Abstract
Key points
Acute exposure and acclimatization to hypoxia are associated with an impairment and partial recovery, respectively, of the capability of the central nervous system to drive muscles during prolonged efforts.
Motoneurones play a vital role in muscle contraction and in fatigue, although the effect of hypoxia on motoneurone excitability during exercise has not been assessed in humans.
We studied the impact of fatigue on motoneurone excitability in normoxia, acute and chronic exposure (5050 m) to hypoxia.
Performance was worse in acute hypoxia but recovered to the normoxic standard in chronic hypoxia, in parallel with an increased excitability of the motoneurones compared to acute exposure to hypoxia.
These findings reveal that prolonged hypoxia causes a heightened motoneurone responsiveness during fatiguing exercise; such an adaptation might favour the restoration of performance where low pressures of oxygen are chronically present.
Abstract
The fatigue‐induced failure of the motor cortex to drive muscles maximally increases in acute hypoxia (AH) compared to normoxia (N) but improves with acclimatization (chronic hypoxia; CH). Despite their importance to muscle output, it is unknown how locomotor motoneurones in humans are affected by hypoxia and acclimatization. Eleven participants performed 16 min of submaximal [25% maximal torque (maximal voluntary contraction, MVC)] intermittent isometric elbow flexions in N, AH (environmental chamber) and CH (7–14 days at 5050 m) (PIO2 = 140, 74 and 76 mmHg, respectively). For each minute of the fatigue protocol, motoneurone responsiveness was measured with cervicomedullary stimulation delivered 100 ms after transcranial magnetic stimulation (TMS) used to transiently silence voluntary drive. Every 2 min, cortical voluntary activation (cVA) was measured with TMS. After the task, MVC torque declined more in AH (∼20%) than N and CH (∼11% and 14%, respectively, P < 0.05), with no differences between N and CH. cVA was lower in AH than N and CH at baseline (∼92%, 95% and 95%, respectively) and at the end of the protocol (∼82%, 90% and 90%, P < 0.05). During the fatiguing task, motoneurone excitability in N and AH declined to ∼65% and 40% of the baseline value (P < 0.05). In CH, motoneurone excitability did not decline and, late in the protocol, was ∼40% higher compared to AH (P < 0.05). These novel data reveal that acclimatization to hypoxia leads to a heightened motoneurone responsiveness during fatiguing exercise. Positive spinal and supraspinal adaptations during extended periods at altitude might therefore play a vital role for the restoration of performance after acclimatization to hypoxia.
Keywords: central fatigue, cervicomedullary motor evoked potential, hypoxia
Key points
Acute exposure and acclimatization to hypoxia are associated with an impairment and partial recovery, respectively, of the capability of the central nervous system to drive muscles during prolonged efforts.
Motoneurones play a vital role in muscle contraction and in fatigue, although the effect of hypoxia on motoneurone excitability during exercise has not been assessed in humans.
We studied the impact of fatigue on motoneurone excitability in normoxia, acute and chronic exposure (5050 m) to hypoxia.
Performance was worse in acute hypoxia but recovered to the normoxic standard in chronic hypoxia, in parallel with an increased excitability of the motoneurones compared to acute exposure to hypoxia.
These findings reveal that prolonged hypoxia causes a heightened motoneurone responsiveness during fatiguing exercise; such an adaptation might favour the restoration of performance where low pressures of oxygen are chronically present.
Introduction
Muscle fatigue is characterized by central and peripheral adaptations, which collectively impair performance output (Enoka & Duchateau, 2016). Severe hypoxia is accompanied by reduced exercise performance (i.e. greater fatigue) in both whole‐body (Amann et al. 2013; Goodall et al. 2014a; Jubeau et al. 2017) and single‐joint (Katayama et al. 2007; Goodall et al. 2010; Millet et al. 2012) iso‐time exercise. Centrally, supraspinal fatigue (measured by pre‐ to post‐exercise changes in voluntary activation) is exacerbated by compromised neuronal function in the brain and lower cerebral oxygenation (Subudhi et al. 2009; Goodall et al. 2010, 2014a). Peripherally, the rate of development of fatigue during maximal whole‐body exercise is increased as a result of the simultaneous effects of reduced arterial oxygen content and increased respiratory muscle work, which, in the face of a finite cardiac output, leads to a redistribution of blood flow away from the locomotor muscles (Harms et al. 1997; Amann et al. 2013). Acclimatization to high‐altitude improves arterial oxygen content (Goodall et al. 2014a) and has been shown to mitigate exercise‐related fatigue during whole‐body exercise compared to acute hypoxia. Supraspinal fatigue is reduced, probably as a result of increased cerebral oxygen delivery during the performance (Goodall et al. 2014a). By contrast, peripheral fatigability (as assessed by the highly labile resting twitch) appears to be unchanged (Amann et al. 2013), possibly as a result of a further redistribution of blood flow in response to a greater work of breathing in chronic compared to acute hypoxia (Amann et al. 2013).
Because fatigue is multifactorial, it is necessary to investigate multiple sites along the motor pathway to identify the sequelae of fatigue. Transcranial magnetic stimulation (TMS) can be used during a maximal voluntary contraction (MVC) to assess the completeness of voluntary drive originating from the motor cortex and above (Todd et al. 2003). An exercise‐induced reduction in this measure of voluntary activation is evidence of supraspinal fatigue. TMS has also been widely used to probe fatigue‐related changes within the central nervous system because the motor evoked potential (MEP) indicates the responsiveness of the corticospinal pathway, provided that one accounts for any changes in peripheral excitability. Cervicomedullary stimulation (CMS) uses subcortical stimuli to study motoneurone pool responsiveness, again, provided that one accounts for peripheral excitability. The size of the cervicomedullary motor evoked potential (CMEP) is influenced strongly by descending drive (Martin et al. 2006), which, given our inability to measure drive to a muscle in humans, makes it difficult to separate fatigue‐induced changes in motoneurone excitability from changes in descending drive (McNeil et al. 2011a). TMS during voluntary contractions induces an interruption of voluntary drive, termed the silent period (SP). Delivery of CMS during the SP has been used to study motoneurone excitability without the confound of unknown descending drive (McNeil et al. 2009, 2011b,c). These studies have shown a marked decline in motoneurone responsiveness with fatigue induced by maximal (McNeil et al. 2009; 2011c) and submaximal (McNeil et al. 2011b) sustained contractions, as well as submaximal intermittent contractions (McNeil et al. 2013a).
To date, few studies have examined motoneurone excitability in hypoxia. At rest in both acute (Szubski et al. 2006) and chronic hypoxia (Kayser et al. 1993; Miscio et al. 2009) or after fatiguing exercise in acute hypoxia (Rupp et al. 2014), it was reported that motoneurone excitability was not different from normoxia. However, each of these studies used a measure of motoneurone excitability (H‐reflex or F‐wave) that is less direct than the CMEP (McNeil et al. 2013b). Because of the important influence that hypoxia has on neuronal function (Amann & Kayser, 2009; Goodall et al. 2014a), it is essential not only to study motoneurone responsiveness during performance in acute and chronic exposure to hypoxia, but also to use the most direct response available in humans (CMEP).
The present study aimed to determine changes in motoneuronal excitability and the time course of supraspinal fatigue (as opposed to pre‐ to post‐exercise changes, as reported previously) during fatiguing exercise in severe acute and chronic hypoxia compared to normoxia. Motoneurone excitability was measured via delivery of CMS during the SP after TMS, whereas supraspinal fatigue was measured using TMS. We hypothesized that the fatigue‐induced reductions in motoneurone excitability, voluntary activation and performance would be exacerbated in acute hypoxia, and would recover to normoxic values in chronic hypoxia.
Methods
Ethical approval
The present study was part of a research expedition to the Ev‐K2‐CNR Pyramid Laboratory (5050 m, Nepal) which took place from October to November 2016. All of the testing procedures were approved by the Clinical Research Ethical Review Board of the University of British Columbia (Application ID: H16‐01028) and conformed with the standards set by the Declaration of Helsinki, except for registration in a database.
Subjects
Twelve healthy male subjects (31 ± 7 years, mean ± SD) participated in the study after providing their informed written consent. All participants were members of the research expedition.
Study design
Participants repeated the same experimental procedures on three occasions: normoxia (N) [Kelowna, Canada, 350 m, barometric pressure (Pb) = 715 ± 15 mmHg, partial pressure of inspired O2 (PIO2) = 140 ± 3 mmHg], acute hypoxia (AH) in an environmental chamber (Kelowna, Canada, Pb = 715 ± 15 mmHg, PIO2 = 74 ± 1 mmHg) and chronic hypoxia (CH) following 7–14 days at 5050 m above sea level (Ev‐K2‐CNR Pyramid Laboratory, Nepal, Pb = 413 ± 4 mmHg, PIO2 = 76 ± 1 mmHg). Sessions in N and AH were counterbalanced and conducted during the 6 weeks prior to departing for Nepal. Participants flew to Kathmandu, where they sojourned 3–7 days, then flew to Lukla (2860 m) and trekked over 9 days to the Ev‐K2‐CNR Pyramid Laboratory at 5050 m, with 2 rest days in both Namche Bazaar (3440 m) and Pheriche (4240 m). No participants used medications to prevent or treat acute mountain sickness (e.g. acetazolamide).
Experimental set‐up
Subjects were seated with the right arm positioned in an isometric myograph and a 90° angle of flexion at the shoulder and elbow. The forearm was supinated and an inelastic strap below the wrist secured the arm to the myograph. Elbow flexor torque was measured with a linear strain gauge (SBO‐300; Transducer techniques, Temecula, CA, USA). The electromyographic activity (EMG) of biceps and triceps brachii was recorded via adhesive Ag‐AgCl electrodes (Cleartrace, Utica, NY, USA), arranged in a monopolar fashion. The recording electrodes were positioned over the belly of each muscle, with the reference electrodes over the respective distal tendons. The ground electrode was positioned on the postero‐lateral aspect of the deltoid. For all experimental sessions, torque and EMG data were recorded using Spike 2, version 7.10 (Cambridge Electronic Design, Cambridge, UK) and a 16‐bit A/D converter (CED 1401‐3; Cambridge Electronic Design). The torque was amplified (× 100) (Neurolog System NL109 module; Digitimer, Welwyn Garden City, UK) and sampled at 1000 Hz. EMG data were amplified (× 100), band‐pass filtered (16‐1000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design) and sampled at 2000 Hz. EMG of the biceps was also rectified and integrated (100 ms moving average window; Neurolog System NL703 module; Digitimer). Integrated EMG activity (iEMG) and torque were continuously displayed on a computer monitor to provide feedback to the subject during the protocol.
Arterial saturation
Arterial saturation (SpO2) and heart rate (HR) were sampled using a pulse oximeter (MD300K1; Beijing Choice Electronic Technology, Beijing, China) placed on the middle finger of the non‐exercising (left) arm. Three readings were taken before the fatiguing task (baseline), whereas a reading was taken once per minute for the remainder of the protocol in N and AH. Because there was no change in SpO2 or HR during either session, only resting measures were recorded in CH and only resting values were compared among sessions. For both the AH and CH sessions, the Lake Louise Questionnaire was used to assess acute mountain sickness symptoms. Mild acute mountain sickness symptoms were reported by seven participants in AH and three in CH (scores of 4.9 ± 3.3 and 3.0 ± 2.0, respectively).
Near‐infrared spectroscopy
Cerebral near‐infrared spectroscopy (cNIRS) signals were recorded using the OxiplexTS tissue oximeter (ISS, Champaign, IL, USA). The OxiplexTS flexible probe has four pairs of near infrared light sources (four emitting at 690 nm and four emitting at 830 nm) located at different distances (2.0, 2.5, 3.0 and 3.5 cm) from the detector. Via phase modulation spectroscopy, the OxiplexTS provides the time course of the absolute concentrations (expressed in μm) of oxygenated haemoglobin (O2Hb), deoxygenated haemoglobin (HHb) and total haemoglobin (tHb = O2Hb + HHb). The tissue oxygenation index (expressed as a percentage; TOI = O2Hb/tHb) is also calculated. Changes in tHb can be interpreted as representing blood volume changes (Millet et al. 2012), whereas TOI represents the dynamic balance between O2 supply and O2 consumption in tissue capillaries, arterioles and venules (Ferrari et al. 2004). An elastic strap held the cNIRS probe over the forehead. To ensure repeatability across sessions, the probe was positioned over the Fp1 position, in accordance with the International 10/20 EEG electrode placement system. The cNIRS signals were sampled at 2 Hz using the OxiplexTS proprietary software. Recordings were initiated 1 min before baseline testing and ended 1 min after the last contraction of the protocol.
Brachial plexus stimulation
Single square‐wave electrical stimuli (200 μs pulse width; 100–400 V) were delivered via a constant current stimulator (DS7AH; Digitimer) to the brachial plexus at Erb's point. The cathode and anode (Ag‐AgCl electrodes; Cleartrace) were placed over the supraclavicular fossa and the acromion, respectively. Intensity of brachial plexus stimulation (BPS) was set at 130% of the current required to obtain the maximal compound muscle action potential (M max) of the biceps brachii to ensure supramaximal stimulation throughout the protocol (30–212 mA).
Transcranial magnetic stimulation
Stimulation of the motor cortex was performed using a circular coil (13.5 cm outside diameter), positioned over the vertex, connected to two Magstim 2002 stimulators via a Bistim unit (Magstim, Whitland, UK). One stimulator delivered the conditioning stimulus 100 ms before the CMS used to measure motoneurone excitability (McNeil et al. 2011a), whereas the other delivered stimuli for the calculation of cortical voluntary activation (cVA). The intensity of the conditioning stimulus (93.6 ± 10.3%, 92.5 ± 10.5% and 94.6 ± 6.9% of stimulator output for N, AH and CH, respectively) was set to produce a SP of ∼200 ms during a contraction to the level of iEMG recorded at 25% MVC. For correct determination of the SP length, participants were asked to momentarily pull ‘hard and fast’ after hearing the discharge of the magnet. The intensity of the stimulus used to calculate cVA was set to the output that elicited the highest elbow flexor twitch torque at 50% MVC but a triceps brachii (antagonist) MEP ≤20% the amplitude of the triceps M max when tested during a brief MVC (Todd et al. 2016). The cVA stimulus intensity was 97.3 ± 6.5%, 89.2 ± 13.8% and 86.8 ± 9.6% of stimulator output for N, AH and CH, respectively, yielding MEPs of 72.5 ± 16.9%, 66.2 ± 16.3% and 66.9 ± 22.4% M max in the biceps brachii, and of 11.7 ± 4.5%, 13.7 ± 3.6% and 15.5 ± 4.2% M max in the triceps brachii during brief elbow flexor MVCs.
Cervicomedullary stimulation
CMEPs were evoked by stimulation of the corticospinal tract at the cervicomedullary level. Single square‐wave electrical stimuli (200 μs pulse width; 100–400 V) were delivered via a constant current stimulator (DS7AH; Digitimer). The cathode and anode (Ag‐AgCl electrodes; Cleartrace) were fixed to the skin ∼1 cm superior and medial to the mastoid processes. Stimulus intensity (105–265 mA) was set to evoke a conditioned CMEP of 33% M max peak‐to‐peak amplitude during control contractions targeting the iEMG at 25% MVC.
Experimental procedures
Data collection began with the determination of the resting M max. Stimulus intensity was raised incrementally until the M max of both biceps and triceps brachii was recorded. Intensity was then increased to 130% of the current required to evoke the M max in biceps brachii and three resting stimuli were delivered. Participants were then asked to perform at least two brief (∼3 s) MVCs to establish peak elbow flexor torque. Strong verbal encouragement and visual feedback were provided during each MVC and a minimum of 90 s of rest was provided between contractions. Additional contractions were performed if maximal torque increased by ≥ 5% with successive MVCs. Peak torque was used to set a torque target on the computer monitor at 25% MVC (henceforth 25% MVC). When the subject matched 25% MVC torque, another cursor was set over the iEMG trace to indicate the iEMG at 25% MVC (henceforth 25% iEMG). Thereafter, participants could continuously see the torque and iEMG traces on the computer screen with their respective 25% MVC and 25% iEMG targets. Three M max (3 s interval) were evoked as the subjects performed one 10 s contraction at 25% iEMG and the mean amplitude was calculated. Next, the TMS intensity for measuring cVA was determined. The magnetic stimulus output was incrementally increased to obtain the maximal superimposed twitch torque as participants performed brief (∼2 s) contractions at 50% MVC. Once the maximal twitch was attained, the same stimulator output was delivered during a brief MVC to confirm a peak‐to‐peak MEP amplitude in the triceps brachii ≤20% M max. If not, the stimulation intensity was decreased until this condition was met. Thereafter, the intensity of the TMS conditioning stimulus to produce a silent period of ∼200 ms when targeting 25% iEMG was set. Paired TMS–CMS stimuli (100 ms interval) were then delivered during brief 25% iEMG contractions. CMS intensity was adjusted until the target amplitude of the conditioned CMEP (33% M max) was obtained. This stimulus intensity was used for the remainder of the experiment.
Baseline measures were obtained for conditioned CMEP, M max and cVA (Fig. 1). Subjects performed one contraction (∼6 s) at 25% iEMG, with paired TMS–CMS and BPS stimuli delivered ∼3 s apart. To determine cVA, 5 s after the 25% iEMG contraction, subjects performed a set of three brief (∼2 s) contractions (100% followed by 75% and 50% MVC) separated by ∼3 s of rest. TMS was delivered during each of the three contractions. The order of the submaximal contractions was randomized. This sequence of four contractions was repeated three times with ≥90 s of rest between sets.
Figure 1. Schematic representation of the protocol.
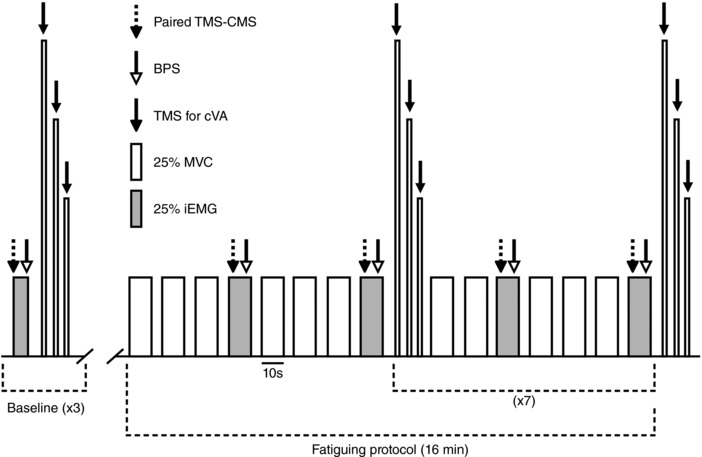
Baseline measures included three sets (90 s rest between) of one 6 s contraction at the level of EMG produced at 25% MVC (25% iEMG, grey rectangle) followed by a series of three brief (∼2 s) contractions (100%, 75% and 50% MVC), separated by ∼3 s of rest. During contractions at 25% iEMG, paired TMS and CMS were delivered (dashed arrow), followed 3 s later by BPS (open arrow). During each contraction at 100%, 75% and 50% MVC, a single TMS pulse was delivered (black arrow) to measure cVA. The fatiguing protocol consisted of 16 min of intermittent (10 s contraction, 5 s rest) isometric elbow flexor contractions at 25% MVC (open rectangles). For the last contraction of each minute, 25% iEMG was targeted, and the same sequence of stimuli used during baseline measures was delivered. After each BPS, and within the 10 s contraction time at 25% iEMG, ratings of perceived effort and perceived pain in the elbow flexors were collected. Every 2 min, and at the end of the protocol (16th min), the series of three contractions was completed to assess cVA.
The fatiguing protocol was 16 min long and consisted of submaximal (25% MVC) intermittent (10 s contraction, 5 s rest) isometric elbow flexor contractions (Fig. 1). For the last contraction of each minute, participants targeted 25% iEMG, and paired TMS–CMS and BPS stimuli were delivered ∼3 s apart. Figure 2 shows raw traces of CMEPs from a single subject, under all conditions, at baseline and at the end of the fatiguing protocol. After the BPS, and within the 10 s contraction time, subjects were asked to provide ratings of perceived exertion (RPE) and perceived pain (in the elbow flexor muscles) using an 11‐point Borg Scale (Borg, 1982). At 2, 4, 6, 8, 10, 12 and 14 min, instead of one contraction at 25% MVC, cVA was measured with the set of three contractions (i.e. 100%, 75% and 50% MVC). To assess cVA at the end of the fatigue protocol, this set of contractions was also performed at 16 min.
Figure 2. Raw individual traces of CMEPs recorded from a single subject, in all conditions, at baseline (three potentials overlaid) and at the end of the fatiguing protocol.
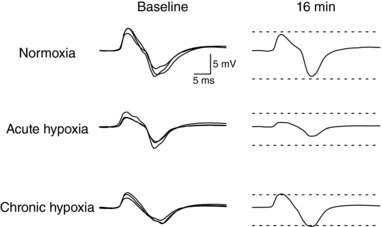
Baseline potentials were set to an amplitude of ∼33% of the maximal compound muscle action potential amplitude collected that session. Dashed lines represent the average amplitude of the baseline potentials.
Data analysis and statistics
All data were analysed off‐line using custom MATLAB scripts (MathWorks, Natick, MA, USA) and Signal, version 5.08 (Cambridge Electronic Design). For cNIRS data, O2Hb, HHb, tHb and TOI were averaged over 1 min time windows (prior to baseline contractions or during the fatigue protocol).
Peak‐to‐peak amplitude and area of CMEPs were measured and then normalized to the amplitude and area of the M max collected during the same contraction to indicate motoneurone responsiveness. The areas of the evoked potentials were measured between cursors marking from the initial deflection from the baseline to the second crossing of the horizontal axis (Martin et al. 2006). Because peak‐to‐peak amplitude and area behaved similarly, only areas are reported for the sake of clarity. Mean torque and iEMG were calculated during the 25% iEMG contractions over 200 ms (in the interval 205 to 5 ms prior to the TMS conditioning stimulus). Mean torque and iEMG were also calculated for the last 25% MVC contraction of each minute, over 500 ms (250 ms on either side of the middle of the contraction). cVA was quantified using the method introduced by Todd et al. (2003). Briefly, a linear regression of the amplitude of the superimposed twitch (SIT) evoked by TMS against voluntary torque (% MVC) was performed for each set of three contractions (100%, 75% and 50% MVC) during and after the protocol. The amplitude of the estimated resting twitch (ERT) was taken as the y‐intercept. The level of voluntary drive was then quantified as cVA = [1 − (SIT at 100% MVC/ERT)] × 100%. Baseline cVA was calculated using the mean SIT at 100% MVC and an ERT determined from a regression that included data from all three sets of contractions. For both fresh and fatigued muscles, only a linear regression between SIT and voluntary torque with a correlation coefficient ≥ 0.9 was considered for calculation of cVA. Two participants repeatedly produced a poor linear regression and were therefore excluded from cVA analysis. Finally, contractile properties of the elbow flexors were assessed using the amplitude of the ERT (Todd et al. 2016).
Normality of data was examined using skewness, kurtosis and the Shapiro–Wilk test. Repeated measures (RM) ANOVAs were used for all comparisons (SPSS, version 23; IBM Corp., Armonk, NY, USA). One‐way RM ANOVAs (session as a factor: N, AH, CH) or Friedman ANOVAs, when conditions for normality were not met, were conducted to compare baseline absolute values of SpO2, HR, TOI, MVC torque, cVA and ERT (both absolute and normalized to the MVC torque) across sessions. When the output was significant, paired samples t tests or Wilcoxon tests (Bonferroni correction of P value) were used to determine which sessions were different. Muscle performance was quantified as pre‐to‐post differences (as a percentage of baseline) in MVC torque, iEMG at 25% MVC and torque at 25% iEMG and then compared across conditions using one‐way RM ANOVAs. When significance was found, paired samples t tests (Bonferroni correction of P value) were used to determine which sessions were different. Data collected at regular intervals during the fatigue protocol were compared with two‐way RM ANOVAs, with session and time as within‐subjects factors. If only a main effect for time was found, a one‐way RM ANOVA was used to assess the effect of time on data pooled across sessions. Paired samples t tests and a Dunnett's table were then used to determine which time points were different from baseline. If a two‐way RM ANOVA had a significant main effect for session or the session × time interaction, one‐way RM ANOVAs were run separately for each session to assess the effect of time. If this main effect was significant, paired samples t tests and a Dunnett's table were used to determine which time points were different from baseline. For those two‐way RM ANOVAs that had a main effect for session, data were compared among sessions at each time point using paired samples t tests (Bonferroni correction of P value).
One participant was not tested at high‐altitude, and another was not tested in normoxia. Therefore, 10 participants were included for all RM ANOVAs (eight for cVA and ERT). When post hoc testing was conducted to compare N, AH and CH, the two participants were added to the sample size for comparisons of the two sessions that they performed, resulting in n = 11 when N and AH, or AH and CH were compared (n = 9 for cVA and ERT). All data are reported as the mean ± SD in the text, and as mean ± SEM in the figures. P < 0.05 was considered statistically significant.
Results
Baseline measures
All baseline measures for each session are reported in Table 1. SpO2 was lower in AH compared to N (−22.6 ± 3.5%) and CH (−10.9 ± 4.6%) and in CH compared to N (−11.7 ± 3.4%; P < 0.01 for all comparisons). No differences among sessions were reported for HR (P = 0.16). TOI was decreased in AH and CH compared to N (−24.9 ± 14.0% and −15.2 ± 8.2%, respectively; P < 0.01), with no difference between AH and CH (P = 0.17). MVC torque was not different between N and AH (P = 0.07) or N and CH (P = 0.25), although it was higher in CH compared to AH (+8.6 ± 8.2%, P < 0.01). The cVA during maximal effort was lower in AH compared to both N (−3.7 ± 2.3%, P < 0.01) and CH (−3.3 ± 3.2%, P < 0.05), whereas it recovered to N values in CH (P = 0.64). ERT (as absolute torque or normalized to the MVC torque) was similar across the three sessions (P > 0.18). The absolute peak‐to‐peak amplitude of M max during baseline contractions at 25% iEMG was lower in CH compared to N (−33.0 ± 27.9%, P < 0.01) and AH (−37.6 ± 44.8%, P < 0.05), whereas no differences were found between N and AH (P = 0.20). Conditioned CMEP amplitude during contractions at 25% iEMG was near to the target of 33% M max and did not differ among sessions (P = 0.39).
Table 1.
Resting arterial saturation, heart rate and neuromuscular measures in all sessions
| Normoxia | Acute hypoxia | Chronic hypoxia | |
|---|---|---|---|
| SpO2 (%) | 97.5 ± 0.9 | 75.5 ± 3.1† | 86.1 ± 3.1* |
| HR (beats min–1) | 64.4 ± 15.6 | 74.3 ± 14.3 | 67.8 ± 13.7 |
| TOI (%) | 66.7 ± 6.9 | 50.2 ± 11.0* | 56.5 ± 7.8* |
| MVC torque (N·m) | 73.4 ± 10.3 | 69.8 ± 7.8# | 75.9 ± 6.8 |
| cVA (%) | 95.4 ± 4.6 | 91.9 ± 4.6† | 94.9 ± 3.3 |
| ERT (N·m) | 15.1 ± 4.0 | 13.8 ± 3.4 | 13.9 ± 4.0 |
| ERT (% MVC) | 20.7 ± 4.3 | 20.0 ± 5.4 | 18.2 ± 4.8 |
| M max amplitude (mV) | 27.2 ± 6.8 | 29.2 ± 8.6# | 20.2 ± 4.7* |
| CMEP (% M max) | 32.6 ± 6.1 | 32.6 ± 4.1 | 34.9 ± 6.0 |
Values are the mean ± SD. † P < 0.05 different vs. normoxia and chronic hypoxia. * P < 0.05 different vs. normoxia. # P < 0.05 different vs. chronic hypoxia.
Fatiguing protocol responses
As intended, muscle output (torque and integrated EMG for the first three and final contractions of each minute, respectively) was targeted consistently during the fatigue protocol (Fig. 3) and was not different across sessions (torque, P = 0.60; iEMG, P = 0.84). When pre‐to‐post changes in MVC torque, iEMG at 25% MVC and torque at 25% iEMG were compared among sessions, each one‐way RM ANOVA showed a main effect for session (F 2,18 > 5.6, P < 0.05). Post hoc comparisons revealed that performance deteriorated more in AH than in CH and N (P < 0.05), as shown in Table 2 from the greater reduction in MVC torque and submaximal torque at 25% iEMG and the greater increase in iEMG during contractions at 25% MVC. For RPE (Fig. 4 A), there were main effects for time (F 1.7,15.4 = 55.9, P < 0.01) and session (F 2,18 = 5.9, P < 0.05). RPE was significantly increased compared to the beginning of the protocol from the 5th min in N and CH (P < 0.05) and from the 4th min (P < 0.01) in AH. In addition, RPE was higher in AH, from the 7th min compared to N, and at the 7th and 16th min compared to CH (P < 0.05). When perceived pain was compared (Fig. 4 B), the two‐way RM ANOVA reported only a main effect of time (F 1.2,11.0 = 19.2, P < 0.01). Post hoc testing on pooled data showed that perceived pain was increased compared to baseline from the 3rd min of the protocol (P < 0.05).
Figure 3. Elbow flexor torque and biceps brachii integrated EMG during the fatiguing protocol.
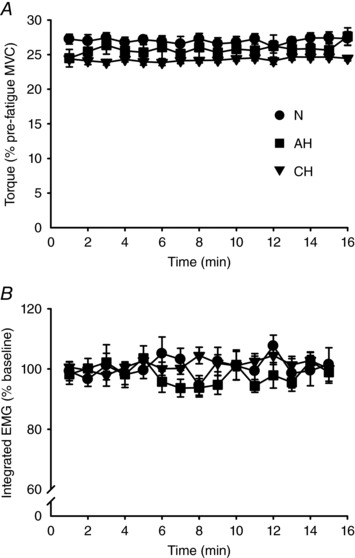
Mean ± SEM values in normoxia (N, ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). A, elbow flexor torque obtained from the last 25% MVC contraction of each minute. B, integrated EMG from the last contraction of each minute expressed as a percentage of the value obtained during baseline contractions at 25% MVC. Torque and iEMG were targeted accurately throughout the protocol and did not differ across sessions (P = 0.60 and P = 0.84 for torque and iEMG, respectively).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). A, elbow flexor torque obtained from the last 25% MVC contraction of each minute. B, integrated EMG from the last contraction of each minute expressed as a percentage of the value obtained during baseline contractions at 25% MVC. Torque and iEMG were targeted accurately throughout the protocol and did not differ across sessions (P = 0.60 and P = 0.84 for torque and iEMG, respectively).
Table 2.
Pre‐ to post‐exercise differences (percent change relative to baseline) in performance indicators, as well as post‐exercise ERT (relative to baseline), in all sessions
| Normoxia | Acute hypoxia | Chronic hypoxia | |
|---|---|---|---|
| ΔMVC torque (%) | −11.5 ± 9.8 | −20.5 ± 8.2† | −14.5 ± 8.1 |
| ΔiEMG at 25% MVC (%) | +22.2 ± 32.5 | +58.0 ± 40.9† | +10.8 ± 6.8 |
| ΔTorque at 25% iEMG (%) | −14.3 ± 14.2 | −30.9 ± 12.2† | −7.0 ± 9.4 |
| ERT (% baseline) | 85.8 ± 19.5 | 89.7 ± 18.1 | 95.4 ± 24.2 |
Values are the mean ± SD. † P < 0.05 different vs. normoxia and chronic hypoxia.
Figure 4. Ratings of perceived effort and muscle pain during the fatiguing protocol.
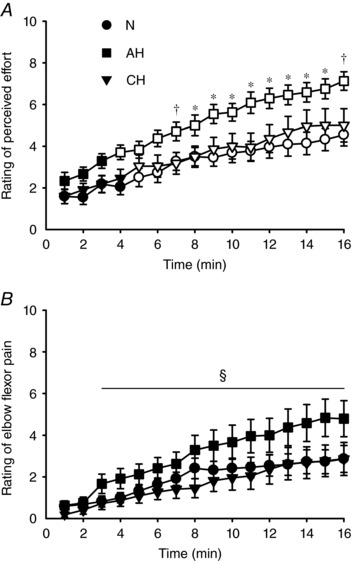
Mean ± SEM values in normoxia (N, ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly higher than baseline values. Rating of perceived effort (A) and perceived pain in the elbow flexors (B) were collected during the last contraction of each minute at 25% iEMG. During the second half of the protocol, the rating of perceived effort was higher in AH compared to N (*
P < 0.05) or both N and CH (†
P < 0.05). For rating of elbow flexor pain, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed an increase from the 3rd min onward (§
P < 0.05).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly higher than baseline values. Rating of perceived effort (A) and perceived pain in the elbow flexors (B) were collected during the last contraction of each minute at 25% iEMG. During the second half of the protocol, the rating of perceived effort was higher in AH compared to N (*
P < 0.05) or both N and CH (†
P < 0.05). For rating of elbow flexor pain, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed an increase from the 3rd min onward (§
P < 0.05).
M max area (normalized to control values) was not altered by the fatigue protocol (i.e. neither the main effects, nor the interaction was significant) (P ≥ 0.08) (Fig. 5 A). Conditioned CMEPs (% M max area, normalized to control values) are depicted in Fig. 5 B. There were main effects of time (F 2.3,20.6 = 12.1, P < 0.01) and session (F 2,18 = 3.6, P < 0.05), as well as an interaction (F 4.3,38.6 = 1.6, P < 0.05). With respect to time, post hoc testing revealed that CMEPs were reduced from the 3rd to the 10th min of the protocol in N (P < 0.05) and from the 3rd min onwards in AH (P < 0.05), whereas no reduction was found in CH. Across sessions, CMEPs were larger at the 10th, 11th, 13th and 15th min in CH compared to AH (P < 0.05).
Figure 5. Maximal compound muscle action potential area, cervicomedullary motor evoked potential area and cortical voluntary activation across the fatiguing protocol.
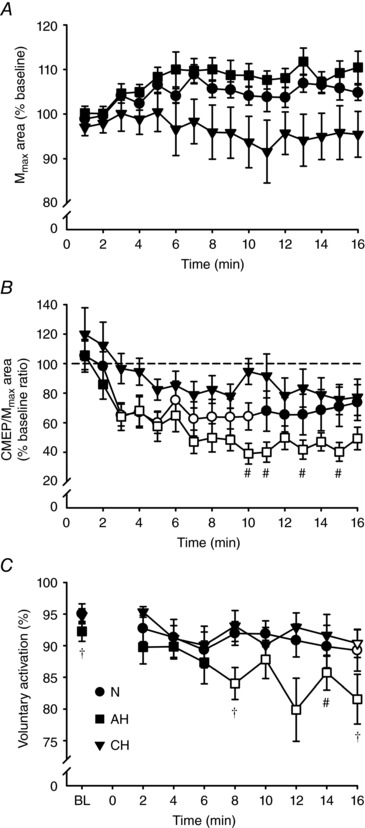
Mean ± SEM values in normoxia (N, ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly lower than baseline values. Area of M
max (A) and area of CMEP (B) normalized to the area of the M
max evoked during the same contraction each minute, expressed as a percentage of the baseline value. C, absolute values of cortical voluntary activation measured at baseline (BL) and every 2 min during the fatiguing protocol. At the 10th, 11th, 13th and 15th min, CMEP area was lower in AH compared to CH (#
P < 0.05). Cortical voluntary activation was lower at BL and the 8th and 16th min in AH compared to both N and CH (†
P < 0.05), and at the 14th min in AH relative to CH (#
P < 0.05).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly lower than baseline values. Area of M
max (A) and area of CMEP (B) normalized to the area of the M
max evoked during the same contraction each minute, expressed as a percentage of the baseline value. C, absolute values of cortical voluntary activation measured at baseline (BL) and every 2 min during the fatiguing protocol. At the 10th, 11th, 13th and 15th min, CMEP area was lower in AH compared to CH (#
P < 0.05). Cortical voluntary activation was lower at BL and the 8th and 16th min in AH compared to both N and CH (†
P < 0.05), and at the 14th min in AH relative to CH (#
P < 0.05).
Absolute values of cVA are depicted in Fig. 5 C. The two‐way RM ANOVA yielded main effects for time (F 2.5,17.4 = 2.8, P < 0.01) and session (F 2,14 = 47.0, P < 0.01), as well as an interaction (F 3.2,22.5 = 1.9, P < 0.05). cVA was lower compared to baseline from the 8th min of the fatiguing task in AH (P < 0.05) and only at task end (16th min) in both N and CH (P < 0.05). When compared across sessions, cVA was lower in AH than N at the 8th and 16th min, and lower in AH compared to CH at the 8th, 14th and 16th min (P < 0.05). The ERT (normalized to control values) did not differ from baseline (i.e. neither the main effects, nor the interaction were significant) (P ≥ 0.09) (Table 2).
Values of TOI, tHb, O2Hb and HHb, normalized to baseline, are represented in Fig. 6. For TOI, there was a significant main effect of time (F 2.3,20.3 = 17.1, P < 0.01) and time × session interaction (F 1.6,14.1 = 1.7, P < 0.05). Specifically, TOI was increased compared to baseline from the 14th min of the protocol in N and from the 12th min in AH and CH (P < 0.05). tHb did not change from baseline for any session and so had neither main effects, nor an interaction (P > 0.14). For O2Hb, there was a main effect of time (F 1.7,15.4 = 6.2, P < 0.05) and time × session interaction (F 1.9,17.4 = 1.6, P < 0.05). Post hoc testing indicated that O2Hb increased only in AH, from the 8th min (P < 0.05). For HHb, RM ANOVA reported only a main effect of time (F 1.4,12.4 = 2.3, P < 0.01); when data were pooled, HHb decreased from the 6th min onwards.
Figure 6. Near‐infrared spectroscopy parameters during the fatiguing protocol.
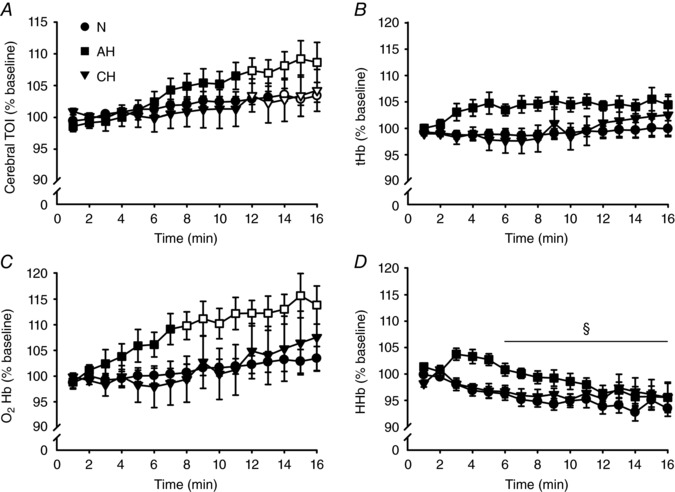
Mean ± SEM values over 1 min windows in normoxia (N, ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). All values are expressed as a percentage of baseline. Open symbols represent data points significantly higher than baseline values. A, cerebral TOI. B, tHb. C, O2Hb. D, HHb. During the second half of the protocol, TOI was higher in all conditions, whereas O2Hb was higher only in AH. For HHb, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed a decrease from the 6th min onward (§
P < 0.05).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). All values are expressed as a percentage of baseline. Open symbols represent data points significantly higher than baseline values. A, cerebral TOI. B, tHb. C, O2Hb. D, HHb. During the second half of the protocol, TOI was higher in all conditions, whereas O2Hb was higher only in AH. For HHb, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed a decrease from the 6th min onward (§
P < 0.05).
Discussion
The aim of the present study was to examine the responsiveness of the motoneurone pool and the time course of supraspinal fatigue during isometric exercise in acute exposure to hypoxia and after acclimatization. Fatigue was induced by 16 min of intermittent isometric elbow flexor contractions, and the neuromuscular system was assessed at the cortical, spinal and peripheral levels. Our data yield two principal novel findings: (i) acclimatization to hypoxia (CH) was accompanied by increased excitability of the spinal motoneurones during the fatiguing protocol compared to AH and (ii) the onset of supraspinal fatigue occurs earlier in AH compared to N and CH. In light of these results, we suggest that spinal factors, in addition to cerebral factors (Goodall et al. 2014a), appear to have contributed to the recovery of performance after acclimatization to hypoxia.
Baseline variables
Immediate exposure to hypoxia resulted in several changes in baseline variables compared to normoxia. As expected, both SpO2 and TOI were lower in AH than N and partially recovered in CH. In the present study, cVA was decreased in AH compared to both N and CH. Previous data were equivocal and so our findings conflict with studies that report no hypoxia‐induced reduction in cVA (Goodall et al. 2010, 2012, 2014a) but support two other previous investigations (Rasmussen et al. 2010; Rupp et al. 2012). Rasmussen et al. (2010) reported a positive correlation between mitochondrial oxygen tension and cVA. Reduced oxygen tension leads to widespread depolarization, depression of synaptic transmission and electrophysiological isolation of corticomotor neurones (Neubauer & Sunderram, 2004; Papadelis et al. 2007; Goodall et al. 2014b). In the present study, SpO2 and TOI fell markedly following exposure to hypoxia. Both measures have been correlated with cerebral capillary oxygen saturation and mitochondrial oxygen tension (Rasmussen et al. 2007), which might explain the reduced cVA in AH, as well as its recovery in CH, in association with partial restoration of both SpO2 and TOI.
Similar to the results of a study conducted at a simulated altitude comparable to ours (Caquelard et al. 2000), M max amplitude showed a significant decline in CH compared to both N and AH, indicating altered neuromuscular propagation. Acclimatization to high‐altitude was found to reduce Na+‐K+‐ATPase concentration in skeletal muscle (Green et al. 2000), possibly as a result of reduced O2 tension or disturbances associated with the prolonged high‐altitude stimulus, such as loss of muscle volume, dietary intake and hormonal changes (Hoppeler & Vogt, 2001; Barnholt et al. 2006).
Fatigue‐related measures
In the present study, all indicators of performance were impaired more in AH compared to N, and similar in N and CH. In addition, participants reported higher perceived effort in AH, whereas no differences were noted between N and CH. This is in accordance with previous investigations (Amann et al. 2013; Goodall et al. 2014a) involving iso‐time exercise.
Although not a traditional measure of peripheral fatigue, the ERT failed to decline under any conditions, including AH. This is in contrast to previous research (Katayama et al. 2007) that reported a higher decline in the potentiated resting twitch after iso‐time single joint exercise in acute hypoxia. The study by Katayama et al. (2007) employed intermittent contractions at 60% of MVC torque, whereas our protocol involved low intensity (25% MVC) 10 s contractions, interspersed by 5 s of rest. This design may have either provided sufficient muscle recovery during the rest periods or failed to induce sufficient metabolic stress for the ERT to be significantly reduced. The ERT, as quantified with the procedures used in the present study (i.e. a set of contractions at 100%, 75% and 50% MVC), has been reported to have a within‐session coefficient of variation of 10.7 ± 5.2% (Todd et al. 2004). Although this variability is sufficient to detect peripheral fatigue for high‐intensity protocols, it may not have been sufficiently sensitive for the magnitude of peripheral fatigue that developed in the present study.
Responsiveness of the motoneurone pool (indicated by the CMEP) was affected by chronic hypoxic exposure. Unlike N and AH, exercise in CH did not lead to a fatigue‐related reduction of the CMEP. Moreover, the CMEP was higher in CH than AH during the last third of the protocol. The period of our measurements in CH corresponds with the window (days 6–18) of maximal sympathetic norepinephrine concentration following exposure to high‐altitude (Barnholt et al. 2006). At the motoneuronal level, norepinephrine acts as a potent neuromodulator, increasing intrinsic motoneurone excitability (Heckman et al. 2009). At least for the elbow flexors, fatigue‐related changes to the CMEP during the silent period are indicative of altered intrinsic properties rather than altered afferent inputs to the motoneurones (McNeil et al. 2011c). Hence, an increased norepinephrine concentration, as previously proposed for the increased MEP seen in chronic hypoxia (Goodall et al. 2014a), appears to be a logical mechanism for the preservation of motoneurone excitability in CH. Given that motoneurone discharge is a critical determinant of muscle contraction, it follows that adaptation of intrinsic motoneurone properties may have contributed to the superior performance in CH compared to AH.
Supraspinal fatigue developed earlier in AH (8th min) compared to N and CH (16th min) and had a greater magnitude (i.e. cVA was lower in AH than N and CH at time points between the 8th and 16th min) (Fig. 5 C). This indicates that acute hypoxic exposure exacerbates the fatigue‐induced failure of motor cortical output (Goodall et al. 2010, 2012, 2014a). Speculation on the mechanism(s) can be aided by our cNIRS data, given that TOI and tHb are considered to be indicators of average capillary oxygenation of brain tissue and blood volume beneath the probe, respectively (Rasmussen et al. 2007; Millet et al. 2012). During the fatiguing task, we observed increased TOI and unchanged tHb under all conditions. Increased TOI is indicative of increased oxygen delivery (blood flow), reduced oxygen utilization, or a combination of both mechanisms. Although indirect, there is evidence that tHb is linked to blood flow (Tachi et al. 2004). Hence, our observed increase in TOI without an increase in tHb, could indicate a reduced cerebral oxygen uptake. This may be particularly true in AH because O2Hb became elevated relative to baseline at the 8th min, the same time point when supraspinal fatigue became evident (cVA was reduced relative to baseline) and 1 min after the effort was perceived to be more difficult in AH than N and CH. The proposition of reduced cerebral oxygen uptake (and any connections to cVA and RPE) is speculative and has a number of potential limitations (see next sentence); nonetheless, the idea warrants further investigation. In addition to the challenge of interpreting TOI vs. tHb data, it is important to note that, in the present study, cNIRS measures were taken over the prefrontal rather than motor cortex; however, haemodynamic changes between prefrontal and motor cortices were shown to be correlated during exercise (Subudhi et al. 2009).
Practical relevance
The findings from the present study suggest that acclimatization to hypoxia is accompanied by increased responsiveness of motoneurones (presumably as a result of chronically increased sympathetic activity). In this case, the adaptation led to a preservation of motoneurone excitability compared to acute hypoxia during a fatiguing task. Preserved motoneurone excitability may be a mechanism that helps to reverse the decreased muscle performance seen in acute hypoxia. This has implications when motor tasks are performed when sojourning at altitude.
Conclusions
Our research showed that motor performance and supraspinal fatigue worsened with acute hypoxic exposure and recovered to normoxic values after acclimatization to high‐altitude. Notably, this recovery of performance was accompanied by a lack of decline in motoneurone responsiveness in chronic hypoxia during the fatiguing protocol. This suggests that motoneuronal properties may play an important role for the restoration of performance in acclimatization to high‐altitude, in association with previously documented supraspinal adaptations.
Additional information
Competing interests
The authors declare that they have no competing interests
Author contributions
LR and CJM contributed to the conception and design of the experiment. All authors contributed to the collection, analysis and interpretation of data. LR drafted the manuscript. All authors revised the manuscript critically for important intellectual content. All authors have read and approved the final submission. Experiments were conducted at the Okanagan campus of The University of British Columbia in Kelowna, Canada, and the Ev‐K2‐CNR Pyramid Laboratory in Nepal.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (DG 435912‐2013) and the Canada Foundation for Innovation/British Columbia Knowledge Development Fund (32260).
Translational perspective.
Hypoxia acutely impairs performance compared to normoxia, increasing supraspinal and peripheral fatigue. With chronic hypoxia, performance and fatigability are partially restored to normoxic values via enhanced cerebral and muscle oxygen delivery. Motoneurones are the final pathway in the brain‐to‐muscle tract and have a pivotal role in regulating muscle contraction. Studying motoneurone excitability modulation during motor tasks is thus paramount. We devised a fatiguing task for the elbow flexors to study the influence of acute hypoxia [partial pressure of inspired O2 (PIO2) = 74 mmHg] and acclimatization (7–14 days at 5050 m, PIO2 = 76 mmHg) on motoneurone excitability, supraspinal fatigue and performance (reduction in muscle torque‐generating capacity). We hypothesized that the fatigue‐induced reductions in motoneurone excitability, voluntary activation and performance would be exacerbated in acute hypoxia, whereas they would recover to normoxic values in chronic hypoxia. As expected, performance and supraspinal fatigue were worst in acute hypoxia and recovered to normoxic values with acclimatization. After acclimatization, compared to acute exposure to hypoxia, motoneurones were more excitable at the end of the fatiguing task. This heightened motoneurone responsiveness with acclimatization to hypoxia (presumably driven from chronically increased sympathetic activity) may favour recovery of performance at altitude. That is, more excitable motoneurones would require less descending drive, which would decrease fatigability and favour successful completion of the task. This research highlights the importance of investigating spinal‐level adaptations to acute and chronic exposure to hypoxia. Understanding and exploiting spinal plasticity may enable enhanced performance of fatiguing motor tasks when sojourning at altitude.
Acknowledgements
We are extremely grateful to ISS for the loan of the OxiplexTS tissue oximeter for the period July to November 2016. We are also grateful to our participants and fellow travellers for helping us to overcome the challenges of an expedition at high‐altitude.
Edited by: Harold Schultz & Frank Powell
This is an Editor's Choice article from the 1 August 2018 issue.
Linked articles This article is highlighted by a Perspective by Goodall. To read this Perspective, visit https://doi.org/10.1113/JP275552. This article is also discussed in a Letter to the Editor by Finn et al. To read this Letter and the response by Ruggiero et al., visit https://doi.org/10.1113/JP275816 and https://doi.org/10.1113/JP275978.
References
- Amann M, Goodall S, Twomey R, Subudhi AW, Lovering AT & Roach RC (2013). AltitudeOmics: on the consequences of high‐altitude acclimatization for the development of fatigue during locomotor exercise in humans. J Appl Physiol 115, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M & Kayser B (2009). Nervous system function during exercise in hypoxia. High Alt Med Biol 10, 149–164. [DOI] [PubMed] [Google Scholar]
- Barnholt KE, Hoffman AR, Rock PB, Musa SR, Fulco CS, Braun B, Holloway L, Mazzeo LS, Cymerman A & Friadlander AL (2006). Endocrine responses to acute and chronic high‐altitude exposure (4,300 meters): modulating effects of caloric restriction. Am J Physiol Endocrinol Metab 290, E1078–E1088. [DOI] [PubMed] [Google Scholar]
- Borg G (1982). Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14, 377–381. [PubMed] [Google Scholar]
- Caquelard F, Burnet H, Tagliarini F, Cauchy E, Richalet JP & Jammes Y (2000). Effects of prolonged hypobaric hypoxia on human skeletal muscle function and electromyographic events. Clin Sci 98, 329–337. [PubMed] [Google Scholar]
- Enoka RM & Duchateau J (2016). Translating fatigue to human performance. Med Sci Sports Exerc 48, 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M, Mottola L & Quaresima V (2004). Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29, 463–487. [DOI] [PubMed] [Google Scholar]
- Goodall S, González‐Alonso J, Ali L, Ross EZ & Romer LM (2012). Supraspinal fatigue after normoxic and hypoxic exercise in humans. J Physiol 590, 2767–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Ross EZ & Romer LM (2010). Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee‐extensor contractions. J Appl Physiol 109, 1842–1851. [DOI] [PubMed] [Google Scholar]
- Goodall S, Twomey R & Amann M (2014b). Acute and chronic hypoxia: implications for cerebral function and exercise tolerance. Fatigue 2, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Twomey R, Amann M, Ross EZ, Lovering AT, Romer LM, Subudhi AW & Roach RC (2014a). AltitudeOmics: exercise‐induced supraspinal fatigue is attenuated in healthy humans after acclimatization to high altitude. Acta Physiol 210, 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H, Roy B, Grant S, Burnett M, Tupling R, Otto C, Pipe A & McKenzie D (2000). Downregulation in muscle Na+‐K+‐ATPase following a 21‐day expedition to 6,194 m. J Appl Physiol 88, 634–640. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB & Dempsey JA (1997). Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82, 1573–1583. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R & Schuster J (2009). Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol 120, 2040–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H & Vogt M (2001). Muscle tissue adaptations to hypoxia. J Exp Biol 204, 3133–3139. [DOI] [PubMed] [Google Scholar]
- Jubeau M, Rupp T, Temesi J, Perrey S, Wuyam B, Millet GY & Verges S (2017). Neuromuscular fatigue during prolonged exercise in hypoxia. Med Sci Sports Exerc 49, 430–439. [DOI] [PubMed] [Google Scholar]
- Katayama K, Amann M, Pegelow DF, Jacques AJ & Dempsey JA (2007). Effect of arterial oxygenation on quadriceps fatigability during isolated muscle exercise. Am J Physiol Regul Integr Comp Physiol 292, R1279–R1286. [DOI] [PubMed] [Google Scholar]
- Kayser B, Bökenkamp R & Binzoni T (1993). Alpha‐motoneuron excitability at high altitude. Eur J Appl Physiol 66, 1–4. [DOI] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC & Taylor JL (2006). Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol 95, 3512–3518. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Kennedy DS, Butler JE, Gandevia SC & Taylor JL (2013a). The responsiveness of knee extensor motoneurons to different fatigue tasks in humans. 615.16/F5. 2013 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2013.
- McNeil CJ, Butler JE, Taylor JL & Gandevia SC (2013b). Testing the excitability of human motoneurons. Front Hum Neurosci 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Gandevia SC & Taylor JL (2011b). Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589, 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Khan SI, Gandevia SC & Taylor JL (2011c). The reduction in human motoneurone responsiveness during muscle fatigue is not prevented by increased muscle spindle discharge. J Physiol 589, 3731–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC & Taylor JL (2009). The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587, 5601–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC & Taylor JL (2011a). A novel way to test human motoneurone behaviour during muscle fatigue. Physiol News 82, 29–31. [Google Scholar]
- Millet GY, Muthalib M, Jubeau M, Laursen PB & Nosaka K (2012). Severe hypoxia affects exercise performance independently of afferent feedback and peripheral fatigue. J Appl Physiol 112, 1335–1344. [DOI] [PubMed] [Google Scholar]
- Miscio G, Milano E, Aguilar J, Savia G, Foffani G, Mauro A, Mordillo‐Mateos L, Romero‐Ganuza J & Oliviero A (2009). Functional involvement of central nervous system at high altitude. Exp Brain Res 194, 157–162. [DOI] [PubMed] [Google Scholar]
- Neubauer JA & Sunderram J (2004). Oxygen‐sensing neurons in the central nervous system. J Appl Physiol 96, 367–374. [DOI] [PubMed] [Google Scholar]
- Papadelis C, Kourtidou‐Papadeli C, Bamidis PD, Maglaveras N & Pappas K (2007). The effect of hypobaric hypoxia on multichannel EEG signal complexity. Clin Neurophysiol 118, 31–52. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Dawson EA, Nybo L, van Lieshout JJ, Secher NH & Gjedde A (2007). Capillary‐oxygenation‐level‐dependent near‐infrared spectrometry in frontal lobe of humans. J Cereb Blood Flow Metab 27, 1082–1093. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Nielsen J, Overgaard M, Krogh‐Madsen R, Gjedde A, Secher NH & Petersen NC (2010). Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol 588, 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp T, Jubeau M, Wuyam B, Perrey S, Levy P, Millet GY & Verges S (2012). Time‐dependent effect of acute hypoxia on corticospinal excitability in healthy humans. J Neurophysiol 108, 1270–1277. [DOI] [PubMed] [Google Scholar]
- Rupp T, Racinais S, Bringard A, Lapole T & Perrey S (2014). Modulation of exercise‐induced spinal loop properties in response to oxygen availability. Eur J Appl Physiol 115, 471–482. [DOI] [PubMed] [Google Scholar]
- Subudhi AW, Miramon BR, Granger ME & Roach RC (2009). Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J Appl Physiol 106, 1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szubski C, Burtscher M & Löscher WN (2006). The effects of short‐term hypoxia on motor cortex excitability and neuromuscular activation. J Appl Physiol 101, 1673–1677. [DOI] [PubMed] [Google Scholar]
- Tachi M, Kouzaki M, Kanehisa H & Fukunaga T (2004). The influence of circulatory difference on muscle oxygenation and fatigue during intermittent static dorsiflexion. Eur J Appl Physiol 91, 682–688. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL & Gandevia SC (2003). Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL & Gandevia SC (2004). Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol 97, 236–242. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL & Gandevia SC (2016). Measurement of voluntary activation based on transcranial magnetic stimulation over the motor cortex. J Appl Physiol 121, 678–686. [DOI] [PubMed] [Google Scholar]


