Abstract
Soil salinity is an important abiotic stress worldwide, and salt-induced oxidative stress can have detrimental effects on the biological nitrogen fixation. We hypothesized that co-inoculation of cowpea plants with Bradyrhizobium and plant growth-promoting bacteria would minimize the deleterious effects of salt stress via the induction of enzymatic and non-enzymatic antioxidative protection. To test our hypothesis, cowpea seeds were inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and plant growth-promoting bacteria and then submitted to salt stress. Afterward, the cowpea nodules were collected, and the levels of hydrogen peroxide; lipid peroxidation; total, reduced and oxidized forms of ascorbate and glutathione; and superoxide dismutase, catalase and phenol peroxidase activities were evaluated. The sodium and potassium ion concentrations were measured in shoot samples. Cowpea plants did not present significant differences in sodium and potassium levels when grown under non-saline conditions, but sodium content was strongly increased under salt stress conditions. Under non-saline and salt stress conditions, plants co-inoculated with Bradyrhizobium and Actinomadura or co-inoculated with Bradyrhizobium and Paenibacillus graminis showed lower hydrogen peroxide content in their nodules, whereas lipid peroxidation was increased by 31% in plants that were subjected to salt stress. Furthermore, cowpea nodules co-inoculated with Bradyrhizobium and plant growth-promoting bacteria and exposed to salt stress displayed significant alterations in the total, reduced and oxidized forms of ascorbate and glutathione. Inoculation with Bradyrhizobium and plant growth-promoting bacteria induced increased superoxide dismutase, catalase and phenol peroxidase activities in the nodules of cowpea plants exposed to salt stress. The catalase activity in plants co-inoculated with Bradyrhizobium and Streptomyces was 55% greater than in plants inoculated with Bradyrhizobium alone, and this value was remarkably greater than that in the other treatments. These results reinforce the beneficial effects of plant growth-promoting bacteria on the antioxidant system that detoxifies reactive oxygen species. We concluded that the combination of Bradyrhizobium and plant growth-promoting bacteria induces positive responses for coping with salt-induced oxidative stress in cowpea nodules, mainly in plants co-inoculated with Bradyrhizobium and P. graminis or co-inoculated with Bradyrhizobium and Bacillus.
Keywords: Plant–bacteria interactions, Reactive oxygen species, Salinity
Introduction
The cowpea plant (Vigna unguiculata [L.] Walp.) is a legume of great agronomic importance worldwide. This crop is traditionally planted in the semi-arid northeast agricultural regions of Brazil and is considered to be adapted to this climatic condition.1, 2 The Brazilian semi-arid northeast has low rainfall combined with high temperatures, which results in soil with low fertility and salinization. Furthermore, the use of water with high salt levels and an inadequate irrigation management system can increase the salinization in semi-arid regions.3 Soil salinity is an abiotic stress that limits plant development worldwide.4 By definition, saline soil displays electrical conductivity superior to 4.0 dS m−1 at 25 °C (approximately 40 mM NaCl) and a concentration of 15% exchangeable sodium (Na+) ion in the root zone. According Jamil et al.,5 more than 50% of arable land will be salinized by 2050.
Soil salinity is an edaphoclimatic limitation that affects crop productivity. In addition to reducing soil use capacity, soil salinity disturbs basic physiological processes, such as photosynthesis and nutrient uptake, and thus decreases plant growth and development.3, 5 Symbiosis and nodule functions are very sensitive to soil salinity, which can reduce nodulation and strongly decrease nitrogenase activity and biological nitrogen fixation (BNF).6 BNF is an important process by which nitrogen is supplied to plants via nitrogen-fixing bacteria (also called rhizobia).7 Cowpea plants establish symbiotic relationships with rhizobia, which form root nodules where BNF occurs. The root nodules can provide approximately 200 kg ha−1 of nitrogen to cowpea plants under optimal conditions and cause a positive soil nitrogen balance up to 92 kg ha−1.1, 8 Therefore, BNF can replace chemical fertilizers and contribute to plant nutrition under unfavorable soil conditions.8
Many free-living microorganisms among different genera, such as Azospirillum, Azotobacter, Actinomadura, Bacillus, Pseudomonas, Streptomyces, Serratia and Xanthomonas, are collectively designated as plant growth-promoting bacteria (PGPB).9 PGPB are commonly used as plant biostimulants to increase root proliferation, promote elongation of the shoots and roots, facilitate nutrient uptake and modulate plant hormones, mainly abscisic acid and ethylene.9, 11 PGPB can enhance plant tolerance to abiotic stressors, especially drought, metal toxicity and salt stress.10 Additionally, combined inoculation of legumes with rhizobia and PGPB can increase the number of nodules compared with those formed by rhizobia alone9 and thereby may improve plant performance and crop yields under stressful conditions.4, 5
Plants under salt stress are vulnerable to ionic toxicity, osmotic stress, and, inevitably, the overproduction of reactive oxygen species (ROS), such as singlet oxygen (1O2), hydroxyl radical (•OH), superoxide anion (O2•−) and hydrogen peroxide (H2O2). Salinity-induced ROS formation causes oxidative damage to lipids, proteins and other cellular components.7, 8, 11 To cope with this situation, plants have developed mechanisms to survive under high-salinity conditions with an emphasis on ionic compartmentalization, synthesis of compatible solutes, and recruitment of antioxidative enzymes and compounds that neutralize and detoxify ROS.4, 6, 11 Superoxide dismutase (SOD), catalase (CAT) and phenol peroxidase (POX) are antioxidative enzymes that are directly involved in defense mechanisms against ROS.6, 11 Antioxidant compounds, such as ascorbate, glutathione, α-tocopherol and carotenoids, also neutralize ROS.6, 11, 12 Overall, increased levels of ROS-scavenging compounds may promote plant tolerance to various stressors, including salinity.3, 4, 10
The use of rhizobia-PGPB as inoculants to mitigate stressful conditions represents a powerful technique in agricultural biotechnology for sustainable crop production. For example, triple inoculation of cowpea with Bradyrhizobium, Pseudomonas graminis and Pseudomonas durus reduced the deleterious effects of the oxidative stress.13 Bradyrhizobium subtilis enhanced nodulation, nutrient uptake and plant growth of Trigonella plants nodulated by Ensifer meliloti and submitted to water stress.14 When the herbaceous legume Galega officinalis L. was grown in salt-amended soil, the combination of Rhizobium and Pseudomonas alleviated salt stress.15 Given this context, this study proposed to test the hypothesis that co-inoculation of cowpea plants with bradyrhizobia and PGPB would minimize the deleterious effects of salt stress by inducing enzymatic and non-enzymatic antioxidative protection and maintaining the BNF.
Material and methods
Microorganisms and preparation of the inoculant
The general aspects and the culture medium utilized to purify and multiply rhizobia and PGPB are described as follow. Bradyrhizobium sp. (UFLA 03-84 strain) was obtained from pasture soil samples collected in Jí-Paraná (Rondônia, Brazil). The PGPB Actinomadura sp. (183-EL strain) was obtained from Caatinga rhizosphere samples (Pernambuco, Brazil), while the PGPB Paenibacillus graminis (MC 04.21 strain) was isolated from corn (Zea mays L.) rhizospheres acquired from Cerrado soil (Brazil). Bacillus sp. (IPACC11 strain) was isolated from sugarcane stalks (Saccharum officinarum L.) obtained from the forest zone of Pernambuco (Brazil), and the PGPB Streptomyces sp. (212 strain) was isolated from arugula rhizospheres (Brazil).
Bradyrhizobium sp. was purified using yeast mannitol agar (YMA) and multiplied in yeast mannitol (YM) medium, both at pH 6.5.16 Bacillus sp. was purified and multiplied in dextrose yeast glucose sucrose (DYGS) medium at pH 6.0.17 Trypticase soy agar (TSA) and trypticase soy broth (TSB) medium, both at pH 7.3, were used to purify and multiply P. graminis. Actinomadura sp. and Streptomyces sp. were purified and multiplied in arginine yeast (AY) and arginine yeast agar (AYA), both at pH 6.4.18 The Bradyrhizobium sp. inoculant was incubated in YM liquid medium for 96 h on a rotating shaker (220 rpm; 28 °C), whereas the PGPB inoculants were maintained on a rotating shaker (200 rpm; 28 °C) for 48–96 h according to the growth requirements of each strain.
Plant cultivation and treatments
The experiment was conducted under axenic conditions in a greenhouse at the Agronomical Institute of Pernambuco (IPA; Recife-PE). Cowpea seeds cv. “IPA-206” were disinfected and sown in Leonard jars containing washed (pH 6.5) and autoclaved (120 °C, 101 kPa, 1 h) sand. During the sowing period, cowpea seeds were simultaneously inoculated with 1.0 mL of a bacterial suspension containing Bradyrhizobium (108 CFU mL−1) or with Bradyrhizobium and PGPB in one of the following combinations: Bradyrhizobium and Actinomadura; Bradyrhizobium and Bacillus; Bradyrhizobium and P. graminis; or Bradyrhizobium and Streptomyces. Co-inoculation was accomplished with 1.0 mL of a bacterial suspension containing Bradyrhizobium and 1.0 mL of a bacterial suspension containing a PGPB strain (107 UFC mL−1). Non-inoculated plants were used as absolute control.
Initially, all cowpea plants were irrigated by capillaries containing a modified nitrogen-free nutrient solution (pH 6.5).19, 20 Thinning of cowpea seedlings was performed four days after germination (DAG), and two plants were retained in each Leonard jar (experimental unit). For salt stress conditions imposed at 15 DAG, the modified nitrogen-free nutrient solution was supplemented with 50 mmol L−1 sodium chloride (NaCl). The electrical conductivity (EC) of the nutrient solution without and with NaCl was 0.99 mS cm−1 and 5.60 mS cm−1, respectively. The nutrient solutions with and without NaCl were changed weekly. During the nutrient solution exchange, the substrate was washed with distilled water, and the pH and the EC of the drainage were measured and compared with the corresponding values of the solution in the vessel. The plants were harvested at 37 DAG (the plants were exposed to NaCl for a total of 23 days). At the time of collection, roots with nodules were frozen in liquid N2 and stored at −80 °C until the biochemical analyses.
Measurements
Sodium and potassium contents were determined according to methods described by Cavalcanti et al.21 and Silveira et al.22 Cowpea nodule samples (∼50 mg) were extracted in a water bath at 95 °C. After cooling and filtering, measurements were performed with a flame photometer (Micronal, Brazil). After extraction with 5% (w/v) trichloroacetic acid, lipid peroxidation was determined by the quantifying the amount of malondialdehyde-thiobarbituric acid (MDA-TBA) complex23; H2O2 content according to the methods described by Brennan and Frenkel24; total, reduced and oxidized ascorbate using methods described by Kampfenkel et al.25; and total, reduced and oxidized glutathione as described by Griffith.26 The redox states of ascorbate and glutathione were calculated and expressed as percentages. Catalase (EC 1.11.1.6),27 phenol peroxidase (EC 1.11.1.7)28 and superoxide dismutase (EC 1.15.1.1)29 were measured after extraction with 100 mM K-phosphate buffer (pH 7.0) containing 1.0 mM EDTA.
Experimental design and statistical analysis
The experimental design was randomized blocks with a factorial design of 5 × 2 + 1, including five bacterial combinations (one inoculation with Bradyrhizobium and four co-inoculations with Bradyrhizobium and PGPB), two salinity levels (0 and 50 mmol L−1 of NaCl), and an absolute control (non-inoculated plants grown without nitrogen and cultivated in non-saline conditions). Two replicates per block were performed. The results obtained were subjected to analysis of variance (ANOVA) preceded by the F test at a 5% probability level. Means were compared using Tukey's test at the 5% probability level. All statistical analyses were performed using ASSISTAT software (version 7.7 beta).30
Results
Cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB did not present significant differences (Tukey test; p < 0.05) in shoot sodium (Na+) levels when grown under non-saline conditions but displayed significant increases in shoot Na+ content when submitted to salt stress conditions (Table 1). In relation to the absolute controls, the shoot Na+ contents in cowpea plants inoculated with Bradyrhizobium and co-inoculated with Bradyrhizobium and PGPB were increased in the non-saline and salt stress conditions (Table 1). Cowpea plants co-inoculated with Bradyrhizobium and P. graminis or co-inoculated with Bradyrhizobium and Streptomyces accumulated higher shoot Na+ contents compared with cowpea plants inoculated with Bradyrhizobium and grown under salt stress conditions (Table 1). In these plants, shoot Na+ content increased by 12% and 23%, respectively, in relation to cowpea plants inoculated with Bradyrhizobium and exposed to salt stress.
Table 1.
Sodium (Na+) and potassium (K+) contents, both in mmol g−1 DW, in the shoots of cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB. Absolute control is non-inoculated plants.
| Treatments | Na+ content |
K+ content |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | Salt stress | Control | Salt stress | |||||
| Absolute control | 0.008 | a | – | 0.39 | a | – | ||
| Bradyrhizobium | 0.012 | aB | 1.40 | bcA | 1.22 | bcA | 1.41 | aA |
| Bradyrhizobium and Actinomadura | 0.014 | aB | 1.31 | cA | 1.26 | bcA | 0.91 | bB |
| Bradyrhizobium and P. graminis | 0.086 | aB | 1.57 | abA | 1.73 | aA | 1.31 | aB |
| Bradyrhizobium and Bacillus | 0.016 | aB | 1.47 | bcA | 1.46 | abA | 1.28 | abA |
| Bradyrhizobium and Streptomyces | 0.016 | aB | 1.73 | aA | 1.00 | cA | 1.08 | abA |
| Coefficient of variance (%) | 15.35 | 14.42 | ||||||
Significant difference (p < 0.05) between absolute control and inoculated plants in non-saline conditions by Dunnett's test. In each column, means followed by the same lowercase letters do not differ statistically by Tukey's test (p < 0.05), whereas uppercase letters represent significant differences between control and salt stress conditions.
Cowpea plants co-inoculated with Bradyrhizobium and P. graminis or co-inoculated with Bradyrhizobium and Bacillus displayed higher shoot potassium (K+) content when grown under non-saline conditions, and these levels were 42% and 20% greater, respectively, compared with those observed in cowpea plants inoculated with Bradyrhizobium (Table 1). As observed with shoot Na+ levels, K+ content was significantly increased in shoots from cowpea plants inoculated with Bradyrhizobium and co-inoculated with Bradyrhizobium and PGPB when compared with the absolute controls (Table 1). When cultivated under salinity conditions, plants co-inoculated with Bradyrhizobium and Actinomadura or Bradyrhizobium and Streptomyces showed reductions of 36% and 23% in shoot K+ content, respectively, when compared with cowpea plants inoculated with Bradyrhizobium (Table 1). Moreover, cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and Bacillus or Bradyrhizobium and Streptomyces were able to maintain the same K+ levels under non-saline and salt stress situations. On the other hand, plants co-inoculated with Bradyrhizobium and Actinomadura, Bradyrhizobium and P. graminis or Bradyrhizobium and Bacillus exhibited decreased shoot K+ levels when submitted to salt stress.
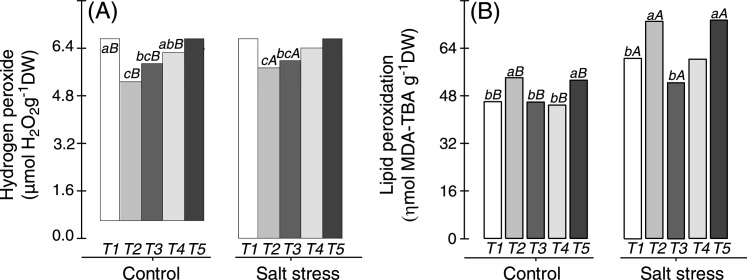
Hydrogen peroxide (H2O2) content was measured in cowpea plant nodules inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB, and the results are shown in Fig. 1. When grown under non-saline or salt stress conditions, plants co-inoculated with Bradyrhizobium and Actinomadura or Bradyrhizobium and P. graminis showed lower H2O2 content in their nodules, whereas plants inoculated with Bradyrhizobium and plants co-inoculated with Bradyrhizobium and Streptomyces displayed higher H2O2 content in their nodules (Fig. 1A). Lipid peroxidation was greater in cowpea plant nodules co-inoculated with Bradyrhizobium and Actinomadura and in plants co-inoculated with Bradyrhizobium and Streptomyces in non-saline and saline conditions, which indicated a lower efficiency of the antioxidant system in the nodules of these plants compared with those of other plants (Fig. 1B). On average, lipid peroxidation increased by 31% when the plants were subjected to salt stress in relation to plants cultivated under non-saline conditions (Fig. 1B).
Fig. 1.
Hydrogen peroxide (A) and lipid peroxidation (B) contents in the nodules of cowpea plants inoculated with Bradyrhizobium (T1) or co-inoculated with Bradyrhizobium and Actinomadura (T2), Bradyrhizobium and Paenibacillus graminis (T3), Bradyrhizobium and Bacillus (T4) or Bradyrhizobium and Streptomyces (T5) in control (non-saline) and salt stress (50 mmol L−1 NaCl) conditions. Means followed by the same lowercase letters do not differ statistically between bacterial combinations, whereas uppercase letters represent significant differences between control and salt stress. All mean comparisons were performed using Tukey's test (p < 0.05).
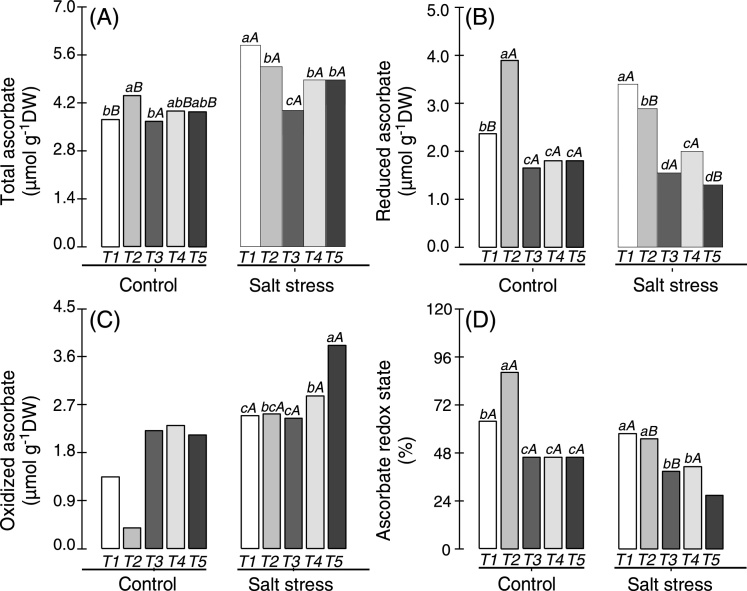
In non-saline and salt stress conditions, total, reduced and oxidized forms of ascorbate and glutathione (non-enzymatic antioxidants) measured in the nodules of cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB showed significant alterations (Fig. 2). Cowpea plants co-inoculated with Bradyrhizobium and Actinomadura showed higher total ascorbate in their nodules when cultivated under non-saline conditions, and this value was 20% greater than that observed in cowpea plants inoculated with Bradyrhizobium (Fig. 2A). Under salt stress, total ascorbate was increased in all treatments compared with non-saline conditions, except in cowpea plants co-inoculated with Bradyrhizobium and P. graminis. In these plants, total ascorbate was decreased by approximately 32% when compared with cowpea plants inoculated with Bradyrhizobium. Overall, the highest total ascorbate levels were observed in the nodules of cowpea plants inoculated with Bradyrhizobium and submitted to salt stress conditions (Fig. 2A).
Fig. 2.
Total (A), reduced (B) and oxidized (C) forms of ascorbate and redox ascorbate (D) in the nodules of cowpea plants inoculated with Bradyrhizobium (T1) or co-inoculated with Bradyrhizobium and Actinomadura (T2), Bradyrhizobium and Paenibacillus graminis (T3), Bradyrhizobium and Bacillus (T4) or Bradyrhizobium and Streptomyces (T5) in control (non-saline) and salt stress (50 mmol L−1 NaCl) conditions. Means followed by the same lowercase letters do not differ statistically between bacterial combinations, whereas uppercase letters represent significant differences between control and salt stress. All mean comparisons were performed using Tukey's test (p < 0.05).
Reduced ascorbate was significantly altered in the nodules of cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB (Fig. 2B). While cowpea plants co-inoculated with Bradyrhizobium and Actinomadura and cultivated in non-saline conditions showed a 65% increase in reduced ascorbate relative to the plants inoculated with Bradyrhizobium, the cowpea plants co-inoculated with Bradyrhizobium and other PGPB (P. graminis, Bacillus or Streptomyces) showed a reduction of approximately 30% when compared with plants inoculated with Bradyrhizobium (Fig. 2B). Under salt stress conditions, all plants co-inoculated with Bradyrhizobium and PGPB showed decreased levels of reduced ascorbate in relation to cowpea plants inoculated with Bradyrhizobium, mainly in plants co-inoculated with Bradyrhizobium and P. graminis (55% reduction) or co-inoculated with Bradyrhizobium and Streptomyces (62% decrease). Plants co-inoculated with Bradyrhizobium and P. graminis or Bradyrhizobium and Bacillus did not exhibit changes in reduced ascorbate when plants cultivated under non-saline and salt stress conditions were compared.
In non-saline conditions, oxidized ascorbate was higher in the nodules of cowpea plants co-inoculated with Bradyrhizobium and P. graminis, Bradyrhizobium and Bacillus or Bradyrhizobium and Streptomyces (Fig. 2C). These plants showed a 65% increase in oxidized ascorbate when compared with plants inoculated with Bradyrhizobium, whereas plants co-inoculated with Bradyrhizobium and Actinomadura exhibited a 71% decrease in oxidized ascorbate. All cowpea plants (inoculated or co-inoculated) showed increased levels of oxidized ascorbate in their nodules when submitted to salt stress (Fig. 2C), especially plants co-inoculated with Bradyrhizobium and Actinomadura, which displayed a 551% increase when the non-saline and salt stress conditions were compared. Overall, under salt stress conditions, the highest value of oxidized ascorbate (3.8 μmol g−1 DW) was observed in the nodules of cowpea plants co-inoculated with Bradyrhizobium and Streptomyces, and these levels were 53% greater than those observed in cowpea plants inoculated with Bradyrhizobium (Fig. 2C).
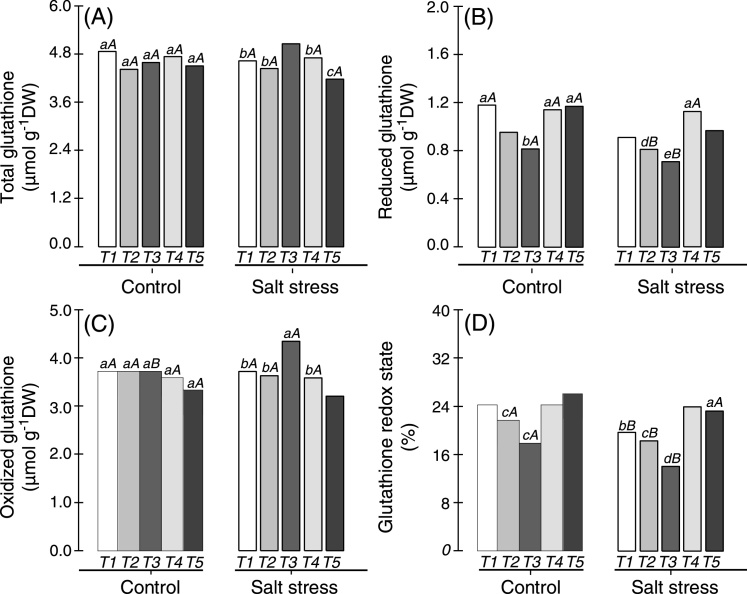
Plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB did not present changes in total glutathione when cultivated under non-saline conditions, but this parameter was altered in cowpea plants exposed to salt stress (Fig. 3A). Cowpea plants co-inoculated with Bradyrhizobium and P. graminis showed the highest levels of total glutathione (5.1 μmol g−1 DW) when exposed to salt stress conditions, whereas the lowest value was registered in cowpea plants co-inoculated with Bradyrhizobium and Streptomyces. Under non-saline conditions, the lowest values of reduced glutathione were observed in plants co-inoculated with Bradyrhizobium and Actinomadura or co-inoculated with Bradyrhizobium and P. graminis (Fig. 3B). All cowpea plants displayed decreases in reduced glutathione when non-saline and salt stress conditions were compared, except cowpea plants co-inoculated with Bradyrhizobium and Bacillus (Fig. 3B). This symbiotic pair showed the highest value of reduced glutathione in relation to other treatments under salt stress conditions, and this value was 24% greater than in plants inoculated with Bradyrhizobium.
Fig. 3.
Total (A), reduced (B) and oxidized (C) forms of glutathione and redox glutathione (D) in the nodules of cowpea plants inoculated with Bradyrhizobium (T1) or co-inoculated with Bradyrhizobium and Actinomadura (T2), Bradyrhizobium and Paenibacillus graminis (T3), Bradyrhizobium and Bacillus (T4) or Bradyrhizobium and Streptomyces (T5) in control (non-saline) and salt stress (50 mmol L−1 NaCl) conditions. Means followed by the same lowercase letters do not differ statistically between bacterial combinations, whereas uppercase letters represent significant differences between control and salt stress. All mean comparisons were performed using Tukey's test (p < 0.05).
The oxidized glutathione in nodules of cowpea plants (inoculated and co-inoculated) did not change significantly under non-saline conditions, but this parameter was increased only in the nodules of cowpea plants co-inoculated with Bradyrhizobium and P. graminis and subjected to salt stress (Fig. 3C). This symbiotic pair showed a higher level of oxidized glutathione (4.3 μmol g−1 DW) when cultivated under salt stress conditions compared with other treatments and was approximately 20% greater than in cowpea plants inoculated with Bradyrhizobium (Fig. 3C). The redox status of glutathione was significantly altered in non-saline and salt stress conditions, especially in cowpea plants co-inoculated with Bradyrhizobium and Streptomyces (26.1%). Under salt stress conditions, the lowest levels of redox glutathione were observed in plants co-inoculated with Bradyrhizobium and P. graminis (14.1%), and the greatest levels were observed in cowpea plants co-inoculated with Bradyrhizobium and Bacillus (23.9%) or Bradyrhizobium and Streptomyces (23.2%) (Fig. 3D).
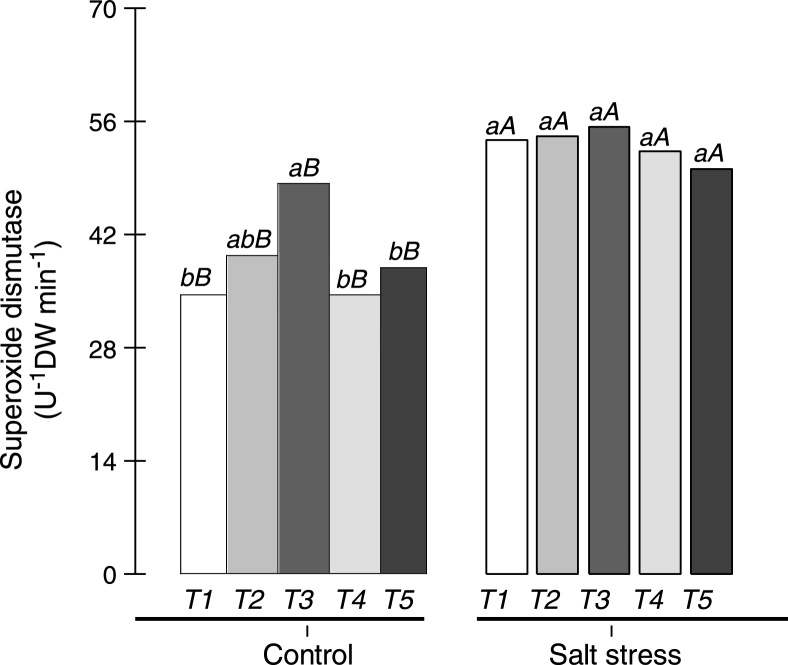
The activities of superoxide dismutase (SOD), catalase (CAT) and phenol peroxidase (POX) were measured in the root nodules of cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB, and the results are shown in Fig. 4, Fig. 5. SOD activity was superior in the root nodules of cowpea plants co-inoculated with Bradyrhizobium and Actinomadura and in cowpea plants co-inoculated with Bradyrhizobium and P. graminis in non-saline conditions. The enzyme activities of plants co-inoculated with these symbiotic pairs were 14% and 40% superior to those of cowpea inoculated with Bradyrhizobium in non-saline conditions. On average, SOD activity increased in the root nodules when cowpea plants were subjected to salt stress, mainly in cowpea plants inoculated with Bradyrhizobium (increase of 55%) or co-inoculated with Bradyrhizobium and Bacillus (increase of 52%). Additionally, no difference was observed between cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and PGPB under salt stress conditions (Fig. 4).
Fig. 4.
Superoxide dismutase in the nodules of cowpea plants inoculated with Bradyrhizobium (T1) or co-inoculated with Bradyrhizobium and Actinomadura (T2), Bradyrhizobium and Paenibacillus graminis (T3), Bradyrhizobium and Bacillus (T4) or with Bradyrhizobium and Streptomyces (T5) in control (non-saline) and salt stress (50 mmol L−1 NaCl) conditions. Means followed by the same lowercase letters do not differ statistically between bacterial combinations, whereas uppercase letters represent significant differences between control and salt stress. All mean comparisons were performed using Tukey's test (p < 0.05).
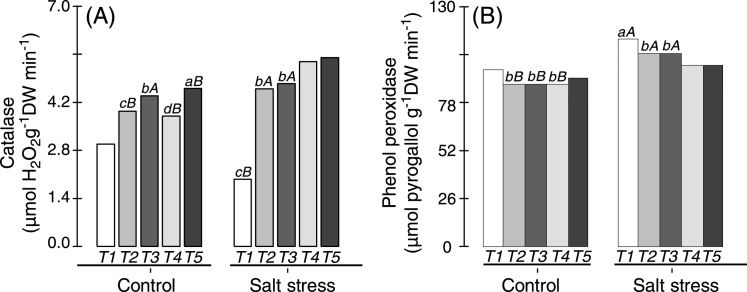
Fig. 5.
Catalase (A) and phenol peroxidase (B) levels in the nodules of cowpea plants inoculated with Bradyrhizobium (T1) or co-inoculated with Bradyrhizobium and Actinomadura (T2), Bradyrhizobium and Paenibacillus graminis (T3), Bradyrhizobium and Bacillus (T4) or with Bradyrhizobium and Streptomyces (T5) in control (non-saline) and salt stress (50 mmol L−1 NaCl) conditions. Means followed by the same lowercase letters do not differ statistically between bacterial combinations, whereas uppercase letters represent significant differences between control and salt stress. All mean comparisons were performed using Tukey's test (p < 0.05).
CAT activity was significantly enhanced in the root nodules of cowpea plants co-inoculated with Bradyrhizobium and PGPB compared with plants inoculated with Bradyrhizobium under non-saline and in salt stress conditions (Fig. 5A). In non-saline conditions, the CAT activity in cowpea plants co-inoculated with Bradyrhizobium and Streptomyces was increased by 55% compared with cowpea plants inoculated with Bradyrhizobium, and this increase was remarkable when compared with the other co-inoculations (Fig. 5A). On the other hand, cowpea plants co-inoculated with Bradyrhizobium and Bacillus or Bradyrhizobium and Streptomyces displays the highest CAT activities under salt stress conditions. When subjected to salt stress, cowpea plants co-inoculated with Bradyrhizobium and P. graminis do not shows significant differences (Tukey test; p < 0.05). Only cowpea plants inoculated with Bradyrhizobium showed reduced CAT activity under salt stress conditions (Fig. 5A).
In non-saline conditions, the highest POX activity was observed in the nodules of cowpea plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and Streptomyces, whereas lower POX activity was observed in cowpea plants co-inoculated with Bradyrhizobium and Actinomadura, Bradyrhizobium and P. graminis or Bradyrhizobium and Bacillus (Fig. 5B). The highest POX activity under salt stress conditions was observed in the nodules of cowpea plants inoculated with Bradyrhizobium (Fig. 5B). Although cowpea plants co-inoculated with PGPB had lower POX activities when compared with cowpea plants inoculated with Bradyrhizobium, these plants showed increased activity of this enzyme when exposed to saline stress in relation to non-saline conditions, especially cowpea plants co-inoculated with Bradyrhizobium and Actinomadura or Bradyrhizobium and P. graminis (increase of approximately 20%).
Discussion
Salt stress is an abiotic stress that negatively affects plant growth and crop production, particularly in arid and semi-arid regions. Some plant roots are able to restrict ion uptake from saline soils, but many plants are limited in this ability, and large quantities of salt (mainly Na+ ions) are carried into the leaves.3, 15 In this study, cowpea plants (inoculated or co-inoculated) exposed to salt stress increased Na+ ion concentrations in their shoots, which indicated a possible ionic stress in these plants. However, plants subjected to salt stress may activate ion compartmentalization using tonoplast cation transporters, such as the tonoplast Na+/H+ antiporter, or activate the Na+ extrusion through Na+/H+ antiporters situated in the plasma membrane.3, 5, 31 These strategies are useful to maintain ionic homeostasis and are considered as mechanisms of stress tolerance.32 On the other hand, K+ and Ca2+ carriers can absorb Na+ ions mainly because these channels are non-selective and because Na+ and K+ shows similar hydrated ionic radii that generate an antagonism between the transport of these two ions.31
K+ is an inorganic solute that contributes to the maintenance of osmotic pressure, ionic strength and osmotic adjustment in plants cultivated under non-saline and salt stress conditions.4 In general, the antagonism between Na+ and K+ ions promotes a decrease in the K+ uptake together with an increased influx of Na+ ions. In this study, K+ ions were unaltered in cowpea plants inoculated with Bradyrhizobium and in plants co-inoculated with Bradyrhizobium and Bacillus or Bradyrhizobium and Streptomyces after salt stress treatment. This response may be related to a decrease in the translocation of this nutrient due to a low demand for plant growth.32, 33 According Hauser and Horie,31 salt-tolerant plants are able to maintain favorable K+ homeostasis during salt stress.
ROS are normally produced at high rates during bacteroid respiration in root nodules and can be overproduced and cause oxidative damage during salt stress conditions.6, 11, 12, 13 The results obtained in this work revealed salt-induced oxidative stress in all cowpea plants, mainly in plants inoculated with Bradyrhizobium or co-inoculated with Bradyrhizobium and Streptomyces. Improved control of H2O2 levels was observed in plants co-inoculated with Bradyrhizobium and Actinomadura or Bradyrhizobium and P. graminis. It is likely that these symbiotic pairs possess a greater efficiency in their nodule antioxidative systems for maintaining lower nodule H2O2 levels.12, 13 H2O2 or others ROS may attack and oxidize polyunsaturated fatty acids in the cell membrane, which results in increased lipid peroxidation.34 In this study, cowpea plants co-inoculated with Bradyrhizobium and P. graminis showed a better control of lipid peroxidation under non-saline and salt stress conditions. Similar results were observed in Trigonella foenum-graecum co-inoculated with E. meliloti and Bacillus and exposed moderate and severe drought14 and in cowpea plants co-inoculated with Bradyrhizobium, P. graminis and P. durus.15
Plants possess a great variety and quantity of antioxidant compounds, such ascorbate and glutathione, to keep ROS levels low and avoid the toxicity of these molecules.34 The nodules of cowpea plants co-inoculated with Bradyrhizobium and Actinomadura showed a greater oxidative protection. This symbiotic pair exhibited 90% of total ascorbate levels as reduced ascorbate, and this feature indicated a greater capacity for oxidative protection of cowpea nodules mediated by PGPB. Reduced ascorbate levels provide membrane protection by reacting directly or indirectly with ROS, mainly H2O2,35, 36 and are an important part of the ascorbate-glutathione cycle.6, 11, 12, 13 Glutathione occurs in reduced and oxidized forms and protects the cell membrane against ROS damage.37 In this study, reduced glutathione and oxidized glutathione displayed responses inversely proportional in cowpea nodules, i.e., while reduced glutathione decreased in the nodules of cowpea plants submitted to salt stress, the oxidized glutathione increased under the same conditions.
CAT and SOD are metalloproteins with important roles in controlling ROS levels. In the nodule, SOD catabolizes O2• into H2O2, which is further detoxified by CAT.34, 35, 36, 37 CAT removes the excess of H2O2 produced in response to salt stress and therefore prevents leakage of H2O2 to other locations in the cell.5, 36 CAT and SOD can be found in mitochondria and bacteroids and provide fine control of ROS levels.6 In this study, the nodules of plants co-inoculated with Bradyrhizobium and PGPB and exposed to salt stress had increased SOD and CAT activities. The SOD and CAT activities were increased and the H2O2 and lipid peroxidation levels decreased in the nodules of plants co-inoculated with Bradyrhizobium and Actinomadura or Bradyrhizobium and P. graminis. These symbiotic pairs also displayed the highest activities of POX, which is an enzyme that catabolize the H2O2 in excess and is linked to lignification and thickening of the cell wall as well as plant defenses against biotic and abiotic stresses, such drought and salinity.6, 36 These results evidence the beneficial effects of PGPB on the positive balance of antioxidative enzymes that detoxify ROS in nodule metabolism.
Conclusions
The combination of Bradyrhizobium and PGPB induces positive responses to cope with the salt-induced oxidative stress in the nodules of cowpea plants, mainly in plants co-inoculated with Bradyrhizobium and P. graminis or Bradyrhizobium and Bacillus, which exhibited the best protection against oxidative damage in the root nodules.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are grateful to the National Council for Scientific and Technological Development (CNPq), Federal Agricultural University of Pernambuco (UFRPE), and Agronomical Institute of Pernambuco (IPA) for their financial support for this research.
Associate Editor: Fernando Andreote
References
- 1.Costa A.F., Vale L.S., Oliveira A.B., Brito Neto J.F., Ribeiro W.S., Cardoso G.D. Evaluation of yield performance in cowpea genotypes (Vigna unguiculata (L.) Walp.) Aust J Crop Sci. 2017;11(3):308–312. [Google Scholar]
- 2.Freire Filho F.R., Ribeiro V.Q., Rocha M.M., Silva K.J.D., Nogueira M.S.R., Rodrigues E.V. Embrapa Meio-Norte; Teresina: 2011. Feijão Caupi no Brasil: Produção, Melhoramento Genético, Avanços e Desafios; p. 84. [Google Scholar]
- 3.Munns R., Gilliham M. Salinity tolerance of crops – what is the cost? New Phytol. 2015;208:668–673. doi: 10.1111/nph.13519. [DOI] [PubMed] [Google Scholar]
- 4.Shrivastava P., Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2014;22(2):123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamil A., Riaz S., Ashraf M., Foolad M.R. Gene expression profiling of plants under salt stress. Crit Rev Plant Sci. 2011;30:435–458. [Google Scholar]
- 6.Silveira J.A.G., Figueiredo M.V.B., Cavalcanti F.R., Ferreira-Silva S.L. Legume nodule oxidative stress and N2 fixation efficiency. In: Araujo A.S.F., Figueiredo M.V.B., editors. Microbial Ecology of Tropical Soils. Nova Science Publishers Inc; 2011. [Google Scholar]
- 7.Figueiredo M.V.B., Bonifacio A., Rodrigues A.C., Araujo F.F. Plant growth-promoting rhizobacteria: key mechanisms of action. In: Choudhary D.K., Varma A., editors. Microbial-mediated Induced Systemic Resistance in Plants. Springer; 2016. pp. 23–37. [Google Scholar]
- 8.Kyei-Boahen S., Savala C.E.N., Chikoye D., Abaidoo R. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front Plant Sci. 2017;8(646) doi: 10.3389/fpls.2017.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peix A., Ramírez-Bahena M.H., Velázquez E., Bedmar E.J. Bacterial associations with legumes. Cr Rev Plant Sci. 2015;34(1–3):17–42. [Google Scholar]
- 10.Bashan Y., de-Bashan L.E., Prabhu S.R., Hernandez J.P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant Soil. 2014;378(1–2):1–33. [Google Scholar]
- 11.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012:26. 10.1155/2012/217037, Article ID 217037. [Google Scholar]
- 12.Locato V., Pinto M.C., Paradiso A., Gara L. Reactive oxygen species and ascorbate glutathione interplay in signaling and stress responses. In: Gupta S.D., editor. Reactive Oxygen Species and Antioxidants in Higher Plants. Science Publishers; Enfield: 2010. pp. 45–64. [Google Scholar]
- 13.Rodrigues A.C., Bonifacio A., Antunes J.E.L., Silveira J.A.G., Figueiredo M.V.B. Minimization of oxidative stress in cowpea nodules by the interrelationship between Bradyrhizobium sp. and plant growth-promoting bacteria. App Soil Ecol. 2013;64:245–251. [Google Scholar]
- 14.Barnawal D., Maji D., Bharti N., Chanotiya C.S., Kalra A. ACC deaminase-containing Bacillus subtilis reduces stress ethylene-induced damage and improves mycorrhizal colonization and rhizobial nodulation in Trigonella foenum-graecum under drought stress. J Plant Growth Regul. 2013;32(4):809–822. [Google Scholar]
- 15.Egamberdieva D., Berg G., Lindström K., Räsänen L.A. Alleviation of salt stress of symbiotic Galega officinalis L (Goat's Rue) by co-inoculation of rhizobium with root colonizing Pseudomonas. Plant Soil. 2013;369(1–2):453–465. [Google Scholar]
- 16.Vincent J.M. International Biological Programme; London: 1970. A Manual for the Practical Study of Root Nodule Bacteria; p. 164. [IBP Handbook, 15] [Google Scholar]
- 17.Rodrigues Neto J., Malavolta Júnior V.A., Victor O. Meio simples para o isolamento e cultivo de Xanthomonas campestris pv. citri tipo B. Summa Phytopathol. 1986;12(1–2):16. [Google Scholar]
- 18.Nonomura H., Ohara Y. Distribution of soil actinomycetes. VI. A culture method effective for both preferential isolation and enumeration of Microbispora and Streptosporangium strains in soil. J Ferment Tech. 1969;47:463–469. [Google Scholar]
- 19.Hoagland D.R., Arnon D.I. 1st ed. University of California; Berkeley: 1950. The Water Culture Method of Growing Plants Without Soil; p. 32. [Google Scholar]
- 20.Silveira J.A.G., Contado J., Rodrigues J., Oliveira J. Phosphoenolpyruvate carboxylase and glutamine synthetase activities in relation to nitrogen fixation in cowpea nodules. Braz J Plant Physiol. 1998;10(1):19–23. [Google Scholar]
- 21.Cavalcanti F.R., Oliveira J.T.A., Martins-Miranda A.S., Viegas R.A., Silveira J.A.G. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol. 2004;163:563–571. doi: 10.1111/j.1469-8137.2004.01139.x. [DOI] [PubMed] [Google Scholar]
- 22.Silveira J.A.G., Araújo S.A.M., Lima J.P.M.S., Viégas R.A. Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex nummularia. Environ Exp Bot. 2009;66:1–8. [Google Scholar]
- 23.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Bioch Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 24.Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kampfenkel K., Montagu M.V., Inzé R. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochem. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- 26.Griffith O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 27.Havir E.A., Mchale N.A. Biochemical and development characterization of multiples forms of catalase in tobacco leaves. Plant Physiol. 1987;84(2):450–455. doi: 10.1104/pp.84.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amako K., Chen G.X., Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504. [Google Scholar]
- 29.Giannopolitis O., Ries S.K. Superoxide dismutase: I. Occurrence in higher plants. Plant Physiol. 1977;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva F.A.S., Azevedo C.A.V. The Assistat Software Version 7.7 and its use in the analysis of experimental data. African J Agri Res. 2016;11(39):3733–3740. [Google Scholar]
- 31.Hauser F., Horie T. A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010;33(4):552–565. doi: 10.1111/j.1365-3040.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 32.Jaarsma R., Vries R.S., Boer A.H. Effect of salt stress on growth Na+ accumulation and proline metabolism in potato (Solanum tuberosum) cultivars. PLOS ONE. 2013;8(3):e60183. doi: 10.1371/journal.pone.0060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutra A.T., Silva E.N., Rodrigues C.R., Vieira S.A., Aragão R.M., Silveira J.A. High temperatures affect ion distribution in NaCl-pretreated cowpea plants. Rev Bras Eng Agríc Ambient. 2011;15(4):403–409. [Google Scholar]
- 34.Pandey S., Fartyal D., Agarwal A. Abiotic stress tolerance in plants: myriad roles of ascorbate peroxidase. Front Plant Sci. 2017;8(581) doi: 10.3389/fpls.2017.00581. [eCollection 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maia J.M., Voigt E.L., Macêdo C.E., Ferreira-Silva S.L., Silveira J.A. Salt-induced changes in antioxidative enzyme activities in root tissues do not account for the differential salt tolerance of two cowpea cultivars. Braz J Plant Physiol. 2010;22(2):113–122. [Google Scholar]
- 36.Conklin P.L., Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the senescence. Plant Cell Environ. 2004;27:959–970. [Google Scholar]
- 37.Sytar O., Kumar A., Latowski D., Kuczynska P., Strzałka K., Prasad M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant. 2013;35(4):985–999. [Google Scholar]