Tocopherols have demonstrated anti-tumor effects in breast cancer. Estrogen is an important positive modulator of cancer stem cell properties in estrogen receptor-positive breast cancer, and tocopherols inhibit estrogen-mediated cancer stemness via regulating OCT4 signaling in breast cancer.
Abstract
Estrogen plays an important role in breast cancer development. While the mechanism of the estrogen effects is not fully elucidated, one possible route is by increasing the stem cell-like properties in the tumors. Tocopherols are known to reduce breast cancer development and progression. The aim of the present study is to investigate the effects of tocopherols on the regulation of breast cancer stemness mediated by estrogen. To determine the effects of tocopherols on estrogen-influenced breast cancer stem cells, the MCF-7 tumorsphere culture system, which enriches for mammary progenitor cells and putative breast cancer stem cells, was utilized. Treatment with estrogen resulted in an increase in the CD44+/CD24− subpopulation and aldehyde dehydrogenase activity in tumorspheres as well as the number and size of tumorspheres. Tocopherols inhibited the estrogen-induced expansion of the breast cancer stem population. Tocopherols decreased the levels of stem cell markers, including octamer-binding transcription factor 4 (OCT4), CD44 and SOX-2, as well as estrogen-related markers, such as trefoil factor (TFF)/pS2, cathepsin D, progesterone receptor and SERPINA1, in estrogen-stimulated tumorspheres. Overexpression of OCT4 increased CD44 and sex-determining region Y-box-2 levels and significantly increased cell invasion and expression of the invasion markers, matrix metalloproteinases, tissue inhibitors of metalloproteinase and urokinase plasminogen activator, and tocopherols inhibited these OCT4-mediated effects. These results suggest a potential inhibitory mechanism of tocopherols in estrogen-induced stemness and cell invasion in breast cancer.
Introduction
Breast cancer stem cells, which represent a subset of tumor cells, are considered responsible for development, growth and progression of tumors (1). In addition, breast cancer stem cells are believed to be the main cause of metastasis and recurrence of cancer because of their strong tumor-initiating abilities and resistance to conventional therapies (1). Therefore, treatment that targets cancer stem cells may be of substantial benefit. Although the importance of estrogen in breast cancer is well established, the mechanism of its effects is not fully understood. Some studies have suggested that estrogen can promote cancer stem cell activity by inducing the secretion of paracrine growth factors from estrogen receptor (ER)-positive cells via fibroblast growth factor/Tbx3, epidermal growth factor and Notch signaling pathways (2,3). In contrast to these findings, estrogen was shown to reduce the self-renewal capacity of breast cancer stem cells by promoting differentiation through downregulation of stem cell genes (4).
Some dietary components and bioactive natural compounds potentially inhibit breast cancer development and progression in experimental systems. It appears that they work by inhibiting breast stem cells through regulation of their self-renewal pathways (5). Tocopherols, the major forms of vitamin E, are particularly active in this regard. Tocopherols are a family of fat-soluble phenolic compounds consisting of a chromanol ring system and a 16-carbon side chain (6). Depending upon the number and position of methyl groups on the chromanol ring, they exist as α-, β-, γ- or δ-tocopherol (6). Many studies have shown that tocopherols inhibit cancer formation and development due to their strong antioxidant properties (7–9). Although α-tocopherol has been the most widely used form of tocopherols for cancer prevention studies, large-scale human trials with α-tocopherol did not find a cancer preventive effect (10,11). γ-Tocopherol is the most abundant tocopherol in the USA diet, mainly from vegetable oils and nuts (12). We have reported previously that treatment with γ- and δ-tocopherols and γ-TmT, a naturally occurring tocopherols mixture, inhibited mammary tumor growth in N-methyl-N-nitrosourea-induced Sprague Dawley rats (13). Recently, we demonstrated that γ- and δ-tocopherols were more active than α-tocopherol in inhibiting mammary tumorigenesis in ACI rats and breast xenograft tumor growth supplemented with estrogen as well as cell proliferation and oxidative stress in estrogen-treated MCF-7 cells (14,15). These studies suggest a potential inhibitory mechanism of tocopherols in estrogen-mediated events in breast cancer.
Recent studies have shown that signaling pathways involved in maintaining the stem cell population are activated in tumor-initiating cells (16). The main transcription factors such as octamer-binding transcription factor 4 (OCT4), sex-determining region Y-box-2 (SOX2) and nuclear factor-κB act as master regulators of pluripotency and maintain the undifferentiated state of cells (17). OCT4, one of the key transcription factors, is associated with the pluripotency and self-renewal properties in embryonic stem cells, germ cells and adult human stem cells (18). Hu et al. (19) reported that ablation of OCT4 expression leads to apoptosis of cancer stem cells through the OCT4/Tcl1/Akt1 pathway in MCF-7 breast cancer cells and inhibition of tumor growth. It is further reported that estrogen increases OCT4 expression and proliferation of tumorspheres as well as expands the breast cancer stem cell population in MCF-7 tumorspheres (20). In addition, SOX2 is expressed in derived spheres, those that have been generated from breast cancer tumors and cell lines (21). Evidence suggests that high levels of OCT4 and SOX2 lead to the activation of other pluripotency genes that aid in the activation of the pluripotency network (22). CD44 is one of the key cell surface markers for tumor-initiating cells in breast cancer (23). Recently, CD44 overexpression was shown to correlate with invasive, metastatic phenotype and nuclear localization of stemness factors in breast cancer (24). Since CD44 does not have intrinsic kinase activity, it modulates multiple intracellular signaling by interacting with other components of signaling transduction (25). Therefore, identification of interacting molecules is important to understand the biological role of OCT4 and CD44 in human breast cancer stem cells.
In the present study, we investigated estrogen as an important positive modulator of cancer stem cell properties in ER-positive breast cancer and examined the effects of tocopherols on estrogen-mediated cancer stemness and OCT4 signaling in breast cancer.
Materials and methods
Cell culture and reagents
Tocopherols were prepared as described previously (14). Briefly, α- and δ-tocopherols were purified to ≥97% purity from the commercial grade α-tocopherol (T3634) and δ-tocopherol (T2028), respectively, from Sigma–Aldrich (St. Louis, MO). γ-Tocopherol was purified from γ-tocopherol-rich mixture of tocopherols (BASF Corporation, Kankakee, IL; Covi-ox T-90, Batch number 0008778732) to ≥97% purity with no other detectable forms of tocopherol. A CombiFlash Companion SL automated flash chromatographic system (Teledyne ISCO, Lincoln, NE) with a Redisep Rf Gold high-performance flash silica gel column (20–40 μm in particle size) was used for the purification. Each tocopherol was dissolved in dimethyl sulfoxide (Sigma–Aldrich). Estrogen was obtained from Sigma–Aldrich (E2758) and dissolved in dimethyl sulfoxide. The MCF-7 cell line was acquired from American Type Culture Collection (ATCC, Manassas, VA) and authenticated by short tandem repeat profiling at ATCC. MCF-7 cells were maintained in DMEM/F12 medium, 10% fetal bovine serum and 1% penicillin/streptomycin at 37oC and 5% CO2. When noted, MCF-7 cells were treated in 10% charcoal-stripped FBS/phenol red-free RPMI medium to examine effects of tocopherols on estrogen-mediated events.
Tumorsphere-forming assay
MCF-7 cells were harvested from monolayer culture using StemPro Accutase® (Life Technologies, Grand Island, NY). Cells were then plated at 10000 cells/ml in six-well ultra-low attachment plates and maintained in a serum-free mammary epithelial basal medium without phenol red (MEBM, Lonza, Walkersville, MD), supplemented with B27 (GIBCO, Carlsbad, CA), 20 ng/ml epithelial growth factor (Sigma–Aldrich), 20 ng/ml basic fibroblast growth factor (GIBCO), 5 µg/ml bovine insulin (Sigma–Aldrich) and 0.5 µg/ml hydrocortisone (Sigma–Aldrich). Cells were incubated with vehicle control (DMSO), estrogen (1 nM) or different forms of tocopherol (1 µM) for 4 days without disturbing the plates and without replenishing the medium. The tumorsphere was gathered at the center of the well by slowly swirling plates, and the pictures were taken to measure number and size of tumorspheres for sphere-forming efficiency (SFE) using a TE200 microscope (Nikon Instrument, Melville, NY).
Flow cytometry
The detailed procedure was reported previously (26). MCF-7 cells were stained with antibodies against CD44-FITC (Cat. 555478) and CD24-PE (Cat. 561893) from BD Biosciences (San Jose, CA). The stained MCF-7 cells were analyzed by flow cytometry using an FC500 Analyzer (Beckman Coulter) to determine the percentage of different CD44−/CD24+, CD44+/CD24+, CD44−/CD24− and CD44+/CD24− subpopulations. For CD44/CD24/aldehyde dehydrogenase (ALDH) staining, tumorspheres were resuspended in 1 ml of Aldefluor buffer containing 5 μl of activated Aldefluor reagent (Stem Cell Technologies, Grenoble, France). One-half of the sample was transferred to a second tube containing 5 μl of the ALDH inhibitor, diethylaminobenzaldehyde. After 30 min of incubation with the Aldefluor reagent, cells were incubated with 15 μl of CD44-APC (Cat. 559945, BD Biosciences) and 15 μl of CD24-PE (Cat. 555428, BD Biosciences) for 30 min at RT in the dark. Then, cells were analyzed by flow cytometry using an FC500 Analyzer (Beckman Coulter).
ALDEFLUOR assay
To measure cells with ALDH activity, the ALDEFLOUR assay was carried out according to manufacturer’s protocol (Stem Cell Technologies). Briefly, MCF-7 tumorspheres were resuspended in 1 ml of ALDEFLUOR assay buffer containing 5 μl of activated Aldefluor reagent for 30 min at 37oC. As a negative control for all experiments, an aliquot of ALDEFLUOR stained cells was immediately quenched with 5 μl diethylaminobenzaldehyde. Cells were analyzed using the green fluorescence channel (FL1) on an FC500 Analyzer (Beckman Coulter).
Quantitative polymerase chain reaction analysis
The procedure was described previously (27); the labeled primers, including OCT4 (Hs00999634), CD44 (Hs01075861), NFKB1 (Hs00765730), SOX2 (Hs01053049), glioma-associated oncogene homolog (GLI; Hs00171790), sonic hedgehog (SHH; Hs00179843), TFF (Hs00907239), cathepsin D (CTSD; Hs00157205), progesterone receptor (PGR; Hs01556702), SERPINA1 (Hs00165475), MMP9 (Hs00957562), MMP13 (Hs00942584), MMP15 (Hs00233997), MMP16 (Hs00234676), TIMP1 (Hs00171558), TIMP2 (Hs00234278), TIMP3 (Hs00165949) and uPA (Hs00958880) were obtained from Applied Biosystems (Foster City, CA).
Nuclear and cytoplasmic fractionation
The nuclear and cytoplasmic fractionation was performed with NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Cat. 78833, Thermo Fisher Scientific, Waltham, MA). The nuclear and cytoplasmic fractions were isolated separately by following the manufacturer’s instruction. The amount of a given protein in each fraction was determined by western blot analysis.
Western blot analysis
The detailed procedures have been described previously (26). The primary antibodies detecting OCT4 (1:1000, 2750S), SOX2 (1:1000, 3579S), p-p65 (1:1000, 3039S) and PGR (1:1000, 8757) were from Cell Signaling Technology (Danvers, MA); CD44 (1:500, sc-7297), ERα (1:500, sc-8002) and LaminA (1:1000, sc-20680) were from Santa Cruz Biotechnology (Santa Cruz, CA); β-actin antibody was from Sigma–Aldrich (1:2000, A1978). Secondary antibodies were from Cell Signaling Technology.
Fluorescence microscopy
The detailed procedure was described previously (28). Cells were incubated in PBS containing 10% goat serum to block non-specific binding and then incubated overnight at 4oC with a combination of primary antibodies against OCT4 (1:100, Cell Signaling Technology) and CD44 (1:100, Santa Cruz). Subsequently, the cells were incubated with fluorophore-conjugated secondary antibodies (Alexa Fluor 488 or 546; 1:200, Life Technologies) and TO-PRO-3 iodide nuclear antibody (1 μM, Life Technologies) at room temperature. The images were taken using confocal microscope (Eclipse Ti, Nikon Instrument) with laser at 488 nm (CD44), 546 nm (OCT4) and 633 nm (TO-PRO-3). The fluorescence was analyzed using ImageJ software (NIH, Bethesda, MD; http://rsbweb.nih.gov/ij).
OCT4 overexpression
The detailed procedures have been described previously (29). In brief, a lentiviral human OCT4 cDNA overexpression vector (pSin-EF2-OCT4-Pur) was obtained from Addgene (Addgene plasmid #16579). The control vector was obtained from Sigma–Aldrich. For the transient transfection of EF2-OCT4 vectors, DNAs were mixed with FuGene HD transfection reagent (Invitrogen, Carlsbad, CA), and this mixture was added to MCF-7 cells in Opti-MEM medium without serum for 24 h. Then, cells were treated with the estrogen (1 nM) or tocopherols (1 μM) for an additional 24 h in 10% charcoal-stripped FBS/phenol red-free RPMI medium.
Cell invasion assay
The inhibitory effect of tocopherols on cells invasion was assessed using Transwell chamber (8 μm pore size) according to the manufacturer’s protocol (Cat. 354480, BD Biosciences). In brief, cell suspensions (8 × 104 cells/ml) were added to the insert chambers in serum-free RPMI medium, and RPMI medium containing 10% FBS was placed in 24-well plates. Following incubation for 22 h in a humidified atmosphere containing 5% CO2 at 37oC, non-invasive cells on the upper surface of the insert chamber were removed with a cotton swab. The invasive cells on the bottom surface of the chamber were fixed with 100% methanol and then stained with H&E stain. For each membrane, images of three different fields at random were captured at ×10 magnification. All experiments were performed in triplicate. Results were presented as images of invading cells and were quantified using ImageJ software (NIH; http://rsbweb.nih.gov/ij).
Statistical analysis
The significance of the difference between control or treatment groups and estrogen groups was evaluated by the Student’s t-test or one-way analysis of variance followed by a Tukey test. The value of P < 0.05 was considered statistically significant.
Results
Effects of estrogen on the CD44/CD24 subpopulations and level of stem cell markers in MCF-7 monolayer culture and tumorspheres
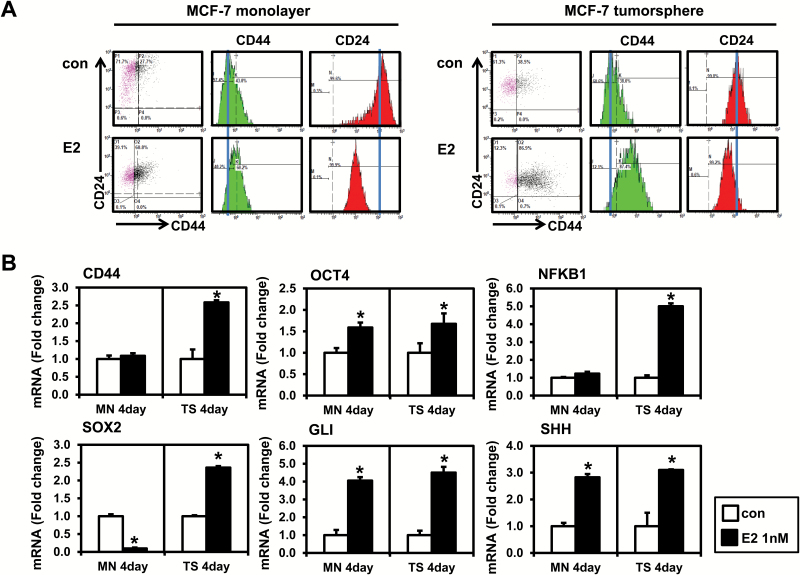
A CD44+/CD24−/low subpopulation of breast cancer cells has been reported to have stem/progenitor cell properties (30). It has also shown that estrogen increases development and/or growth of CD44+/CD24−/low-derived breast tumors (2). We thus determined whether estrogen regulates the cancer stem cell population in MCF-7 monolayer and tumorspheres. Cells were treated with 1 nM estrogen for 4 days, and the expression profile of CD44/CD24 was evaluated by flow cytometry (Figure 1A). In monolayer culture, treatment with estrogen lowered levels of CD24 but had only a modest effect on CD44 levels. In contrast to MCF-7 monolayer cultures, estrogen substantially increased levels of CD44 and decreased levels of CD24 in tumorspheres, resulting in increased CD44+/CD24−/low stem cell-like populations.
Figure 1.
Effects of estrogen (E2) on the CD44/CD24 expression and the level of stem cell markers in MCF-7 monolayer cells and tumorspheres. (A) MCF-7 monolayer cells were plated at a density of 8 × 104 cells/ml in six-well plates and treated with the vehicle control (con) or E2 (1 nM) for 4 days. MCF-7 tumorspheres were formed by plating 10000 cells/ml in ultra-low attachment six-well plates and treated with E2 (1 nM) for 4 days. Cells were stained with combinations of antibodies against CD24 and CD44, and then, flow cytometry was performed. Experiments were performed in triplicate, and representative histograms from flow cytometry are shown. Histograms show the mean fluorescence intensity of CD44 (PE-Green) and CD24 (FITC-Red). (B) qPCR analysis was performed on MCF-7 monolayer cells and tumorspheres treated with E2 and analyzed for markers of cancer stem cells. The data are represented as mean ± standard deviation (SD). Here, n = 3 represents independent experiments, *significantly different from the respective control (P < 0.05). Cycle numbers for genes related to CD44, OCT4, NFKB1, SOX2, GLI and SHH for control group in MCF-7 monolayer cells were 25, 25, 24, 31, 34 and 28 and for control group in MCF-7 tumorspheres cells were 25, 28, 26, 30, 35 and 30, respectively.
We next evaluated the effects of estrogen on mRNA levels of pluripotency and stem cell-related markers including CD44, OCT4, NFKB, SOX2, GLI and SHH in MCF-7 monolayers and tumorspheres (Figure 1B). The levels of CD44 mRNA were increased with estrogen in MCF-7 tumorspheres but not in MCF-7 monolayer cells. The observation was consistent with results from flow cytometry, suggesting that CD44 may be an important target for a functional increase in the breast cancer stem cell population. We next determined the effect of estrogen on mRNA levels of several key transcription factors, such as OCT4, NFKB and SOX2, involved in the regulation of pluripotency and maintenance of self-renewal properties in stem cells (17). OCT4 mRNA was increased by estrogen in MCF-7 monolayer cells as well as tumorspheres. Interestingly, the levels of NFKB and SOX-2 mRNA were increased by estrogen in MCF-7 tumorspheres, not in MCF-7 monolayer cells. For the Hedgehog signaling pathway, the mRNA levels of GLI (a downstream effector) and SHH (a ligand) were determined. Hedgehog signaling is aberrantly activated in cancer stem cells (31). GLI and SHH were increased by estrogen in both MCF-7 monolayer and tumorspheres. Taken together, these results suggest that estrogen is a positive modulator on expression of cancer stem cell-related markers in MCF-7 cells.
Effects of tocopherols on formation of MCF-7 tumorspheres
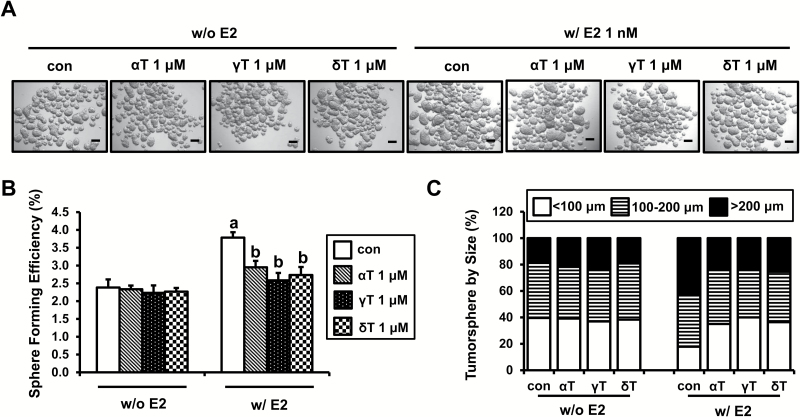
To investigate the effects of tocopherols on formation of tumorspheres, MCF-7 cells were treated with estrogen and/or α-, γ- and δ-tocopherols for 4 days in ultra-low attachment plates. As shown in Figure 2A, α-, γ- and δ-tocopherols without estrogen had little or no effect on the number or size of tumorspheres. However, estrogen markedly increased number and size of tumorspheres, and treatment with tocopherols decreased the number and size of tumorspheres in the estrogen-treated cultures: the SFE was reduced with α-, γ- and δ-tocopherols by 22.0, 31.7 and 27.7%, respectively (Figure 2B). The size of the tumorspheres was increased by estrogen, as shown by significantly increased number of large tumorspheres (>200 μm) to 42.5% in the estrogen treatment group compared with 20.0% in no estrogen control group (Figure 2C). Treatment of MCF-7 tumorspheres with α-, γ- and δ-tocopherols decreased the size of large tumorspheres (>200 μm) by 23.6, 23.3 and 25.4%, respectively (Figure 2C). Since we found significant inhibitory effect of tocopherols on estrogen-induced formation of MCF-7 tumorspheres (Supplementary Figure S3), we tested the effect of tocopherols in another ER-positive human breast cancer line, T47D. Estrogen increased number and size of T47D tumorspheres. While α-, γ- and δ-tocopherols had little or no effect on the number and size of T47D tumorspheres in the absence of estrogen, treatment with α-, γ- and δ-tocopherols decreased the number and size of tumorspheres in estrogen-induced T47D tumorspheres (Supplementary Figure S1, available at Carcinogenesis Online). The results suggest that the effects of tocopherols on breast cancer stem cells were focused principally on the estrogen-mediated increase in the stem cell-like population.
Figure 2.
Effects of tocopherols on formation and size of MCF-7 tumorspheres. (A) MCF-7 cells were plated at a density of 10000 cells/ml in ultra-low attachment six-well plates and grown for 4 days in the presence of E2 (1 nM) or/and α-, γ- and δ-tocopherols (1 μM). Representative pictures of MCF-7 tumorspheres are shown for phenotypic comparison, scale bar 100 μm. (B) SFE of MCF-7 tumorspheres is shown. SFE was calculated by dividing the number of tumorspheres (>100 μm) formed by the number of cells seeded presenting this as a percentage. The data are represented as mean ± SD. Here, n = 3 represents independent experiments in triplicate, a,bsignificantly different from the control and E2, respectively (P < 0.05). (C) The size of tumorspheres was divided into three ranges (<100, 100–200 and >200 μm). Average number of tumorspheres in each size range is shown in the graph.
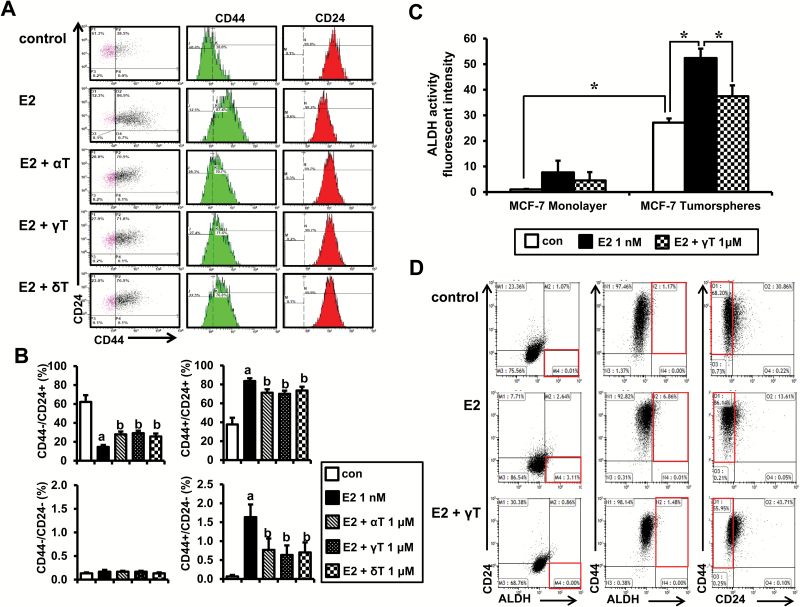
Effects of tocopherols on the CD44/CD24 subpopulations and the ALDH activity in estrogen-treated MCF-7 tumorspheres
To further investigate the effects of tocopherols on the stem cell-like subpopulations in MCF-7 tumorspheres, we examined expression of CD44/CD24 in these cells by flow cytometry. As shown in Figure 3A, expression of CD44 increased and expression of CD24 decreased with estrogen treatment. Expression of these markers returned to near control (no estrogen) levels by treatment with α-, γ- and δ-tocopherols, with significant reduction of the CD44+/ CD24− subpopulation (Figure 3B). Since it has been shown that breast cancer stem cells present high levels of ALDH activity (32), we examined whether tocopherol decreases ALDH activity in estrogen-treated MCF-7 cells. We found that percentage of ALDH-positive cells was higher in MCF-7 tumorspheres (P < 0.05), compared with MCF-7 monolayer culture (Figure 3C). Estrogen increased the ALDH activity further in MCF-7 tumorspheres, and treatment with γ-tocopherol decreased the ALDH activity by 28.5% (Figure 3C). The representative dot plots of the experiments were shown in Supplementary Figure S4, available at Carcinogenesis Online. We next examined the effects of γ-tocopherol on expression of ALDH/CD24/CD44 in MCF-7 tumorspheres (Figure 3D). The percentage of cells with high ALDH and low CD24 was increased by estrogen (3.11%), and this induction was inhibited by γ-tocopherol (0.00%). In addition, the percentage of cells with high ALDH and high CD44 was increased by estrogen (6.86%). Expression of these markers returned to near control levels by treatment with γ-tocopherol (1.48%). MCF-7 tumorspheres treated with estrogen showed lower CD24 expression and higher CD44 expression, which is consistent with CD44/CD24 double staining. These findings suggest that ALDH-positive and the CD44+/CD24−/low subpopulation may be enriched in cancer stem/progenitor cells, and γ-tocopherol reduces estrogen-induced breast cancer stem cell subpopulations.
Figure 3.
Effects of tocopherols on the CD44/CD24 subpopulations and ALDH activity in E2-treated MCF-7 tumorspheres. (A) MCF-7 cells were plated at a density of 10000 cells/ml in ultra-low attachment six-well plates and grown for 4 days in the presence of E2 (1 nM) or/and tocopherols (1 μM). Cells were stained with combinations of antibodies against CD44 and CD24, and then, flow cytometry was performed. Histograms show the mean fluorescence intensity of CD44 (PE-Green) and CD24 (FITC-Red). (B) The average percentage of CD44−/CD24+, CD44+/CD24+, CD44−/CD24− and CD44+/CD24− subpopulations from three independent experiments are represented as a bar graph. The data are represented as mean ± SD. a,bSignificantly different from the control and E2 control, respectively (P < 0.05). (C) The average fluorescent intensities of ALDH in MCF-7 cells treated with E2 (1 nM) and/or γ-tocopherol (1 μM). Data were calculated from immunofluorescence images from three independent experiments. *Significantly different from the respective control (P < 0.05). (D) Representative dot plots of flow cytometry show the expression of ALDH, CD24 and CD44 in MCF-7 tumorspheres treated with E2 (1 nM) and/or γ-tocopherol (1 μM) for 4 days. Cells were stained with ALDH, anti-CD44-APC and/or anti-CD24-PE.
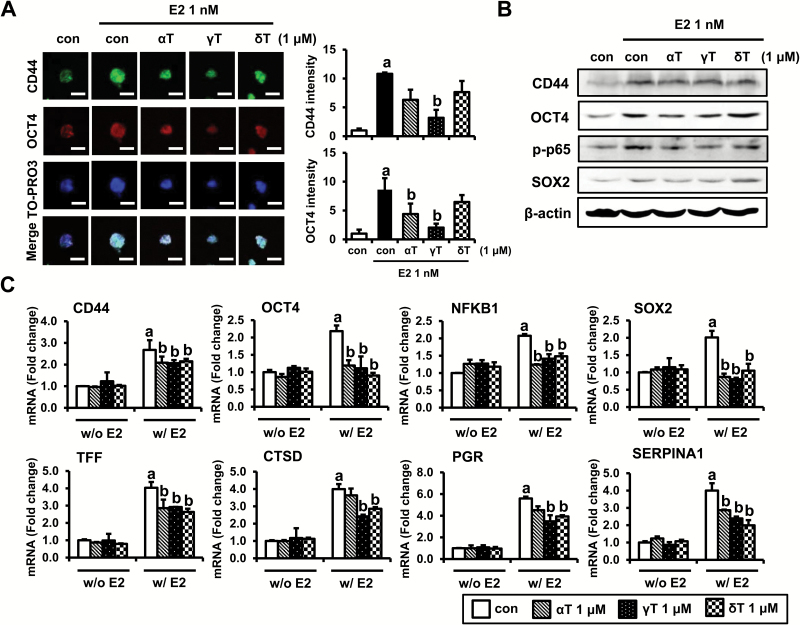
Effects of tocopherols on stem cell markers and estrogen target genes in MCF-7 tumorspheres
As shown above, estrogen increases sphere formation and the levels of stem cell-related markers in MCF-7 tumorspheres. We, therefore, tested the effects of α-, γ- and δ-tocopherols on the expression levels of stem cell-related markers as well as estrogen-related markers in MCF-7 tumorspheres. We first examined CD44 and OCT4 levels in MCF-7 tumorspheres by confocal microscopy. MCF-7 tumorspheres treated with estrogen showed stronger CD44 and OCT4 staining, which was decreased with α-, γ- and δ-tocopherols treatment in MCF-7 tumorsphere. The quantification of CD44 and OCT4 level has shown that γ-tocopherol had the most significant inhibitory effect (Figure 4A, Supplementary Figure S6). Next, we determined the protein levels of OCT4, CD44 as well as stem cell markers, p-p65 and SOX2, in estrogen-treated MCF-7 tumorspheres. As shown in Figure 4B, the protein levels of OCT4, CD44, p-p65 and SOX2 were increased by estrogen, whereas tocopherols reduced estrogen stimulation of these markers in MCF-7 tumorspheres. mRNA levels of OCT4, CD44, NFKB1 and SOX2 were not changed by the treatment with tocopherols in MCF-7 tumorspheres in the absence of estrogen. In contrast, mRNA levels of OCT4, CD44, NFKB1 and SOX2 were significantly increased by estrogen, and this induction was downregulated by α-, γ- and δ-tocopherols (Figure 4C). The mRNA level of Nanog, a transcription factor involved with self-renewal of stem cells, was too low for reliable detection (Supplementary Figure S5, available at Carcinogenesis Online). Since inhibition of tumorsphere formation caused by tocopherols is dependent on estrogen, we investigated the effects of tocopherols on the modulation of the estrogen target genes, including TFF/pS2, CTSD, PGR and SERPINA1, in MCF-7 tumorspheres (Figure 4C). In the absence of estrogen, the mRNA levels of TFF/pS2, CTSD, PGR and SERPINA1 were unaffected by treatment with tocopherols in MCF-7 tumorspheres. However, levels of TFF/pS2, CTSD, PGR and SERPINA1 were significantly upregulated by estrogen treatment, and this stimulation was inhibited by α-, γ-, and δ-tocopherols.
Figure 4.
Effects of tocopherols on expression of E2-target genes and stem cell markers in E2-treated MCF-7 tumorspheres. (A) Immunofluorescence analysis of was performed on MCF-7 tumorspheres collected from 4 days of treatment with control, E2 (1 nM) or tocopherols (1 μM). MCF-7 tumorspheres were fixed using 4% paraformaldehyde and stained with antibodies against CD44 (green) and OCT4 (red). Nuclei were stained with TO-PRO3 (blue). Representative pictures are shown, scale bars: 200 μm. The quantification of CD44 and OCT4 level by ImageJ program from three independent experiments was shown. a,bSignificantly different from the control and E2 control, respectively (P < 0.05). (B) Western blot analysis was performed on MCF-7 tumorspheres collected from 4 days of treatment with control, E2 (1 nM) or tocopherols (1 μM) and analyzed for markers associated with stem cell signaling. β-Actin was used as a loading control. (C) qPCR analysis was performed on tumorspheres collected from 4 days of treatment with control, E2 (1 nM) or tocopherols (1 μM) and analyzed for markers associated with stem cell-related genes, including CD44, OCT4, NFKB1 and SOX-2, and E2-related genes, including TFF, CTSD, PGR and SERPINA1. The data are represented as mean ± SD. Here, n = 3 represents independent experiments, a,bsignificantly different from the control and E2 control, respectively (P < 0.05). Cycle numbers for genes related to CD44, OCT4, NFKB1, SOX-2, TFF, CTSD, PGR and SERPINA1 for control group were 25, 27, 26, 30, 18, 20, 30 and 32, respectively.
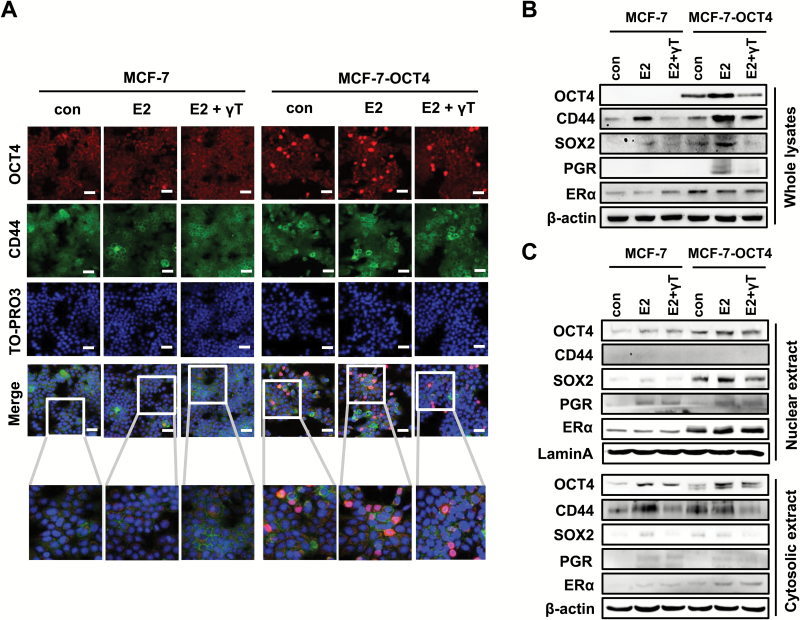
Effects of estrogen and OCT4 overexpression on stem cell markers and the regulation by γ-tocopherol in MCF-7 cells
To further examine the role of OCT4, estrogen and tocopherols on breast cancer cell stemness, we used MCF-7 cells transiently transfected to overexpress OCT4. OCT4 is a well-known transcription factor that plays a crucial role in stem cell self-renewal and pluripotency (24). As γ-tocopherol inhibited the formation of tumorspheres most effectively among tocopherols, we chose γ-tocopherol to investigate further the roles of OCT4 in cancer stemness. MCF-7 cells were transduced with control vector (MCF-7) and lentiviral OCT4-expression vector (MCF-7-OCT4). We first investigated the levels of OCT4 and CD44 in MCF-7 and MCF-7-OCT4 cells treated with γ-tocopherol using confocal microscopy (Figure 5A). Expression of OCT4 was notably upregulated in the nuclei of MCF-7-OCT4 cells, compared with MCF-7 cells, as expected. MCF-7-OCT4 cells treated with estrogen showed stronger OCT4 staining in nuclei, which was decreased with γ-tocopherol treatment (Figure 5A). We found that the level of CD44 was increased by overexpressing OCT4, and the level was reduced by γ-tocopherol treatment (Figure 5A). These changes were further examined by western blot. As shown in Figure 5B, MCF-7-OCT4 cells showed a significantly higher protein level of OCT4, CD44, SOX-2 and ERα than MCF-7 cells. Treatment with estrogen increased the levels of OCT4, CD44, SOX-2 and PGR, and this induction was decreased by γ-tocopherol in MCF-7-OCT4 cells (Figure 5B). We examined the cellular location of OCT4, CD44, SOX-2, PGR and ERα in the treated cells by immunoblotting of subcellular fractions (Figure 5C). We found that MCF-7-OCT4 cells showed a higher level of OCT4 in the nucleus than MCF-7 cells. Treatment with estrogen increased the level of OCT4 in the nucleus, and this stimulation was inhibited by γ-tocopherol in MCF-7-OCT4 cells. The levels of SOX-2 and PGR in the nucleus were further increased by estrogen, and γ-tocopherol downregulated estrogen-stimulated levels of these markers in MCF-7-OCT4 cells (Figure 5C). Since CD44 is a cell surface marker, CD44 was not detected in the nucleus. However, MCF-7-OCT4 cells showed a higher level of CD44 in the cytosol than MCF-7 cells, which was decreased by treatment with γ-tocopherol. The ERα level in the nucleus was higher in MCF-7-OCT4 than MCF-7 cells. The level of ERα was not significantly changed by the treatment with estrogen or tocopherols in either the nucleus or cytosol fractions. Overall, these results suggest that OCT4 is a key regulator of cancer stemness, that estrogen induces OCT4 and its downstream targets, and that γ-tocopherol reduces these effects in breast cancer cells.
Figure 5.
Involvement of OCT4 in the regulation of E2-target genes and stem cell markers by γ-tocopherol. (A) MCF-7 cells were transfected with control vector (MCF-7) and pSin-EF2-OCT4-Pur vector (MCF-7-OCT4) for 24 h, and then, cells were treated with E2 (1 nM) and/or γ-tocopherol (1 μM) for an additional 24 h. MCF-7 and MCF-7-OCT4 cells were fixed using 4% paraformaldehyde and stained with antibodies against OCT4 (red) and CD44 (green). Nuclei were stained with TO-PRO3 (blue). Representative pictures are shown, scale bars: 100 μm. (B) The protein levels of OCT4, CD44, SOX2, PGR and ERα were examined by western blot analysis, with β-actin used as a loading control. (C) The protein levels of OCT4, CD44, SOX2, PGR and ERα in nucleus or in cytoplasm were examined by western blot analysis in MCF-7 and MCF-7-OCT4 cells. Lamin A and β-actin were used as loading controls for nucleus and cytoplasm fractions, respectively.
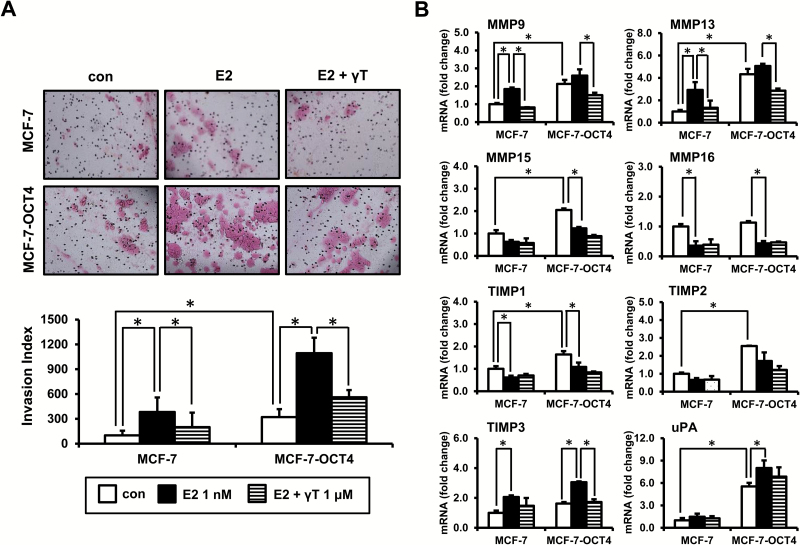
Effects of estrogen and OCT4 overexpression on cell invasion and its regulation by γ-tocopherol
To investigate the possible biological effects of OCT4 expression, its stimulation by estrogen and inhibition by γ-tocopherol, we examined the invasive behavior of tumor cells through a basement membrane matrix barrier using the Matrigel transwell filter assay. As shown in Figure 6A, estrogen significantly increased cell invasion of MCF-7 cells (3.8-fold induction, P < 0.05), and this induction was decreased by γ-tocopherol (47.7% inhibition, P < 0.05). When compared with MCF-7 cells, MCF-7-OCT4 cells showed significantly increased invasive potential (3.2-fold induction, P < 0.05). Treatment with estrogen further increased the invasion potential of MCF-7-OCT4 cells (3.4-fold induction, P < 0.05), and γ-tocopherol effectively suppressed this estrogen-induced cell invasion in MCF-7-OCT4 cells (48.7% inhibition, P < 0.05) (Figure 6A). In addition, all the tocopherols tested, including α- and δ-tocopherols, blocked the cell invasion in estrogen-treated MCF-7 and MCF-7-OCT4 cells (Supplementary Figure S2, available at Carcinogenesis Online). Next, to investigate the molecular changes underlying the changes in cell invasion, we analyzed the mRNA levels of cell invasion markers, including MMP9, MMP13, MMP15, MMP16, TIMP1, TIMP2, TIMP3 and urokinase plasminogen activator (uPA; Figure 6B). The mRNA levels of MMP9, MMP13, MMP15, TIMP1, TIMP2 and uPA were markedly increased by the overexpression of OCT4, while those of MMP16 and TIMP3 were not affected by the overexpression of OCT4. Treatment with estrogen increased the mRNA levels of MMP9, MMP13 and TIMP3 in MCF-7 and MCF-7-OCT4 cells, and this stimulation was inhibited by γ-tocopherol (Figure 6B). The level of uPA was increased by estrogen in MCF-7-OCT4 cells, but the level was not affected by γ-tocopherol. In contrast to these markers, treatment with estrogen decreased the mRNA levels of MMP15, MMP16 and TIMP1, and γ-tocopherol had little or no significant effect on mRNA levels of these genes in MCF-7 and MCF-7-OCT4 cells (Figure 6B). The level of TIMP2 was not significantly changed by treatment with estrogen and tocopherols in MCF-7 and MCF-7-OCT4 cells.
Figure 6.
γ-Tocopherol inhibits cell invasion induced by E2 and OCT4 overexpression. Cells were transfected with control vector (MCF-7) and pSin-EF2-OCT4-Pur vector (MCF-7-OCT4) for 24 h, and then, cells were treated with E2 (1 nM) and/or γ-tocopherol (1 μM) for an additional 24 h. (A) Cells (8 × 104 cells/ml) were loaded onto transwells coated with Matrigel. After 22-h incubation, the transwells were stained with H&E stain (top). Representative pictures are shown, ×10 magnification. The number of invading cells was counted in three representative fields per membrane, and the relative percentage of invasive cells was calculated from three independent experiments (bottom). (B) qPCR analysis was performed to detect genes involved in cell invasion, including MMP9, MMP13, MMP15, MMP16, TIMP1, TIMP2, TIMP3 and uPA. The data are represented as mean ± SD. n = 3 independent experiments. Cycle numbers for genes related to MMP9, MMP13, MMP15, MMP16, TIMP1, TIMP2, TIMP3 and uPA for MCF-7 control group were 27, 28, 25, 23, 22, 23, 24 and 27, respectively. *Significantly different from the respective control (P < 0.05).
Discussion
Accumulating evidence indicates that breast cancer stem cells are responsible for tumor initiation, recurrence, metastasis and progression of breast tumors (33). Hence, targeting breast cancer stem cells with novel agents has become of great interest as a strategy to improve breast cancer treatment and prevention. Our present study demonstrates that tocopherols had significant inhibitory effects on estrogen-induced stemness and cell invasion by regulating OCT4. In these experiments, all forms of tocopherols tested had inhibitory effects, although γ-tocopherol inhibited tumorsphere formation better than α- or δ-tocopherol. In other systems, γ- and δ-tocopherols inhibited azoxymethane-induced colon carcinogenesis and PhIP-induced colon cancer (7,34); δ-tocopherol was more active than γ-tocopherol in inhibiting lung xenograft tumor growth (35); dietary administration of γ- and δ-tocopherols inhibited the N-methyl-N-nitrosourea -induced and estrogen-induced mammary tumorigenesis (13,15). Further studies are needed to understand the basis for the differences in the inhibition of cancer stem cells versus tumorigenesis by different tocopherols.
An earlier study identified the CD44+/CD24−/low subpopulation of breast cancer cells from breast tumors as enriched in cancer stem cells (30). Some in vivo studies indicated that CD44 suppression not only inhibited tumor growth but also reduced the tumor-initiating ability of a human breast cancer cell xenograft (36). In addition, the overexpression of CD44 enhanced both tumorsphere formation and the expression of the stemness factors, Nanog, Sox2 and OCT4 (24). In this study, we found that estrogen increased the CD44+/CD24− stem-like population and expression of CD44, and this was attenuated by treatment with tocopherols in MCF-7 tumorspheres. These findings suggest that CD44 is a useful cancer stem cell marker regulated by estrogen and tocopherols. Since the tumorsphere-forming assay enriches for stem and progenitor cells, the reduction of SFE with tocopherols could be at least in part due to the ability to suppress the putative CD44+/CD24−/low breast cancer stem cell population. In addition, we found that ALDH activity was significantly higher in MCF-7 tumorspheres than MCF-7 monolayer, which was consistent with the previously reported findings by Charafe-Jauffret et al. (37). They demonstrated that the ALDH-positive population showed increased tumorsphere-forming capacity compared with ALDH-negative cells in SUM149 and SUM159 cells (37). Treatment with estrogen increased the ALDH activity, and this induction was decreased by γ-tocopherol, demonstrating that the suppression of ALDH-positive cells and the CD44+/CD24− subpopulation by γ-tocopherol reduced breast cancer stemness. Interestingly, tocopherols without estrogen had little or no effect on the number and size of tumorspheres, suggesting that suppression of tumorspheres by tocopherols occurs at the level of estrogen stimulation of cell stemness. Alternatively, estrogen might selectively induce the replication of stem-like cells, and this might be suppressed by tocopherols.
Coordinated networks of stemness factors are the master regulatory mechanisms of pluripotency and differentiation in stem cells. OCT4, a transcription factor that forms a heterodimer with SOX2, plays a major role in the regulation of stem cell pluripotency and formation in breast cancer stem cells (38,39). OCT4 and SOX2 act synergistically to regulate their own transcription as well as the expression of other key stem cell genes (40). Jung et al. (20) reported that estrogen (1–20 nM) induced increase of OCT4 expression and proliferation of tumorspheres as well as the breast cancer stem cell population in MCF-7 tumorspheres. Our results also confirmed the findings of previous studies indicating that the levels of pluripotency markers, such as OCT4 and SOX2, were increased by estrogen. Progesterone plays an important role in mammary gland pathophysiology, and PGR, TFF/pS2 as well as CTSD have been used as indicators of breast cancer progression and as a predictor for tamoxifen resistance of breast tumors (41,42). Recently, SERPINA1 has been reported to be a direct ER target gene (43). The expression of these genes is decreased by α-, γ- and δ-tocopherols in estrogen-stimulated MCF-7 tumorspheres, suggesting that downregulation of these estrogen-related genes could indicate selective inhibition of ER-regulated transcription by tocopherols. MCF-7 tumorspheres treated with estrogen increased the binding of ERα to the ERE site of POU5F1 (OCT4), suggesting that OCT4 acts through an ER-dependent mechanism in MCF-7 cells (20,44). Estrogen increased the ERE-luciferase activity, and this induction was decreased by γ-tocopherol in MCF-7 cells (Supplementary Figure S7, available at Carcinogenesis Online), suggesting that tocopherols may reduce estrogen-induced OCT4 expression by an ER-dependent mechanism in breast cancer cells.
We further examined the role of OCT4 on expression of stemness- and estrogen-related markers in MCF-7 ER-positive breast cancer cells. Overexpression of OCT4 increased the levels of CD44, SOX-2, PGR and ERα in the presence of estrogen, and this was attenuated by treatment with γ-tocopherol. Moreover, overexpression of OCT4 increased in vitro cell invasion through regulation of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs) and uPA gene expression in MCF-7 cells. MMPs are major players in tumor invasion and metastasis (45,46). MMPs may contribute to the development of breast cancer, while the increase in TIMPs expression may represent a feedback effect (47). In addition, uPA is a critical enzyme for cancer cell invasion converting plasminogen into plasmin, which degrades extracellular matrix and activates multiple MMPs (48). Hu et al. (49) reported that overexpression of OCT4 increases the migration and invasion properties by regulating miR-145 and downstream transcriptional factors, Snail, ZEB1 and ZEB2 in breast cancer cells. Knockdown of OCT4 suppresses the growth and invasion of pancreatic cancer cells through inhibition of Akt pathway-mediated PCNA and MMP2 expression (50) and inhibit colorectal cancer cell motility and invasion (51). Overexpression of OCT4 increases the invasion of breast cancer MCF-7 cells, and tocopherols suppress the estrogen-induced cell invasive properties by regulating these cell invasion markers, suggesting that OCT4 may be a potential target for prevention and/or treatment of breast cancer.
In conclusion, the inhibition of cancer stem-like cell properties and cell invasion by tocopherols in ER-positive breast cancer probably occurs via estrogen-dependent and OCT4-mediated mechanisms. Our findings suggest tocopherols as potentially useful agents for suppressing these estrogen effects on breast cancer development.
Supplementary material
Supplementary Figures S1–S7 can be found at Carcinogenesis online.
Funding
This work was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health [R01 AT007036], the National Institute of Environmental Health Sciences grant [ES005022], Charles and Johanna Busch Memorial Fund at Rutgers University and the Trustees Research Fellowship Program at Rutgers.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement: None declared.
Abbreviations
- CTSD
cathepsin D
- E2
estrogen
- ER
estrogen receptor
- GLI
glioma-associated oncogene homolog
- MMPs
matrix metalloproteinases
- OCT4
octamer-binding transcription factor 4
- PGR
progesterone receptor
- SFE
sphere-forming efficiency
- SHH
sonic hedgehog
- SOX2
sex-determining region Y-box-2
- TFF
trefoil factor
- TIMPs
tissue inhibitors of metalloproteinases
- uPA
urokinase plasminogen activator.
References
- 1. Velasco-Velázquez M.A., et al. (2011)The role of breast cancer stem cells in metastasis and therapeutic implications. Am. J. Pathol., 179, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fillmore C.M., et al. (2010)Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc. Natl. Acad. Sci. USA, 107, 21737–21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison H., et al. (2013)Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and Notch signalling. Breast Cancer Res., 15, R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simões B.M., et al. (2011)Cancer stem cells in the human mammary gland and regulation of their differentiation by estrogen. Future Oncol., 7, 995–1006. [DOI] [PubMed] [Google Scholar]
- 5. Bak M.J., et al. (2016)Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Semin. Cancer Biol., 40–41, 170–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang C.S., et al. (2012)Does vitamin E prevent or promote cancer?Cancer Prev. Res. (Phila), 5, 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J.X., et al. (2017)δ- and γ-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Mol. Carcinog., 56, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Das Gupta S., et al. (2015)Tocopherols inhibit oxidative and nitrosative stress in estrogen-induced early mammary hyperplasia in ACI rats. Mol. Carcinog., 54, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li G., et al. (2012)The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free Radic. Biol. Med., 52, 1151–1158. [DOI] [PubMed] [Google Scholar]
- 10. Lippman S.M., et al. (2009)Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). JAMA, 301, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaziano J.M., et al. (2009)Vitamins E and C in the prevention of prostate and total cancer in men: the physicians’ health study II randomized controlled trial. JAMA, 301, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Das Gupta S., et al. (2016)Tocopherols in cancer: an update. Mol. Nutr. Food Res., 60, 1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolarek A.K., et al. (2012)Dietary administration of δ- and γ-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer Prev. Res. (Phila), 5, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bak M.J., et al. (2017)Inhibitory effects of γ- and δ-tocopherols on estrogen-stimulated breast cancer in vitro and in vivo. Cancer Prev. Res. (Phila), 10, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das Gupta S., et al. (2017)Differential gene regulation and tumor-inhibitory activities of alpha-, delta-, and gamma-tocopherols in estrogen-mediated mammary carcinogenesis. Cancer Prev. Res. (Phila.), 10, 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iqbal W., et al. (2016)Targeting signal transduction pathways of cancer stem cells for therapeutic opportunities of metastasis. Oncotarget, 7, 76337–76353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi K., et al. (2006)Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 18. Pan G.J., et al. (2002)Stem cell pluripotency and transcription factor Oct4. Cell Res., 12, 321–329. [DOI] [PubMed] [Google Scholar]
- 19. Hu T., et al. (2008)Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res., 68, 6533–6540. [DOI] [PubMed] [Google Scholar]
- 20. Jung J.W., et al. (2011)Metformin represses self-renewal of the human breast carcinoma stem cells via inhibition of estrogen receptor-mediated OCT4 expression. PLoS One, 6, e28068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leis O., et al. (2012)Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene, 31, 1354–1365. [DOI] [PubMed] [Google Scholar]
- 22. Buganim Y., et al. (2013)Mechanisms and models of somatic cell reprogramming. Nat. Rev. Genet., 14, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Louderbough J.M., et al. (2011)Understanding the dual nature of CD44 in breast cancer progression. Mol. Cancer Res., 9, 1573–1586. [DOI] [PubMed] [Google Scholar]
- 24. Cho Y., et al. (2015)Cleaved CD44 intracellular domain supports activation of stemness factors and promotes tumorigenesis of breast cancer. Oncotarget, 6, 8709–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zöller M. (2011)CD44: can a cancer-initiating cell profit from an abundantly expressed molecule?Nat. Rev. Cancer, 11, 254–267. [DOI] [PubMed] [Google Scholar]
- 26. So J.Y., et al. (2015)HES1-mediated inhibition of Notch1 signaling by a Gemini vitamin D analog leads to decreased CD44(+)/CD24(-/low) tumor-initiating subpopulation in basal-like breast cancer. J. Steroid Biochem. Mol. Biol., 148, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee H.J., et al. (2006)Gene expression profiling changes induced by a novel Gemini vitamin D derivative during the progression of breast cancer. Biochem. Pharmacol., 72, 332–343. [DOI] [PubMed] [Google Scholar]
- 28. So J.Y., et al. (2013)Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PLoS One, 8, e54020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee H.J., et al. (2009)Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin. Cancer Res., 15, 4242–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Hajj M., et al. (2003)Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA, 100, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu Y., et al. (2015)Salinomycin exerts anticancer effects on human breast carcinoma MCF-7 cancer stem cells via modulation of Hedgehog signaling. Chem. Biol. Interact., 228, 100–107. [DOI] [PubMed] [Google Scholar]
- 32. Kim R.J., et al. (2013)High aldehyde dehydrogenase activity enhances stem cell features in breast cancer cells by activating hypoxia-inducible factor-2α. Cancer Lett., 333, 18–31. [DOI] [PubMed] [Google Scholar]
- 33. Schwarz-Cruz Y.C.A., et al. (2016)Advances in the knowledge of breast cancer stem cells. A review. Histol. Histopathol., 31, 601–612. [DOI] [PubMed] [Google Scholar]
- 34. Guan F., et al. (2012)δ- and γ-tocopherols, but not α-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev. Res. (Phila), 5, 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li G.X., et al. (2011)δ-tocopherol is more active than α - or γ -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. (Phila), 4, 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Godar S., et al. (2008)Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell, 134, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charafe-Jauffret E., et al. (2009)Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res., 69, 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chew J.L., et al. (2005)Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol. Cell. Biol., 25, 6031–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cufi S., et al. (2012)Metformin-induced preferential killing of breast cancer initiating CD44+CD24-/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget, 3, 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu T.M., et al. (2009)Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev., 18, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yao Y., et al. (2010)A novel antiestrogen agent Shikonin inhibits estrogen-dependent gene transcription in human breast cancer cells. Breast Cancer Res. Treat., 121, 233–240. [DOI] [PubMed] [Google Scholar]
- 42. Fan P., et al. (2014)A molecular model for the mechanism of acquired tamoxifen resistance in breast cancer. Eur. J. Cancer, 50, 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chan H.J., et al. (2015)SERPINA1 is a direct estrogen receptor target gene and a predictor of survival in breast cancer patients. Oncotarget, 6, 25815–25827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopes J., et al. (2016)Melatonin decreases estrogen receptor binding to estrogen response elements sites on the OCT4 gene in human breast cancer stem cells. Genes Cancer, 7, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kessenbrock K., et al. (2010)Matrix metalloproteinases: regulators of the tumor microenvironment. Cell, 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gomes L.R., et al. (2012)TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bourboulia D., et al. (2010)Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol., 20, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moirangthem A., et al. (2016)Simultaneous knockdown of uPA and MMP9 can reduce breast cancer progression by increasing cell-cell adhesion and modulating EMT genes. Sci. Rep., 6, 21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu J., et al. (2012)MiR-145 regulates epithelial to mesenchymal transition of breast cancer cells by targeting Oct4. PLoS One, 7, e45965. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Lin H., et al. (2014)Knockdown of OCT4 suppresses the growth and invasion of pancreatic cancer cells through inhibition of the AKT pathway. Mol. Med. Rep., 10, 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dai X., et al. (2013)OCT4 regulates epithelial-mesenchymal transition and its knockdown inhibits colorectal cancer cell migration and invasion. Oncol. Rep., 29, 155–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.