Abstract
Background
Ischemia of the atria and conductive system of the heart results in greater atrial electrophysiological changes and propensity for atrial fibrillation. P wave duration and dispersion have been proposed to be useful for the prediction of paroxysmal atrial fibrillation (PAF). This study aimed to investigate the effect of coronary artery dominance on P wave duration and dispersion.
Methods
The study population included 194 patients with left dominant circulation (LDC) and 200 age- and gender-matched controls with right dominant circulation (RDC) and without coronary artery disease based on invasive coronary angiography findings. P wave dispersion (PWD) was defined as the difference between the maximum and minimum P wave duration. Arrhythmias were identified by 24-hour Holter electrocardiogram at 3 years of follow-up.
Results
PWD was significantly prolonged in the patients with LDC compared to the controls with RDC (p = 0.001). There were positive correlations between PWD and age (r: 0.502, p = 0.009), left ventricular mass (LVM) (r: 0.614, p = 0.001), LVM index (r: 0.727, p < 0.001) and left atrium (LA) diameter (r: 0.558, p = 0.003) in the LDC group. Multivariate logistic regression analysis showed that age, LVM index, LA diameter and LDC were independent predictors of prolonged PWD. At 3 years of follow-up, 7 (3.9%) patients with LDC and 1 (0.5%) patient with RDC had PAF in Holter electrocardiogram (p < 0.001).
Conclusions
LDC could lead to an increased risk of atrial fibrillation through prolonged PWD. We recommend following up these patients to assess the development of atrial fibrillation.
Keywords: Atrial fibrillation, Coronary artery dominance, Holter electrocardiogram, P wave dispersion, P wave duration
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia seen in clinical practice.1 It is linked to several forms of cardiovascular disease, but may also occur in otherwise normal hearts. The normal electrical conduction system of the heart allows for impulses generated by the sinoatrial node (SAN) of the heart to propagate through and stimulate the myocardium. Understanding the circulation in the atria is important, especially with respect to the normal cardiac pacemaker and atrial portion of the conduction system. Atrial coronary circulation plays an important role in the safety and efficacy of ablation procedures, as well as in the pathophysiology of AF itself.2 Ökmen et al. reported that arterial supply to the SAN arose from the right coronary artery (RCA) in 86% of their cases and from the circumflex artery (LCx) in the remaining 14%.3 It is known that more than 90% of coronary blood flow enters the left coronary if it is the dominant artery.4 From this point of view, we speculated that the relative ischemia of the SAN in patients with left-dominant circulation (LDC) may increase the risk of developing AF.
P wave dispersion (PWD), defined as the difference between the longest and shortest P wave duration recorded from 12-lead electrocardiography, is a noninvasive method to assessd is organized atrial repolarization. Improvements in the methodology of recording and analyzing P wave inscriptions may lead to the widespread use of this electrocardiographic marker in various clinical settings and particularly when assessing the risk of AF.5 A previous study demonstrated that PWD values of > 40 ms were correlated with AF, with a sensitivity of 74-83% and specificity of 81-85%.5 Therefore, we hypothesized that the congenitally determined blood supply to the SAN and atria could affect the electrocardiography indices, and that PWD maybe one of the causes of rhythm abnormalities in these patients. Hence, the aim of this study was to investigate the effect of coronary artery dominance on P wave duration and PWD.
METHODS
Patient selection
This observational, case-control study included 394 patients with an indication for invasive coronary angiography (CAG) referred to our clinic between March 2013 and June 2014. Patients with LDC and normal coronary arteries were consecutively selected based on their CAG findings. A total of 194 patients with LDC and 200 age- and gender-matched controls with right-dominant circulation (RDC) and normal coronary arteries were selected for electrocardiographic analysis. Patients with coronary artery disease (CAD), balanced coronary circulation, arrhythmia, valvular heart disease, systemic inflammatory diseases, electrolyte disorders, acute infectious diseases, renal insufficiency and left atrial dilatation were excluded from the study. Demographic data, risk factors, laboratory results including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), fasting blood glucose, blood urea nitrogen and creatinine levels were recorded. All patients underwent transthoracic echocardiography (TTE) before CAG. The electrocardiograms (ECGs) of each patient were recorded before enrollment into the study. Arrhythmias were diagnosed based on ECG obtained at clinical visits at 6, 12 and 24 months, and on 24-hour Holter-ECG monitoring at 3 years of follow-up. Written informed consent was obtained from all patients, and the study was approved by our local Ethics Committee.
Echocardiographic examination
The echocardiographic examinations were carried out using a 2.5- to 3.5-MHz transducer with a Philips cardiovascular ultrasound system (IE33 Echocardiography System, Philips Medical Systems, Eindhoven, the Netherlands) by an experienced cardiologist. M-mode echocardiography and quantitative analysis were conducted using parasternal long axis images according to data provided by the American Society of Echocardiography.6 The left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD) and left atrium (LA) diameter, and interventricular septum (IVS) and posterior wall (PoW) thicknesses were obtained using M-mode echocardiographic tracings under the guidance of 2D imaging. The left ventricular ejection fraction (LVEF) was calculated according to the biplane modified Simpson’s method. Left ventricular (LV) mass was calculated based on the formula: LV mass = 0.8 × (1.04 × ([LVEDD + PoW + IVS]3 – [LVEDD]3)) + 0.6 g.7 LV mass index (LVMI) was determined using the formula: LV mass/body surface area (g/m2). Normal LA volume indexed to body surface area was defined as < 34 mL/m2, and LA dilatation was considered above this value.
Coronary angiography procedure
All patients underwent selective right and left CAG in our catheterization laboratory after an average 3.2 days (range 1-7 days) from the possible diagnosis of CAD. The only medication given before the CAG procedure was acetylsalicylic acid 100 mg/day. Angiography of the RCA was performed in at least two projections, and the branches of the left coronary artery in at least three. Coronary angiograms were saved in a digital format, and each angiogram was analyzed by two experienced cardiologists. CAD was defined as > 50% diameter stenosis in one or more of the three major coronary arteries including left anterior descending (LAD) and LCx arteries and RCA or their major branches. A coronary artery system was classified asbeing right-dominant (RD) if the posterior descending artery (DP) and posterolateral branch originated from the RCA, LD if the DP and the posterolateral branch originated from the LCx artery (Figure 1), and balanced if the DP originated from the RCA in combination with posterolateral branches originating from the LCx artery.8,9
Figure 1.
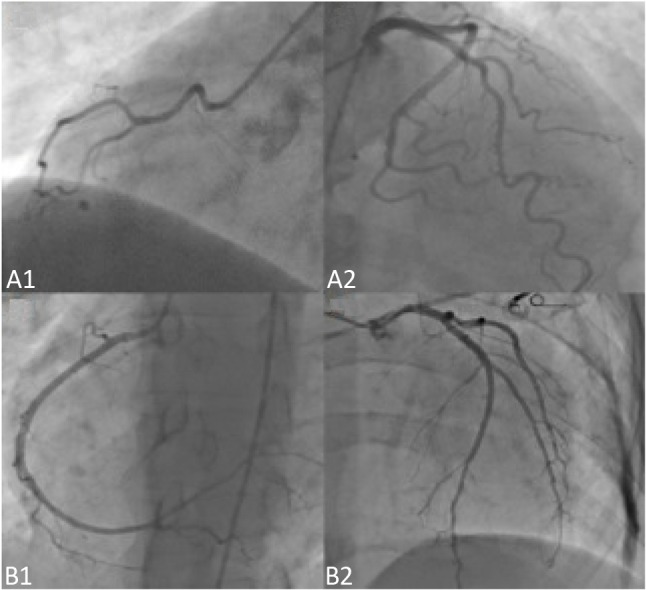
Coronary angiography images of two different patients, (A) patient with left dominant circulation with a non-dominant right coronary artery (A1) and dominant circumflex artery (A2). (B) Patient with right dominant circulation with a dominant right coronary artery (B1) and non-dominant circumflex artery (B2).
P wave duration and dispersion measurements
P wave duration was measured in all simultaneously recorded 12 leads of the surface ECG. All recordings were performed in the same quiet room during spontaneous breathing, following 20 minute of adjustment in the supine position. ECGs were recorded at a standard speed of 100 mm/s, and P wave duration measurements were obtained manually by two independent cardiologists blinded to the CAG results of the patients using calipers and a magnifying lens to accurately define the ECG deflection as described in a previous study.10 The average value of both measurements was taken into consideration. Inter-observer reliability was satisfactory. The concordance correlation coefficients showed close agreement between the two cardiologists, from 0.84 to 0.98. The onset of the P wave was defined as the point of the first visible upward departure of the trace from the bottom of the baseline. The return to baseline of the wave was considered to be the end of the P wave. P maximum in any of the 12 lead surface ECGs was measured and used as a marker of prolonged atrial conduction time. PWD was defined as the difference between the longest and the shortest P wave duration. A normal value of PWD has been reported to be 29 ± 9 ms. Aytemir et al.11 reported a maximum PWD value of 36 ms. A PWD ≥ 40 ms indicates the presence of heterogeneous electrical activity in different regions of the atrium that might cause atrial tachyarrhythmias. Thus, PWD is a strong predictor of atrial tachyarrhythmias and especially AF.12
Holter monitoring
All participants were monitored with a 24-hour two-channel (five-lead) Holter ECG system (CardioMem 3000, Getemed, Teltow, Germany) at baseline and at 3 years (during scheduled follow-up visits). All Holter recordings were analysed at Yıldırım Beyazıt University Hospital by the same experienced technician blinded to the results of CAG. All Holter recordings were scanned using Sentinel Pathfinder Digital (Spacelabs Healthcare) and interpreted using the interactive method. All episodes of AF were recorded during the entire recording period. AF was defined as absolutely irregular RR intervals without any repetitive ECG pattern, lacking a distinct P wave on surface ECG and showing an atrial cycle length of < 200 ms (> 300 beats/min). Only episodes lasting at least 30 s were included.
Statistical analysis
Continuous data were expressed as mean ± standard deviation, while categorical data were presented as number and percentage of patients. Differences in the frequency of characteristics were assessed using the independent sample Student’s t-test for continuous variables. The chi-squared test (or Fisher’s exact test if applicable) was used for categorical variables. Pearson correlation analysis was used to analyze the relationships between PWD and clinical and echocardiographic variables. Multivariate logistic regression analysis was used to identify the independent predictors of a prolonged PWD. The independent variables included clinical and electrocardiographic features that are known to be predictors of AF (age, LA diameter, LV mass and PWD). A value of p < 0.05 was considered to be statistically significant. All data were analyzed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 394 patients without CAD were studied, of whom 194 had LDC and 200 had RDC. The baseline characteristic features of these subjects are shown in Table 1. There were no significant differences between the two groups in mean age, body mass index, gender, smoking rate, frequency of diabetes mellitus, hypertension and hyperlipidemia and lipid profile including TC, LDL-C, HDL-C and TG levels.
Table 1. Baseline demographic and clinical features of the study population.
| Variables | LD patients (n = 194) | RD patients (n = 200) | p value |
| Age, years | 60.4 ± 16.4 | 61.7 ± 14.9 | 0.475 |
| Gender, M/F | 138/56 | 152/48 | 0.156 |
| BMI, kg/m2 | 28.3 ± 4.1 | 28.9 ± 3.7 | 0.327 |
| Smoking rate, % | 42 | 34 | 0.311 |
| DM, % | 21 | 24 | 0.823 |
| HT, % | 48 | 52 | 0.506 |
| HL, % | 37 | 35 | 0.816 |
| TC, mg/dL | 191.6 ± 47.3 | 194.0 ± 44.7 | 0.776 |
| LDL-C, mg/dL | 116.2 ± 32.6 | 118.1 ± 31.8 | 0.812 |
| HDL-C, mg/dL | 43.8 ± 11.3 | 46.0 ± 12.0 | 0.546 |
| TG, mg/dL | 167.4 ± 77.3 | 178.5 ± 147.8 | 0.560 |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
BMI, body mass index; DM, diabetes mellitus; HDL-C, high density lipoprotein cholesterol; HL, hyperlipidemia; HT, hypertension; LD, left dominant; LDL-C, low density lipoprotein cholesterol; M/F, male/female; RD, right dominant; TC, total cholesterol; TG, triglyceride.
The echocardiographic and electrocardiographic results of the study patients are shown in Table 2. There were no statistically significant differences in LVESD, IVS and PoW thicknesses, LA diameter, LVEF, LV mass and LVMI between the two groups. However, the LVEDD was significantly larger in the RDC group compared to the LDC group (4.80 ± 0.35 mm vs. 4.63 ± 0.44 mm, p = 0.012). When we compared the electrocardiographic parameters of both groups including PWD and duration, PWD was significantly prolonged in the LDC group compared to the RDC group (48 ± 14 ms vs. 33 ± 15 ms, p = 0.001). However, there was no significant difference in P wave duration between the two groups (p = 0.124).
Table 2. Electrocardiographic and echocardiographic results of the study population.
| Variables | LD patients (n = 194) | RD patients (n = 200) | p value |
| LVEDD, cm | 4.63 ± 0.44 | 4.80 ± 0.35 | 0.012 |
| LVESD, cm | 3.08 ± 0.42 | 3.15 ± 0.52 | 0.314 |
| IVS, cm | 1.1 ± 0.18 | 1.1 ± 0.12 | 0.916 |
| PoW, cm | 1.08 ± 0.14 | 1.09 ± 0.16 | 0.574 |
| LA diameter, cm | 3.78 ± 0.67 | 3.82 ± 0.36 | 0.422 |
| LVEF, % | 57.8 ± 7.98 | 58.9 ± 8.67 | 0.625 |
| LV mass, g | 187.8 ± 36.8 | 201.4 ± 41.3 | 0.189 |
| LVMI, g/m2 | 99.2 ± 26.3 | 108.2 ± 29.4 | 0.176 |
| P wave duration, msec | 84 ± 21 | 96 ± 24 | 0.124 |
| PWD, mean ± SD | 48 ± 14 | 33 ± 15 | 0.001 |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
IVS, interventricular septum thickness; LA, left atrium; LV, left ventricular; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVMI, left ventricular mass index; PoW, posterior wall thickness; PWD, P wave dispersion.
The correlations of clinical and echocardiographic parameters with PWD are presented in Table 3. PWD was positively correlated with age (r: 0.502, p = 0.009), LV mass (r: 0.614, p = 0.001), LVMI (r: 0.727, p < 0.001) and LA diameter (r: 0.558, p = 0.003) in the LDC group. However, there were no significant correlations between PWD and these parameters in the RDC group. When we evaluated the correlations of P wave duration with demographic and other parameters of both groups, a positive correlation was only detected between P wave duration and LVMI (r: 0.614, p = 0.024). However, no significant correlations were detected between P wave duration and age (p = 0.136), LA diameter (p = 0.144) and LV mass (p = 0.244). In multivariate logistic regression analysis, LDC [odds ratio 0.93, 95% CI 0.91-0.95), p < 0.001] in addition to age, LA diameter and LVMI were found to be independent predictors of prolonged PWD (Table 4).
Table 3. Correlations of P wave dispersion with clinical and echocardiographic parameters.
| Variables | LD patients (n = 194) | RD patients (n = 200) |
| Coefficient (p value) | Coefficient (p value) | |
| Age | 0.502 (0.009) | 0.289 (0.149) |
| BMI | 0.146 (0.320) | 0.138 (0.403) |
| LV mass | 0.614 (0.001) | 0.158 (0.544) |
| LVMI | 0.727 (< 0.001) | 0.406 (0.211) |
| LA diameter | 0.558 (0.003) | 0.314 (0.284) |
| LVEF | -0.284 (0.314) | 0.104 (0.473) |
BMI, body mass index; LA, left atrium; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Table 4. Independent predictors of prolonged P-wave dispersion with multivariate logistic regression analysis.
| Variables | Multivariate analysis | |
| Odds ratio, 95% CI | p | |
| Age | 1.13 (1.00-1.32) | 0.042 |
| Gender | 0.91 (0.76-1.12) | 0.090 |
| BMI | 0.92 (0.76-1.23) | 0.426 |
| Smoking | 0.96 (0.11-2.03) | 0.964 |
| Hyperlipidemia | 0.97 (0.86-1.02) | 0.131 |
| Hypertension | 1.05 (0.91-1.22) | 0.473 |
| Diabetes mellitus | 6.66 (1.12-10.07) | 0.064 |
| LV ejection fraction | 0.75 (0.38-1.45) | 0.390 |
| LV mass | 0.75 (0.38-1.45) | 0.390 |
| LV mass index | 1.15 (1.03-1.28) | 0.010 |
| LA diameter | 1.16 (1.01-1.32) | 0.031 |
| RD circulation | 0.86 (0.44-1.36) | 0.774 |
| LD circulation | 0.93 (0.91-0.95) | < 0.001 |
BMI, body mass index; LA, left atrium; LD, left dominant; LV, left ventricular; RD, right dominant.
At baseline, paroxysmal AF (PAF) was not observed in any patients in ECG and 24-hour Holter ECG monitoring. At 3 years of follow-up, 24-hour Holter ECG monitoring was performed in 178 of the patients with LDC and 183 of the patients with RDC (Table 5). Among them, seven (3.9%) patients with LDC and one (0.5%) patient with RDC had PAF (p < 0.001). We also performed TTE in these patients with PAF to exclude possible valvular or other organic heart diseases, and no significant cardiac pathology was observed. In addition, the number of premature atrial com-plexes (686 ± 1012 vs. 84 ± 165, p = 0.006), premature ventricular complexes (372 ± 716 vs. 32 ± 84, p = 0.004) complexes, sinusal pauses (p < 0.001) and right bundle branch block (p < 0.001) were significantly higher among the patients with LDC compared to those with RDC.
Table 5. 24-h ambulatory electrocardiography results of the patient population at third-year follow-up.
| Findings on Holter ECG | LD patients (n = 178) | RD patients (n = 183) | p value |
| Maximum HR | 94 ± 7.3 | 91 ± 8.2 | 0.421 |
| Average HR | 75 ± 10.4 | 73 ± 12.1 | 0.210 |
| Minimum HR | 54 ± 8.2 | 56 ± 9.5 | 0.112 |
| PACs | 686 ± 1012 | 84 ± 165 | 0.006 |
| PVCs | 372 ± 716 | 32 ± 84. | 0.004 |
| Sinus arrhythmia | 3 (1.6) | 0 (0) | 0.104 |
| Sinusal pause | 13 (7.3) | 2 (1) | < 0.001 |
| First degree AV block | 3 (1.6) | 1 (0.5) | 0.544 |
| LBBB | 4 (2.2) | 6 (3.2) | 0.804 |
| RBBB | 28 (15.7) | 6 (3.2) | < 0.001 |
| SVT | 3 (1.6) | 1 (0.5) | 0.544 |
| AF/flutter | 7 (3.9) | 1 (0.5) | < 0.001 |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
AF, atrial fibrillation; AV, atrioventricular; ECG, electrocardiogram; HR, heart rate; LBBB, left bundle branch block; PAC, premature atrial complex; PVC, premature ventricular complex; RBBB, right bundle branch block.
DISCUSSION
The present study demonstrated an association between left coronary artery dominancy and PWD. Moreover, in the patients with LDC, PWD was positively correlated with age, LA diameter and LV mass, which are known predictors of AF.
The anatomic variations of coronary circulation leading to the concept of coronary dominance were first described by Bianchi in 1904.13 Subsequently, Hettler provided very detailed criteria for the type classification based on the course of the anterior and posterior interventricular branches and defined the following types: left coronary artery dominance, RCA dominance, and co-dominance or balanced13 Accordingly, a coronary artery system can be classified as RD if the DP and posterolateral branch originate from the RCA, which is a common finding in about 87-89% of the general population. In comparison, a coronary artery system can be classified as LD if the DP and the posterolateral branch originate from the LCx artery. The rate of LDC in the general population is about 7-8%. If the DP originates from the RCA and posterolateral branches originate from the LCx artery, it is classified as being balanced.
Heart rhythm is initialized and controlled by the SAN, the primary pacemaker of the heart. Initiation of heart rhythm occurs within specialized cardiomyocytes of the SAN and is propagated throughout the atria and ventricles by the cardiac conduction system. SAN dysfunction, also referred to as sick sinus syndrome, commonly translates into rhythm abnormalities manifesting as bradyarrhythmias or tachycardia-bradycardia syndrome which are frequently associated with cardiac diseases including AF, malignant ventricular arrhythmias, heart failure and cardiac arrest.14 AF, the most common sustained cardiac arrhythmia managed in current cardiology practice, is becoming progressively more prevalent with the aging population.1 It is characterized by rapid and disorganized atrial activation leading to impaired atrial function, which can be diagnosed on an ECG by a lack of a P wave and irregular QRS complexes. It is associated with increased morbidity and mortality and is a risk factor for embolic stroke and worsening heart failure. Therefore, it is important to understand the predisposing factors related to AF and thus take relevant precautions and if needed schedule follow-up for such patients. Current research on AF supports the hypothesis that the initiation and maintenance of AF require pathophysiological remodeling of the atria, either specifically as in lone AF or secondary to other heart diseases as in heart failure-associated AF. Structural and functional remodeling of both atria, which manifest as increased atrial volume and decreased triphasic transport function, have been reported to be more evident in both patients with AF and in those with AF-related stroke.15 Both the left and right atrium possess structural features that contribute to the pathogenesis of AF. Numerous factors can predispose the atria to fibrillation. AF may occur in apparently structurally normal hearts without known precipitating factors, or it may be a complication of underlying cardiac or systemic diseases. The insidious causes of AF are ischemic heart disease, rheumatic valve disease, cor-pulmonale, hypertension, cardiomyopathies, congenital heart disease, and senescent heart disease. CAD involving the atrial branches has been associated with a higher incidence of new-onset AF after myocardial infarction.16
Saremi et al.17 reported that blood supply to the SAN arose from the LCx artery in 27.8% of their cases and the RCA in 65.7%. Furthermore, the atrio-ventricular nodal (AVN) artery arose from the RCA in 87.3% of these hearts. Vieweg et al.18 also determined the origin of the SAN artery and AVN artery for 118 patients with normal coronary arteriograms. The results illustrated that the SAN artery arose from the RCA, left coronary artery and dual origin in 53%, 35% and 11% of the patients, respectively. Moreover, the AVN artery arose from the RCA, left coronary artery and both in 84%, 8% and 8% of patients, respectively. These results demonstrate that the RCA is the main coronary artery supplying the cardiac conduction system. We hypothesize that, in patients with LDC (non-dominant RCA), the blood supply to the SAN is disturbed, and that this predisposes the patients to arrhythmias such as AF due to the sick sinus syndrome.
P wave duration and PWD are widely considered to be non-invasive markers of electrical remodeling within atrial tissue. Increased P wave duration and PWD reflect prolongation of intra-atrial and inter-atrial conduction time with a lack of a well-coordinated conduction system within the atrial muscles. Therefore, a defect within the right or left atrial muscle may disrupt this conduction and increase the PWD. PWD is considered to reflect disrupted and heterogeneous inter-atrial conduction, which is a specific and sensitive marker of AF in a wide variety of conditions. A prolonged P wave duration has been associated with the development of AF.19-21 PWD measured from a single ECG is regarded to be an electrocardiographic marker of inhomogeneous and discontinuous propagation of sinus impulses.22 It reflects the activation of atrial muscles and is influenced by the mass of the excited tissue. It also reflects atrial remodeling, mostly atrial fibrosis. Several studies have shown that PWD has a predictive value for AF, which is characterized by inhomogeneous and discontinuous atrial conduction in patients with various conditions.23-26
This study evaluated for the first time P wave duration and PWD as possible predictors of AF and other atrial arrhythmias in patients with LD and RD coronary circulations and normal coronary arteries. Although P wave duration was similar between the two groups in this study, PWD was significantly prolonged in the LD group compared to the RD group. Moreover, PWD has been positively correlated with age, LA diameter, LV mass and LVMI in patients with LDC. Because of the fact that, aging, LA diameter, LV mass and LVMI are important predisposing factors in the development of AF, these correlations seem reasonable. Furthermore, in multivariate analysis, LDC was a strong and independent predictor of prolonged PWD.
Increased heterogeneity of refractoriness, which is present in ischemia, may be a substrate for AF.27 Accordingly, it has been shown that myocardial ischemia increases P wave duration and PWD.28-30 Thus, one possible mechanism for the increase in PWD may be microvascular ischemia in LD, in other words, right non-dominant patients. The increased frequency of AF after acute myocardial infarction, especially in patients with LDC, also supports these results.
Limitations of the study
There are several limitations to the present study. The most important and major limitation is the absence of long-term (at least 5 years) clinical follow-up to further explore the frequency of AF in the studied patients. The single-center as well as the observational design of the study was another considerable limitation. Furthermore, in the present study, although we did not specifically investigate an association between PWD and age, LV mass and LA diameter, we did identify an association among them. This association should be studied further, perhaps even in a manner similar to the associations between AF and age, LV mass, and LA diameter. Therefore, studies focused on the association between coronary artery dominance and the incidence of AF with clinical follow-up are necessary to confirm our findings.
CONCLUSIONS
Age, LA diameter, LVMI and LDC were independent predictors of prolonged PWD. PWD was significantly prolonged and correlated with age, LV mass, LVMI and LA diameter in the patients with LDC compared to those with RDC. These findings suggest that: 1) atrial blood flow mainly depends on the RCA; 2) the RCA territory is relatively weak in LD patients; 3) left dominancy may increase the risk of AF.
Acknowledgments
All authors read and approved the final version of the manuscript. There was no funding source for this study.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Boppana VS, Castaño A, Avula UM, et al. Atrial coronary arteries: anatomy and atrial perfusion territories. Jour of Atrial Fibril. 2011;3:23–32. doi: 10.4022/jafib.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ökmen AŞ, Ökmen E. Sinoatrial node artery arising from posterolateral branch of right coronary artery: definition by screening consecutive 1500 coronary angiographies. Anadolu Kardiyol Derg. 2009;9:481–485. [PubMed] [Google Scholar]
- 4.Ghaffari S, Kazemi B, Dadashzadeh J, et al. The relation between left coronary dominancy and atherosclerotic involvement of left anterior descending artery origin. J Cardiovasc Thorac Res. 2013;5:1–4. doi: 10.5681/jcvtr.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilaveris PE, Gialafos JE. P-wave dispersion: a novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol. 2001;6:159–165. doi: 10.1111/j.1542-474X.2001.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiller NB, Shah PM, Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards,Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 8.Veltman CE, de Graaf FR, Schuijf JD, et al. Prognostic value of coronary vessel dominance in relation to significant coronary artery disease determined with non-invasive computed tomography coronary angiography. Eur Heart J. 2012;33:1367–1377. doi: 10.1093/eurheartj/ehs034. [DOI] [PubMed] [Google Scholar]
- 9.Fuster V, Alexander RW, O'Rourke RA. Hurst's The Heart (10th ed.) McGraw-Hill; 2001. p. 53. [Google Scholar]
- 10.Dilaveris PE, Gialafos EJ, Sideris SK, et al. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- 11.Aytemir K, Ozer N, Atalar E, et al. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:1109–1112. doi: 10.1111/j.1540-8159.2000.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Riera AR, de Abreu LC, Barbosa-Barros R, et al. P-wave dispersion: an update. Indian Pacing Electrophysiol J. 2016;16:126–133. doi: 10.1016/j.ipej.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gawlikowska-Sroka A, Miklaszewska D, Czerwinski F. Analysis of the influence of heart size and gender on coronary circulation type. Folia Morphol (Warsz) 2010;69:35–41. [PubMed] [Google Scholar]
- 14.Jensen PN, Gronroos NN, Chen LY, et al. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64:531–538. doi: 10.1016/j.jacc.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsao HM, Hu WC, Tsai PH, et al. Functional remodeling of both atria is associated with occurrence of stroke in patients with paroxysmal and persistent atrial fibrillation. Acta Cardiol Sin. 2017;33:50–57. doi: 10.6515/ACS20160411A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James TN. Myocardial infarction and atrial arrhythmias. Circulation. 1961;24:761–776. doi: 10.1161/01.cir.24.4.761. [DOI] [PubMed] [Google Scholar]
- 17.Saremi F, Abolhoda A, Ashikyan O, et al. Arterial supply to sinoatrial and atrioventricular nodes: imaging with multidetector CT. Radiology. 2008;1:99–107. doi: 10.1148/radiol.2461070030. [DOI] [PubMed] [Google Scholar]
- 18.Vieweg WV, Alpert JS, Hagan AD. Origin of the sinoatrial node and atrioventricular node arteries in right, mixed, and left inferior emphasis systems. Cathet Cardiovasc Diagn. 1975;1:361–373. doi: 10.1002/ccd.1810010405. [DOI] [PubMed] [Google Scholar]
- 19.Ariyarajah V, Apiyasawat S, Fernandes J, et al. Association of atrial fibrillation in patients with interatrial block over prospectively followed controls with comparable echocardiographic parameters. Am J Cardiol. 2007;99:390–392. doi: 10.1016/j.amjcard.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons > 60 years old (from the Framingham Heart Study). Am J Cardiol. 2011;107:917–921. doi: 10.1016/j.amjcard.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrikopoulos GK, Dilaveris PE, Richter DJ, et al. Increased variance of P wave duration on the electrocardiogram distinguishes patients with idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:1127–1132. doi: 10.1111/j.1540-8159.2000.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 22.Centurion OA. Clinical implications of the P wave duration and dispersion: relationship between atrial conduction defects and abnormally prolongedand fractionated atrial endocardial electrograms. Int J Cardiol. 2009;134:6–8. doi: 10.1016/j.ijcard.2008.12.072. [DOI] [PubMed] [Google Scholar]
- 23.Michelucci A, Giuseppe B, Colella A, et al. P wave assessment: state of the art update. Card Electrophysiol Rev. 2002;6:215–220. doi: 10.1023/a:1016368723033. [DOI] [PubMed] [Google Scholar]
- 24.Aytemir K, Özer N, Atalar E, et al. P wave dispersion on lead electrocardiography in patients with paroxysmal atrial 12 fibrillation. Pacing Clin Electrophysiol. 2000;23:1109–1112. doi: 10.1111/j.1540-8159.2000.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz R. Effects of alcohol intake on atrial arrhythmias and P-wave dispersion. Anadolu Kardiyol Derg. 2005;5:294–296. [PubMed] [Google Scholar]
- 26.Dilaveris PE, Gialafos EJ, Chrissos D, et al. Detection of hypertensive patients at risk for paroxysmal atrial fibrillation during sinus rhythm by computerassisted P wave analysis. J Hypertens. 1999; 17:1463–1470. doi: 10.1097/00004872-199917100-00015. [DOI] [PubMed] [Google Scholar]
- 27.Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation. 1998;98:2202–2209. doi: 10.1161/01.cir.98.20.2202. [DOI] [PubMed] [Google Scholar]
- 28.Myrianthefs MM, Shandling AH, Startt-Selvester RH, et al. Analysis of the signal-averaged P-wave duration in patients with percutaneous coronary angioplasty-induced myocardial ischemia. Am J Cardiol. 1992;70:728–732. doi: 10.1016/0002-9149(92)90549-e. [DOI] [PubMed] [Google Scholar]
- 29.Yılmaz R, Demirbağ R. P-wave dispersion in patients with stable coronary artery disease and its relationship with severity of the disease. J Electrocardiol. 2005;38:279. doi: 10.1016/j.jelectrocard.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Dilaveris PE, Andrikopoulos GK, Metaxas G, et al. Effects of ischemia on P wave dispersion and maximum P wave duration during spontaneous anginal episodes. Pacing Clin Electrophysiol. 1999;22:1640–1647. doi: 10.1111/j.1540-8159.1999.tb00384.x. [DOI] [PubMed] [Google Scholar]


