Abstract
Bosutinib is a Src/Abl tyrosine kinase inhibitor indicated for adults with newly-diagnosed Philadelphia positive chronic myeloid leukemia or with resistant/intolerant disease. We report the final results of a phase I/II study of second-line bosutinib in chronic phase chronic myeloid leukemia patients after imatinib failure (n=284). Median follow up and treatment durations were 54.8 (range 0.6–96.3) and 25.6 (0.2–96.3) months, respectively. At years 2 and 5, 54% and 40% of patients, respectively, remained on bosutinib. Cumulative major cytogenetic response and complete cytogenetic response rates (newly-attained or maintained from baseline) were 58% and 46%, respectively, by year 2 and 60% and 50% by year 5. Kaplan-Meier probability of maintaining major and complete cytogenetic response was 76% and 78%, respectively, at year 2 and 71% and 69% at year 5. Cumulative incidence of on-treatment disease progression/death was similar at years 5 (19%) and 2 (15%); Kaplan-Meier overall survival was 91% at year 2 and 84% at year 5. Of 169 patients who had discontinued bosutinib by year 5, 38 did so after year 2, most commonly for disease progression (n=11). Most adverse events initially occurred within two years. Overall, gastrointestinal events were the most common (diarrhea 86%, nausea 46%, vomiting 37%); the most common grade 3/4 toxicity was thrombocytopenia (25%). None of the 4 on-treatment deaths in years 3–5 were related to bosutinib. Bosutinib demonstrated durable efficacy and manageable toxicity through year 5 confirming its importance in the treatment of chronic phase chronic myeloid leukemia patients resistant/intolerant to prior imatinib. This trial was registered at clinicaltrials.gov identifier: 00261846.
Introduction
With the success of BCR-ABL1 tyrosine kinase inhibitors (TKIs), patients with Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML) can potentially enjoy a normal life expectancy.1,2 Therefore, information regarding long-term efficacy and tolerability of TKIs is important for informing treatment selection.
Several TKIs are approved for treatment of newly diagnosed chronic phase (CP) CML, each associated with a distinct safety profile. First-line TKI therapies include imatinib3 and the second-generation TKIs, nilotinib,4 dasatinib,5 and, most recently, bosutinib.6 Although response rates are high with TKI therapy, patients develop treatment resistance or experience intolerable toxicities.7,8 Determining the most appropriate therapy following treatment failure is critical to achieve optimal outcomes and prevent disease progression. Second- and third-line treatment decisions are based on the prior therapy, the reason for failure (primary or secondary resistance, intolerance), BCR-ABL1 mutation status, comorbidities, and prior toxicities.8,9
Bosutinib was initially approved in 2012 for the treatment of patients with Ph+ CP, accelerated-phase (AP), or blast-phase (BP) CML resistant or intolerant to prior TKI therapy.6 This approval was based primarily on the results of a pivotal phase I/II trial of bosutinib in CP CML patients following failure of imatinib.10 Results of a preliminary analysis approximately 15 months after the last enrolled patient demonstrated potent activity with second-line bosutinib across a spectrum of BCR-ABL1 mutations and a toxicity profile distinct from those of other TKIs.10 The durability of response was confirmed with subsequent analyses 24 and 48 months after the last enrolled patient.11,12 The final results presented here from the phase I/II trial of bosutinib for imatinib-resistant or imatinib-intolerant CP CML are assessed after at least five years from the time the last patient was enrolled.
Methods
Study design and patients
This phase I/II, open-label, multicenter study was initiated in January 2006; the design has been described previously.10,12 Part 1 (dose-escalation phase) determined the recommended Part 2 starting dose of bosutinib 500 mg/day; in Part 2, the efficacy, safety, and tolerability of bosutinib were evaluated. Patients without a complete hematologic response (CHR) by week 8 or complete cytogenetic response (CCyR) by week 12 were allowed to receive bosutinib 600 mg/day unless treatment-related grade ≥3 adverse events (AEs) occurred. Patients continued bosutinib treatment until disease progression (PD), death, unacceptable toxicity, or withdrawal of consent.
The protocol was approved by central or institutional review boards for each site and was conducted in accordance with Good Clinical Practice principles and the Declaration of Helsinki.
Eligible patients were aged 18 years or over with a confirmed diagnosis of Ph+ CP CML resistant to full-dose imatinib (IM-R; ≥600 mg/day) or intolerant to any dose of imatinib (IM-I). Additional eligibility criteria are provided in the Online Supplementary Methods.
Safety and efficacy analyses
Cytogenetic response was assessed as previously described10 and defined as newly-achieved on study or maintained from baseline for four weeks or more (earliest time point for assessment). Evaluable patients received at least one dose and had a valid baseline assessment for the respective end point. Molecular response data were assessed at a central laboratory; however, the International Scale (IS) was not used. Patients from sites in China, India, Russia, and South Africa were not assessed for molecular response due to logistical constraints. For the purpose of this study, responders for major molecular response (MMR) had a ≥3-log reduction from standardized baseline, a detectable BCR-ABL1 transcript at baseline or postbaseline, and must have maintained or attained a CCyR. Duration of response (date of first response until confirmed response loss or PD/death) was evaluated through 30 days after last dose using the Kaplan-Meier method; patients without events were censored at their last assessment visit. See the Online Supplementary Methods for further details of the statistical methods used for this report.
Disease progression was assessed as described previously.10 Time to PD or death, and time to on-treatment transformation to AP/BP CML, were evaluated through 30 days after the last bosutinib dose using cumulative incidence adjusting for the competing risk of treatment discontinuation without an event; patients without events were censored at their last assessment visit. Progression-free survival (PFS; time to PD or death within 30 days of last bosutinib dose) was analyzed for prognostic factors. Overall survival (OS) was evaluated using the Kaplan-Meier method; patients still alive were censored at the last known date on which they were alive.
Per protocol, patients were followed for OS for up to two years after discontinuation of study treatment; analysis of follow up and OS includes data from patients enrolled in an ongoing extension study (clinicaltrials.gov identifier: 01903733). Safety was assessed in patients who received at least one bosutinib dose; AEs were reported at each visit up to 30 days after last dose and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v.3.0. Treatment-emergent AEs (TEAEs) were assessed overall and by year of first occurrence.
Results
Patients and treatment
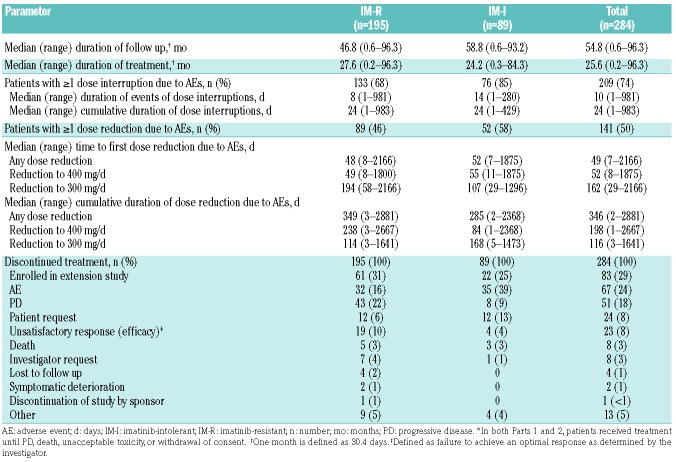
A total of 284 patients with CP CML (IM-R, n=195; IM-I, n=89) were enrolled and treated with second-line bosutinib (Online Supplementary Table S1). The study was closed as of August, 2015; patients still on study were offered enrollment on an extension study. As for the final database lock for this study (2nd October 2015), the time from the last second-line patient’s first dose was 60 months or more. The median (range) duration of OS follow up was 54.8 (0.6–96.3) months, and the median treatment duration was 25.6 (0.2–96.3) months (Table 1). At year 2, 153 (54%) patients were receiving bosutinib and at year 5, 115 (40%; IM-R, n=81 and IM-I, n=34) patients still remained on bosutinib treatment (1 year=48 weeks). Discontinuation from treatment was most common within the first two years, with 131 (46%) patients discontinuing by the end of year 2, and 38 (13%) patients discontinuing treatment in years 3 through 5 (Online Supplementary Table S2). The most common primary reasons for discontinuation from treatment through year 5 were lack of efficacy [categorized by the investigator separately as PD and unsatisfactory response; n=47 (17%) and n=21 (7%), respectively], AE [n=64 (23%)], and patient request [n=19 (7%)]. The most common reasons for discontinuation from treatment in years 3 through 5 were PD and unsatisfactory response in year 3, AE and PD in year 4, and unsatisfactory response and death in year 5. Overall, younger patients (aged <65 years vs. ≥65 years) were less likely to permanently discontinue treatment because of AE (21% vs. 32%), patient request (7% vs. 14%), or death (1% vs. 8%), and more likely to enroll in the extension study (32% vs. 19%) (Online Supplementary Table S3). Ninety-nine (35%) patients completed the 2-year follow up after treatment discontinuation (IM-R, n=60; IM-I, n=39). Thirty-two (11%) patients discontinued treatment after year 5, and 83 (29%) patients continued treatment in the extension study.
Table 1.
Treatment summary.*
Efficacy
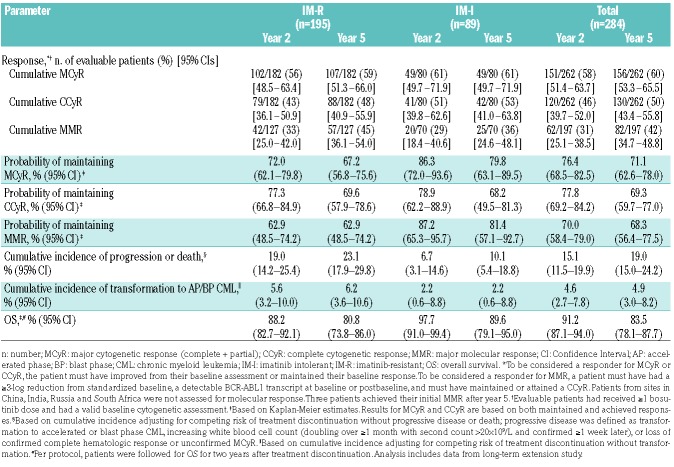
Most cytogenetic responses to bosutinib occurred within two years of initiating treatment (Table 2). By week 12, the cumulative major cytogenetic response (MCyR) rate was 35% (n=93 of 262 evaluable patients), including 22% (n=57) who attained/maintained a CCyR. Of 246 evaluable patients without a CCyR at baseline, 76 (31%) attained an MCyR and 45 (18%) attained a CCyR. The cumulative MCyR rate observed by year 2 was 58%, including 46% with a CCyR. Patients continued to attain a CCyR after two years, with 10 patients having initial on-treatment CCyR during years 3–5. By year 5, the cumulative MCyR CCyR rates were 60% (n=156 of 262 evaluable patients) and 50% (n=130), respectively; 57% (n=141 of 246 evaluable patients) newly-attained an MCyR and 47% (n=116) newly-attained a CCyR. The cumulative MMR rate was 42% (n=82 of 197 evaluable patients). Cytogenetic responses by both two and five years were similar in the IM-R and IM-I subgroups, whereas MMR rates were higher among IM-R patients at both time points (Table 2). Younger patients were more likely to have at least an MCyR (61% vs. 54%); however, rates of CCyR were similar among patients aged under 65 years and 65 years or over (Online Supplementary Table S3).
Table 2.
Efficacy outcomes at two and five years.
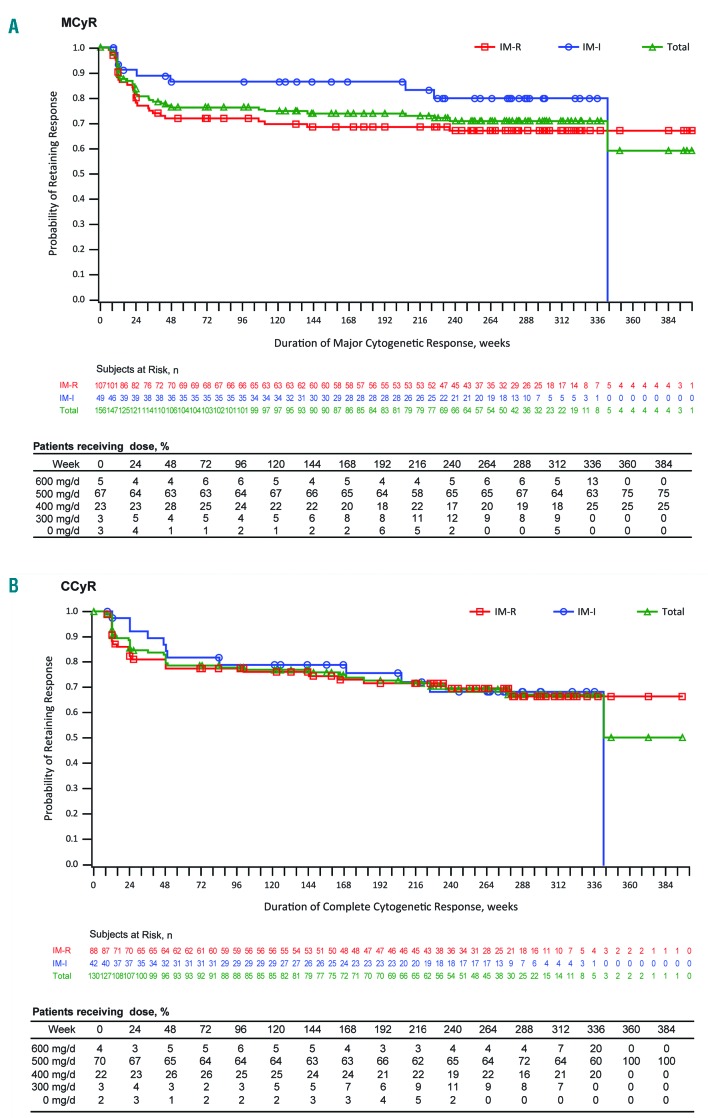
Among responders, the Kaplan-Meier estimated probability of maintaining a response was similar at years 5 and 2: 71% and 76%, respectively, for an MCyR; 69% and 78% for a CCyR; and 68% and 70% for MMR (Table 2 and Figure 1). Overall, 41 of 156 (26%) responders lost MCyR, 37 of 130 (28%) lost CCyR, and 25 of 82 (30%) lost MMR. Few patients lost response after year 2 (7 lost MCyR, 10 lost CCyR, and 2 lost MMR). At the last assessment prior to discontinuation, 81% (n=127) of all 156 responders had an MCyR and 68% (n=106) had a CCyR; of 141 responders without a baseline CCyR, 111 attained an MCyR and 93 attained a CCyR.
Figure 1.
Duration of response. Duration of major cytogenetic response (MCyR) (A) and complete cytogenetic response (CCyR) (B) among responders. Open circles indicate censored observations. IM-I: imatinib-intolerant; IM-R: imatinib-resistant; n: number; d: day.
Among 132 patients (IM-R, n=81; IM-I, n=51) who required a dose reduction to 400 mg/day due to an AE, 81 (61%; IM-R, 63%; IM-I, 59%) had an MCyR, including 67 of 110 (61%) who did not have a CCyR at baseline (Online Supplementary Table S4). Fifty-seven (43%) patients first achieved an MCyR after dose reduction, 19 (14%) achieved an MCyR before and maintained it after dose reduction, and 5 (4%) lost their MCyR after dose reduction. Among 50 patients (IM-R, n=32; IM-I, n=18) who had a dose reduction to 300 mg/day due to an AE, 29 (58%; IM-R, 69%; IM-I, 39%) had an MCyR, including 25 of 43 (58%) without a CCyR at baseline. Twenty (40%) patients achieved an MCyR before and maintained it after dose reduction, 8 (16%) first achieved MCyR after a dose reduction to 300 mg/day, and one (2%) patient lost MCyR. Among patients who achieved an MCyR after a dose reduction, median duration of response (non-Kaplan-Meier) was longer for patients receiving 400 mg/day versus 300 mg/day (167 days vs. 17 days); median MCyR durations (non-Kaplan-Meier) were similar in patients who had a response before and after dose reduction (283 days vs. 260 days) (Online Supplementary Table S4).
Of the 224 (79%) patients with a known baseline mutation status, 79 (35%) had at least one mutation in the BCR-ABL1 kinase domain, most of whom were in the IM-R cohort [IM-R, n=73 of 156 (47%); IM-I, n=6 of 68 (9%)] (Online Supplementary Table S5). Thirteen patients had multiple mutations, all of whom were IM-R. A total of 43 unique BCR-ABL1 mutations were evident, most commonly F359V (n=9), M351T (n=8), M244V (n=6), G250E (n=6), and T315I (n=9). Among evaluable patients with a mutation other than T315I, most (44 of 69, 64%) attained/maintained an MCyR; response rates were similar among patients without a mutation (75 of 130, 58%) and appeared lower among those with multiple mutations (6 of 12; 50%). Among evaluable patients with mutations that are sensitive (n=30), moderately resistant (n=12), and highly resistant (n=12) to bosutinib (see Figure 2 legend for definitions), the cumulative MCyR rates were 67%, 58%, and 33%, respectively, with corresponding CCyR rates of 57%, 42%, and 33%. Among evaluable patients with mutations of unknown sensitivity (n=24) and patients for whom mutation status was unknown (n=54), the MCyR rates were 63% and 65%, respectively, with corresponding CCyR rates of 50% and 57%.
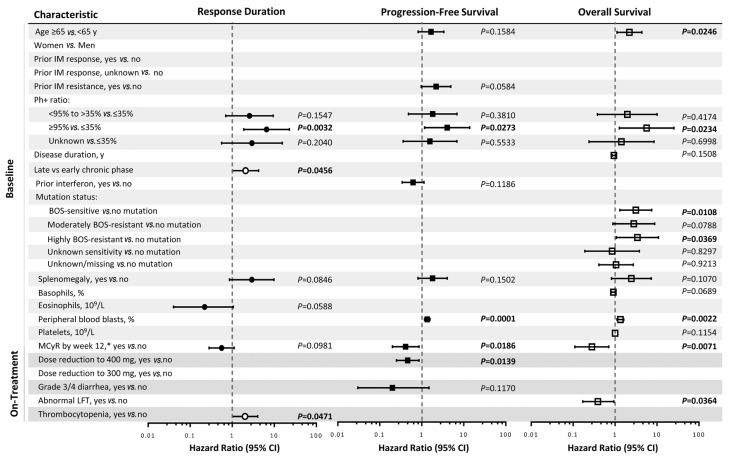
Figure 2.
Predictors of response loss, disease progression, and death. Closed circles represent major cytogenetic response (MCyR) duration and open circles represent complete cytogenetic response. Based on multivariate Cox regression models. Parameters failing to meet elimination criteria (0.20) not shown. Hazard ratios >1 indicate worse outcome. P-values were not adjusted for multiple comparisons; significant P-values are in bold. Definitions of covariates can be found in the Online Supplementary Methods. On-treatment characteristics are Cox time-dependent covariates. *Baseline factor for durable response model. BOS: bosutinib; IM: imatinib; LFT: liver function test; Ph+: Philadelphia chromosome positive; y: years; CI: confidence interval.
Of 104 (37%) patients evaluated for BCR-ABL1 kinase domain mutations before and during bosutinib therapy, 26 had at least one newly-emerging mutation; this was most commonly T315I (n=9), V299L (n=5), and M244V (n=2). Fourteen of the 26 patients with newly-emerging mutations also had at least one BCR-ABL1 mutation present at baseline. Four patients were enrolled in the extension study; the remaining 22 discontinued because of PD (n=12), unsatisfactory response (n=6), AE (grade 2 liver toxicity; n=1), death (sepsis unrelated to bosutinib; n=1), and other (n=2). Four patients achieved a best response of partial cytogenetic response (PCyR) and 8 achieved a CCyR; 12 patients achieved only a CHR as a best response, and 2 patients had no response.
Transformations and survival outcomes
On-treatment transformation to AP/BP CML occurred in 15 (IM-R, n=13; IM-I, n=2) patients overall, with 9 patients transforming to AP (IM-R, n=7; IM-I, n=2), and 6 patients transforming to BP (all IM-R). The cumulative incidence at year 5 was 5% overall (IM-R, 6%; IM-I, 2%); 95% of patients discontinued treatment without transformation. Among the baseline and on-treatment factors examined, only higher baseline peripheral blood blasts was predictive of on-treatment transformation (P=0.0011).
Despite being initially classified as having progressed to AP, 2 patients did well on bosutinib therapy for two years or more after transformation: one patient who transformed on day 14 continued bosutinib treatment for another six years and subsequently continued treatment in the extension study; another patient who transformed on day 246 discontinued treatment two years later for PD. Among 153 patients remaining on treatment after year 2, only 2 (both IM-R) had on-treatment transformation to AP after this time (on days 734 and 2165). Eleven of the 15 patients with on-treatment transformation had responses to bosutinib, including 4 with best responses of MCyR, 3 with CCyR, and 4 with CHR. The cumulative incidence of on-treatment PD/death was higher by 4% at year 5 [19% (IM-R, 23%; IM-I, 10%)] versus year 2 [15% (IM-R, 19%; IM-I, 7%)]; 42% of patients discontinued treatment without on-treatment PD/death before year 5. Long-term outcomes are reported according to age in Online Supplementary Table S3.
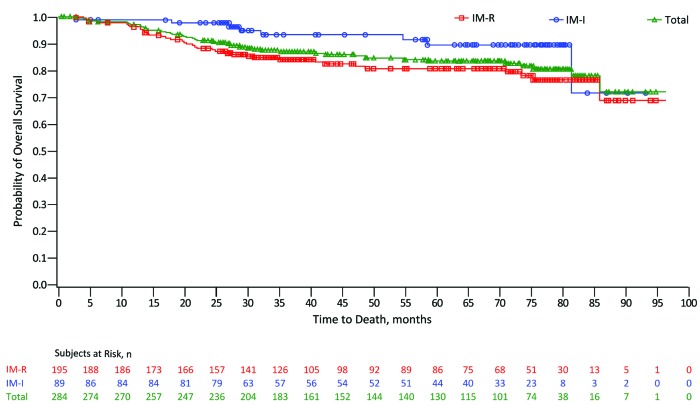
Kaplan-Meier probability of OS at year 5 was 84% (IM-R, 81%; IM-I, 90%) versus 91% (IM-R, 88%; IM-I, 98%) at year 2; 40% of patients were censored prior to year 5, and 31% enrolled in the extension study for continued treatment or follow up for longer-term survival (Figure 3). A total of 45 (16%) deaths occurred on study, 24 through year 2, 5 after year 5, and 10 within 30 days of the last bosutinib dose. Patients aged under 65 years had a higher OS rate compared with those aged 65 years or over (85% vs. 77%) (Online Supplementary Table S3). Causes of death were PD [n=26 (58%); IM-R: n=23; IM-I: n=3], AE unrelated to bosutinib [n=16 (36%); IM-R: n=14; IM-I: n=2], and unknown cause [n=3 (7%); all IM-I]. None of the 45 deaths were assessed as treatment-related. Four deaths occurred within 30 days of the last bosutinib dose through year 2 (all IM-R) and 4 occurred during years 3–5 (2 IM-R and 2 IM-I patients).
Figure 3.
Kaplan-Meier estimated overall survival. Open circles indicate censored observations. Overall survival was calculated as the first date of study dosing until the date of death; patients without events were censored at the last contact. Per protocol, patients were followed for overall survival for two years after treatment discontinuation. Analysis includes data from a long-term extension study. IM-I: imatinib-intolerant; IM-R: imatinib-resistant; n: number.
Predictors of response duration, PFS, and OS
Significant (P<0.05) baseline factors predictive of MCyR or CCyR loss were Ph+ ratio ≥95% versus ≤35% and late versus early disease stage (Figure 2). No on-treatment factors were predictive of MCyR duration; however, experiencing treatment-emergent thrombocytopenia was predictive of loss of CCyR (P=0.0471). Several baseline factors predictive of decreased OS were identified: age ≥65 years versus <65 years, Ph+ ratio ≥95% versus ≤35%, lack of an MCyR by week 12, higher baseline peripheral blood blasts, and having a BCR-ABL1 mutation at baseline that is either sensitive or highly resistant to bosutinib. Among on-treatment factors examined, experiencing an abnormal liver function test (LFT) was predictive of increased OS. Notably, prior response or resistance to imatinib did not predict duration of cytogenetic response or long-term survival outcomes.
Factors predictive of decreased PFS were Ph+ ratio ≥95% versus ≤35%, lack of MCyR by week 12, and higher baseline peripheral blood blasts (Figure 2). The on-treatment factor of receiving a bosutinib dose reduction to 400 mg due to AEs was predictive of increased PFS.
Safety and tolerability
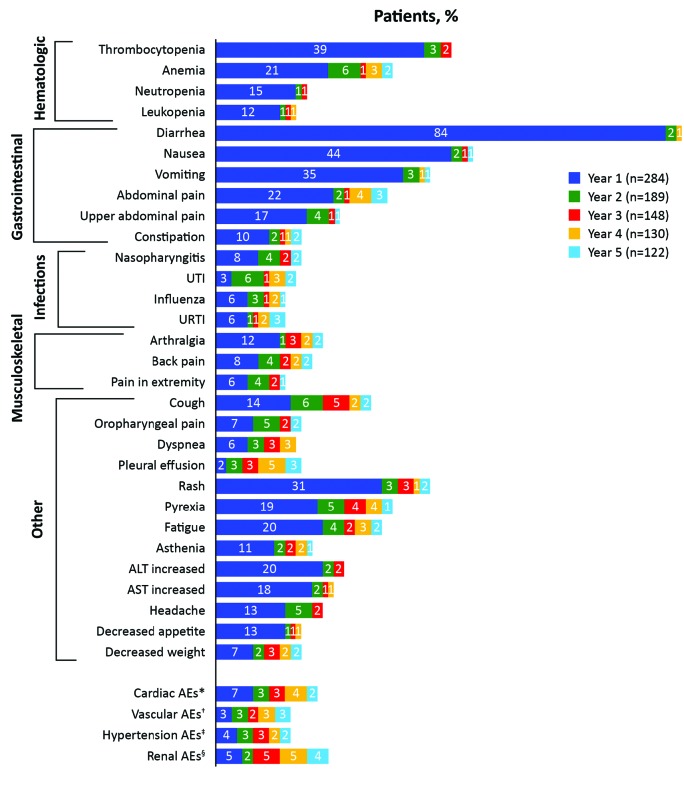
The most common any grade hematologic treatment-emergent AEs (TEAEs) were thrombocytopenia [42% (Grade 3/4, 25%)], anemia [29% (Grade 3/4, 13%)], and neutropenia [16% (Grade 3/4, 10%)] (Online Supplementary Table S6). The most common non-hematologic TEAEs were diarrhea [86% (Grade 3/4, 10%)], nausea [46% (Grade 3/4, 2%)], vomiting [37% (Grade 3/4, 4%)], and rash [36% (Grade 3/4, 9%)]. Most newly-occurring AEs (MedDRA preferred terms not reported for the same patient previously for those on treatment during a given year) were experienced by patients during year 1 (99.6%) of treatment, with rates somewhat lower in years 2 (74%), 3 (68%), 4 (52%), and 5 (57%) (Figure 4). Common AEs (in >5 patients) newly-occurring in year 3 were cough [5% (n=8)], increased blood creatinine [5% (n=7)], and pyrexia [4% (n=6)]; most events were grade 1/2. Common newly-occurring AEs in year 4 were increased blood creatinine [5% (n=6)] and pleural effusion [5% (n=7)]; 2 events (both grade 4 pleural effusion considered probably not related to bosutinib) resulted in hospitalization. No newly-occurring AEs were reported in more than 5 patients in year 5.
Figure 4.
Incidence of newly-occurring adverse events (AEs) over time. Denominators are the number of patients on treatment during the indicated years (NB: incidences of certain AEs appear higher in later years compared with previous years due to a lower number of patients on treatment). Newly-occurring AEs were those not experienced by the same patient previously among patients on treatment during a given year (1 year = 365.25 days). *Includes the high-level group terms (HLGTs) cardiac arrhythmias, pericardial disorders, and heart failures under the cardiac disorders system organ class (SOC); relevant preferred terms (PTs) (cardiac death, sudden cardiac death, sudden death) under the general disorders and administration site SOC conditions; relevant PTs (decreased ejection fraction, abnormal electrocardiogram QT interval, prolonged electrocardiogram QT, long QT syndrome, congenital long QT syndrome, Torsade de pointes, ventricular tachycardia) under the SOC investigations. †HLGTs included: coronary artery disorders, atherosclerosis, stenosis, vascular insufficiency and necrosis, embolism and thrombosis; high-level terms (HLTs) included arterial therapeutic procedures (excluding aortic), central nervous system hemorrhages and cerebrovascular accidents, central nervous system vascular disorders not elsewhere classified (NEC), non-site specific vascular disorders NEC, peripheral vascular disorders NEC (excluding the PTs flushing and hot flash), transient cerebrovascular events, vascular imaging procedures NEC, and vascular therapeutic procedures NEC and all subordinate terms. ‡HLGTs included: vascular hypertensive disorders and cardiac and vascular investigations (excluding enzyme tests), the HLT vascular tests NEC (including blood pressure); PTs included: abnormal blood pressure, abnormal ambulatory blood pressure, increased ambulatory blood pressure, abnormal diastolic blood pressure, increased diastolic blood pressure, increased blood pressure, abnormal systolic blood pressure, and increased systolic blood pressure. §HLT included: renal failure and impairment; PTs included: blood creatinine abnormal, blood creatinine increased, creatinine renal clearance abnormal, creatinine renal clearance decreased, glomerular filtration rate abnormal, glomerular filtration rate decreased. ALT: alanine aminotransferase; AST: aspartate aminotransferase; URTI: upper respiratory tract infection; UTI: urinary tract infection; n: number.
Adverse events led to treatment discontinuation in 69 (24%) patients throughout the study, including one who also discontinued due to PD and another who discontinued due to subject request as the primary reason. AEs resulting in treatment discontinuation in 2% or more of patients overall were thrombocytopenia [6% (n=17)], neutropenia [2% (n=6)], and alanine aminotransferase increased [2% (n=6)]. Of these 69 patients, 28 (41%) discontinued treatment without attempting a dose reduction to less than 500 mg/day. The majority (86%) of discontinuations due to AEs occurred during the first two years of treatment (Online Supplementary Table S2). AEs led to treatment discontinuation in 7 patients in years 3–5: coronary artery disease, scleroderma, and renal failure in year 3; ascites and serositis (same patient), increased blood creatinine, and pulmonary hypertension in year 4; and thrombocytopenia in year 5.
Although diarrhea was the most common AE [IM-R, 86% (n=167); IM-I, 85% (n=76)], in most instances this was grade 1 or 2 [IM-R, 76% (n=149); IM-I, 75% (n=67)] (Online Supplementary Table S6). Only 3 (2%) IM-R and one (1%) IM-I patient discontinued bosutinib treatment primarily because of diarrhea, all within the first two years. Diarrhea (any grade) occurred most frequently within year 1 (84%) with a median (range) time to first occurrence of 2 (1–1330) days; only 4 patients experienced diarrhea for the first time during years 2–5 (Figure 4).
Cardiac AEs occurred in 37 (13%) patients [IM-R, 13% (n=25); IM-I, 14% (n=12)], 12 (32%) of whom had a medical history of these events; maximum grade 3, 4, and 5 cardiac AEs occurred in 11 (4%), 5 (2%), and 2 (1%) patients, respectively (Online Supplementary Table S6). The most common cardiac AEs (occurring in ≥5 patients) were pericardial effusion [3% (grade 3/4, 1%)], congestive cardiac failure [2% (grade 3/4, 2%)], atrial fibrillation [2% (grade 3/4, 1%)], bradycardia [2% (grade 3/4, 1%)], and cardiac failure [2% (grade 3/4, 1%)]. Twelve (4%) patients [IM-R, 3% (n=6); IM-I, 7% (n=6)] experienced cardiac AEs considered by the investigator to be treatment-related, only 3 of whom experienced grade 3/4 events. The median (range) time to first cardiac event was 184 (1-2563) days with the incidence of newly-occurring cardiac AEs decreasing after year 1 (Figure 4). Cardiac AEs led to treatment discontinuation in one patient (cardiac failure in year 2) and death in 2 patients (both congestive heart failure unrelated to bosutinib, occurring in years 3 and 7).
Twenty-two (8%) patients [IM-R, 7% (n=13); IM-I, 10% (n=9)] had vascular AEs including 8 (36%) who had a medical history of vascular events; 11 (4%) patients had grade 3/4 vascular AEs (Online Supplementary Table S6). In 4 (1%) patients [IM-R, 2% (n=3); IM-I, 1% (n=1)], vascular AEs were considered by the investigator to be treatment-related, only one of whom experienced a grade 3/4 vascular event. Most vascular AEs initially occurred within two years with a median (range) time to onset of 548 (47-2452) days. Only one patient discontinued treatment due to vascular AEs (coronary artery disease in year 3). No patients died because of vascular AEs. Twenty-six (9%) patients [IM-R, 10% (n=19); IM-I, 8% (n=7)] experienced hypertension-related AEs, 10 (38%) of whom had a history of vascular events. Events were of low severity in the majority of these patients (maximum grade 1/2, n=18; grade 3, n=8; no grade ≥4) and 5 (2%) experienced events considered by the investigator to be related to treatment; however, no patients discontinued due to hypertension-related AEs. As with vascular AEs, the incidence of newly-occurring hypertension-related AEs did not increase over time (Figure 4).
Renal AEs occurred in 37 (13%) patients [IM-R, 14% (n=27); IM-I, 11% (n=10)], 7 (19%) of whom had a medical history of renal events (Online Supplementary Table S6). Six (2%) patients had maximum grade 3/4 events and 14 (all grade; grade 3/4, n=1) had events considered by the investigator to be treatment related. The median (range) time to first renal AE was 673 (8-2695) days. Renal AEs led to treatment discontinuation in 3 patients (1 each in years 1, 2, and 3) and death in one patient (acute kidney injury in year 1 related to PD and unrelated to bosutinib).
Cross-intolerance
Eighty-nine patients were intolerant to prior imatinib (Online Supplementary Table S7). Of 85 patients with a specific AE reported as the reason for discontinuation of imatinib, 52 (61%) experienced the same AE with bosutinib that led to imatinib discontinuation, most commonly hematologic AEs (thrombocytopenia, n=12; neutropenia, n=5; anemia, n=5) or gastrointestinal AEs (diarrhea, n=6; nausea, n=4); 14 (16%) had cross-intolerance (defined as having discontinued bosutinib due to the same AE that led to prior imatinib discontinuation). Twenty-five (29%) patients experienced the same grade 3/4 AE while on bosutinib. No patient receiving bosutinib died due to the same AE that led to intolerance to prior imatinib.
Discussion
After five years of follow up, the final results of this phase I/II study demonstrated durable efficacy and acceptable long-term safety for second-line bosutinib in patients with CP CML resistant or intolerant to imatinib. The estimated probabilities of responders maintaining an MCyR or CCyR at year 5 (71%, 69%) decreased modestly from the estimated probabilities at year 2 (76%, 78%). Resistance and intolerance to prior imatinib did not appear to result in differences in response durability, as rates observed at years 2 and 5 were similar for both IM-R and IM-I patients. Additionally, late disease progression was uncommon, supporting the observed response durability [although 38 (13%) patients discontinued after year 3, potentially biasing the interpretation of subsequent outcomes]. Cumulative response rates at years 5 and 2 were similar (year 5: MCyR, 60% and CCyR, 50%; year 2: MCyR, 58% and CCyR, 46%). However, it should be noted that results reported here are based on a finalized database resulting in slight differences from previously published data.10
The response rates achieved in this study are comparable to those observed in studies of second-line nilotinib and dasatinib. With similar follow-up durations, CCyR rates of 37% and 49% were reported with nilotinib and dasatinib, respectively, compared with 47% with bosutinib in the present study.4,8,13 Estimated rates of on-treatment PD/death (19%) and transformation to AP/BP CML (5%) remain low with bosutinib; only 2 IM-R patients had on-treatment transformation to AP after year 2, although there is a potential bias from patients lost to follow up. Similar rates of transformation were observed with second-line dasatinib (5%).5,14 The estimated OS rate at 5 years is high, with a modest decrease from the 2-year OS rate (84% vs. 91%). This 5-year rate is also comparable to those reported for dasatinib (91%), nilotinib (87%), and ponatinib (81%) in CP CML patients after prior TKI failure.8
Responses were observed in all but 2 (T315I and M244V) of the 26 patients with newly-emerging BCR-ABL1 mutations. All but one of 14 patients with newly-emerging mutations that are highly resistant to bosutinib15 had a best response of at least CHR; 5 (36%) had a best response of at least PCyR. Effects of dose reductions on response were limited as most patients who dose reduced dose attained/maintained an MCyR. Only 4% and 2% of patients who reduced dose to 400 mg/day and 300 mg/day, respectively, lost their previously achieved MCyR.
Gastrointestinal toxicities remained the most commonly reported AEs overall at the 5-year follow up (diarrhea, 86%; nausea, 46%; vomiting, 37%). Initial events occurred early, with incidences through year 2 of 84% for diarrhea, 45% for nausea, and 37% for vomiting.11 Although diarrhea was common, grade 3 events occurred in only 10% of patients (no grade 4), and only 4 patients discontinued because of this AE, all within two years of initiating bosutinib. Grade 3/4 hematologic AEs, such as thrombocytopenia (25%) and neutropenia (10%), occurred at rates similar to or lower than those observed with second-line dasatinib (24% and 36%), nilotinib (30% and 31%), and ponatinib (35% and 23%).4,5,16 Rates of cross-intolerance between bosutinib and prior imatinib were low, suggesting that most patients intolerant to imatinib therapy may be successfully treated with bosutinib.
Given the long-term nature of TKI therapy, late-emerging toxicities are of concern, particularly cardiac and vascular events. In a study of bosutinib versus imatinib as first-line treatment for CP CML, the incidence of cardiac and vascular AEs with bosutinib was low and similar to that of imatinib.17,18 In the present study, the incidence of newly-occurring cardiac and vascular AEs with second-line bosutinib remained low after year 2. However, most (85%) discontinuations due to AEs as the primary reason occurred within the first two years; thus, patients remaining on treatment after year 2 may have a lower risk of experiencing these events. The incidence of renal AEs, while low, remained similar in years 3–5. Bosutinib has been associated with a decrease in glomerular filtration rate that is typically modest and potentially reversible (similar to what has been reported with imatinib).19,20 Dose adjustments are recommended in patients with baseline and treatment-emergent renal impairment.6,19 Careful monitoring, supportive care, and prompt management of toxicities may allow patients to continue treatment long term.
Most baseline and on-treatment factors examined appeared not to be predictive of response duration, OS, or PFS. Baseline Ph+ ratio ≤35% (vs. ≥95) was associated with all 3 types of long-term outcomes (MCyR duration but not CCyR duration). Lower percentage of peripheral blood blasts at baseline and MCyR by week 12 were associated with both improved OS and PFS. Having a baseline BCR-ABL1 mutation, regardless of sensitivity to bosutinib, was predictive of decreased OS and, interestingly, having an abnormal LFT on-treatment was predictive of increased OS. This unexpected result may be due to increased bosutinib exposure levels resulting from the underlying cause of the abnormal LFT, leading to an increase in efficacy; however, population pharmacokinetics modeling from this study has found no relationship between baseline LFTs and bosutinib pharmacokinetics. Notably, prior response or resistance to IM did not predict any long-term outcomes. Because P-values were not adjusted for multiple comparisons, marginally significant P-values should be interpreted with caution.
The potent and durable activity and distinct toxicity profile of bosutinib confirm it is an important option for treating CML patients in the second-line setting, as demonstrated by its long-term efficacy and safety in these patients; a 10-year follow up is planned for patients enrolled in an ongoing extension study.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. H. Jean Khoury for his extraordinary contributions to the research and treatment of hematologic malignancies. This study was sponsored by Pfizer Inc. Medical writing support was provided by Johna Van Stelten, PhD, of Complete Healthcare Communications, LLC, and was funded by Pfizer Inc. Dr. Jorge Cortes’ participation in this study was supported in part by NCI grants CA016672 and CA049639.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/8/1298
References
- 1.Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553–561. [DOI] [PubMed] [Google Scholar]
- 2.Vigano I, Di Giacomo N, Bozzani S, Antolini L, Piazza R, Gambacorti Passerini C. First-line treatment of 102 chronic myeloid leukemia patients with imatinib: a long-term single institution analysis. Am J Hematol. 2014;89(10):E184–187. [DOI] [PubMed] [Google Scholar]
- 3.GLEEVEC® (imatinib mesylate). Full Prescribing Information. Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, 2016. [Google Scholar]
- 4.TASIGNA® (nilotinib). Full Prescribing Information. Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, 2016. [Google Scholar]
- 5.SPRYCEL® (dasatinib). Full Prescribing Information. Bristol-Myers Squibb Company, Princeton, NJ, USA, 2016. [Google Scholar]
- 6.BOSULIF® (bosutinib). Full Prescribing Information. Pfizer Labs, New York, NY, USA, 2017. [Google Scholar]
- 7.Ramirez P, DiPersio JF. Therapy options in imatinib failures. Oncologist. 2008;13(4): 424–434. [DOI] [PubMed] [Google Scholar]
- 8.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol. 2016;91(2):252–265. [DOI] [PubMed] [Google Scholar]
- 9.Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk. 2015;15(6):323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118(17): 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambacorti-Passerini C, Brummendorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: minimum 24-month follow-up. Am J Hematol. 2014;89(7):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummendorf TH, Cortes JE, Khoury HJ, et al. Factors influencing long-term efficacy and tolerability of bosutinib in chronic phase chronic myeloid leukaemia resistant or intolerant to imatinib. Br J Haematol. 2016; 172(1):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95(2):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NP, Kantarjian H, Kim D-W, et al. Six-year (yr) follow-up of patients (pts) with imatinib-resistant or -intolerant chronic-phase chronic myeloid leukemia (CML-CP) receiving dasatinib. J Clin Oncol. 2012;30(15_suppl):6506. [Google Scholar]
- 15.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27(3):469–471. [DOI] [PubMed] [Google Scholar]
- 16.ICLUSIG® (ponatinib). Full Prescribing Information. ARIAD Pharmaceuticals Inc., Cambridge, MA, USA, 2016. [Google Scholar]
- 17.Cortes JE, Jean Khoury H, Kantarjian H, et al. Long-term evaluation of cardiac and vascular toxicity in patients with Philadelphia chromosome-positive leukemias treated with bosutinib. Am J Hematol. 2016;91(6): 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brummendorf TH, Cortes JE, de Souza CA, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015;168(1):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes JE, Gambacorti-Passerini C, Kim DW, et al. Effects of Bosutinib Treatment on Renal Function in Patients With Philadelphia Chromosome-Positive Leukemias. Clin Lymphoma Myeloma Leuk. 2017;17(10): 684–695. [DOI] [PubMed] [Google Scholar]
- 20.Marcolino MS, Boersma E, Clementino NC, et al. Imatinib treatment duration is related to decreased estimated glomerular filtration rate in chronic myeloid leukemia patients. Ann Oncol. 2011;22(9):2073–2079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.