Abstract
Background
Traditionally, surgical resection is the preferred treatment for typical carcinoids and atypical carcinoids located in the lungs. Recently however, several studies have shown excellent long-term outcome after endobronchial treatment of carcinoid tumors located in the central airways. This study investigates clinical and radiological features as predictors of successful endobronchial treatment in patients with a bronchial carcinoid tumor.
Objectives
To identify clinical and radiological features predictive of successful endobronchial treatment in patients with bronchial carcinoid.
Methods
This analysis was performed in a cohort of patients with typical and atypical bronchial carcinoid referred for endobronchial treatment. Several patient characteristics, radiological features, and histological grade (typical or atypical carcinoid) were tested as predictors of successful endobronchial treatment.
Results
One hundred and twenty-five patients with a diagnosis of bronchial carcinoid underwent endobronchial treatment. On multivariate analysis, a tumor diameter <15 mm (odds ratio 0.09; 95% confidence interval 0.02–0.5; p = <0.01) and purely intraluminal growth on computer tomography (CT scan) (odds ratio, 9.1; 95% confidence interval 1.8–45.8; p = <0.01) were predictive of radical endobronchial treatment. The success rate for intraluminal tumors with a diameter <20 mm was 72%.
Conclusions
Purely intraluminal disease and tumor diameter on CT scan seem to be independent predictors for successful endobronchial treatment in patients with bronchial carcinoid. Based on these data, patients with purely intraluminal carcinoid tumors with a diameter <20 mm on CT scan are good candidates for endobronchial treatment, regardless of histological grade. In contrast, all patients with a tumor diameter ≥20 mm should be directly referred for surgery.
Keywords: Bronchial carcinoid, Endobronchial treatment, Prognostic factors, Bronchoscopy, Neuroendocrine tumor
Introduction
Pulmonary carcinoid tumors are included in the group of neuroendocrine tumors (NETs) and derive from pulmonary neuroendocrine cells [1, 2]. Pulmonary NETs comprise around 20% of all lung tumors and are divided in 2 groups based on specific tumor characteristics. The first group consists of high-grade small cell carcinoma and large cell neuroendocrine carcinoma, both characterized by a high mitotic rate, aggressive behavior, and a predisposition to metastasize early [2]. The second group consists of atypical carcinoid (AC) and typical carcinoid (TC). These tumors have a more benign morphology, are less aggressive, and have a lower tendency to metastasize [3].
Traditionally, surgical resection is the preferred treatment for both TC and AC. Recently, however, several studies have shown excellent long-term outcome after endobronchial treatment (EBT) of carcinoid tumors located in the central airways [4, 4, 5, 6, 7, 8, 9]. When compared with surgery, EBT is a minimally invasive and parenchyma-sparing technique. Not all patients with carcinoid located in the central airways are candidates for EBT because several parts of the bronchial carcinoids have an extraluminal component. In a previous study from our institute, it was reported that EBT is successful in 42% of patients [5]. If curation cannot be achieved by EBT, other advantages of EBT may include deobstruction of the involved bronchus with ensuing resolution of postobstructive pneumonia and reduction of the extent of the subsequent surgical resection. However, these unsuccessful beneficial effects have not been proven. Since serious complications can occur during EBT [5] and because EBT attempts can delay curative treatment, it is critical to assess as soon as possible whether or not patients are good candidates for EBT. Identification of those patients with bronchial carcinoid who are good candidates for EBT would facilitate adequate selection of the most appropriate treatment modality. In the current study, we aimed to identify clinical and radiological factors predictive of successful EBT in patients with bronchial carcinoid.
Material and Methods
Study Design and Methods
With Institutional review board approval (Medical Ethics Review Committee of VU University Medical Center; IRB00002991), a cohort of patients referred to our tertiary referral center for EBT of (suspected) bronchial carcinoids was established between June 1991 and August 2015. Details of this patient cohort and the EBT technique have been described previously [5]. In short, prior to treatment all patients underwent a computed tomography (CT) scan of the chest and bronchoscopy as part of the routine workup. Radiological images were not stored as part of the prospective cohort study. After informed consent, EBT was performed by experienced interventional pulmonologists and procedural data were registered. The procedure was planned based on information obtained from the chest CT scan and the bronchoscopy. Patients were excluded from EBT in the case of evident and significant extraluminal growth, lymph node involvement, or evidence of multifocal/disseminated disease on CT scan. EBT was performed under general anesthesia. At the discretion of the interventional pulmonologist, removal of the carcinoid tissue was established using yttrium aluminum garnet laser, cryo- or electrosurgery, mechanical debulking, or a combination of these techniques. To prevent recurrences we treated the base with cryotherapy. The excised specimen was sent for pathology for definite histology and classification. To detect residual disease, repeat bronchoscopy and CT scan were typically planned 6 weeks after EBT. Biopsies were obtained if residual carcinoid was suspected. Surgery was planned in those patients with extensive residual disease. In patients with minimal residual disease EBT was repeated if deemed feasible. In the absence of residual disease, patients were followed with CT scan and bronchoscopy annually.
For the current analysis, we retrospectively reviewed the patients from the cohort in order to identify clinical and radiological factors predictive of successful EBT. Successful EBT was defined as the absence of residual disease during the first 2 years of follow-up with CT scan and bronchoscopy after EBT. Disease seen during follow-up after 2 years was defined as recurrence of disease. The following potentially predictive factors for successful EBT were considered: sex, age, smoking history, ASA (American Society of Anesthesiologists) classification [10], location (central: trachea and main bronchi; peripheral: segmental branches or more distal), diameter of the carcinoid tumor on CT scan, purely intraluminal disease on CT scan, purely intraluminal disease during bronchoscopy, and final pathology (TC, 0–1 mitosis per mm2 and AC, ≥2 mitosis per mm2). Available CT images of sufficient quality were revised by a senior chest radiologist and a resident. Sufficient quality of CT images was defined as the availability of digital images with an axial slice thickness of ≤5 mm. A structured assessment was made with the following parameters: tumor location, tumor diameter (long axis, axial plane), signs of pneumonia and/or atelectasis, signs of extraluminal disease, enlarged lymph nodes, and distant metastases. Patients in whom tumor extension on CT scan could not be clearly assessed were scored as “indeterminate” by the radiologist and were added to the “possible extraluminal disease” group for the purpose of the statistical analyses.
Analysis
The statistical analyses and calculations were performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as proportions and continuous variables as means and standard deviations. We tested the univariate associations between the independent prognostic factors (sex, age, smoking, ASA score, location, diameter on CT scan, purely intraluminal disease on CT scan, purely intraluminal disease at bronchoscopy, and histology after bronchoscopy – typical/atypical) and the dependent factor “successful EBT,” by either the Fisher exact test (dichotomous/nominal variables) or the t test (continuous variables). Effect sizes for the univariate associations were presented as odds ratios (ORs) or mean differences, both with appropriate 95% confidence intervals. Subsequently, a multivariate logistic regression model with backward selection was fitted. A p value of <0.05 indicated statistical significance.
Results
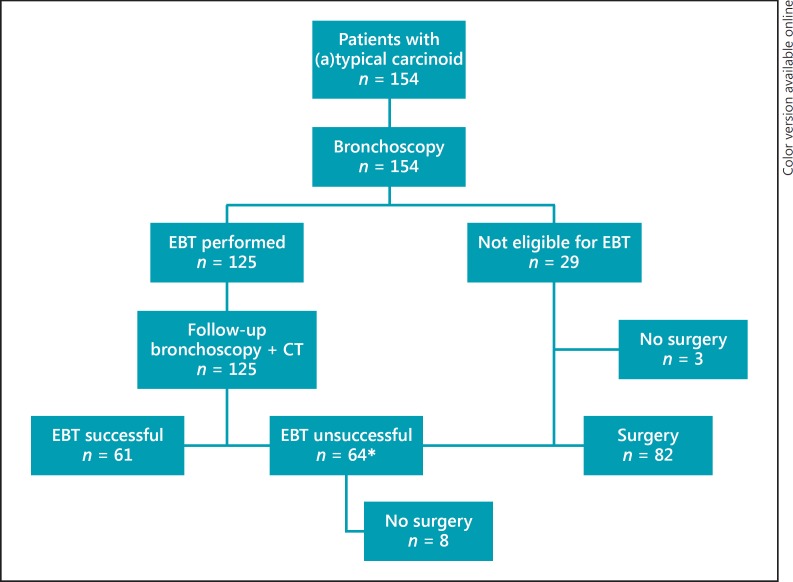
From June 1991 until August 2015, 154 patients were referred for EBT, of whom 125 underwent EBT (Fig. 1). Baseline characteristics of the patients who underwent EBT are presented in Table 1. The mean age was 48.4 years (SD 14–84) and 56% (n = 70) of the patients were female. Patients were classified by the anesthesiologist as ASA I in 48% (n = 60), ASA II in 34% (n = 42) and ASA III in 18% (n = 23). Most patients presented with (recurrent) pneumonia (n = 42, 34%). Other symptoms were cough (n = 24, 20%), hemoptysis (n = 23, 18%), and dyspnea (n = 23, 18%). Twenty-nine patients (19%) were not eligible for EBT due to significant extraluminal disease reported on CT scan or during bronchoscopy (n = 26), or because of the presence of multiple tumor lesions (n = 3) (Fig. 1). Of the 125 patients (81%) who underwent EBT, the majority of patients were treated in 1 session (n = 77, 62%). A total of 2, 3, 4, or even 5 EBTs were necessary in 26% (n = 33), 6% (n = 7), 2% (n = 3), and 4% (n = 5) of patients, respectively. EBT was successful in 61 patients (49%). EBT was unsuccessful in 64 patients (51%) due to extraluminal disease (n = 51, 80%) or histologically confirmed residual disease (n = 13, 20%). Patients not eligible for EBT (n = 29, 19%) and those in whom EBT failed and were subsequently considered not eligible for repeat EBT (n = 64, 42%) were candidates for surgical resection (n = 93, 60%). Eighty-two of these patients (53%) underwent surgical resection. The operations performed included 32 lobectomies, 21 bilobectomies, 6 pneumonectomies, 21 sleeve lobectomies, 1 bronchus sleeve, and 1 segmentectomy. The median time between unsuccessful EBT and surgery was 3 months (interquartile range 9 months). Eleven patients (7%) did not proceed to surgery; 8 patients (5%) were deemed inoperable due to poor physical status/comorbidities and 3 patients (2%) refused surgery. Of all 125 patients treated with EBT, 10 (8%) had residual or recurrence of disease during follow-up. Four (3%) patients showed residual disease ≤2 years after EBT. One (1%) patient was treated with EBT and 3 (2%) received a lobectomy. Only 6 (5%) patients showed a recurrence more than 2 years after the first EBT. Two (2%) patients refused surgery and are alive with disease. The other 4 (3%) patients received a surgical resection and are still alive without disease (see online suppl. Table 4a, b; see www.karger.com/doi/10.1159/000484984 for all online suppl. material).
Fig. 1.
Treatment of patients with bronchial carcinoid. EBT, endobronchial treatment. * Due to residual or extraluminal disease.
Table 1.
Baseline data of patients who received EBT
| Patients | 125 (100) |
| Mean age (SD), years | 48.4 (14–85) |
| Gender | |
| Female | 70 (56) |
| Male | 55 (44) |
| Smoking | |
| Former/current smoker | 61 (49) |
| Never smoker | 57 (46) |
| Missing | 7 (5) |
| Comorbidity | |
| ASA 1 | 60 (48) |
| ASA 2 | 42 (34) |
| ASA 3 | 23 (18) |
| ASA 4 | 0 (0) |
| ASA 5 | 0 (0) |
| Presenting symptoms | |
| Pneumonia | 42 (34) |
| Cough | 24 (20) |
| Dyspnea | 23 (18) |
| Hemoptysis | 23 (18) |
| Asthma-like disease | 3 (2) |
| Incidental finding | 3 (2) |
| Other | 5 (4) |
| Unknown | 2 (2) |
Values are n (%) unless otherwise indicated. EBT, endobronchial treatment; ASA, American Society of Anesthesiologists.
Survival
Of all 61 patients in whom EBT was successful, 52 (85%) are still alive without signs of disease recurrence. Seven (12%) patients died of another cause, 1 patient (2%) died of an unknown cause, and 1 patient (2%) was lost to follow-up. Of the 56 patients who underwent surgery after unsuccessful EBT, 47 (84%) are still alive without disease recurrence. Three (5%) patients had recurrence of disease after surgery but are still alive. Two patients (4%) died of another cause, 3 (5%) patients died due to factors related to the bronchial carcinoid (2 metastatic disease and 1 due to postoperative complications), and 1 (2%) was lost to follow-up (see online suppl. Table 5a, b). The median follow-up time of all included patients was 82 months (interquartile range 98 months).
Complications
EBT remained uncomplicated in 86% (n = 108) of the patients. Complications were bleeding (n = 12, 9%; 1 of which required emergency surgery), bronchospasm (n = 1, 1%), a broken tooth (n = 1, 1%) due to rigid bronchoscopy, vocal cord paralysis (n = 1, 1%), and stricture of the bronchial tree (n = 2, 2%).
Factors Predicting Successful EBT
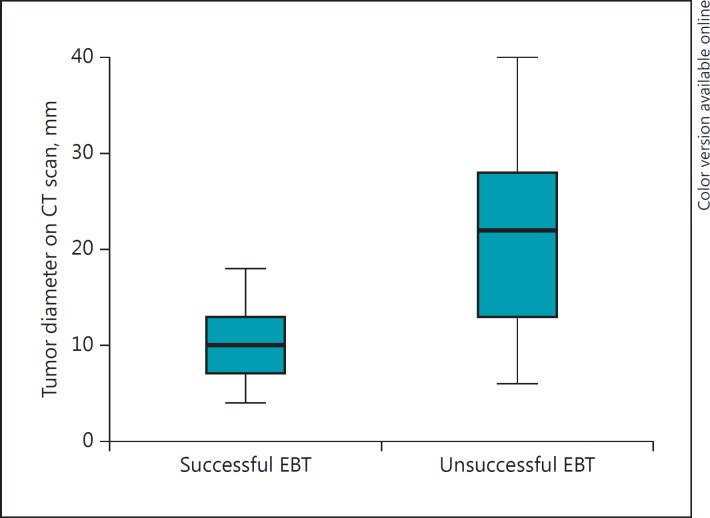
Table 2 shows the univariate and multivariate analysis of prognostic factors of successful EBT. For the purpose of the multivariate analysis, tumor diameter on CT scan was dichotomized by using the median as the cutoff value (15 mm). Smoking (p = 0.01), purely intraluminal disease on CT scan (p = <0.01) or during bronchoscopy (p = <0.01), and tumor size on CT scan (p = <0.01) were significantly associated with successful EBT on univariate analysis. Multivariate analysis revealed tumor size (<15 mm) on CT scan and purely intraluminal growth on CT scan as significant independent predictors for successful EBT. No patient with a tumor size ≥20 mm was successfully treated by EBT (Fig. 2). Agreement between CT scan and bronchoscopy with regard to luminal extension was poor, since 41% of patients with possible extraluminal growth on CT scan were diagnosed as purely intraluminal during bronchoscopy. When combining the significant prognostic factors (tumor diameter and growth pattern on CT), we found that small (<20 mm) purely intraluminal tumors had a success rate of 72% (Table 3).
Table 2.
Prognostic factors of successful (n = 61) and unsuccessful (n = 64) EBT
| Prognostic factors | Successful EBT | Unsuccessful EBT | Univariate analysis, p | OR (95% CI) | Multivariate analysis, p | OR (95% CI) |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 29 (48) | 26 (41) | 0.3 | 0.8 (0.7 to 2.7) | – | – |
| Female | 32 (52) | 38 (59) | ||||
| Smoking | ||||||
| Current or former | 36 (59) | 25 (39) | 0.01 | 2.7 (1.3 to 5.6) | ns | - |
| Never smoker | 20 (33) | 37 (58) | ||||
| Unknown | 5 (8) | 2 (3) | ||||
| Mean age, years | 49.0 | 47.9 | 0.7 | 1.2 (−4.7 to 7.1)b | - | - |
| ASA classification | ||||||
| ASA 1 | 28 (46) | 32 (50) | 0.4 | - | - | - |
| ASA 2 | 19 (31) | 23 (36) | ||||
| ASA 3 | 14 (23) | 9 (14) | ||||
| Location | ||||||
| Central lesion | 47 (77) | 50 (78) | 0.5 | 0.9 (0.5 to 2.5) | - | - |
| Peripheral lesion | 14 (23) | 14 (22) | ||||
| Diameter CT scana (mean 15 mm) | ||||||
| ≤15 mm | 16 (26) | 11 (17) | <0.01 | 11.3 (6.9 to 15.7)b | <0.01 | 0.09 |
| >15 mm | 2 (3) | 25 (39) | (0.02 to 0.5) | |||
| Indeterminate | 18 (30) | 22 (34) | ||||
| Missing | 25 (41) | 6 (9) | ||||
| Purely intraluminal disease on CT scana | ||||||
| Yes | 27 (44) | 46 (72) | <0.01 | 11.5 (4.3 to 30.8) | <0.01 | 9.1 |
| No | 9 (15) | 12 (19) | (1.8 to 45.8) | |||
| Missing | 25 (41) | 6 (9) | ||||
| Purely intraluminal disease at bronchoscopy | ||||||
| Yes | 3 (5) | 27 (42) | <0.01 | 9.6 (3.6 to 25.4) | NS | - |
| No | 55 (90) | 31 (48) | ||||
| Indeterminate | 3 (5) | 6 (10) | ||||
| Histology after bronchoscopy | ||||||
| Typical | 54 (89) | 51 (80) | 0.3 | 1.8 (0.7 to 4.8) | - | - |
| Atypical | 7 (11) | 12 (18) | ||||
| No differentiation possible | 0 (0) | 1 (2) |
Values are n (%) unless otherwise indicated. EBT, endobronchial treatment; ASA, American Society of Anesthesiologists. Significant p values are presented in italics. ns, not significant.
n = 94.
Mean difference (95% CI).
Fig. 2.
Tumor diameter as assessed on initial CT scan in patients with successful and unsuccessful endobronchial treatment (EBT).
Table 3.
Curation rates of EBT for patients groups based on tumor diameter and growth pattern
| Tumor diameter/growth pattern | Successful | % |
|---|---|---|
| <20 mm/purely intraluminal growth | 13/18 | 72 |
| <20 mm/possible extraluminal growth | 5/18 | 28 |
| ≥20 mm/purely intraluminal growth | 0/20 | 0 |
| ≥20 mm/possible extraluminal growth | 0/1 | 0 |
Discussion
We found that purely intraluminal disease on CT scan and tumor diameter on CT scan are independent predictors of successful EBT, whereas patient characteristics, bronchoscopic findings, and histological grade are not. Tumor diameter is the strongest predictor of successful EBT.
In the current study, we show that patients with a carcinoid tumor <20 mm, as identified on CT scan, can be safely treated with EBT regardless of histological grade. For these patients EBT represents a minimally invasive and parenchyma-sparing alternative for surgical resection. Because no patient with a tumor ≥20 mm was successfully treated with EBT, we suggest that these patients are directly referred for surgery. This would also make sense from a biological standpoint since it has been demonstrated earlier that tumor diameter has a prognostic impact. A diameter ≥30 mm is associated with advanced histological grade and poorer prognosis [11, 12, 13, 14, 15].
For patients with carcinoid tumors with possible extraluminal disease on CT scan, EBT is associated with a low success rate. However, it is intriguing that 28% of small tumors (<20 mm) with possible extraluminal growth on CT scan can still be successfully treated with EBT (Table 3). This means that almost 1 in 3 patients with a small bronchial carcinoid and possible extraluminal disease on CT scan might benefit from EBT. There could be several explanations for this finding. First, tumor growth with obstruction of the involved bronchus can cause inflammation or atelectasis, which makes it difficult for the radiologist to definitively rule out extraluminal growth. Indeterminate tumor growth on CT scan, as scored by our radiologist, was classified as possible extraluminal disease in our analysis. As a consequence, the “possible extraluminal disease” group might have contained some purely intraluminal tumors. This is the reason that in practice we schedule a follow-up visit 6 weeks after EBT with bronchoscopy and CT scan. The removal of intraluminal disease and subsequent resolution of atelectasis often makes the second CT scan easier to interpret with regard to extraluminal extension. Radial endobronchial ultrasound can improve the assessment of tumor extent in or beyond the bronchial wall, but this technique was not used in the current study [16, 17]. Second, the tumor can invade the bronchial wall without extending beyond it. It seems feasible to treat bronchial wall involvement or minimal extraluminal disease with techniques such as cryotherapy. Cryotherapy seems a very suitable technique in these patients because it is directed to tissues with high water content, thereby sparing the cartilaginous structures in the bronchial wall. Bertoletti et al. [4] specifically focused on the use of cryotherapy for the treatment of bronchial carcinoid in 18 patients and reported favorable long-term outcome. They proposed to treat the base of the carcinoid with cryotherapy to prevent local recurrence. Over the years, this approach has become standard practice at our institution as well.
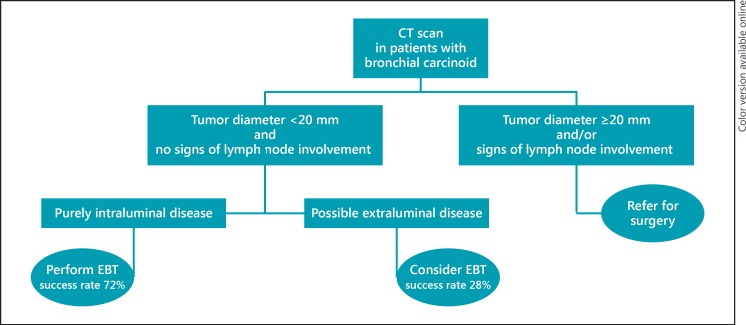
The need for lymph node resection in TC and AC is an issue of ongoing debate. Previous studies showed that parenchyma-sparing therapy (e.g., bronchoscopic resection, bronchial sleeve resections), without lymph node dissection, did not influence survival in patients with TC and AC [4, 5, 8, 18, 19, 20, 20]. In contrast, other studies showed nodal status to be a very important prognostic factor, particularly in AC [11, 13, 14, 15, 21, 22, 26]. With this in mind, preoperative staging of TC and AC, with specific attention to lymph node status, is very important, especially when treating patients with minimal invasive techniques like EBT, as lymph nodes are not sampled during this technique. With a specificity of 90–93% for the assessment of nodal involvement in patients with bronchial carcinoids, a preoperative CT scan is a reliable tool for excluding lymph node involvement before EBT, and is therefore mandatory in the workup for patients with bronchial carcinoid tumors [13, 27]. In this study we used 2 years as a cutoff point to define successful EBT and experienced a very low total recurrence rate of 5%. This can help clinicians in informing patients about EBT for bronchial carcinoids. Furthermore, this serves as a practical surrogate end point for future prospective studies. Figure 3 shows a suggested flowchart to aid the selection of patients for EBT.
Fig. 3.
Suggested treatment protocol of bronchial carcinoid based on assessment of the baseline CT scan. EBT, endobronchial treatment.
The findings of the present study must be interpreted in the context of several potential limitations. Since the patients were referred to our hospital for treatment with EBT, the population included in this study has been exposed to selection bias. We assume that small tumors located in the central airways were referred to our center while larger tumors were directly referred for thoracic surgery. Furthermore, this is a single-center study, with experienced interventional pulmonologists in a tertiary referral center. This means that results cannot be extrapolated to everyday practice in other hospitals. Because EBT of bronchial carcinoids can be technically challenging and complications such as bleeding can occur, we advocate referral to specialized centers with a thoracic surgery department. Another important limitation of the current study is the number of missing CT scans. The cohort was established over a long period of time. We attempted to retrieve CT scans from patients included in the 1990s and early 2000s from referral hospitals; however, most of them were destroyed or of poor quality, resulting in a significant amount of CT scans (57%) not available for revision. Nevertheless, nearly all CT scans of the last 10 years were available, which makes our data reflective of the present era where high-quality CT scans are available almost everywhere. In the reports before revision, specific comments about tumor size and intra-or extraluminal growth were often lacking, which underlines the importance of structured assessment and reporting of CT scans in patients with bronchial carcinoids. This study shows that the CT scan has an important role in directing patients towards the most suitable treatment modality. Additional imaging techniques such as FDG-positron emission tomography (PET)/CT or [68Ga]1,4,7,10-tetraazacyclododecane-N(I-IIII)-tetraacetic acid-(D)-Phe1-Thy3-octreotide (DOTATOC)-PET/CT (68Ga-DOTATATE) are relatively new techniques, and were not evaluated in this study. The clinical role of FDG-PET/CT is related to aerobic glycolysis, which is amplified in fast-growing tumor cells. Slowly growing tumors, for example bronchial carcinoid, have a lower metabolic rate than most other thoracic malignancies (i.e., non-small cell lung carcinoma, small cell lung carcinoma). Multiple studies found that FDG- PET/CT for bronchial carcinoid, and especially TC, is unreliable due to low glucose uptake [28, 29, 30]. More promising is the recently introduced 68Ga-DOTATATE PET/CT scan. Because carcinoid tumors are rich in somatostatin receptors, this scan is more sensitive and more specific than FDG-PET/CT. Recent studies have shown a sensitivity of 90–97% for 68Ga-DOTATATE in the detection of bronchial carcinoid [31, 32, 33, 34, 35]. The 68Ga-DOTATATE PET/CT scan was only recently introduced in our hospital, and we are currently investigating the additional value of this scan in the diagnostic workup of patients with bronchial carcinoid.
To minimize the risk of complications and to maximize the potential of EBT, we highly advocate that this treatment be centralized to referral centers with experienced radiologists for accurate and systematic CT scan evaluation, interventional pulmonologists, and pulmonary surgeons, in order to safely perform EBT and manage complications such as airway hemorrhage.
In conclusion, small tumor size and purely intraluminal disease on CT scan are associated with successful EBT in patients with bronchial carcinoid, independent of the histological grade. Based on these data, CT scan has an important role in directing patients towards the most suitable treatment modality. While patients with a bronchial carcinoid tumor ≥20 mm in diameter should be referred for surgery, purely intraluminal carcinoids with a diameter <20 mm can be treated with EBT with a success rate of 72%. Careful analysis of the baseline CT scan, including lymph node status, enables optimal selection of the appropriate treatment modality.
Financial Disclosure and Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions
E.M.B.P.R., C.D., and J.M.A.D. were responsible for the conception and design of the study and acquisition of data. E.M.B.P.R. and V.M.H.C. performed the analysis and interpretation of the data. The article was written by E.M.B.P.R. and critically revised by the authors, all of whom gave approval for submission. Each author has participated sufficiently in the contributions of this article and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Material
Supplementary data
Acknowledgment
This study was supported by a grant from ORAS (Oncological Research Albert Schweitzer Hospital).
References
- 1.Ito T, Nogawa H, Udaka N, Kitamura H, Kanisawa M. Development of pulmonary neuroendocrine cells of fetal hamster in explant culture. Lab Invest. 1997;77:449–457. [PubMed] [Google Scholar]
- 2.Travis W. Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac Surg Clin. 2014;24:257–266. doi: 10.1016/j.thorsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Filosso PL, Rena O, Guerrera F, Casado PM, Sagan D, Raveglia F, Brunelli A, Welter S, Gust L, Pompili C, Casadio C, Bora G, Alvarez A, Zaluska W, Baisi A, Roesel C, Thomas PA, ESTS NETs-WG Steering Committee Clinical management of atypical carcinoid and large-cell neuroendocrine carcinoma: a multicentre study on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours of the Lung Working Group. Eur J Cardiothorac Surg. 2015;48:55–64. doi: 10.1093/ejcts/ezu404. [DOI] [PubMed] [Google Scholar]
- 4.Bertoletti L, Elleuch R, Kaczmarek D, Jean-Francois R, Vergnon JM. Bronchoscopic cryotherapy treatment of isolated endoluminal typical carcinoid tumor. Chest. 2006;130:1405–1411. doi: 10.1378/chest.130.5.1405. [DOI] [PubMed] [Google Scholar]
- 5.Brokx HA, Paul MA, Postmus PE, Sutedja TG. Long-term follow-up after first-line bronchoscopic therapy in patients with bronchial carcinoids. Thorax. 2015;70:468–472. doi: 10.1136/thoraxjnl-2014-206753. [DOI] [PubMed] [Google Scholar]
- 6.Cavaliere S, Foccoli P, Toninelli C. Curative bronchoscopic laser therapy for surgically resectable tracheobronchial tumors: personal experience. J Bronchology. 2002;9:90–95. [Google Scholar]
- 7.Dalar L, Ozdemir C, Abul Y, Sokucu SN, Karasulu L, Urer HN, Altin S. Endobronchial treatment of carcinoid tumors of the lung. Thorac Cardiovasc Surg. 2016;64:166–171. doi: 10.1055/s-0035-1549274. [DOI] [PubMed] [Google Scholar]
- 8.Fruchter O, Fuks L, Amital A, Fox BD, Abdel Rahman N, Kramer MR. Long-term follow-up of flexible bronchoscopic treatment for bronchial carcinoids with curative intent. Diagn Ther Endosc. 2009;2009:782961. doi: 10.1155/2009/782961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luckraz H, Amer K, Thomas L, Gibbs A, Butchart EG. Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg. 2006;132:113–115. doi: 10.1016/j.jtcvs.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 10.American Society of Anesthesiologists. ASA Physical Status Classification System. Last approved by the ASA House of Delegates on October 15, 2014.
- 11.Aydin E, Yazici U, Gulgosteren M, Agackiran Y, Kaya S, Gulhan E, Tastepe I, Karaoglanoglu N. Long-term outcomes and prognostic factors of patients with surgically treated pulmonary carcinoid: our institutional experience with 104 patients. Eur J Cardiothorac Surg. 2011;39:549–554. doi: 10.1016/j.ejcts.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Belak J, Kudlac M, Zak V, Cavarga I, Kocan P, Boor A, Stebnicky M, Somos A, Tkacova R. Surgical management of bronchopulmonary carcinoid tumors: experience over 8 years and review of the literature. Tumori. 2010;96:84–89. doi: 10.1177/030089161009600114. [DOI] [PubMed] [Google Scholar]
- 13.Chughtai TS, Morin JE, Sheiner NM, Wilson JA, Mulder DS. Bronchial carcinoid - twenty years' experience defines a selective surgical approach. Surgery. 1997;122:801–808. doi: 10.1016/s0039-6060(97)90090-8. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Yuste M, Matilla JM, Alvarez-Gago T, Duque JL, Heras F, Cerezal LJ, Ramos G. Prognostic factors in neuroendocrine lung tumors: a Spanish multicenter study. Spanish Multicenter Study of Neuroendocrine Tumors of the Lung of the Spanish Society of Pneumonology and Thoracic Surgery (EMETNE-SEPAR). Ann Thorac Surg. 2000;70:258–263. doi: 10.1016/s0003-4975(00)01369-2. [DOI] [PubMed] [Google Scholar]
- 15.Jethava A, Codreanu I, Thayer J, Bandyopadhyay T, Ali S, Dasanu CA. Operated bronchial carcinoids: clinical outcomes and long-term follow-up of a single institution series of 30 patients. Conn Med. 2014;78:409–415. [PubMed] [Google Scholar]
- 16.Herth F, Ernst A, Schulz M, Becker H. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest. 2003;123:458–462. doi: 10.1378/chest.123.2.458. [DOI] [PubMed] [Google Scholar]
- 17.Kurimoto N, Murayama M, Yoshioka S, Nishisaka T, Inai K, Dohi K. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest. 1999;115:1500–1506. doi: 10.1378/chest.115.6.1500. [DOI] [PubMed] [Google Scholar]
- 18.El Jamal M, Nicholson AG, Goldstraw P. The feasibility of conservative resection for carcinoid tumours: is pneumonectomy ever necessary for uncomplicated cases? Eur J Cardiothorac Surg. 2000;18:301–306. doi: 10.1016/s1010-7940(00)00519-4. [DOI] [PubMed] [Google Scholar]
- 19.Martini N, Zaman MB, Bains MS, Burt ME, McCormack PM, Rusch VW, Ginsberg RJ. Treatment and prognosis in bronchial carcinoids involving regional lymph nodes. J Thorac Cardiovasc Surg. 1994;107:1–6; discussion 6–7. [PubMed] [Google Scholar]
- 20.Maurizi G, Ibrahim M, Andreetti C, D'Andrilli A, Ciccone AM, Pomes LM, Menna C, Pellegrini M, Venuta F, Rendina EA. Long-term results after resection of bronchial carcinoid tumour: evaluation of survival and prognostic factors. Interact Cardiovasc Thorac Surg. 2014;19:239–244. doi: 10.1093/icvts/ivu109. [DOI] [PubMed] [Google Scholar]
- 21.Bagheri R, Mashhadi M, Haghi SZ, Sadrizadh A, Rezaeetalab F. Tracheobronchopulmonary carcinoid tumors: analysis of 40 patients. Ann Thorac Cardiovasc Surg. 2011;17:7–12. doi: 10.5761/atcs.oa.08.01309. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson MK, Landreneau RJ, Hazelrigg SR, Altorki NK, Naunheim KS, Zwischenberger JB, Kent M, Yim AP. Long-term outcome after resection for bronchial carcinoid tumors. Eur J Cardiothorac Surg. 2000;18:156–161. doi: 10.1016/s1010-7940(00)00493-0. [DOI] [PubMed] [Google Scholar]
- 23.Filosso PL, Guerrera F, Evangelista A, Welter S, Thomas P, Casado PM, Rendina EA, Venuta F, Ampollini L, Brunelli A, Stella F, Nosotti M, Raveglia F, Larocca V, Rena O, Margaritora S, Ardissone F, Travis WD, Sarkaria I, Sagan D. Prognostic model of survival for typical bronchial carcinoid tumours: analysis of 1,109 patients on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours Working Group. Eur J Cardiothorac Surg. 2015;48:441–447; discussion 447. doi: 10.1093/ejcts/ezu495. [DOI] [PubMed] [Google Scholar]
- 24.Herde RF, Kokeny KE, Reddy CB, Akerley WL, Hu N, Boltax JP, Hitchcock YJ. Primary pulmonary carcinoid tumor: a long-term single institution experience. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000221. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machuca TN, Cardoso PFG, Camargo SM, Signori L, Andrade CF, Moreira ALS, Moreira JD, Felicetti JC, Camargo JJ. Surgical treatment of bronchial carcinoid tumors: a single-center experience. Lung Cancer. 2010;70:158–162. doi: 10.1016/j.lungcan.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Rea F, Rizzardi G, Zuin A, Marulli G, Nicotra S, Bulf R, Schiavon M, Sartori F. Outcome and surgical strategy in bronchial carcinoid tumors: single institution experience with 252 patients. Eur J Cardiothorac Surg. 2007;31:186–191. doi: 10.1016/j.ejcts.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 27.Divisi D, Crisci R. Carcinoid tumors of the lung and multimodal therapy. Thorac Cardiovasc Surg. 2005;53:168–172. doi: 10.1055/s-2005-837539. [DOI] [PubMed] [Google Scholar]
- 28.Jindal T, Kumar A, Venkitaraman B, Meena M, Kumar R, Malhotra A, Dutta R. Evaluation of the role of [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT in differentiating typical and atypical pulmonary carcinoids. Cancer Imaging. 2011;11:70–75. doi: 10.1102/1470-7330.2011.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels CE, Lowe VJ, Aubry MC, Allen MS, Jett JR. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest. 2007;131:255–260. doi: 10.1378/chest.06-0711. [DOI] [PubMed] [Google Scholar]
- 30.Kayani I, Conry BG, Groves AM, Win T, Dickson J, Caplin M, Bomanji JB. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med. 2009;50:1927–1932. doi: 10.2967/jnumed.109.066639. [DOI] [PubMed] [Google Scholar]
- 31.Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schaefer M, Schilling T, Haufe S, Herrmann T, Haberkorn U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosini V, Castellucci P, Rubello D, Nanni C, Musto A, Allegri V, Montini GC, Mattioli S, Grassetto G, Al-Nahhas A, Franchi R, Fanti S. 68Ga-DOTA-NOC: a new PET tracer for evaluating patients with bronchial carcinoid. Nucl Med Commun. 2009;30:281–286. doi: 10.1097/MNM.0b013e32832999c1. [DOI] [PubMed] [Google Scholar]
- 33.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 34.Koukouraki S, Strauss LG, Georgoulias V, Eisenhut M, Haberkorn U, Dimitrakopoulou-Strauss A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:1115–1122. doi: 10.1007/s00259-006-0110-x. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Jindal T, Dutta R, Kumar R. Functional imaging in differentiating bronchial masses: an initial experience with a combination of 18F-FDG PET-CT scan and 68Ga DOTA-TOC PET-CT scan. Ann Nucl Med. 2009;23:745–751. doi: 10.1007/s12149-009-0302-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data