Abstract
Background
Sex is one of the most powerful modifiers of disease development. Clear sexual dimorphism exists in cardiometabolic health susceptibility, likely due to differences in sex steroid hormones. Changes in the gut microbiome have been linked with the development of obesity, type 2 diabetes, and atherosclerosis; however, the impact of microbes in sex-biased cardiometabolic disorders remains unclear. The gut microbiome is critical for maintaining a normal estrous cycle, testosterone levels, and reproductive function. Gut microbes modulate the enterohepatic recirculation of estrogens and androgens, affecting local and systemic levels of sex steroid hormones. Gut bacteria can also generate androgens from glucocorticoids.
Scope of review
This review summarizes current knowledge of the complex interplay between sexual dimorphism in cardiometabolic disease and the gut microbiome.
Major conclusions
Emerging evidence suggests the role of gut microbiome as a modifier of disease susceptibility due to sex; however, the impact on cardiometabolic disease in this complex interplay is lacking. Elucidating the role of gut microbiome on sex-biased susceptibility in cardiometabolic disease is of high relevance to public health given its high prevalence and significant financial burden.
Keywords: Sex differences, Steroids, Gut microbiota, Metabolic disease, Cardiovascular disease
Abbreviations: ApoE, apolipoprotein E; BAs, bile acids; CVD, cardiovascular diseases; E1, estrone; E2, estradiol; E3, estriol; ER, estrogen receptor; FCG, four core genotypes; FMO3, flavin monooxygenases 3; FXR, farnesoid X receptor; GI, gastrointestinal; GPR30, G protein-coupled receptor 30; HDL-c, high-density lipoprotein cholesterol; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; LDL-c, low-density lipoprotein cholesterol; LPS, lipopolysaccharides; MUC2, mucin-2; NOD, non-obese diabetic; SHP, small heterodimer partner; T1D, type 1 diabetes; T2D, type 2 diabetes; TLR4, Toll-like receptor 4; TMA, trimethylamine; TUDCA, tauroursodeoxycholic acid; ZO-1, zonula occludens
1. Introduction
Humans harbor over 100 trillion microbes, with the gastrointestinal tract being the most densely populated body habitat [1], [2]. Gut microbial communities include members of the Bacteria, Archaea, and Eukarya (fungi, protozoa) domains, as well as viruses. Their collective genomes encode for metabolic pathways essential for acquiring nutrients that are indigestible to us and for the generation of metabolites that modulate our metabolism. While some of the effects of gut microbes on the immune system and gut physiology have been recognized for a long time, over the last decade, we have developed a deeper appreciation for the many roles these organisms play in virtually every aspect of our biology.
Although there is substantial interpersonal variation in the composition of the distal gut microbiota among unrelated healthy subjects, sequence-based studies have revealed distal gut community patterns associated with different pathological states, including metabolic syndrome. Remarkably, recent studies indicate that the gut microbiota influences the development of cardiometabolic disease:
-
•
Sub-therapeutic antibiotic therapy in young, conventionally-raised mice results in taxonomic changes in the distal gut microbiota and increases adiposity [3].
-
•
Mice of the same genotype, but with different microbiota composition, develop different metabolic phenotypes in response to chronic high-fat/high-sucrose feeding [4].
-
•
Germ-free mice are resistant to diet-induced metabolic disease [5].
-
•
The absence of the gut microbiome differentially impacts the atherosclerosis susceptibility of apolipoprotein E−/− (ApoE−/−) mice compared to conventionally-raised mice (i.e. fully colonized with microorganisms at birth) [6].
-
•
Transplantation of gut microbiota from genetically obese mice or obese humans, to lean, germ-free mice transfers an increased adiposity phenotype [7].
-
•
Transfer of gut microbiota from lean, metabolically healthy human donors to humans with metabolic syndrome increases their insulin sensitivity, and this improvement is linked to changes in plasma metabolites [8]. However, beneficial effects are transient, and response is driven by baseline fecal microbiota composition [9].
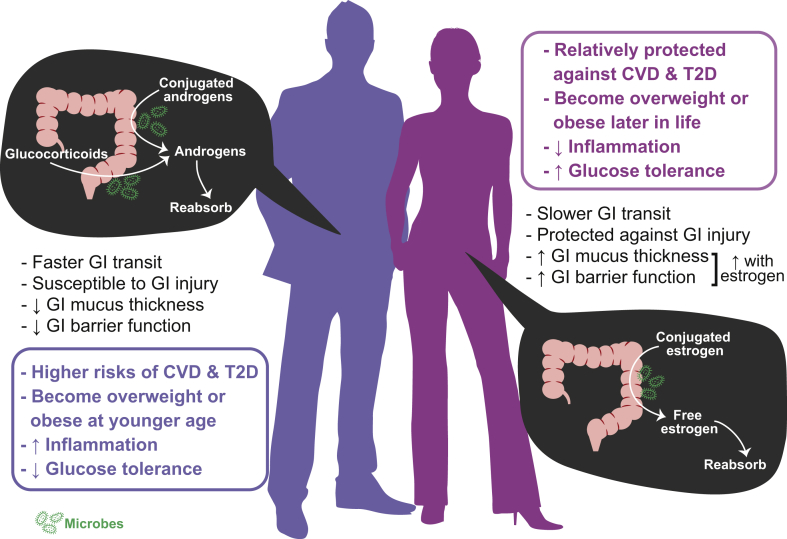
This evidence highlights the importance of the gut microbiome on the etiology of cardiometabolic disease. Additionally, clear sexual dimorphism exists in cardiometabolic disease. Cardiovascular diseases (CVD) are more common in men than age-matched pre-menopausal women. However, this cardioprotection in women is lost once menopause occurs, suggesting the contribution of sex steroid hormones on varying disease susceptibility. Globally, more men are diagnosed with type 2 diabetes (T2D) than women [10]. Men tend to be overweight at a younger age, whereas women tend to be overweight or obese after the age of 45 [11]. Similarly, male mice develop diet-induced obesity and insulin resistance more rapidly than females [12], [13]. Furthermore, obese female mice are more protected against inflammation and glucose intolerance relative to age- and weight-matched males, indicating that the protective effect of estrogen persists in the obese state [12]. Sex steroid hormones also modulate gastrointestinal (GI) health [14], [15]. Irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) are more prevalent in women than in men. These GI disorders have been associated with greater gut permeability due to compromised gut barrier function, which can result in increased levels of pro-inflammatory molecules entering into systemic circulation. Moreover, the symptoms of IBD fluctuate across the menstrual cycle in humans [16] and the mucosal infiltration of immunocytes (i.e., immunologically competent cells) in IBS patients differs between females and males [17]. Data from experimental models show that female rats are more resistant to intestinal injury and inflammation than males [18]. In females, GI permeability fluctuates throughout the estrous cycle, and ovariectomy-induced estrogen deficiency leads to compromised barrier function [19], [20]. This evidence supports that, besides the role in reproductive functions, sex steroid hormones play important roles in GI homeostasis and modulate the susceptibility of diseases, fundamentally contributing to sexual dimorphism (Figure 1).
Figure 1.
Sex differences in cardiometabolic disease and the gut microbiome. GI: gastrointestinal; CVD: cardiovascular disease; T2D: type 2 diabetes.
2. Sexual dimorphism in sterolbiome
Sex steroid hormones and bile acids (BAs) share structural similarity as they are both derivatives of cholesterol which contains cyclic steroid nucleus. They can both be recycled through enterohepatic circulation—a process, in part, regulated by the gut microbiome. Therefore, the action of gut microbes on these steroids is critical in determining whether they are excreted or recycled. Various microbial modifications also alter the cytotoxicity and/or potency of these steroids. The term “sterolbiome” has been recently proposed to describe the genetic potential of the gut microbiome to produce endocrine molecules from endogenous and exogenous steroids [21]. Below, we discuss the current knowledge on the sterolbiome and its impact on sexual dimorphism and cardiometabolic disease.
2.1. Bile acids
Traditionally viewed as surfactants, BAs facilitate the absorption of lipids and fat-soluble vitamins, have antimicrobial effects, and play important signaling roles modulating glucose homeostasis, lipid metabolism, energy expenditure, and intestinal motility. Primary BAs are synthesized in the liver from cholesterol and stored in the gallbladder. Upon consumption of food, primary BA conjugates of taurine (most mammals) and glycine (humans) are secreted into the duodenum, with a large fraction then reabsorbed in the ileum. BAs that escape reabsorption modulate the composition of the gut microbial community at least in part by inhibiting growth of specific microbes [22], [23], and are subjected to microbial modification which generate secondary BAs via deconjugation, dehydrogenation, epimerization, and dehydroxylation of primary BAs. BAs with different modifications vary in their ability to activate receptors, act as antimicrobial agents, and impact host physiology. Changes in the homeostasis of BA have been associated with alterations in metabolic health: (i) a bacterial enzyme responsible for deconjugating BA has been shown to regulate lipid metabolism, adiposity, and cholesterol concentration of the host [24]; (ii) oral supplementation of a primary BA, chenodeoxycholic acid, increases brown adipose tissue activity and whole-body energy expenditure in humans [25]. (iii) a secondary BA, tauroursodeoxycholic acid (TUDCA), exerts a potent metabolic effect by markedly rescuing hyperinsulinema and hepatic steatosis in genetically obese mice and increasing hepatic insulin sensitivity in obese human [26], [27], [28]; (iv) patients with T2D have altered BA profile; (v) supplementation of cholestyramine and other bile acid sequestrants, which increase fecal excretion and modify the composition of BAs, leads to improvement in circulating lipid profile [29]. Altogether, these studies emphasize the critical role of BAs homeostasis on metabolic health and highlight the importance of gut microbial modifications that determine the effects of specific BAs on metabolism.
The BA pool size exhibits sexual dimorphism, although the mechanism and impact of the observed differences remain unclear. This may result in differential bile acid-dependent regulation of metabolic homeostasis and energy utilization in men vs. women. Women have been shown to have smaller BA pools than men [30]. However, BA synthesis and pool size are greatly impacted by diet, a major confounding factor for data collected from humans [31], [32]. In contrast to these observations in humans, female mice have a larger BA pool size as well as hepatic cholesterol concentrations than those observed in age-matched male mice consuming the same diet [33]. Estrogen supplementation in ovariectomized female mice, a model of human menopause, increases the gallbladder volume in wild-type mice and induces hepatic cholesterol synthesis [34]. Similarly, estrogen and progesterone elevate BA synthesis of rat hepatocytes in vitro [35]; however, estrogen represses BA synthesis in male rats [36]. In non-human primates, slower hepatic BA flow has been observed in response to estrogen supplementation [37]. The observed sexual dimorphism in BA regulation and pool size likely affects nutrient absorption, gut microbial composition, and the abundance and profile of primary and secondary bile acids that enter into the large bowel. These differences may ultimately regulate host metabolism and contribute to the sexual dimorphism in metabolic diseases.
Two estrogen-specific receptors, estrogen receptor-α (ERα) and G protein-coupled receptor 30 (GPR30), are expressed in the smooth muscle and epithelial cells of gallbladder, respectively. The disruption of these key receptors impairs gallbladder emptying in response to a fatty meal [34]. Furthermore, recent studies indicate that activation of ERα, similar to farnesoid X receptor (FXR; a central BA sensor), suppresses BA synthesis via induction of the hepatic small heterodimer partner (SHP) transcription in liver [38]. However, the effects of ERα may vary depending on the hormonal status of the hosts. For example, pregnancy leads to elevation of serum and hepatic levels of BAs. In pregnant mice, ERα activation has an inhibitory effect on FXR, which results in downregulation of SHP depression and greater accumulation of BAs. In contrast, as mentioned above, in non-pregnant mice, activation of ERα results in lower BA levels [39]. The interactions between estrogen signaling and BA metabolism emphasize the importance of sex steroid hormones on bile acid-gut microbiome homeostasis that may contribute to sex-specific phenotypes.
2.2. Sex steroid hormones
Similar to BAs, endogenous sex steroid hormones such as androgens, estrogens, and progestogens, are derived from cholesterol. Differences in gut microbiota composition have been described between sexually-mature male and female mice, with male having less diverse gut microbiota than female littermates [40], [41], [42]. This difference is minimized with the castration of males, highlighting the influence of androgens on gut microbiome composition [40]. Although declining gradually with age, concentrations of sex steroid hormones in males are relatively stable after puberty throughout their lifespan. In contrast, women encounter more dramatic fluctuations in sex steroid hormones due to reproductive factors throughout life, which add into the complexity of hormonal effects on metabolism and the gut microbiome. Remarkably, the presence of the gut microbes is critical in maintaining a normal estrous cycle in female mice, testosterone levels in male mice, and reproductive function in both sexes [40], [41], [43].
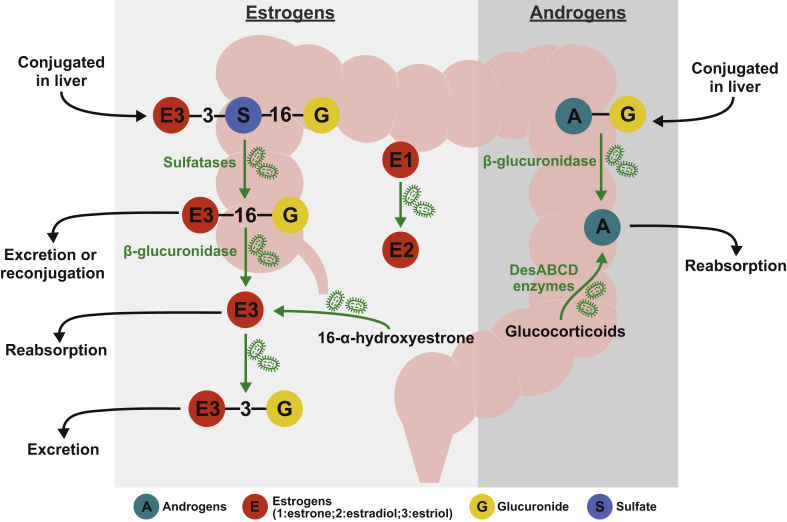
Gut microbial metabolism affects systemic levels, potency, and the half-life of estrogen metabolites. Endogenous estrogens are synthesized by the cytochrome P450 enzyme aromatase. Aromatase converts testosterone into estradiol (17β-estradiol or E2), the most potent estrogen in humans. Estradiol, the most abundant estrogen in pre-menopausal women, is synthesized by the ovaries. Estrone (E1), a less potent estrogen, is produced in the adipose tissue through conversion from either androstenedione or estradiol. Because the ovaries cease to produce estradiol, estrone is the main form of estrogen in post-menopausal women. Estriol (E3), a metabolite derived from estradiol and estrone, is the main estrogen produced by placenta during pregnancy. These three forms of parent estrogens serve as substrates for the production of various metabolites through hydroxylation, oxidation, and conjugation (methylation, glucuronidation, and sulfonation) in the liver. Although ∼50% of conjugated estrogens are subjected to biliary excretion, only a small fraction of these metabolites appears in feces, with the majority being hydrolyzed and reabsorbed in the intestine [44], [45]. Remarkably, administration of antibiotics leads to a 60-fold increase in the fecal excretion of conjugated estrogens in pregnant women, highlighting the central role of the gut microbiome in modulating the reabsorption of estrogens [46], [47]. Bacterial sulfatases and glucuronidases are considered the main deconjugating enzymes. Among the three endogenous forms of estrogens, estriol shows the greatest degree of enterohepatic recycling (Figure 2). Gut microbes are able to cleave off sulfate and glucuronide from conjugated estrogens, allowing these estrogens to be reabsorbed. Bacterial β-glucuronidase is an enzyme largely involved in this enterohepatic recirculation. Bacterial β-glucuronidase activity is encoded by two genes, gus and BG. The Firmicutes phylum accounts for 96% of the gus gene sequences detected in human feces whereas BG gene sequences are detected both in Bacteroidetes and Firmicutes (59% and 41%, respectively) [48], [49]. Besides the deconjugation reactions, in vitro studies suggest that gut microbes are also able to convert estrone into estradiol (aerobically and anaerobically), as well as 16-α-hydroxyestrone into estriol, but this reaction has only been documented under aerobic culture conditions and may not be physiologically relevant in vivo [50]. Several gut bacterial isolates are able to form estradiol—the most potent form of estrogen, from estrone, including Slackia sp. NATTS and Bacteroides fragilis [50], [51], [52]. This interconversion has also been observed in aerobic cultures of Alcaligenes faecalis, Pseudomonas aeruginosa, Staphylococcus aureus, and Mycobacterium smegmatis. Interestingly, estrogen and progesterone have been shown to replace the bacterial needs of vitamin K and promote growth of the oral plaque associated bacterium Bacteroides melaninogenicus, which has been observed at high levels during pregnancy [53]. However, the role of these bacteria in modulating estrogens and the physiological impact on the host is less clear.
Figure 2.
Known microbial transformation on estrogens and androgens. The degree of enterohepatic recirculation of two forms of endogenous estrogens varies greatly among host species [143]. However, estriol exhibits significant enterohepatic recycling. Conjugation of estrogens with sulfate and glucuronide occurs largely in liver, resulting in compounds such as estriol-3-sulfate-16-glucuronide (E3-3-S-16-G). This conjugated estrogenic metabolite is then subjected to excretion via the intestinal tract. Gut microbial sulfatases can hydrolyze E3-3-S-16-G to E3-16-G, which can return to the liver for excretion or reconjugation or be hydrolyzed further into free E3 by bacterial β-glucuronidase for reabsorption. Gut microbes can also reconjugate free E3 into E3-3-G, which is then subjected to excretion. Moreover, in vitro studies suggest that gut microbes are able to convert estrone into estradiol (aerobically and anaerobically), as well as 16-α-hydroxyestrone into estriol, but this reaction has only been shown to occur in the presence of oxygen [50]. Similarly, conjugated androgens can be hydrolyzed in the intestinal tract via bacterial β-glucuronidase into free androgens for reabsorption. Further, glucocorticoids can be converted it into androgens via side-chain cleaving capacity of bacterial desABCD-encoded enzymes.
Several studies have assessed compositional changes in the gut microbiota in response to ovariectomy. Loss of ovarian hormone production leads to distinct gut microbiota composition when fed a high-fat/high-sucrose semi-purified diet in Sprague-Dawley rats and two stains of mice (C57BL/6J and DBA/2J) [54], [55]. Yet, this effect is not observed when mice were fed a natural ingredient based-rodent chow [54]. Similarly, others have found limited signals of ovariectomy on overall gut microbiota composition when feeding natural ingredient-based diets in rats [56], [57]. Nonetheless, Cox-York and colleagues demonstrated ovariectomy effect on gut microbial composition in low-capacity running rats feeding a natural ingredient-based diet [58]. The impact of loss of ovarian hormone production on gut microbiome is likely highly sensitive to diets and specific to host species and strains. For instance, natural ingredients diets commonly contain high and inconsistent level of phytoestrogens, which vary in composition and abundance among production lots. Phytoestrogens are plant-derived compounds that have a structure similar to endogenous estrogens synthesized from the mammalian endocrine system and are generally viewed as natural compounds that exert health benefits with anti-atherosclerotic, anti-cancer, and anti-osteoporotic properties [59]. Due to their structural similarity to endogenous estrogens, phytoestrogens can bind to ER (both ERα and ERβ) and act as estrogen agonists or antagonists. There are three major classes of phytoestrogens, including isoflavones, lignans, and coumestans. Isoflavones usually exist in food as biologically inactive forms, such as genistin and daidzin, that are present in soy as β-D-glycosides, which can be become biologically active aglycone forms via hydrolyzation and deconjugation through bacterial β-glycosidases present in the gut microbiome. Therefore, inclusion of phytoestrogens in the diet may directly and/or indirectly mask the changes of gut microbiome in response to the loss of ovarian hormone production at both compositional and functional level. Alternatively, other components in the diets may also contribute to the inconsistent results, such as variation in the types and abundance of dietary fibers in natural ingredient diets. Altogether, perturbation of ovariectomy-associated gut microbiota on host physiology and metabolism needs to be examined using highly controlled diets free of dietary phytoestrogens.
Supplementation of probiotic Lactobacillus reuteri elevates circulating testosterone, enough to prevent age-associated testicular atrophy in mice [60]. Germ-free non-obese diabetic (NOD) male mice have lower systemic testosterone levels compared to conventionally-raised counterparts [41]. Additionally, inoculation of the gut microbiome from male NOD mice into female NOD mice elevates circulating testosterone levels of the female recipients and exerts a protective effect against type 1 diabetes (T1D) [41]. This evidence highlights the interactions between testosterone and the gut microbiome, which are sufficient to influence host phenotypes. Similar to estrogens, androgens also go through significant enterohepatic recirculation through deconjugation via the gut microbiome. Bacterial β-glucuronidase cleaves off glucuronide from androgen conjugates, releasing free androgens for reabsorption [61], [62]. Furthermore, the gut microbiome also modulates glucocorticoids and androgens through reductive and oxidative reactions [63], [64]. Androgens, such as androstenedione and testosterone, are C-19 steroids derived from C-27 cholesterol through reductive reactions. These androgens can then be converted into C-18 estrogens. The ability of gut microbes to convert steroids anaerobically through side-chain cleavage has been known for decades [65], [66], but the pathways involved have only been recently identified. Work from Riddlon and colleagues has revealed that the side-chain cleavage product of glucocorticoids generated by the human gut bacterium Clostridium scindens is an androgen, 11β-hydroxyandrost-4-ene-3,17-dione (11β-OHA). This work also identified a cortisol-inducible operon (desABCD) encoding (i) the enzyme involved in anaerobic side-chain cleavage (DesC, a 20α-hydroxysteroid dehydrogenase [HSDH]), (ii) a putative transketolase (encoded by desAB) which is hypothesized to have steroid-17,20-desmolase/oxidase activity, and (iii) a possible corticosteroid transporter (encoded by desD) [64]. This conversion may potentially impact testosterone concentrations in vivo. While further work is required to ascertain the host phenotypes affected by these bacterial functions, modulation of sex steroid hormones through bacterial β-glucuronidase and desABCD-encoded enzymes may result in changes in local and systemic levels of steroid compounds that ultimately impact host hormonal homeostasis.
3. Gastrointestinal tract and sexual dimorphism
3.1. GI tract physiology
There are several factors related to the GI tract may contribute to the sex-associated differences observed in gut microbiome composition. These include varying GI motility due to sex hormones that impact GI transit time and the distribution and effector response of the estrogen and/or androgen receptors along the GI tract. Pre-menopausal women have slower gastric emptying and greater GI transit time than men; this difference disappears in post-menopausal women who are not on hormone replacement therapy [67], [68]. Differences in GI transit time likely affects the time gut microbes are in contact with dietary substrates, hence impacting fermentation and accumulation of metabolites. End-products of fermentation can alter the pH within the GI tract and consequently, impact microbiota composition, as pH imposes a selective pressure on microbial growth and metabolism that is differentially tolerated among bacteria (e.g., acidic pH dramatically affects the growth of Bacteroides species, but to a significantly lower extent the growth of Gram-positive bacteria [69]). Sex steroid hormones are thought to be the main contributor to the sexual dimorphism observed in GI transit time [70], [71], [72], [73], [74]. The luteal phase of the menstrual cycles and during the second and third trimester of the pregnancy, when the concentrations of estrogen and progesterone are both elevated, have been associated with prolonged GI transit time; however, these findings have not been consistent among studies [70], [71], [72]. Administration of estrogen inhibits gastric emptying in intact diestrus and ovariectomized female rats, resulting in prolonged GI transit time, whereas progesterone has opposite effects [73]. When administering a combination of estrogen and progesterone to ovariectomized rats, the effect of estrogen dominates, and gastric emptying is inhibited. In contrast, testosterone does not impact the rate of gastric emptying in castrated male rats, suggesting the role of estrogens but not testosterone on GI motility [73]. Interestingly, both estrogen and progesterone inhibit gastric emptying in intact male rats [74]. The mechanisms by which sex steroid hormones modulate GI motility may be multifaceted and segment dependent, possible due to the distribution of ligand-binding receptors.
In both males and females, transcriptional effects of estrogens are thought to be mediated partly by nuclear ER through ligand-binding activation, which then initiates transcription of target genes involved in metabolism, development, reproduction, and homeostasis via recognition of ER (this mode of signaling of estrogens if often referred as the genomic pathway). There are at least two types of ER that are currently known, ERα and ERβ. ERα, the “original ER”, was cloned and characterized in 1985 [75]. ERβ was not discovered until 1996 [76]. Since then, at least five isoforms of ERβ have been identified [77], [78]. ERβ is poorly understood and widely distributed among many tissues that were previously thought to be estrogen-insensitive, including the gastrointestinal tract. The ratio and predominance of ERα and ERβ expression varies along the GI tract, and the mapping of these receptors in the GI tract have been inconsistent among studies, varying among species, sex, and hormonal status. Nonetheless, ERβ has been consistently shown to be the predominant form of the ER in colon of men, women, and developing fetuses [79], [80]. Additionally, while some studies detected greater colonic expression of ERβ in women than men, these differences have not been consistently observed [80], [81], [82]. Given the prevalence of ERβ in the GI tract, elucidating the tissue distribution and functions of ERβ will likely enhance our understanding of sex-dependent GI physiology.
Other than the classic genomic (transcriptional) signaling pathway, GPR30 (also known as G-protein coupled estrogen receptor) is now recognized as the receptor of non-genomic (rapid) signaling of estrogen. GPR30 is expressed in both gastric fundic gland and colonic tissue [83], [84]. Estradiol is a highly specific ligand of GPR30, whereas estrone and estriol exhibit very low affinities [85], [86]. In contrast, progesterone, testosterone and cortisol do not bind to GPR30 [86]. Therefore, the known sex differences in GI motility and disease susceptibility may also be regulated through the non-genomic signaling of sex hormones.
The localization of progesterone and androgen receptors in the GI tract of males and females is poorly defined for both sexes. The expression of progesterone receptors has been observed in the colon of women; whereas the expression of androgen receptors has been detected in the intestine of female and male fetuses [87], [88]. The distribution and localization of the sex hormone receptors and the abundance and potency of differential sex hormones in male and female may both contribute to the observed sexual dimorphism in GI-related disorder and further influence gut microbial communities.
3.2. Gut barrier function
The lining of the GI tract is primarily formed by a layer of epithelial cells held together by the tight junctions, along with a mucus layer coating the top of the epithelium and creating a physical barrier that separates host cells from luminal contents. This dynamic structure is an important first line of defense that prevents inflammatory macromolecules (e.g., lipopolysaccharides [LPS]) and bacteria from entering systemic circulation. The GI tract epithelium has a rapid (2–6 days) turnover in most adult mammals. This process is initiated by the epithelial stem cells located near the base of the crypts. ERβ is preferentially expressed in stem cells and regulates cellular differentiation [89], [90]. Colonic tissue from ERβ−/- mice exhibits epithelial hyperproliferation, incomplete differentiation, and increased shedding, highlighting a key role of sex steroid hormones on maintaining epithelial homeostasis and integrity of the colon [89]. The mucus that coats the colon consists predominantly of mucin-2 (MUC2), which is generated and secreted by intestinal goblet cells. Estrogen has also been shown to dramatically increase mucin content in mucin-producing intestinal epithelial cells in vitro and deletion of ERβ leads to disorganization of the colon mucin layer in mice [89], [91]. Female mice are protected against intestinal injury during proestrus stage (high estrogen) compared to diestrus stage (low and stable estrogen) and compared to male mice [18], [92]. In fact, diestrus female mice and male mice have similar mucus thickness within the colon [93]. Altogether, these studies support the notion that estrogens play an important role in maintaining the health of the intestinal epithelium and integrity of the GI mucus layers through ERβ signaling.
Obesity leads to a compromised intestinal barrier function characterized by disrupted tight junctions, resulting in increased paracellular permeability which allows pro-inflammatory molecules such as bacterial LPS into systemic circulation [94], [95]. As a cellular component of gram-negative bacteria, LPS is a potent inflammatory mediator that is recognized mainly through the Toll-like receptor 4 (TLR4). Subcutaneous infusion of LPS leads to weight gain, adiposity, hepatic insulin resistance, and liver triglycerides in rodent models [96]. Similarly, higher plasma levels of LPS have been reported in obese patients relative to lean and healthy individuals [97], [98]. Estrogens promote gut barrier function not only by increasing mucus production as mentioned above but also through ERβ-mediated upregulation of tight junction transmembrane proteins, in both male and ovariectomized female rodent models and in vitro monolayer cultures [18], [19], [99]. Similar protective effects of estrogen have been observed in the vaginal epithelium [100]. In contrast, a recent study showed that estrogen decreases tight junction protein zonula occludens 1 (ZO-1) expression in primary colonic tissues isolated from post-menopausal women and men [101]. Consistent with this finding, the effect of hormone supplementation in metabolic health of post-menopausal women contrasts to those observed in pre-menopausal women, suggesting that the loss of ovarian function due to menopause may have profound and differential impact on estrogen signaling compared to pre-menopausal women [102], [103], [104]. Temporal and regional changes on GI permeability via tight junction disruption have been recently shown in ovariectomized mice, suggesting that loss of ovarian function alters the homeostasis of GI integrity in time-sensitive manner though dynamic adaptations [105].
4. The role of gut microbiome on sexual dimorphism of cardiometabolic dysfunction
Microbiota transplant experiments in germ-free mice have recently demonstrated that the sex of the recipient animal shapes the composition of the gut microbiota [42]. Additionally, sex influences susceptibility and severity of various diseases, including CVD, obesity, T1D, and gastrointestinal disorders, as discussed above. Emerging evidence suggests that sex-associated differences in gut microbiota composition may contribute to sex-specific susceptibility to disease. For example, females are known to be more susceptible to T1D than males, a phenomenon that has been observed in both humans and rodents. However, this sex-bias in disease susceptibility does not exist in germ-free NOD mice (a mouse model of T1D) [40]. Gut microbiota composition differs between sexually-mature male and female NOD mice with males having greater relative abundance of several genera including Roseburia, Coprococcus, and Bilophilia [41]. Furthermore, cecal microbial transplants from male to female increase the circulating testosterone levels and significantly decrease the incidence of T1D among recipients [41]. While the sexual dimorphism in disease susceptibility may be due to either the distinct sex steroid hormones or sex chromosome, sex hormones appear to be the major component driving the gut microbiota differences between males and females. For instance, male and female NOD mice exhibit similar gut microbiomes up to puberty; differences do not appear until adulthood/postpubescent stage [40], [41]. Removal of endogenous source of androgens through castration of male mice results in a gut microbial community that shows similar composition to those of intact female littermates and increases T1D incidence [40], [106]. Altogether, these results highlight the complex interactions between microbes and sex hormones and support the notion that this microbes-hormonal interplay alters disease susceptibility.
Obesity is one of the major public health crises of the 21st century and the leading contributor of numerous chronic diseases, including insulin resistance, type 2 diabetes, and cardiovascular disease [107]. In obese subjects, sexual dimorphism results in differential distribution of adipose tissue deposition. Women typically have ∼10% higher body fat than men, with most fat deposition occurs in the subcutaneous/peripheral adipose tissues around the hip and thigh area (i.e., pear-shaped body). Men, on the other hand, tend to have an apple-shaped configuration with deposition of more visceral adipose tissues, which is associated with higher risks to obesity-related comorbidities [108]. Female mice are partially protected against high-fat diet induced obesity, whereby the onset of obesity is delayed relative to males [12]. Estrogens reduce inflammation by inhibiting NF-κB translocation into the nucleus, which is typically induced by bacterial LPS [109], [110], [111] and other pro-inflammatory molecules (e.g., bacterial peptidoglycan) [112]. Furthermore, microbial metabolism of sex steroid hormones may impact the potency and affinity of estrogen-derived agonists on ERs, which, in turn, would impact the downstream metabolic and inflammatory pathways. Interestingly, low total testosterone concentrations in older men have been associated with high fasting glucose and insulin resistance, whereas high bioavailable testosterone in older women predicts insulin resistance and incidences of T2D [113]. This sexual dimorphism of testosterone-associated insulin resistance may be due to hormonal homeostasis prior to aging, whereby the androgenic activity is normally low in women but maximized in men. Interactions between the gut microbiome and host metabolism likely contribute to the sex differences in obesity and related comorbidities. Moreover, the inherent hormonal status of the hosts may add another layer of complexity to the etiology of sex-biases in this disease development.
During the third trimester of pregnancy, an increased maternal bacterial load within the gut and significant remodeling of the gut microbiome has been reported [114], [115]. Maternal stress during pregnancy has been associated with lasting sex-specific differential impact on obesity, with male offspring but not females having a significantly higher risk of obesity in adulthood [116]. Smoking during pregnancy has been associated with alteration in meconium microbiota and linked with increased risk of childhood obesity in the male offspring, but not females [117], [118]. Although it is difficult to dissect the direct impact of the maternal gut microbiome on the offspring, modulation of the infant gut microbiota through shaping the prenatal maternal gut microbiome has been demonstrated by administering probiotics to pregnant women 14 days prior to C-section [119].
CVD is the leading cause of death worldwide [120], [121]. As discussed earlier, pre-menopausal women are less likely to have cardiovascular events than men. Women tend to be 10 years older than men at the onset of coronary heart disease and as much as 20 years older than men for cardiac events such as myocardial infarction [122], [123]. However, the prognosis of myocardial infarction is worse in women compared to men [123], [124]. Sex differences in CVD risks have also been demonstrated in rodent models, although the sex-bias differs from humans. The size of atherosclerotic plaque lesions and number of fatty steaks in mice fed a cholesterol-rich diet is significantly greater in females as compared to age-matched and diet-matched males [125], [126]. This sex-dependent difference in atherosclerosis is also observed across many strains of mice [127].
The current knowledge of how the gut microbiome affect sex differences in CVD is quite limited, but evidence supports the contributions of both gut microbiota and the expression of hepatic flavin monooxygenases 3 (FMO3) on sex-dependent development of CVD. Gut microbial metabolism of dietary carnitine and choline (mainly consumed as phosphatidylcholine [lecithin]) results in the production of trimethylamine (TMA), which is rapidly absorbed into portal vein and subsequently converted into TMAO by host hepatic FMO3. Several clinical studies have shown that plasma levels of TMAO are independently associated with incident CVD development and adverse event risks [128], [129], [130], [131], [132]. Recent studies showed that TMAO exacerbates development of CVD, in part by enhancing platelet reactivity and thrombosis potential [128], [129], [130], [131]. Plasma TMAO levels and atherosclerotic lesions are much greater in ApoE−/- female mice relative to their male counterparts [130]. Hepatic FMO3 expression and activity are also significantly higher in female than male mice [133]. Ovariectomized mice show a modest decrease in hepatic FMO3 expression and plasma TMAO levels compared to intact female mice. In contrast, castrated male mice show a more than 100-fold increase in hepatic Fmo3 mRNA levels as well as a 7-fold increase in circulating levels of TMAO compared to intact males, and this response is blunted by treatment with dihydrotestosterone. These effects are likely mediated through FXR because (i) FMO3 expression is an FXR target gene, (ii) FXR activation induces FMO3 and increases plasma TMAO, and (iii) androsterone, a major testosterone degradation product, is a potent FXR agonist [134]. While sex differences of hepatic FMO3 expression are observed in humans, no sex differences in circulating levels of TMAO have been observed in humans [135]. The link between this gut microbial-dependent metabolite and sexual dimorphism of CVD needs to be further examined.
In addition to sex steroid hormones, sex chromosomes could contribute to the observed sex differences in metabolic traits. The presence of female (XX) vs. male (XY) sex chromosome ultimately determines the type and levels of the sex steroid hormones of an individual. Therefore, it is difficult to discern the cause of an observed sex-associated metabolic difference between sex chromosome complement and steroid hormone differences. One of the most widely used models to dissect these two factors is known as the four core genotypes (FCG) mouse model, which includes XX chromosome and XY chromosome on both females and male gonadal background [136]. In both males and females, removal of gonads abolishes the differences in FMO3 gene expression among FCG strains, suggesting that the sex differences on hepatic FMO3 expression are due to sex steroid hormones [135]. On the other hand, sex chromosomes appear to contribute greatly to the sex differences in systemic lipid profiles [137]. In humans, men tend to have greater low-density lipoprotein cholesterol (LDL-c) and triglyceride levels, and lower high-density lipoprotein cholesterol (HDL-c) levels compared to pre-menopausal women. Lipoprotein profiles are altered in women after menopause to levels similar to those of men, which leads to increased risks of CVD and metabolic dysfunction. This cardiometabolic dysfunction is thought to be caused by deficiency of sex steroid hormones, mainly estrogens. Paradoxically, estrogen supplementation in post-menopausal women can lead to unexpected consequences, including an increase in blood clotting and coronary events within the first year of treatment [102], [103], [104], suggesting that the etiology of menopause-associated CVD is complex and multifaceted. Using the FCG mouse model, the role of sex chromosome complement vs. sex steroid hormones on systemic lipid levels were assessed. Regardless of sex or existence of gonads, XX mice have greater HDL-c levels than XY mice, highlighting the importance of sex chromosomes in CVD risks [137]. Much work remains to be done to determine the relevance of sex differences in CVD risks, and how the sex-associated differential lipid profiles are associated with gut microbiome.
The gut microbiome is highly sensitive to environmental perturbation; therefore, variations in diet, hormonal status, exercise (duration and intensity), and stress levels should be considered when designing microbiome studies in humans. Furthermore, genetic variations also shape the human gut microbiome in humans and rodents [138], [139]. A study analyzing the gut microbiome of 89 distinct inbred mouse strains showed that the gut microbial differences between male and females varies as a function of host genotype [54]. For example, female BXD79/RwwJ mice have greater relative abundance of Roseburia than male, but the same taxon is more abundant in the gut microbial community of male BXD85/Rww mice than females, suggesting that host genetics could mask the sex differences on the gut microbial community [54], [93], [140]. Further, it has been shown that gut microbiota differences are mediated by sex hormones and the influences of gonadectomy on bile acid profiles significantly differ between sexes [54]. Of note, the relationship between sex chromosome and the gut microbiome has not been assessed, even though gene variance on the Y chromosome has been associated with sex differences in cardiovascular traits, contributing to dyslipidemia, hypertension, inflammation, atherosclerosis, and coronary heart disease [141], [142]. Thus, the potential influence of gene variants on the sex chromosomes on the colonization of the gut microbiome should not be overlooked.
5. Conclusion and future directions
Sexual dimorphism in cardiometabolic disease is evident, but the mechanisms involved remain largely unknown. In the past decade, many studies have linked cardiometabolic dysfunction with alterations in the gut microbiome. Although emerging evidence suggests a role of the gut microbiome as a modifier of disease, its impact on differential suceptibility to cardiometabolic disease due to sex is lacking. The gut microbiome is critical in maintaining normal estrous cycle, testosterone level, and reproductive function. Elucidating the role of gut microbiome in sex-biased susceptibility to cardiometabolic disease is of high relevance to public health. Metagenomic, metatranscriptomic and metabolomic analyses are warranted to characterize the functional changes in the gut microbiome in response to alterations in sex hormones and to identify sex-specific signals that modulate microbial community composition and activity. Identifying host and microbial genes responsible for selective gut microbial colonization due to sex may contribute to novel approaches in disease prevention. Furthermore, unlike polymorphisms in our human genomes, gut microbial community composition can be rapidly modified through diet or drugs, opening the door to new approaches in translational and personalized medicine.
Acknowledgements
The authors would like to thank Robert L Kerby for constructive criticism of the manuscript. This work was supported in part by grants NIH DK108259-01 (to F.E.R) and by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2016-67017-24416 (to F.E.R.). This work was also supported in part by a Transatlantic Networks of Excellence Award from the Leducq Foundation. T.-W.L.C is supported by the National Institutes of Health, under Ruth L. Kirschstein National Research Service Award T32 HL 007936 from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center. K.K. is supported by Astellas Foundation for Research on Metabolic Disorders, International Atherosclerosis Society, Yamada Science Foundation, and Sumitomo Life Welfare and Culture Foundation..
Contributor Information
Tzu-Wen L. Cross, Email: tlcross@wisc.edu.
Kazuyuki Kasahara, Email: kasahara2@wisc.edu.
Federico E. Rey, Email: ferey@wisc.edu.
Conflict of interest
None declared.
References
- 1.Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Whitman W.B., Coleman D.C., Wiebe W.J. Prokaryotes: the unseen majority. Proceedings of the National Academy of Sciences. 1998;95(12):6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho I., Yamanishi S., Cox L., Methé B.A., Zavadil J., Li K. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serino M., Luche E., Gres S., Baylac A., Bergé M., Cenac C. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61(4):543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasahara K., Tanoue T., Yamashita T., Yodoi K., Matsumoto T., Emoto T. Commensal bacteria at the crossroad between cholesterol homeostasis and chronic inflammation in atherosclerosis. Journal of Lipid Research. 2017;58(3):519–528. doi: 10.1194/jlr.M072165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 8.Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R.S., Bartelsman J.F.W.M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Kootte R.S., Levin E., Salojärvi J., Smits L.P., Hartstra A.V., Udayappan S.D. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metabolism. 2017;26(4):611–619.e6. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Kautzky-Willer A., Harreiter J., Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocrine Reviews. 2016;37(3):278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickelson K.J., Stromsdorfer K.L., Pickering R.T., Liu T.W., Ortinau L.C., Keating A.F. A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice. Experimental Diabetes Research. 2012;2012 doi: 10.1155/2012/859395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stubbins R.E., Holcomb V.B., Hong J., Núñez N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. European Journal of Nutrition. 2012;51(7):861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 14.Drossman D.A., Li Z., Andruzzi E., Temple R.D., Talley N.J., Grant Thompson W. U. S. Householder survey of functional gastrointestinal disorders - prevalence, sociodemography, and health impact. Digestive Diseases and Sciences. 1993;38(9):1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 15.Chang L., Heitkemper M.M. Gender differences in irritable bowel syndrome. Gastroenterology. 2002:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 16.Heitkemper M.M., Jarrett M. Pattern of gastrointestinal and somatic symptoms across the menstrual cycle. Gastroenterology. 1992;102(2):505–513. doi: 10.1016/0016-5085(92)90097-i. [DOI] [PubMed] [Google Scholar]
- 17.Cremon C., Gargano L., Morselli-Labate A.M., Santini D., Cogliandro R.F., De Giorgio R. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. American Journal of Gastroenterology. 2009;104(2):392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 18.Homma H., Hoy E., Xu D.-Z., Lu Q., Feinman R., Deitch E.A. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. AJP Gastrointestinal and Liver Physiology. 2005;288(3):G466–G472. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]
- 19.Braniste V., Leveque M., Buisson-Brenac C., Bueno L., Fioramonti J., Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. The Journal of Physiology. 2009;587(Pt 13):3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J.Y., Chassaing B., Tyagi A.M., Vaccaro C., Luo T., Adams J. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. Journal of Clinical Investigation. 2016;126(6):2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridlon J.M., Bajaj J.S. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics. Acta Pharmaceutica Sinica B. 2015:99–105. doi: 10.1016/j.apsb.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floch M.H., Binder H.J., Filburn B., Gershengoren W. The effect of bile acids on intestinal microflora. American Journal of Clinical Nutrition. 1972;25(12):1418–1426. doi: 10.1093/ajcn/25.12.1418. [DOI] [PubMed] [Google Scholar]
- 23.Binder H.J., Filburn B., Floch M. Bile acid inhibition of intestinal anaerobic organisms. American Journal of Clinical Nutrition. 1975;28(2):119–125. doi: 10.1093/ajcn/28.2.119. [DOI] [PubMed] [Google Scholar]
- 24.Joyce S.A., MacSharry J., Casey P.G., Kinsella M., Murphy E.F., Shanahan F. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proceedings of the National Academy of Sciences. 2014;111(20):7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broeders E.P.M., Nascimento E.B.M., Havekes B., Brans B., Roumans K.H.M., Tailleux A. The bile acid chenodeoxycholic acid increases human Brown adipose tissue activity. Cell Metabolism. 2015;22(3):418–426. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Özcan U., Yilmaz E., Özcan L., Furuhashi M., Vaillancourt E., Smith R.O. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kars M., Yang L., Gregor M.F., Mohammed B.S., Pietka T.A., Finck B.N. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J.-S., Kim J.T., Jeon J., Park H.S., Kang G.H., Park K.S. Changes in hepatic gene expression upon oral administration of taurine-conjugated ursodeoxycholic acid in ob/ob mice. PLoS One. 2010;5(11):e13858. doi: 10.1371/journal.pone.0013858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg A., Grundy S.M. Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Annals of Internal Medicine. 1994;121(6):416–422. doi: 10.7326/0003-4819-121-6-199409150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Bennion L.J., Drobny E., Knowler W.C., Ginsberg R.L., Garnick M.B., Adler R.D. Sex differences in the size of bile acid pools. Metabolism Clinical and Experimental. 1978;27(8):961–969. doi: 10.1016/0026-0495(78)90140-3. [DOI] [PubMed] [Google Scholar]
- 31.Meyer P.D., DenBesten L., Mason E.E. The effects of a high-fiber diet on bile acid pool size, bile acid kinetics, and biliary lipid secretory rates in the morbidly obese. Surgery. 1979;85(3):311–316. [PubMed] [Google Scholar]
- 32.Miyata M., Matsuda Y., Nomoto M., Takamatsu Y., Sato N., Hamatsu M. Cholesterol feeding prevents hepatic accumulation of bile acids in cholic acid-fed farnesoid X receptor (FXR)-null mice: FXR-independent suppression of intestinal bile acid absorption. Drug Metabolism and Disposition the Biological Fate of Chemicals. 2009;37(2):338–344. doi: 10.1124/dmd.108.022590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turley S.D., Schwarz M., Spady D.K., Dietschy J.M. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology. 1998;28(4 I):1088–1094. doi: 10.1002/hep.510280425. [DOI] [PubMed] [Google Scholar]
- 34.de Bari O., Wang T.Y., Liu M., Portincasa P., Wang D.Q.H. Estrogen induces two distinct cholesterol crystallization pathways by activating ERα and GPR30 in female mice. Journal of Lipid Research. 2015;56(9):1691–1700. doi: 10.1194/jlr.M059121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chico Y., Fresnedo O., Botham K., Lacort M., Ochoa B. Regulation of bile acid synthesis by estradiol and progesterone in primary cultures of rat hepatocytes. Experimental and Clinical Endocrinology and Diabetes. 1996;104(2):137–144. doi: 10.1055/s-0029-1211435. [DOI] [PubMed] [Google Scholar]
- 36.Davis R.A., Kern F. Effects of ethinyl estradiol and phenobarbital on bile acid synthesis and biliary bile acid and cholesterol excretion. Gastroenterology. 1976;70(6):1130–1135. [PubMed] [Google Scholar]
- 37.Lynn J., Williams L., O'Brien J., Wittenberg J., Egdahl R.H. Effects of estrogen upon bile: implications with respect to gallstone formation. Annals of Surgery. 1973;178(4):514–524. doi: 10.1097/00000658-197310000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Lu Y., Wang E., Zhang Z., Xiong X., Zhang H. Hepatic estrogen receptor α improves hepatosteatosis through upregulation of small heterodimer partner. Journal of Hepatology. 2015;63(1):183–190. doi: 10.1016/j.jhep.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Milona A., Owen B.M., Cobbold J.F.L., Willemsen E.C.L., Cox I.J., Boudjelal M. Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology (Baltimore Md.) 2010;52(4):1341–1349. doi: 10.1002/hep.23849. [DOI] [PubMed] [Google Scholar]
- 40.Yurkovetskiy L., Burrows M., Khan A.A., Graham L., Volchkov P., Becker L. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markle J.G.M., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle-Kampczyk U. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 42.Wang J.J., Wang J., Pang X.Y., Zhao L.P., Tian L., Wang X.P. Sex differences in colonization of gut microbiota from a man with short-term vegetarian and inulin-supplemented diet in germ-free mice. Scientific Reports. 2016;6:1–9. doi: 10.1038/srep36137. (October) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu K., Muranaka Y., Fujimura R., Ishida H., Tazume S., Shimamura T. 1998. Normalization of reproductive function in germfree mice following bacterial contamination; pp. 151–158. [DOI] [PubMed] [Google Scholar]
- 44.Adlercreutz H. Studies on oestrogen excretion in human bile. Acta Endocrinologica Supplementum. 1962;42(Suppl. 7):1–220. [PubMed] [Google Scholar]
- 45.Sandberg A.A., Slaunwhite W.R. Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women. Journal of Clinical Investigation. 1957;36(8):1266–1278. doi: 10.1172/JCI103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin F., Peltonen J., Laatikainen T., Pulkkinen M., Adlercreutz H. Excretion of progesteone metabolites and estriol in faeces from pregnant women during ampicillin administration. Journal of Steroid Biochemistry. 1975;6(9):1339–1346. doi: 10.1016/0022-4731(75)90363-5. [DOI] [PubMed] [Google Scholar]
- 47.Adlercreutz H., Martin F., Pulkkinen M., Dencker H., Rimer U., Sjoberg N.-O. Intestinal metabolism of estrogens. The Journal of Clinical Endocrinology and Metabolism. 1976;43(3):497–505. doi: 10.1210/jcem-43-3-497. [DOI] [PubMed] [Google Scholar]
- 48.McIntosh F.M., Maison N., Holtrop G., Young P., Stevens V.J., Ince J. Phylogenetic distribution of genes encoding β-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environmental Microbiology. 2012;14(8):1876–1887. doi: 10.1111/j.1462-2920.2012.02711.x. [DOI] [PubMed] [Google Scholar]
- 49.Gloux K., Berteau O., El oumami H., Beguet F., Leclerc M., Dore J. A metagenomic -glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proceedings of the National Academy of Sciences. 2011;108(Suppl. 1):4539–4546. doi: 10.1073/pnas.1000066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Järvenpää P., Kosunen T., Fotsis T., Adlercreutz H. In vitro metabolism of estrogens by isolated intestinal micro-organisms and by human faecal microflora. Journal of Steroid Biochemistry. 1980;13(3):345–349. doi: 10.1016/0022-4731(80)90014-x. [DOI] [PubMed] [Google Scholar]
- 51.Adlercreutz H., Martin F., Järvenpää P., Fotsis T. Steroid absorption and enterohepatic recycling. Contraception. 1979;20(3):201–223. doi: 10.1016/0010-7824(79)90094-5. [DOI] [PubMed] [Google Scholar]
- 52.Tamura M., Hori S., Nakagawa H. Intestinal bacterium TM-30: an S-equol-producing bacterium isolated from human feces is involved in estrogen metabolism in vitro. Food Science and Technology Research. 2014;20(2):309–316. [Google Scholar]
- 53.Kornman K.S., Loesche W.J. Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infection and Immunity. 1982;35(1):256–263. doi: 10.1128/iai.35.1.256-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Org E., Mehrabian M., Parks B.W., Shipkova P., Liu X., Drake T.A. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park S., Kim D.S., Kang E.S., Kim D., Bin Kang S. Low dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. American Journal of Physiology Endocrinology and Metabolism. 2018 doi: 10.1152/ajpendo.00005.2018. ajpendo.00005.2018. [DOI] [PubMed] [Google Scholar]
- 56.Cross T.-W.L., Zidon T.M., Welly R.J., Park Y.-M., Britton S.L., Koch L.G. Soy improves cardiometabolic health and cecal microbiota in female low-fit rats. Scientific Reports. 2017;7(1):9261. doi: 10.1038/s41598-017-08965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreno-Indias I., Sánchez-Alcoholado L., Sánchez-Garrido M.Á., Martín-Núñez G.M., Pérez-Jiménez F., Tena-Sempere M. Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology. 2016;157(12):4888–4898. doi: 10.1210/en.2016-1317. [DOI] [PubMed] [Google Scholar]
- 58.Cox-York K.A., Sheflin A.M., Foster M.T., Gentile C.L., Kahl A., Koch L.G. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiological Reports. 2015;3(8):1–14. doi: 10.14814/phy2.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patisaul H.B., Jefferson W. The pros and cons of phytoestrogens. Frontiers in Neuroendocrinology. 2010:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poutahidis T., Springer A., Levkovich T., Qi P., Varian B.J., Lakritz J.R. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9(1):e84877. doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kreek M.J., Guggenheim F.G., Ross J.E., Tapley D.F. Glucuronide formation in the transport of testosterone and androstenedione by rat intestine. Biochimica et Biophysica Acta. 1963;74:418–427. doi: 10.1016/0006-3002(63)91385-4. [DOI] [PubMed] [Google Scholar]
- 62.Graef V., Furuya E., Nishikaze O. Hydrolysis of steroid glucuronides with beta-glucuronidase preparations from bovine liver, helix pomatia, and E. Coli. Japanese Journal of Clinical Chemistry. 1977;5(2):182–188. [PubMed] [Google Scholar]
- 63.Lombardi P., Goldin B., Boutin E., Gorbach S.L. Metabolism of androgens and estrogens by human fecal microorganisms. Journal of Steroid Biochemistry. 1978;9(8):795–801. doi: 10.1016/0022-4731(78)90203-0. [DOI] [PubMed] [Google Scholar]
- 64.Ridlon J.M., Ikegawa S., Alves J.M.P., Zhou B., Kobayashi A., Iida T. Clostridium scindens : a human gut microbe with a high potential to convert glucocorticoids into androgens. Journal of Lipid Research. 2013;54(9):2437–2449. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gustafsson J.-A. Steroids in germfree and conventional rats 7. Identification of C19 and C21 steroids in faeces from conventional rats. European Journal of Biochemistry. 1968;6:248–255. doi: 10.1111/j.1432-1033.1968.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 66.Eriksson H., Gustafsson J.-A. Excretion of steroid hormones in adults. European Journal of Biochemistry. 1971;18(1):146–150. doi: 10.1111/j.1432-1033.1971.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 67.Datz F., Christian P., Moore J. Gender-related differences in gastric emptying. Journal of Nuclear Medicine Official Publication Society of Nuclear Medicine. 1987;28(7):1204–1207. [PubMed] [Google Scholar]
- 68.Hutson W.R., Roehrkasse R.L., Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96(1):11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 69.Duncan S.H., Louis P., Thomson J.M., Flint H.J. The role of pH in determining the species composition of the human colonic microbiota. Environmental Microbiology. 2009;11(8):2112–2122. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 70.Wald A., Van Thiel D.H., Hoechstetter L., Gavaler J.S., Egler K.M., Verm R. Effect of pregnancy on gastrointestinal transit. Digestive Diseases and Sciences. 1982;27(11):1015–1018. doi: 10.1007/BF01391748. [DOI] [PubMed] [Google Scholar]
- 71.Chiloiro M., Darconza G., Piccioli E., De Carne M., Clemente C., Riezzo G. Gastric emptying and orocecal transit time in pregnancy. Journal of Gastroenterology. 2001;36(8):538–543. doi: 10.1007/s005350170056. [DOI] [PubMed] [Google Scholar]
- 72.Wald A., Van Thiel D., Hoechstetter L., Gavaler J.S., Egler K.M., Verm R. Gastrointestinal Transit : the effect of the menstrual cycle. Gastroenterology. 1981;80(6):1497–1500. [PubMed] [Google Scholar]
- 73.Chen T.S., Doong M.L., Chang F.Y., Lee S.D., Wang P.S. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. American Journal of Physiology Gastrointestinal and Liver Physiology. 1995;268(1):G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 74.Coşkun T., Sevinç A., Tevetoğlu I., Alican I., Kurtel H., Yeğen B.C. Delayed gastric emptying in conscious male rats following chronic estrogen and progesterone treatment. Research in Experimental Medicine. Zeitschrift Für Die Gesamte Experimentelle Medizin Einschliesslich Experimenteller Chirurgie. 1995:49–54. doi: 10.1007/BF02576773. [DOI] [PubMed] [Google Scholar]
- 75.Walter P., Green S., Greenet G., Krust A., Bornert J.-M., Jeltsch J.-M. Cloning of the human estrogen receptor cDNA. Biochemistry. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuiper G.G.J.M., Enmark E., Pelto-Huikkot M., Nilssont S., Gustafsson Ii J.-A., Jensen E.V. vol. 93. 1996. pp. 5925–5930. (Cloning of a novel estrogen receptor expressed in rat prostate and ovary). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanstein B., Liu H., Yancisin M.C., Brown M. Functional analysis of a novel estrogen receptor-beta isoform. Molecular Endocrinology. 1999;13(1):129–137. doi: 10.1210/mend.13.1.0234. [DOI] [PubMed] [Google Scholar]
- 78.Moore J.T., McKee D.D., Slentz-Kesler K., Moore L.B., Jones S.A., Horne E.L. Cloning and characterization of human estrogen receptor beta isoforms. Biochemical and Biophysical Research Communications. 1998;247(1):75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 79.Takeyama J., Suzuki T., Inoue S., Kaneko C., Nagura H., Harada N. Expression and cellular localization of estrogen receptors α and β in the human fetus. Journal of Clinical Endocrinology & Metabolism. 2001;86(5):2258–2262. doi: 10.1210/jcem.86.5.7447. [DOI] [PubMed] [Google Scholar]
- 80.Campbell-Thompson M., Lynch I.J., Bhardwaj N. Expression of estrogen receptor (ER) subtypes and ER-beta isoforms in colon cancer. Cancer Research. 2001;61:632–640. [PubMed] [Google Scholar]
- 81.Kawano N., Koji T., Hishikawa Y., Murase K., Murata I., Kohno S. Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochemistry and Cell Biology. 2004;121(5):399–405. doi: 10.1007/s00418-004-0644-6. [DOI] [PubMed] [Google Scholar]
- 82.Foley E.F., Jazaeri A.A., Shupnik M.A., Jazaeri O., Rice L.W. Selective loss of estrogen receptor β in malignant human colon. Cancer Research. 2000;60:245–248. [PubMed] [Google Scholar]
- 83.Isensee J., Meoli L., Zazzu V., Nabzdyk C., Witt H., Soewarto D. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150(4):1722–1723. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 84.Włodarczyk M., Sobolewska-Włodarczyk A., Cygankiewicz A.I., Jacenik D., Piechota-Polańczyk A., Stec-Michalska K. G protein-coupled receptor 30 (GPR30) expression pattern in inflammatory bowel disease patients suggests its key role in the inflammatory process. A preliminary study. Journal of Gastrointestinal and Liver Diseases. 2017;26(1):29–35. doi: 10.15403/jgld.2014.1121.261.gpr. [DOI] [PubMed] [Google Scholar]
- 85.Thomas P., Pang Y., Filardo E.J., Dong J. Identity of an estrogen membrane receptor coupled to a G Protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 86.Prossnitz E.R., Arterburn J.B., Sklar L.A. GPR30: a G protein-coupled receptor for estrogen. Molecular and Cellular Endocrinology. 2007:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guarino M., Cheng L., Cicala M., Ripetti V., Biancani P., Behar J. Progesterone receptors and serotonin levels in colon epithelial cells from females with slow transit constipation. Neuro Gastroenterology and Motility. 2011;23(6):e210–e575. doi: 10.1111/j.1365-2982.2011.01705.x. [DOI] [PubMed] [Google Scholar]
- 88.Wilson C.M., McPhaul M.J. A and B forms of the androgen receptor are expressed in a variety of human tissues. Molecular and Cellular Endocrinology. 1996;120(1):51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 89.Wada-Hiraike O., Imamov O., Hiraike H., Hultenby K., Schwend T., Omoto Y. Role of estrogen receptor beta in colonic epithelium. Proceedings of the National Academy of Sciences. 2006;103(8):2959–2964. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Imamov O., Morani A., Shim G.-J., Omoto Y., Thulin-Andersson C., Warner M. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(25):9375–9380. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diebel M.E., Diebel L.N., Manke C.W., Liberati D.M. Estrogen modulates intestinal mucus physiochemical properties and protects against oxidant injury. Journal of Trauma and Acute Care Surgery. 2015;78(1):94–99. doi: 10.1097/TA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 92.Sheth S.U., Lu Q., Twelker K., Sharpe S.M., Qin X., Reino D.C. Intestinal mucus layer preservation in female rats attenuates gut injury after trauma-hemorrhagic shock. Journal of Trauma Injury Infection and Critical Care. 2010;68(2):279–287. doi: 10.1097/TA.0b013e3181caa6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elderman M., Sovran B., Hugenholtz F., Graversen K., Huijskes M., Houtsma E. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLoS One. 2017;12(9):1–22. doi: 10.1371/journal.pone.0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cani P.D., Bibiloni R., Knauf C., Neyrinck A.M., Delzenne N.M. Changes in gut microbiota control metabolic diet–induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 95.Cani P.D., Possemiers S., Van De Wiele T., Guiot Y., Everard A., Rottier O. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 97.Trøseid M., Nestvold T.K., Rudi K., Thoresen H., Nielsen E.W., Lappegård K.T. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care. 2013;36(11):3627–3632. doi: 10.2337/dc13-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stoll L.L., Denning G.M., Weintraub N.L. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arteriosclerosis Thrombosis and Vascular Biology. 2004:2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 99.Looijer-van Langen M., Hotte N., Dieleman L.A., Albert E., Mulder C., Madsen K.L. Estrogen receptor-beta signaling modulates epithelial barrier function. AJP Gastrointestinal and Liver Physiology. 2011;300(4):G621–G626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 100.Oh K.-J., Lee H.-S., Ahn K., Park K. Estrogen modulates expression of tight junction proteins in rat vagina. BioMed Research International. 2016;2016:1–6. doi: 10.1155/2016/4394702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Z., Zhang L., Ding M., Luo Z., Yuan S., Bansal M.B. Estrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clinical Immunology. 2017;183:174–180. doi: 10.1016/j.clim.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hulley S., Grady D., Bush T., Furberg C., Herrington D., Riggs B. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Journal of the American Medical Association. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 103.Grodstein F., Manson J.E., Stampfer M.J. Postmenopausal hormone use and secondary prevention of coronary events in the nurses' health study: a prospective, observational study. Annals of Internal Medicine. 2001;135(1):1. doi: 10.7326/0003-4819-135-1-200107030-00003. [DOI] [PubMed] [Google Scholar]
- 104.Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's health initiative randomized controlled trial. JAMA the Journal of the American Medical Association. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 105.Collins F.L., Rios-Arce N.D., Atkinson S., Bierhalter H., Schoenherr D., Bazil J.N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiological Reports. 2017;5(9) doi: 10.14814/phy2.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leiter E.H., Prochazka M., Coleman D.L. The non-obese diabetic (NOD) mouse. The American Journal of Pathology. 1987;128(2):380–383. [PMC free article] [PubMed] [Google Scholar]
- 107.Finkelstein E.A., Trogdon J.G., Cohen J.W., Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs. 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 108.Kuk J.L., Katzmarzyk P.T., Nichaman M.Z., Church T.S., Blair S.N., Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14(2):336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 109.Blasco-Baque V., Serino M., Vergnes J.N., Riant E., Loubieres P., Arnal J.F. High-fat diet induces periodontitis in mice through lipopolysaccharides (LPS) receptor signaling: protective action of estrogens. PLoS One. 2012;7(11):e48220. doi: 10.1371/journal.pone.0048220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riant E., Waget A., Cogo H., Arnal J.F., Burcelin R., Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 111.Ghisletti S., Meda C., Maggi A., Vegeto E. 17 -estradiol inhibits inflammatory gene expression by controlling NF- B intracellular localization. Molecular and Cellular Biology. 2005;25(8):2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schwandner R., Dziarski R., Wesche H., Rothe M., Cj K. Peptidoglycan-and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. Journal of Biological Chemistry. 1999;274(25):17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 113.Oh J.Y., Barrett-Connor E., Wedick N.M., Wingard D.L. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25(1):55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 114.Collado M.C., Isolauri E., Laitinen K., Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. American Journal of Clinical Nutrition. 2008;88(4):894–899. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 115.Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Kling Bäckhed H. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schulz K.M., Pearson J.N., Neeley E.W., Berger R., Leonard S., Adams C.E. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiology and Behavior. 2011;104(2):340–347. doi: 10.1016/j.physbeh.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suzuki K., Kondo N., Sato M., Tanaka T., Ando D., Yamagata Z. Gender differences in the association between maternal smoking during pregnancy and childhood growth trajectories: multilevel analysis. International Journal of Obesity. 2011;35(1):53–59. doi: 10.1038/ijo.2010.198. [DOI] [PubMed] [Google Scholar]
- 118.Gosalbes M.J., Llop S., Vallès Y., Moya A., Ballester F., Francino M.P. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clinical and Experimental Allergy. 2013;43(2):198–211. doi: 10.1111/cea.12063. [DOI] [PubMed] [Google Scholar]
- 119.Rautava S., Collado M.C., Salminen S., Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102(3):178–184. doi: 10.1159/000339182. [DOI] [PubMed] [Google Scholar]
- 120.Murphy S., Xu J., Kochanek K., Curtin S., Arias E. vol. 66. 2017. (National vital statistics reports - Deaths: Final data for 2007). [PubMed] [Google Scholar]
- 121.WHO . 2017. WHO cardiovascular diseases (CVDs)http://www.who.int/mediacentre/factsheets/fs317/en/ [Google Scholar]
- 122.Wenger N.K. You’ve come a long way, baby: cardiovascular health and disease in women problems and prospects. Circulation. 2004:558–560. doi: 10.1161/01.CIR.0000117292.19349.D0. [DOI] [PubMed] [Google Scholar]
- 123.Wenger N.K. Coronary heart disease: the female heart is vulnerable. Progress in Cardiovascular Diseases. 2003:199–229. doi: 10.1016/j.pcad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 124.Dreyer R.P., Ranasinghe I., Wang Y., Dharmarajan K., Murugiah K., Nuti S.V. Sex differences in the rate, timing, and principal diagnoses of 30-day readmissions in younger patients with acute myocardial infarction. Circulation. 2015;132(3):158–166. doi: 10.1161/CIRCULATIONAHA.114.014776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paigen B., Morrow A., Holmes P.A., Mitchell D., Williams R.A. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68(3):231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 126.Paigen B., Holmes P.A., Mitchell D., Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis. 1987;64(2–3):215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- 127.Bennett B.J., Davis R.C., Civelek M., Orozco L., Wu J., Qi H. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLoS Genetics. 2015;11(12) doi: 10.1371/journal.pgen.1005711. e1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang W.H.W., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–65. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brown J.M., Hazen S.L. Microbial modulation of cardiovascular disease. Nature Reviews Microbiology. 2018;16(3):171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Romano K.A., Vivas E.I., Amador-Noguez D., Rey F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6(2):e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caron S., Cariou B., Staels B. FXR: more than a bile acid receptor? Endocrinology. 2006;147(9):4022–4024. doi: 10.1210/en.2006-0701. [DOI] [PubMed] [Google Scholar]
- 135.Bennett B.J., Vallim T.Q.D.A., Wang Z., Shih D.M., Meng Y., Gregory J. Trimethylamine-N-Oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metabolism. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arnold A.P., Burgoyne P.S. Are XX and XY brain cells intrinsically different? Trends in Endocrinology and Metabolism. 2004:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 137.Link J.C., Chen X., Prien C., Borja M.S., Hammerson B., Oda M.N. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arteriosclerosis Thrombosis and Vascular Biology. 2015;35(8):1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kreznar J.H., Keller M.P., Traeger L.L., Rabaglia M.E., Schueler K.L., Stapleton D.S. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Reports. 2017;18(7):1739–1750. doi: 10.1016/j.celrep.2017.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kovacs A., Ben-Jacob N., Tayem H., Halperin E., Iraqi F.A., Gophna U. Genotype is a stronger determinant than sex of the mouse gut microbiota. Microbial Ecology. 2011;61(2):423–428. doi: 10.1007/s00248-010-9787-2. [DOI] [PubMed] [Google Scholar]
- 141.Kren V., Qi N., Krenova D., Zidek V., Sladka M., Jachymova M. Y-chromosome transfer induces changes in blood pressure and blood lipids in SHR. Hypertension. 2001;37(4):1147–1152. doi: 10.1161/01.hyp.37.4.1147. [DOI] [PubMed] [Google Scholar]
- 142.Charchar F.J., Bloomer L.D., Barnes T.A., Cowley M.J., Nelson C.P., Wang Y. Inheritance of coronary artery disease in men: an analysis of the role of the Y chromosome. Lancet. 2012;379(9819):915–922. doi: 10.1016/S0140-6736(11)61453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sandberg A.A., Kirdani R.Y., Back N., Weyman P., Slaunwhite W.R. Biliary excretion and enterohepatic circulation of estrone and estriol in rodents. American Journal of Physiology. 1967;213(5):1138–1142. doi: 10.1152/ajplegacy.1967.213.5.1138. [DOI] [PubMed] [Google Scholar]