045. [DOI] [PubMed] [Google Scholar]
- 48.Kovacs P, Voigt B, Berg S, Vogt L, Kloting I. WOK.1W rats. A potential animal model of the insulin resistance syndrome. Annals of the New York Academy of Sciences. 1997;827:94–9. doi: 10.1111/j.1749-6632.1997.tb51824.x. [DOI] [PubMed] [Google Scholar]
- 49.Aleixandre de Artinano A, Miguel Castro M. Experimental rat models to study the metabolic syndrome. The British journal of nutrition. 2009;102(9):1246–53. doi: 10.1017/S0007114509990729. [DOI] [PubMed] [Google Scholar]
- 50.Vendruscolo JC, Tunstall BJ, Carmack SA, Schmeichel BE, Lowery-Gionta EG, Cole M, et al. Compulsive-Like Sufentanil Vapor Self-Administration in the Rat. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. The European journal of neuroscience. 2008;28(8):1641–53. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holtz NA, Carroll ME. Cocaine self-administration punished by intravenous histamine in adolescent and adult rats. Behavioural pharmacology. 2015;26(4):393–7. doi: 10.1097/FBP.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CANNON B, NEDERGAARD J. Brown Adipose Tissue: Function and Physiological Significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 54.Elattar S, Satyanarayana A. Can Brown Fat Win the Battle Against White Fat? Journal of cellular physiology. 2015;230(10):2311–7. doi: 10.1002/jcp.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, et al. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest. 2002;109(11):1429–36. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mano-Otagiri A, Iwasaki-Sekino A, Nemoto T, Ohata H, Shuto Y, Nakabayashi H, et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regulatory peptides. 2010;160(1–3):81–90. doi: 10.1016/j.regpep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Chondronikola M, Porter C, Malagaris I, Nella AA, Sidossis LS. Brown adipose tissue is associated with systemic concentrations of peptides secreted from the gastrointestinal system and involved in appetite regulation. European journal of endocrinology. 2017;177(1):33–40. doi: 10.1530/EJE-16-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma X, Lin L, Qin G, Lu X, Fiorotto M, Dixit VD, et al. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PloS one. 2011;6(1):e16391. doi: 10.1371/journal.pone.0016391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin L, Sun Y. Thermogenic characterization of ghrelin receptor null mice. Methods in enzymology. 2012;514:355–70. doi: 10.1016/B978-0-12-381272-8.00022-2. [DOI] [PubMed] [Google Scholar]
- 60.Lin L, Lee JH, Bongmba OY, Ma X, Zhu X, Sheikh-Hamad D, et al. The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Aging. 2014;6(12):1019–32. doi: 10.18632/aging.100706. [DOI] [PMC free article] [PubMed] [Google Scholar]